Abstract
This study aimed to evaluate the frequency and clinical characteristics of hydroxychloroquine (HCQ) retinopathy in Korean patients with rheumatologic diseases. We retrospectively reviewed medical records of 310 patients taking HCQ. Ophthalmic examinations included spectral-domain optical coherence tomography (SD-OCT), automated visual field test, and fundus autofluorescence. The severity of retinopathy was categorized as early, moderate, or severe, and the location was categorized as parafoveal, pericentral, or mixed pattern. Among 310 patients, 9 patients (2.9%) were diagnosed as HCQ retinopathy. Among the patients with HCQ use ≥ 5 years (n = 174), the frequency was 5.2%. Only 1 (11.1%) of the 9 patients was symptomatic. The mean daily dose per kilogram of real body weight of the 9 patients was 5.6 mg, and only 3 had used 6.5 mg or more. Four of the 9 patients had severe HCQ retinopathy. Six of the 9 patients showed pericentral or mixed pattern of retinal damage. Consequently, the frequency of HCQ retinopathy in Korean patients was not low, especially when administered at a high cumulative dose and for a long duration. Screening of HCQ retinopathy by the recommended guidelines that include SD-OCT seems useful and should be done to detect retinal damage earlier in patients with chronic exposure to HCQ.
Keywords: Hydroxychloroquine, Hydroxychloroquine Retinopathy, Screening
Graphical Abstract
INTRODUCTION
Hydroxychloroquine (HCQ) is used for the treatment of systemic lupus erythematosus (SLE), rheumatoid arthritis (RA), and other connective tissue disorders (1,2). HCQ retinal toxicity is a well-known complication, characterized by parafoveal photoreceptor loss and retinal pigment epithelial damage. Although retinal toxicity is not common, irreversible retinal damage with visual impairment may occur and the damage can progress even after discontinuation of the drug (3,4,5). Thus, screening for early retinal toxicity is important.
The American Academy of Ophthalmology (AAO) recommendations for screening of HCQ retinopathy was published in 2002 and revised in 2011 and 2016, emphasizing objective measures, such as spectral-domain optical coherence tomography (SD-OCT), fundus autofluorescence (FAF), and multifocal electroretinography (mfERG) along with visual fields (6,7,8). With these objective examinations, retinal toxicity can be detected in its early stages (9,10). Recently, a large group studies have shown that the rate of HCQ retinopathy is much higher than previously recognized, and there are racial differences in terms of the patterns of retinal damages (11,12). However, there is only a few large clinical data for the frequency and patterns of HCQ retinopathy with these objective measures in Asia. In this study, we evaluated the frequency and clinical characteristics of retinal toxicity in Korean patients with rheumatologic diseases.
MATERIALS AND METHODS
Data and study subjects
The medical records of 310 consecutive patients who used HCQ for the treatment of rheumatologic disease and were referred to the ophthalmology department for screening of HCQ retinal toxicity between September 2011 and May 2014 were reviewed. Exclusion criteria included optic nerve diseases or anomalies, glaucoma, other retinal diseases, inflammatory eye diseases, and media opacity that interfered with a high-quality OCT examination.
Ocular examinations
All patients underwent screening examinations including slit-lamp biomicroscopy, dilated fundus examination, automated threshold perimetry (10-2 Humphrey Field Analyzer, Model 750I; Humphrey Instruments Inc., San Leandro, CA, USA), FAF, and SD-OCT (Spectralis® HRA+OCT; Heidelberg Engineering, Heidelberg, Germany) according to the revised recommendations of AAO guidelines for screening for HCQ retinal toxicity (7). SD-OCT was conducted with 6 mm radial scans of 30 degree intervals in addition to 6 mm of horizontal and vertical cross section. The images obtained from SD-OCT, FAF, and automated perimetry were assessed and if abnormalities such as parafoveal thinning, disruption of inner segment/outer segment, or loss of outer segment lines on OCT, reduced or increased autofluorescence on FAF, and decreased visual sensitivity or parafoveal scotoma on 10-2 visual field were present, the cases were determined to be HCQ retinopathy (13,14,15,16). The cases were classified into parafoveal, pericentral, or mixed pattern depending on the location of retinal damage according to a previous report (12). Also, the severity of toxicity was graded into mild, moderate, or severe according to the prior criteria (17). To evaluate the risk factors of HCQ retinopathy, data including age, sex, liver or renal diseases, body weight and height, daily HCQ dosage, and duration of HCQ intake were collected (14,15). Kidney dysfunction was defined as chronic renal disease ≥ stage 3 or mean glomerular filtration rate < 60 mL/min/1.73 m² a period of > 3 months (11,18). Liver dysfunction was defined as the mean liver enzyme levels (aspartate aminotransferase and alanine aminotransferase) being more than twice the normal upper limit (11).
