Abstract
Experiencing early life stress can result in maladjusted stress response via dysregulation of the hypothalamic-pituitary-adrenal axis and serves as a risk factor for developing chronic pelvic pain disorders. We investigated whether neonatal maternal separation (NMS) would increase susceptibility to experimental colitis or exposure to acute or chronic stress. Male mice underwent NMS from postnatal day 1–21 and as adults were assessed for open field behavior, hindpaw sensitivity, and visceromotor response (VMR) to colorectal distension (CRD). VMR was also measured before and after treatment with intracolonic trinitrobenzene sulfonic acid (TNBS) or exposure to acute or chronic water avoidance stress (WAS). Myeloperoxidase (MPO) activity, proinflammatory gene and corticotropin-releasing factor (CRF) receptor expression were measured in distal colon. Baseline VMR was not affected by NMS, but undergoing CRD increased anxiety-like behaviors and mechanical hindpaw sensitivity of NMS mice. Treatment with TNBS dose-dependently decreased body weight and survival only in NMS mice. Following TNBS treatment, IL-6 and artemin mRNA levels were decreased in the distal colon of NMS mice, despite increased MPO activity. A single WAS exposure increased VMR during CRD in NMS mice and increased IL-6 mRNA and CRF2 protein levels in the distal colon of naïve mice, whereas CRF2 protein levels were heightened in NMS colon both at baseline and post-WAS exposure. Taken together, these results suggest that NMS in mice disrupts inflammatory- and stress-induced gene expression in the colon, potentially contributing towards an exaggerated response to specific stressors later in life.
Keywords: Colorectal distension, Experimental colitis, Maternal separation, Stress
Abbreviations: NMS, neonatal maternal separation; VMR, visceromotor response; CRD, colorectal distension; TNBS, trinitrobenzene sulfonic acid; WAS, water avoidance stress; MPO, myeloperoxidase; CRF, corticotropin-releasing factor; IL, interleukin; IBS, irritable bowel syndrome; HPA, hypothalamic-pituitary-adrenal; EMG, electromyographic; NGF, nerve growth factor; IFN, interferon; AUC, area under the curve
Highlights
-
•
Neonatal maternal separation (NMS) altered responses to colonic inflammation and stress later in life.
-
•
NMS mice were dose-dependently susceptible to TNBS colitis.
-
•
A single exposure to water avoidance stress increased colorectal sensitivity in NMS, but not naïve, mice.
-
•
Disruptions in signaling pathways within the colon may contribute to increased susceptibility in NMS mice.
Patients suffering from chronic pelvic pain syndromes, including irritable bowel syndrome (IBS), commonly report symptom onset or increased severity during periods of high stress (Mayer et al., 2001, Blanchard et al., 2008). Many IBS patients also have difficulty coping with stressful situations and suffer from depression, anxiety, and/or panic disorder (Fond et al., 2014). Comorbidity between IBS and mood disorders has been associated with altered functioning of the hypothalamic-pituitary-adrenal (HPA) axis (Chang et al., 2009), which regulates the response to stress and influences the perception of pain (Silverman and Sternberg, 2012). A history of adverse events early in life has been shown to permanently influence the functioning of the HPA axis and also increases the likelihood of developing IBS (Barreau et al., 2007, Chitkara et al., 2008, Videlock et al., 2009).
Corticotropin-releasing factor (CRF) is the primary initiator of the HPA axis and acts both centrally and peripherally in response to an acute or chronic stressor (Ulrich-Lai and Herman, 2009, Pierce and Christianson, 2015). Centrally, CRF is expressed in the paraventricular nucleus of the hypothalamus, and initiates a cascade through the HPA axis to induce a systemic release of glucocorticoids. Two CRF receptors, CRF receptor 1 (CRF1) and CRF receptor 2 (CRF2), largely work in opposition of one another to propagate and diminish HPA axis activation, respectively (Plotsky et al., 1993, Bale and Vale, 2004). These two CRF receptors are also found peripherally, specifically within the mucosal layer of the colon, enteric nervous system, and innate inflammatory cells (O'Malley et al., 2010a). When released peripherally, CRF effects changes in neuroimmune function, partially through mast cell activation and degranulation (Black, 2002), resulting in increased cytokine and growth factor expression, sensitization of nerve endings, and endothelial leakage of associated vasculature (Singh et al., 1999, Tomaszewski et al., 2001, Barbara et al., 2004, Barreau et al., 2008).
Neonatal maternal separation (NMS) is a well-established model of early life stress that has been shown to induce colorectal hypersensitivity (Coutinho et al., 2002, Zhang et al., 2009, O'Malley et al., 2010b, Moloney et al., 2012) and increase colonic permeability (Varghese et al., 2006, Ghia et al., 2008, Veenema et al., 2008), largely driven by dysregulation of the HPA axis (O'Mahony et al., 2011a, O'Mahony et al., 2011b). Rodents exposed to NMS are more susceptible to experimental colitis (Varghese et al., 2006) and have demonstrated increased CRF receptor, growth factor, and cytokine mRNA expression in the adult distal colon and, in particular, IL-6 and CRF have been shown to interactively alter colonic secretory activity (Barreau et al., 2004a, O'Malley et al., 2011b, O'Malley et al., 2013). Introduction of an adult stressor, generally in the form of water avoidance stress (WAS), exacerbates the colorectal hypersensitivity of NMS rodents and can be attenuated by central or peripheral administration of CRF receptor antagonists (Schwetz et al., 2005, van den Wijngaard et al., 2012).
We have previously shown that NMS results in perigenital mechanical hypersensitivity in male mice (Fuentes et al., 2015) and vaginal (Pierce et al., 2014) and bladder (Pierce et al., 2016) hypersensitivity in female mice, with varying adult stress-induced changes. Here we are testing the hypothesis that NMS in male mice will increase susceptibility to colorectal hypersensitivity following adult stress exposure or experimental colitis. We have measured the visceromotor response (VMR) during colorectal distension (CRD) at baseline and following exposure to increasing doses of Trinitrobenzene sulfonic acid (TNBS) or acute or chronic exposure to WAS. We also assayed the distal colon for Interleukin (IL)-6 and artemin mRNA levels and CRF receptor protein levels.
1. Experimental procedures
1.1. Animals
Experiments were performed on male C57Bl/6 mice (Charles River, Wilmington, MA; ages listed in Table 1) born and housed in the Research Support Facility at the University of Kansas Medical Center. Mice were housed on a 12-h light cycle from 600 to 1800 h and received water and food (8604; Harlan Teklad, Madison WI) ad libitum. All research performed conformed to the National Institutes of Health Guide for the Care and Use of Laboratory Animals in accordance with the guidelines specified by the University of Kansas Medical Center Animal Care and Use Protocols.
Table 1.
Age of mice at experimental time points.
| Baseline | Insult | Post-insult measurements | |
|---|---|---|---|
| Colorectal distension | |||
| No insult (n = 10) | 13 | ||
| Saline (n = 6) | 8 | 9 | 10 |
| TNBS 2 mg (n = 6) | 10 | 11 | 12 |
| TNBS 5 mg (n = 6) | 8 | 9 | 10 |
| 1d WAS (n = 6) | 10 | 10 | 11 |
| 7d WAS (n = 6) | 10 | 10–11 | 12 |
| Open Field | |||
| CRD (n = 8) | 6 | 13 | 17 |
| Hindpaw Sensitivity | |||
| CRD (n = 8) | 7 | 13 | 19 |
Naïve and NMS mice underwent behavioral or physiological testing at the above noted ages (in weeks) prior to and/or following introduction of an insult, including colorectal distension (CRD), intracolonic trinitrobenzene sulfonic acid (TNBS), or acute (1d) or chronic (7d) exposure to water avoidance stress (WAS). Repeated tests were performed on the same mice for all CRD experiments involving post-insult measurements. Open field and hindpaw sensitivity tests were performed on separate groups of mice either at baseline or following CRD.
1.2. Neonatal maternal separation
Beginning on postnatal day 1 (P1, date of birth was considered P0), pups were removed daily from their home cages for 180 min (generally 1100–1400 h) and placed as a litter, with a small amount of home bedding material, into a clean glass beaker and held at 34 °C and 50% humidity as previously described (Fuentes et al., 2015). NMS mice were weighed immediately prior to and after the NMS period from P1 through P14 or P21 and were weaned at P22. Naïve mice were born in-house and remained undisturbed in their home cages, with the exception of daily weighing and routine animal husbandry, until weaning at P22. Five separate cohorts of NMS mice were used in this study and each cohort was compared to a corresponding naïve group of mice that were born, housed, and weaned during the same time frame to avoid potential complications arising from variations in prenatal shipping conditions, housing environment, and investigator handling.
