Abstract
Purpose
To investigate the mediators of health-related quality of life (HR-QoL) in colorectal cancer (CRC) patients and effect on overall survival.
Methods
We analyzed baseline (within 1 year of diagnosis) SF-12v1 questionnaire data from 3,734 CRC patients and assessed the differences in mental composite scores (MCS) and physical composite scores (PCS) by socio-demographics and risks of poor HR-QoL by these factors. Hazard ratios were generated using univariate cox regression for MCS and PCS dichotomized using the normalized scoring based mean of 50 and survival estimates generated using the Kaplan-Meier method.
Results
Differences in MCS and PCS were identified by sex, age, education level, alcohol use, tobacco use, and stage. Race, marital status, and cancer site differed only by PCS. Being female, never married, former alcohol user, or with stage IV disease significantly increased risk of a poor HR-QoL, with magnitudes of risk from 1.25- to 1.97-fold. Higher education level had a protective effect (MCS: Ptrend=2.32×10−7; PCS: Ptrend=5.62×10−14). Hispanics and African-Americans had a 1.35- and 1.57-fold risk of poor PCS, and increase in age had a protective effect for risk of poor MCS (Ptrend=1.84×10−7). Poor MCS or PCS were associated with poor prognosis and decreased survival at 5-years (HRMCS=1.57, 95%CI=1.41–1.76 and HRPCS=2.38, 95%CI=2.08–2.72), and both remained significant when adjusting for age, gender, race, education level, tumor stage, and tumor site.
Conclusions
Our findings identify potential mediators for HR-QoL and suggest that baseline HR-QoL assessment may be prognostic for CRC.
Keywords: SF-12, quality of life, MCS, PCS, colorectal cancer
Introduction
Colorectal cancer (CRC) is the third most common cancer diagnosed in the United States, and it is estimated that by the end of 2016, 134,490 new cases will be diagnosed and 49,190 people are expected to die of this disease [1]. The current five-year survival rate for patients with CRC is 64.9% and the 2012 estimated number of individuals in the U.S. living with CRC was 1.2 million [2]. Improvements in CRC survival rates are largely due to advancements in screening, early diagnosis, and treatment modalities [3]. However, even in light of this improved survival, there is still a need for additional prognostic factors to identify those at high risk of a poor outcome, while also better managing overall well-being of CRC patients. Recent evidence has indicated that assessment of health-related quality of life (HR-QoL) may provide valuable information to improve risk assessment in CRC [4]. Evidence-based findings indicate that physical and psychosocial factors other than sociodemographic or clinical variables are associated with increased risk of impaired HR-QoL [5] and may predict CRC survival [6–8]. From a clinical perspective, HR-QoL as complementary data with clinical factors can help to screen for at risk sub-populations with physical or psychosocial issues that may benefit from enhanced monitoring or improved care management. Studies conducted by Cella et al. demonstrated that better (high) HR-QoL is associated with improved survival in cancer patients and that it was highly predictive of patient-reported outcomes associated with overall and progression-free survival times [9, 10].
Health-related assessments are viewed as complimentary measures that when combined with other clinical measures can provide a more complete representation of an individual’s health status [11]. For instance, one study had shown HR-QoL measurement to be a better prognostic measure of survival compared to clinical parameters [12]. Therefore, we set out to investigate baseline (within 1 year of diagnosis) HR-QoL in these patients and identify socio-demographic and behavioral factors that were associated with reduced HR-QoL.
HR-QoL assessments provide valuable information that can aid in predicting CRC patient outcomes [13]. Most HR-QoL studies are limited in that they only explore associations with treatment response or focus on a specific population (those enrolled in clinical trials or undergoing end-of-life palliative care) [14, 15, 13]. Thus, they do not address the impact of baseline HR-QoL outcomes on prognosis of CRC patients. As one of the few studies that has explored baseline HR-QoL, a study by Maisey et al. in 501 CRC patients showed a better survival for patients with above the median global score (≥67) assessed by EORTC-QLQ-C30 and that QoL scales were significant independent predictors of survival [16]. However, this study did not investigate the predictors of QoL in their population. Few studies have explored this relationship at baseline [17, 3]. Thus, there is an important need to develop a better understanding of mediators between HR-QoL measures and CRC patient survival. Our goal was to assess the relationship between baseline MCS/PCS and CRC overall survival. To do this, we used SF-12 HR-QoL MCS and PCS measurements from a cohort of CRC patients to analyze relationships between HR-QoL and patient socio-demographics and overall survival.
Materials and Methods
Colorectal cancer patient study population and sociodemographics
All new patients seen at MD Anderson Cancer Center complete a patient history form, which includes collection of socio-demographic, epidemiology, and risk factor information. This form also includes assessment of HR-QoL using the generic, non-disease specific, validated SF-12v1 questionnaire [18]. Information on cancer diagnosis, previous treatment, tumor characteristics, and follow-up (vital status) was obtained from our institutional Tumor Registry. A total of 4,941 colorectal cancer patients age ≥ 18 years that completed the patient history form within 1 year of their diagnosis were identified. Patients who received prior treatment (N = 238), diagnosed with multiple histology (N = 103), diagnosed with non-adenocarcinomas (N = 658), or had missing race information (N = 208) were excluded. Thus, the final number of patients included in our study population was 3,734, including those with missing survival information (N = 69).
SF-12 quality of life (QoL) health-related questionnaire and scoring
HR-QoL is a multi-dimensional construct that encompasses patients’ negative and positive aspects of the physical, functional, emotional, and social domains [19, 20]. For this study, we used the SF-12v1 tool that consists of twelve items derived from the short-form 36 (SF-36) questionnaire [21] that maps to four domains (physical, functional, emotional, and social) and eight subscales (physical functioning, role-physical, bodily pain, general health, vitality, social functioning, role-emotional, mental health). These subscales are used to generate two composite summary scores, the physical composite summary (PCS) and mental composite summary (MCS). Scoring for the pain interference question of the SF-12 was slightly modified in our questionnaire with responses on a 0–10 scale instead of the SF-12 reported 0–5 scale. Therefore, the scoring was adjusted to match the SF-12 scoring. A norm-based scoring system was used in which the MCS and PCS scores were normalized to a mean score of 50 (SD=10) based on SF-12 data obtained from the US general population [18]. A high MCS or PCS (≥ 50) is indicative of a better HR-QoL compared to the general population, while a low MCS or PCS (< 50) is indicative of a poor HR-QoL compared to the general population. A recall period of 4 weeks was used with the SF-12v1.
Statistical methods
Differences in mean MCS and PCS scores by patient socio-demographics were assessed using analysis of variance (ANOVA) or Wald statistic. A score of 50 was used to dichotomize population by PCS and MCS to evaluate associations between HR-QoL and socio-demographic variables using unconditional logistic regression with corresponding odds ratios (ORs) and 95% confidence intervals (CIs). Hazard models for 5-year survival were generated using univariate and multivariate linear Cox regression analysis adjusting for age, gender, race, tumor stage, and tumor site. The proportional odds assumption was examined for both PCS and MCS under these parameters and found to be valid. Survival estimates were calculated by Kaplan-Meier method with corresponding log-rank P values. Survival was defined as time from diagnosis to date of death or last follow up within 5 years. Multivariate analyses incorporating all variables were performed to identify independent factors associated with risk of a poor HR-QoL and prognosis.
