Abstract
Obsessive‐compulsive disorder (OCD) is often associated with pathological uncertainty regarding whether an action has been performed correctly or whether a bad outcome will occur, leading to compulsive “evidence gathering” behaviors aimed at reducing uncertainty. The current study used event‐related functional magnetic resonance imaging to investigate neural functioning in OCD patients and controls as subjective certainty was rated in response to sequential pieces of evidence for a decision. Uncertainty was experimentally manipulated so that some decisions were associated with no “objective” uncertainty (all observed evidence pointed to one correct choice), whereas other decisions contained calculable but varying levels of objective uncertainty based on displayed probabilities. Results indicated that OCD patients differed from controls on decisions that contained no objective uncertainty, such that patients rated themselves as more uncertain. Patients also showed greater activation in a network of brain regions previously associated with internally‐focused thought and valuation including ventromedial prefrontal cortex, parahippocampus, middle temporal cortex, as well as amygdala and orbitofrontal cortex/ventral anterior insula. In the patient group, a significantly greater number of positive intersubject correlations were found among several of these brain regions, suggesting that this network is more interconnected in patients. OCD patients did not differ from controls on decisions where task parameters led to uncertainty. These results indicate that OCD is associated with hyperactivation in a network of limbic/paralimbic brain regions when making decisions, which may contribute to the greater subjective experience of doubt that characterizes the disorder. Hum Brain Mapp, 2013. © 2012 Wiley Periodicals, Inc.
Keywords: fMRI, anxiety, decision‐making, neuroimaging, default mode network
INTRODUCTION
Obsessive‐compulsive disorder (OCD) is characterized by repetitive intrusive thoughts, ideas, or images that are distressing and cause significant anxiety. In response to obsessions, patients frequently engage in compulsive behaviors that attempt to reduce anxiety. In many cases, these compulsions take the form of “evidence gathering” behaviors attempting to reduce uncertainty regarding whether an action has been performed correctly (i.e., checking that a stove is off or that a door is locked) or whether a bad outcome will or did occur (i.e., whether a disease will be contracted or whether harm has befallen a loved one). When compulsive behaviors are successful in reducing uncertainty and alleviating anxiety, they do so only temporarily; research has indicated that compulsions are more likely to increase uncertainty over the long term [Harkin and Kessler, 2009; van den Hout and Kindt, 2003]. Consistent with clinical observation, behavioral studies have found that OCD patients gather more evidence to reach a decision than controls, even in situations unrelated to OC concerns [Fear and Healy, 1997; Milner et al., 1971; Volans, 1976]. Although the behavioral phenotype of pathological uncertainty is a prominent feature of OCD, little is known about the neural basis of this psychological process.
Neuroimaging studies in OCD have identified hyperactivation during resting state and symptom provocation in a variety of cortical and subcortical regions, with recent meta‐analyses emphasizing the role of orbitofrontal cortex (OFC), medial frontal cortex (MFC), anterior insula/frontal operculum (aI/fO), hippocampus, caudate nucleus, and thalamus [Rotge et al., 2008; Whiteside et al., 2004]. Recent research has linked some of these same brain regions to the experience of uncertainty. Studies in healthy individuals have related greater activity in posterior MFC (pMFC)/dorsal anterior cingulate cortex (Brodmann's areas [BAs] 6, 8, 24, 32) [Grinband et al., 2006; Krain et al., 2006; Volz et al., 2003] and aI/fO (BAs 13, 47) [Preuschoff et al., 2008] to increases in decision uncertainty. These experiments manipulated uncertainty by varying the probabilities associated with two decision outcomes (e.g., the closer both outcome probabilities are to each other and to 0.5, the greater the decision uncertainty), a type of uncertainty that is dictated entirely by task parameters (what will be referred to as “objective” uncertainty). However, probabilistic metrics of uncertainty do not take into account subjective experiences of uncertainty, which are known to vary between individuals with access to the same information [Weber and Milliman, 1997].
To address this issue, we recently examined neural activity while healthy subjects observed evidence for an upcoming decision, relating this activity both to “objective” measures of uncertainty (i.e., those dictated by outcome probabilities) as well as self‐reported “subjective” measures of uncertainty. Results from this study replicated prior findings linking objective uncertainty to pMFC, additionally finding that individuals experiencing relatively greater subjective uncertainty when accumulating evidence exhibited more activity in medial OFC/ventromedial prefrontal cortex (VMPFC; BA 11, 25), particularly for the first piece of information observed [Stern et al., 2010]. This dissociation is interesting considering the divergent cognitive functions that have been attributed to these different brain regions. Posterior MFC and dorsal aI/fO are thought to comprise a brain network involved in detecting salience in the external environment and general monitoring functions necessary to carry out tasks [Dosenbach et al., 2006; Menon and Uddin, 2010; Sridharan et al., 2008]. In contrast, VMPFC is part of the “default mode network” (DMN) of brain regions, which robustly decreases in activity (or deactivates) during externally‐directed cognitive tasks. In addition to VMPFC, DMN includes areas of dorsomedial prefrontal cortex (DMPFC; BAs 8, 9, 10), posterior cingulate cortex (PCC; BAs 7, 23, 31), lateral temporal cortex including middle temporal gyri and temporal pole (BAs 20, 21, 38), hippocampus/parahippocampus, and inferior parietal cortex (BA 39). Unlike pMFC and dorsal aI/fO, DMN regions tend to become active when subjects engage in internally‐focused mental processes such as imagining the future, autobiographical memory, counterfactual thinking and scenario construction, and processing of self and theory of mind [Andrews‐Hanna et al., 2010; Buckner et al., 2008; Schacter et al., 2008]. Together, these findings suggest that, in healthy individuals, greater internal focus associated with VMPFC hyperactivation contributes to the experience of subjective uncertainty when observing evidence.
Prior studies using paradigms that examine neural mechanisms of performance monitoring have shown greater activation of pMFC and aI/fO, as well as a failure to deactivate VMPFC, in response to errors in patients with OCD [Fitzgerald et al., 2005, 2010; Maltby et al., 2005; Stern et al., 2011; Ursu et al., 2003], suggesting altered functioning in both the salience detection network and DMN. In this work, we extend these findings by focusing on a cognitive process relevant for OCD—uncertainty during decision‐making—using an event‐related functional magnetic resonance imaging (fMRI) paradigm in which subjective certainty is rated as sequential pieces of evidence are accumulated for a decision. We hypothesized that OCD patients would experience greater subjective uncertainty than controls when observing evidence for a decision. Furthermore, we predicted that patients would show greater activity when observing evidence in DMN regions, particularly VMPFC, consistent with prior findings in uncertain healthy subjects.
MATERIALS AND METHODS
Subjects
Twenty‐six OCD patients and 30 control subjects participated in the experiment. One OCD patient withdrew because of discomfort in the scanner, and data from one control subject were excluded because of excessive movement, leaving a total of 54 subjects (25 OCD and 29 controls) available for analysis. Nine OCD patients were unmedicated (uOCD) and 16 were medicated (mOCD) with serotonin reuptake inhibitors (SRIs). All patients met DSM‐IV criteria for current OCD, excluding histories of psychosis, bipolar spectrum mood disorders, developmental disorders, and traumatic brain injury. Patients were free of current Tourette's syndrome, chronic or transient tic disorder, and alcohol or substance abuse/dependence, and those with hoarding as the primary symptom complaint were excluded. No patients were in a current major depressive episode. However, because of the high rate of comorbidity between depression and OCD [Overbeek et al., 2002], patients were accepted with current depressive disorder not otherwise specified (NOS) (n = 4) or major depressive disorder in partial remission (n = 4, one uOCD and three mOCD) or full remission (n = 10, four uOCD and six mOCD) according to DSM‐IV criteria, which comprised 81% of mOCD patients and 56% of uOCD patients. Other comorbidity was minimal (generalized anxiety disorder: n = 1; specific phobia: n = 2; tic disorder NOS: n = 1; impulse control disorder NOS: n = 1; prior history of social phobia: n = 2; and prior history of eating disorder NOS, n = 2) and was only allowed if OCD was the patient's primary complaint.
Control subjects included 18 unmedicated healthy controls and 11 medicated patient controls (mPCs). Subjects with any history of OCD were excluded from both control groups. All subjects in the mPC group were on SRIs (see Supporting Information Table 1) due to histories of major depression, and had few comorbidities (prior history of social phobia: n = 1 and prior history of eating disorder NOS, n = 2). As the majority of OCD patients were taking SRIs and had histories of depression, we compared OCD and non‐OCD (control) groups—both of which included medicated participants with histories of depression—to better localize group differences to the presence of OCD. All medicated subjects had been on a stable dose for at least 6 months before testing.
Table I.
Demographic information
| uOCD (n = 9) | mOCD (n = 16) | uHC (n = 18) | mPC (n = 11) | Group differences | Post hoc comparisons | |
|---|---|---|---|---|---|---|
| Age | 23.4 (7.0) | 26.0 (5.5) | 25.9 (8.8) | 29.1 (7.8) | ns | |
| Education (years) | 14.3 (1.7) | 15.7 (2.5) | 15.9 (2.1) | 16.1 (1.4) | ns | |
| Gender | 6 F/3 M | 7 F/9 M | 9 M/9 F | 8 F/3 M | ns | |
| HAM‐A | 11.6 (5.1) | 9.3 (4.4) | 0.72 (1.2) | 3.3 (2.6) | D: F(1,50) = 74.5, P < 0.001 | mOCD > uHC, mPC |
| D × M: F(1,50) = 6.1, P = 0.017 | uOCD > uHC, mPC | |||||
| mPC > uHC all P < 0.005 | ||||||
| HAM‐D | 9.4 (4.7) | 8.1 (4.0) | 0.67 (0.09) | 3.9 (4.0) | D: F(1,50) = 44.6, P < 0.001 | mOCD > uHC, mPC |
| D × M: F(1,50) = 5.5, P = 0.023 | uOCD > uHC, mPC | |||||
| mPC > uHC all P < 0.02 |
uOCD, unmedicated OCD; mOCD, medicated OCD; uHC, unmedicated healthy controls; mPC, medicated patient controls; D, main effect of diagnosis factor; D × M, interaction between diagnosis and medication factors.
