Abstract
Crohn’s disease (CD) and ulcerative colitis (UC) are chronic inflammatory bowel diseases (IBD) characterized by recurrent episodes of mucosal inflammation. This chronic mucosal inflammation has several potential consequences, one of which is the occurrence of colitis associated colorectal cancer (CRC). Over the past decade our understanding of the epidemiology, pathophysiology, and overall approach to diagnosing and managing colitis associated CRC, has grown considerably. In the current review article, we outline these advancements and highlight areas in need of further research.
Keywords: colorectal cancer, dysplasia, colitis, IBD
Epidemiology
The increased risk for colorectal cancer (CRC) among inflammatory bowel disease (IBD) patients with colitis is well established.(1, 2) Earlier studies had suggested a substantial excess risk, with an estimated incidence of nearly 1% per year.(3) More recently, an updated meta-analysis of population based cohort studies has quantified the incidence of CRC among IBD patients to be 1%, 2%, and 5% after 10, 20, and > 20 years of disease duration.(4) Although this would suggest that the burden of this disease is declining, population based data have been conflicting. A Danish study observed that the overall risk for CRC in UC was now similar to that of the general Danish population (Relative Risk [RR] 1.07, 95% CI 0.95 – 1.21), and although the risk of CRC among CD patients had remained stable over time, the risk of CRC among UC patients had declined considerably (1979–1988: RR 1.34, 95% CI 1.13–1.58; 1999–2008: RR 0.57, 95% CI 0.41–0.80).(5)
In contrast, a US based study from the Kaiser Permanente healthcare system observed that the incidence of CRC among both UC and CD patients was 60% higher than the general population, even after accounting for the growth of CRC screening programs. Further, observed incidence remained stable over time for both UC and CD.(6) These variations in observations help to highlight several key issues with prior studies. Dissimilarities in estimates are likely in large part due to differences study designs, outcome classification and ascertainment, an inability to accurately classify disease onset, insufficient study size, and differences in the threshold for performing colectomy.(7) Furthermore, the presence of active inflammation has a substantial impact on the ability to diagnose dysplasia. With advancements in biologic therapies and treatment strategies over time, and improvements in disease control and quality of life, a lead time bias from early dysplasia detection in well-controlled IBD patients without active inflammation may be partially responsible for varying incidence of CRC over-time and across populations, particularly when considering the advancements being made in endoscopic imaging technology. Taken together, colitis associated CRC remains an important consequence of long-standing IBD with an estimated incidence of approximately 5% after 20 years of disease duration, but large-scale high-quality population based studies are still needed to quantify its true burden.
Clinical Risk Factors
Although uncertainty remains as to the true estimate of disease burden, high risk sub-populations and risk factors have consistently been identified, including age of colitis onset, disease extent, duration, and severity, inflammatory complications, primary sclerosing cholangitis (PSC), and family history of CRC. (Table 1(1–30)) For age of disease onset, risk is highest among those diagnosed at a younger age (≤ 15 years), which is perhaps attributable to longer overall disease duration or a more aggressive phenotype among these individuals. An important marker of disease severity and persistence of inflammation may be the development of colonic strictures. Earlier studies suggested that up to 40% of colonic strictures harbored CRC,(8) but more recent studies note much lower, but still substantial, risk, reporting that 2–3.5% of colonic strictures harbor dysplasia or CRC.(9, 10) Furthermore, one these studies suggested that the only factor associated with an increased risk for dysplasia or CRC within colonic strictures was the absence of disease activity at the time of surgery (Odds Ratio [OR] 4.86, 95% CI 1.11 – 21.27).(9) This is a direct contrast to the majority of other risk factors, which are clearly linked to disease activity and inflammation, and highlights the potential gaps in our knowledge and understanding of the pathogenesis of colitis associated CRC. While risk factors have been identified, comparison of the magnitude and significance of risk is a challenge due to variation in study designs, and, in some cases, small sample size. Additionally, the true prevalence of risk factors is difficult to ascertain. A recent population based cohort study demonstrated that the true prevalence of PSC may be much higher than previously estimated.(31) This might suggest that PSC as a risk factor for CRC is only of significance in clinically active PSC. More research on risk factors for colitis associated cancer utilizing population-based data is needed.
Table 1.
Clinical risk factors for colitis associated colorectal cancer
| Age of Onset |
| Increased risk among those diagnosed with IBD at a younger age (≤ 15 years) |
| Disease Extent |
|
Crohn’s Disease: Increased risk when > 30–50% of colonic mucosa involved Ulcerative Colitis: 10–15 fold increased risk with pancolitis throughout disease duration, followed by 2 fold increased risk with left-sided colitis (distal to splenic flexure) until the 4th decade of disease when estimates mirror those of pancolitis, and no risk with proctitis (rectum) |
| Disease Duration and Severity |
| Risk increases with increasing disease severity (endoscopic and histology), and becomes most apparent after 7–10 years with a linear increase thereafter |
| Inflammatory Complications |
| Foreshortened colon, strictures, inflammatory pseudopolyps |
| Primary Sclerosing Cholangitis |
| Predominately right sided lesions, and increased risk present at time of diagnosis as compared to non-PSC IBD patients where risk is apparent after 7–10 years of disease duration. Increased risk remains even after liver transplant and proctocolectomy (i.e. CRC of the pouch). |
| Personal and Family History |
| Additional risk of CRC in IBD patients with a family history of CRC similar to general population. Personal history of dysplasia confers increased risk of synchronous or metachronous CRC |
IBD: Inflammatory bowel disease; PSC: primary sclerosing cholangitis; CRC: colorectal cancer
Pathogenesis
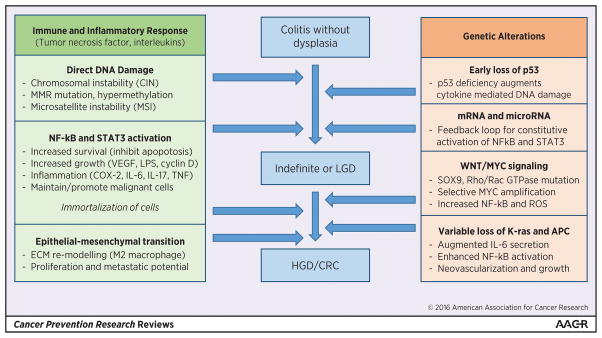
Given the micro-environment within which colitis associated dysplasia and CRC are arising, it is not unexpected that host immune and inflammatory responses play a key role in the pathogenesis. The mechanism through which chronic inflammation results in CRC is felt to be through the induction of cytokines and chemokines, with ensuing alterations in epithelial cell proliferation, survival, and migration. (32–42) (Figure 1) In contrast to sporadic CRC, which is postulated to develop from 1 or 2 foci of dysplasia, colitis associated CRC is hypothesized to develop from multifocal dysplasia where the inflamed colonic mucosa undergoes a field change of cancer-associated molecular alterations before there is any histologic evidence of dysplasia.(2, 24, 43–45) Broadly, two of the most common somatic genetic abnormalities identified in CRC are chromosomal instability (CIN) and microsatellite instability (MSI). These occur with the same frequency in colitis associated CRC as they do with sporadic CRC, but there are differences with respect to the timing and frequency of some alterations in the colitis associated dysplasia-carcinoma sequence.(2, 24, 46–52) For example, p53 mutations (common to both sporadic and colitis associated CRC), appear to occur earlier in carcinogenesis in colitis associated CRC, as these mutations are more commonly observed in colitis associated dysplasia than among sporadic polyps.(47, 51) Mutations in APC and K-ras, which are known to occur much earlier within the adenoma-carcinoma sequence of sporadic CRC, are seen less frequently in colitis associated CRC and are thought to arise much later in the dysplasia-carcinoma sequence where they promote NF-kB mediated cytokine secretion, neovascularization, and maintenance of tumor growth.(24, 51, 53)
Figure 1.
