Abstract
The MET tyrosine kinase, the receptor of hepatocyte growth factor-scatter factor (HGF/SF), is known to be essential for normal development and cell survival. We report that stress stimuli induce the caspase-mediated cleavage of MET in physiological cellular targets, such as epithelial cells, embryonic hepatocytes, and cortical neurons. Cleavage occurs at aspartic residue 1000 within the SVD site of the juxtamembrane region, independently of the crucial docking tyrosine residues Y1001 or Y1347 and Y1354. This cleavage generates an intracellular 40-kDa MET fragment containing the kinase domain. The p40 MET fragment itself causes apoptosis of MDCK epithelial cells and embryonic cortical neurons, whereas its kinase-dead version is impaired in proapoptotic activity. Finally, HGF/SF treatment does not favor MET cleavage and apoptosis, confirming the known survival role of ligand-activated MET. Our results show that stress stimuli convert the MET survival receptor into a proapoptotic factor.
Hepatocyte growth factor-scatter factor (HGF/SF) is a pleiotropic growth factor that acts through the MET tyrosine kinase receptor in a variety of cell types (3, 20, 21, 30). The ligand-activated MET stimulates proliferation, scattering, invasion, and morphogenesis of epithelial cells and has chemoattractant and neurotrophic activities in different types of neurons. Targeted disruption of either the hgf or met gene reveals an essential role of the HGF/SF-MET system during development of the placenta, liver, muscles, and neurons (4, 17, 28, 34).
Whereas HGF/SF-MET signaling mediates a variety of physiological processes, aberrant HGF/SF-MET signaling contributes to tumor progression and metastasis (3). The MET receptor was originally isolated as the cellular counterpart of a transforming gene, tpr-met (22). HGF/SF and MET are overexpressed, often coexpressed, in a significant number of human cancers. In addition, activating MET mutations have been described in various cancers, including sporadic and hereditary papillary renal carcinoma (29). Such activating mutations in lymph nodal and pulmonary metastases further underline MET functions during metastatic progression (7, 16).
The survival property of the HGF/SF-MET couple has been shown with in vitro and in vivo systems. Indeed, phenotypic analysis of hgf or met null mice have shown that HGF/SF-MET signaling is essential for hepatocyte survival, since these mice display a severe reduction in the liver and show massive apoptosis (4, 28, 34). Moreover, in vitro HGF/SF protects a number of cell types against cell toxicity and apoptosis caused by various stimuli, including DNA-damaging agents, serum withdrawal, and activation of death receptors (11, 14, 42). However, HGF/SF was also found to induce apoptosis in sarcoma 180 cells and in some hepatic cell lines (1, 37). This result might be due to the ability of the MET receptor to interact with FAS, a death receptor, with high concentrations of HGF/SF, causing dissociation of the interaction and therefore sensitizing the cells to FAS-mediated apoptosis (37).
Upon HGF/SF binding, the MET receptor is dimerized and its tyrosine kinase activity is stimulated, with autophosphorylation of the receptor (21). Two phosphotyrosine residues located in the noncatalytic C-terminal tail of the receptor (Y1347 and Y1354 of mouse MET) have been identified as multifunctional docking sites, able to interact with several cytoplasmic signal transducers (26, 38). In most cases, phosphorylation of these tyrosine residues is crucial for mediating all biological responses to HGF/SF (8), although this phosphorylation was found to be dispensable for cell scattering in some studies (33). In contrast to the positive signaling triggered by the C-terminal tail, the juxtamembrane region is endowed with several negative regulatory sites, which are involved in down-regulation and/or degradation of the receptor (6, 9, 24, 36).
In this study, we examined the fate of the MET receptor in cells exposed to stress conditions. This resulted in a juxtamembrane caspase-dependent cleavage of the MET receptor, generating an intracellular 40-kDa fragment containing the tyrosine kinase domain. Expression of the 40-kDa MET fragment, displaying intact kinase activity, was found to be sufficient to cause apoptosis. Our data show that stress stimuli convert the antiapoptotic function of MET to a proapoptotic function by caspase-dependent cleavage.
MATERIALS AND METHODS
Cytokines, drugs, and epithelial-cell cultures.
Human recombinant HGF/SF, beta nerve growth factor (β-NGF), and tumor necrosis factor alpha (TNF-α) were purchased from R&D Systems. Anisomycin was purchased from Calbiochem, and cycloheximide was purchased from ICN Biomedicals. The general caspase inhibitor zVAD-FMK was purchased from Promega. Madin-Darby canine kidney (MDCK) epithelial cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Invitrogen) supplemented with 10% fetal calf serum (FCS) and antibiotics at 37°C. NMUMG cells (mammary gland, normal epithelial, mouse; American Type Culture Collection) were cultured in the same medium supplemented with 10 μg of insulin from bovine pancreas (Sigma)/ml.
