Abstract
Inflammatory bowel disease causes chronic, relapsing intestinal inflammation that can lead to the development of colorectal cancer. Members of the TNF superfamily are key regulators of intestinal inflammation. In particular, TNF-like weak inducer of apoptosis (TWEAK) and its receptor, Fn14, are involved in normal and pathologic intestinal tissue remodeling. In this study, we show that the TWEAK/Fn14 signaling complex plays a protective role during the acute stage of intestinal inflammation and contributes to the prevention of colitis-associated cancer during chronic inflammation through its pro-apoptotic effects. Colitis was in Fn14-/- and Fn14+/+ wild type littermates by administering 3% dextran sodium sulfate (DSS) for 7 days followed by 2 weeks recovery; azoxymethane (AOM) administration followed by 2 cycles of DSS/recovery was used to induce tumors. Reciprocal bone marrow chimeric mice were generated to compare hematopoietic and non-hematopoietic-specific effector tissues. Fn14-/- mice had enhanced susceptibility to colitis compared to Fn14+/+ controls as assessed by endoscopic and histological inflammatory scores, daily weight loss, and mortality rates during recovery after DSS administration. Bone marrow transfer experiments showed that both hematopoietic and non-hematopoietic components are involved in protection against colitis. Tumor lesions were found in the colons of most Fn14-/- mice, but not Fn14+/+ controls. AOM/DSS administration enhanced susceptibility to tumorigenesis in Fn14-/- mice. Overall, these findings show that Fn14 plays a protective role during the acute stages of intestinal inflammation, and its absence promotes the development of colitis-associated cancer.
Keywords: Fn14, TWEAK, colitis, cancer, apoptosis
Introduction
Inflammatory bowel disease (IBD) is a chronic, relapsing inflammatory disease of the gastrointestinal tract, wherein inflammation causes significant tissue remodeling and deformity of the intestine. IBD patients with colonic involvement harbor a risk of developing colorectal cancer that is directly proportional to the extent and duration of their disease, since chronic inflammation can lead to tumor initiation and promotion (1, 2). Recently, a number of TNF superfamily members have been considered as potential targets for colorectal cancer therapy, based on their role in mediating apoptotic activity, including TNF-related apoptosis-inducing ligand (TRAIL) (3, 4), TNFα, Fas ligand (FasL) (5, 6) and the TNF-like weak inducer of apoptosis (TWEAK)/fibroblast growth factor (FGF)-inducible molecule 14 (Fn14) ligand/receptor complex (7, 8). In particular, TWEAK is involved in many critical biological processes, including embryo development, organogenesis, tissue repair, and innate and adaptive immune responses (9). It is constitutively expressed in many immune cell types, such as dendritic cells (DC), neutrophils, monocytes/macrophages, and T cells, as well as non-hematopoietic cell types, including epithelial and renal cells (10). TWEAK is believed to exert its effects by binding to its specific receptor, Fn14, which is a highly inducible molecule on epithelial, mesenchymal, and endothelial cells during inflammation. Fn14, the smallest member of the TNF receptor (TNFR) superfamily, is initially synthesized as a 129-amino acid type I transmembrane protein that is then cleaved by signal peptidase into a 102-amino acid cell surface receptor. The binding between TWEAK and Fn14 leads to the activation of the NF-κB signaling cascade, with subsequent downstream production of cytokines, chemokines, adhesion molecules and metalloproteinases (11). Generally, Fn14 is expressed at low levels in normal tissues, but is highly upregulated during tissue injury and regeneration, as well as in tumors, including breast and pancreatic cancer, and glioma (12, 13). Finally, TWEAK-dependent Fn14 signaling is involved in various stages of inflammation and wound repair, including blood clotting, angiogenesis, cell proliferation, and migration (14).
Based on its biological effects, we hypothesized that similar to other members of the TNF superfamily, the TWEAK/Fn14 signaling complex may play dichotomous protective and pathogenic roles within the context of different stages of acute and chronic intestinal inflammation. During early, acute inflammation, its activation may beneficially induce stromal cell activation and fibroblast proliferation, as well as regulation of collagen expression and myofibroblast differentiation, resulting in healing of tissue damage. However, during later, chronic inflammation, increased and persistent levels of Fn14 may result in continuous activation of Fn14-coupled intracellular signaling cascades, leading to pathologic inflammation and fibrosis. We therefore studied the effect of TWEAK/Fn14 during acute colonic inflammation by inducing dextran sodium sulfate (DSS) colitis in Fn14-/- and Fn14+/+ wild type (WT) littermates. We found that Fn14-/- mice were highly susceptible to colitis compared to Fn14+/+ control mice. Fn14-/- mice also showed a significantly higher susceptibility to developing cancer in an inflammation-driven model of colorectal cancer, suggesting a role for TWEAK/Fn14 signaling in the maintenance of gut homeostasis and protection against intestinal inflammation and tumorigenesis.
Materials and Methods
Experimental Animals
Fn14-/- and TWEAK-/- mice on a C57BL/6 background (species: Mus musculus) were generously provided by Linda Burkly (Biogen Idec, Cambridge, MA); Fn14+/+ and TWEAK+/+ WT littermates were used as controls. Mice were propagated in the Animal Resource Center at Case Western Reserve University (CWRU). All mice used in this study were between 15 and 18 weeks of age and were age-matched among experimental groups. An equal number of male and female mice were used, with a mean body weight of 24.1 g. Mice were housed and maintained in ventilated micro-isolator cages (Allentown Inc, Allentown, NJ) with 1/8 inch corn bedding and cotton nestlets for environmental enrichment (Envigo, Indianapolis, IN), and kept on 12 hr light/dark cycles. All mice had ad libitum access to water and standard laboratory rodent diet P3000 (Harlan Teklad, Indianapolis, IN) throughout the experiments. Harem breeding was set up by co-housing one 8-wk-old male with two 8-wk-old females. All procedures were approved by the CWRU Institutional Animal Care and Use Committee (IACUC) and were in accordance with the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC) guidelines. All experiments were conducted in a blinded manner, without prior knowledge of treatments and mouse groups by the experimenter. Mice were randomized to different interventions using a progressive numerical number. The code for each mouse was known only to the animal caretaker and was revealed at the end of the study.
Endoscopy
Colonoscopy was performed using a flexible digital ureteroscope (URF-V, Olympus America, Center Valley, PA) with an 8.5Fr (2.8 mm) tapered-tip design and motion range of 180° in up-angle and 275° in down-angle. The endoscope system included a video system center (Olympus America), a xenon light source (Olympus America), and a video recorder (MediCapture, Philadelphia, PA). Endoscopy images were obtained on an Olympus BX41 microscope (magnification, 100× and 200×; objective, 10×; eyepiece, 10×). Colonoscopy was performed on day 7 and day 21 after DSS administration, and inflammation was evaluated using a previously validated endoscopic scoring system (15). Briefly, the system considers four different parameters to evaluate colonic inflammation: perianal findings (diarrhea, bloody feces or rectal prolapse); wall transparency (based on the ability to see the blood vessels of the colonic mucosa); intestinal bleeding (spontaneous or induced by the endoscope because of the mucosal friability), and focal lesions (edema, erosions and ulcers). Sub-scores for each parameter ranging from 0 (normal colonoscopy) to 3 (maximum severity of colonic changes) were used to evaluate colonic inflammation and to assess the potential presence of tumor lesions. The sum of these sub-scores was used to define colonic health as follows: healthy (0-1), mild colitis (2-4), moderate colitis (5-7), severe colitis (8-13). Mice were anesthetized by isoflurane, USP (Butler Schein Animal Health, Dublin, OH) prior to performing endoscopy; no colonoscopy preparations, such as fasting or laxatives, were required.
