Abstract
PURPOSE
In preclinical studies the poly(ADP-ribose) polymerase (PARP) inhibitor veliparib enhanced the antileukemic action of temozolomide through potentiation of DNA damage. Accordingly, we conducted a phase 1 study of temozolomide with escalating doses of veliparib in patients with relapsed, refractory acute myeloid leukemia (AML) or AML arising from aggressive myeloid malignancies.
EXPERIMENTAL DESIGN
Patients received veliparib (20–200 mg once a day on day 1 and twice daily on days 4–12 in cycle 1 [days 1–8 in cycle ≥2]) and temozolomide (150–200 mg/m2 daily on days 3–9 in cycle 1 [days 1–5 in cycle ≥2]) every 28–56 days. Veliparib pharmacokinetics and pharmacodynamics [ability to inhibit poly(ADP-ribose) polymer (PAR) formation and induce H2AX phosphorylation] were assessed. Pretreatment levels of MGMT and PARP1 protein, methylation of the MGMT promoter and integrity of the Fanconi Anemia pathway were also examined.
RESULTS
Forty-eight patients were treated at seven dose levels. Dose-limiting toxicities were oral mucositis and esophagitis lasting >7 days. The maximum tolerated dose was veliparib 150 mg twice daily with temozolomide 200 mg/m2 daily. The complete response (CR) rate was 17% (8/48 patients). Veliparib exposure as well as inhibition of PAR polymer formation increased dose proportionately. A veliparib-induced increase in H2AX phosphorylation in CD34+ cells was observed in responders. Three of 4 patients with MGMT promoter methylation achieved CR.
CONCLUSIONS
Veliparib plus temozolomide is well tolerated, with activity in advanced AML. Further evaluation of this regimen and of treatment-induced phosphorylation of H2AX and MGMT methylation as potential response predictors appears warranted.
Keywords: PARP inhibition, veliparib, acute myeloid leukemia, temozolomide, DNA repair
INTRODUCTION
The clinical outcome for acute myeloid leukemia (AML) in adults continues to improve but remains a challenge, particularly for older adults and for those with AMLs characterized by genetic complexity and/or evolution from an antecedent hematologic disorder (1). Multiple genetic, epigenetic, and microenvironmental factors contribute to resistant leukemia in these patients (1, 2). Among the mechanisms contributing to drug resistance is the activation of various DNA-damage response (DDR) pathways (3). The poly(ADP-ribose) polymerase (PARP) enzymes are involved in a wide variety of nuclear processes, including DNA damage sensing and repair. Among the 17 PARP family members, PARPs 1, 2, and 3 play active roles in diverse DNA repair processes. Especially germane to the present study, PARP1 plays a major role in base excision repair (BER), while PARP2 cooperates with PARP1 in the formation of poly(ADP-ribose) (PAR) polymers (4). In particular, PARP inhibition not only impedes BER, but also results in trapping of PARP1 on DNA, thereby preventing downstream repair proteins from accessing and fixing the damage (4–7).
PARP inhibition is synthetically lethal when combined with certain defects in double- strand break repair (8, 9). Thus, clinical development of PARP inhibitors as single agents focused initially on epithelial malignancies known to be associated with deficiencies in BRCA genes, including breast, ovarian, pancreatic and prostate cancers (4). While the benefits of single-agent PARP inhibitors have been greatest for patients with germline or somatic BRCA1 or BRCA2 mutations (10, 11), the ability of PARP inhibitors to sensitize malignant cells to DNA- damaging agents has also led to preclinical and clinical testing of these inhibitors with cytotoxic drugs that induce DNA damage (4, 12). One such drug is temozolomide, an alkylating agent that forms three methylpurine adducts, two of which (N7-methylguanine and N3-methyladenine) trigger PARP 1 and 2 and are quickly repaired by BER (13). The third adduct, O6-methylguanine, is not repaired by BER, but instead triggers mismatch repair (MMR) that leads to repetitive cycles of excision and repair due to imprecise base pairing, resulting in apoptosis (13, 14). Temozolomide resistance is mediated by MMR deficiency or by enhanced expression of the DNA repair enzyme O6-methylguanine-DNA methyltransferase (MGMT), which removes O6 methyl adducts from guanine (15). Thus, the net cytotoxicity of temozolomide is determined by the interaction of multiple competing DNA repair pathways. In this context, addition of the PARP inhibitor veliparib (ABT-888) to temozolomide led to enhanced cytotoxicity for AML cell lines and primary human AML cells (16). Interestingly, while this combination was most effective for MMR-deficient cells with low MGMT expression, temozolomide potentiation was also detected in MMR-proficient cells and MMR-deficient cells with MGMT overexpression (16). This potentiation has been attributed to PARP1 and PARP2 trapping on the methylated DNA by the PARP inhibitor (5, 7).
In addition to its well-known clinical activity in advanced gliomas, temozolomide exhibits clinical activity in poor-risk AML (17), particularly newly diagnosed AML in older adults (18, 19). In a phase 2 trial of temozolomide 200 mg/m2/day x 7 days in adults ≥ 60 years, response were observed in 22% of those with newly diagnosed AML and 5% of those with relapsed/refractory AML (19). Responses occurred predominantly in AMLs negative for MGMT, with an overall response rate of 60% in MGMT− AMLs versus 6% in MGMT+ cases. Interestingly, 50% of the newly diagnosed AML, but only 10% of relapsed/refractory AML, were MGMT−. In a subsequent study (18), 53% of newly diagnosed patients with AML or high-risk myelodysplastic syndrome (MDS) with low MGMT expression achieved a response to temozolomide, with higher response rates seen in the settings of MDS, MGMT promoter methylation, and/or non-adverse cytogenetics (18).
Building on these clinical data as well as preclinical studies demonstrating that veliparib enhances the activity of temozolomide in leukemia cells (16), we conducted a phase 1 trial combining veliparib and temozolomide in adults with poor-risk myeloid malignancies, including adults with newly diagnosed AML age ≥ 60 with poor-risk features and/or inability to tolerate intensive induction therapy, secondary AML (arising from MDS or myeloproliferative neoplasm [MPN] or therapy-related), relapsed/refractory AML, and aggressive or transformed chronic myelomonocytic leukemia (CMML). In addition to determining safety, efficacy, and pharmacokinetic parameters, we examined selected aspects of DNA repair in AML samples obtained at baseline and during treatment.
PATIENTS AND METHODS
Patient eligibility and selection
Patients 18 years or older with relapsed or refractory AML; CMML-2 (> 10% or > 5–19% blasts and promonocytes in the bone marrow [BM] or peripheral blood [PB], respectively); newly diagnosed AML arising in the setting of prior MDS, MPN or chemotherapy; or patients age ≥60 years having untreated AML with adverse cytogenetic findings and/or not considered candidates for intensive induction chemotherapy were eligible for the study. Patients could have received any number of prior therapies, including allogeneic stem cell transplant (SCT, ≥60 days previously, no graft-versus-host-disease [GVHD], and off immune suppression for at least 2 weeks) or autologous SCT (≥4 weeks previously), provided that performance status (Eastern Cooperative Oncology Group [ECOG] 0–2) and standard organ function criteria (bilirubin < 2 mg/dl, AST and ALT < 5x upper limit of normal, serum creatinine < 2 mg/dl) were met. Patients were excluded if they had active central nervous system leukemia, uncontrolled infection, or other life-threatening illnesses. A leukemic blast count < 30 x 109/L was required at initiation of study treatment. Hydroxyurea (HU), corticosteroids, or leukapheresis had to be discontinued at least 24 hours prior to initiation of treatment; however, HU was allowed on treatment days 1 through 12 of cycle 1 if it became necessary to control a rising blast count (≥30 x 109/L). The study (NCT01139970) was conducted in accordance with the Declaration of Helsinki after approval by the ethics committee of each participating center. Informed consent was obtained from each participant.