Key alterations within the colitis associated colorectal cancer dysplasia-carcinoma sequence
Recently, whole exome analyses of tumor specimens from colitis associated CRC patients, were compared to exome analyses from tumor specimens from sporadic CRC patients included in The Cancer Genome Atlas (TCGA). The comparisons suggested that colitis associated CRCs have a distinct profile, and are enriched with mutations associated with cell communication, cell to cell signaling, and cell adhesion, all of which may be linked with the dysregulated cytokines and inflammatory mediators associated with IBD.(51, 54) Limitations of this prior work include a paucity of clinical data on the patients who contributed CRC samples, and small sample size (n< 30). Nonetheless, recognition that colitis associated CRC may have a unique genetic profile could offer novel opportunities for chemoprevention and for the development of biomarkers for colitis surveillance. Extension of genetic profiling work to include pre-cancerous dysplasia, and expansion to include additional assessments such as DNA methylation and mucosal microbiome profiles has great potential to expand our understanding of colitis associated CRC pathogenesis and opportunities for surveillance, intervention, and prevention.
Chemoprevention
No primary randomized controlled trials of chemoprevention for colitis associated CRC have been conducted. In observational studies, the two most widely studied anti-inflammatory drugs are 5-aminosalicylic acid (5-ASA) and immunomodulators (methotrexate, azathioprine and 6-mercaptopurine). Although both of these drug categories inhibit NF-kB activation, and to some extent reduce the overall burden of cytokine production,(55–59) clinical data supporting their use as chemoprevention agents has been conflicting. Indeed, the majority of population based studies suggesting no therapeutic benefit exists for this indication.(60–65) Similarly, pooled meta-analyses of observational studies for other non-specific anti-inflammatory agents such as aspirin and non-aspirin non-steroid anti-inflammatory drugs, have also suggested no chemoprevention benefit exists for these agents, despite their demonstrated efficacy as chemoprevention agents for sporadic CRC.(66) Among a high-risk population of patients suffering from PSC, early data suggested a substantial chemoprevention benefit with ursodexycholic acid.(67) However, more recent meta-analyses of population based studies have suggested that a chemoprevention benefit may not exist for all patients, particularly when considering the dose of UCDA used.(68, 69) This lack of benefit with prior chemoprevention studies may be in part due to variability in disease activity, extent, and presence of established risk factors, or the non-specific mechanism through which these agents inhibit inflammation and modulate cancer risk.
Given the known importance of TNF and interleukins within the pathogenesis of colitis associated CRC, more targeted inhibition of these pathways may offer an opportunity to prevent colitis associated CRC, particularly among high-risk individuals who have developed early dysplastic lesions where these cytokines serve to stabilize the cancer microenvironment. In non-CRC malignancy, such as ovarian and renal cell cancer, the use of TNF-antagonists in early phase clinical trials has been shown to stabilize disease and prevent further progression among those with advanced cancer.(70) Within colitis associated CRC, although animal models have suggested that TNF-antagonists may prevent the development or progression of dysplasia and cancer,(71) and some population based data within IBD has demonstrated a lower frequency of CRC among those treated with infliximab,(72, 73) non-IBD data has suggested a potential increased frequency of CRC with infliximab treatment.(74) This has created uncertainty as to whether TNF-antagonist and biologics are effective chemoprevention agents for colitis associated CRC.(75)
Overall, chemoprevention against colitis associated CRC is understudied. Well-designed randomized controlled trials for candidate agents are needed, and large population based registries or healthcare databases may help guide the identification of these candidate agents. An example of this can be seen within the Boston healthcare network where statin use was observed to be inversely associated with CRC risk and thus may be a potential candidate chemoprevention agent worthy of future research.(76) As alluded to above, expanding understandings of the genetic and molecular profiles of colitis associated dysplasia and CRC offers potential to engage in a new era of chemoprevention research in IBD. For example, mutations in Rac GTP may be more common in IBD associated neoplasia, and Rac1 inhibition has been shown in animals to prevent CRC carcinogenesis.(51) The potential impact of such genetic observations is highlighted by a recent successful pilot trial against adenomas in patients with familial adenomatous polyps, where observations of EGFR up-regulation in FAP associated polyps led to a successful placebo controlled proof of concept human trial of erlotinib that markedly reduced adenoma burden and progression.(77)
Screening and Surveillance
Although a great deal of research has been conducted to understand the pathogenesis of colitis associated CRC, and attempts have been made to off-set the natural course of this disease through chemoprevention, the timing of these dysplastic transitions can vary, and tumor progression can skip one or more of these steps.(78) This creates a great deal of uncertainty with regards to screening and surveillance, and efforts have therefore now focused on the early detection of dysplastic changes through endoscopic surveillance with the intent of reducing the risk of progression through early colectomy when colitis associated dysplasia is found. This approach has been associated with a reduction in CRC related mortality,(79–84) and is now considered standard of care by several GI societies. The optimal approach and timing to surveillance, however, continues to be debated.
Surveillance Intervals
When considering endoscopic surveillance intervals, societies vary in the manner in which they stratify patients and the intervals they recommend. Broadly, surveillance can be classified as risk-stratified or non-stratified intervals. (Table 3(78, 85–89)) European societies have suggested a more stratified approach to surveillance, after taking into account the number of risk factors and strength of each risk factor, whereas US societies have suggested a more aggressive and non-stratified approach to surveillance assuming an equal degree of risk across sub-populations. A recent cost-effectiveness analysis suggested that the European based risk-profiling approach may be more cost-effective as compared to the non-stratified US approach, but this cost-effectiveness was largely driven by the cost of colonoscopy and it is unclear if the different strategies are equal in their ability to improve CRC related morbidity and mortality.(90) Furthermore, the overall utilization of surveillance colonoscopy programs at the population level has been demonstrated to be low, with only a quarter of patients undergoing surveillance at recommended intervals.(91, 92) Even among high risk individuals (PSC), adherence to guidelines is reported to be less than 40%.(91–94) Thus, irrespective of the interval or strategy followed, adherence to any surveillance program would be considered an optimization of current practices.
