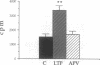Abstract
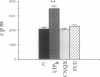
Several studies suggest that protein kinase C and type II Ca2+/calmodulin-dependent protein kinase are activated during induction of long-term potentiation (LTP). We now report that casein kinase II (CK-II), which is present in high concentration in the hippocampus, is also activated in the CA1 region during LTP. CK-II activity increased within 2 min after a train of high-frequency electrical stimulations and reached a maximum (2-fold increase) 5 min later before returning to baseline value. The stimulated protein kinase activity, which was blocked by a selective antagonist of N-methyl-D-aspartate receptors, exhibited specific properties of CK-II, including phosphorylation of the specific substrates of CK-II, marked inhibition by a low heparin concentration, and the use of GTP as a phosphate donor. CK-II activity was also selectively and rapidly augmented in another form of LTP produced by bath application of tetraethylammonium; this LTP (called LTPk) is Ca2+ dependent but N-methyl-D-aspartate independent. Phosphorylation of casein that was not inhibited by heparin (i.e., casein kinase I) remained unchanged. We suggest that an increase in CK-II activity is important in LTP induction.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc Natl Acad Sci U S A. 1990 Jan;87(2):821–825. doi: 10.1073/pnas.87.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akers R. F., Lovinger D. M., Colley P. A., Linden D. J., Routtenberg A. Translocation of protein kinase C activity may mediate hippocampal long-term potentiation. Science. 1986 Feb 7;231(4738):587–589. doi: 10.1126/science.3003904. [DOI] [PubMed] [Google Scholar]
- Alexander K. A., Cimler B. M., Meier K. E., Storm D. R. Regulation of calmodulin binding to P-57. A neurospecific calmodulin binding protein. J Biol Chem. 1987 May 5;262(13):6108–6113. [PubMed] [Google Scholar]
- Aniksztejn L., Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991 Jan 3;349(6304):67–69. doi: 10.1038/349067a0. [DOI] [PubMed] [Google Scholar]
- Bliss T. V., Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973 Jul;232(2):331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Santoro N., Marshak D. R. Regulating cell growth: casein-kinase-II-dependent phosphorylation of nuclear oncoproteins. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):91–95. doi: 10.1101/sqb.1988.053.01.014. [DOI] [PubMed] [Google Scholar]
- Díaz-Nido J., Serrano L., Méndez E., Avila J. A casein kinase II-related activity is involved in phosphorylation of microtubule-associated protein MAP-1B during neuroblastoma cell differentiation. J Cell Biol. 1988 Jun;106(6):2057–2065. doi: 10.1083/jcb.106.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman A. M., Blumenthal D. K., Krebs E. G. Protein serine/threonine kinases. Annu Rev Biochem. 1987;56:567–613. doi: 10.1146/annurev.bi.56.070187.003031. [DOI] [PubMed] [Google Scholar]
- Girault J. A., Hemmings H. C., Jr, Williams K. R., Nairn A. C., Greengard P. Phosphorylation of DARPP-32, a dopamine- and cAMP-regulated phosphoprotein, by casein kinase II. J Biol Chem. 1989 Dec 25;264(36):21748–21759. [PubMed] [Google Scholar]
- Girault J. A., Hemmings H. C., Jr, Zorn S. H., Gustafson E. L., Greengard P. Characterization in mammalian brain of a DARPP-32 serine kinase identical to casein kinase II. J Neurochem. 1990 Nov;55(5):1772–1783. doi: 10.1111/j.1471-4159.1990.tb04968.x. [DOI] [PubMed] [Google Scholar]
- Gispen W. H., De Graan P. N., Chan S. Y., Routtenberg A. Comparison between the neural acidic proteins B-50 and F1. Prog Brain Res. 1986;69:383–386. doi: 10.1016/s0079-6123(08)61072-9. [DOI] [PubMed] [Google Scholar]
- Hathaway G. M., Traugh J. A. Cyclic nucleotide-independent protein kinases from rabbit reticulocytes. Purification of casein kinases. J Biol Chem. 1979 Feb 10;254(3):762–768. [PubMed] [Google Scholar]
- Hemmings H. C., Jr, Greengard P., Tung H. Y., Cohen P. DARPP-32, a dopamine-regulated neuronal phosphoprotein, is a potent inhibitor of protein phosphatase-1. Nature. 1984 Aug 9;310(5977):503–505. doi: 10.1038/310503a0. [DOI] [PubMed] [Google Scholar]
