Abstract
We examined the extent to which the arginine vasopressin receptor 1a (AVPR1a) and dopamine receptor d4 (DRD4) were related to sensitive maternal behavior directly or indirectly via maternal social cognition. Participants were 207 (105 European American, 102 African American) mothers and their children (52% female). Sensitive maternal behavior was rated and aggregated across a series of tasks when infants were 6 months, 1 year, and 2 years old. At 6 months, mothers were interviewed about their empathy, attributions about infant behavior, and beliefs about crying to assess their parenting-related social cognition. Mothers with long alleles for AVPR1a and DRD4 engaged in more mother-oriented social cognition (i.e., negative attributions and beliefs about their infants’ crying), β = .13, p < .05 and β = .16, p < .05, respectively, which in turn predicted less sensitive maternal behavior, β = −.23, p < .01. Both indirect effects were statistically significant independent of one another and covariates; 95% CI [−.22, −.03] and β = −.03 for AVPR; 95% CI [−.20, −.03] and β = −.04 for DRD4. There were no significant direct effects of AVPR1a or DRD4 on maternal sensitivity, β = .02, ns and β = −.10, ns, respectively. The results did not vary for African American and European American mothers (Δχ2 = 18.76, Δdf = 16, p = .28). Results support the view that one mechanism by which maternal genes are associated with parental behavior is via social cognition.
Keywords: mothers, infants, sensitivity, social cognition, arginine vasopressin, AVPR1a, dopamine, DRD4
In the last decade, interest in the genetic underpinnings of individual differences in parenting behavior has increased, largely influenced by compelling evidence that neurological and hormonal factors are associated with parenting behavior among humans and other mammals (Barrett & Fleming, 2011; Mileva-Seitz & Fleming, 2011; Numan, 2010). Although several studies have demonstrated associations between specific genotypes and parenting outcomes, relatively few investigators have examined the role of dopamine receptor D4 (DRD4) and arginine vasopressin receptor 1A (AVPR1a) in relation to parenting (see Mileva-Seitz, Bakermans-Kranenburg & van IJzendoorn, 2016 for a review). Thus, additional examination of associations between these genes and parenting is warranted. Moreover, prior studies did not directly test the purported mechanism, or endophenotypes, by which these genes are associated with sensitive maternal behavior. In this paper, we directly test the possibility that these genes are related to maternal sensitivity via their association with maternal social cognition.
Maternal Sensitivity and Underlying Skills
Sensitive mothers respond to their infants’ signals promptly and consistently, and do so in a manner that is well matched to their infants’ current state, developmental level, and the context. According to Ainsworth, in order to respond sensitively a mother must be aware of her infants’ signals and interpret them from her infants’ point of view rather than distorting them based on her own mood, needs or desires (Ainsworth, Blehar, Water's and Whall, 1978). Thus, maternal social cognitive skills should underlie sensitive maternal behavior. In our prior work, we have demonstrated that mothers who engage in more infant-oriented social information processing, characterized by accurate identification of infant distress, appropriate attributions about the causes of crying, empathy for the infant, and the endorsement of positive and infant-oriented beliefs about crying (i.e., crying communicates infant needs) were observed to be more sensitive (Leerkes, Su, Calkins, Supple & O'Brien, 2016). In contrast, mothers who engage in more mother-oriented social information processing, characterized by negative and non-emotional attributions about the causes of crying and negative, self-oriented beliefs about crying (e.g., crying should be minimized, responding to crying spoils infants) were observed to be less sensitive. Mothers’ ability to engage in infant-oriented social-cognition and related sensitive behavioral responses may be influenced by neural and hormonal processes which are tied to specific genotypes as elaborated below (Mileva-Seitz & Fleming, 2011; Numan, 2010).
The Role of AVPR1a
The neuropeptide arginine vasopressin has been implicated as critically important in relation to social motivation and social cognition, pair bonding, and parenting behavior among rodents and humans (Donaldson & Young, 2008; Heinrichs, von Dawans, & Domes, 2009). Thus, arginine vasopressin receptor genes are particularly relevant to our social-information processing perspective on maternal sensitivity. In humans, allele variation in RS3, one of 3 microsatellites in the promotor region of AVPR1a, has received particular attention. RS3 is a complex repeat located 3625 base pairs (bp) from the transcription start site. Sixteen alleles that vary in length have been identified, and carrying long alleles (i.e., 327 or 334 depending on genotyping method, or longer) has been associated with negative outcomes (Kim et al., 2002). Specifically, carrying 1 or 2 copies of these long alleles has been linked with deficits in social cognition as indexed by autism (Kim et al., 2002), lower generosity (Avinum et al., 2011), lower empathy (Uzefovsky et al., 2015), greater amygdala arousal in response to emotion matching tasks, a correlate of social avoidance (Meyer-Lindenberg et al., 2008), and relationship difficulties as evidenced by greater marital problems (Walum et al., 2008).