Table 3.
Unanswered questions and future research
| Epidemiology and Risk Factors |
|
| Molecular basis and targets of carcinogenesis |
|
| Surveillance and Endoscopic Management |
|
Surveillance Techniques and Management of Dysplasia
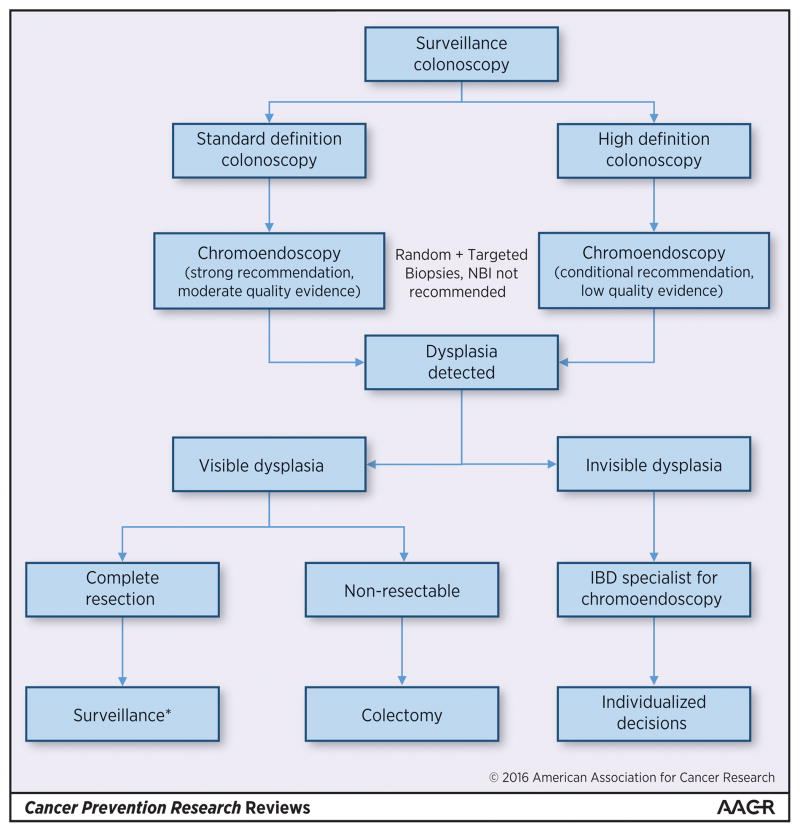
Uncertainty and variability surrounds surveillance techniques and the optimal management of dysplastic lesions. Providers have traditionally failed to comply with biopsy protocols, with some reports documenting adherence to be less than 5%.(92, 93, 95, 96) It is unclear if this variation in practice and adherence to surveillance techniques is due to patient preferences, provider practice preferences, or technical challenges (i.e. time required to submit multiple biopsies in multiple sample jars),(97) but it may help to explain why the rate of early/missed CRC after colonoscopy is reported to be nearly 15% among IBD patients as compared to only 5% in non-IBD patients.(98) In an effort to address this gap, a group of experts recently came together and prepared the Surveillance for Colorectal Endoscopic Neoplasia Detection and Management in Inflammatory Bowel Disease Patients Internal Consensus Recommendations (SCENIC Recommendations).(99) (Figure 2) Within these recommendations a key point to note is that not all dysplastic lesions require colectomy, and certain patients with focal LGD or visible lesions can be monitored endoscopically or undergo focal endoscopic resection.
Figure 2.
Clinical approach to endoscopic dysplasia surveillance and management
When performing surveillance, the SCENIC recommendations place a great deal of emphasis on the use of chromoendoscopy to optimize surveillance techniques. However, it should be noted that this recommendation is a conditional recommendation when using newer high definition colonoscopy equipment, as it is based largely upon on a single observational study and no randomized trials currently exist in this arena. Given the increased effort and time required to perform chromoendoscopy, with an unclear added benefit, several authors have brought into question the optimal use of chromoendoscopy and whether it is truly required in all patients.(100, 101) Furthermore, if chromoendoscopy is to be used, it is unclear if random biopsies are still needed beyond targeted biopsies, and what the incremental yield of this might be. Nonetheless, although several of the recommendations are conditional and/or have low quality of evidence supporting them, this consensus recommendation statement helps to highlight the need for a unified approach to diagnosing, characterizing, and treating these lesions in routine practice, and represents a step towards a more integrated approach to dealing with colitis associated dysplasia and CRC.(102)
Emerging Surveillance Techniques
Recently, the use of stool based surveillance has been considered and the detection of CpG island methylation in human DNA isolated from stool has been proposed for noninvasive screening.(103) Kisiel et al.(104) demonstrated that methylated gene markers BMP3, vimentin, EYA4 and NDRG4 showed a high discrimination between neoplastic and non-neoplastic tissue (ROC curve of 0.91, 0.91, 0.85 and 0.84 for total IBD-neoplasia and of 0.97, 0.97, 0.95 and 0.85 for cancer). Azuara et al.(105) found that SLIT2 gene methylation was more frequently seen in patients at high risk of dysplasia or cancer as compared to those at low risk (25% vs. 0%, p < 0.01), suggesting this may also be a potential stool based surveillance biomarker. Several other studies have since been conducted to evaluate the potential of stool based testing for colitis associated CRC surveillance, but to date no well validated panels are available for routine clinical use in IBD and further studies are needed to understand the optimized use of these stool based biomarkers, and the comparative effectiveness of this approach as compared to currently accepted standards – high definition colonoscopy with chromoendoscopy.(103)
Summary
Significant advances have been made in our understanding and approach to managing colitis associated CRC. Despite this, several important gaps remain which will need to be addressed. (Table 3) Quantifying the true burden of disease, impact of changing treatment paradigms on disease risk, and identifying sub-populations at greatest risk is of utmost importance as we transition to personalized risk profiling and surveillance. New insights into the pathogenesis of colitis associated CRC, and rigorous, comprehensive analyses of genetic and molecular profiles associated with colitis associated dysplasia and CRC, could pave the way for targeted chemoprevention and surveillance strategies. Although a great deal of work has been done to optimize the surveillance and management of dysplasia and CRC, questions remain regarding the optimal integration of novel optical technology, endoscopic therapeutics, and changing risk profiles over time. Well-designed population level comparative effectiveness studies are needed to optimize the value and utility of CRC surveillance and screening in IBD.
Table 2.