- Kennedy M. B. Regulation of synaptic transmission in the central nervous system: long-term potentiation. Cell. 1989 Dec 1;59(5):777–787. doi: 10.1016/0092-8674(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Klarlund J. K., Czech M. P. Insulin-like growth factor I and insulin rapidly increase casein kinase II activity in BALB/c 3T3 fibroblasts. J Biol Chem. 1988 Nov 5;263(31):15872–15875. [PubMed] [Google Scholar]
- Krebs E. G., Eisenman R. N., Kuenzel E. A., Litchfield D. W., Lozeman F. J., Lüscher B., Sommercorn J. Casein kinase II as a potentially important enzyme concerned with signal transduction. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 1):77–84. doi: 10.1101/sqb.1988.053.01.012. [DOI] [PubMed] [Google Scholar]
- Kuenzel E. A., Krebs E. G. A synthetic peptide substrate specific for casein kinase II. Proc Natl Acad Sci U S A. 1985 Feb;82(3):737–741. doi: 10.1073/pnas.82.3.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lovinger D. M., Colley P. A., Akers R. F., Nelson R. B., Routtenberg A. Direct relation of long-term synaptic potentiation to phosphorylation of membrane protein F1, a substrate for membrane protein kinase C. Brain Res. 1986 Dec 10;399(2):205–211. doi: 10.1016/0006-8993(86)91510-6. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Mauk M. D., Kelly P. T., Nicoll R. A., Waxham M. N. An essential role for postsynaptic calmodulin and protein kinase activity in long-term potentiation. Nature. 1989 Aug 17;340(6234):554–557. doi: 10.1038/340554a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C., Kauer J. A., Perkel D. J., Nicoll R. A. The impact of postsynaptic calcium on synaptic transmission--its role in long-term potentiation. Trends Neurosci. 1989 Nov;12(11):444–450. doi: 10.1016/0166-2236(89)90094-5. [DOI] [PubMed] [Google Scholar]
- Malinow R., Madison D. V., Tsien R. W. Persistent protein kinase activity underlying long-term potentiation. Nature. 1988 Oct 27;335(6193):820–824. doi: 10.1038/335820a0. [DOI] [PubMed] [Google Scholar]
- Malinow R., Schulman H., Tsien R. W. Inhibition of postsynaptic PKC or CaMKII blocks induction but not expression of LTP. Science. 1989 Aug 25;245(4920):862–866. doi: 10.1126/science.2549638. [DOI] [PubMed] [Google Scholar]
- Miller S. G., Kennedy M. B. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986 Mar 28;44(6):861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Morris R. G., Anderson E., Lynch G. S., Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP5. 1986 Feb 27-Mar 5Nature. 319(6056):774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Mulner-Lorillon O., Cormier P., Labbé J. C., Dorée M., Poulhe R., Osborne H., Bellé R. M-phase-specific cdc2 protein kinase phosphorylates the beta subunit of casein kinase II and increases casein kinase II activity. Eur J Biochem. 1990 Oct 24;193(2):529–534. doi: 10.1111/j.1432-1033.1990.tb19368.x. [DOI] [PubMed] [Google Scholar]
- Nairn A. C., Hemmings H. C., Jr, Greengard P. Protein kinases in the brain. Annu Rev Biochem. 1985;54:931–976. doi: 10.1146/annurev.bi.54.070185.004435. [DOI] [PubMed] [Google Scholar]
- Nielander H. B., Schrama L. H., van Rozen A. J., Kasperaitis M., Oestreicher A. B., Gispen W. H., Schotman P. Mutation of serine 41 in the neuron-specific protein B-50 (GAP-43) prohibits phosphorylation by protein kinase C. J Neurochem. 1990 Oct;55(4):1442–1445. doi: 10.1111/j.1471-4159.1990.tb03159.x. [DOI] [PubMed] [Google Scholar]
- Pisano M. R., Hegazy M. G., Reimann E. M., Dokas L. A. Phosphorylation of protein B-50 (GAP-43) from adult rat brain cortex by casein kinase II. Biochem Biophys Res Commun. 1988 Sep 30;155(3):1207–1212. doi: 10.1016/s0006-291x(88)81268-3. [DOI] [PubMed] [Google Scholar]
- Sommercorn J., Krebs E. G. Induction of casein kinase II during differentiation of 3T3-L1 cells. J Biol Chem. 1987 Mar 15;262(8):3839–3843. [PubMed] [Google Scholar]
- Teyler T. J., DiScenna P. Long-term potentiation. Annu Rev Neurosci. 1987;10:131–161. doi: 10.1146/annurev.ne.10.030187.001023. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Krogsgaard-Larsen P., Honoré T. Structure-activity relationships in the development of excitatory amino acid receptor agonists and competitive antagonists. Trends Pharmacol Sci. 1990 Jan;11(1):25–33. doi: 10.1016/0165-6147(90)90038-a. [DOI] [PubMed] [Google Scholar]