Abstract
Many NK cells express inhibitory receptors that bind self MHC class I molecules and prevent killing of self-cells, while enabling killing of MHC I-deficient cells. But tolerance also occurs for NK cells that lack inhibitory receptors for self MHC I, and for all NK cells in MHC I-deficient animals. In both cases, NK cells are unresponsive to MHC I-deficient cells and hyporesponsive when stimulated through activating receptors, suggesting that hyporesponsiveness is responsible for self tolerance. We generated irradiation chimeras, or carried out adoptive transfers, with WT and/or MHC I-deficient hematopoietic cells in WT or MHC I-deficient C57BL/6 host mice. Unexpectedly, in WT hosts, donor MHC I-deficient hematopoietic cells failed to induce hyporesponsiveness to activating receptor stimulation, but did induce tolerance to MHC I-deficient grafts. Therefore, these two properties of NK cells are separable. Both tolerance and hyporesponsiveness occurred when the host was MHC I-deficient. Interestingly, infections of mice or exposure to inflammatory cytokines reversed the tolerance of NK cells that was induced by MHC I-deficient hematopoietic cells, but not the tolerance induced by MHC I-deficient non-hematopoietic cells. These data have implications for successful bone marrow transplantation, and suggest that tolerance induced by hematopoietic cells versus non-hematopoietic cells may be imposed by distinct mechanisms.
Introduction
An important role of NK cells is to eliminate cells that extinguish or diminish expression of self MHC class I molecules, which commonly occurs as a result of viral infection or cellular transformation (1-5). NK cells recognize MHC I molecules using various inhibitory receptor families including KIRs (in humans), Ly49s (in mice) and CD94/NKG2A (in both humans and mice) (4, 6, 7). When an NK cell encounters a cell with normal MHC I expression, engagement of the inhibitory receptors conveys signals that counteract stimulatory signaling, and therefore the cell is spared. When the target cell lacks one or more self-MHC I molecule, in contrast, inhibitory signaling is diminished and lysis may occur. Lysis occurs because even normal cells often present ligands that engage activating receptors on NK cells, but the intensity of stimulation is typically insufficient to over-ride inhibitory signaling by KIRs or Ly49 receptors. However, other activating ligands are often upregulated on infected or transformed cells, and in some cases are sufficiently potent to over-ride inhibitory signals conveyed by KIRs or Ly49 receptors.
NK cells vary in the number and specificity of MHC I-specific inhibitory receptors they express (4, 6-9). NK cells undergo an education process that depends on the set of MHC I-specific inhibitory receptors expressed by a given NK cell and the MHC molecules expressed in the environment. The education process determines how well the NK cell responds to stimulation by otherwise normal MHC I–deficient cells or to engagement of activating receptors (10-12). Cells with several self MHC I-specific receptors exhibit the greatest basal responsiveness, and mediate the greatest activity against MHC I-deficient cells. NK cells that lack all self MHC I-specific receptors are the least responsive, and fail to attack MHC I-deficient cells. These data suggest that the responsiveness set point of individual NK cells is tuned depending on the balance of inhibitory and stimulatory ligands that each NK cell encounters on other cells in the steady state environment (13, 14).
As one model of NK cell education, NK cells from MHC I-deficient mice have been studied in detail. Such NK cells, which have never encountered MHC I, fail to kill, or reject, cells from MHC-I-deficient mice (2, 15, 16), and also exhibit many other deficient responses, including reduced tumor cell killing (15), reduced antibody-dependent cellular cytotoxicity (17), and lower cytokine responses when stimulated with immobilized antibodies that bind activating receptors (18, 19). The available evidence suggests that signaling pathways that activate NK cells are dampened in such NK cells in a direct or indirect fashion, resulting in poor activation of the cells despite normal amounts of activating receptor occupancy. In these respects, such NK cells are very similar to NK cells in normal MHC I+ mice that lack receptors for self MHC I molecules (18, 19). In both cases, the low responsiveness of the cells to stimulatory receptor activation is thought to help prevent autoreactivity mediated by NK cells despite the absence of inhibitory receptor engagement by MHC I.
The low responsiveness that occurs when NK cells do not encounter MHC I molecules was initially assumed to be the consequence of developmental processes, but we observed that even mature NK cells can be rapidly induced to become hyporesponsive when the cells are transplanted to MHC I-deficient mice (20). Within a few days after transfer the donor NK cells gave much reduced responses when restimulated in vitro, and the reconstituted mice were unable to reject grafts of MHC I-deficient spleen cells. Conversely, when mature NK cells from MHC I-deficient mice were transferred to wild-type mice, the donor NK cells were induced to undergo a significant increase in responsiveness, when tested 7-10 days later (20, 21). These data suggested that NK cell responsiveness is highly plastic, and that mature NK cells can undergo “re-education”, which allows them to re-set their responsiveness thresholds. Thus, the processes that determine NK cell responsiveness allow even mature NK cells to continually adapt to the MHC environment.
Many questions remain concerning the role of MHC I and KIR/Ly49 receptors in NK cell education. One important question is whether NK cell education and re-education are driven by interactions with specialized cell types as opposed to many different cell types. Different aspects of T cell education are known to be driven by distinct cell types, and the knowledge of this has led to a deeper understanding of the molecular mechanisms of positive selection in the thymus and T cell self-tolerance. An equally important issue to be addressed concerns whether the tolerance of NK cells that fail to engage self-MHC I is determined exclusively by the responsiveness set point of NK cells to activating receptor aggregation, or involves other mechanisms as well. In studies thus far, NK cell responsiveness to antibodies that aggregate activating receptors has been well correlated with their capacity to reject MHC-deficient cells, consistent with the conclusion that self-tolerance depends on low responsiveness. If exceptions exist, it would suggest that multiple mechanisms may be responsible for self-tolerance.
In this study, we attempted to define the cell compartment that is responsible for induction of NK cell tolerance, and for induction of low responsiveness of NK cells to activating receptor stimuli. We demonstrate that hematopoietic cells that lack expression of MHC class I induce tolerance to MHC-deficient target cells but fail to induce hyporesponsiveness to activating receptor stimulation. In contrast, MHC I-negative non-hematopoietic cells induce unresponsiveness to target cells as well as hyporesponsiveness to stimulatory receptor engagement. Notably, tolerance imparted by MHC I-deficient hematopoietic cells (i.e. associated with high responsiveness to receptor stimulation) can be broken in the presence of infection or treatment with inflammatory cytokines. In contrast, tolerance imparted by MHC I-deficient non-hematopoietic cells (i.e. associated with hyporesponsiveness to receptor stimulation) is quite refractory to inflammatory signals. These results suggest that multiple mechanisms of tolerance may exist and furthermore work together to shape NK cell functionality depending on the state of the immune environment.
Materials and Methods
Mice
Mice were bred at the University of CA, Berkeley from breeders obtained from Jackson Laboratories (C57BL/6J), Charles River (B6-Ly5.2/Cr, which express Ly5.1), W. Yokoyama (Washington University in St. Louis, St. Louis, MO; NKD mice) and Dr. Lewis Lanier (University of CA, San Francisco; Raet1e transgenic mice (22)). B2m−/− mice (23) on the B6 background (B6-B2m−/−) had been backcrossed more than 10 times to B6. B6 B2m−/−-Ly5.1 mice were bred in our facilities.
Antibodies and flow cytometry analysis
The following antibodies were purchased from eBioscience: anti-NK1.1 (PK136), anti-CD45.1 (A20), anti-CD45.2 (104), anti-H-2Db (28-14-8), anti-CD107a (1D4B), anti-IFN-γ (XMG1.2), anti-NKG2D (MI-6, (24)), anti-H-2Kb (AF6-88.5), and anti-CD107a (1D4B), anti-FoxP3 (150D/E4), and anti-CD226 (10E5). From Biolegend we purchased anti-CD3-ε (145-2C11), and anti-NKp46 (29A1.4). From R&D systems we purchased anti-pan-RAE-1 (199215).
Biotin-conjugated mAbs were detected with streptavidin Pacific Blue (Invitrogen) or streptavidin Brilliant Violet 421 (BioLegend). Before staining, cells were preincubated for 20 min with 2.4G2 hybridoma supernatant to block FcγRII/III receptors. Flow cytometry was performed on a cytometer (LSR II, LSR Fortessa, or LSR Fortessa X-20, BD Biosciences), and data were analyzed with the FlowJo software (Tree Star, Inc.).
