Abstract
Freezing of gait (FoG) in people with Parkinson’s disease (PD) is an environmentally sensitive, intermittent problem that occurs most often during turning. FoG is difficult for clinicians to evaluate and treat because it can be difficult to elicit during a clinical visit. Here, we aimedto develop a clinically valid objective measure of freezing severity during a 2-minute 360 degrees turning-in-place.
Twenty-eight subjects with PD (16 freezers, FoG+, and 12 nonfreezers, FoG−) in the “off” state and 14 healthy control subjects were tested. Subjects wore 3 inertial sensors (one on each shin and one on the waist) while 1) turning in place for 2 minutes (alternating 360 degrees to the right with 360 degrees to the left) and 2) performing an Instrumented 7m Timed Up and Go test (ITUG). Performance was videotaped, and clinical severity of FoG was independently rated by two movement disorders specialists (co-authors).
Turning in place consistently resulted in FoG (13 out of 16 subjects with PD) while FoG was clinically observed in only 2 subjects with PD during the ITUG test. The Freezing Ratio during the turning test was significantly correlated with the clinical ratings (ρ=0.7, p=0.003) and with score on the new freezing of gait questionnaire (ρ=0.5, p=0.03). After correcting for symptom severity (UPDRS-III), out of the 4 objective measures of the turning test (total number of turns, average turn peak speed and average turn smoothness), only the Freezing Ratio was significantly different across groups (p=0.04).
Freezing can be well quantified with body-worn inertial sensors during a 2-minute turning-in-place protocol.
Keywords: Freezing of Gait, Inertial measurement unit, turning in place
INTRODUCTION
Freezing of gait (FoG) is one of the major causes of mobility impairment in Parkinson’s disease (PD) (Moore et al., 2007, Morris et al., 2008). It is one of the major reasons for falls and loss of freedom of movement, leading to increased admission to nursing homes, increased social isolation, and decreased quality of life (Perez-Lloret et al., 2014, Delval et al., 2015, Nonnekes et al., 2015, Walton et al., 2015). Over half of patients with PD develop this intermittent failure to initiate or maintain walking (Hely et al., 2008).
Typically, FoG episodes are brief (1 s or less) and are associated with a subjective feeling of “the feet being glued to the floor” (Bloem et al., 2004, Nutt et al., 2011). Although patients may report that they have FoG, it might not be observed. (Snijders et al., 2008, Nutt et al., 2011, Heremans et al., 2013) This might be because FoG is triggered in specific conditions, such as multi-tasking in challenging environments that do not take place at a clinical appointment. Without observing FoG, clinicians can’t be confident about what type of gait problem they are treating. Furthermore, assessment of FoG in the clinic may offer little indication about frequency of FoG away from the stimulating clinic environment.
The “gold standard” of FoG assessment is clinical observation to determine the conditions eliciting FoG and the severity of FoG. However even clinical observation is still subjective, and inter-rater reliability is variable, ranging from low to good (Morris et al., 2012).
To date, several studies have objectively characterized FoG in a research laboratory, using either instruments available in traditional gait laboratories, such as motion analysis (Delval et al., 2010b), foot switches (Hausdorff et al., 2003, Plotnik et al., 2005a), force plates (Nantel et al., 2011), sEMG (Nieuwboer et al., 2004), or more recently with inertial sensors (Moore et al., 2007, Moore et al., 2008, Bachlin et al., 2010, Moore et al., 2011, Zach et al., 2015). The majority of these studies validated objective FoG measures, such as number and duration of episodes, with the gold standard, experienced clinical judgment. However, as described in a recent review, despite objective measures having high sensitivity and specificity in discriminating subjects with and without FoG, the gold standard still remains the clinical expert opinion (Delval et al., 2015).
A major drawback of some objective approaches to quantifying FoG is that they depend upon laboratory motion analysis that is not practical for clinical trials or clinical practice. Therefore, the emerging studies utilizing inertial sensors to capture the FoG hallmark “trembling of knees”, with a frequency analysis approach, are particularly promising because they could be used easily in the clinic. This promising approach has demonstrated a sensitivity of 75–83% and specificity of >95% in identifying single freezing episodes compared to clinical observations (Hausdorff et al., 2003, Schaafsma et al., 2003b, Moore et al., 2008, Delval et al., 2010a). Moore et al and Hausdorff et al pioneered the use of leg movement frequency to quantify trembling of the legs during FoG with wearable sensors (foot switches or accelerometers) during simple walking or Timed-Up and Go (TUG) (Hausdorff et al., 2003, Moore et al., 2008) to identify the occurrence (start and end) of freezing episodes and measure their duration. In a recent short paper, we modified that approach to quantify overall freezing severity rather than identify the occurrence and duration of single freezing episodes. Indeed we calculated a Frequency Ratio for the whole duration of a 7m TUG test finding that this objective measure was significantly correlated with self-perceived severity of FoG and with gait and balance confidence (Mancini et al., 2012).
