Abstract
We aimed to investigate epidemiology and host- and pathogen-related factors associated with clinical severity of acute gastroenteritis (AGE) in children after rotavirus vaccination introduction. Factors assessed included age, co-infection with more than 2 viruses, and virus-toxigenic Clostridium difficile co-detection. Fecal samples and clinical information, including modified Vesikari scores, were collected from hospitalized children with AGE. The presence of enteric viruses and bacteria, including toxigenic C. difficile, was detected by polymerase chain reaction (PCR). Among the 415 children included, virus was detected in stool of 282 (68.0%) children. Co-infection with more than 2 viruses and toxigenic C. difficile were found in 24 (8.5%) and 26 (9.2%) children with viral AGE, respectively. Norovirus (n = 130) infection, including norovirus-associated co-infection, was the most frequent infection, especially in children aged < 24 months (P < 0.001). In the severity-related analysis, age < 24 months was associated with greater diarrheal severity (P < 0.001) and modified Vesikari score (P = 0.001), after adjustment for other severity-related factors including rotavirus status. Although the age at infection with rotavirus was higher than that for other viruses (P = 0.001), rotavirus detection was the most significant risk factor for all severity parameters, including modified Vesikari score (P < 0.001). Viral co-infection and toxigenic C. difficile co-detection were not associated with any severity-related parameter. This information will be helpful in the management of childhood AGE in this era of rotavirus vaccination and availability of molecular diagnostic tests, which often lead to the simultaneous detection of multiple pathogens.
Keywords: Acute Gastroenteritis, Clinical Severity, Age, Rotavirus, Norovirus, Co-Infection, Clostridium difficile, Children
Graphical Abstract
INTRODUCTION
Introduction of the rotavirus vaccine significantly decreased the incidence of acute gastroenteritis (AGE) in children (1,2). Nevertheless, among children, AGE remains a major cause of hospitalization. Recently, evidence-based guidelines for the management of AGE in children have been published in Europe (3). According to this report, the major risk factors affecting gastroenteritis severity are both pathogen- and host-related. Among the pathogens, rotavirus has been reported to be the main cause of persistent or severe diarrhea in Europe (4,5). Among the host-related factors, age, underlying chronic disease, and immune deficiencies have been reported to be associated with specific diarrheal pathogens and the severity of diarrhea (6,7).
However, previous studies on the severity of viral gastroenteritis were conducted mostly before the introduction of rotavirus vaccination; hence, rotavirus infection was most prevalent and mostly occurred in those less than 24 months of age. The clinical epidemiology of viral gastroenteritis, including prevalence and vulnerable age, has been changing since the introduction of rotavirus vaccination (1,2,8). This might affect both clinical severity and the factors related to clinical severity of viral gastroenteritis. In addition, the association between age and severity of diarrhea was not consistent among previous studies performed before the introduction of rotavirus vaccination. An age of less than 6 months was associated with less severe diarrhea in a large-scale prospective study in Europe (9), but in studies in developing countries, infants less than 6 months of age tended towards persistent or severe diarrhea (6). It has been thought that the high incidence of dehydration in infants less than 6 months is related to a high exposure to rotavirus.
Among pathogen-related factors that affect the clinical severity of gastroenteritis, the influence of co-infections with different viruses also varies among studies (10,11). Toxigenic Clostridium difficile infection, which is known to be mostly asymptomatic in infants and young toddlers, could also affect the severity of viral gastroenteritis as C. difficile co-infections in viral gastroenteritis have been reported to undergo a more severe clinical course in a few case reports (12,13). Currently, with the increasing use of diagnostic multiplex polymerase chain reaction (PCR) testing for viruses and enteric bacteria, multiple pathogens have often been simultaneously detected in the feces of children with AGE. Hence, the assessment of clinical severity of virus-virus or virus-C. difficile co-infection is important for the determination of management for viral AGE in children.
This study aimed to investigate the epidemiology and host- and pathogen-related factors, including host age, co-infection with multiple viruses, and toxigenic C. difficile co-detection, associated with the clinical severity of gastroenteritis in hospitalized Korean children in the era of rotavirus vaccination and molecular diagnosis of multiple pathogens. To assess the clinical severity of gastroenteritis, a modified Vesikari scoring system was used according to recent European guidelines (3,14).
MATERIALS AND METHODS
Patients and clinical information
This study was conducted in the pediatric ward of the Seoul Metropolitan Government-Seoul National University Boramae Medical Center. From January 2012 to December 2013, i.e., 5–6 years after the introduction of rotavirus vaccination in Korea, stool samples were collected within 3 days of admission from children under 16 years of age who were hospitalized with a clinical suspicion of AGE. The patients were admitted via the emergency department or outpatient clinics. Children with known immunosuppressive underlying chronic illnesses, including inflammatory bowel diseases or malnutrition, were excluded. Informed consent was obtained from the guardians of the children at enrolment. At discharge, subjects were further excluded based on the following criteria: failure to obtain permission, insufficient amount of fecal samples to complete the tests, and having a final diagnosis of an acute or chronic illness that mimics AGE, such as sepsis, cyclic vomiting syndrome, viral infection with mucocutaneous manifestations, allergic or eosinophilic enterocolitis, or inflammatory bowel disease.
Clinical information including the age of onset, date of hospitalization, anthropometry (including weight and height/length), fever, duration and maximum number of episodes of diarrhea before and during hospitalization, duration and maximum number of vomiting episodes before and during hospitalization, respiratory symptoms (including cough and rhinorrhea), and medications (including antibiotics taken in the 4 weeks before admission and during hospitalization) were collected. From these records, weight-for-height Z-scores (WHZ) and modified Vesikari scores were calculated (3). Laboratory examinations, including complete blood cell count and albumin, blood urea nitrogen, and calcium level measurement, were performed on all of the children in the hospital laboratory. A stool test for white blood cells, hemoglobin, Rotavirus antigen (Bioline rotavirus®; SD standard diagnostics, Youngin, Korea), Salmonella species (spp.), Shigella spp., and Vibrio spp. were also conducted for all included children. The C. difficile toxin immunoassay (Tox A/B QUIK CHEK®; TechLab, Blacksburg, VA, USA) was performed for the selected children: those with a history of taking antibiotics within 2 weeks prior to the onset of diarrhea. For children who tested positive for rotavirus stool antigen, a vaccination history was obtained from the individual vaccination cards and the Korea Centers for Disease Control and Prevention website (http://is.cdc.go.kr), which records histories as part of the national vaccination program.
