Abstract
Purpose
Cetuximab, an EGFR-specific antibody (mAb), modestly improves clinical outcome in head and neck cancer (HNC) patients. Cetuximab mediates natural killer (NK) cell:dendritic cell (DC) cross-talk by cross-linking FcγRIIIa, which is important for inducing anti-tumor cellular immunity. Cetuximab activated NK cells upregulate the costimulatory receptor CD137 (4-1BB) which, when triggered by agonistic mAb urelumab, might enhance NK cell functions, to promote T cell based immunity.
Experimental design
CD137 expression on tumor infiltrating lymphocytes was evaluated in a prospective cetuximab neoadjuvant trial, and CD137 stimulation was evaluated in a phase Ib trial, in combining agonistic urelumab with cetuximab. Flow cytometry and cytokine release assays using NK cells and DC were employed in vitro, testing the addition of urelumab to cetuximab-activated NK, DC, and cross presentation to T cells.
Results
CD137 agonist mAb urelumab enhanced cetuximab-activated NK cell survival, DC maturation and tumor antigen cross-presentation. Urelumab boosted DC maturation markers, CD86 and HLA DR, and antigen processing machinery (APM) components TAP1/2, leading to increased tumor antigen cross-presentation. In neoadjuvant cetuximab treated HNC patients, upregulation of CD137 by intratumoral, cetuximab-activated NK cells correlated with FcγRIIIa V/F polymorphism and predicted clinical response. Moreover, immune biomarker modulation was observed in an open label, phase Ib clinical trial, of HNC patients treated with cetuximab plus urelumab.
Conclusion
These results suggest a beneficial effect of combination immunotherapy using cetuximab and CD137 agonist in HNC.
Keywords: Cetuximab, CD137, EGFR, cytotoxic T cells, ADCC, head and neck cancer, immunotherapy
Introduction
Immunotherapy against head and neck cancer (HNC) is an important and rapidly expanding field (1). Using a phase II clinical trial of neoadjuvant cetuximab we studied the therapeutically relevant, anti-tumor immune effects of this EGFR-targeted mAb (2, 3). Indeed, cetuximab treatment induced innate and adaptive immunity in a subset of patients who generated objective clinical responses (4, 5). Previous studies have shown that cetuximab-coated HNC cells induce NK cells (4, 5), promote NK cell-dendritic cell (DC) cross-talk (2,6), and expand EGFR-specific cytotoxic T cells (CTL) (2, 7, 8, 9, 10). Combining the tumor targeting effects of cetuximab with a specific, immune cell targeting mAb may be a useful therapeutic strategy (11).
CD137 (4-1BB), a member of the TNF-receptor superfamily, is broadly induced on activated CD4+ T cells (12), CD8+ T cells (13), B cells (14), NK cells (15), monocytes (16) and DC (17). The introduction of the fully human, clinical grade CD137-agonist mAb, urelumab (BMS-663513) has enabled modulation of CD137 function in immune-oncology, including evaluating its role in combination with tumor targeting mAb (11).
Previous studies have established the effects of CD137 pathway on activated T and B lymphocytes (13,18,19), however, the potential mechanism of action of CD137 targeting to enhance NK: DC cross-talk is only recently emerging (15). NK cells are subdivided in to CD56dim and CD56bright subsets, which differ significantly in their effector function. Cetuximab-activated CD56dim NK cells appear to upregulate CD137 receptor in higher magnitude than CD5bright NK cells (2, 6, 15). Depletion of DC abrogates CD137 agonist mAb induced therapeutic benefits in mice, however, antigen presentation, cross-presentation of TA were not tested in HNC patients (20, 21). Here, we have investigated the factors which modulate CD137 expression on cetuximab-activated NK cells (2, 4). We additionally investigated the effect of stimulating CD137 expressed by cetuximab activated NK:DC cross-talk in the tumor microenvironment using clinical specimens from a neoadjuvant cetuximab clinical trial (NCT01218048). These findings have important implications for biomarkers of response to cetuximab based immunotherapy (18, 22).
Materials and Methods
Lymphocyte isolation, DC generation, and HNC cell lines culture
Following Institutional Review Board (UPCI protocol 99-069) approval and informed consent, blood was obtained from healthy donors (Western PA blood bank) or HNC patients treated with cetuximab (NCI-2011-02479, NCT01218048). Lymphocytes were purified by Ficoll-Paque™ PLUS centrifugation (Amersham Biosciences, Uppsala, Sweden) and stored frozen. DC were generated as described previously (2). NK cells were purified using EasySep kits (Stem cell technologies, Vancouver, BC, Canada) and purity was >95% CD16+, CD56+, CD3− evaluated with flow cytometry (23).
The HNC cell lines PCI-15B (HLA-A2−EGFR+) was isolated from patient treated at the University of Pittsburgh Cancer Institute (Pittsburgh, PA) through the explant/culture method. JHU-029 (HLA-A2−EGFR+ and MAGE-3+) cell line was a kind gift from Dr. James Rocco (Harvard Medical School, Boston, MA) in January 2007. All cell lines were authenticated, and validated as unique using STR profiling and HLA genotyping every 6 months (24, 25). Cell lines were grown in IMDM (Sigma, St. Louis, MO) supplemented with 10% FBS (Cellgro, Manassas, VA), 2% L-glutamine and 1% penicillin/streptomycin (Invitrogen, Carlsbad, CA) at 37°C in a 5% CO2, 95% humidity atmosphere. Adherent tumor cells were detached by warm Trypsin-EDTA (0.25%) solution (Invitrogen, Carlsbad, CA).