OCD and control groups were matched on age, education, and gender. Differences in scores from the Hamilton Anxiety Rating Scale (HAM‐A) and Hamilton Depression Rating Scale (HAM‐D) were evaluated with separate 2 × 2 ANOVAs using diagnosis (OCD and control) and medication status (unmedicated and medicated) as between‐subjects factors. Values in parentheses represent standard deviations. Only those effects significant at P ≤ 0.05 are shown and followed up with post hoc comparisons using independent samples t‐tests.
Subjects provided written informed consent and were evaluated by a trained clinician using the Structured Clinical Interview for DSM‐IV [First et al., 1996]. Symptoms of anxiety and depression were quantified using Hamilton Ratings Scales for Anxiety (HAM‐A) [Hamilton, 1959] and Depression (HAM‐D) [Hamilton, 1960]. OC symptom severity (current and lifetime) was quantified using the Yale‐Brown Obsessive‐Compulsive Scale [Y‐BOCS, Goodman et al., 1989]. Table I shows demographic and clinical information for all groups. Both OCD groups showed significantly more generalized anxiety and depression than either control group, but were not statistically different from each other. Table II shows clinical information specific to the OCD group.
Table II.
Characteristics of OCD group
| uOCD (n = 9) | mOCD (n = 16) | Group differences | |
|---|---|---|---|
| Lifetime Y‐BOCS | 27.7 (3.4) | 28.8 (4.3) | ns |
| Current Y‐BOCS | 22.7 (4.9) | 20.1 (4.1) | ns |
| Age of onset (years) | 11.2 (5.6) | 11.8 (4.9) | ns |
| OCI‐R washing | 4.5 (4.0) | 3.7 (2.8) | ns |
| OCI‐R obsessing | 7.3 (3.6) | 7.2 (3.0) | ns |
| OCI‐R hoarding | 4.2 (3.6) | 3.6 (2.9) | ns |
| OCI‐R ordering | 8.2 (3.9) | 3.8 (3.4) | t (15.7) = 2.7, P < 0.01 |
| OCI‐R checking | 8.3 (3.0) | 3.8 (2.1) | t (13.4) = 4.0, P < 0.001 |
| OCI‐R neutralizing | 4.6 (4.7) | 3.7 (3.7) | ns |
Independent samples t‐tests were used to compare uOCD and mOCD patients. Severity scores from six symptom dimensions were obtained by summing responses to relevant questions (three per each symptom dimension) on the Obsessive‐Compulsive Inventory‐Revised [OCI‐R; Foa et al., 2002]. Summed scores could range from 0 (no endorsement for any question) to 12 (maximum endorsement for all three questions). Values in parentheses represent standard deviations. Only those effects significant at P ≤ 0.05 are shown. OCI‐R scores were missing from three mOCD patients.
Task
Task instructions and practice trials were presented before scanning. While in the scanner, subjects received 72 sequences presenting four pieces of evidence used for an upcoming decision. On 18 sequences, rectangles representing two decks containing red and blue cards were displayed at the top of the screen. Deck A (left side of screen) contained 100% blue cards and 0% red cards, whereas Deck B (right side of screen) contained 0% blue cards and 100% red cards (certain sequences; Fig. 1a). On 54 sequences, Deck A contained 80% blue cards and 20% red cards, whereas Deck B contained 20% blue cards and 80% red cards (uncertain sequences; Fig. 1b). Percentages of red and blue cards contained in each deck were graphically represented as the proportion of space colored in red and blue in each rectangle. Subjects were told that the percentages were 20%/80%, so that all participants entered into the task with the same information about probability. At the start of each sequence (regardless of whether it was a certain or uncertain sequence), the experimenter selected one of the decks, the identity of which was kept hidden from subjects. Subjects needed to figure out which deck had been selected by viewing four sequential colored cards (evidence) drawn from the (hidden) selected deck. On each draw, subjects rated their subjective certainty regarding which of the two decks (Deck A or Deck B) was supplying the draws. Ratings were made on a nine‐point scale with “uncertain” at the center and “certain” at either end (e.g., “certain” for Deck A on left end and “certain” for Deck B on right end). Each subject was informed that the choices located in‐between “uncertain” and “certain” should be interpreted as evenly spaced intermediate categories between uncertainty and certainty. Responses were made using an MRI‐compatible fiber‐optic button response system (PST, Sharpsburg, PA) attached to both hands. After viewing the four draws of cards, subjects were given the option to choose which deck was supplying the cards or to decline to make a choice. After the decision, feedback was presented and a new sequence was started.
Figure 1.
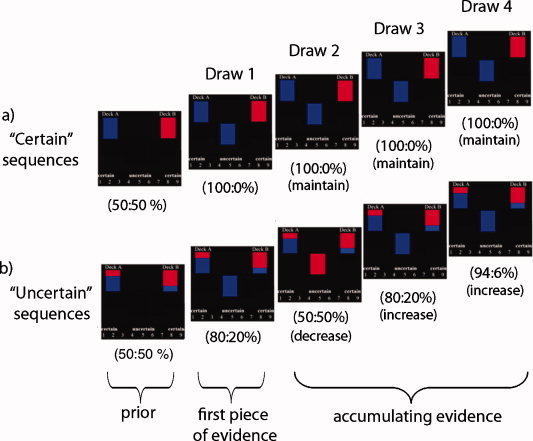
Sequences of trials in the task. Subjects viewed two decks containing equal but opposite ratios of red and blue cards. One of the decks was selected at the beginning of each sequence, and subjects determined which deck was selected by observing four draws of cards from the chosen deck. “Objective certainty” was defined as the difference between the two posterior probabilities calculated for the decks using a 50:50% prior (e.g., the further away the deck probabilities are from 50%, the greater the objective certainty regarding the identity of the deck supplying the draws). “Subjective certainty” was determined by individual ratings (made on each draw) regarding the identity of the deck supplying the cards. After observing all evidence, subjects decided which deck was supplying the draws and feedback was provided (not shown). (a) On “certain” sequences, the deck on the left contained 100% blue cards and the deck on the right contained 100% red cards. Thus, probabilities were furthest away from 50:50% after the first piece of evidence (at 100:0%), with accumulating evidence serving to maintain maximal certainty without providing any new information. (b) On “uncertain” sequences, the deck on the left contained 80% blue cards and 20% red cards, whereas the deck on the right contained 80% red cards and 20% blue cards. Objective certainty varied as evidence accumulated, with some trials increasing certainty (e.g., trials where the probabilities moved further away from 50:50%, e.g., from 50:50% to 80:20%) and others decreasing certainty (e.g., trials where probabilities moved closer to 50:50%, e.g., from 80:20% to 50:50%). Numbers in parentheses represent posterior probabilities for both decks on each draw.
On each sequence, the likelihood of Deck A or Deck B supplying the observed draws was updated as evidence accumulated. Subjects were told that both decks had equal likelihood of being selected to supply the draws (i.e., the prior was 50:50), with posterior probabilities for both decks computed after each draw using Bayes' theorem [see methods in Stern et al., 2010]. “Objective” certainty was defined as the difference between the calculated posterior probabilities for the two decks (based on observed evidence) after each card was drawn (throughout this article, we report probabilities as percentages). A larger difference in posterior probabilities (i.e., the further away that both deck probabilities are from 50:50%, the closer the probability for one deck is to 100% and the other to 0%) is associated with greater objective certainty (and lesser objective uncertainty) regarding the identity of the desk supplying the draws. By contrast, “subjective” certainty was determined by subjects' individual ratings and was not required to be based on deck probabilities.
For certain sequences (Fig. 1a), the first draw observed updated the posterior probabilities from the 50:50% prior probability to 100:0% (and thus increased objective certainty, 18 trials), with accumulating evidence (draws 2–4) providing no additional information (draws that “maintained” maximal certainty, 54 trials). For uncertain sequences (Fig. 1b), the first draw updated posterior probabilities from the 50:50% prior probability to 80:20% (54 trials), with accumulating evidence serving to either increase or decrease certainty (108 and 54 trials, respectively) depending on the color of the current and prior cards. Subjects were informed that observed evidence would fairly represent what would occur by chance based on the probability distributions of the decks and that the number of cards contained in each deck was “infinite” (i.e., counting of cards would not aid performance).
Each sequence began by displaying the two decks for 500 ms, followed by the four draws (1,750 ms each) and then the decision (1,750 ms). All stimuli remained on‐screen until 1,750 ms had elapsed regardless of when individual certainty ratings were made. If responses were not made on time, a message asked subjects to speed up. An interstimulus‐interval blank screen was presented for 500–3,000 ms after each draw and after the final decision, allowing for the isolation of the BOLD signal for each event. Feedback was jittered between 500 and 1,500 ms after the decision, followed immediately by the start of the next sequence. Timings were selected by a design optimization program written in Matlab by RCW, which minimized mulitcollinearity between events for contrasts of interest.