Statistical analysis
Mann-Whitney U test was used to compare the 2 patient groups using PASW Statistics 21 (SPSS Inc., Chicago, IL, USA) and the significance level was accepted at P value < 0.05.
Ethics statement
This study followed the Declaration of Helsinki and was approved by the Institutional Review Board of the Samsung Medical Center (IRB No. 2014-06-064-001). Informed consent was waived by the board.
RESULTS
The demographics and clinical characteristics are shown in Table 1. Among 310 patients, 9 patients (2.9%) showed HCQ retinopathy. Among the patients with HCQ use ≥ 5 years, the frequency was 5.2% (9 of 174 patients). The mean duration of HCQ use in patients with retinal toxicity was 108.9 ± 31.9 months and mean total intake of HCQ was 952.0 ± 519.2 g. Mean daily dose per kilogram of real body weight of these 9 patients was 5.6 ± 2.6 mg and 3 of the 9 patients had used ≥ 6.5 mg/kg of their real body weight of a day. Total duration of HCQ intake and cumulative dose were significantly different (P = 0.005 and 0.008, respectively) between the 2 patient groups. In a univariate analysis, cumulative dose ≥ 600 g was a significant risk factor for HCQ retinopathy (odds ratio [OR], 9.3; 95% confidence interval [CI], 1.152 to 75.453; P = 0.036). Also, total duration of HCQ intake ≥ 72 months was a significant risk factor (OR, 21.3; 95% CI, 1.227 to 368.694; P = 0.036).
Table 1. Demographics and clinical characteristics of the included patients with chronic exposure to HCQ.
| Parameters | All patients | HCQ retinopathy | No HCQ retinopathy | P value* |
|---|---|---|---|---|
| No. of subjects | 310 | 9 | 301 | NA |
| No. of patients whose duration ≥ 5 yr | 175 (31.3) | 9 (100.0) | 166 (55.1) | NA |
| Age, yr | 44.5 ± 12.4 | 48.7 ± 15.3 | 44.4 ± 12.4 | 0.308 |
| Female | 286 (92.3) | 8 (88.9) | 278 (92.4) | 0.701 |
| Body weight, kg | 56.2 ± 8.5 | 53.0 ± 7.8 | 56.3 ± 8.5 | 0.251 |
| BMI, kg/m2 | 22.3 ± 3.1 | 21.6 ± 3.2 | 22.3 ± 3.1 | 0.527 |
| Duration of use, mon | 71.7 ± 40.4 (range, 2–174) | 108.9 ± 31.9 (range, 76–174) | 70.5 ± 40.1 (range, 2–160) | 0.005 |
| Cumulative dose, g | 625.5 ± 376.2 (range, 24–2,088) | 952.0 ± 519.2 (range, 558–2,088) | 615.1 ± 368.1 (range, 24–1,680) | 0.008 |
| Daily dose, mg | 302.9 ± 83.3 | 284.1 ± 96.3 | 303.3 ± 83.1 | 0.495 |
| Daily dose per real body weight, mg/kg | 5.5 ± 1.8 | 5.6 ± 2.6 | 5.5 ± 1.7 | 0.84 |
| No. of patients whose cumulative dose ≥ 1,000 g | 44 (14.2) | 2 (22.2) | 42 (14.0) | 0.485 |
| No. of patients whose daily dose ≥ 6.5 mg/kg real body weight | 103 (33.2) | 3 (33.3) | 100 (33.2) | 0.841 |
| Diagnosis of patients† | ||||
| SLE | 224 (72.3) | 5 (55.6) | 219 (72.6) | NA |
| Sjögren syndrome | 45 (14.5) | 1 (11.1) | 44 (14.6) | NA |
| RA | 32 (10.3) | 3 (33.3) | 29 (9.6) | NA |
| APLS | 10 (3.2) | 1 (11.1) | 9 (3.0) | NA |
| Others | 13 (4.2) | 0 (0) | 13 (4.3)‡ | NA |
| Systemic disease | ||||
| Kidney dysfunction | 10 (3.2) | 0 (0) | 10 (3.3) | NA |
| Liver dysfunction | 0 (0) | 0 (0) | 0 (0) | NA |
Data are shown as mean ± SD or number (%).