1.3. Trinitrobenzene sulfonic acid (TNBS) treatment
Naïve and NMS mice were anesthetized with inhaled isoflurane (4% induction, 2% maintenance) and secured on a platform that elevated the pelvic region approximately 5 cm above the working surface. A water-based lubricant (KY Jelly, Johnson & Johnson, New Brunswick, NJ) was liberally applied to the perianal region to avoid sensitization of surrounding somatic tissues. Mice received an intracolonic instillation of TNBS (0.1 ml of 50 mg ml−1 or 20 mg ml−1 in 50% EtOH) or saline (0.1 ml) using an oral feeding needle attached to a 50 μl Hamilton syringe. Mice remained in an elevated position for 5 min to prevent leakage. Mice were then allowed to recover from anesthesia and were returned to their home cages.
1.4. Water avoidance stress
Water avoidance stress (WAS) was performed for 1 h, within the first 6 h of the light cycle, for 1 day or 7 consecutive days. Mice were placed individually on a round platform (5 cm diameter) centrally affixed to the bottom of a container (36 cm length x 31 cm width x 27 cm height) filled with room temperature tap water up to 1 cm below the top of the platform.
1.5. Electromyographic electrode implantation and colorectal distension
The visceromotor response (VMR) to colorectal distension (CRD) was evaluated in naïve and NMS mice. Electrode implantation was performed as previously described (Christianson and Gebhart, 2007). Under inhaled isoflurane (4% induction, 2.5% maintenance) and aseptic conditions, the bare ends of two Teflon-coated stainless steel wires (3 mm; Grass Technologies, West Warwick, RI) were inserted into the right lateral abdominal musculature, secured via 5-0 prolene sutures, tunneled subcutaneously to a small incision made in the nape of the neck, and externalized for access during testing. Skin incisions were closed using 5-0 silk suture. Following recovery from anesthesia, mice were housed singly and allowed to recover for a minimum of 4 days before undergoing testing.
To facilitate balloon insertion and obtain proper restraint during CRD, mice were briefly sedated with inhaled isoflurane (4% induction, 2.5% maintenance) and a custom-made, catheterized polyethylene plastic balloon (1.5 cm length × 0.8 cm diameter) was inserted through the anus until the proximal end of the balloon was 0.5 cm from the anal verge (total balloon insertion, 2 cm) and secured to the base of the tail with tape. The mouse was then placed into a Broome-style rodent restraint (Kent Scientific, Torrington, CT), the free ends of the electrode wires were attached to a differential amplifier (Model 1700, A-M Systems, Sequim, WA), and the mice were allowed to recover from anesthesia for 30 min. The balloon was inflated with air from a compressed nitrogen tank equipped with a dual-stage low delivery pressure regulator (Matheson-Linweld, Kansas City, MO) and a separate pressure monitor (World Precision Instruments, Sarasota, FL) was used to manually regulate the pressure inside of the balloon. Each pressure (15, 30, 45, 60, and 75 mmHg) was applied three times for 20 s with a 4-min rest period in between. A custom-made distension control device (The University of Iowa Medical Instruments, Iowa City, IA) was used to control the gas flow through the system. Electromyographic (EMG) activity was amplified, filtered, and recorded on a personal computer with Spike 2 software (Cambridge Electronic Design, Cambridge, UK) for off-line analysis. The VMR was quantified by measuring the area under the curve for the entire distension period divided by the duration of the distension and expressed as a percent of baseline activity (10s prior to CRD).
1.6. Behavioral analysis
All mice underwent a 30-min acclimatization period within the testing room for at least one day prior to each behavioral test. For both thermal and mechanical hindpaw sensitivity testing, the mice were allowed to acclimate to the apparatus for 30 min prior to testing and the experimenter was blinded to the group status of the mice.
1.6.1. Open field testing
Activity in naïve and NMS mice was measured using a Force Plate Actimeter (BASi, San Diego, CA), which consists of a rigid, low-mass horizontal plate (44 cm × 44 cm) coupled to high sensitivity force transducers on each corner. A Plexiglas enclosure rests a few millimeters above the plate to create a transparent enclosure, all of which rests within a light and sound-attenuated box. Animals were individually placed into the middle of the testing arena and allowed to move freely for 10 min. During this time, the software recorded the distance traveled and position of the mouse. The total distance traveled and percent of time spent in the perimeter (outermost 8.25 cm; increased time spent in the perimeter is indicative of anxiety (Bailey and Crawley, 2009)) was calculated and binned and the data from the second 5 min bin is reported here.
1.6.2. Thermal analgesiometer testing
Naïve and NMS mice were placed in individual clear plastic chambers (11 × 5 × 3.5 cm) on the 30 °C heated glass surface of a thermal analgesiometer (UARDG; Department of Anesthesiology, University of California San Diego, La Jolla, CA). A high intensity light (4.25 A) was directed at the plantar aspect of the hindpaw and the latency to withdrawal from the stimulus was automatically recorded within 0.01 s. Alternating hindpaws were tested for a total of three times per side with a minimum of 5 min between applications. The stimulus terminated automatically at 20 s to avoid tissue damage. Individual responses were averaged and group means were determined as previously described (Christianson et al., 2003).
1.6.3. Von Frey monofilament testing
Naïve and NMS mice were placed into individual clear plastic chambers (11 × 5 × 3.5 cm) on a wire mesh screen elevated 55 cm above a table. The up-down method was performed to test mechanical sensitivity using a standard set of von Frey monofilaments (1.65, 2.36, 2.83, 3.22, 3.61, 4.08, 4.31, 4.74 g; Stoelting, Wood Dale, IL)(Dixon, 1980). Beginning with the 3.22 g monofilament, mice received a single application to the plantar surface of the right hindpaw. A negative response was followed by the next larger filament and a positive response (considered a brisk withdrawal of the paw) was followed by the next smaller filament. The experimenter continued to move up or down the series, depending on the previously elicited response, for an additional four applications after the first positive response was observed for a minimum of five or a maximum of nine total monofilament applications. The value in log units of the final von Frey monofilament applied in the trial series was used to calculate a 50% g threshold for each mouse and group means were determined as previously described (Chaplan et al., 1994).
1.7. Myeloperoxidase assay
Mice were overdosed with inhaled isoflurane (>5%) and the distal 1.5 cm of colon was dissected and the fecal matter, excess blood vessels, and mesentery were removed. The tissue was weighed, added to a beaker containing 1 ml 0.5% hexadecyltrumethylammonium bromide (HTAB; Sigma), and finely minced using spring scissors. The solution was transferred to a 15 ml centrifuge tube, along with another 2 ml HTAB, and sonicated for 10 s before being homogenized for 30 s. Another 2 ml HTAB was added and the tube was placed on dry ice until all samples were similarly processed. The samples underwent three freeze-thaw cycles, were centrifuged twice, and loaded in triplicate, along with MPO standards (Calbiochem, San Diego, CA), onto a 96-well plate. The standards and samples were reacted with O-dianisidine dihydrochloride (Sigma) and read on a plate reader at 460 nm every 20 s for 15 min. The slope for each standard reading was calculated and plotted and the slope of those values was used to calculate the units of MPO activity/tissue weight for each sample.
1.8. mRNA extraction and qRT-PCR
Mice were overdosed with inhaled isoflurane (>5%) and transcardially perfused with ice cold 0.9% saline. The 1.5 cm distal segment of the colon was removed, bisected longitudinally (to facilitate both mRNA and protein [see below] analysis), snap frozen in liquid nitrogen, and stored at −80 °C. Total RNA was isolated using Trizol reagent (Ambion, Austin, TX) and RNeasy Mini Kit (Qiagen, Valencia, CA). The concentration and purity were determined using a 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA) and cDNA was synthesized from total RNA (0.63 μg) using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). Quantitative RT-PCR was performed using SsoAdvanced SYBR Green Supermix (Bio-Rad) and a Bio-Rad iCycler IQ real time PCR system with indicated 20 μM primers (Integrated DNA Technologies, Coralville, IA) for interleukin-6 (IL-6; forward: CTGCCAGAGACTTCCATCCAGTT, reverse: GAAGTAGGGAAGGCCGTGG), artemin (forward: GGCCAACCCTAGCTGTTCT, reverse: TGGGTCCAGGGAAGCTT), with β-actin (forward: AGTGTGACGTTGACATCCGTA, reverse: GCCAGAGCAGTAATCTCCTTCT) used as a control. Samples were run in triplicate and negative control reactions were run with each amplification series. To reduce variability among efficiency due to fluctuations in baseline fluorescence, the raw (i.e. non-baseline corrected) PCR data was imported to the LinRegPCR software (version 2012.3) (Ramakers et al., 2003, Ruijter et al., 2009, Tuomi et al., 2010) and PCR efficiency values were derived for each individual sample by fitting a regression line to a subset of data points within the sample's log-linear phase. Threshold cycle (Ct) values were subtracted from that of the selected housekeeping gene and the percentage of fold change over naïve controls was calculated using the Pfaffl method (Pfaffl, 2001).