Functional MRI Acquisition and Preprocessing
MRI scanning occurred on a GE 3 T Signa scanner (LX [8.3] release). A T1‐weighted image was acquired in the same prescription as functional images to facilitate coregistration. Functional images were acquired with a T2*‐weighted, reverse spiral acquisition sequence (gradient echo (GRE), time of repetition = 2,000, echo time = 30, flip angle (FA) = 90°, field of view = 20, 40 slices, 3.0/0, matrix diameter 71—equivalent to 64 × 64) sensitive to signal in ventral frontal regions [Yang et al., 2002]. Subjects underwent six runs, each consisting of 127 volumes plus four initial, discarded volumes to allow for thermal equilibration of scanner signal. After acquisition of functional volumes, a high‐resolution T1 SPGR scan was obtained for anatomic normalization.
Images were presented by a BrainLogics (PST, Pittsburgh, PA) digital MR projector, which provides high‐resolution video (1,024 × 768) by back projection.
Preprocessing was performed using the Statistical Parametric Mapping (SPM) 2 package (Wellcome Institute of Cognitive Neurology, London), with the exception of realignment and slice‐time correction, which used MCFLIRT [Jenkinson et al., 2002] and slicetimer (interpolated with an eight‐point sinc kernel multiplied by a Hanning window), respectively (FSL, Analysis Group, FMRIB, Oxford). Realignment parameters were inspected to ensure that movement did not exceed either 3 mm translation or 1° rotation within each run. Parameters for anatomic normalization to the MNI152 brain, an average of 152 T1 images from the Montreal Neurological Institute, were derived from the high resolution SPGR T1 image and applied to the time series of co‐registered, functional volumes, which were re‐sliced (voxel size: 3 × 3 × 3 mm) and smoothed with a 5 mm isotropic Gaussian smoothing kernel.
Data Analysis
Primary analysis
Behavioral
The main behavioral analysis of interest compared certainty ratings between the groups. Because of prior work in healthy subjects suggesting that individual differences in subjective certainty predicted neural activity most robustly for the first piece of evidence [Stern et al., 2010], we examined draw 1 separately from accumulating evidence on subsequent draws 2–4. Draws were also segregated based on sequence type (certain or uncertain) and, for uncertain sequences, based on whether they increased or decreased objective certainty, resulting in a total of five different trial types (first piece of evidence for certain sequences, first piece of evidence for uncertain sequences, accumulating evidence that maintained maximal objective certainty, accumulating evidence that increased objective certainty, and accumulating evidence that decreased objective certainty). Analyses of subjective ratings were based on level of certainty reported irrespective of which deck was selected (Deck A or Deck B), resulting in five potential levels of certainty that a subject could endorse, ranging from complete uncertainty (a “5” response on the scale) to complete certainty (“1” or “9” responses on the scale).
Subjective ratings for the first piece of evidence were examined with separate mixed‐model (repeated and nonrepeated measures) ANOVAs using sequence type (certain and uncertain) as the within‐subject variable and diagnosis (OCD and control) and medication (unmedicated and medicated) as between‐subject variables. Similarly, ratings for accumulating evidence were examined using change in certainty (“maintain,” “increase,” or “decrease,” see Fig. 1), diagnosis (OCD and controls), and medication (unmedicated and medicated) as factors. All main effects and interactions were examined. Significant interactions were followed‐up with one‐way ANOVAs looking at simple main effects.
Functional MRI
All analyses of fMRI BOLD signal were event‐related. In parallel with the five trial types used in the behavioral analyses, five regressors of interest were created for neuroimaging analyses, specifying: (1) onsets for the first piece of evidence (draw 1) on certain sequences, (2) onsets for the first piece of evidence on uncertain sequences, (3) onsets for subsequent accumulating evidence (draws 2–4) that maintained maximal certainty (certain sequences), (4) onsets for accumulating evidence that increased objective certainty (uncertain sequences), and (5) onsets for accumulating evidence that decreased objective certainty (uncertain sequences). Regressors specifying onset times for the final decision and feedback were included in the model to account for variance but are not the focus of the current analysis. Each regressor was convolved with the canonical hemodynamic response function using the general linear model as implemented in SPM2. Omission trials where subjects did not make a certainty rating within the deadline were infrequent (5.6 ± 4.5 trials) and not modeled.
Five first‐level contrasts examined activity for the five trial types in the task versus implicit baseline (brain responses occurring during blank screens): (1) first piece of evidence for certain sequences, (2) first piece of evidence for uncertain sequences, (3) accumulating evidence (draws 2–4) that maintained maximal certainty, (4) accumulating evidence that increased objective certainty, and (5) accumulating evidence that decreased objective certainty. Results from direct comparisons between event types (first piece of evidence for certain vs. uncertain sequences, accumulating evidence that increased vs. maintained objective certainty, accumulating evidence that decreased vs. maintained objective certainty, and accumulating evidence that increased vs. decreased objective certainty) can be found in Supporting Information.
Within SPM2, one‐sample t‐tests examined activations (e.g., draw > baseline) and deactivations (e.g., baseline > draw) for each of the contrasts within each group (OCD and controls) separately, with group‐level analyses comparing patients and controls using two‐sample t‐tests. Tests were thresholded at an alpha of 0.05, cluster‐level corrected for multiple comparisons within whole‐brain gray matter as implemented by AlphaSim in AFNI [Cox, 1996; Ward, 2000]. This method uses Monte‐Carlo simulations to determine the cluster size required based on voxelwise threshold, size of search area, and data smoothness. Based on the average smoothness for comparisons made in the contrasts examined (FWHM: 11.27 mm) and using a whole‐brain search region, a stringent cluster size of 86 voxels with a voxelwise threshold of P < 0.005 was required to reach corrected significance (corrected P = 0.049).
We also sought to examine correlations between activations in brain regions that exhibited group differences, with the prediction that OCD patients would show greater interregional correlations among hyperactive brain regions than controls. Parameter estimates were extracted from 6‐mm‐radius spheres surrounding peak coordinates of clusters that differed between the groups for a given contrast. Correlations between brain regions could only be examined for contrasts where there were two or more regions differing between patients and controls (i.e., draw 1 on certain sequences vs. baseline, see Results and Table IV). Partial correlations (controlling for effects of medication status and combined HAM‐A/HAM‐D scores, see Exploratory analyses section below) were performed on activations from pairs of brain regions separately for OCD patients and controls, resulting in a correlation matrix for each group. Note that this analysis focused on BOLD signal correlations between brain regions across subjects, which differs from connectivity analyses that examine correlations between time‐series within subjects. Correlation matrices were compared between the groups using a Box M (omnibus) test (alpha of 0.05) to determine whether the overall pattern of correlations between pairs of brain regions was significantly different between OCD patients and controls. Significant matrix differences were followed‐up with an examination of group differences in correlation coefficients for each pair of brain regions using a Fisher r‐to‐z transformation with two‐tailed t‐tests. Although our step of first requiring significant differences between correlation matrices before examining individual pairs of correlations helps control for Type I error associated with comparisons between multiple pairs of correlation coefficients [see Levin et al., 1994], we further controlled for false positives by using a pairwise significance threshold of P ≤ 0.01.
Table IV.
OCD patients > control subjects
| Region | BA | OCD > Control | ||||
|---|---|---|---|---|---|---|
| k | x | y | z | Z | ||
| First piece of evidence, certain > baseline | ||||||
| Temporal pole (R) | 38 | 416 | 36 | 24 | −30 | 4.52 |
| Amygdala (R) | 28, 34 | 24 | 3 | −21 | 4.35 | |
| VMPFC (B) | 11 | 9 | 33 | −21 | 3.69 | |
| OFC/aI/fO (R) | 11, 47 | 33 | 15 | −12 | 3.35 | |
| PG/uncus (R) | 28, 34 | 27 | −9 | −24 | 2.76 | |
| OFC/aI/fO (L) | 47 | 194 | −18 | 12 | −18 | 4.28 |
| ITG/MTG (L) | 20, 21 | 133 | −60 | −9 | −27 | 3.87 |
| ITG/MTG (R) | 20, 21 | 310 | 57 | −27 | −21 | 3.85 |
| TPJ/IPL (L)± | 39, 40 | 82 | −54 | −42 | 18 | 3.82 |
| Accumulating evidence, maintaining maximal certainty > baseline | ||||||
| SPL/IPL (R) | 40 | 175 | 36 | −72 | 48 | 3.81 |
Regions listed are corrected for multiple comparisons across the whole brain at P < 0.05. Coordinates are in MNI space. ± Significant at trend level (P < 0.1). There were no regions where controls > OCD at the current threshold.
BA, Brodmann's area(s); k, number of voxels; Z, maximum z‐score; R, right; B, bilateral; L, left; VMPFC, ventromedial prefrontal cortex; OFC, orbitofrontal cortex; aI/fO, anterior insula/frontal operculum; PG, parahippocampal gyrus; ITG, inferior temporal gyrus; MTG, middle temporal gyrus; TPJ, temporoparietal junction; IPL, inferior parietal lobule; SPL, superior parietal lobule.