Culture of cortical neurons.
Neocortices were removed from 15.5-day-old Cd1 mouse embryos, incubated for 15 min in trypsin, and then mechanically dissociated by pipetting them in DMEM-F-12 medium (Invitrogen) supplemented with antibiotics and 10% horse serum (Sigma). The cells were then submitted to electroporation as described below. For biochemistry, the dissociated cells were seeded on poly-dl-ornithine-coated 6-cm-diameter dishes (Falcon) at a density of 6 × 106/dish in DMEM-horse serum. Four hours later, the medium was removed and replaced by neurobasal medium supplemented with B27 nutrient (Invitrogen).
Culture of hepatocytes.
Embryonic hepatocyte cultures were performed as previously described (18). Briefly, dissected embryonic day 15.5 livers were treated with collagenase, debris was removed by filtration, and cells were collected by centrifugation. Hepatocytes were seeded on collagen-coated dishes in hepatocyte attachment medium (Invitrogen) supplemented with 2% FCS, 10 μg of insulin/ml, and 50 ng of epidermal growth factor/ml. The cells were starved for 16 h before biochemical studies were performed.
Plasmid construction.
The LEX-MET fusions were constructed as follows. The HindIII/EcoRI restriction fragment, corresponding to the dimerization domain of LEX of pLEXA (Clontech Laboratories), was cloned in the mammalian expression vector pcDNA3 (Invitrogen). The full-length cytoplasmic domain of mouse MET was amplified by PCR using the primer 5′ TATCCGGGGAATTCCTGTGGATG 3′, containing the EcoRI restriction site, and primer 5′ TCATCTAGACCCCTAGCCATCAATG 3′, containing the XbaI restriction site. The full cytoplasmic domain of MET was cloned in frame with LEX in pcDNA3 using the EcoRI/XbaI sites. The LEX-MET protein in which aspartic acid 1000 was mutated to glutamic acid (LEX-MET D1000N) was created using the QuickChange site-directed mutagenesis system from Stratagene, using wild-type (WT) LEX-MET as a template and the following primers: 5′ TCAAATGAGTCTGTAAACTACAGAGCTACT 3′ and 5′ AGTAGCTCTGTAGTTTACAGACTCATTTGA 3′. Insertion of the mutation was verified by sequencing.
The TRK-MET chimeras were previously described (40). We modified these vectors by introducing a C-terminal V5 epitope. The TRK-MET expression vector allow the expression of an extracellular TRKA receptor fused to transmembrane and cytoplasmic regions of mouse MET, an activity inducible by NGF. The original TRK-MET chimeras in the pBAT expression vector were cloned in the pcDNA 3.1 V5-His C plasmid (Invitrogen) as follows. The TRK sequence, up to the BamHI restriction site, was cut out by digestion using HindIII and BamHI restriction enzymes and cloned into the pcDNA 3.1 V5-His plasmid. The remaining TRK-MET sequence, from the BamHI restriction site to the 3′ end, was amplified by PCR using the primer 5′ GCAGGCCGGCTGGATCCTCACAGAGCTGG 3′, containing the BamHI restriction site, and primer 5′ GGGCCTCTCATTCCTCGAGTGTTCCCC 3′, containing the XhoI restriction site, using pBAT TRK-MET WT, Y1347-1354F, Y1001F, or K1108A as a template. The PCR product was subcloned into a pGEM plasmid (pGEM Easy kit; Stratagene). MET in which aspartic acid 1000 was mutated to glutamic acid was created using the QuickChange site-directed mutagenesis system from Stratagene, using pGEM MET WT as a template and the following primers: 5′ TCAAATGAGTCTGTAAACTACAGAGCTACT 3′ and 5′ AGTAGCTCTGTAGTTTACAGACTCATTTGA 3′. Insertion of the mutation was verified by sequencing. Finally, MET sequence from PGEM was inserted in pcDNA3 TRK using BamHI and XhoI restriction sites.
The N-terminal Flag-cleaved MET (p40 MET) and the kinase-dead (KD) version (p40 MET K1108A) were constructed as follows. Mouse MET from the caspase site to the C-terminal end was amplified by PCR using TRK-MET WT and TRK-MET K1108A, described previously (40), as templates and the primer 5′ GAGTCTGTAGGATCCAGAGCTAC 3′, containing the BamHI restriction site, and the primer 5′ GGGCCCTCTAGACCCCTAGCCATC 3′, containing the XbaI restriction site. The sequence obtained was cloned using BamHI/XbaI in frame with Flag sequence in the pcDNA3 vector.