Antibodies, cytokines and FcγR IIIa genotyping
The EGFR-specific chimeric IgG1 mAb cetuximab (Erbitux™), and CD137-specific human IgG4 mAb urelumab (BMS-663513) were obtained from Bristol-Myers Squibb (Imclone, Princeton, NJ). FITC-CD56, AF-700 CD56, PE-Texas Red-CD56, PercpCy5.5-CD3, AF700-CD3, PE-CRTAM, PECy5-CD137, PE-Texas Red-CD16, APC-PD-1, FITC-CD69, PE-Cy7-NKG2D, BV421-NKp46, APC-Cy7 IFN-γ, AF-700 TNF-α, FITC-Ki67, PE-CD107a, AF647-Granzyme B, PE-Cy5–CD8, APC-Cy7–CD8, PerCPCy5.5-CD4, BV421-TIM3, APC-CD14, BV-421-PD-L1 were purchased from Biolegend. FITC or PE-Cy7-anti-CD11c mAb (R & D systems), anti-CD80 mAb, anti-CD86 mAb, anti-PD-L1 mAb, anti-HLA-DR mAb, and EPCAM mAb (BD Biosciences Pharmingen) were purchased. PE-Cy5 anti-CD137 Ab and PE- and FITC- conjugated IgG isotypes for flow cytometry were purchased from BD Biosciences. FITC- goat-anti-human Fc specific IgG and FITC-goat anti-mouse IgG were purchased from Invitrogen. The antigen processing machinery components (APM) TAP-1-specific mAb (clone NOB1) and TAP-2 specific mAb (clone NOB2) were developed and characterized as described (26). Recombinant human GM-CSF and recombinant human IL-4, were purchased from R&D Systems Inc (Minneapolis, MN). Frozen patient PBMC were thawed and subjected to viability testing by using Zombie-aqua™ fixable viability kit (Biolegend) for multicolor flow cytometry.
FcγR IIIa-158 genotype was determined using a quantitative PCR-based assay kit from Applied Biosystems (Framingham, MA). Briefly, genomic DNA was extracted using the DNeasy Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. 5 to 50 ng of genomic DNA were added to a 25-μL reaction using 2× Taqman master mix (Applied Biosystems). Plates were run and analyzed for allelic expression using an ABI prism 7700 sequence detection system (2, 4).
Flow cytometry
Lymphocytes, NK cells were prepared for flow cytometry by washing with PBS (Sigma-Aldrich, St. Louis, MO) and FACS buffer (2% FBS in PBS). Cells were analyzed on BD LSR Fortessa™ flow cytometer (Beckman Dickinson) using FACS DIVA software, and data were analyzed by using Flowjo software. Intracellular APM component (TAP-1, TAP-2, LMP-2 specific mAbs, 1:500 dilutions) staining of DC was performed by using cytofix/cytoperm fixation/permeabilization kit (BD Bioscience) (26).
Tumor and lymphocyte specimens
Peripheral venous blood samples were obtained from HNC patients with stage III/IVA disease (Table 1), receiving neoadjuvant cetuximab on a prospective phase II clinical trial (UPCI 08-013, NCT 01218048). Tumors were biopsied immediately before, and again after 4 weeks, of cetuximab therapy. Clinical response was analyzed by comparing paired CT scans pre/post cetuximab, and quantifying tumor measurement by a dedicated head and neck radiologist blinded to patient status. Anatomic tumor measurements were recorded in two dimensions and the cohort segregated into clinical responders, or non-responders. Peripheral venous blood samples were obtained from HNC patients receiving cetuximab plus urelumab on a phase Ib, open-label trial (UPCI-14-049, NCT02110082).
Table 1.
Demographics table of cetuximab, cetuximab plus urelumab treated cohorts
Statistical Analysis
Data were analyzed statistically using GraphPAD Prism 4.0. A two way ANOVA, A two-tailed unpaired or paired t-test was used to calculate whether observed differences were statistically significant, defined as p≤0.05*, p≤ 0.01**, p≤ 0.001***, p≤ 0.0001****.
Results
Cetuximab-mediated NK cell expression of CD137 is dependent on FcγRIIIa polymorphism
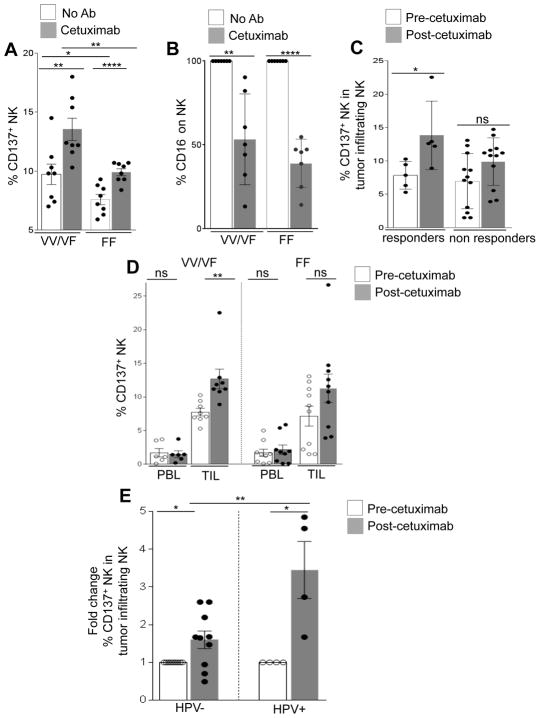
CD137 is an activation marker for NK cells after exposure to cetuximab. Although cetuximab coated HNC cells significantly enhanced CD137 expression on all NK cells, CD137 expression was significantly higher on NK cells expressing FcγRIIIa VV/VF (n=8) compared with FcγRIIIa FF (n=8) genotype (Fig. 1A). Upregulation of CD137 on both groups coincided with decreased expression of surface CD16 (Fig. 1B). To validate the clinical importance of these in vitro findings, we used specimens from a phase II clinical trial (UPCI 08-013) in which tumors from HNC patients were biopsied before and after 4 weeks of single-agent neoadjuvant cetuximab. CD137 expression was measured on intratumoral CD56+CD3− NK cells by flow cytometry from freshly isolated TIL, and correlated with radiological clinical response. Following cetuximab therapy, CD137 expression was significantly upregulated in clinical responders (n=5, p=0.02) but not in non-responders (n=12) (Fig. 1C).
