Abstract
A broad spectrum of autoimmunity is now well described in patients with primary immunodeficiencies (PIDs). Management of autoimmune disease in the background of PID is particularly challenging given the seemingly discordant goals of immune support and immune suppression. Our growing ability to define the molecular underpinnings of immune dysregulation has facilitated novel targeted therapeutics. This review focuses on mechanism-based treatment strategies for the most common autoimmune and inflammatory complications of PID including autoimmune cytopenias, rheumatologic disease, and gastrointestinal disease. We aim to provide guidance regarding the rational use of these agents in the complex PID patient population.
Keywords: primary immunodeficiencies (PIDs), treatment, autoimmunity, cytopenias, arthritis, vasculitis, lupus, autoimmune enteropathy (AIE), inflammatory bowel disease (IBD)
Autoimmune and inflammatory diseases can complicate the course of primary immunodeficiency (PID) and the complex care of these patients (1). The clinical spectrum is broad and frequently includes autoimmune cytopenias, rheumatologic disease, and gastrointestinal (GI) disease (2, 3). The pathogenesis of immune dysregulation leading to autoimmunity in PIDs was recently comprehensively reviewed (4). In light of mechanistic understanding, it is timely to review management strategies.
Balancing immunosuppressive therapy in patients with susceptibility to infection is a clinical challenge. Treatment success hinges upon correcting the underlying immune dysregulation while minimizing nonspecific immune suppression. Herein we will review management of PID-associated autoimmunity by therapeutic mechanism: targeting B cell, T cell, or innate immune pathology or using hematopoietic stem cell transplantation (HSCT) to reconstitute the immune system.
1. Treatment of autoimmune cytopenias in primary immunodeficiencies
While autoimmune cytopenias, including autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), and autoimmune neutropenia (AN), occur in the general population, they are particularly common in patients with PID. As an example, PID was uncovered in 13% of children with AIHA (5) and up to 50% of children with multi-lineage cytopenias (Evans syndrome) (6). Autoimmune cytopenias have been described in both innate and adaptive immune deficiencies (3, 7) and may be the first sign of immune dysregulation that precedes the classical presentation of PID with recurrent or opportunistic infections (8, 9). Clinical warning signs that may prompt the clinician to consider PID at an earlier stage include: multi-lineage cytopenias, AIHA with no response to first-line therapy, persistent/chronic ITP, and AN in a patient > two years of age and/or persistent for > 24 months (10-14).
Corticosteroids are the mainstay of treatment for AIHA with a high response rate around 80% in the general population (15). For ITP, corticosteroids or high-dose intravenous immunoglobulin (IVIG) are considered first-line (16). In the fraction of patients who relapse following these therapies, splenectomy has been the traditional second-line approach. With the advance of biologics, anti-CD20 antibody (rituximab) is now considered an effective second-line approach although randomized clinical trials are lacking. In general, clinical approach in treatment-resistant cases is one of therapeutic trial and error in the absence of a guiding underlying immunophenotype or biomarkers to direct care. By contrast, second-line treatment strategies for PID-associated autoimmune cytopenias are increasingly being targeted to the underlying mechanism of immunopathology.
1.1 Targeting B cell pathology
Several studies address the approach to autoimmune cytopenias in the background of common variable immunodeficiency (CVID), a heterogeneous condition defined by decreased serum immunoglobulins (low IgG with low IgM and/or IgA), frequent infections, and poor antigen-specific antibody titers (17). Classical CVID is considered to be a primary disorder of B cells. However, improved genetic discovery and immunophenotyping has led to reclassification of a growing CVID subset as de facto combined immunodeficiency (CID) (18).
The link between CVID and autoimmunity was first established in the 1990s (19) and has been greatly expanded since that time (Table I) (20, 21). Initial treatment regimens for autoimmune cytopenias included combinations of corticosteroids, high-dose IVIG, and anti-Rho(D) in the case of ITP. These guidelines were extrapolated from the standard of care in the general population. Initial response rates to corticosteroids were reasonable, 85% for ITP (22) and 81% for AIHA (23); however, prolonged use was often required, which increased risk for infection as a secondary complication. Before the era of biologics, nearly half of these autoimmune cytopenia cases ultimately required second-line splenectomy (response rates of 60-80%), which was in contrast to the majority of first-line treatment responders seen in the general population (8, 22, 23). Other agents such as vinca-alkaloids, danazol, cyclophosphamide, azathioprine, and cyclosporine did not show long-term success and are now rarely used.
Table I.
Primary immunodeficiencies associated with autoimmune disease.
| PID | Immunologic Defect | AI Cytopenias Prevalence (%) | Rheum Disease Prevalence (%) | GI Disease Prevalence (%) | Other Noninfectious Manifestations | Refs. |
|---|---|---|---|---|---|---|
| CVID |
Polygenic low IgG (low IgA or IgM), low vaccine titers, low sm B cells, high CD19hi21lo B cells |
ITP (5.6-14.2) AIHA (2.7-7) AN (<1-2.7) Evans (4.2) |
RA (3.2) vasculitis & SLE (<1-2.7) |
diarrhea (14-23) malabsorption/AIE (6-9) IBD (4.2) |
Lymphoproliferative pathology (LAD, HSM, GLILD, NRH, leukemia, lymphoma) Other autoimmunity (hepatitis, alopecia, thyroiditis, vitiligo) |
(20, 21, 144-146) |
| XLA |
BTK Low/absent circulating B cells, loss of germinal centers, pan low immunoglobulins, impaired innate immune signaling, decreased Tfh cells |
ITP (2.7*) AIHA (9.8*) |
RA/JIA (1.8*-16) | diarrhea (8*-29) IBD (3.6*-3.8) |
Neutropenia in the setting of overwhelming infection | (86, 147-150) |
| ALPS |
TNFRSF6 (FAS), TNFSF6 (FASL), CASP10 high DN T cells, IL-10, IL-18, vit B12, FAS; decreased FAS-mediated apoptosis |
ITP (26-39) AIHA (29-36) AN (8-37) Evans (10-23) |
uveitis (1-10) vasculitis (4) arthritis (case reported) |
IBD (case reported) | Lymphoproliferative pathology (LAD, HSM, lymphoma) Other autoimmunity (hepatitis, PBC, GBS, GN) |
(34, 151-155) |
| pDGS |
22q11.2 impaired thymic development, decreased T cell number & function, variably decreased IgG/A/M & sm B cells |
ITP (3.1-6.3) AIHA (0.5-3.1) Evans (0.5-3.1) |
vasculitis (3.1) arthritis (2.5-3.1) |
IBD (0.5) | Craniofacial anomalies, hypoplastic thymus, conotruncal cardiac anomalies, hypocalcemia Other autoimmunity (thyroiditis) |
(44, 156-158) |
| CTLA4 |
CTLA4 (haploinsuficiency) impaired FOXP3+ Tregs, activated effector & decreased naïve T cells, low IgG, low B cells, high CD21lo B cells |
ITP (35) AIHA (28) |
arthritis (14) | Diarrhea/AIE (78) | Lymphoproliferative pathology (LAD, HSM, GLILD) Other autoimmunity (thyroiditis) |
(47, 83, 119) |
| LRBA |
LRBA decreased/impaired FOXP3+ Tregs, activated T effector cells, low IgG, low B cells (sm B cells and plasmablasts) |
ITP (29-52) AIHA (39-57) AN (24) |
arthritis (26) uveitis (10) |
Diarrhea/AIE (61-62) | Growth retardation, eczema Lymphoproliferative pathology (LAD, HSM, GLILD, lymphoma) Other autoimmunity (T1DM, thyroiditis, hepatitis, alopecia) |
(48, 49, 159) |
| IPEX |
FOXP3 impaired FOXP3+ Tregs, high IgE, high eosinophils, low Th1 cytokines, high Th2 cytokines |
AIHA or ITP or AN (31) | arthritis (1) | Diarrhea/AIE (92) | FTT, severe dermatitis Lymphoproliferative pathology (LAD, HSM) Other autoimmunity (early-onset T1DM, thyroiditis, hepatitis) |
(160) |
| STAT3-GOF |
STAT3 decreased/impaired FOXP3+ Tregs, increased DN T cells, variably low IgG |
ITP (62) AIHA (69) AN (46) Evans (46) |
arthritis (15-20) | AIE (38-60) | Short stature, eczema Lymphoproliferative pathology (LAD, HSM, GLILD, lymphoma) Other autoimmunity (T1DM, thyroiditis, alopecia, scleroderma, hepatitis) |
(84, 85) |
| STAT1-GOF |
STAT1 augmented Th1, decreased/impaired Th17, low memory B cells, low IgG2/IgG4 |
AIHA or ITP (4) | SLE (2) | AIE (4) | Aneurysms, eczema, carcinomas Other autoimunity (thyroiditis, T1DM, alopecia, vitiligo, psoriasis, hepatitis) |
(161, 162) |
| WAS |
WAS decreased T cell number & function, low IgG/A/M, high IgE, low vaccine titers |
ITP (32) AIHA (14-36) Evans (20) |
vasculitis (13) arthritis (10) |
IBD (3) | Microthrombocytes with low count & poor function, eczema, mucosal bleeding, lymphoma, renal disease | (163) |
| CGD |
CYBB (x), CYBA, NCF1, NCF2, NCF4 dysfunctional NADPH oxidase, impaired phagocytosis, aseptic hyper-inflammation |
ITP (1.4) | DLE (2.7) chorioretinitis (2.2) SLE, APLS, vasculitis, & arthritis (<1) |
IBD (17-88) | Lymphoproliferative pathology with severe multi-organ granulomatous disease (GI tract, lungs, kidneys, eyes) | (96, 99, 128, 164) |
Abbreviations: patient self-reported (*), primary immunodeficiency (PID), autoimmune (AI), rheumatologic (rheum), gastrointestinal (GI), common variable immunodeficiency (CVID), autoimmune lymphoproliferative syndrome (ALPS), partial DiGeorge syndrome (pDGS), cytotoxic T-lymphocyte antigen 4 (CTLA4), LPS-responsive vesicle trafficking, beach and anchor containing protein (LRBA), immune dysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX), signal transducer and activator of transcription (STAT), gain-of-function (GOF), Wiskott-Aldrich syndrome (WAS), chronic granulomatous disease (CGD), regulatory T cell (Treg), helper T cell (Th), follicular helper T cell (Tfh), double negative (DN), switched memory (sm), autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), autoimmune neutropenia (AN), rheumatoid arthritis (RA), juvenile idiopathic arthritis (JIA), systemic lupus erythematosus (SLE), discoid lupus erythematosus (DLE), anti-phospholipid syndrome (APLS), myasthenia gravis (MG), glomerulonephritis (GN), primary biliary cirrhosis (PBC), type I diabetes mellitus (T1DM), Guillain-Barre syndrome (GBS), autoimmune enteropathy (AIE), inflammatory bowel disease (IBD), lymphadenopathy (LAD), hepatosplenomegaly (HSM), granulomatous and lymphocytic interstitial lung disease (GLILD), nodular regenerative hyperplasia (NRH), failure to thrive (FTT)
In 2004, rituximab was introduced as second-line therapy for CVID-associated AIHA (24). In a subsequent multicenter study of 33 CVID patients with refractory autoimmune cytopenias, which included steroid-dependence (56%), immunomodulatory therapy (44%), and prior splenectomy (21%), rituximab was demonstrated to have a durable response rate of 59% (25). The authors proposed that rituximab be considered standard second-line therapy, prior to splenectomy and/or other immunomodulatory therapy, in CVID-associated autoimmune cytopenias. Although 24% of patients developed severe bacterial infections after rituximab treatment, half of these cases were off of immunoglobulin replacement therapy and/or had undergone splenectomy (25). While concerning, the rate of severe bacterial infections was not significantly different than that observed in CVID patients with ITP treated by the more traditional approach of corticosteroids with or without high-dose IVIG (22). Therefore, risk for infection with rituximab use needs to be considered primarily in CVID patients not receiving immunoglobulin replacement therapy.