Results
Study population
Our study population consisted of non-Hispanic White (77.7%), Hispanic (9.8%), African-American (8.5%), and Asian/Pacific Islander (4.1%) colorectal cancer patients with slightly more than half of the overall population being male (58.0%) (Table 1). A significant proportion of the patients were diagnosed at <50 years old (30.7%) and 50–59 years old (30.9%) compared to older patients that were 60–69 (24.5%) and 70+ years old (13.8%). A majority of patients were married (76.4%) compared to those that were widowed (5.4%), separated (0.4%), divorced (6.9%), or never married (10.8%). By education level, most of our study population had at least a high school level (48.5%) or some college level education (39.2%). For the behavioral factors, most of the patients had never used alcohol (42.8%) or tobacco (51.8%), while less reported being former (17.7%) or current (38.5%) alcohol users and former (37.1%) and current tobacco users (9.9%). Most patients’ tumors were found in the colon (69.8%) with only 30.2% of patients’ tumors in the rectum. According to stage, there was a higher percentage of patients with stage III and IV (25.2%) tumors than stage I and II (11.6%) tumors.
Table 1.
Patient socio-demographics by MCS and PCS.
| N (%)a | Mean MCS (SD) |
P value | Mean PCS (SD) |
P value | |
|---|---|---|---|---|---|
| Total | 3,734 (100.00) | 48.5 (10.5) | 43.1 (11.1) | ||
| Race | |||||
| Non-Hispanic Whites | 2,900 (77.7) | 48.7 (10.4) | 43.5 (10.9) | ||
| Hispanics | 366 (9.8) | 47.7 (10.8) | 41.9 (11.1) | ||
| African-Americans | 316 (8.5) | 47.9 (11.3) | 40.0 (11.9) | ||
| Asians/PI | 152 (4.1) | 48.7 (10.1) | 0.23 | 44.8 (10.5) | 9.33 × 10−8 |
| Sex | |||||
| Male | 2,168 (58.0) | 49.5 (10.2) | 44.2 (10.9) | ||
| Female | 1,566 (41.9) | 47.1 (10.7) | 1.47 × 10−12 | 43.1 (11.1) | 6.12 × 10−13 |
| Age | |||||
| <50 | 1,147 (30.7) | 47.5 (10.3) | 43.2 (11.0) | ||
| 50–59 | 1,155 (30.9) | 48.1 (10.7) | 43.0 (11.0) | ||
| 60–69 | 916 (24.5) | 49.4 (10.0) | 43.7 (11.2) | ||
| 70+ | 516 (13.8) | 50.0 (10.5) | 1.27 × 10−6 | 41.9 (11.1) | 0.030 |
| Marital status | |||||
| Married | 2,851 (76.4) | 48.7 (10.4) | 43.7 (10.9) | ||
| Widowed | 201 (5.4) | 47.7 (11.6) | 41.0 (11.2) | ||
| Separated | 16 (0.4) | 43.6 (11.9) | 44.2 (11.3) | ||
| Divorced | 258 (6.9) | 47.9 (10.9) | 41.7 (11.3) | ||
| Never Married | 405 (10.8) | 47.7 (10.4) | 40.7 (11.6) | ||
| Missing | 3 (0.1) | 47.4 (7.5) | 0.050 | 42.3 (9.7) | 1.03 × 10−7 |
| Education level | |||||
| < High School | 308 (8.3) | 46.1 (11.6) | 39.1 (11.4) | ||
| High School/AA/VOC | 1,811 (48.5) | 48.2 (10.6) | 42.0 (11.1) | ||
| Some college | 1,465 (39.2) | 49.4 (9.8) | 45.1 (10.6) | ||
| Missing | 150 (4.0) | 48.5 (10.5) | 2.49 × 10−7 | 44.0 (11.2) | 1.91 × 10−24 |
| Alcohol use | |||||
| Never | 1,598 (42.8) | 48.3 (10.6) | 41.9 (11.0) | ||
| Yes, but quit | 662 (17.7) | 46.8 (11.2) | 38.9 (11.5) | ||
| Yes, currently | 1,437 (38.5) | 49.5 (9.9) | 46.3 (10.0) | ||
| Missing | 37 (1.0) | 48.9 (10.5) | 3.49 × 10−6 | 43.2 (11.8) | 1.17 × 10−33 |
| Tobacco use | |||||
| Never | 1,935 (51.8) | 49.0 (10.2) | 43.3 (10.9) | ||
| Yes, but quit | 1,386 (37.1) | 48.5 (10.5) | 43.3 (11.0) | ||
| Yes, currently | 369 (9.9) | 45.6 (11.3) | 41.0 (11.5) | ||
| Missing | 44 (1.2) | 49.1 (10.0) | 5.29 × 10−6 | 42.2 (11.6) | 0.00090 |
| Cancer site | |||||
| Rectum | 1,126 (30.2) | 48.4 (10.3) | 44.8 (11.2) | ||
| Colon | 2,608 (69.8) | 48.5 (10.6) | 0.83 | 42.3 (10.9) | 2.40 × 10−10 |
| Stage | |||||
| I | 146 (3.9) | 50.1 (10.5) | 46.9 (9.6) | ||
| II | 286 (7.7) | 49.5 (10.5) | 45.7 (10.6) | ||
| III | 568 (15.2) | 48.9 (10.2) | 46.9 (10.1) | ||
| IV | 372 (10.0) | 46.0 (10.9) | 40.8 (11.8) | ||
| Not available | 2,362 (63.3) | 48.6 (10.4) | 4.88 × 10−6 | 41.9 (11.0) | 5.79 × 10−30 |
PI, Pacific Islanders; AA, associate of arts, VOC, vocational
Percentage may not equal 100% due to rounding
PCS and MCS score distribution among colorectal cancer patients
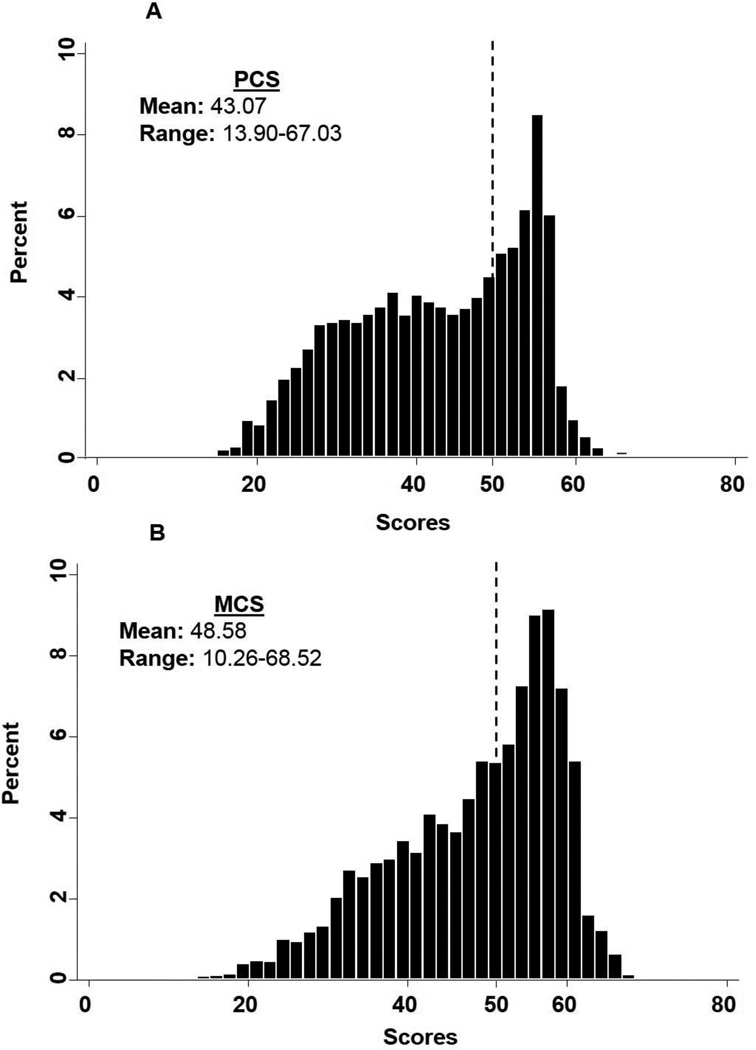
The mean PCS reported by our study population was 43.07 (range: 13.90–67.03) (Figure 1A). The total percent of those that reported PCS scores that were worse than the general population (< 50; 65%) was greater than those that reported PCS scores that were better than the general population (≥ 50; 35%). For MCS, patients had a reported mean of 48.58 (range: 10.26–68.52), with 47% being the total percent of patients with a score worse than the general population (< 50) and 53% the total percent of those with a score better than the general population (≥ 50) (Figure 1B). The mean MCS and mean PCS in this population were significantly different (P<0.01).
