Abstract
Advances in neuroimaging have helped illuminate our understanding of how the brain works in the presence of chronic pain, which often persists with unknown etiology or after the painful stimulus has been removed and any wounds have healed. Neuroimaging has enabled us to make great progress in identifying many of the neural mechanisms that contribute to chronic pain, and to pinpoint the specific regions of the brain that are activated in the presence of chronic pain. It has provided us with a new perception of the nature of chronic pain in general, leading researchers to move toward a whole-brain approach to the study and treatment of chronic pain, and to develop novel technologies and analysis techniques, with real potential for developing new diagnostics and more effective therapies. We review the use of neuroimaging in the study of chronic pain, with particular emphasis on magnetic resonance imaging.
Keywords: brain-based therapy, chronic low back pain, CNS, fibromyalgia, fMRI, MRI, MVPA, real-time fMRI, resting state fMRI
Chronic pain is a widespread and growing problem in the USA, affecting more than 100 million adults at some point in their lives, and accounting for about US$600 billion annually in medical costs and lost productivity [1].
Chronic pain is complex, and the neural mechanisms that underlie chronic pain have been poorly understood. However, the evolution of various neuroimaging techniques has opened new windows into the brain and spurred new avenues of pain research that hold real promise for developing new, more effective treatments. Neuroimaging has shown us that chronic pain is different from acute pain, and that it can become a separate disease entity that may occur, in part, following changes in the entire CNS that cause chronicity and the development of comorbid symptoms. However, it is imperative to remember that despite the involvement of brain changes in chronic pain, the nervous system is not solely responsible for the initiation and/or maintenance of chronic pain, as addressed in a series of recent commentaries [2,3].
Nonetheless, neuroimaging has become an increasingly important and popular means of studying how the brain perceives and processes chronic pain. Various neuroimaging modalities have been used, including PET, EEG [4], magnetoencephalography (MEG), single-photon-emission computed tomography (SPECT/CT) [5] and MRI. These techniques have been used to study several chronic pain states, including, most commonly, chronic low back pain (cLBP) [6], fibromyalgia (FM) [7], osteoarthritis [8], complex regional pain syndrome (CRPS) [9,10], phantom-limb pain, chronic migraine [11], chronic pelvic pain (CPP) [12,13] and peripheral neuropathy [14], among others [15]. Experiments have evaluated acute pain processing mechanisms in healthy volunteers [16–18] and in animals [19] and in animal models of chronic pain [20]. Neuroimaging has helped elucidate many of the neural correlates regarding factors well known to modulate the experience of pain, including attention [21], anticipation [22], empathy [23,24], placebo [25], meditation [26], fear/anxiety [18] and reward [15]. Each factor impacts how we perceive pain, and an increasing number of functional neuroimaging studies are investigating how these factors affect pain perception and activity in the brain. Current pain research also uses various neuroimaging techniques to investigate a broad range of translational science that can eventually be tested in clinical trials.
The present review focuses specifically on the use of neuroimaging, and especially MRI, to study CNS changes in patients with a variety of chronic pain states.
How MRI has been used to image chronic pain
The many variations of MRI technology
MRI is one of the most widely used modalities for the study of chronic pain. It combines a strong magnetic field with radiofrequency pulses to display high-spatial-resolution structural images. These images can be used to measure the density and distribution of gray matter (voxel-based morphometry, cortical thickness analysis), and white matter (diffusion tensor imaging, functional anisotropy). Functional MRI (fMRI) allows for an indirect measurement of brain activity by tracking changes in blood oxygenation levels (referred to as the BOLD signal) [27]. Additional techniques include magnetic resonance spectroscopy (MRS), which measures relative concentrations of metabolites in the brain [28,29], and arterial spin labeling fMRI, which uses magnetically labeled protons in the blood as an endogenous tracer to measure changes in global and regional blood flow [30,31].
Neuroimaging allows us to study neural activity in individuals with chronic pain when they are either completely at rest, or while they are subjected to various tasks, interventions and procedures. These can include applying physical stimuli, such as heat pain, pressure or body movement, in a block design and exposing the patient to event-related stimuli, such as emotion-evoking images, working memory tasks and auditory stimuli. Neuroimaging experiments using combinations of imaging modalities and these various techniques are rapidly advancing our knowledge of how chronic pain affects brain structure and activity.
What MRI has taught us about chronic pain
Keeping the CNS in perspective: how pain (as nociceptive information) gets to the brain
Pain processing typically involves transmission and modulation of nociceptive signals along a predictable pathway. Noxious stimuli trigger signals in the peripheral nerves. A-delta nerve fibers transmit the ‘first-pain’ signals, the pricking, sharp sensations felt immediately after the painful stimulus is applied. C fibers transmit ‘second-pain’ signals, the dull, aching and throbbing pain felt after a 1–2-s delay [32]. These peripheral nerve fibers synapse in the dorsal horn of the spinal cord, where interneurons cause inhibitory/excitatory modulation. Secondary spinal projection neurons then transmit the information to two areas of the brainstem – the rostral ventral medulla and periaqueductal gray, where they are further modulated and relayed first to the thalamus and then to the somatosensory cortex in the cerebrum, where they are interpreted as pain (for review [33]).
In chronic pain states, inflammatory factors and sensitized receptors in the skin are thought to cause an abnormal increase in the transmission of nociceptive signals from the periphery as well as either a lack of inhibition or increased excitation, or both, at the spinal cord, brainstem or cortical levels, called ‘central sensitization’ [34,35].
Specific brain & brainstem regions implicated in chronic pain
Neuroimaging provides a means of noninvasively studying altered activity levels in the CNS. Specifically, neuroimaging can be used to study the brain, brainstem and spinal cord, where central sensitization and pain modulation occur and contribute to the ongoing experience of chronic pain and related symptoms [35,36]. Much of neuroimaging research has focused on identifying the brain regions that demonstrate altered structure and activity in chronic pain states. A major goal of this research is to identify specific brain regions as future targets for chronic pain therapy.
Several key brain regions have been identified as potentially playing a role in chronic pain. These regions are primarily implicated in sensory and affective components of pain processing and perception, motor function and higher order brain processing and integration, as reviewed below.
Structural changes
Differences in brain structure have been widely assessed in individuals with chronic pain, typically using voxel-based morphology [37] and cortical thickness analysis [38]. Regional increases and decreases in cortical thickness and gray matter density have been observed across several types of chronic pain, including CRPS [10], fibromyalgia [39,40], migraine [41–43], temporomandibular disorders (TMD) [15] and cLBP [6,44–46] and in visceral pain states, such as irritable bowel syndrome (IBS) [47]. One study demonstrated these changes simultaneously among different chronic pain syndromes, including CRPS, knee osteoarthritis and cLBP [48]. These studies indicate that the key areas of observed gray matter change include regions within the insular, somatosensory, motor and associated cortices; in subcortical structures, including the thalamus and basal ganglia, and parietal cortices; in regions within the prefrontal cortex; and in structures implicated in memory and emotion regulation, such as the hippocampus and amygdala, respectively.
It was initially thought that changes in gray matter, primarily decreased gray matter density, were associated with increased rates of age-related gray matter atrophy [49,50]. However, this theory is being questioned because several chronic pain studies have shown a mixture of regional increases and decreases in gray matter density [15,51], as well as reversal of gray matter change following effective therapy [52]. The exact nature and cause of these changes are currently unknown. Moreover, we do not know whether the observed changes represent existing differences in brain structure that predispose individuals to chronic pain, whether they occur as a result of the presence of chronic pain (e.g., due to additional stress and pain experience itself), or whether they are functionally linked to the maintenance of chronic pain. In addition, it is unclear whether these detected differences in gray matter structure are specifically due to chronic pain, or whether they are more complex in nature and result from multiple factors linked to chronic pain (such as depression or the medications that the patient is taking for pain). For example, a meta-analysis of several studies of structural brain changes in patients with FM indicated that the depression score accounted for most, if not all, of the changes in gray matter structure in individuals with FM as compared with healthy volunteers [53,54]. However, gray matter changes have also been shown to occur in patients with cLBP with little to no emotional distress [6].