Histology
Colons from experimental mice were removed, flushed of fecal contents, opened longitudinally, and placed in Bouin's fixative. Tissues were embedded in paraffin and stained with hematoxylin and eosin. Inflammation was evaluated by a trained pathologist in a blinded fashion using an established histological scoring system (16). Briefly, scores ranging from 0 (normal histology) to 3 (maximum severity of histologic changes) were used to evaluate four individual histologic sub-indices for (1) active inflammation (infiltration with neutrophils), (2) chronic inflammation (lymphocytes, plasma cells, and macrophages in the mucosa and submucosa), (3) percent re-epithelialization, and (4) percent ulceration.
Induction of Colitis
Induction of acute colitis was achieved by exposing mice to sterilized 3% (w/v) DSS (TdB Consultancy AB, Uppsala, Sweden; Batch no. DB001-31) in drinking water for 7 days. Our protocol for induction of DSS colitis includes renewal of the DSS solution every 3 days. Fn14-/- or TWEAK-/- mice, as well as their WT littermates were allowed ad libitum access to water. Mice returned to normal drinking water after 7 days of DSS and were allowed to recover for 2 weeks. Daily monitoring was performed for body weight, fecal bleeding and the presence of loose stools. In a separate protocol used to test cell proliferation activity or to induce inflammation-driven colorectal tumors, mice were administered two separate 7-day cycles of 2.5% DSS in drinking water, each of which were followed by a 14-day recovery period (42 days total). In order to control for cage-to-cage variability that could be attributable to gut microbiota changes, fresh fecal samples from the experimental cages were mixed and equally distributed back to the original cages, 7 days before the induction of DSS colitis.
Cells Isolation and Cytokine ELISA
Mesenteric lymph nodes (MLNs) from mice with DSS-induced colitis were removed aseptically at the time of sacrifice and cells were gently dispersed through a 70-μm cell strainer to obtain single-cell suspensions. 1 × 106 resulting cells were cultured in RPMI 1640 with 10% FBS and 1% penicillin/streptomycin for 72 hrs in the presence of αCD3/αCD28 monoclonal antibodies (1μg/mL) as previously described (17). After the incubation period, supernatants were collected for TNFα and IFNγ analysis by ELISA (eBioscience, San Diego, CA). All ELISAs were performed according to manufacturer's instructions.
Bone Marrow Transfer Experiments
Prior to transplant, WT recipient mice were irradiated twice (600 rad), with a 6-hr interval between the first and the second dose. Bone marrow (BM) was then harvested from femurs and tibias of 12-wk-old Fn14-/- or Fn14+/+ mice in RPMI medium (10% FCS), and cell suspensions were washed and diluted to a concentration of 30 × 106 cells/mL in HBSS. Immediately following the second dose of 600 rad, a total of 7.5 × 106 cells/250 μL were then injected i.v. into the lateral tail veins of recipient Fn14-/- or Fn14+/+ mice, resulting in four different groups of chimeric mice: 1) Fn14-/- BM→ Fn14-/- mice, 2) Fn14+/+ BM→ Fn14-/- mice, 3) Fn14-/- BM→ Fn14+/+ mice, 4) Fn14+/+ BM→ Fn14+/+ mice. BM chimera recipient mice were placed on antibiotic water (0.7 mM neomycin sulfate, 80 mM sulfamethoxazole, and 0.37 mM trimethoprim) for 2 wks after irradiation, and then given autoclaved water for 6 wks to reconstitute normal gut microbiota. After the recovery period, mice were treated with 3% (w/v) DSS in their drinking water for 7 days and then sacrificed.
BrdU Assay
In order to test if cell proliferation activity was higher in the Fn14-/- vs. Fn14+/+ mice, on day 42 at the end of two consecutive 21-day cycles (each 7 days of 2.5% DSS in their drinking water, followed by 14 days of recovery), mice were administered 5-bromo-2′-deoxyuridine (BrdU) (Life Technologies, Carlsbad, CA; Cat. 00-0103) by intraperitoneal injection (200 μL in PBS solution), using a dose of 100 mg/kg of body weight and sacrificed 2 hr after injection. Actively replicating cells were detected by immunohistochemistry for BrdU using the antibody kit by Invitrogen (Carlsbad, CA, Ref 933943). The biotinylated mouse anti-BrdU antibody was incubated for 1 hr at a ready-to-use concentration already provided by the kit.
AOM-DSS Induction of Colorectal Cancer
Mice were administered a single intraperitoneal injection of azoxymethane (AOM, Sigma-Aldrich, St. Louis, MO; Cat. A5486-25MG) (7.4 mg/kg body weight), and were allowed to recover for two weeks. Two separate cycles of 2.5% DSS (TdB Consultancy AB, Uppsala, Sweden; Batch no. DB001-31) were then administered for 7 days, each followed by a 14-day recovery period. Mice were sacrificed at day 56 of treatment.
Stereomicroscopy
Three-dimensional stereomicroscopy assessment and pattern profiling serve to map and quantify the intestinal health in mice and to determine the extent of mucosal involvement that occurs with acute and chronic inflammation (18). To analyze the variable patterns of stereomicroscopic abnormalities, stereomicroscopy photographs were analyzed using ImageJ public domain software (imagej.nih.gov/ij/). We measured single tumoral lesions and plotted the areas for visualization and circular dimension analysis.
TUNEL Assay
The level of apoptosis in colonic tissues was determined using the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine triphosphate (dUTP)-biotin nick end labeling (TUNEL) method. Paraffin-embedded tissue sections (4 μm thick) from the distal colon were processed using the ApopTag peroxidase in situ apoptosis detection kit (TUNEL; EMD Millipore) according to manufacturer's instructions. After staining, sections were counterstained with 0.5% (w/v) Methyl Green (Sigma-Aldrich, St. Louis, MO; Cat. 323829-5G).
Treatment with Fn14-specific blocking antibody
C57BL/6 mice were treated with an Fn14-specific blocking human immunoglobulin G1 antibody variant (18D1-dead), generously provided by Harald Wajant of University Hospital Würzburg, Würzburg, Germany (19). Control mice were treated with Rituximab, a therapeutic human IgG1 antibody recognizing human, but not murine, CD20 as an isotype control antibody. Mice were administered a daily intraperitoneal injection of antibody for 21 d (100μg/200 μl of PBS) during a cycle of 7d 3% (w/v) DSS followed by 14 d of recovery with water. Mice were sacrificed at day 21 of treatment.
Statistical Analysis
Experiments were conducted at a minimum in duplicate. Univariate and multivariate analyses were conducted using the collective data from replicated experiments. When the data fulfilled the assumptions for parametric statistics, comparisons of continuous data across experimental groups were conducted using the Student's t tests, one-way or two-way ANOVA, or linear regression. Alternative nonparametric tests were used for data with unfulfilled distributional assumptions regarding the normality of the data. Hazard ratios between treatment groups in survival experiments were estimated and tested with log-rank statistics. Data were expressed as SEMs, and 95% confidence intervals were reported when appropriate. An alpha level of 0.05 was considered significant. All analyses were conducted with STATA and GraphPad software (San Diego, CA).