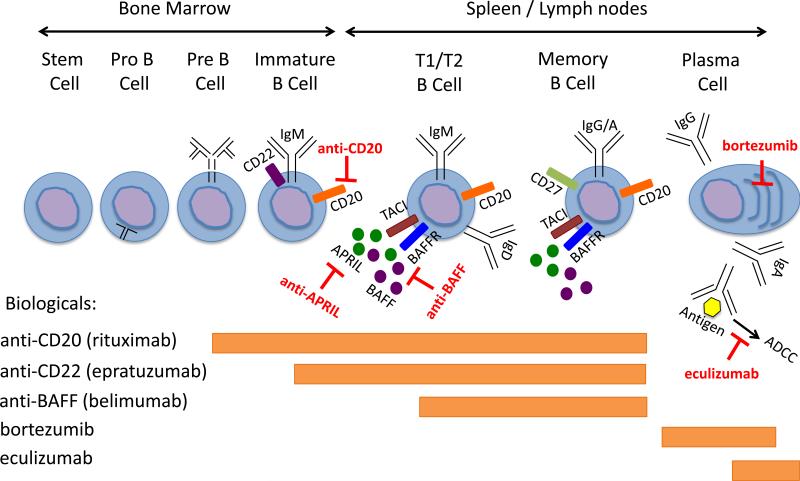
Response to B cell depletion therapy in most cases of CVID-associated autoimmune cytopenias localized the immunopathology to the B cell compartment and suggested that other therapies targeting this compartment may also be efficacious. It should be emphasized that rituximab depletes only maturing B cells and does not target long-lived plasma cells that can sustain autoantibody production in lymphoid niches for some time (months) after treatment. Alternative B cell-directed therapy may include bortezomib, a proteasome inhibitor that is approved for the treatment of multiple myeloma and preferentially causes apoptosis of antibody-producing plasma cells through activation of the unfolded protein response (UPR) (26). Bortezomib has shown promising results in peri-transplant cases of PID-associated refractory autoimmune cytopenias specifically (four of five patients with PID responded to treatment and only two patients required transition to alternative therapy (27)). Additional B cell-directed therapies currently in clinical trial include an anti-CD22 antibody (epratuzumab) and an anti-APRIL antibody. Both show promise in severe refractory autoimmune diseases including cytopenias (28-31), but have yet to be trialed in PID specifically. Finally, the terminal complement inhibitor eculizumab (anti-C5) has been utilized to rescue a patient from fatal complications related to treatment-refractory AIHA (32). Since it acts distal to the B cell in autoantibody mediated diseases, it could in theory be applied in combination with B cell depleting therapies to more completely control disease. The mechanism of action for these biologics is reviewed in Figure 1.
Figure 1.
Mechanisms of targeting B cell pathology in the treatment of autoimmune and inflammatory diseases associated with PID.
1.2 Targeting T cell pathology
PID patients with prominent T cell dysfunction may not fully benefit from the removal of autoreactive B cells. In autoimmune lymphoproliferative syndrome (ALPS), the accumulation of pathognomonic TCRαβ+CD4−CD8− (double negative, DN) T cells occurs secondary to defective apoptosis. While autoimmune cytopenias are a key feature of the disease (Table I), rituximab is a therapy of last resort given the associated finding of profound and prolonged hypogammaglobulinemia up to 4 years post-treatment (33). Similarly, splenectomy is less preferred as it may result in unfavorable outcomes with recurrent cytopenias and high rates of sepsis (41%) in ALPS patients (34).
The conventional first-line therapy for ALPS-associated autoimmune cytopenias has been corticosteroids, but second-line therapies including mycophenolate mofetil (MMF, a prodrug of mycophenolic acid that inhibits inosine monophosphate dehydrogenase and suppresses T and B cells) and sirolimus (an mTOR inhibitor) that more effectively target DN T cells are increasingly being used as primary therapy (35, 36). Sirolimus was first trialed in four corticosteroid-refractory ALPS patients in 2009 and resulted in marked improvements in both autoimmune cytopenias and associated systemic inflammatory features (arthritis, colitis, lymphadenopathy, and splenomegaly) (10). In a subsequent trial of 30 patients with refractory autoimmune cytopenias across multiple PIDs (CVID and ALPS), sirolimus resulted in a complete and durable remission in the majority of patients (37). Treatment response in ALPS has been shown to coincide with a specific reduction in DN T cells, which are particularly dependent on an intact mTOR pathway (37-40).
Autoimmune cytopenias have been associated with partial DiGeorge syndrome (pDGS), occasionally preceding diagnosis of the underlying genetic defect (Table I) (41-43). Breaks in both central T cell tolerance (e.g., thymic aplasia/dysplasia) and peripheral T cell tolerance (e.g., T cell proliferation to low-affinity self antigens) have been proposed to induce autoimmunity (44). To date, large studies do not exist as to the optimal therapeutic approach. Steroids and azathioprine have been anecdotally used to treat ITP with benefit (42). Progression despite rituximab has been reported in two cases of severe autoimmune cytopenias associated with pDGS, one requiring HSCT for definitive treatment (45), the other requiring plasmaphoresis in combination with splenectomy for stabilization (46).
Autoimmune cytopenias can also occur in the setting of regulatory T cell (Treg) dysfunction. CTLA4 haploinsufficiency is a novel autosomal-dominant immunodeficiency where decreased CTLA4 cell surface expression results in impaired Treg suppressor function. It has been associated with a broad clinical spectrum of autoimmunity including high rates of ITP and AIHA (Table I). Here, direct complementation of the underlying immunoregulatory defect with CTLA4-Ig (abatacept) has been anecdotally reported to treat pancytopenia and associated life-threatening autoimmunity otherwise refractory to corticosteroids, tacrolimus, azathioprine, cyclophosphamide, and sirolimus (47). LRBA deficiency is an associated autosomal-recessive PID where Treg impairment occurs secondary to aberrant recycling of CTLA4 to the cell surface (48). It is strongly associated with systemic autoimmunity including cytopenias (Table I). Major treatment modalities have included corticosteroids (39%), IVIG (39%), MMF (22%), abatacept (15%), tacrolimus/sirolimus (11%), and HSCT (11%) (49). Interestingly, inhibition of lysosomal degradation via chloroquine/hydroxychloroquine rescued CTLA4 expression in LRBA deficient cells in vitro (48) and improved lymphoproliferative lung pathology in a patient with LRBA mutation in vivo (50), however, improvement in autoimmune cytopenias specifically has yet to be described.
Finally, patients with STAT1-GOF mutations develop chronic mucocutaneous candidiasis and autoimmunity including cytopenias in the background of prominent T cell dysregulation (Table I). Specifically, naïve CD4+ T cells are uniquely biased towards IFN-γ production irrespective of polarizing conditions and expansion of follicular helper T cells relative to Tregs has been shown (51). T cell targeting with cyclosporine has been anecdotally used to treat AIHA in STAT1-GOF with benefit (52). More recently, a janus kinase (JAK) 1/2 inhibitor (ruxolitinib) was used to treat two distinct cases of STAT1-GOF with associated autoimmunity including autoimmune cytopenias (51) and refractory alopecia areata (53). Ruxolitinib was shown to reduce hyper-responsiveness to IFN-γ, restore Th17 and Treg counts, induce long-lasting control of autoimmunity (up to 6 months post-treatment (53)), and had the unexpected benefit of reducing occurrence of mucocutaneous candidiasis in both cases.
1.3 Immune Reconstitution
Patients with severe immunodeficiency may require progression to HSCT for definitive treatment. Wiskott-Aldrich syndrome (WAS) is a well-described PID where autoimmune cytopenias occur beyond abnormal platelet number, size, and function (54). AIHA is severe, early-onset, and poorly responsive to corticosteroids, and ITP mainly occurs post-splenectomy (Table I). The presence of autoimmunity increases disease severity and contributes to the indication for HSCT. Unfortunately, even after HSCT and/or gene therapy autoimmune cytopenias may resurface and become refractory (55-58), as demonstrated by the 55% of WAS patients who developed autoimmune cytopenias in the post-transplant period (59). Thrombopoietin receptor agonists such as romiplostim and eltrombopag are emerging therapies for ITP, mainly by promoting platelet production. Since these agents are not immunosuppressive, they could be particularly useful in the treatment of ITP on a background of PID going forward (60-62).
Finally, autoimmunity is increasingly recognized among patients with CIDs secondary to classical severe combined immunodeficiency (SCID)-related gene defects. Patients with recombination activating gene (RAG) mutations can have broad clinical heterogeneity ranging from early-onset severe infections (SCID phenotype) to delayed-onset autoimmune and inflammatory complications such as cytopenias, vasculitis and granulomas (CID-AI/G phenotype) (63). Specific RAG mutation, RAG activity, and ultimately the resultant B and T cell repertoire correlate well with these distinct phenotypes (64). Several checkpoints of B and T cell tolerance are impaired in RAG deficiency, which results in impaired removal of autoreactive cells (abnormal thymic selection, dysfunctional Tregs, impaired B cell receptor editing in the bone marrow, and elevated B cell activating factor (BAFF) expression) (65-67). However the relative contribution of these mechanisms in driving autoimmunity is still unclear. Treatment outcomes data in our RAG deficient cohort suggest that second-line therapy with biologics is not standardized and often ineffective. Progression to HSCT for definitive treatment was ultimately required in 20% of CID-AI/G patients with autoimmune cytopenias (68).
Autoimmune cytopenias have been anecdotally reported in other CIDs (PIK3CD (PI3K-D), TPP2, and DOCK8) as well as in hypomorphic SCID variants (DCLRE1 (ARTEMIS), ADA, PNP, RMRP, and ORAI1) (62) (Table II). The largest review to date details 14 hypomorphic ARTEMIS cases, where 6 of 14 patients (45%) had autoimmune cytopenias (69). For the other PIDs in this group, autoimmune cytopenias are more sporadically reported, and treatment strategies have not been discussed in depth.
Table II.
Combined immunodeficiencies associated with autoimmune cytopenias.
| Gene | Function | Autoimmune Cytopenias | Treatment Strategies | Associated Autoimmunity | References |
|---|---|---|---|---|---|
| RAG1, RAG2 | dsDNA cleavage during V(D)J recombination | AIHA, ITP, AN | steroids, IVIG, rituximab, HSCT | vasculitis, GBS, MG, psoriasis, vitiligo | (63, 70, 165) |
| DCLRE1 (ARTEMIS) | non-homologous end joining, opening the hairpins | AIHA, ITP, AN | n.a. | (69, 166) | |
| ADA | deamination of adenosine and 2′-deoxyadenosine | AIHA, ITP | PEG-ADA, HSCT | AI thyroiditis, T1DM | (167) |
| PNP | conversion of inosine and guanosine to hypoxanthine | AIHA, ITP | steroids, rituximab, azathioprine, cyclosporine, HSCT | (168) | |
| RMRP | RNA component of the mitochondrial RNA processing (RMRP) endoribonuclease complex | AIHA, ITP post-HSCT | steroids, IVIG, rituximab, HSCT | granulomas | (169) |
| TRAC | loss of TCR (transmembrane & intra-cytoplasmic domains) | AIHA | treatment is not discussed, s/p HSCT | vitiligo, alopecia areata, pityriasis rubra pilaris | (170) |
| IL-7R | signaling through the IL-7 receptor ensures the development of mature B cells & T cells | AIHA, ITP | treatment is not discussed, s/p HSCT | (171) | |
| CD3ɣ | TCR signal transduction | AIHA, ITP | steroids | AI hepatitis & thyroiditis, minimal change disease | (172) |
| ZAP70 | CD3ζ binding, T cell activation | ITP | IVIG | arthritis, nephritis in the mouse model | (173) |
| LCK/p56 | TCR signaling, associated with CD4 and CD8, upon activation mediates phosphorylation of CD3 and ZAP70 | ITP | steroids, HSCT | retinal vasculitis, sterile septal and lobular neutrophillic panniculitis, sterile arthritis | (174) |
| MST1/STK4 | interacts with Foxo1 that controls IL-7Ra expression in naive T cells and T cell homeostasis | AIHA, ITP, AN | steroids, IVIG, rituximab, cyclosporine, azathioprine | (175-177) | |
| ORAI1 (CRACM1) | store operated calcium entry, interaction with STIM1, T cell activation | ITP, AN | n.a. | (178) | |
| STIM1 | ER-resident calcium sensor, activates ORAI1 to promote store operated calcium entry | AIHA, ITP | steroids | (179) | |
| MAGT1 | magnesium-specific transporter and immune regulator | unspecified cytopenias | n.a. | (180) | |
| PIK3CD (PI3K-D) | Akt-mTOR pathway activation, generation of short lived effector CD8+ cells | AIHA, ITP | n.a. | (181, 182) | |
| TPP2 | cell proliferation and survival, anti-apoptotic | AIHA, ITP | steroids, IVIG, cyclosporine, MMF, rituximab, sirolimus, HSCT | (183) | |
| DOCK8 | intracellular signal transduction | AIHA | n.a. | thyroiditis | (184-187) |
| MHCII | antigen presentation | unspecified cytopenias | n.a. | (7, 188) |
Abbreviations: not annotated (n.a.), autoimmune (AI), autoimmune hemolytic anemia (AIHA), immune thrombocytopenic purpura (ITP), autoimmune neutropenia (AN), intravenous immunoglobulin (IVIG), hematopoietic stem cell transplantation (HSCT), T cell receptor (TCR), endoplasmic reticulum (ER), myasthenia gravis (MG), type I diabetes mellitus (T1DM), Guillain-Barre syndrome (GBS), mycophenolate mofetil (MMF)
2. Treatment of rheumatologic disease in primary immunodeficiencies
PIDs are now known to be associated with a spectrum of rheumatologic disease including inflammatory arthritis, vasculitis, systemic lupus erythematosus (SLE), and SLE-like disorders (Table I). It is not uncommon that rheumatologic disease is treated prior to the discovery of an underlying PID, which can result in substantial infectious complications. Indeed, delay in immunophenotyping and definitive treatment has resulted in increased morbidity and/or fatal outcomes in cases recently reported (70-72). Therefore, clinicians must consider risk for infection when approaching therapeutic options for rheumatologic disease in PID. Here, we discuss PID-associated rheumatologic diseases with polyautoimmunity. There are a significant number of important PIDs that cause primarily rheumatologic disease, for example complement deficiencies and monogenic disorders of dysregulated IL-1 production, that have been reviewed elsewhere (73-75).
