Abstract
Stress-induced plasticity in the brain requires a precisely orchestrated sequence of cellular events involving novel as well as well known mediators. We have previously demonstrated that tissue plasminogen activator (tPA) in the amygdala promotes stress-induced synaptic plasticity and anxiety-like behavior. Here, we show that tPA activity in the amygdala is up-regulated by a major stress neuromodulator, corticotropin-releasing factor (CRF), acting on CRF type-1 receptors. Compared with WT, tPA-deficient mice responded to CRF treatment with attenuated expression of c-fos (an indicator of neuronal activation) in the central and medial amygdala but had normal c-fos responses in paraventricular nuclei. They exhibited reduced anxiety-like behavior to CRF but had a sustained corticosterone response after CRF administration. This effect of tPA deficiency was not mediated by plasminogen, because plasminogen-deficient mice demonstrated normal behavioral and hormonal changes to CRF. These studies establish tPA as an important mediator of cellular, behavioral, and hormonal responses to CRF.
Keywords: stress, c-fos, corticosterone
Aversive experiences produce an array of endocrine, autonomic, and behavioral responses aimed to maintain homeostasis and avoid threatening environmental stimuli. These adaptive changes cause a precisely orchestrated sequence of cellular events called experience-dependent plasticity. Although several important mediators of this process have been identified, its precise mechanism awaits clarification.
The amygdala is a key element of the neuroanatomical circuits that coordinate stress responses by processing inputs from other brain areas and relaying signals to structures involved in the hormonal, behavioral, and autonomic components of this response (1-3). Corticotropin-releasing factor (CRF) is a major integrator of these processes (4). CRF is a 41-aa peptide that was originally discovered in the endocrine hypothalamus (5) as a factor triggering activation of the hypothalamic-pituitary-adrenal (HPA) axis (4, 5). Release of CRF from the hypothalamic paraventricular nucleus (PVN) to the median eminence elicited by diverse stressors leads to secretion of corticotropin by the pituitary gland and subsequent liberation of corticosteroids from the adrenal glands (4). In addition to this classic role, CRF modulates the behavioral component of the stress response by its neurotropic action on extrahypothalamic targets. Central administration of CRF leads to activation of stress-related brain regions (6) and produces behavioral effects akin to those elicited by stress (7). Similarly, overproduction of CRF in transgenic mice increases anxiety-like behavior (8). Two CRF receptors, CRF-R1 and CRF-R2, have been identified (9), and most of the behavioral and hormonal effects of CRF can be ascribed to CRF-R1, whose disruption or inhibition decreases anxiety and blunts hormonal response to stress (7, 10-12). In contrast, disruption of CRF-R2 produces an opposite action (13-15), pointing to its possible role in limiting the effects of CRF during stress response.
Tissue plasminogen activator (tPA) is a serine protease that acts in the circulation to convert inactive zymogen plasminogen to plasmin, the key enzyme of the fibrinolytic cascade (16). tPA is also present in certain brain structures, like the hippocampus, amygdala, hypothalamus, and cerebellum (17). tPA is released from neurons upon neuronal excitation (18-20) and has been implicated in mechanisms of neuronal plasticity (18, 21) and learning (22-24). In the amygdala, tPA activity is critical for stress-induced synaptic plasticity and is required for expression of anxiety-like behavior in response to stress (25). Here, we show that tPA in the amygdala acts downstream of CRF-R1, facilitating neuronal activation and behavioral changes induced by CRF.
Materials and Methods
Animals. Experiments were performed on 3-month-old WT C57BL/6 and tPA or plasminogen (plg) knockout mice (tPA-/- and plg-/-; generous gift of P. Carmeliet and D. Collen, University of Leuven, Leuven, Belgium) backcrossed to C57BL/6 for nine generations. The animals were housed three to five per cage in a colony room with a 12-h light/12-h dark cycle and ad libitum access to commercial chow and tap water. All procedures were approved by the Institutional Animal Care and Use Committee of The Rockefeller University. All efforts were made to minimize any potential suffering and the number of animals used.
Cannulas Implantation and Intracerebroventricular (i.c.v.) Injections. Mice were injected with atropine (0.6 mg/kg i.p.), anesthetized with 2.5% tribromoethanol (Avertin, 0.02 ml/g of body weight, i.p.) and placed in a Kopf stereotaxic apparatus (Kopf Instruments, Tujunga, CA). The skull was exposed, a burr hole overlying the implantation coordinates was drilled, and a 26-gauge guide cannula (Plastics One, Roanoke, VA) was lowered into the right lateral ventricle. Stereotaxic coordinates of the cannula tip in relation to bregma (anteroposterior, -0.3; dorsoventral, 2.5; and mediolateral, 1.0 mm) were selected in reference to the Paxinos and Franklin mouse brain atlas (26). The guide cannula was attached to the skull with dental cement, and a dummy cannula was inserted to maintain patency. The animals were allowed to recover for 7 days and were handled daily during this period. i.c.v. injections were performed by using a 33-gauge injection cannula fitted to a 10-μl Hamilton syringe.
