Abstract
Although episodic and semantic memory share overlapping neural mechanisms, it remains unclear how our pre-existing semantic associations modulate the formation of new, episodic associations. When freely recalling recently studied words, people rely on both episodic and semantic associations, shown through temporal and semantic clustering of responses. We asked whether orienting participants toward semantic associations interferes with or facilitates the formation of episodic associations. We compared electroencephalographic (EEG) activity recorded during the encoding of subsequently recalled words that were either temporally or semantically clustered. Participants studied words with or without a concurrent semantic orienting task. We identified a neural signature of successful episodic association formation whereby high frequency EEG activity (HFA, 44 – 100 Hz) overlying left prefrontal regions increased for subsequently temporally clustered words, but only for those words studied without a concurrent semantic orienting task. To confirm that this disruption in the formation of episodic associations was driven by increased semantic processing, we measured the neural correlates of subsequent semantic clustering. We found that HFA increased for subsequently semantically clustered words only for lists with a concurrent semantic orienting task. This dissociation suggests that increased semantic processing of studied items interferes with the neural processes that support the formation of novel episodic associations.
Keywords: episodic, semantic, free recall, electroencephalography
Introduction
Episodic memory, the memory for contextually rich personal experiences, is typically distinguished from semantic memory, the memory for general knowledge and facts (Tulving, 1972). Despite this qualitative distinction, it is clear that semantic and episodic memory interact (Tulving, 1983; Squire & Zola, 1998; McClelland & Rogers, 2003), given that episodic retrieval leads to activation of the same neural substrates which support semantic memory (Polyn et al., 2005; Martin, 2007; Patterson et al., 2007; Binder & Desai, 2011; Rissman & Wagner, 2012; Kuhl & Chun, 2014). Although these lines of evidence show a broad interaction between semantic and episodic memory systems, it remains unclear how pre-existing semantic knowledge impacts the formation of new, episodic associations.
The goal of the current study was to measure the influence of semantic associations during the encoding of items in a free recall task (Figure 1A). In free recall, participants study a list of items and, either immediately or after a brief delay, must recall those items in any order. Participants often consecutively recall study neighbors, a phenomena known as temporal clustering (Kahana, 1996; Sederberg et al., 2010), which may be the result of an association formed between a study item and a slowly updating context representation, as posited by retrieved context theory (Howard & Kahana, 2002a; Sederberg et al., 2008; Polyn et al., 2009; Lohnas et al., 2015). Semantic processing could either facilitate or interfere with the formation of these episodic associations. Semantic processing could be facilitative by enhancing the context representation. In this view, any additional information incorporated in the memory trace, including semantic associations, will provide a better retrieval cue. Behavioral evidence has shown enhanced priming for items that are both semantically and episodically related (McKoon & Ratcliff, 1979) and has shown increased probability of transitioning between these items during free recall (Howard & Kahana, 2002b). Alternatively, if semantic and episodic associations compete for position in the context representation, increased semantic processing will interfere with the formation of episodic associations. The context maintenance and retrieval model of Polyn et al., (2009) inversely weights the input of semantic and episodic associations in context, which predicts that each will interfere with the other. Behavioral studies have shown that presenting many semantically related items on a study list reduces temporal clustering (Greene & Crowder, 1984), suggesting that focused processing of semantic associations might negatively impact the formation of episodic associations.
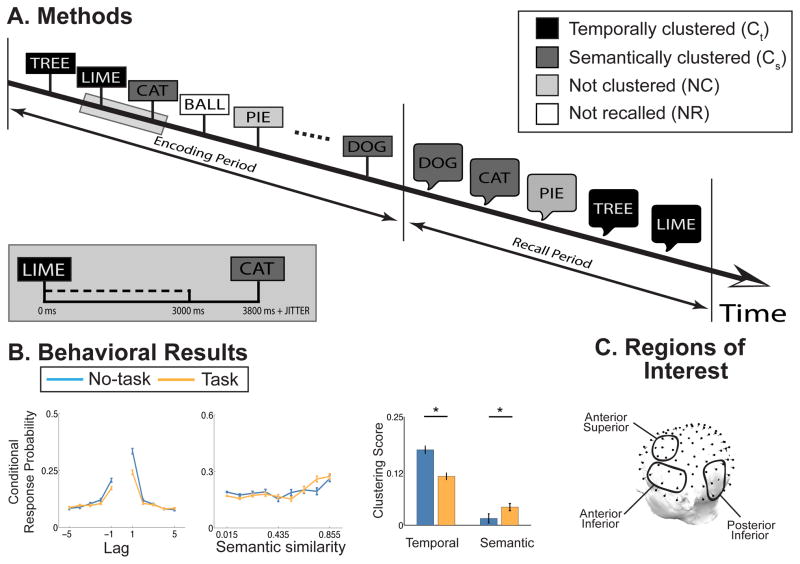
Figure 1. Methods and behavioral results.
(A) Methods. During the encoding period, participants viewed words presented for 3000 ms and separated by a variable interstimulus interval. Following the last item on the list, participants recall the study items in any order. A subset of study items were semantic associates, e.g. cat and dog in this figure. Semantic relatedness was determined using Word Association Space (WAS) values (see Methods). Encoding items were divided into four conditions based on how they were recalled: temporally clustered (Ct, black) or recalled preceding or following a study neighbor, e.g. tree and lime; semantically clustered (Cs, dark grey) or recalled preceding or following a semantic associate; not clustered (NC, light grey) or recalled preceding and following non-neighboring and non-semantically related items; or not recalled (NR, white). (B) Behavioral results. Participants show a tendency to both temporally and semantically cluster their recalls. The lag contiguity analysis (first panel) shows that participants are more likely to make transitions between study neighbors, those items separated by a lag of +/− 1, than between non-neighboring study items. Likewise, the semantic contiguity analysis (second panel) shows that participants are more likely to make transitions between semantically associated items, where increased association corresponds to increased WAS values. These effects are consistent for both no-task (blue) and task (orange) lists. The third panel shows the quantification of these contiguity effects. Temporal clustering scores are reliably greater on no-task compared to task lists, whereas semantic clustering scores are reliably greater on task compared to no-task lists. Errorbars are standard error of the mean. (C) Regions of interest. We analyzed three a priori defined ROIs, left Anterior Superior (AS), left Anterior Inferior (AI) and left Posterior Inferior (PI).
As memory formation and retrieval processes are difficult to dissociate using behavioral methods alone, we assessed the impact of semantic processing on episodic formation using neuro-imaging methods. We measured the spectral correlates of subsequent temporal clustering, a proxy of episodic association formation, using scalp electroencephalographic (EEG) recordings. These EEG signals can be analyzed in terms of specific time-varying oscillatory or spectral components of neural activity. High frequency activity (HFA, 44 – 100 Hz) in particular may reflect general cortical activation (Manning et al., 2009; Jacobs & Kahana, 2009; Lachaux et al., 2012; Burke et al., 2015; Johnson & Knight, 2015) as increases in HFA correlate with single and multi-unit activity (Rasch, Gretton, Murayama, Maass, & Logothetis, 2008; Manning et al., 2009) as well as the blood oxygenated level dependent effect observed using functional magnetic resonance imaging (Mukamel et al., 2005; Kilner, Mattout, Henson, & Friston, 2005; Niessing et al., 2005; Lachaux et al., 2007; Ojemann, Ojemann, & Ramsey, 2013). Changes in HFA track a variety of cognitive processes, including successful memory formation (Long et al., 2014) and retrieval (Burke, Sharan, et al., 2014), suggesting that differences in episodic association formation might be reflected by changes in HFA.
