Abstract
Background and Purpose
Crosstalk between thyrotropin (TSH) receptors and insulin‐like growth factor 1 (IGF‐1) receptors initiated by activation of TSH receptors could be important in the development of Graves' ophthalmopathy (GO). Specifically, TSH receptor activation alone is sufficient to stimulate hyaluronic acid (HA) secretion, a major component of GO, through both IGF‐1 receptor‐dependent and ‐independent pathways. Although an anti‐IGF‐1 receptor antibody is in clinical trials, its effectiveness depends on the relative importance of IGF‐1 versus TSH receptor signalling in GO pathogenesis.
Experimental Approach
TSH and IGF‐1 receptor antagonists were used to probe TSH/IGF‐1 receptor crosstalk in primary cultures of Graves' orbital fibroblasts (GOFs) following activation with monoclonal TSH receptor antibody, M22. Inhibition of HA secretion following TSH receptor stimulation was measured by modified HA elisa.
Key Results
TSH receptor antagonist, ANTAG3 (NCGC00242364), inhibited both IGF‐1 receptor ‐dependent and ‐independent pathways at all doses of M22; whereas IGF‐1 receptor antagonists linsitinib and 1H7 (inhibitory antibody) lost efficacy at high M22 doses. Combining TSH and IGF‐1 receptor antagonists exhibited Loewe additivity within the IGF‐1 receptor‐dependent component of the M22 concentration‐response. Similar effects were observed in GOFs activated by autoantibodies from GO patients' sera.
Conclusions and Implications
Our data support TSH and IGF‐1 receptors as therapeutic targets for GO, but reveal putative conditions for anti‐IGF‐1 receptor resistance. Combination treatments antagonizing both receptors yield additive effects by inhibiting crosstalk triggered by TSH receptor stimulatory antibodies. Combination therapy may be an effective strategy for dose reduction and/or compensate for any loss of anti‐IGF‐1 receptor efficacy.
Abbreviations
- GO‐Igs
autoantibodies from GO patients
- CI
combination index
- GOB
GO Bethesda sample
- GD
Graves' disease
- GO
Graves' ophthalmopathy
- GOFs
Graves' orbital fibroblasts
- HA
hyaluronic acid
- IRBs
Institutional review boards
- IGF‐1
insulin‐like growth factor 1
- NIDDK
National Institute of Diabetes and Digestive and Kidney Diseases
- TSH
thyroid‐stimulating hormone
- TSAb
TSH receptor‐stimulating antibody
Tables of Links
| TARGETS | |
|---|---|
| Other protein targets a | Enzymes e |
| FABP4 | Acetyl CoA carboxylase |
| TNF‐α | Adenylate cyclase |
| GPCRs b | Akt (PKB) |
| GLP‐1 receptor | ERK1 |
| Nuclear hormone receptors c | ERK2 |
| PPARγ | FASN |
| Transporters d | Hormone sensitive lipase (HSL) |
| GLUT4 | PKA |
| LIGANDS | |
|---|---|
| Adiponectin | IBMX |
| cAMP | IL‐6 |
| Dexamethasone | Indomethacin |
| Exenatide (exendin‐4) | Insulin |
| Exendin (9‐39) | Liraglutide |
| GLP‐1 | Metformin |
These Tables list key protein targets and ligands in this article that are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,b,c,d,eAlexander et al., 2015a,b,c,d,e).
Introduction
Graves' ophthalmopathy (GO) is an orbital manifestation of autoimmune Graves' disease (GD), the most common cause of hyperthyroidism (Bahn, 2010). A major component of GO is accumulation of hyaluronan (hyaluronic acid; HA) in the extracellular matrix of retro‐orbital tissue resulting in exophthalmos and, in severe cases, visual impairment. Although other antigens have been proposed as participating in GO pathogenesis, auto‐antibodies that stimulate the thyrotropin (thyroid‐stimulating hormone; TSH) receptor on retro‐orbital cells play a major role in GO pathogenesis, in part, by stimulating HA secretion. Unlike hyperthyroidism of GD, GO is not treatable by reducing thyroid hormone levels. At present, there is no medical therapy for GO directed at its pathogenesis; rather, severe signs and symptoms are managed with corticosteroids or orbital decompression surgery.
A clinical trial, now in progress, uses an insulin‐like growth factor 1 (IGF‐1) receptor inhibitory antibody to treat GO (https://clinicaltrials.gov/ct2/show/NCT01868997). This approach is based on the idea that IGF‐1 receptors may be an auto‐antigen and a target for stimulatory antibodies, although evidence for this is inconclusive (Pritchard et al., 2003; Minich et al., 2013; Varewijck et al., 2013). Increased IGF‐1 receptor expression has been found in thyroid tissue of GD patients and retro‐orbital tissue of GO patients (Smith et al., 2008). In addition, IGF‐1 receptor inhibition has been shown to block stimulation of HA secretion by autoantibodies from GO patients (GO‐Igs) in vitro using primary orbital fibroblasts derived from donors with GO (GOFs) (Chen et al., 2014; Krieger et al., 2015). However, it is controversial whether IGF‐1 receptor‐stimulating antibodies are present in the blood of GO patients. In fact, no definitive evidence of IGF‐1 receptor‐stimulating antibodies has been presented for any human disease. While direct stimulation of IGF‐1 receptors is capable of up‐regulating cytokine production (another component of GO) and HA secretion, these pathways may not be initiated by GO‐Igs binding to and activating IGF‐1 receptors (Krieger et al., 2016).
Crosstalk between TSH receptors and IGF‐1 receptors is a recently reported phenomenon that may explain previous data suggesting a role for IGF‐1 receptors in GO pathogenesis. Evidence suggests that TSH receptors and IGF‐1 receptors form a physical complex in GOFs (Tsui et al., 2008). We have previously shown that TSH receptor /IGF‐1 receptor crosstalk is initiated by a monoclonal TSH receptor‐stimulating antibody (TSAb), M22, and purified GO‐Igs in the absence of IGF‐1 receptor activation; however, certain IGF‐1 receptor blocking antibodies and IGF‐1 receptor kinase inhibitors were efficacious at attenuating TSH receptor signalling (Krieger et al., 2016). Given the apparent importance of TSH receptor /IGF‐1 receptor crosstalk in the mechanism used by TSAbs to up‐regulate HA, we hypothesize that treatment targeting both TSH receptors and IGF‐1 receptors would be a more effective strategy to treat GO than either agent alone.
In our previous study, we showed that M22 stimulation of HA secretion from GOFs was bi‐phasic representing two pathways in M22 signalling including (i) an IGF‐1 receptor‐dependent pathway at lower doses of M22, and (ii) an IGF‐1 receptor‐independent pathway at higher M22 doses (Krieger et al., 2015). Here, we have taken a pharmacological approach and show that IGF‐1 receptor antagonists are fully efficacious at blocking the more potent phase (lower doses) of the M22 concentration‐response, but lose efficacy as M22 doses increase and become reliant on IGF‐1 receptor‐independent signalling. On the other hand, TSH receptor antagonism is fully efficacious at blocking M22 stimulation at both phases of the concentration‐response. Drug combination studies reveal inhibitory effects are additive in the IGF‐1 receptor‐dependent component of the M22 concentration response. Taken together, we suggest that lower doses of both inhibitors in combination may provide a better therapeutic index for treatment of GO particularly for patients in which anti‐IGF‐1 receptor therapies are efficacious. In addition, the pharmacology lends further support to a role for TSH receptor /IGF‐1 receptor crosstalk in GO pathogenesis.
Footnote 1 – The term TSAb is typically used when an antibody has been shown to stimulate cAMP production by activating TSH receptors. In this paper, we use TSAb to signify any antibody that stimulates HA production by activating TSH receptors even though some of these antibodies may not generate a significant cAMP response.
Methods
Isolation and culture of primary Graves' orbital fibroblasts.
