Abstract
During inflammation, leukocytes influx into lung compartments and interact with extracellular matrix (ECM). Two ECM components, versican and hyaluronan, increase in a range of lung diseases. The interaction of leukocytes with these ECM components controls leukocyte retention and accumulation, proliferation, migration, differentiation, and activation as part of the inflammatory phase of lung disease. In addition, bronchial epithelial cells from asthmatic children co-cultured with human lung fibroblasts generate an ECM that is adherent for monocytes/macrophages. Macrophages are present in both early and late lung inflammation. Matrix metalloproteinase 10 (MMP10) is induced in alveolar macrophages with injury and infection and modulates macrophage phenotype and their ability to degrade collagenous ECM components. Collectively, studies outlined in this review highlight the importance of specific ECM components in the regulation inflammatory events in lung disease. The widespread involvement of these ECM components in the pathogenesis of lung inflammation make them attractive candidates for therapeutic intervention.
Keywords: Asthma, Extracellular matrix, Fibrosis, Hyaluronan, Immunity, Inflammation, Macrophage, Matrix metalloproteinase 10, Versican
1. Introduction
The extracellular matrix (ECM) is a critical component of normal lung tissue that not only provides structural support for cells and tissue architecture of the airways and lung parenchyma, but also is a major effector of cell behavior and fate. Indeed, we now know that the ECM has considerable control over cellular function during lung development, homeostasis, normal repair, immunity, inflammation, and disease. The airways, blood vessels, interlobular septa, and visceral pleura are bordered and embedded in specialized ECM structures. As for all visceral organs, the lung ECM consist of two distinct compartments. One compartment is the basement membrane (basal lamina), which is a thin, organized layer of laminins, type IV collagen, nidogen/entactin, and perlecan, a heparan sulfate proteoglycan. Basement membrane is the substratum on which endothelial and epithelial cells reside and is well established as a key driver of differentiation and cell survival. The second compartment is the interstitium, which is mostly a loose connective tissue composed of an array of structural and nonstructural ECM components such as fibrillar collagens (e.g., types I and III), elastin, fibronectin, fibrillins, various proteoglycans, matricellular proteins (e.g., CCN proteins, SPARC, tenascins, thrombospondins) and polysaccharides, such as hyaluronan, an abundant and physiologically important glycosaminoglycan (GAG) [1, 2]. Within the interstitium are blood and lymph vessels, airway smooth muscle bundles and cartilage, and a range of cells types, including fibroblasts, pericytes, and resident leukocytes. Furthermore, the ECM includes numerous related proteins, such as the enzymes that form fibers, proteinases that remodel ECM, cytokines and growth factors that are stored within the ECM, and more.
Recent studies have further indicated that specific individual components of the ECM can impact developmental and pathological events within the lung. For the purpose of this review, we will focus on versican and hyaluronan, two interstitial ECM components [3] that can serve as ligands for leukocytes and impact immune and inflammatory responses in lung disease [3–7]. In addition, we will discuss how specific leukocytes, such as the macrophage, interact with the ECM and the importance of a specific matrix metalloproteinase (MMP), MMP10, in controlling the state of macrophage activation in lung disease.
2. The ECM as a regulator of the innate immune response
Inflammatory responses as a result of tissue infection require the emigration of leukocytes from the vasculature to the infected area as part of the innate immune response. Upon extravasation into the subendothelial and/or subepithelial compartments, leukocytes encounter an ECM enriched in versican and hyaluronan that functions as a scaffold or “landing strip” for cell adhesion and subsequent retention and activation [8] (Figure 1). These components are highly interactive and bind chemokines, growth factors, proteases, and receptors on the surface of the immune cells to provide intrinsic signals and influence immune cell phenotype [9–11]. We recently demonstrated that hyaluronan interacts with the surface of T-regulatory cells through CD44 and promotes their differentiation [12–14]. Furthermore, once bound, these leukocytes modify the ECM in such a way as to generate pro-inflammatory ECM fragments to further drive the inflammatory response [15, 16]. Fragments of ECM affect multiple functional properties of inflammatory and immune cells [17]. Since different types of infection may demand extravasation of certain immune cell types, the ECM often undergoes compositional changes which regulate the appropriate cellular responses. Such compositional changes may enrich for specific ECM molecules that actively participate in the recruitment and activation of specific immune cell types to either promote or inhibit the inflammatory cascade [18]. Such findings suggest that the ECM may be a useful therapeutic target to control various aspects of the immune response associated with inflammation in a variety of diseases [19].
Figure 1.
Extravasation of leukocytes across the endothelium and/or epithelium (E) into the interstitium of the tissue during an inflammatory response. The leukocytes interact with specific ECM components, such as versican and hyaluronan, generated by resident cells of the tissue, such as endothelia and epithelia, and stromal cells, such as fibroblasts and smooth muscle cells. This interaction involves receptor-mediated interactions with hyaluronan and versican via cell surface receptors such as PSGL-1, TLR2, and CD44. These interactions in turn influence leukocyte phenotype by stimulating intracellular signals that promote their adhesion, proliferation, migration, differentiation, and activation. Furthermore, the leukocytes themselves may produce versican and hyaluronan in response to inflammatory stimuli to further enrich the matrix with these specific components. Such matrices, depending on their interactive partners, may exhibit either pro-inflammatory or anti-inflammatory properties. Figure from: Thomas N. Wight, Inkyung Kang, Mervyn J. Merrilees, Matrix Biology. 35:152–161, 2014, http://dx.doi.org/10.1016/j.matbio.2014.01.015. Reuse permitted under Creative Commons Attribution-NonCommercial-No Derivatives License (CC BY NC ND).
3. ECM components: interaction with leukocytes
A number of different ECM components interact with leukocytes and it has become clear that this interaction is a critical part of the inflammatory response [20]. It has also become clear that the ECM exhibits specificity for binding leukocytes and impacting their phenotype [6]. We have become interested in versican and hyaluronan, which increase during inflammation. Versican is a proteoglycan that exists in at least four different isoforms due to alternative splicing of the major exons that code for the attachment regions of the chondroitin sulfate (CS) GAGs attached to the core protein [21, 22]. Versican interacts with a number of other molecules, many of which are involved in promoting tissue inflammation [6]. For example, versican interacts with hyaluronan [23, 24], link protein, TSG-6, and CD44 through a common structural domain in each of the proteins called the link module [25, 26]. These macromolecules form higher ordered macromolecular complexes that increase as part of the inflammatory response [27–31]. Known functions for versican include controlling tissue space due to its ability to entrap water such as observed in the lung [32, 33], as well as influencing cell adhesion, proliferation, migration, and survival [21, 34–36]. Versican is highly interactive due to the negatively charged CS side chains. For example, versican regulates the availability and activity of several inflammatory chemokines [37–41]. In addition, the CS chains of versican interact with MMPs [42], influencing their catalytic activity [43–45]. As versican accumulates in diseased tissues, it can be degraded by a number of proteases including five members of the a disintegrin and metalloproteinase with a thrombospondin type-1 motif (ADAMTS) family of proteases [46, 47]. Cleavage of versican generates biologically active fragments that have been associated with inflammatory cytokine release and cell death through apoptosis [48, 49]. In addition, the G3 domain of versican can interact with P-selectin glycoprotein-1 (PSGL-1) and cause macrophage aggregation [50]. Several studies indicate that versican is a danger-associated molecular pattern (DAMP) molecule that interacts with toll-like receptors (TLRs), such as TLR2 on alveolar macrophages to promote production of inflammatory cytokines such as tumor necrosis factor α (TNFα), IL-6, and other pro-inflammatory cytokines [51–57]. As such, versican has been implicated in regulating several key events in the inflammatory response [3, 6, 36, 58, 59].
Several studies have demonstrated that hyaluronan, a binding partner of versican, influences inflammatory responses [60–62]. Hyaluronan interacts with the surface of many myeloid and non-myeloid cells through CD44, a major cell surface receptor for hyaluronan [63], to affect their phenotype. Lack of CD44 leads to excessive accumulation of hyaluronan in the lungs of bleomycin-treated animals due to the inability of lung cells to clear hyaluronan via CD44 [64]. As hyaluronan accumulates, hyaluronan fragments also accumulate and interact with immune cells to promote the expression of specific inflammatory cytokines and chemokines to drive the immune response. Low molecular weight hyaluronan (LMW-HA) activates inflammatory gene expression in epithelial cells, dendritic cells, endothelial cells, fibroblasts, and macrophages [65–71]. Lack of CD44 limits macrophage recruitment to the lung when mice are challenged with lipopolysaccharide (LPS) [72]. Interestingly, alveolar macrophages isolated from CD44-deficient, LPS-treated mice secrete lower levels of TNFα, suggesting a key role for CD44 in the innate immune response to LPS. Recently, it has been recognized that it is possible to stimulate chemokine expression by immune cells in the absence of CD44 [73–75]. This stimulation involves the TLRs as part of the innate immune response [76]. Using a series of TLR−/− mice, it has been demonstrated that hyaluronan fragments stimulate elicited alveolar macrophages to express inflammatory chemokines via TLR2 and TLR4 pathways [75, 77]. TLRs are found not only on myeloid cells, but also on non-myeloid cells of the lung [78, 79]. While the above studies implicate TLR2 and TLR4 in the hyaluronan innate immune response, other studies suggest that TLR3 is involved since viral infection of synoviocytes causes hyaluronan synthase 1 (HAS1) activation with no effect on HAS2 or HAS3 [80]. Interestingly, overexpression of HAS2 in epithelial cells is associated with decreased hyaluronan cable structures and reduced monocyte binding to the ECM [81]. These findings, plus our own data, suggest that HAS1, together with versican, may be critical for the formation of this proinflammatory ECM following lung infection. In fact, a number of studies demonstrate that these ECMs are important for leukocyte adhesion during inflammation in the lung [64, 82], colon [83–85], kidney [86, 87], and skin [88, 89].
4. Versican and hyaluronan as components in lung disease
The observation that versican, a CS proteoglycan (CSPG), is abundantly expressed during lung development, is expressed at very low levels in lungs of healthy adults, but is re-expressed and accumulates in a number of lung diseases, including bacterial infection (Figure 2), suggests that versican functions in a number of overlapping processes in lung development, injury, and repair [32, 90–92]. Recent studies show that versican is expressed and accumulates in the lungs of mice in studies of gram-negative lung infection [59, 90], acute lung injury [93], allergen-induced airway inflammation [94], fibrosis [95], cancer [51, 55, 96, 97], and emphysema[98]. Similarly, in humans, versican accumulates in chronic lung diseases such as pulmonary fibrosis [99–101], acute respiratory distress syndrome [102, 103], asthma [104, 105], cancer [106], lymphangioleiomyomatosis [107], and chronic obstructive pulmonary disease (COPD) [108, 109]. Whereas numerous studies show that the re-expression and accumulation of versican is a common observation in lung disease, very little is known about the regulation of versican expression or the role of versican in the pathogenesis of lung disease.
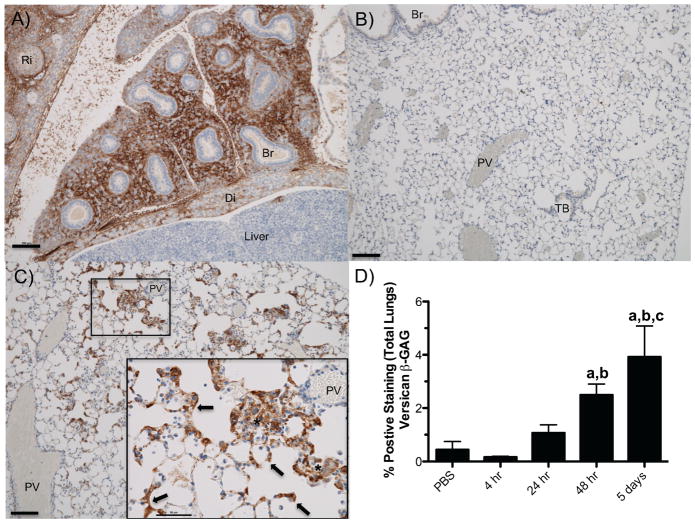
Figure 2.
Versican accumulation during embryonic mouse development and in lungs of a mouse with Pseudomonas aeruginosa lung infection. (A) Versican accumulation in fetal lung tissue at E14.5 days. (B) Versican accumulation in the lung of a 16-week-old mouse treated with PBS as a vehicle control. (C) Versican accumulation from a 16-week-old mouse infected with live Pseudomonas aeruginosa for 5 days. Brown indicates positive staining for versican β-GAG; blue, hematoxylin counterstain. Br, bronchiole; Di, diaphragm; Ri, rib; PV, postcapillary Vein; TB, terminal bronchiole. Arrows indicate versican staining in the alveolar septa; * marks an area of positive staining of the alveolar septa; cells in alveolar space makes it difficult to distinguish these two anatomical compartments. (D) The amount of versican-stained lung tissue as a percentage of total lung tissue in control mice (PBS) and those exposed to live P. aeruginosa for up to 5 days. Values are the mean β SEM (n = 3–6). asignificantly different from PBS, bsignificantly different from 4 hr, csignificantly different from 24 hr. p<00001 using a one-way ANOVA with Tukey’s multiple comparison test. Scale (A–C) 100 μm; (C inset) 50 μm. Figure reused with permission from: Jessica M. Snyder, Ida M. Washington, Timothy Birkland, Mary Y. Chang, Charles W. Frevert, Journal of Histochemistry & Cytochemistry (Volume 63 Issue 12) pp. 952–967, copyright c 2015 by The Authors. Reprinted by Permission of SAGE Publications, Inc.
Our recent work showing that versican expression and accumulation is rapidly increased in lungs of mice with gram-negative pneumonia suggests an important role for this CSPG in the innate immune response to lung infection (Figure 2) [59, 90]. There are multiple binding domains on versican for a number of cytokines, chemokines, adhesion molecules and growth factors, many of which are involved in the innate immune response [3, 36, 110]. The ability of versican to bind to chemokines is due in large part to the negatively charged CS side chains associated with the α- and β-GAG domains. For example, versican regulates the availability and activity of several chemokines including CXCL2, CXCL10, CCL2, CCL5, CCL8, CCL20, and CCL21 [37–41, 111]. We previously showed that GAGs provide fine-tune control of the innate immune response in lungs by controlling the kinetics of chemokine-GAG interactions, chemokine diffusion, and leukocyte migration [9, 10, 112, 113]. Chemokine-GAG interactions are also known to regulate the oligomerization of chemokines in tissues [114, 115] and the ability of chemokines to bind to their high affinity receptor on leukocytes [116, 117]. More recently, studies show that the binding of versican to TLR2 reprograms macrophages and dendritic cells [51, 118]. The reprograming of dendritic cells by versican in a TLR2-dependent manner increases the amount of IL-10 and IL-6 receptors on the cell surface, resulting in a immunosuppressive phenotype [118]. As such, versican has been implicated in regulating several key events in the innate immune response [3, 6, 36, 58, 59]. The observation that versican is observed in a number of lung diseases and is able to modify the innate immune response in studies performed in vitro makes it an attractive target for therapeutic intervention. However, to advance these concepts into preclinical studies, we need to learn much more about the mechanisms whereby versican modifies outcomes in fundamental/experimental studies of lung disease.