Exploratory analyses
Additional exploratory analyses were performed to supplement information obtained from primary analyses. Behaviorally, reaction times (RTs) to make certainty ratings on each draw were examined using the same mixed‐model ANOVAs that were used for the investigation of ratings. For analysis of brain activation, we sought to determine whether medication status or generalized anxiety and depression could be influencing group effects, as our sample contained more mOCD patients than medicated controls subjects (mPCs), and because OCD patients showed significantly greater levels of generalized anxiety and depression (as indexed by the HAM‐A and HAM‐D, see Table I). Parameter estimates derived from events/trial types that were associated with significantly different activations between OCD patients and control subjects (see results section) were averaged across all voxels in the region showing group difference (except where noted in results) and submitted to multiple regression analyses. Predictor variables in these regressions were diagnosis (OCD and control), medication (unmedicated and medicated), and a combined HAM‐A/HAM‐D score (average of the two measures for each subject, due to the high collinearity between HAM‐A and HAM‐D scores), which allowed us to determine not only whether diagnosis would remain a significant predictor of brain activity when controlling for these other factors, but also whether medication status or generalized anxiety/depression would account for neural activity in these regions over and above any effects of diagnosis.
Finally, we also performed multiple regressions to determine whether OC symptom severity as measured by the Y‐BOCS was related to brain activations in regions showing group differences, while controlling for effects of medication status and generalized anxiety/depression. For each regression model, the dependent variable was the extracted parameter estimate taken from a region of group difference (or sphere around peak coordinate, see results) and predictors were Y‐BOCS scores, medication status, and combined HAM‐A/D score. Separate models were run for current and lifetime Y‐BOCS scores.
RESULTS
Behavioral
Results from ANOVAs examining subjective certainty ratings and RT can be found in Table III. As can be seen in Figure 2, OCD patients rated themselves as significantly less certain than control subjects on certain sequences, both when observing the first piece of evidence (left) and when accumulating evidence that maintained maximal certainty (right).
Table III.
Behavioral data
| Dependent measure | Within‐subjects factor | Significance of within‐subjects factor | Between‐subjects factors | Significance of between‐subjects factors | Significance of interactions |
|---|---|---|---|---|---|
| Subjective certainty ratings | |||||
| Draw 1 | Sequence type (certain vs. uncertain) | F(1,50) = 138.7, P < 0.001 Certain > uncertain (Fig. 2a) | Diagnosis (OCD vs. control) and medication (unmedicated vs. medicated) | ns | Diagnosis × sequence type: F(1,50) = 4.9, P = 0.03 Controls > OCD for certain sequences, no differences for uncertain sequences |
| Accumulating evidence | Change in certainty (maintain maximal vs. increase vs. decrease) | F(2, 100) = 476.5, P < 0.001 | Diagnosis (OCD vs. control) and medication (unmedicated vs. medicated) | ns | nsa |
| Maintain maximal > increase > decrease (Fig. 2b) | |||||
| Reaction times | |||||
| Draw 1 | Sequence type (certain vs. uncertain) | F(1,50) = 123.2, P < 0.001 Uncertain > certain | Diagnosis (OCD vs. control) and medication (unmedicated vs. medicated) | ns | Diagnosis × sequence type: F(1,50) = 14.2, P < 0.001 Controls > OCD for uncertain sequences, no differences for certain sequences |
| Accumulating evidence | Change in certainty (maintain maximal vs. increase vs. decrease) | F(2, 100) = 423.9, P < 0.001 | Diagnosis (OCD vs. control) and medication (unmedicated vs. medicated) | ns | ns |
| Decrease > increase > maintain maximal | |||||
Four mixed‐model ANOVAs (each with one within‐subjects factor and two between‐subjects factors) examined subjective certainty ratings and reaction times (RTs) for the first piece of evidence (draw 1) and accumulating evidence (draws 2–4) separately. Note that greater values for subjective certainty ratings reflect increased certainty, whereas greater values for RT reflect slower response times.
Despite this lack of interaction, controls rated themselves as significantly more certain than OCD patients for accumulating evidence that maintained maximal certainty on certain sequences (one‐way ANOVA: F(1, 50) = 8.7, P = 0.005), with no group differences in ratings for accumulating evidence that increased or decreased certainty on uncertain sequences.
Figure 2.
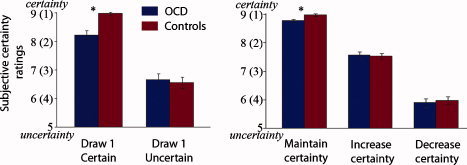
Subjective certainty ratings. Ratings for the first piece of evidence (draw 1) and accumulating evidence (draws 2–4) on certain and uncertain sequences. OCD patients (n = 25) are shown in blue and controls (n = 29) are shown in red. Bars represent standard error of the mean. Asterisks denote conditions showing group differences. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Functional MRI
First piece of evidence (draw 1) for certain and uncertain sequences
Activations within and between groups for the first draw of certain and uncertain sequences are illustrated in Figure 3 (also see Supporting Information Fig. 1). When evaluating the first piece of evidence on certain sequences When evaluating the first piece of evidence on certain sequences as compared with implicit baseline, OCD patients showed significantly greater activation than controls in several regions of DMN, including VMPFC, right parahippocampal gyrus and amygdala, right temporal pole, bilateral inferior/middle temporal gyri, and, at trend level (P = 0.06), left inferior parietal cortex/angular gyrus (Table IV, Fig. 3). In addition, OCD patients exhibited hyperactivity in bilateral OFC and adjacent regions of ventral aI/fO. There were no regions where controls showed greater activation than OCD patients at the current threshold. There were no significant differences between patients and controls in response to the first draw for uncertain sequences compared with implicit baseline (Fig. 3, right panel).
Figure 3.
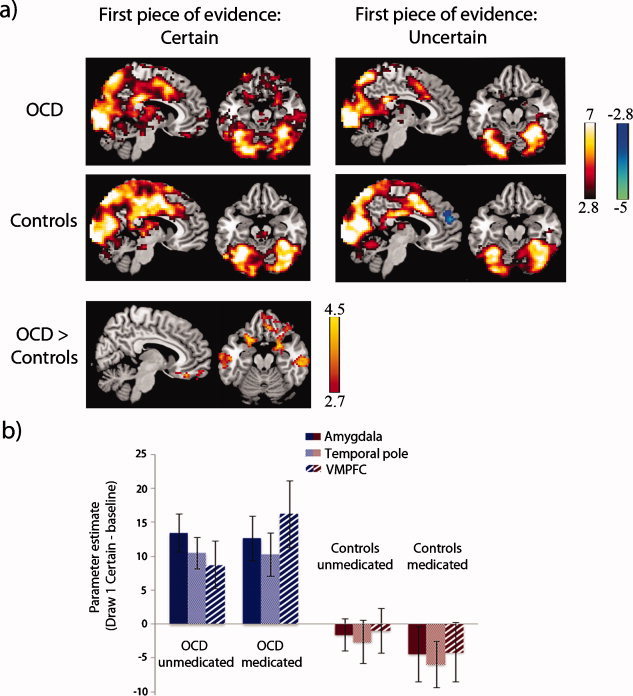
First piece of evidence on certain and uncertain sequences. (a) Activations (warm colors) and deactivations (cool colors) for OCD patients and controls in response to observing the first piece of evidence (draw 1) on certain sequences (left panel) and uncertain sequences (right panel). For certain sequences only, OCD patients exhibited greater activity than controls in ventromedial prefrontal cortex (VMPFC), anterior parahippocampus, amygdala, lateral orbitofrontal cortex (OFC)/ventral anterior insula/frontal operculum (aI/fO), and lateral temporal regions (middle temporal cortex and temporal pole; bottom left). There were no differences between the groups when observing the first piece of evidence for uncertain sequences, and no regions where controls showed greater activation than OCD patients. Images are displayed at x = 6 and z = −20 in the sagittal and axial planes (MNI coordinates), with a voxelwise threshold of P < 0.005, cluster‐level corrected for multiple comparisons at P < 0.05. Color bars represent t scores. (b) Parameter estimates for draw 1 on certain sequences are shown separately for medicated and unmedicated subjects in the OCD and control groups to illustrate the absence of medication effects. Estimates were averaged across voxels located in 6‐mm‐radius spheres placed around peak coordinates in regions of the amygdala, temporal pole, and VMPFC showing group differences (see Table IV).
Accumulating evidence (draws 2–4) on certain and uncertain sequences
Within‐ and between‐group effects for accumulating evidence that maintained maximal objective certainty (i.e., deck probabilities of 100:0% on certain sequences) are illustrated in Figure 4 (also see Supporting Information Fig. 2). OCD patients showed significantly greater activation than controls in a right hemisphere region encompassing superior and inferior parietal cortex (Table IV) compared with implicit baseline, with no regions exhibiting greater activity in controls. Considering that, behaviorally, OCD patients exhibited greater subjective uncertainty both in response to the first piece of evidence and when accumulating subsequent pieces of evidence on certain sequences, we wished to determine whether any of the brain regions in DMN showing hyperactivity in patients to the first piece of evidence (VMPFC, parahippocampus, amygdala, OFC/aI/fO, inferior parietal/angular gyrus, temporal pole, and lateral temporal cortex, see Table IV) would also exhibit group differences on these subsequent draws. Because of our interest in DMN, we interrogated activity in these regions using a reduced threshold (P < 0.005, k = 20). Results from this analysis revealed greater activity in VMPFC/subgenual cingulate (x = 9, y = 30, z = −18; k = 47) and right temporal pole (x = 33, y = 27, z = −33; k = 37) in OCD patients when compared with controls for accumulating evidence on certain sequences (Fig. 4, bottom panel).