Of most relevance, two prior studies have demonstrated associations between AVPR1a and maternal sensitivity. In one, White mothers with two copies of the long allele were observed to be less sensitive interacting with their children (a toddler and older sibling, observed separately) than mothers with one or zero copies of the long allele (Bisceglia et al., 2012). In the other, Israeli mothers who carried 1 or 2 copies of the 327/334 bp allele were observed to be less sensitive (i.e., engaged in less structuring and gentle guidance) during play episodes with their 3.5 year old twins than mothers with no copies of the target allele (Avinun et al., 2012). Importantly, this difference held across both twins and while controlling for the children's AVPR1a genotype thus eliminating the possibility that observed differences are a function of evocative child effects. Thus, we predict that carriers of long AVPR1a alleles (327/334 bp or longer) will engage in more mother-oriented cry processing, less infant-oriented cry processing, and behave less sensitively when interacting with their infants than non-carriers.
The Role of DRD4
Numan (2010) proposed that dopamine activates a motivational system controlled by the nucleus accumbens that promotes appropriate responses to significant stimuli and is critically important to caregiving behavior. Consistent with this view, there is evidence that dopamine is released in the nucleus accumbens during maternal behavior and that injections of dopamine receptor (DR) agonists in the nucleus accumbens activate maternal behavior whereas injections of DR antagonists disrupt maternal responses in postpartum rats (Lonstein, Levy, & Fleming, 2015; Numan, 2010). In humans, DRD4 is one of several dopamine related genes that has received a good deal of attention in relation to prosocial behavior. DRD4 includes a 48 bp variable nucleotide repeat polymorphism in exon III of chromosome 11. Individuals may carry 2 to 11 repeat units, and individuals with 1 or 2 long alleles (7 repeat or more) demonstrate lower gene expression (Schoots & Van Tol, 2003), which may in turn undermine social cognition and prosocial behavior. In fact, individuals who carry the long allele demonstrate lower altruism (Anacker, Enge, Reif & Strobel, 2013) and theory of mind (Lackner, Sabbagh, Hallinan, Liu & Holden, 2012) and higher psychopathy, which is characterized in part by limited empathy for others (Wu & Barnes, 2013). On the other hand, in one study carrying the long DRD4 allele was unrelated to women's self-reported emotional empathy but was associated with their heightened cognitive empathy (Uzefovsky et al., 2014).
To our knowledge three published studies have examined associations between DRD4 and parenting and none have reported main effects of this genotype on parenting outcomes (Beach et al., 2012; Beaver, Shutt, Vaughn, DeLisis, & Wright, 2012; van Ijzendoorn et al., 2008). However, moderating effects of DRD4 on parenting were apparent in two of these studies. Specifically, in a sample of African American parents of adolescents, parental negative mood was associated with more negative parent-child interaction only among parents with DRD4 long alleles (Beach et al., 2012). In a sample of Dutch mothers of children aged 1 to 3 years, daily hassles were associated with lower maternal sensitivity only among mothers with DRD4 long alleles coupled with another dopamine risk polymorphism (COMTval158met) (van Ijzendoorn et al., 2008). Thus, carriers of the long DRD4 alleles appeared to be at elevated risk for compromised parenting under certain conditions. Given that parenting is a complex phenotype influenced by biological, psychological and contextual factors, the absence of simple direct effects of single genes is not entirely surprising. Based on the prior literature, we predict that DRD4 has indirect effects on maternal sensitivity via its associations with mothers’ social cognition, but we test a direct pathway between DRD4 and sensitivity as well.
Proposed Pathways Linking Genes to Sensitivity
In sum, we examine three pathways by which AVPR1a and DRD4 may be linked with maternal sensitivity. The first is a direct pathway in which carrying the long allele for AVPR1a or DRD4 will be associated with lower maternal sensitivity. Then, we consider indirect pathways in which carrying the long allele of AVPR1a or DRD4 will be associated with a) lower infant-oriented crying processing and/or b) higher mother-oriented cry processing which in turn would predict lower maternal sensitivity. We control for race, adult attachment coherence, and maternal education as prior research has demonstrated that each is related to cry processing and/or maternal sensitivity in this and other samples (Leerkes et al., 2016). In addition, we control for infant's DRD4 and AVPR1a genotypes to ensure observed associations are not a function of infant evocative effects. As a final step, we test race as a moderator of proposed pathways given half of our participants are African American and half are European American and differences in the frequency distribution of specific genotypes across groups can have implications for associations between genotypes and phenotypes (Haberstick et al., 2015).
Material and Methods
Participants
Participants in the current study were 209 primiparous mothers (106 European American, 103 African American) and their children from the southeastern United States drawn from a larger sample of 259 mothers initially recruited during the prenatal period. Mothers in the analytic sample ranged in age from 18 to 44 years (M = 25.5) at recruitment. Twenty-three percent had a high school diploma or less, 31% had attended but not completed college, and 46% had a 4-year college degree. The majority (59%) of mothers were married or living with their child's father, 23% were in a relationship but not living with their child's father, and 16% were single. Annual family income ranged from less than $2,000 to over $100,000 (median =$35,000). All participating infants were healthy; 52% were female. Initial participants who did not provide DNA (due to attrition, not refusal) were younger, less educated and rated somewhat less sensitive at 6 months than mothers who did provide DNA, but they did not differ on race, income, measures of cry processing, adult attachment or maternal sensitivity at 1 year.