Fig. 1. Distribution of HR-QoL scores in the CRC study population (N = 3,734).
A) PCS, 1B) MCS. Score of 50 represents the mean score of the general US population as marked by the dashed line.
Differences in baseline mental and physical HR-QoL by socio-demographic factors
We observed socio-demographic factors to be statistically different by MCS and PCS (Table 1). Significant differences in PCS scores by racial groups (P = 9.33 × 10−8) were identified, but this was not observed for MCS scores (P = 0.23). Males had a significantly better MCS (P = 1.47 × 10−12) and PCS (P = 6.12 × 10−13) than females. MCS significantly differed among age groups (P = 1.27 × 10−6). Improvements paralleled increases in age with those being 70+ years old having the best mental health QoL. For PCS, significant differences by age were identified (P = 0.030), however the finding was opposite with the 70+ age group reporting the poorest physical HR–QoL. There was no statistical difference in mean MCS by marital status (P = 0.050). In contrast, significant differences in mean PCS were observed by marital status (P = 1.03 × 10−7). Both mean MCS (P = 2.49 × 10−7) and PCS (P = 1.19 × 10−24) were significantly different by education level, in which those with a college level education had a better HR-QoL than those with less than a high school level education. A poorer mean MCS and PCS was observed for former alcohol users (MCS: P = 3.49 × 10−6; PCS: P = 1.17 × 10−33) and for current tobacco users (MCS: P = 5.29 × 10−6; PCS: P = 0.00090). We also observed a significant difference in mean PCS (P = 2.40 × 10−10) by cancer site, but not for MCS (P = 0.83). By stage, there was a significant difference in mean MCS (P = 4.88 × 10−6) and mean PCS (P = 5.79 × 10−30) with stage IV patients, as expected, having the poorest HR-QoL.
Socio-demographic predictors of mental and physical HR-QoL
We evaluated the relationship between stratified PCS (+/− 50) and socio-demographics (Table 2) and found females carried a 1.65-fold risk of poor PCS (95% CI: 1.44–1.90, P = 1.47 × 10−12). By race, Hispanics and African-Americans had a 1.35-fold risk (95% CI: 1.06–1.70, P = 0.013) and 1.57-fold (95% CI: 1.21–2.04, P = 0.0010) increased risk of poor PCS compared to non-Hispanic White CRC patients, respectively. When we assessed risk by age, there was no association with PCS (ORPCS: 1.00, 95% CI: 0.93–1.06, Ptrend = 0.91). Widowed individuals had a 1.65-fold risk (95% CI: 1.19–2.28, P = 0.0020) and never married individuals had a 1.44-fold risk (95% CI: 1.14–1.80, P = 0.0020) of poor PCS when compared to those who were married.
Table 2.
Risk of poor PCS by socio-demographic factors
| Low, N (%)a | High, N (%)a | OR (95% CI) | P value | |
|---|---|---|---|---|
| Race | ||||
| Non-Hispanic White | 1,837 (76.2) | 1,063 (80.4) | 1 | |
| Hispanic | 256 (10.6) | 110 (8.3) | 1.35 (1.06–1.70) | 0.013 |
| African-American | 231 (9.6) | 85 (6.4) | 1.57 (1.21–2.04) | 0.0010 |
| Asian/PI | 88 (3.7) | 64 (4.8) | 0.80 (0.57–1.11) | 0.18 |
| Sex | ||||
| Male | 1,298 (53.8) | 870 (65.8) | 1 | |
| Female | 1,114 (46.2) | 452 (34.2) | 1.65 (1.44–1.90) | 1.47 × 10−12 |
| Age, years | ||||
| <50 | 749 (31.1) | 398 (30.1) | 1 | |
| 50–59 | 750 (31.1) | 405 (30.6) | 0.98 (0.83–1.17) | 0.85 |
| 60–69 | 563 (23.3) | 353 (26.7) | 0.84 (0.71–1.01) | 0.072 |
| 70+ | 350 (14.5) | 166 (12.6) | 1.12 (0.90–1.40) | 0.31 |
| P for trend | 1.00 (0.93–1.06) | 0.91 | ||
| Marital Status | ||||
| Married | 1,793 (74.4) | 1,058 (80.0) | 1 | |
| Widowed | 148 (6.1) | 53 (4.0) | 1.65 (1.19–2.28) | 0.0020 |
| Separated | 10 (0.42) | 6 (0.5) | 0.98 (0.36–2.71) | 0.97 |
| Divorced | 171 (7.1) | 87 (6.6) | 1.16 (0.89–1.52) | 0.28 |
| Never Married | 287 (11.9) | 118 (8.3) | 1.44 (1.14–1.80) | 0.0020 |
| Education Level | ||||
| < High School | 237 (10.2) | 71 (5.6) | 1 | |
| High School/AA/VOC | 1231 (53.0) | 580 (45.9) | 0.64 (0.48–0.84) | <0.0001 |
| At least some college | 853 (36.8) | 612 (48.5) | 0.42 (0.31–0.56) | <0.0001 |
| P for trend | 0.65 (0.58–0.73) | 5.62 × 10−14 | ||
| Alcohol Use | ||||
| Never | 1,118 (46.80) | 480 (36.70) | 1 | |
| Yes, but quit | 510 (21.35) | 152 (11.62) | 1.44 (1.17–1.78) | 0.0010 |
| Yes, currently | 761 (31.85) | 676 (51.68) | 0.48 (0.42–0.56) | <0.0001 |
| P for trend | 0.69 (0.64–0.75) | 5.84 × 10−22 | ||
| Tobacco Use | ||||
| Never | 1,245 (52.25) | 690 (52.79) | 1 | |
| Yes, but quit | 876 (36.76) | 510 (39.02) | 0.95 (0.82–1.10) | 0.50 |
| Yes, currently | 262 (10.99) | 107 (8.19) | 1.36 (1.06–1.73) | 0.014 |
| P for trend | 1.08 (0.97–1.19) | 0.14 | ||
| Cancer Site | ||||
| Rectum | 634 (26.3) | 492 (37.2) | 1 | |
| Colon | 1778 (73.7) | 830 (62.8) | 1.66 (1.44–1.92) | 4.18 × 10−12 |
| Stage | ||||
| I | 80 (10.3) | 66 (11.0) | 1 | |
| II | 150 (19.4) | 136 (22.7) | 0.91 (0.61–1.36) | 0.64 |
| III | 282 (36.4) | 286 (47.8) | 0.81 (0.56–1.17) | 0.27 |
| IV | 262 (33.9) | 110 (18.4) | 1.97(1.32–2.92) | <0.0001 |
| P for trend | 1.26 (1.12–1.41) | 7.71 × 10−5 |
PI, Pacific Islanders; AA, associate of arts, VOC, vocational
Percentage may not equal 100% due to rounding
When we assessed the relationship between stratified MCS and socio-demographics (Table 3), female individuals had a 1.55-fold risk of poor MCS (95% CI: 1.36–1.77, P = 4.79 × 10−11). Interestingly, the risk of poor MCS decreased linearly with age in which there was a 0.84fold reduction for 50–59 year olds (95% CI: 0.71–0.99, P = 0.034), 0.71-fold reduction for 60–69 year olds (95% CI: 0.60–0.85, P = 1.45 × 10−4), and a 0.61-fold reduction for 70+ year olds (95% CI: 0.49–0.75, P = 3.46 × 10−6).