In Situ Zymography and Plasminogen Activator Inhibitor 1 (PAI-1) Immunohistochemistry. The animals received i.c.v. injection of CRF (30 ng in 1 μl; Bachem), whereas control mice received the same volume of the vehicle artificial cerebrospinal fluid (ACSF) (composition: 148 mM NaCl/3 mM KCl/1.4 mM CaCl2/1 mM MgCl2/0.8 mM Na2HPO4/0.2 mM NaH2PO4). Thirty or 120 min after injections, the animals were anesthetized with Avertin and perfused transcardially with ice-cold PBS, and their brains were removed, frozen, and embedded in optimal cutting temperature compound (OCT, Tissue-Tek, Sakura USA, Torrance, CA). Coronal brain sections (15 μm) were cut on a cryostat, collected on silane-coated slides, immediately frozen (for in situ zymography) or allowed to air-dry (for PAI-1 immunohistochemistry), and stored at -80°C until analyzed. In situ zymography was performed according to a previously published protocol (25) by overlaying the sections with a mixture containing 2.5% commercial instant nonfat milk and 25 μg/ml purified human plg (27). The zymograms were developed in a humidified chamber at 37°C for 2.5 h and photographed in 8-bit grayscale under dark-field illumination, and the areas of lytic zones were measured by using imagej 1.30 (a Java version of nih image, publicly available at http://rsb.info.nih.gov/ij). All zymograms used for comparisons were processed and photographed at the same time. PAI-1 immunohistochemistry was performed on adjacent brain sections as described (25) by using rabbit primary antibodies [rabbit anti-PAI-1, 1:1,000 (American Diagnostica, Greenwich, CT) and rabbit anti-PAI-1, 1:1000 (a gift from D. Loskutoff, The Scripps Research Institute, La Jolla, CA)] followed by rhodamine red X-coupled donkey anti-rabbit IgG (1:400, Jackson ImmunoResearch). For quantification of the PAI-1 fluorescent signal, images were photographed and converted to 8-bit grayscale, and the signal intensity was measured by using imagej.
Determination of CRF Receptor Involved in Up-Regulation of tPA Activity. This procedure was performed in live brain slices maintained in Ringer's solution (RS) (composition: 115 mM NaCl/3.3 mM KCl/2 mM CaCl2/1 mM MgSO4/25.5 mM NaHCO3/1.2 mM NaH2PO4/25 mM glucose) equilibrated with carbogen (95% O2/5% CO2). The animals were anesthetized with metoxyflurane (Metofane) and transcardially perfused with ice-cold RS, and the brain was rapidly removed and submerged in ice-cold RS. Four-hundred-micrometer coronal slices containing the amygdala were cut on a vibratome into halves in the midline. Both halves were transferred to separate superfusion chambers and perfused at 32-33°C with carbogenated RS with a flow rate of 1 ml/min. To examine whether CRF has any effect on tPA activity under these conditions, after a 15-min equilibration, one-half of the slice was treated with CRF (100 nM) added to the perfusate, and the perfusion was continued for 30 min, whereas the other half was maintained in RS for the same period. To determine the type of CRF receptor involved, one-half of the slice was treated with CRF only, whereas the other half was treated with CRF in the presence of CRF type 1 (1 μM antalarmin, Sigma) or type 2 (1 μM Antisauvagine 30, PolyPeptide Laboratories, Wolfenbüttel, Germany) receptor antagonist. The antagonists were applied 5 min before addition of CRF and remained in the perfusate throughout the experiment, whereas antagonist vehicle (DMSO and distilled water for antalarmin and Antisauvagine 30, respectively) was added to the RS supplying the control chamber. The perfusion chambers and halves used for each treatment (left vs. right) were alternated in each experimental group. After perfusion, the slices were quickly frozen, embedded in optimal cutting temperature compound, and cut into 15-μm coronal sections, and zymography was performed as described above. The area of lysis was measured by using imagej and compared between halves of the same slice.
c-fos Immunohistochemistry. Animals were injected with CRF (30 ng in 1 μl, i.c.v) or ACSF, and 2 h later anesthetized with Avertin and transcardially perfused with ice-cold PBS followed by 4% paraformaldehyde in PBS. The brains were removed, postfixed in the same fixative for 16-24 h, and cryoprotected in 30% sucrose for 48 h at 4°C. c-fos immunodetection was performed in a one-in-six series of 30-μm free-floating microtome sections. After quenching endogenous peroxidase activity [1% hydrogen peroxide in PBS, 30 min at room temperature (RT)], sections were blocked for 2 h at RT in PBS containing 5% normal goat serum plus 0.3% Triton X-100 and incubated with primary antibody (rabbit anti-c-fos, 1:2,500, Santa Cruz Biotechnology) at 4°C overnight. Detection was performed by using biotinylated goat anti-rabbit secondary antibody (1:200), followed by streptavidin-horseradish peroxidase complex (Elite ABC) and NovaRED substrate (all from Vector Laboratories). The sections were transferred onto gelatin-coated slides, air-dried, dehydrated through an ascending alcohol series, and coverslipped. The slides were coded to conceal the treatment applied, and sections containing the regions of interest were identified with reference to the Paxinos and Franklin mouse brain atlas (26). Regions of interest were photographed in 8-bit grayscale at a magnification of ×100. c-fos immunoreactive cells were counted by using image analysis software as described by others (28). In brief, the background for all photographs was normalized, density thresholds were set to 85 (minimum) and 255 (maximum), and the image was inverted; thus, labeled cells were visualized in black on a white background. Counting was performed by using imagej particle analysis algorithm with the acceptable range of particle size between 2 and 50 pixels. For each region of interest, a spot check was performed by visually counting the cells under the microscope, and the consistency of results obtained with both methods was found to exceed 95%.