Where applicable, donor cells were gated based on the expression of a congenic CD45 molecule. NK cells were defined as CD3−NK1.1+ or CD3−NKp46+ cells.
In-vitro NK stimulation assay
Stimulation assays of spleen cells were performed using flat-bottom, high-binding 96-well plates (Thermo Fisher) pre-coated with the relevant antibody: MI6 specific for NKG2D (24), eBio244F4 specific for 2B4 (eBioscience), 13G3-19D specific for Ly108 (eBioscience) and 29A1.4 specific for NKp46 (eBioscience). Plates were coated by applying 100 μl of the indicated antibody diluted in PBS, and incubating overnight at 4°C. Prior to stimulation, the plates were washed 4 times with 200 μl of PBS/well. Wells coated with isotype control antibody or PBS (as indicated) served as controls. 1×106 splenocytes/well were stimulated for 5 hours in the presence of 1 μg/ml of Brefeldin A and 1000 U/ml of recombinant human IL-2 (from the National Cancer Institute, to optimize viability). In assays where intracellular IFN-γ and degranulation (i.e. cell surface display of CD107a) were simultaneously analyzed, 1 μg/ml of monensin was also added to the samples. In some experiments, where indicated, mice were pre-treated with 200 μg of HMW poly(I:C) (Invivogen cat # tlrl-pic) IP 24-36 hours prior to collecting spleen cells for assay.
Spleen cell rejection assay in vivo
Spleen cells from B2m−/− or Raet1e-transgenic mice were labeled with 10 μM CFSE for 10 minutes at 37°C. For use as an internal control, WT B6 splenocytes were similarly labeled with 1 μM CFSE. A mixture of 5×106 cells of each genotype were injected intravenously into recipient mice. Graft rejection was assessed 18-42 hours later (specified in Figure Legends) by harvesting recipient spleens and determining the percentages of CFSEhi and CFSElo cells among CFSE+ cells by flow cytometry. Percent graft rejection was defined as: 100 × [1−(%B2m−/− cells/ %WT cells)] after normalizing to the mean rejection obtained when B2m−/− mice were challenged with the same mixture. When rejection of Raet1e-transgenic splenocytes was measured, the data were normalized to the starting percentages, determined by flow cytometry after mixing the cells.
Adoptive Transfers
WT or B2m−/− splenocytes (containing 2-2.5×6 NK cells) were injected intravenously into mice that had received 6 Gy of irradiation from a 137Cs source 4-5 hours prior. Adoptive transfer recipients were kept on water containing antibiotics (Sulfamethoxazole and Trimethoprim) following irradiation until the end of the experiment.
Radiation chimeras
Chimera hosts were irradiated with either a 137Cs source or using an X-ray irradiator. For the 137Cs source, mice received 9 Gy 4-5 hours before injecting fetal liver cells. For the X-ray irradiator, mice received a split dose of 6 Gy 16 hours before receiving a second dose of 4 Gy, which was followed 3-4 hours later by the fetal liver cell injection. Each recipient received 1 × 107 donor fetal liver cells from CD45-congenic, embryonic day 14-17 embryos. For mixed chimeras, 1 × 107 fetal liver cells of each genotype were coinjected. WT hosts that were to receive B2m−/− donor cells were pretreated with 200 μg of anti-NK1.1 antibody (PK136, purified in-house) i.p. 2 days prior to reconstitution, in order to deplete NK cells and prevent rejection of B2m−/− donor cells. Chimeras were kept on antibiotic (Sulfamethoxazole and Trimethoprim) – containing water for at least 8 weeks following irradiation and reconstitution. Chimeras were analyzed 13-22 weeks post reconstitution.
Infections
MCMV
Mice were infected intraperitoneally with 1 − 104 PFU of 3rd passage salivary gland-derived MCMV in 100-200 μl of PBS. For preparation of salivary gland MCMV, 4-week-old BALB/c female mice were infected with 1 × 103 PFU of tissue-culture derived MCMV (obtained from ATCC). 14-17 days later, salivary glands were harvested, dissociated using the gentleMACS device (Miltenyi biotec), and sonicated 5 times, alternating between 30 seconds of sonication and 30 seconds of incubation at 4°C. The resulting viral extract was filtered through a 0.4 μM filter and used to infect a second group of naïve BALB/c mice (1 × 103 PFU was used). After this amplification step, the salivary gland virus was prepared again after 14-17 days and used for another round of infection (for a total of 3 passages).
Listeria
Mice were infected intraperitoneally with 1 × 104 CFU of WT Listeria monocytogenes.
In-vivo cytokine treatment
Mice were injected intraperitoneally every 2 days with the indicated cytokines, for a total of 3 injections. The cytokine doses used are indicated in the Figure Legends. Recombinant murine IL-12 (rmIL-12, cat # 210-12) and IL-15 (rmIL-15, cat # 210-15) were purchased from Peprotech. Recombinant murine IL18 (rmIL-18, cat # B004) and IL-15Rα (rm IL-15Rα, cat # 551-MR) were purchased from R&D systems. IL-15 and IL-15Rα were pre-complexed by co-incubation at 37°C for 30 minutes at a concentration of 5 ug/ml of IL-15 and 30 ug/ml of IL-15Rα.
Results
Cell types determining responsiveness and tolerance of NK cells in fetal liver chimeras
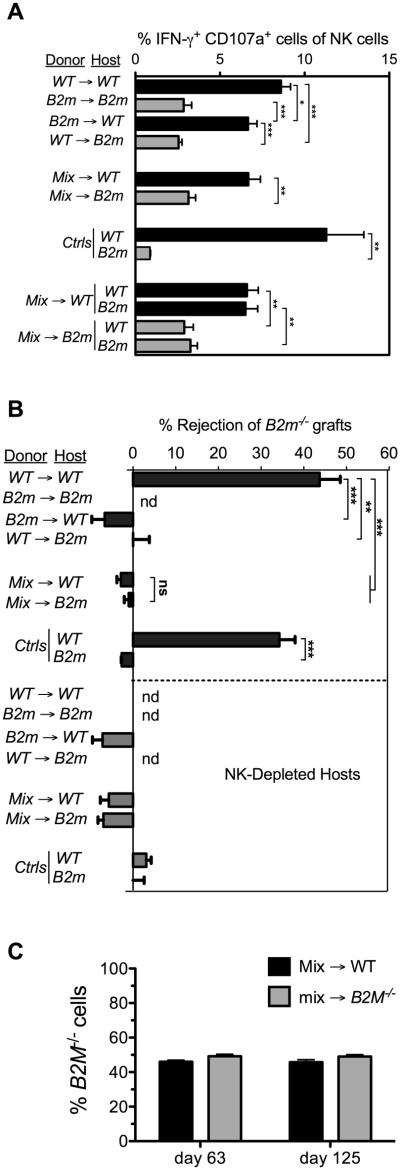
We employed fetal liver irradiation chimeras as a means to distinguish the roles of non-hematopoietic versus hematopoietic cells in educating NK cells. We generated chimeras in which wild-type (WT) or MHC I-deficient (B2m−/−) NK cells developed from hematopoietic precursor cells in B2m−/− or WT hosts. This protocol allowed us to determine the origin (hematopoietic versus non-hematopoietic) of cells that interact with NK cells and regulate their responsiveness and tolerance. The donor cells differed from the host in the allele of CD45 expressed, allowing us to identify the genotype of the NK cells. NK cell responsiveness was analyzed 13-20 weeks following reconstitution with fetal liver cells, by stimulating splenic NK cells from the chimeras with plate-bound antibodies against different activating NK receptors followed by staining for CD107a to assess degranulation, and intracellular staining of IFN-γ to assess cytokine production. The responsiveness of the NK cells from each type of chimera in these assays was compared to the capacity of similar chimeras to reject spleen cell grafts from B2m−/− mice in vivo.
The results indicated that WT NK cells that developed in MHC I-deficient hosts were hyporesponsive to immobilized NKG2D antibody (Fig. 1A). In fact, these NK cells (WT→B2m−/− NK cells) were as hyporesponsive as NK cells from control B2m−/−→B2m−/− chimeras. In comparison, WT→WT NK cells gave substantially stronger responses. Interestingly, however, B2m−/− NK cells that developed in WT hosts (from B2m−/−→WT chimeras) gave high responses to plate bound antibody stimulation. These findings were replicated in 5 independent experiments. The responsiveness of B2m−/−→WT NK cells, in terms of the percentage of responding NK cells, was only slightly lower than that observed with NK cells from control WT→WT chimeras. Therefore, despite the prevalence of B2m−/− cells among hematopoietic cells in the B2m−/−→WT chimeras, the NK cells attained a relatively high level of responsiveness to activating receptor stimuli.