5. Chronic changes to airway ECM in asthma
Bronchial biopsies from asthmatic adults and children demonstrate features of airway remodeling, including excessive subepithelial ECM protein/proteoglycan deposition that are already present by early childhood [119–122]. Excessive ECM production by human lung fibroblasts (HLFs) and the role of epithelial regulation of fibroblasts have been extensively studied in the context of pulmonary fibrosis [123]; however, there is less data on the role of epithelial regulation of ECM production by fibroblasts in asthmatic airway remodeling [124, 125]. Previous work has demonstrated that mesenchymal cells are the predominate source of many ECM constituents. This is particularly true for fibroblasts that have undergone transforming growth factor-β (TGF-β)-dependent fibroblast-to-myofibroblast transition (FMT), differentiating into a phenotype expressing cytoskeletal alpha smooth muscle actin (α-SMA) [126, 127]. Myofibroblasts are the primary source of types I and III collagen in fibrotic lesions [128–130]. Additionally, myofibroblasts represent a contractile phenotype that may directly participate in scar formation and contraction in the asthmatic airway [131]. Other important ECM constituents such as fibronectin, hyaluronan, and versican are also primarily secreted by fibroblasts and may play important roles in airway remodeling. Hyaluronan is major component of the ECM and its clearance is essential for resolution of local inflammation during acute injury [132, 133]. Furthermore, expression of airway hyaluronan [134, 135] and versican [136, 137] is higher in asthmatics and correlates with asthma severity [138]. Hyaluronan has also been linked to localized collagen deposition in animal models of asthma [139]. Interestingly, fibroblasts from patients with airway hyper-responsiveness demonstrate greater overall ECM production than those from healthy individuals [140, 141]. Collagens I and III, hyaluronan, and versican are, therefore, potentially important constituents of altered basement membranes and may be differentially regulated in asthma [128, 132, 142, 143].
5.1. Role of the airway epithelium in regulating ECM in asthma
The current mainstay of therapy for persistent asthma is suppression of airway inflammation using corticosteroids. However, clinical trials in asthmatic children show that although inhaled corticosteroids improve symptoms and prevent exacerbations, they do not alter the natural course of asthma [144, 145]. Because the airway epithelium undergoes significant structural changes early in asthma, and is the first contact point between the host airways and the environment, a new paradigm of asthma pathogenesis has emerged to partially explain asthma pathogenesis and airway remodeling, wherein ongoing injury, irritation, and/or viral infection of airway epithelial cells results in disordered wound repair in asthmatics, including disordered regulation of lung fibroblast and airway smooth muscle activity and altered airway ECM deposition (Figure 3) [146]. In vivo animal models of airway remodeling [147–149] and descriptive data from human bronchial biopsies [150–152] suggest the airway epithelium secretes proteins that regulate lung fibroblasts and airway remodeling with increased ECM deposition, including TGF-β [150, 153–155], VEGF [151, 156–159], periostin [160, 161], activin A [162–164], and follistatins [165, 166]. Furthermore, recent ex vivo investigations using primary bronchial epithelial cells (BECs) from asthmatic and healthy children demonstrated that when co-cultured with BECs from healthy children, lung fibroblast expression of types I and III collagen, hyaluronan, as well as expression of α-SMA, indicative of a myofibroblast phenotype, are downregulated [167, 168]. This downregulation is diminished in fibroblasts co-cultured with asthmatic BECs [167, 168], suggesting that, in addition to stimulatory signals, there must be epithelial-derived factors that inhibit fibroblasts and FMT, such that in normal airways, a balance of epithelial-secreted stimulatory and inhibitory factors that regulate fibroblasts and ECM deposition. Other studies have shown that airway epithelial cells synthesize hyaluronan and its degradative enzymes, the hyaluronidases (Hyals), in response to oxidative stress and other injurious agents [169–174] and that hyaluronan-enriched ECM synthesized by respiratory epithelial cells can impact monocyte adhesion [175].
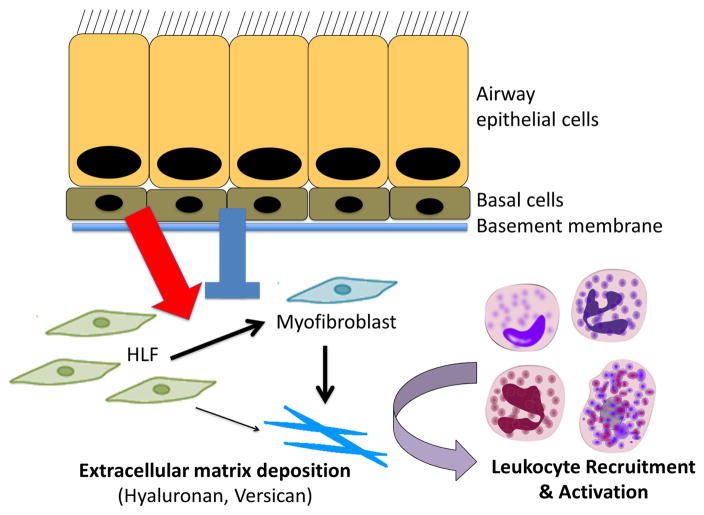
Figure 3.
Working hypothesis of leukocyte/ECM interaction in asthma suggesting that ongoing injury, irritation, and/or bacterial/viral infection of the epithelial cells results in signals that promote disordered wound repair resulting in altered ECM remodeling and the formation of a versican-/hyaluronan-rich ECM that promotes leukocyte recruitment and activation. Blue arrow indicates agonists that promote ECM accumulation and red arrow indicates antagonists the prevent ECM accumulation.
5.2. Effects of airway ECM on immune cells in asthma
In addition to its role as a structural component of the airway, the ability of the ECM to modulate the recruitment and adhesion of inflammatory cells in the airway is an emerging area of investigation [20]. ECM, enriched in hyaluronan and versican, is synthesized by various lung cells in response to allergens, inflammatory cytokines, and other infectious agents [3]. Hyaluronan and its degradation products, are key ECM components that are believed to be involved in modifying immune cell recruitment, activation, and retention during inflammation [176]. Additional studies have demonstrated that hyaluronan binding partners such as versican [59] are also critical to the recruitment and activation of leukocytes in hyaluronan-enriched matrices. Versican accumulation in the subepithelial layer in airways of atopic asthmatics has been described and correlates with the degree of airway hyper-responsiveness [99].
In recent years, there has been an emerging appreciation for the role of ECM in leukocyte trafficking and modulation of local airway inflammation [20, 177, 178]. Evidence from both animal and human cell culture models has demonstrated that modulation of hyaluronan occurs in the setting of infection and/or viral mimetics [84, 179]. Hyaluronan levels in lung tissue and bronchoalveolar lavage fluid (BALF) are elevated and correlate with the degree of inflammation in animal models of lung injury [139, 180, 181]. Furthermore, concentration of airway hyaluronan is higher in asthmatics and correlates with asthma severity [105, 134, 135, 138]. Blockade of the major hyaluronan receptor, CD44, reduced hyaluronan and eosinophil accumulation in animal models of antigen-induced eosinophilia [177]; however, CD44-deficient mice suffer from increased inflammation and increased deposition of hyaluronan suggesting that CD44 is critical for hyaluronan turnover [64, 182]. Indeed, Hyal1 and Hyal2, two principal hyaluronidases, have been shown to be dependent on an association with CD44 for their activity [183]. Turnover of hyaluronan by Hyals is important in fibrotic lung disease and diverse biological activity can be stimulated by differing sizes of hyaluronan fragments [31, 132, 184]. For example, high molecular weight hyaluronan (HMW-HA) has been shown to stabilize inflammatory cell activation, inhibit scar formation, and suppress inflammation [31, 185]. In contrast, LMW-HA has been found to stimulate gene expression in macrophages, endothelial cells, and epithelial cells and to enhance scar formation [66, 186–188]. Furthermore, LMW-HA has been found to increase production of TGF-β by eosinophils and prolong their survival in a dose-dependent, CD44-mediated fashion [189]. The latter finding is of significant interest in the context of activation of resident lung fibroblasts.
Given that a compelling argument for a critical role of hyaluronan in the establishment and regulation of airway inflammation is building, the study of hyaluronan binding partners has also become an important area of investigation. Versican content in normal lung is typically low; however, it increases dramatically in the context of disease and inflammation and is known to interact closely with hyaluronan, TSG-6, and CD44 [25, 26]. Additional studies have confirmed that increases in versican expression influence cell adhesion, proliferation, migration, and survival, as well as regulation of key inflammatory responses [6, 34, 59]. Accumulation of versican in the subepithelial layer in airways of atopic asthmatics has been described previously and correlates with the degree of airway hyper-responsiveness to methacholine challenge [99]. In addition, increased accumulation of versican has been associated with both small and large airway remodeling seen in autopsy specimens following fatal asthma exacerbations [136]. Subjects with uncontrolled asthma demonstrate increased accumulation of versican in biopsy specimens from their central airways compared to healthy subjects with well controlled asthma [104]. Interestingly, these same subjects also demonstrated a greater number of myofibroblasts per unit area in tissue samples. Of note, HLFs obtained from asthmatic adults produce greater amounts of proteoglycans, including versican, in vitro compared to HLFs obtained from healthy adults [141]. Additional studies have confirmed the presence of versican in the sputum of adults with severe asthma, which correlates negatively with their forced expiratory volume over one second (FEV1), indicating a correlation with airway obstruction [105]. In animal studies, rats sensitized with ovalbumin displayed increased deposition of proteoglycans in the airways and BALF. Increased staining for versican was observed in the airways and blood vessels of the ovalbumin-exposed rats, which co-localized with α-SMA staining, suggesting an association with myofibroblasts in these tissues. Furthermore, these changes were not reversible following treatment with budesonide, a commonly used inhaled corticosteroid [190]. More recently, in a mouse model of allergic airway inflammation using cockroach antigen (CRA), we found increased subepithelial accumulation of versican and hyaluronan that paralleled monocyte/macrophage infiltration (Figure 4) [94], supporting a role for these ECM components in leukocyte retention. Of interest in this same study, differentiated primary human airway epithelial cells from asthmatic children expressed elevated levels of versican and hyaluronan when compared to epithelial cells from healthy children, suggesting that epithelial cells may also be a source of these ECM components and that their production may be dysregulated in asthma.
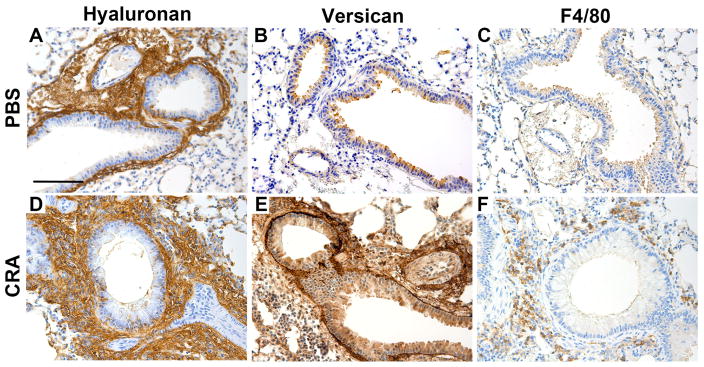
Figure 4.
Hyaluronan (A,D), versican (B,E ) and macrophage (C,F) involvement (brown color) in control- and CRA-treated mouse lungs showing increases in hyaluronan and versican content in the subepithelial region of airway bronchioles in the CRA-treated lungs. These areas were also enriched in F4/80 positive macrophages. Figure adapted with permission from: Stephen R. Reeves, Gernot Kaber, Alyssa Sheih, Georgiana Cheng, Mark A. Aronica, Mervyn J. Merrilees, Jason S. Debley, Charles W. Frevert, Steven F. Ziegler, Thomas N. Wight. Journal of Histochemistry & Cytochemistry (Volume 64, Issue 6) pp. 364–380, copyright c 2016 by The Histochemical Society. Reprinted by Permission of SAGE Publications, Inc.
Respiratory viruses also play a significant role in asthma inception and exacerbation and are a major cause of morbidity in asthma [191]. Our group [59, 176, 179] and others [84] have shown that HLFs and smooth muscle cells treated with respiratory syncytial virus and/or the viral mimetic, poly I:C, produce a complex viscous ECM that is enriched in hyaluronan and versican, and displays extensive hyaluronan- and versican-enriched “cables” extending into the ECM. Monocytes, eosinophils, and lymphocytes specifically adhere in much larger numbers to this enriched ECM rather than directly to the cell surface [179]. Furthermore, we have shown that formation of a monocyte-retaining ECM can be blocked by the presence of anti-versican antibodies [179]. Since viral infection is the most common trigger of acute asthma exacerbations, these exacerbations may be caused by changes in ECM remodeling that take place in the lung, creating a microenvironment that supports inflammatory cell invasion and adhesion. Presently, data regarding the regulation of HLF deposition of ECMs enriched in hyaluronan and versican, and the role that viral infection and/or aeroallergen stimulation of airway epithelial cells may play in modifying ECM in asthmatic lungs is lacking, but may offer valuable insight into the regulation of airway inflammation.
6. The macrophage, ECM and lung inflammation
6.1. Macrophage heterogeneity
Macrophages play essential, yet distinct, roles in both promoting and resolving inflammation as well as in both in facilitating tissue repair and contributing to its destruction [192]. That a single cell type can serve opposing functions may seem counterintuitive, but dramatic phenotypic changes occur when macrophages respond to local stimuli [192–197]. Based on patterns of gene and protein expression and function, macrophages are commonly classified as classically activated (M1) or alternatively activated (M2) cells (as well as sub-M2 types) [192–194, 197]. The M1 phenotype is induced by infection and pro-inflammatory TH1 cytokines [196]. M1 macrophages are effective at killing bacteria and release pro-inflammatory cytokines, such as IL-1β, IL-12, and TNFα. In contrast, the M2 phenotype is induced by the TH2 cytokines IL-4 and IL-13 and other factors [196, 197]. M2 macrophages release anti-inflammatory factors, such as IL-10 and TGF-β1, are weakly microbicidal, and promote repair [196]. However, dividing macrophages into M1 vs. M2 classes oversimplifies the complex continuum of functional and reversible states that these immune cells exist in in vivo [198, 199].
6.2. Macrophages and fibrosis
Macrophages present early in inflammation are functionally distinct from those at later stages [197, 200–207]. Depletion of macrophages in the early phases of wound repair significantly impairs scar formation [208, 209], whereas depletion of macrophages during later stages leads to an inability to resolve scars [204, 210]. Hence, early phase macrophages, which are predominately M1-biased cells, contribute to ECM deposition and fibrosis likely by producing profibrotic cytokines that promote the activation of resident fibroblasts and pericytes into ECM-producing myofibroblasts [197, 200–203, 211–215]. During the later resolution phase, macrophages tend to be alternatively activated, remodeling-competent M2-biased macrophages [202, 213, 216] (Figure 5). Although far from being fully understood, resolution of scarring and fibrosis appears to be – not surprisingly – the responsibility of macrophages and, in particular, M2 macrophages [202, 204, 217–221].