Elevated Plus-Maze. The elevated plus-maze apparatus was made of four wooden arms (two enclosed arms, 67 × 7 × 17 cm, that formed a cross shape with the two open arms, 67 × 7 cm). The maze was 55 cm above the floor and dimly illuminated. Experiments were performed during the light period of the circadian cycle. One group of animals received i.c.v injection of CRF (30, 100, or 300 ng in 1 μl), whereas the control mice received the same volume of ACSF. The test was performed 30 min after injections. The mice were placed on the central platform facing an open arm and allowed to explore the apparatus for 5 min. Sessions were videotaped, and the numbers of entries into open and closed arms and head dips into open arms were counted by an investigator unaware of the treatment applied.
Corticosterone Concentration Measurements. The blood for corticosterone level measurements was collected in heparinized tubes from the right heart chamber before PBS perfusion 30 and 120 min after CRF (30 ng) or ACSF injection. Plasma was separated and analyzed by using an EIA-based kit (OCTEIA Corticosterone EIA, Alpco Diagnostics, Windham, NH).
Statistical Analysis. Data are presented as mean ± SEM. Between-groups comparisons were performed with a Mann-Whitney U test. In multiple group analysis, a Kruskal-Wallis test followed by Dunn's test was used. P values <0.05 were considered significant. Numbers of animals in each experiment and the level of statistical significance are presented in the figure legends.
Results and Discussion
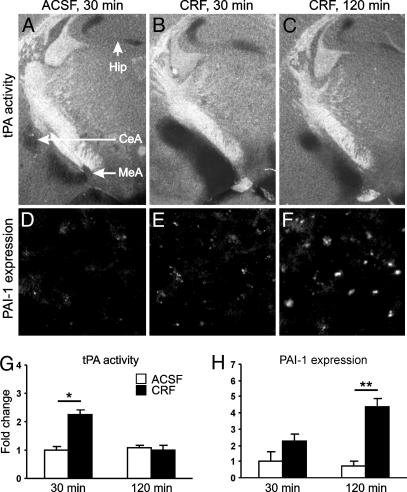
Acute stress leads to increased tPA activity in the central and medial amygdala (25). To examine whether this up-regulation is mediated by CRF, we performed in situ zymography after i.c.v. injection of this neuropeptide. Within 30 min after CRF delivery, the activity of tPA in the central and medial amygdala increased >2-fold in comparison with that in ACSF-injected control animals (Fig. 1 A and B) and 90 min later returned to basal values (Fig. 1C and quantification in 1G). No changes in tPA activity between the 30- and 120-min time points were observed in ACSF-injected animals (Fig. 1G). CRF injection did not result in any significant changes in tPA activity in the hippocampus (Fig. 1 A-C). These CRF-induced changes in tPA activity in the amygdala closely follow those previously observed after acute restraint stress (25), demonstrating a role for CRF in mediating this effect.
Fig. 1.
CRF increases tPA activity in the amygdala in vivo. Thirty minutes after CRF injection, tPA activity in the amygdala (dark lytic areas in B) was significantly up-regulated in comparison with ACSF-injected control mice (A) and returned to basal values 90 min later (C). (D-F) Immunohistochemistry performed on adjacent brain sections showed increased PAI-1 expression in the same region. (G and H) Quantification of changes shown in A-C and D-F, respectively (n = 3-5 mice per group). CeA, central amygdala; MeA, medial amygdala; Hip, hippocampal mossy fiber pathway. The labels “30 min” and “120 min” indicate the time after injection. *, P < 0.05; **, P < 0.01.
The up-regulation of tPA activity in the amygdala in response to restraint stress is followed by its inhibition by PAI-1 (25), a serpin family inhibitor of tPA widely expressed in the murine brain (29). To examine whether similar regulation is observed during CRF-induced changes, we performed PAI-1 immunostaining on adjacent brain sections. We found that the decrease in tPA activity at later time points was indeed accompanied by enhanced PAI-1 expression in the same region (Fig. 1 D-F and quantification in 1H), suggesting that PAI-1 is involved in the normalization of tPA activity in the amygdala. No PAI-1 expression was observed in the hippocampus (data not shown).