Oncomine, a cancer microarray database and web-based data-mining platform was utilized to determine the translational relevance of TWEAK and Fn14 expression in different type of human cancers including colon cancer (20). We used the search terms “TNFSF12” and “TNFRSF12A” for TWEAK and Fn14 respectively in the Oncomine dataset (www.oncomine.org). The expression levels of Fn14 and TWEAK were then compared in clinical specimens of cancer vs. normal patient datasets. In order to reduce our false discovery rate, we selected P < 1×10-4 as a threshold. We analyzed the results for their P-values, fold-change, and cancer subtype. In many instances we found several significant correlations in different cancer datasets, but we show detailed analyses for the colon cancer dataset.
Results
The TWEAK/Fn14 pathway protects C57BL/6 mice from acute DSS-induced colitis
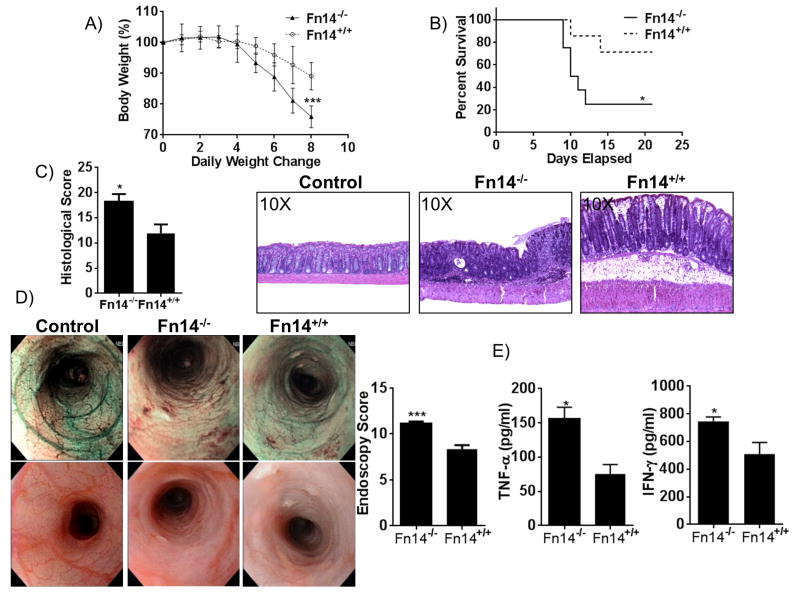
Acute colitis was induced in Fn14+/+ and Fn14-/- C57BL/6 mice by administration of 3% DSS in drinking water for 7 days. Fn14-/- mice lost more weight compared to Fn14+/+ controls after 7 days of DSS administration (mean weight expressed as percentage of weight before DSS treatment: Fn14-/-: 75.92% ± 1.34, Fn14+/+: 89.09% ± 1.67, P < 0.0001, Figure 1A). All mice survived the 7 days of DSS administration, but during the subsequent 14-day recovery phase, Fn14-/- mice experienced a four-fold increased rate of mortality compared to Fn14+/+ controls (95% CI: 1.083 – 17.82, P < 0.05, Figure 1B). Histological analysis of the colonic tissues revealed that Fn14-/- mice had significantly more severe colitis compared to Fn14+/+ mice (mean total inflammatory score: Fn14-/-: 18.17 ± 1.51, Fn14+/+: 11.88 ± 1.78, P < 0.02), with Fn14-/- mice experiencing a greater loss of crypts, more prominent infiltration of leukocytes, and a higher percentage of tissue ulceration (Figure 1C). This result was confirmed through endoscopic examination (endoscopy score: Fn14-/-: 11.14 ± 0.22, Fn14+/+: 8.35 ± 0.44; P < 0.001, Figure 1D). MLN lymphocytes isolated from Fn14-/- mice on day 8 and stimulated with anti-CD3 and anti-CD28 secreted elevated levels of both TNFα (Fn14-/-: 155.7 ± 16.8 pg/ml, Fn14+/+: 75.2 ± 14.0 pg/ml, P = 0.002) and IFNγ (Fn14-/-: 743.9 ± 31.7 pg/ml, Fn14+/+: 507.4 ± 84.4 pg/ml, P < 0.05) compared to MLN from Fn14+/+ mice (Figure 1E).
Figure 1. Fn14 alleviates DSS-induced colitis in mice.
(A) Time-course of changes in body weights expressed as percentages of pre-DSS body weights on day 0. Fn14-/- mice lose more body weight compared to Fn14+/+ mice (two-way ANOVA, P < 0.001; n = 7/group). (B) Percent survival of mice after 7 days of 3% DSS treatment and 14 days of recovery with drinking water. (Gehan-Breslow-Wilcoxon test, hazard ratio for Fn14-/- = 3.988, 95% CI= 1.083 - 17.82, P < 0.05). (C) Representative histopathological sections show active, severe ulcers, adjacent regenerative crypts, active cryptitis and increased inflammatory cells in the lamina propria of Fn14-/- mice (middle panel). Sections from Fn14+/+ mice treated with DSS (right panel) show regenerative colonic mucosa with focal, mild, active cryptitis and minimal inflammatory cells compared with Fn14-/- (unpaired t test; P < 0.05; n ≥ 12/group). Control mice without DSS treatment (left panel) show absence of ulcers, no infiltration of inflammatory cells in the lamina propria and no damaged epithelium. (D) High-resolution endoscopic images of the distal colon after 7 days of DSS treatment show severe inflammation in Fn14-/- mice (middle panels) and mild inflammation in Fn14+/+ mice (right panels) (unpaired t test; P < 0.001; n = 17/group). Control mice without DSS treatment (left panels) show absence of ulcers and erosions, and a visible vascular pattern without inflammation. (E) MLN lymphocytes isolated at day 8 of 3% DSS treatment and incubated for 72 hr with RPMI medium, anti-CD3 and anti-CD28 antibody (1 μg/ml). Cell free supernatants were analyzed by ELISA for production of TNF-α and IFN-γ. Fn14-/--derived cells responded by producing significantly increased levels of TNF-α (unpaired t test; P < 0.05; n ≥ 9/group) and IFN-γ (unpaired t test, 743.9 ± 31.7 vs. 507.4 ± 84.4; P < 0.05; n ≥ 8/group). Data are represented as mean ± SEM. * P < 0.05, *** P < 0.001. Results are representative of two independent experiments, performed in duplicate.