Figure 4.
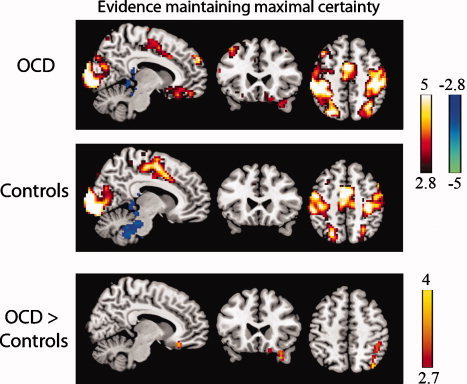
Accumulating evidence that maintained maximal certainty on certain sequences. Activations (warm colors) and deactivations (cool colors) for draws 2–4 on certain sequences in OCD patients and control subjects. Group differences emerged in right parietal cortex, as well as VMPFC/subgenual cingulate and right temporal pole (bottom panel) at an uncorrected threshold (P < 0.005, k > 20). Images are displayed at x = 8, y = 24, and z = 50 in the sagittal, coronal, and axial planes (MNI coordinates) with a voxelwise threshold of P < 0.005, k > 20. Color bars represent t scores.
There were no significant differences between OCD patients and controls for accumulating evidence that increased certainty compared with baseline (see Supporting Information Fig. 3 for effects within each group) or decreased certainty compared with baseline (see Supporting Information Fig. 4 for effects within each group).
Relationships between brain regions
Partial correlations (controlling for effects of medication status and HAM‐A/HAM‐D scores) were performed to examine relationships between activations in different brain regions across subjects. We examined the following eight foci: left OFC/ventral aI/fO, left MTG, right MTG, VMPFC, right temporal pole, right amygdala, right parahippocampus, and right OFC/ventral aI/fO (these last five foci were significant subregions in the 416‐voxel cluster, see Table IV), which represent those regions that were hyperactive in OCD patients in response to the first piece of evidence on certain sequences. The correlation matrix for OCD patients revealed a pattern of highly correlated subject‐level peak activity between many of the activation clusters (Fig. 5, top). Controls also showed correlations between clusters, but these were mostly confined to the right hemisphere, with notably fewer cross‐hemispheric correlations (Fig. 5, bottom). A Box M omnibus test of differences in matrices between the groups was highly significant (χ2 (36) = 105.33, P < 0.001). Follow‐up comparisons of correlation coefficients between the groups revealed significantly more correlated activity among OCD patients when compared with controls (P ≤ 0.01, two‐tailed) for the following pairs: left OFC/aI/fO–right temporal pole, left OFC/aI/fO–right parahippocampus, left OFC/aI/fO–VMPFC, right amygdala–left MTG, and right temporal pole–right parahippocampus (Fig. 5, lines in red).
Figure 5.
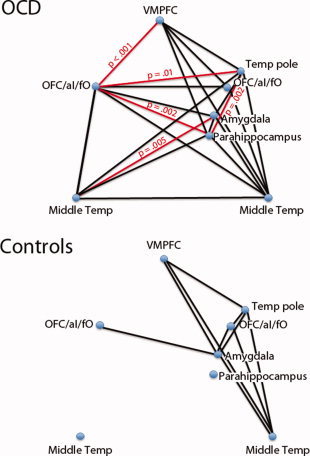
Relationships between brain regions. A Box M test revealed significant group differences among patterns of intersubject correlations between brain regions for the first piece of evidence on certain sequences. Positive correlations between individual pairs of brain regions are shown for each group (P < 0.01, black lines). Red lines represent correlations that are greater in OCD patients than controls (P < 0.01). VMPFC, ventromedial prefrontal cortex; OFC/aI/fO, orbitofrontal cortex/anterior insula/frontal operculum; Temp, temporal. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Exploratory analyses
Effect of medication and HAM‐A/HAM‐D variables
Multiple regression analyses investigated whether brain activation in regions showing group differences (dependent variable) was related to effects of medication status and HAM‐A/HAM‐D scores (independent variables), which also differed between groups. To separately examine activity in subregions comprising the 416 voxel cluster that was hyperactive in OCD patients on the first draw of certain sequences (peak coordinate located in right temporal pole), we submitted extracted parameter estimates from 6‐mm‐radius spheres located around five subpeaks of this cluster (Table IV) to the multiple regression analysis (instead of using one average parameter estimate for the entire cluster). For the majority of regions, diagnosis remained a significant predictor when controlling for effects of medication status and generalized anxiety/depression. Further, these factors did not uniquely predict neural activity when controlling for effects of diagnosis. Two exceptions were found in the right parahippocampus subregion for draw 1 on certain sequences and VMPFC/subgenual cingulate for accumulating evidence that maintained maximal certainty. For these two regions, none of the individual predictors reached significance even though the full models explained variance in brain activations (trend for right parahippocampus subregion, P = 0.08, and significant for VMPFC/subgenual, P = 0.003). This effect is likely due to collinearity between diagnosis and HAM‐A/HAM‐D scores (r = 0.75), which may have weakened the ability to detect unique variance when all predictors were entered simultaneously. Follow‐up simple regressions indicated that diagnosis by itself significantly predicted activity in the right parahippocampus subregion (t = 2.7, P = 0.009) and VMPFC/subgenual cingulate (t = 3.5, P = 0.001), as would be expected given that these regions were derived from whole‐brain results of group differences. Of interest, HAM‐A/D scores also predicted activity in these regions (right parahippocampus: t = 2.1, P = 0.04; VMPFC/subgenual cingulate: t = 3.8, P < 0.001), whereas medication alone did not predict activity in these regions (P > 0.1 for both).
Relationship with OC symptom severity
Lifetime Y‐BOCS scores were not related to brain activation in any of the regions showing group differences. Current Y‐BOCS scores showed a negative relationship with activity in the left OFC/aI/fO cluster (k = 194; t = −2.1, P = 0.04) and in the right parahippocampal subregion (taken from the 416‐voxel cluster, see Table IV; t = −2.2, P = 0.04). However, visual inspection of scatterplots depicting these effects suggested that they may have been overly influenced by one subject. Analysis using Cook's distance statistic [Cook and Weisberg, 1999] indicated that this subject was above the critical threshold for influencing the identified relationships (4/N = 0.16) for both brain regions (left OFC‐Y‐BOCS: 0.232 and right parahippocampal‐Y‐BOCS: 0.191). Indeed, when this subject was removed from the analyses, these relationships were no longer significant.
DISCUSSION
The current study examined how OCD patients experience uncertainty as evidence is accumulated for a decision. Behaviorally, patients exhibited less certainty than controls when data were unequivocal. Although controls showed essentially no uncertainty when the observed evidence pointed to a single correct answer (on certain sequences), OCD patients remained subjectively uncertain even in the face of clear evidence. When accumulating subsequent pieces of evidence on certain sequences, patients remained significantly less certain than controls, although this effect was smaller than for the first piece of information. Although prior behavioral research in OCD has found that patients report greater subjective uncertainty than healthy individuals when making a decision in the presence of uncertainty [Volans, 1976], to our knowledge this is the first experimental report of greater subjective uncertainty in OCD despite unequivocal evidence. However, this finding is consistent with the clinical phenomenology of the disorder, where patients appear to remain uncertain regarding whether a behavior has achieved a desired goal (e.g., locking the door or turning off the stove) despite clear evidence that the goal has been attained (i.e., the patient can visually perceive that the door is locked or that the knob on the stove is in the “off” position).
Linking behavior to brain activity, when observing the first piece of evidence on certain sequences, OCD patients exhibited hyperactivation in several brain regions including lateral OFC/ventral aI/fO (BAs 11, 47), amygdala and anterior parahippocampus (BAs 28, 34), bilateral middle temporal cortex and temporal pole (BAs 20, 21 and 38), and VMPFC (BA 11). Furthermore, patients showed greater activity in VMPFC/subgenual cingulate and temporal pole when accumulating subsequent evidence on certain sequences, although group differences in neural activity only appeared at a reduced threshold. We did not find group differences in brain areas involved in detecting external salience and monitoring task set, such as pMFC and dorsal aI/fO [Dosenbach et al., 2006; Menon and Uddin, 2010; Sridharan et al., 2008], suggesting that these executive functions may be relatively unimpaired in OCD when observing evidence for a decision.
Many of the areas where OCD patients exhibited hyperactivation when observing evidence on certain sequences—VMPFC, parahippocampus, middle temporal cortex, and temporal poles—are nodes of the “DMN” of brain regions that activate during self‐focused internal thought processes such as imagining and predicting the future, autobiographical memory, scenario construction, and theory of mind [Andrews‐Hanna et al., 2010; Buckner et al., 2008; Schacter et al., 2008]. As might be expected, DMN decreases in activation, in some cases deactivating from baseline, when attention becomes increasingly focused on external stimuli, such as when a task becomes more difficult or requires greater cognitive effort [McKiernan et al., 2003; Pallesen et al., 2009]. Hyperactivation of default mode network in OCD may reflect an overengagement of internally‐focused mental processes to the detriment of external task demands; in particular, frequent or persistent imagining of negative scenarios and their relationship to the self may contribute to the characteristic pattern of persistent worry about a potential bad event that is often found in OCD. Interestingly, DMN activation in patients was not significantly different from controls for trials on uncertain sequences (both groups exhibited less DMN activity for uncertain when compared with certain trials), suggesting that the greater cognitive effort associated with making a decision in the presence of actual uncertainty dictated by probability in the task—supported by the overall slower RTs for uncertain when compared with certain sequences—is sufficient to promote the disengagement of internally‐focused mental processes, even in OCD patients. It may be only when there is relatively less cognitive demand (i.e., on certain sequences) that default mode processing emerges and interferes with patients' functioning. The current results complement prior findings of aberrant VMPFC, insula, and parahippocampal activity in OCD during symptom provocation and in tasks focusing on other aspects of the OCD phenotype, such as response inhibition and performance monitoring [for reviews, see Menzies et al., 2008; Rotge et al., 2008]. Here, we not only link hyperactivation in these regions to specific conditions where patients experience heightened uncertainty during decision‐making, but also identify altered activation in additional regions that are not as frequently associated with OCD, such as the amygdala and lateral temporal cortex.