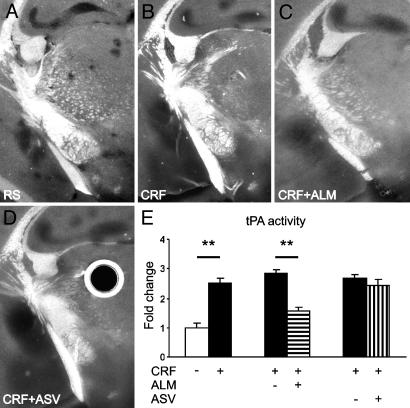
Because CRF activates a number of other stress-related pathways (30), it was possible that the action of CRF on tPA activity was indirect and mediated by some other neurotransmitter. Particularly, because tPA colocalizes with catecholamines in neuronal cell lines (19, 20), indirect action could involve the noradrenergic pathway originating in the locus coeruleus and providing input to the central amygdala (7, 30). To examine this possibility, we applied CRF to live coronal brain slices devoid of hindbrain connections. Addition of CRF to the perfusate resulted in a >2-fold increase in tPA activity in the central and medial amygdala (Fig. 2 A, B, and E), demonstrating that CRF acts on the level of forebrain to exert this effect. To establish which CRF receptor mediates its effect on tPA activity, we superfused brain slices with CRF in the absence or presence of selective CRF-R1 and CRF-R2 antagonists. When the slices were incubated with CRF in the presence of the CRF-R1 antagonist, antalarmin, activity of tPA in amygdala was significantly decreased compared with CRF alone and was similar to that obtained in the absence of CRF (Fig. 2 C and E). In contrast, no difference in tPA activity was observed in slices treated with CRF in the presence or absence of the CRF-R2 antagonist, Antisauvagine 30 (Fig. 2 D and E). No significant changes in tPA activity were observed in the hippocampus. Taken together, these results demonstrate that CRF acts directly to increase tPA activity in the amygdala and that this action is mediated by CRF-R1 receptor.
Fig. 2.
CRF increases tPA activity in the amygdala in brain slices by means of the CRF-R1 receptor. CRF caused a significant up-regulation of tPA in the central and medial amygdala in comparison with the slice perfused with RS only (dark lytic areas in B vs. A). This up-regulation was inhibited by the CRF-R1 antagonist, antalarmin (ALM, C), but not the CRF-R2 antagonist Antisauvagine 30 (ASV, D). Slices perfused with CRF with addition of ALM or ASV vehicle (corresponding to C and D) are not shown. Round dark area with bright halo in D is an artifact caused by an air bubble in the overlay gel. (E) Quantification of changes shown in A-D (n = 5 slices per group) in relation to the mean area of lysis in slices perfused with RS only. **, P < 0.05.
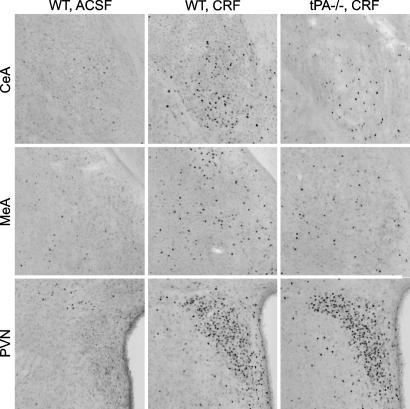
To examine whether tPA could influence activation of stress-related regions by CRF, the number of c-fos-positive cells in the central and medial amygdala was compared between WT and tPA-/- mice; this analysis also included the PVN, a structure critical for the integration of the endocrine component of the stress response (31). c-fos is an immediate early gene whose expression in the brain is transiently induced by a variety of stimuli (32), and the level of c-fos protein is widely used to assess neuronal activity (6, 33-36). c-fos-positive cells were counted 2 h after CRF injection, when there is peak expression of c-fos protein (6). In control WT (Fig. 3 Left and Table 1) and tPA-/- animals injected with ACSF (Table 1), the number of c-fos-positive cells was low and uniform, with the exception of the thalamic PVN and pyriform cortex, previously identified as sites of constitutive c-fos expression (6, 35, 37). CRF induced up-regulation of c-fos expression in WT mice in all regions studied (Fig. 3 Center and Table 1). Up-regulation of c-fos expression in response to CRF also was evident in tPA-/- mice; however, the number of c-fos-positive cells in the central and medial amygdala was significantly lower than in WT mice (Fig. 3 Right and Table 1). Expression of c-fos in the PVN did not differ between the two genotypes (Fig. 3 and Table 1), demonstrating that tPA does not play a role in its activation.
Fig. 3.
tPA is necessary for neuronal activation in the central and medial amygdala. Examples of c-fos immunoreactive cells (visible as black dots) in the central (CeA) and medial (MeA) amygdala and PVN of WT and tPA-/- mice. For quantification and statistical analysis, see Table 1.
