Abstract
Bv8 and endocrine-gland-derived VEGF (EG-VEGF), or prokineticins, are two highly related, secreted proteins that we previously described as selective angiogenic mitogens. Here we describe the expression and functional characterization of Bv8 in peripheral blood cells, notably monocytes, neutrophils, and dendritic cells, and in the bone marrow. In human and mouse, the two Bv8 G protein-coupled receptors are expressed in hematopoietic stem cells and specific mature blood cells, including lymphocytes. Bv8 is highly expressed by neutrophils at sites of inflammation and can stimulate migration of monocytes, in a pertussis toxin-sensitive manner. Bv8, or EG-VEGF that shares the same receptors, increased numbers of colony-forming units granulocytic and monocytic in cultures of human or mouse hematopoietic stem cells. Systemic in vivo exposure to Bv8 or EG-VEGF resulted in significant increases in total leukocyte, neutrophil, and monocyte counts. Additionally, adenovirus (Av)Bv8 or AvEG-VEGF delivered just before 5-fluorouracil injury promoted the survival of hematopoietic cells and enhanced progenitor mobilization. In conclusion, Bv8 can promote survival and differentiation of the granulocytic and monocytic lineages. Bv8 potentially modulates growth, survival, and function of cells of the innate and adaptive immune systems, possibly through autocrine or paracrine signaling mechanisms.
Keywords: prokineticins, angiogenesis, G protein-coupled receptor
Bv8 (1, 2) and endocrine gland-derived VEGF (EG-VEGF) (3), secreted proteins also referred to as prokineticin-2 and -1, respectively (4, 5), are structurally related to a larger class of peptides that are defined by a five disulphide-bridged motif called a colipase fold (6–8). We previously demonstrated that these ligands promote proliferation of endothelial cells derived from the adrenal cortex and stimulate angiogenic responses in the ovary (3) and testis (2). A variety of additional activities have been reported, including effects on gastrointestinal motility (4), neuronal survival (9), pain sensation (10), and circadian locomotor rhythm (5). Expression and signal transduction of the two EG-VEGF/Bv8 G protein-coupled receptors (GPCRs) have been characterized. In specific endothelial cells (11), neurons (9) and transfected lines (12–14), receptor activation stimulates calcium mobilization, phosphoinositol turnover, and mitogen-activated protein kinase and Akt pathway activation.
Endothelial cell responses mediated by EG-VEGF receptor (EG-VEGFR) activation in selective primary cultures and in the gonads were comparable to those induced by VEGF. A well characterized, potent angiogenic factor and inducer of permeability, VEGF has been more recently evaluated for its ability to mobilize and promote the proliferation and survival of bone marrow-derived cell types (15). A link between endothelial and hematopoietic cell development was suggested by the common anatomical site for development of the progenitor cells of these systems in the embryo. Moreover, gene ablation experiments in the mouse indicated that VEGF (16, 17) and VEGFR (18) were required for endothelial cell and blood island development. Both autocrine- (15, 19) and paracrine-signaling mechanisms appear to account for VEGF function in progenitor and mature blood cell types.
In addition to the recognized role of VEGF in solid tumor progression, the contribution of VEGF to hematological malignancies has been investigated. Inhibition of VEGF or VEGFR in cultured leukemia cell lines inhibited proliferation or chloroma and leukemia progression in mouse models (19, 20)
In the present study, we characterized the distribution of Bv8 and its receptors, EG-VEGFR-1/prokineticin R-1/GPCR73 and EG-VEGFR-2 in hematopoietic cell types. Ligand and receptors expression are differentially regulated in activated monocytes, and Bv8 or the homologous protein, EG-VEGF, stimulates migration. Bv8 or EG-VEGF increased colony formation of granulocytic and monocytic lineages in human and mouse hematopoietic stem cell (HSC) cultures. Finally, we determined that overexpression of these ligands increased circulating leukocyte numbers in intact or 5-fluorouracil (5-FU)-challenged mice.
Materials and Methods
Expression Analyses. Reagents and conditions for Taqman for EG-VEGF, Bv8, EG-VEGFR-1, EG-VEGFR-2, VEGF, VEGFR-1, and VEGFR-2 have been described (2). RNA was prepared from low-density human cell lines purchased from the American Type Culture Collection; human primary cell RNA was from AllCells (Berkeley, CA) and human tissue RNA was from Becton Dickinson.
Tissues were processed for in situ hybridization, and 33P-UTP-labeled RNA probes were generated as described (21). Sequences for sense and antisense probes for Bv8 have been reported (2).
Recombinant Protein and Adenovirus (Av) Production. Baculovirus production of EG-VEGF and Bv8 has been described (2, 3). Avs encoding LacZ, Bv8, EG-VEGF, or Fltsel (VEGF mutant selective for VEGFR-1) were generated and titered as described (2, 22). Av granulocyte/macrophage-colony-stimulating factor (GM-CSF) was purchased from Qbiogene (Quebec).
Colony Formation Assays. Presorted CD34+ human bone marrow cells were purchased from AllCells. Cells (5,000) were seeded into methylcellulose culture media (complete Methocult GF H4434 or basic H4230) from Stem Cell Technologies (Vancouver) as recommended by the manufacturer. Human HSC experiments were replicated with three independent donors. Colony numbers and phenotypes were determined after 7–14 days.