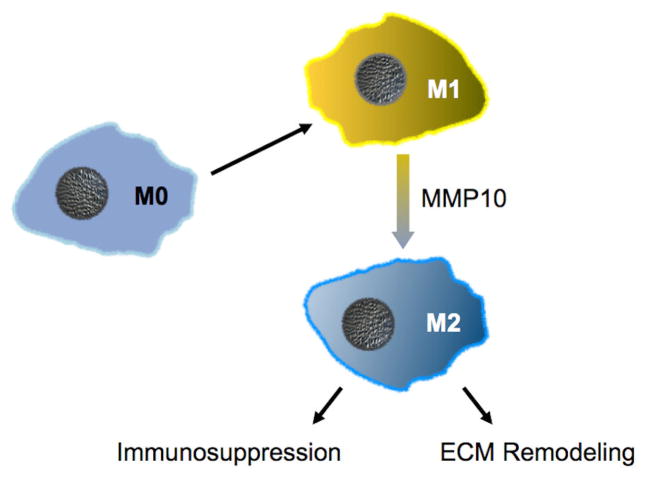
Figure 5.
MMP10 functions in a cell-autonomous manner to control the state of macrophage activation. Likely via shedding of a yet-to-be-identified surface protein, MMP10 drives the conversion of pro-inflammatory M1-biased macrophages towards immunosuppressive M2-biased cells. In addition, MMP10 controls the activation of ECM degrading activity in M2 macrophages, such as by promoting expression of MMP13, a collagenolytic proteinase.
Despite the compelling data in various tissue models with macrophage-depletion and direct proteolysis strategies, M2 macrophages – or specific subsets of M2 macrophages – have been considered to be profibrotic [222] for two key reasons. One, M2-like macrophages (or M2 markers) are present in scars and fibrotic tissue. However, these are mostly correlative data, whereas functional studies – such as our data below – indicate that M2-biased macrophages are working to resolve fibrosis, not promote it. A couple of studies concluded that M2 macrophages are profibrotic in interstitial lung disease, including idiopathic pulmonary fibrosis (IPF). One study relied on one M2 marker [223] and the other on three, including CD163 [224], but no M1 markers. Although the use of M1 and M2 makers may be convenient – and provides a reasonably good lexicon for discussion of macrophage subtypes – we hold that assessing functional read outs is more critical to understanding macrophage biology.
The second reason why M2 macrophages are thought to be profibrotic is because they express known or suspected profibrotic factors, particularly TGF-β1 and arginase-1, a cytosolic enzyme that functions in the synthesis of proline, an abundant amino acid in collagens. However, depletion of TGF-β1 or arginase-1 from macrophages does not affect fibrosis [225, 226]. It is likely that macrophage-derived TGF-β1 is a functionally distinct pool from the well-established profibrotic TGF-β1 produced by resident epithelium and interstitial cells.
6.3. Collagen degradation
Current models indicate that ECM turnover involves two sequential steps: limited extracellular proteolysis followed by uptake and lysosomal degradation [227, 228]. For the first step, some MMPs cleave the large collagen fibrils into fragments that are then endocytosed and degraded intracellularly [218, 229–231]. However, because MMPs act on much more than ECM, they can contribute to resolution of fibrosis by directly degrading ECM or indirectly by shaping the proteolytic phenotype of cells [232–234]. Based on published data [218, 221], we propose that MMP10 is a critical effector controlling the ability of M2-like macrophages to clear fibrotic ECM.
6.4. MMPs: effectors of immunity
Several proteins influence macrophage behavior, including some MMPs. For example, MMP12 and MMP28, both macrophage products, either promote or restrict macrophage influx into lung [235, 236], and MMP28 and TIMP3 regulate M1 activation of macrophages in lung [237, 238]. As their name (matrix metalloproteinases) implies, MMPs are thought to degrade ECM proteins, a function that is indeed performed by some members [239–242]. However, ECM degradation is neither the sole, nor predominant function of these enzymes. Findings from several groups demonstrate that individual MMPs regulate specific immune processes, such as leukocyte influx and activation [243–248]. MMPs control immune functions typically by gain-of-function processing of non-ECM proteins, such as cytokines, chemokines, surface proteins, etc. [248–253]. Two other important concepts, both supported by many observations with gene-targeted mice [243, 245, 247], are that i) individual MMPs perform specific, non-redundant functions with no evidence of functional compensation by other MMPs; and ii) in normal processes, such as repair and immunity, MMPs typically serve beneficial roles. However, if their expression is prolonged or misregulated, then their catalytic activity can lead to disease.
Recent findings suggest that MMP10 impacts macrophage functions with different outcomes in different conditions and at different stages. In an acute setting, MMP10 moderates the proinflammatory activity of macrophages, which appears to be a beneficial effect [254]. Later on, MMP10 facilitates scar resolution and limits fibrosis by activating the ability of M2-biased macrophages to degrade ECM. This remodeling activity is beneficial in a setting with excess ECM, such as a wound (scar) or fibrotic tissue, as found in IPF. However, this MMP10-dependent ECM degrading activity of macrophages can be damaging when sustained in an otherwise structurally normal lung, such as the development of emphysema after many years of smoking. For example, blocking MMP10 activity or the pathways it controls or altering macrophage activation status could reduce the destructive potential of M2 cells in chronic conditions (e.g., COPD), whereas stimulating these mechanisms could be beneficial in IPF.
The importance of MMP10 in human lung disease is being recognized by others. Both Sokai et al. [255] and Vuga et al. [256] proposed that MMP10 is a predictor of outcomes in IPF, complementing earlier work showing that MMP10 is among the genes that are over-expressed in acute exacerbations of IPF [257]. In addition, MMP10 is expressed by lung macrophages in human smokers with emphysema [258], and MMP10 is one of two genes whose levels are significantly related to a decline in FEV1 in human smokers with COPD [259], findings we validated with functional studies as part of large genome-wide association study (GWAS) on obstructive lung disease (discussed below) [260]. Furthermore, because macrophages and MMPs are important effectors in many conditions, such as asthma, vascular disease, cancer, and more, MMP10’s control of macrophage activation may be relevant to a wide range of models and diseases.
6.5. MMP10 promotes ECM degradation by M2 macrophages
In models of excess ECM deposition in lung (bleomycin fibrosis; WCP, unpublished observations) and skin (scarring in wounds) [221], macrophage MMP10 functions to reduce collagen accumulation. In both models, levels of deposited collagen were greater in bleomycin-treated lungs and skin wounds in Mmp10−/− mice than in wildtype mice, with no differences in collagen expression or other synthetic endpoints between genotypes [221].
Net collagen deposition is the sum of collagen production minus turnover, and we determined that significantly less collagenase activity is released from Mmp10−/− macrophages. The missing activity is not that of MMP10; it cannot cleave fibrillar collagens [243, 261]. Depletion of macrophages in wildtype tissue reduced collagenase activity to the levels seen in Mmp10−/− samples, but ablation did not further lower the activity in Mmp10−/− tissue. Selective ablation of M2 cells [262] led to decreased collagenase activity in wildtype explants, but not in Mmp10−/− samples. In addition, whereas M2 polarization in culture increased the collagenolytic activity released from wildtype macrophages, it had no effect on the activity released from Mmp10−/− macrophages.
We compared the expression of MMPs with known or suspected macrophage-derived collagenase activity (i.e., MMP2, 8, 9, 13, 14, 16 [263, 264]) between wildtype and Mmp10−/− tissue and M0-, M1-, and M2-polarized macrophages. Consistently, we found reduced expression of MMP8 (collagenase-2) and MMP13 (collagenase-3) in Mmp10−/− samples and M2-biased macrophages. We found no expression difference between wildtype and Mmp10−/− M0-and M1-biased cells. We assessed the relative contributions of MMP8 and MMP13 to M2 collagenase activity. Whereas anti-MMP13 removed essentially all activity, anti-MMP8 removed none [221]. Overall, these data indicate that MMP10 functions in M1 macrophages to moderate their pro-inflammatory behavior and to transition them into ECM remodeling-competent M2 cells (Fig. 5)
6.6. MMP10 and emphysema
If MMP10 controls the ECM remodeling activity of M2 macrophages, then this MMP could be detrimental in long-term conditions, such as cigarette smoke-induced emphysema. Indeed, a multi-center study identified MMP10 as a candidate gene for COPD in humans [260]. Using a model of chronic (6-mo) exposure to cigarette smoke, we found that Mmp10−/− mice are fully resistant to the development of emphysema. As stated above, MMP10 is produced by macrophages from human smokers with emphysema [258] and is one of two genes whose expression correlates with reduced lung function in smokers [259].
These findings indicate that macrophage MMP10 contributes to disease progression in emphysema, which is seemingly opposed to the protective role for this MMP in acute models, such as bleomycin fibrosis. However, there are important differences between these models, especially with respect to macrophage biology. As discussed above, macrophages that function early in inflammation are functionally distinct from those that function late in inflammation or in a persistent inflammatory response, like long-term smoke exposure. Whereas acute infection and injury bias macrophages toward an M1 phenotype [197], cigarette smoke promotes expansion of M2 macrophages [265]. Macrophages are considered to be the destructive cell in emphysema [266, 267], and our findings indicate that MMP10 promotes the ECM-degrading activity of M2 macrophages [221]. Thus, in acute or fibrotic settings, MMP10 is beneficial by moderating the pro-inflammatory activity of M1-biased macrophages and by stimulating the ability of M2-biased macrophages to remodel scar tissue. But in a chronic setting, MMP10-driven ECM remodeling could be excessive and detrimental, as suggested in our smoke-exposure studies. Still, the common conclusion among these models is that MMP10 functions to control macrophage behavior.
7. Conclusions
The ECM serves as a template for adhesion once leukocytes invade tissue in immune and inflammatory responses in diseases of the lung. Within the lung, there is a complementary set of ECM components that characterize each cellular compartment and any disturbance in the composition and/or organization of these components disrupts lung architecture and destroys lung function. Specific components of the ECM, such as versican and hyaluronan, are dramatically altered in all forms of lung disease, including bacterial and viral infection as well as asthma. These changes promote leukocyte invasion and retention and significantly affect normal tissue architecture and lung function. The macrophage is a critical player in lung disease. These cells come into contact with the ECM through a specific set of ECM receptors on the cell surface. Such interactions impact the ability of these cells to proliferate, migrate, and degrade the ECM via a specific set of proteases including MMP10. Defining precise roles for these specific ECM components in lung disease is critical if effective therapeutic interventions are to be developed in the future.
Highlights.
Leukocytes interact with lung extracellular matrix (ECM) during inflammation
This interaction affects how leukocytes accumulate, migrate, and differentiate
Versican and hyaluronan are ECM components that regulate leukocyte phenotype
Versican and hyaluronan increase in a range of lung diseases
Macrophages can both promote and resolve inflammation and are influenced by MMP10
Acknowledgments
This work was supported by National Institutes of Health grants HL098067 and AI125378 (TNW, CWF, JSD, WCP, SFZ), HL128361 (JSD, TNW), HL128995, HL089455 (WCP), AI068731 (SFZ), and a Parker B. Francis fellowship (SSR). We thank Dr. Virginia M. Green for careful editing and preparation of the manuscript.