Treatment plan and study design
Veliparib was administered orally once on day 1 and then twice daily on days 4 – 12 of cycle 1. In subsequent cycles (cycle 2+), veliparib was administered twice daily on days 1 – 8. Temozolomide was administered orally once a day on days 3 – 9 in cycle 1 and days 1 – 5 in cycle 2+. Each cycle lasted 28 days except for cycle 1, which lasted 30 days (day 28 from the start of temozolomide). Patients who achieved complete remission (CR) or CR with incomplete count recovery (CRi) could receive up to 5 additional cycles of therapy. Patients who achieved partial remission (PR) or hematologic improvement (HI) could continue to receive treatment as long as they derived a clinical benefit and had acceptable toxicities. Peripheral count recovery to absolute neutrophil count (ANC) ≥ 1 x 109/L and platelets ≥ 100 x 109/L (≥80% pre-cycle baseline for CRi) was required for patients in CR/PR prior to next cycle administration. Patients with baseline pancytopenia who achieved anti-tumor effect (HI; > 50% reduction in PB or BM blasts or extramedullary disease) without marrow aplasia (cellularity ≥20%) on day 30 of cycle 1 could receive the next cycle of therapy without full hematologic recovery. A treatment delay of up to 6 weeks was allowed; however, for > 4-week delay because of myelosuppression or in case of dose-limiting toxicity (DLT), the administration of both veliparib and temozolomide was shortened by 1 day in subsequent cycles (maximum twice).
Using a traditional “3+3” design, the temozolomide dose was initially escalated from 150 mg/m2/day (dose level [DL] 1A) to 200 mg/m2/day (DL 1B) given with veliparib 20 mg twice daily (BID). Subsequently, the temozolomide dose was fixed at 200 mg/m2/day and veliparib was dose-escalated as follows: 40 mg BID (DL 2), 80 mg BID (DL 3), 120 mg BID (DL 4), 150 mg BID (DL 5), and 200 mg BID (DL 6). The maximum tolerated dose (MTD) was determined as the highest dose level at which no more than 1/6 patients experienced DLT. Once the MTD was defined, twelve additional patients were enrolled at the recommended phase 2 dose (RP2D) to better define toxicities and to document responses. No intra-patient dose escalation was allowed.
Toxicity evaluation
Clinical and laboratory monitoring of the study participants was performed according to the standards of practice for adults with leukemia undergoing chemotherapy (20). Toxicity was described and graded using NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. DLT was assessed during cycle 1 with the following considered to be DLTs: (i) any grade 4 drug-related non-hematologic toxicity excluding infection; (ii) any grade 3 drug-related non-hematologic toxicity excluding infection, not resolving to ≤ grade 2 within 48 hours, with the following exceptions: grade 3 elevations in bilirubin, AST, ALT or alkaline phosphatase that improved to ≤ grade 2 in ≤ 7 days, or grade 3 mucositis, diarrhea, nausea or vomiting that improved to ≤ grade 2 in ≤7 days; (iii) grade 3 neurotoxicity or nephrotoxicity of any duration; (iv) myelosuppression (ANC < 0.5 x 109/L and platelets < 20 x 109/L) with BM cellularity <5% more than 58 days from the start of therapy without residual leukemia.
Response
Bone marrow aspirate and biopsy were obtained at the time of hematologic recovery following the first cycle of chemotherapy (day 30–42), every 2–3 cycles, at any time leukemia re-growth was suspected, or if persistent pancytopenia more than 58 days from the start of therapy. Responses were assessed according to the International Working Group criteria for CR, CRi, PR (21). HI was defined as at least 50% decrease in marrow or circulating blasts (or extramedullary disease), improvement in ANC (to ≥0.5 x 109/L), platelets (to ≥20 x 109/L) or improved transfusion needs.
Laboratory correlates
Pharmacokinetic studies
Serial blood samples were obtained on days 1 (veliparib alone), 3 (temozolomide alone) and 8 (veliparib and temozolomide) prior to and at 0.25, 0.5, 1, 1.5, 2, 4, 6, 8, and 24 hours after drug administration. Plasma concentrations of veliparib were quantitated using a validated LC-MS method (22). Temozolomide was quantitated in plasma with an HPLC-UV assay modified from Kim et al. (23, 24). Parameters estimated by non-compartment analysis with PK Solution 2.0 (Summit Research Services, Montrose, CO) were: area under the concentration vs. time curve (AUC) from time 0 to infinity; apparent oral clearance (Cl/F); elimination half-life (t1/2); and apparent volume of distribution (V/F). Peak plasma concentrations (Cmax) were the observed values. Differences in the pharmacokinetic parameters between study periods were evaluated using a Wilcoxon matched-pairs signed-rank test. Differences in dose-normalized pharmacokinetic parameters across groups were evaluated using a 2-sided Kruskal-Wallis test. Mann-Whitney U tests were used to assess correlations between veliparib exposure and toxicity. The a priori level of significance was set at P < 0.05.
Pharmacodynamic studies
Fanconi Anemia (FA) pathway integrity
As a readout for integrity of the FA pathway, pretreatment leukemic BM samples were treated with the DNA cross-linker melphalan and FANCD2 ubiquitylation was assessed by immunoblotting (25).
Pretreatment PARP1 and MGMT
Marrow mononuclear cells from Ficoll-Hypaque step gradients (density 1.077 gm/cm3) were washed with serum-free RPMI 1640 medium containing 10 mM HEPES (pH 7.4 at 21 °C) and prepared for electrophoresis as previously described (26). Aliquots containing protein from 5 x 105 cells were subjected to SDS-PAGE and immunoblotting with enhanced chemiluminescent detection (27) using goat anti-MGMT from R & D Systems (Minneapolis, MN), C-2-10 anti-PARP1 (28) from Guy Poirier (Laval University, Ste. Foy, Quebec) and rabbit anti-histone H1 from Active Motif (Carlsbad, CA).
Gene methylation
Methylation analysis of the MGMT, BRCA1 and MLH1 genes was performed by methylation-specific polymerase chain reaction (MS-PCR) on DNA extracted from pretreatment marrow and/or PB blasts as previously described (29, 30).
Suppression of PAR formation
PB specimens were collected on day 1 of veliparib alone and on day 8 in combination with temozolomide (0, 2, 6 and 24 hours after drug administration). BM specimens were collected pretreatment and on day 5–8 in patients who agreed to second BM procedure. PAR was assayed by ELISA as previously described (31).
DNA damage in viable peripheral blood mononuclear cells and CD34+ populations induced by treatment with veliparib and veliparib/temozolomide
PB specimens were collected pre-treatment, on day 1 at 6 hours after treatment and on day 8 prior to veliparib/temozolomide administration. BM aspirates were collected pre-treatment and on day 5–8. Assessments of Ser139-phospho-H2AX (γH2AX) were performed according to previously described methods, using CD34+ cell fractions when appropriate, to distinguish leukemic population from other cells (32).
Statistical considerations
Overall survival (OS) was defined from first day of the treatment to death or censored at the last follow-up date. Leukemia-free survival (LFS) was measured from the date of achievement of CR to the date of relapse or death. Progression-free survival (PFS) was calculated from first day of the treatment to the date that progressive or recurrent disease was objectively documented or date of death. Patients who remained progression-free for PFS and/or leukemia-free for LFS were censored at their last known follow-up date. OS, LFS, and PFS were estimated using the Kaplan-Meier method, along with medians and 95% confidence intervals. Survival data were analyzed as of October 1, 2015.
RESULTS
Patient characteristics
As depicted in Table 1, 48 adults with AML (1 bi-lineage AML/T-ALL) and median age of 69 years were enrolled between June 2010 and February 2014. This was a high-risk group of patients, with 65% having secondary AML and 56% an adverse karyotype. The median number of prior therapies for MDS and AML was 2 (range 0–6). The majority had not responded to the most recent AML therapy (36/39; 92%). Among the 9 patients who had not received prior therapy for AML, 7 had progressed during or after therapy for MDS. Overall, 69% of patients had had prior treatment with high-intensity chemotherapy and 63% with demethylating agents (median 7 cycles, range 1–36; 20% [6/30] received both azacitidine and decitabine). Among 17 patients with prior CR, nine (53%) received allogenic SCT in CR1, with median CR1 duration of 10 months.
Table 1.