Hematopoietic and non-hematopoietic components of the TWEAK/Fn14 pathway participate in promoting a protective effect during acute colitis
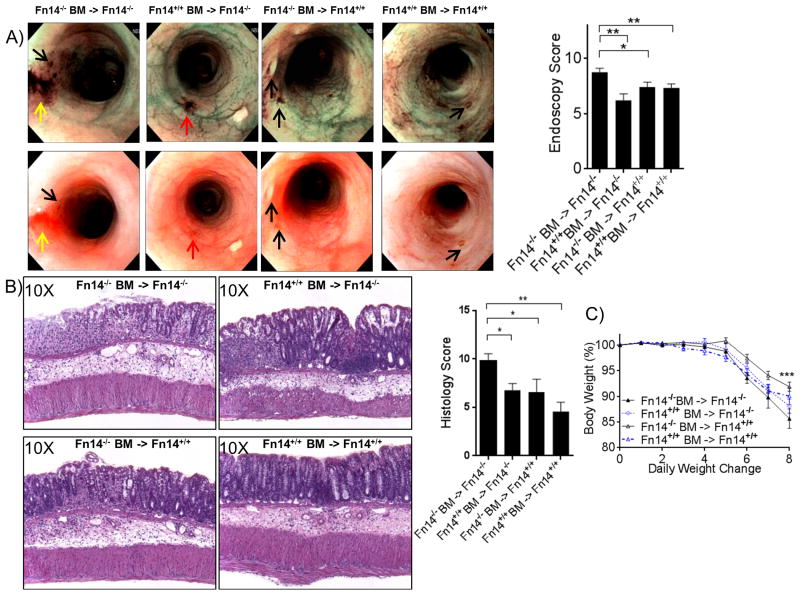
In order to assess which components of the intestinal immune system (hematopoietic or non-hematopoietic) are responsible for TWEAK/Fn14-mediated protection during acute colitis, we used BM chimera experiments to generate four groups of mice that expressed either: (1) no components of the Fn14 receptor (Fn14-/- BM→ Fn14-/- mice), (2) only hematopoietic-derived Fn14 receptors (Fn14+/+ BM→ Fn14-/- mice), (3) only non-hematopoietic-derived Fn14 receptor (Fn14-/- BM→ Fn14+/+ mice), or (4) both hematopoietic and non-hematopoietic-derived Fn14 receptors (Fn14+/+ BM→ Fn14+/+ mice). After the subsequent 8-wk recovery phase, acute colitis was induced by administration of 3% DSS in drinking water for 7 days. Endoscopic evaluation of macroscopic colitis severity showed that Fn14-/- mice (Group 1) were affected by a higher number of ulcers, more prominent intestinal bleeding, and a thicker colonic mucosa compared to the other three groups of chimeric mice (mean endoscopic inflammatory score, Group 1: 8.74 ± 0.36; Group 2: 6.19 ± 0.59 [P = 0.0033, vs. Group 1]; Group 3: 7.41 ± 0.43 [P = 0.0102, vs. Group 1]; Group 4: 7.32 ± 0.36 [ P= 0.0042, vs. Group 1]. No endoscopic differences were observed among three groups of chimeric mice that expressed some source of Fn14 (Groups 2-4) (Figure 2A). These results were confirmed by histological assessment, which showed that Fn14-/- mice (Group 1) were more susceptible to DSS-induced acute colitis compared to the other three groups (mean total inflammatory score, Group 1: 9.88 ± 0.69; Group 2: 6.78 ± 0.70 [P = 0.0109, vs. Group 1]; Group 3: 6.58 ± 1.34 [P = 0.0361, vs. Group 1]; Group 4: 4.58 ± 0.94 [P = 0.002, vs. Group 1]; Figure 2B). Weight changes were monitored daily, and by day 8 the mean weight loss for mice completely lacking Fn14 receptors (Group 1) was significantly higher than for that for mice with no deficiency in Fn14 (Group 4), but not significantly different compared to the groups that expressed only hematopoietic or non-hematopoietic-derived Fn14 receptors (mean weight expressed as percentage of weight before DSS treatment: Fn14-/- BM→ Fn14-/-: 84.74% ± 1.90, Fn14+/+ BM→ Fn14-/-: 87.74% ± 1.36, Fn14-/- BM→ Fn14+/+: 91.14 ± 0.88, Fn14+/+ BM→ Fn14+/+: 90.04 ± 1.66, P < 0.001, Figure 2C). These data suggest that, during the acute phase of DSS-induced colitis, both Fn14 expression by either the hematopoietic or non-hematopoietic compartments are sufficient to decrease susceptibility to colonic inflammation.
Figure 2. Both hematopoietic and epithelial Fn14 participate in promoting a protecting effect during acute colitis.
Fn14-/- mice and Fn14+/+ mice (n=6 per group) were transplanted with Fn14-/- and Fn14+/+ BM, respectively (n=4 per group). (A) High-resolution endoscopic images with white light and NBI of the distal colon after 7 days of DSS treatment show severe inflammation in Fn14-/- mice injected with Fn14-/- BM and mild inflammation in the other 3 groups (one-way ANOVA; P < 0.001, P < 0.05, P < 0.05; n ≥ 9/group); black arrows: ulcer; yellow arrows: erosion; red arrows: bleeding. (B) Representative histopathological sections show increased inflammation and severe ulcers in Fn14-/- mice injected with Fn14-/- BM, compared to the other 3 groups (one-way ANOVA; P < 0.05, P < 0.05, P < 0.02; n ≥ 6/group). There were no significant differences between Fn14-/- mice injected with Fn14+/+ BM and Fn14+/+ mice injected with Fn14-/- BM (6.78 ± 0.70 vs. 6.58 ± 1.34; P = ns; n ≥ 6). (C) Time-course of changes in body weights expressed as percentages of pre-DSS body weights on day 0. Fn14-/- mice injected with Fn14-/- BM showed more weight lost compared to the other 3 groups at day 7 (two-way ANOVA; P = 0.0008; n = 6/group). Data are represented as mean ± SEM. * P < 0.05, ** P < 0.01, *** P < 0.001. Results are representative of two independent experiments.