Abbreviations
- ECM
extracellular matrix
- GAG
glycosaminoglycan
- MMP
matrix metalloproteinase
- CS
chondroitin sulfate
- ADAMTS
a disintegrin and metalloproteinase with a thrombospondin type-1 motif
- PSGL-1
P-selectin glycoprotein-1
- TLR
toll-like receptors
- TNFα
tumor necrosis factor α
- LMWHA
low molecular weight hyaluronan
- LPS
lipopolysaccharide
- HAS1
hyaluronan synthase 1
- CSPG
chondroitin sulfate proteoglycan
- COPD
chronic obstructive pulmonary disease
- HLF
human lung fibroblast
- TGF-β
transforming growth factor-β
- FMT
fibroblast-to-myofibroblast transition
- α-SMA
alpha smooth muscle actin
- BEC
bronchial epithelial cell
- Hyal
hyaluronidase
- BALF
bronchoalveolar lavage fluid
- HMW-HA
high molecular weight hyaluronan
- FEV1
forced expiratory volume over one second
- CRA
cockroach antigen
- M1
classically activated macrophages
- M2
alternatively activated macrophages
- IPF
idiopathic pulmonary fibrosis
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Booth AJ, Hadley R, Cornett AM, Dreffs AA, Matthes SA, Tsui JL, Weiss K, Horowitz JC, Fiore VF, Barker TH, Moore BB, Martinez FJ, Niklason LE, White ES. Acellular normal and fibrotic human lung matrices as a culture system for in vitro investigation. Am J Respir Crit Care Med. 2012;186:866–76. doi: 10.1164/rccm.201204-0754OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weibel ER, Crystal RG. Structural organization of the pulmonary interstitium. In: Crystal RG, West JB, editors. The Lung: Scientific Foundations. Raven Press; New York: 1991. pp. 369–380. [Google Scholar]
- 3.Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec (Hoboken) 2010;293:968–81. doi: 10.1002/ar.21094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nastase MV, Iozzo RV, Schaefer L. Key roles for the small leucine-rich proteoglycans in renal and pulmonary pathophysiology. Biochim Biophys Acta. 2014;1840:2460–70. doi: 10.1016/j.bbagen.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Tormanen K, Bjermer L, Malmstrom A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: The impact on lung disorders. Glycobiology. 2014 doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wight TN, Kang I, Merrilees MJ. Versican and the control of inflammation. Matrix Biol. 2014;35:152–61. doi: 10.1016/j.matbio.2014.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Burgess JK, Mauad T, Tjin G, Karlsson JC, Westergren-Thorsson G. The extracellular matrix - the under-recognized element in lung disease? J Pathol. 2016 doi: 10.1002/path.4808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vaday GG, Franitza S, Schor H, Hecht I, Brill A, Cahalon L, Hershkoviz R, Lider O. Combinatorial signals by inflammatory cytokines and chemokines mediate leukocyte interactions with extracellular matrix. J Leukoc Biol. 2001;69:885–92. [PubMed] [Google Scholar]
- 9.Frevert CW, Goodman RB, Kinsella MG, Kajikawa O, Ballman K, Clark-Lewis I, Proudfoot AE, Wells TN, Martin TR. Tissue-specific mechanisms control the retention of IL-8 in lungs and skin. J Immunol. 2002;168:3550–6. doi: 10.4049/jimmunol.168.7.3550. [DOI] [PubMed] [Google Scholar]
- 10.Frevert CW, Kinsella MG, Vathanaprida C, Goodman RB, Baskin DG, Proudfoot A, Wells TN, Wight TN, Martin TR. Binding of interleukin-8 to heparan sulfate and chondroitin sulfate in lung tissue. Am J Respir Cell Mol Biol. 2003;28:464–72. doi: 10.1165/rcmb.2002-0084OC. [DOI] [PubMed] [Google Scholar]
- 11.Taylor KR, Gallo RL. Glycosaminoglycans and their proteoglycans: host-associated molecular patterns for initiation and modulation of inflammation. Faseb J. 2006;20:9–22. doi: 10.1096/fj.05-4682rev. [DOI] [PubMed] [Google Scholar]
- 12.Bollyky PL, Falk BA, Long SA, Preisinger A, Braun KR, Wu RP, Evanko SP, Buckner JH, Wight TN, Nepom GT. CD44 costimulation promotes FoxP3+ regulatory T cell persistence and function via production of IL-2, IL-10, and TGF-β. J Immunol. 2009;183:2232–41. doi: 10.4049/jimmunol.0900191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollyky PL, Falk BA, Wu RP, Buckner JH, Wight TN, Nepom GT. Intact extracellular matrix and the maintenance of immune tolerance: high molecular weight hyaluronan promotes persistence of induced CD4+CD25+ regulatory T cells. J Leukoc Biol. 2009;86:567–72. doi: 10.1189/jlb.0109001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bollyky PL, Lord JD, Masewicz SA, Evanko SP, Buckner JH, Wight TN, Nepom GT. Cutting edge: high molecular weight hyaluronan promotes the suppressive effects of CD4+CD25+ regulatory T cells. J Immunol. 2007;179:744–7. doi: 10.4049/jimmunol.179.2.744. [DOI] [PubMed] [Google Scholar]
- 15.Schor H, Vaday GG, Lider O. Modulation of leukocyte behavior by an inflamed extracellular matrix. Dev Immunol. 2000;7:227–38. doi: 10.1155/2000/51902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vaday GG, Lider O. Extracellular matrix moieties, cytokines, and enzymes: dynamic effects on immune cell behavior and inflammation. J Leukoc Biol. 2000;67:149–59. doi: 10.1002/jlb.67.2.149. [DOI] [PubMed] [Google Scholar]
- 17.Adair-Kirk TL, Senior RM. Fragments of extracellular matrix as mediators of inflammation. Int J Biochem Cell Biol. 2008;40:1101–10. doi: 10.1016/j.biocel.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wight T, Evanko S, Kinsella MG, Chang MY, Yeop Han C, Sakr S, Huang R, Merrilees M, Rosenfeld ME, Chait A. The pro-inflammatory nature of the extracellular matrix. In: Matsuzawa Y, Kita T, Nagai R, Teramoto T, editors. Atherosclerosis XIII. Elsevier B.V; Amsterdam: 2004. pp. 404–406. [Google Scholar]
- 19.Jarvelainen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–23. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 21.Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Opin Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- 22.Zimmermann D. Versican. In: Iozzo R, editor. Proteoglycans: Structure, Biology and Molecular Interactions. Marcel Dekker, Inc; New York: 2000. pp. 327–341. [Google Scholar]
- 23.LeBaron RG, Zimmermann DR, Ruoslahti E. Hyaluronate binding properties of versican. J Biol Chem. 1992;267:10003–10010. [PubMed] [Google Scholar]
- 24.Matsumoto K, Shionyu M, Go M, Shimizu K, Shinomura T, Kimata K, Watanabe H. Distinct interaction of versican/PG-M with hyaluronan and link protein. J Biol Chem. 2003;278:41205–12. doi: 10.1074/jbc.M305060200. [DOI] [PubMed] [Google Scholar]
- 25.Kohda D, Morton CJ, Parkar AA, Hatanaka H, Inagaki FM, Campbell ID, Day AJ. Solution structure of the link module: a hyaluronan-binding domain involved in extracellular matrix stability and cell migration. Cell. 1996;86:767–75. doi: 10.1016/s0092-8674(00)80151-8. [DOI] [PubMed] [Google Scholar]
- 26.Day AJ, Prestwich GD. Hyaluronan-binding proteins: tying up the giant. J Biol Chem. 2002;277:4585–8. doi: 10.1074/jbc.R100036200. [DOI] [PubMed] [Google Scholar]
- 27.Lundell A, Olin AI, Morgelin M, al-Karadaghi S, Aspberg A, Logan DT. Structural basis for interactions between tenascins and lectican C-type lectin domains: evidence for a crosslinking role for tenascins. Structure (Camb) 2004;12:1495–506. doi: 10.1016/j.str.2004.05.021. [DOI] [PubMed] [Google Scholar]
- 28.Day AJ, de la Motte CA. Hyaluronan cross-linking: a protective mechanism in inflammation? Trends Immunol. 2005;26:637–43. doi: 10.1016/j.it.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Wang A, de la Motte C, Lauer M, Hascall V. Hyaluronan matrices in pathobiological processes. FEBS J. 2011;278:1412–8. doi: 10.1111/j.1742-4658.2011.08069.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evanko SP, Potter-Perigo S, Johnson PY, Wight TN. Organization of hyaluronan and versican in the extracellular matrix of human fibroblasts treated with the viral mimetic poly I:C. J Histochem Cytochem. 2009;57:1041–60. doi: 10.1369/jhc.2009.953802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Petrey AC, de la Motte CA. Hyaluronan, a crucial regulator of inflammation. Front Immunol. 2014;5:101. doi: 10.3389/fimmu.2014.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Faggian J, Fosang AJ, Zieba M, Wallace MJ, Hooper SB. Changes in versican and chondroitin sulphate proteoglycans during structural development of the lung. Am J Physiol Regul Integr Comp Physiol. 2007;293:R784–92. doi: 10.1152/ajpregu.00801.2006. [DOI] [PubMed] [Google Scholar]
- 33.Roberts CR, Wight TN, Hascall VC. Proteoglycans. In: Crystal R, West JB, editors. The Lung: Scientific Foundations. Lippincott Raven Publishers; Philadelphia, PA: 1997. pp. 757–767. [Google Scholar]
- 34.Evanko SP, Angello JC, Wight TN. Formation of hyaluronan- and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- 35.Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit Rev Eukaryot Gene Expr. 2004;14:203–34. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- 36.Wight TN, Kinsella MG, Evanko SP, Potter-Perigo S, Merrilees MJ. Versican and the regulation of cell phenotype in disease. Biochim Biophys Acta. 2014;1840:2441–51. doi: 10.1016/j.bbagen.2013.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kawashima H, Hirose M, Hirose J, Nagakubo D, Plaas AH, Miyasaka M. Binding of a large chondroitin sulfate/dermatan sulfate proteoglycan, versican, to L-selectin, P-selectin, and CD44. J Biol Chem. 2000;275:35448–56. doi: 10.1074/jbc.M003387200. [DOI] [PubMed] [Google Scholar]
- 38.Kawashima H, Li YF, Watanabe N, Hirose J, Hirose M, Miyasaka M. Identification and characterization of ligands for L-selectin in the kidney. I. Versican, a large chondroitin sulfate proteoglycan, is a ligand for L-selectin. Int Immunol. 1999;11:393–405. doi: 10.1093/intimm/11.3.393. [DOI] [PubMed] [Google Scholar]
- 39.Hirose J, Kawashima H, Yoshie O, Tashiro K, Miyasaka M. Versican interacts with chemokines and modulates cellular responses. J Biol Chem. 2001;276:5228–34. doi: 10.1074/jbc.M007542200. [DOI] [PubMed] [Google Scholar]
- 40.Wu YJ, La Pierre DP, Wu J, Yee AJ, Yang BB. The interaction of versican with its binding partners. Cell Res. 2005;15:483–94. doi: 10.1038/sj.cr.7290318. [DOI] [PubMed] [Google Scholar]
- 41.Masuda A, Yasuoka H, Satoh T, Okazaki Y, Yamaguchi Y, Kuwana M. Versican is upregulated in circulating monocytes in patients with systemic sclerosis and amplifies a CCL2-mediated pathogenic loop. Arthritis Res Ther. 2013;15:R74. doi: 10.1186/ar4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malla N, Berg E, Theocharis AD, Svineng G, Uhlin-Hansen L, Winberg JO. In vitro reconstitution of complexes between pro-matrix metalloproteinase-9 and the proteoglycans serglycin and versican. FEBS J. 2013;280:2870–87. doi: 10.1111/febs.12291. [DOI] [PubMed] [Google Scholar]
- 43.Ra HJ, Harju-Baker S, Zhang F, Linhardt RJ, Wilson CL, Parks WC. Control of promatrilysin (MMP7) activation and substrate-specific activity by sulfated glycosaminoglycans. J Biol Chem. 2009;284:27924–32. doi: 10.1074/jbc.M109.035147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ra HJ, Parks WC. Control of matrix metalloproteinase catalytic activity. Matrix Biol. 2007;26:587–96. doi: 10.1016/j.matbio.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tocchi A, Parks WC. Functional interactions between matrix metalloproteinases and glycosaminoglycans. FEBS J. 2013;280:2332–41. doi: 10.1111/febs.12198. [DOI] [PubMed] [Google Scholar]
- 46.Apte SS. A disintegrin-like and metalloprotease (reprolysin-type) with thrombospondin type 1 motif (ADAMTS) superfamily: functions and mechanisms. J Biol Chem. 2009;284:31493–7. doi: 10.1074/jbc.R109.052340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kenagy RD, Plaas AH, Wight TN. Versican degradation and vascular disease. Trends Cardiovasc Med. 2006;16:209–15. doi: 10.1016/j.tcm.2006.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kenagy RD, Min SK, Clowes AW, Sandy JD. Cell death-associated ADAMTS4 and versican degradation in vascular tissue. J Histochem Cytochem. 2009;57:889–97. doi: 10.1369/jhc.2009.953901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McCulloch DR, Nelson CM, Dixon LJ, Silver DL, Wylie JD, Lindner V, Sasaki T, Cooley MA, Argraves WS, Apte SS. ADAMTS metalloproteases generate active versican fragments that regulate interdigital web regression. Dev Cell. 2009;17:687–98. doi: 10.1016/j.devcel.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zheng PS, Vais D, Lapierre D, Liang YY, Lee V, Yang BL, Yang BB. PG-M/versican binds to P-selectin glycoprotein ligand-1 and mediates leukocyte aggregation. J Cell Sci. 2004;117:5887–95. doi: 10.1242/jcs.01516. [DOI] [PubMed] [Google Scholar]
- 51.Kim S, Takahashi H, Lin WW, Descargues P, Grivennikov S, Kim Y, Luo JL, Karin M. Carcinoma-produced factors activate myeloid cells through TLR2 to stimulate metastasis. Nature. 2009;457:102–6. doi: 10.1038/nature07623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang W, Xu GL, Jia WD, Ma JL, Li JS, Ge YS, Ren WH, Yu JH, Liu WB. Ligation of TLR2 by versican: a link between inflammation and metastasis. Arch Med Res. 2009;40:321–3. doi: 10.1016/j.arcmed.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 53.Bogels M, Braster R, Nijland PG, Gul N, van de Luijtgaarden W, Fijneman RJ, Meijer GA, Jimenez CR, Beelen RH, van Egmond M. Carcinoma origin dictates differential skewing of monocyte function. Oncoimmunology. 2012;1:798–809. doi: 10.4161/onci.20427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li D, Wang X, Wu JL, Quan WQ, Ma L, Yang F, Wu KY, Wan HY. Tumor-produced versican V1 enhances hCAP18/LL-37 expression in macrophages through activation of TLR2 and vitamin D3 signaling to promote ovarian cancer progression in vitro. PLoS One. 2013;8:e56616. doi: 10.1371/journal.pone.0056616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Said N, Sanchez-Carbayo M, Smith SC, Theodorescu D. RhoGDI2 suppresses lung metastasis in mice by reducing tumor versican expression and macrophage infiltration. J Clin Invest. 2012;122:1503–18. doi: 10.1172/JCI61392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Said N, Theodorescu D. RhoGDI2 suppresses bladder cancer metastasis via reduction of inflammation in the tumor microenvironment. Oncoimmunology. 2012;1:1175–1177. doi: 10.4161/onci.20594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zhang Z, Miao L, Wang L. Inflammation amplification by versican: the first mediator. Int J Mol Sci. 2012;13:6873–82. doi: 10.3390/ijms13066873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wight TN, Potter-Perigo S. The extracellular matrix: an active or passive player in fibrosis. Am J Physiol Gastrointest Liver Physiol. 2011;301:G950–5. doi: 10.1152/ajpgi.00132.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang MY, Tanino Y, Vidova V, Kinsella MG, Chan CK, Johnson PY, Wight TN, Frevert CW. A rapid increase in macrophage-derived versican and hyaluronan in infectious lung disease. Matrix Biol. 2014;34:1–12. doi: 10.1016/j.matbio.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mummert ME. Immunologic roles of hyaluronan. Immunol Res. 2005;31:189–206. doi: 10.1385/IR:31:3:189. [DOI] [PubMed] [Google Scholar]