Although the majority of differences between patients and controls remained significant when controlling for medication status and generalized anxiety/depression, no factors uniquely contributed to activity in VMPFC/subgenual cingulate in response to observing evidence on certain sequences. Neither diagnosis nor HAM‐A/D (generalized anxiety/depression) scores uniquely contributed to activity in VMPFC/subgenual cingulate in response to observing evidence on certain sequences. This occurred despite the fact that both diagnosis and HAM‐A/D scores significantly predicted activity in this region when examined alone, which suggests that VMPFC/subgenual activity on these trials can be accounted for by variance that is shared between these two variables. This is particularly interesting given prior studies suggesting a prominent role for subgenual cingulate in the pathogenesis of major depression [Mayberg et al., 1999; Price and Drevets, 2010], and suggests that OCD and generalized negative affect are both related to subgenual hyeractivation.
The current results are consistent with prior studies identifying a relationship between VMPFC activity and uncertainty during decision‐making. In a previous study using the same paradigm in healthy individuals only, we found a similarly positive correlation between VMPFC activity in response to the first piece of evidence and subjective ratings of uncertainty [Stern et al., 2010]. However, in this prior study, the correlation between brain and behavior emerged when participants observed evidence on uncertain, rather than certain, sequences [Stern et al., unpublished data]. Furthermore, hyperactivity in VMPFC (as well as amygdala and OFC) has been found when adolescents with generalized anxiety disorder or social phobia with high intolerance of uncertainty make decisions under complete uncertainty [Krain et al., 2008]. This suggests that DMN/limbic activity may be generally associated with subjective experiences of certainty, and that the type of experimental context that elicits the relationship between DMN and uncertainty may vary based on disorder.
The current study also found greater activity in OCD patients when observing the first piece of evidence on certain sequences in regions linked to emotional‐motivational functions, such as amygdala and OFC/ventral aI/fO. The importance of the amygdala in emotion processing is well documented [for a review, see Phelps and LeDoux, 2005], particularly for aversive emotions such as fear and sadness [Blair et al., 1999; Phan et al., 2002; but see Murray, 2007 for the amygdala's role in positive emotions]. Relatively less work has been dedicated to understanding the cognitive processes subserved by ventral regions of anterior insula, which are located inferior and medial to areas associated with the detection of external salience [Menon and Uddin, 2010; Sridharan et al., 2008] and decision uncertainty related to probability [Grinband et al., 2006; Preuschoff et al., 2008]. Although it remains unclear whether ventral and dorsal aI/fO are involved in similar functions, a meta‐analysis suggested that ventral aI/fO activity was related more to experienced emotion, whereas dorsal aI/fO activity was involved in executive functions [Wager and Barret, 2004].
Particularly relevant for the present findings, the amygdala and OFC/ventral aI/fO interact with key nodes of DMN. The amygdala is both structurally [Amaral and Price, 1984] and functionally [Roy et al., 2009; Stein et al., 2007] connected to several DMN structures, including medial and lateral temporal regions and VMPFC. Although the spatial resolution of fMRI may not be able to distinguish neighboring but architectonically distinct regions, the ventral aI/fO activations found in the current study are located in the vicinity of a region of caudolateral OFC described by Price and coworkers as “intermediate agranular insula.” In monkeys and humans, this area is separate from dorsal insula and other areas of lateral OFC, exhibiting dense interconnections with VMPFC, PCC, temporal pole, and medial temporal lobe regions [Carmichael and Price, 1996; Ongur et al., 2003; Price, 2007; Price and Drevets, 2010]. It is thus possible that hyperactivations of amygdala and OFC/ventral aI/fO in OCD reflect heightened emotional responses, and that coactivation with DMN may be linked to internal mentation with a particularly negative focus, although such a suggestion is speculative and remains to be tested.
Not only did several limbic/paralimbic brain regions exhibit hyperactivation in the OCD group, the amount of coactivation between regions was more correlated across patients. When looking at the relationship between brain activity on the first draw of certain sequences, significant correlations were found in both OCD and control groups, suggesting the presence of a coherent network involving OFC/aI/fO, amygdala, and DMN across diagnostic categories. Patients, however, showed significantly more interregional between‐subject correlations across hemispheres and between nodes associated with emotion (amygdala and OFC/ventral aI/fO) and DMN (temporal pole, VMPFC, middle temporal, and parahippocampus) on these trials, suggesting that greater linkage between these regions may contribute to the overall pattern of hyperactivation seen in OCD.
There are several limitations of the current study. First, certain sequences were less frequent than uncertain sequences, which may have influenced findings. Even though we provide evidence against this critique (see discussion in Supporting Information), the current results should be interpreted with caution until they can be replicated in a task using equal numbers of both sequence types. In addition, although our inclusion criteria for current comorbidities were strict, we did allow for the concurrent presence of GAD (n = 1), tic disorder NOS (n = 1), and specific phobia (n = 1) in the OCD group, which may have impacted results. Similarly, although the current manuscript focuses on the primary comparison of OCD and non‐OCD participants, data suggest that patients with MDD in remission exhibit differences with healthy individuals in both brain activity and behavior [Neumeister et al., 2006; Norbury et al., 2010; Okada et al., 2009; Thomas et al., 2011]. As such, future work focusing on OCD may seek to further delineate neural markers of disorders that are frequently comorbid with OCD, such as MDD. Despite these limitations, the current findings of behavioral and brain abnormalities during evidence accumulation highlight the importance of internally‐focused mental processes and their relation to decision‐making uncertainty in OCD.
Supporting information
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table 1. List of medications and dosages (mg/day) for medicated OCD (mOCD) and medicated patient control (mPC) groups. SSRIs = selective‐serotonin reuptake inhibitors; SNRIs = serotonin‐norepinephrine reuptake inhibitors; TCAs = tricycle antidepressants. All subjects were taking a serotonin reuptake inhibitor (SSRI or SNRI). Five mOCD and 4 mPC subjects were taking more than one medication (not including benzodiazepines, which were taken as needed and omitted on the day of testing). ± Dosing information not available for one subject.
Supporting Information Table 2. Within‐group effects for the first piece of evidence on Certain vs. Uncertain sequences. There were no significantly active regions for the contrast of Uncertain > Certain trials for OCD patients. aI/fO = anterior insula/frontal operculum; DMPFC = dorsomedial prefrontal cortex; MFG = middle frontal gyrus; SFG = superior frontal gyrus; IFG = inferior frontal gyrus; aPFC = anterior prefrontal cortex; IPL = inferior parietal lobule; dACC = dorsal anterior cingulate cortex; SMA = supplementary motor area; PCC = posterior cingulate cortex; STG = superior temporal gyrus, PG = parahippocampal gyrus; OFC = orbitofrontal cortex; VMPFC = ventromedial prefrontal cortex; MTG = middle temporal cortex, aMFC = anterior medial frontal cortex. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 3. Within‐group effects for accumulating evidence that increases vs. decreases certainty. There were no regions uniquely activated by controls for the increase > decrease contrast or for OCD patients for the decrease > increase contrast. IPL = inferior parietal lobule; STG = superior temporal gyrus; SMA = supplementary motor area; aMFC = anterior medial frontal cortex; VMPFC = ventromedial prefrontal cortex; MTG = middle temporal gyrus; MFG = middle frontal gyrus; IFG = inferior frontal gyrus; dACC = dorsal anterior cingulate cortex; SPL = superior parietal lobule; aI/fO = anterior insula/frontal operculum; ITG = inferior temporal gyrus. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 4. Within‐group effects for accumulating evidence that increases vs. maintains certainty. There were no regions uniquely activated by OCD patients for the increase > maintain contrast. IPL = inferior parietal lobule; SFG = superior frontal gyrus; MFG = middle frontal gyrus; sSMA = supplementary motor area; aI/fO = anterior insula/frontal operculum; IFG = inferior frontal gyrus; PCC = posterior cingulate cortex; MTG = middle temporal cortex; DMPFC = dorsomedial prefrontal cortex; aMFC = anterior medial frontal cortex; VMPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex; PG = parahippocampal gyrus; STG = superior temporal gyrus; ITG = inferior temporal gyrus; OFC = orbitofrontal cortex. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 5. Within‐group effects for accumulating evidence that decreases vs. maintains certainty. There were no regions uniquely activated by OCD patients for the decrease > maintain contrast. aPFC = anterior prefrontal cortex; MFG = middle frontal gyrus; aI/fO = anterior insula/frontal operculum; SMA = supplementary motor area; dorsal anterior cingulate cortex = dACC; IPL = inferior parietal lobule; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; MTG = middle temporal cortex; PCC = posterior cingulate cortex; PG = parahippocampal gyrus; DMPFC = dorsomedial prefrontal cortex; aMFC = anterior medial frontal cortex; VMPFC = ventromedial prefrontal cortex; STG = superior temporal gyrus; OFC = orbitofrontal cortex. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 6. Regions where OCD patients > control subjects for the first piece of evidence on Certain as compared to Uncertain sequences, corrected for multiple comparisons across the whole‐brain at p < .05. BA = Brodmann's area(s); k = number of voxels; Z = maximum z‐score; R = right; L = left; PG = Parahippocampal gyrus;, MTG = middle temporal gyrus; STG = superior temporal gyrus; IPL = inferior parietal lobule; xcoordinates are in MNI space. ± Significant at trend level (p < .1). There were no regions where controls > OCD at the current threshold.