Demographics of 48 Adults Receiving Veliparib/Temozolomide
| Age (median, range) | 69 (20–88) |
| Male/Female | 23/25 |
| Disease Category | |
| AML de novo | 17 (35%; 1 T-ALL/AML) |
| Secondary AML | 31 (65%) |
| MDS | 13 (27%) |
| t-MDS/AML | 9 (19%) |
| MPN | 3 (6%) |
| CMML | 6 (13%) |
| Prior Anti-Leukemia (MDS) Therapy* | |
| None | 2 (4%) |
| Cytotoxic Chemotherapy | 33 (69%; 3*) |
| Demethylating Agents | 30 (63%; 4*) |
| Allogeneic SCT | 9 (19%) |
| Disease Status (n=39; previously treated for AML) | |
| 1st Relapse | 12 (31%; 10#) |
| ≥ 2 Relapse | 5 (13%; 4#) |
| 1° Refractory | 12 (31%; 7&) |
| Multiply Refractory | 10 (25%) |
| Median duration CR1 | 10 (1.5–41) months |
| Genetics | |
| Favorable | 1 (2%; inv16, c-KIT+) |
| Intermediate | 20 (42%; 13 normal karyotype) |
| FLT3-ITD | 4** (10%; 40 tested) |
| NPM1 | 2** (7%; 29 tested) |
| Adverse | 27 (56%) |
| Single | 6 |
| Complex (≥ 3) | 9 |
| Monosomal karyotype | 2 |
7 patients received only therapy for MDS or MPN;
refractory relapse;
received only demethylating agents;
1 both FLT-3 and NPM1 mutation
DLTs, MTD, and safety
No DLT was observed at veliparib doses from 20 to 150 mg twice a day and temozolomide 200 mg/m2; 2 of 4 patients experienced a DLT at veliparib 200 mg twice a day consisting of grade 3 oropharyngeal mucositis/esophagitis lasting more than 7 days. Thus, the MTD and RP2D was defined as temozolomide 200 mg/m2/day for 7 days along with veliparib 150 mg twice a day for 9 days beginning on the day after temozolomide initiation. Overall 9 patients required cytoreduction with HU prior to study to meet eligibility criterion for circulating blast count, with 5 of those 9 patients requiring HU again during the first 6–10 days of treatment on the study. Administration of HU did not appear to exacerbate regimen-related toxicities and none of the patients receiving HU developed DLT. The median number of cycles administered was 1 (range, 0.4 – 7).
The most common non-hematologic adverse events of any grade associated with the temozolomide/veliparib regimen were nausea/vomiting (36%; 40% cycle 1), fatigue (24%; 29% cycle 1), mucositis (13%; 17% cycle 1), diarrhea (10%; 10% cycle 1) and constipation (11%; 15% cycle 1). All non-hematologic adverse events are listed in Table 2. Only one patient discontinued study treatment due to an adverse event, specifically worsening fungal pneumonia and respiratory failure on day 8. An additional patient discontinued treatment on day 5 per patient preference. The most common serious adverse events were infections (Table 3), including grade ≥3 bacteremia, pneumonia and sepsis (40% cycle 1; 24% all cycles); febrile neutropenia (25% cycle 1; 20% all cycles); and oropharyngeal mucositis/esophagitis (4%), representing the DLT. While mucositis and infections were seen at all dose levels, they increased in severity and frequency, respectively, at the highest dose. Constitutional symptoms also increased in frequency with higher veliparib doses, but did not meet criteria for DLT.
Table 2.
Regimen-Related Non-Hematologic Toxicities During Veliparib/Temozolomide
| Dose level | Cy 1 (n=48) | 1A/B (n=8) | 2 (n=3) | 3 (n=3) | 4 (n=11) | 5 (n=19) | 6 (n=4) | Cy ≥2 (n=36) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||||||
| Toxicities | Grade | ≤2 | ≥3 | ≤2 | ≥3 | ≤2 | ≥3 | ≤2 | ≥3 | ≤2 | ≥3 | ≤2 | ≥3 | ≤2 | ≥3 |
|
| |||||||||||||||
| Total | |||||||||||||||
|
| |||||||||||||||
| Constitutional | |||||||||||||||
| Fatigue/Weakness | 14 | 2 | - | - | - | - | - | 5 | - | 4 | - | 3 | - | 6 | - |
| Anorexia | 3 | 1 | - | - | - | - | - | 1 | - | 1 | - | - | - | 3 | - |
| Flushing/Insomnia/Weight loss/Chills | 3 | 1 | - | - | - | - | - | 2 | - | - | - | - | - | 2 | - |
| Gastrointestinal | |||||||||||||||
| Altered taste | 2 | - | - | - | - | - | - | 2 | - | - | - | - | - | - | - |
| Nausea/Vomiting | 19 | 3 | - | - | - | 1 | - | 3 | - | 9 | 1 | 2 | - | 11 | - |
| Oropharyngeal mucositis | 8 | 1 | - | 1 | - | - | - | 1 | - | 2 | - | 1 | 2 | 3 | - |
| Esophagitis | 2 | - | - | - | - | - | - | - | - | - | - | - | 2 | - | - |
| Diarrhea | 5 | 1 | - | 1 | - | - | - | 2 | - | 1 | - | - | - | 3 | - |
| Constipation | 7 | 2 | - | - | - | 2 | - | 2 | - | 1 | - | - | - | 2 | - |
| Abdominal pain | 2 | 1 | - | - | - | - | - | 1 | - | - | - | - | - | 1 | - |
| Hepatic | |||||||||||||||
| (↑Alk Phos or Bilirubin) | 3 | - | - | - | - | 1 | - | 2 | - | - | - | - | - | - | - |
| Cardiac (edema) | 1 | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - |
| Pulmonary (dyspnea) | 0 | - | - | - | - | - | - | - | - | - | - | - | - | 1 | - |
| Neuro (dizziness) | 1 | - | - | - | - | - | - | - | - | 1 | - | - | - | - | - |
| Musculoskeletal Back, joint or body pain | - | - | - | - | |||||||||||
| 3 | - | - | 1 | - | - | - | 1 | - | 1 | - | - | - | - | - | |
| Dermatologic | |||||||||||||||
| Skin rash/dry skin | 1 | 1 | - | - | - | - | - | - | - | - | - | - | - | 2 | - |
| Chemistry/Laboratory | |||||||||||||||
| ↑UA; ↑/↓Phos; ↓Na; ↑Glucose | 5 | 2 | - | 1 | - | - | - | 2 | - | - | - | - | - | 1 | - |
| Decreased albumin or protein | 1 | - | - | - | - | - | - | 1 | - | - | - | - | - | - | - |
Table 3.