Surveillance intervals and strategies
| High Risk (Annually) | Intermediate Risk (every 3 years) | Low Risk (every 5 years) | |
|---|---|---|---|
| Risk Stratified Intervals | |||
| NICE, ECCO, and BSG(85–87) | Pancolitis with moderate-severe inflammation; strictures or dysplasia within past 5 years (± surgery), PSC, or family history of CRC in first degree relative > 50 years | Pancolitis with active inflammation (endoscopic or histologic); presence of pseudopolyps, or family history of CRC in first degree relative > 50 years | Pancolitis without inflammation (endoscopic or histology); left sided UC or CD of similar extent (i.e. < 50% mucosa involved)* |
| Non-Stratified Intervals | |||
| ASGE, ACG and AGA(78, 88, 89) | Active inflammation (any severity), anatomic abnormalities (foreshortened colons, strictures or pseudopolyps), history of dysplasia, PSC, or family history of CRC in first degree relative (irrespective of age) | Extension to 1–3 years considered after 2 consecutive negative surveillance colonoscopies and no inflammation (no specification on how to lengthen interval) | No recommendation to extend to 5 year intervals |
ECCO guidelines do not have specific low-risk criteria. Low risk is those without high or intermediate risk.
NICE: National Institute of Health and Clinical Excellence; ECCO: European Crohn’s and Colitis Organization; BSG: British Society of Gastroenterology; ASGE: American Society of Gastrointestinal Endoscopy; ACG: American College of Gastroenterology; AGA: American Gastroenterology Association; PSC: Primary sclerosing cholangitis; CRC: colorectal cnacer; UC: ulcerative colitis; CD: Crohn’s disease
Acknowledgments
Financial Support: Parambir S. Dulai is supported by the NIDDK training grant 5T32DK007202. Samir Gupta is supported in part by NCI 2 U54CA132379-06A1, as well as Merit Review Award number 1 I01 HX001574-01A1 from the United States Department of Veterans Affairs Health Services Research & Development Service of the VA Office of Research and Development.
Footnotes
Disclosures: The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
References
- 1.Ekbom A, Helmick C, Zack M, Adami HO. Ulcerative colitis and colorectal cancer. A population-based study. The New England journal of medicine. 1990;323:1228–33. doi: 10.1056/NEJM199011013231802. [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. The New England journal of medicine. 2015;372:1441–52. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 3.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–35. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lutgens MW, van Oijen MG, van der Heijden GJ, Vleggaar FP, Siersema PD, Oldenburg B. Declining risk of colorectal cancer in inflammatory bowel disease: an updated meta-analysis of population-based cohort studies. Inflammatory bowel diseases. 2013;19:789–99. doi: 10.1097/MIB.0b013e31828029c0. [DOI] [PubMed] [Google Scholar]
- 5.Jess T, Simonsen J, Jorgensen KT, Pedersen BV, Nielsen NM, Frisch M. Decreasing risk of colorectal cancer in patients with inflammatory bowel disease over 30 years. Gastroenterology. 2012;143:375–81. doi: 10.1053/j.gastro.2012.04.016. [DOI] [PubMed] [Google Scholar]
- 6.Herrinton LJ, Liu L, Levin TR, Allison JE, Lewis JD, Velayos F. Incidence and mortality of colorectal adenocarcinoma in persons with inflammatory bowel disease from 1998 to 2010. Gastroenterology. 2012;143:382–9. doi: 10.1053/j.gastro.2012.04.054. [DOI] [PubMed] [Google Scholar]
- 7.Adami HO, Bretthauer M, Emilsson L, Hernan MA, Kalager M, Ludvigsson JF, et al. The continuing uncertainty about cancer risk in inflammatory bowel disease. Gut. 2016;65:889–93. doi: 10.1136/gutjnl-2015-311003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lashner BA, Turner BC, Bostwick DG, Frank PH, Hanauer SB. Dysplasia and cancer complicating strictures in ulcerative colitis. Digestive diseases and sciences. 1990;35:349–52. doi: 10.1007/BF01537413. [DOI] [PubMed] [Google Scholar]
- 9.Fumery M, Pineton de Chambrun G, Stefanescu C, Buisson A, Bressenot A, Beaugerie L, et al. Detection of Dysplasia or Cancer in 3.5% of Patients With Inflammatory Bowel Disease and Colonic Strictures. Clinical gastroenterology and hepatology. 2015;13:1770–5. doi: 10.1016/j.cgh.2015.04.185. [DOI] [PubMed] [Google Scholar]
- 10.Sonnenberg A, Genta RM. Epithelial Dysplasia and Cancer in IBD Strictures. Journal of Crohn’s & colitis. 2015;9:769–75. doi: 10.1093/ecco-jcc/jjv108. [DOI] [PubMed] [Google Scholar]
- 11.Peyrin-Biroulet L, Phelip JM, Roblin X. Is ulcerative colitis proctitis associated with an increased risk of colorectal cancer? Gastroenterology. 2009;137:1857–8. doi: 10.1053/j.gastro.2009.05.072. [DOI] [PubMed] [Google Scholar]
- 12.Soderlund S, Brandt L, Lapidus A, Karlen P, Brostrom O, Lofberg R, et al. Decreasing time-trends of colorectal cancer in a large cohort of patients with inflammatory bowel disease. Gastroenterology. 2009;136:1561–7. doi: 10.1053/j.gastro.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 13.Heuschen UA, Hinz U, Allemeyer EH, Stern J, Lucas M, Autschbach F, et al. Backwash ileitis is strongly associated with colorectal carcinoma in ulcerative colitis. Gastroenterology. 2001;120:841–7. doi: 10.1053/gast.2001.22434. [DOI] [PubMed] [Google Scholar]
- 14.Biancone L, Michetti P, Travis S, Escher JC, Moser G, Forbes A, et al. European evidence-based Consensus on the management of ulcerative colitis: Special situations. Journal of Crohn’s & colitis. 2008;2:63–92. doi: 10.1016/j.crohns.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 15.Itzkowitz SH, Present DH. Consensus conference: Colorectal cancer screening and surveillance in inflammatory bowel disease. Inflammatory bowel diseases. 2005;11:314–21. doi: 10.1097/01.mib.0000160811.76729.d5. [DOI] [PubMed] [Google Scholar]
- 16.Beaugerie L, Svrcek M, Seksik P, Bouvier AM, Simon T, Allez M, et al. Risk of colorectal high-grade dysplasia and cancer in a prospective observational cohort of patients with inflammatory bowel disease. Gastroenterology. 2013;145:166–75. doi: 10.1053/j.gastro.2013.03.044. [DOI] [PubMed] [Google Scholar]