Table 1. CRF-induced c-fos expression in the central and medial amygdala is decreased in tPA−/− mice.
| Region | Genotype | ACSF | CRF |
|---|---|---|---|
| CeA | WT | 9 ± 2 | 28 ± 3* |
| tPA−/− | 5 ± 1 | 16 ± 4*† | |
| plg−/− | 7 ± 4 | 30 ± 2* | |
| MeA | WT | 10 ± 1 | 35 ± 2* |
| tPA−/− | 10 ± 2 | 23 ± 3*† | |
| plg−/− | 13 ± 3 | 39 ± 6* | |
| PVN | WT | 6 ± 3 | 157 ± 11 |
| tPA−/− | 7 ± 2 | 146 ± 17 | |
| plg−/− | 9 ± 2 | 147 ± 13 |
The number of c-fos-positive cells in the central (CeA) and medial (MeA) amygdala and PVN in WT, tPA−/−, and plg−/− mice. *, P < 0.05 vs. respective control (ACSF-injected) animals. †, P < 0.05 tPA−/− vs. WT, n = 4-5 per group.
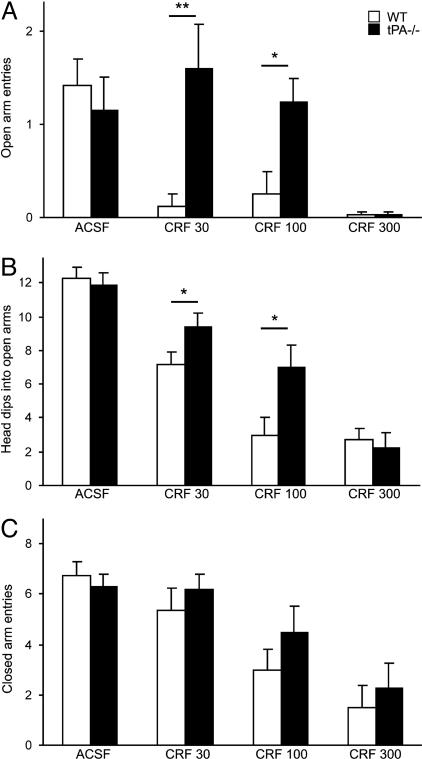
Given the increase in tPA activity and neuronal activation in the amygdala in response to CRF, we next evaluated whether tPA contributes to the behavioral effect of this neuropeptide. To this end, the behavior of WT and tPA-/- mice was examined in the elevated plus-maze. This test relies on the natural avoidance of open spaces by rodents, which is exacerbated when level of anxiety is high (38, 39). Testing in the elevated plus-maze allows assessment of anxiety (number of entries into open arms), exploration (head dips into open arms), and activity (entries into closed arms) during the same trial (39) and has been previously shown to be a sensitive measure of anxiogenic action of CRF (7). WT and tPA-/- mice injected with ACSF showed similar numbers of open arms entries (Fig. 4A) and head dips into open arms (Fig. 4B). The basal level of locomotor activity (closed arms entries) was also comparable between genotypes (Fig. 4C), consistent with similar horizontal locomotor activity in WT and tPA-deficient animals in the open field test (23, 24, 40). In WT mice, CRF completely abolished exploration of the open arms (Fig. 4A) at all doses tested, indicating a high level of anxiety in these animals, and caused a dose-dependent decrease in their exploratory behavior (Fig. 4B). In contrast, no significant anxiety was observed in tPA-/- mice injected with either the low or middle dose of CRF (Fig. 4A) and the number of head dips was higher than in WT animals (Fig. 4B). These differences were abolished by the highest dose of CRF, which also produced almost complete suppression of activity in both WT and tPA-/- mice (Fig. 4C). The number of closed arms entries, however, was similar between the two genotypes (Fig. 4C), indicating that the differences in CRF-induced anxiety and exploration were not due to differences in locomotor activity. These results demonstrate that tPA-/- mice show reduced anxiety in response to CRF, consistent with previous results showing lack of stress-induced anxiety in these animals (25).
Fig. 4.
tPA-/- mice show a lower level of CRF-induced anxiety in the elevated plus-maze. (A) Injection of CRF strongly inhibited the exploration of open arms by WT animals, whereas tPA-/- mice failed to explore the open arms only at the highest dose of CRF. (B) tPA-/- animals more often dipped their heads into open arms. (C) Overall level of activity measured by closed arms entries was similar in both genotypes (n = 4-13 mice per group) *, P < 0.05; **, P < 0.01. CRF 30, CRF 100, and CRF 300 indicate dose of CRF in ng per animal.