Mouse HSC, from femurs and tibia, were purified through a series of enrichment steps as described (15). In brief, bone marrow cells were collected by flushing bones with 2% FCS. After erythrocyte lysis, other mature cells were depleted by using conjugated lineage-specific antigen Abs [CD4/L3T3, CD8/Ly2.3, B-220/CD45 (Caltag), Gr-1, Mac-1, and Ter-119 (Pharmingen)], magnetic beads, and the AutoMacs system (Miltenyi Biotec). This depleted fraction was further sorted (Elite ESP FACS) to isolate the double-positive (Sca+Kit+Linlo) population by using anti-Sca-1-FITC, anti-cKit-biotin, and avidin-CyChrome. Cultures were initiated with 2,000 cells in Methocult media (complete media GF M3434, media without CSFs M3334). Additional cytokines included in assays were IL-3, IL-6 (10 ng/ml), G-CSF, or stem cell factor (0.1–5 ng/ml each) all purchased from Stem Cell Technologies.
To determine spleen-colony-forming unit (CFU), single cells were isolated after disrupting the tissue. Cells were counted and 1 × 104 cells (n = 3) from each of two animals per group were plated in GF M3434 media. The colony phenotypes were determined after 7–14 days. Experiments were replicated at least twice for all treatment groups.
Monocyte Assays. Human primary monocytes were isolated from heparinized blood by using the Miltenyi Biotec kit according to the manufacturer's instructions. RNA was purified from cells before or after 3 h in culture, with or without 1 μg/ml LPS, 055:B5 (Sigma).
For migration assays, 5 × 105 cells were added to the upper chamber of 4-μm transwell inserts and incubated for 2.5 h at 37°C. Cells in the lower chamber were counted by using a Coulter counter. Two independent donors were used in each experiment, and experiments were replicated four times. Cells were pretreated for 15 min with pertussis toxin (Ptx) (200 ng/ml) (BioMol) for two independent donors. Phosphorylated extracellular signal-regulated kinase (ERK) (E10) and pan-ERK immunblots (clone16, Transduction Laboratories, Lexington, KY) were performed for monocyte samples pretreated with media or 200 ng/ml Ptx and stimulated with 10% FCS or Bv8.
Animal Experiments. Virus (5 × 108) was delivered by tail-vein injection into nude mice (Charles River Breeding Laboratories). Blood was collected into EDTA from the orbital sinus before and for a time course after virus dosing. Peripheral blood was analyzed by using a Cell Dyn 3700 (Abbott). For bone marrow injury, 150 mg/kg 5-FU (Pharmacia) was administered i.p., 12 h after virus dosing. All animal studies were performed according to institutional guidelines.
Statistics. Results are expressed as the mean ± SD, with the exception of peripheral blood cell counts, mean ± SEM. Data were analyzed by using the unpaired two-tailed Student t test and the log-rank test, and significance was accepted when P < 0.05.
Results
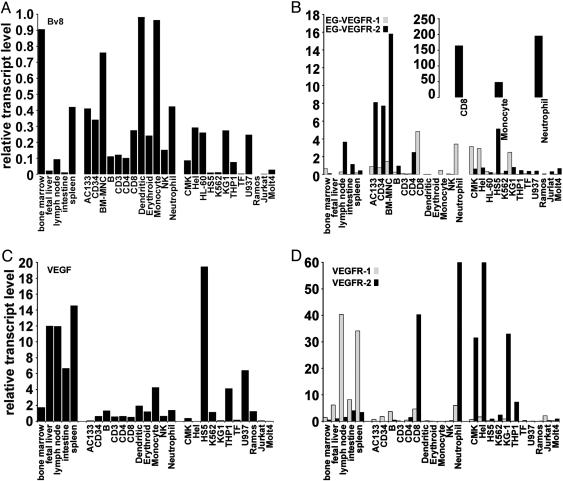
Bv8, EG-VEGFR-1, and EG-VEGFR-2 Are Expressed by Progenitor and Mature Blood Cells. Bv8 RNA was detected in peripheral blood leukocytes and bone marrow (2). Given the difficulty of examining expression of the relatively low abundance GPCRs and to quantitate relative transcript levels of Bv8 and receptors, Taqman analysis was used to examine the expression profiles in a human RNA panel derived from tissues, primary cells, and cell lines. The standard curves for all transcripts were generated by using testis RNA, because all transcripts evaluated are represented in this tissue. Bv8 expression is highest in spleen, bone marrow, and lymph node tissue samples (Fig. 1A). EG-VEGFR-1 and R-2 are also expressed in these tissues (Fig. 1B).
Fig. 1.
Expression of Bv8, VEGF, and receptors in hematopoietic and lymphocytic tissues, cells, and cell lines. (A) Bv8 mRNA levels relative to GAPDH transcript in HSC, within the total population of bone marrow-derived mononuclear cells (BM-MNC), tissues, primary cells, and hematopoietic lines. (B–D) Expression of EG-VEGFR-1 and EG-VEGFR-2 (B), VEGF (C), and VEGFR-1, and VEGFR-2 (D). Transcript data were confirmed in independently prepared RNA samples, and representative results are shown. Testis RNA was used to generate all standard curves, and data were normalized to GAPDH levels.