We sought to assess the impact of semantic processing on the formation of episodic associations by measuring these spectral signals. To manipulate semantic processing, we included lists with and without a semantic orienting task (henceforth task and no-task lists). On task lists, participants evaluated the size or animacy of each item. With these semantic tasks, we were able to direct processing to item-specific semantic features as well as obtain a measure of performance in order to ensure that participants were engaged with the task. We predict that HFA will increase during the encoding of subsequently temporally clustered items and that this HFA increase will vary as a function of orienting task. If semantic processing is facilitative, these effects should be greater for task lists than no-task lists, as the semantic processing on task lists should enhance contextual encoding. If semantic processing interferes with episodic processing, subsequent temporal clustering effects should be greater for no-task lists, as semantic processing will disrupt episodic processes specifically on task lists.
Materials and Methods
Participants
152 (86 female) paid volunteers (ages 18 – 29) were recruited via fliers posted around the University of Pennsylvania campus. Participants were provided with a base monetary compensation plus an additional performance-based monetary incentive to ensure full effort. Monetary incentive was based on responding within 3000 ms for trials with a concurrent encoding task and on minimizing blinking during word presentations. Participants could earn up to $5 per session for responding in time on all task list trials. Participants could earn up to $5 per session for blinking on fewer than 15% of trials. The Institutional Review Board at the University of Pennsylvania approved our research protocol, and informed consent was obtained from all participants.
Free recall task
The data reported in this manuscript were collected as part the Penn Electrophysiology of Encoding and Retrieval Study (PEERS), involving three multi-session experiments that were sequentially administered. The data reported below are from Experiment 1 and can be accessed at the following lab website (http://memory.psych.upenn.edu/Publications).
Participants performed an immediate free recall experiment consisting of seven sessions of 16 lists of 16 words presented one at a time on a computer screen (Figure 1A). Each word was drawn from a pool of 1638 words taken from the University of South Florida free association norms (Nelson et al., 2004, available at http://memory.psych.upenn.edu/files/wordpools/PEERS_wordpool.zip). Semantic relatedness was determined using the word association space (WAS) model (Steyvers et al., 2004). WAS similarity values were used to group words into four similarity bins (high similarity, cos θ > 0.7; medium-high, 0.4 < cos θ < 0.7; medium-low, 0.14 < cos θ < 0.4; low similarity, cos θ < 0.14). Two pairs of items from each of the four groups were arranged such that one pair occurred at adjacent serial positions and the other pair was separated by at least two other items. All randomly generated word lists conformed to this structure. The same word was not repeated in a session.
Words were presented concurrently either with a task cue, indicating the judgment that the participant should make for that word, or with no encoding task. The two encoding tasks were a size judgment (“Will this item fit into a shoebox?”) and an animacy judgment (“Does this word refer to something living or not living?”), and the current task was indicated by the color and typeface of the presented item. Participants indicated their response by pressing one of four labeled buttons on the keyboard. During no-task lists participants were instructed to read and remember the words without making any overt responses. There were four no-task lists (participants did not have to perform judgments with the presented items), six single-task lists (all items were presented with the same task, three of each task), and six task-shift lists (items were presented with either task). List and task order were counterbalanced across sessions and participants. As we had no predictions of how task-switching would interact with semantic processing to impact the formation of episodic associations, we did not analyze task-shift lists.
For each list, there was a 1500 ms delay before the first word appeared on the screen. Each item was on the screen for 3000 ms, followed by a jittered 800 to 1200 ms interstimulus interval (uniform distribution). After the last item in the list, there was a 1200 to 1400 ms jittered delay, after which a tone sounded, a row of asterisks appeared, and the participant was given 75 s to attempt to recall any of the just-presented items.
We excluded all recency items (serial positions 13 – 16) to minimize recency effects (Murdock, 1962) as temporal clustering of these items might be due to either episodic formation during encoding or the result of the high degree of similarity between the end-of-list and test contexts, which may reflect separate processes. Additionally, we excluded the last three sessions from our analyses to minimize the number of words that repeated across session per condition. With this criterion, on average ten or fewer items within each condition of interest repeated across all sessions. This resulted in a total of 40 lists per participant.
Electrophysiological recordings and data processing
EEG measurements were recorded using Geodesic Sensor Nets (GSN; Netstation 4.3 acquisition environment, from Electrical Geodesics, Inc.). The GSN provided 129 standardized electrode placements across participants. All channels were digitized at a sampling rate of 500 Hz, and the signal from the caps was amplified via either the Net Amps 200 or 300 amplifier. Recordings were initially referenced to Cz and later converted to an average reference. Channels that demonstrated high impedance or poor contact with the scalp were excluded from the average reference. For each participant and electrode, a fourth order 2 Hz stopband butterworth notch filter was applied to the raw EEG signal at 60 Hz to eliminate electrical line noise.
To identify epochs contaminated with eyeblink and other movement artifacts, electrooculogram (EOG) activity was monitored bipolarly using right and left electrode pairs (electrodes 25, 127 and 8 and 126 on the GSN). An individual word presentation event was rejected from subsequent analyses if the weighted running average for either the right or the left EOG pair exceeded a 100 μV threshold.
Data analyses and spectral power
We applied the Morlet wavelet transform (wave number 6) to all electrode EEG signals from 500 ms preceding to 3000 ms following word presentation, across 46 logarithmically spaced frequencies (2–100 Hz). We included a 1000 ms buffer on both sides of the data to minimize edge effects. After log transforming the power, we downsampled the data by taking a moving average across 100 ms time windows and sliding the window every 50 ms, resulting in 69 time intervals (35 non-overlapping) from −500 ms to 3000 ms surrounding stimulus presentation. Power values were then Z-transformed within session by subtracting the mean and dividing by the standard deviation power. Mean and standard deviation power were calculated across all encoding events and time points in a session for each frequency. We split the Z-transformed power into six distinct frequency bands (θL, 3–4 Hz; θH, 6–8 Hz; α, 10–14 Hz; β, 16–26 Hz; γL, 28–42 Hz; γH, 44–100 Hz; Sederberg et al., 2006), by taking the mean of the Z-transformed power in each frequency band and across the 0 to 3000 ms presentation interval. We collapsed our analyses across the encoding interval as we had no a priori hypotheses about the time course of subsequent temporal and semantic clustering processes.