Stool examination for viruses
Stool samples were collected in sterile tubes, transferred to the laboratory, and stored at −70°C until further processing. Stool samples were diluted to 10% (w/v) with phosphate-buffered saline and centrifuged. Viral double stranded RNA and DNA were extracted from the supernatant using the QIAamp MiniElute Virus Spin Kit (Qiagen, Hilden, Germany). PCR was performed to detect adenovirus (15) and reverse transcription PCR (RT-PCR) was performed to detect rotavirus (8), norovirus (16), and astrovirus (17) as described previously (18). Multiplex PCR using a Seeplex Diarrhea-V ACE detection kit (Seegene Inc., Seoul, Korea) was also performed to detect astrovirus, group A rotavirus, enteric adenovirus, and norovirus, according to the manufacturer's instructions (19). Amplification products were examined by electrophoresis on 1.5% agarose gels and documented with the Bio-Rad Gel Doc 1000 Documentation System (BioRad, Hercules, CA, USA). The samples were scored as positive for rotavirus if the rotavirus antigen test and/or PCR reactions were positive. The samples were scored as positive for norovirus, adenovirus, or astrovirus if the PCR reactions were positive.
Stool examination for bacteria including toxigenic C. difficile
For bacterial DNA extraction, a portion of the 10% faucal slush was incubated for 10 minutes at 95°C and then centrifuged. Bacterial DNA was extracted from the supernatant using a QIAamp DNA Mini Kit (Qiagen). Multiplex PCR using the Seeplex Diarrhea-B1 and -B2 ACE detection kits (Seegene Inc.) was performed on stool samples from included children in accordance with the manufacturer's instructions to detect Vibrio spp., C. difficile toxin B, Salmonella spp., Shigella spp., Campylobacter spp., Clostridium perfringens, Yersinia enterocolitica, Escherichia coli O157, E. coli H7, VTEC, and Aeromonas spp. (20). Samples were scored as positive for toxigenic C. difficile if either C. difficile toxin immunoassay or the PCR reaction was positive.
Clinical severity analysis
In clinical severity related analyses of AGE, children with the following conditions were excluded: children with presence of enteric bacterial pathogen other than C. difficile, gross frequent hematochezia or the presence of tenesmus without toxigenic C. difficile being detected, diagnosis of co-morbid acute lower respiratory infection, documented respiratory virus or enterovirus co-infection, and suspicion of co-morbid focal or systemic bacterial infection requiring ongoing antibiotic use.
Statistical analysis
We examined the data with the Kolmogorov-Smirnov test for normality. For age-related analyses, four age groups, < 6 months, 6–23 months, 24–59 months, and ≥ 60 months, or 2 age groups, < 24 months, and ≥ 24 months were used. Comparisons of categorical data were evaluated using the χ2 test. Continuous variables were summarized using the median and interquartile range (IQR). Comparisons of continuous data were evaluated using the Mann-Whitney test or the Kruskal-Wallis test. Data were analyzed using ordered logit models to determine the cumulative odds ratio (c-OR) for clinical severity-related factors. Potential clinical severity-related factors including age, virus status, viral co-infection status, C. difficile status, presence of upper respiratory tract infection-related symptoms, and previous use of antibiotics for more than 1 day within 2 weeks prior to the onset of diarrhea were chosen as the explanatory variables for ordinal logistic regression. All analyses were performed using SPSS version 20.0 (IBM Corp., Chicago, IL, USA).
Ethics statement
The study protocol was approved by the Institutional Review Board of Seoul Metropolitan Government-Seoul National University Boramae Medical Center (IRB No. 02-2011-11, 06-2012-151, 16-2015-78). Informed consent was confirmed by the IRB.
RESULTS
Clinical characteristics of subjects
During the study period, a total of 1,023 children (2012: n = 496, 2013: n = 527) were diagnosed with AGE according to electronic medical records. Of those, 415 children (40.6%) met the inclusion criteria and were enrolled in this study. The median age of these 415 children was 27.2 (IQR 13.8–56.0) months and there were 244 males (58.8%). Of the 415 children, 319 (76.9%) were under 5 years of age and 190 (45.8%) were under 2 years of age (Table 1). There were no significant differences between the patients admitted in 2012 (n = 179, 43.1%) and 2013 (n = 236, 56.9%) in terms of demographic and anthropometric characteristics.
Table 1. Distribution of enteric pathogens according to the age group in 415 children with AGE.
| Subjects/pathogens | Age, mon, median (IQR) | Age group, mon, No. (%) | P* | P† | ||||
|---|---|---|---|---|---|---|---|---|
| < 6 | 6–23 | 24–59 | ≥ 60 | |||||
| Total | 415 (100.0) | 27.2 (13.8–56.3) | 19 (100.0) | 171 (100.0) | 129 (100.0) | 96 (100.0) | - | - |
| Pathogen positive | 335 (80.7) | 27.4 (14.2–55.4) | 12 (63.2) | 140 (81.9) | 108 (83.7) | 75 (78.1) | 0.166 | 0.732 |
| Virus positive | 282 (68.0) | 25.2 (13.8–48.3) | 10 (52.6) | 126 (73.7) | 91 (70.5) | 55 (57.3) | 0.018 | 0.146 |
| Norovirus | 130 (31.3) | 19.0 (11.8–40.9) | 4 (21.1) | 73 (42.7) | 30 (23.3) | 23 (24.0) | 0.001 | < 0.001 |
| Rotavirus | 103 (24.8) | 30.5 (20.3–57.3) | 4 (21.1) | 30 (17.5) | 44 (34.1) | 25 (26.0) | 0.012 | 0.003 |
| Adenovirus | 56 (13.5) | 19.7 (12.0–40.1) | 3 (15.8) | 31 (18.1) | 16 (12.4) | 6 (6.3) | 0.009 | 0.016 |
| Astrovirus | 20 (4.8) | 26.2 (14.5–55.9) | 0 (0.0) | 10 (5.8) | 7 (5.4) | 3 (3.1) | 0.679 | 0.698 |
| Virus coinfection | 24 (5.8) | 17.7 (12.5–38.6) | 1 (5.3) | 15 (8.8) | 6 (4.7) | 2 (2.1) | 0.033 | 0.034 |
| Clostridium difficile | 38 (9.2) | 13.4 (9.9–21.5) | 1 (5.3) | 29 (17.0) | 5 (3.9) | 3 (3.1) | < 0.001 | < 0.001 |
| Other enteric bacteria | 80 (19.3) | 38.2 (15.2–79.4) | 2 (10.5) | 25 (14.6) | 26 (20.2) | 27 (28.1) | 0.012 | 0.016 |
The values were calculated using the χ2 test.
AGE = acute gastroenteritis, IQR = interquartile range.
*Among the 4 age groups; †Between children aged less than 24 months and those older than 24 months.