Along with different instruments used to objectively characterize FoG, a variety of motor tasks have been proposed to evoke FoG in the laboratory. Among the most promising ones, tasks requiring bilateral limb coordination, such as stepping in place (Nantel et al., 2011), walking and turning on the TUG test (Mancini et al., 2012, Moore et al., 2013), and turning in place (Spildooren et al., 2010, Snijders et al., 2012, Zach et al., 2015) are the most reliable methods to elicit FoG in the laboratory. Although the recent trend in objective measures of FoG is to assess the percentage of time frozen and the number of FoG episodes (Delval et al., 2015), it is also necessary to find an overall objective measures of freezing severity that is related to clinical severity, with the long term goal of deploying such a measure over long periods of time to investigate how much of a problem FoG poses in the home environment. In addition, a recent consensus review of FoG shows a most pressing need to characterize FoG with quantitative, instrumented measures both to understand the pathophysiology and to facilitate clinical trials (Nutt et al., 2011). In the current study, we scored the presence of FoG with a clinical rating scale and with wearable technology quantify the frequency of leg movements during a 7m TUG and two-minute, turning-in-place protocol. We hypothesized that objective measures of freezing severity would correlate with the clinical ratings.
EXPERIMENTAL PROCEDURES
Participants
Twenty-eight subjects with PD and 14 healthy control subjects of similar age (mean age 66±7.3 years) were recruited through the Parkinson’s Center of Oregon clinic at Oregon Health & Science University. Individuals were excluded if they could not safely walk 20 feet without walking aids, or if they had any musculoskeletal or vestibular disorder, dementia, severe tremor, or metal in their bodies (another aspect of this study included neuroimaging). Of the 28 PD patients, 16 (age 67±5.3 years) were classified as FoG+ based on a score of >3 on the New Freezing of Gait Questionnaire (NFOGQ). Twelve patients scoring ≤3 were classified as FoG− (age 65±6.8 years). All PD patients were tested in the “OFF” state, after 12–18 hour overnight withdrawal from anti-parkinsonian medications.
All gave informed, written consent to a protocol approved by the Institutional Review Board of Oregon Health and Science University.
Protocol
After explaining the study and obtaining consent, the researchers administered the motor section of the UPDRS to quantify disease severity (Fahn and Elton, 1987) and the NFOGQ (Nieuwboer et al., 2009) to assess the clinical presence of freezing from the patient’s experience. Subjects then completed two trials of the instrumented mobility protocol, the 7m Instrumented TUG (Mancini et al., 2012) and turning in place, while wearing wireless, synchronized inertial sensors (Opals by APDM, Inc) on both shanks and on the posterior trunk (around L2). For the turning in place test, subjects stood and turned in place alternating 360° turns to their right, then 360° to their left, repeating this sequence at a self-selected pace for 2 minutes. The inertial sensors recorded 3-D linear accelerations and 3-D angular velocities at 128 Hz.
Data were stored for offline analysis with Matlab.
Clinical observation assessment
Turning trials were also video recorded; two internationally recognized movement disorders specialist (JGN and NG) subsequently reviewed the videos (blinded to group allocation) and rated freezing severity on a 0 to 4 scale. Freezing was rated as: − 0, Absent (no freezing episodes), − 1, Mild (hesitation or episodic slowing), − 2, Moderate (at least one arrest), − 3, Severe (multiple arrests), − 4, Unable (required assistance). Clinical severity of FoG was scored separately for the ITUG and turning trials.
Objective measure of the clinical phenomenon
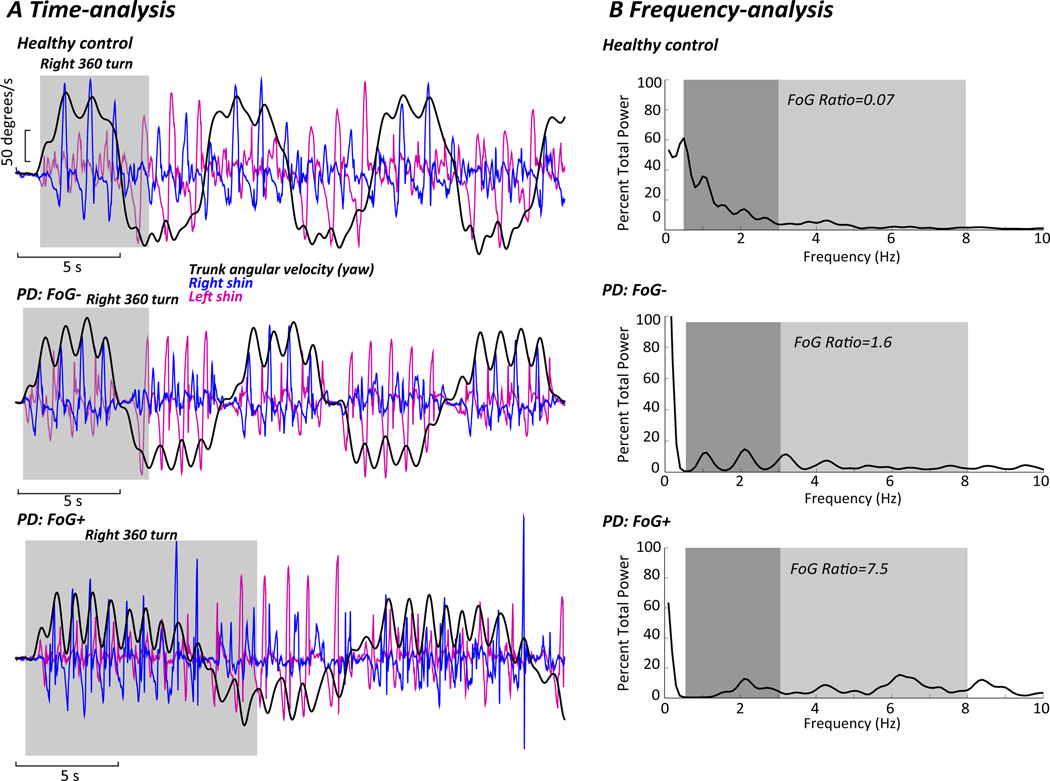