Antibodies.
Mouse monoclonal antibody directed against the C-terminal tail of mouse c-MET (B-2) and rabbit polyclonal antibody against Erk2 (C-14) were purchased from Santa Cruz. Mouse monoclonal antibody directed against the kinase domain of MET (25H2) and rabbit polyclonal antibody against cleaved caspase 3 were purchased from Cell Signaling Technology. Rabbit polyclonal antibody against phosphorylated tyrosines 1234 and 1235 of the MET kinase domain was purchased from Upstate Biotechnology. Mouse monoclonal antibody against Flag sequence (M5) was purchased from Sigma. Peroxidase-, fluorescein-, and rhodamine-conjugated antibodies directed against rabbit and mouse immunoglobulin G (IgG) were purchased from Jackson Immunoresearch Laboratories.
In vitro translation and caspase cleavage reaction.
In vitro translations and caspase cleavage assays of LEX-MET and the corresponding mutant LEX-MET D1000N were performed as previously described (19) using TNT reticulocyte lysate (Promega). Purified active caspases were generously provided by G. S. Salvesen (The Burnham Institute, La Jolla, Calif.).
Transfection.
Transient transfections of MDCK cells were performed as described previously using the lipofection method (33). Cortical neurons (8 × 104 cells) were electroporated in cold phosphate-buffered saline in the presence of 4 μg of DNA. The parameters for electroporation were 270 V and five pulses of 3 ms each. The cells were then placed on ice for 10 min and then seeded on poly-dl-ornithine-coated wells (Lab-Tek). Four hours later, the medium was removed and replaced by neurobasal medium supplemented with B27 nutrient medium.
Western blotting.
MDCK or NMUMG cells (4 × 105/35-mm-diameter dish), transfected or not, were cultured for 1 day in DMEM-10% FCS and treated with TNF-α-cycloheximide, anisomycin, or HGF/SF in serum-free DMEM or left untreated. Mouse embryonic hepatocytes and cortical neurons were cultured for 3 days and treated with 5 μM camptothecin, actinomycin D, or TNF-α or left untreated. The cells were then lysed, and the protein concentration was determined by a Bio-Rad protein assay. Western blotting was performed as described previously (23). The results presented are representative of at least two experiments.
Immunofluorescence.
MDCK cells (105/24-mm-diameter dish) were cultured for 1 day in DMEM-10% FCS on glass coverslips and transiently transfected as described above. At the end of the experiment, the cells were washed, fixed with 3% paraformaldehyde, permeabilized with 0.1% Triton X-100, and blocked with 10% goat serum for 20 min. The cells were incubated for 30 min with a combination of mouse anti-Flag antibody (10 μg/ml) and rabbit anti-active caspase 3 antibody (1:100). The cells were then washed and incubated with a combination of fluorescein-conjugated anti-mouse IgG (7 μg/ml) and rhodamine-conjugated anti-rabbit IgG (7 μg/ml). The cell nuclei were counterstained using Hoechst 33258. Coverslips were mounted with Glycergel mounting medium (Dako), and fluorescence was examined using a Zeiss Axioplan2. The results presented are representative of three experiments.
Caspase 3 activity.
Caspase 3 activity was monitored using Ac-DEVD-pNA as a substrate (caspase 3 cellular activity assay kit; Calbiochem). The activity was determined according to the manufacturer's instructions.
TUNEL staining.
Thirty-six hours after electroporation, cortical neurons were fixed with 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling (TUNEL) staining (Apoptag in situ apoptosis detection kit; Serologicals Corp.) was then performed according to the manufacturer's instructions. The coverslips were mounted in Vectashield-DAPI (4′,6′-diamidino-2-phenylindole) (Vector).
RESULTS
Stress stimuli induce the generation of an intracellular 40-kDa C-terminal fragment of MET.
In order to check the fate of MET exposed to stress conditions, primary cultures of embryonic day 15.5 mouse hepatocytes and cortical neurons were treated with TNF-α-actinomycin D or camptothecin, respectively. These stress stimuli were found to cause activation of caspase 3 and increase of TUNEL-positive cells in both cell types within 12 to 18 h (Fig. 1B and D). Under these proapoptotic conditions, an additional protein of ∼40 kDa (p40 MET) was detected by using an anti-mouse MET antibody (Fig. 1A and C).
FIG. 1.