Fn14 deficiency leads to increased tumorigenesis following induction of DSS colitis
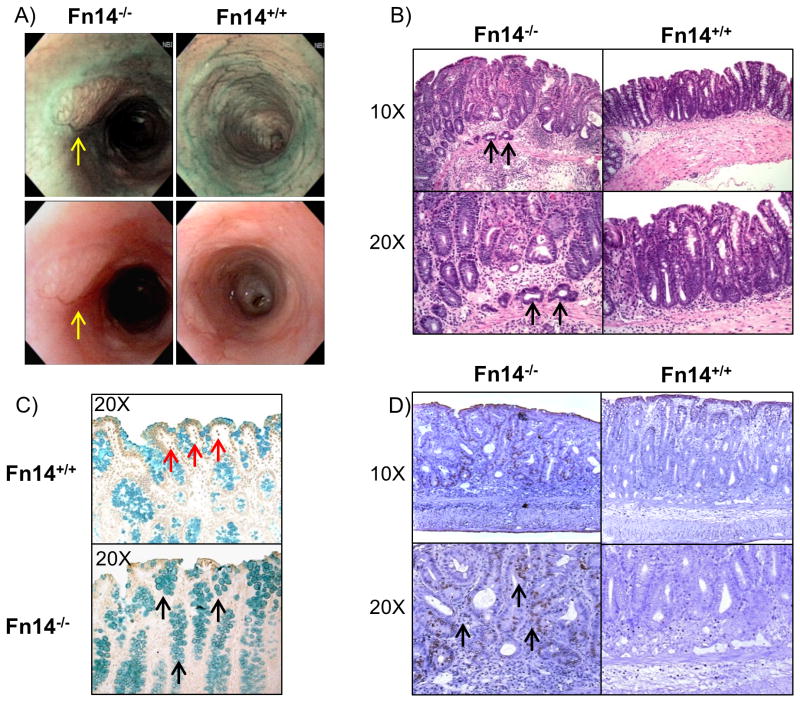
We then evaluated the severity of colitis during the recovery phase of DSS colitis (day 21). To our surprise, after 14 days of DSS recovery with normal drinking water, endoscopic examination revealed tumor masses in the colons of the Fn14-/- survivors, but not in Fn14+/+ mice (Figure 3A). Histological evaluation by a blinded pathologist showed the presence of cancerous lesions at the submucosal level in the colons of Fn14-/-, but not Fn14+/+ mice (Figure 3B). Due to the high mortality observed in the Fn14-/- mice, we then repeated this experiment using 2 cycles of 7 days 2.5 % DSS administration followed by 14 days of drinking water. At the end of the experiment (day 42) we observed the presence of cancerous lesions in 70% of Fn14-/- mice (7/10) compared to 0% in Fn14+/+ (0/10), P < 0.0001. In order to test if the increased tumor development was caused by a reduction in apoptosis, we performed a TUNEL assay on the colon sections of Fn14-/- and Fn14+/+ mice. TUNEL staining was higher in the Fn14+/+ group, showing increased DNA fragmentation in the inflamed colons of Fn14+/+ compared to the Fn14-/- mice; furthermore, methyl green counterstaining showed an increased number of mucus-secreting goblet cells (commonly observed during cancer development) in Fn14-/- mice compared to Fn14+/+ controls (Figure 3C). In order to test if the cell proliferation activity was higher in Fn14-/- mice compared to Fn14+/+ mice, we performed a BrdU proliferation assay. Histological analysis of colonic sections showed that Fn14-/- mice had a higher number of cells that were actively undergoing DNA replication compared to their Fn14+/+ controls, especially within the lamina propria (Figure 3D). Interestingly, TWEAK-/- mice did not reveal any tumors or show any difference in severity of DSS-colitis compared to their TWEAK+/+ WT controls (Supplementary Fig. 1). In order to test whether pharmacological blockade of the Fn14 signaling pathway had similar effects, we treated C57BL/6 mice with an Fn14-specific variant blocking antibody for 21 days during the acute induction of 3% DSS colitis (7 days) and the recovery phase (14 days). Our results show that at 7 days after colitis induction, anti-Fn14 treated mice lost more weight compared to isotype antibody-treated control mice (mean weight expressed as percentage of weight before DSS treatment: Anti-Fn14: 94.28% ± 1.78, Isotype: 99.20% ± 0.87, P < 0.05, Supplementary Fig. 2D). In addition, after the recovery phase (day 21), 3/5 mice in the anti-Fn14 treated group show tumor-like lesions detected by colonoscopy compared to 0/5 in the isotype antibody-treated control group (Supplementary Fig. 2A-B). Histologically, anti-Fn14 treated mice show tubular adenomas, areas of increased regenerative lesions with enlarged lymphoid aggregates and increased ulceration (mean total inflammatory score: 9.30 ± 1.38 vs. 5.50 ± 1.66; P=0.1258, Supplementary Fig. 2C). Taken together, these data indicate that lack of Fn14 could lead to increased colonic tumorigenesis following acute intestinal injury.
Figure 3. Fn14 protects from carcinogenesis induced by DSS-colitis.
(A) High-resolution endoscopic images of the distal colon during the recovery phase of DSS colitis (day 21) show tumor lesions in Fn14-/- mice, but no masses in Fn14+/+ mice (tumor lesions indicated by yellow arrows). (B) Representative histopathological sections show that cancer is present in Fn14-/- mice at the submucosal level (cancer cells indicated by black arrows). (C) TUNEL assay for apoptotic cells in colon sections of mice show that Fn14+/+ mice have an increased number of TUNEL-positive cells in comparison with Fn14-/- (apoptotic cells indicated by red arrows). The methyl green counterstaining shows that in the Fn14-/- mice there is an increased number of mucus secreting goblet cells (indicated by black arrows) in comparison with the Fn14+/+. (D) Mice were injected with BrdU 2 hr before sacrifice. Histological sections treated with BrdU antibody show that a higher number of cells were actively replicating their DNA at the level of the lamina propria (black arrows). Results are representative of two independent experiments.
Fn14 deficiency enhances carcinogenesis in the AOM-DSS model of inflammation-driven tumorigenesis
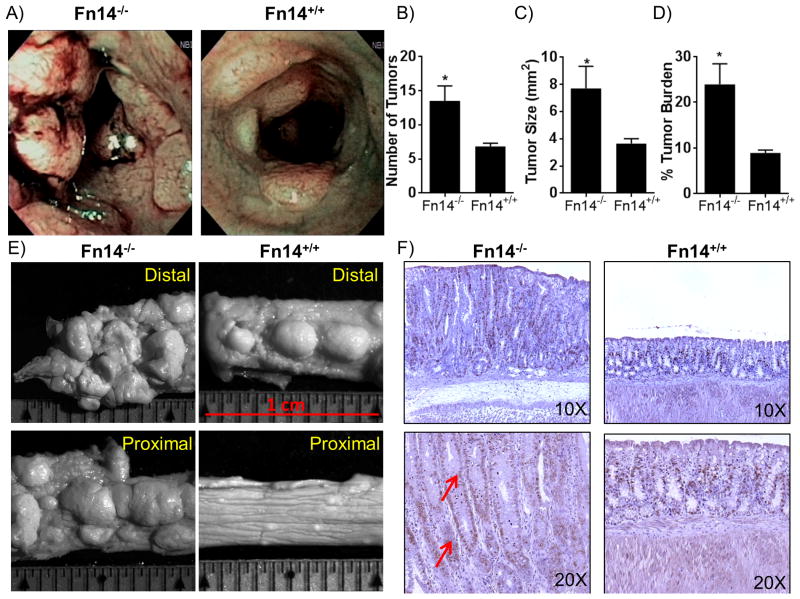
We next tested the effects of Fn14 deficiency in the AOM-DSS model of inflammation-driven colorectal carcinoma. Mice were administered two cycles of 2.5% DSS, each followed by a 14-day rest period, and then were injected with BrdU. After 2 hr, endoscopic examinations were performed. Both Fn14-/- and Fn14+/+ mice had tumor masses in the distal portions of their colons (Figure 4A), but Fn14-/- mice clearly had an increased number and size of tumor masses (mean number of tumors: Fn14-/- =3.5 ± 2.2 vs. Fn14+/+ =6.8 ± 0.5; P = 0.0262; n = 6, Figure 4B), (mean tumor size: Fn14-/- =7.7 ± 1.6 vs. Fn14+/+ = 3.6 ± 0.4; P = 0.0368; n = 6, Figure 4C). The tumor burden, expressed as percentage of the space occupied by tumors in respect to the whole colon, was analyzed by stereomicroscopy, and results showed that the burden was significantly higher in Fn14-/- mice (% tumor burden: Fn14-/- = 23.9 ± 4.5 vs. Fn14+/+ = 8.9 ± 0.6; P = 0.0206; n = 6, Figure 4D, E). Finally, Fn14-/- colons showed a higher level of cell proliferation and DNA replication activity compared to colons from Fn14+/+ mice (Figure 4F). Altogether, these data indicate that the lack of Fn14 receptors increases the risk of inflammation-driven colorectal carcinogenesis via decreased apoptosis, which leads to enhanced survival of cancer cells.
Figure 4. Susceptibility to carcinogenesis is enhanced in Fn14-/- mice in the AOM-DSS inflammation-driven colon cancer model.