We based our approach to developing a “Freezing Ratio” on a study by Moore et al.(Moore et al., 2008), and on our preliminary findings(Mancini et al., 2012). We examined the 3-D accelerations and angular velocities of the shins and found the clearest freezing ratios came from the power spectral density (PSD) of antero-posterior, and not vertical, shin accelerations, unlike Moore (Moore et al., 2008). The PSD was calculated for each trial using a 4-s Hanning window with 50% overlap (using the Welch method). The total power was then normalized to the area under the PSD for each subject. A Freezing Ratio was calculated as the square of the total power in the 3–8 Hz band, divided by the square of the total power in the 0.5–3 Hz band. Figure 1 shows time-series (A) of trunk and shank angular velocity during the turning task, as well as the frequency analysis (B) with PSDs and the Freezing Ratio calculated for 3 representative subjects performing the continuous turning-in-place task. We calculated the Freezing Ratio for both the 7m ITUG and for the turning in place tests. In addition, for the turning in place test, we computed from the yaw angular velocity of the posterior trunk sensor: 1) the total numbers of turns in 2 minutes, 2) the average peak speed of the turns completed; and from the mediolateral acceleration of the posterior trunk sensor 3) the average jerkiness of the turns, quantifying fluidity of turning.
Figure 1.
A) Time series of trunk (black line) and shins (blue and pink lines) angular velocities during the turning task. The grey shaded area identifies the first 360-degree turn. Representative examples in a healthy control subject, PD subject without freezing (FoG−), and PD subject with freezing (FoG+). B) Frequency analysis of the shin anteroposterior acceleration PSDs in a healthy control subject, PD subject without freezing (FoG−), and PD subject with freezing (FoG+) during the turning in place task. Dark shading indicates the locomotor band; light shading indicates the freezing band. Freezing ratios are provided.
Statistical Analysis
Inter-rater reliability of the clinical FoG rating was assessed using the Intraclass Correlation Coefficient (ICC(1,1)).The Shapiro-Wilk normality test was used to check the data distribution; for the variables that were not normally distributed (Average jerkiness, Freezing Ratio), a natural logarithm transform was used. One-way Analysis of Variance (ANOVA) was then used to determine whether differences in the objective measures of turning in place and 7m TUG existed among the three groups (healthy controls, FoG−, and FoG+). In addition, we analyzed the data with disease severity (UPDRS-III) as a covariate for the analysis (ANCOVA).
Non-parametric (Spearman) correlations were performed to investigate the associations between objective measures of turning in place and clinical FoG severity. Statistical analyses were performed using SPSS (V.22).
RESULTS
Turning, but not ITUG, consistently resulted in FoG
The ICC between the two raters for scoring FoG during the turning task was 0.97 (95% CI: 0.94– 0.98). During the 360-degree turns, clinicians identified freezing in 13 of 16 FoG+ subjects; none of the FoG− subjects froze. The 3 FoG+ subjects who did not show FoG had a similar NFOGQ score and UPDRS III compared to the rest of subjects who showed freezing during the test. However, two out of three had a PIGD subscore of 1, the lowest among FoG+ subjects.
In contrast, clinicians identified freezing in only 2 FoG+ subjects during the ITUG; none of the FoG− subjects froze. The ICC between the two raters for the ITUG test was 0.98 (95% CI: 0.96– 0.99). The 2 FoG+ subjects who did show freezing during the TUG test had similar NFOGQ score and UPDRS III compared to the rest of subjects who did not show freezing during the ITUG test. However, their PIGD subscores were among the highest (6 and 8).
Since only 2 out of 16 FoG+ subjects froze in the ITUG test, we concentrate the following analysis only on the turning test.
