Abstract
Many adolescents struggle with overweight/obesity, which exponentially increases in the transition to adulthood. Overweight/obesity places youth at risk for serious health conditions, including type 2 diabetes. In adults, neural substrates implicated in addiction (e.g., orbitofrontal cortex (OFC), striatum, amygdala, and ventral tegmental area) have been found to be relevant to risk for overweight/obesity. In this study, we examined three hypotheses to disentangle the potential overlap between addiction and overweight/obesity processing by examining (1) brain response to high vs. low calorie beverages, (2) the strength of correspondence between biometrics, including body mass index (BMI) and insulin resistance, and brain response and (3) the relationship between a measure of food addiction and brain response using an established fMRI gustatory cue exposure task with a sample of overweight/obese youth (M age = 16.46; M BMI = 33.1). Greater BOLD response was observed across the OFC, inferior frontal gyrus (IFG), nucleus accumbens, right amygdala, and additional frontoparietal and temporal regions in neural processing of high vs. low calorie beverages. Further, BMI scores positively correlated with BOLD activation in the high calorie > low calorie contrast in the right postcentral gyrus and central operculum. Insulin resistance positively correlated with BOLD activation across the bilateral middle/superior temporal gyrus, left OFC, and superior parietal lobe. No relationships were observed between measures of food addiction and brain response. These findings support the activation of parallel addiction-related neural pathways in adolescents’ high calorie processing, while also suggesting the importance of refining conceptual and neurocognitive models to fit this developmental period.
Keywords: Adolescents, Overweight/obesity, Cue exposure, fMRI, Addiction
Background
Obesity is epidemic for children and adolescents within the United States (U.S.) (Lee et al. 2011). Recent surveys estimate that 34.5 % of 12–19 year olds meet criteria for overweight/obesity, defined as a body mass index (BMI) between the 85th and 95th percentile (overweight) or ≥95th percentile (obese) (Ogden et al. 2014). Importantly, the values are not static; as adolescents transition into young adulthood, these rates double (Harris et al. 2006). One reason for this includes the stressors and challenges inherent to adolescents’ transition into more independent roles, such as leaving home, taking over meal preparation, and undertaking new responsibilities at work and school. Susceptibility to weight gain has been tied to difficulty maintaining healthy eating habits (Poobalan et al. 2014) and prioritizing time for regular physical activity (Poobalan et al. 2012), which are often exacerbated in this transition.
The development of type 2 diabetes mellitus is one of the most worrisome manifestations of adolescent overweight and obesity. Medically, type 2 diabetes is preceded by a pre-diabetic state marked by insulin resistance. This state occurs when the body continues to produce insulin but does not successfully process it; the result is that glucose continues to accumulate in the blood rather than being successfully utilized by cells (National Institute of Diabetes and Digestive and Kidney Diseases 2014). Insulin resistance is associated with a constellation of problems in metabolic functioning, including hypertension, dyslipidemia, and impaired glucose metabolism; this array of risk factors increases risk for heart disease, diabetes, and stroke and has been termed “metabolic syndrome” (Sinaiko and Caprio 2012). While there is some degree of controversy regarding metabolic syndrome in children and adolescents (Weiss et al. 2013), 12–44 % of obese adolescents in the U.S. currently meet clinical diagnostic criteria for metabolic syndrome (Cook et al. 2008). In turn, some medical professionals posit that adolescents with metabolic syndrome are more likely to transition into type 2 diabetes (DeBoer et al. 2015; Morrison et al. 2008). Notably, Magnussen et al. (2010) assert that high BMI scores perform equally well as, if not better than, metabolic syndrome scores in predicting risk for type 2 diabetes in adulthood. In particular, there continues to be substantive controversy regarding the nature and utility of two biometric markers of potential transition to type 2 diabetes: BMI and insulin resistance. In light of this, the current study included both BMI and insulin resistance to help disentangle their potential empirical value in determining the relationship between adolescent brain response and physical health.
Primarily examined with adults, one framework used to explain chronic patterns of overeating is via neurocognitive processes, which continue to highlight commonalities between “addictive” processes in substance use disorders and overeating (Filbey et al. 2012; Gearhardt et al. 2011a, b; Volkow et al. 2013a). Interestingly, this link has also been established in the behavioral intervention literature with many successful treatment approaches for overweight/obesity (e.g., Weight Watchers) informing interventions for problem substance use (e.g., Controlled Drinking) (Miller and Heather 1986). Despite clinically and scientifically intuitive synergy between behavioral undercontrol processes that potentially drive both overweight/overeating and addiction, empirical studies are only beginning to explore these relationships at a neural level with adolescents (Burger and Stice 2014; Stice and Yokum 2014; Yokum et al. 2014). This has substantive public health value, as adolescents represent one of the highest-risk demographics for whom the epidemic of overweight/obesity is not decreasing (Ogden et al. 2014).
Therefore, the goal of this study was to explicitly examine and replicate, with an overweight/obese adolescent sample, an empirically established cue exposure paradigm. This gustatory cue-exposure paradigm has shown functional overlap with what we term here “neural addiction-related reward pathways” in adults, including the orbitofrontal cortex (OFC), striatum, amygdala, and ventral tegmental area (VTA) (Filbey et al. 2012). Thus, following this study and others (Filbey et al. 2012; Stice and Yokum 2014; Yokum et al. 2014), in terms of the first hypothesis, we expected to observe the same pattern: greater activation of neural addiction-related reward pathways in our sample of adolescents (e.g., OFC, striatum, amygdala, VTA) in response to exposure to high vs. low calorie beverage tastants. For the second aim, we sought to determine degree of correlation between two predominant biomarkers for health risk in adolescent overweight/obesity (BMI, insulin resistance) and functional brain response during this fMRI-based calorie beverage task. In terms of anticipated loci of activation, the relationship between BMI and brain response has not been consistent across studies. At this time, prior studies have shown correlations between BMI with operculum and insula activation in female adolescents (Batterink et al. 2010) along with null findings in adults (Filbey et al. 2012). Further, we could find no published studies on the relationship between insulin resistance and neural response during cue exposure in this age group. Thus, for the second hypothesis, we posited that we would find a correlation between BMI with BOLD activation in the insula and operculum. However, given the extant literature, we had no a priori hypotheses for areas of activation for insulin resistance. For the final aim, given the growing literature on the commonalities between addiction symptoms and overweight/obesity in the brain (Volkow and Baler 2015; Volkow et al. 2013b), we believed a unique contribution of this empirical study would be to include a behavioral metric of food addiction, and examine its impact as a correlate of BOLD response in this age group. To our knowledge, food addiction scores and neural response has only been assessed in adult females, with greater BOLD response observed across the anterior cingulate cortex (ACC), OFC, and amygdala (Gearhardt et al. 2011a, b). We could find no published data examining the neural correlates of food addiction via this measure in youth. Thus, for the third hypothesis, we proposed that we would observe a relationship between food addiction scores with BOLD response in the ACC, OFC, and amygdala in the adolescent brain.