Enteric pathogen distribution
A total of 355 (80.7%) children tested positive for enteric pathogens. Viruses, toxigenic C. difficile, and other enteric bacteria were detected in 282 (68.0%), 38 (9.2%), and 80 (19.3%) children, respectively (Table 1). The age distribution was not significantly different between those pathogen positive vs. negative, however, it was significantly different between those with virus positive vs. negative status (P = 0.018). Toxigenic C. difficile infection was significantly more common in children less than 24 months of age (P < 0.001). In contrast, enteric bacterial infection other than toxigenic C. difficile was significantly more frequent in children older than 24 months (P = 0.016).
Virus distribution
Of the 282 children (68.0%) who tested positive for viral infection by PCR, norovirus was the most commonly detected (n = 130), followed by rotavirus (n = 103), adenovirus (n = 56), and astrovirus (n = 20) (Table 1). The age distributions of the children differed significantly according to virus type (P = 0.008): norovirus was found mostly in those aged 19.0 (IQR 12.0–40.1) months, rotavirus in those aged 30.5 (IQR 20.3–57.3) months, adenovirus in those aged 19.7 (IQR 12.0–40.0) months, and astrovirus in those aged 26.2 (IQR 14.5–55.9) months. Rotavirus infection was significantly more frequent in children more than 24 months of age (P < 0.001) and showed a preference for older age groups compared with other enteric viruses (P = 0.001). Among children with rotavirus infection, 14 (13.6%) had been vaccinated with more than 2 doses of rotavirus vaccines. The age of vaccinated children was significantly younger than that of unvaccinated children (22.9 [IQR 15.7–29.7] months vs. 34.0 [IQR 21.9–63.1] months, P = 0.010).
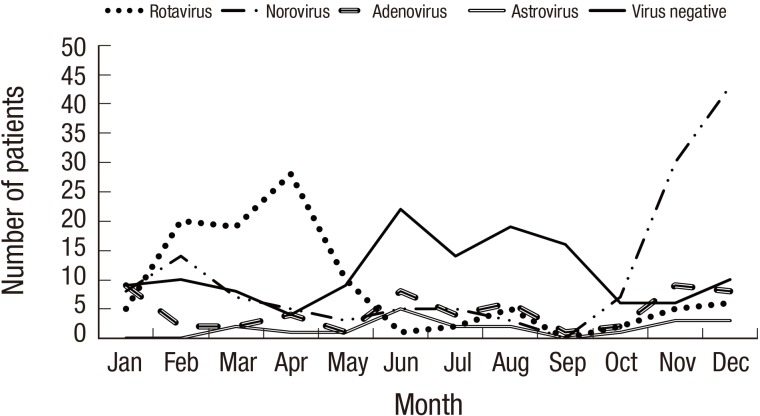
Seasonal differences were also observed among the viruses (Fig. 1). Mixed viral infections were found in 8.5% of children with AGE (Table 2). Norovirus co-infection (n = 18) occurred most frequently, followed by adenovirus and rotavirus co-infection. Median age of virus–virus co-infection was 17.7 (IQR 12.5–38.6) months: viral co-infection tended to be more frequent in children less than 24 months of age, although this was not statistically significant (P = 0.059).
Fig. 1.
Monthly distribution of enteric viruses in 415 children.
Table 2. Distribution enteric pathogens involved in coinfections according to the age group in 282 children with viral AGE.
| Subjects/Pathogens | No. (%) | Age group, mon | P* | P† | |||
|---|---|---|---|---|---|---|---|
| < 6 | 6–23 | 24–59 | ≥ 60 | ||||
| Total | 282 (100.0) | 10 (100.0) | 126 (100.0) | 91 (100.0) | 55 (100.0) | - | - |
| Viral coinfection | 24 (8.5) | 1 (10.0) | 15 (11.9) | 6 (6.6) | 2 (3.6) | 0.261 | 0.059 |
| NoroV + AdenoV | 7 | 1 | 4 | 2 | - | ||
| NoroV + RotaV | 5 | - | 3 | 1 | 1 | ||
| NoroV + AstroV | 3 | - | 2 | 1 | - | ||
| RotaV + AdenoV | 3 | - | 1 | 1 | 1 | ||
| RotaV + AstroV | 1 | - | - | 1 | - | ||
| AdenoV + AstroV | 2 | - | 2 | - | - | ||
| NoroV + RotaV + AdenoV | 2 | - | 2 | - | - | ||
| NoroV + AdenoV + AstroV | 1 | - | 1 | - | - | ||
| Virus + Clostridium difficile | 26 (9.2) | 1 (10.0) | 21 (16.7) | 4 (4.4) | 0 (0.0) | 0.001 | < 0.001 |
| NoroV + C. difficile | 13 | - | 12 | 1 | - | ||
| RotaV + C. difficile | 7 | 1 | 3 | 3 | - | ||
| AdenoV + C. difficile | 3 | - | 3 | - | - | ||
| NoroV + AdenoV + C. difficile | 3 | - | 3 | - | - | ||
| Virus + other enteric bacteria | 36 (12.8) | 0 | 17(13.5) | 9 (9.9) | 10 (18.2) | 0.302 | 0.897 |
| NoroV + C. perfringens | 9 | - | 7 | 2 | - | ||
| NoroV + EHEC | 3 | - | - | 3 | - | ||
| NoroV + Salmonella | 3 | - | - | 2 | 1 | ||
| NoroV + Campylobacter | 1 | - | - | - | 1 | ||
| NoroV + C. perfringens + C. difficile | 3 | - | 3 | - | - | ||
| RotaV + C. perfringens | 8 | - | 3 | - | 5 | ||
| RotaV + EHEC | 1 | - | 1 | - | - | ||
| RotaV + C. perfringens + C. difficile | 1 | - | 1 | - | - | ||
| AdenoV + EHEC | 2 | - | - | 1 | 1 | ||
| AdenoV + Salmonella | 1 | - | - | - | 1 | ||
| AstroV + C. perfringens | 1 | - | - | 1 | - | ||
| NoroV + AdenoV + C. perfringens | 1 | - | 1 | - | - | ||
| NoroV + AstroV + C. perfringens | 1 | - | 1 | - | - | ||
| RotaV + AdenoV + C. perfringens | 1 | - | - | - | 1 | ||
The values were calculated using the χ2 test.
AGE = acute gastroenteritis, NoroV = Norovirus, AdenoV = Adenovirus, RotaV = Rotavirus, AstroV = Astrovirus, EHEC = enterohemorrhagic Escherichia coli.
*Among the 4 age groups; †Between children less than 24 months and those older than 24 months.
Co-infection of C. difficile and other bacteria in viral AGE
Co-infection by toxigenic C. difficile and other enteric bacteria in viral AGE was present in 26 (9.2%) and 36 (12.8%) children, respectively (Table 2). Median age of virus-toxigenic C. difficile co-infection was 12.7 (IQR 10.2–20.6) months, which was younger than that of virus–virus co-infection, albeit not statistically significant. Toxigenic C. difficile co-infection with viruses was significantly more frequent in those less than 24 months of age (P < 0.001). Toxigenic C. difficile co-infection with viruses was most frequently associated with norovirus infection (n = 19), followed by rotavirus infection. However, there was no significant association between presence of toxigenic C. difficile and norovirus detection.