HCQ = hydroxychloroquine, BMI = body mass index, SLE = systemic lupus erythematosus, RA = rheumatoid arthritis, APLS = antiphospholipid antibody syndrome, NA = not applicable, SD = standard deviation.
*Mann-Whitney U test, which compared the 2 patient groups, “HCQ retinopathy” and “No HCQ retinopathy” group. †Fourteen patients were diagnosed with more than 1 disease. ‡Twelve patients were diagnosed with mixed connective tissue disease and one of them had vasculitis.
Table 2 summarizes the clinical findings in patients with HCQ retinopathy. According to the AAO recommended objective examinations, we found abnormalities based on SD-OCT in 9 cases, which included retinal inner segment/outer segment defect or retinal pigmented epithelial (RPE) damage. Of those, 6 patients also had evidence of HCQ retinopathy based on FAF, and 7 based on the automated visual field test. All of these abnormalities were shown in both eyes. Only 1 out of the 9 patients was symptomatic and her best corrected visual acuity was 20/200 in the right eye and 20/50 in the left eye. The cumulative HCQ dose in the 7 patients was ≥ 500 g per patient, and 2 with cumulative doses ≥ 1,000 g. Only 3 patients (33.3%) had used 6.5 mg/kg or more per kilogram of real body weight daily. Three patients with HCQ retinopathy showed a parafoveal pattern of retinal damage that was in the ring 2° to 6° from the center of the fovea, 4 patients had mixed pattern, and 2 patients showed a pericentral pattern with retinal damage that was ≥ 8° from the center of the fovea (12).
Table 2. Summary of clinical findings in 9 patients with HCQ retinopathy.
| No. | Sex/Age, yr | Diagnosis | CDVA (RE, LE) | Duration of use, mon | Total HCQ dose, g | Daily dose/RBW, mg/kg | SD-OCT | FAF | HVF | Pattern | Severity |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F/48 | SLE, APLS | 20/20, 18/20 | 120 | 1,440 | 7.74 | Outer retinal thinning | NS | NS | Pericentral | Mild |
| 2 | F/33 | SLE | 20/20, 20/20 | 174 | 2,088 | 10.05 | Outer retinal thinning, CME | Abnormal | Field loss | Mixed | Severe |
| 3 | M/68 | RA | 20/25, 20/25 | 119 | 636 | 2.69 | Outer retinal thinning | Abnormal | Field loss | Mixed | Mod |
| 4 | F/74 | RA | 20/200, 20/50 | 76 | 912 | 8.33 | Outer retinal thinning | Abnormal | Field loss | Parafoveal | Severe |
| 5 | F/44 | Sjögren | 20/20, 20/20 | 93 | 558 | 3.70 | Outer retinal thinning | NS | Field loss | Parafoveal | Mild |
| 6 | F/60 | RA | 18/20, 18/20 | 116 | 768 | 4.38 | Outer retinal thinning | Abnormal | Field loss | Mixed | Severe |
| 7 | F/31 | SLE | 20/20, 20/20 | 119 | 786 | 3.52 | Outer retinal thinning | Abnormal | Field loss | Pericentral | Severe |
| 8 | F/39 | SLE | 20/20, 20/20 | 87 | 612 | 4.11 | Outer retinal thinning | NS | NS | Parafoveal | Mild |
| 9 | F/67 | SLE | 20/20, 18/20 | 125 | 888 | 4.08 | Outer retinal thinning | Abnormal | Field loss | Mixed | Mod |
CDVA = corrected distance visual acuity, RE = right eye, LE = left eye, RBW = real body weight, SD-OCT = spectral-domain optical coherence tomography, FAF = fundus autofluorescence, HVF = Humphrey visual field test, SLE = systemic lupus erythematosus, APLS = antiphospholipid antibody syndrome, RA = rheumatoid arthritis, IS/OS = inner segment/outer segment, CME = cystoid macular edema, NS = not significant, Mod = moderate.