Figure 1. Cetuximab-mediated NK cell expression of CD137 is dependent on FcγRIIIa polymorphism.
Expression of CD137 (VV/VF n=8, FF n=8) (A), and CD16 (VV/VF n=7, FF n=7) (B) on healthy donor peripheral blood NK cells from high (VV/VF) and low affinity (FF) FcγRIIIa genotypes was determined after co-culture with PCI-15B cells in the presence of cetuximab (10 μg/ml, 24h) or no Ab. Flow cytometric analysis of CD137 expression in tumor infiltrating NK cells from HNC patients before and after cetuximab neoadjuvant therapy (cetuximab IV 400 mg/m2 day 1 then 250 mg/m2 alone days 8, 15, and 22 (UPCI 08-013). Patients defined as responders (n=5 responders, n=12 non responders), demonstrated upregulation of CD137 on tumor infiltrating NK cells following cetuximab therapy compared with non-responders (C). Frequency of CD137 in peripheral blood NK cells (PBL VV/VF n=7, PBL FF n=9) and tumor infiltrating NK cells (TINK VV/VF n=8, TINK FF n=10) were determined in VV/VF and FF HNC patients before and after cetuximab neoadjuvant therapy (UPCI 08-013) (D). Percentage of CD137 in tumor infiltrating NK cells was determined before and after cetuximab neoadjuvant therapy (UPCI 08-013) and correlated with HPV status of HNC patients HPV (−) n=10, HPV (+) n=4 (E). A two tailed unpaired or paired t test was performed for statistical analysis, collective data are representative of ± SEM.
We then measured the expression of CD137 in peripheral blood and intratumoral NK cells before and after therapy with prospective neoadjuvant cetuximab clinical trial (UPCI 08-013). Significant induction of CD137 was observed on intratumoral, but not circulating NK cells, primarily in FcγRIIIa VV/VF patients (p=0.03) (Fig. 1D). In contrast, no significant increase in CD137 expression was observed on intratumoral or peripheral blood NK cells in FcγRIIIa FF patients (Fig. 1D) consistent with the correlation of CD137 upregulation with clinical response to neoadjuvant cetuximab. We next compared CD137 expression on intratumoral NK cells in HPV (+) and HPV (−) HNC patients treated on the neoadjuvant cetuximab trial. Although cetuximab treatment raised CD137 expression on both HPV (−) and HPV (+) patients NK cells, the mean induction of CD137 on HPV (+) patients was significantly higher than HPV (−) patients (Fig. 1E).
Urelumab enhances cetuximab-mediated DC maturation
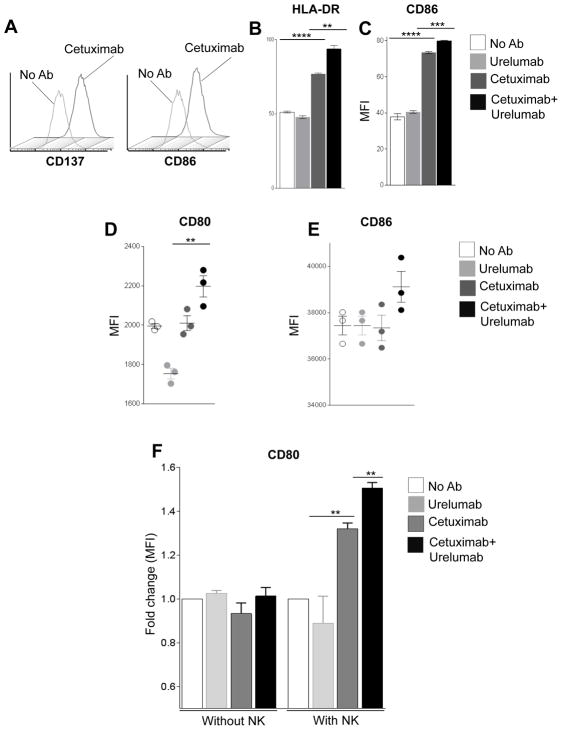
In addition to stimulating better innate immunity through activated NK cells, we investigated whether cetuximab modulates the expression of CD137 on DC during cetuximab-mediated NK: DC crosstalk. We measured surface activation/maturation markers on DC co-cultured with PCI-15B cells and NK cells in the presence of cetuximab. FACS analysis of DC showed significant upregulation of CD137 and CD86 in the presence of cetuximab plus NK cells (Fig. 2A, supplementary fig. S1). Furthermore, we examined whether the addition of urelumab could enhance cetuximab-mediated DC maturation in the presence of NK cells. Compared to cetuximab alone, the addition of urelumab significantly enhanced HLA-DR and CD86 expression (Fig. 2B–C). Since both DC and NK express CD137, we investigated whether enhanced DC maturation is the direct effect of urelumab, or if this was mediated by NK cells activated by urelumab. Purified NK cells were incubated with cetuximab-coated PCI-15B cells (24h) then isolated. These cetuximab-activated NK cells were then co-cultured with autologous immature DC and PCI-15B cells in the presence of urelumab, cetuximab or a combination of both mAbs for 48h. Although urelumab alone failed to induce CD80 (Fig. 2D), CD86 (Fig. 2E), the combination of urelumab and cetuximab augmented cetuximab-mediated DC maturation markers (Fig. 2D–E). To determine the additive effect of urelumab on cetuximab-mediated NK:DC cross talk we co-cultured DC and PCI-15B cells, in absence or presence of NK cells. Whole PBMC was incubated with cetuximab-coated HNC cells for 24h. NK cells were then purified and co-cultured with autologous DC and PCI-15B cells for 48h without mAb, or co-cultured with autologous DC and PCI-15B cells for 48h in the presence of cetuximab, urelumab or a combination of both mAbs. Urelumab alone failed to upregulate CD80 on DC co-cultured with or without NK cells. However, urelumab in combination increased cetuximab-mediated CD80 expression on DC, when NK cells were present in co-culture (Fig. 2F). These results suggest that cetuximab-induced DC maturation, important for NK:DC cross-talk, is enhanced by urelumab, and dependent on NK cell activation.