Retro‐orbital adipose tissue was obtained from GO patients who underwent orbital decompression surgery. Informed consent was obtained from patients prior to inclusion in these studies. Use of human tissues was approved by the Johns Hopkins and National Institute of Diabetes and Digestive and Kidney Diseases Institutional review boards (IRBs). Tissue explants were minced and plated in culture dishes containing complete growth media comprised of high‐glucose DMEM with FBS (10% vol/vol), penicillin (100 U·mL−1), and streptomycin (100 μg·mL−1). Resulting monolayer outgrowths of adherent cells were serially passaged with trypsin/EDTA and cultured in F‐media composed of DMEM with FBS (10% vol/vol), penicillin (100 U·mL−1), streptomycin (100 μg·mL−1), L‐glutamine (2 mM), Ham's F‐12 nutrient mixture (25% vol/vol), hydrocortisone (25 ng·mL−1), epithelial growth factor (0.125 ng·mL−1), insulin (5 μg·mL−1), cholera toxin (11.7 nM), gentamicin (10 μg·mL−1), Fungizone (250 ng·mL−1), and Y‐27632 (5 μM). Cells were maintained in a humidified 7% CO2 incubator at 37°C. Cells from 12 GO patients were used in this study. However, the rapid loss of TSH receptor expression in GOFs in culture required all experiments be performed at passage 3, limiting cell numbers and affecting the capacity of experiments performed with each GOF strain (i.e. all experiments could not be conducted with cells from all 12 patients). As such, the number of donors used is specified in the figure legends. The exception is the experiment using purified GO‐Igs to stimulate HA secretion, where three of the strains were used at passage 4.
Stimulation and inhibition of HA secretion in GOFs
M22 is a monoclonal stimulatory antibody of TSH receptors used to model GD‐Igs in vitro (Sanders et al., 2003). GOF cells were grown to confluence in F‐media. Pre‐existing HA was removed by incubating GOF monolayers with hyaluronidase (1 U·mL−1 in HBSS) for 1 h at 37°C. GOFs were activated with M22 or IGF‐1 in F‐media and incubated for 4–5 days in 7% CO2 at 37°C. For experiments inhibiting TSH receptors and/or IGF‐1 receptors, cells were pretreated with antagonist(s) in low‐serum DMEM (1% FBS) at 37°C for 1 h before treatment with M22 or IGF‐1 in F‐media. Conditioned media were collected, stored at −20°C, and assayed for secreted HA using a modified Corgenix HA elisa kit as previously described (Krieger and Gershengorn, 2014). Standard curves were generated using dilutions of 1 MDa HA in solution.
Median‐effect analysis for dose equivalence
Dose equivalence was analysed according to the median‐effect method of Chou and Talaly (Chou and Talaly, 1977). IC50 values were calculated for ANTAG3, linsitinib and 1H7 in GOF cells following stimulation with M22 ECmed to define median effect in context to inhibiting HA stimulation. Combination treatments at fixed ratios were calculated using the combination index (CI) theorem as previously described and analysed via the isobologram method of Loewe in which CI values of <1, =1 or >1 define synergy, additivity or antagonism respectively (Loewe, 1953; Chou and Talaly, 1977; Chou, 2010).
Patient selection for GO serum collection
Five patients with clinically active GO (GO Bethesda samples GOB8, GOB9, GOB10, GOB12 and GOB13) and one patient with a history of exposure keratopathy associated with lid retraction (GOB11) were identified out of a cohort of patients with GD at the Diabetes, Endocrinology, and Obesity Branch, National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), National Institutes of Health, Bethesda, MD, USA. Blood samples (clinical trial identifier: NCT00001159) were obtained under NIDDK/National Institute of Arthritis and Musculoskeletal and Skin Diseases IRB approved protocols after informed consent was obtained from patients.
Purification of GO‐Igs from whole serum
Antibodies were isolated from whole serum by thiophilic affinity chromatography. Briefly, a 2 mL gravity flow column was packed with Thiophilic‐Superflow resin and equilibrated according to the manufacturer's directions. Samples were diluted 1:10 in sample buffer (50 mM sodium phosphate, 0.55 M sodium sulfate, pH 7.0) and applied to the column. Unbound proteins were washed with equilibrium buffer (50 mM sodium phosphate, 0.55 M sodium sulfate, pH 7.0). GO‐Igs were eluted with 2–3 column volumes of elution buffer (20 mM sodium phosphate, 20% glycerol, pH 7.0). Eluent samples were combined and concentrated to their original volume using Corning Spin‐X UF Concentrators with a 100 K molecular weight cutoff. Samples were dialysed with Tube‐O‐Dialyzer (G‐Biosciences, St. Louis, MO, USA) in HBSS containing 10 mM HEPES, pH 7.4. Final protein concentration was measured by bicinchoninic acid protein assay using bovine γ globulin as a standard. For experiments using purified GO‐Igs to stimulate HA, GO‐Igs were stored in separate vials and individually used to treat cells. No GO‐Ig samples were mixed during this study.
Experimental design
Group size
Group size was restricted by the number of available GO patient cell strains. Some experiments describe exploratory studies, and increasing their group size was impractical for the following reasons: (i) cell culture passage could not exceed three; (ii) these experiments required a large number of cells; (iii) previously used patient strains were completely exhausted of cells; and (iv) new donations of GO patient tissue were not available. The experiments where group size was less than five are clearly stated in the figure legends and detailed below.
For experiments determining IC50 of ANTAG3 and linsitinib, group size was seven. Group size was six in experiments verifying additivity with combinations of ANTAG3/linsitinib and ANTAG3/1H7 in regards to HA secretion. For dose analyses at fixed‐ratio combinations of ANTAG3/linsitinib and ANTAG3/1H7, group size was less than five. However, these experiments were necessary to the overall study because they provided preliminary evidence that antagonist combinations were additive and allowed us to design subsequent experiments. They were included for those reasons.
Experiments demonstrating the loss of linsitinib efficacy at M22 [ECmax] had group size equal to five. Experiments comparing linsitinib and ANTAG3 IC50s at 1 nM and M22 [ECmed] were preliminary with group size less than five. These experiments, however, support the statistically significant finding that linsitinib loses efficacy rather than potency. They confirmed that the empirically determined linsitinib [ICmax] was valid at high M22 concentrations. We have included this preliminary data because without them, we could not have been confident in the design of the experiments depicted in Figure 3A,B.
Experiments determining the IC50 of 1H7 and the partial efficacy of 1H7 ICmax used group size equal to five. Group size less than five was used in experiments testing the efficacy of AF305 and 1H7 against IGF‐1. However, the IGF‐1 receptor inhibitory properties of these antibodies have previously been established. The purpose of this experiment was to demonstrate that AF305 and 1H7 function similarly in our experimental system as they do in previously published ones. These preliminary data were necessary to the design and implementation of subsequent experiments and are included for those reasons.
Experiments testing combination treatments against stimulation with GO‐Igs used antibodies from six GO patients. Each GO‐Ig isolate was tested separately amongst five different GOF cell strains. Their individual responses for each treatment condition were averaged and the combined data is shown in Figure 6. Statistical analysis was performed on the combined data.
Figure 6.