(A) High-resolution endoscopic images of the distal colon after 1 injection of AOM followed by 2 cycles of 7 days of DSS plus 14 days of recovery show enhanced susceptibility of Fn14-/- mice (left panel) to carcinogenesis compared to Fn14+/+ mice (right panel), which showed fewer masses. (B) Quantification of the number of tumors showed that Fn14-/- mice develop a significant higher number of tumor compared to Fn14+/+ mice (unpaired t test; P < 0.05; n = 6/group). (C) The assessment of the size of the tumors shows that Fn14-/- mice developed tumors that were significantly bigger than Fn14+/+ mice (unpaired t test; P < 0.05; n = 6/group). (D) Tumor area expressed as a percentage of the whole colon shows that Fn14-/- mice develop a significantly greater tumor burden in comparison to Fn14+/+ mice (unpaired t test; P < 0.05; n = 6/group). (E) Images from stereomicroscopy examination of distal and proximal parts of colon of Fn14-/- and Fn14+/+ mice show a higher number of tumors in the Fn14-/- mice compared to the Fn14+/+ mice. (F) Histological sections treated with BrdU antibody show that a higher number of cells are actively replicating their DNA at the level of the lamina propria in Fn14-/- mice (red arrows) compared to Fn14+/+ mice. * P < 0.05, ** P < 0.01, *** P < 0.001. Results are representative of two independent experiments.
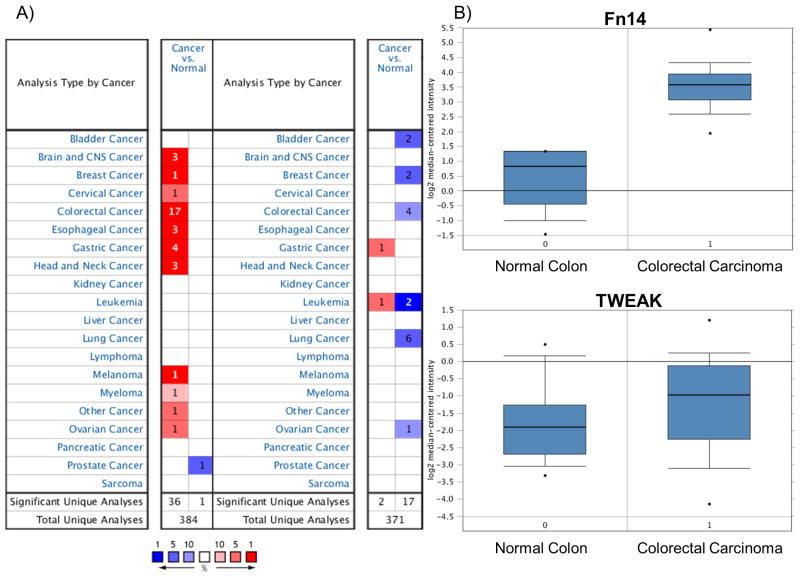
Patients with cancer and colorectal cancer show overexpression of the Fn14 gene
We next utilized the Oncomine database to determine the translational relevance of TWEAK and Fn14 expression in different type of cancers. Oncomine™ is a cancer microarray database and web-based data-mining platform. It contains gene expression datasets from over 18,000 cancer gene expression microarrays, spanning the majority of cancer types and subtypes (20). We used this platform to investigate how TWEAK and Fn14 expression modulates the tumorigenesis process, particularly in colorectal cancers. We found that Fn14 overexpression correlated with several types of cancers, including cancer of the brain and central nervous system, breast, cervix, esophagus, stomach, head and skin. The most relevant correlation, though, was found with colorectal cancer, with 17 out of 35 analyses meeting the 1×10-4 threshold for the Fn14 gene in 9 out of 13 datasets (Figure 5A). Conversely, the analysis of the correlation between TWEAK expression and tumorigenesis did not generate the same results. Specifically, TWEAK has been found to be mildly overexpressed only in gastric tumors and leukemia compared to normal healthy tissues, with only 1 out of 19 analyses meeting the 1×10-4 threshold for TWEAK expression in 1 out of 5 datasets. Four studies of colorectal carcinoma in particular analyzed both TWEAK and Fn14 expression in colons from patients with and without colorectal carcinoma (21-25). Specifically, in the study performed by Hong et al (21), Fn14 is potently upregulated in colorectal cancer by 8.531-fold compared to healthy colons (Log2 median centered intensity average: 3.59 ± 1.86 vs. 0.83 ± 0.52 normal; N ≥ 12; P = 6.98×10-8). In contrast, TWEAK did not show a significant difference of expression between colorectal carcinoma and normal healthy colons, with a fold-change of 1.459 (Log2 median centered intensity average: -0.98 ± 3.16 vs. -1.90 ± 1.42; n ≥ 12; P = 0.085) (Figure 5B).
Figure 5. Human patients with cancer and colorectal carcinoma show overexpression of the Fn14 gene.
(A) Oncomine dataset of analyses on different types of cancer and related correlation with TWEAK/Fn14 expression. Fn14 overexpression has been correlated with several types of cancers, including cancer of the brain and central nervous system, breast, cervix, esophagus, stomach, head and skin. The strongest correlation was found with colorectal carcinoma, with 17 out of 35 analyses meeting the 1×10-4 threshold for the Fn14 gene in 9 out of 13 datasets. TWEAK has been found to be mildly overexpressed only in gastric tumors and leukemia, compared to normal healthy tissues, with only 1 out of 19 analyses meeting the 1×10-4 threshold for the TWEAK expression in 1 out of 5 datasets. (B) Comparative study on TWEAK and Fn14 expression in colorectal carcinoma compared to normal colon. Fn14 in colorectal carcinoma is potently upregulated 8.531-fold compared to healthy colons (Log2 median centered intensity average; P = 6.98 × 10-8; n ≥ 12/group). TWEAK did not show a significant difference of expression between colorectal cancer and normal healthy colon, with a fold change of 1.459 (Log2 median centered intensity average; P = 0.085; n ≥ 12/group).
Discussion
In this study, we demonstrated a novel protective role for the TWEAK/Fn14 ligand/receptor pair in acute DSS colitis in mice. Lack of Fn14 was associated with increased severity of colitis and tumorigenesis, even in the absence of exposure to the carcinogen AOM; lack of Fn14 also led to enhanced tumor development in the AOM-DSS model of inflammation-driven colorectal cancer. These effects were mediated through inhibition of TWEAK/Fn14-induced apoptosis and increased intestinal epithelial cell proliferation. Altogether these results indicate that lack of Fn14 may lead to increased colonic tumorigenesis following acute intestinal injury. In order to guarantee reproducibility and robustness of our experiments, our study was performed in accordance with recently published stringent guidelines for preclinical investigation (26).