Figure 2. Urelumab enhances cetuximab-induced DC maturation.
Representative histograms demonstrating upregulation of CD86 and CD137 on DC co-cultured with NK cells and PCI-15B (1:1:1 ratio) in the presence of cetuximab (10 μg/ml, 24h) or no Ab (A). Whole PBMC was incubated with cetuximab-coated PCI-15B, and then NK cells were purified and incubated with DC and PCI-15B cells, in the presence of urelumab (50μg/ml), cetuximab (10 μg/ml), or cetuximab (10 μg/ml) plus urelumab (50μg/ml). FACS analysis of upregulation of maturation markers, HLA-DR (B) and CD86 (C) on DC co-cultured with cetuximab-activated NK cells (in PBMC) and PCI-15B cells in the presence of cetuximab plus urelumab. Expression level of CD80 (D), CD86 (E) on DC was analyzed by FACS indicates that cetuximab-mediated NK-induced DC maturation is increased by addition of urelumab. Whole PBMC were incubated with cetuximab-coated PCI-15B cells, and then NK cells were purified and incubated with DC, PCI15B, and expression level of CD80 (F) on DC was evaluated after co-culture with PCI-15B cells with or without NK cells treated with cetuximab, urelumab or both. A two tailed paired t test was performed for statistical analysis, collective data are representative of ± SEM.
Urelumab enhances cetuixmab-mediated cross-presentation of TA in the presence of NK cells by augmenting antigen processing machinery in DC
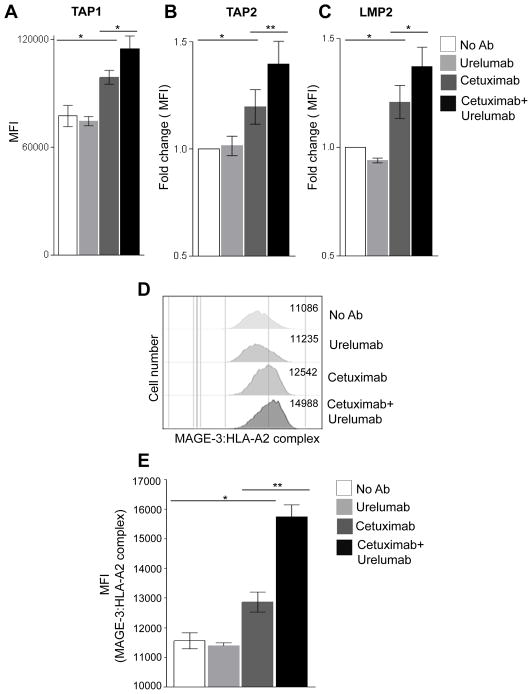
We observed that urelumab augments cetuximab-mediated DC maturation (Fig. 2B–F), a crucial mechanism for cross-priming of TA specific T cells (2). Moreover, type I DC have previously been shown to secrete high levels of Th1 cytokines and chemokines (26), which may augment the expression of certain APM components, such as TAP-1/2 and LMP-2, which are important for TA derived peptide presentation to cognate CTL. Thus, investigated whether intracellular APM components were upregulated in DC incubated with cetuximab-activated NK cells plus HNC cells (JHU-029) in the presence of urelumab. Interestingly, the addition of urelumab enhanced expression of TAP-1 (Fig. 3A), TAP-2 (Fig. 3B), and LMP-2 (Fig. 3C) by DC that were co-cultured with cetuximab-activated NK cells.
Figure 3. Enhancement of DC APM pathway by urelumab.
Intracellular levels of antigen processing machinery components TAP1 (A), TAP2 (B), LMP-2 (C) on DC were evaluated after co-culture with NK cells and PCI-15B cells in presence of urelumab (50μg/ml), cetuximab (10 μg/ml) or cetuximab (10 μg/ml) plus urelumab (50μg/ml) (48h at 1:1:1 ratio). (D–E) Cell surface expression of MAGE-3:HLA-A2 complex on DC was determined after co-culture with DC: NK: JHU-029 (1:1:1 ratio, 48h co-culture) with MAGE-3:HLA-A2 complex specific mAb 12b6. A two tailed unpaired or paired t test was performed for statistical analysis, collective data are representative of ± SEM.
We then utilized a novel mAb (clone 12B6), recognizing the HLA-A2:MAGE-3271–279 complex (27), to investigate whether the enhanced HLA class I APM components resulted in elevated levels of surface HLA-TA complexes. Indeed, cetuximab treatment enhanced HLA-A2:MAGE-3271–279 complexes on DC in the presence of JHU-029 and NK cells (Fig. 3D–E), but not in urelumab alone-treated cells, or those co-cultured without MAGE-3271–279 positive HNC cells (data not shown). Furthermore, the combination of urelumab plus cetuximab further augmented TA presentation in a quantitative function (Fig. 3D–E) (p=0.02).