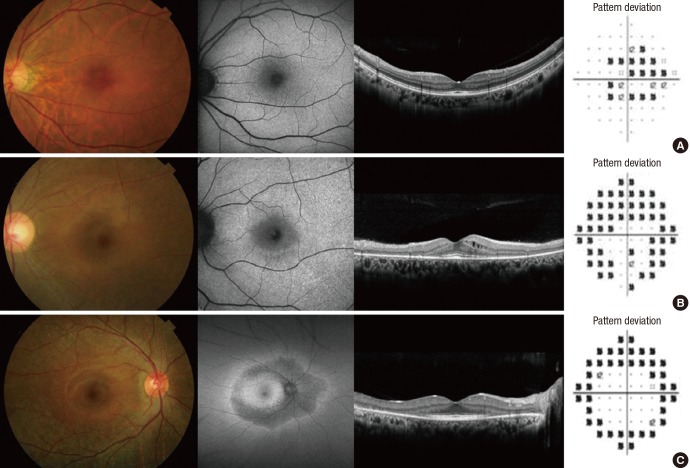
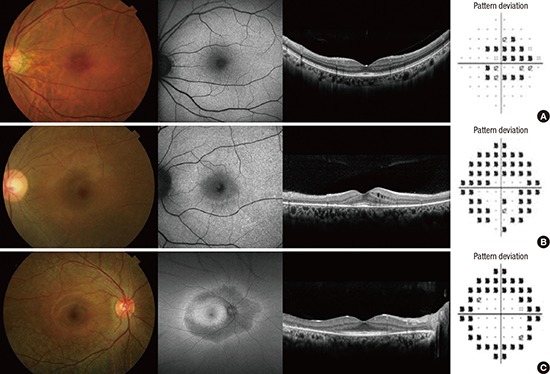
Table 3 summarizes the severity and pattern of HCQ retinal damage. The proportion of mixed and pericentral pattern was 66.7%. Four of 9 patients showed severe severity of retinal damage and 3 of them showed mixed and pericentral pattern. The representative patterns of retinal damage are shown in Fig. 1.
Table 3. Severity and pattern of HCQ retinopathy (n = 9).
| Parameters | Pattern | |||
|---|---|---|---|---|
| Parafoveal | Pericentral | Mixed | Total | |
| No. of patients | 3 | 2 | 4 | 9 |
| Severity | ||||
| Mild | 2 | 1 | 0 | 3 |
| Moderate | 0 | 0 | 2 | 2 |
| Severe | 1 | 1 | 2 | 4 |
| HCQ use | ||||
| Duration, mon | 85.3 | 119.5 | 133.5 | 108.9 |
| Cumulative dose, g | 694.0 | 1,113.0 | 1,095.0 | 952.0 |
HCQ = hydroxychloroquine.
Fig. 1.
Photographs showing pattern of HCQ retinopathy. (A) Parafoveal. (B) Mixed. (C) Pericentral pattern.
HCQ = hydroxychloroquine.