Procedure
Expectant mothers were recruited at childbirth classes. Upon enrollment in the study, women provided written consent. Women completed the Adult Attachment Interview prenatally in our laboratory. Mothers and infants visited our laboratory for a videotaped observation of mother-infant interaction when infants were about 6 months (M = 6.39 months), 1 year (M = 13.90 months), and 2 years old (M = 27.32 months). At each visit, mothers and infants engaged in a 7-minute free play, followed by 2 to 3 tasks designed to elicit infant distress (frustration and fear) further described in the supplementary materials. Immediately after the 6-month observation, mothers participated in an audiotaped video-recall interview in which they viewed the videotapes of each distress task, and answered a series of questions to assess cry processing. Mothers’ and infants’ DNA was collected via saliva samples during the 2 year visit. Procedures were approved by the University of North Carolina at Greensboro's Institutional Review Board.
Measures
Covariates
At the prenatal visit, mothers were administered the Adult Attachment Interview (AAI; George, Kaplan, & Main, 1984-1996), a semi structured interview in which participants describe their early childhood relationships with their primary caregivers and the influences they perceive those experiences have had on them. The coherence of mind rating (1 = not at all coherent to 9 = very coherent), a summary measure of participants’ ability to describe early attachment experiences and their influence on current functioning in an organized manner, was our criterion measure of adult attachment security (Main & Goldwyn, 1998;2003). Interrater reliability was good, intraclass correlation = .75, p < .001, based on 50 double coded transcripts. In addition, mothers self-reported their highest level of education and their race.
Cry processing
During the 6 month video-recall interview, mothers were asked to rate how strongly they felt 17 emotions (e.g., sad, concerned, sympathetic) during each interactive task on a 4-point scale (1 = not at all; 4 = very strongly). Then, mothers were asked to describe why they felt each emotion. Their reasons were coded as infant-oriented or mother-oriented (Dix, Gershoff, Meunier, & Miller, 2004); kappa based on 40 double coded transcripts was .94. Empathy was calculated by averaging mothers’ intensity ratings for infant-oriented empathy, sympathy and sadness across the 3 tasks to yield a single score.
Second, mothers were asked to indicate how frequently infants were distressed during each interactive task on a 7-point scale from never to the whole time and to indicate all emotions the infant displayed during each task using a list of 20 emotion terms (e.g., happy, sad, angry). Mothers’ responses were compared to ratings made by reliably trained infant affect coders. If an infant was distressed according to our raters, and the mother rated the infant as never distressed (under-rating) or failed to indicate the infant felt specific negative emotions like sadness, fear, anger (under-identification), the number of seconds the infants was rated as distressed by us was recorded to reflect the egregiousness of her detection error. That is, not noting an infant was distressed if they cried for 30 seconds is a bigger error than not noting they only cried for 5 seconds. Mothers who did not make these errors were scored as 0. These scores were calculated for each caregiving task and then summed across tasks. The two types of detection errors correlated, r (206) = .20, p < .01, and were averaged. This score was multiplied by −1 so high scores reflect more accurate distress detection.
Third, mothers rated the extent to which they agreed with 18 statements about why their infant behaved as he or she did during each task on a 4-point scale ranging from strongly disagree to strongly agree to assess their causal attributions. Situational/emotional attributions is the mean of 4 items (upset by the situation, no one was helping my baby, trying to show he/she needs help; had no way to feel better) averaged across the 3 tasks. Emotion minimizing attributions is the mean of 5 items (having a bad day, in a bad mood, tired, hungry, not feeling well) averaged across the 3 tasks. Negative/internal attributions is the mean of 7 items (spoiled, difficult temperament, trying to make my life difficult, unreasonable, crying on purpose, selfish, just wanted attention) averaged across the 3 tasks.
Mothers completed the Infant Crying Questionnaire (Haltigan et al, 2012), a single time, to assess their beliefs about infant crying by rating the extent to which they believed 43 statements on a 5-point scale ranging from never (1) to always (5). Infant-oriented cry beliefs is the average of 2 subscales: Attachment (8 items; e.g., when my baby cries, I want to make my baby feel secure) and Crying as Communication (3 items; e.g., when my baby cries, I think my baby is trying to communicate). Mother-oriented cry beliefs is the average of 2 subscales: Minimization (9 items; when my baby cries, I want my baby to stop because I can't get anything else done) and Spoiling (3 items; how I respond when my baby cries could spoil my baby).
We created two manifest variables based on analyses presented in Leerkes et al. (2016) by standardizing and averaging the relevant scores. Infant-oriented cry processing is the average of empathy, distress detection, situational/emotional cry attributions, and infant-oriented cry beliefs (Chronbach's alpha = .62) and mother-oriented cry processing is the average of negative and minimizing cry attributions and mother-oriented cry beliefs (Chronbach's alpha = .61).