Combination of cetuximab and urelumab enhances anti-apoptotic proteins on cetuximab-activated NK cells
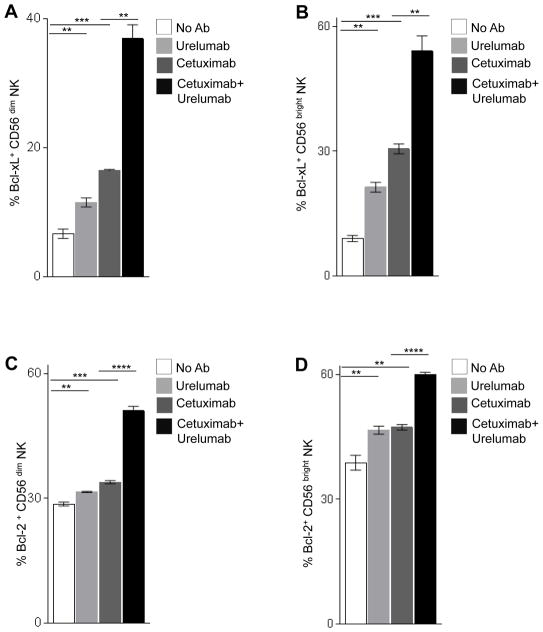
We investigated the role of CD137 stimulation in boosting the survival of NK cells in co-culture with HNC cells (18, 22). We measured the expression level of the anti-apoptotic mitochondrial proteins, Bcl-xL and Bcl-2 on NK cells as an indicator of cell survival. First, we stimulated healthy donor PBMC with cetuximab-coated JHU-029 for 24h and purified NK cells. NK cells were then co-cultured with urelumab, cetuximab, or a combination of urelumab and cetuximab, in the presence of JHU-029 cells and autologous DC for 36h. Intracellular staining of CD56+ Bcl-xL+ and CD56+ Bcl-2+ NK cells was analyzed by FACS. Urelumab alone enhances Bcl-xL level in both CD56low NK (Fig. 4A) and CD56bright NK (Fig. 4B). Higher levels of Bcl-xL were observed in NK cells activated by cetuximab alone. Interestingly, the combination of urelumab and cetuximab further increased the levels of Bcl-xL on NK cells (Fig. 4A–B). Similarly, urelumab and cetuximab alone enhanced Bcl-2 expression in CD56dim, and CD56bright NK cells. Again, the combination of urelumab and cetuximab significantly increased Bcl-2 expression on both NK cell subsets, and addition of urelumab enhanced viability of NK cells (Fig. 4C–D, supplemental figure S 2A, B).
Figure 4. The combination of cetuximab and urelumab enhances anti-apoptotic proteins on cetuximab-activated NK cells.
The levels of expression of intracellular anti-apoptotic proteins Bcl-xL CD56dim NK (A), and CD56bright NK (B) was analyzed by intracellular FACS after co-culture with DC:NK:PCI-15B (1:1:1 ratio, 36h) in the presence of urelumab (50μg/ml), cetuximab (10μg/ml) or cetuximab (10 μg/ml) plus urelumab (50μg/ml). The expression level of intracellular anti-apoptotic proteins Bcl-2 in CD56low NK (C), and CD56bright NK (D), was analyzed by intracellular FACS after co-culture with DC:NK:PCI-15B (1:1:1 ratio, 36h) in presence of urelumab (50μg/ml), cetuximab (10μg/ml), cetuximab (10 μg/ml) plus urelumab (50μg/ml). A two tailed unpaired or paired t test was performed for statistical analysis, collective data are representative of ± SEM.
Immunophenotypic analysis of urelumab in combination with cetuximab in HNC patients
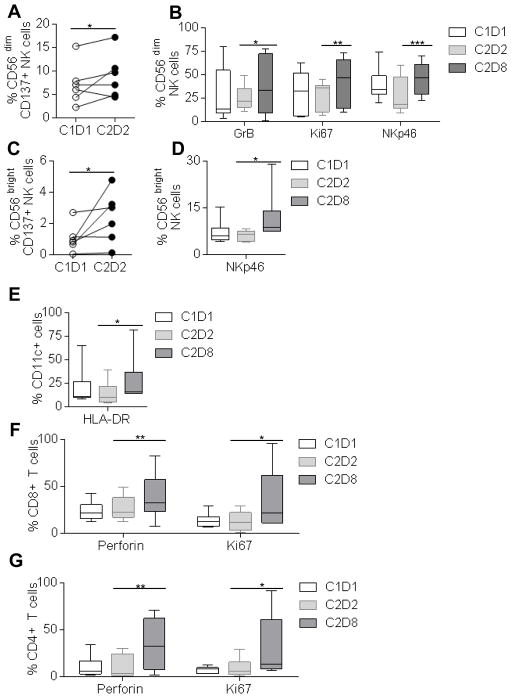
To identify modulation of biomarkers in innate and adaptive immune cell types, we performed multi-color flow cytometry in PBMC obtained from advanced stage HNC patients treated on a phase IB trial of cetuximab plus urelumab (UPCI-14-049, NCT02110082) (Table 1). PBMC were tested before and 24h after cetuximab treatment, and after two cycles of cetuximab plus urelumab treatment (Schema, Table 1). We observed enhancement of CD137 receptor on CD56low and CD56bright NK cells at 24h after cetuximab treatment (Fig. 5A, C). Enhancement of cytotoxic marker granzyme B, proliferation marker Ki67, and natural cytotoxic receptor NKp46 upregulation was apparent after the combination of cetuximab and urelumab in CD56low NK cells (Fig. 5B), whereas, NKp46 upregulation is seen in CD56bright NK cells (Fig. 5D), however no changes in the expression level of, TNF-α, CRTAM, IFN-γ, PD-1, CD69, NKG2D, CD107a, and CD16 were observed in CD56low cells, similarly no changes in the expression level of TNF-α, CRTAM, IFN-γ, PD-1, CD69, NKG2D, CD107a, Gr-B, Ki67 and CD16 was observed in CD56bright NK cells (Supplementary Fig. S3A–B). Furthermore, in accordance with our in vitro results (Fig. 2), we also observed upregulation of HLA-DR in CD11c+ myeloid cells (Fig. 5E), whereas no changes in the expression level of CD80, CD86, PD-L1, CD14, CD11c was observed in CD11c+ myeloid immune cells (Supplementary Fig. S3C). Interestingly, we also observed upregulation of perforin, and Ki67 expression level in CD8+ T cells, and CD4+ T cells (Fig. 5F–G), whereas no changes in the expression of TNF-α, IFN-γ, CRTAM, TIM-3, PD-1, Gr-B, CD69 were observed in these T cells (Supplementary Fig. S3D–E).
Figure 5. Immunophenotypic analysis of CD56dim, CD56bright, CD11c+ myeloid cells, CD8+ T cells, and CD4+ T cells in PBMC isolated from UPCI-14-049, an open-label phase Ib clinical trial.