Table 3.
Risk of poor MCS by socio-demographic factors
| Low, N(%)a | High, N(%)a | OR (95% CI) | P value | |
|---|---|---|---|---|
| Race | ||||
| Non-Hispanic White | 1,337 (76.6) | 1,563 (78.6) | 1 | |
| Hispanic | 186 (10.7) | 180 (9.05) | 1.21 (0.97–1.50) | 0.089 |
| African-American | 152 (8.71) | 164 (8.25) | 1.08 (0.86–1.37) | 0.50 |
| Asian/PI | 70 (4.01) | 82 (4.12) | 1.00 (0.72–1.38 | 0.99 |
| Sex | ||||
| Male | 914 (52.4) | 1,254 (63.1) | 1 | |
| Female | 831 (47.62) | 735 (36.9) | 1.55 (1.36–1.77) | 4.79 × 10−11 |
| Age, years | ||||
| <50 | 595 (34.1) | 552 (27.8) | 1 | |
| 50–59 | 548 (31.4) | 607 (30.5) | 0.84 (0.71–0.99) | 0.034 |
| 60–69 | 398 (22.8) | 518 (26.0) | 0.71 (0.60–0.85) | <0.0001 |
| 70+ | 204 (11.7) | 312 (15.7) | 0.61 (0.49–0.75) | <0.0001 |
| P for trend | 0.85 (0.79–0.90) | 1.84 × 10−7 | ||
| Marital Status | ||||
| Married | 1,301 (74.64) | 1,550 (78.0) | 1 | |
| Widowed | 96 (5.51) | 105 (5.28) | 1.09 (0.82–1.45) | 0.56 |
| Separated | 11 (0.63) | 5 (0.25) | 2.62 (0.91–7.56) | 0.075 |
| Divorced | 128 (7.34) | 130 (6.54) | 1.17 (0.91–1.51) | 0.22 |
| Never Married | 207 (11.9) | 198 (9.96) | 1.25 (1.01–1.53) | 0.039 |
| Education Level | ||||
| < High School | 176 (10.5) | 132 (6.90) | 1 | |
| High School/AA/VOC | 876 (52.4) | 935 (48.9) | 0.70 (0.55–0.90) | <0.0001 |
| At least some college | 619 (37.0) | 846 (44.2) | 0.55 (0.43–0.70) | <0.0001 |
| P for trend | 0.76 (0.68–0.84) | 2.32 × 10−7 | ||
| Alcohol Use | ||||
| Never | 766 (44.30) | 832 (42.28) | 1 | |
| Yes, but quit | 348 (20.13) | 314 (15.96) | 1.20 (1.00–1.44) | 0.045 |
| Yes, currently | 615 (35.57) | 822 (41.77) | 0.81 (0.70–0.94) | 0.0050 |
| P for trend | 0.90 (0.84–0.97) | 0.0060 | ||
| Tobacco Use | ||||
| Never | 862 (49.94) | 1,073 (54.63) | 1 | |
| Yes, but quit | 649 (37.60) | 737 (37.53) | 1.10 (0.95–1.26) | 0.19 |
| Yes, currently | 215 (12.46) | 154 (7.84) | 1.74 (1.39–2.18) | <0.0001 |
| P for trend | 1.23 (1.12–1.36) | 2.32 × 10−5 | ||
| Cancer Site | ||||
| Rectum | 518 (29.7) | 608 (30.6) | 1 | |
| Colon | 1,227 (70.3) | 1,381 (69.4) | 1.04 (0.91–1.20) | 0.56 |
| Stage | ||||
| I | 57 (8.91) | 89 (12.2) | 1 | |
| II | 123 (19.2) | 163 (22.3) | 1.18 (0.78–1.77) | 0.43 |
| III | 254 (39.7) | 314 (42.9) | 1.26 (0.87–1.83) | 0.22 |
| IV | 206 (32.2) | 166 (22.7) | 1.94 (1.31–2.86) | <0.0001 |
| P for trend | 1.24 (1.11–1.40) | 1.69 × 10−4 |
PI, Pacific Islanders; AA, a ssociate of arts, VOC, vocational
Percentage may not equal 100% due to rounding
For both PCS and MCS, we observed an education level greater than high school or some college conferred a protective effect on HR-QoL (PCS: Ptrend =5.62 × 10−14; MCS: Ptrend = 2.32 × 10−7). Interestingly, in current alcohol users, we observed a protective effect on poor PCS (OR: 0.48, 95% CI: 0.42–0.56, P = 1.00 × 10−24) and MCS (OR: 0.81, 95% CI: 0.70–0.94, P = 0.005). In contrast, current tobacco users had an increased risk of poor PCS (OR: 1.36, 95% CI: 1.06–1.73, P = 0.014) and MCS (OR: 1.74, 95% CI: 1.39–2.18, P = 1.56 × 10−6). Although CRC patients with colon tumors had only an increased risk for poor PCS (ORPCS: 1.66, 95% CI: 1.44–1.92, P = 4.18 × 10−12; ORMCS: 1.04, 95% CI: 0.91–1.20, P = 0.56), individuals with stage IV disease were at a significant risk of poor HR-QoL (ORPCS: 1.97, 95% CI: 1.32–2.92, P = 7.96 × 10−4; ORMCS: 1.94, 95% CI: 1.31–2.86, P = 8.95 × 10−4).