- 61.Hascall VC, Majors AK, De La Motte CA, Evanko SP, Wang A, Drazba JA, Strong SA, Wight TN. Intracellular hyaluronan: a new frontier for inflammation? Biochim Biophys Acta. 2004;1673:3–12. doi: 10.1016/j.bbagen.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 62.Jiang D, Liang J, Noble PW. Hyaluronan in tissue injury and repair. Annu Rev Cell Dev Biol. 2007;23:435–61. doi: 10.1146/annurev.cellbio.23.090506.123337. [DOI] [PubMed] [Google Scholar]
- 63.Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61:1303–13. doi: 10.1016/0092-8674(90)90694-a. [DOI] [PubMed] [Google Scholar]
- 64.Teder P, Vandivier RW, Jiang D, Liang J, Cohn L, Pure E, Henson PM, Noble PW. Resolution of lung inflammation by CD44. Science. 2002;296:155–8. doi: 10.1126/science.1069659. [DOI] [PubMed] [Google Scholar]
- 65.Hodge-Dufour J, Noble PW, Horton MR, Bao C, Wysoka M, Burdick MD, Strieter RM, Trinchieri G, Pure E. Induction of IL-12 and chemokines by hyaluronan requires adhesion-dependent priming of resident but not elicited macrophages. J Immunol. 1997;159:2492–500. [PubMed] [Google Scholar]
- 66.McKee CM, Penno MB, Cowman M, Burdick MD, Strieter RM, Bao C, Noble PW. Hyaluronan (HA) fragments induce chemokine gene expression in alveolar macrophages. The role of HA size and CD44. J Clin Invest. 1996;98:2403–2413. doi: 10.1172/JCI119054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Termeer CC, Hennies J, Voith U, Ahrens T, Weiss JM, Prehm P, Simon JC. Oligosaccharides of hyaluronan are potent activators of dendritic cells. J Immunol. 2000;165:1863–70. doi: 10.4049/jimmunol.165.4.1863. [DOI] [PubMed] [Google Scholar]
- 68.Beck-Schimmer B, Oertli B, Pasch T, Wuthrich RP. Hyaluronan induces monocyte chemoattractant protein-1 expression in renal tubular epithelial cells. J Am Soc Nephrol. 1998;9:2283–90. doi: 10.1681/ASN.V9122283. [DOI] [PubMed] [Google Scholar]
- 69.Montesano R, Kumar S, Orci L, Pepper MS. Synergistic effect of hyaluronan oligosaccharides and vascular endothelial growth factor on angiogenesis in vitro. Lab Invest. 1996;75:249–262. [PubMed] [Google Scholar]
- 70.Mascarenhas MM, Day RM, Ochoa CD, Choi WI, Yu L, Ouyang B, Garg HG, Hales CA, Quinn DA. Low molecular weight hyaluronan from stretched lung enhances interleukin-8 expression. Am J Respir Cell Mol Biol. 2004;30:51–60. doi: 10.1165/rcmb.2002-0167OC. [DOI] [PubMed] [Google Scholar]
- 71.Scheibner KA, Lutz MA, Boodoo S, Fenton MJ, Powell JD, Horton MR. Hyaluronan fragments act as an endogenous danger signal by engaging TLR2. J Immunol. 2006;177:1272–81. doi: 10.4049/jimmunol.177.2.1272. [DOI] [PubMed] [Google Scholar]
- 72.Hollingsworth JW, Li Z, Brass DM, Garantziotis S, Timberlake SH, Kim A, Hossain I, Savani RC, Schwartz DA. CD44 regulates macrophage recruitment to the lung in lipopolysaccharide-induced airway disease. Am J Respir Cell Mol Biol. 2007;37:248–53. doi: 10.1165/rcmb.2006-0363OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Khaldoyanidi S, Moll J, Karakhanova S, Herrlich P, Ponta H. Hyaluronate-enhanced hematopoiesis: two different receptors trigger the release of interleukin-1beta and interleukin-6 from bone marrow macrophages. Blood. 1999;94:940–9. [PubMed] [Google Scholar]
- 74.Fieber C, Baumann P, Vallon R, Termeer C, Simon JC, Hofmann M, Angel P, Herrlich P, Sleeman JP. Hyaluronan-oligosaccharide-induced transcription of metalloproteases. J Cell Sci. 2004;117:359–67. doi: 10.1242/jcs.00831. [DOI] [PubMed] [Google Scholar]
- 75.Jiang D, Liang J, Fan J, Yu S, Chen S, Luo Y, Prestwich GD, Mascarenhas MM, Garg HG, Quinn DA, Homer RJ, Goldstein DR, Bucala R, Lee PJ, Medzhitov R, Noble PW. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat Med. 2005;11:1173–9. doi: 10.1038/nm1315. [DOI] [PubMed] [Google Scholar]
- 76.Kopp EB, Medzhitov R. The Toll-receptor family and control of innate immunity. Curr Opin Immunol. 1999;11:13–8. doi: 10.1016/s0952-7915(99)80003-x. [DOI] [PubMed] [Google Scholar]
- 77.Jiang D, Liang J, Li Y, Noble PW. The role of Toll-like receptors in non-infectious lung injury. Cell Res. 2006;16:693–701. doi: 10.1038/sj.cr.7310085. [DOI] [PubMed] [Google Scholar]
- 78.Sukkar MB, Xie S, Khorasani NM, Kon OM, Stanbridge R, Issa R, Chung KF. Toll-like receptor 2, 3, and 4 expression and function in human airway smooth muscle. J Allergy Clin Immunol. 2006;118:641–8. doi: 10.1016/j.jaci.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 79.Kleinman ME, Yamada K, Takeda A, Chandrasekaran V, Nozaki M, Baffi JZ, Albuquerque RJ, Yamasaki S, Itaya M, Pan Y, Appukuttan B, Gibbs D, Yang Z, Kariko K, Ambati BK, Wilgus TA, DiPietro LA, Sakurai E, Zhang K, Smith JR, Taylor EW, Ambati J. Sequence- and target-independent angiogenesis suppression by siRNA via TLR3. Nature. 2008;452:591–7. doi: 10.1038/nature06765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Stuhlmeier KM. Hyaluronan production in synoviocytes as a consequence of viral infections: HAS1 activation by Epstein-Barr virus and synthetic double- and single-stranded viral RNA analogs. J Biol Chem. 2008;283:16781–9. doi: 10.1074/jbc.M801669200. [DOI] [PubMed] [Google Scholar]
- 81.Selbi W, Day AJ, Rugg MS, Fulop C, de la Motte CA, Bowen T, Hascall VC, Phillips AO. Overexpression of hyaluronan synthase 2 alters hyaluronan distribution and function in proximal tubular epithelial cells. J Am Soc Nephrol. 2006;17:1553–67. doi: 10.1681/ASN.2005080879. [DOI] [PubMed] [Google Scholar]
- 82.Savani RC, Hou G, Liu P, Wang C, Simons E, Grimm PC, Stern R, Greenberg AH, DeLisser HM, Khalil N. A role for hyaluronan in macrophage accumulation and collagen deposition after bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2000;23:475–84. doi: 10.1165/ajrcmb.23.4.3944. [DOI] [PubMed] [Google Scholar]
- 83.de la Motte C, Hascall VC, Drazba JA, Strong SA. Poly I:C induces mononuclear leukocyte-adhesive hyaluronan structures on colon smooth muscle cells: IαI and versican facilitate adhesion. In: Kennedy JF, Phillips GO, Williams PA, Hascall VC, editors. Hyaluronan: Chemical, Biochemical and Biological Aspects. Woodhead Publishing Limited; Cambridge, England: 2002. pp. 381–388. [Google Scholar]
- 84.de la Motte CA, Hascall VC, Calabro A, Yen-Lieberman B, Strong SA. Mononuclear leukocytes preferentially bind via CD44 to hyaluronan on human intestinal mucosal smooth muscle cells after virus infection or treatment with poly(I.C) J Biol Chem. 1999;274:30747–30755. doi: 10.1074/jbc.274.43.30747. [DOI] [PubMed] [Google Scholar]
- 85.de la Motte CA, Hascall VC, Drazba J, Bandyopadhyay SK, Strong SA. Mononuclear leukocytes bind to specific hyaluronan structures on colon mucosal smooth muscle cells treated with polyinosinic acid:polycytidylic acid: inter-α-trypsin inhibitor is crucial to structure and function. Am J Pathol. 2003;163:121–133. doi: 10.1016/s0002-9440(10)63636-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selbi W, de la Motte C, Hascall V, Phillips A. BMP-7 modulates hyaluronan-mediated proximal tubular cell-monocyte interaction. J Am Soc Nephrol. 2004;15:1199–211. doi: 10.1097/01.asn.0000125619.27422.8e. [DOI] [PubMed] [Google Scholar]
- 87.Wang A, Hascall VC. Hyaluronan structures synthesized by rat mesangial cells in response to hyperglycemia induce monocyte adhesion. J Biol Chem. 2004;279:10279–85. doi: 10.1074/jbc.M312045200. [DOI] [PubMed] [Google Scholar]
- 88.Mummert ME, Mohamadzadeh M, Mummert DI, Mizumoto N, Takashima A. Development of a peptide inhibitor of hyaluronan-mediated leukocyte trafficking. J Exp Med. 2000;192:769–79. doi: 10.1084/jem.192.6.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Milinkovic M, Antin JH, Hergrueter CA, Underhill CB, Sackstein R. CD44-hyaluronic acid interactions mediate shear-resistant binding of lymphocytes to dermal endothelium in acute cutaneous GVHD. Blood. 2004;103:740–2. doi: 10.1182/blood-2003-05-1500. [DOI] [PubMed] [Google Scholar]
- 90.Snyder JM, Washington IM, Birkland T, Chang MY, Frevert CW. Correlation of versican expression, accumulation, and degradation during embryonic development by quantitative immunohistochemistry. J Histochem Cytochem. 2015;63:952–67. doi: 10.1369/0022155415610383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Beers MF, Morrisey EE. The three R’s of lung health and disease: repair, remodeling, and regeneration. J Clin Invest. 2011;121:2065–73. doi: 10.1172/JCI45961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kotton DN, Morrisey EE. Lung regeneration: mechanisms, applications and emerging stem cell populations. Nat Med. 2014;20:822–32. doi: 10.1038/nm.3642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Xu L, Xue T, Zhang J, Qu J. Knockdown of versican V1 induces a severe inflammatory response in LPS-induced acute lung injury via the TLR2-NF-kappaB signaling pathway in C57BL/6J mice. Mol Med Rep. 2016;13:5005–12. doi: 10.3892/mmr.2016.5168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Reeves SR, Kaber G, Sheih A, Cheng G, Aronica MA, Merrilees MJ, Debley JS, Frevert CW, Ziegler SF, Wight TN. Subepithelial accumulation of versican in a cockroach antigen-induced murine model of allerigic asthma. J Histochem Cytochem. 2016;64:364–80. doi: 10.1369/0022155416642989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Spanjer AI, Baarsma HA, Oostenbrink LM, Jansen SR, Kuipers CC, Lindner M, Postma DS, Meurs H, Heijink IH, Gosens R, Konigshoff M. TGF-beta-induced profibrotic signaling is regulated in part by the WNT receptor Frizzled-8. FASEB J. 2016;30:1823–35. doi: 10.1096/fj.201500129. [DOI] [PubMed] [Google Scholar]
- 96.Khare P, Bose A, Singh P, Singh S, Javed S, Jain SK, Singh O, Pal R. Gonadotropin and tumorigenesis: Direct and indirect effects on inflammatory and immunosuppressive mediators and invasion. Mol Carcinog. 2016 doi: 10.1002/mc.22499. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z, Li Z, Wang Y, Cao D, Wang X, Jiang M, Li M, Yan X, Li Y, Liu Y, Luo F. Versican silencing improves the antitumor efficacy of endostatin by alleviating its induced inflammatory and immunosuppressive changes in the tumor microenvironment. Oncol Rep. 2015;33:2981–91. doi: 10.3892/or.2015.3903. [DOI] [PubMed] [Google Scholar]
- 98.Takahashi A, Majumdar A, Parameswaran H, Bartolak-Suki E, Suki B. Proteoglycans maintain lung stability in an elastase-treated mouse model of emphysema. Am J Respir Cell Mol Biol. 2014;51:26–33. doi: 10.1165/rcmb.2013-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Huang J, Olivenstein R, Taha R, Hamid Q, Ludwig M. Enhanced proteoglycan deposition in the airway wall of atopic asthmatics. Am J Respir Crit Care Med. 1999;160:725–9. doi: 10.1164/ajrccm.160.2.9809040. [DOI] [PubMed] [Google Scholar]
- 100.Roberts C. Versican in the cell biology of pulmonary fibrosis. In: Garg H, Roughley P, Hales C, editors. Proteoglycans and Lung Disease. Marcel Dekker; New York: 2003. pp. 191–212. [Google Scholar]
- 101.Araujo BB, Dolhnikoff M, Silva LF, Elliot J, Lindeman JH, Ferreira DS, Mulder A, Gomes HA, Fernezlian SM, James A, Mauad T. Extracellular matrix components and regulators in the airway smooth muscle in asthma. Eur Respir J. 2008;32:61–9. doi: 10.1183/09031936.00147807. [DOI] [PubMed] [Google Scholar]
- 102.Morales MM, Pires-Neto RC, Inforsato N, Lancas T, da Silva LF, Saldiva PH, Mauad T, Carvalho CR, Amato MB, Dolhnikoff M. Small airway remodeling in acute respiratory distress syndrome: a study in autopsy lung tissue. Crit Care. 2011;15:R4. doi: 10.1186/cc9401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Bensadoun ES, Burke AK, Hogg JC, Roberts CR. Proteoglycan deposition in pulmonary fibrosis. Am J Respir Crit Care Med. 1996;154:1819–28. doi: 10.1164/ajrccm.154.6.8970376. [DOI] [PubMed] [Google Scholar]
- 104.Weitoft M, Andersson C, Andersson-Sjoland A, Tufvesson E, Bjermer L, Erjefalt J, Westergren-Thorsson G. Controlled and uncontrolled asthma display distinct alveolar tissue matrix compositions. Respir Res. 2014;15:67. doi: 10.1186/1465-9921-15-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ayars AG, Altman LC, Potter-Perigo S, Radford K, Wight TN, Nair P. Sputum hyaluronan and versican in severe eosinophilic asthma. Int Arch Allergy Immunol. 2013;161:65–73. doi: 10.1159/000343031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–45. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- 107.Merrilees MJ, Hankin EJ, Black JL, Beaumont B. Matrix proteoglycans and remodelling of interstitial lung tissue in lymphangioleiomyomatosis. J Pathol. 2004;203:653–60. doi: 10.1002/path.1577. [DOI] [PubMed] [Google Scholar]