1.9. Western blot
Total protein was isolated from approximately 50 mg of snap-frozen colon using Cell Extraction Buffer (Invitrogen, Grand Island, NY) containing Halt protease and phosphatase inhibitors (ThermoFisher Scientific, Waltham, MA) and Na3VO4 (Sigma, St. Louis, MO). Protein concentrations were determined using a DC protein assay (ThermoFisher). Samples were reduced by heating to 95 °C for 5 min in the presence of 2-mercaptoethanol, subjected to SDS-PAGE (Criterion 4%–12% Bis-Tris gels; Bio-Rad, Hercules, CA), and transferred to Nitrocellulose transfer membrane (Whatman GmbH, Dassel, Germany) by Criterion Blotter wet transfer (Bio-Rad). The membranes were blocked for 1 h at room temperature in 5% milk in Tris-buffered saline with Tween-20 (TBST) and incubated overnight at 4 °C with antisera to CRF1 (1:250; Millipore, Billerica, MA), CRF2 (1:1000; Millipore), and GAPDH (1:2000; Cell Signaling Technology, Danvers, MA) diluted in 5% milk in TBST. Membranes were then washed with TBST and incubated for 1 h with anti-rabbit secondary antibody (1:2000; Cell Signaling, Danvers, MA). Densitometry was performed using Quantity One 4.6.9 software (Bio-Rad).
1.10. Statistics
Calculations were made using Microsoft Excel and statistical analysis was performed using Student's t-test or 2-way (with or without repeated measures) analysis of variance (ANOVA) followed by Bonferroni's posttest (GraphPad Prism 6, GraphPad Software, La Jolla, CA), as denoted in the manuscript. All data are expressed as mean ± SEM. A p value of less than 0.05 was considered significant.
2. Results
2.1. NMS increases hindpaw sensitivity, but not baseline colorectal sensitivity or anxiety measures
Male mice underwent NMS or remained unhandled (naïve) for the first 21 days of life and were assessed as adults for susceptibility to colorectal inflammation and acute or chronic exposure to WAS (Table 1). At baseline, naïve and NMS mice were not remarkably different in regards to colorectal sensitivity, measured as the VMR during CRD (Fig. 1A). Open field exploratory behavior and hindpaw mechanical and thermal sensitivity were also measured in naïve and NMS mice at baseline and following CRD. The percent of time spent in the perimeter of the open field was not significantly different between naïve and NMS mice at baseline; however, exposure to CRD significantly increased overall time spent in the perimeter, specifically in NMS mice (Fig. 1B). The withdrawal threshold to application of von Frey monofilaments to the plantar surface of the hindpaw was significantly reduced in NMS mice, particularly post-CRD (Fig. 1C). Thermal withdrawal latency to a radiant heat source on the plantar surface of the hindpaw was significantly shorter in NMS mice at baseline, but not following CRD (Fig. 1D).
Fig. 1.
The visceromotor response (VMR) during colorectal distension (CRD) was measured, as well as open field activity and mechanical and thermal hindpaw sensitivity prior to and following CRD, in male mice that did and did not (naïve) undergo neonatal maternal separation (NMS). A) No difference in VMR, measured as the electromyographic (EMG) activity of the abdominal muscles during CRD, was observed between naïve and NMS mice. B) At baseline, naïve and NMS mice spent a similar percent of time in the perimeter of an open field; however, following exposure to CRD, there was an overall significant increase in the percent of time spent in the perimeter, specifically in NMS mice. C) Mechanical hindpaw withdrawal thresholds were significantly reduced in NMS mice across both measurements, particularly post-CRD. D) Thermal hindpaw withdrawal latencies were significantly shorter in NMS mice at baseline, compared to naïve, but not post-CRD. An overall effect of NMS and an NMS/CRD interaction was observed across both measurements. Brackets indicate a significant effect of NMS (*, **p < 0.05, 0.01), CRD (##p < 0.01), or an NMS/CRD interaction (+p < 0.05) two-way RM ANOVA; *, **p < 0.05, 0.01 vs. naïve, #p < 0.05 vs. BL, Bonferroni posttest.
2.2. NMS increases susceptibility to TNBS
To determine whether NMS increased susceptibility to experimental colitis, two different concentrations of TNBS were applied intracolonically. Application of 2mg/mouse TNBS did not significantly alter body weight, survival, or colorectal sensitivity of naïve or NMS mice, compared to saline-treated counterparts, over the 4 days immediately following treatment (Fig. 2). However, 5mg/mouse TNBS resulted in significant weight loss in NMS mice, compared to both 5 mg TNBS-treated naïve mice and saline-treated NMS mice (Fig. 2B) and also decreased survival rate in NMS mice, compared to 5 mg TNBS-treated naïve mice (16.7% ± 15.2 vs. 100%; p = 0.0006, Log-rank Mantel-Cox test). Neutrophil activation was measured by MPO activity and NMS mice that were separated for only the first 14 days of life and treated with 5 mg TNBS had significantly higher MPO at 4 d post-treatment compared to baseline NMS measurements (Table 2). NMS had a significant impact on colorectal sensitivity in both the saline-treated and 5 mg TNBS-treated groups; however, TNBS treatment showed no significant impact on colorectal sensitivity in either NMS or naïve mice (Fig. 2A).
Fig. 2.
Mice that underwent neonatal maternal separation (NMS) were dose-dependently susceptible to intracolonic administration of trinitrobenzene sulfonic acid (TNBS). Mice were assessed for colorectal sensitivity and weighed to determine baseline (BL) measurements and then treated with saline, 2 mg TNBS, or 5 mg TNBS. Body weight was measured daily and colorectal distension (CRD) was performed on day 4 post-TNBS. A) The total electromyographic (EMG) activity is expressed as the area under the curve (AUC) for the entire CRD pressure series. No treatment effects were observed for saline, 2 mg TNBS, or 5 mg TNBS; however, NMS significantly impacted VMR in the saline-treated and 5 mg TNBS-treated groups. B) NMS mice given 5 mg TNBS lost significantly more body weight over three subsequent days than either naïve mice treated with 5 mg TNBS or saline-treated NMS mice. Brackets indicate a significant effect of NMS (*p < 0.05), two-way RM ANOVA; *, **p < 0.05, 0.01 vs. naïve, #, ##p < 0.05, 0.01 vs. saline, Bonferroni posttest.
Table 2.
Myeloperoxidase activity following Trinitrobenzene sulfonic acid treatment.
| Naïve | NMS‡ | |
|---|---|---|
| Baseline (n = 3) | 0.852 ± 0.20 | 0.606 ± 0.25 |
| 1d post (n = 4) | 6.43 ± 1.64 | 8.06 ± 0.77 |
| 4d post (n = 4) | 4.75 ± 1.81 | 12.12 ± 3.81## |
Myeloperoxidase (MPO) activity (expressed as U mg−1 tissue weight) was measured in distal colon from naïve and NMS mice prior to (baseline) and following intracolonic instillation of 5 mg trinitrobenzene sulfonic acid (TNBS). Treatment with TNBS significantly increased MPO levels in both groups (p = 0.0071, two-way ANOVA). Naïve MPO levels peaked at 1d post-TNBS, while NMS MPO levels continued to increase at 4d post-TNBS. ##p < 0.01 vs. baseline, Bonferroni posttest. ‡ denotes NMS (neonatal maternal separation) mice that were separated from postnatal day 1–14.
2.3. NMS enhances acute WAS-induced colorectal hypersensitivity
Colorectal sensitivity was measured prior to and 1 d after either a single exposure to 1 h of WAS (1d WAS) or 7 consecutive daily exposures to 1 h of WAS (7d WAS) to determine susceptibility to acute or chronic stress, respectively. The VMR during the entire CRD series was significantly higher in NMS mice exposed to 1d WAS compared to their baseline measurements (Fig. 3A). In particular, the VMR at 60 and 75 mmHg was significantly higher in NMS mice after 1d WAS than both their baseline measurements and that of their naïve counterparts (Fig. 3A). Exposure to 1d WAS did not significantly impact the VMR of naïve mice (Fig. 3A). Exposure to 7d WAS did not significantly impact the VMR of either naïve or NMS mice (Fig. 3B).