From cell analysis, Bv8 is expressed by HSCs (AC133 or CD34+) and within the total population of bone marrow-derived mononuclear cells (Fig. 1A). Bv8 is expressed by cells of the innate immune system; specifically, dendritic cells, monocytes, and neutrophils. The highest expression of EG-VEGFR-1 is detected in total mononuclear cells, CD8+ lymphocytes, and HSCs. EG-VEGFR-2 transcript level is high in CD8+ lymphocytes, neutrophils, and monocytes and also detected in B cells, CD4+ lymphocytes, and AC133 or CD34+ cells (Fig. 1B). Mouse HSCs (Sca+Kit+Linlo) also express Bv8 and its receptors. The relative transcript levels in a representative experiment were Bv8, 9.10; EG-VEGFR-1, 4.30; and EG-VEGFR-2 2.05, using testis RNA to generate standard curves (data not shown).
Cell lines including Hel (erythroleukemia), U937 (histiocytic lymphoma) and KG-1 (acute myelogenous leukemia) express Bv8 mRNA (Fig. 1A). Hel, CMK (megakaryoblast), and KG-1 cell lines express EG-VEGFR-1 and EG-VEGFR-2 transcripts (Fig. 1B). Coexpression of ligand and receptors in several of these lines, including HEL and TF (erythroleukemia), HL60 (promyelomonocytic), CMK, U937, and KG-1 suggest that this system may contribute in an autocrine manner to the survival or growth of these cells. Additionally, EG-VEGFR-2 expression is high in the HS-5 bone marrow stromal cell line.
We also examined expression of VEGF and its receptors because Bv8 or EG-VEGF can induce similar angiogenic responses in specific contexts, and the VEGF system has been implicated in bone marrow cell survival, mobilization (15, 23), and leukemias. VEGF (Fig. 1C) and VEGFR-1 (Fig. 1D) expression are highest in tissues examined, including fetal liver, lymph node, small intestine, and spleen. VEGF is also detected in HS-5, TF, and U937 lines. VEGFR-2 is most highly expressed in neutrophils, HEL, CMK, and KG-1 lines (Fig. 1D). Transcripts for VEGF and its receptors are also detectable in human HSC (Fig. 1 C and D) and in mouse Sca+Kit+Linlo cells (24). Relative transcript levels in mouse HSC were VEGF, 0.74; VEGFR-1, 0.017; and VEGFR-2, 0.025, with standard curves derived from mouse testis RNA.
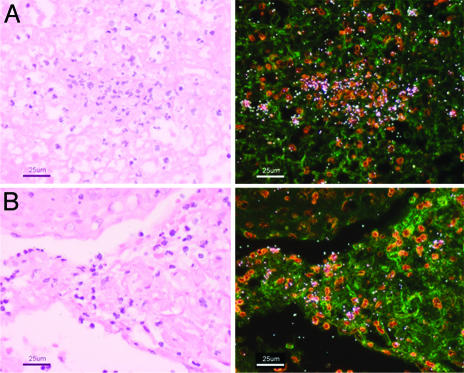
Bv8 Expression at Sites of Inflammation. To further evaluate Bv8 expression, in situ hybridization experiments were performed by using samples from inflamed tonsil or appendix. Clearly, Bv8 signal is associated with the infiltrating cells, predominantly neutrophils (Fig. 2).
Fig. 2.
Human Bv8 is expressed by infiltrating cells at sites of inflammation. Light field (Left) and dark field (Right) micrographs of tonsillitis (A) and appendicitis (B) samples from in situ hybridization studies with a Bv8 antisense probe. Transcript is associated with infiltrating cells, predominantly neutrophils. Sense probe did not generate specific signal (data not shown).
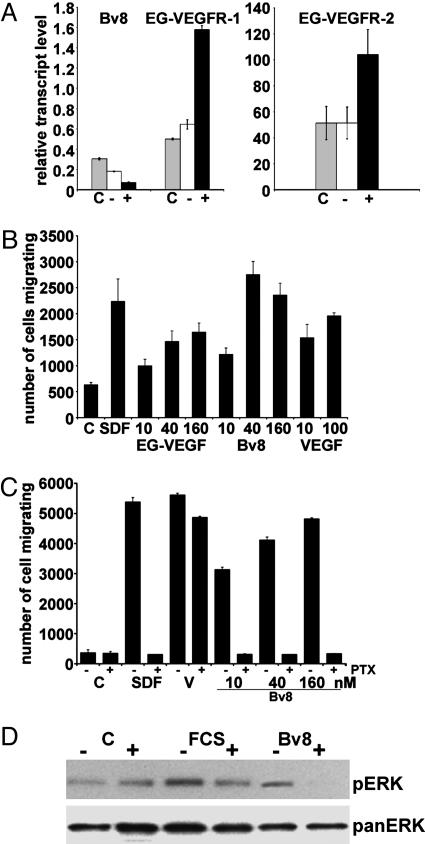
Bv8 or EG-VEGF Induce Monocyte Migration. To assess Bv8 and receptor regulation in monocytes, we exposed primary cells to endotoxin. LPS induced a 100–150% increase in EG-VEGFR-1 and R-2 expression, and a coincident, ≈4-fold decrease in Bv8 expression within 3 h (Fig. 3A). To investigate EG-VEGFR activation in these cells, we tested the ability of ligands to induce migration. Bv8 or EG-VEGF induced significant, dose-dependent increases in numbers of monocytes migrating, 1.9- to 4.3- fold and 1.6- to 2.6-fold, respectively, above control media (Fig. 3B). SDF-1 (25) or VEGF, a previously characterized chemoattractant (26), also significantly stimulated migration. Ptx specifically modifies the heterotrimeric G protein Gαi, blocking downstream signaling of these GPCRs. EG-VEGFR-mediated signaling is Ptx sensitive (11, 12). Consistent with this finding, the Bv8 (or SDF-1) response was significantly inhibited by pretreatment with Ptx (Fig. 3C). VEGF-induced migration, mediated by receptor tyrosine kinase activation, was insensitive. Bv8-induced ERK phosphorylation was also Ptx-sensitive (Fig. 3D), further supporting a direct effect on monocytes.