The prevalence of co-infection with other enteric bacteria and viruses was 12.8%, which was slightly higher than the rate of virus–virus co-infection or virus-toxigenic C. difficile co-infection. Norovirus (n = 21) was the most frequent virus involved in this type of co-infection. The median age of children with virus–other enteric bacterial co-infection was 28.4 (IQR 11.4–64.4) months, which was significantly higher than those with virus-C. difficile co-infection (P = 0.010).
Association of age and clinical severity of AGE
Among 415 children, 313 children were included in the severity analysis, after excluding 102. The exclusion criteria were as follows: presence of enteropathogens other than C. difficile on multiplex PCR or stool culture (n = 80), gross frequent hematochezia or presence of tenesmus without toxigenic C. difficile detection (n = 4), or presence of co-diagnosis of an acute lower respiratory infection, documentation of a respiratory virus or enterovirus co-infection or other suspicious focal or systemic bacterial co-infections with previous and/or ongoing antibiotic use (n = 18).
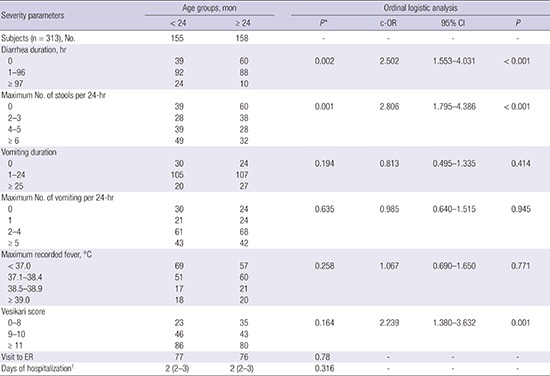
In the ordinal logistic regression, age less than 24 months was significantly associated with greater diarrheal severity in terms of duration and frequency and a higher modified Vesikari score than age more than 24 months, after adjustment for other potential severity-related factors (Table 3). Age less than 6 months was significantly associated with less vomiting severity in terms of duration (cumulative odds ratio [c-OR], 0.16; 95% confidence interval [CI], 0.06–0.49; P = 0.001) and frequency (c-OR, 0.29; 95% CI, 0.09–0.90; P = 0.032) compared to other age groups, after adjustment for other potential severity-related factors.
Table 3. Clinical severity of AGE according to the age group in 313 children.
| Severity parameters | Age groups, mon | Ordinal logistic analysis | ||||
|---|---|---|---|---|---|---|
| < 24 | ≥ 24 | P* | c-OR | 95% CI | P | |
| Subjects (n = 313), No. | 155 | 158 | - | - | - | - |
| Diarrhea duration, hr | 0.002 | 2.502 | 1.553–4.031 | < 0.001 | ||
| 0 | 39 | 60 | ||||
| 1–96 | 92 | 88 | ||||
| ≥ 97 | 24 | 10 | ||||
| Maximum No. of stools per 24-hr | 0.001 | 2.806 | 1.795–4.386 | < 0.001 | ||
| 0 | 39 | 60 | ||||
| 2–3 | 28 | 38 | ||||
| 4–5 | 39 | 28 | ||||
| ≥ 6 | 49 | 32 | ||||
| Vomiting duration | 0.194 | 0.813 | 0.495–1.335 | 0.414 | ||
| 0 | 30 | 24 | ||||
| 1–24 | 105 | 107 | ||||
| ≥ 25 | 20 | 27 | ||||
| Maximum No. of vomiting per 24-hr | 0.635 | 0.985 | 0.640–1.515 | 0.945 | ||
| 0 | 30 | 24 | ||||
| 1 | 21 | 24 | ||||
| 2–4 | 61 | 68 | ||||
| ≥ 5 | 43 | 42 | ||||
| Maximum recorded fever, ℃ | 0.258 | 1.067 | 0.690–1.650 | 0.771 | ||
| < 37.0 | 69 | 57 | ||||
| 37.1–38.4 | 51 | 60 | ||||
| 38.5–38.9 | 17 | 21 | ||||
| ≥ 39.0 | 18 | 20 | ||||
| Vesikari score | 0.164 | 2.239 | 1.380–3.632 | 0.001 | ||
| 0–8 | 23 | 35 | ||||
| 9–10 | 46 | 43 | ||||
| ≥ 11 | 86 | 80 | ||||
| Visit to ER | 77 | 76 | 0.78 | - | - | - |
| Days of hospitalization† | 2 (2–3) | 2 (2–3) | 0.316 | - | - | - |
AGE = acute gastroenteritis, c-OR = cumulative odds ratio, CI = confidence interval, ER = emergency room.
*P values were calculated using the χ2 test; †The values are presented as median (interquartile range).
Association of viral pathogen and clinical severity of AGE
In the severity analyses of 313 children, virus detection was significantly associated with vomiting severity in terms of duration and frequency (c-OR, 2.5; 95% CI, 1.4–4.4; P = 0.002; c-OR, 1.8; 95% CI, 1.1–2.9; P = 0.028) and with the modified Vesikari score (c-OR, 2.3; 95% CI, 1.4–3.8; P = 0.001). Rotavirus infection (including 13 vaccinated cases) was significantly associated with severity of diarrhea, vomiting, fever, and overall clinical severity (Table 4). Detection of norovirus was associated with vomiting severity, and less severe fever (Table 5). However, viral co-infection (n = 20) was not significantly associated with any of the clinical severity-related parameters.