The Freezing Ratio was related to clinically observed and self-reported FoG
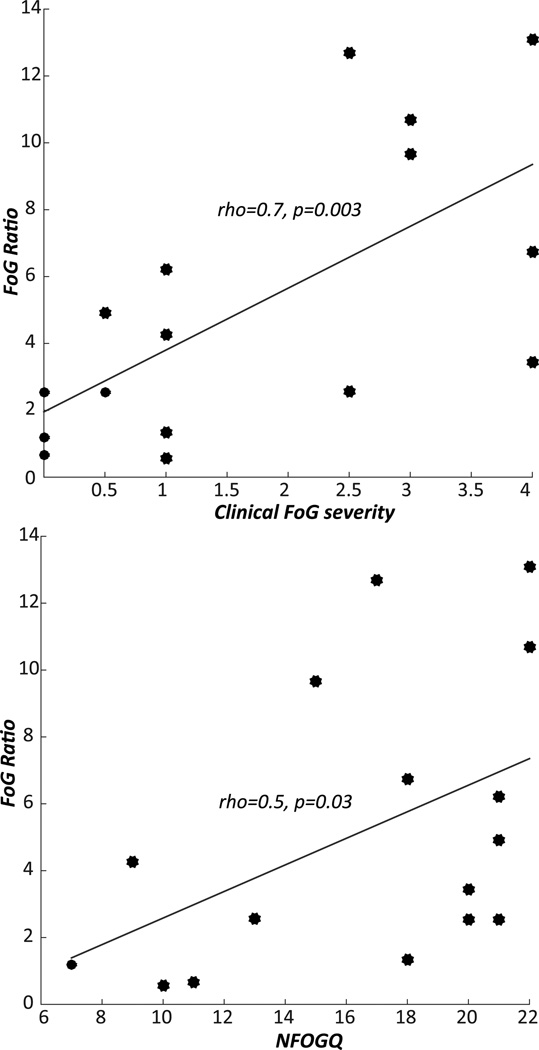
The Freezing Ratio during the turning test was significantly correlated with clinical ratings (ρ=0.7, p=0.003) and with scores on the NFOGQ (ρ=0.5, p=0.03), as shown in Figure 2.
Figure 2.
Associations between the Freezing Ratio during turning and the clinical FoG severity (left), FoG questionnaire (right).
Freezing Ratio was significantly larger in FoG+ than in FoG
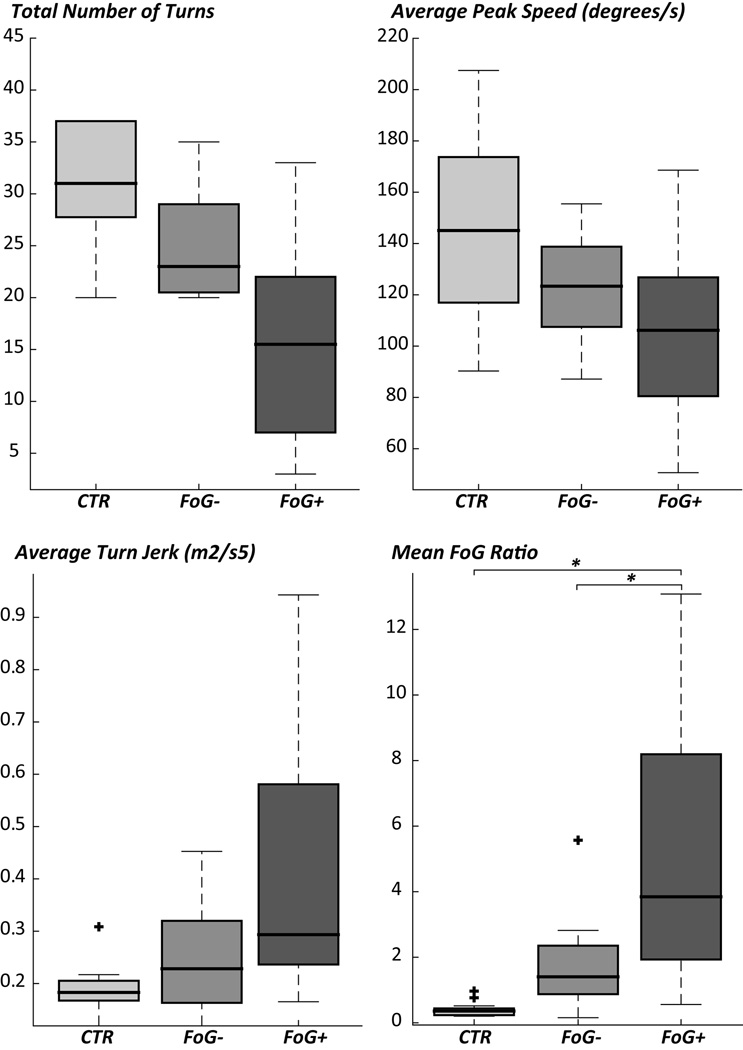
Disease severity and PIGD, as measured by the UPDRS-III, were significantly worse in PD FoG+ compared to PD FoG− and controls, as shown in Table I. Therefore, in Table I we report turning performance measures and statistics from both the ANOVA and the ANCOVA correcting for disease severity. After correcting for symptom severity (UPDRS-III), out of the 4 objective measures of the turning test, only the Freezing Ratio was significantly different across groups (Figure 3).
Table I.