- 108.Merrilees MJ, Ching PST, Beaumont B, Hinek A, Wight TN, Black PN. Changes in elastin, elastin binding protein and versican in alveoli in chronic obstructive pulmonary disease. Respir Res. 2008;18:41–50. doi: 10.1186/1465-9921-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Andersson-Sjoland A, Hallgren O, Rolandsson S, Weitoft M, Tykesson E, Larsson-Callerfelt AK, Rydell-Tormanen K, Bjermer L, Malmstrom A, Karlsson JC, Westergren-Thorsson G. Versican in inflammation and tissue remodeling: the impact on lung disorders. Glycobiology. 2015;25:243–51. doi: 10.1093/glycob/cwu120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kang I, Yoon DW, Braun KR, Wight TN. Expression of versican V3 by arterial smooth muscle cells alters TGFβ-, EGF-, and NFκB-dependent signaling pathways, creating a microenvironment that resists monocyte adhesion. J Biol Chem. 2014;289:15393–15404. doi: 10.1074/jbc.M113.544338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Maeda N. Proteoglycans and neuronal migration in the cerebral cortex during development and disease. Front Neurosci. 2015;9:98. doi: 10.3389/fnins.2015.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tanino Y, Coombe DR, Gill SE, Kett WC, Kajikawa O, Proudfoot AE, Wells TN, Parks WC, Wight TN, Martin TR, Frevert CW. Kinetics of chemokine-glycosaminoglycan interactions control neutrophil migration into the airspaces of the lungs. J Immunol. 2010;184:2677–85. doi: 10.4049/jimmunol.0903274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Tanino Y, Chang MY, Wang X, Gill SE, Skerrett S, McGuire JK, Sato S, Nikaido T, Kojima T, Munakata M, Mongovin S, Parks WC, Martin TR, Wight TN, Frevert CW. Syndecan-4 regulates early neutrophil migration and pulmonary inflammation in response to lipopolysaccharide. Am J Respir Cell Mol Biol. 2012;47:196–202. doi: 10.1165/rcmb.2011-0294OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gangavarapu P, Rajagopalan L, Kolli D, Guerrero-Plata A, Garofalo RP, Rajarathnam K. The monomer-dimer equilibrium and glycosaminoglycan interactions of chemokine CXCL8 regulate tissue-specific neutrophil recruitment. J Leukoc Biol. 2012;91:259–65. doi: 10.1189/jlb.0511239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Joseph PR, Mosier PD, Desai UR, Rajarathnam K. Solution NMR characterization of chemokine CXCL8/IL-8 monomer and dimer binding to glycosaminoglycans: structural plasticity mediates differential binding interactions. Biochem J. 2015;472:121–33. doi: 10.1042/BJ20150059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Singh A, Kett WC, Severin IC, Agyekum I, Duan J, Amster IJ, Proudfoot AE, Coombe DR, Woods RJ. The Interaction of Heparin Tetrasaccharides with Chemokine CCL5 Is Modulated by Sulfation Pattern and pH. J Biol Chem. 2015;290:15421–36. doi: 10.1074/jbc.M115.655845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Monneau Y, Arenzana-Seisdedos F, Lortat-Jacob H. The sweet spot: how GAGs help chemokines guide migrating cells. J Leukoc Biol. 2016;99:935–53. doi: 10.1189/jlb.3MR0915-440R. [DOI] [PubMed] [Google Scholar]
- 118.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS. Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling. Cell Rep. 2015;13:2851–64. doi: 10.1016/j.celrep.2015.11.053. [DOI] [PubMed] [Google Scholar]
- 119.Barbato A, Turato G, Baraldo S, Bazzan E, Calabrese F, Panizzolo C, Zanin ME, Zuin R, Maestrelli P, Fabbri LM, Saetta M. Epithelial damage and angiogenesis in the airways of children with asthma. Am J Respir Crit Care Med. 2006;174:975–81. doi: 10.1164/rccm.200602-189OC. [DOI] [PubMed] [Google Scholar]
- 120.Malmstrom K, Pelkonen AS, Malmberg LP, Sarna S, Lindahl H, Kajosaari M, Turpeinen M, Saglani S, Bush A, Haahtela T, Jeffery PK, Makela MJ. Lung function, airway remodelling and inflammation in symptomatic infants: outcome at 3 years. Thorax. 2011;66:157–62. doi: 10.1136/thx.2010.139246. [DOI] [PubMed] [Google Scholar]
- 121.Payne DN, Rogers AV, Adelroth E, Bandi V, Guntupalli KK, Bush A, Jeffery PK. Early thickening of the reticular basement membrane in children with difficult asthma. Am J Respir Crit Care Med. 2003;167:78–82. doi: 10.1164/rccm.200205-414OC. [DOI] [PubMed] [Google Scholar]
- 122.Saglani S, Payne DN, Zhu J, Wang Z, Nicholson AG, Bush A, Jeffery PK. Early detection of airway wall remodeling and eosinophilic inflammation in preschool wheezers. Am J Respir Crit Care Med. 2007;176:858–64. doi: 10.1164/rccm.200702-212OC. [DOI] [PubMed] [Google Scholar]
- 123.Kis K, Liu X, Hagood JS. Myofibroblast differentiation and survival in fibrotic disease. Expert Rev Mol Med. 2011;13:e27. doi: 10.1017/S1462399411001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Al-Muhsen S, Johnson JR, Hamid Q. Remodeling in asthma. J Allergy Clin Immunol. 2011;128:451–62. doi: 10.1016/j.jaci.2011.04.047. quiz 463–4. [DOI] [PubMed] [Google Scholar]
- 125.Cho JY. Recent advances in mechanisms and treatments of airway remodeling in asthma: a message from the bench side to the clinic. Korean J Intern Med. 2011;26:367–83. doi: 10.3904/kjim.2011.26.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Phan SH. The myofibroblast in pulmonary fibrosis. Chest. 2002;122:286S–289S. doi: 10.1378/chest.122.6_suppl.286s. [DOI] [PubMed] [Google Scholar]
- 127.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis. A combined immunohistochemical and in situ hybridization study. Am J Pathol. 1994;145:114–25. [PMC free article] [PubMed] [Google Scholar]
- 128.Phan SH. Biology of fibroblasts and myofibroblasts. Proc Am Thorac Soc. 2008;5:334–7. doi: 10.1513/pats.200708-146DR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Petrov VV, Fagard RH, Lijnen PJ. Stimulation of collagen production by transforming growth factor-beta1 during differentiation of cardiac fibroblasts to myofibroblasts. Hypertension. 2002;39:258–63. doi: 10.1161/hy0202.103268. [DOI] [PubMed] [Google Scholar]
- 130.Lambrecht BN, Hammad H. The airway epithelium in asthma. Nat Med. 2012;18:684–92. doi: 10.1038/nm.2737. [DOI] [PubMed] [Google Scholar]
- 131.Nam YH, Lee SK, Sammut D, Davies DE, Howarth PH. Preliminary study of the cellular characteristics of primary bronchial fibroblasts in patients with asthma: expression of alpha-smooth muscle actin, fibronectin containing extra type III domain A, and smoothelin. J Investig Allergol Clin Immunol. 2012;22:20–7. [PubMed] [Google Scholar]
- 132.Liang J, Jiang D, Jung Y, Xie T, Ingram J, Church T, Degan S, Leonard M, Kraft M, Noble PW. Role of hyaluronan and hyaluronan-binding proteins in human asthma. J Allergy Clin Immunol. 2011;128:403–411 e3. doi: 10.1016/j.jaci.2011.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wilkinson TS, Potter-Perigo S, Tsoi C, Altman LC, Wight TN. Pro- and anti-inflammatory factors cooperate to control hyaluronan synthesis in lung fibroblasts. Am J Respir Cell Mol Biol. 2004;31:92–9. doi: 10.1165/rcmb.2003-0380OC. [DOI] [PubMed] [Google Scholar]
- 134.Soderberg M, Lundgren R, Bjermer L, Stjernberg N, Rosenhall L. Inflammatory response in bronchoalveolar lavage fluid after inhaling histamine. Allergy. 1989;44:98–102. doi: 10.1111/j.1398-9995.1989.tb02231.x. [DOI] [PubMed] [Google Scholar]
- 135.Vignola AM, Chanez P, Campbell AM, Souques F, Lebel B, Enander I, Bousquet J. Airway inflammation in mild intermittent and in persistent asthma. Am J Respir Crit Care Med. 1998;157:403–9. doi: 10.1164/ajrccm.157.2.96-08040. [DOI] [PubMed] [Google Scholar]
- 136.de Medeiros Matsushita M, da Silva LF, dos Santos MA, Fernezlian S, Schrumpf JA, Roughley P, Hiemstra PS, Saldiva PH, Mauad T, Dolhnikoff M. Airway proteoglycans are differentially altered in fatal asthma. J Pathol. 2005;207:102–10. doi: 10.1002/path.1818. [DOI] [PubMed] [Google Scholar]
- 137.Pini L, Hamid Q, Shannon J, Lemelin L, Olivenstein R, Ernst P, Lemiere C, Martin JG, Ludwig MS. Differences in proteoglycan deposition in the airways of moderate and severe asthmatics. Eur Respir J. 2007;29:71–7. doi: 10.1183/09031936.00047905. [DOI] [PubMed] [Google Scholar]
- 138.Bousquet J, Chanez P, Lacoste JY, Enander I, Venge P, Peterson C, Ahlstedt S, Michel FB, Godard P. Indirect evidence of bronchial inflammation assessed by titration of inflammatory mediators in BAL fluid of patients with asthma. J Allergy Clin Immunol. 1991;88:649–60. doi: 10.1016/0091-6749(91)90159-l. [DOI] [PubMed] [Google Scholar]
- 139.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Correlation of hyaluronan deposition with infiltration of eosinophils and lymphocytes in a cockroach-induced murine model of asthma. Glycobiology. 2013;23:43–58. doi: 10.1093/glycob/cws122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Jeffery PK. Remodeling in asthma and chronic obstructive lung disease. Am J Respir Crit Care Med. 2001;164:S28–38. doi: 10.1164/ajrccm.164.supplement_2.2106061. [DOI] [PubMed] [Google Scholar]
- 141.Westergren-Thorsson G, Chakir J, Lafreniere-Allard MJ, Boulet LP, Tremblay GM. Correlation between airway responsiveness and proteoglycan production by bronchial fibroblasts from normal and asthmatic subjects. Int J Biochem Cell Biol. 2002;34:1256–67. doi: 10.1016/s1357-2725(02)00058-4. [DOI] [PubMed] [Google Scholar]
- 142.Burgess JK. The role of the extracellular matrix and specific growth factors in the regulation of inflammation and remodelling in asthma. Pharmacol Ther. 2009;122:19–29. doi: 10.1016/j.pharmthera.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 143.Hinz B, Phan SH, Thannickal VJ, Prunotto M, Desmouliere A, Varga J, De Wever O, Mareel M, Gabbiani G. Recent developments in myofibroblast biology: paradigms for connective tissue remodeling. Am J Pathol. 2012;180:1340–55. doi: 10.1016/j.ajpath.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Long-term effects of budesonide or nedocromil in children with asthma. The Childhood Asthma Management Program Research Group. N Engl J Med. 2000;343:1054–63. doi: 10.1056/NEJM200010123431501. [DOI] [PubMed] [Google Scholar]
- 145.Guilbert TW, Morgan WJ, Zeiger RS, Mauger DT, Boehmer SJ, Szefler SJ, Bacharier LB, Lemanske RF, Jr, Strunk RC, Allen DB, Bloomberg GR, Heldt G, Krawiec M, Larsen G, Liu AH, Chinchilli VM, Sorkness CA, Taussig LM, Martinez FD. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354:1985–97. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
- 146.Holgate ST, Holloway J, Wilson S, Bucchieri F, Puddicombe S, Davies DE. Epithelial-mesenchymal communication in the pathogenesis of chronic asthma. Proc Am Thorac Soc. 2004;1:93–8. doi: 10.1513/pats.2306034. [DOI] [PubMed] [Google Scholar]
- 147.Kelly MM, Leigh R, Bonniaud P, Ellis R, Wattie J, Smith MJ, Martin G, Panju M, Inman MD, Gauldie J. Epithelial expression of profibrotic mediators in a model of allergen-induced airway remodeling. Am J Respir Cell Mol Biol. 2005;32:99–107. doi: 10.1165/rcmb.2004-0190OC. [DOI] [PubMed] [Google Scholar]
- 148.Kumar RK, Herbert C, Foster PS. Expression of growth factors by airway epithelial cells in a model of chronic asthma: regulation and relationship to subepithelial fibrosis. Clin Exp Allergy. 2004;34:567–75. doi: 10.1111/j.1365-2222.2004.1917.x. [DOI] [PubMed] [Google Scholar]
- 149.Baluk P, Lee CG, Link H, Ator E, Haskell A, Elias JA, McDonald DM. Regulated angiogenesis and vascular regression in mice overexpressing vascular endothelial growth factor in airways. Am J Pathol. 2004;165:1071–85. doi: 10.1016/S0002-9440(10)63369-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Torrego A, Hew M, Oates T, Sukkar M, Fan Chung K. Expression and activation of TGF-beta isoforms in acute allergen-induced remodelling in asthma. Thorax. 2007;62:307–13. doi: 10.1136/thx.2006.063487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Chetta A, Zanini A, Foresi A, D’Ippolito R, Tipa A, Castagnaro A, Baraldo S, Neri M, Saetta M, Olivieri D. Vascular endothelial growth factor up-regulation and bronchial wall remodelling in asthma. Clin Exp Allergy. 2005;35:1437–42. doi: 10.1111/j.1365-2222.2005.02360.x. [DOI] [PubMed] [Google Scholar]
- 152.Amishima M, Munakata M, Nasuhara Y, Sato A, Takahashi T, Homma Y, Kawakami Y. Expression of epidermal growth factor and epidermal growth factor receptor immunoreactivity in the asthmatic human airway. Am J Respir Crit Care Med. 1998;157:1907–12. doi: 10.1164/ajrccm.157.6.9609040. [DOI] [PubMed] [Google Scholar]
- 153.Chu HW, Balzar S, Seedorf GJ, Westcott JY, Trudeau JB, Silkoff P, Wenzel SE. Transforming growth factor-beta2 induces bronchial epithelial mucin expression in asthma. Am J Pathol. 2004;165:1097–106. doi: 10.1016/s0002-9440(10)63371-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Howell JE, McAnulty RJ. TGF-beta: its role in asthma and therapeutic potential. Curr Drug Targets. 2006;7:547–65. doi: 10.2174/138945006776818692. [DOI] [PubMed] [Google Scholar]
- 155.Zhang S, Smartt H, Holgate ST, Roche WR. Growth factors secreted by bronchial epithelial cells control myofibroblast proliferation: an in vitro co-culture model of airway remodeling in asthma. Lab Invest. 1999;79:395–405. [PubMed] [Google Scholar]
- 156.Bhandari V, Choo-Wing R, Chapoval SP, Lee CG, Tang C, Kim YK, Ma B, Baluk P, Lin MI, McDonald DM, Homer RJ, Sessa WC, Elias JA. Essential role of nitric oxide in VEGF-induced, asthma-like angiogenic, inflammatory, mucus, and physiologic responses in the lung. Proc Natl Acad Sci U S A. 2006;103:11021–6. doi: 10.1073/pnas.0601057103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Hoshino M, Nakamura Y, Hamid QA. Gene expression of vascular endothelial growth factor and its receptors and angiogenesis in bronchial asthma. J Allergy Clin Immunol. 2001;107:1034–8. doi: 10.1067/mai.2001.115626. [DOI] [PubMed] [Google Scholar]
- 158.Asai K, Kanazawa H, Kamoi H, Shiraishi S, Hirata K, Yoshikawa J. Increased levels of vascular endothelial growth factor in induced sputum in asthmatic patients. Clin Exp Allergy. 2003;33:595–9. doi: 10.1046/j.1365-2222.2003.01576.x. [DOI] [PubMed] [Google Scholar]
- 159.Voelkel NF, Vandivier RW, Tuder RM. Vascular endothelial growth factor in the lung. Am J Physiol Lung Cell Mol Physiol. 2006;290:L209–21. doi: 10.1152/ajplung.00185.2005. [DOI] [PubMed] [Google Scholar]
- 160.Takayama G, Arima K, Kanaji T, Toda S, Tanaka H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K. Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol. 2006;118:98–104. doi: 10.1016/j.jaci.2006.02.046. [DOI] [PubMed] [Google Scholar]