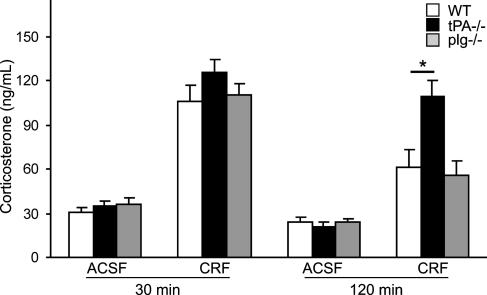
To investigate whether the lack of CRF-induced anxiety in tPA-/- animals could be explained by abnormal stimulation of the HPA axis, we compared the concentration of corticosterone between WT and tPA-/- mice. We did not find any difference in this parameter at the time point at which the animals were tested in the elevated plus-maze (Fig. 5), indicating that tPA is not necessary for the CRF-induced activation of the HPA axis. This result is in line with similar activation of PVN in both genotypes and with observations of other authors who demonstrated that the behavioral component of the response to CRF is independent on the activation of the HPA axis and glucocorticoid level (12, 41, 42). However, tPA-/- mice exhibited sustained elevation of corticosterone 90 min later (Fig. 5), corroborating our previous finding that tPA participates in regulating the duration of the hormonal component of stress response (25). Negative feedback regulation of the PVN is in large part provided by the hippocampus. However, it is unlikely that tPA had an effect on the hippocampus itself, because the activity of tPA in this region was not changed by CRF. It will require further investigation to determine whether the delayed shutoff of the HPA axis in tPA-/- animals has any consequences on the central nervous system, especially in conditions of chronic stress.
Fig. 5.
tPA-/- mice show normal up-regulation of corticosterone in response to CRF but sustained elevation of corticosterone level during recovery. Shown is corticosterone concentration in WT, tPA-/-, and plg-/- mice (n = 4-5 per group) 30 or 120 min after CRF or ACSF injection. *, P < 0.05.
These results raise the question of the mechanism by which tPA promotes CRF-induced effects. The main substrate of tPA is plg, which is converted to the broad-spectrum protease plasmin. Because plasmin can modulate neuronal activity (43, 44) and some of the previously characterized effects of tPA in the central nervous system require plg (45), we examined whether the action of tPA was mediated by plg activation. Although plg-/- mice reportedly exhibit reduced response in certain behavioral paradigms (46) and show alterted processing of corticotropin precursor (47), we did not find any abnormalities in CRF-induced expression of c-fos within examined regions (Table 1), behavior in the elevated plus-maze (data not shown), and corticosterone response in these animals (Fig. 5). Thus, we conclude that tPA-mediated facilitation of CRF-induced responses does not require plg activation.
Alternatively, the effect of tPA could be mediated by a proteolytic or nonproteolytic interaction with other substrates or receptors. It has been demonstrated that neuronal activation within central and medial amygdala involves N-methyl-d-aspartate (NMDA) receptor signaling (48), which can be potentiated by tPA (49, 50). Although the mechanism of the latter awaits clarification (51), potentiation of NMDA signaling by tPA could thus be involved in the activation of stress-related regions and subsequent anxiety-like behavior. Another likely candidate is low-density lipoprotein receptor-related protein (LRP), whose interaction with tPA has been implicated in long-term potentiation in the hippocampus (52). Finally, tPA released in response to CRF could in turn modulate its effect by means of modification of CRF-R1 receptor signaling. Such a possibility is supported by a striking similarity of the behavioral and hormonal response to CRF of tPA-/- animals to that with selective disruption of limbic, but not hypothalamic, CRF-R1 receptors (12).
The observation that the up-regulation of tPA is accompanied by enhanced expression of PAI-1 also raises the question about a role of this protein in the effect of tPA. Because PAI-1 inhibits the proteolytic activity of tPA, it would limit the effect of this protease if it were mediated by proteolysis. On the other hand, if the effect of tPA is nonproteolytic and involves binding to other proteins, PAI-1 could play either a limiting or enhancing role in such interactions. The latter possibility is supported by an observation that deletion of PAI-1 has the same consequences as tPA deficiency in some behavioral tasks (53), suggesting that both proteins might act in concert to mediate this effect. This question needs to be addressed by using PAI-1-deficient animals in similar experimental paradigm.
In conclusion, our studies implicate tPA in the cascade of events initiated by CRF, a major integrator of the stress response. Up-regulation of tPA activity in the amygdala by CRF is necessary for subsequent neuronal activation and anxiety-like behavior elicited by this neuropeptide. Identification of the mechanisms by which tPA participates in this response could aid in the development of strategies aimed at the treatment of affective disorders.
Acknowledgments
We thank Y. Keptsi for technical assistance and the members of the Strickland laboratory for discussion. This study was supported by National Institutes of Health Grants NS-35704 and NS-38472.
Abbreviations: CRF, corticotropin-releasing factor; tPA, tissue plasminogen activator; HPA, hypothalamic-pituitary-adrenal; PVN, paraventricular nucleus; plg, plasminogen; i.c.v., intracerebroventricular; PAI-1, plasminogen activator inhibitor 1; ACSF, artificial cerebrospinal fluid; RS, Ringer's solution.