Connections between brain regions are now under study as well. Diffusion tensor imaging and fractional anisotropy have been used to investigate differences in white matter structure seen in various chronic pain states, including TMD [55], and IBS [56,57]. Changes in brain structure have also been observed using combined voxel-based morphology and diffusion tensor imaging to analyze interactions between regions of gray matter change and white matter change in patients with CRPS [58] and FM [59,60].
Functional changes
Alterations in brain function have been demonstrated in multiple chronic pain syndromes, and many of the identified regions of functional change overlap with regions of structural change [61]. Investigations of brain function in the presence of chronic pain typically involve protocols to assess brain function in response to pain evoked by noxious or innocuous stimulation [62–64], in the presence of emotional or cognitive tasks [65,66] or stress [67], or while patients rate their ongoing chronic pain symptoms [68]. Ultimately, no one region within the brain, brainstem or spinal cord is singularly responsible for chronic pain: all neuroimaging studies have shown that chronic pain and its comorbid symptoms cause neurological changes across several brain regions [69]. Moreover, these studies repeatedly demonstrate altered function in several key regions within the CNS, as described below.
Altered activity within the primary somatosensory cortex and posterior insular cortex has been observed when noxious stimuli are applied in individuals with cLBP, FM, CPP and CRPS [70]. These are regions typically associated with intensity coding (which measures how painful a stimulus is), and these functional alterations suggest altered intensity processing of pain in chronic pain states. Similarly, the secondary somatosensory cortex (SII) is a region of higher order sensory processing and integration, and has shown both structural and functional alterations [66,71].
The primary motor cortex, premotor cortex and supplementary motor areas also play a role in chronic pain. Alterations within these motor regions may be related to the changes seen within the cerebellum, which have been historically reported, yet minimally discussed, in the literature. Currently, however, insights into cerebellar changes in chronic pain are accumulating and may add to the known function of the cerebellum and how it coordinates with altered sensory motor and emotional processing in the presence of chronic pain [72,73].
Several investigators have examined the relationship between cognitive processes and chronic pain [74]. These studies primarily identify functional changes in higher order regions within the prefrontal cortex (PFC), including the ventromedial PFC, dorsolateral PFC and orbitofrontal PFC [75–77]. Regions within the parietal cortex, including the temporo-parietal junction, precuneus and posterior cingulate cortex, also demonstrate functional changes in individuals with chronic pain. These regions are involved in introspection, mind wandering and self-referential thought processes [78], which may be more highly integrated with pain processing and experience in chronic pain states.
Brain regions related to the affective aspects of pain processing (such as the level of unpleasantness, negative context), including the anterior insular cortex [79] and anterior cingulate cortex [80], demonstrate altered function in chronic pain states [81]. Studies investigating the psychological aspects of chronic pain, including altered fear and emotional processing, have identified scale-based correlations of altered emotion processing with altered brain structure and function in emotion and fear-processing regions, including the amygdala [82], and in memory-related processing regions including the hippocampus [83]. While changes in the amygdala have been observed, fear avoidance (of movement) is not indicated as being responsible for these changes [84]. Therefore, these alterations are more likely due to general changes in limbic and memory networks.
Altered function within subcortical, midbrain and brainstem regions suggests that chronic pain modifies brain circuits and modulation. Thalamic lesions have been implicated in central pain [85], and they frequently accompany altered activity and structure in other chronic pain states as well. Functional alterations within the basal ganglia [86,87] suggest altered motor and general connectivity of the brain. Altered activity within midbrain regions, in particular in the ventral tegmental area [88,89], may signal that chronic pain disrupts the mechanisms of reward, punishment and dopamine function.
Altered activity within brainstem regions, especially involving the periaqueductal gray [90–92], may signify disrupted regulatory control of pain [93]. However, further research is needed because the small size and highly complex, multifunctional heterogeneity of the brainstem has thus far limited study within this region.
Although invasive electrophysiology studies of chronic pain and chronic pain models have observed altered activity within the spinal cord, MRI imaging of the cervical spinal cord has to date been conducted only in healthy individuals [94,95]. This technology is evolving and may soon be useful for the study of chronic pain. However, technological advances are necessary to improve the S/N in cervical spinal cord imaging, which is greatly diminished by local pulsation and physiological noise [96].
Brain network-based approach: resting state fMRI
Recent methods of resting-state fMRI have focused on multiple regions in the brain, targeting inherent and altered measures of connectivity between regions and within brain ‘networks’ [97]. Resting-state fMRI has the advantage of enabling neuroimaging data to be collected while individuals with chronic pain simply rest in the MRI scanner. Moreover, it provides information about the natural state of brain activity in chronic pain without having to apply any external sensory or cognitive stimulation. Resting-state fMRI methods investigate the degree of functional connectivity, seen as changes in correlation of low-frequency oscillations in neural activity between brain regions. These changes can provide information about altered resting-state brain activity in chronic pain states [98]. Chronic pain has been noted to alter several networks, or groups, of individual brain regions with similar low-frequency oscillatory activity and an increase or decrease in the presence or absence of external stimulation [99]. These primarily include the default-mode networks (DMN) [100], which are more active at rest; salience and executive control networks, which are more active during stimulation of the senses or tasks; and sensory motor networks, which are related to sensory and motor processing. Notably, altered DMN function in chronic pain has also been demonstrated in a study using arterial spin labeling [31].
Decreased DMN connectivity, specifically within the medial PFC, posterior cingulate cortex and amygdala, has been observed in cLBP [101]. Conversely, greater connectivity within the default mode and executive attention networks has been observed in FM [102]. Greater connectivity between the DMN and insular cortex has also been observed, indicating that these regions function together differently in FM as compared with healthy states [102]. Additional studies in FM have noted similar alterations between the insular cortex and other cortical regions [103]. Low-frequency fluctuations within brain regions are also altered in chronic pain, specifically within the primary somatosensory cortex, supplementary motor area, dorsolateral prefrontal cortex and amygdala [104]. Conversely, in CRPS, reduced resting-state functional connectivity has been observed within the DMN, and greater connectivity has been noted in the sensory and motor regions with other pain-processing-related regions [105]. Altered resting state activity within sensory and motor network regions [106] and within the DMN have been demonstrated in CPP [107]. Several studies have shown that altered functional connectivity of the brainstem [108], basal ganglia [109] and other regions within the frontal and temporal cortices [110] may underlie chronic migraine. Diabetic neuropathic pain also shows similar alterations in resting state activity [111].
Longitudinal changes & limitations
The majority of investigations mentioned thus far are nonlongitudinal, and none of these observational studies track individuals before the onset and through the development of chronic pain. This is a major limitation for all neuroimaging studies of chronic pain: the observed functional and structural changes cannot specifically be determined to be caused by the presence of chronic pain. Typically, in order to gain some sense of the longitudinal progression of structural and functional brain changes in chronic pain, the observed alterations are assessed for correlations with the duration and intensity of pain within the studied population. However, more recently, a growing number of longitudinal investigations have been conducted, in particular for cLBP [112] and IBS [113]. A recent study that tracked patients who transitioned from subacute to cLBP noted changes in the structure of white matter [114]. A few interesting studies have also shown that brain changes reverse when chronic pain is reduced by means of various effective therapies [52,115], including psychological therapy [116]. This indicates that although CNS abnormalities are highly implicated in chronic pain states, they may not have to be permanent – the use of appropriate, effective therapy may be able to restore normal brain function, at least in part.