Figure 1. Responsiveness of NK cells in fetal liver chimeras to activating receptor triggering and MHC I-deficient target cells in fetal liver chimeras.
Lethally irradiated and NK-depleted hosts were reconstituted with WT, B2m−/−, or a 1:1 mix of WT and B2m−/− fetal liver cells. Black bars indicate WT hosts; gray bars indicate B2m−/− hosts. Data represent means ± SEM. (A) Responsiveness of splenic NK cells from chimeras was tested 13 weeks after reconstitution, by stimulating for 5 h with 5μg/ml of plate-bound anti-NKG2D antibody (MI6 clone). The percentages of donor NK cells co-expressing intracellular IFN-γ and surface CD107a were determined by flow cytometry. In analyzing mix→ WT and mix→B2m−/− chimeras, we gated on the congenic CD45 markers and MHC I during the analysis to determine the separate responses of WT and B2m−/− NK cells. Data are representative of 5 independent experiments performed 13-21 weeks after reconstitution. (B) Rejection of B2m−/− spleen cell grafts by chimeras 9–17 weeks after reconstitution. Rejection of CFSE-labeled B2m−/− spleen cells, mixed with internal control B6 spleen cells, was determined by flow cytometry of spleen cells 18 h after injection of the cells. Unmanipulated B6 and B2m−/− mice were tested in parallel as controls for rejection and tolerance, respectively. To establish the role of NK cells, some groups of chimeras were pretreated intraperitoneally twice (on days -2 and -1) with 200 μg PK136 (NK1.1) antibody (NK depleted) in PBS, as indicated. n = 3-4 in all groups except NK-depleted B6 controls, where n=2. Data are representative of 3 independent experiments. nd=not done. (C) Chimerism was determined on the days shown by flow cytometry of PBLs stained for H-2Kb (in this experiment), or the congenic CD45 marker (in experiments not shown), 63 and 125 days following reconstitution. Statistical significance was determined with a two-tailed unpaired Student’s t test (*p < 0.05, **p < 0.005, ***p < 0.0005).
To isolate the impact on NK cell education of non-hematopoietic cells we created chimeras in which WT or B2m−/− hosts were reconstituted with a mixture of WT and B2m−/− hematopoietic cells. Both types of mixed chimeras contained roughly 50% B2m−/− cells and 50% WT cells and there was little change in these fractions over a period of months (Fig. 1C; also evident in Fig. 5A, below). Notably, NK cells from mix→WT chimeras produced high responses whereas NK cells from mix→B2m−/− chimeras produced low responses, demonstrating that radioresistant host non-hematopoietic cells determine the responsiveness of NK cells in the chimeras (Fig. 1A). The responsiveness of NK cells from mix→WT chimeras was again only slightly lower than that observed with control WT→WT NK cells. This small difference pertained even when the stimulatory antibody was present at limiting concentrations (Supplemental Fig. 1), arguing against a threshold effect. When gating on the responding NK cells, the intensity of staining of activated NK cells with anti-IFN-γ or anti-CD107a differed very little in the different chimeras (Supplemental Fig. 2), consistent with previous findings that NK cell education primarily impacts the probability that an NK cell will trigger, but not the magnitude of its individual response. Thus, the presence of B2m−/− cells in the hematopoietic cell mixture had only a minor effect on the responsiveness of NK cells from the chimeras, whereas host non-hematopoietic cells had a substantial impact.
Figure 5. Infections break self-tolerance to MHC I-deficient donor cells in mix→WT chimeras but not in mix→B2m−/− chimeras.
(A) The chimerism of peripheral blood cells (expressed as percentage of blood hematopoietic cells from the B2m−/− donor) was stable in mixed chimeras until infection with MCMV on day 156. MCMV infection caused an abrupt loss of B2m−/− donor cells in mix→WT chimeras but not in mix→B2m−/− chimeras. Loss of chimerism stabilized 7 days after infection, around the time virus is expected to be cleared in the acute infection. Chimerism was determined by staining peripheral blood cells with H-2Kb antibody. n=3-4. Data in panel A are representative of 3 independent experiments. (B) Data in panel A calculated to show % loss of Kb+ cells on each day after infection with MCMV. The percentage loss of Kb+ cells was calculated as 100 × [1−(%Kb-negative cells at a given timepoint/%Kb-negative cells on day 0)]. (C) 13 weeks after reconstitution, chimeras were infected with Listeria monocytogenes. Peripheral blood B2m−/− cells were lost preferentially from mix→WT chimeras in comparison to mix→B2m−/− chimeras. % loss of chimerism is shown. Data are representative of 2 independent experiments. Data represent means ± SEM. N=3-4. Statistical significance (comparing mix→WT with mix→B2m−/−) was determined with a two-tailed unpaired Student’s t test (*p < 0.05, **p < 0.005, ***p < 0.0005).
An important issue is whether rejection of MHC I-deficient cells is always correlated with low responsiveness to stimulation via activating receptors. The pattern of rejection of B2m−/− spleen cell grafts by chimeras, which has been investigated previously in the case of both spleen cell and bone marrow cell grafts, is shown for comparison (Fig. 1B and (20, 25)). Strikingly, this pattern differed from the pattern of responsiveness in one critical respect. Whereas the WT→B2m−/− and mix→B2m−/− chimeras failed to reject the grafts, consistent with the low responsiveness of their NK cells after stimulation through activating receptors, the B2m−/−→WT and mix→WT chimeras also failed to reject the grafts, despite the high responsiveness of their NK cells (Fig. 1B). These data showed that unresponsiveness to B2m−/− cells can occur despite the high responsiveness of the NK cells to activating receptor aggregation. As expected, the rejection of B2m−/− spleen cell grafts by WT mice was entirely NK cell-dependent, as it was abrogated when the mice were pretreated with NK1.1 antibody to deplete NK cells (Fig. 1B). Finally, the differences in the chimeras in graft rejection or responsiveness cannot be attributed to alterations in the Treg/NK cell ratios, which were similar in the different chimeras (Supplemental Fig. 3).
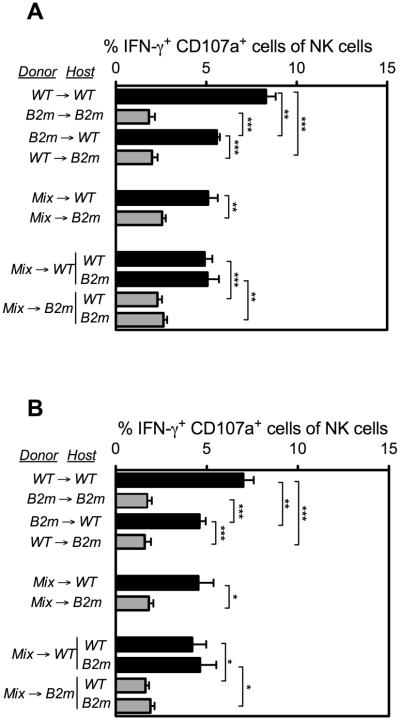
SLAM family receptor signaling is essential for responses of NK cells to MHC I-deficient hematopoietic cell grafts, and is therefore directly relevant to rejection of MHC I-deficient spleen cell grafts (26). The pattern of NK cell responsiveness observed in chimeras with immobilized NKG2D antibodies was replicated when the cells were stimulated with immobilized antibodies specific for other receptors, including two different SLAM family receptors, 2B4 and Ly108 (Fig. 2). Most notably, NK cells from B2m−/−→WT and mix→WT chimeras exhibited high responsiveness to SLAM family receptor crosslinking with antibodies (Fig. 2), despite the finding that these chimeras were unresponsive to B2m−/− spleen cell (and bone marrow cell, data not shown) grafts (Fig. 1B). Therefore, tolerance induced by MHC I-deficient hematopoietic cells is not associated with hyporesponsiveness to stimulation via SLAM family receptors.
Figure 2. Responsiveness of NK cells in fetal liver chimeric mice to antibody-mediated stimulation of SLAM-family activating receptors.