Maternal sensitivity
Maternal sensitivity during each interactive task at each time point was rated using Ainsworth's 9 point sensitivity scale from (1) highly insensitive to (9) highly sensitive (Ainsworth et al., 1978). At each time point, 15 to 20% of videos were double coded to assess inter-rater reliability via interclass correlation coefficients (ICC). Mean ICC across all waves and tasks was .88. Maternal sensitivity correlated significantly across tasks and time, r ranged from .51 to .85, all p < .001. Thus, a single measure of maternal sensitivity was created by averaging maternal sensitivity across all tasks and time points (Chronbach's alpha = .91).
Genotyping
Mothers’ DNA was obtained using Oragene kits (DNAgenotek, Ottawa, Ontario, Canada). Mothers deposited two ml of saliva into a vial (#OG-500), that then capped released a stabilizing lysis buffer. All saliva samples were sealed and given a bar coded label before sending the tubes for DNA processing. Genotyping was conducted at the Institute for Behavioral Genetics at the University of Colorado under the supervision of Andrew Smolen. The RS3 site in AVPR1a was genotyped using the method of Walum et al (2008). The primer sequences were forward: 5′-6FAM’-CCT GTA GAG ATG TAA GTG CT-3’; and reverse: 5′-gtttcttTCTGGAAGAGACTTAGATGG -3’, which yielded polymerase chain reaction (PCR) products of 317 to 355 bp, amplicons that are 7 bp larger than those given in Knafo et al (2007). Our most frequent allele (332 bp) is equivalent to the most frequent (325 bp) allele reported by Knafo et al (2007). For the primary analysis, alleles were grouped as 334 bp or longer (long) versus 333 bp or shorter (short), and AVPR1a genotypes were classified in two groups according to the absence (coded as 0) or presence of the long allele (coded as 1).
The 48 bp Variable Number Tandem Repeat (VNTR) polymorphism in the third exon of the DRD4 gene (van Tol et al., 1992) was genotyped following the approach of Anchordoquy et al. (2003). The primer sequences were forward: 5′-VIC-GCT CAT GCT GCT GCT CTA CTG GGC-3′; and reverse: 5′-CTG CGG GTC TGC GGT GGA GTC TGG-3’, which yielded PCR products from 279 (2R) to 663 (10R) bp. We followed previous strategies (Hutchison et al, 2002; Lerman et al., 1998) to classify DRD4 genotypes into two groups as presence (coded as 1) or absence of the long allele (i.e.,7 repeat or longer) (coded as 0).
To address reliability, 10% of samples were randomly duplicated with 100% concordance. In addition samples that did not amplify well (approximately 5%) were duplicated and resolved via consensus if needed. Among mothers in the analytic sample, DRD4 was successfully genotyped for 100%, and AVPR1a for 99% (all but 2). Among infants in the analytic sample, 2 provided insufficient DNA for any genotyping. Of the remaining 207 infants, DRD4 was successfully genotyped for all but 1 (99%) and AVPR1a for all but 4 (98%).
Analysis
Preliminary analyses were performed to examine the frequencies of AVPR1a and DRD4 genotypes for mothers and infants. We conducted chi-square tests using SPSS version 23 to examine whether genotype frequencies varied across racial groups. We also conducted chi-square test to examine deviations from Hardy-Weinberg Equilibrium (HWE). Descriptive statistics and correlations between study variables were also examined. Path analysis was conducted using Mplus version 7 (Muthén & Muthén, 2012) to evaluate the pathways through which AVPR1a and DRD4 affect maternal sensitivity. In the path model, AVPR1a and DRD4 were specified as exogenous variables that predicted infant-oriented and mother-oriented cry processing and maternal sensitivity. Infant-oriented and mother-oriented cry processing were specified as predicting maternal sensitivity. Maternal education and coherence of mind were specified as exogenous control variables linked to maternal sensitivity. Race was specified as a covariate associated with infant-oriented and mother-oriented cry processing and maternal sensitivity to account for potential population stratification effects. Infants’ AVPR1a and DRD4 genotypes were also included as covariates associated with infant-oriented and mother-oriented cry processing and maternal sensitivity to take into account potential child effects due to evocative gene-environment correlation. Infants’ genotypes were specified to be correlated with mothers’ genotypes. Hypotheses related to indirect associations were evaluated using bias-corrected bootstrapped 95% confidence intervals (CI) (MacKinnon, Lockwood, & Williams, 2004). To examine possible differences in path coefficients between European American and African American mothers, multigroup analysis was conducted by removing race from the path model and then comparing a model with all remaining paths constrained to equality with one that had all paths freely estimated across African American and European American women.
Results
Preliminary analysis
Genotype frequencies for mothers and infants for the whole sample and by maternal racial groups are presented in Table 1. Chi-square tests indicated that genotype frequencies did not vary across racial groups for either AVPR1a (χ2 = 2.17, df = 2, p = .34 for mothers; χ2 = 1.14, df = 2, p = .57 for infants) or DRD4 (χ2 = 1.44, df = 2, p = .49 for mothers; χ2 = 1.63, df = 2, p = .44 for infants). Genotype frequencies for both AVPR1a and DRD4 were in Hardy-Weinberg Equilibrium for the whole sample and for each racial group (p ranged from .07 to .96) for mothers and infants. Descriptive statistics and intercorrelations are presented in Table 2.