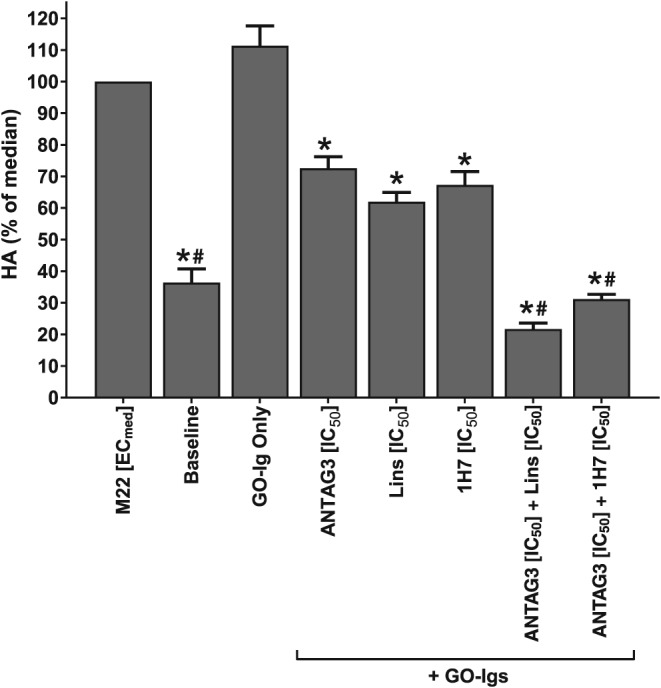
Combination treatments on HA stimulation by purified GO‐Igs from six donor patients. Primary GOF cells in culture were stimulated with GO‐Igs (3.5–4.5 mg·mL−1) purified from the sera of six different donors (GOB8‐13) and co‐treated with IC50 concentrations of ANTAG3, linsitinib (Lins), 1H7, or combinations thereof for 5 days. Total HA levels were quantified in culture media by elisa. Data represents mean response ± SEM from 5 different donor cell strains plotted as percent HA levels relative to the M22 median response. * P <0.001, significantly different fromM22 [ECmed]. #P <0.001, significantly different from ANTAG3 [IC50].
Randomization
While formal randomization was not employed, access to GOF strains was, practically speaking, randomized by which strains were available based on the timing of patient donation. Because experiments were constrained to passage 3, tissue from a single donor did not produce an adequate number of cells to perform all the experiments depicted in this study. New strains were continuously introduced as the study progressed.
Blinding
Blinding was not included in the experimental design due to practical considerations. Experiments were complicated by large number of treatment conditions using multiple drug concentrations at different ratios in conjunction with TSH receptor stimulation in primary GOF cells, which have limited replication potential. No step in the treatment process was automated and blinding could potentially lead to an increase in human error. Minimizing mistakes is a responsibility to the patients and clinicians who donated these specimens.
Normalization
Because experiments were conducted in primary cells, the absolute values of the minimal and maximal HA responses would vary from strain to strain. As such, every experiment, at a minimum, included baseline, M22 [ECmed] and M22 [ECmax] controls. M22 ECmed is the projected 50% response concentration extrapolated from a force‐fit monophasic concentration‐response curve, which also turns out to be the median between both low and high EC50 points. This concentration was empirically verified and shown in Figure S1. M22 [ECmed] or [ECmax] was set to 100% as specified in the figure legend. Concentration‐response curves were additionally normalized with 0% corresponding to baseline HA levels. Normalization was done using GraphPad Prism 5. Zero, and 100% was defined as the mean of the technical replicates, and the SEM was normalized appropriately.
Data analysis
Data and statistical analysis in this study comply with the recommendations of Curtis et al., (2015). Statistical analysis using GraphPad Prism version 5.04 Windows, GraphPad Software, San Diego, CA, USA, www.graphpad.com. For one‐way ANOVA, equality of variance was validated with Bartlett's test. Where specified, means were compared using Dunnett's test. For all statistical tests, P < 0.001 was considered significant.
Materials
Monoclonal autoantibody that stimulates human TSH receptors (M22) was purchased from Kronus (Star, Idaho, USA). Recombinant human insulin‐like growth factor 1 (IGF‐1) was purchased from Peprotech (Rocky Hill, New Jersey, USA). The TSH receptor antagonist NCGC00242364 (ANTAG3) was synthesized by the National Center for Advancing Translational Science, National Institutes of Health (Bethesda, Maryland, USA) as previously reported (Turcu et al., 2013). The IGF‐1 receptor kinase inhibitor linsitinib was purchased from Selleckchem (Houston, Texas, USA). Mouse monoclonal antibody directed at human IGF‐1 receptors (1H7) and goat polyclonal antibody directed at human/mouse IGF‐1 receptors (AF305) were purchased from AbD Serotec (Raleigh, North Carolina, USA) and R&D Systems (Minneapolis, Minnesota, USA), respectively. HA ELISA kits were purchased from Corgenix (Broomfield, Colorado, USA). One MDa HA was purchased from Lifecore Biomedical (Chaska, Minnesota, USA). Y‐27632, a Rho kinase inhibitor, was purchased from R&D Systems (Minneapolis, Minnesota, USA). Thiophilic‐Superflow resin and Corning Spin‐X UF Concentrators were purchased from Clontech (Mountain View, CA) and Sigma‐Aldrich (St. Louis, MO, USA) respectively. Retro‐orbital adipose tissue was generously supplied by Drs. Neil Miller, Prem Subramanian, Nicholas Mahoney and Shannath Merbs (Johns Hopkins School of Medicine, Baltimore, MD, USA).
Results
ANTAG3 and linsitinib both inhibit HA production following TSH receptor stimulation by M22
M22 stimulates HA production in a biphasic manner in GOFs with a median effective concentration (ECmed) of 0.25 ± 0.04 nM located at the boundary of the high and low potency phases of the concentration‐response curve (Figure S1). In order to evaluate the pharmacological effects of ANTAG3 or linsitinib on HA production, donor GOF cells were treated with either inhibitor in a concentration dependent manner following stimulation with M22 ECmed. As shown in Figure 1A, B, both ANTAG3 and linsitinib function as full antagonists of M22 ECmed stimulation. These data suggest that both agents may function in combination to cooperatively interfere with HA production by M22.
Figure 1.
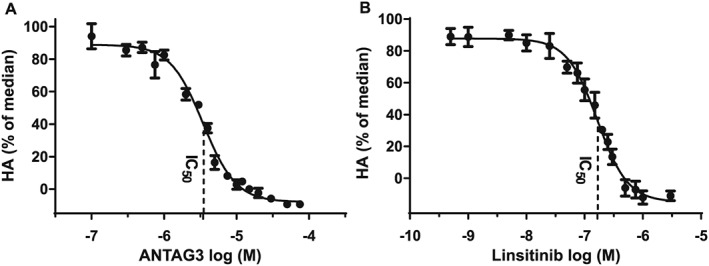
Concentration‐response curves for ANTAG3 and linsitinib in GOF cells following stimulation by M22 ECmed. (A) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with increasing doses of ANTAG3 for 5 days. Total HA was measured in culture media by elisa. Data represent mean ± SEM of seven different donor cell strains plotted as percent HA levels relative to the M22 median response (0% corresponding to HA levels at baseline). Indicated is the ANTAG3 IC50 concentration (3.34 μM). (B) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with increasing doses of linsitinib for 5 days. Total HA was measured in culture media as described in A. The linsitinib IC50 concentration is indicated at 151 nM. Data represent mean ± SEM of seven different donor cell strains.
ANTAG3 and linsitinib function additively for dose reduction
In an exploratory study to characterize the effects of ANTAG3 and linsitinib in combination for inhibiting HA production in GOF cells, we used the fixed‐ratio isobologram method to determine median‐effect dose equivalence. Dosing pairs were calculated using the CI theorem of Chou‐Talalay for specified fixed‐ratio constants (Table S1). Dose equivalency was defined as any dosing pair that inhibited HA stimulation by M22 ECmed equal to the effects of ANTAG3 and linisitinib IC50 treatments. As shown in Figure 2A, results indicate that dosing pairs with CI values equal to 1 (CI = 1) yielded inhibition of HA production comparable with the IC50 treatment by either agent alone, while efficacy of other combinations was progressively reduced as CI values decreased. Further analysis evaluating the averaged inhibitory effect grouped by CI value confirmed dose equivalency at CI = 1 (Figure 2B). The isobologram shown in Figure 2C graphically summarizes the dosing pairs of ANTAG3 and linsitinib with activity equivalent to IC50 treatments; all plotted combinations conform to a straight line defining Loewe additivity. In order to substantiate these findings, six different GOF strains were stimulated with M22 ECmed and co‐treated with IC50 doses of ANTAG3 and linsitinib to test for dose reduction. As shown in Figure 2D, treatments of ANTAG3 and linsitinib at IC50 doses were maximally efficacious at inhibiting HA production compared to baseline when used in combination.
Figure 2.