As part of our investigations, we hypothesized that one effect of Fn14 deficiency would be a reduction in apoptosis, which could result in enhanced colonic tumorigenesis. In support of this hypothesis, our results revealed that even in the absence of exposure to a specific carcinogen, 70% of Fn14-/- mice developed colorectal cancer after two cycles of DSS treatment, compared to no tumors occurring in DSS-treated Fn14+/+ mice. Thus, Fn14 deficiency in C57BL/6 with DSS-colitis may represent a spontaneous model of colitis-associated adenocarcinoma. We also studied the effects of Fn14 deficiency in the AOM/DSS model of inflammation-driven colorectal carcinoma. Our data confirmed the correlation between Fn14 deficiency and tumor initiation observed in the DSS model alone. In addition, we found that Fn14-/- colitic mice pre-treated with an injection of AOM were more susceptible to colorectal cancer compared to the Fn14-/- who were not administered AOM treatment, in terms of size and quantity of invasive carcinomas. Based on the known correlation between the TWEAK/Fn14 pathway and apoptosis, we compared the amount of apoptotic bodies present in Fn14-/- and Fn14+/+ mice after DSS treatment by means of the TUNEL assay. The assay was performed on the colons of DSS-treated mice and showed apoptosis in Fn14+/+, but not in Fn14-/- mice. It has been shown that defects in the apoptotic pathway can lead to a wide variety of diseases, such as atrophy (excessive apoptosis) or cancer (insufficient apoptosis and uncontrolled cell proliferation) (27). Insufficient apoptosis in Fn14-/- mice is likely the reason for tumor development in the Fn14-/- mice after two cycles of DSS administration, a model of repeated intestinal injury as well as enhanced tumorigenesis when the carcinogen AOM is injected before the DSS treatment. In addition, our BrdU assay data clearly demonstrate that the lack of activation of the TWEAK/Fn14 apoptotic pathway (including NF-κB and Fas) in Fn14-/- mice prevents programmed cell death of DSS-damaged intestinal epithelial cells, thus allowing the damaged cells in the colonic epithelium to survive and replicate their altered DNA, eventually leading to tumor development.
In this study, we also treated C57BL/6 mice with an Fn14-specific blocking antibody for 21 days following the induction of 3% DSS colitis. Despite the fact that anti-Fn14 treated mice developed tubular adenomas and showed increased intestinal epithelial damage, we did not observe the presence of cancer-like lesions similar to those observed in DSS-treated Fn14-/- mice. These findings may be explained by incomplete Fn14 blockade by antibody treatment (dosage and tissue concentration), and the short duration of treatment (21 days) compared to Fn14 genetic deletion, that did not allow the development of colonic dysplasia and cancer.
Our bone marrow chimera experiments show that Fn14 expressed on cells found in both hematopoietic and non-hematopoietic compartments is important for the protective effects against DSS-induced colitis. These results are not surprising due to the ubiquitous expression of Fn14 in multiple cell types such as endothelial, epithelial and mesenchymal cells (10). In fact, Kawashima et al. (28) showed that Fn14 is expressed in intestinal epithelial cells. In addition, production of TWEAK/Fn14 from granulocytes, macrophages and intestinal epithelial cells has been well documented (29). These results strongly suggest that the TWEAK/Fn14 pair represents an important cytokine pathway that mediates the inflammatory process in the gut by acting on both hematopoietic and non-hematopoietic cells.
The anti-inflammatory role of TWEAK/Fn14 described in our study was unexpected and rather surprising. However, several TNF superfamily members have been found to perform seemingly dichotomous roles within different environments of intestinal inflammation. During early, acute stages of intestinal inflammation, these cytokines, along with their cognate receptors beneficially contribute to maintenance of homeostasis and tissue repair; however, during established disease or chronic intestinal inflammatory settings, they promote pro-inflammatory conditions and tissue damage (30). Our group has found a similar dichotomous pattern of protective vs. pro-inflammatory effects with the TNF-like cytokine TL1A and its cognate receptor DR3, which represent another ligand/receptor complex within the TNF superfamily (31).
In humans, Fn14 overexpression is linked to different types of tumors, including brain, esophageal, gastric and skin cancer, but the most significant correlation has been observed with colorectal cancer (data from www.oncomine.org). Nine studies have reported that Fn14 is overexpressed in the colons of patients affected by colon adenocarcinoma compared to healthy controls (21, 25, 32). Three of these nine studies also measured TWEAK expression and did not find a corresponding significant correlation between TWEAK overexpression and colorectal cancer. These findings are consistent with our data showing that TWEAK-/- mice do not have enhanced susceptibility to colitis-associated cancer compared to TWEAK+/+ mice (Supplementary Figure 1). This different DSS response in Fn14 deficient mice compared to TWEAK-/- mice suggests the possible existence of an additional ligand for Fn14 that has yet to be identified.
The human data are not necessarily in conflict with our murine results, as we believe that during tumorigenesis the increased expression of TWEAK/Fn14 likely represents a protective response aimed at increasing apoptosis in the cancerous tissue and decreasing tumor growth. Interestingly, TWEAK and Fn14 were also found to be significantly upregulated in patients with ulcerative colitis compared to patients with normal colonic mucosa. Specifically, Kawahisma et al. (28) reported that both TWEAK and Fn14 are upregulated during intestinal inflammation in ulcerative colitis. However, for the small number of colitis patients that develop colon cancer, Fn14 overexpression may not be enough to prevent progression to colitis-associated cancer, as they may have other genomic alterations that overcome the effects of Fn14 protection.
In conclusion, our study shows that Fn14 expression is not only significantly correlated with protection against DSS-colitis in mice, but it is also important for prevention of colitis-associated carcinogenesis. Therefore, activation of the TWEAK/Fn14 signaling pathway by stimulating anti-Fn14 antibodies or TWEAK-like ligands may represent a new potential treatment approach for colitis-associated cancer. This concept is supported by several studies showing that the anti-tumor effects of anti-Fn14 antibodies are based on their activation of NF-κB and other intracellular pathways (33, 34). In this context, recombinant agonistic Fn14 antibodies could be also used to activate tumor cell death specifically in colon cancer therapy. Finally, based on our results, caution should be used when administering inhibitors of the TWEAK/Fn14 pathway in patients with chronic inflammatory conditions.
Supplementary Material
Acknowledgments
We thank Sandy Markowitz for his critical discussion of the manuscript. The authors thank Mitchell Guanzon, Dennis Gruszka, and Joshua Webster for their technical support. The authors finally acknowledge the services of the Mouse Models and Histology/Imaging Cores and Clinical Component of the NIH Cleveland Digestive Diseases Research Core Center.