Fig. 3.
EG-VEGFR-1 and EG-VEGFR-2 induction and activation in monocytes. (A) Expression of Bv8, EG-VEGFR-1, and EG-VEGFR-2 in freshly isolated human monocytes (C), and cells incubated without (–) or with (+) LPS for 3 h. (B) Migration of primary monocytes in response to SDF-1 (500 ng/ml), VEGF (ng/ml), Bv8, or EG-VEGF (nM). Heat-denatured proteins did not induce migration (data not shown). (C) Migration of monocytes pretreated in media alone (–) or 200 ng/ml Ptx (+), in response to SDF-1 (500 ng/ml), VEGF (500 ng/ml), and Bv8. (D) Bv8 stimulates ERK phosphorylation in freshly isolated monocytes. Cells were preincubated in RPMI or Ptx. Representative immunoblot for phosphorylated ERK(Upper); membrane after stripping and blotting for total ERK (Lower).
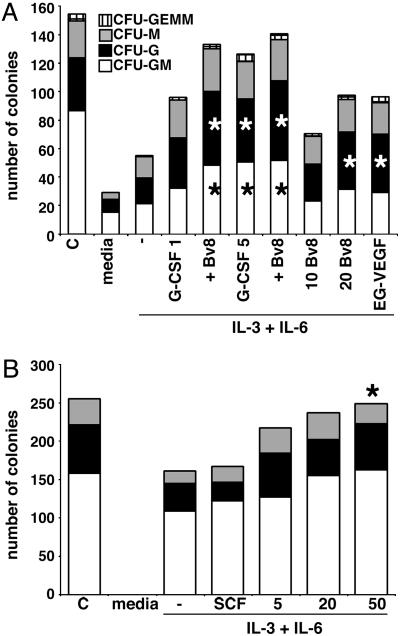
Bv8 or EG-VEGF Stimulates Production of Granulocytic and Monocytic Colonies in Vitro. The expression of Bv8 and receptors in the bone marrow prompted an assessment of the ability of Bv8 or EG-VEGF to stimulate hematopoietic colony formation in vitro. Human colonies were morphologically phenotyped under a light microscope as CFU-GM, CFU-G, CFU-M, and CFU-granulocyte/erythroid/monocyte/megakaryocyte (CFU-erythroid or blast forming unit-erythroid, data not shown). Bv8 or EG-VEGF (20 nM; 5 or 50 nM, data not shown) increased CFU-GM (P < 0.05), CFU-G (<0.07), and CFU-M (<0.2; 0.36) above that of the IL-3 plus IL-6 control media (Fig. 4A). The numbers of granulocytic and monocytic colonies were comparable to those in the positive control G-CSF-treated cultures, although the colonies in Bv8 or EG-VEGF treatment groups contained 40–55% fewer cells. Addition of VEGF did not increase colony numbers (data not shown), as reported (27). Combining 10 nM of Bv8 and 1 ng/ml G-CSF significantly increased the total number of colonies, CFU-GM (P = 0.03) and CFU-G (P = 0.05), over either factor alone (Fig. 4A).
Fig. 4.
Bv8 or EG-VEGF increases granulocytic and monocytic colonies in cultures of human and mouse HSCs. (A) Colony formation increased in human CD34+ HSC cultures after the addition of 20 nM Bv8 or EG-VEGF with IL-3 and IL-6. Combining 10 nM Bv8 and 1 ng/ml G-CSF increased colony formation above either condition alone in human CD34+ cultures. G-CSF (5 ng/ml) served as control. Media control (–) does not contain cytokines. (B) Colony formation in mouse Sca+Kit+Linlo cultures treated with Bv8. Complete methylcellulose media contained stem cell factor, GM-CSF, IL-3, and Epo for human; IL-3, IL-6, stem cell factor, and Epo for mouse. n = 3 for each treatment. *, P ≤ 0.05.
For mouse HSCs, Bv8 induced dose-dependent increases of CFU-GM at 5 nM (P = 0.07), 20 nM (P = 0.06), and 50 nM (P = 0.008) (Fig. 4B). EG-VEGF had essentially identical effects (data not shown). These data demonstrate that Bv8 and EG-VEGF can function as hematopoietic cytokines.