Table 1.
Genotype Frequencies and Hardy-Weinberg Equilibrium Tests
| Mothers |
Infants |
|||||
|---|---|---|---|---|---|---|
| Genotypes | Whole sample | European American | African American | Whole sample | European American | African American |
| AVPR1a | ||||||
| L/L | 76 (36.7%) | 41 (39.0%) | 35 (34.3%) | 74 (36.5%) | 39 (37.5%) | 35 (35.4%) |
| L/S | 100 (48.3%) | 52 (49.5%) | 48 (47.1%) | 90 (44.3%) | 48 (46.2%) | 42 (42.4%) |
| S/S | 31 (15.0%) | 12 (11.4%) | 19 (18.6%) | 39 (19.2%) | 17 (16.3%) | 22 (22.2%) |
| DRD4 | ||||||
| L/L | 10 (4.8%) | 5 (4.7%) | 5 (4.9%) | 14 (6.8%) | 8 (7.6%) | 6 (5.9%) |
| L/S | 72 (34.4%) | 32 (30.2%) | 40 (38.8%) | 61 (29.6%) | 27 (25.7%) | 34 (33.7%) |
| S/S | 127 (60.8%) | 69 (65.1%) | 58 (56.3%) | 131 (63.6%) | 70 (66.7%) | 61 (60.4%) |
| HWE test | ||||||
| AVPR1a | .84 | .46 | .72 | .22 | .73 | .17 |
| DRD4 | .96 | .61 | .56 | .07 | .09 | .75 |
Note. n = 207 for mothers' AVPR1a, n = 209 for mothers DRD4, n = 203 for infants' AVPR1a, and n = 206 for infants' DRD4. Genotype frequencies are provided by maternal race. p values from Hardy-Weinberg Equilibrium tests are presented.
Table 2.
Descriptive Statistics and Intercorrelations
| M or % | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Race (European American) | 51% | -- | -- | |||||||||
| 2. Maternal Education | 3.94 | 1.78 | .37** | -- | ||||||||
| 3. Coherence of Mind | 5.39 | 1.43 | .30** | .37** | -- | |||||||
| 4. Infant AVPR1a risk allele | 81% | -- | .08 | −.01 | −.02 | -- | ||||||
| 5. Infant DRD4 risk allele | 36% | -- | −.07 | .02 | −.01 | −.03 | -- | |||||
| 6. Mother AVPR1a risk allele | 85% | -- | .10 | −.11 | .03 | .20** | −.12 | -- | ||||
| 7. Mother DRD4 risk allele | 39% | -- | −.08 | −.12 | −.04 | −.10 | .40** | .00 | -- | |||
| 8. IO Cry Processing 6M | .00 | .64 | .13 | .16* | .10 | −.00 | −.04 | −.09 | −.04 | -- | ||
| 9. MO Cry processing 6M | .00 | .73 | −.27** | −.28** | −.17* | .11 | .03 | .14 | .16* | −.10 | -- | |
| 10. Maternal Sensitivity 6M-2Y | 5.58 | 1.35 | .53** | .58** | .38** | .02 | −.14* | −.02 | −.18* | .26** | −.42** | -- |
Note. n ranges from 196 to 209
p < .05.
p < .01
Predicting Maternal Sensitivity
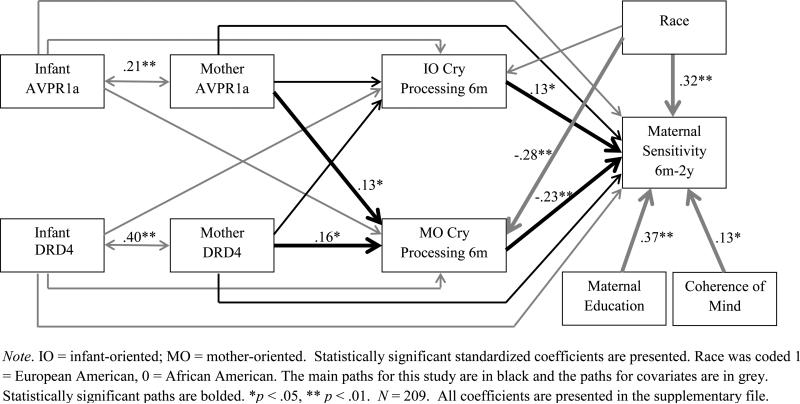
Path coefficients are presented in Figure 1. Consistent with preliminary analysis and prior research, higher coherence of mind and maternal education were associated with higher maternal sensitivity, B = .11, SE =.05, β = .13, p <.05 and B = .26, SE = .04, β = .37, p < .01, respectively. Consistent with prediction, mothers’ long allele of AVPR1a and DRD4 were associated positively with mother-oriented cry processing, B = .27, SE = .14, β = .13, p < .05 andB = .24, SE = .11, β = .16, p < .05, respectively. That is, mothers who carried the long allele of AVPR1a and/or of DRD4 were more likely to focus on their own needs and endorse negative cognitions about their infants during distressing tasks. In contrast, neither mother's AVPR1a nor DRD4 genotype was associated with infant-oriented cry processing, B =−.19, SE = .13, β = −.11, p = .13 and B = −.02, SE = .10, β = −.01, p = .87, respectively. Consistent with hypothesis, infant-oriented cry processing was associated with higher maternal sensitivity, B = .26, SE = .11, β = .13, p < .05, whereas mother-oriented cry processing was associated with lower maternal sensitivity B = −.39, SE = .10, β = −.23, p < .01, above and beyond the effects of covariates. There were no direct effects of AVPR1a or DRD4 on maternal sensitivity, B = .07, SE = .19, β = .02, p= .73 and B = −.08, SE = .15, β = −.03, p= .57, respectively. However, results indicated that the indirect effects of AVPR1a and DRD4 on maternal sensitivity via mother-oriented cry processing were significant, 95% CI [−.22, −.03], B = −.11, SE = .06, β=−.03 for AVPR1a; 95% CI [−.20, −.03], B = −.09, SE = .05, β=−.04 for DRD4. Thus, maternal AVPR1a and DRD4 “risk” genotypes were linked with mothers’ heightened focus on their own needs, which in turn predicted lower sensitivity to infant distress. There were no statistically significant associations between infants’ AVPR1a and DRD4 genotypes and mothers’ infant-oriented cry processing, mother-oriented cry processing, and maternal sensitivity in the path model. Results of the multigroup analysis indicated that path coefficients did not differ significantly across racial groups (Δχ2 = 18.76, Δdf = 16, p = .28).