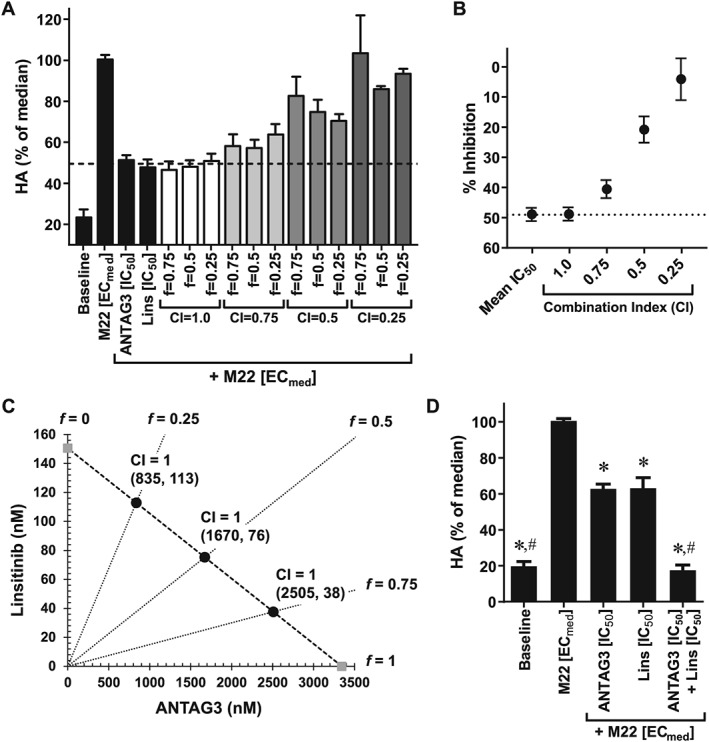
ANTAG3 and linsitinib function additively in combination to inhibit HA production by M22 ECmed. (A) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with ANTAG3 and linsitinib (Lins) at different fixed‐ratios (f) for the defined CI values. All drug combinations were evaluated for their inhibitory effects on HA production by elisa analysis. Data represents mean ± SEM from three donor cell strains plotted as percent HA levels relative to the M22 median response. The dashed line delineates the mean IC50 response of ANTAG3 and linsitinib in order to visualize dose equivalency for any of the indicated combination treatments. (B) The CI‐effect plot displays the averaged inhibitory effect grouped by CI value compared with the mean IC50 response of ANTAG3 and linsitinib. Data represents mean ± SEM from three donor cell strains. (C) The IC50 isobologram depicts the combinational effect of ANTAG3 and linsitinib on HA production. Cultured GOF cells were stimulated with M22 ECmed and co‐treated with ANTAG3 and linsitinib at the indicated fixed‐ratios (f) for 4 days. The Chou–Talalay theorem was used to define dosing pairs based on different CI values at intersecting f ratios. All defined dosing pairs were evaluated for inhibitory effects on HA production by elisa analysis. Only those dosing pairs with activity equivalent to ANTAG3 and linsitinib IC50 activity are plotted on the isobologram. (D) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with ANTAG3 IC50 (3.34 μM), linsitinib (Lins) IC50 (151 nM) or both in combination for 4 days. Total HA was measured in culture media by elisa. Data represent mean ± SEM from six different donor cell strains plotted as percent HA levels relative to the M22 median response. * P <0.001, significantly different from M22 [ECmed]; # P <0.001, significantly different from ANTAG3 [IC50].
Linsitinib efficacy is diminished at elevated M22 concentrations
To determine if ANTAG3 and linsitinib efficacies are conserved at higher M22 concentrations, we treated donor GOFs with maximal doses (ICmax) of either inhibitor and stimulated HA production with increasing concentrations of M22. As shown in Figure 3A, ANTAG3 remained fully efficacious in all concentrations of M22, whereas linsitinib became less efficacious as M22 concentrations increased. Analysis of HA levels at maximal doses (ECmax) of M22 indicated that linsitinib activity was not completely abolished; rather, it remained ~20% efficacious relative to maximal stimulation (Figure 3B). In order to determine if a reduction in maximal efficacy or a shift in potency accounted for the effects of linsitinib at high doses of M22, full concentration‐response curves were generated comparing linsitinib activity at M22 ECmed and near maximal (1 nM) stimulation – note 1 nM M22 was used here (as opposed to maximally efficacious doses) to improve concentration‐response resolution over a possible small range of activity. Data indicated that the reduction in linsitinib activity was the result of diminished maximal efficacy without a significant shift in potency (Figure 3C). In comparison, both concentration‐response curves for ANTAG3 were fully efficacious with similar potencies regardless of M22 concentration (Figure 3D).
Figure 3.
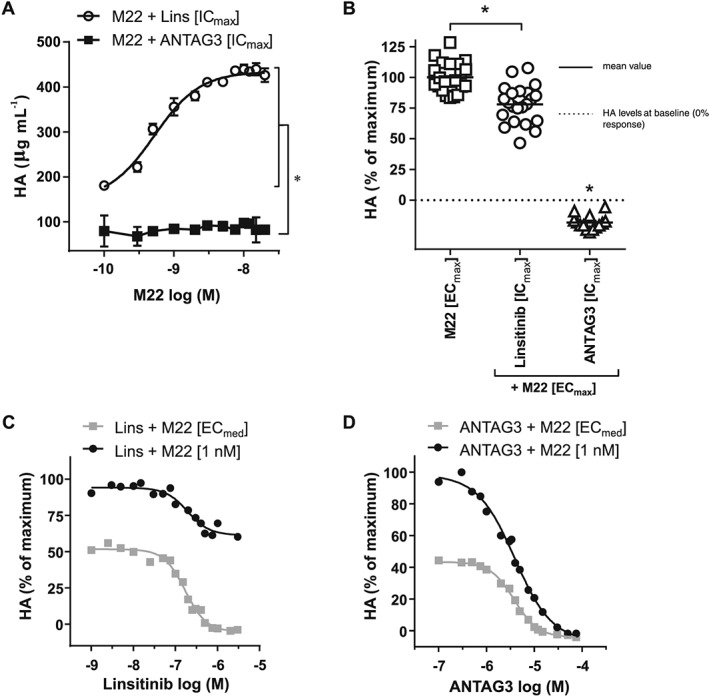
Increasing concentrations of M22 reduces linsitinib efficacy in GOF cells. (A) Cultured GOF cells were treated with a maximal concentration (ICmax) of linsitinib (1 μM) or ANTAG3 (10 μM) and stimulated with increasing concentrations of M22 for 4 days. Total HA was measured in culture media by elisa. Data represents mean ± SEM from five different donor cell strains. Two‐way ANOVA confirms M22 + Lins [ICmax] and M22 + ANTAG3 [ICmax] compensation curves are significantly different. (B) All data points corresponding to maximal doses (ECmax) of M22 (≥10 nM) alone or in combination with linsitinib or ANTAG3 at ICmax are visualized by scatted plot. Data represents five different donor cell strains. One‐way ANOVA analysis confirms the partial reduction in HA production by linsitinib (Lins [ICmax]) is statistically significant. (C) Cultured GOF cells were stimulated with 1 nM M22 or M22 ECmed and co‐treated with increasing doses of linsitinib for 4 days. Total HA was measured in culture media by elisa. Data represents the mean of two donor cell strains plotted as percent HA levels relative to 1 nM M22 maximal response. (D) Cultured GOF cells were stimulated with 1 nM M22 or M22 ECmed and co‐treated with increasing doses of ANTAG3 for 4 days. Total HA was measured in culture media as described in (C). Data represents the mean of two donor cell strains plotted as percent HA levels relative to 1 nM M22 maximal response.