We defined four conditions of interest (Figure 1A), items subsequently recalled and temporally clustered (Ct), items subsequently recalled and semantically clustered (Cs), items subsequently recalled and not clustered (NC), and items subsequently not recalled (NR). Ct items were study items recalled either preceding or following the recall of a study neighbor (absolute lag between serial position of items was 1), but not recalled preceding or following a semantic associate. Cs items were study items recalled either preceding or following the recall of a semantic associate (WAS value for the pair of items was >= .4), but not recalled preceding or following a study neighbor. NC items were study items recalled preceding and following non-neighboring and non-semantically associated study items. We analyzed these conditions separately for no-task lists (no encoding task performed) and task lists (single encoding task, either animacy or size judgment, performed for all items in a list).
ROI selection and analysis
Z-power values were averaged across electrodes within a region of interest (ROI) as we were interested in effects consistent across an ROI and not regional differences within an ROI. Therefore, each participant contributed a single Z-power value for each condition for each ROI. Our three ROIs (Figure 1) were selected a priori based on previous scalp EEG studies (Weidemann et al., 2009; Long et al., 2014) and were intended to cover left prefrontal (Anterior Superior, AS; Anterior Inferior, AI) and left temporal cortex (Posterior Inferior, PI) as subsequent memory effects are predominantly left lateralized (Kim, 2011; Burke, Long, et al., 2014). Conditions were compared across participants within an ROI and frequency using a paired t-test.
Results
The goal of our study was to assess the influence of semantic processing on episodic memory formation. We first measured response accuracy on task lists. Participants gave an accurate response on 86% of size trials and 88% of animacy trials where we defined the correct size or animacy judgment based on the modal response of an independently collected dataset of 42 participants (Polyn et al., 2012). We then measured the tendency of participants to temporally and semantically cluster their recalls (Figure 1B) separately for task and no-task lists. Difference in serial position, or lag, determined temporal relatedness. Word Association Space (WAS) values (Steyvers et al., 2004) determined semantic relatedness. WAS values ranged from −.2 to 1, where low values indicate weak semantic relatedness (e.g. tree and lime, WAS = .17) and high values indicate strong semantic relatedness (e.g. dog and cat, WAS = .95). We crossed episodic and semantic relatedness by designing study lists such that at least two pairs of high semantically related items (WAS > .4) were temporally contiguous, while another two pairs of highly semantically related items were separated by at least two intervening items. All temporally contiguous high semantic items were excluded from these analyses as it is impossible to know if such items are clustered based semantic or episodic associations.
Participants recalled on average 66% (SD = 13%) of studied items on no-task lists and 58% (SD = 11%) of studied items on task lists, with recall performance reliably greater on no-task relative to task lists (t(151) = 14.1, p < .001). Participants were more likely to make recall transitions between neighboring than distal study items (Figure 1B, first panel) and were more likely to make recall transitions between semantically related study items than non-semantically related study items (Figure 1B, second panel). We quantified the tendency to cluster study neighbors with a temporal clustering (tc) score: the probability of making a transition of absolute lag of 1 minus the average probability of making a transition of absolute lag of 3 through 5 (Kahana, 1996). Likewise, we quantified the tendency to cluster semantic associates with a semantic clustering (sc) score: the probability of making a transition to another item with a WAS value of .4 or greater minus the average probability of making a transition to an item with a WAS value less than .4. Across both list types, tc scores were reliably greater than zero (Figure 1B, third panel, ts > 13.0, ps < .001). sc scores were only reliably greater than zero for task lists (Figure 1B, third panel, t(151) = 5.3, ps < .001). sc scores were reliably greater than zero for both size (t(151) = 5.1, p < .01) and animacy (t(151) = 2.8, p < .01) lists, and did not differ between tasks (t(151) = 1.7, p = .10), thus we collapse all following analyses across both task list types. tc scores reliably differed between no-task and task lists (t(151) = 6.3, p < .001), as did sc scores (t(151) = 2.2, p < .05).
The behavioral results suggest that the semantic orienting task might interfere with episodic encoding as temporal clustering was reliably decreased in task lists. However, clustering is likely the result of both encoding and retrieval processes, thus the decrease in temporal clustering might be due to interference at retrieval. As behavioral measures alone cannot dissociate these two processes and our goal was to measure how semantic processing impacts episodic association formation, we turn to the neural data to measure the processes at encoding. We extracted spectral signals across six frequency bands, low theta (3–4 Hz), high theta (6–8 Hz), alpha (10–14 Hz), beta (16–26 Hz), low gamma (28–42 Hz) and high frequency activity (44–100 Hz) and three regions of interest (ROIs), left Anterior Superior (AS), left Anterior Inferior (AI) and left Posterior Inferior (PI; Figure 1C). We made comparisons across items subsequently temporally clustered, but not semantically clustered (Ct), items subsequently semantically clustered, but not temporally clustered (Cs), and items subsequently recalled, but not clustered (NC) separately for no-task and task lists. Ct items were recalled preceding or following a study neighbor, Cs items were recalled preceding or following a semantic associate, and NC items were recalled preceding and following non-neighboring and non-related study items. Semantic associates were pairs of study list items with WAS values of .4 or greater.
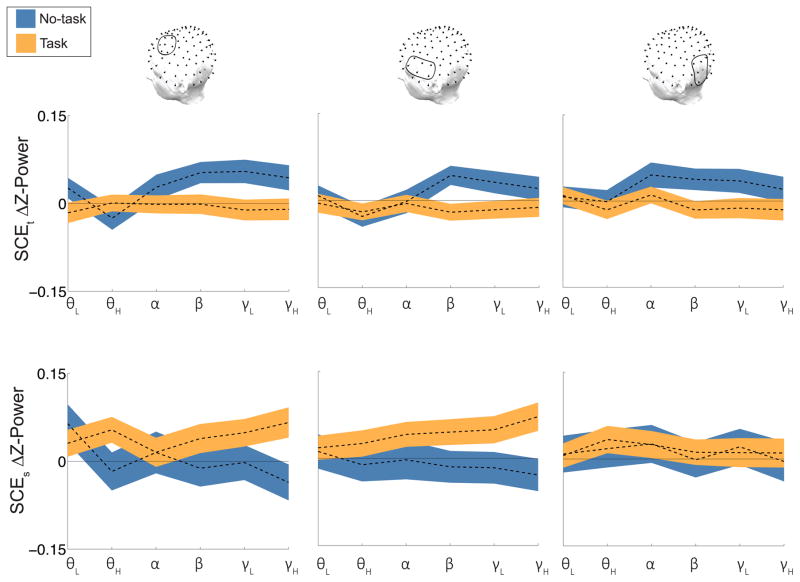
Our first goal was to identify a neural subsequent temporal clustering effect. In a previous study (Long & Kahana, 2015) we found that high frequency activity (HFA, 44 – 100Hz) increases as a function of subsequent temporal clustering; however, as we did not control for semantic relatedness among study words, temporally clustered words could also have been clustered based on semantic associations. Therefore, we were unable to dissociate the contributions of episodic and semantic processing. To measure the signals unique to episodic association formation, we analyzed the subsequent temporal clustering effect (SCEt) by comparing Ct items to NC items on no-task lists. All semantically clustered items were excluded. This SCEt analysis revealed several power increases across ROIs (Figure 2, top row, blue line). Beta power increases were significant in all ROIs (AS, t(151) = 3.0, p < .01; AI, t(151) = 2.8, p< .01; PI, t(151) = 2.3, p = .02). Low gamma and HFA power increases were significant in AS (low gamma, t(151) = 2.7, p < .01; HFA, t(151) = 2.0, p= .04). These results suggest that the formation of episodic associations is characterized by increases in HFA over left prefrontal regions. This is the first demonstration in scalp EEG of HFA increases related to episodic association formation.