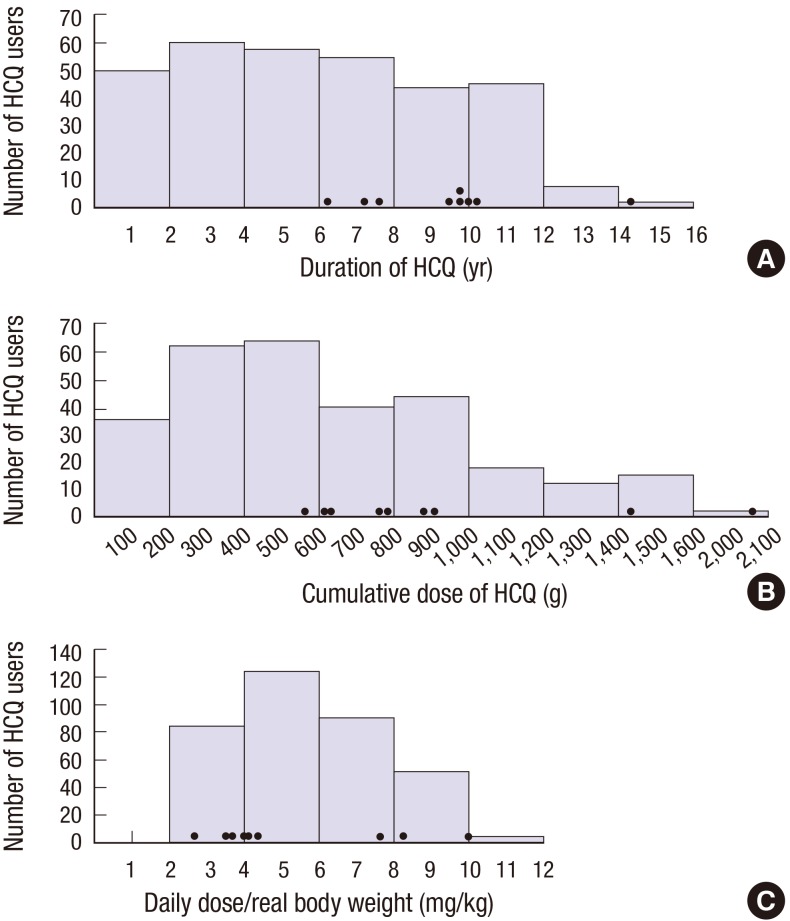
The distribution of HCQ retinopathy based on the duration of HCQ use is shown in Fig. 2. There was no retinal toxicity in 136 patients who took HCQ less than 5 years. HCQ retinopathy occurred in 9 of 174 (5.2%) patients who took HCQ for at least 5 years, and in 3 of 53 (5.7%) patients who took it ≥ 10 years. Also, the distribution of HCQ retinopathy according to the dose of HCQ is shown in Fig. 2. There was no HCQ retinal toxicity in patients who took under 500 g of the cumulative dose. Among the 179 patients whose total HCQ intake was ≥ 500 g, 9 patients (5.0%) showed HCQ retinopathy. Also, 2 (4.5%) out of 44 patients whose cumulative dose ≥ 1,000 g showed retinal toxicity.
Fig. 2.
Graphs showing distribution of patients with HCQ retinopathy according to the duration of HCQ (A), cumulative dose of HCQ intake (B), and daily dose/real body weight (C). Black dots indicate each value of patients with HCQ retinopathy.
HCQ = hydroxychloroquine.
DISCUSSION
In this study, we evaluated the frequency and clinical characteristics of retinal toxicity in Korean patients with chronic exposure to HCQ for the treatment of rheumatologic diseases. According to the revised AAO recommendations on screening for HCQ retinopathy in 2011, we obtained data from the visual field test, SD-OCT, and FAF as well as fundus examination in 310 consecutive patients. The frequency of HCQ retinopathy in Korean patients is comparable to that of European populations (11). However, 6 of the 9 patients showed pericentral or mixed patterns of retinal damages, which is a different finding compared to Caucasian patients (12).
The first case of chloroquine retinopathy was reported by Cambiaggi (19) in 1957, and there were many subsequent studies that demonstrated retinal toxicity from chloroquine. However, after the treatment for malaria and rheumatologic disease was changed from chloroquine to HCQ, chloroquine/HCQ retinopathy decreased (20). Many previous studies have shown that retinal toxicity from HCQ is uncommon (21,22,23,24,25). However, in rheumatologic diseases such as SLE, RA or Sjögren's syndrome, HCQ has been used for a long time, and once retinopathy occurs, damage to the retina may continue even after discontinuation of the drug because it takes many months for HCQ to be eliminated from the body (26). Also, the structural and functional changes can be irreversible, so that the screening and early detection of HCQ retinopathy is important.