In multivariate analysis (Table 4), sex, education level, current smoking, current tobacco use, colon tumors, and stage IV were independent predictors of risk of poor PCS. Interestingly, age at diagnosis was only a significantly independent predictor of MCS with older age having a larger effect on predicting decreased risk of a poor MCS. Other independent variables were similar to PCS: sex, education level, tobacco use, and stage IV cancer.
Table 4.
Multivariate analysis risk of poor HR-QoL by socio-demographic factors
| PCS |
MCS |
|||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Race | ||||
| Non-Hispanic White | 1.00 | 1.00 | ||
| Hispanic | 1.36 (0.88–2.09) | 0.16 | 1.27 (0.85–1.90) | 0.24 |
| African-American | 1.22 (0.78–1.92) | 0.38 | 0.93 (0.62–1.42) | 0.75 |
| Asian/PI | 0.69 (0.40–1.20) | 0.19 | 1.39 (0.80–2.42) | 0.25 |
| Sex | ||||
| Male | 1.00 | 1.00 | ||
| Female | 1.48 (1.14–1.91) | 0.0030 | 1.62 (1.27–2.08) | <0.0001 |
| Age, years | ||||
| <50 | 1.00 | 1.00 | ||
| 50–59 | 1.02 (0.75–1.40) | 0.88 | 0.71 (0.53–0.97) | 0.029 |
| 60–69 | 0.93 (0.66–1.31) | 0.68 | 0.64 (0.46–0.89) | 0.0080 |
| 70+ | 1.08 (0.72–1.63) | 0.70 | 0.35 (0.23–0.52) | <0.0001 |
| Marital Status | ||||
| Married | 1.00 | 1.00 | ||
| Widowed | 1.44 (0.84–2.44) | 0.18 | 1.13 (0.69–1.86) | 0.62 |
| Separated | 0.91 (0.15–5.54) | 0.92 | 0.85 (0.16–4.51) | 0.85 |
| Divorced | 1.20 (0.76–1.88) | 0.44 | 1.23 (0.80–1.89) | 0.34 |
| Never Married | 1.15 (0.76–1.73) | 0.50 | 1.28 (0.87–1.88) | 0.22 |
| Education Level | ||||
| < High School | 1.00 | 1.00 | ||
| High School/AA/VOC | 0.56 (0.35–0.90) | 0.017 | 0.62 (0.40–0.94) | 0.26 |
| At least some college | 0.36 (0.22–0.59) | 0.00 | 0.54 (0.35–0.85) | 0.0070 |
| Alcohol Use | ||||
| Never | 1.00 | 1.00 | ||
| Yes, but quit | 1.38 (0.93–2.06) | 0.11 | 1.35 (0.94–1.95) | 0.11 |
| Yes, currently | 0.52 (0.39–0.68) | <0.0001 | 0.84 (0.64–1.09) | 0.19 |
| Tobacco Use | ||||
| Never | 1.00 | 1.00 | ||
| Yes, but quit | 1.13 (0.86–1.49) | 0.37 | 1.21 (0.93–1.58) | 0.15 |
| Yes, currently | 1.89 (1.26–2.84) | 0.0020 | 1.61 (1.11–2.35) | 0.013 |
| Cancer site | ||||
| Rectum | 1.00 | 1.00 | ||
| Colon | 1.48 (1.16–1.90) | 0.0020 | 0.97 (0.76–1.23) | 0.79 |
| Stage | ||||
| I | 1.00 | 1.00 | ||
| II | 0.85 (0.55–1.33) | 0.46 | 1.17 (0.76–1.82) | 0.47 |
| III | 0.81 (0.54–1.21) | 0.30 | 1.16 (0.78–1.73) | 0.47 |
| IV | 1.66 (1.08–2.57) | 0.022 | 1.65 (1.08–2.51) | 0.020 |
PI, Pacific Islanders; AA, associate of arts, VOC, vocational
Poor mental and physical HR-QoL as a prognostic factor in CRC
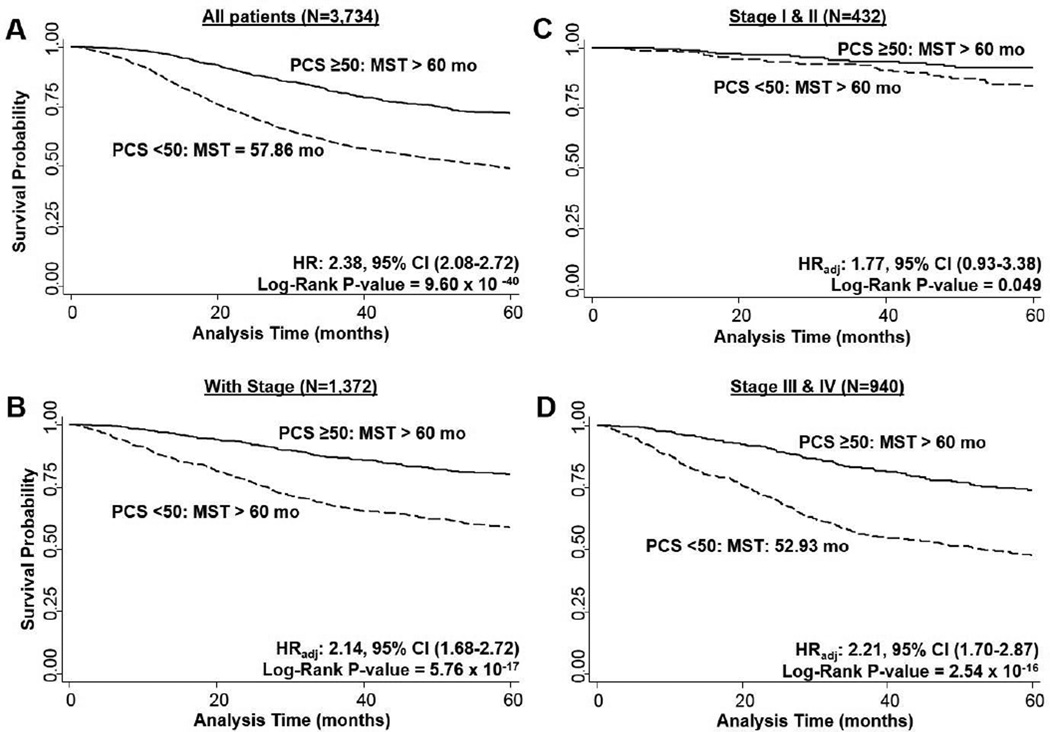
We assessed differences in overall survival of CRC patients (N = 3,665 with vital status available) by PCS or MCS (Figures 2 and 3). We observed that CRC patients reporting a poor PCS compared to the general population (< 50) had a significant reduction in survival time at 5-years compared to those with a better PCS than the general population (≥ 50; log-rank P = 9.60 × 10−40; Figure 2A). This corresponded to a 2.38-fold increase in risk of dying in those with a PCS score < 50 (95% CI: 2.08–2.72, P < 0.0001). A large portion of our CRC patient population had missing stage information (63.3%). Therefore, we repeated the analysis restricting to those patients with stage information available (N = 1,360) and included adjustment for age, gender, education level, race, tumor stage, and tumor site. A similar highly significant effect (log-rank P = 5.76 × 10−17) was observed by PCS (HRadj: 2.14, 95% CI: 1.68–2.72, P < 0.0001; Figure 2B. When further stratified by stage at diagnosis, this effect by PCS was borderline significant for stage I/II patients (log-rank P = 0.049) and increase in risk (HR: 1.77, 95% CI: 0.93–3.38, P = 0.082) for those with poor PCS (Figure 2C). The effect was highly significant in the stage III/IV patients with a poor PCS being associated with a 2.21-fold increase risk of dying (95% CI: 1.70–2.87, P < 0.0001) and reduction in median survival time (log-rank P = 2.54 × 10−16; Figure 2D).