Greatest future potential for neuroimaging in the study of chronic pain
The use of neuroimaging technology to study chronic pain continues to gain interest and momentum. Noninvasive imaging techniques, including MRI, EEG, MEG and others, are being used with increasing frequency; this may largely be a function of the fact that neuroimaging can gather large amounts of data without requiring study subjects to engage in activity that could aggravate their pain – they are allowed to simply rest while the images are passively obtained.
In future, developments in three main areas hold the most promise to add to our understanding of CNS involvement in chronic pain, which will spur the subsequent development of novel therapies: combining imaging technologies to obtain simultaneous high-spatial and high-temporal resolution scans; identifying neurological signature patterns and prediction potential; and continuing to develop clinical neuroimaging-based interventions.
Good qualities: noninvasive & high-spatial & high-temporal resolution
Researchers have begun to combine functional imaging technologies for use in some medical research, but few studies have used this technique to study chronic pain. For example, MRI can be combined with EEG or MEG, which achieves measures of both high spatial resolution from the MRI/fMRI scans and high temporal resolution from the EEG/MEG scans [117]. The technology for simultaneous acquisition of MRI and EEG scans and for combining the images still needs to be developed. However, future studies using combined neuroimaging may provide invaluable insight into the brain changes in chronic pain states.
Multivariate pattern analysis: machine learning technology
New advances in the technology for neuroimaging data analysis are gaining momentum and showing promise, specifically in the case of multivariate pattern analysis (MVPA) (for review: [118]). MVPA is a machine-learning technology that can be applied as an algorithm to analyze large data sets and identify signature patterns that represent subgroups. Moreover, MVPA can function as a predictive tool; once a signature pattern has been identified in individuals with chronic pain versus healthy controls, data from a single individual can be classified as belonging to one of the groups, based on that individual’s pattern of brain structure or activity and its similarity to the signature patterns of the group [119,120]. MVPA technology has already been applied to identify acute pain related changes in healthy human volunteers [17,121], and has been extended to differentiate patients with chronic pain from healthy volunteers based on brain structure [6]. Ultimately, it is anticipated that MVPA technology will advance neuroimaging to the next level, allowing it to be useful as a diagnostic tool to predict an individual’s prognosis and define the appropriate therapies based on an individual’s brain structure and activity patterns. In the future, this technology could also be combined with big data, such as phenotype and genetic information, to create a more personalized approach for diagnosing and treating the each patient. Longitudinal studies using MVPA may also provide scientific grounds for assessing the transition from acute to chronic pain. Overall, MVPA technology is a powerful tool that is expected to improve the clinical utility of neuroimaging for chronic pain and to advance neuroimaging analyses from the current standard of group comparisons to an individualized approach.
Brain-based therapies: real-time fMRI neurofeedback & neurostimulation
Neuroimaging continues to advance our understanding of how the CNS is affected by and involved in chronic pain, and neuroimaging interventions are being and gaining momentum as an alternative or supplement to pharmaceutical therapy, or both [122,123]. Several studies of real-time neurofeedback for chronic pain have been conducted [124,125], but further research and additional clinical trials are still needed. The efficacy and benefits of real-time neurofeedback for an individual may be better harnessed in the future by the combining real-time neurofeedback fMRI and machine-learning classifiers (MVPA) to identify spatiotemporal brain maps ideal for individualized, real-time manipulation for each patient [126].
Although neurostimulation is invasive and is only implicated for use in the most severe, intractable cases of chronic pain, novel tools are being developed to better select patients who are most likely to benefit from this intervention [127]. Implantation of neurostimulators is still an option for targeted manipulation of brain activity within specific brain regions, and there have been great advances in this technology since its inception [128]. Current techniques use adaptive models [129] and target brain regions, such as the motor cortex, that have the potential to activate multiple downstream effects [130].
Transcranial magnetic stimulation is also gaining popularity as an interventional and alternative method for reducing the symptoms of chronic pain (for review, see [131,132]). Preliminary clinical trials of transcranial magnetic stimulation have demonstrated effective pain reduction that persists days to weeks after treatment [133–136]. However, current investigations continue to search for ideal brain region targets and delivery specifications (such as parameters and treatment frequency).
Additional exciting advancements for the future use of neuroimaging in chronic pain-related therapy include the development of brain–computer interfaces using electrocorticography and visual feedback, which has been tested as a potential therapy for phantom limb pain [137]. Advancements in the use of PET imaging are making it possible to use this technique to predict the efficacy of motor cortex stimulation, in particular using opioid binding and receptor density to predict the efficacy of motor cortex stimulation [138]. Advances in present technology and combinations of old and new neuroimaging modalities will continue to help pain researchers decode the mysteries of the brain’s response to chronic pain, which will enable the development of new and improved therapies for this complex and often disabling condition.
Conclusion
Neuroimaging has provided evidence of structural and functional brain changes in the majority of chronic pain syndromes. To date, cLBP, FM, neuropathic pain and TMD have been the most widely studied pain syndromes using this technology. The expression that ‘pain is in a patient’s head’ no longer reflects the idea that chronic pain is a largely psychological problem. Rather, it can now be taken more literally, because neuroimaging studies have repeatedly demonstrated extensive alterations in brain structure and function in chronic pain states. To date, we have accumulated a large amount of somewhat variable, yet overlapping, evidence indicating that altered brain mechanisms may, in many cases, greatly contribute to, if not wholly underlie, real pain sensations. Moreover, neuroimaging has shown that multiple regions of the brain are involved in a range of pain, sensory, motor, cognitive, motivational, memory, emotion and fear processes. Individual variability in the pain experience remains a challenge in the clinical care of chronic pain. Continued research and advances in neuroimaging technology are needed to further clarify brain mechanisms involved in chronic pain and to further develop novel brain-based treatment approaches for patients with chronic pain.
Future perspective
Neuroimaging of chronic pain has largely focused on identifying individual regions of the brain implicated in chronic pain, and determining what these regions contribute to the development and persistence of chronic pain and its comorbid symptoms. Neuroimaging has demonstrated that we need a more network-based approach to the study of chronic pain, with a particular focus on how the various regions in the brain interact with each other and with other regions of the CNS, such as the cervical spinal cord. Neuroimaging has shown us that no specific pain center exists in the brain, and the quest to find this conceptual single pain center responsible for chronic pain may have ended. However, all of the regions that have been found to play specific roles in chronic pain will continue to be useful targets for brain-based therapies. Eventually, neuroimaging of chronic pain will evolve into a therapy-driven field. We are building a large knowledge-base about regional alterations seen in chronic pain states, and we are redirecting research efforts to examine networks and combinations of regions that are altered in the presence of chronic pain (Figure 1).
Figure 1. Illustration of the advancement of chronic pain neuroimaging technology and methods.