Infectious Complications, Death, and Hematologic Toxicities During Veliparib/Temozolomide Treatment
| Infections | Cycle 1 (n=48) | All cycles (n=84) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Grade | ≤ 2 | 3–4 | 5 | ≤ 2 | 3–4 | 5 | |
|
| |||||||
| Febrile Neutropenia (FN) only | - | 12 | - | - | 17 | - | |
|
| |||||||
| *#Pneumonia | 2 | 8 | 2 | 3 | 9 | 2°,°° | |
|
| |||||||
| *&Bacteremia/sepsis | 1 | 13 | - | 1 | 14 | - | |
|
| |||||||
| *Pneumonia and bacteremia | 5 cycles both | 6 cycles both | |||||
|
| |||||||
| # 9 of 14 pneumonia presumed or documented fungal | |||||||
| & 7 of 15 bacteremias considered catheter-related | |||||||
| Frequency of pneumonia, bacteremia, sepsis according to dose level (DL): | |||||||
| DL 1A/B 38%; DL2 0%; DL3 33%; DL4 36%; DL5 42%; DL6 75%. | |||||||
|
| |||||||
| Other grade 3 infections (All cycles): cellulitis (1); cholecystitis (2); tooth infection (1); | |||||||
| Other not regimen related ≥ grade 3 toxicities in ≥ 5% patients (All cycles): | |||||||
| Hypophosphatemia (7); hypokalemia (3); hyperbilirubinemia (3) | |||||||
|
| |||||||
| °Death ≤ 30 days: | |||||||
| 1 patient (veliparib [V] 20 mg) died on day 15 due to worsening of pre-existing fungal pneumonia in the setting of progressive disease; completed 8 days of treatment. | |||||||
| °°Deaths ≤ 60 days: | |||||||
| 1 patient (V 120 mg, day 31) died of Aspergillus pneumonia in the absence of count recovery, neutropenic for months before enrollment on the study. | |||||||
| In addition, 9 patients died of documented refractory/progressive disease on days 36 (V 150 mg), 36 (V 150 mg; plus sepsis and multi-organ failure), 39 (V 20 mg; plus aspiration pneumonia), 45 (V 150 mg), 48 (V 150 mg), 55 (V 40 mg), 56 (V 150 mg; plus ileus/irreducible inguinal hernia), 58 (veliparib 120 mg), and 60 (veliparib 150 mg; patient withdraw on day 5 per patient preference). An additional patient died on day 60 while undergoing salvage treatment with clofarabine. | |||||||
|
| |||||||
| Hematologic Toxicities | |||||||
|
| |||||||
| Count Recovery | ANC > 0.5 × 109/L (range) | ANC > 1 × 109/L (range) | Platelets > 20 × 109/L (range) | Platelets > 100 × 109/L (range) | |||
|
| |||||||
| Cycle 1, days | 38 (20–49) | 43 (30–56) | 35 (29–45) | 42 (33–56) | |||
|
| |||||||
| Cycle ≥ 2, days | 8 (0–49) | 32 (0–52) | 21 (0–38) | 37 (29–77) | |||
The 30- and 60-day all-cause mortality rates were 2.1% and 22.9%, respectively (Table 3). With the exception of a single patient who died of Aspergillus pneumonia in the absence of count recovery, all patients had documented progressive/refractory disease at the time of death. The most common non-regimen related ≥ grade 3 toxicities occurring in ≥ 5% patients were hypophosphatemia (7; grade 3), hypokalemia (4; grade 3); and increased bilirubin (3; grade 3).
In patients who achieved CR, the median time to recovery of ANC > 0.5 x 109/L and platelets > 20 x 109/L was 38 (range, 20–49) and 35 days (range, 29–45), respectively (Table 3). In subsequent cycles administered in remission, the median time to recovery of counts was shorter, the duration of neutropenia (ANC < 0.5 x 109/L) and thrombocytopenia (platelets ≤ 20 x 109/L) were a median of only 4 (range, 0–25) and 3 days (range, 0–21), respectively, and in 50% and 36% of subsequent cycles, ANC and platelets did not nadir below those levels.
Responses
CRs were attained in 8 of 48 patients (16.6%), with 7 of 8 achieving CR after a single cycle. An additional 8 patients achieved HI/disease stabilization as manifested by decrease or absence of circulating blasts or reduction in marrow blasts. Responses were observed at all dose levels, across different AML subtypes, and in those previously treated with demethylating agents and/or allo SCT (Table 4). Among 5 patients requiring additional HU to control blast count during the first 10 days of therapy, 3 developed disease progression within less than 30 days while 2 patients having AML arising from CMML achieved durable HI/SD.
Table 4.
Characteristics of Patients Responding to Veliparib/Temozolomide Treatment
| DL | Age/Sex | Disease | Prior treatment | Karyotype | Response | Cy | Off Study |
|---|---|---|---|---|---|---|---|
| 1A | 71F | AML | 7+3, HiDACx3-CR1 of 33 months (mos); Relapse-ara-C+Chk1 inhibitor-NR | 46, XX; FLT3-ITD and NPM1 mut | CR | 4 | For alloSCT but 4 mos delay -Relapse |
| 2 | 70F | AML from MDS | MDS-AZAx19; DECx2-SD; transformed to AML- 7+3- NR; SAHA-HiDAC+etoposide-NR | 46, XX, t(3;5) (q25;q33) | CR | 3 | Relapse |
| 3 | 76F | t-AML from MDS | Hx of t(8;21)AML s/p 7+3 and tipifarnib but developed t-AML from MDS 19 mos later | 46, XX | CR | 4 | Relapse |
| 4 | 64F | AML | AcDVP-16-NR AZAx5-NR |
46, XX, del(7)(q32) | CR | 3 | NMA alloSCT- still in CR |
| 4 | 61M | AML from MDS | Flavopiridol+ara-C-NR | Complex (>5) | CR | 2 | NMA alloSCT-Relapse 3 mos later |
| 5 | 63M | AML from CMMoL | Induction chemo and NMA alloHSCT-CR; relapse 39 mos later-AZAx20 and DLIx2 | 46, XY | CRp post Cy1 but CR post Cy2 | 2 | DLI and then NMA alloSCT |
| 5 | 64M | AML | AcDVP-16/NMA alloHSCT-CR1 of 41 mos; AcDVP-16 + AZA-CR2 of 3 mos; 2nd relapse | 46,XY,t(2;21)(p11.2;p11.2),t(13;15)(q34;q22) | CR post Cy2 | 2 | Due to histoplasmosis; received anti-CD123 Abx6 in CR |
| 5 | 70M | AML from MDS | AZA+etinostatx2-NR | Complex (>5) Inv(3) |
CR | 4 | PD |
| 1A | 62M | AML from MDS | AZAx18 for MDS then transformed to AML | 46, XY | HI-clearance of PB and decrease in BM blasts; stable counts | 6 | PD |
| 1B | 73F | AML from CMML | Triapine+Fludarabinex4-PD on HU | 46, XX | SD/HI-reduction and stabilization of WBC; clearance of circulating blasts | 3 | PD |
| 2 | 71F | AML from CMML | Triapine+Fludarabinex2-PD on HU | 46, XX | SD/HI-reduction and stabilization of WBC; reduction in circulating blasts | 7 | PD |
| 3 | 73M | AML from MDS | MTX/dexamethasone/G-CSF for MDS-transformed to AML-AZAx5-NR; Clofarabine ×1-NR | 46, XY | SD-WBC count stabilization | 3 | PD |
| 4 | 70F | AML from MDS | AZAx2 for MDS-transformed to AML on HU | 47, XX, +8 | HI-marrow PR and clearance of circulating blasts | 2 | PD |
| 4 | 70M | AML from MPN/MDS | AZAx15, Revlimid × 1, Onconova × 6 for MDS-transformed to AML on HU | 46, XY | HI-clearance of circulating blasts, increase ANC, platelets stable | 3 | PD |
| 6 | 75F | AML from MDS | Epogen/G-CSF; AZAx10 for MDS-transformed to AML | 46, XX | HI- marrow CR | 2 | PD |
| 5 | 75M | AML from MDS | AZAx12 for MDS-transformed to AML-Revlimid-NR; Clofarabinex1-NR | 47, XY, del(3)(q12q21), +8 | SD/HI-disease stabilization and decrease in BM blasts | 2 | PD |
Abbreviations: M-male; F-female; HiDAC-high dose cytarabine; AZA-azacitidine; DEC-decitabine; ACDVP-16-cytarabine, daunorubicin, etoposide; MTX-methotrexate; HU- hydroxyurea; DLI- donor lymphocyte infusion; CR-complete remission; CRp-CR with incomplete platelet recovery; NR-no response; HI-hematologic improvement; SD-stable disease; PD- progressive disease; NMA- non myeloablative; WBC- white blood cell
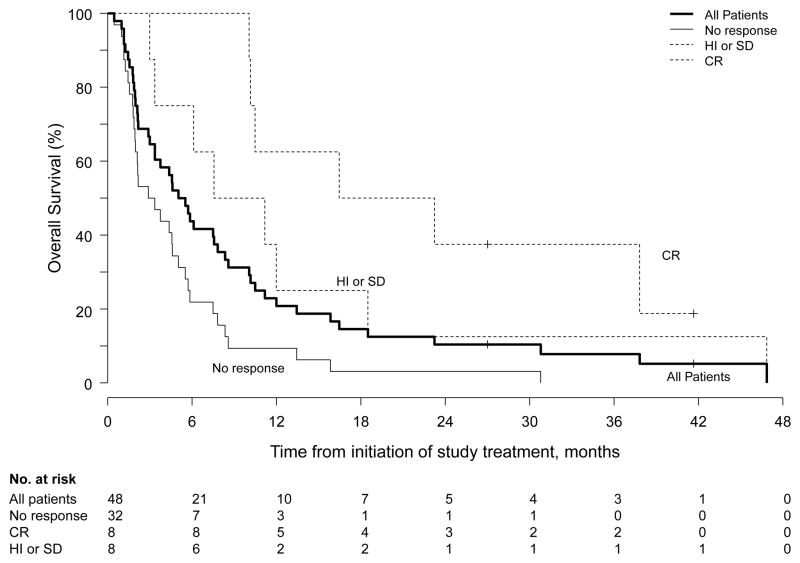
The median follow-up for the entire cohort was 5.3 months (range, 0.5–47). In the 8 patients who achieved CR, the median LFS was 6.4 months (95% CI, 5.4–40+ months) and the median PFS was 9.1 months (95% CI, 7.2–42+ months). Patients having responses of HI/SD had a median PFS of 3.1 months (95% CI; 3–42+ months). The median OS for all patients was 5.3 months (95% CI, 3.3–8.3 months) (Figure 1). Patients who achieved CR had a median OS of 20 months (95% CI, 10–47+ months), while median OS for patients who achieved HI/SD was 9.4 months (95% CI, 6.1–47+ months). Median OS for all responders was 12 months (95% CI, 10–47+ months). Five patients underwent allogeneic SCT; 3 of those patients had achieved remission with the study treatment. One patient remains in CR at 3.5 years follow-up.