Fig. 3.
Acute, but not chronic, exposure to water avoidance stress (WAS) selectively increased colorectal sensitivity in NMS mice. A) The electromyographic (EMG) activity of the abdominal musculature was measured to determine the visceromotor response (VMR) to colorectal distension (CRD) both prior to (baseline) and 1 d after a single exposure to WAS (1d WAS) in naïve and NMS mice. Exposure to 1d WAS significantly increased VMR of NMS mice compared to their baseline measurements. This was particularly evident at the highest balloon pressures applied, 60 and 75 mmHg. B) Chronic daily exposure to WAS (7d WAS) had no impact on VMR during CRD in either naïve or NMS mice. Brackets indicate a significant effect of WAS (#p < 0.05), two-way RM ANOVA; **p < 0.01 vs. naïve, #, ###p < 0.05, 0.001 vs. baseline, Bonferroni posttest.
2.4. NMS blunted TNBS and WAS-induced inflammatory gene expression and increased CRF receptor expression in the colon
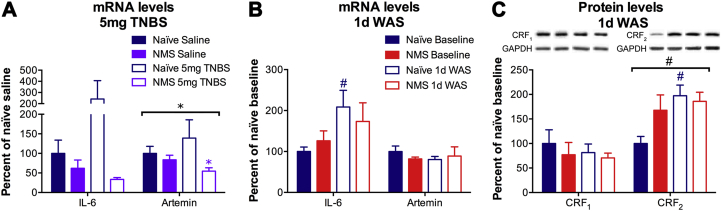
The impact of NMS, TNBS, and WAS on pro-inflammatory gene expression in the distal colon was evaluated by RT-PCR. Treatment with 5 mg TNBS induced a trend toward increased IL-6 and Artemin mRNA levels in the distal colon of naïve mice, which was not present in NMS mice (Fig. 4A). Indeed, artemin mRNA levels were significantly lower in NMS colon compared to naïve, particularly following TNBS treatment (Fig. 4A). Exposure to 1d WAS significantly increased IL-6 mRNA levels in naïve colon and the mRNA levels of IL-6 trended toward a significant increase in NMS mice, (p = 0.0525, two-way ANOVA; Fig. 4B). Exposure to 1d WAS had no impact on artemin mRNA levels in the colon of either naïve or NMS mice (Fig. 4B).
Fig. 4.
Neonatal maternal separation (NMS) alters localized gene and protein expression changes in response to inflammation and acute stress. A) Real-time PCR was performed on mRNA from naïve and NMS distal colon following saline or 5 mg trinitrobenzene sulfonic acid (TNBS) treatment. A non-significant increase in interleukin (IL)-6 mRNA levels was observed in naïve colon following TNBS treatment, with a corresponding decrease in both IL-6 and artemin mRNA levels in NMS colon. B) Exposure to 1d WAS significantly increased IL-6 mRNA levels in naïve colon and NMS effected a trend toward increased IL-6 mRNA levels (p = 0.0525, two-way ANOVA). Artemin mRNA levels were unaffected by either NMS or WAS. C) Representative Western blots are shown for CRF1, CRF2, and corresponding GAPDH protein with bands at 55, 49, and 35kD, respectively. Exposure to 1d WAS had no effect on CRF1 protein levels in either naïve or NMS colon. At baseline, CRF2 protein levels were increased in NMS colon and exposure to 1d WAS significantly increased CRF2 protein levels, particularly in naïve colon. Brackets indicate a significant effect of NMS (*p < 0.05) or WAS (#p < 0.05), two-way RM ANOVA; *p < 0.05 vs. naïve, #p < 0.05 vs. baseline, Bonferroni posttest.
To determine the impact of 1d WAS on peripheral CRF receptor levels, Western blot was performed to measure CRF1 and CRF2 protein levels in the distal colon. Protein levels of CRF1 in the distal colon were not affected by NMS or 1d WAS (Fig. 4C). In contrast, 1d WAS exposure significantly increased CRF2 protein levels overall, specifically in naïve colon post-1d WAS compared to baseline levels (Fig. 4C). CRF2 protein levels were increased, though not significantly, in NMS colon both at baseline and post-1d WAS compared to baseline naïve levels (Fig. 4C).
3. Discussion
Exposure to early life stress or trauma is a significant risk factor for developing several chronic pain syndromes, including IBS (Barreau et al., 2007, Chitkara et al., 2008, Videlock et al., 2009). NMS has long been used in rodents to model both psychological disturbances and heightened colorectal sensitivity commonly experienced by IBS patients. Here, we performed a prolonged NMS paradigm in male mice and provided evidence of increased susceptibility of the distal colon to experimental colitis using TNBS and acute stress exposure, with accompanying alterations in pro-inflammatory gene/CRF receptor expression.
Rodent models of NMS (Faure et al., 2007, Veenema et al., 2007, Aisa et al., 2008, Kawano et al., 2008) and stress-sensitive strains of rodents (Overstreet, 2012) have been shown to display behaviors indicative of increased anxiety and depression. We previously reported a significant decrease in baseline anxiety-like behavior and thermal and mechanical thresholds in the hindpaw of female NMS mice at baseline (Pierce et al., 2014). In the current study, male NMS mice displayed no significant change in anxiety-like behaviors, yet did have significantly shorter thermal withdrawal latencies at baseline. Further exposure to stress, in the form of CRD, significantly increased anxiety-like behaviors, similar to the increase observed in female NMS mice following vaginal balloon distension (Pierce et al., 2014). Interestingly, mechanical withdrawal thresholds were significantly lower in NMS than naïve mice, post-CRD; however, thermal withdrawal latencies were no longer significantly shorter in NMS mice. Exposure to VBD maintained decreased hindpaw thresholds in our previous study of female NMS mice (Pierce et al., 2014) and sex differences in behavioral outcomes following NMS have been previously reported (Rosztoczy et al., 2003, Desbonnet et al., 2008) and likely contribute to the more robust response in the female NMS mice, compared to the male.
Increased colorectal sensitivity is a commonly reported outcome of NMS and other manipulations of the stress-response system. High-anxiety strains of rats demonstrate significantly greater colorectal sensitivity than non-anxious strains (Gunter et al., 2000, Greenwood-Van Meerveld et al., 2005, O'Mahony et al., 2011a, O'Mahony et al., 2011b)). Several studies of NMS in rats have reported increased colorectal sensitivity measured as either increased VMR (Coutinho et al., 2002, Tjong et al., 2010, Chen et al., 2015) or visualized abdominal withdrawal reflexes (Rosztoczy et al., 2003, Chung et al., 2007, Ren et al., 2007, Gosselin et al., 2010) during CRD. In the current study, as well as in our previous study of female NMS mice (Pierce et al., 2016), we did not observe any evidence of colorectal sensitivity during CRD in NMS mice at baseline. This is in contrast to the significant perigenital mechanical sensitivity exhibited by male NMS mice (Fuentes et al., 2015) and vaginal (Pierce et al., 2014) and bladder (Pierce et al., 2016) hypersensitivity exhibited by female NMS mice, suggesting that the NMS paradigm employed in our studies selectively sensitizes the urogenital system at baseline. To our knowledge, only one previous study has investigated colorectal sensitivity in NMS mice and reported a significant increase in VMR during CRD (Moloney et al., 2012). This study used BALB/c mice, which have been shown to be a higher anxiety strain than C57Bl/6 mice (Belzung and Griebel, 2001), and incorporated NMS from P1-14 with concurrent maternal stress (forced swimming or restraint) during the separation period.
Evidence of increased susceptibility to experimental colitis was observed in NMS mice, despite the lack of increased colorectal sensitivity four days after treatment with either 2 or 5 mg TNBS. Previous studies of NMS in mice reported increased MPO and cytokine production, as well as elevated histological scores (Varghese et al., 2006, Ghia et al., 2008, Veenema et al., 2008). Here, we demonstrated a greater loss of body weight and decreased survival in NMS mice following 5 mg TNBS. Colonic MPO activity was continuing to increase at 4d post-TNBS in NMS mice, while MPO activity had decreased from its peak at 1d post-TNBS in naïve mice, as has been shown previously (Malin et al., 2011), suggesting an exaggerated inflammatory response in NMS mice. Cytokine and growth factor production have also been shown to simultaneously increase in rodent models of colitis (Barada et al., 2007) and ulcerative colitis patients have an increased production of pro-inflammatory cytokines (Johansson et al., 2008), as well as greater expression of neurotrophin receptors in peripheral nerve endings innervating the diseased colon (Johansson et al., 2007). Naïve mice treated with 5 mg TNBS showed a trend toward increased colonic IL-6 mRNA levels, which was not apparent in NMS colon. Two growth factors, nerve growth factor (NGF) and artemin, have been shown to dramatically increase following acute colitis in rodents (Constantinou et al., 1994, Amaya et al., 2004, Malin et al., 2006). Here we observed a slight, but not significant, increase in artemin mRNA levels in naïve mice following 5 mg TNBS and NMS colon actually showed a significant decrease in artemin mRNA levels compared to naïve colon. Colonic NGF mRNA was not significantly altered by 5 mg TNBS in either naïve or NMS mice (data not shown).