Figure 1.
Effect of AVPR and DRD4 on maternal sensitivity via maternal cry processing while caregiving
We examined statistical power by conducting post hoc Monte Carlo simulations in Mplus with the obtained model estimates used as population values and 10,000 replications. The power to detect direct and indirect effects of DRD4 and AVPR1a on maternal social cognition and sensitivity was well below the threshold of .80 (all values less than are equal to .64).
Discussion
The goal of this paper was to examine the extent to which AVPR1a and DRD4 predicted maternal sensitivity directly and indirectly via mothers’ social cognition. Consistent with prediction, the results demonstrated that mothers who carried long alleles of AVPR1a or DRD4 were more likely to engage in mother-oriented cry processing, characterized by negative beliefs and attributions about their infants’ crying, which in turn predicted lower maternal sensitivity, and both indirect effects were significant independent of one another, infants’ genotypes, and important covariates. That mothers with long alleles of these two genes engaged in more negative social cognition about their infants is consistent with prior research linking these alleles with deficits in theory of mind (Lackner et al., 2012) and with negative personality traits (Wu & Barnes, 2013). These two indirect pathways are consistent with the view that genes related to the vasopressin and dopamine systems, both part of the proposed maternal circuit (Numan, 2010), are in fact related to mothers’ social cognition. It seems mothers with these two risk alleles have greater difficulty taking their infants’ perspective and instead focus on their own needs in the moment undermining their ability to respond sensitively.
That neither DRD4 nor AVPR1a were significantly associated with infant-oriented cry processing may be a function of the inclusion of mother's immediate emotional empathy which may be highly context specific. That is, the extent to which a mother feels empathy may be more strongly driven by her infant's state in the moment than in her own genetically driven dispositions. In contrast, mothers’ negative attributions and beliefs about crying may reflect a more stable tendency to minimize or downplay infant distress (Leerkes et al., 2016). The fact that women's DRD4 and global emotional empathy were not significantly associated in a prior study (Usefovsky et al., 2014) buttresses this argument.
The lack of direct effects of either gene on maternal sensitivity is not entirely surprising given the complexity of maternal sensitivity as a phenotype, and is consistent with prior research on DRD4 and parenting in which main effects were not significant (Beach et al., 2012; Beaver, et al., 2012; van Ijzendoorn et al., 2008). However, the non-significant direct effect of AVPR1a on parenting is inconsistent with prior research (Avinun et al., 2012; Bisceglia et al., 2012). This difference is not attributable to our more conservative analytic approach which included multiple covariates given inspection of the zero-order correlations indicates the association between AVPR1a and sensitivity was near zero prior to considering covariates. Discrepancies across studies could also result from differences in approaches to creating AVPR1a groups. But, post hoc analyses demonstrate that there are no differences in maternal sensitivity as a function of homozygozity versus heterozygosity for the long allele (Bisceglia et al., 2012) or when only the 334 bp alleles are considered risk rather than 334 bp and longer alleles (Avinun et al., 2012). Thus, this does not explain the discrepancy either suggesting additional research on the direct association between AVPR1a and maternal sensitivity is warranted.
Strengths of this research include the inclusion and simultaneous examination of two distinct genotypes related to different neural systems and our efforts to identify specific endophenotypes (two patterns of social cognition) that may explain associations between these genes and parenting behavior. To our knowledge, we are the first to present evidence that social cognition plays a role in linking specific genes to maternal behavior, although numerous others have proposed it would (e.g., Avinun et al., 2012; Barrett & Fleming, 2011; van IJzendoorn et al., 2008). Additionally, that we collapsed ratings of maternal sensitivity across time and tasks with varying demands likely yielded a highly reliable indicator of maternal sensitivity in contrast to relying on observations from a single time point or task. Finally, we took a conservative analytic approach with multiple covariates. Controlling for maternal education and adult attachment coherence, both of which correlated highly with observed sensitivity, demonstrates the robustness of the indirect effects of AVPR1a and DRD4 over and above other predictors of sensitivity. Likewise, controlling for infants’ genotypes eliminates the possibility that observed associations were a function of evocative gene-environment correlations.