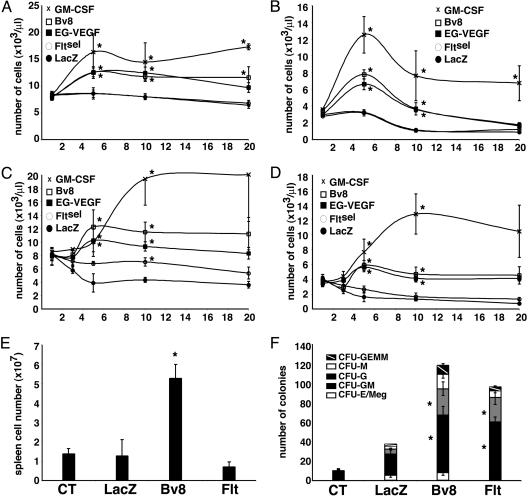
Bv8 or EG-VEGF Stimulates Blood Cell Mobilization in Vivo. Systemic expression of Bv8 or EG-VEGF in the mouse was accomplished by Av injection. To avoid the virus-induced inflammatory response in these long-term studies, nude mice were used. Previous experiments that assessed Av-delivered VEGF in hematopoiesis also used immunocompromised (SCID) mice (28). Exogenous expression of Bv8, EG-VEGF, and GM-CSF transcripts was assessed in liver tissues to confirm transduction (data not shown). Numbers of peripheral blood cells were monitored over the course of 3–20 days. Although no significant changes were observed in red blood cells, treatment with Bv8 or EG-VEGF increased numbers of circulating leukocytes at all time points (Fig. 5A). Bv8 and EG-VEGF induced the same effects in all in vivo studies. At day 5, Bv8- or EG-VEGF- and GM-CSF-treated mice exhibited 1.5- and 1.9-fold higher, respectively, leukocyte counts than LacZ control mice. Correlated with this increase, absolute neutrophil counts were ≈2.4-fold (P < 0.005) higher for Bv8 and EG-VEGF-treated mice at day 5 relative to the control, LacZ-treated group: GM-CSF induced a 3.8-fold increase (P = 0.002) by day 5. Peripheral monocytes also increased with treatment, peak values were observed at day 5 (Bv8 2.8 ± 0.3-fold; EG-VEGF 1.9 ± 0.4-fold; GM-CSF 2.3 ± 0.7-fold). Notably, GM-CSF induced the greatest increases in leukocyte numbers after day 5, so the kinetics, as well as the magnitude, of the mobilization is distinct. Interestingly, peripheral platelet counts were 20–34% higher in the Bv8 and EG-VEGF (P < 0.06) treatment groups, whereas GM-CSF treatment decreased counts relative to control or LacZ mice at all time points, by as much as 60% (P ≤ 0.003) (data not shown).
Fig. 5.
Bv8 or EG-VEGF increases circulating leukocytes in mice and increases peripheral cells and spleen-derived colony forming units after 5-FU injury. Peripheral leukocyte (A) and absolute neutrophil (B) counts in animals treated with Bv8, EG-VEGF, GM-CSF, or control LacZ. Leukocyte (C) and neutrophil (D) counts in 5-FU-injured animals are shown. Bv8, EG-VEGF, GM-CSF, or control LacZ was administered 12 h before cytotoxin. (E) Spleen cell number in control, LacZ, Bv8, and Fltsel mice at day 10 after 5-FU. (F) Colony formation for 1 × 104 spleen cells from control, LacZ, Bv8, and Fltsel mice. Representative experiments are shown; n = 6–10 mice per group. *, P ≤ 0.05.
VEGF has been demonstrated to mobilize stem cells (23), and this effect is mediated by VEGFR-1. For this reason, and to avoid VEGFR-2-mediated systemic toxicities, virus encoding a mutant of VEGF that selectively binds VEGFR-1 was used. In steady-state conditions, effects of Fltsel and LacZ viruses were indistinguishable.
To further evaluate the capacity of these molecules to mobilize and stimulate survival or proliferation of hematopoietic cells, we induced an acute bone marrow injury with the cytotoxic drug 5-FU. Bv8, EG-VEGF, or GM-CSF increased leukocyte numbers >3-fold relative to control LacZ (Fig. 5C). At day 5, when the nadir is achieved in control (data not shown) or LacZ-treated animals to an equivalent level, effects of GM-CSF, Bv8, and EG-VEGF were essentially the same. However, counts continued to increase in the GM-CSF-treated mice, as also observed in the uninjured groups (Fig. 5A). Increases in the GM-CSF-treated animals peaked at day 10, with a 4.4-fold increase. Absolute neutrophil counts significantly increased, 3.5- to 4.7-fold (P < 0.05), in the Bv8-, EG-VEGF-, and GM-CSF-treated mice by day 5, and the GM-CSF counts continued to increase to 9.6-fold by day 10 (Fig. 5D). At day 5, peripheral monocyte counts were 4.7-fold higher in Bv8- and EG-VEGF-treated animals and 2.8-fold higher in the GM-CSF group (data not shown) (Bv8, P = 0.18; EG-VEGF, P = 0.13; GM-CSF, P = 0.09). In Fltsel-treated mice, leukocyte counts were elevated to a significant level at day 10 (P = 0.027). This effect is in contrast to that observed in experiments without 5-FU administration, demonstrating distinct activity in stress or injury-induced mobilization versus steady-state conditions.
Increases in spleen to body ratios for Bv8- and EG-VEGF-treated groups indicated an infiltration of hematopoietic cells. The spleen is a site of extramedullary hematopoiesis. Initially, spleen cellularity was determined after 10 days for two animals in each of control, LacZ, Bv8, EG-VEGF, and Fltsel groups, all having received the single dose of 5-FU. Relative to untreated (1.36 ± 0.28 × 107) or LacZ (1.26 ±.84) controls; Bv8 (5.25 ± 0.71) or EG-VEGF (4.86 ± 0.52) significantly increased spleen cellularity. Fltsel (0.693 ± 0.25) did not increase spleen cell number relative to LacZ control (Fig. 5E). To assay for the presence of hematopoietic precursors, the same absolute number of cells from each sample was plated for colony formation. Interestingly, Fltsel spleen cultures contained high numbers of granulocytic and monocytic progenitors, although the spleen cell number was not increased relative to controls. Therefore, VEGFR-1 activation efficiently, and distinctly, mobilized progenitors from the marrow dependent on injury. Bv8 spleen cultures contained increased numbers of CFU-GM, G, M, and granulocyte/erythroid/monocyte/megakaryocyte (Fig. 5F). Given that the Bv8 spleens contained approximately five times the cell number of other treatment groups, the absolute number of hematopoietic precursors in this tissue was substantial.