Methods
Participants
This study was a component of a larger trial with overweight/obese youth. To evaluate specific aims herein, all analyses were restricted to adolescents who completed the neuroimaging paradigm. Following other studies in this area (Kong et al. 2012), participants were recruited from a public charter high school in the southwest; demographics of this sample, including race/ethnicity and socioeconomic status, paralleled the broader representation of public school students in this geographic region. Students were invited to participate in this project during standard academic programming periods. Research staff introduced the project, informing youth that participation was voluntary and separate from their academic requirements. All youth completed informed written assent. As with other studies (e.g. Schmiege et al. 2009), audio-recorded informed parent/guardian consent was obtained via telephone following youth assent. All procedures were conducted with university institutional review board approval. To participate, youth had to: (1) be 14–19 years old; (2) have a BMI ≥25; (3) be right handed; (4) have infomed consent of a parent/legal guardian (if under age 18); (5) provide own informed consent/assent; and (6) be willing to complete a 1 month follow-up. Exclusion criteria included: (1) type 1 or 2 diabetes; (2) history of injury to the brain or brain-related medical problems; (3) presence of MRI contraindications (e.g., non-removable implants or braces); (4) stage 2 hypertension; (5) systemic corticosteroid use during past 2 weeks; (6) neuroleptic/antipsychotic medications; (7) inability to perform moderate to vigorous physical activity, and (8) pregnancy. To maximize generalizability, youth were neither evaluated for, nor excluded on co-occurring substance use or Axis I diagnoses.
Procedures
All youth completed baseline evaluations of demographics (age, gender, education) and an estimate of food addiction (Yale Food Addiction Scale; YFAS; scores ≥3 indicate food addiction symptoms in line with DSM-IV-TR substance dependence) (Gearhardt et al. 2011a, b). The YFAS has been validated and successfully used in previous empirical studies with adolescents (Chen et al. 2015; Meule et al. 2015). In this protocol, we also included anthropometric measures including height and weight (Bodo et al. 2015), and a biomarker of insulin resistance (homeostatic model assessment insulin resistance index; HOMA-IR = product of fasting insulin mU/L and fasting glucose mmol/L/22.5) (Katz et al. 2000). All anthropomorphic measures were taken to the nearest 0.1 of the utilized scale. Height was measured using a vertical measuring rod (Seca Model 213, Chino, CA, USA), and weight was measured with a portable strain-gauge digital scale (Seca Model 770, Chino, CA, USA). All measures were performed twice. BMI was calculated as kilograms per meters squared, and Z-scores and BMI percentiles were generated from the participants’ measured BMI using the Center for Disease Control and Prevention’s equation and age- and sex-specific LMS parameters for the BMI-for-age charts, ages 2–20 years (Centers for Disease Control and Prevention 2009). Overweight was defined as a BMI percentile of 85 % to 94.9 %, and obese as a BMI percentile ≥95 %. To calculate insulin resistance, blood samples were drawn after a 12-hour fast. Serum samples were prepared by incubating whole blood at room temperature for 30 minutes, followed by refrigerated centrifugation at 1000×gram for 10 minutes. Supernatants (serum) were transferred to clean tubes and stored at 4 °C until assayed. All samples were centrifuged on-site and transported in an ice chest with freezer packs to the university laboratory for processing. Insulin concentration was determined by capture ELISA using a Siemens Immulite 1000 Immunoassay System (Siemens Healthcare, Malvern, PA, USA). THOMA-IR was calculated as the product of fasting insulin mU/L and fasting glucose mmol/L/22.5 (Katz et al. 2000). Higher HOMA-IR values correspond with increased levels of insulin resistance. All youth on this protocol were on a standardized meal and snack schedule through their school program. All scans occurred during the daytime hours. In order to assess the impact of hunger during the scan session, all youth were queried about their urge to eat during the fMRI protocol. All youth received $15 for completion of the baseline assessment and scan session.
Gustatory high-calorie exposure task
In line with the paradigm developed by Filbey et al. (2012), during each trial participants were initially presented with a 2-second (s) “ready” prompt prior to receiving each tastant, followed by receipt of 1 milliliter (ml) of either water or 1 ml of the participant’s preferred high-calorie beverage [e.g., Coca Cola (12 calories, 3.33 g of sugar, and 2.67 mg caffeine per ounce), Sprite (12.35 cal and 3.21 g of sugar per ounce), orange Fanta (14.81 cal and 3.70 g of sugar per ounce)] over the course of 24 s. To minimize variability across conditions due to differential swallowing rates, all youth received a prompt on the instruction screen to swallow at 8 s and at 18 s. Following delivery of each tastant, a 16 s washout period was included in which no tastant stimuli were presented. This was followed by an urge rating screen instructing participants to: “Please rate your desire to eat right now.” Participants were shown an 11-point Likert scale with the anchors “No Desire” (0) and “Very Strong Desire” (10). The current urge rating was highlighted in red. Button presses with the index finger moved the rating towards 0, and button presses with the middle finger moved the rating towards 10. Each trial started with the highlighted rating set to “5”. Each run included 6 trials of each beverage type, for a total of 12 trials per beverage condition. Visual stimuli were presented using E-prime (Psychology Software Tools, Inc.). Tastant stimuli were delivered with a gustometer controlled using custom code within E-prime. The task was synced with the first trigger pulse from the MRI during each functional scan. Subjective ratings of urge to eat were extracted from the E-prime behavioral files and used for subsequent analyses. Specifically, we compared the mean urge ratings after presentation of the high calorie beverage versus the water stimulus via paired t-test.
MRI acquisition
MRI data was collected on a 3 T Siemens Trio (Erlangen, Germany) whole body scanner. An echo-planar gradient-echo pulse sequence (TR = 2000 ms, TE = 29, flip angle = 75°, 33 axial slices, 64 × 64 matrix, 3.75 × 3.75 mm2, 3.5 mm thickness, 1 mm gap) was acquired with a 12-channel head coil, and images were acquired parallel to the ventral surface of a participant’s orbitofrontal cortex to reduce signal dropout and distortion in this region (Deichmann et al. 2003). Two hundred seventy-two EPI volumes were acquired in each of the 2 runs of the gustatory exposure task, for a total scan time of 9:04 min per run. A high resolution T1-weighted MPRAGE anatomical image was also acquired (TR = 2530 ms, TE = 1.64 ms, flip angle = 7°, 192 sagittal slices, 256 × 256 matrix, slice thickness = 1 mm, no gap).