Figure 2. Subsequent clustering effects.
The top panel shows the subsequent temporal clustering effect (SCEt) and the bottom panel shows the subsequent semantic clustering effect (SCEs) for no-task (blue) and task (orange) lists. Each line shows the difference in Z-Power between subsequently clustered and subsequently recalled, but not clustered, items, for 6 frequency bands (θL, 3–4 Hz; θH, 6–8 Hz; α, 10–14 Hz; β, 16–26 Hz; γL, 28–42 Hz; γH, 44–100 Hz). Z-Power is averaged across the encoding interval (0 – 3000 ms). Errorbars are standard error of the mean.
Next, we sought to test the two hypotheses that orienting participants to semantic associations could either facilitate or interfere with the formation of episodic associations. If semantic processing facilitates episodic memory formation, we should observe an increased SCEt on task relative to no-task lists. Semantic processing may increase contextual encoding by strengthening the context representation. Alternatively, if semantic processing interferes with episodic memory formation, we should observe a decreased SCEt on task relative to no-task lists. The task may decrease contextual encoding by directing processing toward semantic associations at the expense of episodic associations. We tested these competing hypotheses by comparing Ct and NC items on task lists. This SCEt analysis revealed no consistent effects in our ROIs (Figure 2, top row, orange line) and specifically no significant HFA effects (ts < 1.5, ps > .10).
Our results suggest that semantic processing interferes with episodic encoding mechanisms. However, this interpretation assumes that the semantic orienting task fully focuses processing on semantic features and although we see behavioral evidence for semantic clustering on task lists only, this may be due to processes at retrieval rather than encoding. It is thus unclear whether the task increases semantic processing. If the semantic orienting task directs resources away from episodic association formation in favor of semantic processing, there should be a neural subsequent semantic clustering effect for task lists. To test this hypothesis, we analyzed the subsequent semantic clustering effect (SCEs) by comparing Cs and NC items exclusively for task lists. All temporally clustered items were excluded. This SCEs analysis revealed broad increases in power across both anterior ROIs (Figure 2, bottom row, orange line). High theta increases were significant in AS (t(148) = 2.1, p = .03) and beta increases were significant in AI (t(148) = 2.0, p= .04). Low gamma and HFA increases were significant in both AS (low gamma, t(148) = 2.3, p = .02; HFA, t(148) = 2.7, p < .01) and AI (low gamma, t(148) = 2.3, p = .02; HFA, t(148) = 2.9, p < .01).
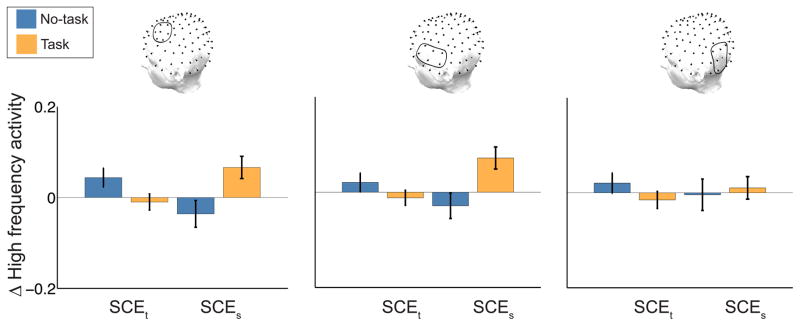
We have shown that for task lists the SCEt decreases whereas the SCEs increases, suggesting that participants are processing semantic features during the orienting task. However, participants may process those semantic features regardless of the orienting task. If semantic processing interferes with episodic encoding, a reliable SCEt should not co-occur with a SCEs. To test this hypothesis, we measured the SCEs for no-task lists. This SCEs analysis revealed no consistent effects in our ROIs (Figure 2, bottom row, blue line) and specifically no significant HFA effects (ts < 2, ps > .05). As our neural results suggest that semantic processing interferes with episodic processing, we should observe an interaction between the type of clustering effect and the list type. We compared HFA for the SCEt and SCEs for no-task and task lists (Figure 3) using a 2 × 2 repeated measures ANOVA and found a reliable clustering effect type × list type interaction in AS (F(1,143) = 14.5, p < .001) and AI (F(1,143) = 11.7, p < .001), but not PI (F(1,143) = .85, p = .36).
Figure 3. HFA as a function of clustering and list type.
Subsequent temporal and semantic clustering effects (SCEt and SCEs) separately for no-task (blue) and task (orange) lists. Each bar shows the difference in high frequency activity between subsequently clustered (temporal or semantic) and subsequently recalled, but not clustered words. There is a reliable interaction in AS and AI such that the SCEt is specific to no-task lists and the SCEs is specific to task lists. Error bars are standard errors of the mean.
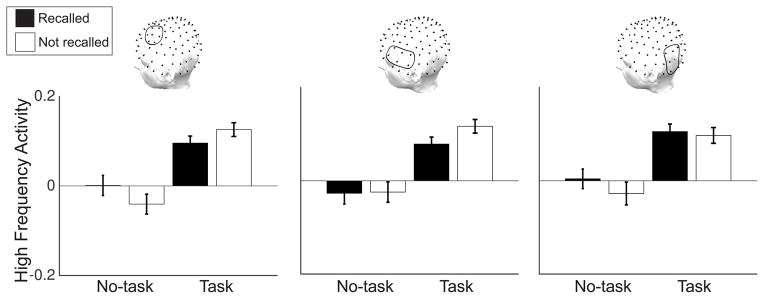
The dissociation in clustering effect type and list type suggests that semantic processing interferes with episodic processing. As HFA increases for both of these processes, it might reflect a control mechanism which biases retrieval of task-relevant features. Although HFA increases are typically associated with successful memory formation (Sederberg et al., 2003; Burke, Long, et al., 2014; Long et al., 2014), an implication of such a control mechanism is that when task-relevant associations are incongruent with episodic memory demands, HFA should be negatively related to memory performance. We can test this prediction by measuring the encoding activity of semantically isolated items, those items with no semantic neighbors on the study list (pairs of study list items with WAS values < .4). Increased semantic processing of these items, reflected through increases in HFA, should be detrimental to their subsequent recall, as no other semantic associate will be available as a retrieval cue, and the necessary episodic associations will not have been formed. We measured HFA during the encoding of semantically isolated items which were later recalled or not recalled, for both task and no-task lists. This analysis revealed a negative subsequent memory effect exclusively for task lists (Figure 4). We ran a 2 × 2 repeated measures ANOVA comparing recall status (recalled or not recalled) and list type (no-task or task) to test the significance of this result. We found a reliable recall status × list type interaction in AS (F(1,151) = 6.4, p = .01), but not AI or PI (AI, F(1,151) = 1.8, p = .19; PI, F(1,151) = .79, p = .37).