Symptom onset or exacerbation is often triggered by stress in patients suffering from IBS (Fond et al., 2014). Clinically, peripheral administration of CRF has been shown to reduce the threshold to CRD and increase rectal compliance in human subjects (Lembo et al., 1996). Likewise, administration of CRF1 antagonist produced inhibitory effects within the emotional-arousal circuit of female IBS patients that were anticipating a painful stimulus (Hubbard et al., 2011). A single exposure to WAS has previously been shown to increase VMR in NMS rats, but not in naïve rats (Coutinho et al., 2002, van den Wijngaard et al., 2009, van den Wijngaard et al., 2012), whereas repeated exposure to WAS has been shown to significantly increase colorectal sensitivity in non-NMS rats (Bradesi et al., 2005, Hong et al., 2011). In the current study, colorectal sensitivity in NMS mice was only increased by a single exposure to WAS and not following chronic exposure. The VMR of naïve mice was completely unaffected by either WAS treatment. Considering the paucity of studies investigating the impact of NMS or WAS on colorectal sensitivity in mice, it is possible that these stressors do not have the same impact across species. This theory is supported by our previous studies showing an impact of NMS on urogenital sensitivity in mice (Pierce et al., 2014, Pierce et al., 2016, Fuentes et al., 2015, Pierce et al., 2016), which has largely not been observed in rat models of NMS.
Rodents exposed to NMS demonstrate increased growth factor and cytokine expression, including NGF, IL-6, IL-1β, IL-2, IL-4, IL-10, and interferon (IFN)-γ (Barreau et al., 2004a, Barreau et al., 2004b, O'Malley et al., 2011a, O'Malley et al., 2011b), as well as infiltration of mast cells (van den Wijngaard et al., 2009, van den Wijngaard et al., 2012, Lennon et al., 2013), in the distal colon, all of which can sensitize peripheral nociceptors and enhance visceral perception (Barreau et al., 2004a, Barreau et al., 2004b, Daniels et al., 2009, O'Malley et al., 2011a, O'Malley et al., 2011b). Mast cell infiltration and hypertrophy of sensory innervation has also been reported in biopsies from patients with IBS (Barbara et al., 2004, Barbara et al., 2007, Akbar et al., 2008). A single exposure to WAS elicited a significant increase in IL-6 mRNA levels in naïve colon, confirming that, despite the lack of physiological evidence, 1d WAS exposure did indeed significantly impact naïve colon. The mRNA levels of IL-6 in NMS mice trended toward a significant increase; however, the blunted effect of WAS on increasing IL-6 expression in NMS colon further suggests that neuroimmune responses to stress exposure are imbalanced following NMS exposure in mice.
A potential role for CRF in mediating comorbidity between psychological and chronic pain disorders has been investigated in both clinical and preclinical settings. Overactivity of central CRF1 signaling has been proposed to contribute towards comorbid anxiety/depression in female diarrhea-predominant IBS patients (Tache et al., 2005). Associations between anxiety and voiding disorders have also been reported (Klausner and Steers, 2004) and interstitial cystitis (IC) patients with fibromyalgia, chronic fatigue syndrome, or rheumatoid arthritis had higher mean afternoon cortisol levels and increased pain during bladder filling than IC patients with no additional diagnoses (Lutgendorf et al., 2002). In a study using repeated WAS exposure to induce colorectal sensitivity in non-NMS rats, treatment with a CRF1 antagonist treatment prior to, but not following, WAS prevented an increase in VMR (Larauche et al., 2008). However, treatment with astressin, a non-selective CRF1/CRF2 antagonist, only reduced the effect of WAS, suggesting that activation of CRF2 mediates colonic hypoalgesia. This observation is supported by studies showing that activation of CRF2 decreases CRF1-driven colorectal hypersensitivity (Million et al., 2005, Million et al., 2006), cFos induction in the colonic myenteric ganglia, and increased colonic motility (Gourcerol et al., 2011). In the current study, protein levels of CRF2 were increased in NMS colon at baseline, similar to previous studies investigating mRNA levels in both male NMS rats (O'Malley et al., 2010a) and female NMS mice (Pierce et al., 2014). The protein levels of CRF2 were also increased in naïve colon following a single exposure to WAS. Urocortin signaling through CRF2 has been shown to increase IL-6 expression/release in cardiomyocytes and aortic smooth muscle cells (Kageyama et al., 2006, Huang et al., 2009), and could be playing a similar mechanistic role here. The baseline increase in CRF2 protein in NMS colon may have primed the response to WAS, thereby increasing colorectal sensitivity, compared to naïve mice. Further investigation will be required to understand how the CRF receptors mediate colorectal sensitivity in mice and how stress at both early and late stages of life will impact their expression and activation patterns.
4. Conclusions
We have tested the hypothesis that early life stress in male mice increases susceptibility to experimental colitis and adult stress exposure. Mice exposed to NMS show increased thermal hindpaw sensitivity at baseline with an increase in anxiety-like behaviors and mechanical hindpaw sensitivity following exposure to CRD. Intracolonic instillation of TNBS induces a dose-dependent loss in body weight and decreased survival rate in NMS mice. A single exposure to WAS, but not a 7 day repeated exposure, increased the VMR during CRD of NMS mice, and revealed evidence of improper neuroimmune response within the colon. Taken together, these results suggest that NMS in mice disrupts inflammatory- and stress-induced gene expression in the colon, potentially contributing towards an exaggerated response to specific stressors later in life.
Funding
This work was supported by NIH grants R03 DK088011 (JAC), T32 HD057850 (IMF), Center of Biomedical Research Excellence (COBRE) grant P20 GM104936 (JAC), start-up funds, student fellowship, and core support from the Kansas Institutional Development Award (IDeA) P20 GM103418 (JAC and NKW), and The Madison and Lila Self Fellowship Program (ANP).
Conflicts of interest
The authors have no competing interests.
Author contribution
IMF and NKW contributed equally to this study; JAC designed the research study; IMF, NKW, ANP, BRH, and ERD performed the experiments; IMF, NKW, ANP, and JAC analyzed the data; IMF and JAC wrote the paper.
Acknowledgments
We would like to thank Bilal Hassan, Ruipeng Wang, and Janelle Ryals for technical assistance.