2.1 Targeting B cell pathology
CVID has been associated with rheumatologic complications including inflammatory arthritis, vasculitis, and SLE (Table I). The majority of patients will require therapy beyond IVIG. Case reports have demonstrated successful use of rituximab to treat both CVID-associated SLE (76) and ANCA-positive vasculitis (77). These data localize pathology to the B cell compartment and suggest that other B cell targeting strategies may be efficacious. Belimumab is a novel therapeutic uniquely targeting BAFF that just gained FDA approval for the treatment of SLE (78). Rationale for its use originated in the notion that autoreactive B cells have less BAFF-R on their surface and reside in an anergic state when BAFF levels are normal (79). In inflammatory conditions, BAFF levels may elevate and contribute to the survival of autoreactive cells (80). While promising, belimumab has yet to be trialed in CVID specifically and may need special consideration in patients with BAFF receptor deficiencies (TACI and BAFF-R). Other potential mechanisms of targeting B cell pathology that may prove efficacious in CVID-associated rheumatologic disease have already been reviewed (Figure 1).
2.2 Targeting T cell pathology
The predominance of rheumatologic complications seen in patients with Treg dysfunction including CTLA4haploinsufficiency, LRBA deficiency, and STAT3-GOF (Table I) converges on the hypothesis that FOXP3+CD25+CD4+ Tregs play a critical role in host defense against the development of rheumatologic diseases including inflammatory arthritis (81). Consistent with this hypothesis, CTLA4-Ig therapy (abatacept) is FDA approved for the treatment of rheumatoid arthritis (RA) and juvenile idiopathic arthritis (JIA) in the general population. More recently, abatacept has shown benefit in PID. In LRBA deficiency, two children with inflammatory arthritis and uveitis (clinically consistent with JIA) demonstrated robust response to abatacept therapy (48, 82). Inflammatory arthritis can also complicate the course of CTLA4 haploinsufficiency (83), and it has yet to be determined whether abatacept will be additionally beneficial in these cases. Finally, inflammatory arthritis has been reported in several patients with STAT3-GOF (84, 85). Immunophenotype is notable for decreased Treg numbers and functional expression of FOXP3 and CD25, potentially mediated by increased STAT3-dependent SOCS3 expression driving decreased STAT5 phosphorylation (84, 85). As Treg inhibition in STAT3-GOF is indirect, clinicians hypothesized that use of an anti-IL6R antibody (tocilizumab) might be beneficial via blocking upstream IL-6-induced STAT3 activation. To date, one patient with STAT3-GOF complicated by arthritis and scleroderma-like skin changes refractory to treatment with TNF-α inhibitors, anti-IL-1 therapy, and rituximab demonstrated sustained response to tocilizumab over a one year follow-up period (84).
Inflammatory arthritis is also a known complication of x-linked agammaglobulinemia (XLA), a PID where autoreactive B cells are effectively absent due to maturation arrest at the pre-B cell stage. While infectious joint inflammation resolving on immunoglobulin replacement therapy is frequently seen in XLA (86), aseptic arthritis has also been described including presentations of RA (87), JIA (88, 89), and enthesitis-related arthritis (ERA) (90). Infiltrating CD8+ T cells can be seen on joint cytology (87). Underlying mechanisms of T cell-driven autoimmunity (90) and/or innate immune hyperactivation (91, 92) have been proposed. In these cases, IVIG alone can be insufficient management (87, 90), progression despite methotrexate has been described (87), nonsteroidal anti-inflammatories (NSAIDs) may provide some benefit (89, 90), and there is no systematic guidance for the use of T cell or innate immune targeted strategies to date.
2.3 Targeting innate immune pathology
In contrast to the PIDs previously presented, patients with chronic granulomatous disease (CGD) develop systemic autoimmunity in the background of a primary innate immune deficiency. Here decreased NADPH oxidase results in defective phagocytosis. Profound aseptic hyper-inflammatory responses are seen in CGD, characterized by loss of anti-inflammatory mediators (93), impaired clearance of apoptotic cells (94), and downstream CD4+ T cell skewing that can drive autoimmune arthritis in the mouse model (95). In patients, CGD has been associated with cutaneous discoid lupus erythematosus (DLE), chorioretinitis, inflammatory arthritis, vasculitis, and SLE as well as DLE in female carriers of x-linked disease (Table I) (96-99). A single case series on treatment of rheumatologic manifestations in CGD recently demonstrated clinical stabilization with systemic corticosteroids (one case of DLE), methotrexate (one case of antiphospholipid syndrome (APLS)), and etanercept (one case of JIA) (96). While these anecdotal data are promising, anti-TNF-α therapies have been associated with invasive fungal disease even in immunocompetent hosts and should be used cautiously in these and other PID patients with significant susceptibility to infection.
2.4 Immune Reconstitution
HSCT has the potential to be curative for PID with autoimmunity in terms of reconstitution of the immune system and reduced susceptibility to infection. However, autoimmune disease can sometimes persist or even broaden post-transplant. 70% of WAS patients have associated autoimmunity that can include inflammatory arthritis and vasculitis (Table I). Although arthritis and vasculitis generally improve following HSCT or gene therapy, there are several cases where autoimmunity has persisted or even newly arisen (56-58). RAG deficiency has also been associated with rheumatologic and autoimmune diseases including vitiligo, myasthenia gravis (MG), and vasculitis (Table II) (63). Progression of vasculitis in RAG deficiency despite treatment with corticosteroids, IVIG, and rituximab has been described (70). In contrast, HSCT in RAG deficiency has been case reported to be curative/preventative for polyautoimmunity (70, 100). As fewer post-transplant auto-inflammatory complications were observed in patients with RAG deficiency compared to patients with impaired ARTEMIS (101), benefit of HSCT may be PID-specific. However, additional clinical evidence is required to determine whether HSCT is truly curative for rheumatologic disease in PID. Optimal timing for transplantation, regimen for conditioning, and goal for donor chimerism have yet to be determined.
3. Treatment of GI disease in primary immunodeficiencies
PIDs have been associated with a broad clinical spectrum of autoimmune GI disorders including gastritis (pernicious anemia), celiac disease, autoimmune enteropathy (AIE), and inflammatory bowel disease (IBD) (Table I) (102). In the background of frequent infections (e.g., Giardia, Campylobacter, Salmonella, rotavirus, enterovirus, norovirus) diagnosis of nonspecific GI symptoms such as nausea, vomiting, diarrhea, and weight loss becomes particularly challenging. However, elucidating the underlying pathophysiology is critical given the associated finding of increased mortality in the PID subgroup with GI complications specifically (20).
3.1 Targeting T cell pathology
Gastritis, AIE, and IBD have all been described in CVID (103). Small intestinal biopsy frequently demonstrates villous atrophy that resembles sprue apart from the absence of plasma cells (104, 105). Lymphocytic infiltrates and occasional granulomas can occur both in the small intestine and the colon, consisting predominantly of CD 8+ T cells (104-106). Unfortunately, GI inflammatory disease in CVID has been notoriously difficult to treat. Despite benefit from combination rituximab/azathioprine therapy to manage granulomatous lung pathology (107), a similar response has not been seen in the inflamed GI tract (108). TNF-α inhibitors (109, 110) as well as the anti-α4β7 integrin monoclonal vedolizumab, which may inhibit Treg trafficking to the GI mucosa (103), have been anecdotally reported as successful. We have a case of severe CVID-associated AIE with negative genetic testing for CTLA4 and LRBA mutations currently improving after 4 months of treatment with abatacept (weight gain, decreased stool output, decreased infiltrating T cells on biopsy) (Walter, JE and Farmer, JR; unpublished data). Therefore, GI inflammatory disease may be a unique complication of CVID where B cell targeting is insufficient and directed T cell targeting is required to effectively manage this often life-threatening complication.
Mounting data are converging on the importance of Tregs in host defense against auto-inflammation in the GI tract. Immune dysregulation polyendocrinopathy enteropathy X-linked syndrome (IPEX) is a profound disorder of FOXP3+CD25+CD4+ Tregs caused by mutations in FOXP3. The pathognomonic clinical features of IPEX are severe and early-onset dermatitis, type I diabetes mellitus, and failure to thrive secondary to refractory diarrhea starting in infancy (111, 112). A demonstrated break in peripheral B cell tolerance leading to the production of auto-antibodies to the brush border proteins villin and AIE-75 has been described (113, 114). However, the role of anti-villin and anti-AIE-75 in disease pathogenesis is entirely unclear. AIE on biopsy is characterized by villous atrophy with infiltrating lymphocytes and eosinophils. Histopathologic patterns of “graft-versus-host disease-like,” “celiac disease-like,” and “depletion of the intestinal goblet cells” have all been described (115). Most single targeted immunosuppressive agents have been disappointing in the management of the profound autoimmunity and failure to thrive. However, T cell targeted therapeutics including tacrolimus, cyclosporine, and sirolimus have shown benefit in reducing the burden of IPEX-related autoimmune disease in the pre-transplant period (116-118).
Beyond intrinsic Treg defects secondary to abnormal FOXP3, CD25 or STAT5b; interestingly, AIE and IBD are shared complications of other Treg disorders including CTLA4 haploinsufficiency (83, 119), LBRA deficiency (120), STAT1-GOF (121) STAT3-GOF (84), mutated RAG1 (122) or DOCK8 (123), and ITCH deficiency (124, 125). Furthermore, autoimmune GI disease can be robustly induced (27-54% symptomatic with watery diarrhea) upon treatment with anti-CTLA4 biologics (126). These data again converge on the hypothesis that Tregs are critical in gut homeostasis (127). To this end, infiltrating T cells have been demonstrated on intestinal biopsy in CTLA4 haploinsufficiency (83), and lack of response to traditional therapeutics including TNF-α inhibitors has been demonstrated in LBRA deficiency (120). By contrast, sirolimus has been reliably efficacious in CTLA-4 haploinsufficient patients, and immune reconstitution with abatacept has been shown to markedly reduce AIE (47).
3.2 Targeting innate immune pathology
Profound autoimmune GI disease can also occur in the setting of innate immune deficiency. Classic is CGD, where multi-organ granulomatous inflammatory pathology occurs, most prominently affecting the GI tract in up to 73-88% of patients (Table I) (128, 129). Biopsy demonstrates skip lesions most frequently affecting the ano-rectum and consisting of crypt abscesses, large pigment-containing macrophages, and noncaseating granulomas, which can be indistinguishable from Crohn's disease (128-131). Despite the predisposition towards infection, no causative pathogens were identified in up to 93% of CGD-associated inflammatory GI disease cases (128), suggesting an underlying mechanism of aseptic autoimmunity. Treatment outcomes to date demonstrate limited benefit from corticosteroids (63-86% relapse rate) and/or NSAIDs (50-100% relapse rate) (128). Immunomodulation with methotrexate, azathioprine, cyclosporine, and thalidomide have been case reported as successful (128, 129, 132, 133). Finally, despite efficacy in colitis management, TNF-α inhibitors should be avoided given the high rate of complicating deadly infections (two deaths out of five infliximab-treated CGD patients (134)).