Identification of an IGF‐1 receptor inhibitory antibody that reduces HA production by M22
IGF‐1 stimulates HA production in a monophasic, dose‐dependent manner with a mean EC50 equivalent to 2.05 ± 1.59 nM in GOF cells (S4). In order to validate target specificity, GOF cells were treated with IGF‐1 receptor inhibitory antibodies AF305 or 1H7 in order to block IGF‐1‐mediated stimulation. As shown in Figure 4A, both AF305 and 1H7 completely abolished HA production by IGF‐1 EC50. However, only 1H7 was capable of blocking stimulation by M22 ECmed in a manner similar to linsitinib (Figure 4B). Note AF305 and 1H7 were maximally efficacious at 1 μg·mL−1 following stimulation by IGF‐1 EC50, while 1H7 required concentrations greater than 5 μg·mL−1 to block HA production by M22 ECmed. The differences in effective concentrations are indicative of different potencies depending on the type of stimulation used to trigger HA production in GOFs.
Figure 4.
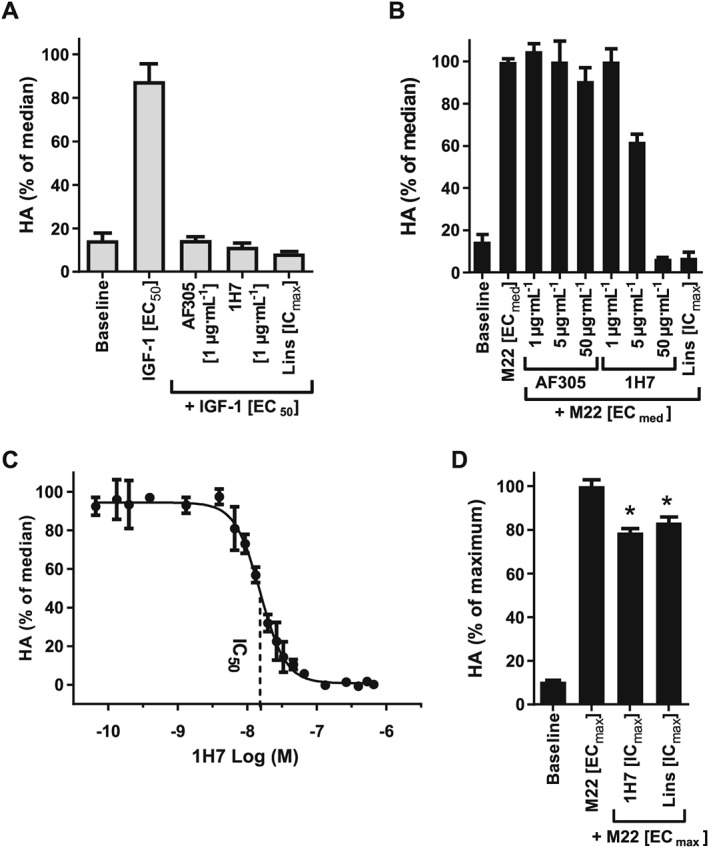
Identifying IGF‐1 receptors inhibitory antibodies with activity against M22 stimulation. (A) Cultured GOF cells were stimulated with IGF‐1 EC50 and co‐treated with anti‐IGF‐1 receptor inhibitory antibodies AF305 or 1H7 at 1 μg·mL−1 concentrations in comparison to 1 μM linsitinib (Lins [ICmax]) for 4 days. Total HA was measured in culture media by elisa. Data bars represent mean ± SEM from three donor cell strains plotted as percent HA levels relative to the M22 median response. (B) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with anti‐IGF‐1 receptor blocking antibodies AF305 or 1H7 at the indicated concentrations for 4 days. Total HA was measured by elisa as described in (A). Data represent mean ± SEM from three donor cell strains plotted as percent HA levels relative to the M22 median response. (C) Cultured GOF cells were stimulated with M22 ECmed and treated with increasing doses of 1H7 for 5 days. Total HA was measured by elisa assay as described in (A). Data represent mean ± SEM of five donor cell strains plotted as percent HA levels relative to the M22 median response (0% corresponding to HA levels at baseline). Indicated is the approximate 1H7 IC50 concentration (14.3 nM). (D) GOF cells were treated with maximal doses (ICmax) of linsitinib (1 μM) or 1H7 (0.3 μM) and stimulated with a maximal concentration (ECmax) of M22 (10 nM). Total HA was measured in culture media by elisa. Data represent mean ± SEM of five donor cell strains plotted as percent HA levels relative to maximal response. * P <0.001, significantly different from M22 [ECmax].
In order to better resolve potency in GOF cells, a full concentration‐response curve was generated for 1H7 following stimulation of HA production by M22 ECmed. As shown in Figure 4C, 1H7 functioned as a full antagonist of M22 ECmed. Moreover, much like linsitinib, 1H7 also lost efficacy at maximal M22 concentrations (Figure 4D). Taken together, both 1H7 and linsitinib share similar pharmacological features in regards to their inhibitory effects on M22.
ANTAG3 and 1H7 function additively for dose reduction
To define the behaviour of ANTAG3 and 1H7, given in combination, in inhibiting HA production in GOF cells, we again conducted a preliminary study using the fixed‐ratio isobologram method to determine median‐effect dose equivalence. All tested dosing pairs calculated by the CI theorem are listed in Table S2. Dose equivalency was defined as any dosing pair equal to the effects of ANTAG3 and 1H7 IC50 treatments alone. Results indicate that dosing pairs at CI = 1 inhibited HA production comparable to the IC50 treatments of either agent; whereas efficacy was progressively reduced in dosing pairs as CI values decreased (Figure 5A). The averaged inhibitory effect of all dosing pairs grouped by CI value also confirmed dose equivalency at CI = 1 (Figure 5B). The isobologram shown in Figure 5C graphically summarizes the dosing pairs of ANTAG3 and 1H7 with activity equivalent to IC50 treatments in which all plotted combinations conform to a straight line defining Loewe additivity. To substantiate these findings and demonstrate dose‐reduction, treatments of ANTAG3 and 1H7 at IC50 doses were maximally efficacious at inhibiting HA production relative to baseline when used in combination on average across 6 different GOF strains (Figure 5D).
Figure 5.
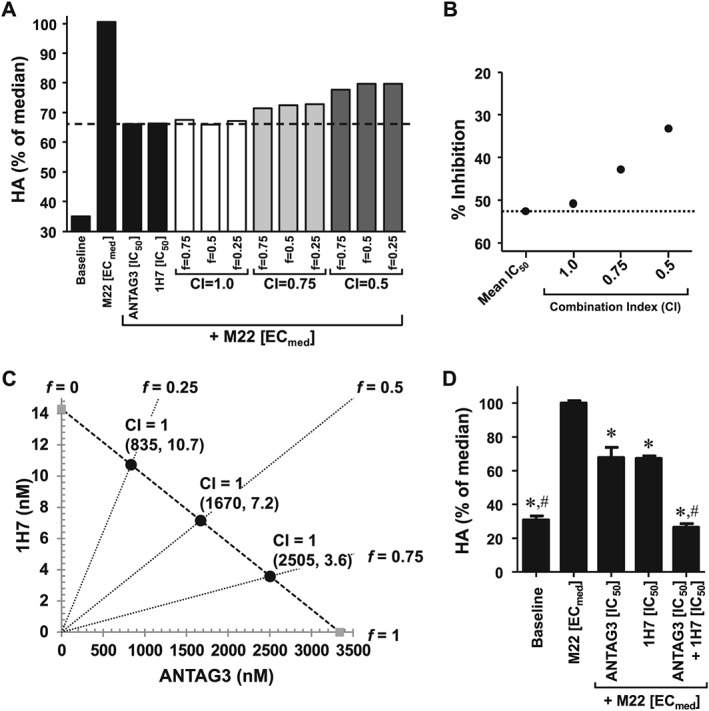
ANTAG3 and 1H7 function additively in combination to inhibit HA production by M22 ECmed. (A) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with ANTAG3 and 1H7 at different fixed‐ratios (f) for the defined CI values. All drug combinations were evaluated for their inhibitory effects on HA production by elisa analysis. Data represents mean from two donor cell strains plotted as percent HA levels relative to the M22 median response. The dashed line delineates the mean IC50 response of ANTAG3 and 1H7 in order to visualize dose equivalency for any of the indicated combination treatments. (B) The CI‐effect plot displays the averaged inhibitory effect grouped by CI value compared to the mean IC50 response of ANTAG3 and 1H7 (dotted line). Data represents mean from two donor cell strains. (C) The IC50 isobologram depicts the combinational effect of ANTAG3 and 1H7 on HA production. Cultured GOF cells were stimulated with M22 ECmed and co‐treated with ANTAG3 and 1H7 at the indicated fixed ratios (f) for 4 days. The Chou‐Talalay theorem was used to define dosing pairs based on different CI values at intersecting f ratios. All defined dosing pairs were evaluated for inhibitory effects on HA production by elisa. Only those dosing pairs with activity equivalent to ANTAG3 and 1H7 IC50 activity are plotted on the isobologram. (D) Cultured GOF cells were stimulated with M22 ECmed and co‐treated with ANTAG3 IC50 (3.34 μM), 1H7 IC50 (14.3 nM), or both in combination for 4 days. Total HA was measured in culture media by elisa. Data represents mean ± SEM from six different donor cell strains plotted as percent HA levels relative to the M22 median response. * P <0.001, significantly different from M22 [ECmed]. # P <0.001, significantly different from ANTAG3 [IC50].