- 17.Nuako KW, Ahlquist DA, Mahoney DW, Schaid DJ, Siems DM, Lindor NM. Familial predisposition for colorectal cancer in chronic ulcerative colitis: a case-control study. Gastroenterology. 1998;115:1079–83. doi: 10.1016/s0016-5085(98)70077-0. [DOI] [PubMed] [Google Scholar]
- 18.Askling J, Dickman PW, Karlen P, Brostrom O, Lapidus A, Lofberg R, et al. Family history as a risk factor for colorectal cancer in inflammatory bowel disease. Gastroenterology. 2001;120:1356–62. doi: 10.1053/gast.2001.24052. [DOI] [PubMed] [Google Scholar]
- 19.Soetikno RM, Lin OS, Heidenreich PA, Young HS, Blackstone MO. Increased risk of colorectal neoplasia in patients with primary sclerosing cholangitis and ulcerative colitis: a meta-analysis. Gastrointestinal endoscopy. 2002;56:48–54. doi: 10.1067/mge.2002.125367. [DOI] [PubMed] [Google Scholar]
- 20.Broome U, Lofberg R, Veress B, Eriksson LS. Primary sclerosing cholangitis and ulcerative colitis: evidence for increased neoplastic potential. Hepatology. 1995;22:1404–8. doi: 10.1002/hep.1840220511. [DOI] [PubMed] [Google Scholar]
- 21.Devroede GJ, Taylor WF, Sauer WG, Jackman RJ, Stickler GB. Cancer risk and life expectancy of children with ulcerative colitis. The New England journal of medicine. 1971;285:17–21. doi: 10.1056/NEJM197107012850103. [DOI] [PubMed] [Google Scholar]
- 22.Ekbom A, Helmick C, Zack M, Adami HO. Increased risk of large-bowel cancer in Crohn’s disease with colonic involvement. Lancet. 1990;336:357–9. doi: 10.1016/0140-6736(90)91889-i. [DOI] [PubMed] [Google Scholar]
- 23.Rutter M, Saunders B, Wilkinson K, Rumbles S, Schofield G, Kamm M, et al. Severity of inflammation is a risk factor for colorectal neoplasia in ulcerative colitis. Gastroenterology. 2004;126:451–9. doi: 10.1053/j.gastro.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 24.Ullman TA, Itzkowitz SH. Intestinal inflammation and cancer. Gastroenterology. 2011;140:1807–16. doi: 10.1053/j.gastro.2011.01.057. [DOI] [PubMed] [Google Scholar]
- 25.Gupta RB, Harpaz N, Itzkowitz S, Hossain S, Matula S, Kornbluth A, et al. Histologic inflammation is a risk factor for progression to colorectal neoplasia in ulcerative colitis: a cohort study. Gastroenterology. 2007;133:1099–105. doi: 10.1053/j.gastro.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rubin DT, Huo D, Kinnucan JA, Sedrak MS, McCullom NE, Bunnag AP, et al. Inflammation is an independent risk factor for colonic neoplasia in patients with ulcerative colitis: a case-control study. Clinical gastroenterology and hepatology. 2013;11:1601–8. doi: 10.1016/j.cgh.2013.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jess T, Rungoe C, Peyrin-Biroulet L. Risk of colorectal cancer in patients with ulcerative colitis: a meta-analysis of population-based cohort studies. Clinical gastroenterology and hepatology. 2012;10:639–45. doi: 10.1016/j.cgh.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 28.Greenstein AJ, Sachar DB, Smith H, Pucillo A, Papatestas AE, Kreel I, et al. Cancer in universal and left-sided ulcerative colitis: factors determining risk. Gastroenterology. 1979;77:290–4. [PubMed] [Google Scholar]
- 29.Gilat T, Fireman Z, Grossman A, Hacohen D, Kadish U, Ron E, et al. Colorectal cancer in patients with ulcerative colitis. A population study in central Israel. Gastroenterology. 1988;94:870–7. doi: 10.1016/0016-5085(88)90541-0. [DOI] [PubMed] [Google Scholar]
- 30.Torres J, Pineton de Chambrun G, Itzkowitz S, Sachar DB, Colombel JF. Review article: colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2011;34:497–508. doi: 10.1111/j.1365-2036.2011.04753.x. [DOI] [PubMed] [Google Scholar]
- 31.Lunder AK, Hov JR, Borthne A, Gleditsch J, Johannesen G, Tveit K, et al. Prevalence of Sclerosing Cholangitis, Detected by Magnetic Resonance Cholangiography, in Patients with Long-term Inflammatory Bowel Disease. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.06.021. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 32.Popivanova BK, Kitamura K, Wu Y, Kondo T, Kagaya T, Kaneko S, et al. Blocking TNF-alpha in mice reduces colorectal carcinogenesis associated with chronic colitis. The Journal of clinical investigation. 2008;118:560–70. doi: 10.1172/JCI32453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer research. 2008;68:323–8. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chan IH, Jain R, Tessmer MS, Gorman D, Mangadu R, Sathe M, et al. Interleukin-23 is sufficient to induce rapid de novo gut tumorigenesis, independent of carcinogens, through activation of innate lymphoid cells. Mucosal immunology. 2014;7:842–56. doi: 10.1038/mi.2013.101. [DOI] [PubMed] [Google Scholar]
- 35.Tong Z, Yang XO, Yan H, Liu W, Niu X, Shi Y, et al. A protective role by interleukin-17F in colon tumorigenesis. PloS one. 2012 doi: 10.1371/journal.pone.0034959. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer cell. 2009;15:103–13. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brighenti E, Calabrese C, Liguori G, Giannone FA, Trere D, Montanaro L, et al. Interleukin 6 downregulates p53 expression and activity by stimulating ribosome biogenesis: a new pathway connecting inflammation to cancer. Oncogene. 2014;33:4396–406. doi: 10.1038/onc.2014.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang S, Liu Z, Wang L, Zhang X. NF-kappaB signaling pathway, inflammation and colorectal cancer. Cellular & molecular immunology. 2009;6:327–34. doi: 10.1038/cmi.2009.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Agoff SN, Brentnall TA, Crispin DA, Taylor SL, Raaka S, Haggitt RC, et al. The role of cyclooxygenase 2 in ulcerative colitis-associated neoplasia. The American journal of pathology. 2000;157:737–45. doi: 10.1016/S0002-9440(10)64587-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.De Simone V, Franze E, Ronchetti G, Colantoni A, Fantini MC, Di Fusco D, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–503. doi: 10.1038/onc.2014.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu S, Sun X, Wang M, Hou Y, Zhan Y, Jiang Y, et al. A microRNA 221- and 222-mediated feedback loop maintains constitutive activation of NFkappaB and STAT3 in colorectal cancer cells. Gastroenterology. 2014;147:847–59. doi: 10.1053/j.gastro.2014.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shi C, Yang Y, Xia Y, Okugawa Y, Yang J, Liang Y, et al. Novel evidence for an oncogenic role of microRNA-21 in colitis-associated colorectal cancer. Gut. 2015;65:1470–81. doi: 10.1136/gutjnl-2014-308455. [DOI] [PubMed] [Google Scholar]