Subject characteristics and objective measures of freezing.
| Mean | STD | Mean | STD | Mean | STD | F-value | p-value | F-value | p-value | |
|---|---|---|---|---|---|---|---|---|---|---|
| UPDRS III | 5.9 | 3.9 | 29.2 | 6.9 | 36.9 | 9.0 | 75.0 | <0.0001 | - | - |
| PIGD | 0.4 | 0.6 | 1.8 | 1.2 | 3.5 | 1.9 | 19.0 | <0.0001 | - | - |
| # of turns | 28.79 | 9.94 | 25.08 | 5.63 | 15.38 | 8.54 | 10.00 | <0.0001 | 2.70 | 0.08 |
| Average peak speed | 136.02 | 51.46 | 124.23 | 20.91 | 106.08 | 30.85 | 2.50 | 0.09 | 0.30 | 0.70 |
| Average jerkiness | 0.17 | 0.07 | 0.24 | 0.11 | 0.41 | 0.23 | 4.90 | 0.01 | 1.70 | 0.20 |
| Freezing Ratio Turn | 0.38 | 0.24 | 1.75 | 1.45 | 5.19 | 4.24 | 25.00 | <0.0001 | 3.50 | 0.04 |
| Freezing Ratio TUG | 0.19 | 0.14 | 0.39 | 0.48 | 0.75 | 1.06 | 2.3 | 0.1 | 0.60 | 0.5 |
ANCOVA tests are corrected for UPDRS-III
Figure 3.
Objective measures of turning in controls (CTR), nonfreezers (FoG−), and freezers (FoG+). Boxes indicate the interquartile range, middle lines the median, whiskers the min-max value, outliers are plotted with +. * p<0.01.
DISCUSSION
The aim of this study was to validate an objective measure of FoG with clinical ratings of FoG severity, during a turning in place task that frequently provokes FoG in the clinic.
The results demonstrate that 1) turning in place provoked freezing more than a 7m ITUG task; 2) both the ratings from expert movement disorders specialists and patients’ own judgment of their freezing severity were associated with an objective measure of FoG severity, the, Freezing Ratio, a ratio of high “trembling” frequencies (3–8 Hz) divided by gait frequencies (.5–3 Hz) during the turning task; and 3) the Freezing Ratio was significantly larger in self-identified FoG+ compared to FoG− subjects during the turning test, while other objective measures of turning, such as turning speed, were not significantly different between groups.
Consistent with recent findings (Moore et al., 2008, Snijders et al., 2012, Zach et al., 2015), asking patients to repeatedly make 360-degrees turns is an efficient protocol to elicit FoG. Turning is often impaired in PD even in the early, untreated phase of the disease (Zampieri et al., 2010). In fact, subjects with PD often show longer turn durations and a greater number of steps to complete a turn, even when straight ahead walking speed is normal (review (Earhart, 2013)). These turning impairments might be associated with a compromised ability to change motor patterns from straight walking to turning (Earhart, 2013). Interestingly, we only observed a slight increase in the Freezing Ratio and clinical ratings in FoG+ compared to FoG− during the ITUG test, indicating that 180-degree turns in the ITUG are not sufficient to elicit FoG. Continuously turning for 2 minutes with direction reversals every 360 degrees may induce FoG because it imposes temporal and spatial asymmetry of steps (step length decreases on the inner side of the turn more than on the outer side) (Plotnik et al., 2008, Snijders et al., 2012). In line with this, Plotnik et al (Plotnik et al., 2005b, Plotnik et al., 2008), associated FoG pathophysiology to asymmetric gait performance and reduced bilateral motor coordination. Generalized problems with coordination of rhythmic movement may reflect abnormalities in gait pattern generation (Nutt et al., 2011). This would suggest that freezing is a disruption of spinal cord pattern generators rather than disruption of frontal cortex including motor cortex and supplementary motor area (SMA).
However, we did not observe FoG during the straight-ahead gait of the ITUG, and turning is a more complex task compared to straight-ahead gait also requiring tight coupling between anticipatory postural adjustments to unload the stepping leg and the locomotor pattern, mediated by the SMA. A recent study showed a reduced medial and increased anterior position of the center of mass during turning prior to a FoG episode (Bengevoord et al., 2016). The results are consistent with previous findings showing a high association between weight shifting difficulties in the mediolateral direction and freezing severity during a repetitive stepping in place task (Nantel et al., 2011), and an associations between the occurrence of multiple anticipatory postural adjustments and freezing when subjects were asked to respond to a backward platform perturbation (Jacobs et al., 2009) or during gait initiation (Delval et al., 2014). These findings favor an abnormal coupling of posture with gait as the basis of freezing, in which the breakdown in coupling posture by the SMA, and step initiation by the motor cortex might occur in the pontomedullary reticular formation (Drew and Rossignol, 1984, Nutt et al., 2011).