Inhibitors of TSH receptors and IGF‐1 receptors function in combination ex vivo for dose‐reduction
GO‐Igs comprise a heterogeneous mixture of polyclonal antibodies that may not be mimicked by M22 stimulation. Therefore, we purified GO‐Igs from the serum of six patients with active GO (GOB8‐13) to stimulate HA production in primary GOF cultures to validate the dose‐reduction data. As shown in Figure 6, all inhibitor combinations were maximally efficacious and functioned at least additively to block HA stimulation by GO‐Igs. The mean responses indicate that dose‐reduction and individual inhibitor treatments behaved in a manner predictive of the pharmacology data generated using M22 (Figure 6).
Discussion and conclusions
Previous studies have implicated a relationship between TSH receptors and IGF‐1 receptors in the pathogenesis of GO. Earlier work suggested that IGF‐1 receptors served as an auto‐antigen in a manner much like TSH receptors leading to stimulatory antibodies and disease progression (Chen et al., 2014). This has been the basis for the clinical development of anti‐IGF‐1 receptor therapy for the treatment of GO. However, data supporting circulating stimulatory GO‐Igs targeting IGF‐1 receptors have been inconclusive (Minich et al., 2013; Varewijck et al., 2013). More recently, evidence has been presented implicating IGF‐1 receptors in an alternative mechanism of GO pathogenesis by functioning with TSH receptors in crosstalk independent of an IGF‐1 receptor agonist (Krieger et al., 2015; Krieger et al., 2016). Regardless of mechanism, their general relationship warrants evaluating co‐inhibition of TSH receptors and IGF‐1 receptors as possible future treatments for GO.
In this study, we performed basic in vitro pharmacological analyses to characterize the interactions and individual properties of TSH receptor and IGF‐1 receptor inhibitors in response to TSAb stimulation of HA secretion in GOF cells. We found that despite targeting completely different domains of IGF‐1 receptors, linsitinib (a low MW IGF‐1 receptor kinase inhibitor) and 1H7 (an anti‐IGF‐1 receptor inhibitory antibody) have similar pharmacological phenotypes: (i) they are fully efficacious at inhibiting HA secretion by M22 ECmed; (ii) they lose efficacy as M22 concentrations are increased; and (iii) they display Loewe additivity in combination with ANTAG3. These results support IGF‐1 receptor antagonism as a possible therapeutic approach for GO as it has efficacy against TSAb stimulation in our cell model. However, it also suggests that IGF‐1 receptor inhibitors may become less efficacious at elevated titers of TSAbs in GO patients. The anti‐IGF‐1 receptor antibody, teprotumumab, is currently under evaluation for clinical efficacy against GO (https://clinicaltrials.gov/ct2/show/NCT01868997). It appears to exhibit characteristics similar to 1H7 in attenuating TSH receptor signalling independent of an IGF‐1 receptor agonist (Chen et al., 2014; Chen et al., 2015). While the results have not yet been made public, it will be interesting if any correlation can be linked between therapeutic response to teprotumumab and TSAb titre in treated patients.
In comparison, TSH receptors may be a better target for GO treatment. Our results reveal that ANTAG3 retains efficacy at all concentrations of the M22 concentration‐response. Although there is no therapy targeting TSH receptors currently under clinical evaluation, there are low MW compounds in preclinical development (Gershengorn and Neumann, 2012; Davies and Latif, 2015).
This study only investigates HA stimulation and does not directly measure TSH receptor‐mediated cytokine synthesis or (pre)adipocyte proliferation/differentiation; both of which may be important pathological processes in GO (Zhang et al., 2006; Bahn, 2010; Kumar et al., 2012). However, HA secretion correlates with inflammation (Petrey and de la Motte, 2014) and adipogenesis (Ji et al., 2014; Zhang et al., 2016). As such, measurement of HA secretion allows us to indirectly account for these processes.
Dose‐reduction analyses revealed IC50 treatments with ANTAG3, linsitinib and 1H7 alone partly inhibited HA secretion by M22 ECmed, whereas ANTAG3 in combination with either linsitinib or 1H7 was fully efficacious. While it is not possible to reproduce all the experiments performed with M22 using patient blood samples, we did extend our study to include dose‐reduction analyses with GO‐Igs isolated from the blood of several GO patients. GO‐Igs offer a more relevant model for TSH receptor stimulation as they contain natural varieties of polyclonal TSAbs and TSH receptor inhibitory/blocking autoantibodies present in patients (Kampmann et al., 2015). Our results indicate that the effects of dose‐reduction were also conserved in GOFs activated by donor GO‐Igs. Taken together, our data demonstrate proof‐of‐concept that combination therapy targeting TSH receptors and IGF‐1 receptors may be an effective strategy for GO treatment in the future, particularly if teprotumumab proves to be efficacious in the clinic.
Pharmacological approaches for analysing drug combinations represent black‐box techniques for generating meaningful data on drug interactions in the absence of mechanistic insight. However, the results can capture complex signalling dynamics that can be used to understand signalling pathways. For instance, drugs inhibiting different parts of the same linear pathway typically act according to Loewe additivity (Fitzgerald et al., 2006). In our previous studies, we characterized the biphasic nature of the M22 concentration‐response curve in GOF cells and found IGF‐1 receptor antagonists abolish its high potency phase (Krieger et al., 2015; Krieger et al., 2016). This allowed us to identify the IGF‐1 receptor‐dependent and IGF‐1 receptor‐independent components of TSH receptor signalling. We found that drug combinations targeted at TSH receptors and IGF‐1 receptors demonstrated Loewe additivity, in the isobolograms, for inhibiting HA secretion at M22 ECmed. Taken together with our pharmacological data, we can model the M22 signalling pathway to include targeted inhibition of TSH receptor /IGF‐1 receptor crosstalk.
As shown in Figure 7A, concentrations of M22 within the high potency phase of the concentration‐response curve (e.g. M22 ECmed) bind TSH receptors and stimulate HA secretion primarily through crosstalk. Both ANTAG3 and IGF‐1 receptor antagonists (i.e. linsitinib and 1H7) function as full inhibitors acting on different components within the same linear pathway; consistent with Loewe additivity in combination.
Figure 7.