Table 4. Clinical severity of AGE according to the rotavirus infection status in 313 children.
| Severity parameters | Rotavirus | Ordinal logistic analysis | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | P* | c-OR | 95% CI | P | |
| Subject (n = 313), No. | 90 | 223 | - | - | - | - |
| Diarrhea duration, hr | 0.051 | 1.959 | 1.133–3.387 | 0.016 | ||
| 0 | 13 | 86 | ||||
| 1–96 | 73 | 107 | ||||
| ≥ 97 | 4 | 30 | ||||
| Maximum No. of stools per 24-hr | 0.002 | 2.582 | 1.545–4.317 | < 0.001 | ||
| 1 | 13 | 86 | ||||
| 2–3 | 27 | 39 | ||||
| 4–5 | 20 | 47 | ||||
| ≥ 6 | 30 | 51 | ||||
| Vomiting duration | 0.048 | 2.221 | 1.224–4.030 | 0.009 | ||
| 0 | 5 | 49 | ||||
| 1–24 | 73 | 139 | ||||
| ≥ 25 | 12 | 35 | ||||
| Maximum no. of vomiting per 24-hr | 0.004 | 2.447 | 1.463–4.094 | 0.001 | ||
| 0 | 5 | 49 | ||||
| 1 | 12 | 33 | ||||
| 2–4 | 47 | 82 | ||||
| ≥ 5 | 26 | 59 | ||||
| Maximum recorded fever, ℃ | < 0.001 | 3.216 | 1.896–5.455 | < 0.001 | ||
| < 37.0 | 13 | 113 | ||||
| 37.1–38.4 | 38 | 73 | ||||
| 38.5–38.9 | 23 | 15 | ||||
| ≥ 39.0 | 16 | 22 | ||||
| Vesikari score | < 0.001 | 6.962 | 3.655–13.262 | < 0.001 | ||
| 0–8 | 3 | 55 | ||||
| 9–10 | 16 | 73 | ||||
| ≥ 11 | 71 | 95 | ||||
| Visit to ER | 51 | 102 | 0.08 | - | - | - |
| Days of hospitalization† | 3 (2–4) | 2 (2–3) | 0.001* | - | - | - |
AGE = acute gastroenteritis, c-OR = cumulative odds ratio, CI = confidence interval, ER = emergency room.
*P values were calculated using the χ2 test; †The values are presented as median (interquartile range).
Table 5. Clinical severity of AGE according to the norovirus infection status in 313 children.
| Severity parameters | Norovirus | Ordinal logistic analysis | ||||
|---|---|---|---|---|---|---|
| Positive | Negative | P* | c-OR | 95% CI | P | |
| Subject (n = 313), No. | (103) | (210) | - | - | - | - |
| Diarrhea duration, hr | 0.094 | 0.737 | 0.430–1.261 | 0.265 | ||
| 0 | 41 | 58 | ||||
| 1–96 | 51 | 129 | ||||
| ≥ 97 | 11 | 23 | ||||
| Maximum No. of stools per 24-hr | 0.020 | 0.807 | 0.496–1.312 | 0.386 | ||
| 1 | 41 | 58 | ||||
| 2–3 | 21 | 45 | ||||
| 4–5 | 21 | 46 | ||||
| ≥ 6 | 20 | 61 | ||||
| Vomiting duration | 0.029 | 2.514 | 1.397–4.525 | 0.002 | ||
| 0 | 11 | 43 | ||||
| 1–24 | 73 | 139 | ||||
| ≥ 25 | 19 | 28 | ||||
| Maximum No. of vomiting per 24-hr | 0.006 | 2.437 | 1.480–4.012 | < 0.001 | ||
| 0 | 11 | 43 | ||||
| 1 | 11 | 34 | ||||
| 2–4 | 47 | 82 | ||||
| ≥ 5 | 34 | 51 | ||||
| Maximum recorded fever, ℃ | < 0.001 | 0.575 | 0.343–0.961 | 0.035 | ||
| < 37.0 | 57 | 69 | ||||
| 37.1–38.4 | 36 | 75 | ||||
| 38.5–38.9 | 3 | 35 | ||||
| ≥ 39.0 | 7 | 31 | ||||
| Vesikari score | 0.138 | 1.168 | 0.705–1.933 | 0.546 | ||
| 0–8 | 21 | 37 | ||||
| 9–10 | 35 | 54 | ||||
| ≥ 11 | 47 | 119 | ||||
| Visit to ER | 49 | 104 | 0.746 | - | - | - |
| Days of hospitalization† | 2 (2–3) | 2 (2–3.3) | 0.032* | - | - | - |
AGE = acute gastroenteritis, c-OR = cumulative odds ratio, CI = confidence interval, ER = emergency room.
*P values were calculated using the χ2 test; †The values are presented as median (interquartile range).
Association of C. difficile co-infection and the clinical severity of viral AGE
The severity assessment for toxigenic C. difficile co-detection was performed with a subpopulation of children who tested positive for viral infections (n = 237). C. difficile co-infection (n = 22) was not significantly associated with any clinical severity-related parameters after adjustment for other potential severity-related factors. In this subgroup analysis, younger age, infection with rotavirus, and infection with norovirus were severity-related factors associated with AGE, as they were in the previous analysis of all 313 children.
DISCUSSION
During the study period, norovirus infection was the leading cause of AGE in hospitalized children, exceeding rotavirus infection. This has been observed in several recent studies from various countries after implementing rotavirus vaccination (1,21,22,23). The prevalence of adenovirus was also relatively high compared with that reported by previous Korean studies from the pre-vaccine era, which enrolled hospitalized children (16,24,25). In some recent multi-center Asian studies conducted in inpatient or outpatient settings, an increased prevalence of adenovirus or enteric adenovirus infection was not demonstrated (26,27). However, recent studies from Korea (16.6% of children with documented viral AGE) and Brazil (12.5% of AGE) conducted with inpatient and outpatient children reported a prevalence similar to that found in the present study (19.9% of children with documented viral AGE and 13.5% of children with AGE). These findings suggest the increasing importance of other viruses in cases of severe childhood AGE after the introduction of rotavirus vaccination, at least in some regions (21,28).
The prevalence of mixed viral infections (5.8% of AGE) was similar to that noted by previous studies from various countries, which reported a prevalence of 3%–6% in hospitalized children with AGE. However, the prevalence of co-infections varied across studies (18,24,26,29,30) and seemed to be higher (up to 15%) in recent studies conducted in outpatient settings (27,31). The dominant types of co-infection were quite different even among studies, partly because of variations in the study inclusion criteria, such as those related to age, hospitalization, season, or year, and variations in the diagnostic methods used to detect enteric viruses. Mostly, rotavirus co-infection was the most frequent type in studies conducted in the pre-vaccine period (29,30,32,33). However, norovirus co-infection has become most prevalent in a few studies conducted in the era of rotavirus vaccination (21,27,31), similar to the findings of this study. Few large-scale studies have examined the co-detection rate of toxigenic C. difficile and enteric virus in childhood viral gastroenteritis. In a recent single center European study with a modest number of fecal samples, a prevalence of 14.4% was reported (10). The overall prevalence of toxigenic C. difficile (including both mono-infection and co-infection) was approximately 5%–20% in hospitalized children with diarrhea, no different from the prevalence in hospitalized children without diarrhea (34,35,36,37). The diversity in prevalence may be largely due to the different age distribution of included children, which could be deduced from the results of this study: there was a 17.0% prevalence of toxigenic C. difficile in children aged 6–23 months in contrast to 4.4% in those 24–59 months (P < 0.001). Toxigenic C. difficile co-infection was also most frequently associated with norovirus infection in this study population, which is in contrast to the results of previous studies conducted in the pre-vaccine era. In those previous studies, rotavirus-toxigenic C. difficile co-infection was the most frequent virus-toxigenic C. difficile co-infection (10,36). However, the association between the norovirus and toxigenic C. difficile was not statistically significant.