References
- Aisa B., Tordera R., Lasheras B., Del Rio J., Ramirez M.J. Effects of maternal separation on hypothalamic-pituitary-adrenal responses, cognition and vulnerability to stress in adult female rats. Neuroscience. 2008;154:1218–1226. doi: 10.1016/j.neuroscience.2008.05.011. [DOI] [PubMed] [Google Scholar]
- Akbar A., Yiangou Y., Facer P., Walters J.R., Anand P., Ghosh S. Increased capsaicin receptor TRPV1-expressing sensory fibres in irritable bowel syndrome and their correlation with abdominal pain. Gut. 2008;57:923–929. doi: 10.1136/gut.2007.138982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaya F., Shimosato G., Nagano M., Ueda M., Hashimoto S., Tanaka Y., Suzuki H., Tanaka M. NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur. J. Neurosci. 2004;20:2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x. [DOI] [PubMed] [Google Scholar]
- Bailey K.R., Crawley J.N. Anxiety-related behaviors in mice. In: Buccafusco J.J., editor. Methods of Behavior Analysis in Neuroscience. 2009. Boca Raton (FL) [Google Scholar]
- Bale T.L., Vale W.W. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu. Rev. Pharmacol. Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- Barada K.A., Mourad F.H., Sawah S.I., Khoury C., Safieh-Garabedian B., Nassar C.F., Tawil A., Jurjus A., Saade N.E. Up-regulation of nerve growth factor and interleukin-10 in inflamed and non-inflamed intestinal segments in rats with experimental colitis. Cytokine. 2007;37:236–245. doi: 10.1016/j.cyto.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Barbara G., Stanghellini V., De Giorgio R., Cremon C., Cottrell G.S., Santini D., Pasquinelli G., Morselli-Labate A.M., Grady E.F., Bunnett N.W., Collins S.M., Corinaldesi R. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126:693–702. doi: 10.1053/j.gastro.2003.11.055. [DOI] [PubMed] [Google Scholar]
- Barbara G., Wang B., Stanghellini V., de Giorgio R., Cremon C., Di Nardo G., Trevisani M., Campi B., Geppetti P., Tonini M., Bunnett N.W., Grundy D., Corinaldesi R. Mast cell-dependent excitation of visceral-nociceptive sensory neurons in irritable bowel syndrome. Gastroenterology. 2007;132:26–37. doi: 10.1053/j.gastro.2006.11.039. [DOI] [PubMed] [Google Scholar]
- Barreau F., Cartier C., Ferrier L., Fioramonti J., Bueno L. Nerve growth factor mediates alterations of colonic sensitivity and mucosal barrier induced by neonatal stress in rats. Gastroenterology. 2004;127:524–534. doi: 10.1053/j.gastro.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Barreau F., Ferrier L., Fioramonti J., Bueno L. Neonatal maternal deprivation triggers long term alterations in colonic epithelial barrier and mucosal immunity in rats. Gut. 2004;53:501–506. doi: 10.1136/gut.2003.024174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barreau F., Ferrier L., Fioramonti J., Bueno L. New insights in the etiology and pathophysiology of irritable bowel syndrome: contribution of neonatal stress models. Pediatr. Res. 2007;62:240–245. doi: 10.1203/PDR.0b013e3180db2949. [DOI] [PubMed] [Google Scholar]
- Barreau F., Salvador-Cartier C., Houdeau E., Bueno L., Fioramonti J. Long-term alterations of colonic nerve-mast cell interactions induced by neonatal maternal deprivation in rats. Gut. 2008;57:582–590. doi: 10.1136/gut.2007.126680. [DOI] [PubMed] [Google Scholar]
- Belzung C., Griebel G. Measuring normal and pathological anxiety-like behaviour in mice: a review. Behav. Brain Res. 2001;125:141–149. doi: 10.1016/s0166-4328(01)00291-1. [DOI] [PubMed] [Google Scholar]
- Black P.H. Stress and the inflammatory response: a review of neurogenic inflammation. Brain, Behav. Immun. 2002;16:622–653. doi: 10.1016/s0889-1591(02)00021-1. [DOI] [PubMed] [Google Scholar]
- Blanchard E.B., Lackner J.M., Jaccard J., Rowell D., Carosella A.M., Powell C., Sanders K., Krasner S., Kuhn E. The role of stress in symptom exacerbation among IBS patients. J. Psychosom. Res. 2008;64:119–128. doi: 10.1016/j.jpsychores.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Bradesi S., Schwetz I., Ennes H.S., Lamy C.M., Ohning G., Fanselow M., Pothoulakis C., McRoberts J.A., Mayer E.A. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G42–G53. doi: 10.1152/ajpgi.00500.2004. [DOI] [PubMed] [Google Scholar]
- Chang L., Sundaresh S., Elliott J., Anton P.A., Baldi P., Licudine A., Mayer M., Vuong T., Hirano M., Naliboff B.D., Ameen V.Z., Mayer E.A. Dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis in irritable bowel syndrome. Neurogastroenterol. Motil. 2009;21:149–159. doi: 10.1111/j.1365-2982.2008.01171.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplan S.R., Bach F.W., Pogrel J.W., Chung J.M., Yaksh T.L. Quantitative assessment of tactile allodynia in the rat paw. J. Neurosci. Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen A., Bao C., Tang Y., Luo X., Guo L., Liu B., Lin C. Involvement of protein kinase zeta in the maintenance of hippocampal long-term potentiation in rats with chronic visceral hypersensitivity. J. Neurophysiol. 2015;113:3047–3055. doi: 10.1152/jn.00929.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chitkara D.K., van Tilburg M.A., Blois-Martin N., Whitehead W.E. Early life risk factors that contribute to irritable bowel syndrome in adults: a systematic review. Am. J. Gastroenterol. 2008;103:765–774. doi: 10.1111/j.1572-0241.2007.01722.x. quiz 775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christianson J.A., Gebhart G.F. Assessment of colon sensitivity by luminal distension in mice. Nat. Protoc. 2007;2:2624–2631. doi: 10.1038/nprot.2007.392. [DOI] [PubMed] [Google Scholar]
- Christianson J.A., Ryals J.M., McCarson K.E., Wright D.E. Beneficial actions of neurotrophin treatment on diabetes-induced hypoalgesia in mice. J. Pain. 2003;4:493–504. doi: 10.1016/j.jpain.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Chung E.K., Zhang X.J., Xu H.X., Sung J.J., Bian Z.X. Visceral hyperalgesia induced by neonatal maternal separation is associated with nerve growth factor-mediated central neuronal plasticity in rat spinal cord. Neuroscience. 2007;149:685–695. doi: 10.1016/j.neuroscience.2007.07.055. [DOI] [PubMed] [Google Scholar]
- Constantinou J., Reynolds M.L., Woolf C.J., Safieh-Garabedian B., Fitzgerald M. Nerve growth factor levels in developing rat skin: upregulation following skin wounding. Neuroreport. 1994;5:2281–2284. doi: 10.1097/00001756-199411000-00019. [DOI] [PubMed] [Google Scholar]
- Coutinho S.V., Plotsky P.M., Sablad M., Miller J.C., Zhou H., Bayati A.I., McRoberts J.A., Mayer E.A. Neonatal maternal separation alters stress-induced responses to viscerosomatic nociceptive stimuli in rat. Am. J. Physiol. Gastrointest. Liver Physiol. 2002;282:G307–G316. doi: 10.1152/ajpgi.00240.2001. [DOI] [PubMed] [Google Scholar]
- Daniels W.M., Fairbairn L.R., van Tilburg G., McEvoy C.R., Zigmond M.J., Russell V.A., Stein D.J. Maternal separation alters nerve growth factor and corticosterone levels but not the DNA methylation status of the exon 1(7) glucocorticoid receptor promoter region. Metab. Brain Dis. 2009;24:615–627. doi: 10.1007/s11011-009-9163-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L., Garrett L., Daly E., McDermott K.W., Dinan T.G. Sexually dimorphic effects of maternal separation stress on corticotrophin-releasing factor and vasopressin systems in the adult rat brain. Int. J. Dev. Neurosci. Off. J. Int. Soc. Dev. Neurosci. 2008;26:259–268. doi: 10.1016/j.ijdevneu.2008.02.004. [DOI] [PubMed] [Google Scholar]
- Dixon W.J. Efficient analysis of experimental observations. Annu. Rev. Pharmacol. Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Faure J., Uys J.D., Marais L., Stein D.J., Daniels W.M. Early maternal separation alters the response to traumatization: resulting in increased levels of hippocampal neurotrophic factors. Metab. Brain Dis. 2007;22:183–195. doi: 10.1007/s11011-007-9048-3. [DOI] [PubMed] [Google Scholar]
- Fond G., Loundou A., Hamdani N., Boukouaci W., Dargel A., Oliveira J., Roger M., Tamouza R., Leboyer M., Boyer L. Anxiety and depression comorbidities in irritable bowel syndrome (IBS): a systematic review and meta-analysis. Eur. Arch. Psychiatry Clin. Neurosci. 2014;264:651–660. doi: 10.1007/s00406-014-0502-z. [DOI] [PubMed] [Google Scholar]