Figure 4. Subsequent memory effect for semantically isolated items.
Semantically isolated items are those items which do not have a strong semantic study associate (WAS > .4). The figure shows HFA for semantically isolated items that are subsequently recalled (recalled, black) or subsequently not recalled (not recalled, white), separately for no-task and task lists. There is a reliable interaction in AS such that HFA is increased for not recalled relative to recalled items specifically for task lists. Error bars are standard errors of the mean.
Discussion
The goal of the current study was to measure the impact of semantic processing on the formation of episodic associations. Our study demonstrates three key findings. First, there is a subsequent temporal clustering effect (SCEt), characterized by increased high frequency activity (HFA, 44 – 100 Hz) over left prefrontal (PFC) regions, specific to lists without a concurrent semantic orienting task (no-task lists). Second, there is a subsequent semantic clustering effect (SCEs), characterized by increased HFA over left PFC, specific to lists with a concurrent semantic encoding task (task lists). Finally, during task lists, semantically isolated items show a negative subsequent memory effect (SME). That is, HFA over PFC was greater for subsequently forgotten compared to subsequently recalled items, specifically for items which were only weakly semantically related to other study list items. These results suggest that orienting processing toward pre-existing semantic associations, as in the task lists, interferes with the formation of new, episodic associations.
We found increased HFA over left PFC regions during the encoding of subsequently temporally clustered items, specifically for no-task lists (Figure 2, top row, blue line). Retrieved context theory posits that clustering is the result of items forming associations with a slowly updating context representation (Howard & Kahana, 2002a; Polyn et al., 2009; Lohnas et al., 2015). This context representation is a weighted sum of both pre-experimental semantic associations and newly formed episodic associations. We hypothesize that increased HFA over PFC reflects a cognitive control mechanism which determines the relative weight of each of these associations. Specifically, PFC may alternate between maintaining the previously studied items or retrieving semantic associations. Substantial evidence has shown that PFC is critical for a variety of control processes, including maintenance and manipulation of information in working memory (Petrides, 2000; Howard et al., 2003; Hazy et al., 2006; Chatham et al., 2014) as well as controlled retrieval, selection, and associative encoding processes (Thompson-Schill et al., 1998; Otten et al., 2001; Thompson-Schill, 2003; Bunge et al., 2005; Badre & Wagner, 2007; Blumenfeld & Ranganath, 2007; Park & Rugg, 2011; Rodd et al., 2012). Our finding that the SCEt is specific to no-task lists suggests that HFA increases on no-task lists might reflect maintenance of previous study list items and that the orienting task interferes with this process.
HFA over left PFC increased during the encoding of subsequently semantically clustered items specifically for task lists (Figure 2, bottom row, orange line). This result confirmed that during encoding, the semantic orienting task increased semantic processing on task lists relative to the no-task lists. Furthermore, this result is consistent with the interference hypothesis, as we would not expect to concurrently observe both an SCEs and an SCEt on no-task lists. That the SCEs and SCEt both show increased HFA over left PFC suggests a control mechanism which directs processing based on task demands. During no-task lists, left PFC may direct participants to maintain previous study items, promoting the formation of episodic associations. During task lists, left PFC may direct participants to retrieve item-specific semantic features which interfere with the maintained representations of previously studied items, diminishing the formation episodic associations. The interpretation that left PFC exerts control in this manner is consistent with previous work showing that left PFC can bias content-specific processing in posterior regions (Miller & Cohen, 2001; Thompson-Schill, 2003; Noppeney et al., 2006; Bedny et al., 2008; Kuhl et al., 2013).
The dissociation of subsequent clustering effect and list type suggests that the processing of semantic and episodic associations may be inversely related as predicted by retrieved context models (Polyn et al., 2009). However, one might have predicted that semantic associations would facilitate the formation of episodic associations, as there is evidence that items related both episodically and semantically show the most facilitative priming (McKoon & Ratcliff, 1979). Our experiment was ideally suited to test these competing hypotheses as the design intentionally included semantically isolated items, items paired with a study list item of low semantic similarity (Word Association Score < .4). According to the facilitative hypothesis, increased semantic processing should always benefit a given study item, whether or not that item is semantically isolated. Alternatively, according to the interference hypothesis, increased semantic processing of a semantically isolated item should diminish its probability of being recalled. That is, semantic processing will not be beneficial because there are no semantic associates which can cue retrieval of the semantic isolate. It is furthermore detrimental because it prevents episodic association formation. Our results support the interference hypothesis. We found increased HFA for subsequently forgotten relative to recalled semantically isolated items, specifically for task lists (Figure 4).
HFA increases typically predict successful memory formation. Across both intracranial and scalp EEG, as well as free recall, cued recall and recognition paradigms, electrophysiological activity above 30 Hz tends to increase for items that are later remembered, compared to those that are later forgotten (Sederberg et al., 2003, 2006; Nyhus & Curran, 2010; Düzel et al., 2010; Matsumoto et al., 2013; Burke, Long, et al., 2014; Long et al., 2014; Johnson & Knight, 2015; Burke et al., 2015). In light of this large body of research, it may seem surprising to find a negative subsequent memory effect whereby HFA decreases for subsequently remembered items during task lists. Our interpretation is that when task demands emphasize stimulus features that will not be useful at retrieval, HFA increases could predict memory failures (Blumenfeld & Ranganath, 2007). Thus, during the task lists, left PFC biased processing towards task-relevant information that was both not beneficial for later memory and impaired processing that was necessary for later memory. This interpretation is in line with previous work suggesting that encoding tasks may direct resources away from processes supporting subsequent memory (Otten & Rugg, 2001). Together our results show that accessing semantic associations can interfere with the formation of episodic associations.
An alternative account of the present results is that the signals we observe may reflect differences in item and relational processes. During task lists, participants may direct processing to item-specific semantic features and thus reduce relational processing across study items within a list, enhancing semantic clustering at the expense of temporal clustering. A further possibility is that increased HFA reflects cognitive effort directed to temporal or semantic associations and the degree of match between encoding and retrieval processes determines which type of clustering prevails, in line with a transfer-appropriate processing account (Morris, Bransford, & Franks, 1977). The current study design cannot adjudicate between these interpretations and it is possible that increased HFA reflects increased cognitive effort directed to either item-specific or relational processing. Although future studies will be needed to dissociate these accounts, our results show that the interaction between episodic and semantic processing at encoding warrants further investigation. Ultimately, computational models that make direct contact with these types of data will be necessary to understand the nature of encoding and retrieval processes.