An earlier study reported the incidence of HCQ retinopathy in 1,207 patients, and they found just 6 patients whose daily dosage of HCQ was > 6.5 mg/kg or took it for over 10 years (4). The AAO recommendations of screening for HCQ retinopathy in 2002 included criteria for early detection of HCQ retinopathy (6). Of those criteria, daily dosage > 6.5 mg/kg of HCQ and a duration of use > 5 years put patients at higher risk for retinopathy. Baseline studies of a complete ophthalmologic examination, Amsler grid or Humphrey 10-2 field testing with optional tests such as color testing, fundus photography, fluorescein angiography, or multifocal ERG were recommended within first year of starting HCQ. Also, annual these examinations with periodically Humphrey 10-2 testing or fundus photography were recommended for higher risk patients. However, Wolfe and Marmor (3) reported that the rate of HCQ retinal toxicity was 6.5 cases per 1,000 HCQ users, but the risk increased markedly after 5–7 years and exceeded 1%. Although advanced retinal toxicity from HCQ such as bull's eye maculopathy is easily determined on a fundus examination, paracentral visual field defect in automated threshold perimetry or localized depression in mfERG may be present before fundus photography (27). Also, SD-OCT can demonstrate early objective signs of parafoveal damage. For these reasons, the AAO revised recommendations on screening for HCQ retinopathy. According to the revised recommendations, SD-OCT, FAF, and mfERG can be more sensitive in detecting HCQ retinopathy than visual fields. With these examinations, we can aware of retinal toxicity before a bull's eye maculopathy is seen on a fundus examination. For these revised recommendations, many retinal specialists have conducted these objective tests, and recent studies looking at the frequency of HCQ retinopathy found them to be higher than previously reported. Melles and Marmor (11) reported the risk of toxic retinopathy from HCQ therapy in the patients who took the medication more than 5 years and found that the overall frequency was 7.5%. In this study, most patients showed retinal toxicity before a bull's eye maculopathy was detected. Also, the risk markedly increases with the HCQ intake > 5.0 mg/kg of real body weight and with duration > 20 years. Our study also showed that HCQ retinopathy was more common than previously recognized. However, real body weight was not associated with the HCQ retinopathy in this study.
Before Melles and Marmor (12) investigated the pattern of HCQ retinopathy and racial differences, most studies reported retinal toxicity of HCQ occurred in parafoveal region. However, they showed a new pattern of retinal damage in pericentral region, and this pericentral retinal damage was more common in Asian patients. In that study, pericentral damage alone was seen in 50% of Asian patients with HCQ retinopathy, but in only 2% of Caucasian patients. Mixed and pericentral pattern was detected in 83% of Asians and 9% of Caucasians. According to the study of Lee et al. (18) on HCQ retinopathy in Korean patients, 8 (89.0%) of 9 patients with HCQ retinopathy showed pericetnral pattern. However, in our study, 6 of 9 (66.7%) patients were determined as pericentral (n = 2) or mixed pericentral and parafoveal pattern (n = 4). Although the proportion of pericetnral pattern was lower than that seen in previous study, our study also suggests there are racial differences in HCQ retinopathy. Therefore, to detect HCQ retinopathy earlier in Asian patients, more extensive examinations such as the 24- or 30-degree visual field test or SD-OCT scan with coverage of pericentral area may be necessary because the current screening examinations of retinal damage were focused on central fovea. In particular, we could detect these all pericentral types of HCQ retinopathy by 6 mm radial scans of SD-OCT in our study, even though only 10-degree visual field tests were performed.
This study has several limitations, including its retrospective nature. Although this study investigated screening results in consecutive patients who were referred to Department of Ophthalmology, not all the patients taking HCQ in our hospital had screening examinations. Thus, there might be a selection bias. In addition, because only 9 of 310 patients showed HCQ retinopathy, there is a limitation in analyzing risk factors for HCQ retinopathy. Further larger studies are needed to evaluate the risk factors for HCQ retinopathy and other prognostic factors.
In conclusion, this study showed that the frequency of HCQ retinal toxicity evaluated by the revised AAO recommendations was higher than in previous reports. Screening of HCQ retinopathy by the revised guidelines that include SD-OCT seems useful and should be done to detect retinal damage earlier in patients with chronic exposure to HCQ. In addition, pericentral or mixed pattern of retinal toxicity was common in Korean patients, which re-emphasizes the need for different screening algorithm that can cover the wider area of central retina in Korean patients.