3.3 Immune Reconstitution
In CGD, HSCT has been shown to be curative both in terms of the recurrent infections and the multi-organ granulomatous pathology (135). However, using full myeloablative conditioning, patients with peri-transplant comorbidities including colitis had increased mortality (135), bringing up controversy as to the optimal timing and conditioning for transplant. More recently, reduced-intensity conditioning using high-dose fludarabine, serotherapy, and low-dose busulfan in high-risk CGD was shown to be both safe and effective (89% event-free survival at 21 month follow-up (136)). As this study included 33% of patients with active peri-transplant colitis, the data suggest that this reduced-intensity conditioning HSCT can be considered in severe CGD cases complicated by IBD.
Finally, while directed immunosuppression in IPEX can help to reduce the burden of multi-organ inflammatory pathology, HSCT is the only definitive treatment. Improved outcomes are seen with earlier age and fewer comorbidities at time of transplant and with the use of reduced-toxicity conditioning regimens (137-143). Even in the case of partial donor chimerism, clinical disease remission has been reported, coinciding with the presence of full donor Tregs (139, 141). The selective advantage of wild-type Tregs is consistent with the underlying pathophysiology of IPEX and may dictate Treg-sparing therapies for graft-versus-host disease in the post-transplant period (112).
Summary
Autoimmune and inflammatory diseases can greatly complicate the care of PID patients. Treatment strategies in PID should be targeted not only to the clinical spectrum of autoimmunity (cytopenias, rheumatologic disease, and/or GI disease) but to the underlying molecular cause of immune dysregulation (B cell, T cell, and/or innate immune pathology). As we advance our understanding of mechanisms that mediate autoimmunity in PID, we inherently improve the care of our PID patients and broaden our basic understanding of autoimmune and inflammatory disease.
Abbreviations
- PIDs
primary immunodeficiencies
- GI
gastrointestinal
- HSCT
hematopoietic stem cell transplantation
- AIHA
autoimmune hemolytic anemia
- ITP
immune thrombocytopenic purpura
- AN
autoimmune neutropenia
- IVIG
intravenous immunoglobulin
- CVID
common variable immunodeficiency
- CID
combined immunodeficiency
- SCID
severe combined immunodeficiency
- ALPS
autoimmune lymphoproliferative syndrome
- WAS
Wiskott-Aldrich syndrome
- CGD
chronic granulomatous disease
- pDGS
partial DiGeorge syndrome
- XLA
x-linked agammaglobulinemia
- IPEX
immune dysregulation polyendocrinopathy enteropathy X-linked syndrome
- SLE
systemic lupus erythematosus
- DLE
discoid lupus erythematosus
- RA
rheumatoid arthritis
- JIA
juvenile idiopathic arthritis
- ERA
enthesitis-related arthritis
- MG
myasthenia gravis
- APLS
antiphospholipid syndrome
- AIE
autoimmune enteropathy
- IBD
inflammatory bowel disease
- BAFF
B cell activating factor
- CTLA4
cytotoxic T-lymphocyte antigen 4
- LRBA
LPS-responsive vesicle trafficking, beach and anchor containing protein
- RAG
recombination activating gene
- STAT
signal transducer and activator of transcription
- GOF
gain-of-function
- Treg
regulatory T cell
- DN
double negative
- UPR
unfolded protein response
- JAK
janus kinase
- MMF
mycophenolate mofetil
- NSAID
nonsteroidal anti-inflammatory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Picard C, Al-Herz W, Bousfiha A, Casanova JL, Chatila T, Conley ME, et al. Primary Immunodeficiency Diseases: an Update on the Classification from the International Union of Immunological Societies Expert Committee for Primary Immunodeficiency 2015. J Clin Immunol. 2015;35(8):696–726. doi: 10.1007/s10875-015-0201-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Allenspach E, Torgerson TR. Autoimmunity and Primary Immunodeficiency Disorders. J Clin Immunol. 2016 doi: 10.1007/s10875-016-0294-1. [DOI] [PubMed] [Google Scholar]
- 3.Maggadottir SM, Sullivan KE. The intersection of immune deficiency and autoimmunity. Curr Opin Rheumatol. 2014;26(5):570–8. doi: 10.1097/BOR.0000000000000091. [DOI] [PubMed] [Google Scholar]
- 4.Grimbacher B, Warnatz K, Yong PF, Korganow AS, Peter HH. The crossroads of autoimmunity and immunodeficiency: Lessons from polygenic traits and monogenic defects. J Allergy Clin Immunol. 2016;137(1):3–17. doi: 10.1016/j.jaci.2015.11.004. quiz 8. [DOI] [PubMed] [Google Scholar]
- 5.Aladjidi N, Leverger G, Leblanc T, Picat MQ, Michel G, Bertrand Y, et al. New insights into childhood autoimmune hemolytic anemia: a French national observational study of 265 children. Haematologica. 2011;96(5):655–63. doi: 10.3324/haematol.2010.036053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Teachey DT, Manno CS, Axsom KM, Andrews T, Choi JK, Greenbaum BH, et al. Unmasking Evans syndrome: T-cell phenotype and apoptotic response reveal autoimmune lymphoproliferative syndrome (ALPS). Blood. 2005;105(6):2443–8. doi: 10.1182/blood-2004-09-3542. [DOI] [PubMed] [Google Scholar]
- 7.Arkwright PD, Abinun M, Cant AJ. Autoimmunity in human primary immunodeficiency diseases. Blood. 2002;99(8):2694–702. doi: 10.1182/blood.v99.8.2694. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Cunningham-Rundles C. Treatment and outcome of autoimmune hematologic disease in common variable immunodeficiency (CVID). J Autoimmun. 2005;25(1):57–62. doi: 10.1016/j.jaut.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 9.Savasan S, Warrier I, Buck S, Kaplan J, Ravindranath Y. Increased lymphocyte Fas expression and high incidence of common variable immunodeficiency disorder in childhood Evans' syndrome. Clin Immunol. 2007;125(3):224–9. doi: 10.1016/j.clim.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 10.Teachey DT, Greiner R, Seif A, Attiyeh E, Bleesing J, Choi J, et al. Treatment with sirolimus results in complete responses in patients with autoimmune lymphoproliferative syndrome. Br J Haematol. 2009;145(1):101–6. doi: 10.1111/j.1365-2141.2009.07595.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Heitink-Polle KM, Nijsten J, Boonacker CW, de Haas M, Bruin MC. Clinical and laboratory predictors of chronic immune thrombocytopenia in children: a systematic review and meta-analysis. Blood. 2014;124(22):3295–307. doi: 10.1182/blood-2014-04-570127. [DOI] [PubMed] [Google Scholar]
- 12.Teachey DT, Lambert MP. Diagnosis and management of autoimmune cytopenias in childhood. Pediatr Clin North Am. 2013;60(6):1489–511. doi: 10.1016/j.pcl.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miano M. How I manage Evans Syndrome and AIHA cases in children. Br J Haematol. 2016;172(4):524–34. doi: 10.1111/bjh.13866. [DOI] [PubMed] [Google Scholar]
- 14.Farruggia P, Fioredda F, Puccio G, Porretti L, Lanza T, Ramenghi U, et al. Autoimmune neutropenia of infancy: Data from the Italian neutropenia registry. Am J Hematol. 2015;90(12):E221–2. doi: 10.1002/ajh.24187. [DOI] [PubMed] [Google Scholar]
- 15.Lechner K, Jager U. How I treat autoimmune hemolytic anemias in adults. Blood. 2010;116(11):1831–8. doi: 10.1182/blood-2010-03-259325. [DOI] [PubMed] [Google Scholar]
- 16.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr., Crowther MA, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117(16):4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 17.Bonilla FA, Barlan I, Chapel H, Costa-Carvalho BT, Cunningham-Rundles C, de la Morena MT, et al. International Consensus Document (ICON): Common Variable Immunodeficiency Disorders. J Allergy Clin Immunol Pract. 2016;4(1):38–59. doi: 10.1016/j.jaip.2015.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Buchbinder D, Baker R, Lee YN, Ravell J, Zhang Y, McElwee J, et al. Identification of patients with RAG mutations previously diagnosed with common variable immunodeficiency disorders. J Clin Immunol. 2015;35(2):119–24. doi: 10.1007/s10875-014-0121-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sneller MC, Strober W, Eisenstein E, Jaffe JS, Cunningham-Rundles C. NIH conference. New insights into common variable immunodeficiency. Ann Intern Med. 1993;118(9):720–30. doi: 10.7326/0003-4819-118-9-199305010-00011. [DOI] [PubMed] [Google Scholar]
- 20.Resnick ES, Moshier EL, Godbold JH, Cunningham-Rundles C. Morbidity and mortality in common variable immune deficiency over 4 decades. Blood. 2012;119(7):1650–7. doi: 10.1182/blood-2011-09-377945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gathmann B, Mahlaoui N, Ceredih, Gerard L, Oksenhendler E, Warnatz K, et al. Clinical picture and treatment of 2212 patients with common variable immunodeficiency. J Allergy Clin Immunol. 2014;134(1):116–26. doi: 10.1016/j.jaci.2013.12.1077. [DOI] [PubMed] [Google Scholar]
- 22.Michel M, Chanet V, Galicier L, Ruivard M, Levy Y, Hermine O, et al. Autoimmune thrombocytopenic purpura and common variable immunodeficiency: analysis of 21 cases and review of the literature. Medicine (Baltimore) 2004;83(4):254–63. doi: 10.1097/01.md.0000133624.65946.40. [DOI] [PubMed] [Google Scholar]
- 23.Seve P, Bourdillon L, Sarrot-Reynauld F, Ruivard M, Jaussaud R, Bouhour D, et al. Autoimmune hemolytic anemia and common variable immunodeficiency: a case-control study of 18 patients. Medicine (Baltimore) 2008;87(3):177–84. doi: 10.1097/MD.0b013e31817a90ba. [DOI] [PubMed] [Google Scholar]
- 24.Wakim M, Shah A, Arndt PA, Garratty G, Weinberg K, Hofstra T, et al. Successful anti-CD20 monoclonal antibody treatment of severe autoimmune hemolytic anemia due to warm reactive IgM autoantibody in a child with common variable immunodeficiency. Am J Hematol. 2004;76(2):152–5. doi: 10.1002/ajh.20072. [DOI] [PubMed] [Google Scholar]
- 25.Gobert D, Bussel JB, Cunningham-Rundles C, Galicier L, Dechartres A, Berezne A, et al. Efficacy and safety of rituximab in common variable immunodeficiency-associated immune cytopenias: a retrospective multicentre study on 33 patients. Br J Haematol. 2011;155(4):498–508. doi: 10.1111/j.1365-2141.2011.08880.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raedler L. Velcade (Bortezomib) Receives 2 New FDA Indications: For Retreatment of Patients with Multiple Myeloma and for First-Line Treatment of Patients with Mantle-Cell Lymphoma. Am Health Drug Benefits. 2015;8(Spec Feature):135–40. [PMC free article] [PubMed] [Google Scholar]
- 27.Khandelwal P, Davies SM, Grimley MS, Jordan MB, Curtis BR, Jodele S, et al. Bortezomib for refractory autoimmunity in pediatrics. Biol Blood Marrow Transplant. 2014;20(10):1654–9. doi: 10.1016/j.bbmt.2014.06.032. [DOI] [PubMed] [Google Scholar]
- 28.Lee WJ, Lee ST, Moon J, Sunwoo JS, Byun JI, Lim JA, et al. Tocilizumab in Autoimmune Encephalitis Refractory to Rituximab: An Institutional Cohort Study. Neurotherapeutics. 2016 doi: 10.1007/s13311-016-0442-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wallace DJ, Hobbs K, Clowse ME, Petri M, Strand V, Pike M, et al. Long-Term Safety and Efficacy of Epratuzumab in the Treatment of Moderate-to-Severe Systemic Lupus Erythematosus: Results From an Open-Label Extension Study. Arthritis Care Res (Hoboken) 2016;68(4):534–43. doi: 10.1002/acr.22694. [DOI] [PubMed] [Google Scholar]