The level of expression of CD137 receptor in baseline PBMC samples (C1D1), 24h after cetuximab treatment (C2D2) were analyzed in CD56low CD3− NK cells (A). The levels of expression of intracellular granzymeB, Ki67, and surface molecule NKp46 was analyzed in baseline PBMC samples (C1D1), 24h after cetuximab treatment (C2D2), cetuximab plus two cycles of urelumab treatment (C2D8) in CD56dim CD3− NK cells (B). The level of expression of CD137 receptor in baseline PBMC samples (C1D1), 24h after cetuximab treatment (C2D2) were analyzed in CD56dim CD3− NK cells (C). The levels of expression of surface molecule NKp46 was analyzed in baseline PBMC samples (C1D1), 24h after cetuximab treatment (C2D2), cetuximab plus two cycles of urelumab treatment (C2D8) in CD56bright CD3− NK cells (D), The expression level of HLA-DR in CD11c+ myeloid cells was analyzed in baseline PBMC samples (C1D1), 24h after cetuximab treatment (C2D2), cetuximab plus two cycles of urelumab treatment (C2D8) (E). The expression level of perforin, and Ki-67 was analyzed in CD8+ T cells (F), and CD4+ T cells (G), in baseline PBMC samples (C1D1), 24h after cetuximab treatment (C2D2), cetuximab plus two cycles of urelumab treatment (C2D8). One-tailed Wilcoxon matched-pair signed rank test was performed for statistical analysis (A, C). A Two-way ANOVA, Tukey’s multiple comparison test was performed for statistical analysis, collective data are representative of six different donors at different time points, ± SEM (n=6).
Discussion
This is the first study to analyze combined triggering of CD137 after cetuximab induced activation of NK cells, and effects on DC processing and presentation to TA-specific T cells. In this study, we investigated the effect of harnessing CD137 expression on NK, DC, and stimulation to enhance innate and adaptive anti-tumor immune responses. Upregulated expression of CD107a (tumor infiltrating NK cells), perforin (peripheral blood NK cells), granzymeB (peripheral blood NK cells) (data not shown), and CD137 (tumor infiltrating NK cells) was observed in a cetuximab neo-adjuvant trial (UPCI-08-013, NCT01218048) and addition of urelumab to cetuximab treatment in a combinatorial clinical trial, (UPCI-14-049, NCT02110082), showed enhancement in NK cell, DC, and T cell functionality. We observed that cetuximab-activated NK cells express surface CD137, which correlated with clinical response to neoadjuvant cetuximab. Interestingly, eetuximab-activated CD56dim NK cells upregulate CD137 receptor to a greater extent than CD5bright NK cells. These NK cells could be triggered using agonistic anti-CD137 mAb to potentiate the cytotoxic and helper function of NK cells, thus improving their role in anti-tumor immunity. We additionally demonstrate that urelumab can uniquely enhance survival of distinct immune cell types by upregulating mitochondrial anti-apoptotic proteins Bcl-xL, Bcl-2, modulating the NF-κB pathway (18, 22, 28).
Given the presence of CD137 on cetuximab-activated NK cells (Fig. 1, 2) and on DC (Fig. 2) and the importance of cetuximab-induced NK:DC crosstalk on the expansion of CTL(2), we tested the impact of CD137 on the function of DC in the tumor microenvironment. The stimulatory CD137 mAb, urelumab alone failed to elevate DC maturation markers or cross-presentation of TA by DC even in the presence of NK cells. Moreover, we see an unexpected decrease in the CD80 expression in urelumab alone treated co-culture (Fig. 2D) Altogether, this inability of the CD137 agonist mAb alone to control HNC had been recently proves in a mouse HPV+ HNC model, where stimulatory CD137 neither affected tumor growth nor survival of experimental animals (29, 30). We observed that cetuximab-mediated NK cell activation in the presence of other lymphocytes, i.e in unfractionated PBMC, are sensitive to a second dose of cetuximab, and the cetuximab plus urelumab combination, to induce DC maturation. Interestingly, cetuximab-mediated NK activation in absence of other immune cells are refractory to a second dose of cetuximab, whereas the combination of cetuximab plus urelumab promotes DC maturation (Fig. 2D–F).
Previously, we showed that cetuximab-HNC cell (JHU-029) complexes, in the presence of NK and DC generate polyclonal TA (MAGE-3, and EGFR) which are then processed and presented to CTL (2). This is facilitated by DC maturation as identified by upregulation of the maturation/activation molecules, HLA-DR, CD80, CD86, and CD137 (Fig. 2) (6). Interestingly, the addition of urelumab enhanced DC maturation and upregulation of APM components in the presence of cetuximab-activated NK cells. This observation supports the hypothesis of boosting the “vaccinal effect” of cetuximab by cross-linking CD137 mAb to their receptors (Fig. 2–3) (20). Using a novel neoadjuvant trial of single agent cetuximab and associated paired specimens, we demonstrate that CD137 upregulation is associated with clinical response.
Cetuximab-coated HNC cells induce a higher magnitude of CD137 induction on healthy donor NK cells carrying high-affinity FcγRIIIa VV/VF than low-affinity FcγRIIIa FF (Fig. 1D). This correlates with enhancement of cetuximab-mediated ADCC by urelumab (data not shown). In vivo, CD137 induction in tumor infiltrating NK cells predicted clinical outcomes to neoadjuvant cetuximab therapy and our data suggest better clinical outcomes might be seen in patients if urelumab is added to cetuximab treatment regimens. In circulating NK cells from patients on UPCI-08-013 trial, we did not see induction of CD137 post cetuximab therapy in peripheral blood lymphocytes but only in tumor infiltrating lymphocytes. We did observe CD137 induction in NK cells post cetuximab therapy in the UPCI-14-049 trial, when blood was available 24h after cetuximab treatment. This discrepancy in CD137 induction in two distinct trial could be affected by distinct regimens, where blood collection was performed on different time intervals (30days vs 24 hr, ref. 15). Cetuximab-induced tumor infiltrating NK cells showed CD137 induction in FcγRIIIa VV/VF patients, but not in FcγRIIIa FF patients (Fig. 1D). This suggests that NK cell recruitment, contact to HNC, and FcγRIIIa affinity are major players in determining overall NK cell function in response to cetuximab at the tumor site.