Discussion
The present studies demonstrate that Bv8 functions as a monocyte chemoattractant and a hematopoietic cytokine. Although we do not detect EG-VEGF in the same tissue and cell samples as Bv8, this protein does elicit the same effects in cultures and in vivo as those stimulated by Bv8. These ligands bind their shared receptors with similar affinities and activate overlapping signal transduction pathways (11, 12, 14).
Neutrophils, monocytes, and dendritic cells are effectors of innate immunity and are essential coactivators in the acquired immune response. Neutrophils are rapidly recruited to sites of infection, and although they predominate in the early response, monocytes/macrophages eventually infiltrate and release cytokines involved in the initiation, resolution, and repair processes of tissue inflammation (reviewed in ref. 29). The expression of Bv8 in tonsillitis and appendicitis samples suggests that Bv8 can be highly concentrated in inflamed tissues. Predicted Bv8 isoforms are very basic, indicating that they may be regulated through sequestration to extracellular matrix. The potential localization of Bv8 in tissues that express its receptors may represent an important regulatory mechanism, given the pleiotropic activities of Bv8 in hematopoietic, CNS, and endocrine tissue contexts.
The ability of Bv8 to induce monocyte migration, and the expression of receptors on lymphocytes, suggest that Bv8 may directly contribute to the immune response by recruiting additional effectors and mediating B and T cell responses in vivo.
Hematopoietic tissue is regenerated throughout the lifetime of an organism. There has been great interest in identifying growth and survival factors for these cells, specifically to use as adjuncts in chemotherapeutic strategies. In our studies we included the established therapeutic molecules, G-CSF or GM-CSF for comparative purposes (30, 31). Although Bv8 or EG-VEGF increased the granulocytic and monocytic colonies formed in culture to levels comparable to G-CSF, the colonies contained fewer numbers of cells. Possibly, this is the result of more differentiated cell population becoming nonresponsive or at least differently responsive to the growth factor stimulus. It will be important to evaluate responses in subsets of cells at different stages of differentiation to further delineate function. Combining Bv8 with G-CSF in culture increased colony formation. Potentially, these factors will also cooperate in vivo.
At early time points, Bv8, EG-VEGF, and GM-CSF induced similar in vivo effects, yet peak effects for GM-CSF followed a different course, again indicating that these cytokines activate distinct responses. In intact or 5-FU-treated mice, an additional distinction between Bv8 or EG-VEGF- and GM-CSF-treated animals was the impact on peripheral platelet counts. Bv8- or EG-VEGF-treated mice trended to have higher counts throughout experiments. Treatment with GM-CSF has been associated with mild to more severe or protracted thrombocytopenia. However, in methylcellulose culture conditions (which lacks thrombopoietin), Bv8 did not increase CFU-Meg. The effects of Bv8 on megakaryocyte development require additional analyses.
We propose that Bv8 and EG-VEGF act to directly promote the survival and development of granulocytes and monocytes. However, it is possible that the in vivo mechanism for increased leukocytes, and platelets, is in part indirect, mediated by responses of the bone marrow endothelium and stroma. It has been a challenge to examine expression of the EG-VEGF/Bv8 receptors in situ. Therefore, at this point we cannot exclude the potential contribution of stromal cell-derived paracrine signals. Interestingly, Fltsel induced distinct effects and mobilization in animals treated with 5-FU. The spleens of the 5-FU-treated Fltsel mice contained significantly high numbers of progenitors. This result supports that VEGFR-1 activation is most effective when additional, potentially synergistic, chemokines and proteases (23) are induced after bone marrow injury.
Hematopoietic stem cells and some more mature blood cell types express several growth factors and their cognate receptors, making both autocrine and paracrine signaling possible in these populations. This is also the case in many leukemic cells, which may be a reflection of the stage of cell differentiation, or indicate a role for these ligand/receptor systems in malignancy (reviewed in refs. 15 and 32). Several studies have investigated VEGF or placenta growth factor (PlGF) functions in normal or neoplastic blood cells. Initially, contributions of the VEGFR system appeared to be indirect, due to bone marrow angiogenesis and endothelial cell paracrine signaling (33, 34). In vitro, exogenous VEGF or PlGF had no direct effects on HSC survival or colony formation (27), although VEGFR activation promoted cell migration (26, 28). However, inhibition of VEGFR-1 compromised HSC recruitment and mobilization (23) and recent data point to a VEGF internal autocrine loop in regulating hematopoietic repopulation (15). It will be of great interest to investigate the physiological role and the potential requirements for Bv8 receptor activation in blood cell and immune pathologies with neutralizing reagents and receptor antagonists.