- 30.Gao Q, Li Q, Xue Z, Wu P, Yang X. In vitro and in vivo evaluation of a humanized anti-APRIL antibody. Curr Mol Med. 2013;13(3):464–5. [PubMed] [Google Scholar]
- 31.Liu XG, Hou M. Immune thrombocytopenia and B-cell-activating factor/a proliferation-inducing ligand. Semin Hematol. 2013;50(Suppl 1):S89–99. doi: 10.1053/j.seminhematol.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 32.Ma K, Caplan S. Refractory IgG Warm Autoimmune Hemolytic Anemia Treated with Eculizumab: A Novel Application of Anticomplement Therapy. Case Rep Hematol. 2016;2016:9181698. doi: 10.1155/2016/9181698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rao VK, Price S, Perkins K, Aldridge P, Tretler J, Davis J, et al. Use of rituximab for refractory cytopenias associated with autoimmune lymphoproliferative syndrome (ALPS). Pediatr Blood Cancer. 2009;52(7):847–52. doi: 10.1002/pbc.21965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Price S, Shaw PA, Seitz A, Joshi G, Davis J, Niemela JE, et al. Natural history of autoimmune lymphoproliferative syndrome associated with FAS gene mutations. Blood. 2014;123(13):1989–99. doi: 10.1182/blood-2013-10-535393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miano M, Scalzone M, Perri K, Palmisani E, Caviglia I, Micalizzi C, et al. Mycophenolate mofetil and Sirolimus as second or further line treatment in children with chronic refractory Primitive or Secondary Autoimmune Cytopenias: a single centre experience. Br J Haematol. 2015 doi: 10.1111/bjh.13533. [DOI] [PubMed] [Google Scholar]
- 36.Rao VK, Oliveira JB. How I treat autoimmune lymphoproliferative syndrome. Blood. 2011;118(22):5741–51. doi: 10.1182/blood-2011-07-325217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bride KL, Vincent T, Smith-Whitley K, Lambert MP, Bleesing JJ, Seif AE, et al. Sirolimus is effective in relapsed/refractory autoimmune cytopenias: results of a prospective multi-institutional trial. Blood. 2016;127(1):17–28. doi: 10.1182/blood-2015-07-657981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato H, Perl A. Mechanistic target of rapamycin complex 1 expands Th17 and IL-4+ CD4-CD8-double-negative T cells and contracts regulatory T cells in systemic lupus erythematosus. J Immunol. 2014;192(9):4134–44. doi: 10.4049/jimmunol.1301859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Volkl S, Rensing-Ehl A, Allgauer A, Schreiner E, Lorenz MR, Rohr J, et al. Hyperactive mTOR pathway promotes lymphoproliferation and abnormal differentiation in autoimmune lymphoproliferative syndrome. Blood. 2016 doi: 10.1182/blood-2015-11-685024. [DOI] [PubMed] [Google Scholar]
- 40.Teachey DT. New advances in the diagnosis and treatment of autoimmune lymphoproliferative syndrome. Curr Opin Pediatr. 2012;24(1):1–8. doi: 10.1097/MOP.0b013e32834ea739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Akar NA, Adekile AD. Chromosome 22q11.2 deletion presenting with immune-mediated cytopenias, macrothrombocytopenia and platelet dysfunction. Med Princ Pract. 2007;16(4):318–20. doi: 10.1159/000102157. [DOI] [PubMed] [Google Scholar]
- 42.Hernandez-Nieto L, Yamazaki-Nakashimada MA, Lieberman-Hernandez E, Espinosa-Padilla SE. Autoimmune thrombocytopenic purpura in partial DiGeorge syndrome: case presentation. J Pediatr Hematol Oncol. 2011;33(6):465–6. doi: 10.1097/MPH.0b013e31821b0915. [DOI] [PubMed] [Google Scholar]
- 43.DePiero AD, Lourie EM, Berman BW, Robin NH, Zinn AB, Hostoffer RW. Recurrent immune cytopenias in two patients with DiGeorge/velocardiofacial syndrome. J Pediatr. 1997;131(3):484–6. doi: 10.1016/s0022-3476(97)80085-6. [DOI] [PubMed] [Google Scholar]
- 44.McLean-Tooke A, Spickett GP, Gennery AR. Immunodeficiency and autoimmunity in 22q11.2 deletion syndrome. Scand J Immunol. 2007;66(1):1–7. doi: 10.1111/j.1365-3083.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 45.Soldatou A, Anastassiou T, Vougiouka O, Goussetis E, Kossiva L. Transient effect of anti-CD20 therapy in a child with 22q11.2 deletion syndrome and severe steroid refractory cytopenias: a case report. J Pediatr Hematol Oncol. 2013;35(4):311–4. doi: 10.1097/MPH.0b013e31828be602. [DOI] [PubMed] [Google Scholar]
- 46.Damlaj M, Seguin C. Refractory autoimmune hemolytic anemia in a patient with DiGeorge syndrome treated successfully with plasma exchange: a case report and review of the literature. Int J Hematol. 2014;100(5):494–7. doi: 10.1007/s12185-014-1648-1. [DOI] [PubMed] [Google Scholar]
- 47.Lee S, Moon JS, Lee CR, Kim HE, Baek SM, Hwang S, et al. Abatacept alleviates severe autoimmune symptoms in a patient carrying a de novo variant in CTLA-4. J Allergy Clin Immunol. 2016;137(1):327–30. doi: 10.1016/j.jaci.2015.08.036. [DOI] [PubMed] [Google Scholar]
- 48.Lo B, Zhang K, Lu W, Zheng L, Zhang Q, Kanellopoulou C, et al. AUTOIMMUNE DISEASE. Patients with LRBA deficiency show CTLA4 loss and immune dysregulation responsive to abatacept therapy. Science. 2015;349(6246):436–40. doi: 10.1126/science.aaa1663. [DOI] [PubMed] [Google Scholar]
- 49.Gamez-Diaz L, August D, Stepensky P, Revel-Vilk S, Seidel MG, Noriko M, et al. The extended phenotype of LPS-responsive beige-like anchor protein (LRBA) deficiency. J Allergy Clin Immunol. 2016;137(1):223–30. doi: 10.1016/j.jaci.2015.09.025. [DOI] [PubMed] [Google Scholar]
- 50.Mustillo PJ, editor. Response to hydroxychloroquine in CVID with granulomatous interstitial lung disease (GL-ILD).. Clinical Immunology Society (CIS) Annual Meeting; Boston, MA.. 2016. [Google Scholar]
- 51.Weinacht KG, Charbonnier Lm, Plant A, Torgerson T, Rosenzweig S, Fleisher T, et al., editors. Successful Therapy of a Patient with a Novel STAT1 Gain of Function Mutation and Life-Threatening Cytopenias with Janus Kinase Inhibitor Ruxolitinib.. American Society of Hematology (ASH) Annual Meeting; Orlando, FL.. 2015. [Google Scholar]
- 52.Mizoguchi Y, Tsumura M, Okada S, Hirata O, Minegishi S, Imai K, et al. Simple diagnosis of STAT1 gain-of-function alleles in patients with chronic mucocutaneous candidiasis. J Leukoc Biol. 2014;95(4):667–76. doi: 10.1189/jlb.0513250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Higgins E, Al Shehri T, McAleer MA, Conlon N, Feighery C, Lilic D, et al. Use of ruxolitinib to successfully treat chronic mucocutaneous candidiasis caused by gain-of-function signal transducer and activator of transcription 1 (STAT1) mutation. J Allergy Clin Immunol. 2015;135(2):551–3. doi: 10.1016/j.jaci.2014.12.1867. [DOI] [PubMed] [Google Scholar]
- 54.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, et al. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111(5 Pt 1):e622–7. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 55.Moratto D, Giliani S, Bonfim C, Mazzolari E, Fischer A, Ochs HD, et al. Long-term outcome and lineage-specific chimerism in 194 patients with Wiskott-Aldrich syndrome treated by hematopoietic cell transplantation in the period 1980-2009: an international collaborative study. Blood. 2011;118(6):1675–84. doi: 10.1182/blood-2010-11-319376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ozsahin H, Cavazzana-Calvo M, Notarangelo LD, Schulz A, Thrasher AJ, Mazzolari E, et al. Long-term outcome following hematopoietic stem-cell transplantation in Wiskott-Aldrich syndrome: collaborative study of the European Society for Immunodeficiencies and European Group for Blood and Marrow Transplantation. Blood. 2008;111(1):439–45. doi: 10.1182/blood-2007-03-076679. [DOI] [PubMed] [Google Scholar]
- 57.Conyers RK, Cole TS. Successful second bone marrow transplantation in a Wiskott-Aldrich syndrome patient with systemic vasculitis. J Allergy Clin Immunol. 2016;137(5):1615–6. doi: 10.1016/j.jaci.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 58.Hacein-Bey Abina S, Gaspar HB, Blondeau J, Caccavelli L, Charrier S, Buckland K, et al. Outcomes following gene therapy in patients with severe Wiskott-Aldrich syndrome. JAMA. 2015;313(15):1550–63. doi: 10.1001/jama.2015.3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shin CR, Kim MO, Li D, Bleesing JJ, Harris R, Mehta P, et al. Outcomes following hematopoietic cell transplantation for Wiskott-Aldrich syndrome. Bone Marrow Transplant. 2012;47(11):1428–35. doi: 10.1038/bmt.2012.31. [DOI] [PubMed] [Google Scholar]
- 60.Seidel MG, Urban C, Sipurzynski J, Beham-Schmid C, Lackner H, Benesch M. High response rate but short-term effect of romiplostim in paediatric refractory chronic immune thrombocytopenia. Br J Haematol. 2014;165(3):419–21. doi: 10.1111/bjh.12766. [DOI] [PubMed] [Google Scholar]
- 61.Gerrits AJ, Leven EA, Frelinger AL, 3rd, Brigstocke SL, Berny-Lang MA, Mitchell WB, et al. Effects of eltrombopag on platelet count and platelet activation in Wiskott-Aldrich syndrome/X-linked thrombocytopenia. Blood. 2015;126(11):1367–78. doi: 10.1182/blood-2014-09-602573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Seidel MG. Autoimmune and other cytopenias in primary immunodeficiencies: pathomechanisms, novel differential diagnoses, and treatment. Blood. 2014;124(15):2337–44. doi: 10.1182/blood-2014-06-583260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Walter JE, Rosen LB, Csomos K, Rosenberg JM, Mathew D, Keszei M, et al. Broad-spectrum antibodies against self-antigens and cytokines in RAG deficiency. J Clin Invest. 2015;125(11):4135–48. doi: 10.1172/JCI80477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Notarangelo LD, Kim MS, Walter JE, Lee YN. Human RAG mutations: biochemistry and clinical implications. Nat Rev Immunol. 2016;16(4):234–46. doi: 10.1038/nri.2016.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Villa A, Marrella V, Rucci F, Notarangelo LD. Genetically determined lymphopenia and autoimmune manifestations. Curr Opin Immunol. 2008;20(3):318–24. doi: 10.1016/j.coi.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 66.Walter JE, Rucci F, Patrizi L, Recher M, Regenass S, Paganini T, et al. Expansion of immunoglobulin-secreting cells and defects in B cell tolerance in Rag-dependent immunodeficiency. J Exp Med. 2010;207(7):1541–54. doi: 10.1084/jem.20091927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kreuzaler M, Rauch M, Salzer U, Birmelin J, Rizzi M, Grimbacher B, et al. Soluble BAFF levels inversely correlate with peripheral B cell numbers and the expression of BAFF receptors. J Immunol. 2012;188(1):497–503. doi: 10.4049/jimmunol.1102321. [DOI] [PubMed] [Google Scholar]
- 68.Foldvari Z, Walter JE, editors. Clinical Spectrum and Outcome of Treatment for Autoimmune Cytopenias in RAG Deficiency.; Clinical Immunology Society Annual Meeting. Boston, MA.. 2016. [Google Scholar]