Fetal liver chimeras were generated as described in Figure 1. Responsiveness of splenic NK cells from chimeras, tested 13 weeks after reconstitution, to stimulation for 5 h with 5μg/ml of plate-bound (A) anti-2B4 or (B) anti-Ly108 antibodies. The percentages of donor NK cells co-expressing IFN-γ and CD107a were determined by flow cytometry. Black bars indicate WT hosts; gray bars indicate B2m−/− hosts. Data represent means ± SEM. Data are representative of 3 independent experiments performed 13-21 weeks after reconstitution. For some comparisons, statistical significance was determined with a two-tailed unpaired Student’s t test (*p < 0.05, **p < 0.005, ***p < 0.0005).
In contrast to the high responsiveness of NK cells from B2m−/−→WT and mix→WT chimeras when activating receptors were aggregated, these chimeras failed to reject B2m−/− spleen cell grafts (Fig. 1B, and (20, 25). These data confirmed that B2m−/− cells of hematopoietic origin are capable of inducing tolerance to B2m−/− cell grafts. Thus, B2m−/−→WT and mix→WT chimeras represent situations where NK cells exhibit a form of tolerance with unresponsiveness to MHC I-deficient grafts despite high responsiveness to activating receptor stimuli.
Hyporesponsiveness of NK cells is imparted in trans by MHC-deficient non-hematopoietic cells
It was possible to separately test the responsiveness of WT and B2m−/− NK cells from the mixed chimeras by gating the two populations based on congenic markers and MHC I expression. The results showed that both WT and B2m−/− NK were hyporesponsive in the mix→B2m−/− chimeras, when tested by stimulating the cells with plate-bound antibodies for NKG2D (Fig. 1A), 2B4 (Fig. 2A) or Ly108 (Fig. 2B). Conversely, both WT and B2m−/− NK were similarly responsive in the mix→WT chimeras (Fig. 1A, 2A,B). Therefore, the responsiveness of NK cells in the mixed chimeras was independent of MHC I expression by the NK cells themselves or by hematopoietic cells generally, but was instead determined in trans by the MHC I expressed on host non-hematopoietic cells.
Cell types determining responsiveness of adoptively transferred NK cells
We previously reported that when mature NK cells are transferred to hosts that differ in MHC I expression, the transferred NK cells rapidly re-set their responsiveness based on the new environment (20, 21). Thus, WT NK cells transferred to B2m−/− hosts were induced to become hyporesponsive to stimulation through the NKG2D and NKR-P1C activating receptors (20), whereas B2m−/− NK cells transferred to WT hosts were induced to become responsive to these stimuli (20, 21).
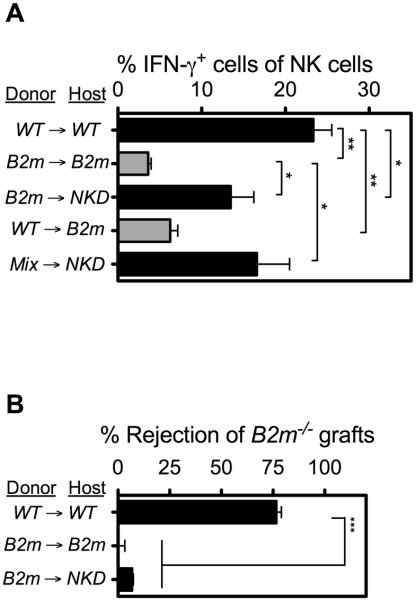
We corroborated and extended those earlier results by showing that WT NK cells transferred to B2m−/− adoptive transfer recipients exhibited low responsive 10 days later when stimulated with immobilized antibodies specific for a distinct activating receptor, NKp46 (Fig. 3A). We also examined responses of B2m−/− NK cells transferred to MHC I+ recipients. In order to prevent the donor cells from being rejected by host NK cells in these transfers, we used as recipients NKD mice, which are NK-deficient due to a transgene insertion (27, 28). Donor NK cells from B2m−/−→NKD or mix→NKD adoptive transfer recipients exhibited high responsiveness to NKp46 stimulation (Fig. 3A). To assess the impact of adoptive transfer on the reactivity of NK cells to MHC I-deficient cells, we determined whether similar adoptive transfer recipients were capable of rejecting B2m−/− spleen cell grafts. As shown in Fig. 3B, MHC I+ (NKD) hosts reconstituted with B2m−/− donor cells failed to reject B2m−/− spleen cell grafts (Fig. 3B). Control WT hosts reconstituted with donor WT cells potently rejected the grafts whereas B2m−/− hosts reconstituted with donor B2m−/− cells did not, as expected. We previously reported that B2m−/− hosts reconstituted with WT donor cells also failed to reject B2m−/− spleen cell grafts (20). Therefore both chimera and adoptive transfer studies show that NK cells exposed concurrently in vivo to WT non-hematopoietic cells and B2m−/− hematopoietic cells were responsive to activating receptor stimuli yet unresponsive to B2m−/− hematopoietic cell grafts. To the contrary, NK cells exposed in vivo to B2m−/− non-hematopoietic cells and WT hematopoietic cells were hyporesponsive to both activating receptor triggering and B2m−/− hematopoietic cell grafts.
Figure 3. Responsiveness of adoptively transferred NK cells to activating receptor triggering and MHC I-deficient target cells.
Spleen cells containing mature NK cells from the donor strains shown were transferred to the indicated irradiated hosts. Black bars indicate WT or NKD hosts; gray bars indicate B2m−/− hosts. (A) 10 d after transfer, the responsiveness of splenic NK cells was determined after stimulating the cells for 5 h with 5 μg/ml of plate-bound NKp46 antibody. The percentages of donor NK cells expressing IFN-γ were determined by flow cytometry. Data are representative of 3 independent experiments. (B) 10 days after transfer, groups of adoptive transfer recipients (n = 3–4) were tested for rejection of CFSE-labeled B2m−/− spleen cell grafts, mixed with internal control B6 spleen cells, 18 hrs after injection of the cells. Data are representative of 3 independent experiments, with n = 3–4 mice for each. For relevant comparisons, statistical significance was determined with a two-tailed unpaired Student’s t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005). Data represent means ± SEM.
Cell types in chimeras determining responsiveness of NK cells in vivo to target cells displaying NKG2D ligands
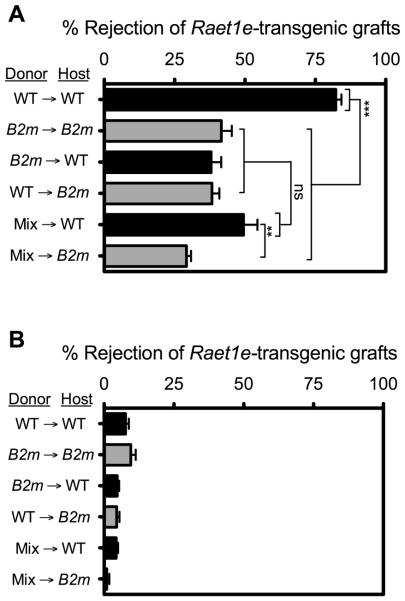
Having assessed the impact of host environment on the responsiveness of NK cells to activating receptor aggregation with plate-bound antibodies, we determined whether responses to target cells expressing activating ligands were similarly affected. This issue was examined by determining the capacity of NK cells in the various chimeras to reject Raet1e-transgenic spleen cells. These transgenic spleen cells express the NKG2D ligand RAE-1ε (22), which is absent from normal spleen cells. In preliminary experiments, we observed that rejection of Raet1e-transgenic spleen cells by non-chimeric WT mice, or by any of the chimeras, did not occur significantly unless the mice were pre-treated with poly(I:C), which enhances NK responses in other contexts as well (compare Fig. 4A and B). When pretreated with poly(I:C), WT→WT chimeras rejected Raet1e-transgenic spleen cells, whereas B2m−/−→B2m−/− , WT→B2m−/−, and mix→B2m−/− chimeras rejected the Raet1e-transgenic spleen cells substantially less efficiently, as expected (Fig. 4A). Surprisingly, chimeras prepared with B2m−/− hematopoietic cells in WT hosts (i.e. B2m−/−→WT or mix→WT chimeras) were similarly defective in rejecting the Raet1e-transgenic spleen cells (Fig. 4A), despite harboring responsive NK cells as tested by stimulating with antibodies against NKG2D (Fig. 1A), the same receptor that RAE-1ε engages. Therefore, the presence of B2m−/− cells in either the hematopoietic or non-hematopoietic compartments of fetal liver chimeras resulted in deficient responses to Raet1e-transgenic spleen cell grafts, mimicking the lack of reactivity towards B2m−/− hematopoietic cell grafts. Thus, defective responses to Raet1e-expressing cells can occur even when the cells exhibit high responses against plate-bound NKG2D antibodies.