A growing body of literature is using a neuroimaging approach to investigate whether functional and structural locomotor network differences are associated with freezing, see (Lewis and Shine, 2016, Snijders et al., 2016) for comprehensive reviews. Our group previously showed a pattern of altered structural and functional connectivity in FoG+ compared to FoG− and healthy controls, in a smaller set of the subjects that participated in the present study (Fling et al., 2013, Fling et al., 2014). Specifically, FoG+ exhibited higher functional connectivity between the SMA and the mesencephalic (MLR) and cerebellar (CLR) locomotor regions compared to FoG− and healthy controls. In addition, FoG+ exhibited a reduction in functional connectivity between the right subthalamic nucleus (STN) and SMA compared to FoG−. Such findings might explain the abnormal coupling of posture with gait as inability to inhibit competing motor programs and to initiate movement in FoG+. However, as recently summarized by (Lewis and Shine, 2016) freezing is a paroxysmal phenomenon unlikely to be due to a focused specific region of the brain. In the current study, we focused on the clinical severity of freezing episodes scored by two movement disorders specialists rather than counting the number or duration of the freezing episodes. The association we found between the objective Freezing Ratio and clinical scores during turning may be very useful for assessing the impact of pharmacological or rehabilitation treatments on freezing (the latter is a currently ongoing project).
When correcting for disease duration, the only objective measure differentiating between FoG+ and FoG− was the Freezing Ratio. The other objectively quantified turning characteristics, including number of turns, average peak speed, and average jerkiness, may deteriorate with disease progression rather than being specifically related to freezing, and therefore may be a good marker of disease progression. In keeping with our preliminary data (Mancini et al., 2012), we found the clearest difference in freezing ratio from the antero-posterior acceleration on the shins rather than from the vertical shank acceleration as reported by Moore (Schaafsma et al., 2003a, Moore et al., 2008). Such differences between studies might be attributable to a less severely impaired cohort in our study, or to the fact that festination (rapid, small steps while falling forward) is generally not present during turning in place. A limitation of this study is that we did not compare the Freezing Ratio derived from sensors on various locations on the body (shin versus lumbar segment), as has been previously reported (Bachlin et al., 2010, Morris et al., 2012). We based our approach on using the sensors on the shins to quantify the “trembling of knees” and the sensor on the lumbar segment to characterize turning characteristics (number of turns, peak velocity and fluidity). Future studies could determine if one accelerometer on the more affected leg could be enough to obtain a clinically valuable measure. In addition, the Freezing Ratio needs to be examined in the home situation where freezing may be occurring without 360-degree turns and with other situations such as passing through doorways.
In summary, we showed that a two-minute turning-in-place task elicits freezing and that our objectively measured Freezing Ratio correlates with the “gold standard” clinical assessment of the severity of freezing. The Freezing Ratio based on body-worn movement monitors during turning is a practical way to objectively evaluate freezing severity in clinical practice, where FoG is often very difficult to elicit.
Highlights.
The validity of an objective measure of freezing during turning in place and TUG is investigated.
Turning in place consistently elicited freezing, but not the TUG test.
Only the Freezing Ratio during the turning in place test discriminated PD with and without freeing of gait.
The objective Freezing Ratio was significantly correlated to the clinical judgment of freezing severity.
Acknowledgments
This work has been supported by grants from National Institute of Health via Career Development Award K99 HD078492 0IAI (PI, Mancini), Morris K. Udall Center for Excellence in Parkinson's Disease Research at the University of Washington, Portland Veterans Affairs Medical Center (VA Merit Award: E1075-R, Horak PI), R01 AG006457, (PI, Horak).
The authors are grateful to the participants in this study for their time, Mari Nomura for study management, and Michael Fleming for data collection.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
OHSU and Dr. Horak have a significant financial interest in APDM, a company that may have a commercial interest in the results of this research and technology. This potential institutional and individual conflict has been reviewed and managed by OHSU.
References
- Bachlin M, Plotnik M, Roggen D, Maidan I, Hausdorff JM, Giladi N, Troster G. Wearable assistant for Parkinson's disease patients with the freezing of gait symptom. IEEE Trans Inf Technol Biomed. 2010;14:436–446. doi: 10.1109/TITB.2009.2036165. [DOI] [PubMed] [Google Scholar]