The EG-VEGFRs appear to be coexpressed on the same populations of endothelial cells (2, 13). However, within distinct contexts, the receptors may be differentially regulated. For example, monocytes and CD8+ lymphocytes express higher levels of EG-VEGFR-2 than EG-VEGFR-1. A new appreciation for the intricacy of peptidergic GPCRs is developing, as the ligands for orphan receptors are identified, and as distinct signaling and functional responses for multiple ligands and oligomerized receptors are demonstrated (reviewed in ref. 35). Thus the distinct or overlapping expression of EG-VEGF and Bv8 (three isoforms), and the possible heterooligomerization of receptors may increase the functional complexity of this system.
Bv8 peptides were purified from the skin secretions of toads (10, 36). Interest in such exotic factors stem from the identification of proteins related to mammalian hormones, neurotransmitters, and antimicrobial peptides. Lai et al. (36) suggested that the Bv8 peptides are potentially mediators of the amphibian innate immune system. Given the reported expression patterns and activities of the mammalian orthologs in brain (5, 9), reproductive tract (1, 2), and now cells of the immune system, the molecules seem to have subserved this function across evolution.
In conclusion, activation of EG-VEGFRs by Bv8 or EG-VEGF stimulates hematopoiesis, directly increasing granulocyte and monocyte production. Indirect effects within the bone marrow environment are also possible. In peripheral blood and tissues, Bv8 produced by cells of the innate immune system may influence infiltration and activities of both innate cell types and lymphocytes. Future studies should continue to examine the function of this ligands-receptors system in hematopoiesis and reproduction and probe the potential contribution of these molecules to hematological, inflammatory, and gynecological disorders.
Acknowledgments
We are grateful for advice from Ajay Malik and Kirsten Schmidt for cell culture and migration assays and for assistance from Jose Zavala for hematology.
Author contributions: J.L.C., F.P., and N.F. designed research; J.L.C., C.Z., M.T., and F.P. performed research; J.L.C., F.P., and N.F. analyzed data; and J.L.C. and N.F. wrote the paper.
Abbreviations: GPCR, G protein-coupled receptor; GM-CSF, granulocyte/macrophage colony-stimulating factor; HSC, hematopoietic stem cell; ERK, extracellular signal-regulated kinase; Ptx, pertussis toxin; Av, adenovirus; 5-FU, 5-fluorouracil; EG-VEGF, endocrine gland-derived VEGF; EG-VEGFR, EG-VEGF receptor; VEGFR, VEGF receptor.
References
- 1.Wechselberger, C., Puglisi, R., Engel, E., Lepperdinger, G., Boitani, C. & Kreil, G. (1999) FEBS Lett. 462, 177–181. [DOI] [PubMed] [Google Scholar]
- 2.LeCouter, J., Lin, R., Tejada, M., Frantz, G., Peale, F., Hillan, K. & Ferrara, N. (2003) Proc. Natl. Acad. Sci. USA 100, 2685–2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.LeCouter, J., Kowalski, J., Foster, J., Hass, P., Zhang, Z., Dillard-Telm, L., Frantz, G., Rangell, L., DeGuzman, L., Keller, G.-A., et al. (2001) Nature 412, 877–884. [DOI] [PubMed] [Google Scholar]
- 4.Li, M., Bullock, C. M., Knauer, D. J., Ehlert, F. J. & Zhou, Q. Y. (2001) Mol. Pharmacol. 59, 692–698. [DOI] [PubMed] [Google Scholar]
- 5.Cheng, M. Y., Bullock, C. M., Li, C., Lee, A. G., Bermak, J. C., Belluzzi, J., Weaver, D. R., Leslie, F. M. & Zhou, Q. Y. (2002) Nature 417, 405–410. [DOI] [PubMed] [Google Scholar]
- 6.Aravind, L. & Koonin, E. V. (1998) Curr. Biol. 8, R477–R478. [DOI] [PubMed] [Google Scholar]
- 7.Boisbouvier, J., Albrand, J. P., Blackledge, M., Jaquinod, M., Schweitz, H., Lazdunski, M. & Marion, D. (1998) J. Mol. Biol. 283, 205–219. [DOI] [PubMed] [Google Scholar]
- 8.Kaser, A., Winklmayr, M., Lepperdinger, G. & Kreil, G. (2003) EMBO Rep. 4, 469–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melchiorri, D., Bruno, V., Besong, G., Ngomba, R. T., Cuomo, L., De Blasi, A., Copani, A., Moschella, C., Storto, M., Nicoletti, F., et al. (2001) Eur. J. Neurosci. 13, 1694–1702. [DOI] [PubMed] [Google Scholar]
- 10.Mollay, C., Wechselberger, C., Mignogna, G., Negri, L., Melchiorri, P., Barra, D. & Kreil, G. (1999) Eur. J. Pharmacol. 374, 189–196. [DOI] [PubMed] [Google Scholar]