References
- 1.Davis, M. (1992) Annu. Rev. Neurosci. 15 353-375. [DOI] [PubMed] [Google Scholar]
- 2.Herman, J. P. & Cullinan, W. E. (1997) Trends Neurosci. 20 78-84. [DOI] [PubMed] [Google Scholar]
- 3.Rogan, M. T. & LeDoux, J. E. (1996) Cell 85 469-475. [DOI] [PubMed] [Google Scholar]
- 4.Owens, M. J. & Nemeroff, C. B. (1991) Pharmacol. Rev. 43 425-473. [PubMed] [Google Scholar]
- 5.Vale, W., Spiess, J., Rivier, C. & Rivier, J. (1981) Science 213 1394-1397. [DOI] [PubMed] [Google Scholar]
- 6.Bittencourt, J. C. & Sawchenko, P. E. (2000) J. Neurosci. 20 1142-1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koob, G. F. & Heinrichs, S. C. (1999) Brain Res. 848 141-152. [DOI] [PubMed] [Google Scholar]
- 8.Stenzel-Poore, M. P., Heinrichs, S. C., Rivest, S., Koob, G. F. & Vale, W. W. (1994) J. Neurosci. 14 2579-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bale, T. L. & Vale, W. W. (2004) Annu. Rev. Pharmacol. Toxicol. 44 525-557. [DOI] [PubMed] [Google Scholar]
- 10.Timpl, P., Spanagel, R., Sillaber, I., Kresse, A., Reul, J. M., Stalla, G. K., Blanquet, V., Steckler, T., Holsboer, F. & Wurst, W. (1998) Nat. Genet. 19 162-166. [DOI] [PubMed] [Google Scholar]
- 11.Smith, G. W., Aubry, J. M., Dellu, F., Contarino, A., Bilezikjian, L. M., Gold, L. H., Chen, R., Marchuk, Y., Hauser, C., Bentley, C. A., et al. (1998) Neuron 20 1093-1102. [DOI] [PubMed] [Google Scholar]
- 12.Muller, M. B., Zimmermann, S., Sillaber, I., Hagemeyer, T. P., Deussing, J. M., Timpl, P., Kormann, M. S., Droste, S. K., Kuhn, R., Reul, J. M., et al. (2003) Nat. Neurosci. 6 1100-1107. [DOI] [PubMed] [Google Scholar]
- 13.Bale, T. L., Contarino, A., Smith, G. W., Chan, R., Gold, L. H., Sawchenko, P. E., Koob, G. F., Vale, W. W. & Lee, K. F. (2000) Nat. Genet. 24 410-414. [DOI] [PubMed] [Google Scholar]
- 14.Coste, S. C., Kesterson, R. A., Heldwein, K. A., Stevens, S. L., Heard, A. D., Hollis, J. H., Murray, S. E., Hill, J. K., Pantely, G. A., Hohimer, A. R., et al. (2000) Nat. Genet. 24 403-409. [DOI] [PubMed] [Google Scholar]
- 15.Kishimoto, T., Radulovic, J., Radulovic, M., Lin, C. R., Schrick, C., Hooshmand, F., Hermanson, O., Rosenfeld, M. G. & Spiess, J. (2000) Nat. Genet. 24 415-419. [DOI] [PubMed] [Google Scholar]
- 16.Collen, D. (1999) Thromb. Haemostasis 82 259-270. [PubMed] [Google Scholar]
- 17.Sappino, A. P., Madani, R., Huarte, J., Belin, D., Kiss, J. Z., Wohlwend, A. & Vassalli, J. D. (1993) J. Clin. Invest. 92 679-685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baranes, D., Lederfein, D., Huang, Y. Y., Chen, M., Bailey, C. H. & Kandel, E. R. (1998) Neuron 21 813-825. [DOI] [PubMed] [Google Scholar]
- 19.Gualandris, A., Jones, T. E., Strickland, S. & Tsirka, S. E. (1996) J. Neurosci. 16 2220-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Parmer, R. J., Mahata, M., Mahata, S., Sebald, M. T., O'Connor, D. T. & Miles, L. A. (1997) J. Biol. Chem. 272 1976-1982. [DOI] [PubMed] [Google Scholar]
- 21.Neuhoff, H., Roeper, J. & Schweizer, M. (1999) Eur. J. Neurosci. 11 4241-4250. [DOI] [PubMed] [Google Scholar]
- 22.Madani, R., Hulo, S., Toni, N., Madani, H., Steimer, T., Muller, D. & Vassalli, J. D. (1999) EMBO J. 18 3007-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Calabresi, P., Napolitano, M., Centonze, D., Marfia, G. A., Gubellini, P., Teule, M. A., Berretta, N., Bernardi, G., Frati, L., Tolu, M. & Gulino, A. (2000) Eur. J. Neurosci. 12 1002-1012. [DOI] [PubMed] [Google Scholar]
- 24.Pawlak, R., Nagai, N., Urano, T., Napiorkowska-Pawlak, D., Ihara, H., Takada, Y., Collen, D. & Takada, A. (2002) Neuroscience 113 995-1001. [DOI] [PubMed] [Google Scholar]