We focused our analyses on HFA as this spectral signal is thought to reflect cortical activation (Jacobs & Kahana, 2009; Lachaux et al., 2012; Burke et al., 2015). Changes in HFA are unlikely to exclusively reflect either memory encoding or clustering mechanisms as fluctuations in HFA correlate with a variety of cognitive processes (Crone, Miglioretti, Gordon, & Lesser, 1998a; Crone et al., 1998b; Crone, Boatman, Gordon, & Hao, 2001; Ray, Niebur, Hsiao, Sinai, & Crone, 2008; Ball, Schulze-Bonhage, Aertsen, & Mehring, 2009; Jerbi et al., 2009). Instead, HFA is likely indexing the activity of a large population of neurons, given that HFA correlates with single unit activity, multiunit activity, and fMRI signals (Mukamel et al., 2005; Ray, Crone, Niebur, Franaszczuk, & Hsiao, 2008; Manning et al., 2009; Ojemann et al., 2013). Previous work has suggested that HFA increases are best understood in terms of when and where they occur, not simply whether they occur (Burke et al., 2015). In the present study we interpret the observed HFA increases over PFC as reflecting cognitive control processes. Although the majority of our effects occurred in the high frequency range, we might have expected to observe both theta (3 – 8 Hz) and beta (16 – 26 Hz) effects. Evidence has shown that theta increases during order and contextual processing (Hsieh et al., 2011; Staudigl & Hanslmayr, 2013). We did not find theta effects, outside of a single contrast in a single ROI. As appears to be the case with HFA, variations in task demands, e.g. explicitly encoding item order, may modulate theta effects. Also, recent work has suggested that beta decreases reflect semantic processing (Hanslmayr et al., 2009; Hanslmayr & Staudigl, 2014). We found beta increases for both subsequent temporal and semantic clustering. This discrepancy could be the result of differences in encoding single items as opposed to pairs of items or associations (Hanslmayr et al., 2012). It is also possible that the increases we observed reflect broadband asynchronous high frequency effects (Manning et al., 2009; Burke et al., 2015) that obscure narrowband effects.
An important future question to address is how semantic and episodic processes interact during retrieval. Unlike episodic information, semantic information does not need to be accessed during encoding in order to be available at retrieval. Thus, there could be an interaction between episodic and semantic processing during recall as well, potentially explaining how the most likely recall transitions are to those items that are both semantically and episodically related (Howard & Kahana, 2002b). An additional critical next step will be to understand how our univariate results relate to recent multivariate work showing that episodic and semantic information is present in context representations during encoding and retrieval (Manning et al., 2011, 2012). Univariate HFA increases should correlate with the degree to which patterns reflect episodic vs. semantic associations and such an effect should be modulated by task demands, given our conclusion that focused semantic processing can interfere with the formation of episodic associations.
Since the term “episodic memory” was coined by Endel Tulving, researchers have endeavored to understand how the episodic and semantic memory systems interact. A critical question has been how our pre-existing associations influence our ability to form new episodic associations. By measuring the impact of a semantic orienting judgment in an episodic task, we have provided evidence suggesting that focused semantic processing interferes with the formation of episodic associations.
Acknowledgments
We thank Elizabeth Crutchley, Joel Kuhn, and Kylie Hower for help with data collection and Youssef Ezzyat and Karl Healey for helpful discussion and input.
Funding
This work was supported by the National Institutes of Health (grant number MH055687).
Footnotes
Conflict of Interest: The authors declare no competing financial interests.
References
- Badre D, Wagner A. Left ventrolateral prefrontal cortex and the cognitive control of memory. Neuropsychologia. 2007;45(13):2883–2901. doi: 10.1016/j.neuropsychologia.2007.06.015. [DOI] [PubMed] [Google Scholar]
- Ball T, Schulze-Bonhage A, Aertsen A, Mehring C. Differential representation of arm movement direction in relation to cortical anatomy and function. Journal of neural engineering. 2009;6(1):016006. doi: 10.1088/1741-2560/6/1/016006. [DOI] [PubMed] [Google Scholar]
- Bedny M, McGill M, Thompson-Schill SL. Semantic adaptation and competition during word comprehension. Cerebral Cortex. 2008;18(11):2574–2585. doi: 10.1093/cercor/bhn018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Desai RH. The neurobiology of semantic memory. Trends in Cognitive Sciences. 2011;15(11):527–536. doi: 10.1016/j.tics.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld R, Ranganath C. Prefrontal cortex and long-term memory encoding: An integrative review of findings from neuropsychology and neuroimaging. The Neuroscientist. 2007;13(3):280. doi: 10.1177/1073858407299290. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cerebral cortex. 2005;15(3):239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Burke JF, Long NM, Zaghloul KA, Sharan AD, Sperling MR, Kahana MJ. Human intracranial high-frequency activity maps episodic memory formation in space and time. NeuroImage. 2014;85(Pt. 2):834–843. doi: 10.1016/j.neuroimage.2013.06.067. doi:0.1016/j.neuroimage.2013.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JF, Ramayya AG, Kahana MJ. Human intracranial high-frequency activity during memory processing: Neural oscillations or stochastic volatility? Current Opinion in Neurobiology. 2015;31:104–110. doi: 10.1016/j.conb.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke JF, Sharan AD, Sperling MR, Ramayya AG, Evans JJ, Healey MK, … Kahana MJ. Theta and high–frequency activity mark spontaneous recall of episodic memories. Journal of Neuroscience. 2014;34(34):11355–11365. doi: 10.1523/JNEUROSCI.2654-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatham CH, Frank MJ, Badre D. Corticostriatal output gating during selection from working memory. Neuron. 2014;81(4):930–942. doi: 10.1016/j.neuron.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crone NE, Boatman D, Gordon B, Hao L. Induced electrocorticographic gamma activity during auditory perception. Clinical Neurophysiology. 2001;112(4):565–582. doi: 10.1016/s1388-2457(00)00545-9. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. II. Event-related synchronization in the gamma band. Brain. 1998a;121(12):2301. doi: 10.1093/brain/121.12.2301. [DOI] [PubMed] [Google Scholar]
- Crone NE, Miglioretti DL, Gordon B, Sieracki JM, Wilson MT, Uematsu S, Lesser RP. Functional mapping of human sensorimotor cortex with electrocorticographic spectral analysis. I. Alpha and beta event-related desynchronization. Brain. 1998b;121:2271–2299. doi: 10.1093/brain/121.12.2271. [DOI] [PubMed] [Google Scholar]