Image analysis
The first 3 volumes of each functional run were discarded to allow the magnet to reach steady state. MCFLIRT (Motion Correction using FMRIB’s Linear Image Registration Tool) (Jenkinson et al. 2002) was used to motion correct images within a run. Each volume was aligned to the first volume in the run. Images were deskulled using BET (Brain Extraction Tool) (Smith 2002), spatially smoothed with a 5 mm full-width half-max Gaussian kernel, temporally filtered using a high-pass filter of 100 s, and grand mean intensity normalized. All of these steps were performed using FMRIB’s Expert Analysis Tool (FEAT) (Smith et al. 2004).
Custom timing files were created for each participant with the following conditions: high calorie beverage presentation, low calorie beverage (water) presentation, high calorie urge, and low calorie urge. The beverage presentation condition was modeled as a 28 s boxcar that started at the first “swallow” prompt and continued through the washout period. The urge rating was modeled as a 6 s “on” period that immediately followed the beverage presentation. The remaining time in each trial was not modeled and thus included in the baseline. Regressors were convolved with a double-gamma hemodynamic response function. In addition, temporal derivatives were included with each regressor to allow for variation in the timing of the hemodynamic response function.
In terms of planned analyses, to evaluate Hypothesis 1, the primary contrast of interest compared parameter estimates for the high calorie beverage presentation to the low calorie beverage presentation. Group analyses were conducted using FMRIB’s Local Analysis of Mixed Effects (FLAME) Stage 1, and the main contrasts of interest (e.g., HCB > LCB, LCB > HCB) were corrected for multiple comparisons using a voxelwise threshold of z > 2.3 and a cluster threshold of p < 0.05. To examine Hypothesis 2 and Hypothesis 3, we evaluated the correlation between BMI and insulin resistance, respectively, with BOLD response (during HCB > LCB and LCB > HCB), and the correlation between food addiction symptoms (YFAS) and BOLD response (during HCB > LCB and LCB > HCB). Each correlate of interest (e.g. BMI, insulin resistance, YFAS) was entered into the model with the group mean regressor, and the correlation was computed across the whole brain. Maps were corrected for multiple comparisons using cluster-based thresholding (z > 1.96, cluster p < .05). Finally, we attempted a parametric modulation analysis to examine the impact of in-scanner urge on neural response (during HCB > LCB and LCB > HCB). Several participants had limited variability in their urge ratings (e.g., ratings were comparable across both the HCB and LCB presentations); thus, we had too few subjects to meaningfully examine urge ratings to neural response for this final comparison.
Results
Participants’ ethnicity, socio-demographics, and baseline health risk
Representative of the larger geographic region from where this school is located, this sample (N = 24) was between 14 and 19 years old (M = 16.46; SD = 1.4), self-identified as Hispanic (79 %; N = 19) or Caucasian (21 %; N = 5), and was predominantly male (83.33 %; N = 20). Socioeconomic status at this public charter school paralleled the southwest region where these data were collected. All students were overweight/obese as defined by both the adult BMI cutscore used in medical settings (BMI ≥ 25) and BMI percentile (adjusted for age and gender; BMI ≥ 85th percentile = overweight; BMI >95th percentile = obesity) defined criteria. In this sample, the average BMI = 33.1 (SD = 5.9, range 25.74–45.62), and the average BMI percentile = 96.3 (SD = 4.2; range 85.2–99.9) falling in the obese range. For the estimate of food addiction symptoms (YFAS), scores ≥3 indicate food addiction symptoms in line with DSM-IV-TR substance dependence. In this study, only 4 youth endorsed clinically significant levels of food addiction, with the average YFAS score below the clinical cut-score of 3 (M = 1.84; SD = 1.42; range = 0.5).
Beverage selection and urge ratings
Of the 3 high calorie beverage options, most selected Sprite (N = 12), followed by Coca Cola (N = 6), and Fanta (N = 2). Beverage selection for n = 4 individuals was missing. T-tests were conducted for the full sample (N = 24) to compare mean in-scanner urge rating after the HCB vs. LCB. In line with expectations, adolescents reported greater in-scanner urge after presentation of the HCB (mean (SD) rating = 3.73 (2.71)) as compared with the LCB tastant [mean (SD) rating = 3.08 (2.46); t(23) = 2.20, p = 0.04)].
Main effects for high calorie beverage (HCB) and low calorie beverage (LCB)
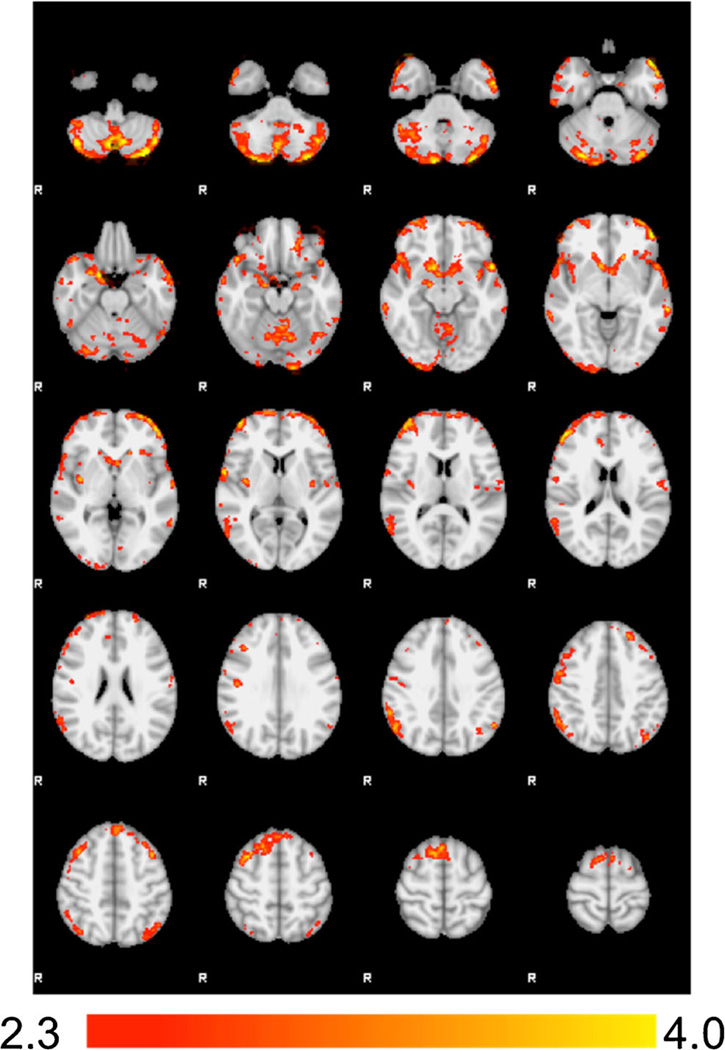
For Hypothesis 1, comparison of the HCB > LCB contrast revealed significantly increased BOLD activation in regions including the bilateral orbitofrontal cortex (OFC), inferior frontal gyrus (IFG), cerebellum, nucleus accumbens, and right amygdala. Additional activation was also observed across the medial superior frontal gyrus, supplemental motor area (SMA), right middle frontal gyrus, right frontal pole, left temporal pole, superior temporal gyrus, right angular gyrus, and inferior parietal lobe. Interestingly, we found no areas of significant BOLD response during the LCB > HCB contrast (Fig 1; Table 1).