- 25.Pawlak, R., Magarinos, A. M., Melchor, J., McEwen, B. & Strickland, S. (2003) Nat. Neurosci. 6 168-174. [DOI] [PubMed] [Google Scholar]
- 26.Paxinos, G. & Franklin, K. B. J. (2001) The Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 27.Deutsch, D. G. & Mertz, E. T. (1970) Science 170 1095-1096. [DOI] [PubMed] [Google Scholar]
- 28.Gammie, S. C. & Nelson, R. J. (2001) Brain Res. 898 232-241. [DOI] [PubMed] [Google Scholar]
- 29.Masos, T. & Miskin, R. (1997) Brain Res. Mol. Brain Res. 47 157-169. [DOI] [PubMed] [Google Scholar]
- 30.Millan, M. J. (2003) Prog. Neurobiol. 70 83-244. [DOI] [PubMed] [Google Scholar]
- 31.Sawchenko, P. E., Li, H. Y. & Ericsson, A. (2000) Prog. Brain Res. 122 61-78. [DOI] [PubMed] [Google Scholar]
- 32.Hughes, P. & Dragunow, M. (1995) Pharmacol. Rev. 47 133-178. [PubMed] [Google Scholar]
- 33.Sagar, S. M., Sharp, F. R. & Curran, T. (1988) Science 240 1328-1331. [DOI] [PubMed] [Google Scholar]
- 34.Ferguson, J. N., Aldag, J. M., Insel, T. R. & Young, L. J. (2001) J. Neurosci. 21 8278-8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sinnayah, P., Blair-West, J. R., McBurnie, M. I., McKinley, M. J., Oldfield, B. J., Rivier, J., Vale, W. W., Walker, L. L., Weisinger, R. S. & Denton, D. A. (2003) Eur. J. Neurosci. 18 373-382. [DOI] [PubMed] [Google Scholar]
- 36.Nomura, M., Saito, J., Ueta, Y., Muglia, L. J., Pfaff, D. W. & Ogawa, S. (2003) J. Neuroendocrinol. 15 1054-1061. [DOI] [PubMed] [Google Scholar]
- 37.Ryabinin, A. E., Criado, J. R., Henriksen, S. J., Bloom, F. E. & Wilson, M. C. (1997) Mol. Psychiatry 2 32-43. [DOI] [PubMed] [Google Scholar]
- 38.File, S. E. (2001) Behav. Brain Res. 125 151-157. [DOI] [PubMed] [Google Scholar]
- 39.Rodgers, R. J. & Dalvi, A. (1997) Neurosci. Biobehav. Rev. 21 801-810. [DOI] [PubMed] [Google Scholar]
- 40.Horwood, J. M., Ripley, T. L. & Stephens, D. N. (2004) Behav. Brain Res. 150 127-138. [DOI] [PubMed] [Google Scholar]
- 41.Berridge, C. W. & Dunn, A. J. (1989) Pharmacol. Biochem. Behav. 34 517-519. [DOI] [PubMed] [Google Scholar]
- 42.Britton, K. T., Lee, G., Dana, R., Risch, S. C. & Koob, G. F. (1986) Life Sci. 39 1281-1286. [DOI] [PubMed] [Google Scholar]
- 43.Mizutani, A., Saito, H. & Matsuki, N. (1996) Brain Res. 739 276-281. [DOI] [PubMed] [Google Scholar]
- 44.Nakagami, Y., Abe, K., Nishiyama, N. & Matsuki, N. (2000) J. Neurosci. 20 2003-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chen, Z. L. & Strickland, S. (1997) Cell 91 917-925. [DOI] [PubMed] [Google Scholar]
- 46.Hoover-Plow, J., Skomorovska-Prokvolit, O. & Welsh, S. (2001) Brain Res. 898 256-264. [DOI] [PubMed] [Google Scholar]
- 47.Wang, N., Zhang, L., Miles, L. & Hoover-Plow, J. (2004) J. Thromb. Haemost. 2 785-796. [DOI] [PubMed] [Google Scholar]
- 48.Hattori, K., Yagi, T., Maekawa, M., Sato, T. & Yuasa, S. (2001) Brain Res. 905 188-198. [DOI] [PubMed] [Google Scholar]
- 49.Centonze, D., Saulle, E., Pisani, A., Bonsi, P., Tropepi, D., Bernardi, G. & Calabresi, P. (2002) NeuroReport 13 115-118. [DOI] [PubMed] [Google Scholar]
- 50.Nicole, O., Docagne, F., Ali, C., Margaill, I., Carmeliet, P., MacKenzie, E. T., Vivien, D. & Buisson, A. (2001) Nat. Med. 7 59-64. [DOI] [PubMed] [Google Scholar]
- 51.Matys, T. & Strickland, S. (2003) Nat. Med. 9 371-372, and author reply, 372-373, and [DOI] [PubMed] [Google Scholar]; erratum (2003) 9 975. [Google Scholar]
- 52.Zhuo, M., Holtzman, D. M., Li, Y., Osaka, H., DeMaro, J., Jacquin, M. & Bu, G. (2000) J. Neurosci. 20 542-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horwood, J. M., Ripley, T. L. & Stephens, D. N. (2001) Behav. Pharmacol. 12 487-496. [DOI] [PubMed] [Google Scholar]