Figure 1.
Overall survival for all patients and according to response.
Veliparib and temozolomide pharmacokinetics
Pharmacokinetics of veliparib and temozolomide (Table 5) were available for 37 patients. There was no significant change in veliparib tmax (P=0.18) and t1/2 (P=0.91) when administered alone or in combination with temozolomide. As indicated by the accumulation index for Cmax (1.66 ± 0.62) and AUC (1.38 ± 0.52), there was significant accumulation of veliparib which corresponds with a decrease in both Cl/F (P=0.0001) and V/F (P=0.01). Due to the large variability in exposure, there was only a significant difference noted in dose-normalized Cmax when administered alone (P=0.03) but in no other pharmacokinetic parameters across dose levels. At the highest administered dose of 200 mg, there was a 2.5-fold increase in Cmax with only a 1.4-fold increase in AUC when veliparib was administered alone. It was also noted that there was a decrease in veliparib V/F (alone P=0.003; combination P=0.012), and t1/2 (alone P=0.014) when administered alone in female patients, but not in male patients (data not shown).
Table 5.
Plasma Pharmacokinetic Parameters1 of Veliparib or Temozolomide when Administered Alone and in Combination
| Veliparib Dose (mg) | Temozolomide Dose (mg/m2/day) | Alone or in Combination | Cmax (μg/mL) | tmax (h) | AUCINF or Tau (μg*h/mL) | t1/2 (h) | Cl/F (L/h) | V/F (L) |
|---|---|---|---|---|---|---|---|---|
| Veliparib Pharmacokinetics | ||||||||
| 20 | 150 or 200 | Alone | 0.09±0.03 (4) | 2.8 (0.5–4.0; 4) | 0.7±0.4 (3) | 5.9±0.4 (3) | 33.2±18.4 (3) | 285±175 |
| Combination | 0.14±0.006 (3) | 2.0 (1.0–4.0; 3) | 0.9±0.1 (3) | 5.6±2.1 (3) | 22.9±3.6 (3) | 180±49 (3) | ||
| 40 | 200 | Alone | 0.45 (1) | 2.0 (1) | 4.7 (1) | 7.3 (1) | 8.6 (1) | 90 (1) |
| Combination | n/a | n/a | n/a | n/a | n/a | n/a | ||
| 80 | 200 | Alone | 0.26, 0.98 (2) | 1.0, 1.5 (2) | 3.1, 5.1 (2) | 3.2, 8.9 (2) | 15.6, 25.6 (2) | 72, 328 (2) |
| Combination | 0.69, 1.0 (2) | 2.0, 2.0 (2) | 4.6, 8.1 (2) | 5.3, 6.9 (2) | 9.9, 17.4 (2) | 76, 173 (2) | ||
| 120 | 200 | Alone | 0.74±0.21 (10) | 1.5 (0.5–6.0; 10) | 6.3±1.7 (1) | 6.7±3.2 (10) | 20.2±4.9 (10) | 181±50 (10) |
| Combination | 1.2±0.25 (8) | 2.0 (1.5–4.0; 8) | 8.4±1.8 (8) | 9.1±8.0 (8) | 15.0±3.6 (8) | 191±165 (8) | ||
| 150 | 200 | Alone | 1.0±0.31 (16) | 1.5 (0.5–4.0; 16) | 10.4±7.5 (16) | 9.0±7.3 (16) | 18.4±7.5 (16) | 190±72 (16) |
| Combination | 1.5±0.75 (12) | 1.3 (0.3–4.0; 12) | 12.4±6.1 (10) | 5.3±1.7 (10) | 14.6±6.8 (10) | 109±45 (10) | ||
| 200 | 200 | Alone | 2.5±0.99 (4) | 1.8 (1.0–2.0; 4) | 15.1±5.1 (4) | 4.1±1.2 (4) | 14.6±5.1 (4) | 84±26 (4) |
| Combination | 3.9±3.0 (3) | 1.5 (1.0–2.0; 3) | 14.5±4.1 (3) | 3.6±2.8 (3) | 14.6±4.1 (3) | 77±59 (3) | ||
| Temozolomide Pharmacokinetics | ||||||||
| 20 | 150 | Alone | 5.9, 10.2 (2) | 1.0, 4.0 (2) | 27.4, 33.8 (2) | 1.9, 2.3 (2) | Pred. 10.3, 13.2 (2) Obs. 8.6, 13.1 (2) |
Pred. 32, 37 (2) Obs. 29, 35 (2) |
| Combination | 6.3, 8.3 (2) | 1.0, 2.0 (2) | 25.6, 36.0 (2) | 1.6, 2.9 (2) | 8.1, 14.1 (2) | 33, 33 (2) | ||
| 20–200 | 200 | Alone | 13.1±5.3 (34) | 1.0 (0.3–4.0; 34) | 41.8±12.4 (34) | 1.9±0.2 (34) | Pred. 11.1±1.32 (34) Obs. 9.2±3.2 (34) |
Pred. 31±3.2 (34) Obs. 25±9.1 (34) |
| Combination | 12.0±3.8 (25) | 1.0 (0.3–4.0, 25) | 37.4±10.5 (25) | 1.9±0.4 (25) | 10.1±2.7 (25) | 28±10 (25) | ||
Data were obtained from n patients and are presented in the table as mean values ± SD (n). tmax is presented as median (range; n). If n<3, the actual values are reported.
There was no significant change in temozolomide Cmax (P=0.06), tmax (P=0.24) or t1/2 (P=0.86) when administered alone versus in combination with veliparib. However, as indicated by the accumulation index for AUC (0.89 ± 0.26), there was a significant decrease in temozolomide exposure corresponding to an increase in both Cl/F (P=0.007) and V/F (P=0.01). There were no significant differences noted in temozolomide pharmacokinetic parameters across the veliparib dose levels (P>0.05). Lastly, there was a correlation between the DLT of mucositis and single dose exposure (Cmax P=0.005; AUC P=0.009) and multiple dose exposure (Cmax P = 0.02; AUC P=0.03).
Pharmacodynamics
Pretreatment FA pathway integrity
Because i) FA pathway defects are reportedly common in AML (33) and ii) loss of FA pathway function is associated with enhanced sensitivity to PARP inhibitors (34), we examined functional integrity of the FA pathway in pretreatment blasts ex vivo. Impaired FANCD2 monoubiquitination following melphalan exposure was detected in blasts from all 19 patients examined, thus providing no correlation with the response to therapy (Supplemental Fig. 1).