Fig. 1. Main Contrast Effects.
Significant differences were found in the HCB > LCB contrast such that the regions below showed greater activation during presentation of the high calorie beverage cue compared to the low calorie (water) cue. No differences emerged in the LCB > HCB contrast. Images are presented in radiological convention
Table 1.
Regions of increased BOLD response in the HCB > LCB contrast, and correlations with overweight/obese measures (N = 24). No significant findings were present in the LCB > HCB contrast, and no correlations were observed with YFAS scores
| # voxels | Localization | BA | x (mm) | y (mm) | z (mm) | Max z |
|---|---|---|---|---|---|---|
| High calorie beverage (HCB) > Low calorie beverage (LCB) | ||||||
| 8736 | Bilateral Cerebellum | - | −16 | −68 | −54 | 4.61 |
| 4388 | Bilateral IFG/OFC/Bilateral Nucleus Accumbens/Right Amygdala |
10/47 | −36 | 62 | 4 | 4.31 |
| 1688 | Medial Superior Frontal Gyrus/Supplemental Motor Area/Right Middle Frontal Gyrus |
6 | 10 | 16 | 62 | 3.97 |
| 1576 | Right Frontal Pole/Lateral OFC | 10/46 | 48 | 44 | 16 | 4.04 |
| 1338 | Left Temporal Pole/Superior Temporal Gyrus | 221/22 | −50 | 20 | −28 | 4.16 |
| 1107 | Right Angular Gyrus/Inferior Parietal Lobe | 40 | 56 | −54 | 42 | 3.58 |
| 507 | Right Occipital Pole | 18 | 28 | −100 | −12 | 3.48 |
| Body mass index (BMI) | ||||||
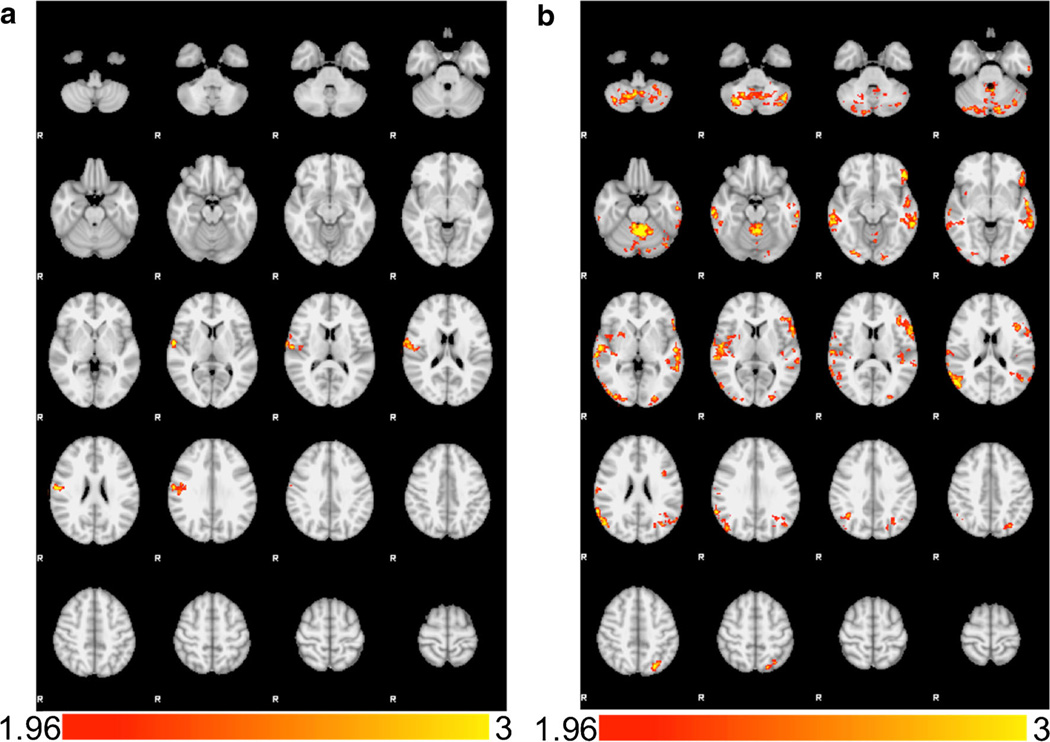
| 735 | Right Postcentral gyrus/Central Operculum | 43 | 60 | −4 | 6 | 3.61 |
| Insulin Resistance (HOMA-IR) | ||||||
| 3814 | Bilateral Cerebellum | - | −2 | −52 | −26 | 3.89 |
| 1689 | Right Middle/Superior Temporal Gyrus | 20/21 | 66 | −22 | −16 | 3.5 |
| 1541 | Left Middle/Superior Temporal Gyrus | 20/21 | −60 | −42 | −10 | 3.77 |
| 1288 | Right Lateral Occipital Cortex | 39 | 62 | −50 | 28 | 3.51 |
| 1075 | Left Orbitofrontal Cortex | 47 | −48 | 36 | −12 | 3.24 |
| 729 | Left Superior Lateral Occipital Cortex/Superior Parietal Lobe |
7/39 | −30 | −66 | 52 | 2.99 |
The HCB > LCB map was corrected for multiple comparisons using a voxelwise threshold of 2.3 and a cluster level threshold of p < 0.05. Correlation maps (i.e., BMI and insulin resistance) were corrected for multiple comparisons using cluster-based thresholding (voxel z > 1.96, cluster p < 0.05)
Correlations with BMI, insulin resistance (HOMA-IR), and Yale food addiction scale (YFAS)
Prior to analyzing the data for Hypothesis 2, we confirmed that BMI and insulin resistance were not correlated (r = 0.02, p = 0.92). BMI and insulin resistance were then examined in two separate models. There was a positive correlation between BMI and the HCB > LCB contrast in right postcentral gyrus/central operculum (Fig. 2a; Table 1), such that adolescents with higher BMI showed greater BOLD response in these areas during the HCB > LCB contrast. However, as with the main effects, we found no relationships between BMI and BOLD response during the LCB > HCB contrast. For insulin resistance, we found significant positive correlation with brain response across the dorsolateral PFC, left OFC, bilateral inferior parietal lobe, bilateral cerebellum, bilateral middle/superior temporal gyrus, and left superior occipital cortex/superior parietal lobe (Fig. 2b; Table 1). As with BMI, we found no significant correlations between insulin resistance and BOLD response during the LCB > HCB contrast. For Hypothesis 3, we found no significant correlations between brain response and food addiction symptoms (YFAS) during either the HCB > LCB or the LCB > HCB comparisons.