To our knowledge, the association that we found between younger age (less than 24 months) and the clinical severity of diarrhea after adjustment for rotavirus infection may be one of the first observed in a non-developing country. Previous European studies showed that rotavirus infection is associated with severe clinical manifestations of AGE (4,5). However, these studies were performed in the pre-vaccine era in which most children with rotavirus infections were less than 24 months of age. Hence, age-related severity may, at least partly, be attributed to rotavirus-related severity (9). In contrast, in this study, the median age of children with rotavirus infection was 31 months. In spite of the higher age of rotavirus-infected children, we found that an age of less than 24 months was a significant severity-related host factor. Additionally, we found that rotavirus infection (in mostly unvaccinated children in this study) was the primary pathogenic cause of severe clinical gastroenteritis. Because this study was performed between 2012 and 2013, which corresponds to 5–6 years after the introduction of the rotavirus vaccine in Korea (the rate of rotavirus vaccination more than 2 times was reported to be 65.6% in Seoul, and nationwide rate 52.4% in 2013 by Korean National Immunization Survey; http://www.cdc.go.kr), the age of children vulnerable to rotavirus infection seems to have transiently increased over the study period (8).
In this study, children less than 6 months of age, as well as children aged between 6 and 23 months, had more severe diarrhea than the older age groups. These findings are contrary to previous European studies but similar to results from previous studies from developing countries (6,9). Although other host-related factors, such as breastfeeding, were not investigated in this study, WHZ and macronutrient status (including albumin, blood urea nitrogen, and serum calcium) were examined to exclude malnutrition. Therefore, malnutrition-associated immune deficiencies were not likely to have caused the age-related difference in diarrheal severity. Given that an age of less than 24 months was significantly associated with toxigenic C. difficile detection and also tended to be associated with a higher incidence of viral co-infection, the greater diarrheal severity in these age groups might, at least in part, be related to the relative instability of the intestinal microbiota in these age groups in comparison with those in older age groups. In other words, intestinal mucosal immunity might play a role in this age-related diarrheal severity.
It is controversial whether gastroenteritis cases with co-infection with multiple viruses are more severe than gastroenteritis cases with mono-infection (10,11,30). A previous study showed greater severity in co-infection cases, but did not separate viral co-infections from bacterial co-infections (10). In our study, bacterial co-infection was excluded in the severity-related analyses. In this condition, viral co-infection was not associated with greater clinical severity, which was compatible with the results of some previous studies (11,29,30). Because rotavirus infection cases showed the most severe clinical manifestations, and norovirus infection was associated with greater vomiting severity, one could expect more severe clinical symptoms in co-infection cases with rotavirus and norovirus than in mono-infection cases, if both viruses play roles as infecting agents. Although evaluation of the severity of gastroenteritis resulting from this co-infection was limited, as only 7 of our patients were co-infected with rotavirus and norovirus, these patients did not show any greater clinical severity compared to mono-infection cases with rotavirus or norovirus or co-infection cases with other viruses. Further studies that include a sufficient number of rotavirus and norovirus co-infection cases may be needed to determine the clinical significance of this co-infection and to determine whether this co-infection doubles the clinical manifestations or whether 1 virus functions as the main pathogen and the other as a bystander.
Regarding the clinical severity of co-infection with C. difficile, 2 contradictory results were also found in the literature. In one study, viral co-infection with C. difficile was clinically indistinguishable from C. difficile mono-infection, although the bacterial burden of C. difficile was significantly higher in viral co-infection cases than in cases without viral co-infection (38). However, in another study, the severity of viral gastroenteritis in children co-infected with C. difficile was significantly greater than viral or bacterial mono-infection (10). In our study, clinical severity of viral AGE was not affected by presence of toxigenic C. difficile: Vomiting severity and the modified Vesikari score, but not diarrheal severity, were significantly different between virus-C. difficile co-infection and C. difficile mono-infection cases. Because these associations were also found in children with viral gastroenteritis without C. difficile, it seems that co-infection with C. difficile may not further contribute to severity of childhood gastroenteritis in most cases. This is in line with the previous opinion that detection of C. difficile toxin cannot be assumed to be the causative agent for diarrhea in most children between 1–3 years of age (39,40). Thus, in most such cases, if toxigenic C. difficile is detected unexpectedly in typical viral gastroenteritis cases, it may not be necessary to treat C. difficile.
A limitation of this study is that this was a single-center study that enrolled approximately half of the children hospitalized with gastroenteritis during the study period. However, a considerable number of epidemiologic findings share similar trends with the results of previous multicenter or national studies from other countries, as mentioned previously. Moreover, the number of samples examined is not that small, compared with previous epidemiologic studies. Additionally, the patients included in the severity analysis were strictly chosen to exclude potential confounders that may affect the clinical severity of gastroenteritis such as presence of co-morbid acute illnesses.
In summary, this study captured the changing prevalence and age-related distribution of viruses and their co-infections after implementing rotavirus vaccination in Seoul: norovirus infection, including norovirus-associated co-infection, was the leading cause of AGE in hospitalized children, especially in those less than 24 months of age. Age-dependent clinical severity was also observed: age less than 24 months was associated with high-grade diarrheal severity and overall severity of gastroenteritis independent of rotavirus status. Co-infection with multiple viruses may not affect clinical severity, but this needs to be investigated further with a sufficient number of children co-infected with rotavirus and norovirus. Mostly, the clinical severity of viral gastroenteritis may not be affected by the co-existence of toxigenic C. difficile. This study provides information on the changing epidemiology and clinical severity-related factors of childhood gastroenteritis. It will aid in the management of childhood AGE in this era of rotavirus vaccination and molecular diagnostic techniques to detect multiple pathogens.
ACKNOWLEDGMENT
We thank Kyung Mi Choi (Seoul Metropolitan Government Seoul National University Boramae Medical Center) for the expert technical assistance.
Footnotes
Funding: This work supported by a grant from a Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT & Future Planning (NRF-2012R1A1A3010058).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Chang JY, Yi H. Data curation: Kim A, Chang JY, Shin S, Oh S. Formal analysis: Kim A, Chang JY, Shin S, Oh S. Investigation: Kim A, Chang JY, Shin S.Writing - original draft: Kim A, Chang JY. Writing - review & editing: Moon JS, Ko JS.