- 11.Lin, R., LeCouter, J., Kowalski, J. & Ferrara, N. (2002) J. Biol. Chem. 277, 8724–8729. [DOI] [PubMed] [Google Scholar]
- 12.Lin, D. C., Bullock, C. M., Ehlert, F. J., Chen, J. L., Tian, H. & Zhou, Q. Y. (2002) J. Biol. Chem. 277, 19276–19780. [DOI] [PubMed] [Google Scholar]
- 13.Masuda, Y., Takatsu, Y., Terao, Y., Kumano, S., Ishibashi, Y., Suenaga, M., Abe, M., Fukusumi, S., Watanabe, T., Shintani, Y., et al. (2002) Biochem. Biophys. Res. Commun. 293, 396–402. [DOI] [PubMed] [Google Scholar]
- 14.Soga, T., Matsumoto, S., Oda, T., Saito, T., Hiyama, H., Takasaki, J., Kamohara, M., Ohishi, T., Matsushime, H. & Furuichi, K. (2002) Biochim. Biophys. Acta 1579, 173–179. [DOI] [PubMed] [Google Scholar]
- 15.Gerber, H.-P., Malik, A., Solar, G. P., Sherman, D., Liang, X. H., Meng, G., Hong, K., Marsters, J. C. & Ferrara, N. (2002) Nature 417, 954–958. [DOI] [PubMed] [Google Scholar]
- 16.Ferrara, N., Carver-Moore, K., Chen, H., Dowd, M., Lu, L., O'Shea, K. S., Powell-Braxton, L., Hillan, K. J. & Moore, M. W. (1996) Nature 380, 439–442. [DOI] [PubMed] [Google Scholar]
- 17.Carmeliet, P., Ferreira, V., Breier, G., Pollefeyt, S., Kieckens, L., Gertsenstein, M., Fahrig, M., Vandenhoeck, A., Harpal, K., Eberhardt, C., et al. (1996) Nature 380, 435–439. [DOI] [PubMed] [Google Scholar]
- 18.Shalaby, F., Rossant, J., Yamaguchi, T. P., Gertsenstein, M., Wu, X. F., Breitman, M. L. & Schuh, A. C. (1995) Nature 376, 62–66. [DOI] [PubMed] [Google Scholar]
- 19.Dias, S., Hattori, K., Zhu, Z., Heissig, B., Choy, M., Lane, W., Wu, Y., Chadburn, A., Hyjek, E., Gill, M., et al. (2000) J. Clin. Invest. 106, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schuch, G., Machluf, M., Bartsch, G., Jr., Masashi, N., Richard, H., Atala, A. & Soker, S. (2002) Blood 100, 4622–4628. [DOI] [PubMed] [Google Scholar]
- 21.Phillips, H. S., Hains, J. M., Laramee, G. R., Rosenthal, A. & Winslow, J. W. (1990) Science 250, 290–294. [DOI] [PubMed] [Google Scholar]
- 22.LeCouter, J., Moritz, D. R., Li, B., Phillips, G., Liang, X. H., Gerber, H. P., Hillan, K. J. & Ferrara, N. (2003) Science 299, 890–893. [DOI] [PubMed] [Google Scholar]
- 23.Hattori, K., Heissig, B., Wu, Y., Dias, S., Tejada, R., Ferris, B., Hicklin, D. J., Zhu, Z., Bohlen, P., Witte, L., et al. (2002) Nat. Med. 8, 841–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ziegler, B. L., Valtieri, M., Porada, G. A., De Maria, R., Muller, R., Masella, B., Gabbianelli, M., Casella, I., Pelosi, E., Bock, T., et al. (1999) Science 285, 1553–1558. [DOI] [PubMed] [Google Scholar]
- 25.Bleul, C. C., Fuhlbrigge, R. C., Casasnovas, J. M., Aiuti, A. & Springer, T. A. (1996) J. Exp. Med. 184, 1101–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barleon, B., Sozzani, S., Zhou, D., Weich, H. A., Mantovani, A. & Marme, D. (1996) Blood 87, 3336–3343. [PubMed] [Google Scholar]
- 27.Ratajczak, M. Z., Ratajczak, J., Machalinski, B., Majka, M., Marlicz, W., Carter, A., Pietrzkowski, Z. & Gewirtz, A. M. (1998) Br. J. Haematol. 103, 969–979. [DOI] [PubMed] [Google Scholar]
- 28.Hattori, K., Dias, S., Heissig, B., Hackett, N. R., Lyden, D., Tateno, M., Hicklin, D. J., Zhu, Z., Witte, L., Crystal, R. G., et al. (2001) J. Exp. Med. 193, 1005–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beutler, B. (2004) Mol. Immunol. 40, 845–859. [DOI] [PubMed] [Google Scholar]
- 30.Lieschke, G. J. & Burgess, A. W. (1992) N. Engl. J. Med. 327, 28–35. [DOI] [PubMed] [Google Scholar]
- 31.Lieschke, G. J. & Burgess, A.W. (1992) N. Engl. J. Med. 327, 99–106. [DOI] [PubMed] [Google Scholar]
- 32.Majka, M., Janowska-Wieczorek, A., Ratajczak, J., Ehrenman, K., Pietrzkowski, Z., Kowalska, M. A., Gewirtz, A. M., Emerson, S. G. & Ratajczak, M. Z. (2001) Blood 97, 3075–3085. [DOI] [PubMed] [Google Scholar]
- 33.Perez-Atayde, A. R., Sallan, S. E., Tedrow, U., Connors, S., Allred, E. & Folkman, J. (1997) Am. J. Pathol. 150, 815–821. [PMC free article] [PubMed] [Google Scholar]
- 34.Fiedler, W., Graeven, U., Ergun, S., Verago, S., Kilic, N., Stockschlader, M. & Hossfeld, D. K. (1997) Blood 89, 1870–1875. [PubMed] [Google Scholar]
- 35.Rashid, A. J., O'Dowd, B. F. & George, S. R. (2004) Endocrinology 145, 2645–2652. [DOI] [PubMed] [Google Scholar]
- 36.Lai, R., Liu, H., Lee, W. H. & Zhang, Y. (2003) Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 134, 509–514. [DOI] [PubMed] [Google Scholar]