Pretreatment PARP1 and MGMT protein levels
High MGMT protein levels are associated with resistance to single-agent temozolomide (18, 19) and low PARP1 expression is associated with resistance to the temozolomide/veliparib combination (16, 35), consistent with model in which sensitization to temozolomide involves trapping of PARP1 at sites of DNA methylation, thereby diminishing repair (6, 7). Accordingly, we examined expression of PARP1 as well as MGMT by immunoblotting in pretreatment marrow samples from patients treated on the expansion cohort. This analysis demonstrated that PARP1 levels varied widely among samples, with samples from patients 19 and 35 lacking detectable levels (Supplemental Fig. 2, lane 6 and data not shown). MGMT expression also varied, being undetectable in samples from patients 16 and 19, high in samples from patients 29, 30 and 38, and low but detectable in the others (Supplemental Fig. 2, lanes 6–11 and data not shown). Patient 19, with undetectable PARP1 and MGMT levels, achieved a CR.
MGMT, BRCA1 and MLH promoter methylation
Cells with MGMT promoter methylation are hypersensitive to temozolomide (15); and cells with BRCA1 promoter hypermethylation are hypersensitive to PARP inhibitors (4). Accordingly, promoter CpG island methylation status of MGMT, BRCA1, and MLH1 was examined using quantitative methylation-specific PCR (MSP) in pre-treatment AML samples (Supplemental Fig. 3). The frequencies of methylation of all three genes were similar to those previously reported (29), with 4 of 38 patients (10.5%) patients having MGMT, 2 (5.3%) having BRCA1 and 1 (3%) having MLH1 promoter gene methylation. Interestingly, among 4 patients with MGMT promoter methylation, 3 achieved CR and one achieved marrow PR after a single cycle, with response lasting 6 months without subsequent treatment. One patient with BRCA1 methylation achieved HI and received 6 cycles of treatment.
Veliparib inhibits PAR formation in circulating and bone marrow leukemia cells
To determine whether inhibition of PAR formation might be predictive of response, PAR was assessed at baseline and following veliparib on day 1 and veliparib/temozolomide on day 8 in all 48 patients. The average baseline PAR levels in PB and BM cells were 457.2 pg/107 cells (48 patients) and 1418.2 pg/107 cells (13 patients), respectively. For the 15 patients who had baseline PAR levels < 116 pg/107 cells, limitations of the assay made it difficult to demonstrate significant diminution of PAR, if present. For the remaining 33 patients, PAR levels decreased from baseline by ≥ 80% and ≥ 50% in PBMCs from 25 (76%) and 31 (94%) patients after a single veliparib dose on day 1. Similar polymerase inhibition was observed on Day 8. In general, the % decrease in PAR levels after veliparib and veliparib/temozolomide relative to baseline was lower in patients treated at veliparib ≤ 40 mg dose compared to patients treated at ≥150 mg dose (Supplemental Fig. 4), indicating more complete inhibition of PARP at higher doses. However, neither the degree of PARP inhibition nor the baseline PARP activity correlated with response. Among 16 patients responding to therapy, one HI and 3 CR patients had baseline PAR levels < 116 pg/107 cells. Two of only 3 patients (DL5) who had BM specimens collected on day 8 demonstrated 14% and 81% PARP inhibition compared to pre-treatment BM, while one patient had an increase in PARP activity on day 8.
Veliparib alone or given with temozolomide induces DNA damage in peripheral blood cells and CD34+ blasts as measured by H2AX phosphorylation
The accumulation of DNA damage as measured by phosphorylation of H2AX was assessed in viable peripheral blood cells (median 75% viability) and in the CD34+ subset by flow cytometry prior to and 6 hours after the initial veliparib dose, as well as on day 8 prior to the daily dose of veliparib/temozolomide in 17 patients (DL5 = 150 mg, 14 patients; DL6 = 200 mg, 3 patients). However, only 13 patients had paired samples (8 for all 3 time points). As depicted in Supplemental Table 1, H2AX phosphorylation in PB cells and the CD34+ subset increased following a single dose of veliparib relative to pretreatment (day 0) levels in 4 of 12 and 8 of 12 patients, respectively. Increases were also noted in PB cells and CD34+ subset during veliparib/temozolomide administration in 5 of 9 and 4 of 9 patients, respectively. Two patients who achieved CR had ≥ 2-fold increase in H2AX phosphorylation in PB cells and ≥1.2-fold (2.3, 1.3) increase in phopspho-H2AX positive CD34+ cells on day 1 (6 hours post- versus pre-treatment) and day 8 (1.5; versus pre-treatment); 1 patient with HI had 2.3-fold increase in phospho-H2AX positive CD34+ cells on day 1.
DISCUSSION
The present study, which is among the first to test a PARP inhibitor in AML, demonstrates that the oral combination regimen of veliparib/temozolomide has clinical activity in myeloid malignancies with multiple poor-risk features, including secondary AML, relapsed/refractory AML, aggressive or transformed CMML, and adverse genetics. Further, the regimen is well tolerated in older adults and can be administered for multiple cycles without cumulative toxicity. Responses, including CRs, occurred at all veliparib dose levels and in patients who were previously treated with hypomethylating agents and/or allogeneic SCT. Patients who achieved CR had improved survival relative to those having HI/SD or no response (Figure 1).
The MTD for veliparib when given with temozolomide at 200 mg/m2 daily for 7 days was 150 mg twice daily, which is less than half of the MTD for veliparib as a single agent (36). There was no clinically relevant pharmacokinetic interaction between veliparib and temozolomide and the pharmacokinetics appeared to be linear, similar to single-agent studies in solid malignancies (37, 38). This is in contrast to our study of veliparib plus topotecan/carboplatin in poor-risk myeloid malignancies, where non-linear pharmacokinetics were observed above veliparib doses of 80 mg twice daily for 9–14 days (39). A possible explanation for the difference in pharmacokinetics between the two regimens could be an impact of topotecan/carboplatin on renal elimination, resulting in prolonged veliparib exposure (38, 40) that is not seen with temozolomide. Nonetheless, the DLT in both combination studies was oral or esophageal mucositis that correlated with veliparib exposure and reflected the ability of veliparib to exacerbate the inherent toxicity of the traditional chemotherapeutic agents.
We interrogated pretreatment tumor cells from peripheral blood and/or bone marrow for selected aspects of various DNA repair pathways and their potential relationship to clinical response. Interestingly, 19 of 19 AMLs exhibited impaired FANCD2 ubiquitination, consistent with the notion that defects in FA pathway activity are common in poor-risk myeloid malignancies.
Methylation of the promoters of pivotal DNA repair genes was uncommon, with only 2 (5%) of 38 samples displaying BRCA1 promoter hypermethylation and 4 (10%) MGMT hypermethylation. The low frequency of MGMT hypermethylation in these AMLs is similar to the frequency of low MGMT expression found in a relapsed/refractory cohort (19). It is possible that prior use of hypomethylating agents in the majority of patients might have contributed to low frequency of methylation of these and other genes. Nonetheless, while 3 of the 4 with MGMT hypermethylation achieved CR, responses also occurred in those without MGMT methylation, including patients in whom multiple therapies had failed and/or who had secondary AML.
In pharmacodynamic studies, veliparib at all dose levels suppressed PAR formation in peripheral blood cells with different percentages of circulating blasts. There was significant intra-and inter-patient variability and no clear difference between responders and non-responders. Nonetheless, more potent PARP inhibition was observed with increasing veliparib dose, consistent with earlier studies (37).
Pretreatment CD34+ cell populations from bone marrow and peripheral blood exhibited varying degrees of DNA damage, with evidence of H2AX phosphorylation in >10% of the CD34+ cells in 8 of 15 bone marrows and 11 of 17 circulating leukemia cell populations. Quantitation of γH2AX in viable CD34+ cells from sequential peripheral blood samples demonstrated that veliparib caused an increase in γH2AX in 8 of 12 patients. Interestingly, both patients who achieved CR exhibited veliparib-induced DNA damage within 6 hours of the first veliparib dose. Although there was a DNA damage signal, the relatively low amplitude of γH2AX increase relative to baseline may have been due to population dilution or selective kill of dividing cells compared to cells in G0/G1. It is worth noting that the time point at which a maximum γH2AX signal can be measured is unknown in this patient population. This signal needs to be validated in larger numbers of patients and in bone marrow leukemic cells. Furthermore, recent studies suggest that high levels of γH2AX at baseline with increase following PARP inhibition confer sensitivity to PARP inhibition only in those AMLs carrying specific oncogenic fusion proteins (AML1-ETO; PML-RARα) and having defective DDR, whereas high levels of HOXA9 expression and proficient DDR confer resistance to PARP inhibition in MLL-AF9 leukemia (41). None of our CR patients with veliparib-induced γH2AX had these abnormalities; one had a complex karyotype with inv(3), while others had two rare chromosomal translocations, t(2;21) and t(13;15).