References
- 1.Wikswo ME, Desai R, Edwards KM, Staat MA, Szilagyi PG, Weinberg GA, Curns AT, Lopman B, Vinjé J, Parashar UD, et al. Clinical profile of children with norovirus disease in rotavirus vaccine era. Emerg Infect Dis. 2013;19:1691–1693. doi: 10.3201/eid1910.130448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choi UY, Lee SY, Ma SH, Jang YT, Kim JY, Kim HM, Kim JH, Kim DS, Kim YS, Kang JH. Epidemiological changes in rotavirus gastroenteritis in children under 5 years of age after the introduction of rotavirus vaccines in Korea. Eur J Pediatr. 2013;172:947–952. doi: 10.1007/s00431-013-1974-y. [DOI] [PubMed] [Google Scholar]
- 3.Guarino A, Ashkenazi S, Gendrel D, Lo Vecchio A, Shamir R, Szajewska H, European Society for Pediatric Gastroenterology, Hepatology, and Nutrition European Society for Pediatric Infectious Diseases. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition/European Society for Pediatric Infectious Diseases evidence-based guidelines for the management of acute gastroenteritis in children in Europe: update 2014. J Pediatr Gastroenterol Nutr. 2014;59:132–152. doi: 10.1097/MPG.0000000000000375. [DOI] [PubMed] [Google Scholar]
- 4.Friesema IH, de Boer RF, Duizer E, Kortbeek LM, Notermans DW, Norbruis OF, Bezemer DD, van Heerbeek H, van Andel RN, van Enk JG, et al. Etiology of acute gastroenteritis in children requiring hospitalization in the Netherlands. Eur J Clin Microbiol Infect Dis. 2012;31:405–415. doi: 10.1007/s10096-011-1320-0. [DOI] [PubMed] [Google Scholar]
- 5.Gimenez-Sanchez F, Delgado-Rubio A, Martinon-Torres F, Bernaola-Iturbe E, Rotascore Research Group Multicenter prospective study analysing the role of rotavirus on acute gastroenteritis in Spain. Acta Paediatr. 2010;99:738–742. doi: 10.1111/j.1651-2227.2010.01684.x. [DOI] [PubMed] [Google Scholar]
- 6.Mathew A, Rao PS, Sowmyanarayanan TV, Kang G. Severity of rotavirus gastroenteritis in an Indian population: report from a 3 year surveillance study. Vaccine. 2014;32(Suppl 1):A45–8. doi: 10.1016/j.vaccine.2014.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Strand TA, Sharma PR, Gjessing HK, Ulak M, Chandyo RK, Adhikari RK, Sommerfelt H. Risk factors for extended duration of acute diarrhea in young children. PLoS One. 2012;7:e36436. doi: 10.1371/journal.pone.0036436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shim JO, Chang JY, Shin S, Moon JS, Ko JS. Changing distribution of age, clinical severity, and genotypes of rotavirus gastroenteritis in hospitalized children after the introduction of vaccination: a single center study in Seoul between 2011 and 2014. BMC Infect Dis. 2016;16:287. doi: 10.1186/s12879-016-1623-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Albano F, Bruzzese E, Bella A, Cascio A, Titone L, Arista S, Izzi G, Virdis R, Pecco P, Principi N, et al. Rotavirus and not age determines gastroenteritis severity in children: a hospital-based study. Eur J Pediatr. 2007;166:241–247. doi: 10.1007/s00431-006-0237-6. [DOI] [PubMed] [Google Scholar]
- 10.Valentini D, Vittucci AC, Grandin A, Tozzi AE, Russo C, Onori M, Menichella D, Bartuli A, Villani A. Coinfection in acute gastroenteritis predicts a more severe clinical course in children. Eur J Clin Microbiol Infect Dis. 2013;32:909–915. doi: 10.1007/s10096-013-1825-9. [DOI] [PubMed] [Google Scholar]
- 11.Matthijnssens J, Zeller M, Heylen E, De Coster S, Vercauteren J, Braeckman T, Van Herck K, Meyer N, Pirçon JY, Soriano-Gabarro M, et al. Higher proportion of G2P[4] rotaviruses in vaccinated hospitalized cases compared with unvaccinated hospitalized cases, despite high vaccine effectiveness against heterotypic G2P[4] rotaviruses. Clin Microbiol Infect. 2014;20:O702–10. doi: 10.1111/1469-0691.12612. [DOI] [PubMed] [Google Scholar]
- 12.Bignardi GE, Staples K, Majmudar N. A case of norovirus and Clostridium difficile infection: casual or causal relationship? J Hosp Infect. 2007;67:198–200. doi: 10.1016/j.jhin.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Lukkarinen H, Eerola E, Ruohola A, Vainionpää R, Jalava J, Kotila S, Ruuskanen O. Clostridium difficile ribotype 027-associated disease in children with norovirus infection. Pediatr Infect Dis J. 2009;28:847–848. doi: 10.1097/INF.0b013e31819d1cd9. [DOI] [PubMed] [Google Scholar]
- 14.Ruuska T, Vesikari T. Rotavirus disease in Finnish children: use of numerical scores for clinical severity of diarrhoeal episodes. Scand J Infect Dis. 1990;22:259–267. doi: 10.3109/00365549009027046. [DOI] [PubMed] [Google Scholar]
- 15.Allard A, Girones R, Juto P, Wadell G. Polymerase chain reaction for detection of adenoviruses in stool samples. J Clin Microbiol. 1990;28:2659–2667. doi: 10.1128/jcm.28.12.2659-2667.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koh H, Baek SY, Shin JI, Chung KS, Jee YM. Coinfection of viral agents in Korean children with acute watery diarrhea. J Korean Med Sci. 2008;23:937–940. doi: 10.3346/jkms.2008.23.6.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Belliot G, Laveran H, Monroe SS. Detection and genetic differentiation of human astroviruses: phylogenetic grouping varies by coding region. Arch Virol. 1997;142:1323–1334. doi: 10.1007/s007050050163. [DOI] [PubMed] [Google Scholar]
- 18.Yang HR, Jee YM, Ko JS, Seo JK. Detection and genotyping of viruses detected in children with benign afebrile seizures associated with acute gastroenteritis. Korean J Pediatr Gastroenterol Nutr. 2009;12:183–193. [Google Scholar]