Fig. 2. Overall survival of CRC patients by PCS scores.
2A) overall population (N = 3,665), 2B) with stage information (N = 1,360), 2C) by stage I/II (N = 425), 2D) by stage III/IV (N = 935). MST: median survival time.
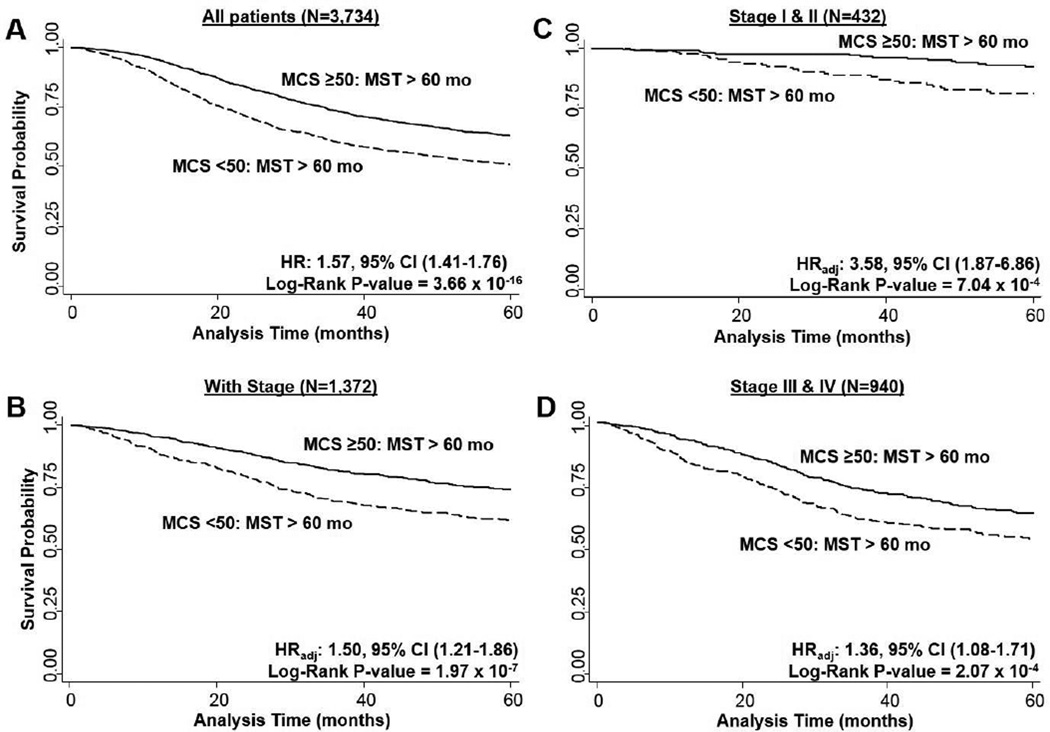
Fig. 3. Overall survival of CRC patients by MCS scores.
3A) overall population (N = 3,665), 3B) with stage information (N = 1,360), 3C) by stage I/II (N = 425), 3D) by stage III/IV (N = 935). MST: median survival time.
Survival time in the overall population was also significantly decreased for those with a MCS worse than the general population compared to those with a MCS better than the general population (log-rank P = 3.66 × 10−16; Figure 3A). CRC patients with a poor MCS were at increased risk of dying (HR: 1.57, 95% CI: 1.41–1.76, P < 0.0001), and we observed comparable results when restricting to those with stage information and adjusting for adjustment for age, gender, race, education level, tumor stage, and tumor site: HR: 1.50, 95% CI: 1.21–1.86, P = 0.0002 (Figure 3B). This effect of MCS on overall survival was consistent when stratified by stage at diagnosis. Both early stage (I/II; Figure 3C) and late (III/IV; Figure 3D) stage patients had significantly increased risk and reduced survival durations. When PCS and MCS were analyzed as continuous variables in the adjusted Cox model for 5-year survival, each unit decrease in the score was associated with a significant increase in risk of dying. For PCS the HR was 1.04 (95% CI: 1.03–1.05) and for MCS it was 1.01 (1.00–1.02).
Discussion
The goal of this study was to explore the differences in PCS/MCS by socio-demographic factors, assess associations between MCS/PCS and socio-demographic factors, and determine the effect on overall survival in CRC patients. To our knowledge, this is the first baseline HR-QoL study using the SF-12 questionnaire that evaluated MCS/PCS association with socio-demographics and as a predictor of prognosis in CRC patients.
Our findings showed that African-Americans have the worst HR-QoL when measured by PCS or MCS compared to other racial groups. From data obtained by the Behavioral Risk Factor Surveillance System (BRFSS), minority populations (Hispanic and African-American) in the U.S. were found to have a higher number of individuals who reported fair or poor health compared to non-Hispanic Whites [22]. In another study, African-Americans and Hispanics in the general population, have higher rates of depression compared to non-Hispanic Whites [23]. However, despite these similarities with the general population, CRC patients have poorer HR-QoL than the general population that may be attributed by existing racial disparities for CRC patients, including for later stage at diagnose in which African-American men have a 18% higher odds of late-stage CRC at diagnosis than non-Hispanic Whites [24]. Thus, these important findings indicate a critical need for future evaluations of HR-QoL racial disparities among CRC patients as a step to improve overall well-being and prognosis for African-American and Hispanic CRC patients.
By stratified PCS or MCS scores, those that were widowed, never married, or had less than a high school level education, were at risk of poor PCS or MCS. This is in concordance with results from other studies that highlights an association between married/living as married and having better physical and psychosocial well-being and a protective effect for mental health in cancer patients with higher education [25]. Based on the general U.S. population, there is a higher percentage of reported fair and poor health observed for females, widowed, separated or divorced individuals, compared to married individuals, and the percentage of adults that reported fair or poor health was greater among those with less than a high school education compared to those with a college level education [26, 22]. Marital status and education are often used as surrogate markers for socioeconomic status and thus, access to care and overall health status. Further studies would be of interest to explore the specific mediators of HR-QoL in these populations.
When we assessed patient socio-demographics by stratified MCS, surprisingly, we found younger patients were at risk of a poor MCS compared to older patients. In comparison to our study results, another study observed a better global health mean score of 62.8 among older CRC patients compared to younger patients [27]. However, this is in contrast to what is observed in the general population for which there is increased reported numbers of poor HR-QoL with an increase in age [28]. This observation in the general population is likely the cause of physical pain/discomfort, depression and anxiety linked to chronic conditions related to aging [29]. It has also been noted that illness acceptance for cancer patients is linearly associated with patient income [30]. Therefore, it can be hypothesized that a better self-reported HR-QoL among older CRC patients is reflective of more acceptance of the disease and having lower financial stress/concerns.