- Düzel E, Penny WD, Burgess N. Brain oscillations and memory. Current Opinion in Neurobiology. 2010;20(2):143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- Greene RL, Crowder RG. Effects of semantic similarity on long-term recency. American Journal of Psychology. 1984;97:441–449. [Google Scholar]
- Hanslmayr S, Spitzer B, Bauml K. Brain oscillations dissociate between semantic and nonsemantic encoding of episodic memories. Cerebral Cortex. 2009;19(7):1631–1640. doi: 10.1093/cercor/bhn197. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T. How brain oscillations form memoriesa processing based perspective on oscillatory subsequent memory effects. Neuroimage. 2014;85:648–655. doi: 10.1016/j.neuroimage.2013.05.121. [DOI] [PubMed] [Google Scholar]
- Hanslmayr S, Staudigl T, Fellner M. Oscillatory power decreases and long-term memory: the information via desynchronization hypothesis. Frontiers in Human Neuroscience. 2012;6(74) doi: 10.3389/fnhum.2012.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazy TE, Frank MJ, OReilly RC. Banishing the homunculus: making working memory work. Neuroscience. 2006;139(1):105–118. doi: 10.1016/j.neuroscience.2005.04.067. [DOI] [PubMed] [Google Scholar]
- Howard MW, Kahana MJ. A distributed representation of temporal context. Journal of Mathematical Psychology. 2002a;46(3):269–299. [Google Scholar]
- Howard MW, Kahana MJ. When does semantic similarity help episodic retrieval? Journal of Memory and Language. 2002b;46:85–98. [Google Scholar]
- Howard MW, Rizzuto DS, Caplan JC, Madsen JR, Lisman J, Aschenbrenner-Scheibe R, … Kahana MJ. Gamma oscillations correlate with working memory load in humans. Cerebral Cortex. 2003;13:1369–1374. doi: 10.1093/cercor/bhg084. [DOI] [PubMed] [Google Scholar]
- Hsieh LT, Ekstrom AD, Ranganath C. Neural oscillations associated with item and temporal order maintenance in working memory. The Journal of Neuroscience. 2011;31(30):10803–10810. doi: 10.1523/JNEUROSCI.0828-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ. Neural representations of individual stimuli in humans revealed by gamma-band ECoG activity. Journal of Neuroscience. 2009;29(33):10203–10214. doi: 10.1523/JNEUROSCI.2187-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jerbi K, Freyermuth S, Minotti L, Kahane P, Berthoz A, Lachaux J. Watching Brain TV and Playing Brain Ball:: Exploring Novel BCI Strategies using Real-Time Analysis of Human Intracranial Data. International Review of Neurobiology. 2009:159–168. doi: 10.1016/S0074-7742(09)86012-1. [DOI] [PubMed] [Google Scholar]
- Johnson EL, Knight RT. Intracranial recordings and human memory. Current opinion in Neurobiology. 2015;31:18–25. doi: 10.1016/j.conb.2014.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahana MJ. Associative retrieval processes in free recall. Memory & Cognition. 1996;24(1):103–109. doi: 10.3758/BF03197276. [DOI] [PubMed] [Google Scholar]
- Kilner J, Mattout J, Henson R, Friston K. Hemodynamic correlates of eeg: a heuristic. Neuroimage. 2005;28(1):280–286. doi: 10.1016/j.neuroimage.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Kim H. Neural activity that predicts subsequent memory and forgetting: a meta-analysis of 74 fMRI studies. NeuroImage. 2011;54(3):2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- Kuhl BA, Chun MM. Successful remembering elicits event-specific activity patterns in lateral parietal cortex. Journal Of Neuroscience. 2014;34(23):8051–8060. doi: 10.1523/JNEUROSCI.4328-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhl BA, Johnson MK, Chun MM. Dissociable neural mechanisms for goal-directed versus incidental memory reactivation. The Journal of Neuroscience. 2013;33(41):16099–16109. doi: 10.1523/JNEUROSCI.0207-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Axmacher N, Mormann F, Halgren E, Crone NE. High-frequency neural activity and human cognition: Past, present, and possible future of intracranial EEG research. Progress in Neurobiology. 2012;98:279–301. doi: 10.1016/j.pneurobio.2012.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachaux JP, Fonlupt P, Kahane P, Minotti L, Hoffmann D, Bertrand O, Baciu M. Relationship between task-related gamma oscillations and bold signal: New insights from combined fMRI and intracranial EEG. Human Brain Mapping. 2007;28(12):1368–1375. doi: 10.1002/hbm.20352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohnas LJ, Polyn SM, Kahana MJ. Expanding the scope of memory search: Intralist and interlist effects in free recall. Psychological Review. 2015;122(2):337–363. doi: 10.1037/a0039036. [DOI] [PubMed] [Google Scholar]
- Long NM, Burke JF, Kahana MJ. Subsequent memory effect in intracranial and scalp EEG. NeuroImage. 2014;84:488–494. doi: 10.1016/j.neuroimage.2013.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long NM, Kahana MJ. Successful memory formation is driven by contextual encoding in the core memory network. NeuroImage. 2015;119:332–337. doi: 10.1016/j.neuroimage.2015.06.073. [DOI] [PubMed] [Google Scholar]
- Manning JR, Jacobs J, Fried I, Kahana MJ. Broadband shifts in LFP power spectra are correlated with single-neuron spiking in humans. Journal of Neuroscience. 2009;29(43):13613–13620. doi: 10.1523/JNEUROSCI.2041-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Polyn SM, Baltuch G, Litt B, Kahana MJ. Oscillatory patterns in temporal lobe reveal context reinstatement during memory search. Proceedings of the National Academy of Sciences, USA. 2011;108(31):12893–12897. doi: 10.1073/pnas.1015174108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JR, Sperling MR, Sharan A, Rosenberg EA, Kahana MJ. Spontaneously reactivated patterns in frontal and temporal lobe predict semantic clustering during memory search. Journal of Neuroscience. 2012;32(26):8871–8878. doi: 10.1523/JNEUROSCI.5321-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin A. The representation of object concepts in the brain. Psychology. 2007;58(1):25. doi: 10.1146/annurev.psych.57.102904.190143. [DOI] [PubMed] [Google Scholar]
- Matsumoto JY, Stead M, Kucewicz MT, Matsumoto AJ, Peters PA, Brinkmann BH, et al. Network oscillations modulate interictal epileptiform spike rate during human memory. Brain. 2013;136(8) doi: 10.1093/brain/awt159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Rogers TT. The parallel distributed processing approach to semantic cognition. Nature Reviews Neuroscience. 2003;4(4):310–322. doi: 10.1038/nrn1076. [DOI] [PubMed] [Google Scholar]
- McKoon G, Ratcliff R. Priming in episodic and semantic memory. Journal of Verbal Learning and Verbal Behavior. 1979;18(4):463–480. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Morris CD, Bransford JD, Franks JJ. Levels of processing versus transfer appropriate processing. Journal of Verbal Learning and Verbal Behavior. 1977;16:519–533. [Google Scholar]
- Mukamel R, Gelbard H, Arieli A, Hasson U, Fried I, Malach R. Coupling between neuronal firing, field potentials, and fMRI in human auditory cortex. Science. 2005;309(5736):951–954. doi: 10.1126/science.1110913. [DOI] [PubMed] [Google Scholar]
- Murdock BB. The serial position effect of free recall. Journal of Experimental Psychology. 1962;64:482–488. doi: 10.1037/h0045106. [DOI] [Google Scholar]