- 43.Triantafillidis JK, Nasioulas G, Kosmidis PA. Colorectal cancer and inflammatory bowel disease: epidemiology, risk factors, mechanisms of carcinogenesis and prevention strategies. Anticancer research. 2009;29:2727–37. [PubMed] [Google Scholar]
- 44.Willenbucher RF, Zelman SJ, Ferrell LD, Moore DH, 2nd, Waldman FM. Chromosomal alterations in ulcerative colitis-related neoplastic progression. Gastroenterology. 1997;113(3):791–801. doi: 10.1016/s0016-5085(97)70173-2. [DOI] [PubMed] [Google Scholar]
- 45.Befrits R, Hammarberg C, Rubio C, Jaramillo E, Tribukait B. DNA aneuploidy and histologic dysplasia in long-standing ulcerative colitis. A 10-year follow-up study. Diseases of the colon and rectum. 1994;37:313–9. doi: 10.1007/BF02053590. [DOI] [PubMed] [Google Scholar]
- 46.Kim ER, Chang DK. Colorectal cancer in inflammatory bowel disease: the risk, pathogenesis, prevention and diagnosis. World journal of gastroenterology. 2014;20:9872–81. doi: 10.3748/wjg.v20.i29.9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burmer GC, Rabinovitch PS, Haggitt RC, Crispin DA, Brentnall TA, Kolli VR, et al. Neoplastic progression in ulcerative colitis: histology, DNA content, and loss of a p53 allele. Gastroenterology. 1992;103:1602–10. doi: 10.1016/0016-5085(92)91184-6. [DOI] [PubMed] [Google Scholar]
- 48.Itzkowitz S. Colon carcinogenesis in inflammatory bowel disease: applying molecular genetics to clinical practice. Journal of clinical gastroenterology. 2003;36:S70–4. doi: 10.1097/00004836-200305001-00012. [DOI] [PubMed] [Google Scholar]
- 49.Issa JP, Ahuja N, Toyota M, Bronner MP, Brentnall TA. Accelerated age-related CpG island methylation in ulcerative colitis. Cancer research. 2001;61:3573–7. [PubMed] [Google Scholar]
- 50.Fleisher AS, Esteller M, Harpaz N, Leytin A, Rashid A, Xu Y, et al. Microsatellite instability in inflammatory bowel disease-associated neoplastic lesions is associated with hypermethylation and diminished expression of the DNA mismatch repair gene, hMLH1. Cancer research. 2000;60:4864–8. [PubMed] [Google Scholar]
- 51.Robles AI, Traverso G, Zhang M, Roberts NJ, Khan MA, Joseph C, et al. Whole-Exome Sequencing Analyses of Inflammatory Bowel Disease-Associated Colorectal Cancers. Gastroenterology. 2016;150:931–43. doi: 10.1053/j.gastro.2015.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Svrcek M, El-Bchiri J, Chalastanis A, Capel E, Dumont S, Buhard O, et al. Specific clinical and biological features characterize inflammatory bowel disease associated colorectal cancers showing microsatellite instability. Journal of clinical oncology. 2007;25:4231–8. doi: 10.1200/JCO.2007.10.9744. [DOI] [PubMed] [Google Scholar]
- 53.Klampfer L. Cytokines, inflammation and colon cancer. Current cancer drug targets. 2011;11:451–64. doi: 10.2174/156800911795538066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaeger R, Shah MA, Miller VA, Kelsen JR, Wang K, Heins ZJ, et al. Genomic Alterations Observed in Colitis-associated Cancers are Distinct from Those Found in Sporadic Colorectal Cancers and Vary by Type of Inflammatory Bowel Disease. Gastroenterology. 2016 doi: 10.1053/j.gastro.2016.04.001. [epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weber CK, Liptay S, Wirth T, Adler G, Schmid RM. Suppression of NF-kappaB activity by sulfasalazine is mediated by direct inhibition of IkappaB kinases alpha and beta. Gastroenterology. 2000;119:1209–18. doi: 10.1053/gast.2000.19458. [DOI] [PubMed] [Google Scholar]
- 56.Majumdar S, Aggarwal BB. Methotrexate suppresses NF-kappaB activation through inhibition of IkappaBalpha phosphorylation and degradation. Journal of immunology. 2001;167:2911–20. doi: 10.4049/jimmunol.167.5.2911. [DOI] [PubMed] [Google Scholar]
- 57.Minghetti PP, Blackburn WD., Jr Effects of sulfasalazine and its metabolites on steady state messenger RNA concentrations for inflammatory cytokines, matrix metalloproteinases, and tissue inhibitors of metalloproteinase in rheumatoid synovial fibroblasts. The Journal of rheumatology. 2000;27:653–60. [PubMed] [Google Scholar]
- 58.Gerards AH, de Lathouder S, de Groot ER, Dijkmans BA, Aarden LA. Inhibition of cytokine production by methotrexate. Studies in healthy volunteers and patients with rheumatoid arthritis. Rheumatology. 2003;42:1189–96. doi: 10.1093/rheumatology/keg323. [DOI] [PubMed] [Google Scholar]
- 59.Hildner K, Marker-Hermann E, Schlaak JF, Becker C, Germann T, Schmitt E, et al. Azathioprine, mycophenolate mofetil, and methotrexate specifically modulate cytokine production by T cells. Annals of the New York Academy of Sciences. 1998;859:204–7. doi: 10.1111/j.1749-6632.1998.tb11129.x. [DOI] [PubMed] [Google Scholar]
- 60.Velayos FS, Terdiman JP, Walsh JM. Effect of 5-aminosalicylate use on colorectal cancer and dysplasia risk: a systematic review and metaanalysis of observational studies. The American journal of gastroenterology. 2005;100:1345–53. doi: 10.1111/j.1572-0241.2005.41442.x. [DOI] [PubMed] [Google Scholar]
- 61.Zhao LN, Li JY, Yu T, Chen GC, Yuan YH, Chen QK. 5-Aminosalicylates reduce the risk of colorectal neoplasia in patients with ulcerative colitis: an updated meta-analysis. PloS one. 2014;9:e94208. doi: 10.1371/journal.pone.0094208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bernstein CN, Nugent Z, Blanchard JF. 5-aminosalicylate is not chemoprophylactic for colorectal cancer in IBD: a population based study. The American journal of gastroenterology. 2011;106:731–6. doi: 10.1038/ajg.2011.50. [DOI] [PubMed] [Google Scholar]