- 19.Higgins RR, Beniprashad M, Cardona M, Masney S, Low DE, Gubbay JB. Evaluation and verification of the Seeplex Diarrhea-V ACE assay for simultaneous detection of adenovirus, rotavirus, and norovirus genogroups I and II in clinical stool specimens. J Clin Microbiol. 2011;49:3154–3162. doi: 10.1128/JCM.00599-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onori M, Coltella L, Mancinelli L, Argentieri M, Menichella D, Villani A, Grandin A, Valentini D, Raponi M, Russo C. Evaluation of a multiplex PCR assay for simultaneous detection of bacterial and viral enteropathogens in stool samples of paediatric patients. Diagn Microbiol Infect Dis. 2014;79:149–154. doi: 10.1016/j.diagmicrobio.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 21.Jin HI, Lee YM, Choi YJ, Jeong SJ. Recent viral pathogen in acute gastroenteritis: a retrospective study at a tertiary hospital for 1 year. Korean J Pediatr. 2016;59:120–125. doi: 10.3345/kjp.2016.59.3.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Doll MK, Gagneur A, Tapiéro B, Charest H, Gonzales M, Buckeridge DL, Quach C. Temporal changes in pediatric gastroenteritis after rotavirus vaccination in Quebec. Pediatr Infect Dis J. 2016;35:555–560. doi: 10.1097/INF.0000000000001077. [DOI] [PubMed] [Google Scholar]
- 23.Lopman BA, Steele D, Kirkwood CD, Parashar UD. The vast and varied global burden of norovirus: prospects for prevention and control. PLoS Med. 2016;13:e1001999. doi: 10.1371/journal.pmed.1001999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung JY, Huh K, Kim SW, Shin BM, Han TH, Lee JI, Song MO. Molecular epidemiology of human astrovirus infection in hospitalized children with acute gastroenteritis. Korean J Pediatr Gastroenterol Nutr. 2006;9:139–146. [Google Scholar]
- 25.Lee JI, Lee GC, Chung JY, Han TH, Lee YK, Kim MS, Lee CH. Detection and molecular characterization of adenoviruses in Korean children hospitalized with acute gastroenteritis. Microbiol Immunol. 2012;56:523–528. doi: 10.1111/j.1348-0421.2012.00469.x. [DOI] [PubMed] [Google Scholar]
- 26.Chen CJ, Wu FT, Huang YC, Chang WC, Wu HS, Wu CY, Lin JS, Huang FC, Hsiung CA. Clinical and epidemiologic features of severe viral gastroenteritis in children: a 3-year surveillance, multicentered study in Taiwan with partial rotavirus immunization. Medicine (Baltimore) 2015;94:e1372. doi: 10.1097/MD.0000000000001372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thongprachum A, Takanashi S, Kalesaran AF, Okitsu S, Mizuguchi M, Hayakawa S, Ushijima H. Four-year study of viruses that cause diarrhea in Japanese pediatric outpatients. J Med Virol. 2015;87:1141–1148. doi: 10.1002/jmv.24155. [DOI] [PubMed] [Google Scholar]
- 28.Reis TA, Assis AS, do Valle DA, Barletta VH, de Carvalho IP, Rose TL, Portes SA, Leite JP. da Rosa e Silva ML. The role of human adenoviruses type 41 in acute diarrheal disease in Minas Gerais after rotavirus vaccination. Braz J Microbiol. 2016;47:243–250. doi: 10.1016/j.bjm.2015.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran A, Talmud D, Lejeune B, Jovenin N, Renois F, Payan C, Leveque N, Andreoletti L. Prevalence of rotavirus, adenovirus, norovirus, and astrovirus infections and coinfections among hospitalized children in northern France. J Clin Microbiol. 2010;48:1943–1946. doi: 10.1128/JCM.02181-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Román E, Wilhelmi I, Colomina J, Villar J, Cilleruelo ML, Nebreda V, Del Alamo M, Sánchez-Fauquier A. Acute viral gastroenteritis: proportion and clinical relevance of multiple infections in Spanish children. J Med Microbiol. 2003;52:435–440. doi: 10.1099/jmm.0.05079-0. [DOI] [PubMed] [Google Scholar]
- 31.Lu L, Jia R, Zhong H, Xu M, Su L, Cao L, Dong Z, Dong N, Xu J. Molecular characterization and multiple infections of rotavirus, norovirus, sapovirus, astrovirus and adenovirus in outpatients with sporadic gastroenteritis in Shanghai, China, 2010-2011. Arch Virol. 2015;160:1229–1238. doi: 10.1007/s00705-015-2387-1. [DOI] [PubMed] [Google Scholar]
- 32.Marie-Cardine A, Gourlain K, Mouterde O, Castignolles N, Hellot MF, Mallet E, Buffet-Janvresse C. Epidemiology of acute viral gastroenteritis in children hospitalized in Rouen, France. Clin Infect Dis. 2002;34:1170–1178. doi: 10.1086/339807. [DOI] [PubMed] [Google Scholar]
- 33.González GG, Liprandi F, Ludert JE. Molecular epidemiology of enteric viruses in children with sporadic gastroenteritis in Valencia, Venezuela. J Med Virol. 2011;83:1972–1982. doi: 10.1002/jmv.22185. [DOI] [PubMed] [Google Scholar]
- 34.Cerquetti M, Luzzi I, Caprioli A, Sebastianelli A, Mastrantonio P. Role of Clostridium difficile in childhood diarrhea. Pediatr Infect Dis J. 1995;14:598–603. doi: 10.1097/00006454-199507000-00009. [DOI] [PubMed] [Google Scholar]
- 35.Mårdh PA, Helin I, Colleen I, Oberg M, Holst E. Clostridium difficile toxin in faecal specimens of healthy children and children with diarrhoea. Acta Paediatr Scand. 1982;71:275–278. doi: 10.1111/j.1651-2227.1982.tb09414.x. [DOI] [PubMed] [Google Scholar]
- 36.de Graaf H, Pai S, Burns DA, Karas JA, Enoch DA, Faust SN. Co-infection as a confounder for the role of Clostridium difficile infection in children with diarrhoea: a summary of the literature. Eur J Clin Microbiol Infect Dis. 2015;34:1281–1287. doi: 10.1007/s10096-015-2367-0. [DOI] [PubMed] [Google Scholar]
- 37.Rexach CE, Tang-Feldman YJ, Cantrell MC, Cohen SH. Epidemiologic surveillance of Clostridium difficile diarrhea in a freestanding pediatric hospital and a pediatric hospital at a university medical center. Diagn Microbiol Infect Dis. 2006;56:109–114. doi: 10.1016/j.diagmicrobio.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 38.El Feghaly RE, Stauber JL, Tarr PI, Haslam DB. Viral co-infections are common and are associated with higher bacterial burden in children with Clostridium difficile infection. J Pediatr Gastroenterol Nutr. 2013;57:813–816. doi: 10.1097/MPG.0b013e3182a3202f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schutze GE, Willoughby RE, Committee on Infectious Diseases American Academy of Pediatrics. Clostridium difficile infection in infants and children. Pediatrics. 2013;131:196–200. doi: 10.1542/peds.2012-2992. [DOI] [PubMed] [Google Scholar]
- 40.Borali E, De Giacomo C. Clostridium difficile infection in children: a review. J Pediatr Gastroenterol Nutr. 2016;63:e130–40. doi: 10.1097/MPG.0000000000001264. [DOI] [PubMed] [Google Scholar]