- 69.Lee PP, Woodbine L, Gilmour KC, Bibi S, Cale CM, Amrolia PJ, et al. The many faces of Artemis-deficient combined immunodeficiency - Two patients with DCLRE1C mutations and a systematic literature review of genotype-phenotype correlation. Clin Immunol. 2013;149(3):464–74. doi: 10.1016/j.clim.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 70.Henderson LA, Frugoni F, Hopkins G, de Boer H, Pai SY, Lee YN, et al. Expanding the spectrum of recombination-activating gene 1 deficiency: a family with early-onset autoimmunity. J Allergy Clin Immunol. 2013;132(4):969–71. e1–2. doi: 10.1016/j.jaci.2013.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Ravin SS, Cowen EW, Zarember KA, Whiting-Theobald NL, Kuhns DB, Sandler NG, et al. Hypomorphic Rag mutations can cause destructive midline granulomatous disease. Blood. 2010;116(8):1263–71. doi: 10.1182/blood-2010-02-267583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mathieu AL, Verronese E, Rice GI, Fouyssac F, Bertrand Y, Picard C, et al. PRKDC mutations associated with immunodeficiency, granuloma, and autoimmune regulator-dependent autoimmunity. J Allergy Clin Immunol. 2015;135(6):1578–88. e5. doi: 10.1016/j.jaci.2015.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Aksentijevich I, Kastner DL. Genetics of monogenic autoinflammatory diseases: past successes, future challenges. Nat Rev Rheumatol. 2011;7(8):469–78. doi: 10.1038/nrrheum.2011.94. [DOI] [PubMed] [Google Scholar]
- 74.ter Haar NM, Oswald M, Jeyaratnam J, Anton J, Barron KS, Brogan PA, et al. Recommendations for the management of autoinflammatory diseases. Annals of the rheumatic diseases. 2015;74(9):1636–44. doi: 10.1136/annrheumdis-2015-207546. [DOI] [PubMed] [Google Scholar]
- 75.Sturfelt G, Truedsson L. Complement in the immunopathogenesis of rheumatic disease. Nat Rev Rheumatol. 2012;8(8):458–68. doi: 10.1038/nrrheum.2012.75. [DOI] [PubMed] [Google Scholar]
- 76.Al Hamzi H, Al Shaikh A, Arnaout RK. Poor specific antibody response immunodeficiency (dysgammaglobulinemia) predates systemic lupus erythematosus. Lupus. 2013;22(9):961–6. doi: 10.1177/0961203313497820. [DOI] [PubMed] [Google Scholar]
- 77.Hill F, Yonkof J, Chaitanya Arudra SK, Thomas J, Altorok N. Successful Treatment of ANCA-Associated Vasculitis in the Setting of Common Variable Immunodeficiency Using Rituximab. Am J Ther. 2015 doi: 10.1097/MJT.0000000000000323. [DOI] [PubMed] [Google Scholar]
- 78.Lutalo PM, D'Cruz DP. Update on belimumab for the management of systemic lupus erythematosus. Expert Opin Biol Ther. 2014;14(11):1701–8. doi: 10.1517/14712598.2014.959924. [DOI] [PubMed] [Google Scholar]
- 79.Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32(8):388–94. doi: 10.1016/j.it.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, et al. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190(11):1697–710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miyara M, Ito Y, Sakaguchi S. TREG-cell therapies for autoimmune rheumatic diseases. Nat Rev Rheumatol. 2014;10(9):543–51. doi: 10.1038/nrrheum.2014.105. [DOI] [PubMed] [Google Scholar]
- 82.Levy E, Stolzenberg MC, Bruneau J, Breton S, Neven B, Sauvion S, et al. LRBA deficiency with autoimmunity and early onset chronic erosive polyarthritis. Clin Immunol. 2016 doi: 10.1016/j.clim.2016.03.006. [DOI] [PubMed] [Google Scholar]
- 83.Schubert D, Bode C, Kenefeck R, Hou TZ, Wing JB, Kennedy A, et al. Autosomal dominant immune dysregulation syndrome in humans with CTLA4 mutations. Nat Med. 2014;20(12):1410–6. doi: 10.1038/nm.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milner JD, Vogel TP, Forbes L, Ma CA, Stray-Pedersen A, Niemela JE, et al. Early-onset lymphoproliferation and autoimmunity caused by germline STAT3 gain-of-function mutations. Blood. 2015;125(4):591–9. doi: 10.1182/blood-2014-09-602763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Flanagan SE, Haapaniemi E, Russell MA, Caswell R, Lango Allen H, De Franco E, et al. Activating germline mutations in STAT3 cause early-onset multi-organ autoimmune disease. Nat Genet. 2014;46(8):812–4. doi: 10.1038/ng.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee PP, Chen TX, Jiang LP, Chan KW, Yang W, Lee BW, et al. Clinical characteristics and genotype phenotype correlation in 62 patients with X-linked agammaglobulinemia. J Clin Immunol. 2010;30(1):121–31. doi: 10.1007/s10875-009-9341-5. [DOI] [PubMed] [Google Scholar]
- 87.Verbruggen G, De Backer S, Deforce D, Demetter P, Cuvelier C, Veys E, et al. X linked agammaglobulinaemia and rheumatoid arthritis. Ann Rheum Dis. 2005;64(7):1075–8. doi: 10.1136/ard.2004.030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu Z, Kang Y, Lin Z, Huang Y, Lv H, Li Y. X-linked agammaglobulinemia combined with juvenile idiopathic arthritis and invasive Klebsiella pneumoniae polyarticular septic arthritis. Clin Rheumatol. 2015;34(2):397–401. doi: 10.1007/s10067-014-2537-y. [DOI] [PubMed] [Google Scholar]
- 89.Vancsa A, Toth B, Szekanecz Z. BTK gene mutation in two non-identical twins with X-linked agammaglobulinemia associated with polyarticular juvenile idiopathic arthritis. Isr Med Assoc J. 2011;13(9):579–80. [PubMed] [Google Scholar]
- 90.Sukumaran S, Marzan K, Shaham B, Church JA. A child with x-linked agammaglobulinemia and enthesitis-related arthritis. Int J Rheumatol. 2011;2011:175973. doi: 10.1155/2011/175973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lopez-Herrera G, Vargas-Hernandez A, Gonzalez-Serrano ME, Berron-Ruiz L, Rodriguez-Alba JC, Espinosa-Rosales F, et al. Bruton's tyrosine kinase--an integral protein of B cell development that also has an essential role in the innate immune system. J Leukoc Biol. 2014;95(2):243–50. doi: 10.1189/jlb.0513307. [DOI] [PubMed] [Google Scholar]
- 92.Nyhoff LE, Barron BL, Johnson EM, Bonami RH, Maseda D, Fensterheim BA, et al. Bruton's Tyrosine Kinase Deficiency Inhibits Autoimmune Arthritis in Mice but Fails to Block Immune Complex-Mediated Inflammatory Arthritis. Arthritis Rheumatol. 2016;68(8):1856–68. doi: 10.1002/art.39657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Brown JR, Goldblatt D, Buddle J, Morton L, Thrasher AJ. Diminished production of anti-inflammatory mediators during neutrophil apoptosis and macrophage phagocytosis in chronic granulomatous disease (CGD). J Leukoc Biol. 2003;73(5):591–9. doi: 10.1189/jlb.1202599. [DOI] [PubMed] [Google Scholar]
- 94.Fernandez-Boyanapalli RF, Frasch SC, McPhillips K, Vandivier RW, Harry BL, Riches DW, et al. Impaired apoptotic cell clearance in CGD due to altered macrophage programming is reversed by phosphatidylserine-dependent production of IL-4. Blood. 2009;113(9):2047–55. doi: 10.1182/blood-2008-05-160564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.George-Chandy A, Nordstrom I, Nygren E, Jonsson IM, Postigo J, Collins LV, et al. Th17 development and autoimmune arthritis in the absence of reactive oxygen species. Eur J Immunol. 2008;38(4):1118–26. doi: 10.1002/eji.200737348. [DOI] [PubMed] [Google Scholar]
- 96.De Ravin SS, Naumann N, Cowen EW, Friend J, Hilligoss D, Marquesen M, et al. Chronic granulomatous disease as a risk factor for autoimmune disease. J Allergy Clin Immunol. 2008;122(6):1097–103. doi: 10.1016/j.jaci.2008.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Manzi S, Urbach AH, McCune AB, Altman HA, Kaplan SS, Medsger TA, Jr., et al. Systemic lupus erythematosus in a boy with chronic granulomatous disease: case report and review of the literature. Arthritis Rheum. 1991;34(1):101–5. doi: 10.1002/art.1780340116. [DOI] [PubMed] [Google Scholar]
- 98.Battersby AC, Cale AM, Goldblatt D, Gennery AR. Clinical manifestations of disease in X-linked carriers of chronic granulomatous disease. J Clin Immunol. 2013;33(8):1276–84. doi: 10.1007/s10875-013-9939-5. [DOI] [PubMed] [Google Scholar]
- 99.Winkelstein JA, Marino MC, Johnston RB, Jr., Boyle J, Curnutte J, Gallin JI, et al. Chronic granulomatous disease. Report on a national registry of 368 patients. Medicine (Baltimore) 2000;79(3):155–69. doi: 10.1097/00005792-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 100.Chen K, Wu W, Mathew D, Zhang Y, Browne SK, Rosen LB, et al. Autoimmunity due to RAG deficiency and estimated disease incidence in RAG1/2 mutations. J Allergy Clin Immunol. 2014;133(3):880–2. e10. doi: 10.1016/j.jaci.2013.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Schuetz C, Neven B, Dvorak CC, Leroy S, Ege MJ, Pannicke U, et al. SCID patients with ARTEMIS vs RAG deficiencies following HCT: increased risk of late toxicity in ARTEMIS-deficient SCID. Blood. 2014;123(2):281–9. doi: 10.1182/blood-2013-01-476432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Agarwal S, Mayer L. Diagnosis and treatment of gastrointestinal disorders in patients with primary immunodeficiency. Clin Gastroenterol Hepatol. 2013;11(9):1050–63. doi: 10.1016/j.cgh.2013.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Uzzan M, Ko HM, Mehandru S, Cunningham-Rundles C. Gastrointestinal Disorders Associated with Common Variable Immune Deficiency (CVID) and Chronic Granulomatous Disease (CGD). Curr Gastroenterol Rep. 2016;18(4):17. doi: 10.1007/s11894-016-0491-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Malamut G, Verkarre V, Suarez F, Viallard JF, Lascaux AS, Cosnes J, et al. The enteropathy associated with common variable immunodeficiency: the delineated frontiers with celiac disease. Am J Gastroenterol. 2010;105(10):2262–75. doi: 10.1038/ajg.2010.214. [DOI] [PubMed] [Google Scholar]
- 105.Agarwal S, Smereka P, Harpaz N, Cunningham-Rundles C, Mayer L. Characterization of immunologic defects in patients with common variable immunodeficiency (CVID) with intestinal disease. Inflamm Bowel Dis. 2011;17(1):251–9. doi: 10.1002/ibd.21376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Daniels JA, Lederman HM, Maitra A, Montgomery EA. Gastrointestinal tract pathology in patients with common variable immunodeficiency (CVID): a clinicopathologic study and review. Am J Surg Pathol. 2007;31(12):1800–12. doi: 10.1097/PAS.0b013e3180cab60c. [DOI] [PubMed] [Google Scholar]
- 107.Chase NM, Verbsky JW, Hintermeyer MK, Waukau JK, Tomita-Mitchell A, Casper JT, et al. Use of combination chemotherapy for treatment of granulomatous and lymphocytic interstitial lung disease (GLILD) in patients with common variable immunodeficiency (CVID). J Clin Immunol. 2013;33(1):30–9. doi: 10.1007/s10875-012-9755-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Boursiquot JN, Gerard L, Malphettes M, Fieschi C, Galicier L, Boutboul D, et al. Granulomatous disease in CVID: retrospective analysis of clinical characteristics and treatment efficacy in a cohort of 59 patients. J Clin Immunol. 2013;33(1):84–95. doi: 10.1007/s10875-012-9778-9. [DOI] [PubMed] [Google Scholar]
- 109.Chua I, Standish R, Lear S, Harbord M, Eren E, Raeiszadeh M, et al. Anti-tumour necrosis factor-alpha therapy for severe enteropathy in patients with common variable immunodeficiency (CVID). Clin Exp Immunol. 2007;150(2):306–11. doi: 10.1111/j.1365-2249.2007.03481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Vazquez-Moron JM, Pallares-Manrique H, Martin-Suarez IJ, Benitez-Rodriguez B, Ramos-Lora M. Crohn's-like disease in a patient with common variable immunodeficiency treated with azathioprine and adalimumab. Rev Esp Enferm Dig. 2013;105(5):299–302. doi: 10.4321/s1130-01082013000500010. [DOI] [PubMed] [Google Scholar]