Limitations of this research include the sample size. Although our sample is relatively large for developmental studies with extensive observational measures, it is quite small for molecular genetic research in which much larger sample sizes are desirable to detect small effects of specific genes on complex phenotypes such as maternal sensitivity. Post hoc power analyses demonstrate that we in fact had limited statistical power to identify genetic effects. In addition, although a diverse sample is often preferred for generalizability, in molecular genetic research, homogenous samples are preferred given concerns about potential confounding effects due to population stratification (Cardon & Palmer, 2003). Thus our sample is not ideal in this regard as it is composed of equal numbers of African American and European American mothers. But that genotype frequencies of AVPR1a and DRD4 in our sample did not significantly differ across race, and that we included race as a covariate, reduce concern for population stratification in this study. On the other hand, our sample allowed us to formally test race as a moderator of genetic effects, and our multigroup analyses indicated the pathways did not vary across racial groups. Future work with non-Caucasian samples and with fathers in addition to mothers is needed. Such work should also consider gene-environment interaction (G X E) effects in which specific genes are examined as susceptibility factors that alter the links between mothers’ childhood experiences, social cognition, and maternal sensitivity, as there is more evidence for G X E effects than main effects of genes in relation to parenting outcomes (Mileva-Seitz et al., 2016). These efforts will require larger samples. Finally, the extent to which any gene is expressed is also dependent on epigenetics, which is determined in part by environmental experiences. Future work on the epigenetics of human parenting is still needed.
In conclusion, our results demonstrate that arginine vasopressin and dopamine-related “risk” genes are related to mothers’ compromised social cognition about their infants which in turn predicts less sensitive maternal behavior. These findings support the view that parenting is in part controlled by biological systems related to affect and cognition, and this is one way in which genes are linked with individual differences in maternal behavior.
Supplementary Material
Acknowledgements
This project was supported by R01HD058578 and R21HD073594. The contents of this article are the sole responsibility of the authors and do not necessarily reflect the views of the Eunice Kennedy Shriver National Institute for Child Health and Human Development. We are grateful to the participants for their time and Dr. Regan Burney and project staff for their dedication.
Footnotes
There are no conflicts of interest to report.
References
- Ainsworth MDS, Blehar MC, Waters E, Wall S. Patterns of attachment. Erlbaum; Hillsdale, NJ.: 1978. [Google Scholar]
- Anacker K, Enge S, Reif A, Lesch K, Strobel A. Dopamine D4 receptor gene variation impacts self-reported altruism. Mol Psychiatry. 2013;18:402–403. doi: 10.1038/mp.2012.49. [DOI] [PubMed] [Google Scholar]
- Avinun R, Ebstein RP, Knafo A. Human maternal behaviour is associated with arginine vasopressin receptor 1A gene. Biol Lett. 2012;8:894–896. doi: 10.1098/rsbl.2012.0492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avinun R, Israel S, Shalev I, Gritsenko I, Bornstein G, Ebstein RP, Knafo A. AVPR1A variant associated with preschoolers' lower altruistic behavior. PLoS ONE. 2011;6:e25274. doi: 10.1371/journal.pone.0025274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van IJzendoorn MH. Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci. 2008;3:128–134. doi: 10.1093/scan/nsn004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS. Annual research review: All mothers are not created equal: Neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry. 2011;52:368–397. doi: 10.1111/j.1469-7610.2010.02306.x. [DOI] [PubMed] [Google Scholar]
- Beach SH, Lei MK, Brody GH, Simons RL, Cutrona C, Philibert RA. Genetic moderation of contextual effects on negative arousal and parenting in African-American parents. J Fam Psychol. 2012;26:46–55. doi: 10.1037/a0026236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaver KM, Shutt JE, Vaughn MG, DeLisi M, Wright JP. Genetic influences on measures of parental negativity and childhood maltreatment: An exploratory study testing for gene × environment correlations. J Contemp Crim Justice. 2012;28:273–292. [Google Scholar]
- Bisceglia R, Jenkins JM, Wigg KG, O'Connor TG, Moran G, Barr CL. Arginine vasopressin 1a receptor gene and maternal behavior: Evidence of association and moderation. Genes Brain Behav. 2012;11:262–268. doi: 10.1111/j.1601-183X.2012.00769.x. [DOI] [PubMed] [Google Scholar]