Figure 4. Rejection of spleen cells expressing NK-activating ligand RAE-1ε by fetal liver chimeras.
Chimeras were generated as described in Figure 1. 20 weeks post-reconstitution, chimeras were challenged with CFSE-labeled grafts of spleen cells from Raet1e-transgenic mice (22) mixed with internal control B6 spleen cells. Rejection was tested by flow cytometry 42 hours later. Mice were either pretreated i.p. (on day −1 relative to the time of engraftment) with 200 μg poly(I:C) to enhance NK responses (A), or left untreated (B). Data in panel A are representative of 3 independent experiments performed 13-21 weeks after reconstitution with n = 3-5 mice for each. Panel B was performed only once. Black bars indicate WT hosts; gray bars indicate B2m−/− hosts. Data represent means ± SEM. For some comparisons, statistical significance was determined with a two-tailed unpaired Student’s t test (**p < 0.005, ***p < 0.0005).
Impact of infections and cytokines on NK cell tolerance
It was previously reported that MCMV infection breaks tolerance of NK cells in mix→WT chimeras, resulting in elimination of B2m−/− cells (29). Therefore, we next addressed the impact of immune system activation associated with infections on the maintenance of self-tolerance to MHC I-deficient donor cells in chimeras, and whether the stability of self-tolerance correlated with the in vitro hyporesponsiveness of NK cells. Mix→WT and mix→B2m−/− chimeras were compared, and loss of tolerance was inferred from the disappearance of B2m−/− cells in the blood after initiating the infection. As mentioned previously, hematopoietic cell chimerism was consistently maintained long term in both types of chimeras if they were not further manipulated (note sustained chimerism between days 60 and 157 in Fig. 5A). Infection of mix→WT chimeras with mouse cytomegalovirus (MCMV) resulted in a rapid 30-40% decrease in the representation of B2m−/− cells among blood cells in the chimeras (Fig. 5A, B). After 7-8 days, at which time the acute MCMV infection is typically resolved, the populations stabilized and remained more or less constant for the remainder of the experiment. These data suggested that elimination of B2m−/− hematopoietic cells in the chimeras only occurs during the acute stage of infection when inflammation is ongoing, and ceases once inflammation subsides. Strikingly, however, MCMV infection of mix→B2m−/− chimeras, prepared and tested in parallel, had little or no effect on chimerism (Fig. 5A, B). These data indicated that NK cell self-tolerance in mix→B2m−/− chimeras is refractory to inflammation associated with MCMV infection.
MCMV induces inflammatory cytokines and also causes NK cell activation through expression in infected cells of a virus-encoded cell surface ligand for the NK cell activating receptor Ly49H. Infections with the gram+ bacterial pathogen Listeria monocytogenes also induce various inflammatory cytokines but are not known to induce expression in infected cells of activating ligands for NK receptors. Similar to infections with MCMV, infections with Listeria resulted in a depletion of approximately 50% of the B2m−/− donor cells among blood cells in the mix→WT chimeras, which ceased after 5-10 days (Fig. 5C). Substantially less (though still significant) depletion occurred in infected mix→B2m−/− chimeras. These data support the conclusion that tolerance associated with high responsiveness of NK cells to activating receptor stimuli can be readily broken upon infection, but tolerance associated with low responsiveness to activating receptor stimuli, as in mix→B2m−/− chimeras, is considerably more refractory to such effects.
MCMV and Listeria infections are accompanied by production of large amounts of inflammatory cytokines. We therefore tested the possibility that provision of cytokines that activate NK cells, in the absence of infections, could also break tolerance. Injections every other day of a mixture of IL-12, IL-18 and IL-15 (the latter pre-complexed with IL-15Rα protein in vitro, to effect trans-presentation of IL-15) (30), resulted in a depletion of up to 80% of the B2m−/− donor cells in mix→WT chimeras (Fig. 6A). In contrast, the same doses of IL-12 + IL-18 alone, or IL-15 alone, had almost no effect compared to mock-treated chimeras, indicating that these cytokines synergistically break NK cell tolerance. By itself, however, a higher dose of IL-15 had nearly the same effect as the mixture of the three cytokines (Fig. 6A). The depletion of B2m−/− donor cells in cytokine-treated chimeras, compared to mock-treated chimeras, was abolished if the mice were pretreated with NK1.1 antibody to deplete NK cells before application of the cytokines (Fig. 6A). These data indicated that high doses of IL-15, or lower doses of IL-15 in combination with IL-12+IL-18, break NK cell self-tolerance in mix→WT chimeras, resulting in depletion of B2m−/− donor cells. The cytokine mixture did not cause increases in NKp46, NKG2D or NK1.1 activating receptor expression on NK cells in either WT or B2m−/− mice, suggesting that any changes in NK activity in vivo are unlikely to be mediated by increases in expression of these activating receptors (Supplemental Figure 4).
Figure 6. Treatments with pro-inflammatory cytokines break self tolerance of NK cells to B2m-deficient donor cells in mix→WT chimeras but in not mix→B2m−/− chimeras.
(A, B) % loss of B2m−/− cells in chimeras treated on days 0, 2 and 4 with the cytokine mixtures shown. In panel A, mix→WT chimeras were analyzed, and in panel B, mix→WT and mix→B2m−/− chimeras were compared. The cytokine mix group in panel A was also tested in mice that were depleted of NK cells by injecting 200 μg PK136 antibody one day prior to and one day after the first cytokine treatment. The IL-12+IL-18+IL15 cytokine mixture in both panels consisted of 200 ng each IL-12 and IL-18 plus a pre-complexed low dose of IL-15 + IL15Rα (2 μg + 0.6 μg each/mouse, respectively). The corresponding doses of IL-12+IL-18, or low dose IL-15 alone were also tested. In addition, a high dose of IL-15 + IL15Rα (1 μg + 3 μg each/mouse, respectively) was tested. N=5 mice in each group. Data represent means ± SEM. Data are representative of 3 independent experiments comparing different cytokine treatments, and 2 independent experiments comparing cytokine-treated mix→WT and mix→B2m−/− groups. Statistical significance comparisons in panel A were determined with a Dunnett’s multiple comparison test (*, P < 0.05; **, P < 0.01; ***, P < 0.001). In panel B, the mix, cytokine-treated group was compared with the mix→B2m−/− cytokine treated group with a two-tailed unpaired Student’s t test (*, P < 0.05; **, P < 0.005; ***, P < 0.0005).
In contrast to the results in mix→WT chimeras, the IL-12+IL-18+IL-15 mixture failed to cause significant depletion of B2m−/− donor cells in mix→B2m−/− chimeras (Fig. 6B), consistent with the infection results (Fig. 5). Together, these findings indicated that self-tolerance of NK cells in mix→WT chimeras is readily reversed as a result of inflammation induced by cytokines or associated with viral or bacterial infections, whereas self-tolerance in mix→B2m−/− chimeras is relatively refractory to such inflammation. These data support the hypothesis that tolerance that is accompanied by high responsiveness to plate-bound activating receptor antibodies (as in mix→WT chimeras) is more fragile than tolerance accompanied by low responsiveness (as in mix→ B2m−/− chimeras).
Discussion
Differential responsiveness induced by non-hematopoietic and hematopoietic cells
One of the most significant findings of this paper is that the absence of MHC I ligands on non-hematopoietic cells results in low NK cell responsiveness to activating receptor stimuli, whereas in contrast, the absence of MHC I ligands solely on hematopoietic cells does not lead to hyporesponsiveness to activating receptor engagement. In both cases, the NK cells lack reactivity against MHC I-deficient spleen cell grafts, which demonstrates that tolerance does not solely rely on the lack of responsiveness of NK cells to activation receptor stimuli. These findings suggest that encounters with non-hematopoietic cells plays the principal role in determining the responsiveness “set-point” of NK cells to activating receptor stimuli. Although the identity of the specific non-hematopoietic cell types responsible for imparting high vs low responsiveness is not known, it is tempting to speculate that they are the same cell types that aid in the differentiation of NK cells. For example, they may correspond to the non-hematopoietic cells that produce the cytokine IL-15 which is required for NK cell development and survival (31-34), or possibly to the non-hematopoietic cell types that express ligands for the Tyro3 family of tyrosine kinase receptors, which have been shown to be necessary for NK cell maturation and the acquisition of functional activity (35, 36).