Fig. 2.
a Correlations with Body Mass Index (BMI). BMI was positively correlated with >BOLD activation in the HCB > LCB contrast in the right postcentral gyrus/central operculum. b Correlations with Insulin Resistance (HOMA-IR). Insulin resistance was positively correlated with BOLD activation in the HCB > LCB contrast in several regions including bilateral cerebellum, bilateral middle/superior temporal gyri, bilateral occipital poles, left orbitofrontal cortex, and left superior parietal lobe. Images are presented in radiological convention
Finally, as the risk of elevated BMI increases with age, and neural patterns may differ over the course of development, we conducted a post hoc examination of each analysis presented herein with age as a covariate. We did not observe any significant differences and thus retained the original analyses.
Discussion
This is, to our knowledge, one of the first explorations of adolescent brain response to gustatory high calorie vs. low calorie beverage cues in an overweight/obese sample. Our goal was to utilize an established neuroimaging paradigm to contribute to the emerging empirical discussion regarding whether existing neural addiction paradigms (Volkow et al. 2013a) offer a good fit for understanding patterns of overweight and obesity during this critical developmental period. It was also our goal to examine how adolescent brain response may (or may not) correlate with a constellation of related, unique factors integral to the nature of adolescent overweight/obesity, including BMI, insulin resistance, and food addiction symptomology.
For our first hypothesis, we projected that during the HCB > LCB beverage contrast, we would find engagement of neural addiction-related reward pathways, including orbitofrontal cortex (OFC), striatum, amygdala, and ventral tegmental area (VTA) (Filbey et al. 2012; Stice and Yokum 2014; Yokum et al. 2014). For our second hypothesis, we examined the correlation between BMI and insulin resistance, respectively; here we anticipated observing a correlation between BMI and BOLD response in the insula and operculum (Batterink et al. 2010). Given the extant literature on insulin resistance and fMRI -based cue exposure in youth, we had no a priori hypotheses for that comparison. Finally, for Hypothesis 3, we expected to observe relationship between food addiction scores and BOLD response across the ACC, OFC, and amygdala in this sample of adolescents (Gearhardt et al. 2011a, b). Broadly, results from this study map onto neural addiction-related reward pathways implicated in overweight/obesity (Burger and Stice 2014; Filbey et al. 2012).
For Hypothesis 1, adolescents had differential subjective responses to the presentation of the high calorie gustatory cue as compared with the low calorie gustatory cue, as reflected by significantly higher urge to eat following high calorie beverage presentation. During the high vs. low-calorie beverage comparison, we found a compelling pattern of neural response that mirrors the gustatory cue response found by Filbey et al. (2012) in binge eating adults (e.g., IFG, medial frontal gyrus, superior frontal gyrus, orbitofrontal regions, and amygdala). The areas of activation observed within this sample are also precisely the regions that have been implicated in the neurobiological phenotype of substance use severity [e.g., PFC, OFC, IFG, SMA, amygdala, cerebellum; (Claus et al. 2011)]. In contrast to expectations, adolescents’ pattern of brain response did not necessarily overlap with the prior overweight/obesity literature. To this end, our observation of significantly greater response in bilateral OFC during the high calorie beverage processing was interesting, as this region has not been associated with overweight/obesity in other adolescent studies (Yokum et al. 2014), but has been found to be a prominent neural substrate across adolescent addictive behaviors (e.g., cannabis use) (Filbey et al. 2014). Our results suggest that the OFC, for example, may represent an integration center in appetitive reward processing, a neural substrate commonly observed within problem substance use (Goldstein and Volkow 2002).
Further, we also found significantly increased BOLD response in the bilateral IFG, bilateral nucleus accumbens, SMA, frontal and temporal gyri, and cerebellum. These results also map on to the existing addiction cue exposure literature, which continues to support the role of these areas in neural processing exposure to these substances of abuse (Karoly et al. 2014). These areas have also been implicated in metrics of behavioral control and substance use severity (Claus et al. 2013). Another interesting area that emerged in the high calorie comparison was the right amygdala. While the amygdala has been less prominent throughout the adult addiction literature, the developmental adolescent and young adult health risk literature increasingly supports the amygdala in cannabis and other illicit substance use (Filbey and Dunlop 2014; Schacht et al. 2012; Yip et al. 2016). In other words, there may be something about the nature of appetitive rewards or cues during adolescence, that particularly activates limbic processing relative to adulthood (Feldstein Ewing et al. 2016a, b, c).
In terms of Hypothesis 2, BMI did not generate the substantive brain response expected. BMI was significantly correlated with BOLD response only in the right postcentral gyrus/central operculum. Yet, this region aligns with previous examinations of BMI during visual presentations of rewarding food stimuli in female adolescents (Batterink et al. 2010; Stice et al. 2010). Arguably, to some degree, these findings are also consistent with prior null findings for BMI and this gustatory cue exposure paradigm in adults (Filbey et al. 2012). It, therefore, might the case that the pattern of neural response to rewarding cues, including food, and potentially even substance cues, is unique to this developmental period (Feldstein Ewing et al. 2013b).
In contrast, our measure of insulin resistance generated much more robust findings, with bilateral activation across the middle/superior temporal gyri, left OFC, left superior parietal lobe, and cerebellum. Previous work has demonstrated that insulin resistance (HOMA-IR) has mediated the relationship between functional activation in the ventral striatum during rewarding food anticipation and BMI (Simon et al. 2014). Due to our focus on the neural substrates of cue response among overweight/obese youth in particular, we did not enroll youth with a range of BMI. Therefore, we were unable to evaluate potential mediational roles for insulin resistance within this sample. At the same time, our findings provide preliminary support that insulin resistance, over and above BMI, is a relevant metric for estimating differential processing of high calorie taste cues.