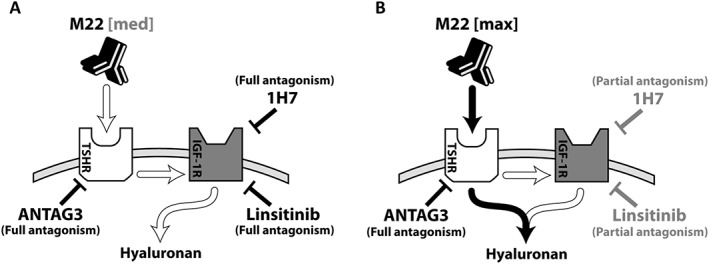
Possible pathways for targeted inhibition of TSH receptor /IGF‐1 receptor (TSHR, IGF‐1R) crosstalk in GOF cells. (A) At M22 concentrations (e.g. M22 ECmed) within the high potency phase of its concentration‐response curve (M22 [med]), HA stimulation is dependent on IGF‐1 receptors as a downstream mediator of TSH receptor activation (white arrows). ANTAG3 and the indicated IGF‐1 receptor inhibitors (linsitinib and 1H7) function as full antagonists by acting on different components within the same linear pathway. (B) As M22 concentrations approach maximal efficacy (M22 [max]), M22 activates a secondary pathway, independent of IGF‐1 receptors, contributing to HA production (black arrows). ANTAG3 retains full efficacy by blocking all feed‐forward pathways at TSH receptors, while linisitinib and 1H7 behave as partial antagonists inhibiting only the IGF‐1 receptor‐dependent component of HA stimulation by M22.
Because M22 is not an IGF‐1 receptor agonist (Krieger et al., 2016), we can infer that IGF‐1 receptors function downstream of TSH receptors . However, as M22 concentrations approach maximal efficacy, the TSH receptors activate a secondary pathway that circumvents IGF‐1 receptors and eliminates most of the inhibitory effects of linisitinib and 1H7 (Figure 7B). In this model, ANTAG3 retains full efficacy by blocking all feed‐forward pathways, while linsitinib and 1H7 become partial antagonists inhibiting only the contributions of IGF‐1 receptor ‐dependent signalling to HA production.
In addition to blocking IGF‐1 signalling, we have demonstrated that IGF‐1 receptor inhibitory antibodies can also attenuate TSH receptor stimulation via crosstalk in the absence of IGF‐1 receptor ligand. However, this feature is not conserved amongst all anti‐IGF‐1 receptor antibodies. We show here and previously (Krieger et al., 2016) that AF305 did not interfere with TSH receptor stimulation, while 1H7 was fully efficacious at low dose M22 (i.e. M22 ECmed). The methodology used to differentiate 1H7 from AF305 can also have broader application in drug development for GO. For instance, it could be implemented to screen all types of IGF‐1 receptor inhibitors as possible candidates for GO therapy.
In conclusion, we provide pharmacological evidence supporting both TSH receptors and IGF‐1 receptors as therapeutic targets for GO. TSH receptor inhibitors (e.g. ANTAG3), IGF‐1 receptor kinase inhibitors (e.g. linsitinib) and select IGF‐1 receptor blocking antibodies (e.g. 1H7) all have variable efficacies for treatment of GO by inhibiting TSH receptor signalling stimulated by TSAbs. Our model for drug intervention indicates anti‐IGF‐1 receptor therapy can function to block TSH receptor signalling by inhibiting crosstalk through IGF‐1 receptors. However, it also highlights a mechanism for possible anti‐IGF‐1 receptor resistance in patients with elevated concentrations of TSAbs. Based on the heterogeneous progression of GO and correlation of disease with TSAb titre, we believe many patients are likely to fall within the high‐potency phase (IGF‐1 receptor‐dependent component) of TSAb signalling, making our findings regarding crosstalk highly relevant for targeted intervention. We showed that combinations of ANTAG3 with linsitinib or 1H7 functioned co‐operatively for dose‐reduction in regard to inhibiting both M22 and GO‐Ig signalling in our cell model. This offers proof‐of‐concept for developing potential drug combinations with a greater therapeutic index than either drug alone. For instance, TSH receptor antagonists combined with selected IGF‐1 receptor inhibitors could allow for lower doses of both drugs to reduce prevalence of any potential side effects, while maximizing therapeutic efficacy. Alternatively, drug combinations may allow TSH receptor antagonists to compensate for any potential loss in anti‐IGF‐1 receptor therapies. While combination therapies are ideal for minimizing potential side effects and improving therapeutic indices at reduced doses, it is also important to remember that drug combinations could lead to additional untoward effects especially when considering the ubiquitous expression of IGF‐1 receptors.
Author contributions
R.F.P. made the following substantial contributions to the work: (i) the conception and design; (ii) the acquisition, analysis and interpretation of the data; (iii) drafting and revising critically for important intellectual content; and (iv) final approval of the version to be published. C.C.K. made the following substantial contributions to the work: (i) the conception and design; (ii) the acquisition, analysis and interpretation of the data; (iii) drafting and revising critically for important intellectual content; and (iv) final approval of the version to be published. S.N. made the following substantial contributions to the work: (i) the conception and design; (ii) revising critically for important intellectual content; and (iii) final approval of the version to be published. M.C.G. made the following substantial contributions to the work: (i) the conception and design; (ii) drafting and revising critically for important intellectual content; and (iii) final approval of the version to be published. R.F.P., C.C.K., S.N. and M.C.G. agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflict of interest
C.C.K., R.F.P., S.N. and M.C.G. have filed a patent pertaining to drug combinations targeting TSH receptors and IGF‐1 receptors. Nova Therapeutics LLC holds licences to intellectual properties applicable in the development of GO therapies. R.F.P. acknowledges support from Nova Therapeutics, LLC, for earlier work on low MW TSH receptor ligand technologies.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Fixed‐ratio combinations of ANTAG3 and linsitinib for median‐effect analysis of dose equivalence.
Table S2 Fixed‐ratio combinations of ANTAG3 and 1H7 for median‐effect analysis of dose equivalence.
Table S3 Patient Characteristics.
Figure S1 M22 dose response in primary Graves' orbital fibroblasts. Cultured GOF cells were stimulated with increasing concentrations of M22 for 5 days in order to generate a dose response curve. Total HA was measured in culture media by elisa. Data represents mean ± SE from 3 different donor cell strains plotted as percent HA levels relative to maximal response. The curve fits to a biphasic model with an ECmed concentration estimated at 0.25 ± 0.04 nM. The grey dotted line corresponds to a forced‐fit monophasic model for comparison to the biphasic curve (solid black line). As previously described (Krieger et al., 2015), fitting to the biphasic model was conducted by GraphPad Prism. The extra‐sum‐of‐squares F test was conducted to discriminate between a monophasic and biphasic models and found the biphasic fit to be significantly better (P < 0.0001). Above data was generated from 3 different donor cell strains, which were not used in the previous publication.
Figure S2 IGF‐1 dose response in primary Graves' orbital fibroblasts. Cultured GOF cells were stimulated with increasing concentrations of IGF‐1 for 5 days in order to generate a dose response curve. Total HA was measured in culture media by elisa. Data represents mean ± SE from 3 different donor cell strains plotted as percent HA levels relative to maximal response. The curve is monophasic with an EC50 concentration estimated at 2.05 ± 1.59 nM. Please note ECmax values of IGF‐1 in patient strains were comparatively ~70–80% of M22 ECmax for HA secretion (data not shown). This is consistent with previously published results (Krieger et al., 2016).
Acknowledgements
We would wish to thank Ms. Bernice Marcus‐Samuels for her assistance with experiments. We would like to acknowledge the support Drs. Neil Miller, Prem Subramanian, Nicholas Mahoney and Shannath Merbs (Johns Hopkins School of Medicine, Baltimore, MD, USA) who have kindly provided materials for our experiments, as well as Dr. Monica Skarulin, Mr. Brent Abel, Ms. Anula Bhusry, Dr. Mary Walter and the members of the NIDDK Clinical Core for providing and processing the blood samples from GO patients. This work was supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, USA (DK011006 to MCK).
Place, R. F. , Krieger, C. C. , Neumann, S. , and Gershengorn, M. C. (2017) Inhibiting thyrotropin/insulin‐like growth factor 1 receptor crosstalk to treat Graves' ophthalmopathy: studies in orbital fibroblasts in vitro . British Journal of Pharmacology, 174: 328–340. doi: 10.1111/bph.13693.