- Cardon LR, Palmer LJ. Population stratification and spurious allelic association. Lancet. 2003;361:598–604. doi: 10.1016/S0140-6736(03)12520-2. [DOI] [PubMed] [Google Scholar]
- Dix T, Gershoff ET, Meunier LN, Miller PC. The affective structure of supportive parenting: Depressive symptoms, immediate emotions, and child-oriented motivation. Dev Psychol. 2004;40:1212–1227. doi: 10.1037/0012-1649.40.6.1212. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Young LJ. Oxytocin, vasopressin, and the neurogenetics of sociality. Science. 2008;322:900–904. doi: 10.1126/science.1158668. [DOI] [PubMed] [Google Scholar]
- George C, Kaplan N, Main M. Adult attachment interview protocol. University of California; Berkeley: 1984-1996. Unpublished manuscript. [Google Scholar]
- Haberstick BC, Smolen A, Williams RB, Bishop GD, Foshee VA, Thornberry TP, Harris KM. Population frequencies of the triallelic 5HTTLPR in six ethnicially diverse samples from North America, Southeast Asia, and Africa. Behav Genetics. 2015;45:255–261. doi: 10.1007/s10519-014-9703-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haltigan JD, Leerkes EM, Burney RV, O'Brien M, Supple AJ, Calkins SD. The infant crying questionnaire: Initial factor structure and validation. Infant Behav Dev. 2012;35:876–883. doi: 10.1016/j.infbeh.2012.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrichs M, von Dawans B, Domes G. Oxytocin, vasopressin, and human social behavior. Frontiers Neuroendocrinol. 2009;30:548–557. doi: 10.1016/j.yfrne.2009.05.005. [DOI] [PubMed] [Google Scholar]
- Lackner C, Sabbagh MA, Hallinan E, Liu X, Holden JA. Dopamine receptor D4 gene variation predicts preschoolers’ developing theory of mind. Dev Science. 2012;15:272–280. doi: 10.1111/j.1467-7687.2011.01124.x. [DOI] [PubMed] [Google Scholar]
- Leerkes EM, Su J, Calkins SD, Supple AJ, O'Brien M. Pathways by which mothers’ physiological arousal and regulation while caregiving predict sensitivity to infant distress. J Fam Psychol. 2016 doi: 10.1037/fam0000185. doi:10.1037/fam0000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonstein JS, Lévy F, Fleming AS. Common and divergent psychobiological mechanisms underlying maternal behaviors in non-human and human mammals. Horm Behav. 2015;73:156–185. doi: 10.1016/j.yhbeh.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Lockwood CM, Williams J. Confidence limits for the indirect effect: Distribution of the product and resampling methods. Multivariate Behav Res. 2004;39:99–128. doi: 10.1207/s15327906mbr3901_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mileva-Seitz VR, Bakermans-Kranenburg MJ, van IJzendoorn MH. Genetic mechanisms of parenting. Horm Behav. 2016;77:211–223. doi: 10.1016/j.yhbeh.2015.06.003. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Fleming AS. How mothers are born: A psychobiological analysis of mothering. In: Booth A, McHale SM, Landale NS, editors. Biosocial Foundations of Family Processes. Springer Science + Business Media; New York: 2011. pp. 3–34. [Google Scholar]
- Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Fleming AS. Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants. Genes Brain Behav. 2011;10:325–333. doi: 10.1111/j.1601-183X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Kennedy J, Atkinson L, Steiner M, Levitan R, Matthews SG, Fleming AS. 'Serotonin transporter allelic variation in mothers predicts maternal sensitivity, behavior and attitudes toward 6-month-old infants': Corrigendum. Genes Brain Behav. 2012;11:125. doi: 10.1111/j.1601-183X.2010.00671.x. [DOI] [PubMed] [Google Scholar]
- Muthén K, Muthén BO. Mplus User's guide. Seventh Edition. Múthen & Múthen; Los Angeles, CA.: 1998-2012. [Google Scholar]
- Numan M. Parental behavior. In: Koob GF, Le Moal M, Thompson RF, editors. Encyclopedia of Behavioral Neuroscience. Vol. 3. Academic Press; Oxford: 2010. pp. 14–23. [Google Scholar]
- Schoots O, Van Tol HH. The human dopamine D4 receptor repeat sequences modulate expression. Pharmacogenomics J. 2003;3:343–348. doi: 10.1038/sj.tpj.6500208. [DOI] [PubMed] [Google Scholar]
- Taylor SE. Genetic contributions to sensitive parenting. Soc Cogn Affect Neurosci. 2008;3:89–90. doi: 10.1093/scan/nsn015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzefovsky F, Shalev I, Israel S, Edelman S, Raz Y, Perach-Barzilay N, Ebstein RP. The Dopamine D4 receptor gene shows a gender-sensitive association with cognitive empathy: Evidence from two independent samples. Emotion. 2014;14:712–721. doi: 10.1037/a0036555. [DOI] [PubMed] [Google Scholar]
- van Ijzendoorn MH, Bakermans-Kranenburg MJ, Mesman J. Dopamine system genes associated with parenting in the context of daily hassles. Genes Brain & Behav. 2008;7:403–410. doi: 10.1111/j.1601-183X.2007.00362.x. [DOI] [PubMed] [Google Scholar]
- Wu T, Barnes JC. Two dopamine receptor genes (DRD2 and DRD4) predict psychopathic personality traits in a sample of American adults. J Crim Justice. 2013;41:188–195. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.