HPV (+) HNC are commonly believed to be a separate disease entity and correlates with better prognosis than HPV (−) HNC. Although in vivo both HPV(+) and HPV(−) tumors display higher expression of CD137 on tumor infiltrating NK cells compared with peripheral NK cells post cetuximab, we notice a higher magnitude of CD137 induction on NK cells infiltrating HPV(+) HNC. Increased susceptibility of HPV (+) HNC to NK cells could be attributed to the previously established antiviral functionality of NK cells (29), which may be differentially boosted by cetuximab in HPV (+) tumors. Thus, HPV (+) HNC may result in better clinical outcomes than HPV (−) HNC when treated with a combination of cetuximab and urelumab.
In phase1b, open label, urelumab in combination with cetuximab trial, we observed enhancement in cytotoxic and proliferation markers in NK cells, and HLA-DR upregulation in myeloid cells, similarly we show upregulation of proliferation marker Ki67, and perforin expression in CD8, CD4 T cells. Although we see enhancement in immune activity with urelumab treatment after cetuximab, the clinical outcome for the sequential combination of urelumab, and cetuximab should be tested in bigger cohort.
In agreement with several reports (17, 31–34), our findings support the notion of strengthening anti-tumor immunity with urelumab, albeit in the presence of NK cells already activated by cetuximab in the tumor microenvironment. Taken together, these results suggest that CD137 may present a biomarker of immune and clinical response to cetuximab treatment and provide a novel mechanism of enhancement of cetuximab.
Supplementary Material
Translational Relevance.
The anti-epidermal growth factor receptor (EGFR) mAb cetuximab acts through blocking oncogenic signals and by inducing Fcγ receptor (FcγR) mediated cytotoxicity and cross-priming of T cell responses. However, cetuximab only modestly improves clinical outcome in head and neck cancer (HNC) patients. Therefore, a suitable combinatorial agent, which can boost cetuximab mediated anti-tumor cellular immunity is warranted. Stimulation of CD137 delivers a robust co-stimulatory signal to both NK and DC, potentially improving adaptive, T cell based anti-tumor immune responses. The implication of these finding includes identification of biomarkers for combination therapy.
Acknowledgments
Financial support: National Institute of Health grants R01 DE019727, P50 CA097190, University of Pittsburgh Cancer Center Support Grant P30CA047904.
Abbreviations used in this article
- HNC
head and neck cancer
- EGFR
Epidermal growth factor receptor
- TA
tumor-antigen
Footnotes
Conflict of interest: Robert L. Ferris has received research grants from Bristol Myers Squibb and AZ/Medimmune and has been a consultant/advisory board member for Celgene, Merck, BMS and AZ/Medimmune.
References
- 1.Ferris RL. Immunology and immunotherapy of head and neck cancer. J Clin Oncol. 2015;33(29):3293–304. doi: 10.1200/JCO.2015.61.1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. 2013;19(7):1858–72. doi: 10.1158/1078-0432.CCR-12-2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Trivedi S, Concha-Benavente F, Srivastava RM, Jie HB, Gibson SP, Schmitt NC, et al. Immune biomarkers of anti-EGFR monoclonal antibody therapy. Ann Oncol. 2015;26(1):40–7. doi: 10.1093/annonc/mdu156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. 2009;58(11):1853–64. doi: 10.1007/s00262-009-0697-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lopez-Albaitero A, Ferris RL. Immune activation by epidermal growth factor receptor specific monoclonal antibody therapy for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2007;133(12):1277–81. doi: 10.1001/archotol.133.12.1277. [DOI] [PubMed] [Google Scholar]
- 6.Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. 2011;50(2–3):248–54. doi: 10.1007/s12026-011-8231-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andrade Filho PA, Lopez-Albaitero A, Gooding W, Ferris RL. Novel immunogenic HLA-A*0201-restricted epidermal growth factor receptor-specific T-cell epitope in head and neck cancer patients. J Immunother. 2010;33(1):83–91. doi: 10.1097/CJI.0b013e3181b8f421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schuler PJ, Boeckers P, Engers R, Boelke E, Bas M, Greve J, et al. EGFR-specific T cell frequencies correlate with EGFR expression in head and neck squamous cell carcinoma. J Transl Med. 2011;9:168. doi: 10.1186/1479-5876-9-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Comprehensive genomic characterization of head and eck squamous cell carcinomas. Nature. 2015;517(7536):576–82. doi: 10.1038/nature14129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferris RL, Whiteside TL, Ferrone S. Immune escape associated with functional defects in antigen-processing machinery in head and neck cancer. Clin Cancer Res. 2006;12(13):3890–5. doi: 10.1158/1078-0432.CCR-05-2750. [DOI] [PubMed] [Google Scholar]
- 11.Ascierto PA, Kalos M, Schaer DA, Callahan MK, Wolchok JD. Biomarkers for immunostimulatory monoclonal antibodies in combination strategies for melanoma and other tumor types. Clin Cancer Res. 2013;19(5):1009–20. doi: 10.1158/1078-0432.CCR-12-2982. [DOI] [PubMed] [Google Scholar]