- 63.Terdiman JP. The prevention of colitis-related cancer by 5-aminosalicylates: an appealing hypothesis that remains unproven. The American journal of gastroenterology. 2011;106:737–40. doi: 10.1038/ajg.2011.56. [DOI] [PubMed] [Google Scholar]
- 64.Velayos FS, Loftus EV, Jr, Jess T, Harmsen WS, Bida J, Zinsmeister AR, et al. Predictive and protective factors associated with colorectal cancer in ulcerative colitis: A case-control study. Gastroenterology. 2006;130:1941–9. doi: 10.1053/j.gastro.2006.03.028. [DOI] [PubMed] [Google Scholar]
- 65.Matula S, Croog V, Itzkowitz S, Harpaz N, Bodian C, Hossain S, et al. Chemoprevention of colorectal neoplasia in ulcerative colitis: the effect of 6-mercaptopurine. Clinical gastroenterology and hepatology. 2005;3:1015–21. doi: 10.1016/s1542-3565(05)00738-x. [DOI] [PubMed] [Google Scholar]
- 66.Burr NE, Hull MA, Subramanian V. Does aspirin or non-aspirin non-steroidal anti-inflammatory drug use prevent colorectal cancer in inflammatory bowel disease? World journal of gastroenterology. 2016;22:3679–86. doi: 10.3748/wjg.v22.i13.3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pardi DS, Loftus EV, Jr, Kremers WK, Keach J, Lindor KD. Ursodeoxycholic acid as a chemopreventive agent in patients with ulcerative colitis and primary sclerosing cholangitis. Gastroenterology. 2003;124:889–93. doi: 10.1053/gast.2003.50156. [DOI] [PubMed] [Google Scholar]
- 68.Hansen JD, Kumar S, Lo WK, Poulsen DM, Halai UA, Tater KC. Ursodiol and colorectal cancer or dysplasia risk in primary sclerosing cholangitis and inflammatory bowel disease: a meta-analysis. Digestive diseases and sciences. 2013;58:3079–87. doi: 10.1007/s10620-013-2772-0. [DOI] [PubMed] [Google Scholar]
- 69.Singh S, Khanna S, Pardi DS, Loftus EV, Jr, Talwalkar JA. Effect of ursodeoxycholic acid use on the risk of colorectal neoplasia in patients with primary sclerosing cholangitis and inflammatory bowel disease: a systematic review and meta-analysis. Inflammatory bowel diseases. 2013;19:1631–8. doi: 10.1097/MIB.0b013e318286fa61. [DOI] [PubMed] [Google Scholar]
- 70.Balkwill F. Tumour necrosis factor and cancer. Nature reviews Cancer. 2009;9:361–71. doi: 10.1038/nrc2628. [DOI] [PubMed] [Google Scholar]
- 71.Kim YJ, Hong KS, Chung JW, Kim JH, Hahm KB. Prevention of colitis-associated carcinogenesis with infliximab. Cancer prevention research. 2010;3:1314–33. doi: 10.1158/1940-6207.CAPR-09-0272. [DOI] [PubMed] [Google Scholar]
- 72.Fidder H, Schnitzler F, Ferrante M, Noman M, Katsanos K, Segaert S, et al. Long-term safety of infliximab for the treatment of inflammatory bowel disease: a single-centre cohort study. Gut. 2009;58:501–8. doi: 10.1136/gut.2008.163642. [DOI] [PubMed] [Google Scholar]
- 73.Biancone L, Orlando A, Kohn A, Colombo E, Sostegni R, Angelucci E, et al. Infliximab and newly diagnosed neoplasia in Crohn’s disease: a multicentre matched pair study. Gut. 2006;55:228–33. doi: 10.1136/gut.2005.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bongartz T, Sutton AJ, Sweeting MJ, Buchan I, Matteson EL, Montori V. Anti-TNF antibody therapy in rheumatoid arthritis and the risk of serious infections and malignancies: systematic review and meta-analysis of rare harmful effects in randomized controlled trials. Jama. 2006;295:2275–85. doi: 10.1001/jama.295.19.2275. [DOI] [PubMed] [Google Scholar]
- 75.Biancone L, Petruzziello C, Calabrese E, Zorzi F, Naccarato P, Onali S, et al. Long-term safety of Infliximab for the treatment of inflammatory bowel disease: does blocking TNFalpha reduce colitis-associated colorectal carcinogenesis? Gut. 2009;58:1703. doi: 10.1136/gut.2008.176461. [DOI] [PubMed] [Google Scholar]
- 76.Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Churchill S, et al. Statin Use Is Associated With Reduced Risk of Colorectal Cancer in Patients With Inflammatory Bowel Diseases. Clinical gastroenterology and hepatology. 2016;14:973–9. doi: 10.1016/j.cgh.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Samadder NJ, Neklason DW, Boucher KM, Byrne KR, Kanth P, Samowitz W, et al. Effect of Sulindac and Erlotinib vs Placebo on Duodenal Neoplasia in Familial Adenomatous Polyposis: A Randomized Clinical Trial. Jama. 2016;315:1266–75. doi: 10.1001/jama.2016.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farraye FA, Odze RD, Eaden J, Itzkowitz SH. AGA technical review on the diagnosis and management of colorectal neoplasia in inflammatory bowel disease. Gastroenterology. 2010;138:746–74. doi: 10.1053/j.gastro.2009.12.035. [DOI] [PubMed] [Google Scholar]
- 79.Nugent FW, Haggitt RC, Gilpin PA. Cancer surveillance in ulcerative colitis. Gastroenterology. 1991;100:1241–8. [PubMed] [Google Scholar]
- 80.Eaden J, Abrams K, Ekbom A, Jackson E, Mayberry J. Colorectal cancer prevention in ulcerative colitis: a case-control study. Alimentary pharmacology & therapeutics. 2000;14:145–53. doi: 10.1046/j.1365-2036.2000.00698.x. [DOI] [PubMed] [Google Scholar]
- 81.Karlen P, Kornfeld D, Brostrom O, Lofberg R, Persson PG, Ekbom A. Is colonoscopic surveillance reducing colorectal cancer mortality in ulcerative colitis? A population based case control study. Gut. 1998;42:711–4. doi: 10.1136/gut.42.5.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lofberg R, Brostrom O, Karlen P, Tribukait B, Ost A. Colonoscopic surveillance in long-standing total ulcerative colitis--a 15-year follow-up study. Gastroenterology. 1990;99:1021–31. doi: 10.1016/0016-5085(90)90622-8. [DOI] [PubMed] [Google Scholar]