- 161.Woodruff PG, Boushey HA, Dolganov GM, Barker CS, Yang YH, Donnelly S, Ellwanger A, Sidhu SS, Dao-Pick TP, Pantoja C, Erle DJ, Yamamoto KR, Fahy JV. Genome-wide profiling identifies epithelial cell genes associated with asthma and with treatment response to corticosteroids. Proc Natl Acad Sci U S A. 2007;104:15858–63. doi: 10.1073/pnas.0707413104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Karagiannidis C, Hense G, Martin C, Epstein M, Ruckert B, Mantel PY, Menz G, Uhlig S, Blaser K, Schmidt-Weber CB. Activin A is an acute allergen-responsive cytokine and provides a link to TGF-beta-mediated airway remodeling in asthma. J Allergy Clin Immunol. 2006;117:111–8. doi: 10.1016/j.jaci.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 163.Kariyawasam HH, Pegorier S, Barkans J, Xanthou G, Aizen M, Ying S, Kay AB, Lloyd CM, Robinson DS. Activin and transforming growth factor-beta signaling pathways are activated after allergen challenge in mild asthma. J Allergy Clin Immunol. 2009;124:454–62. doi: 10.1016/j.jaci.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kariyawasam HH, Semitekolou M, Robinson DS, Xanthou G. Activin-A: a novel critical regulator of allergic asthma. Clin Exp Allergy. 2011;41:1505–14. doi: 10.1111/j.1365-2222.2011.03784.x. [DOI] [PubMed] [Google Scholar]
- 165.Hardy CL, O’Connor AE, Yao J, Sebire K, de Kretser DM, Rolland JM, Anderson GP, Phillips DJ, O’Hehir RE. Follistatin is a candidate endogenous negative regulator of activin A in experimental allergic asthma. Clin Exp Allergy. 2006;36:941–50. doi: 10.1111/j.1365-2222.2006.02523.x. [DOI] [PubMed] [Google Scholar]
- 166.Hedger MP, de Kretser DM. The activins and their binding protein, follistatin-Diagnostic and therapeutic targets in inflammatory disease and fibrosis. Cytokine Growth Factor Rev. 2013;24:285–95. doi: 10.1016/j.cytogfr.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 167.Reeves SR, Kolstad T, Lien TY, Elliott M, Ziegler SF, Wight TN, Debley JS. Asthmatic airway epithelial cells differentially regulate fibroblast expression of extracellular matrix components. J Allergy Clin Immunol. 2014;134:663–670 e1. doi: 10.1016/j.jaci.2014.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Reeves SR, Kolstad T, Lien TY, Herrington-Shaner S, Debley JS. Fibroblast-myofibroblast transition is differentially regulated by bronchial epithelial cells from asthmatic children. Respir Res. 2015;16:21. doi: 10.1186/s12931-015-0185-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Casalino-Matsuda SM, Monzon ME, Conner GE, Salathe M, Forteza RM. Role of hyaluronan and reactive oxygen species in tissue kallikrein-mediated epidermal growth factor receptor activation in human airways. J Biol Chem. 2004;279:21606–16. doi: 10.1074/jbc.M309950200. [DOI] [PubMed] [Google Scholar]
- 170.Forteza R, Lieb T, Aoki T, Savani RC, Conner GE, Salathe M. Hyaluronan serves a novel role in airway mucosal host defense. FASEB J. 2001;15:2179–86. doi: 10.1096/fj.01-0036com. [DOI] [PubMed] [Google Scholar]
- 171.Bhagat R, Forteza RM, Calcote CB, Williams WT, Bigler SA, Dwyer TM. Pulmonary emboli from therapeutic sodium hyaluronate. Respir Care. 2012;57:1670–3. doi: 10.4187/respcare.01666. [DOI] [PubMed] [Google Scholar]
- 172.Casalino-Matsuda SM, Monzon ME, Forteza RM. Epidermal growth factor receptor activation by epidermal growth factor mediates oxidant-induced goblet cell metaplasia in human airway epithelium. Am J Respir Cell Mol Biol. 2006;34:581–91. doi: 10.1165/rcmb.2005-0386OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Monzon ME, Forteza RM, Casalino-Matsuda SM. MCP-1/CCR2B-dependent loop upregulates MUC5AC and MUC5B in human airway epithelium. Am J Physiol Lung Cell Mol Physiol. 2011;300:L204–15. doi: 10.1152/ajplung.00292.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Casalino-Matsuda SM, Monzon ME, Day AJ, Forteza RM. Hyaluronan fragments/CD44 mediate oxidative stress-induced MUC5B up-regulation in airway epithelium. Am J Respir Cell Mol Biol. 2009;40:277–85. doi: 10.1165/rcmb.2008-0073OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Lauer ME, Erzurum SC, Mukhopadhyay D, Vasanji A, Drazba J, Wang A, Fulop C, Hascall VC. Differentiated murine airway epithelial cells synthesize a leukocyte-adhesive hyaluronan matrix in response to endoplasmic reticulum stress. J Biol Chem. 2008;283:26283–96. doi: 10.1074/jbc.M803350200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Evanko SP, Potter-Perigo S, Bollyky PL, Nepom GT, Wight TN. Hyaluronan and versican in the control of human T-lymphocyte adhesion and migration. Matrix Biol. 2012;31:90–100. doi: 10.1016/j.matbio.2011.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Katoh S, Matsumoto N, Kawakita K, Tominaga A, Kincade PW, Matsukura S. A role for CD44 in an antigen-induced murine model of pulmonary eosinophilia. J Clin Invest. 2003;111:1563–70. doi: 10.1172/JCI16583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Kai Y, Yoneyama H, Koyama J, Hamada K, Kimura H, Matsushima K. Treatment with chondroitinase ABC alleviates bleomycin-induced pulmonary fibrosis. Med Mol Morphol. 2007;40:128–40. doi: 10.1007/s00795-007-0370-y. [DOI] [PubMed] [Google Scholar]
- 179.Potter-Perigo S, Johnson PY, Evanko SP, Chan CK, Braun KR, Wilkinson TS, Altman LC, Wight TN. Polyinosine-polycytidylic acid stimulates versican accumulation in the extracellular matrix promoting monocyte adhesion. Am J Respir Cell Mol Biol. 2010;43:109–20. doi: 10.1165/rcmb.2009-0081OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Venkatesan N, Ouzzine M, Kolb M, Netter P, Ludwig MS. Increased deposition of chondroitin/dermatan sulfate glycosaminoglycan and upregulation of beta1,3-glucuronosyltransferase I in pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2011;300:L191–203. doi: 10.1152/ajplung.00214.2010. [DOI] [PubMed] [Google Scholar]
- 181.Cheng G, Swaidani S, Sharma M, Lauer ME, Hascall VC, Aronica MA. Hyaluronan deposition and correlation with inflammation in a murine ovalbumin model of asthma. Matrix Biol. 2011;30:126–34. doi: 10.1016/j.matbio.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Wang Q, Teder P, Judd NP, Noble PW, Doerschuk CM. CD44 deficiency leads to enhanced neutrophil migration and lung injury in Escherichia coli pneumonia in mice. Am J Pathol. 2002;161:2219–28. doi: 10.1016/S0002-9440(10)64498-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Harada H, Takahashi M. CD44-dependent intracellular and extracellular catabolism of hyaluronic acid by hyaluronidase-1 and -2. J Biol Chem. 2007;282:5597–607. doi: 10.1074/jbc.M608358200. [DOI] [PubMed] [Google Scholar]
- 184.Monzon ME, Manzanares D, Schmid N, Casalino-Matsuda SM, Forteza RM. Hyaluronidase expression and activity is regulated by pro-inflammatory cytokines in human airway epithelial cells. Am J Respir Cell Mol Biol. 2008;39:289–95. doi: 10.1165/rcmb.2007-0361OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.West DC, Shaw DM, Lorenz P, Adzick NS, Longaker MT. Fibrotic healing of adult and late gestation fetal wounds correlates with increased hyaluronidase activity and removal of hyaluronan. Int J Biochem Cell Biol. 1997;29:201–10. doi: 10.1016/s1357-2725(96)00133-1. [DOI] [PubMed] [Google Scholar]
- 186.Oertli B, Beck-Schimmer B, Fan X, Wuthrich RP. Mechanisms of hyaluronan-induced upregulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-kappa B and activating protein-1. J Immunol. 1998;161:3431–7. [PubMed] [Google Scholar]
- 187.Slevin M, Krupinski J, Kumar S, Gaffney J. Angiogenic oligosaccharides of hyaluronan induce protein tyrosine kinase activity in endothelial cells and activate a cytoplasmic signal transduction pathway resulting in proliferation. Lab Invest. 1998;78:987–1003. [PubMed] [Google Scholar]
- 188.Jiang D, Liang J, Noble PW. Hyaluronan as an immune regulator in human diseases. Physiol Rev. 2011;91:221–64. doi: 10.1152/physrev.00052.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ohkawara Y, Tamura G, Iwasaki T, Tanaka A, Kikuchi T, Shirato K. Activation and transforming growth factor-beta production in eosinophils by hyaluronan. Am J Respir Cell Mol Biol. 2000;23:444–51. doi: 10.1165/ajrcmb.23.4.3875. [DOI] [PubMed] [Google Scholar]
- 190.Venkatesan N, Siddiqui S, Jo T, Martin JG, Ludwig MS. Allergen-induced airway remodeling in brown norway rats: structural and metabolic changes in glycosaminoglycans. Am J Respir Cell Mol Biol. 2012;46:96–105. doi: 10.1165/rcmb.2011-0014OC. [DOI] [PubMed] [Google Scholar]
- 191.Jackson DJ, Sykes A, Mallia P, Johnston SL. Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol. 2011;128:1165–74. doi: 10.1016/j.jaci.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 193.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–84. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Laskin DL, Weinberger B, Laskin JD. Functional heterogeneity in liver and lung macrophages. J Leukoc Biol. 2001;70:163–70. [PubMed] [Google Scholar]
- 195.Mantovani A, Sica A, Locati M. Macrophage polarization comes of age. Immunity. 2005;23:344–6. doi: 10.1016/j.immuni.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 196.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 197.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–9. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 198.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege JL, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity. 2014;41:14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Kratz M, Coats BR, Hisert KB, Hagman D, Mutskov V, Peris E, Schoenfelt KQ, Kuzma JN, Larson I, Billing PS, Landerholm RW, Crouthamel M, Gozal D, Hwang S, Singh PK, Becker L. Metabolic dysfunction drives a mechanistically distinct proinflammatory phenotype in adipose tissue macrophages. Cell Metab. 2014;20:614–25. doi: 10.1016/j.cmet.2014.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Porcheray F, Viaud S, Rimaniol AC, Leone C, Samah B, Dereuddre-Bosquet N, Dormont D, Gras G. Macrophage activation switching: an asset for the resolution of inflammation. Clin Exp Immunol. 2005;142:481–9. doi: 10.1111/j.1365-2249.2005.02934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Stout RD, Jiang C, Matta B, Tietzel I, Watkins SK, Suttles J. Macrophages sequentially change their functional phenotype in response to changes in microenvironmental influences. J Immunol. 2005;175:342–9. doi: 10.4049/jimmunol.175.1.342. [DOI] [PubMed] [Google Scholar]
- 202.Lichtnekert J, Kawakami T, Parks WC, Duffield JS. Changes in macrophage phenotype as the immune response evolves. Curr Opin Pharmacol. 2013;13:555–64. doi: 10.1016/j.coph.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Aggarwal NR, King LS, D’Alessio FR. Diverse macrophage populations mediate acute lung inflammation and resolution. Am J Physiol Lung Cell Mol Physiol. 2014;306:L709–25. doi: 10.1152/ajplung.00341.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Duffield JS, Forbes SJ, Constandinou CM, Clay S, Partolina M, Vuthoori S, Wu S, Lang R, Iredale JP. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tabas I, Bornfeldt KE. Macrophage phenotype and function in different stages of atherosclerosis. Circ Res. 2016;118:653–67. doi: 10.1161/CIRCRESAHA.115.306256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Nahrendorf M, Swirski FK, Aikawa E, Stangenberg L, Wurdinger T, Figueiredo JL, Libby P, Weissleder R, Pittet MJ. The healing myocardium sequentially mobilizes two monocyte subsets with divergent and complementary functions. J Exp Med. 2007;204:3037–47. doi: 10.1084/jem.20070885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ricardo SD, van Goor H, Eddy AA. Macrophage diversity in renal injury and repair. J Clin Invest. 2008;118:3522–30. doi: 10.1172/JCI36150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Lucas T, Waisman A, Ranjan R, Roes J, Krieg T, Muller W, Roers A, Eming SA. Differential roles of macrophages in diverse phases of skin repair. J Immunol. 2010;184:3964–77. doi: 10.4049/jimmunol.0903356. [DOI] [PubMed] [Google Scholar]
- 209.Goren I, Allmann N, Yogev N, Schurmann C, Linke A, Holdener M, Waisman A, Pfeilschifter J, Frank S. A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol. 2009;175:132–47. doi: 10.2353/ajpath.2009.081002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Ramachandran P, Pellicoro A, Vernon MA, Boulter L, Aucott RL, Ali A, Hartland SN, Snowdon VK, Cappon A, Gordon-Walker TT, Williams MJ, Dunbar DR, Manning JR, van Rooijen N, Fallowfield JA, Forbes SJ, Iredale JP. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci USA. 2012;109:E3186–95. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Song E, Ouyang N, Horbelt M, Antus B, Wang M, Exton MS. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell Immunol. 2000;204:19–28. doi: 10.1006/cimm.2000.1687. [DOI] [PubMed] [Google Scholar]
- 212.Duffield JS. The inflammatory macrophage: a story of Jekyll and Hyde. Clin Sci (Lond) 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 213.Wynn TA, Barron L. Macrophages: master regulators of inflammation and fibrosis. Semin Liver Dis. 2010;30:245–57. doi: 10.1055/s-0030-1255354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Mahdavian Delavary B, van der Veer WM, van Egmond M, Niessen FB, Beelen RH. Macrophages in skin injury and repair. Immunobiology. 2011;216:753–62. doi: 10.1016/j.imbio.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 215.Pellicoro A, Ramachandran P, Iredale JP, Fallowfield JA. Liver fibrosis and repair: immune regulation of wound healing in a solid organ. Nat Rev Immunol. 2014;14:181–94. doi: 10.1038/nri3623. [DOI] [PubMed] [Google Scholar]