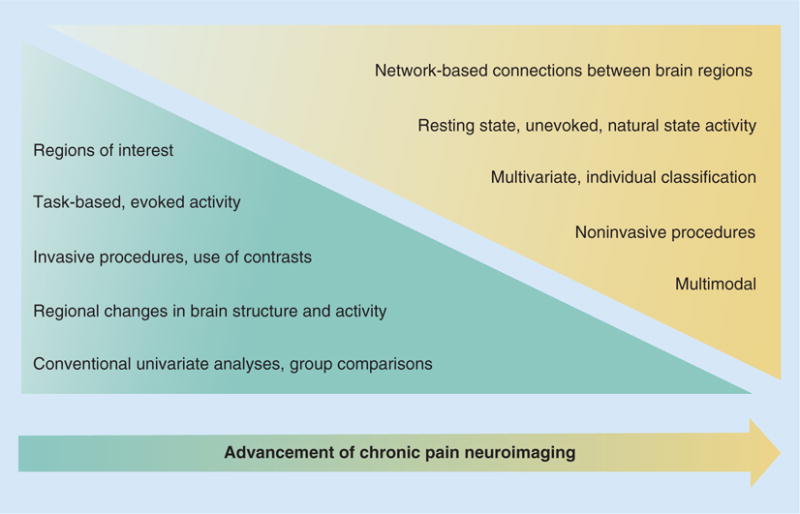
Over the next several years, it is expected that neuroimaging methods will shift from predominantly conventional analyses (blue triangle) toward newer methods (yellow triangle) offering multiple benefits for analysis and interpretative power. Analyses of localized regions of interest will shift toward network-based assessments involving multiple brain regions. Task-based imaging of evoked activity (typical block design and event-related designs) will give way to more natural, resting state imaging of the individual patient in their unprovoked condition of chronic pain. Invasive procedures and contrasts are expected to continue to decline in popularity in favor of more comfortable and noninvasive techniques. Traditional group analyses will eventually be replaced with individual assessment through the continued development of classification and other technology that will enhance the level of power from an individual’s brain scan. Multivariate analyses will replace univariate analyses, and these multivariate analyses will also be able to incorporate genetic and biomarker data into their models. Analyses of single modality (e.g., morphometry, functional MRI) will eventually be improved through the ability to combine across data modalities (e.g., combined morphometry, resting state and diffusion tensor imaging; combined functional MRI, EEG, PET) to enhance and validate findings across data types and signals. Ultimately, improved methods and technology will be used to assess a broader scope of data types and modalities, and together these will provide enhance statistical power for understanding CNS alterations in the individual.
Additional integration of pain medicine with other fields, such as psychology, physical and occupational therapy, immunology and other chronic pain-related fields will continue to increase the potential for us to develop interventions that modulate response of the CNS to chronic pain. The ultimate goal is to prevent and reverse the maladaptive processes that take place in the CNS in the presence of chronic pain.
EXECUTIVE SUMMARY.
Pain-related changes in brain structure and activity have been observed across several regions of the brain. These most notably include the anterior cingulate cortex, insular cortex, prefrontal cortex, primary and secondary somatosensory cortices (S1 and S2), motor cortex (M1) and supplementary motor area, thalamus, basal ganglia, amygdala, hippocampus and cerebellum.
Ultimately, no one region within the brain, brainstem or spinal cord is singularly responsible for chronic pain; across all neuroimaging studies of chronic pain, the general consensus is that neurological changes across several brain regions are implicated in the presence of chronic pain and its comorbid symptoms.
Resting-state functional MRI allows researchers to focus on network-based changes and has revealed changes within the default mode network salience network, executive control network and sensory motor network in chronic pain.
Multivariate pattern analysis, which focuses on a whole-brain approach to identify differences in brain structure and activity, is gaining momentum as a new method of analysis for MRI studies of chronic pain.
Advancements in real-time functional MRI, transcranial magnetic stimulation and other neuroimaging-based therapies continue to promise novel and more effective treatments for chronic pain.
Acknowledgments
Special thanks to Maureen Donohue, Editor for the Department of Anesthesiology, Pain and Perioperative Medicine at Stanford, for editing the manuscript.
Financial disclosure
Writing of this review article was supported by the Chris Redlich Pain Research Endowment (to S Mackey), NIH K24 DA029262 (to S Mackey), NIH P01 AT006651 (to S Mackey) and NIH T32 GM89626 (KT Martucci).
Footnotes
Competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
•• of considerable interest.
- 1.IOM. IOM (Institute of Medicine) Report: Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education, and Research. doi: 10.7205/MILMED-D-16-00012. www.iom.edu/~/media/Files/Report. [DOI] [PubMed]
- 2.Sullivan MD, Cahana A, Derbyshire S, Loeser JD. What does it mean to call chronic pain a brain disease? J Pain. 2013;14(4):317–322. doi: 10.1016/j.jpain.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 3.Mackey SC. Central neuroimaging of pain. J Pain. 2013;14(4):328–331. doi: 10.1016/j.jpain.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Diers M, Koeppe C, Diesch E, et al. Central processing of acute muscle pain in chronic low back pain patients: an EEG mapping study. J Clin Neurophysiol. 2007;24(1):76–83. doi: 10.1097/01.wnp.0000241093.00844.0e. [DOI] [PubMed] [Google Scholar]
- 5.Harisankar CN, Mittal BR, Bhattacharya A, Singh P, Sen R. Utility of single photon emission computed tomography/computed tomography imaging in evaluation of chronic low back pain. Indian J Nucl Med. 2012;27(3):156–163. doi: 10.4103/0972-3919.112720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6••.Ung H, Brown JE, Johnson KA, Younger J, Hush J, Mackey S. Multivariate classification of structural MRI data detects chronic low back pain. Cereb Cortex. 2012;24(4):1037–1044. doi: 10.1093/cercor/bhs378. First investigation using multivariate pattern analysis to distinguish chronic pain patients from healthy volunteers based on brain gray matter density. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Staud R. Brain imaging in fibromyalgia syndrome. Clin Exp Rheumatol. 2011;29(6 Suppl 69):S109–S117. [PubMed] [Google Scholar]
- 8.Howard MA, Sanders D, Krause K, et al. Alterations in resting-state regional cerebral blood flow demonstrate ongoing pain in osteoarthritis: an arterial spin-labeled magnetic resonance imaging study. Arthritis Rheumat. 2012;64(12):3936–3946. doi: 10.1002/art.37685. [DOI] [PubMed] [Google Scholar]
- 9.Schwenkreis P, Maier C, Tegenthoff M. Functional imaging of central nervous system involvement in complex regional pain syndrome. Am J Neuroradiol. 2009;30(7):1279–1284. doi: 10.3174/ajnr.A1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barad MJ, Ueno T, Younger J, Chatterjee N, Mackey S. Complex regional pain syndrome is associated with structural abnormalities in pain-related regions of the human brain. J Pain. 2014;15(2):197–203. doi: 10.1016/j.jpain.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiapparini L, Ferraro S, Grazzi L, Bussone G. Neuroimaging in chronic migraine. Neurol Sci. 2010;31(Suppl 1):S19–S22. doi: 10.1007/s10072-010-0266-9. [DOI] [PubMed] [Google Scholar]
- 12.Farmer MA, Chanda ML, Parks EL, Baliki MN, Apkarian AV, Schaeffer AJ. Brain functional and anatomical changes in chronic prostatitis/chronic pelvic pain syndrome. J Urol. 2011;186(1):117–124. doi: 10.1016/j.juro.2011.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kairys AE, Schmidt-Wilcke T, Puiu T, et al. Increased brain gray matter in the primary somatosensory cortex is associated with increased pain and mood disturbance in interstitial cystitis/painful bladder syndrome patients. J Urol. 2014 doi: 10.1016/j.juro.2014.08.042. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moisset X, Bouhassira D. Brain imaging of neuropathic pain. Neuroimage. 2007;37(Suppl 1):S80–S88. doi: 10.1016/j.neuroimage.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 15.Younger JW, Shen YF, Goddard G, Mackey SC. Chronic myofascial temporomandibular pain is associated with neural abnormalities in the trigeminal and limbic systems. Pain. 2010;149(2):222–228. doi: 10.1016/j.pain.2010.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ohara S, Crone NE, Weiss N, Lenz FA. Analysis of synchrony demonstrates ‘pain networks’ defined by rapidly switching, task-specific, functional connectivity between pain-related cortical structures. Pain. 2006;123(3):244–253. doi: 10.1016/j.pain.2006.02.012. [DOI] [PubMed] [Google Scholar]