- 83.Ananthakrishnan AN, Cagan A, Cai T, Gainer VS, Shaw SY, Churchill S, et al. Colonoscopy is associated with a reduced risk for colon cancer and mortality in patients with inflammatory bowel diseases. Clinical gastroenterology and hepatology. 2015;13:322–9. doi: 10.1016/j.cgh.2014.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Choi CH, Rutter MD, Askari A, Lee GH, Warusavitarne J, Moorghen M, et al. Forty-Year Analysis of Colonoscopic Surveillance Program for Neoplasia in Ulcerative Colitis: An Updated Overview. The American journal of gastroenterology. 2015;110:1022–34. doi: 10.1038/ajg.2015.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Centre for Clinical Practice at NICE (UK) Colonoscopic Surveillance for Prevention of Colorectal Cancer in People with Ulcerative Colitis, Crohn’s Disease or Adenomas. London: National Institute for Health and Clinical Excellence (UK); 2011. (NICE Clinical Guidelines, No. 118). Available from http://www.ncbi.nlm.nih.gov/books/NBK82209/ [PubMed] [Google Scholar]
- 86.Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, et al. European evidence based consensus for endoscopy in inflammatory bowel disease. Journal of Crohn’s & colitis. 2013;7:982–1018. doi: 10.1016/j.crohns.2013.09.016. [DOI] [PubMed] [Google Scholar]
- 87.Cairns SR, Scholefield JH, Steele RJ, Dunlop MG, Thomas HJ, Evans GD, et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002) Gut. 2010;59:666–89. doi: 10.1136/gut.2009.179804. [DOI] [PubMed] [Google Scholar]
- 88.Sengupta N, Yee E, Feuerstein JD. Colorectal Cancer Screening in Inflammatory Bowel Disease. Digestive diseases and sciences. 2016;61:980–9. doi: 10.1007/s10620-015-3979-z. [DOI] [PubMed] [Google Scholar]
- 89.Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American College Of Gastroenterology, Practice Parameters Committee. The American journal of gastroenterology. 2010;105:501–23. doi: 10.1038/ajg.2009.727. [DOI] [PubMed] [Google Scholar]
- 90.Lutgens M, van Oijen M, Mooiweer E, van der Valk M, Vleggaar F, Siersema P, et al. A risk-profiling approach for surveillance of inflammatory bowel disease-colorectal carcinoma is more cost-effective: a comparative cost-effectiveness analysis between international guidelines. Gastrointestinal endoscopy. 2014;80:842–8. doi: 10.1016/j.gie.2014.02.1031. [DOI] [PubMed] [Google Scholar]
- 91.Velayos FS, Liu L, Lewis JD, Allison JE, Flowers N, Hutfless S, et al. Prevalence of colorectal cancer surveillance for ulcerative colitis in an integrated health care delivery system. Gastroenterology. 2010;139:1511–8. doi: 10.1053/j.gastro.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 92.van Rijn AF, Fockens P, Siersema PD, Oldenburg B. Adherence to surveillance guidelines for dysplasia and colorectal carcinoma in ulcerative and Crohn’s colitis patients in the Netherlands. World journal of gastroenterology. 2009;15:226–30. doi: 10.3748/wjg.15.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Eaden JA, Ward BA, Mayberry JF. How gastroenterologists screen for colonic cancer in ulcerative colitis: an analysis of performance. Gastrointestinal endoscopy. 2000;51:123–8. doi: 10.1016/s0016-5107(00)70405-6. [DOI] [PubMed] [Google Scholar]
- 94.Kaplan GG, Heitman SJ, Hilsden RJ, Urbanski S, Myers RP, Lee SS, et al. Population-based analysis of practices and costs of surveillance for colonic dysplasia in patients with primary sclerosing cholangitis and colitis. Inflammatory bowel diseases. 2007;13:1401–7. doi: 10.1002/ibd.20204. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez SA, Collins JM, Knigge KL, Eisen GM. Surveillance and management of dysplasia in ulcerative colitis. Gastrointestinal endoscopy. 2007;65:432–9. doi: 10.1016/j.gie.2006.07.034. [DOI] [PubMed] [Google Scholar]
- 96.Gearry RB, Wakeman CJ, Barclay ML, Chapman BA, Collett JA, Burt MJ, et al. Surveillance for dysplasia in patients with inflammatory bowel disease: a national survey of colonoscopic practice in New Zealand. Diseases of the colon and rectum. 2004;47:314–22. doi: 10.1007/s10350-003-0049-y. [DOI] [PubMed] [Google Scholar]
- 97.Marion JF, Sands BE. The SCENIC consensus statement on surveillance and management of dysplasia in inflammatory bowel disease: praise and words of caution. Gastroenterology. 2015;148:462–7. doi: 10.1053/j.gastro.2015.01.029. [DOI] [PubMed] [Google Scholar]
- 98.Wang YR, Cangemi JR, Loftus EV, Jr, Picco MF. Rate of early/missed colorectal cancers after colonoscopy in older patients with or without inflammatory bowel disease in the United States. The American journal of gastroenterology. 2013;108:444–9. doi: 10.1038/ajg.2012.429. [DOI] [PubMed] [Google Scholar]
- 99.Laine L, Kaltenbach T, Barkun A, McQuaid KR, Subramanian V, Soetikno R. SCENIC international consensus statement on surveillance and management of dysplasia in inflammatory bowel disease. Gastrointestinal endoscopy. 2015;81:489–501. doi: 10.1016/j.gie.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 100.Vaziri H, Anderson JC. White light endoscopy versus chromoendoscopy for the detection of dysplasia during inflammatory bowel disease surveillance with colonoscopy. Gastroenterology. 2015;149:1630–2. doi: 10.1053/j.gastro.2015.09.022. [DOI] [PubMed] [Google Scholar]
- 101.Mooiweer E, Oldenburg B. Reply: To PMID 25823770. Gastroenterology. 2015;149:1632. doi: 10.1053/j.gastro.2015.09.023. [DOI] [PubMed] [Google Scholar]
- 102.Soetikno R, Kaltenbach T, McQuaid KR, Subramanian V, Laine L, Kumar R, et al. A Paradigm Shift in the Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Digestive endoscopy. 2016 doi: 10.1111/den.12634. [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 103.Ahlquist DA. Molecular detection of colorectal neoplasia. Gastroenterology. 2010;138:2127–39. doi: 10.1053/j.gastro.2010.01.055. [DOI] [PubMed] [Google Scholar]
- 104.Kisiel JB, Yab TC, Nazer Hussain FT, Taylor WR, Garrity-Park MM, Sandborn WJ, et al. Stool DNA testing for the detection of colorectal neoplasia in patients with inflammatory bowel disease. Alimentary pharmacology & therapeutics. 2013;37:546–54. doi: 10.1111/apt.12218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Azuara D, Rodriguez-Moranta F, de Oca J, Sanjuan X, Guardiola J, Lobaton T, et al. Novel methylation panel for the early detection of neoplasia in high-risk ulcerative colitis and Crohn’s colitis patients. Inflammatory bowel diseases. 2013;19:165–73. doi: 10.1002/ibd.22994. [DOI] [PubMed] [Google Scholar]