Supporting Information Figure 1. Within‐group activations for a) first piece of evidence > implicit baseline on Certain sequences, b) first piece of evidence > implicit baseline on Uncertain sequences, and c) first piece of evidence on Certain > Uncertain sequences. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 2. Within‐group a) activations and b) deactivations for accumulating evidence (draws 2, 3, and 4) that maintains maximal certainty on Certain sequences compared to implicit baseline. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 3. Within‐group a) activations and b) deactivations for accumulating evidence (draws 2, 3, and 4) that increases certainty on Uncertain sequences compared to implicit baseline. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 4. Within‐group a) activations and b) deactivations for accumulating evidence (draws 2, 3, and 4) that decreases certainty on Uncertain sequences compared to implicit baseline. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 5. Within‐group activations for accumulating evidence (draws 2, 3, and 4) that increases > decreases certainty and b) decreases > increases certainty on Uncertain sequences. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information
REFERENCES
- Amaral DG, Price JL ( 1984): Amygdalo‐cortical projections in the monkey (Macaca fascicularis). J Comp Neurol 230: 465–496. [DOI] [PubMed] [Google Scholar]
- Andrews‐Hanna JR, Reidler JS, Sepulcre J, Poulin R, Buckner RL ( 2010): Functional‐anatomic fractionation of the brain's default network. Neuron 65: 550–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ ( 1999): Dissociable neural responses to facial expressions of sadness and anger. Brain 122 ( Part 5): 883–893. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Andrews‐Hanna JR, Schacter DL ( 2008): The brain's default network: Anatomy, function, and relevance to disease. Ann N Y Acad Sci 1124: 1–38. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL ( 1996): Connectional networks within the orbital and medial prefrontal cortex of macaque monkeys. J Comp Neurol 371: 179–207. [DOI] [PubMed] [Google Scholar]
- Cook RD, Weisberg S ( 1999): Applied Regression Including Computing and Graphics. Hoboken, NJ: Wiley. [Google Scholar]
- Cox RW ( 1996): AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29: 162–173. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Burgund ED, Grimes AL, Schlaggar BL, Petersen SE ( 2006): A core system for the implementation of task sets. Neuron 50: 799–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fear CF, Healy D ( 1997): Probabilistic reasoning in obsessive‐compulsive and delusional disorders. Psychol Med 27: 199–208. [DOI] [PubMed] [Google Scholar]
- First M, Spitzer R, Gibbon M, Williams J ( 1996): Structured Clinical Interview for DSM‐IV Axis Disorders (SCID), Clinician Version: User's Guide. Washington, DC: American Psychiatric Press. [Google Scholar]
- Foa EB, Huppert JD, Leiberg S, Langner R, Kichic R, Hajcak G, Salkovskis PM ( 2002): The Obsessive‐Compulsive Inventory: Development and validation of a short version. Psychol Assess 14: 485–496. [PubMed] [Google Scholar]
- Fitzgerald KD, Welsh RC, Gehring WJ, Abelson JL, Himle JA, Liberzon I, Taylor SF ( 2005): Error‐related hyperactivity of the anterior cingulate cortex in obsessive‐compulsive disorder. Biol Psychiatry 57: 287–294. [DOI] [PubMed] [Google Scholar]
- Fitzgerald KD, Stern ER, Angstadt M, Nicholson‐Muth KC, Maynor MR, Welsh RC, Hanna GL, Taylor SF ( 2010): Altered function and connectivity of the medial frontal cortex in pediatric obsessive‐compulsive disorder. Biol Psychiatry 68: 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman WK, Price LH, Rasmussen SA, Mazure C, Fleischmann RL, Hill CL, Heninger GR, Charney DS ( 1989): The Yale‐Brown Obsessive Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 46: 1006–1011. [DOI] [PubMed] [Google Scholar]
- Grinband J, Hirsch J, Ferrera VP ( 2006): A neural representation of categorization uncertainty in the human brain. Neuron 49: 757–763. [DOI] [PubMed] [Google Scholar]
- Hamilton M ( 1959): The assessment of anxiety states by rating. Br J Med Psychol 32: 50–55. [DOI] [PubMed] [Google Scholar]
- Hamilton M ( 1960): A rating scale for depression. J Neurol Neurosurg Psychiatry 23: 56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harkin B, Kessler K ( 2009): How checking breeds doubt: Reduced performance in a simple working memory task. Behav Res Ther 47: 504–512. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S ( 2002): Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage 17: 825–841. [DOI] [PubMed] [Google Scholar]
- Krain AL, Hefton S, Pine DS, Ernst M, Castellanos FX, Klein RG, Milham MP ( 2006): An fMRI examination of developmental differences in the neural correlates of uncertainty and decision‐making. J Child Psychol Psychiatry 47: 1023–1030. [DOI] [PubMed] [Google Scholar]
- Krain AL, Gotimer K, Hefton S, Ernst M, Castellanos FX, Pine DS, Milham MP ( 2008): A functional magnetic resonance imaging investigation of uncertainty in adolescents with anxiety disorders. Biol Psychiatry 63: 563–568. [DOI] [PubMed] [Google Scholar]
- Levin JR, Serlin RC, Seaman MA ( 1994): A controlled, powerful multiple‐comparison strategy for several situations. Psychol Bull 115: 153–159. [Google Scholar]
- Maltby N, Tolin DF, Worhunsky P, O'Keefe TM, Kiehl KA ( 2005): Dysfunctional action monitoring hyperactivates frontal‐striatal circuits in obsessive‐compulsive disorder: An event‐related fMRI study. Neuroimage 24: 495–503. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurin RK, Jerabek PA, Silva JA, Tekell JL, Martin CC, Lancaster JL, Fox PT. ( 1999): Reciprocal limbic‐cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156: 675–682. [DOI] [PubMed] [Google Scholar]
- McKiernan KA, Kaufman JN, Kucera‐Thompson J, Binder JR ( 2003): A parametric manipulation of factors affecting task‐induced deactivation in functional neuroimaging. J Cogn Neurosci 15: 394–408. [DOI] [PubMed] [Google Scholar]
- Menon V, Uddin LQ ( 2010): Saliency, switching, attention and control: A network model of insula function. Brain Struct Funct 214: 655–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzies L, Chamberlain SR, Laird AR, Thelen SM, Sahakian BJ, Bullmore ET ( 2008): Integrating evidence from neuroimaging and neuropsychological studies of obsessive‐compulsive disorder: The orbitofronto‐striatal model revisited. Neurosci Biobehav Rev 32: 525–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner AD, Beech HR, Walker VJ ( 1971): Decision processes and obsessional behavior. Br J Soc Clin Psychol 10: 88–89. [DOI] [PubMed] [Google Scholar]
- Murray EA ( 2007): The amygdala, reward and emotion. Trends Cogn Sci 11: 489–497. [DOI] [PubMed] [Google Scholar]
- Neumeister A, Drevets WC, Belfer I, Luckenbaugh DA, Henry S, Bonne O, Herscovitch P, Goldman D, Charney DS ( 2006): Effects of a alpha 2C‐adrenoreceptor gene polymorphism on neural responses to facial expressions in depression. Neuropsychopharmacology 31: 1750–1756. [DOI] [PubMed] [Google Scholar]
- Norbury R, Selvaraj S, Taylor MJ, Harmer C, Cowen PJ ( 2010): Increased neural response to fear in patients recovered from depression: A 3T functional magnetic resonance imaging study. Psychol Med 40: 425–432. [DOI] [PubMed] [Google Scholar]
- Okada G, Okamoto Y, Yamashita H, Ueda K, Takami H, Yamawaki S ( 2009): Attenuated prefrontal activation during a verbal fluency task in remitted major depression. Psychiatry Clin Neurosci 63: 423–425. [DOI] [PubMed] [Google Scholar]
- Ongur D, Ferry AT, Price JL ( 2003): Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol 460: 425–449. [DOI] [PubMed] [Google Scholar]
- Overbeek T, Schruers K, Vermetten E, Griez E ( 2002): Comorbidity of obsessive‐compulsive disorder and depression: Prevalence, symptom severity, and treatment effect. J Clin Psychiatry 63: 1106–1112. [DOI] [PubMed] [Google Scholar]
- Pallesen KJ, Brattico E, Bailey CJ, Korvenoja A, Gjedde A ( 2009): Cognitive and emotional modulation of brain default operation. J Cogn Neurosci 21: 1065–1080. [DOI] [PubMed] [Google Scholar]
- Phan KL, Wager T, Taylor SF, Liberzon I ( 2002): Functional neuroanatomy of emotion: A meta‐analysis of emotion activation studies in PET and fMRI. Neuroimage 16: 331–348. [DOI] [PubMed] [Google Scholar]
- Phelps EA, LeDoux JE ( 2005): Contributions of the amygdala to emotion processing: From animal models to human behavior. Neuron 48: 175–187. [DOI] [PubMed] [Google Scholar]
- Preuschoff K, Quartz SR, Bossaerts P ( 2008): Human insula activation reflects risk prediction errors as well as risk. J Neurosci 28: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL ( 2007): Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Ann N Y Acad Sci 1121: 54–71. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC ( 2010): Neurocircuitry of mood disorders. Neuropsychopharmacology 35: 192–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotge JY, Guehl D, Dilharreguy B, Cuny E, Tignol J, Bioulac B, Allard M, Burbaud P, Aouizerate B ( 2008): Provocation of obsessive‐compulsive symptoms: A quantitative voxel‐based meta‐analysis of functional neuroimaging studies. J Psychiatry Neurosci 33: 405–412. [PMC free article] [PubMed] [Google Scholar]