- 17.Brown JE, Chatterjee N, Younger J, Mackey S. Towards a physiology-based measure of pain: patterns of human brain activity distinguish painful from non-painful thermal stimulation. PLoS ONE. 2011;6(9):e24124. doi: 10.1371/journal.pone.0024124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ochsner KN, Ludlow DH, Knierim K, et al. Neural correlates of individual differences in pain-related fear and anxiety. Pain. 2006;120(1–2):69–77. doi: 10.1016/j.pain.2005.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jeffrey-Gauthier R, Guillemot JP, Piche M. Neurovascular coupling during nociceptive processing in the primary somatosensory cortex of the rat. Pain. 2013;154(8):1434–1441. doi: 10.1016/j.pain.2013.04.042. [DOI] [PubMed] [Google Scholar]
- 20.Thompson SJ, Millecamps M, Aliaga A, et al. Metabolic brain activity suggestive of persistent pain in a rat model of neuropathic pain. Neuroimage. 2014;1(91):344–352. doi: 10.1016/j.neuroimage.2014.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lawrence JM, Hoeft F, Sheau KE, Mackey SC. Strategy-dependent dissociation of the neural correlates involved in pain modulation. Anesthesiology. 2011;115(4):844–851. doi: 10.1097/ALN.0b013e31822b79ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fairhurst M, Wiech K, Dunckley P, Tracey I. Anticipatory brainstem activity predicts neural processing of pain in humans. Pain. 2007;128(1–2):101–110. doi: 10.1016/j.pain.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 23.Zaki J, Ochsner KN, Hanelin J, Wager TD, Mackey SC. Different circuits for different pain: patterns of functional connectivity reveal distinct networks for processing pain in self and others. Soc Neurosci. 2007;2(3–4):276–291. doi: 10.1080/17470910701401973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ochsner KN, Zaki J, Hanelin J, et al. Your pain or mine? Common and distinct neural systems supporting the perception of pain in self and other. Soc Cogn Affect Neurosci. 2008;3(2):144–160. doi: 10.1093/scan/nsn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Watson A, El-Deredy W, Iannetti GD, et al. Placebo conditioning and placebo analgesia modulate a common brain network during pain anticipation and perception. Pain. 2009;145(1–2):24–30. doi: 10.1016/j.pain.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeidan F, Martucci KT, Kraft RA, Gordon NS, McHaffie JG, Coghill RC. Brain mechanisms supporting the modulation of pain by mindfulness meditation. J Neurosci. 2011;31(14):5540–5548. doi: 10.1523/JNEUROSCI.5791-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Logothetis NK. The underpinnings of the BOLD functional magnetic resonance imaging signal. J Neurosci. 2003;23(10):3963–3971. doi: 10.1523/JNEUROSCI.23-10-03963.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Widerstrom-Noga E, Pattany PM, Cruz-Almeida Y, et al. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain. 2013;154(2):204–212. doi: 10.1016/j.pain.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grachev ID, Fredrickson BE, Apkarian AV. Abnormal brain chemistry in chronic back pain: an in vivo proton magnetic resonance spectroscopy study. Pain. 2000;89(1):7–18. doi: 10.1016/S0304-3959(00)00340-7. [DOI] [PubMed] [Google Scholar]
- 30.Williams DS. Quantitative perfusion imaging using arterial spin labeling. Methods Mol Med. 2006;124:151–173. doi: 10.1385/1-59745-010-3:151. [DOI] [PubMed] [Google Scholar]
- 31.Loggia ML, Kim J, Gollub RL, et al. Default mode network connectivity encodes clinical pain: an arterial spin labeling study. Pain. 2013;154(1):24–33. doi: 10.1016/j.pain.2012.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Price DD, Hu JW, Dubner R, Gracely RH. Peripheral suppression of first pain and central summation of second pain evoked by noxious heat pulses. Pain. 1977;3(1):57–68. doi: 10.1016/0304-3959(77)90035-5. [DOI] [PubMed] [Google Scholar]
- 33.Willis WD, Westlund KN. Neuroanatomy of the pain system and of the pathways that modulate pain. J Clin Neurophysiol. 1997;14(1):2–31. doi: 10.1097/00004691-199701000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35•.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl):S2–S15. doi: 10.1016/j.pain.2010.09.030. Excellent overview of central sensitization related CNS mechanisms in chronic pain. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zambreanu L, Wise RG, Brooks JC, Iannetti GD, Tracey I. A role for the brainstem in central sensitisation in humans. Evidence from functional magnetic resonance imaging. Pain. 2005;114(3):397–407. doi: 10.1016/j.pain.2005.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 38.Chung MK, Worsley KJ, Robbins S, et al. Deformation-based surface morphometry applied to gray matter deformation. Neuroimage. 2003;18(2):198–213. doi: 10.1016/s1053-8119(02)00017-4. [DOI] [PubMed] [Google Scholar]
- 39.Robinson ME, Craggs JG, Price DD, Perlstein WM, Staud R. Gray matter volumes of pain-related brain areas are decreased in fibromyalgia syndrome. J Pain. 2011;12(4):436–443. doi: 10.1016/j.jpain.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuchinad A, Schweinhardt P, Seminowicz DA, Wood PB, Chizh BA, Bushnell MC. Accelerated brain gray matter loss in fibromyalgia patients: premature aging of the brain? J Neurosci. 2007;27(15):4004–4007. doi: 10.1523/JNEUROSCI.0098-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmidt-Wilcke T, Ganssbauer S, Neuner T, Bogdahn U, May A. Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia. 2008;28(1):1–4. doi: 10.1111/j.1468-2982.2007.01428.x. [DOI] [PubMed] [Google Scholar]
- 42.Kim JH, Suh SI, Seol HY, et al. Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia. 2008;28(6):598–604. doi: 10.1111/j.1468-2982.2008.01550.x. [DOI] [PubMed] [Google Scholar]
- 43.Valfre W, Rainero I, Bergui M, Pinessi L. Voxel-based morphometry reveals gray matter abnormalities in migraine. Headache. 2008;48(1):109–117. doi: 10.1111/j.1526-4610.2007.00723.x. [DOI] [PubMed] [Google Scholar]
- 44.Apkarian AV, Sosa Y, Sonty S, et al. Chronic back pain is associated with decreased prefrontal and thalamic gray matter density. J Neurosci. 2004;24(46):10410–10415. doi: 10.1523/JNEUROSCI.2541-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt-Wilcke T, Leinisch E, Ganssbauer S, et al. Affective components and intensity of pain correlate with structural differences in gray matter in chronic back pain patients. Pain. 2006;125(1–2):89–97. doi: 10.1016/j.pain.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 46.Ivo R, Nicklas A, Dargel J, et al. Brain structural and psychometric alterations in chronic low back pain. Eur Spine J. 2013;22(9):1958–1964. doi: 10.1007/s00586-013-2692-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Davis KD, Pope G, Chen J, Kwan CL, Crawley AP, Diamant NE. Cortical thinning in IBS: implications for homeostatic, attention, and pain processing. Neurology. 2008;70(2):153–154. doi: 10.1212/01.wnl.0000295509.30630.10. [DOI] [PubMed] [Google Scholar]