- 111.Bennett CL, Ochs HD. IPEX is a unique X-linked syndrome characterized by immune dysfunction, polyendocrinopathy, enteropathy, and a variety of autoimmune phenomena. Curr Opin Pediatr. 2001;13(6):533–8. doi: 10.1097/00008480-200112000-00007. [DOI] [PubMed] [Google Scholar]
- 112.Bacchetta R, Barzaghi F, Roncarolo MG. From IPEX syndrome to FOXP3 mutation: a lesson on immune dysregulation. Ann N Y Acad Sci. 2016 doi: 10.1111/nyas.13011. [DOI] [PubMed] [Google Scholar]
- 113.Kinnunen T, Chamberlain N, Morbach H, Choi J, Kim S, Craft J, et al. Accumulation of peripheral autoreactive B cells in the absence of functional human regulatory T cells. Blood. 2013;121(9):1595–603. doi: 10.1182/blood-2012-09-457465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kobayashi I, Kubota M, Yamada M, Tanaka H, Itoh S, Sasahara Y, et al. Autoantibodies to villin occur frequently in IPEX, a severe immune dysregulation, syndrome caused by mutation of FOXP3. Clin Immunol. 2011;141(1):83–9. doi: 10.1016/j.clim.2011.05.010. [DOI] [PubMed] [Google Scholar]
- 115.Patey-Mariaud de Serre N, Canioni D, Ganousse S, Rieux-Laucat F, Goulet O, Ruemmele F, et al. Digestive histopathological presentation of IPEX syndrome. Mod Pathol. 2009;22(1):95–102. doi: 10.1038/modpathol.2008.161. [DOI] [PubMed] [Google Scholar]
- 116.Bindl L, Torgerson T, Perroni L, Youssef N, Ochs HD, Goulet O, et al. Successful use of the new immune-suppressor sirolimus in IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome). J Pediatr. 2005;147(2):256–9. doi: 10.1016/j.jpeds.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 117.Yong PL, Russo P, Sullivan KE. Use of sirolimus in IPEX and IPEX-like children. J Clin Immunol. 2008;28(5):581–7. doi: 10.1007/s10875-008-9196-1. [DOI] [PubMed] [Google Scholar]
- 118.Gambineri E, Perroni L, Passerini L, Bianchi L, Doglioni C, Meschi F, et al. Clinical and molecular profile of a new series of patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome: inconsistent correlation between forkhead box protein 3 expression and disease severity. J Allergy Clin Immunol. 2008;122(6):1105–12. e1. doi: 10.1016/j.jaci.2008.09.027. [DOI] [PubMed] [Google Scholar]
- 119.Kuehn HS, Ouyang W, Lo B, Deenick EK, Niemela JE, Avery DT, et al. Immune dysregulation in human subjects with heterozygous germline mutations in CTLA4. Science. 2014;345(6204):1623–7. doi: 10.1126/science.1255904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lopez-Herrera G, Tampella G, Pan-Hammarstrom Q, Herholz P, Trujillo-Vargas CM, Phadwal K, et al. Deleterious mutations in LRBA are associated with a syndrome of immune deficiency and autoimmunity. Am J Hum Genet. 2012;90(6):986–1001. doi: 10.1016/j.ajhg.2012.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Uzel G, Sampaio EP, Lawrence MG, Hsu AP, Hackett M, Dorsey MJ, et al. Dominant gain-of-function STAT1 mutations in FOXP3 wild-type immune dysregulation-polyendocrinopathy-enteropathy-X-linked-like syndrome. J Allergy Clin Immunol. 2013;131(6):1611–23. doi: 10.1016/j.jaci.2012.11.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Schuetz C, Pannicke U, Jacobsen EM, Burggraf S, Albert MH, Honig M, et al. Lesson from hypomorphic recombination-activating gene (RAG) mutations: Why asymptomatic siblings should also be tested. J Allergy Clin Immunol. 2014;133(4):1211–5. doi: 10.1016/j.jaci.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 123.Sanal O, Jing H, Ozgur T, Ayvaz D, Strauss-Albee DM, Ersoy-Evans S, et al. Additional diverse findings expand the clinical presentation of DOCK8 deficiency. J Clin Immunol. 2012;32(4):698–708. doi: 10.1007/s10875-012-9664-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lohr NJ, Molleston JP, Strauss KA, Torres-Martinez W, Sherman EA, Squires RH, et al. Human ITCH E3 ubiquitin ligase deficiency causes syndromic multisystem autoimmune disease. Am J Hum Genet. 2010;86(3):447–53. doi: 10.1016/j.ajhg.2010.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jin HS, Park Y, Elly C, Liu YC. Itch expression by Treg cells controls Th2 inflammatory responses. J Clin Invest. 2013;123(11):4923–34. doi: 10.1172/JCI69355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bertrand A, Kostine M, Barnetche T, Truchetet ME, Schaeverbeke T. Immune related adverse events associated with anti-CTLA-4 antibodies: systematic review and meta-analysis. BMC Med. 2015;13:211. doi: 10.1186/s12916-015-0455-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pedros C, Duguet F, Saoudi A, Chabod M. Disrupted regulatory T cell homeostasis in inflammatory bowel diseases. World J Gastroenterol. 2016;22(3):974–95. doi: 10.3748/wjg.v22.i3.974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Magnani A, Brosselin P, Beaute J, de Vergnes N, Mouy R, Debre M, et al. Inflammatory manifestations in a single-center cohort of patients with chronic granulomatous disease. J Allergy Clin Immunol. 2014;134(3):655–62. e8. doi: 10.1016/j.jaci.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 129.Marks DJ, Miyagi K, Rahman FZ, Novelli M, Bloom SL, Segal AW. Inflammatory bowel disease in CGD reproduces the clinicopathological features of Crohn's disease. Am J Gastroenterol. 2009;104(1):117–24. doi: 10.1038/ajg.2008.72. [DOI] [PubMed] [Google Scholar]
- 130.Alimchandani M, Lai JP, Aung PP, Khangura S, Kamal N, Gallin JI, et al. Gastrointestinal histopathology in chronic granulomatous disease: a study of 87 patients. Am J Surg Pathol. 2013;37(9):1365–72. doi: 10.1097/PAS.0b013e318297427d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Khangura SK, Kamal N, Ho N, Quezado M, Zhao X, Marciano B, et al. Gastrointestinal Features of Chronic Granulomatous Disease Found During Endoscopy. Clin Gastroenterol Hepatol. 2016;14(3):395–402. e5. doi: 10.1016/j.cgh.2015.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Noel N, Mahlaoui N, Blanche S, Suarez F, Coignard-Biehler H, Durieu I, et al. Efficacy and safety of thalidomide in patients with inflammatory manifestations of chronic granulomatous disease: a retrospective case series. J Allergy Clin Immunol. 2013;132(4):997–1000. e1–4. doi: 10.1016/j.jaci.2013.04.059. [DOI] [PubMed] [Google Scholar]
- 133.Rosh JR, Tang HB, Mayer L, Groisman G, Abraham SK, Prince A. Treatment of intractable gastrointestinal manifestations of chronic granulomatous disease with cyclosporine. J Pediatr. 1995;126(1):143–5. doi: 10.1016/s0022-3476(95)70519-8. [DOI] [PubMed] [Google Scholar]
- 134.Uzel G, Orange JS, Poliak N, Marciano BE, Heller T, Holland SM. Complications of tumor necrosis factor-alpha blockade in chronic granulomatous disease-related colitis. Clin Infect Dis. 2010;51(12):1429–34. doi: 10.1086/657308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Seger RA, Gungor T, Belohradsky BH, Blanche S, Bordigoni P, Di Bartolomeo P, et al. Treatment of chronic granulomatous disease with myeloablative conditioning and an unmodified hemopoietic allograft: a survey of the European experience, 1985-2000. Blood. 2002;100(13):4344–50. doi: 10.1182/blood-2002-02-0583. [DOI] [PubMed] [Google Scholar]
- 136.Gungor T, Teira P, Slatter M, Stussi G, Stepensky P, Moshous D, et al. Reduced-intensity conditioning and HLA-matched haemopoietic stem-cell transplantation in patients with chronic granulomatous disease: a prospective multicentre study. Lancet. 2014;383(9915):436–48. doi: 10.1016/S0140-6736(13)62069-3. [DOI] [PubMed] [Google Scholar]
- 137.Burroughs LM, Torgerson TR, Storb R, Carpenter PA, Rawlings DJ, Sanders J, et al. Stable hematopoietic cell engraftment after low-intensity nonmyeloablative conditioning in patients with immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome. J Allergy Clin Immunol. 2010;126(5):1000–5. doi: 10.1016/j.jaci.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kucuk ZY, Bleesing JJ, Marsh R, Zhang K, Davies S, Filipovich AH. A challenging undertaking: Stem cell transplantation for immune dysregulation, polyendocrinopathy, enteropathy, X-linked (IPEX) syndrome. J Allergy Clin Immunol. 2016;137(3):953–5. e4. doi: 10.1016/j.jaci.2015.09.030. [DOI] [PubMed] [Google Scholar]
- 139.Horino S, Sasahara Y, Sato M, Niizuma H, Kumaki S, Abukawa D, et al. Selective expansion of donor-derived regulatory T cells after allogeneic bone marrow transplantation in a patient with IPEX syndrome. Pediatr Transplant. 2014;18(1):E25–30. doi: 10.1111/petr.12184. [DOI] [PubMed] [Google Scholar]
- 140.Kasow KA, Morales-Tirado VM, Wichlan D, Shurtleff SA, Abraham A, Persons DA, et al. Therapeutic in vivo selection of thymic-derived natural T regulatory cells following non-myeloablative hematopoietic stem cell transplant for IPEX. Clin Immunol. 2011;141(2):169–76. doi: 10.1016/j.clim.2011.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Seidel MG, Fritsch G, Lion T, Jurgens B, Heitger A, Bacchetta R, et al. Selective engraftment of donor CD4+25high FOXP3-positive T cells in IPEX syndrome after nonmyeloablative hematopoietic stem cell transplantation. Blood. 2009;113(22):5689–91. doi: 10.1182/blood-2009-02-206359. [DOI] [PubMed] [Google Scholar]
- 142.Zhan H, Sinclair J, Adams S, Cale CM, Murch S, Perroni L, et al. Immune reconstitution and recovery of FOXP3 (forkhead box P3)-expressing T cells after transplantation for IPEX (immune dysregulation, polyendocrinopathy, enteropathy, X-linked) syndrome. Pediatrics. 2008;121(4):e998–1002. doi: 10.1542/peds.2007-1863. [DOI] [PubMed] [Google Scholar]
- 143.Mazzolari E, Forino C, Fontana M, D'Ippolito C, Lanfranchi A, Gambineri E, et al. A new case of IPEX receiving bone marrow transplantation. Bone Marrow Transplant. 2005;35(10):1033–4. doi: 10.1038/sj.bmt.1704954. [DOI] [PubMed] [Google Scholar]
- 144.Chapel H, Lucas M, Lee M, Bjorkander J, Webster D, Grimbacher B, et al. Common variable immunodeficiency disorders: division into distinct clinical phenotypes. Blood. 2008;112(2):277–86. doi: 10.1182/blood-2007-11-124545. [DOI] [PubMed] [Google Scholar]
- 145.Quinti I, Soresina A, Spadaro G, Martino S, Donnanno S, Agostini C, et al. Long-term follow-up and outcome of a large cohort of patients with common variable immunodeficiency. J Clin Immunol. 2007;27(3):308–16. doi: 10.1007/s10875-007-9075-1. [DOI] [PubMed] [Google Scholar]
- 146.Atarod L, Raissi A, Aghamohammadi A, Farhoudi A, Khodadad A, Moin M, et al. A review of gastrointestinal disorders in patients with primary antibody immunodeficiencies during a 10-year period (1990-2000), in children hospital medical center. Iran J Allergy Asthma Immunol. 2003;2(2):75–9. [PubMed] [Google Scholar]