- Nelson DL, McEvoy CL, Schreiber TA. The University of South Florida free association, rhyme, and word fragment norms. Behavior Research Methods, Instruments and Computers. 2004;36(3):402–407. doi: 10.3758/BF03195588. [DOI] [PubMed] [Google Scholar]
- Niessing J, Ebisch B, Schmidt KE, Niessing M, Singer W, Galuske RAW. Hemodynamic signals correlate tightly with synchronized gamma oscillations. Science. 2005 Aug;309(5736):948–951. doi: 10.1126/science.1110948. [DOI] [PubMed] [Google Scholar]
- Noppeney U, Price CJ, Penny WD, Friston KJ. Two distinct neural mechanisms for category-selective responses. Cerebral Cortex. 2006;16(3):437–445. doi: 10.1093/cercor/bhi123. [DOI] [PubMed] [Google Scholar]
- Nyhus E, Curran T. Functional role of gamma and theta oscillations in episodic memory. Neuroscience & Biobehavioral Reviews. 2010 Jun;34(7):1023–1035. doi: 10.1016/j.neubiorev.2009.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojemann GA, Ojemann J, Ramsey NF. Relation between functional magnetic resonance imaging (fmri) and single neuron, local field potential (lfp) and electrocorticography (ecog) activity in human cortex. Front Hum Neurosci. 2013;7:34. doi: 10.3389/fnhum.2013.00034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otten LJ, Henson RNA, Rugg MD. Depth of processing effects on neural correlates of memory encoding: relationship between findings from across- and within-task comparisons. Brain. 2001 Feb;124(Pt 2):399–412. doi: 10.1093/brain/124.2.399. [DOI] [PubMed] [Google Scholar]
- Otten LJ, Rugg MD. When more means less: neural activity related to unsuccessful memory encoding. Current Biology. 2001;11(19):1528–1530. doi: 10.1016/s0960-9822(01)00454-7. [DOI] [PubMed] [Google Scholar]
- Park H, Rugg MD. Neural correlates of encoding within-and across-domain inter-item associations. Journal of cognitive neuroscience. 2011;23(9):2533–2543. doi: 10.1162/jocn.2011.21611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson K, Nestor PJ, Rogers TT. Where do you know what you know? The representation of semantic knowledge in the human brain. Nature Reviews Neuroscience. 2007;8(12):976–987. doi: 10.1038/nrn2277. [DOI] [PubMed] [Google Scholar]
- Petrides M. Dissociable roles of mid-dorsolateral prefrontal and anterior inferotemporal cortex in visual working memory. Journal of Neuroscience. 2000;20(19):7496. doi: 10.1523/JNEUROSCI.20-19-07496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polyn SM, Kragel JE, Morton NW, McCluey JD, Cohen ZD. The neural dynamics of task context in free recall. Neuropsychologia. 2012;50:447–457. doi: 10.1016/j.neuropsychologia.2011.08.025. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Natu VS, Cohen JD, Norman KA. Category-specific cortical activity precedes retrieval during memory search. Science. 2005;310:1963–1966. doi: 10.1126/science.1117645. [DOI] [PubMed] [Google Scholar]
- Polyn SM, Norman KA, Kahana MJ. A context maintenance and retrieval model of organizational processes in free recall. Psychological Review. 2009;116:129–156. doi: 10.1037/a0014420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch MJ, Gretton A, Murayama Y, Maass W, Logothetis NK. Inferring spike trains from local field potentials. Journal of Neurophysiology. 2008;99:1461–1476. doi: 10.1152/jn.00919.2007. [DOI] [PubMed] [Google Scholar]
- Ray S, Crone N, Niebur E, Franaszczuk P, Hsiao S. Neural Correlates of High-Gamma Oscillations (60–200 Hz) in Macaque Local Field Potentials and Their Potential Implications in Electrocorticography. Journal of Neuroscience. 2008;28(45):11526. doi: 10.1523/JNEUROSCI.2848-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray S, Niebur E, Hsiao SS, Sinai A, Crone NE. High-frequency gamma activity (80–150hz) is increased in human cortex during selective attention. Clinical Neurophysiology. 2008;119(1):116–133. doi: 10.1016/j.clinph.2007.09.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Wagner AD. Distributed representations in memory: Insights from functional brain imaging. Annual Review of Psychology. 2012;63:101–128. doi: 10.1146/annurev-psych-120710-100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd JM, Johnsrude IS, Davis MH. Dissociating frontotemporal contributions to semantic ambiguity resolution in spoken sentences. Cerebral Cortex. 2012;22(8):1761–1773. doi: 10.1093/cercor/bhr252. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Gauthier LV, Terushkin V, Miller JF, Barnathan JA, Kahana MJ. Oscillatory correlates of the primacy effect in episodic memory. NeuroImage. 2006;32(3):1422–1431. doi: 10.1016/j.neuroimage.2006.04.223. [DOI] [PubMed] [Google Scholar]
- Sederberg PB, Howard MW, Kahana MJ. A context-based theory of recency and contiguity in free recall. Psychological Review. 2008;115(4):893–912. doi: 10.1037/a0013396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Kahana MJ, Howard MW, Donner EJ, Madsen JR. Theta and gamma oscillations during encoding predict subsequent recall. Journal of Neuroscience. 2003;23(34):10809–10814. doi: 10.1523/JNEUROSCI.23-34-10809.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sederberg PB, Miller JF, Howard WH, Kahana MJ. The temporal contiguity effect predicts episodic memory performance. Memory & Cognition. 2010;38(6):689–699. doi: 10.3758/MC.38.6.689. [DOI] [PubMed] [Google Scholar]
- Squire LS, Zola SM. Episodic memory, semantic memory and amnesia. Hippocampus. 1998;8:205–211. doi: 10.1002/(SICI)1098-1063(1998)8:3<205::AID-HIPO3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Staudigl T, Hanslmayr S. Theta oscillations at encoding mediate the context-dependent nature of human episodic memory. Current Biology. 2013;23(12):1101–1106. doi: 10.1016/j.cub.2013.04.074. [DOI] [PubMed] [Google Scholar]
- Steyvers M, Shiffrin RM, Nelson DL. Word association spaces for predicting semantic similarity effects in episodic memory. In: Healy AF, editor. Cognitive psychology and its applications: Festschrift in honor of Lyle Bourne, Walter Kintsch, and Thomas Landauer. Washington, DC: American Psychological Association; 2004. [Google Scholar]
- Thompson-Schill SL. Neuroimaging studies of semantic memory: inferring “how” from “where”. Neuropsychologia. 2003;41(3):280–292. doi: 10.1016/s0028-3932(02)00161-6. [DOI] [PubMed] [Google Scholar]
- Thompson-Schill SL, Swick D, Farah MJ, D’Esposito M, Kan IP, Knight RT. Verb generation in patients with focal frontal lesions: A neuropsychological test of neuroimaging findings. Proceedings of the National Academy of Sciences. 1998;95(26):15855–15860. doi: 10.1073/pnas.95.26.15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulving E. Episodic and semantic memory. In: Tulving E, Donaldson W, editors. Organization of memory. New York: Academic Press; 1972. pp. 381–403. [Google Scholar]
- Tulving E. Elements of episodic memory. New York: Oxford; 1983. [Google Scholar]
- Weidemann CT, Mollison MV, Kahana MJ. Electrophysiological correlates of high-level perception during spatial navigation. Psychonomic Bulletin & Review. 2009;16(2):313–319. doi: 10.3758/PBR.16.2.313. [DOI] [PMC free article] [PubMed] [Google Scholar]