Footnotes
Funding: This work was supported by a grant of the Korean Health Technology R & D Project, Ministry of Health & Welfare, Republic of Korea (HI13C1826).
DISCLOSURE: The authors have no potential conflicts of interest to disclose.
AUTHOR CONTRIBUTION: Conceptualization: Eo DR, Kim SJ. Data curation: Eo DR, Lee MG, Ham DI, Kang SW, Lee J, Cha HS, Koh E, Kim SJ. Formal analysis: Eo DR. Funding acquisition: Kim SJ. Investigation: Eo DR, Kim SJ. Writing - original draft: Eo DR, Kim SJ.
References
- 1.Dörner T. Therapy: hydroxychloroquine in SLE: old drug, new perspectives. Nat Rev Rheumatol. 2010;6:10–11. doi: 10.1038/nrrheum.2009.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cansu DU, Korkmaz C. Hypoglycaemia induced by hydroxychloroquine in a non-diabetic patient treated for RA. Rheumatology (Oxford) 2008;47:378–379. doi: 10.1093/rheumatology/kem378. [DOI] [PubMed] [Google Scholar]
- 3.Wolfe F, Marmor MF. Rates and predictors of hydroxychloroquine retinal toxicity in patients with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2010;62:775–784. doi: 10.1002/acr.20133. [DOI] [PubMed] [Google Scholar]
- 4.Levy GD, Munz SJ, Paschal J, Cohen HB, Pince KJ, Peterson T. Incidence of hydroxychloroquine retinopathy in 1,207 patients in a large multicenter outpatient practice. Arthritis Rheum. 1997;40:1482–1486. doi: 10.1002/art.1780400817. [DOI] [PubMed] [Google Scholar]
- 5.Mavrikakis I, Sfikakis PP, Mavrikakis E, Rougas K, Nikolaou A, Kostopoulos C, Mavrikakis M. The incidence of irreversible retinal toxicity in patients treated with hydroxychloroquine: a reappraisal. Ophthalmology. 2003;110:1321–1326. doi: 10.1016/S0161-6420(03)00409-3. [DOI] [PubMed] [Google Scholar]
- 6.Marmor MF, Carr RE, Easterbrook M, Farjo AA, Mieler WF, American Academy of Ophthalmology Recommendations on screening for chloroquine and hydroxychloroquine retinopathy: a report by the American Academy of Ophthalmology. Ophthalmology. 2002;109:1377–1382. doi: 10.1016/s0161-6420(02)01168-5. [DOI] [PubMed] [Google Scholar]
- 7.Marmor MF, Kellner U, Lai TY, Lyons JS, Mieler WF, American Academy of Ophthalmology Revised recommendations on screening for chloroquine and hydroxychloroquine retinopathy. Ophthalmology. 2011;118:415–422. doi: 10.1016/j.ophtha.2010.11.017. [DOI] [PubMed] [Google Scholar]
- 8.Marmor MF, Kellner U, Lai TY, Melles RB, Mieler WF, American Academy of Ophthalmology Recommendations on screening for chloroquine and hydroxychloroquine retinopathy (2016 revision) Ophthalmology. 2016;123:1386–1394. doi: 10.1016/j.ophtha.2016.01.058. [DOI] [PubMed] [Google Scholar]
- 9.Browning DJ, Lee C. Relative sensitivity and specificity of 10-2 visual fields, multifocal electroretinography, and spectral domain optical coherence tomography in detecting hydroxychloroquine and chloroquine retinopathy. Clin Ophthalmol. 2014;8:1389–1399. doi: 10.2147/OPTH.S66527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Asensio-Sánchez VM. SD-OCT As screening test for hydroxychloroquine retinopathy: the «flying saucer» sign. Arch Soc Esp Oftalmol. 2015;90:338–340. doi: 10.1016/j.oftal.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Melles RB, Marmor MF. The risk of toxic retinopathy in patients on long-term hydroxychloroquine therapy. JAMA Ophthalmol. 2014;132:1453–1460. doi: 10.1001/jamaophthalmol.2014.3459. [DOI] [PubMed] [Google Scholar]