- 48.Baliki MN, Schnitzer TJ, Bauer WR, Apkarian AV. Brain morphological signatures for chronic pain. PLoS ONE. 2011;6(10):e26010. doi: 10.1371/journal.pone.0026010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.May A. Chronic pain may change the structure of the brain. Pain. 2008;137(1):7–15. doi: 10.1016/j.pain.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 50.Rodriguez-Raecke R, Niemeier A, Ihle K, Ruether W, May A. Brain gray matter decrease in chronic pain is the consequence and not the cause of pain. J Neurosci. 2009;29(44):13746–13750. doi: 10.1523/JNEUROSCI.3687-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schmidt-Wilcke T, Luerding R, Weigand T, et al. Striatal grey matter increase in patients suffering from fibromyalgia–a voxel-based morphometry study. Pain. 2007;132(Suppl 1):S109–S116. doi: 10.1016/j.pain.2007.05.010. [DOI] [PubMed] [Google Scholar]
- 52.Seminowicz DA, Wideman TH, Naso L, et al. Effective treatment of chronic low back pain in humans reverses abnormal brain anatomy and function. J Neurosci. 2011;31(20):7540–7550. doi: 10.1523/JNEUROSCI.5280-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53••.Smallwood RF, Laird AR, Ramage AE, et al. Structural brain anomalies and chronic pain: a quantitative meta-analysis of gray matter volume. J Pain. 2013;14(7):663–675. doi: 10.1016/j.jpain.2013.03.001. Meta-analysis, in which the authors point out that there is significant overlap of effects on brain structure due to pain symptoms and psychological alterations, therefore psychological measures need to be consistently accounted for in structural brain analyses in the future. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hsu MC, Harris RE, Sundgren PC, et al. No consistent difference in gray matter volume between individuals with fibromyalgia and age-matched healthy subjects when controlling for affective disorder. Pain. 2009;143(3):262–267. doi: 10.1016/j.pain.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Moayedi M, Weissman-Fogel I, Salomons TV, et al. White matter brain and trigeminal nerve abnormalities in temporomandibular disorder. Pain. 2012;153(7):1467–1477. doi: 10.1016/j.pain.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 56.Chen JY, Blankstein U, Diamant NE, Davis KD. White matter abnormalities in irritable bowel syndrome and relation to individual factors. Brain Res. 2011;1392:121–131. doi: 10.1016/j.brainres.2011.03.069. [DOI] [PubMed] [Google Scholar]
- 57.Ellingson BM, Mayer E, Harris RJ, et al. Diffusion tensor imaging detects microstructural reorganization in the brain associated with chronic irritable bowel syndrome. Pain. 2013;154(9):1528–1541. doi: 10.1016/j.pain.2013.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Geha PY, Baliki MN, Harden RN, Bauer WR, Parrish TB, Apkarian AV. The brain in chronic CRPS pain: abnormal gray-white matter interactions in emotional and autonomic regions. Neuron. 2008;60(4):570–581. doi: 10.1016/j.neuron.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lutz J, Jager L, De Quervain D, et al. White and gray matter abnormalities in the brain of patients with fibromyalgia: a diffusion-tensor and volumetric imaging study. Arthritis Rheumat. 2008;58(12):3960–3969. doi: 10.1002/art.24070. [DOI] [PubMed] [Google Scholar]
- 60.Luerding R, Weigand T, Bogdahn U, Schmidt-Wilcke T. Working memory performance is correlated with local brain morphology in the medial frontal and anterior cingulate cortex in fibromyalgia patients: structural correlates of pain-cognition interaction. Brain. 2008;131(Pt 12):3222–3231. doi: 10.1093/brain/awn229. [DOI] [PubMed] [Google Scholar]
- 61.Jensen KB, Srinivasan P, Spaeth R, et al. Overlapping structural and functional brain changes in patients with long-term exposure to fibromyalgia pain. Arthritis Rheumat. 2013;65(12):3293–3303. doi: 10.1002/art.38170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Staud R, Craggs JG, Perlstein WM, Robinson ME, Price DD. Brain activity associated with slow temporal summation of C-fiber evoked pain in fibromyalgia patients and healthy controls. Eur J Pain. 2008;12(8):1078–1089. doi: 10.1016/j.ejpain.2008.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Peyron R, Schneider F, Faillenot I, et al. An fMRI study of cortical representation of mechanical allodynia in patients with neuropathic pain. Neurology. 2004;63(10):1838–1846. doi: 10.1212/01.wnl.0000144177.61125.85. [DOI] [PubMed] [Google Scholar]
- 64.Derbyshire SW, Jones AK, Creed F, et al. Cerebral responses to noxious thermal stimulation in chronic low back pain patients and normal controls. Neuroimage. 2002;16(1):158–168. doi: 10.1006/nimg.2002.1066. [DOI] [PubMed] [Google Scholar]
- 65.Glass JM, Williams DA, Fernandez-Sanchez ML, et al. Executive function in chronic pain patients and healthy controls: different cortical activation during response inhibition in fibromyalgia. J Pain. 2011;12(12):1219–1229. doi: 10.1016/j.jpain.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Eck J, Richter M, Straube T, Miltner WH, Weiss T. Affective brain regions are activated during the processing of pain-related words in migraine patients. Pain. 2011;152(5):1104–1113. doi: 10.1016/j.pain.2011.01.026. [DOI] [PubMed] [Google Scholar]
- 67.Vachon-Presseau E, Martel MO, Roy M, et al. Acute stress contributes to individual differences in pain and pain-related brain activity in healthy and chronic pain patients. J Neurosci. 2013;33(16):6826–6833. doi: 10.1523/JNEUROSCI.4584-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baliki MN, Chialvo DR, Geha PY, et al. Chronic pain and the emotional brain: specific brain activity associated with spontaneous fluctuations of intensity of chronic back pain. J Neurosci. 2006;26(47):12165–12173. doi: 10.1523/JNEUROSCI.3576-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Apkarian AV, Bushnell MC, Treede RD, Zubieta JK. Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain. 2005;9(4):463–484. doi: 10.1016/j.ejpain.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 70.Vartiainen N, Kirveskari E, Kallio-Laine K, Kalso E, Forss N. Cortical reorganization in primary somatosensory cortex in patients with unilateral chronic pain. J Pain. 2009;10(8):854–859. doi: 10.1016/j.jpain.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 71.Rodriguez-Raecke R, Ihle K, Ritter C, Muhtz C, Otte C, May A. Neuronal differences between chronic low back pain and depression regarding long-term habituation to pain. Eur J Pain. 2013;18(5):701–711. doi: 10.1002/j.1532-2149.2013.00407.x. [DOI] [PubMed] [Google Scholar]
- 72.Rosenberger C, Thurling M, Forsting M, Elsenbruch S, Timmann D, Gizewski ER. Contributions of the cerebellum to disturbed central processing of visceral stimuli in irritable bowel syndrome. Cerebellum. 2013;12(2):194–198. doi: 10.1007/s12311-012-0413-3. [DOI] [PubMed] [Google Scholar]
- 73.Moulton EA, Elman I, Pendse G, Schmahmann J, Becerra L, Borsook D. Aversion-related circuitry in the cerebellum: responses to noxious heat and unpleasant images. J Neurosci. 2011;31(10):3795–3804. doi: 10.1523/JNEUROSCI.6709-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weissman-Fogel I, Moayedi M, Tenenbaum HC, Goldberg MB, Freeman BV, Davis KD. Abnormal cortical activity in patients with temporomandibular disorder evoked by cognitive and emotional tasks. Pain. 2011;152(2):384–396. doi: 10.1016/j.pain.2010.10.046. [DOI] [PubMed] [Google Scholar]
- 75.Apkarian AV, Thomas PS, Krauss BR, Szeverenyi NM. Prefrontal cortical hyperactivity in patients with sympathetically mediated chronic pain. Neurosci Lett. 2001;311(3):193–197. doi: 10.1016/s0304-3940(01)02122-x. [DOI] [PubMed] [Google Scholar]