- 12.Morris GP, Chen L, Kong YC. CD137 signaling interferes with activation and function of CD4+CD25+ regulatory T cells in induced tolerance to experimental autoimmune thyroiditis. Cell Immunol. 2003;226(1):20–9. doi: 10.1016/j.cellimm.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 13.Choi BK, Lee SC, Lee MJ, Kim YH, Kim YW, Ryu KW, et al. 4-1BB-based isolation and expansion of CD8+ T cells specific for self-tumor and non-self-tumor antigens for adoptive T-cell therapy. J Immunother. 2014;37(4):225–36. doi: 10.1097/CJI.0000000000000027. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X, Voskens CJ, Sallin M, Maniar A, Montes CL, Zhang Y, et al. CD137 promotes proliferation and survival of human B cells. J Immunol. 2010;184(2):787–95. doi: 10.4049/jimmunol.0901619. [DOI] [PubMed] [Google Scholar]
- 15.Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. 2014;124(6):2668–82. doi: 10.1172/JCI73014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Langstein J, Becke FM, Sollner L, Krause G, Brockhoff G, Kreutz M, et al. Comparative analysis of CD137 and LPS effects on monocyte activation, survival, and proliferation. Biochem Biophys Res Commun. 2000;273(1):117–22. doi: 10.1006/bbrc.2000.2889. [DOI] [PubMed] [Google Scholar]
- 17.Wilcox RA, Chapoval AI, Gorski KS, Otsuji M, Shin T, Flies DB, et al. Cutting edge: Expression of functional CD137 receptor by dendritic cells. J Immunol. 2002;168(9):4262–7. doi: 10.4049/jimmunol.168.9.4262. [DOI] [PubMed] [Google Scholar]
- 18.Lee HW, Park SJ, Choi BK, Kim HH, Nam KO, Kwon BS. 4-1BB promotes the survival of CD8+ T lymphocytes by increasing expression of Bcl-xL and Bfl-1. J Immunol. 2002;169(9):4882–8. doi: 10.4049/jimmunol.169.9.4882. [DOI] [PubMed] [Google Scholar]
- 19.Vinay DS, Kwon BS. 4-1BB (CD137), an inducible costimulatory receptor, as a specific target for cancer therapy. BMB Rep. 2014;47(3):122–9. doi: 10.5483/BMBRep.2014.47.3.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Houot R, Kohrt H. CD137 stimulation enhances the vaccinal effect of anti-tumor antibodies. Oncoimmunology. 2014;3(7):e941740. doi: 10.4161/21624011.2014.941740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murillo O, Dubrot J, Palazon A, Arina A, Azpilikueta A, Alfaro C, et al. In vivo depletion of DC impairs the anti-tumor effect of agonistic anti-CD137 mAb. European journal of immunology. 2009;39(9):2424–36. doi: 10.1002/eji.200838958. [DOI] [PubMed] [Google Scholar]
- 22.Kuang Y, Weng X, Liu X, Zhu H, Chen Z, Chen H. Effects of 4-1BB signaling on the biological function of murine dendritic cells. Oncol Lett. 2012;3(2):477–81. doi: 10.3892/ol.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jie HB, Gildener-Leapman N, Li J, Srivastava RM, Gibson SP, Whiteside TL, et al. Intratumoral regulatory T cells upregulate immunosuppressive molecules in head and neck cancer patients. British journal of cancer. 2013;109(10):2629–35. doi: 10.1038/bjc.2013.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao M, Sano D, Pickering CR, Jasser SA, Henderson YC, Clayman GL, et al. Assembly and initial characterization of a panel of 85 genomically validated cell lines from diverse head and neck tumor sites. Clinical cancer research: an official journal of the American Association for Cancer Research. 2011;17(23):7248–64. doi: 10.1158/1078-0432.CCR-11-0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heo DS, Snyderman C, Gollin SM, Pan S, Walker E, Deka R, et al. Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 1989;49:5167–75. [PubMed] [Google Scholar]
- 26.Lopez-Albaitero A, Mailliard R, Hackman T, Andrade Filho PA, Wang X, Gooding W, et al. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother. 2009;32(5):465–73. doi: 10.1097/CJI.0b013e3181a1c24e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. STAT1-Induced HLA Class I Upregulation Enhances Immunogenicity and Clinical Response to Anti-EGFR mAb Cetuximab Therapy in HNC Patients. Cancer Immunol Res. 2015;3(8):936–45. doi: 10.1158/2326-6066.CIR-15-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kroon HM, Li Q, Teitz-Tennenbaum S, Whitfield JR, Noone AM, Chang AE. 4-1BB costimulation of effector T cells for adoptive immunotherapy of cancer: involvement of Bcl gene family members. J Immunother. 2007;30(4):406–16. doi: 10.1097/CJI.0b013e31802eecc6. [DOI] [PubMed] [Google Scholar]
- 29.Macdonald DC, Hotblack A, Akbar S, Britton G, Collins MK, Rosenberg WC. 4-1BB ligand activates bystander dendritic cells to enhance immunization in trans. J Immunol. 2014;193(10):5056–64. doi: 10.4049/jimmunol.1301723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucido CT, Vermeer PD, Wieking BG, Vermeer DW, Lee JH. CD137 enhancement of HPV positive head and neck squamous cell carcinoma tumor clearance. Vaccines. 2014;2(4):841–53. doi: 10.3390/vaccines2040841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Srivastava RM, Savithri B, Khar A. Activating and inhibitory receptors and their role in natural killer cell function. Indian J Biochem Biophys. 2003;40(5):291–9. [PubMed] [Google Scholar]
- 32.Futagawa T, Akiba H, Kodama T, Takeda K, Hosoda Y, Yagita H, et al. Expression and function of 4-1BB and 4-1BB ligand on murine dendritic cells. Int Immunol. 2002;14(3):275–86. doi: 10.1093/intimm/14.3.275. [DOI] [PubMed] [Google Scholar]
- 33.Chester C, Marabelle A, Houot R, Kohrt HE. Dual antibody therapy to harness the innate anti-tumor immune response to enhance antibody targeting of tumors. Curr Opin Immunol. 2015;33C:1–8. doi: 10.1016/j.coi.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 34.Ito F, Li Q, Shreiner AB, Okuyama R, Jure-Kunkel MN, Teitz-Tennenbaum S, et al. Anti-CD137 monoclonal antibody administration augments the antitumor efficacy of dendritic cell-based vaccines. Cancer research. 2004;64(22):8411–9. doi: 10.1158/0008-5472.CAN-04-0590. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.