- Fuentes I.M., Pierce A.N., O'Neil P.T., Christianson J.A. Assessment of perigenital sensitivity and prostatic mast cell activation in a mouse model of neonatal maternal separation. J. Vis. Exp. JoVE. 2015;(102):e53181. doi: 10.3791/53181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghia J.E., Blennerhassett P., Collins S.M. Impaired parasympathetic function increases susceptibility to inflammatory bowel disease in a mouse model of depression. J. Clin. Investig. 2008;118:2209–2218. doi: 10.1172/JCI32849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosselin R.D., O'Connor R.M., Tramullas M., Julio-Pieper M., Dinan T.G., Cryan J.F. Riluzole normalizes early-life stress-induced visceral hypersensitivity in rats: role of spinal glutamate reuptake mechanisms. Gastroenterology. 2010;138:2418–2425. doi: 10.1053/j.gastro.2010.03.003. [DOI] [PubMed] [Google Scholar]
- Gourcerol G., Wu S.V., Yuan P.Q., Pham H., Miampamba M., Larauche M., Sanders P., Amano T., Mulak A., Im E., Pothoulakis C., Rivier J., Tache Y., Million M. Activation of corticotropin-releasing factor receptor 2 mediates the colonic motor coping response to acute stress in rodents. Gastroenterology. 2011;140:1586–1596. doi: 10.1053/j.gastro.2011.01.039. e1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood-Van Meerveld B., Johnson A.C., Cochrane S., Schulkin J., Myers D.A. Corticotropin-releasing factor 1 receptor-mediated mechanisms inhibit colonic hypersensitivity in rats. Neurogastroenterol. Motil. 2005;17:415–422. doi: 10.1111/j.1365-2982.2005.00648.x. [DOI] [PubMed] [Google Scholar]
- Gunter W.D., Shepard J.D., Foreman R.D., Myers D.A., Greenwood-Van Meerveld B. Evidence for visceral hypersensitivity in high-anxiety rats. Physiol. Behav. 2000;69:379–382. doi: 10.1016/s0031-9384(99)00254-1. [DOI] [PubMed] [Google Scholar]
- Hong S., Zheng G., Wu X., Snider N.T., Owyang C., Wiley J.W. Corticosterone mediates reciprocal changes in CB 1 and TRPV1 receptors in primary sensory neurons in the chronically stressed rat. Gastroenterology. 2011;140:627–637. doi: 10.1053/j.gastro.2010.11.003. e624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M., Kempuraj D., Papadopoulou N., Kourelis T., Donelan J., Manola A., Theoharides T.C. Urocortin induces interleukin-6 release from rat cardiomyocytes through p38 MAP kinase, ERK and NF-kappaB activation. J. Mol. Endocrinol. 2009;42:397–405. doi: 10.1677/JME-08-0120. [DOI] [PubMed] [Google Scholar]
- Hubbard C.S., Labus J.S., Bueller J., Stains J., Suyenobu B., Dukes G.E., Kelleher D.L., Tillisch K., Naliboff B.D., Mayer E.A. Corticotropin-releasing factor receptor 1 antagonist alters regional activation and effective connectivity in an emotional-arousal circuit during expectation of abdominal pain. J. Neurosci. 2011;31:12491–12500. doi: 10.1523/JNEUROSCI.1860-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson M., Jonsson M., Norrgard O., Forsgren S. New aspects concerning ulcerative colitis and colonic carcinoma: analysis of levels of neuropeptides, neurotrophins, and TNFalpha/TNF receptor in plasma and mucosa in parallel with histological evaluation of the intestine. Inflamm. Bowel Dis. 2008;14:1331–1340. doi: 10.1002/ibd.20487. [DOI] [PubMed] [Google Scholar]
- Johansson M., Norrgard O., Forsgren S. Study of expression patterns and levels of neurotrophins and neurotrophin receptors in ulcerative colitis. Inflamm. Bowel Dis. 2007;13:398–409. doi: 10.1002/ibd.20072. [DOI] [PubMed] [Google Scholar]
- Kageyama K., Hanada K., Nigawara T., Moriyama T., Terui K., Sakihara S., Suda T. Urocortin induces interleukin-6 gene expression via cyclooxygenase-2 activity in aortic smooth muscle cells. Endocrinology. 2006;147:4454–4462. doi: 10.1210/en.2006-0008. [DOI] [PubMed] [Google Scholar]
- Kawano K., Morinobu S., Sawada T., Tsuji S., Erabi K., Fuchikami M., Kozuru T., Yamawaki S., Hisaoka K., Takebayashi M. Prior neonatal isolation reduces induction of NGF mRNA and decreases GDNF mRNA in the hippocampus of juvenile and adult rodents subjected to immobilization stress. Synapse. 2008;62:259–267. doi: 10.1002/syn.20487. New York, NY. [DOI] [PubMed] [Google Scholar]
- Klausner A.P., Steers W.D. Corticotropin releasing factor: a mediator of emotional influences on bladder function. J. Urol. 2004;172:2570–2573. doi: 10.1097/01.ju.0000144142.26242.f3. [DOI] [PubMed] [Google Scholar]
- Larauche M., Bradesi S., Million M., McLean P., Tache Y., Mayer E.A., McRoberts J.A. Corticotropin-releasing factor type 1 receptors mediate the visceral hyperalgesia induced by repeated psychological stress in rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;294:G1033–G1040. doi: 10.1152/ajpgi.00507.2007. [DOI] [PubMed] [Google Scholar]
- Lembo T., Plourde V., Shui Z., Fullerton S., Mertz H., Tache Y., Sytnik B., Munakata J., Mayer E. Effects of the corticotropin-releasing factor (CRF) on rectal afferent nerves in humans. Neurogastroenterol. Motil. 1996;8:9–18. doi: 10.1111/j.1365-2982.1996.tb00237.x. [DOI] [PubMed] [Google Scholar]
- Lennon E.M., Maharshak N., Elloumi H., Borst L., Plevy S.E., Moeser A.J. Early life stress triggers persistent colonic barrier dysfunction and exacerbates colitis in adult IL-10-/- mice. Inflamm. Bowel Dis. 2013;19:712–719. doi: 10.1097/MIB.0b013e3182802a4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutgendorf S.K., Kreder K.J., Rothrock N.E., Hoffman A., Kirschbaum C., Sternberg E.M., Zimmerman M.B., Ratliff T.L. Diurnal cortisol variations and symptoms in patients with interstitial cystitis. J. Urol. 2002;167:1338–1343. [PubMed] [Google Scholar]
- Malin S., Molliver D., Christianson J.A., Schwartz E.S., Cornuet P., Albers K.M., Davis B.M. TRPV1 and TRPA1 function and modulation are target tissue dependent. J. Neurosci. 2011;31:10516–10528. doi: 10.1523/JNEUROSCI.2992-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malin S.A., Molliver D.C., Koerber H.R., Cornuet P., Frye R., Albers K.M., Davis B.M. Glial cell line-derived neurotrophic factor family members sensitize nociceptors in vitro and produce thermal hyperalgesia in vivo. J. Neurosci. 2006;26:8588–8599. doi: 10.1523/JNEUROSCI.1726-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer E.A., Naliboff B.D., Chang L., Coutinho S.V. V. Stress and irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2001;280:G519–G524. doi: 10.1152/ajpgi.2001.280.4.G519. [DOI] [PubMed] [Google Scholar]
- Million M., Maillot C., Adelson D.A., Nozu T., Gauthier A., Rivier J., Chrousos G.P., Bayati A., Mattsson H., Tache Y. Peripheral injection of sauvagine prevents repeated colorectal distension-induced visceral pain in female rats. Peptides. 2005;26:1188–1195. doi: 10.1016/j.peptides.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Million M., Wang L., Wang Y., Adelson D.W., Yuan P.Q., Maillot C., Coutinho S.V., McRoberts J.A., Bayati A., Mattsson H., Wu V., Wei J.Y., Rivier J., Vale W., Mayer E.A., Tache Y. CRF2 receptor activation prevents colorectal distension induced visceral pain and spinal ERK1/2 phosphorylation in rats. Gut. 2006;55:172–181. doi: 10.1136/gut.2004.051391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney R.D., O'Leary O.F., Felice D., Bettler B., Dinan T.G., Cryan J.F. Early-life stress induces visceral hypersensitivity in mice. Neurosci. Lett. 2012;512:99–102. doi: 10.1016/j.neulet.2012.01.066. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Coelho A.M., Fitzgerald P., Lee K., Winchester W., Dinan T.G., Cryan J.F. The effects of gabapentin in two animal models of co-morbid anxiety and visceral hypersensitivity. Eur. J. Pharmacol. 2011;667:169–174. doi: 10.1016/j.ejphar.2011.05.055. [DOI] [PubMed] [Google Scholar]
- O'Mahony S.M., Hyland N.P., Dinan T.G., Cryan J.F. Maternal separation as a model of brain-gut axis dysfunction. Psychopharmacology. 2011;214:71–88. doi: 10.1007/s00213-010-2010-9. [DOI] [PubMed] [Google Scholar]
- O'Malley D., Cryan J.F., Dinan T.G. Crosstalk between interleukin-6 and corticotropin-releasing factor modulate submucosal plexus activity and colonic secretion. Brain Behav. Immun. 2013;30:115–124. doi: 10.1016/j.bbi.2013.01.078. [DOI] [PubMed] [Google Scholar]