The data are equally compatible with two proposals that differ in certain predictions: one is that encounters of NK cells with B2m−/− non-hematopoietic cells induce low responsiveness, and the other is that encounters with WT non-hematopoietic cells are necessary to induce high responsiveness. Distinguishing between these proposals will require generating animals in which the relevant non-hematopoietic cells are a mixture of WT and B2m−/− cells.
Our data conflict with a recent study by Ebihara et al (37) where the authors utilized an inducible MHC class I transgene to address the roles of MHC expression on non-hematopoietic and hematopoietic cells in NK cell education. This study suggested that MHC I expression on hematopoietic cells, but not non-hematopoietic cells, is critical for inducing responsiveness. One possible explanation for this discrepancy is that the transgene was not expressed on the relevant non-hematopoietic cell types. Another possibility is that the interaction between the transgene-encoded MHC molecule used (a covalently linked peptide-MHC complex) and the cognate inhibitory receptor (Ly49C in this case) does not adequately represent the interactions between cells expressing WT MHC and NK cells. Further studies addressing the in-vivo interactions between NK cells and the “educating” cells are required to address these possibilities.
Beyond our findings concerning the impact of different cell types in imparting responsiveness of NK cells to activating receptor stimuli, our data, as well as previously published data, investigated the impact of these cell types in imparting NK cell self tolerance. The results show that the absence of MHC I ligands on either hematopoietic cells or non-hematopoietic cells results in lack of reactivity against MHC I-deficient spleen cell grafts (20, 25, 38), and low rejection of Raet1e-transgenic spleen cell grafts (this paper). As previously noted, the finding that unresponsiveness to B2m−/− grafts occurs in mix→WT chimeras but not WT→WT chimeras demonstrates that B2m−/− hematopoietic cells actively induce tolerance to B2m−/− grafts. Mix→WT chimeras also were relatively deficient in rejecting Raet1e-transgenic spleen cell grafts, indicating that the impact of interactions with B2m−/− hematopoietic cells extended to responses to cells displaying NKG2D ligands.
Published studies have emphasized the role of hematopoietic cells in educating NK cells especially in the context of anti-tumor responses. Hence, donor allogeneic NK cells transferred with bone marrow grafts are tolerant to healthy host cells but reactive against host leukemic cells (39). These findings do not necessarily conflict with the present results, given that they represent the activity of NK cells in the context of a tumor, where inflammation is ongoing and where the tumor may express strong activating ligands for NK cells (40). With respect to NK cell responsiveness, one study reported that long after transplantation, high NK cell responsiveness was determined by MHC molecules expressed by donor cells (41). In contrast, another report arrived at the conclusion that recipient MHC molecules can confer high responsiveness after marrow transplantation in humans (42), consistent with our results. Additional studies to address these issues are warranted.
Two distinct types of tolerance
Our findings are significant in light of previous studies that have reported that NK cell tolerance to MHC I – deficient grafts is generally correlated with hyporesponsiveness to stimulation with plate-bound activating receptor antibodies (10, 11, 18, 19). Based on these findings, it has been generally assumed that self-tolerance is accounted for by the reduced responsiveness of the NK cells (43-45). As far as we know, our new data are the first demonstration that responsiveness and tolerance can in some instances be separated. NK cells in mix→WT or B2m−/−→WT chimeras, or in WT recipients of transferred B2m−/− NK cells, exhibited high responsiveness when stimulated with antibodies against activating receptors, but were tolerant of MHC I-deficient cells. Because we have dissociated responsiveness to immobilized activating receptor antibodies, and responsiveness to NK sensitive target cells, the term “hyporesponsive” needs to be better defined when used in future publications.
Notably, no converse example has yet been reported of NK cells that exhibit low responsiveness to anti-receptor stimulation but react against MHC I-deficient hematopoietic cell grafts. The apparent discrepancy between responsiveness to activating receptor stimulation and responses to target cells expressing ligands for these activating receptors is likely due to regulation of additional interactions that are necessary for cell-cell interactions. These include the action of inhibitory ligands on target cells (including non-MHC I inhibitory ligands), and the activity of ligands on target cells that impart adhesive and costimulatory signals. As it is known that productive target cell interactions require engagement of accessory receptors including LFA-1, DNAM-1 and other adhesion receptors, an interesting hypothesis is that NK cells in mix→WT or B2m−/−→WT chimeras are impaired in signaling by one or more of these accessory molecules, or others that have yet to be defined.
Thus, we hypothesize two distinct mechanisms of NK cell self tolerance, though they may have partially overlapping features. One mechanism desensitizes signaling via primary activating receptors and we propose that a second mechanism acts at another level, such as adhesion or other accessory receptors. Both mechanisms may be operative in the same NK cells, as is likely the case for NK cells in B2m−/− mice, as well as NK cells in normal mice that lack inhibitory receptors for self MHC I. But the second mechanism is apparently sufficient to account for the tolerance of NK cells to B2m−/− cells in mix→WT or B2m−/−→WT chimeras, as these NK cells exhibit high responsiveness to activating receptor antibodies. Consistent with the idea that such NK cells are impaired in a distinct locus of activation, they also respond poorly to spleen cells displaying RAE-1, despite the relatively high responses to antibodies that crosslink the very receptor that RAE-1 engages, NKG2D. The existence of two or more mechanisms of self tolerance in NK cells should not be unexpected, given the evidence that multiple mechanisms account for self tolerance of T cells and B cells.
Other hypotheses to account for the results remain viable, one being that there are different depths of hyporesponsiveness at the same step in the activation process. According to this idea, NK cells in the mix→WT chimeras are partially desensitized, sufficient to remain unresponsive to MHC I-deficient cells, but are still more sensitive than mix→B2m−/− NK cells when activating receptors are aggregated with antibodies. Although we often observed slightly lower responsiveness with mix→WT NK cells as compared to WT →WT NK cells, the difference was so small that it seems unlikely to account for the major defects in rejection of B2m−/− or Raet1e-transgenic spleen cells. The magnitude of the difference was, if anything, smaller at more limiting concentrations of stimulatory antibodies. These data rule out the possibility that we underestimated the difference in responsiveness between WT→WT and mix→WT NK cells due to the use of high antibody concentrations. Furthermore, the similar CD107a and IFN-γ staining intensities of NK cells from the different chimeras rules out the possibility that we underestimated the difference because WT→WT NK cells responded better on a per cell basis.
Breaking tolerance
A previous study showed that NK cell self-tolerance to B2m−/− cells is unstable in mix→WT chimeras, as shown by the induced rejection of B2m−/− donor cells after infection with MCMV (46). In the present study we made important additional findings concerning the stability of NK cell self-tolerance. First, we showed that infections with the bacterial pathogen Listeria have a similar effect as MCMV infections in breaking NK cell self-tolerance in mix→WT chimeras. Interestingly, in both infections, the destruction of B2m−/− donor cells following infection ceased after a few days, around the time the pathogens should be cleared, suggesting that tolerance was rapidly re-established once the inflammatory stimulus abated. We extended these findings by showing that tolerance was broken simply by injecting uninfected mix→WT chimeras with inflammatory cytokines, such as a high dose of IL-15, or lower doses of IL-15 combined with IL-12 and IL-18 (which were ineffective when injected separately). These findings raise the possibility that cytokines induced during infections may be sufficient to break NK cell self-tolerance and lead to rejection of hematopoietic cell grafts, as opposed to the possibility that breaking tolerance requires the engagement of cell surface ligands for NK activating receptors, which are induced in certain infected cells (47, 48).
Interestingly, tolerance was broken in mix→WT chimeras by a combination of IL-12, IL-18, and a relatively low dose of IL-15 complexed with its soluble receptor, IL-15Rα. In contrast, the same doses of IL-15/IL-15Rα alone, or IL-12 + IL18 alone, were ineffective, suggesting that the cytokines work synergistically. IL-15 trans-presentation by dendritic cells has been shown to be crucial for priming the responses of NK cells (49), whereas IL-12 and IL-18 can enhance both elimination of target cells and cytokine production by NK cells (50-52). A possible mechanism of action is that NK cells are primed through IL-15 trans-presented by DCs, and IL-12 and IL-18 amplify the killing of B2m−/− donor cells by the primed NK cells. On the other hand, a high dose of IL-15/IL-15Rα was sufficient by itself to cause rejection of B2m−/− donor cells in these mixed chimeras.