For Hypothesis 3, the role of food addiction symptoms in adolescent brain response to gustatory cues, all youth in this sample met criteria for overweight/obesity, with the average adolescent being obese. Interestingly, despite their high BMI, few adolescents in this sample passed the threshold for clinically significant food addiction (N = 4). Our adolescent results stand in contrast to prior adult work in this area (Gearhardt et al. 2011a, b). One reason for this might be that our study population was dissimilar to Gearhardt’s studies (2011a; b); Gearhardts’ studies included only females, while our study was predominantly male (83.33 %). This is relevant because females are twice as likely to receive a food addiction diagnosis, as compared with males (Pursey et al. 2014). In addition, while Gearheardt and colleagues observed a connection between YFAS and neural response in the ACC, OFC, and amygdala in adults (Gearhardt et al. 2011a, b), we did not find any evidence supporting the correlation of this measure of food addiction with youth brain response during the cue exposure. One potential explanation is that the YFAS may not be a sufficiently sensitive and/or specific marker of neural response for young males within this age group.
In terms of response to the low calorie beverage condition, we found no areas where neural response was significantly greater during the low calorie vs. high calorie beverage presentation. Further, we found no ecorrelation between BMI and/or insulin resistance, during the LCB > HCB contrast. This parallels prior empirical studies which also did not report any areas of significant activation during the low calorie condition (Filbey et al. 2012). While interpretations of direction of neural response, and what that might mean behaviorally, remain an area of high discussion in the adolescent neurodevelopmental literature (Feldstein Ewing et al. 2014), differences in the neural response for the high vs. low calorie beverage support the specificity of the relationships between health biomarkers (BMI, insulin resistance) and adolescents’ neural response to the high calorie context.
Enhanced neural addiction-related reward pathways have been highly implicated in both overweight/obesity and substance use, but one critical consideration of these overlapping neural findings is that the relationship may not be causal. In other words, in a recent longitudinal examination, youth with elevated striatal reward response transitioned into more problematic substance use, but not to overweight/obesity (Stice et al. 2013). Thus, future work must take steps to understand the nature of neural response (Burger and Stice 2011) to better understand how a general marker for risk translates into a specific phenotypic manifestation (substance use vs. overweight/obesity). Much like current questions explored in the larger adolescent neurodevelopment literature, we are still very much at foreground of disentangling how differential neural response may impact future health risk behaviors.
Ultimately, this study supports two critical next steps in this line of research: one examining the robustness of addictive pathways in the transition into behavioral under control in the context of eating for adolescents specifically, and the second examining the potential role of these neural substrates in adolescent treatment response. These steps are fundamental to guiding appropriate and developmentally-responsive methods in prevention and intervention efforts. For example, while the integration of neuroimaging into treatment devleopment is an emerging area (Feldstein Ewing et al. 2016a, b; c; Feldstein Ewing et al. 2016a, b; c), the significant involvement of brain behavioral control areas (e.g., OFC, IFG) and emotion regulation areas (e.g., amygdala) suggest that effective prevention and intervention efforts for adolescent overweight/obesity may need to include content articulated to improving control over urges and emotionally-driven decision-making around food consumption, presented in a manner that is sensitive and responsive to this age group. However, while individual-level efforts for adolescent overweight/obesity exist (Kong et al. 2013), successful treatment efforts for adolescent overweight/obesity are complicated by the nature in which food is purchased and offered in adolescent living situations, which are inherently family-based (Rhee et al. 2016). Continuing dialogue on the relationship between neural response and adolescent decision-making around food is fundamental in guiding appropriate and developmentally-responsive methods in prevention and intervention efforts.
Limitations and future directions
It is worthwhile to interpret study findings with an eye to the following considerations. All results were conducted with a primarily male sample with low food addiction scores, thus replication with a more demographically balanced sample with a greater range of food addiction scores, using more developmentally-sensitive metrics of food addiction represents an integral next step. Interestingly, since the completion of this study, a new measure of child and adolescent addiction has emerged: the YFAS-C (Gearhardt et al. 2013). While we have every reason to believe that the YFAS was a valid measure in this age group, in the future, this may be an additional avenue to explore food addiction assessment. Though meal times were standardized within the sample as part of their routine, scan times varied throughout the day based on scheduling needs; however, we separately analyzed participant urge ratings to flush out additional information regarding participant desire to consume food at the time of the scan. Differences in behavioral urge ratings suggest that participants generally responded in the expected direction in response to gustatory stimuli despite a lack of hunger assessment at the time of scan. Thus, as with Filbey et al. (2012), between youths’ standardized meal schedule, and our own assessments of their hunger during the scan, we have confidence that state-based differences in hunger did not significantly impact the neural response to the paradigm. A small number (N = 6) of participants selected Coca Cola as their high calorie beverage. Because Coca Cola was the only beverage offered that contained caffeine, it is possible that results were driven by the psychostimulant properties of that beverage. However, because the 1 ml of Coca Cola presented over 24 s contains less than a tenth of a milligram of caffeine, and caffeinated and alcoholic beverages have been used in established cue exposure tasks previously (Feldstein Ewing et al. 2010; Filbey et al. 2008; Filbey et al. 2012), it is unlikely that current findings can be attributed to differences in beverage selection.
Further, motion is a large issue with adolescents (Feldstein Ewing et al. 2013a), and has the potential to inadvertently introduce artifact into neuroimaging findings despite motion correction. While the goal of this study was to generate results that had the maximum potential for generalization, replication with a larger sample with additional examinations of the role of substance use and psychopathology would strengthen study findings, as smoking and depression rates tend to be elevated in overweight and obese adolescents (Lanza et al. 2015). Additionally, our interest in this examination was to examine consummatory response, following Filbey et al. (2012); however, future work would benefit from refining the model to examine anticipatory response as well, given the role that anticipatory response to food cues has played in prior studies related to overweight/obesity (Stice et al. 2015). Finally, the inclusion of a healthy weight comparison group could provide additional insight into the reward regions (e.g., OFC, nucleus accumbens, amygdala) involved during cue exposure across the BMI spectrum, and the degree to which those regions differentially respond to gustatory high calorie exposure.
Acknowledgments
The authors would like to thank Dustin Truitt for his contribution to this project.
Sources of support This research was supported by the University of New Mexico Pediatric Research Allocations Committee, the La Tierra Sagrada Society, and the Mind Research Network.
Footnotes
Compliance with ethical standards
Financial disclosures The authors declare that they have no competing financial or other conflicts of interest relating to the data included in the manuscript.