- Bengevoord A, Vervoort G, Spildooren J, Heremans E, Vandenberghe W, Bloem BR, Nieuwboer A. Center of mass trajectories during turning in patients with Parkinson's disease with and without freezing of gait. Gait Posture. 2016;43:54–59. doi: 10.1016/j.gaitpost.2015.10.021. [DOI] [PubMed] [Google Scholar]
- Bloem BR, Hausdorff JM, Visser JE, Giladi N. Falls and freezing of gait in Parkinson's disease: a review of two interconnected, episodic phenomena. Mov Disord. 2004;19:871–884. doi: 10.1002/mds.20115. [DOI] [PubMed] [Google Scholar]
- Delval A, Moreau C, Bleuse S, Tard C, Ryckewaert G, Devos D, Defebvre L. Auditory cueing of gait initiation in Parkinson's disease patients with freezing of gait. Clin Neurophysiol. 2014;125:1675–1681. doi: 10.1016/j.clinph.2013.12.101. [DOI] [PubMed] [Google Scholar]
- Delval A, Snijders AH, Weerdesteyn V, Duysens JE, Defebvre L, Giladi N, Bloem BR. Objective detection of subtle freezing of gait episodes in Parkinson's disease. Mov Disord. 2010a;25:1684–1693. doi: 10.1002/mds.23159. [DOI] [PubMed] [Google Scholar]
- Delval A, Snijders AH, Weerdesteyn V, Duysens JE, Defebvre L, Giladi N, Bloem BR. Objective detection of subtle freezing of gait episodes in Parkinson's disease. Mov Disord. 2010b;25:1684–1693. doi: 10.1002/mds.23159. [DOI] [PubMed] [Google Scholar]
- Delval A, Tard C, Rambour M, Defebvre L, Moreau C. Characterization and quantification of freezing of gait in Parkinson's disease: Can detection algorithms replace clinical expert opinion? Clin Neurophysiol. 2015;45:305–313. doi: 10.1016/j.neucli.2015.09.009. [DOI] [PubMed] [Google Scholar]
- Drew T, Rossignol S. Phase-dependent responses evoked in limb muscles by stimulation of medullary reticular formation during locomotion in thalamic cats. J Neurophysiol. 1984;52:653–675. doi: 10.1152/jn.1984.52.4.653. [DOI] [PubMed] [Google Scholar]
- Earhart GM. Dynamic control of posture across locomotor tasks. Mov Disord. 2013;28:1501–1508. doi: 10.1002/mds.25592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahn S, Elton R. The UPDRS Development Committee. Unified Parkinson's disease rating scale. In: Fahn S, et al., editors. Recent Developments in Parkinson's Disease. Florham Park, New Jersey: Macmillan Healthcare Information; 1987. pp. 153–163. [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Carpenter SD, Fair DA, Nutt JG, Horak FB. Functional reorganization of the locomotor network in Parkinson patients with freezing of gait. PLoS One. 2014(9):e100291. doi: 10.1371/journal.pone.0100291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fling BW, Cohen RG, Mancini M, Nutt JG, Fair DA, Horak FB. Asymmetric pedunculopontine network connectivity in parkinsonian patients with freezing of gait. Brain. 2013;136:2405–2418. doi: 10.1093/brain/awt172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausdorff JM, Balash Y, Giladi N. Time series analysis of leg movements during freezing of gait in Parkinson's disease: akinesia, rhyme or reason? Physica A. 2003;321:565–570. [Google Scholar]
- Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson's disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- Heremans E, Nieuwboer A, Vercruysse S. Freezing of gait in Parkinson's disease: where are we now? Curr Neurol Neurosci Rep. 2013;13:350. doi: 10.1007/s11910-013-0350-7. [DOI] [PubMed] [Google Scholar]
- Jacobs JV, Nutt JG, Carlson-Kuhta P, Stephens M, Horak FB. Knee trembling during freezing of gait represents multiple anticipatory postural adjustments. Exp Neurol. 2009;215:334–341. doi: 10.1016/j.expneurol.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis SJ, Shine JM. The Next Step: A Common Neural Mechanism for Freezing of Gait. Neuroscientist. 2016;22:72–82. doi: 10.1177/1073858414559101. [DOI] [PubMed] [Google Scholar]
- Mancini M, Priest KC, Nutt JG, Horak FB. Quantifying freezing of gait in Parkinson's disease during the instrumented timed up and go test. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1198–1201. doi: 10.1109/EMBC.2012.6346151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore O, Peretz C, Giladi N. Freezing of gait affects quality of life of peoples with Parkinson's disease beyond its relationships with mobility and gait. Mov Disord. 2007;22:2192–2195. doi: 10.1002/mds.21659. [DOI] [PubMed] [Google Scholar]
- Moore ST, Dilda V, Hakim B, Macdougall HG. Validation of 24-hour ambulatory gait assessment in Parkinson's disease with simultaneous video observation. Biomed eng online. 2011;10:82. doi: 10.1186/1475-925X-10-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore ST, MacDougall HG, Ondo WG. Ambulatory monitoring of freezing of gait in Parkinson's disease. J Neurosci Methods. 2008;167:340–348. doi: 10.1016/j.jneumeth.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Moore ST, Yungher DA, Morris TR, Dilda V, MacDougall HG, Shine JM, Naismith SL, Lewis SJ. Autonomous identification of freezing of gait in Parkinson's disease from lower-body segmental accelerometry. J Neuroeng Rehabil. 2013;10:19. doi: 10.1186/1743-0003-10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ME, Iansek R, Galna B. Gait festination and freezing in Parkinson's disease: pathogenesis and rehabilitation. Mov Disord. 2008;23(Suppl 2):S451–S460. doi: 10.1002/mds.21974. [DOI] [PubMed] [Google Scholar]