An important finding of the present paper is that the stability of tolerance differed dramatically between mix→WT and mix→B2m−/− chimeras. Infections and cytokine treatments caused much less or no loss of chimerism in mix→B2m−/− chimeras as compared to the loss of chimerism in mix→WT chimeras, suggesting that self-tolerance accompanying the low responsiveness state is much more stable than self-tolerance accompanying high responsiveness. One potential explanation for this effect is that the tolerance mechanism operative in mix→WT chimeras is readily reversed by cytokines while the mechanism operative in mix→B2m−/− chimeras is less readily reversed. Regardless of the explanation, we did observe a modest loss of B2m−/− cells in mix→B2m−/− chimeras after Listeria infections in some experiments, suggesting that intense inflammation may enable NK cells to exhibit autoreactivity in some circumstances. It is interesting to speculate that such autoreactivity could represent one component of NK cell-induced immunopathology that accompanies some infections in vivo (52-54).
Our results represent a substantial revision of current thinking regarding NK cell self tolerance and responsiveness. As molecular mechanisms of self-tolerance are elucidated, it will be essential to account for the dissociation of responsiveness and tolerance uncovered here. The results concerning the stability of hematopoietic cell tolerance in mixed chimeras are important because they raise the possibility that in some transplant scenarios, NK cell self tolerance may be broken as a result of infections or other sources of intense inflammation, resulting in NK-mediated rejection of donor allogeneic or partially allogeneic human bone marrow grafts. Extrapolating from our findings, this problem is predicted to be more acute in the case of marrow transplants that lack one or more of the recipient’s MHC I alleles.
Supplementary Material
Acknowledgements
We thank L. Zhang and K. Pestal and for excellent assistance in the laboratory and Raulet Lab members for useful discussions. Hector Nolla and Alma Valeros provided expert help with flow cytometry. We thank Lewis Lanier and Wayne Yokoyama for providing transgenic mouse strains. NTS, MA, DK, NTJ and DR designed the experiments, which were performed and analyzed by NTS, MA, DK, and NTJ. DHR directed the study, proposed and helped interpret experiments, and with NTS, DK and MA, prepared the manuscript. All authors critically read the manuscript.
Footnotes
This work was supported by NIH grant R01-AI039642 and California Institute for Regenerative Medicine (CIRM) grant RM1-01730 to DHR.
References
- 1.Karre K, Ljunggren HG, Piontek G, Kiessling R. Selective rejection of H-2-deficient lymphoma variants suggests alternative immune defense strategy. Nature. 1986;319:675–678. doi: 10.1038/319675a0. [DOI] [PubMed] [Google Scholar]
- 2.Bix M, Liao NS, Zijlstra M, Loring J, Jaenisch R, Raulet D. Rejection of class I MHC-deficient haemopoietic cells by irradiated MHC-matched mice. Nature. 1991;349:329–331. doi: 10.1038/349329a0. [DOI] [PubMed] [Google Scholar]
- 3.Karre K. NK cells, MHC class I molecules and the missing self. Scand J Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 4.Raulet DH, Held W, Correa I, Dorfman J, Wu M-F, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol. Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 5.Garrido F, Cabrera T, Lopez-Nevot MA, Ruiz-Cabello F. HLA class I antigens in human tumors. Adv Cancer Res. 1995;67:155–195. doi: 10.1016/s0065-230x(08)60713-7. [DOI] [PubMed] [Google Scholar]
- 6.Moretta A, Bottino C, Vitale M, Pende D, Cantoni C, Mingari MC, Biassoni R, Moretta L. Activating receptors and coreceptors involved in human natural killer cell-mediated cytolysis. Annu Rev Immunol. 2001;19:197–223. doi: 10.1146/annurev.immunol.19.1.197. [DOI] [PubMed] [Google Scholar]
- 7.Lanier LL. Missing self, NK cells, and The White Album. J Immunol. 2005;174:6565. doi: 10.4049/jimmunol.174.11.6565. [DOI] [PubMed] [Google Scholar]
- 8.Vivier E, Raulet DH, Moretta A, Caligiuri MA, Zitvogel L, Lanier LL, Yokoyama WM, Ugolini S. Innate or adaptive immunity? The example of natural killer cells. Science. 2011;331:44–49. doi: 10.1126/science.1198687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Raulet DH, Vance RE, McMahon CW. Regulation of the natural killer cell receptor repertoire. Annu Rev Immunol. 2001;19:291–330. doi: 10.1146/annurev.immunol.19.1.291. [DOI] [PubMed] [Google Scholar]
- 10.Joncker NT, Fernandez NC, Treiner E, Vivier E, Raulet DH. NK cell responsiveness is tuned commensurate with the number of inhibitory receptors for self-MHC class I: the rheostat model. J Immunol. 2009;182:4572–4580. doi: 10.4049/jimmunol.0803900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 12.Yawata M, Yawata N, Draghi M, Partheniou F, Little AM, Parham P. MHC class I-specific inhibitory receptors and their ligands structure diverse human NK-cell repertoires toward a balance of missing self-response. Blood. 2008;112:2369–2380. doi: 10.1182/blood-2008-03-143727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joncker NT, Raulet DH. Regulation of NK cell responsiveness to achieve self-tolerance and maximal responses to diseased target cells. Immunol Rev. 2008;224:85–97. doi: 10.1111/j.1600-065X.2008.00658.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31:413–441. doi: 10.1146/annurev-immunol-032712-095951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liao N, Bix M, Zijlstra M, Jaenisch R, Raulet D. MHC class I deficiency: susceptibility to natural killer (NK) cells and impaired NK activity. Science. 1991;253:199–202. doi: 10.1126/science.1853205. [DOI] [PubMed] [Google Scholar]
- 16.Hoglund P, Glas R, Ohlen C, Ljiunggren H-G, Karre K. Alteration of the natural killer cell repertoire in H-2 transgenic mice: Specificity of rapid lymphoma cell clearance determined by the H-2 phenotype of the target. J. Exp. Med. 1991;174:327–334. doi: 10.1084/jem.174.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorfman JR, Zerrahn J, Coles MC, Raulet DH. The basis for self-tolerance of natural killer cells in beta2m− and TAP-1− mice. J Immunol. 1997;159:5219–5225. [PubMed] [Google Scholar]
- 18.Fernandez NC, Treiner E, Vance RE, Jamieson AM, Lemieux S, Raulet DH. A subset of natural killer cells achieves self-tolerance without expressing inhibitory receptors specific for self-MHC molecules. Blood. 2005;105:4416–4423. doi: 10.1182/blood-2004-08-3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 20.Joncker NT, Shifrin N, Delebecque F, Raulet DH. Mature natural killer cells reset their responsiveness when exposed to an altered MHC environment. J Exp Med. 2010;207:2065–2072. doi: 10.1084/jem.20100570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elliott JM, Wahle JA, Yokoyama WM. MHC class I-deficient natural killer cells acquire a licensed phenotype after transfer into an MHC class I-sufficient environment. J Exp Med. 2010;207:2073–2079. doi: 10.1084/jem.20100986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ehrlich LI, Ogasawara K, Hamerman JA, Takaki R, Zingoni A, Allison JP, Lanier LL. Engagement of NKG2D by cognate ligand or antibody alone is insufficient to mediate costimulation of human and mouse CD8+ T cells. J Immunol. 2005;174:1922–1931. doi: 10.4049/jimmunol.174.4.1922. [DOI] [PubMed] [Google Scholar]
- 23.Zijlstra M, Li E, Sajjadi F, Subramani S, Jaenisch R. Germ-line transmission of a disrupted b2-microglobulin gene produced by homologous recombination in embryonic stem cells. Nature. 1989;342:435–438. doi: 10.1038/342435a0. [DOI] [PubMed] [Google Scholar]