- O'Malley D., Dinan T.G., Cryan J.F. Alterations in colonic corticotropin-releasing factor receptors in the maternally separated rat model of irritable bowel syndrome: differential effects of acute psychological and physical stressors. Peptides. 2010;31:662–670. doi: 10.1016/j.peptides.2010.01.004. [DOI] [PubMed] [Google Scholar]
- O'Malley D., Dinan T.G., Cryan J.F. Altered expression and secretion of colonic interleukin-6 in a stress-sensitive animal model of brain-gut axis dysfunction. J. Neuroimmunol. 2011;235:48–55. doi: 10.1016/j.jneuroim.2011.04.003. [DOI] [PubMed] [Google Scholar]
- O'Malley D., Julio-Pieper M., Gibney S.M., Dinan T.G., Cryan J.F. Distinct alterations in colonic morphology and physiology in two rat models of enhanced stress-induced anxiety and depression-like behaviour. Stress. 2010;13:114–122. doi: 10.3109/10253890903067418. (Amsterdam, Netherlands) [DOI] [PubMed] [Google Scholar]
- O'Malley D., Liston M., Hyland N.P., Dinan T.G., Cryan J.F. Colonic soluble mediators from the maternal separation model of irritable bowel syndrome activate submucosal neurons via an interleukin-6-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 2011;300:G241–G252. doi: 10.1152/ajpgi.00385.2010. [DOI] [PubMed] [Google Scholar]
- Overstreet D.H. Modeling depression in animal models. Methods Mol. Biol. 2012;829:125–144. doi: 10.1007/978-1-61779-458-2_7. [DOI] [PubMed] [Google Scholar]
- Pfaffl M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A.N., Christianson J.A. Stress and chronic pelvic pain. Prog. Mol. Biol. Transl. Sci. 2015;131:509–535. doi: 10.1016/bs.pmbts.2014.11.009. [DOI] [PubMed] [Google Scholar]
- Pierce A.N., Di Silvestro E.R., Eller O.K., Wang R., Ryals J.M., Christianson J.A. Urinary bladder hypersensitivity and dysfunction in female mice following early life and adult stress. Brain Res. 2016;1639:58–73. doi: 10.1016/j.brainres.2016.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce A.N., Ryals J.M., Wang R., Christianson J.A. Vaginal hypersensitivity and hypothalamic-pituitary-adrenal axis dysfunction as a result of neonatal maternal separation in female mice. Neuroscience. 2014;263:216–230. doi: 10.1016/j.neuroscience.2014.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky P.M., Thrivikraman K.V., Meaney M.J. Central and feedback regulation of hypothalamic corticotropin-releasing factor secretion. Ciba Found. Symp. 1993;172:59–75. doi: 10.1002/9780470514368.ch4. discussion 75–84. [DOI] [PubMed] [Google Scholar]
- Ramakers C., Ruijter J.M., Deprez R.H., Moorman A.F. Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci. Lett. 2003;339:62–66. doi: 10.1016/s0304-3940(02)01423-4. [DOI] [PubMed] [Google Scholar]
- Ren T.H., Wu J., Yew D., Ziea E., Lao L., Leung W.K., Berman B., Hu P.J., Sung J.J. Effects of neonatal maternal separation on neurochemical and sensory response to colonic distension in a rat model of irritable bowel syndrome. Am. J. Physiol. Gastrointest. Liver Physiol. 2007;292:G849–G856. doi: 10.1152/ajpgi.00400.2006. [DOI] [PubMed] [Google Scholar]
- Rosztoczy A., Fioramonti J., Jarmay K., Barreau F., Wittmann T., Bueno L. Influence of sex and experimental protocol on the effect of maternal deprivation on rectal sensitivity to distension in the adult rat. Neurogastroenterol. Motil. 2003;15:679–686. doi: 10.1046/j.1350-1925.2003.00451.x. [DOI] [PubMed] [Google Scholar]
- Ruijter J.M., Ramakers C., Hoogaars W.M., Karlen Y., Bakker O., van den Hoff M.J., Moorman A.F. Amplification efficiency: linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwetz I., McRoberts J.A., Coutinho S.V., Bradesi S., Gale G., Fanselow M., Million M., Ohning G., Tache Y., Plotsky P.M., Mayer E.A. Corticotropin-releasing factor receptor 1 mediates acute and delayed stress-induced visceral hyperalgesia in maternally separated Long-Evans rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2005;289:G704–G712. doi: 10.1152/ajpgi.00498.2004. [DOI] [PubMed] [Google Scholar]
- Silverman M.N., Sternberg E.M. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann. N. Y. Acad. Sci. 2012;1261:55–63. doi: 10.1111/j.1749-6632.2012.06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh L.K., Boucher W., Pang X., Letourneau R., Seretakis D., Green M., Theoharides T.C. Potent mast cell degranulation and vascular permeability triggered by urocortin through activation of corticotropin-releasing hormone receptors. J. Pharmacol. Exp. Ther. 1999;288:1349–1356. [PubMed] [Google Scholar]
- Tache Y., Million M., Nelson A.G., Lamy C., Wang L. Role of corticotropin-releasing factor pathways in stress-related alterations of colonic motor function and viscerosensibility in female rodents. Gend. Med. 2005;2:146–154. doi: 10.1016/s1550-8579(05)80043-9. [DOI] [PubMed] [Google Scholar]
- Tjong Y.W., Ip S.P., Lao L., Wu J., Fong H.H., Sung J.J., Berman B., Che C.T. Neonatal maternal separation elevates thalamic corticotrophin releasing factor type 1 receptor expression response to colonic distension in rat. Neuro Endocrinol. Lett. 2010;31:215–220. [PubMed] [Google Scholar]
- Tomaszewski J.E., Landis J.R., Russack V., Williams T.M., Wang L.P., Hardy C., Brensinger C., Matthews Y.L., Abele S.T., Kusek J.W., Nyberg L.M. Biopsy features are associated with primary symptoms in interstitial cystitis: results from the interstitial cystitis database study. Urology. 2001;57:67–81. doi: 10.1016/s0090-4295(01)01166-9. [DOI] [PubMed] [Google Scholar]
- Tuomi J.M., Voorbraak F., Jones D.L., Ruijter J.M. Bias in the Cq value observed with hydrolysis probe based quantitative PCR can be corrected with the estimated PCR efficiency value. Methods. 2010;50:313–322. doi: 10.1016/j.ymeth.2010.02.003. San Diego, Calif. [DOI] [PubMed] [Google Scholar]
- Ulrich-Lai Y.M., Herman J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Wijngaard R.M., Klooker T.K., Welting O., Stanisor O.I., Wouters M.M., van der Coelen D., Bulmer D.C., Peeters P.J., Aerssens J., de Hoogt R., Lee K., de Jonge W.J., Boeckxstaens G.E. Essential role for TRPV1 in stress-induced (mast cell-dependent) colonic hypersensitivity in maternally separated rats. Neurogastroenterol. Motil. 2009;21:1107–e1194. doi: 10.1111/j.1365-2982.2009.01339.x. [DOI] [PubMed] [Google Scholar]
- van den Wijngaard R.M., Stanisor O.I., van Diest S.A., Welting O., Wouters M.M., de Jonge W.J., Boeckxstaens G.E. Peripheral alpha-helical CRF (9-41) does not reverse stress-induced mast cell dependent visceral hypersensitivity in maternally separated rats. Neurogastroenterol. Motil. 2012;24:274–282. doi: 10.1111/j.1365-2982.2011.01840.x. e111. [DOI] [PubMed] [Google Scholar]
- Varghese A.K., Verdu E.F., Bercik P., Khan W.I., Blennerhassett P.A., Szechtman H., Collins S.M. Antidepressants attenuate increased susceptibility to colitis in a murine model of depression. Gastroenterology. 2006;130:1743–1753. doi: 10.1053/j.gastro.2006.02.007. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Bredewold R., Neumann I.D. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology. 2007;32:437–450. doi: 10.1016/j.psyneuen.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Veenema A.H., Reber S.O., Selch S., Obermeier F., Neumann I.D. Early life stress enhances the vulnerability to chronic psychosocial stress and experimental colitis in adult mice. Endocrinology. 2008;149:2727–2736. doi: 10.1210/en.2007-1469. [DOI] [PubMed] [Google Scholar]
- Videlock E.J., Adeyemo M., Licudine A., Hirano M., Ohning G., Mayer M., Mayer E.A., Chang L. Childhood trauma is associated with hypothalamic-pituitary-adrenal axis responsiveness in irritable bowel syndrome. Gastroenterology. 2009;137:1954–1962. doi: 10.1053/j.gastro.2009.08.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.J., Li Z., Chung E.K., Zhang H.Q., Xu H.X., Sung J.J., Bian Z.X. Activation of extracellular signal-regulated protein kinase is associated with colorectal distension-induced spinal and supraspinal neuronal response and neonatal maternal separation-induced visceral hyperalgesia in rats. J. Mol. Neurosci. 2009;37:274–287. doi: 10.1007/s12031-008-9134-y. [DOI] [PubMed] [Google Scholar]