In summary, veliparib/temozolomide is a well-tolerated oral regimen with clinical activity in patients whose AML exhibits multiple poor-risk features. These results support the development of a phase 2 trial accompanied by studies aimed at identifying biomarkers of response. The regimen may have its greatest applicability to older adults with secondary AML who cannot tolerate more intensive approaches, particularly those who are resistant to previous cytarabine or demethylating agent therapy. Of special interest would be older adults with newly diagnosed AML having methylated MGMT.
Supplementary Material
TRANSLATIONAL RELEVANCE.
We hypothesize that PARP inhibitors, acting by inhibiting base excision repair as well as trapping PARP1 and PARP2 on damaged DNA and thereby inhibiting downstream repair, may enhance the anti-leukemic effects of temozolomide. In this phase 1 study, we examined the safety and efficacy of this novel strategy by combining the PARP inhibitor veliparib with temozolomide in patients with relapsed or refractory acute myeloid leukemia (AML). The combination was tolerable at higher doses of veliparib than previously administered with temozolomide in solid tumors. Clinical responses, which were observed irrespective of prior AML treatment, karyotype, or evolution, seemed to correlate with pretreatment MGMT promoter hypermethylation and treatment-induced H2AX phosphorylation. Veliparib exposure correlated with toxicity, but not with response. These results suggest that the temozolomide/veliparib regimen, along with treatment-induced H2AX phosphorylation and pre-treatment MGMT methylation as potential predictors of response, warrants further investigation in AML
Acknowledgments
We are grateful to the patients who participated in this study and the nurses and physicians who cared for them. We would like to thank Robert G. Fenton for contribution to study design, Karen Flatten, Kevin Peterson, Paula Schneider and Cathy Huntoon for technical assistance with the correlative studies, and research nurses Jackie Greer and Stephanie Fleckinger for their effort on this study.
Financial Support: This work is supported by NIH grants U01CA070095, U01CA099168, UM1CA186690, UM1CA186691 and N01CM5701716. The project described was also supported by the Cancer Pharmacokinetics and Pharmacodynamics Facility at University of Pittsburgh Cancer Institute (P30CA047904) and the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins (NIH grants P30 CA006973 and UL1 TR 001079). Frederick National Laboratory Support was provided by NCI, NIH, under Contract No. HHSN261200800001E. Merck & Co., Inc. provided temozolomide for this study.
Footnotes
Conflict of Interest Statement: The authors of this manuscript report no relationship to disclose. The data in this manuscript were in part presented in the abstract/poster form at the American Society of Hematology Meeting, December 5–8, 2015, Orlando, FL
AUTHOR CONTRIBUTIONS
Conception and design: I Gojo, JH Beumer, A Chen, JE Karp
Provision of study materials or patients: I Gojo, KW Pratz, MA McDevitt, MR Baer, BD Smith, SD Gore, HE Carraway, MM Showel, MJ Levis, A Dezern, DE Gladstone, JE Karp
Acquisition of data: All authors
Analysis and interpretation of data: I Gojo, KW Pratz, MA McDevitt, JH Beumer, A Blackford, J Ji, RJ Kinders, MA Rudek, J Herman, L Karnitz, SH Kaufmann, and JE Karp
Writing, review and/or revision of the manuscript: All authors.
Study supervision: I Gojo, JE Karp
References
- 1.Kadia TM, Ravandi F, Cortes J, Kantarjian H. Toward individualized therapy in acute myeloid leukemia: A contemporary review. JAMA Oncol. 2015;1:820–8. doi: 10.1001/jamaoncol.2015.0617. [DOI] [PubMed] [Google Scholar]
- 2.Gojo I, Karp JE. New strategies in acute myelogenous leukemia: leukemogenesis and personalized medicine. Clinical Cncer Res. 2014;20:6233–41. doi: 10.1158/1078-0432.CCR-14-0900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Esposito MT, So CW. DNA damage accumulation and repair defects in acute myeloid leukemia: implications for pathogenesis, disease progression, and chemotherapy resistance. Chromosoma. 2014;123:545–61. doi: 10.1007/s00412-014-0482-9. [DOI] [PubMed] [Google Scholar]
- 4.Scott CL, Swisher EM, Kaufmann SH. Poly (ADP-ribose) polymerase inhibitors: recent advances and future development. J Clin Oncol. 2015;33:1397–406. doi: 10.1200/JCO.2014.58.8848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hopkins TA, Shi Y, Rodriguez LE, Solomon LR, Donawho CK, DiGiammarino EL, et al. Mechanistic dissection of PARP1 trapping and the impact on in vivo tolerability and efficacy of PARP inhibitors. Mol Cancer Res. 2015;13:1465–77. doi: 10.1158/1541-7786.MCR-15-0191-T. [DOI] [PubMed] [Google Scholar]
- 6.Murai J, Huang SY, Das BB, Renaud A, Zhang Y, Doroshow JH, et al. Trapping of PARP1 and PARP2 by clinical PARP inhibitors. Cancer Res. 2012;72:5588–99. doi: 10.1158/0008-5472.CAN-12-2753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murai J, Zhang Y, Morris J, Ji J, Takeda S, Doroshow JH, et al. Rationale for poly(ADP-ribose) polymerase (PARP) inhibitors in combination therapy with camptothecins or temozolomide based on PARP trapping versus catalytic inhibition. J Pharmacol Exp Ther. 2014;349:408–16. doi: 10.1124/jpet.113.210146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bryant HE, Schultz N, Thomas HD, Parker KM, Flower D, Lopez E, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–7. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 9.Farmer H, McCabe N, Lord CJ, Tutt AN, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–21. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 10.Audeh MW, Carmichael J, Penson RT, Friedlander M, Powell B, Bell-McGuinn KM, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: a proof-of-concept trial. Lancet. 2010;376:245–51. doi: 10.1016/S0140-6736(10)60893-8. [DOI] [PubMed] [Google Scholar]
- 11.Fong PC, Boss DS, Yap TA, Tutt A, Wu P, Mergui-Roelvink M, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. New Engl J Med. 2009;361:123–34. doi: 10.1056/NEJMoa0900212. [DOI] [PubMed] [Google Scholar]
- 12.De Lorenzo SB, Patel AG, Hurley RM, Kaufmann SH. The elephant and the blind men: Making sense of PARP inhibitors in homologous recombination deficient tumor cells. Front Oncol. 2013;3:228. doi: 10.3389/fonc.2013.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Shi Y, Guan R, Donawho C, Luo Y, Palma J, et al. Potentiation of temozolomide cytotoxicity by poly(ADP)ribose polymerase inhibitor ABT-888 requires a conversion of single-stranded DNA damages to double-stranded DNA breaks. Mol Cancer Res. 2008;6:1621–9. doi: 10.1158/1541-7786.MCR-08-0240. [DOI] [PubMed] [Google Scholar]
- 14.D'Atri S, Tentori L, Lacal PM, Graziani G, Pagani E, Benincasa E, et al. Involvement of the mismatch repair system in temozolomide-induced apoptosis. Mol Pharmacol. 1998;54:334–41. doi: 10.1124/mol.54.2.334. [DOI] [PubMed] [Google Scholar]
- 15.Gerson SL. Clinical relevance of MGMT in the treatment of cancer. J Clin Oncol. 2002;20:2388–99. doi: 10.1200/JCO.2002.06.110. [DOI] [PubMed] [Google Scholar]