Financial Support: This work was supported by National Institutes of Health grants DK042191, DK055812, DK091222 and DK097948 to Fabio Cominelli
Footnotes
Conflict of Interest: The authors declare no conflicts of interest
References
- 1.Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14 e5. doi: 10.1053/j.gastro.2010.01.058. [DOI] [PubMed] [Google Scholar]
- 2.Beaugerie L, Itzkowitz SH. Cancers complicating inflammatory bowel disease. N Engl J Med. 2015;372:1441–52. doi: 10.1056/NEJMra1403718. [DOI] [PubMed] [Google Scholar]
- 3.Dai X, Zhang J, Arfuso F, Chinnathambi A, Zayed ME, Alharbi SA, et al. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp Biol Med (Maywood) 2015;240:760–73. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trivedi R, Mishra DP. Trailing TRAIL Resistance: Novel Targets for TRAIL Sensitization in Cancer Cells. Front Oncol. 2015;5:69. doi: 10.3389/fonc.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhong B, Ma G, Sato A, Shimozato O, Liu H, Li Q, et al. Fas ligand DNA enhances a vaccination effect by coadministered DNA encoding a tumor antigen through augmenting production of antibody against the tumor antigen. J Immunol Res. 2015;2015:743828. doi: 10.1155/2015/743828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abusamra AJ, Zhong Z, Zheng X, Li M, Ichim TE, Chin JL, et al. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol Dis. 2005;35:169–73. doi: 10.1016/j.bcmd.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 7.Trebing J, Lang I, Chopra M, Salzmann S, Moshir M, Silence K, et al. A novel llama antibody targeting Fn14 exhibits anti-metastatic activity in vivo. MAbs. 2014;6:297–308. doi: 10.4161/mabs.26709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vince JE, Chau D, Callus B, Wong WW, Hawkins CJ, Schneider P, et al. TWEAK-FN14 signaling induces lysosomal degradation of a cIAP1-TRAF2 complex to sensitize tumor cells to TNFalpha. J Cell Biol. 2008;182:171–84. doi: 10.1083/jcb.200801010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burkly LC. Regulation of Tissue Responses: The TWEAK/Fn14 Pathway and Other TNF/TNFR Superfamily Members That Activate Non-Canonical NFkappaB Signaling. Front Immunol. 2015;6:92. doi: 10.3389/fimmu.2015.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Winkles JA. The TWEAK-Fn14 cytokine-receptor axis: discovery, biology and therapeutic targeting. Nat Rev Drug Discov. 2008;7:411–25. doi: 10.1038/nrd2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim SH, Kang YJ, Kim WJ, Woo DK, Lee Y, Kim DI, et al. TWEAK can induce pro-inflammatory cytokines and matrix metalloproteinase-9 in macrophages. Circ J. 2004;68:396–9. doi: 10.1253/circj.68.396. [DOI] [PubMed] [Google Scholar]
- 12.Winkles JA, Tran NL, Brown SA, Stains N, Cunliffe HE, Berens ME. Role of TWEAK and Fn14 in tumor biology. Front Biosci. 2007;12:2761–71. doi: 10.2741/2270. [DOI] [PubMed] [Google Scholar]
- 13.Tran NL, McDonough WS, Donohue PJ, Winkles JA, Berens TJ, Ross KR, et al. The human Fn14 receptor gene is up-regulated in migrating glioma cells in vitro and overexpressed in advanced glial tumors. Am J Pathol. 2003;162:1313–21. doi: 10.1016/S0002-9440(10)63927-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Burkly LC, Michaelson JS, Hahm K, Jakubowski A, Zheng TS. TWEAKing tissue remodeling by a multifunctional cytokine: role of TWEAK/Fn14 pathway in health and disease. Cytokine. 2007;40:1–16. doi: 10.1016/j.cyto.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Kodani T, Rodriguez-Palacios A, Corridoni D, Lopetuso L, Di Martino L, Marks B, et al. Flexible colonoscopy in mice to evaluate the severity of colitis and colorectal tumors using a validated endoscopic scoring system. J Vis Exp. 2013:e50843. doi: 10.3791/50843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Corridoni D, Kodani T, Rodriguez-Palacios A, Pizarro TT, Xin W, Nickerson KP, et al. Dysregulated NOD2 predisposes SAMP1/YitFc mice to chronic intestinal inflammation. Proc Natl Acad Sci U S A. 2013;110:16999–7004. doi: 10.1073/pnas.1311657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosiewicz MM, Nast CC, Krishnan A, Rivera-Nieves J, Moskaluk CA, Matsumoto S, et al. Th1-type responses mediate spontaneous ileitis in a novel murine model of Crohn's disease. J Clin Invest. 2001;107:695–702. doi: 10.1172/JCI10956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rodriguez-Palacios A, Kodani T, Kaydo L, Pietropaoli D, Corridoni D, Howell S, et al. Stereomicroscopic 3D-pattern profiling of murine and human intestinal inflammation reveals unique structural phenotypes. Nat Commun. 2015;6:7577. doi: 10.1038/ncomms8577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chopra M, Brandl A, Siegmund D, Mottok A, Schafer V, Biehl M, et al. Blocking TWEAK-Fn14 interaction inhibits hematopoietic stem cell transplantation-induced intestinal cell death and reduces GVHD. Blood. 2015;126:437–44. doi: 10.1182/blood-2015-01-620583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes DR, Kalyana-Sundaram S, Mahavisno V, Varambally R, Yu J, Briggs BB, et al. Oncomine 3.0: genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–80. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 22.Kurashina K, Yamashita Y, Ueno T, Koinuma K, Ohashi J, Horie H, et al. Chromosome copy number analysis in screening for prognosis-related genomic regions in colorectal carcinoma. Cancer Sci. 2008;99:1835–40. doi: 10.1111/j.1349-7006.2008.00881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–75. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 24.Kaiser S, Park YK, Franklin JL, Halberg RB, Yu M, Jessen WJ, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, et al. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 2010;5(10) doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Omary MB, Cohen DE, El-Omar EM, Jalan R, Low MJ, Nathanson MH, et al. Not all mice are the same: Standardization of animal researech data presentation. Gastroenterology. 2016;63:1752–4. doi: 10.1002/hep.28608. [DOI] [PubMed] [Google Scholar]
- 27.Cotter TG. Apoptosis and cancer: the genesis of a research field. Nat Rev Cancer. 2009;9:501–7. doi: 10.1038/nrc2663. [DOI] [PubMed] [Google Scholar]
- 28.Kawashima R, Kawamura YI, Oshio T, Son A, Yamazaki M, Hagiwara T, et al. Interleukin-13 damages intestinal mucosa via TWEAK and Fn14 in mice-a pathway associated with ulcerative colitis. Gastroenterology. 2011;141:2119–29.e8. doi: 10.1053/j.gastro.2011.08.040. [DOI] [PubMed] [Google Scholar]
- 29.Burkly LC, Michaelson JS, Zheng TS. TWEAK/Fn14 pathway: an immunological switch for shaping tissue responses. Immunol Rev. 2011;244:99–114. doi: 10.1111/j.1600-065X.2011.01054.x. [DOI] [PubMed] [Google Scholar]
- 30.Bamias G, Corridoni D, Pizarro TT, Cominelli F. New insights into the dichotomous role of innate cytokines in gut homeostasis and inflammation. Cytokine. 2012;59:451–9. doi: 10.1016/j.cyto.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jia LG, Bamias G, Arseneau KO, Burkly LC, Wang EC, Gruszka D, et al. A Novel Role for TL1A/DR3 in Protection against Intestinal Injury and Infection. J Immunol. 2016;197:377–86. doi: 10.4049/jimmunol.1502466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ki DH, Jeung HC, Park CH, Kang SH, Lee GY, Lee WS, et al. Whole genome analysis for liver metastasis gene signatures in colorectal cancer. Int J Cancer. 2007;121:2005–12. doi: 10.1002/ijc.22975. [DOI] [PubMed] [Google Scholar]
- 33.Culp PA, Choi D, Zhang Y, Yin J, Seto P, Ybarra SE, et al. Antibodies to TWEAK receptor inhibit human tumor growth through dual mechanisms. Clin Cancer Res. 2010;16:497–508. doi: 10.1158/1078-0432.CCR-09-1929. [DOI] [PubMed] [Google Scholar]
- 34.Zhou H, Marks JW, Hittelman WN, Yagita H, Cheung LH, Rosenblum MG, et al. Development and characterization of a potent immunoconjugate targeting the Fn14 receptor on solid tumor cells. Mol Cancer Ther. 2011;10:1276–88. doi: 10.1158/1535-7163.MCT-11-0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.