References
- Batterink L, Yokum S, Stice E. Body mass correlates inversely with inhibitory control in response to food among adolescent girls: an fMRI study. NeuroImage. 2010;52(4):1696–1703. doi: 10.1016/j.neuroimage.2010.05.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodo MJ, Jimenez EY, Conn C, Dye A, Pomo P, Kolkmeyer D, et al. Association between circulating CCL2 levels and modifiable behaviors in overweight and obese adolescents: a cross-sectional pilot study. Journal of Pediatric Endocrinology & Metabolism. 2015 doi: 10.1515/jpem-2015-0260. [DOI] [PubMed] [Google Scholar]
- Burger KS, Stice E. Variability in reward response and obesity: evidence from brain imaging studies. Current Drug Abuse Reviews. 2011;4:182–189. doi: 10.2174/1874473711104030182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger KS, Stice E. Neural responsivity during soft drink intake, anticipation, and advertisement exposure in habitually consuming youth. Obesity (Silver Spring) 2014;22:441–450. doi: 10.1002/oby.20563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Percentile data files with LMS values. 2009 Retrieved from http://www.cdc.gov/growthcharts/percentile_data_files.htm.
- Chen G, Tang Z, Guo G, Liu X, Xiao S. The Chinese version of the Yale food addiction scale: an examination of its validation in a sample of female adolescents. Eating Behaviors. 2015;18:97–102. doi: 10.1016/j.eatbeh.2015.05.002. [DOI] [PubMed] [Google Scholar]
- Claus ED, Ewing SW, Filbey FM, Sabbineni A, Hutchison KE. Identifying neurobiological phenotypes associated with alcohol use disorder severity. Neuropsychopharmacology. 2011;36(10):2086–2096. doi: 10.1038/npp.2011.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claus ED, Feldstein Ewing SW, Filbey FM, Hutchison KE. Behavioral control in alcohol use disorders: relationships with severity. Journal of Studies on Alcohol and Drugs. 2013;74(1):141–151. doi: 10.15288/jsad.2013.74.141. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3517257/pdf/jsad141.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and nutrition examination survey, 1999–2002. The Journal of Pediatrics. 2008;152(2):165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- DeBoer MD, Gurka MJ, Woo JG, Morrison JA. Severity of the metabolic syndrome as a predictor of type 2 diabetes between childhood and adulthood: the Princeton lipid research cohort study. Diabetologia. 2015;58(12):2745–2752. doi: 10.1007/s00125-015-3759-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichmann R, Gottfried JA, Hutton C, Turner R. Optimized EPI for fMRI studies of the orbitofrontal cortex. NeuroImage. 2003;19:430–441. doi: 10.1016/s1053-8119(03)00073-9. [DOI] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Filbey FM, Chandler LD, Hutchison KE. Exploring the relationship between depressive and anxiety symptoms and neuronal response to alcohol cues. Alcoholism, Clinical and Experimental Research. 2010;34(3):396–403. doi: 10.1111/j.1530-0277.2009.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents' response to a cannabis intervention. Psychology of Addictive Behaviors. 2013a;27:510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, McEachern AD, Yezhuvath U, Bryan AD, Hutchison KE, Filbey FM. Integrating brain and behavior: evaluating adolescents' response to a cannabis intervention. Psychology of Addictive Behaviors. 2013b;27(2):510–525. doi: 10.1037/a0029767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Sakhardande A, Blakemore SJ. The effect of alcohol consumption on the adolescent brain: a systematic review of MRI and fMRI studies of alcohol-using youth. NeuroImage: Clinical. 2014;5:420–437. doi: 10.1016/j.nicl.2014.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Apodaca TR, Gaume J. Ambivalence: prerequisite for success in motivational interviewing with adolescents? Addiction. 2016a doi: 10.1111/add.13286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Ryman SG, Gillman AS, Weiland BJ, Thayer RE, Bryan AD. Developmental cognitive neuroscience of adolescent sexual risk and alcohol use. AIDS and Behavior. 2016b;20(Suppl 1):97–108. doi: 10.1007/s10461-015-1155-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldstein Ewing SW, Tapert SF, Molina BS. Uniting adolescent neuroimaging and treatment research: recommendations in pursuit of improved integration. Neuroscience and Biobehavioral Reviews. 2016c;62:109–114. doi: 10.1016/j.neubiorev.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Dunlop J. Differential reward network functional connectivity in cannabis dependent and non-dependent users. Drug and Alcohol Dependence. 2014;140:101–111. doi: 10.1016/j.drugalcdep.2014.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Claus E, Audette AR, Niculescu M, Banich MT, Tanabe J, et al. Exposure to the taste of alcohol elicits activation of the mesocorticolimbic neurocircuitry. Neuropsychopharmacology. 2008;33(6):1391–1401. doi: 10.1038/sj.npp.1301513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filbey FM, Myers US, DeWitt SJ. Reward circuit function in high BMI indivduals iwth compulsive overeating: similarities with addiction. NeuroImage. 2012;63:1800–1806. doi: 10.1016/j.neuroimage.2012.08.073. [DOI] [PubMed] [Google Scholar]
- Filbey FM, Aslan S, Calhoun VD, Spence JS, Damaraju E, Caprihan A, et al. Long-term effects of marijuana use on the brain. Proceedings of the National Academy of Sciences of the United States of America. 2014;25:16913–16918. doi: 10.1073/pnas.1415297111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Grilo CM, DiLeone RJ, Brownell KD, Potenza MN. Can food be addictive? Public health and policy implications. Addiction. 2011a;106:1208–1212. doi: 10.1111/j.1360-0443.2010.03301.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Yokum AN, Orr PT, Stice E, Corbin WR, Brownell KD. Neural correlates of food addiction. Archives of General Psychiatry. 2011b;68:808–816. doi: 10.1001/archgenpsychiatry.2011.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearhardt AN, Roberto CA, Seamans MJ, Corbin WR, Brownell KD. Preliminary validation of the Yale food addiction scale for children. Eating Behaviors. 2013;14(4):508–512. doi: 10.1016/j.eatbeh.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. Drug addiction and its underlying neurobiological basis: neuroimaging evidence for the involvement of the frontal cortex. The American Journal of Psychiatry. 2002;159(10):1642–1652. doi: 10.1176/appi.ajp.159.10.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM, Gordon-Larsen P, Chantala K, Udry JR. Longitudinal trends in race/ethnic disparities in leading health indicators from adolescence to young adulthood. Archives of Pediatrics & Adolescent Medicine. 2006;160(1):74–81. doi: 10.1001/archpedi.160.1.74. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Karoly HC, Weiland BJ, Sabbineni A, Hutchison KE. Preliminary functional MRI results from a combined stop-signal alcohol-cue task. Journal of Studies on Alcohol and Drugs. 2014;75(4):664–673. doi: 10.15288/jsad.2014.75.664. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4108606/pdf/jsad664.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz A, Nambi SS, Mather K. Quantitative insulin sensitivity check index: a simple, accurate method for assessing insulin sensitivity in humans. The Journal of Clinical Endocrinology and Metabolism. 2000;85:2402–2410. doi: 10.1210/jcem.85.7.6661. [DOI] [PubMed] [Google Scholar]