- Morris TR, Cho C, Dilda V, Shine JM, Naismith SL, Lewis SJ, Moore ST. A comparison of clinical and objective measures of freezing of gait in Parkinson's disease. Parkinsonism Relat Disord. 2012;18:572–577. doi: 10.1016/j.parkreldis.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Nantel J, de Solages C, Bronte-Stewart H. Repetitive stepping in place identifies and measures freezing episodes in subjects with Parkinson's disease. Gait Posture. 2011;34:329–333. doi: 10.1016/j.gaitpost.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Dom R, De Weerdt W, Desloovere K, Janssens L, Stijn V. Electromyographic profiles of gait prior to onset of freezing episodes in patients with Parkinson's disease. Brain. 2004;127:1650–1660. doi: 10.1093/brain/awh189. [DOI] [PubMed] [Google Scholar]
- Nieuwboer A, Rochester L, Herman T, Vandenberghe W, Emil GE, Thomaes T, Giladi N. Reliability of the new freezing of gait questionnaire: agreement between patients with Parkinson's disease and their carers. Gait Posture. 2009;30:459–463. doi: 10.1016/j.gaitpost.2009.07.108. [DOI] [PubMed] [Google Scholar]
- Nonnekes J, Snijders AH, Nutt JG, Deuschl G, Giladi N, Bloem BR. Freezing of gait: a practical approach to management. Lancet Neurol. 2015;14:768–778. doi: 10.1016/S1474-4422(15)00041-1. [DOI] [PubMed] [Google Scholar]
- Nutt JG, Bloem BR, Giladi N, Hallett M, Horak FB, Nieuwboer A. Freezing of gait: moving forward on a mysterious clinical phenomenon. Lancet Neurol. 2011;10:734–744. doi: 10.1016/S1474-4422(11)70143-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destee A, Meissner WG, Schelosky L, Tison F, Rascol O. Prevalence, determinants, and effect on quality of life of freezing of gait in Parkinson disease. JAMA neurology. 2014;71:884–890. doi: 10.1001/jamaneurol.2014.753. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol. 2005a;57:656–663. doi: 10.1002/ana.20452. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Balash Y, Peretz C, Hausdorff JM. Is freezing of gait in Parkinson's disease related to asymmetric motor function? Ann Neurol. 2005b;57:656–663. doi: 10.1002/ana.20452. [DOI] [PubMed] [Google Scholar]
- Plotnik M, Giladi N, Hausdorff JM. Bilateral coordination of walking and freezing of gait in Parkinson's disease. Eur J Neurosci. 2008;27:1999–2006. doi: 10.1111/j.1460-9568.2008.06167.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Balash Y, Gurevich T, Bartels AL, Hausdorff JM, Giladi N. Characterization of freezing of gait subtypes and the response of each to levodopa in Parkinson's disease. Eur J Neurol. 2003a;10:391–398. doi: 10.1046/j.1468-1331.2003.00611.x. [DOI] [PubMed] [Google Scholar]
- Schaafsma JD, Giladi N, Balash Y, Bartels AL, Gurevich T, Hausdorff JM. Gait dynamics in Parkinson's disease: relationship to Parkinsonian features, falls and response to levodopa. J Neurol Sci. 2003b;212:47–53. doi: 10.1016/s0022-510x(03)00104-7. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Haaxma CA, Hagen YJ, Munneke M, Bloem BR. Freezer or non-freezer: clinical assessment of freezing of gait. Parkinsonism Relat Disord. 2012;18:149–154. doi: 10.1016/j.parkreldis.2011.09.006. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Nijkrake MJ, Bakker M, Munneke M, Wind C, Bloem BR. Clinimetrics of freezing of gait. Mov Disord. 2008;23(Suppl 2):S468–S474. doi: 10.1002/mds.22144. [DOI] [PubMed] [Google Scholar]
- Snijders AH, Takakusaki K, Debu B, Lozano AM, Krishna V, Fasano A, Aziz TZ, Papa SM, Factor SA, Hallett M. Physiology of freezing of gait. Ann Neurol. 2016 doi: 10.1002/ana.24778. [DOI] [PubMed] [Google Scholar]
- Spildooren J, Vercruysse S, Desloovere K, Vandenberghe W, Kerckhofs E, Nieuwboer A. Freezing of gait in Parkinson's disease: the impact of dual-tasking and turning. Mov Disord. 2010;25:2563–2570. doi: 10.1002/mds.23327. [DOI] [PubMed] [Google Scholar]
- Walton CC, Shine JM, Hall JM, O'Callaghan C, Mowszowski L, Gilat M, Szeto JY, Naismith SL, Lewis SJ. The major impact of freezing of gait on quality of life in Parkinson's disease. J Neurol. 2015;262:108–115. doi: 10.1007/s00415-014-7524-3. [DOI] [PubMed] [Google Scholar]
- Zach H, Janssen AM, Snijders AH, Delval A, Ferraye MU, Auff E, Weerdesteyn V, Bloem BR, Nonnekes J. Identifying freezing of gait in Parkinson's disease during freezing provoking tasks using waist-mounted accelerometry. Parkinsonism Relat Disord. 2015;21:1362–1366. doi: 10.1016/j.parkreldis.2015.09.051. [DOI] [PubMed] [Google Scholar]
- Zampieri C, Salarian A, Carlson-Kuhta P, Aminian K, Nutt JG, Horak FB. The instrumented timed up and go test: potential outcome measure for disease modifying therapies in Parkinson's disease. J Neurol Neurosurg Psychiatry. 2010;81:171–176. doi: 10.1136/jnnp.2009.173740. [DOI] [PMC free article] [PubMed] [Google Scholar]