In our study we found current alcohol use to be inversely associated with a poor MCS and PCS, and former users associated with poor MCS and PCS. In a study by Owusu et al. they reported associations between CRC screening rates and depression, alcohol use, and smoking. Stratified by race, past alcohol use was associated with an increase in CRC screening in African-Americans [31]. Indicating that there are racial disparities in the relationship between CRC screening and alcohol use and smoking [31]. The use of alcohol and tobacco may influence emotions and behavioral attitudes about CRC screening, which may also influence a patient’s perspective or acceptance of their disease at diagnosis. Alcohol use could also be serving as a coping mechanism among CRC patients, and thus improving their perceived HR-QoL. As modifiable risk factors, alcohol use and tobacco use could be potential areas for intervention to improve overall survival of CRC patients.
PCS and MCS remained associated with overall survival in our overall population (N = 3,665), stage-restricted population (N = 1,360), and stratified by stage (I/II, N = 425 and III/IV, N = 935). Our results support PCS and MCS as prognostic factors for CRC, an effect that appears to be independent of stage. Interestingly, the prognostic effect of PCS was muted in early stage CRC patients with borderline significant effects on survival time and risk (P = 0.063 and 0.065, respectively). It is possible the early stage patients would not be experiencing the physical effects of CRC due to the limited symptoms that are present at this stage. In contrast, MCS was a significant prognostic factor in early stage patients – potentially suggesting that the effects of a cancer diagnosis on mental well-being overwhelms the specific knowledge regarding stage.
One of the strengths of this study is the use of a large, diverse CRC patient cohort (N = 3,734), while other previous studies used relatively small cohort sizes [25, 32, 33]. Another strength was that we assessed HR-QoL at baseline in which patient reported outcomes were not influenced by the effect of treatment regimens. This is in contrast to many studies in the literature that assess HR-QoL in cancer patient populations focusing on periods during or immediately following treatment [13–15]. We utilized the SF-12 questionnaire in obtaining our HR-QoL measurements, which provides an overall composite score for mental health and physical health, allowing us to assess these two composites separately as opposed to the limitations presented with using other HR-QoL assessments that provide a single overall HR-QoL score [34]. Furthermore, because of our large patient population, we were able to include those with early stage (I and II) CRC in our analysis, while other studies have focused on patients with advanced or metastatic disease [35, 36, 16].
Although we consider the diversity of our population to be one of the strengths of this study, the use of a hospital-based cohort may limit the generalizability of our findings to other CRC populations. The assessment of HR-QoL in this study was based on a generic HR-QoL questionnaire, and not a disease-specific quality of life tool. Several previous studies have used cancer-specific measures such as the EORTC QLQ-C30 [37] or the FACT-C [38, 13], making direct comparisons with other studies using these tools difficult. However, since a gold-standard for HR-QoL instruments has not yet been established for the assessment of CRC QoL outcomes, it remains to be determined which instrument would be most appropriate [34]. Further studies that incorporate multiple institutions with subsequent controlled trials would be necessary to validate these findings and determine the generalizability to the CRC population more broadly. Despite these limitations, our results clearly indicate a strong relationship between poor PCS and MCS at baseline and prognosis of CRC patients. This is similar to another study that supports baseline HR-QoL as a significant independent prognostic indicator in advanced CRC patients in which they showed QoL scales to be significant independent predictors of survival [16]. Contrary to our study, they had a smaller patient cohort (N=501) with most patients (82%) diagnosed with metastatic disease. Our findings can be generalized to both early and late stage CRC patients.
The findings from this study highlighted a shorter survival time for CRC patients who had scored poorly for physical and mental HR-QoL at baseline, suggesting that HR-QoL could serve as a prognostic factor in these patients. If validated, utilizing HR-QoL assessment as a prognostic tool prior to initiation of treatment could help to identify sub-populations of CRC patients who are at risk for a poor outcome. These high-risk patients would be candidates for potential interventions to improve their quality of life (such as counseling, tobacco cessation, and others), while also providing additional information that could be used to help inform decisions regarding treatment. The information regarding HR-QoL would be complementary with other existing prognostic factors based on tumor and patient characteristics.
Acknowledgments
Funding support: Funding support for this study was provided in part by the NIH through MD Anderson’s Cancer Center Support Grant CA016672.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016 doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.SEER cancer statistics review, 1975–2012, National Cancer Institute. SEER. 2015 http://seer.cancer.gov/csr/1975_2012/
- 3.Schag CA, Ganz PA, Wing DS, Sim MS, Lee JJ. Quality of life in adult survivors of lung, colon and prostate cancer. Qual Life Res. 1994;3(2):127–141. doi: 10.1007/BF00435256. [DOI] [PubMed] [Google Scholar]
- 4.Sales PM, Carvalho AF, McIntyre RS, Pavlidis N, Hyphantis TN. Psychosocial predictors of health outcomes in colorectal cancer: a comprehensive review. Cancer Treat Rev. 2014;40(6):800–809. doi: 10.1016/j.ctrv.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Mols F, Thong MS, van de Poll-Franse LV, Roukema JA, Denollet J. Type D (distressed) personality is associated with poor quality of life and mental health among 3080 cancer survivors. J Affect Disord. 2012;136(1–2):26–34. doi: 10.1016/j.jad.2011.08.034. [DOI] [PubMed] [Google Scholar]
- 6.Zhang JK, Fang LL, Zhang DW, Jin Q, Wu XM, Liu JC, et al. Type D personality is associated with delaying patients to medical assessment and poor quality of life among rectal cancer survivors. Int J Colorectal Dis. 2016;31(1):75–85. doi: 10.1007/s00384-015-2333-4. [DOI] [PubMed] [Google Scholar]
- 7.Kroz M, Reif M, Bussing A, Zerm R, Feder G, Bockelbrink A, et al. Does self-regulation and autonomic regulation have an influence on survival in breast and colon carcinoma patients? results of a prospective outcome study. Health Qual Life Outcomes. 2011;9:85. doi: 10.1186/1477-7525-9-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallance JK, Boyle T, Courneya KS, Lynch BM. Associations of objectively assessed physical activity and sedentary time with health-related quality of life among colon cancer survivors. Cancer. 2014;120(18):2919–2926. doi: 10.1002/cncr.28779. [DOI] [PubMed] [Google Scholar]
