Abstract
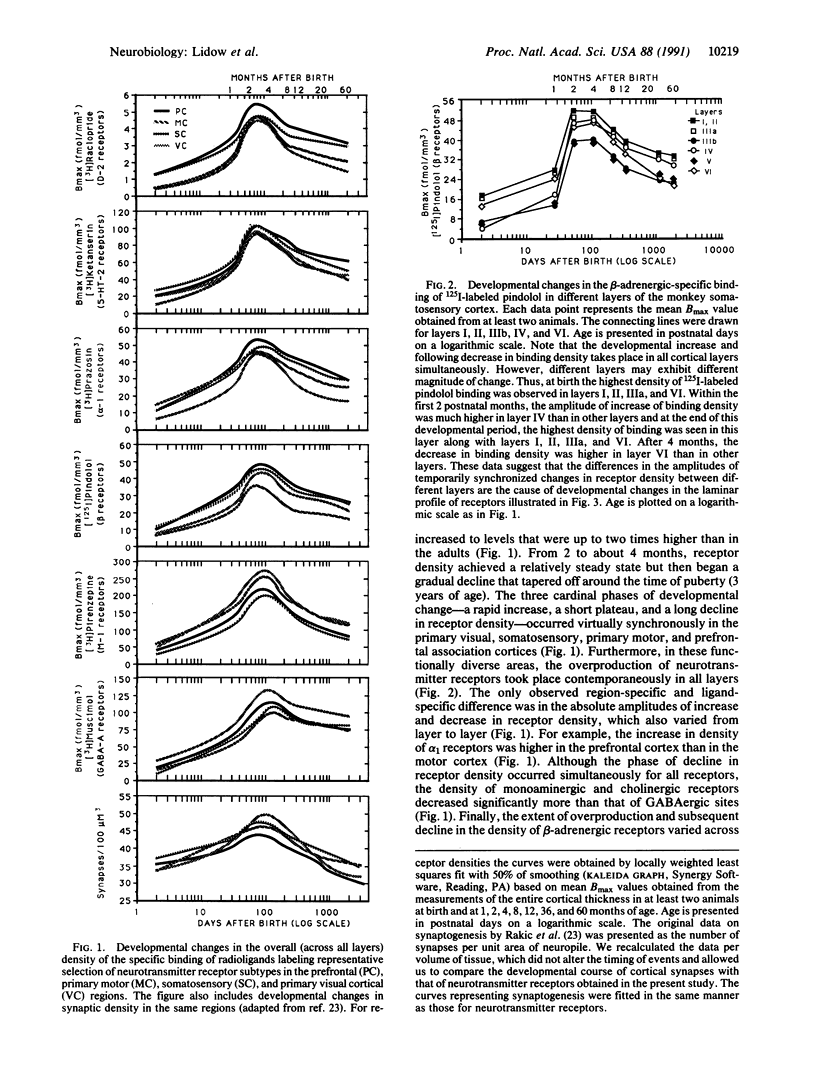
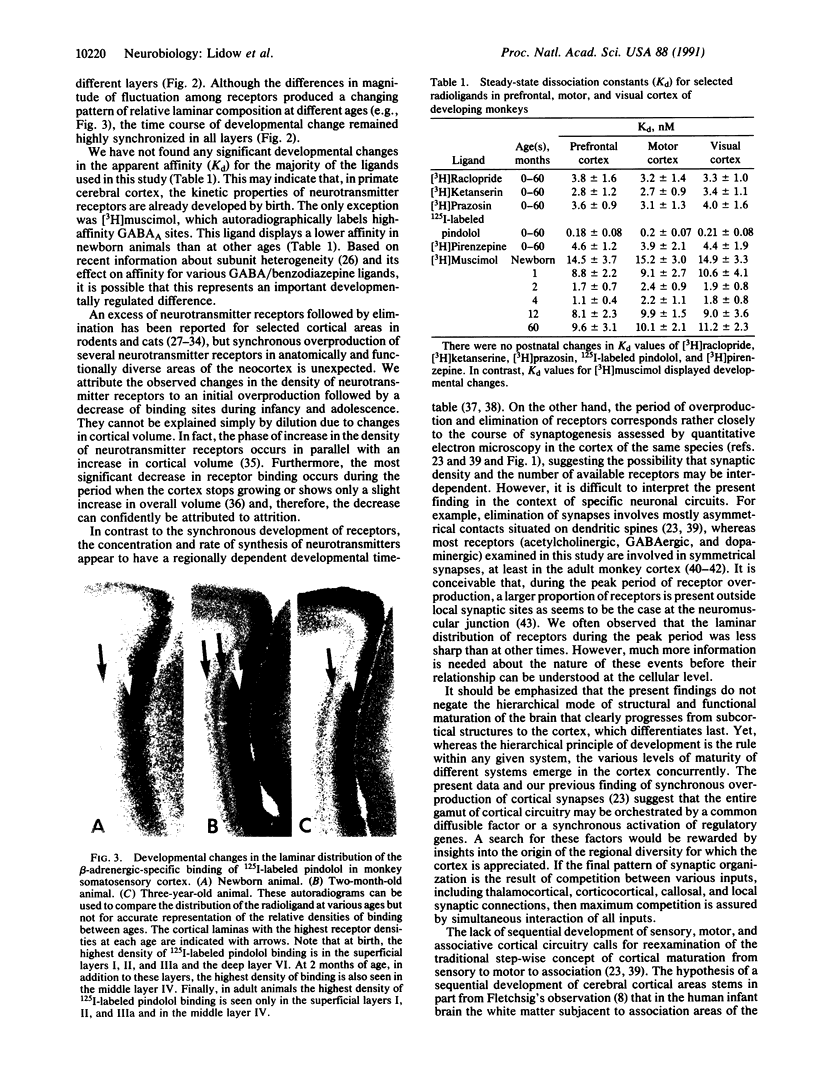
A remarkable diversity of neurotransmitter receptors develops concurrently in disparate areas of the primate cerebral cortex. The density of dopaminergic, adrenergic, serotonergic, cholinergic, and GABAergic receptors (where GABA is gamma-aminobutyric acid) in rhesus monkey reaches a maximum level between 2 and 4 months of age and then declines gradually to adult levels in all layers of sensory, motor, and association regions. The synchronized development of neurotransmitter receptors in diverse layers and regions of the neocortex occurs pari passu with synaptogenesis, demonstrating unusual coordination of biochemical and structural maturation and supporting the hypothesis that the entire cerebral cortex matures as an integrated network, rather than as a system-by-system cascade.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Betz H., Bourgeois J. P., Changeux J. P. Evolution of cholinergic proteins in developing slow and fast skeletal muscles in chick embryo. J Physiol. 1980 May;302:197–218. doi: 10.1113/jphysiol.1980.sp013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chugani H. T., Phelps M. E., Mazziotta J. C. Positron emission tomography study of human brain functional development. Ann Neurol. 1987 Oct;22(4):487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Development and modifiability of the cerebral cortex. Neurosci Res Program Bull. 1982 Apr;20(4):429–611. [PubMed] [Google Scholar]
- ELLINGSON R. J., WILCOTT R. C. Development of evoked responses in visual and auditory cortices of kittens. J Neurophysiol. 1960 Jul;23:363–375. doi: 10.1152/jn.1960.23.4.363. [DOI] [PubMed] [Google Scholar]
- Fiedler E. P., Marks M. J., Collins A. C. Postnatal development of cholinergic enzymes and receptors in mouse brain. J Neurochem. 1987 Sep;49(3):983–990. doi: 10.1111/j.1471-4159.1987.tb00990.x. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. S., Brown R. M. Postnatal development of monoamine content and synthesis in the cerebral cortex of rhesus monkeys. Brain Res. 1982 Jul;256(3):339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P. S. Development of cortical circuitry and cognitive function. Child Dev. 1987 Jun;58(3):601–622. [PubMed] [Google Scholar]