- Kong AS, Dalen J, Negrete S, Sanders SG, Keane PC, Davis SM. Interventions for treating overweight and obesity in adolescents. 2012;23:544–570. [PubMed] [Google Scholar]
- Kong AS, Sussman AL, Yahne C, Skipper BJ, Burge MR, Davis SM. School-based health center intervention improves body mass index in overweight and obese adolescents. Journal of Obesity. 2013;2013:575016. doi: 10.1155/2013/575016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza HI, Grella CE, Chung PJ. Adolescent obesity and future substance use: incorporating the psychosocial context. Journal of Adolescence. 2015;45:20–30. doi: 10.1016/j.adolescence.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Lee D, Guo G, Harris KM. Trends in body mass index in adolescence and young adulthood in the United States: 1959–2002. The Journal of Adolescent Health. 2011;49(6):601–608. doi: 10.1016/j.jadohealth.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnussen CG, Koskinen J, Chen W, Thomson R, Schmidt MD, Srinivasan SR, et al. Pediatric metabolic syndrome predicts adulthood metabolic syndrome, subclinical atherosclerosis, and type 2 diabetes mellitus but is no better than body mass index alone: the Bogalusa heart study and the cardiovascular risk in young Finns study. Circulation. 2010;122(16):1604–1611. doi: 10.1161/CIRCULATIONAHA.110.940809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meule A, Hermann T, Kubler A. Food addiction in overweight and obese adolescents seeking weight-loss treatment. European Eating Disorders Review. 2015;23(3):193–198. doi: 10.1002/erv.2355. [DOI] [PubMed] [Google Scholar]
- Miller WR, Heather N, editors. Treating Addictive Behaviors: Processes of Change. 1. Springer US; 1986. [Google Scholar]
- Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. The Journal of Pediatrics. 2008;152(2):201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- N. I. o. Health, editor. National Institute of Diabetes and Digestive and Kidney Diseases. Insulin resistance and prediabetes. 2014. [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poobalan AS, Aucott LS, Clarke A, Smith WC. Physical activity attitudes, intentions and behaviour among 18–25 year olds: a mixed method study. BMC Public Health. 2012;12:640. doi: 10.1186/1471-2458-12-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poobalan AS, Aucott LS, Clarke A, Smith WC. Diet behaviour among young people in transition to adulthood (18–25 year olds): a mixed method study. Health Psychol Behav Med. 2014;2(1):909–928. doi: 10.1080/21642850.2014.931232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pursey KM, Stanwell P, Gearhardt AN, Collins CE, Burrows TL. The prevalence of food addiction as assessed by the Yale food addiction scale: a systematic review. Nutrients. 2014;6(10):4552–4590. doi: 10.3390/nu6104552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KE, Jelalian E, Boutelle K, Dickstein S, Seifer R, Wing R. Warm parenting associated with decreasing or stable child BMI during treatment. Child Obes. 2016;12(2):94–102. doi: 10.1089/chi.2015.0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37(11):2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmiege SJ, Broaddus MR, Levin M, Bryan AD. Randomized trial of group interventions to reduce HIV/STD risk and change theoretical mediators among detained adolescents. Journal of Consulting and Clinical Psychology. 2009;77(1):38–50. doi: 10.1037/a0014513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon JJ, Skunde M, Hamze Sinno M, Brockmeyer T, Herpertz SC, Bendszus M, et al. Impaired cross-talk between mesolimbic food reward processing and metabolic signaling predicts body mass index. Frontiers in Behavioral Neuroscience. 2014;8:359. doi: 10.3389/fnbeh.2014.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinaiko AR, Caprio S. Insulin resistance. The Journal of Pediatrics. 2012;161(1):11–15. doi: 10.1016/j.jpeds.2012.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, et al. Advances in functional and structuralMR image analysis and implementation as FSL. NeuroImage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Stice E, Yokum S. Brain reward region responsivity of adolescents with and without parental substance use disorders. Psychology of Addictive Behaviors. 2014;28:805–815. doi: 10.1037/a0034460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Bohon C, Marti N, Smolen A. Reward circuitry responsivity to food predicts future increases in bodymass: moderating effects of DRD2 and DRD4. NeuroImage. 2010;50(4):1618–1625. doi: 10.1016/j.neuroimage.2010.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Yokum S, Burger KS. Elevated reward region responsivity predicts future substance use onset but not overweight/obesity onset. Biological Psychiatry. 2013;73:896–876. doi: 10.1016/j.biopsych.2012.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stice E, Burger KS, Yokum S. Reward region responsivity predicts future weight gain and moderating effects of the TaqIA allele. The Journal of Neuroscience. 2015;35(28):10316–10324. doi: 10.1523/JNEUROSCI.3607-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Baler RD. NOW vs LATER brain circuits: implications for obesity and addiction. Trends in Neurosciences. 2015;38(6):345–352. doi: 10.1016/j.tins.2015.04.002. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. The addictive dimension of obesity. Biological Psychiatry. 2013a;73:811–818. doi: 10.1016/j.biopsych.2012.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Tomasi D, Baler RD. Obesity and addiction: neurobiological overlaps. Obesity Reviews. 2013b;14(1):2–18. doi: 10.1111/j.1467-789X.2012.01031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R, Bremer AA, Lustig RH. What is metabolic syndrome, and why are children getting it? Annals of the New York Academy of Sciences. 2013;1281:123–140. doi: 10.1111/nyas.12030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip SW, Lacadie CM, Sinha R, Mayes LC, Potenza MN. Prenatal cocaine exposure, illicit-substance use and stress and craving processes during adolescence. Drug and Alcohol Dependence. 2016;158:76–85. doi: 10.1016/j.drugalcdep.2015.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokum S, Gearhardt AN, Harris JL, Brownell KD, Stice E. Individual differences in striatum activity to food commercials predict weight gain in adolescents. Obesity (Silver Spring) 2014;22:2544–2551. doi: 10.1002/oby.20882. [DOI] [PMC free article] [PubMed] [Google Scholar]