References
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Overview. Br J Pharmacol 172: 5734–5143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Cidlowski JA, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015c). The Concise Guide to PHARMACOLOGY 2015/16: Nuclear hormone receptors. Br J Pharmacol 172: 5956–5978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Kelly E, Marrion N, Peters JA, Benson HE, Faccenda E et al. (2015d). The Concise Guide to PHARMACOLOGY 2015/16: Transporters. Br J Pharmacol 172: 6110–6202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015e). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahn RS (2010). Graves' ophthalmopathy. N Engl J Med 362: 726–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Mester T, Raychaudhuri N, Kauh CY, Gupta S, Smith TJ et al. (2014). Teprotumumab, an IGF‐1R blocking monoclonal antibody inhibits TSH and IGF‐1 action in fibrocytes. J Clin Endocrinol Metab 99: E1635–E1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Shan SJ, Mester T, Wei YH, Douglas RS (2015). TSH‐mediated TNFalpha production in human fibrocytes is inhibited by teprotumumab, an IGF‐1R antagonist. PLoS One 10: e0130322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC (2010). Drug combination studies and their synergy quantification using the Chou‐Talalay method. Cancer Res 70: 440–446. [DOI] [PubMed] [Google Scholar]
- Chou TC, Talaly P (1977). A simple generalized equation for the analysis of multiple inhibitions of Michaelis–Menten kinetic systems. J Biol Chem 252: 6438–6442. [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies TF, Latif R (2015). Targeting the thyroid‐stimulating hormone receptor with small molecule ligands and antibodies. Expert Opin Ther Targets 19: 835–847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald JB, Schoeberl B, Nielsen UB, Sorger PK (2006). Systems biology and combination therapy in the quest for clinical efficacy. Nat Chem Biol 2: 458–466. [DOI] [PubMed] [Google Scholar]
- Gershengorn MC, Neumann S (2012). Update in TSH receptor agonists and antagonists. J Clin Endocrinol Metab 97: 4287–4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji E, Jung MY, Park JH, Kim S, Seo CR, Park KW et al. (2014). Inhibition of adipogenesis in 3T3‐L1 cells and suppression of abdominal fat accumulation in high‐fat diet‐feeding C57BL/6J mice after downregulation of hyaluronic acid. Int J Obes (Lond) 38: 1035–1043. [DOI] [PubMed] [Google Scholar]
- Kampmann E, Diana T, Kanitz M, Hoppe D, Kahaly GJ (2015). Thyroid stimulating but not blocking autoantibodies are highly prevalent in severe and active thyroid‐associated orbitopathy: a prospective study. Int J Endocrinol 2015: 678194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger CC, Gershengorn MC (2014). A modified ELISA accurately measures secretion of high molecular weight hyaluronan (HA) by Graves' disease orbital cells. Endocrinology 155: 627–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger CC, Neumann S, Place RF, Marcus‐Samuels B, Gershengorn MC (2015). Bidirectional TSH and IGF‐1 receptor cross talk mediates stimulation of hyaluronan secretion by Graves' disease immunoglobins. J Clin Endocrinol Metab 100: 1071–1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger CC, Place RF, Bevilacqua C, Marcus‐Samuels B, Abel BS, Skarulis MC et al. (2016). Thyrotropin/IGF‐1 receptor crosstalk in Graves' ophthalmopathy pathogenesis. J Clin Endocrinol Metab 101: 2340–2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Iyer S, Bauer H, Coenen M, Bahn RS (2012). A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in graves' orbital fibroblasts: inhibition by an IGF‐I receptor blocking antibody. J Clin Endocrinol Metab 97: 1681–1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewe S (1953). The problem of synergism and antagonism of combined drugs. Arzneimittelforschung 3: 285–290. [PubMed] [Google Scholar]
- Minich WB, Dehina N, Welsink T, Schwiebert C, Morgenthaler NG, Kohrle J et al. (2013). Autoantibodies to the IGF1 receptor in Graves' orbitopathy. J Clin Endocrinol Metab 98: 752–760. [DOI] [PubMed] [Google Scholar]
- Petrey AC, de la Motte CA (2014). Hyaluronan, a crucial regulator of inflammation. Front Immunol 5: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard J, Han R, Horst N, Cruikshank WW, Smith TJ (2003). Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves' disease is mediated through the insulin‐like growth factor I receptor pathway. J Immunol 170: 6348–6354. [DOI] [PubMed] [Google Scholar]
- Sanders J, Evans M, Premawardhana LD, Depraetere H, Jeffreys J, Richards T et al. (2003). Human monoclonal thyroid stimulating autoantibody. Lancet 362: 126–128. [DOI] [PubMed] [Google Scholar]
- Smith TJ, Tsai CC, Shih MJ, Tsui S, Chen B, Han R et al. (2008). Unique attributes of orbital fibroblasts and global alterations in IGF‐1 receptor signaling could explain thyroid‐associated ophthalmopathy. Thyroid 18: 983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui S, Naik V, Hoa N, Hwang CJ, Afifiyan NF, Sinha Hikim A et al. (2008). Evidence for an association between thyroid‐stimulating hormone and insulin‐like growth factor 1 receptors: a tale of two antigens implicated in Graves' disease. J Immunol 181: 4397–4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turcu AF, Kumar S, Neumann S, Coenen M, Iyer S, Chiriboga P et al. (2013). A small molecule antagonist inhibits thyrotropin receptor antibody‐induced orbital fibroblast functions involved in the pathogenesis of Graves ophthalmopathy. J Clin Endocrinol Metab 98: 2153–2159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varewijck AJ, Boelen A, Lamberts SW, Fliers E, Hofland LJ, Wiersinga WM et al. (2013). Circulating IgGs may modulate IGF‐I receptor stimulating activity in a subset of patients with Graves' ophthalmopathy. J Clin Endocrinol Metab 98: 769–776. [DOI] [PubMed] [Google Scholar]
- Zhang L, Baker G, Janus D, Paddon CA, Fuhrer D, Ludgate M (2006). Biological effects of thyrotropin receptor activation on human orbital preadipocytes. Invest Ophthalmol Vis Sci 47: 5197–5203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ji QH, Ruge F, Lane C, Morris D, Tee AR et al. (2016). Reversal of pathological features of graves' orbitopathy by activation of forkhead transcription factors, FOXOs. J Clin Endocrinol Metab 101: 114–122. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Fixed‐ratio combinations of ANTAG3 and linsitinib for median‐effect analysis of dose equivalence.
Table S2 Fixed‐ratio combinations of ANTAG3 and 1H7 for median‐effect analysis of dose equivalence.
Table S3 Patient Characteristics.
Figure S1 M22 dose response in primary Graves' orbital fibroblasts. Cultured GOF cells were stimulated with increasing concentrations of M22 for 5 days in order to generate a dose response curve. Total HA was measured in culture media by elisa. Data represents mean ± SE from 3 different donor cell strains plotted as percent HA levels relative to maximal response. The curve fits to a biphasic model with an ECmed concentration estimated at 0.25 ± 0.04 nM. The grey dotted line corresponds to a forced‐fit monophasic model for comparison to the biphasic curve (solid black line). As previously described (Krieger et al., 2015), fitting to the biphasic model was conducted by GraphPad Prism. The extra‐sum‐of‐squares F test was conducted to discriminate between a monophasic and biphasic models and found the biphasic fit to be significantly better (P < 0.0001). Above data was generated from 3 different donor cell strains, which were not used in the previous publication.
Figure S2 IGF‐1 dose response in primary Graves' orbital fibroblasts. Cultured GOF cells were stimulated with increasing concentrations of IGF‐1 for 5 days in order to generate a dose response curve. Total HA was measured in culture media by elisa. Data represents mean ± SE from 3 different donor cell strains plotted as percent HA levels relative to maximal response. The curve is monophasic with an EC50 concentration estimated at 2.05 ± 1.59 nM. Please note ECmax values of IGF‐1 in patient strains were comparatively ~70–80% of M22 ECmax for HA secretion (data not shown). This is consistent with previously published results (Krieger et al., 2016).