- 76.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 77.Neugebauer V, Galhardo V, Maione S, Mackey SC. Forebrain pain mechanisms. Brain Res Rev. 2009;60(1):226–242. doi: 10.1016/j.brainresrev.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kucyi A, Salomons TV, Davis KD. Mind wandering away from pain dynamically engages antinociceptive and default mode brain networks. Proc Natl Acad Sci USA. 2013;110(46):18692–18697. doi: 10.1073/pnas.1312902110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gu X, Gao Z, Wang X, et al. Anterior insular cortex is necessary for empathetic pain perception. Brain. 2012;135(Pt 9):2726–2735. doi: 10.1093/brain/aws199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277(5328):968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 81.Noll-Hussong M, Otti A, Wohlschlaeger AM, et al. Neural correlates of deficits in pain-related affective meaning construction in patients with chronic pain disorder. Psychosom Med. 2013;75(2):124–136. doi: 10.1097/PSY.0b013e31827e60f3. [DOI] [PubMed] [Google Scholar]
- 82.Simons LE, Moulton EA, Linnman C, Carpino E, Becerra L, Borsook D. The human amygdala and pain: evidence from neuroimaging. Hum Brain Mapp. 2014;35(2):527–538. doi: 10.1002/hbm.22199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aoki Y, Inokuchi R, Suwa H. Reduced N-acetylaspartate in the hippocampus in patients with fibromyalgia: a meta-analysis. Psychiatry Res. 2013;213(3):242–248. doi: 10.1016/j.pscychresns.2013.03.008. [DOI] [PubMed] [Google Scholar]
- 84.Barke A, Baudewig J, Schmidt-Samoa C, Dechent P, Kroner-Herwig B. Neural correlates of fear of movement in high and low fear-avoidant chronic low back pain patients: an event-related fMRI study. Pain. 2012;153(3):540–552. doi: 10.1016/j.pain.2011.11.012. [DOI] [PubMed] [Google Scholar]
- 85.Nandi D, Liu X, Joint C, Stein J, Aziz T. Thalamic field potentials during deep brain stimulation of periventricular gray in chronic pain. Pain. 2002;97(1–2):47–51. doi: 10.1016/s0304-3959(01)00486-9. [DOI] [PubMed] [Google Scholar]
- 86.Baliki MN, Geha PY, Fields HL, Apkarian AV. Predicting value of pain and analgesia: nucleus accumbens response to noxious stimuli changes in the presence of chronic pain. Neuron. 2010;66(1):149–160. doi: 10.1016/j.neuron.2010.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Becerra L, Morris S, Bazes S, et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J Neurosci. 2006;26(42):10646–10657. doi: 10.1523/JNEUROSCI.2305-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Loggia ML, Berna C, Kim J, et al. Disrupted brain circuitry for pain-related reward/punishment in fibromyalgia. Arthritis Rheumat. 2013;66(1):203–212. doi: 10.1002/art.38191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wood PB. Stress and dopamine: implications for the pathophysiology of chronic widespread pain. Med Hypotheses. 2004;62(3):420–424. doi: 10.1016/j.mehy.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 90.Berman SM, Naliboff BD, Suyenobu B, et al. Reduced brainstem inhibition during anticipated pelvic visceral pain correlates with enhanced brain response to the visceral stimulus in women with irritable bowel syndrome. J Neurosci. 2008;28(2):349–359. doi: 10.1523/JNEUROSCI.2500-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Welch KM, Nagesh V, Aurora SK, Gelman N. Periaqueductal gray matter dysfunction in migraine: cause or the burden of illness? Headache. 2001;41(7):629–637. doi: 10.1046/j.1526-4610.2001.041007629.x. [DOI] [PubMed] [Google Scholar]
- 92.Dunckley P, Wise RG, Fairhurst M, et al. A comparison of visceral and somatic pain processing in the human brainstem using functional magnetic resonance imaging. J Neurosci. 2005;25(32):7333–7341. doi: 10.1523/JNEUROSCI.1100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heinricher MM, Tavares I, Leith JL, Lumb BM. Descending control of nociception: specificity, recruitment and plasticity. Brain Res Rev. 2009;60(1):214–225. doi: 10.1016/j.brainresrev.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nash P, Wiley K, Brown J, et al. Functional magnetic resonance imaging identifies somatotopic organization of nociception in the human spinal cord. Pain. 2013;154(6):776–781. doi: 10.1016/j.pain.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 95.Summers PE, Ferraro D, Duzzi D, Lui F, Iannetti GD, Porro CA. A quantitative comparison of BOLD fMRI responses to noxious and innocuous stimuli in the human spinal cord. Neuroimage. 2010;50(4):1408–1415. doi: 10.1016/j.neuroimage.2010.01.043. [DOI] [PubMed] [Google Scholar]
- 96.Summers PE, Iannetti GD, Porro CA. Functional exploration of the human spinal cord during voluntary movement and somatosensory stimulation. Magn Reson Imaging. 2010;28(8):1216–1224. doi: 10.1016/j.mri.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 97.Fox MD, Snyder AZ, Vincent JL, Corbetta M, Van Essen DC, Raichle ME. The human brain is intrinsically organized into dynamic, anticorrelated functional networks. Proc Natl Acad Sci USA. 2005;102(27):9673–9678. doi: 10.1073/pnas.0504136102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fox MD, Raichle ME. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nat Rev Neurosci. 2007;8(9):700–711. doi: 10.1038/nrn2201. [DOI] [PubMed] [Google Scholar]
- 99.Smith SM, Fox PT, Miller KL, et al. Correspondence of the brain’s functional architecture during activation and rest. Proc Natl Acad Sci USA. 2009;106(31):13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA. 2003;100(1):253–258. doi: 10.1073/pnas.0135058100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Baliki MN, Geha PY, Apkarian AV, Chialvo DR. Beyond feeling: chronic pain hurts the brain, disrupting the default-mode network dynamics. J Neurosci. 2008;28(6):1398–1403. doi: 10.1523/JNEUROSCI.4123-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102••.Napadow V, Lacount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheumat. 2010;62(8):2545–2555. doi: 10.1002/art.27497. Interesting findings of altered resting state functional connectivity in fibromyalgia patients, suggesting that these alterations may underlie chronic pain symptoms. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cifre I, Sitges C, Fraiman D, et al. Disrupted functional connectivity of the pain network in fibromyalgia. Psychosom Med. 2012;74(1):55–62. doi: 10.1097/PSY.0b013e3182408f04. [DOI] [PubMed] [Google Scholar]
- 104.Kim JY, Kim SH, Seo J, et al. Increased power spectral density in resting-state pain-related brain networks in fibromyalgia. Pain. 2013;154(9):1792–1797. doi: 10.1016/j.pain.2013.05.040. [DOI] [PubMed] [Google Scholar]
- 105.Bolwerk A, Seifert F, Maihofner C. Altered resting-state functional connectivity in complex regional pain syndrome. J Pain. 2013;14(10):1107–1115 e1108. doi: 10.1016/j.jpain.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 106.Kilpatrick LA, Kutch JJ, Tillisch K, et al. Alterations in resting state oscillations and connectivity within sensory and motor networks in women with interstitial cystitis/painful bladder syndrome. J Urol. 2014;192(3):947–955. doi: 10.1016/j.juro.2014.03.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Martucci KT, Johnson KA, Bagarinao E, et al. Altered functional connectivity in chronic pelvic pain: dual regression resting state fMRI analysis. Presented at: 19th Annual Meeting of the Organization for Human Brain Mapping; Seattle, WA, USA. Jun, 2013. pp. 16–20. Abstract 1132. [Google Scholar]