- 12.Melles RB, Marmor MF. Pericentral retinopathy and racial differences in hydroxychloroquine toxicity. Ophthalmology. 2015;122:110–116. doi: 10.1016/j.ophtha.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 13.Elder M, Rahman AM, McLay J. Early paracentral visual field loss in patients taking hydroxychloroquine. Arch Ophthalmol. 2006;124:1729–1733. doi: 10.1001/archopht.124.12.1729. [DOI] [PubMed] [Google Scholar]
- 14.Michaelides M, Stover NB, Francis PJ, Weleber RG. Retinal toxicity associated with hydroxychloroquine and chloroquine: risk factors, screening, and progression despite cessation of therapy. Arch Ophthalmol. 2011;129:30–39. doi: 10.1001/archophthalmol.2010.321. [DOI] [PubMed] [Google Scholar]
- 15.Bergholz R, Schroeter J, Rüther K. Evaluation of risk factors for retinal damage due to chloroquine and hydroxychloroquine. Br J Ophthalmol. 2010;94:1637–1642. doi: 10.1136/bjo.2009.174458. [DOI] [PubMed] [Google Scholar]
- 16.Lai TY, Ngai JW, Chan WM, Lam DS. Visual field and multifocal electroretinography and their correlations in patients on hydroxychloroquine therapy. Doc Ophthalmol. 2006;112:177–187. doi: 10.1007/s10633-006-9006-0. [DOI] [PubMed] [Google Scholar]
- 17.Marmor MF. Comparison of screening procedures in hydroxychloroquine toxicity. Arch Ophthalmol. 2012;130:461–469. doi: 10.1001/archophthalmol.2011.371. [DOI] [PubMed] [Google Scholar]
- 18.Lee DH, Melles RB, Joe SG, Lee JY, Kim JG, Lee CK, Yoo B, Koo BS, Kim JT, Marmor MF, et al. Pericentral hydroxychloroquine retinopathy in Korean patients. Ophthalmology. 2015;122:1252–1256. doi: 10.1016/j.ophtha.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 19.Cambiaggi A. Unusual ocular lesions in a case of systemic lupus erythematosus. AMA Arch Opthalmol. 1957;57:451–453. doi: 10.1001/archopht.1957.00930050463019. [DOI] [PubMed] [Google Scholar]
- 20.Finbloom DS, Silver K, Newsome DA, Gunkel R. Comparison of hydroxychloroquine and chloroquine use and the development of retinal toxicity. J Rheumatol. 1985;12:692–694. [PubMed] [Google Scholar]
- 21.Yam JC, Kwok AK. Ocular toxicity of hydroxychloroquine. Hong Kong Med J. 2006;12:294–304. [PubMed] [Google Scholar]
- 22.Silman A, Shipley M. Ophthalmological monitoring for hydroxychloroquine toxicity: a scientific review of available data. Br J Rheumatol. 1997;36:599–601. doi: 10.1093/rheumatology/36.5.599. [DOI] [PubMed] [Google Scholar]
- 23.Tehrani R, Ostrowski RA, Hariman R, Jay WM. Ocular toxicity of hydroxychloroquine. Semin Ophthalmol. 2008;23:201–209. doi: 10.1080/08820530802049962. [DOI] [PubMed] [Google Scholar]
- 24.Hickley NM, Al-Maskari A, McKibbin M. Chloroquine and hydroxychloroquine toxicity. Arch Ophthalmol. 2011;129:1506–1507. doi: 10.1001/archophthalmol.2011.321. [DOI] [PubMed] [Google Scholar]
- 25.Mills PV, Beck M, Power BJ. Assessment of the retinal toxicity of hydroxychloroquine. Trans Ophthalmol Soc U K. 1981;101:109–113. [PubMed] [Google Scholar]
- 26.Farrell DF. Retinal toxicity to antimalarial drugs: chloroquine and hydroxychloroquine: a neurophysiologic study. Clin Ophthalmol. 2012;6:377–383. doi: 10.2147/OPTH.S27731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moschos MN, Moschos MM, Apostolopoulos M, Mallias JA, Bouros C, Theodossiadis GP. Assessing hydroxychloroquine toxicity by the multifocal ERG. Doc Ophthalmol. 2004;108:47–53. doi: 10.1023/b:doop.0000018385.99215.0d. [DOI] [PubMed] [Google Scholar]