- Roy AK, Shehzad Z, Margulies DS, Kelly AM, Uddin LQ, Gotimer K, Biswal BB, Castellanos FX, Milham MP ( 2009): Functional connectivity of the human amygdala using resting state fMRI. Neuroimage 45: 614–626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacter DL, Addis DR, Buckner RL ( 2008): Episodic simulation of future events: Concepts, data, and applications. Ann N Y Acad Sci 1124: 39–60. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V ( 2008): A critical role for the right fronto‐insular cortex in switching between central‐executive and default‐mode networks. Proc Natl Acad Sci USA 105: 12569–12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein JL, Wiedholz LM, Bassett DS, Weinberger DR, Zink CF, Mattay VS, Meyer‐Lindenberg A ( 2007): A validated network of effective amygdala connectivity. Neuroimage 36: 736–745. [DOI] [PubMed] [Google Scholar]
- Stern ER, Gonzalez R, Welsh RC, Taylor SF ( 2010): Updating beliefs for a decision: Neural correlates of uncertainty and underconfidence. J Neurosci 30: 8032–8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern ER, Welsh RC, Fitzgerald KD, Gehring WJ, Lister JJ, Himle JA, Abelson JL, Taylor SF ( 2011): Hyperactive error responses and altered connectivity in ventromedial and frontoinsular cortices in obsessive‐compulsive disorder. Biol Psychiatry 69: 583–591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas EJ, Elliott R, McKie S, Arnone D, Downey D, Juhasz G, Deakin JF, Anderson IM ( 2011): Interaction between a history of depression and rumination on neural response to emotional faces. Psychol Med 41: 1845–1855. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS ( 2003): Overactive action monitoring in obsessive‐compulsive disorder: Evidence from functional magnetic resonance imaging. Psychol Sci 14: 347–353. [DOI] [PubMed] [Google Scholar]
- van den Hout M, Kindt M ( 2003): Repeated checking causes memory distrust. Behav Res Ther 41: 301–316. [DOI] [PubMed] [Google Scholar]
- Volans PJ ( 1976): Styles of decision‐making and probability appraisal in selected obsessional and phobic patients. Br J Soc Clin Psychol 15: 305–317. [DOI] [PubMed] [Google Scholar]
- Volz KG, Schubotz RI, von Cramon DY ( 2003): Predicting events of varying probability: Uncertainty investigated by fMRI. Neuroimage 19( 2 Part 1): 271–280. [DOI] [PubMed] [Google Scholar]
- Wager T, Barret LF ( 2004): From affect to control: Functional specialization of the insula in motivation and regulation. Published online at PsycNET.
- Ward BD ( 2000): Simultaneous inference for fMRI data. Avilable at: afni.nimh.nih.gov/pub/dist/doc/manual/AlphaSim.pdf.
- Weber EU, Milliman RA ( 1997): Perceived risk attitudes: Relating risk perception to risky choice. Manage Sci 43: 123–144. [Google Scholar]
- Whiteside SP, Port JD, Abramowitz JS ( 2004): A meta‐analysis of functional neuroimaging in obsessive‐compulsive disorder. Psychiatry Res 132: 69–79. [DOI] [PubMed] [Google Scholar]
- Yang Y, Gu H, Zhan W, Xu S, Silbersweig DA, Stern E ( 2002): Simultaneous perfusion and BOLD imaging using reverse spiral scanning at 3T: Characterization of functional contrast and susceptibility artifacts. Magn Reson Med 48: 278–289. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting Information may be found in the online version of this article.
Supporting Information Table 1. List of medications and dosages (mg/day) for medicated OCD (mOCD) and medicated patient control (mPC) groups. SSRIs = selective‐serotonin reuptake inhibitors; SNRIs = serotonin‐norepinephrine reuptake inhibitors; TCAs = tricycle antidepressants. All subjects were taking a serotonin reuptake inhibitor (SSRI or SNRI). Five mOCD and 4 mPC subjects were taking more than one medication (not including benzodiazepines, which were taken as needed and omitted on the day of testing). ± Dosing information not available for one subject.
Supporting Information Table 2. Within‐group effects for the first piece of evidence on Certain vs. Uncertain sequences. There were no significantly active regions for the contrast of Uncertain > Certain trials for OCD patients. aI/fO = anterior insula/frontal operculum; DMPFC = dorsomedial prefrontal cortex; MFG = middle frontal gyrus; SFG = superior frontal gyrus; IFG = inferior frontal gyrus; aPFC = anterior prefrontal cortex; IPL = inferior parietal lobule; dACC = dorsal anterior cingulate cortex; SMA = supplementary motor area; PCC = posterior cingulate cortex; STG = superior temporal gyrus, PG = parahippocampal gyrus; OFC = orbitofrontal cortex; VMPFC = ventromedial prefrontal cortex; MTG = middle temporal cortex, aMFC = anterior medial frontal cortex. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 3. Within‐group effects for accumulating evidence that increases vs. decreases certainty. There were no regions uniquely activated by controls for the increase > decrease contrast or for OCD patients for the decrease > increase contrast. IPL = inferior parietal lobule; STG = superior temporal gyrus; SMA = supplementary motor area; aMFC = anterior medial frontal cortex; VMPFC = ventromedial prefrontal cortex; MTG = middle temporal gyrus; MFG = middle frontal gyrus; IFG = inferior frontal gyrus; dACC = dorsal anterior cingulate cortex; SPL = superior parietal lobule; aI/fO = anterior insula/frontal operculum; ITG = inferior temporal gyrus. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 4. Within‐group effects for accumulating evidence that increases vs. maintains certainty. There were no regions uniquely activated by OCD patients for the increase > maintain contrast. IPL = inferior parietal lobule; SFG = superior frontal gyrus; MFG = middle frontal gyrus; sSMA = supplementary motor area; aI/fO = anterior insula/frontal operculum; IFG = inferior frontal gyrus; PCC = posterior cingulate cortex; MTG = middle temporal cortex; DMPFC = dorsomedial prefrontal cortex; aMFC = anterior medial frontal cortex; VMPFC = ventromedial prefrontal cortex; ACC = anterior cingulate cortex; PG = parahippocampal gyrus; STG = superior temporal gyrus; ITG = inferior temporal gyrus; OFC = orbitofrontal cortex. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 5. Within‐group effects for accumulating evidence that decreases vs. maintains certainty. There were no regions uniquely activated by OCD patients for the decrease > maintain contrast. aPFC = anterior prefrontal cortex; MFG = middle frontal gyrus; aI/fO = anterior insula/frontal operculum; SMA = supplementary motor area; dorsal anterior cingulate cortex = dACC; IPL = inferior parietal lobule; IFG = inferior frontal gyrus; ITG = inferior temporal gyrus; MTG = middle temporal cortex; PCC = posterior cingulate cortex; PG = parahippocampal gyrus; DMPFC = dorsomedial prefrontal cortex; aMFC = anterior medial frontal cortex; VMPFC = ventromedial prefrontal cortex; STG = superior temporal gyrus; OFC = orbitofrontal cortex. Superscript letters designate coordinates located within the same cluster.
Supporting Information Table 6. Regions where OCD patients > control subjects for the first piece of evidence on Certain as compared to Uncertain sequences, corrected for multiple comparisons across the whole‐brain at p < .05. BA = Brodmann's area(s); k = number of voxels; Z = maximum z‐score; R = right; L = left; PG = Parahippocampal gyrus;, MTG = middle temporal gyrus; STG = superior temporal gyrus; IPL = inferior parietal lobule; xcoordinates are in MNI space. ± Significant at trend level (p < .1). There were no regions where controls > OCD at the current threshold.
Supporting Information Figure 1. Within‐group activations for a) first piece of evidence > implicit baseline on Certain sequences, b) first piece of evidence > implicit baseline on Uncertain sequences, and c) first piece of evidence on Certain > Uncertain sequences. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 2. Within‐group a) activations and b) deactivations for accumulating evidence (draws 2, 3, and 4) that maintains maximal certainty on Certain sequences compared to implicit baseline. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 3. Within‐group a) activations and b) deactivations for accumulating evidence (draws 2, 3, and 4) that increases certainty on Uncertain sequences compared to implicit baseline. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 4. Within‐group a) activations and b) deactivations for accumulating evidence (draws 2, 3, and 4) that decreases certainty on Uncertain sequences compared to implicit baseline. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information Figure 5. Within‐group activations for accumulating evidence (draws 2, 3, and 4) that increases > decreases certainty and b) decreases > increases certainty on Uncertain sequences. OCD patients are shown in blue, controls are shown in red, and overlap shown in pink. Color bars represent t scores.
Supporting Information