- Goldman-Rakic P. S., Leranth C., Williams S. M., Mons N., Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic P. S., Lidow M. S., Gallager D. W. Overlap of dopaminergic, adrenergic, and serotoninergic receptors and complementarity of their subtypes in primate prefrontal cortex. J Neurosci. 1990 Jul;10(7):2125–2138. doi: 10.1523/JNEUROSCI.10-07-02125.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman P. S., Nauta W. J. Columnar distribution of cortico-cortical fibers in the frontal association, limbic, and motor cortex of the developing rhesus monkey. Brain Res. 1977 Feb 25;122(3):393–413. doi: 10.1016/0006-8993(77)90453-x. [DOI] [PubMed] [Google Scholar]
- Harden T. K., Wolfe B. B., Sporn J. R., Poulos B. K., Molinoff P. B. Effects of 6-hydroxydopamine on the development of the beta adrenergic receptor/adenylate cyclase system in rat cerebral cortex. J Pharmacol Exp Ther. 1977 Oct;203(1):132–143. [PubMed] [Google Scholar]
- Hayashi M., Yamashita A., Shimizu K., Oshima K. Ontogeny of cholecystokinin-8 and glutamic acid decarboxylase in cerebral neocortex of macaque monkey. Exp Brain Res. 1989;74(2):249–255. doi: 10.1007/BF00248857. [DOI] [PubMed] [Google Scholar]
- Jonsson G., Kasamatsu T. Maturation of monoamine neurotransmitters and receptors in cat occipital cortex during postnatal critical period. Exp Brain Res. 1983;50(2-3):449–458. doi: 10.1007/BF00239212. [DOI] [PubMed] [Google Scholar]
- Kennedy C., Sakurada O., Shinohara M., Miyaoka M. Local cerebral glucose utilization in the newborn macaque monkey. Ann Neurol. 1982 Oct;12(4):333–340. doi: 10.1002/ana.410120404. [DOI] [PubMed] [Google Scholar]
- Killackey H. P., Chalupa L. M. Ontogenetic change in the distribution of callosal projection neurons in the postcentral gyrus of the fetal rhesus monkey. J Comp Neurol. 1986 Feb 15;244(3):331–348. doi: 10.1002/cne.902440306. [DOI] [PubMed] [Google Scholar]
- LaMantia A. S., Rakic P. Axon overproduction and elimination in the corpus callosum of the developing rhesus monkey. J Neurosci. 1990 Jul;10(7):2156–2175. doi: 10.1523/JNEUROSCI.10-07-02156.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidow M. S., Goldman-Rakic P. S., Gallager D. W., Geschwind D. H., Rakic P. Distribution of major neurotransmitter receptors in the motor and somatosensory cortex of the rhesus monkey. Neuroscience. 1989;32(3):609–627. doi: 10.1016/0306-4522(89)90283-2. [DOI] [PubMed] [Google Scholar]
- Morris M. J., Dausse J. P., Devynck M. A., Meyer P. Ontogeny of alpha 1 and alpha 2-adrenoceptors in rat brain. Brain Res. 1980 May 19;190(1):268–271. doi: 10.1016/0006-8993(80)91178-6. [DOI] [PubMed] [Google Scholar]
- O'Kusky J., Colonnier M. Postnatal changes in the number of neurons and synapses in the visual cortex (area 17) of the macaque monkey: a stereological analysis in normal and monocularly deprived animals. J Comp Neurol. 1982 Sep 20;210(3):291–306. doi: 10.1002/cne.902100308. [DOI] [PubMed] [Google Scholar]