- 147.Chun JK, Lee TJ, Song JW, Linton JA, Kim DS. Analysis of clinical presentations of Bruton disease: a review of 20 years of accumulated data from pediatric patients at Severance Hospital. Yonsei Med J. 2008;49(1):28–36. doi: 10.3349/ymj.2008.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Garcia-Garcia E, Staines-Boone AT, Vargas-Hernandez A, Gonzalez-Serrano ME, Carrillo-Tapia E, Mogica-Martinez D, et al. Clinical and mutational features of X-linked agammaglobulinemia in Mexico. Clin Immunol. 2016;165:38–44. doi: 10.1016/j.clim.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 149.Bateman EA, Ayers L, Sadler R, Lucas M, Roberts C, Woods A, et al. T cell phenotypes in patients with common variable immunodeficiency disorders: associations with clinical phenotypes in comparison with other groups with recurrent infections. Clin Exp Immunol. 2012;170(2):202–11. doi: 10.1111/j.1365-2249.2012.04643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Hernandez-Trujillo VP, Scalchunes C, Cunningham-Rundles C, Ochs HD, Bonilla FA, Paris K, et al. Autoimmunity and inflammation in X-linked agammaglobulinemia. J Clin Immunol. 2014;34(6):627–32. doi: 10.1007/s10875-014-0056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Neven B, Magerus-Chatinet A, Florkin B, Gobert D, Lambotte O, De Somer L, et al. A survey of 90 patients with autoimmune lymphoproliferative syndrome related to TNFRSF6 mutation. Blood. 2011;118(18):4798–807. doi: 10.1182/blood-2011-04-347641. [DOI] [PubMed] [Google Scholar]
- 152.Sneller MC, Dale JK, Straus SE. Autoimmune lymphoproliferative syndrome. Curr Opin Rheumatol. 2003;15(4):417–21. doi: 10.1097/00002281-200307000-00008. [DOI] [PubMed] [Google Scholar]
- 153.Ucar D, Kim JS, Bishop RJ, Nussenblatt RB, Rao VK, Sen HN. Ocular Inflammatory Disorders in Autoimmune Lymphoproliferative Syndrome (ALPS). Ocul Immunol Inflamm. 2016:1–7. doi: 10.1080/09273948.2016.1175637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Rieux-Laucat F, Blachere S, Danielan S, De Villartay JP, Oleastro M, Solary E, et al. Lymphoproliferative syndrome with autoimmunity: A possible genetic basis for dominant expression of the clinical manifestations. Blood. 1999;94(8):2575–82. [PubMed] [Google Scholar]
- 155.Jackson CE, Fischer RE, Hsu AP, Anderson SM, Choi Y, Wang J, et al. Autoimmune lymphoproliferative syndrome with defective Fas: genotype influences penetrance. Am J Hum Genet. 1999;64(4):1002–14. doi: 10.1086/302333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gennery AR, Barge D, O'Sullivan JJ, Flood TJ, Abinun M, Cant AJ. Antibody deficiency and autoimmunity in 22q11.2 deletion syndrome. Arch Dis Child. 2002;86(6):422–5. doi: 10.1136/adc.86.6.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Jawad AF, McDonald-Mcginn DM, Zackai E, Sullivan KE. Immunologic features of chromosome 22q11.2 deletion syndrome (DiGeorge syndrome/velocardiofacial syndrome). J Pediatr. 2001;139(5):715–23. doi: 10.1067/mpd.2001.118534. [DOI] [PubMed] [Google Scholar]
- 158.Tison BE, Nicholas SK, Abramson SL, Hanson IC, Paul ME, Seeborg FO, et al. Autoimmunity in a cohort of 130 pediatric patients with partial DiGeorge syndrome. J Allergy Clin Immunol. 2011;128(5):1115–7. e1–3. doi: 10.1016/j.jaci.2011.06.043. [DOI] [PubMed] [Google Scholar]
- 159.Alkhairy OK, Abolhassani H, Rezaei N, Fang M, Andersen KK, Chavoshzadeh Z, et al. Spectrum of Phenotypes Associated with Mutations in LRBA. J Clin Immunol. 2016;36(1):33–45. doi: 10.1007/s10875-015-0224-7. [DOI] [PubMed] [Google Scholar]
- 160.Barzaghi F, Passerini L, Bacchetta R. Immune dysregulation, polyendocrinopathy, enteropathy, x-linked syndrome: a paradigm of immunodeficiency with autoimmunity. Front Immunol. 2012;3:211. doi: 10.3389/fimmu.2012.00211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Baris S, Alroqi F, Kiykim A, Karakoc-Aydiner E, Ogulur I, Ozen A, et al. Severe Early-Onset Combined Immunodeficiency due to Heterozygous Gain-of-Function Mutations in STAT1. J Clin Immunol. 2016 doi: 10.1007/s10875-016-0312-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Toubiana J, Okada S, Hiller J, Oleastro M, Lagos Gomez M, Aldave Becerra JC, et al. Heterozygous STAT1 gain-of-function mutations underlie an unexpectedly broad clinical phenotype. Blood. 2016;127(25):3154–64. doi: 10.1182/blood-2015-11-679902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Sullivan KE, Mullen CA, Blaese RM, Winkelstein JA. A multiinstitutional survey of the Wiskott-Aldrich syndrome. J Pediatr. 1994;125(6 Pt 1):876–85. doi: 10.1016/s0022-3476(05)82002-5. [DOI] [PubMed] [Google Scholar]
- 164.Song E, Jaishankar GB, Saleh H, Jithpratuck W, Sahni R, Krishnaswamy G. Chronic granulomatous disease: a review of the infectious and inflammatory complications. Clin Mol Allergy. 2011;9(1):10. doi: 10.1186/1476-7961-9-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Dutmer CM, Asturias EJ, Smith C, Dishop MK, Schmid DS, Bellini WJ, et al. Late Onset Hypomorphic RAG2 Deficiency Presentation with Fatal Vaccine-Strain VZV Infection. J Clin Immunol. 2015;35(8):754–60. doi: 10.1007/s10875-015-0207-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Moshous D, Pannetier C, Chasseval Rd R, Deist Fl F, Cavazzana-Calvo M, Romana S, et al. Partial T and B lymphocyte immunodeficiency and predisposition to lymphoma in patients with hypomorphic mutations in Artemis. J Clin Invest. 2003;111(3):381–7. doi: 10.1172/JCI16774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Sauer AV, Brigida I, Carriglio N, Aiuti A. Autoimmune dysregulation and purine metabolism in adenosine deaminase deficiency. Front Immunol. 2012;3:265. doi: 10.3389/fimmu.2012.00265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Delicou S, Kitra-Roussou V, Peristeri J, Goussetis E, Vessalas G, Rigatou E, et al. Successful HLA-identical hematopoietic stem cell transplantation in a patient with purine nucleoside phosphorylase deficiency. Pediatr Transplant. 2007;11(7):799–803. doi: 10.1111/j.1399-3046.2007.00772.x. [DOI] [PubMed] [Google Scholar]
- 169.McCann LJ, McPartland J, Barge D, Strain L, Bourn D, Calonje E, et al. Phenotypic variations of cartilage hair hypoplasia: granulomatous skin inflammation and severe T cell immunodeficiency as initial clinical presentation in otherwise well child with short stature. J Clin Immunol. 2014;34(1):42–8. doi: 10.1007/s10875-013-9962-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Morgan NV, Goddard S, Cardno TS, McDonald D, Rahman F, Barge D, et al. Mutation in the TCRalpha subunit constant gene (TRAC) leads to a human immunodeficiency disorder characterized by a lack of TCRalphabeta+ T cells. J Clin Invest. 2011;121(2):695–702. doi: 10.1172/JCI41931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Yu GP, Nadeau KC, Berk DR, de Saint Basile G, Lambert N, Knapnougel P, et al. Genotype, phenotype, and outcomes of nine patients with T-B+NK+ SCID. Pediatr Transplant. 2011;15(7):733–41. doi: 10.1111/j.1399-3046.2011.01563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Tokgoz H, Caliskan U, Keles S, Reisli I, Guiu IS, Morgan NV. Variable presentation of primary immune deficiency: two cases with CD3 gamma deficiency presenting with only autoimmunity. Pediatr Allergy Immunol. 2013;24(3):257–62. doi: 10.1111/pai.12063. [DOI] [PubMed] [Google Scholar]
- 173.Fischer A, Picard C, Chemin K, Dogniaux S, le Deist F, Hivroz C. ZAP70: a master regulator of adaptive immunity. Semin Immunopathol. 2010;32(2):107–16. doi: 10.1007/s00281-010-0196-x. [DOI] [PubMed] [Google Scholar]
- 174.Hauck F, Randriamampita C, Martin E, Gerart S, Lambert N, Lim A, et al. Primary T-cell immunodeficiency with immunodysregulation caused by autosomal recessive LCK deficiency. J Allergy Clin Immunol. 2012;130(5):1144–52. e11. doi: 10.1016/j.jaci.2012.07.029. [DOI] [PubMed] [Google Scholar]
- 175.Nehme NT, Pachlopnik Schmid J, Debeurme F, Andre-Schmutz I, Lim A, Nitschke P, et al. MST1 mutations in autosomal recessive primary immunodeficiency characterized by defective naive T-cell survival. Blood. 2012;119(15):3458–68. doi: 10.1182/blood-2011-09-378364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Abdollahpour H, Appaswamy G, Kotlarz D, Diestelhorst J, Beier R, Schaffer AA, et al. The phenotype of human STK4 deficiency. Blood. 2012;119(15):3450–7. doi: 10.1182/blood-2011-09-378158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Halacli SO, Ayvaz DC, Sun-Tan C, Erman B, Uz E, Yilmaz DY, et al. STK4 (MST1) deficiency in two siblings with autoimmune cytopenias: A novel mutation. Clin Immunol. 2015;161(2):316–23. doi: 10.1016/j.clim.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 178.McCarl CA, Picard C, Khalil S, Kawasaki T, Rother J, Papolos A, et al. ORAI1 deficiency and lack of store-operated Ca2+ entry cause immunodeficiency, myopathy, and ectodermal dysplasia. J Allergy Clin Immunol. 2009;124(6):1311–8. e7. doi: 10.1016/j.jaci.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Picard C, McCarl CA, Papolos A, Khalil S, Luthy K, Hivroz C, et al. STIM1 mutation associated with a syndrome of immunodeficiency and autoimmunity. N Engl J Med. 2009;360(19):1971–80. doi: 10.1056/NEJMoa0900082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Li FY, Chaigne-Delalande B, Su H, Uzel G, Matthews H, Lenardo MJ. XMEN disease: a new primary immunodeficiency affecting Mg2+ regulation of immunity against Epstein-Barr virus. Blood. 2014;123(14):2148–52. doi: 10.1182/blood-2013-11-538686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Crank MC, Grossman JK, Moir S, Pittaluga S, Buckner CM, Kardava L, et al. Mutations in PIK3CD can cause hyper IgM syndrome (HIGM) associated with increased cancer susceptibility. J Clin Immunol. 2014;34(3):272–6. doi: 10.1007/s10875-014-0012-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lucas CL, Kuehn HS, Zhao F, Niemela JE, Deenick EK, Palendira U, et al. Dominant-activating germline mutations in the gene encoding the PI(3)K catalytic subunit p110delta result in T cell senescence and human immunodeficiency. Nat Immunol. 2014;15(1):88–97. doi: 10.1038/ni.2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Stepensky P, Rensing-Ehl A, Gather R, Revel-Vilk S, Fischer U, Nabhani S, et al. Early-onset Evans syndrome, immunodeficiency, and premature immunosenescence associated with tripeptidyl-peptidase II deficiency. Blood. 2015;125(5):753–61. doi: 10.1182/blood-2014-08-593202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Su HC, Jing H, Zhang Q. DOCK8 deficiency. Ann N Y Acad Sci. 2011;1246:26–33. doi: 10.1111/j.1749-6632.2011.06295.x. [DOI] [PubMed] [Google Scholar]
- 185.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ, et al. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. 2009;361(21):2046–55. doi: 10.1056/NEJMoa0905506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Alsum Z, Hawwari A, Alsmadi O, Al-Hissi S, Borrero E, Abu-Staiteh A, et al. Clinical, immunological and molecular characterization of DOCK8 and DOCK8-like deficient patients: single center experience of twenty-five patients. J Clin Immunol. 2013;33(1):55–67. doi: 10.1007/s10875-012-9769-x. [DOI] [PubMed] [Google Scholar]
- 187.Engelhardt KR, Gertz ME, Keles S, Schaffer AA, Sigmund EC, Glocker C, et al. The extended clinical phenotype of 64 patients with dedicator of cytokinesis 8 deficiency. J Allergy Clin Immunol. 2015;136(2):402–12. doi: 10.1016/j.jaci.2014.12.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Griscelli C, Lisowska-Grospierre B, Mach B. Combined immunodeficiency with defective expression in MHC class II genes. Immunodefic Rev. 1989;1(2):135–53. [PubMed] [Google Scholar]