- 16.Horton TM, Jenkins G, Pati D, Zhang L, Dolan ME, Ribes-Zamora A, et al. Poly(ADP-ribose) polymerase inhibitor ABT-888 potentiates the cytotoxic activity of temozolomide in leukemia cells: influence of mismatch repair status and O6-methylguanine-DNA methyltransferase activity. Mol Cancer Ther. 2009;8:2232–42. doi: 10.1158/1535-7163.MCT-09-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seiter K, Liu D, Loughran T, Siddiqui A, Baskind P, Ahmed T. Phase I study of temozolomide in relapsed/refractory acute leukemia. J Clin Oncol. 2002;20:3249–53. doi: 10.1200/JCO.2002.01.030. [DOI] [PubMed] [Google Scholar]
- 18.Brandwein JM, Kassis J, Leber B, Hogge D, Howson-Jan K, Minden MD, et al. Phase II study of targeted therapy with temozolomide in acute myeloid leukaemia and high-risk myelodysplastic syndrome patients pre-screened for low O(6) -methylguanine DNA methyltransferase expression. Br J Haematol. 2014;167:664–70. doi: 10.1111/bjh.13094. [DOI] [PubMed] [Google Scholar]
- 19.Brandwein JM, Yang L, Schimmer AD, Schuh AC, Gupta V, Wells RA, et al. A phase II study of temozolomide therapy for poor-risk patients aged >or=60 years with acute myeloid leukemia: low levels of MGMT predict for response. Leukemia. 2007;21:821–4. doi: 10.1038/sj.leu.2404545. [DOI] [PubMed] [Google Scholar]
- 20.Bolanos-Meade J, Guo C, Gojo I, Karp JE. A phase II study of timed sequential therapy of acute myelogenous leukemia (AML) for patients over the age of 60: two cycle timed sequential therapy with topotecan, ara-C and mitoxantrone in adults with poor-risk AML. Leuk Res. 2004;28:571–7. doi: 10.1016/j.leukres.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 21.Cheson BD, Bennett JM, Kopecky KJ, Buchner T, Willman CL, Estey EH, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 22.Parise RA, Shawaqfeh M, Egorin MJ, Beumer JH. Liquid chromatography-mass spectrometric assay for the quantitation in human plasma of ABT-888, an orally available, small molecule inhibitor of poly(ADP-ribose) polymerase. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;872:141–7. doi: 10.1016/j.jchromb.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim H, Likhari P, Parker D, Statkevich P, Marco A, Lin CC, et al. High-performance liquid chromatographic analysis and stability of anti-tumor agent temozolomide in human plasma. J Pharm Biomed Anal. 2001;24:461–8. doi: 10.1016/s0731-7085(00)00466-0. [DOI] [PubMed] [Google Scholar]
- 24.Tawbi HA, Beumer JH, Tarhini AA, Moschos S, Buch SC, Egorin MJ, et al. Safety and efficacy of decitabine in combination with temozolomide in metastatic melanoma: a phase I/II study and pharmacokinetic analysis. Ann Oncol. 2013;24:1112–9. doi: 10.1093/annonc/mds591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geng L, Huntoon CJ, Karnitz LM. RAD18-mediated ubiquitination of PCNA activates the Fanconi anemia DNA repair network. J Cell Biol. 2010;191:249–57. doi: 10.1083/jcb.201005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaufmann SH, Svingen PA, Gore SD, Armstrong DK, Cheng YC, Rowinsky EK. Altered formation of topotecan-stabilized topoisomerase I-DNA adducts in human leukemia cells. Blood. 1997;89:2098–104. [PubMed] [Google Scholar]
- 27.Kaufmann SH. Reutilization of immunoblots after chemiluminescent detection. Anal Biochem. 2001;296:283–6. doi: 10.1006/abio.2001.5313. [DOI] [PubMed] [Google Scholar]
- 28.Lamarre D, Talbot B, de Murcia G, Laplante C, Leduc Y, Mazen A, et al. Structural and functional analysis of poly(ADP ribose) polymerase: an immunological study. Biochim Biophys Acta. 1988;950:147–60. doi: 10.1016/0167-4781(88)90007-3. [DOI] [PubMed] [Google Scholar]
- 29.Griffiths EA, Gore SD, Hooker CM, Mohammad HP, McDevitt MA, Smith BD, et al. Epigenetic differences in cytogenetically normal versus abnormal acute myeloid leukemia. Epigenetics. 2010;5:590–600. doi: 10.4161/epi.5.7.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veeck J, Ropero S, Setien F, Gonzalez-Suarez E, Osorio A, Benitez J, et al. BRCA1 CpG island hypermethylation predicts sensitivity to poly(adenosine diphosphate)-ribose polymerase inhibitors. J Clin Oncol. 2010;28:e563–4. doi: 10.1200/JCO.2010.30.1010. [DOI] [PubMed] [Google Scholar]
- 31.Ji J, Kinders RJ, Zhang Y, Rubinstein L, Kummar S, Parchment RE, et al. Modeling pharmacodynamic response to the poly(ADP-Ribose) polymerase inhibitor ABT-888 in human peripheral blood mononuclear cells. PloS One. 2011;6:e26152. doi: 10.1371/journal.pone.0026152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Karp JE, Ricklis RM, Balakrishnan K, Briel J, Greer J, Gore SD, et al. A phase 1 clinical-laboratory study of clofarabine followed by cyclophosphamide for adults with refractory acute leukemias. Blood. 2007;110:1762–9. doi: 10.1182/blood-2007-03-081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xie Y, de Winter JP, Waisfisz Q, Nieuwint AW, Scheper RJ, Arwert F, et al. Aberrant Fanconi anaemia protein profiles in acute myeloid leukaemia cells. Br J Haematol. 2000;111:1057–64. [PubMed] [Google Scholar]
- 34.McCabe N, Turner NC, Lord CJ, Kluzek K, Bialkowska A, Swift S, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66:8109–15. doi: 10.1158/0008-5472.CAN-06-0140. [DOI] [PubMed] [Google Scholar]
- 35.Liu X, Han EK, Anderson M, Shi Y, Semizarov D, Wang G, et al. Acquired resistance to combination treatment with temozolomide and ABT-888 is mediated by both base excision repair and homologous recombination DNA repair pathways. Mol Cancer Res. 2009;7:1686–92. doi: 10.1158/1541-7786.MCR-09-0299. [DOI] [PubMed] [Google Scholar]
- 36.Coleman RL, Sill MW, Bell-McGuinn K, Aghajanian C, Gray HJ, Tewari KS, et al. A phase II evaluation of the potent, highly selective PARP inhibitor veliparib in the treatment of persistent or recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer in patients who carry a germline BRCA1 or BRCA2 mutation - An NRG Oncology/Gynecologic Oncology Group study. Gynecol Oncol. 2015;137:386–91. doi: 10.1016/j.ygyno.2015.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kummar S, Ji J, Morgan R, Lenz HJ, Puhalla SL, Belani CP, et al. A phase I study of veliparib in combination with metronomic cyclophosphamide in adults with refractory solid tumors and lymphomas. Clin Cancer Res. 2012;18:1726–34. doi: 10.1158/1078-0432.CCR-11-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Salem AH, Giranda VL, Mostafa NM. Population pharmacokinetic modeling of veliparib (ABT-888) in patients with non-hematologic malignancies. Clin Pharmacokinet. 2014;53:479–88. doi: 10.1007/s40262-013-0130-1. [DOI] [PubMed] [Google Scholar]
- 39.Pratz KW, Kaufmann SH, Litzow MR, Ji J, Chen A, Rudek MA, et al. Phase I Trial of the Oral Poly (ADP-ribose) Polymerase (PARP) Inhibitor Veliparib (ABT-888, V) Combined Wtih Topoecan (T) and Carboplatin (C) for Adults with Relapsed and Refractory Acute Leukemias. Blood. 2011;118:3634. [Google Scholar]
- 40.Li J, Kim S, Sha X, Wiegand R, Wu J, LoRusso P. Complex disease-, gene-, and drug-drug interactions: impacts of renal function, CYP2D6 phenotype, and OCT2 activity on veliparib pharmacokinetics. Clin Cancer Res. 2014;20:3931–44. doi: 10.1158/1078-0432.CCR-14-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Esposito MT, Zhao L, Fung TK, Rane JK, Wilson A, Martin N, et al. Synthetic lethal targeting of oncogenic transcription factors in acute leukemia by PARP inhibitors. Nat Med. 2015;21:1481–90. doi: 10.1038/nm.3993. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.