- 24.Jamieson AM, Diefenbach A, McMahon CW, Xiong N, Carlyle JR, Raulet DH. The role of the NKG2D immunoreceptor in immune cell activation and natural killing. Immunity. 2002;17:19–29. doi: 10.1016/s1074-7613(02)00333-3. [DOI] [PubMed] [Google Scholar]
- 25.Wu M-F, Raulet DH. Class I-deficient hematopoietic cells and non-hematopoietic cells dominantly induce unresponsiveness of NK cells to class I-deficient bone marrow grafts. J Immunol. 1997;158:1628–1633. [PubMed] [Google Scholar]
- 26.Dong Z, Cruz-Munoz ME, Zhong MC, Chen R, Latour S, Veillette A. Essential function for SAP family adaptors in the surveillance of hematopoietic cells by natural killer cells. Nat Immunol. 2009;10:973–980. doi: 10.1038/ni.1763. [DOI] [PubMed] [Google Scholar]
- 27.Kim S, Iizuka K, Aguila HL, Weissman IL, Yokoyama WM. In vivo natural killer cell activities revealed by natural killer cell-deficient mice. Proc Natl Acad Sci U S A. 2000;97:2731–2736. doi: 10.1073/pnas.050588297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim S, Song YJ, Higuchi DA, Kang HP, Pratt JR, Yang L, Hong CM, Poursine-Laurent J, Iizuka K, French AR, Sunwoo JB, Ishii S, Reimold AM, Yokoyama WM. Arrested natural killer cell development associated with transgene insertion into the Atf2 locus. Blood. 2006;107:1024–1030. doi: 10.1182/blood-2005-04-1493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J Exp Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dubois S, Patel HJ, Zhang M, Waldmann TA, Muller JR. Preassociation of IL-15 with IL-15R alpha-IgG1-Fc enhances its activity on proliferation of NK and CD8+/CD44high T cells and its antitumor action. J Immunol. 2008;180:2099–2106. doi: 10.4049/jimmunol.180.4.2099. [DOI] [PubMed] [Google Scholar]
- 31.Mrozek E, Anderson P, Caligiuri MA. Role of interleukin-15 in the development of human CD56+ natural killer cells from CD34+ hematopoietic progenitor cells. Blood. 1996;87:2632–2640. [PubMed] [Google Scholar]
- 32.Mingari MC, Vitale C, Cantoni C, Bellomo R, Ponte M, Schiavetti F, Bertone S, Moretta A, Moretta L. Interleukin-15-induced maturation of human natural killer cells from early thymic precursors: selective expression of CD94/NKG2-A as the only HLA class I-specific inhibitory receptor. Eur. J. Immunol. 1997;27:1374–1380. doi: 10.1002/eji.1830270612. [DOI] [PubMed] [Google Scholar]
- 33.Lodolce JP, Boone DL, Chai S, Swain RE, Dassopoulos T, Trettin S, Ma A. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity. 1998;9:669–676. doi: 10.1016/s1074-7613(00)80664-0. [DOI] [PubMed] [Google Scholar]
- 34.Kennedy MK, Glaccum M, Brown SN, Butz EA, Viney JL, Embers M, Matsuki N, Charrier K, Sedger L, Willis CR, Brasel K, Morrissey PJ, Stocking K, Schuh JC, Joyce S, Peschon JJ. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. Journal of Experimental Medicine. 2000;191:771–780. doi: 10.1084/jem.191.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Caraux A, Lu Q, Fernandez N, Riou S, Di Santo JP, Raulet DH, Lemke G, Roth C. Natural killer cell differentiation driven by Tyro3 receptor tyrosine kinases. Nat Immunol. 2006;7:747–754. doi: 10.1038/ni1353. [DOI] [PubMed] [Google Scholar]
- 36.Roth C, Rothlin C, Riou S, Raulet DH, Lemke G. Stromal-cell regulation of natural killer cell differentiation. J Mol Med. 2007;85:1047–1056. doi: 10.1007/s00109-007-0195-0. [DOI] [PubMed] [Google Scholar]
- 37.Ebihara T, Jonsson AH, Yokoyama WM. Natural killer cell licensing in mice with inducible expression of MHC class I. Proc Natl Acad Sci U S A. 2013;110:E4232–4237. doi: 10.1073/pnas.1318255110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hoglund P, Ohlen C, Carbone E, Franksson L, Ljunggren H, Latour A, Koller B, Karre K. Recognition of b2-microglobulin-negative (b2m−) T-cell blasts by natural killer cells from normal but not from b2m− mice: nonresponsiveness controlled by b2m− bone marrow in chimeric mice. Proc. Natl. Acad. Sci. USA. 1991;88:10332–10336. doi: 10.1073/pnas.88.22.10332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, Velardi A. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- 40.Yu J, Venstrom JM, Liu XR, Pring J, Hasan RS, O'Reilly RJ, Hsu KC. Breaking tolerance to self, circulating natural killer cells expressing inhibitory KIR for non-self HLA exhibit effector function after T cell-depleted allogeneic hematopoietic cell transplantation. Blood. 2009;113:3875–3884. doi: 10.1182/blood-2008-09-177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Haas P, Loiseau P, Tamouza R, Cayuela JM, Moins-Teisserenc H, Busson M, Henry G, Falk CS, Charron D, Socie G, Toubert A, Dulphy N. NK-cell education is shaped by donor HLA genotype after unrelated allogeneic hematopoietic stem cell transplantation. Blood. 2011;117:1021–1029. doi: 10.1182/blood-2010-02-269381. [DOI] [PubMed] [Google Scholar]
- 42.Cooley S, Foley B, Verneris MR, McKenna D, Luo X, Dusenbery KE, Blazar BE, Weisdorf DJ, Miller JS. Haploidentical Natural Killer (NK) Cells Expanding In Vivo After Adoptive Transfer Exhibit Hyperfunction That Partially Overcomes Self Tolerance and Leads to Clearance of Refractory Leukemia. Blood. 2011;118:355. [Google Scholar]
- 43.Raulet DH, Vance RE. Self-tolerance of natural killer cells. Nat Rev Immunol. 2006;6:520–531. doi: 10.1038/nri1863. [DOI] [PubMed] [Google Scholar]
- 44.Yokoyama WM, Kim S. How do natural killer cells find self to achieve tolerance? Immunity. 2006;24:249–257. doi: 10.1016/j.immuni.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 45.Johansson MH, Hoglund P. The dynamics of natural killer cell tolerance. Semin Cancer Biol. 2006;16:393–403. doi: 10.1016/j.semcancer.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 46.Sun JC, Lanier LL. Cutting edge: viral infection breaks NK cell tolerance to "missing self". J Immunol. 2008;181:7453–7457. doi: 10.4049/jimmunol.181.11.7453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 48.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 49.Mortier E, Woo T, Advincula R, Gozalo S, Ma A. IL-15R{alpha} chaperones IL-15 to stable dendritic cell membrane complexes that activate NK cells via trans presentation. J Exp Med. 2008;205:1213–1225. doi: 10.1084/jem.20071913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ortaldo JR, Young HA. Expression of IFN-gamma upon triggering of activating Ly49D NK receptors in vitro and in vivo: costimulation with IL-12 or IL-18 overrides inhibitory receptors. J Immunol. 2003;170:1763–1769. doi: 10.4049/jimmunol.170.4.1763. [DOI] [PubMed] [Google Scholar]
- 51.Farhadi N, Lambert L, Triulzi C, Openshaw PJ, Guerra N, Culley FJ. Natural killer cell NKG2D and granzyme B are critical for allergic pulmonary inflammation. J Allergy Clin Immunol. 2014;133:827–835. doi: 10.1016/j.jaci.2013.09.048. e823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hyodo Y, Matsui K, Hayashi N, Tsutsui H, Kashiwamura S, Yamauchi H, Hiroishi K, Takeda K, Tagawa Y, Iwakura Y, Kayagaki N, Kurimoto M, Okamura H, Hada T, Yagita H, Akira S, Nakanishi K, Higashino K. IL-18 up-regulates perforin-mediated NK activity without increasing perforin messenger RNA expression by binding to constitutively expressed IL-18 receptor. J Immunol. 1999;162:1662–1668. [PubMed] [Google Scholar]
- 53.Kash JC, Tumpey TM, Proll SC, Carter V, Perwitasari O, Thomas MJ, Basler CF, Palese P, Taubenberger JK, Garcia-Sastre A, Swayne DE, Katze MG. Genomic analysis of increased host immune and cell death responses induced by 1918 influenza virus. Nature. 2006;443:578–581. doi: 10.1038/nature05181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdul-Careem MF, Mian MF, Yue G, Gillgrass A, Chenoweth MJ, Barra NG, Chew MV, Chan T, Al-Garawi AA, Jordana M, Ashkar AA. Critical role of natural killer cells in lung immunopathology during influenza infection in mice. J Infect Dis. 2012;206:167–177. doi: 10.1093/infdis/jis340. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.