- 108.Mainero C, Boshyan J, Hadjikhani N. Altered functional magnetic resonance imaging resting-state connectivity in periaqueductal gray networks in migraine. Ann Neurol. 2011;70(5):838–845. doi: 10.1002/ana.22537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yuan K, Zhao L, Cheng P, et al. Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain. 2013;14(8):836–844. doi: 10.1016/j.jpain.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 110.Schwedt TJ, Larson-Prior L, Coalson RS, et al. Allodynia and descending pain modulation in migraine: a resting state functional connectivity analysis. Pain Med. 2014;15(1):154–165. doi: 10.1111/pme.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cauda F, D’agata F, Sacco K, et al. Altered resting state attentional networks in diabetic neuropathic pain. J Neurol Neurosurg Psychiatry. 2010;81(7):806–811. doi: 10.1136/jnnp.2009.188631. [DOI] [PubMed] [Google Scholar]
- 112.Hashmi JA, Baliki MN, Huang L, et al. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain. 2013;136(Pt 9):2751–2768. doi: 10.1093/brain/awt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Naliboff BD, Berman S, Suyenobu B, et al. Longitudinal change in perceptual and brain activation response to visceral stimuli in irritable bowel syndrome patients. Gastroenterology. 2006;131(2):352–365. doi: 10.1053/j.gastro.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 114.Mansour AR, Baliki MN, Huang L, et al. Brain white matter structural properties predict transition to chronic pain. Pain. 2013;154(10):2160–2168. doi: 10.1016/j.pain.2013.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gwilym SE, Filippini N, Douaud G, Carr AJ, Tracey I. Thalamic atrophy associated with painful osteoarthritis of the hip is reversible after arthroplasty: a longitudinal voxel-based morphometric study. Arthritis Rheumat. 2010;62(10):2930–2940. doi: 10.1002/art.27585. [DOI] [PubMed] [Google Scholar]
- 116.Jensen KB, Kosek E, Wicksell R, et al. Cognitive behavioral therapy increases pain-evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain. 2012;153(7):1495–1503. doi: 10.1016/j.pain.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 117.Christmann C, Koeppe C, Braus DF, Ruf M, Flor H. A simultaneous EEG-fMRI study of painful electric stimulation. Neuroimage. 2007;34(4):1428–1437. doi: 10.1016/j.neuroimage.2006.11.006. [DOI] [PubMed] [Google Scholar]
- 118•.Orru G, Pettersson-Yeo W, Marquand AF, Sartori G, Mechelli A. Using support vector machine to identify imaging biomarkers of neurological and psychiatric disease: a critical review. Neurosci Biobehav Rev. 2012;36(4):1140–1152. doi: 10.1016/j.neubiorev.2012.01.004. Excellent review article of the use of multivariate pattern classification methods in neuroimaging. [DOI] [PubMed] [Google Scholar]
- 119.Westman E, Aguilar C, Muehlboeck JS, Simmons A. Regional magnetic resonance imaging measures for multivariate analysis in Alzheimer’s disease and mild cognitive impairment. Brain Topogr. 2013;26(1):9–23. doi: 10.1007/s10548-012-0246-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120••.Iuculano T, Rosenberg-Lee M, Supekar K, et al. Brain organization underlying superior mathematical abilities in children with autism. Biol Psychiatry. 2014;75(3):223–230. doi: 10.1016/j.biopsych.2013.06.018. Interesting study highlighting the hidden potential of brain-based markers. In this study, brain-based markers were more highly predictive of which students would benefit from tutoring, as compared with several standard testing measures (e.g., IQ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med. 2013;368(15):1388–1397. doi: 10.1056/NEJMoa1204471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kim SH, Lee Y, Lee S, Mun CW. Evaluation of the effectiveness of pregabalin in alleviating pain associated with fibromyalgia: using functional magnetic resonance imaging study. PLoS ONE. 2013;8(9):e74099. doi: 10.1371/journal.pone.0074099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Harris RE, Napadow V, Huggins JP, et al. Pregabalin rectifies aberrant brain chemistry, connectivity, and functional response in chronic pain patients. Anesthesiology. 2013;119(6):1453–1464. doi: 10.1097/ALN.0000000000000017. [DOI] [PubMed] [Google Scholar]
- 124.Decharms RC, Maeda F, Glover GH, et al. Control over brain activation and pain learned by using real-time functional MRI. Proc Natl Acad Sci USA. 2005;102(51):18626–18631. doi: 10.1073/pnas.0505210102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Chapin H, Bagarinao E, Mackey S. Real-time fMRI applied to pain management. Neurosci Lett. 2012;520(2):174–181. doi: 10.1016/j.neulet.2012.02.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Laconte SM, Peltier SJ, Hu XP. Real-time fMRI using brain-state classification. Hum Brain Mapp. 2007;28(10):1033–1044. doi: 10.1002/hbm.20326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Baron R, Backonja MM, Eldridge P, et al. Refractory Chronic Pain Screening Tool (RCPST): a feasibility study to assess practicality and validity of identifying potential neurostimulation candidates. Pain Med. 2014;15(2):281–291. doi: 10.1111/pme.12272. [DOI] [PubMed] [Google Scholar]
- 128.Richardson DE, Akil H. Pain reduction by electrical brain stimulation in man. Part 1: acute administration in periaqueductal and periventricular sites. J Neurosurg. 1977;47(2):178–183. doi: 10.3171/jns.1977.47.2.0178. [DOI] [PubMed] [Google Scholar]
- 129.Schultz DM, Webster L, Kosek P, Dar U, Tan Y, Sun M. Sensor-driven position-adaptive spinal cord stimulation for chronic pain. Pain Phys. 2012;15(1):1–12. [PubMed] [Google Scholar]
- 130.Garcia-Larrea L, Peyron R. Motor cortex stimulation for neuropathic pain: from phenomenology to mechanisms. Neuroimage. 2007;37(Suppl 1):S71–S79. doi: 10.1016/j.neuroimage.2007.05.062. [DOI] [PubMed] [Google Scholar]
- 131.Treister R, Lang M, Klein MM, Oaklander AL. Non-invasive transcranial magnetic stimulation (TMS) of the motor cortex for neuropathic pain-at the tipping point? Rambam Maimonides Med J. 2013;4(4):e0023. doi: 10.5041/RMMJ.10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Moreno-Duarte I, Morse LR, Alam M, Bikson M, Zafonte R, Fregni F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage. 2014;85(Pt 3):1003–1013. doi: 10.1016/j.neuroimage.2013.05.097. [DOI] [PubMed] [Google Scholar]
- 133.Passard A, Attal N, Benadhira R, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain. 2007;130(Pt 10):2661–2670. doi: 10.1093/brain/awm189. [DOI] [PubMed] [Google Scholar]
- 134.Lefaucheur JP, Hatem S, Nineb A, et al. Somatotopic organization of the analgesic effects of motor cortex rTMS in neuropathic pain. Neurology. 2006;67(11):1998–2004. doi: 10.1212/01.wnl.0000247138.85330.88. [DOI] [PubMed] [Google Scholar]
- 135.Tzabazis A, Aparici CM, Rowbotham MC, Schneider MB, Etkin A, Yeomans DC. Shaped magnetic field pulses by multi-coil repetitive transcranial magnetic stimulation (rTMS) differentially modulate anterior cingulate cortex responses and pain in volunteers and fibromyalgia patients. Mol Pain. 2013;9(1):33. doi: 10.1186/1744-8069-9-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Mhalla A, Baudic S, Ciampi De Andrade D, et al. Long-term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain. 2011;152(7):1478–1485. doi: 10.1016/j.pain.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 137.Walter A, Naros N, Roth A, Rosenstiel W, Gharabaghi A, Bogdan M. A brain-computer interface for chronic pain patients using epidural ECoG visual feedback. Presented at: Proceedings of the 2012 IEEE 12th International Conference on Bioinformatics & Bioengineering (BIBE); Larnaca, Cyprus. Nov, 2012. pp. 11–13. [Google Scholar]
- 138••.Maarrawi J, Peyron R, Mertens P, et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain. 2013;154(11):2563–2568. doi: 10.1016/j.pain.2013.07.042. Novel paper which shows how brain-derived measures (e.g., opioid receptor density) can be used to predict efficacy of brain-based therapies. [DOI] [PubMed] [Google Scholar]