- 9.Cella D, Cappelleri JC, Bushmakin A, Charbonneau C, Li JZ, Kim ST, et al. Quality of life predicts progression-free survival in patients with metastatic renal cell carcinoma treated with sunitinib versus interferon alfa. J Oncol Pract. 2009;5(2):66–70. doi: 10.1200/JOP.0922004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cella D, Bushmakin AG, Cappelleri JC, Charbonneau C, Michaelson MD, Motzer RJ. Baseline quality of life as a prognostic survival tool in patients receiving sunitinib for metastatic renal cell carcinoma. Br J Cancer. 2012;106(4):646–650. doi: 10.1038/bjc.2011.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinten C, Coens C, Mauer M, Comte S, Sprangers MA, Cleeland C, et al. Baseline quality of life as a prognostic indicator of survival: a meta-analysis of individual patient data from EORTC clinical trials. Lancet Oncol. 2009;10(9):865–871. doi: 10.1016/S1470-2045(09)70200-1. [DOI] [PubMed] [Google Scholar]
- 12.Efficace F, Bottomley A, Coens C, Van Steen K, Conroy T, Schoffski P, et al. Does a patient’s self-reported health-related quality of life predict survival beyond key biomedical data in advanced colorectal cancer? Eur J Cancer. 2006;42(1):42–49. doi: 10.1016/j.ejca.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 13.Conroy T, Bleiberg H, Glimelius B. Quality of life in patients with advanced colorectal cancer: what has been learnt? Eur J Cancer. 2003;39(3):287–294. doi: 10.1016/s0959-8049(02)00664-0. [DOI] [PubMed] [Google Scholar]
- 14.Mauer M, Bottomley A, Coens C, Gotay C. Prognostic factor analysis of health-related quality of life data in cancer: a statistical methodological evaluation. Expert Rev Pharmacoecon Outcomes Res. 2008;8(2):179–196. doi: 10.1586/14737167.8.2.179. [DOI] [PubMed] [Google Scholar]
- 15.Gotay CC, Kawamoto CT, Bottomley A, Efficace F. The prognostic significance of patient-reported outcomes in cancer clinical trials. J Clin Oncol. 2008;26(8):1355–1363. doi: 10.1200/JCO.2007.13.3439. [DOI] [PubMed] [Google Scholar]
- 16.Maisey NR, Norman A, Watson M, Allen MJ, Hill ME, Cunningham D. Baseline quality of life predicts survival in patients with advanced colorectal cancer. Eur J Cancer. 2002;38(10):1351–1357. doi: 10.1016/s0959-8049(02)00098-9. [DOI] [PubMed] [Google Scholar]
- 17.Whynes DK, Neilson AR, Robinson MH, Hardcastle JD. Colorectal cancer screening and quality of life. Qual Life Res. 1994;3(3):191–198. doi: 10.1007/BF00435384. [DOI] [PubMed] [Google Scholar]
- 18.Ware J, Jr, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233. doi: 10.1097/00005650-199603000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Cella DF, Tulsky DS. Quality of life in cancer: definition, purpose, and method of measurement. Cancer Invest. 1993;11(3):327–336. doi: 10.3109/07357909309024860. [DOI] [PubMed] [Google Scholar]
- 20.Conroy T, Blazeby JM. Health-related quality of life in colorectal cancer patients. Expert Rev Anticancer Ther. 2003;3(4):493–504. doi: 10.1586/14737140.3.4.493. [DOI] [PubMed] [Google Scholar]
- 21.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 22.Zack MM, Centers for Disease C, Prevention Health-related quality of life -United States, 2006 and 2010. MMWR Surveill Summ. 2013;62(Suppl 3):105–111. [PubMed] [Google Scholar]
- 23.Dunlop DD, Song J, Lyons JS, Manheim LM, Chang RW. Racial/ethnic differences in rates of depression among preretirement adults. Am J Public Health. 2003;93(11):1945–1952. doi: 10.2105/ajph.93.11.1945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tawk R, Abner A, Ashford A, Brown CP. Differences in Colorectal Cancer Outcomes by Race and Insurance. Int J Environ Res Public Health. 2015;13(1) doi: 10.3390/ijerph13010048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yost KJ, Hahn EA, Zaslavsky AM, Ayanian JZ, West DW. Predictors of health-related quality of life in patients with colorectal cancer. Health Qual Life Outcomes. 2008;6:66. doi: 10.1186/1477-7525-6-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zahran HS, Kobau R, Moriarty DG, Zack MM, Holt J, Donehoo R, et al. Health-related quality of life surveillance--United States, 1993–2002. MMWR Surveill Summ. 2005;54(4):1–35. [PubMed] [Google Scholar]
- 27.Arndt V, Merx H, Stegmaier C, Ziegler H, Brenner H. Quality of life in patients with colorectal cancer 1 year after diagnosis compared with the general population: a population-based study. J Clin Oncol. 2004;22(23):4829–4836. doi: 10.1200/JCO.2004.02.018. [DOI] [PubMed] [Google Scholar]
- 28.Kind P, Dolan P, Gudex C, Williams A. Variations in population health status: results from a United Kingdom national questionnaire survey. BMJ. 1998;316(7133):736–741. doi: 10.1136/bmj.316.7133.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barile JP, Mitchell SA, Thompson WW, Zack MM, Reeve BB, Cella D, et al. Patterns of Chronic Conditions and Their Associations With Behaviors and Quality of Life, 2010. Prev Chronic Dis. 2015;12:E222. doi: 10.5888/pcd12.150179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Religioni U, Czerw A, Deptala A. Acceptance of Cancer in Patients Diagnosed with Lung, Breast, Colorectal and Prostate Carcinoma. Iran J Public Health. 2015;44(8):1135–1142. [PMC free article] [PubMed] [Google Scholar]
- 31.Owusu D, Quinn M, Wang KS. Alcohol Consumption, Depression, Insomnia and Colorectal Cancer Screening: Racial Differences. Int J High Risk Behav Addict. 2015;4(2):e23424. doi: 10.5812/ijhrba.4(2)2015.23424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheithauer W, Rosen H, Kornek GV, Sebesta C, Depisch D. Randomised comparison of combination chemotherapy plus supportive care with supportive care alone in patients with metastatic colorectal cancer. BMJ. 1993;306(6880):752–755. doi: 10.1136/bmj.306.6880.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akyol M, Ulger E, Alacacioglu A, Kucukzeybek Y, Bayoglu V, Yildiz Y, et al. Quality of life in colorectal cancer patients: an Izmir Oncology Group (IZOG) study. J BUON. 2015;20(4):1015–1022. [PubMed] [Google Scholar]
- 34.Sloan JA, Loprinzi CL, Kuross SA, Miser AW, O’Fallon JR, Mahoney MR, et al. Randomized comparison of four tools measuring overall quality of life in patients with advanced cancer. J Clin Oncol. 1998;16(11):3662–3673. doi: 10.1200/JCO.1998.16.11.3662. [DOI] [PubMed] [Google Scholar]
- 35.Hilgenfeld RU, Streit M, Thiel E, Kreuser ED. Current treatment modalities in advanced colorectal carcinoma. Recent Results Cancer Res. 1996;142:353–380. doi: 10.1007/978-3-642-80035-1_20. [DOI] [PubMed] [Google Scholar]
- 36.Earlam S, Glover C, Fordy C, Burke D, Allen-Mersh TG. Relation between tumor size, quality of life, and survival in patients with colorectal liver metastases. J Clin Oncol. 1996;14(1):171–175. doi: 10.1200/JCO.1996.14.1.171. [DOI] [PubMed] [Google Scholar]
- 37.Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al. The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst. 1993;85(5):365–376. doi: 10.1093/jnci/85.5.365. [DOI] [PubMed] [Google Scholar]
- 38.Ward WL, Hahn EA, Mo F, Hernandez L, Tulsky DS, Cella D. Reliability and validity of the Functional Assessment of Cancer Therapy-Colorectal (FACT-C) quality of life instrument. Qual Life Res. 1999;8(3):181–195. doi: 10.1023/a:1008821826499. [DOI] [PubMed] [Google Scholar]