- 216.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–69. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Atabai K, Jame S, Azhar N, Kuo A, Lam M, McKleroy W, Dehart G, Rahman S, Xia DD, Melton AC, Wolters P, Emson CL, Turner SM, Werb Z, Sheppard D. Mfge8 diminishes the severity of tissue fibrosis in mice by binding and targeting collagen for uptake by macrophages. J Clin Invest. 2009;119:3713–22. doi: 10.1172/JCI40053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Madsen DH, Leonard D, Masedunskas A, Moyer A, Jurgensen HJ, Peters DE, Amornphimoltham P, Selvaraj A, Yamada SS, Brenner DA, Burgdorf S, Engelholm LH, Behrendt N, Holmbeck K, Weigert R, Bugge TH. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J Cell Biol. 2013;202:951–66. doi: 10.1083/jcb.201301081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Iredale JP, Bataller R. Identifying molecular factors that contribute to resolution of liver fibrosis. Gastroenterology. 2014;146:1160–4. doi: 10.1053/j.gastro.2014.03.019. [DOI] [PubMed] [Google Scholar]
- 220.Vannella KM, Barron L, Borthwick LA, Kindrachuk KN, Narasimhan PB, Hart KM, Thompson RW, White S, Cheever AW, Ramalingam TR, Wynn TA. Incomplete deletion of IL-4Ralpha by LysM(Cre) reveals distinct subsets of M2 macrophages controlling inflammation and fibrosis in chronic schistosomiasis. PLoS Pathog. 2014;10:e1004372. doi: 10.1371/journal.ppat.1004372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Rohani MG, McMahan RS, Razumova MV, Hertz AL, Cieslewicz M, Pun SH, Regnier M, Wang Y, Birkland TP, Parks WC. MMP-10 regulates collagenolytic activity of alternatively activated resident macrophages. J Invest Dermatol. 2015;135:2377–84. doi: 10.1038/jid.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Duffield JS, Lupher M, Thannickal VJ, Wynn TA. Host responses in tissue repair and fibrosis. Annu Rev Pathol. 2013;8:241–76. doi: 10.1146/annurev-pathol-020712-163930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Prasse A, Pechkovsky DV, Toews GB, Jungraithmayr W, Kollert F, Goldmann T, Vollmer E, Muller-Quernheim J, Zissel G. A vicious circle of alveolar macrophages and fibroblasts perpetuates pulmonary fibrosis via CCL18. Am J Respir Crit Care Med. 2006;173:781–92. doi: 10.1164/rccm.200509-1518OC. [DOI] [PubMed] [Google Scholar]
- 224.Gibbons MA, MacKinnon AC, Ramachandran P, Dhaliwal K, Duffin R, Phythian-Adams AT, van Rooijen N, Haslett C, Howie SE, Simpson AJ, Hirani N, Gauldie J, Iredale JP, Sethi T, Forbes SJ. Ly6Chi monocytes direct alternatively activated profibrotic macrophage regulation of lung fibrosis. Am J Respir Crit Care Med. 2011;184:569–81. doi: 10.1164/rccm.201010-1719OC. [DOI] [PubMed] [Google Scholar]
- 225.Barron L, Smith AM, El Kasmi KC, Qualls JE, Huang X, Cheever A, Borthwick LA, Wilson MS, Murray PJ, Wynn TA. Role of arginase 1 from myeloid cells in th2-dominated lung inflammation. PLoS One. 2013;8:e61961. doi: 10.1371/journal.pone.0061961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Huen SC, Moeckel GW, Cantley LG. Macrophage-specific deletion of transforming growth factor-beta1 does not prevent renal fibrosis after severe ischemia-reperfusion or obstructive injury. Am J Physiol Renal Physiol. 2013;305:F477–84. doi: 10.1152/ajprenal.00624.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Everts V, van der Zee E, Creemers L, Beertsen W. Phagocytosis and intracellular digestion of collagen, its role in turnover and remodelling. Histochem J. 1996;28:229–45. doi: 10.1007/BF02409011. [DOI] [PubMed] [Google Scholar]
- 228.Madsen DH, Ingvarsen S, Jurgensen HJ, Melander MC, Kjoller L, Moyer A, Honore C, Madsen CA, Garred P, Burgdorf S, Bugge TH, Behrendt N, Engelholm LH. The non-phagocytic route of collagen uptake: a distinct degradation pathway. J Biol Chem. 2011;286:26996–7010. doi: 10.1074/jbc.M110.208033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Lee H, Overall CM, McCulloch CA, Sodek J. A critical role for the membrane-type 1 matrix metalloproteinase in collagen phagocytosis. Mol Biol Cell. 2006;17:4812–26. doi: 10.1091/mbc.E06-06-0486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Madsen DH, Engelholm LH, Ingvarsen S, Hillig T, Wagenaar-Miller RA, Kjoller L, Gardsvoll H, Hoyer-Hansen G, Holmbeck K, Bugge TH, Behrendt N. Extracellular collagenases and the endocytic receptor, urokinase plasminogen activator receptor-associated protein/Endo180, cooperate in fibroblast-mediated collagen degradation. J Biol Chem. 2007;282:27037–45. doi: 10.1074/jbc.M701088200. [DOI] [PubMed] [Google Scholar]
- 231.Wagenaar-Miller RA, Engelholm LH, Gavard J, Yamada SS, Gutkind JS, Behrendt N, Bugge TH, Holmbeck K. Complementary roles of intracellular and pericellular collagen degradation pathways in vivo. Mol Cell Biol. 2007;27:6309–22. doi: 10.1128/MCB.00291-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Giannandrea M, Parks WC. Diverse functions of matrix metalloproteinases during fibrosis. Dis Model Mech. 2014;7:193–203. doi: 10.1242/dmm.012062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Craig VJ, Zhang L, Hagood JS, Owen CA. Matrix metalloproteinases as therapeutic targets for idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2015 doi: 10.1165/rcmb.2015-0020TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Pardo A, Selman M, Kaminski N. Approaching the degradome in idiopathic pulmonary fibrosis. Int J Biochem Cell Biol. 2008;40:1141–55. doi: 10.1016/j.biocel.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 235.Hautamaki RD, Kobayashi DK, Senior RM, Shapiro SD. Requirement for macrophage elastase for cigarette smoke-induced emphysema. Science. 1997;277:2002–2004. doi: 10.1126/science.277.5334.2002. [DOI] [PubMed] [Google Scholar]
- 236.Manicone AM, Birkland TP, Lin M, Betsuyaku T, van Rooijen N, Lohi J, Keski-Oja J, Wang Y, Skerrett SJ, Parks WC. Epilysin (MMP-28) restrains early macrophage recruitment in Pseudomonas aeruginosa pneumonia. J Immunol. 2009;182:3866–76. doi: 10.4049/jimmunol.0713949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Gharib SA, Johnston LK, Huizar I, Birkland TP, Hanson J, Wang Y, Parks WC, Manicone AM. MMP28 promotes macrophage polarization toward M2 cells and augments pulmonary fibrosis. J Leukoc Biol. 2014;95:9–18. doi: 10.1189/jlb.1112587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Gill SE, Gharib SA, Bench EM, Sussman SW, Wang RT, Rims C, Birkland TP, Wang Y, Manicone AM, McGuire JK, Parks WC. Tissue inhibitor of metalloproteinases-3 moderates the proinflammatory status of macrophages. Am J Respir Cell Mol Biol. 2013;49:768–77. doi: 10.1165/rcmb.2012-0377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Holmbeck K, Bianco P, Caterina J, Yamada S, Kromer M, Kuznetsov SA, Mankani M, Robey PG, Poole AR, Pidoux I, Ward JM, Birkedal-Hansen H. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- 240.Hotary K, Allen E, Punturieri A, Yana I, Weiss SJ. Regulation of cell invasion and morphogenesis in a three-dimensional type I collagen matrix by membrane-type matrix metalloproteinases 1, 2, and 3. J Cell Biol. 2000;149:1309–23. doi: 10.1083/jcb.149.6.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hotary KB, Allen ED, Brooks PC, Datta NS, Long MW, Weiss SJ. Membrane type I matrix metalloproteinase usurps tumor growth control imposed by the three-dimensional extracellular matrix. Cell. 2003;114:33–45. doi: 10.1016/s0092-8674(03)00513-0. [DOI] [PubMed] [Google Scholar]
- 242.Filippov S, Caras I, Murray R, Matrisian LM, Chapman HA, Jr, Shapiro S, Weiss SJ. Matrilysin-dependent elastolysis by human macrophages. J Exp Med. 2003;198:925–35. doi: 10.1084/jem.20030626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Parks WC, Wilson CL, Lopez-Boado YS. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat Rev Immunol. 2004;4:617–629. doi: 10.1038/nri1418. [DOI] [PubMed] [Google Scholar]
- 244.Yong VW. Metalloproteinases: mediators of pathology and regeneration in the CNS. Nat Rev Neurosci. 2005;6:931–44. doi: 10.1038/nrn1807. [DOI] [PubMed] [Google Scholar]
- 245.Cauwe B, Van den Steen PE, Opdenakker G. The biochemical, biological, and pathological kaleidoscope of cell surface substrates processed by matrix metalloproteinases. Crit Rev Biochem Mol Biol. 2007;42:113–85. doi: 10.1080/10409230701340019. [DOI] [PubMed] [Google Scholar]
- 246.Hu J, Van Den Steen P, Sang Q, Opdenakker G. Matrix metalloproteinase inhibitors as therapy for inflammatory and vascular diseases. Nat Rev Drug Discov. 2007;6:480–98. doi: 10.1038/nrd2308. [DOI] [PubMed] [Google Scholar]
- 247.Greenlee KJ, Werb Z, Kheradmand F. Matrix metalloproteinases in lung: multiple, multifarious, and multifaceted. Physiol Rev. 2007;87:69–98. doi: 10.1152/physrev.00022.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Van Lint P, Libert C. Chemokine and cytokine processing by matrix metalloproteinases and its effect on leukocyte migration and inflammation. J Leukoc Biol. 2007;82:1375–81. doi: 10.1189/jlb.0607338. [DOI] [PubMed] [Google Scholar]
- 249.McQuibban GA, Gong JH, Tam EM, McCulloch CA, Clark-Lewis I, Overall CM. Inflammation dampened by gelatinase A cleavage of monocyte chemoattractant protein-3. Science. 2000;289:1202–6. doi: 10.1126/science.289.5482.1202. [DOI] [PubMed] [Google Scholar]
- 250.Wilson CL, Ouellette AJ, Satchell DP, Ayabe T, Lopez-Boado YS, Stratman JL, Hultgren SJ, Matrisian LM, Parks WC. Regulation of intestinal a-defensin activation by the metalloproteinase matrilysin in innate host defense. Science. 1999;286:113–117. doi: 10.1126/science.286.5437.113. [DOI] [PubMed] [Google Scholar]
- 251.Ochieng J, Fridman R, Nangia-Makker P, Kleiner DE, Liotta LA, Stetler-Stevenson WG, Raz A. Galectin-3 is a novel substrate for human matrix metalloproteinases-2 and -9. Biochemistry. 1994;33:14109–14114. doi: 10.1021/bi00251a020. [DOI] [PubMed] [Google Scholar]
- 252.Levi E, Fridman R, Miao HQ, Ma YS, Yayon A, Vlodavsky I. Matrix metalloproteinase 2 releases active soluble ectodomain of fibroblast growth factor receptor 1. Proc Natl Acad Sci USA. 1996;93:7069–7074. doi: 10.1073/pnas.93.14.7069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Fitzgerald ML, Wang Z, Park PW, Murphy G, Bernfield M. Shedding of syndecan-1 and -4 ectodomains is regulated by multiple signaling pathways and mediated by a TIMP-3-sensitive metalloproteinase. J Cell Biol. 2000;148:811–824. doi: 10.1083/jcb.148.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.McMahan RS, Birkland TP, Smigiel KS, Vandivort TC, Rohani MG, Manicone AM, McGuire JK, Gharib SA, Parks WC. Stromelysin-2 (MMP10) Moderates Inflammation by Controlling Macrophage Activation. J Immunol. 2016;197:899–909. doi: 10.4049/jimmunol.1600502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Sokai A, Handa T, Tanizawa K, Oga T, Uno K, Tsuruyama T, Kubo T, Ikezoe K, Nakatsuka Y, Tanimura K, Muro S, Hirai T, Nagai S, Chin K, Mishima M. Matrix metalloproteinase-10: a novel biomarker for idiopathic pulmonary fibrosis. Respir Res. 2015;16:120. doi: 10.1186/s12931-015-0280-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Vuga LJ, Herzog E, Lindell KO, Zhang Y, Aminski N, Tan J, Kass D, Gibson KF, Duncan S. MMp10 is a predictor of outcomes in IPF (Abstract). International Colloquim on Lung and Airway Fibrosis; Mont-Tremblant, Quebec Canada. 2014. [Google Scholar]
- 257.Konishi K, Gibson KF, Lindell KO, Richards TJ, Zhang Y, Dhir R, Bisceglia M, Gilbert S, Yousem SA, Song JW, Kim DS, Kaminski N. Gene expression profiles of acute exacerbations of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;180:167–75. doi: 10.1164/rccm.200810-1596OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Kaner R, Santiago F, Crystal R. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1+ smokers with early emphysema. J Leukoc Biol. 2009;86:913–922. doi: 10.1189/jlb.0408240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Gosselink JV, Hayashi S, Elliott WM, Xing L, Chan B, Yang L, Wright C, Sin D, Pare PD, Pierce JA, Pierce RA, Patterson A, Cooper J, Hogg JC. Differential expression of tissue repair genes in the pathogenesis of COPD. Am J Respir Crit Care Med. 2010;181:1329–35. doi: 10.1164/rccm.200812-1902OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Gharib SA, Loth DW, Soler Artigas M, Birkland TP, Wilk JB, Wain LV, Brody JA, Obeidat M, Hancock DB, Tang W, Rawal R, Boezen HM, Imboden M, Huffman JE, Lahousse L, Alves AC, Manichaikul A, Hui J, Morrison AC, Ramasamy A, Smith AV, Gudnason V, Surakka I, Vitart V, Evans DM, Strachan DP, Deary IJ, Hofman A, Glaser S, Wilson JF, North KE, Zhao JH, Heckbert SR, Jarvis DL, Probst-Hensch N, Schulz H, Barr RG, Jarvelin MR, O’Connor GT, Kahonen M, Cassano PA, Hysi PG, Dupuis J, Hayward C, Psaty BM, Hall IP, Parks WC, Tobin MD, London SJ, SpiroMeta C Consortium C. Integrative pathway genomics of lung function and airflow obstruction. Hum Mol Genet. 2015;24:6836–48. doi: 10.1093/hmg/ddv378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Cieslewicz M, Tang J, Yu JL, Cao H, Zavaljevski M, Motoyama K, Lieber A, Raines EW, Pun SH. Targeted delivery of proapoptotic peptides to tumor-associated macrophages improves survival. Proc Natl Acad Sci USA. 2013;110:15919–15924. doi: 10.1073/pnas.1312197110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Sabeh F, Li X, Saunders TL, Rowe RG, Weiss SJ. Secreted versus membrane-anchored collagenases: Relative roles in fibroblast-dependent collagenolysis and invasion. J Biol Chem. 2009;284:23001–23011. doi: 10.1074/jbc.M109.002808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Shi J, Son MY, Yamada S, Szabova L, Kahan S, Chrysovergis K, Wolf L, Surmak A, Holmbeck K. Membrane-type MMPs enable extracellular matrix permissiveness and mesenchymal cell proliferation during embryogenesis. Dev Biol. 2008;313:196–209. doi: 10.1016/j.ydbio.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Shaykhiev R, Krause A, Salit J, Strulovici-Barel Y, Harvey B-G, O’Connor TP, Crystal RG. Smoking-dependent reprogramming of alveolar macrophage polarization: implication for pathogenesis of chronic obstructive pulmonary disease. J Immunol. 2009;183:2867–83. doi: 10.4049/jimmunol.0900473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Shapiro SD. The macrophage in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1999;160:S29–32. doi: 10.1164/ajrccm.160.supplement_1.9. [DOI] [PubMed] [Google Scholar]
- 267.Tetley TD. Macrophages and the pathogenesis of COPD. Chest. 2002;121:156S–159S. doi: 10.1378/chest.121.5_suppl.156s. [DOI] [PubMed] [Google Scholar]