- Pittman R. N., Minneman K. P., Molinoff P. B. Ontogeny of beta 1- and beta 2-adrenergic receptors in rat cerebellum and cerebral cortex. Brain Res. 1980 Apr 28;188(2):357–368. doi: 10.1016/0006-8993(80)90037-2. [DOI] [PubMed] [Google Scholar]
- Preuss T. M., Goldman-Rakic P. S. Myelo- and cytoarchitecture of the granular frontal cortex and surrounding regions in the strepsirhine primate Galago and the anthropoid primate Macaca. J Comp Neurol. 1991 Aug 22;310(4):429–474. doi: 10.1002/cne.903100402. [DOI] [PubMed] [Google Scholar]
- Pritchett D. B., Lüddens H., Seeburg P. H. Type I and type II GABAA-benzodiazepine receptors produced in transfected cells. Science. 1989 Sep 22;245(4924):1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- Rakic P., Bourgeois J. P., Eckenhoff M. F., Zecevic N., Goldman-Rakic P. S. Concurrent overproduction of synapses in diverse regions of the primate cerebral cortex. Science. 1986 Apr 11;232(4747):232–235. doi: 10.1126/science.3952506. [DOI] [PubMed] [Google Scholar]
- Rakic P., Goldman-Rakic P. S. The development and modifiability of the cerebral cortex. Overview. Neurosci Res Program Bull. 1982 Apr;20(4):433–438. [PubMed] [Google Scholar]
- Rakic P. Prenatal development of the visual system in rhesus monkey. Philos Trans R Soc Lond B Biol Sci. 1977 Apr 26;278(961):245–260. doi: 10.1098/rstb.1977.0040. [DOI] [PubMed] [Google Scholar]
- Rakic P. Prenatal genesis of connections subserving ocular dominance in the rhesus monkey. Nature. 1976 Jun 10;261(5560):467–471. doi: 10.1038/261467a0. [DOI] [PubMed] [Google Scholar]
- Ravikumar B. V., Sastry P. S. Muscarinic cholinergic receptors in human foetal brain: characterization and ontogeny of [3H]quinuclidinyl benzilate binding sites in frontal cortex. J Neurochem. 1985 Jan;44(1):240–246. doi: 10.1111/j.1471-4159.1985.tb07136.x. [DOI] [PubMed] [Google Scholar]
- Schliebs R., Rothe T. Development of GABAA receptors in the central visual structures of rat brain. Effect of visual pattern deprivation. Gen Physiol Biophys. 1988 Jun;7(3):281–291. [PubMed] [Google Scholar]
- Shaw C., Wilkinson M., Cynader M., Needler M. C., Aoki C., Hall S. E. The laminar distributions and postnatal development of neurotransmitter and neuromodulator receptors in cat visual cortex. Brain Res Bull. 1986 May;16(5):661–671. doi: 10.1016/0361-9230(86)90137-1. [DOI] [PubMed] [Google Scholar]
- Zecevic N., Bourgeois J. P., Rakic P. Changes in synaptic density in motor cortex of rhesus monkey during fetal and postnatal life. Brain Res Dev Brain Res. 1989 Nov 1;50(1):11–32. doi: 10.1016/0165-3806(89)90124-7. [DOI] [PubMed] [Google Scholar]