Stress stimuli generate a p40 MET fragment in primary cultures of mouse hepatocytes and cortical neurons. (A) Hepatocytes were cultured for 3 days and treated for 12 or 18 h with actinomycin D or actinomycin D plus TNF-α (100 ng/ml) or left untreated (−). For each condition, the same amount of protein was resolved by SDS-10% PAGE and analyzed by Western blotting using antibodies directed against the C-terminal tail of mouse MET. The filter was stripped and reprobed using an anti-Erk2 antibody to assess comparable loading. (B) Percentages of TUNEL-positive hepatocytes over total number of hepatocytes were determined (n = 3; plus standard deviation [SD]). Protein was resolved by SDS-10% PAGE and analyzed by Western blotting using anti-cleaved caspase 3 antibody. The filter was stripped and reprobed using an anti-α-tubulin antibody to assess comparable loading. (C) Cortical neurons were cultured for 3 days and treated for 12 h with camptothecin (Campto.; 5 μM) or left untreated (−). For each condition, the same amount of protein was resolved by SDS-10% PAGE and analyzed by Western blotting using antibodies directed against the C-terminal tail of mouse MET. The filter was stripped and reprobed using an anti-Erk2 antibody to assess comparable loading. (D) Percentages of TUNEL-positive cells over total number of cells were determined (n = 3; plus SD). Protein was resolved by SDS-10% PAGE and analyzed by Western blotting using anti-cleaved caspase 3 antibody. The filter was stripped and reprobed using an anti-α-tubulin antibody to assess comparable loading. The arrowheads indicate the positions of the MET precursor p170, MET β subunit p140, and MET p40 fragment. Although expression of p140 MET is higher than that of p170 MET, it appears that degradation of full-length MET is more pronounced for the p170 MET precursor than for the fully glycosylated p140 MET β subunit, suggesting that mature glycosylated receptors are more resistant to degradation.
We also investigated the generation of p40 MET in epithelial cell lines, such as MDCK renal cells and NMUMG mammary cells, treated with either TNF-α-cycloheximide or anisomycin. These stress stimuli were found to cause massive cell detachment and activation of caspase 3 in both cell types within 12 to 24 h. These effects correlated with the generation of p40 MET and a decrease in full-length MET receptor detection in MDCK cells (Fig. 2A). In a time course experiment, the earliest detection of p40 MET occurred 4 h after anisomycin treatment, in correlation with activated caspase 3 (data not shown). Decreased MET expression and generation of p40 MET were also obtained in NMUMG cells, albeit in a less pronounced manner (Fig. 2B). Since the 40-kDa fragment was recognized by antibodies directed against either the MET kinase domain (Fig. 2A) or the MET C-terminal tail (Fig. 1A and C and 2B), we can conclude that p40 MET is an intracellular C-terminal fragment of MET.
FIG. 2.
Caspase inhibitor prevents p40 MET generation. (A) MDCK epithelial cells were treated for 12 h with either TNF-α (30 ng/ml)-cycloheximide (Chx; 10 μg/ml) or anisomycin (Ani; 50 μM) in the presence or absence of zVAD-FMK (zVAD; 20 μM) or left untreated (−). For each condition, the same amount of protein was resolved by SDS-10% PAGE and analyzed by Western blotting using an anti-MET antibody directed against its kinase domain. The filter was stripped and reprobed sequentially using an anti-cleaved caspase 3 antibody and an anti-Erk2 antibody to assess loading. (B) NMUMG epithelial cells were treated as for panel A. Protein extracts were analyzed by Western blotting using antibody directed against the C-terminal tail of mouse MET. The filter was stripped and reprobed using an anti-Erk2 antibody. The same extracts were analyzed by Western blotting using an anti-cleaved caspase 3 antibody. The arrowheads indicate the positions of the MET precursor p170, MET subunit p140, and MET p40 fragment.
Furthermore, treatment of both epithelial-cell types with the general caspase inhibitor Z-VAD-FMK abolished generation of the p40 MET fragment and partially restored expression of MET, showing that p40 MET generation is caspase dependent (Fig. 2).
Taken together, these results show that the MET receptor is cleaved by apoptotic proteases under stress conditions, leading to the generation of an intracellular 40-kDa MET C-terminal fragment.
p40 MET is generated by caspase cleavage both in vitro and in living cells.
The size of the p40 MET fragment suggests that the MET receptor is cleaved within its juxtamembrane domain. This region contains several negative regulatory sites involved in down-regulation and/or degradation of the receptor, including a protein kinase C phosphorylation site (Ser 983) (9), a PEST sequence (amino acids 986 to 1001) (6), a phosphatase-binding region (amino acids 993 to 1007) (36), and a c-CBL binding site (Tyr-1001) (24) (Fig. 3).
FIG. 3.
Schematic representation of the α and β subunits of the mature mouse MET receptor. The tyrosine-phosphorylated docking sites Y1001 and Y1347-Y1354 located in the juxtamembrane and the C-terminal tail, respectively, are shown, as well as the protein kinase C phosphorylation site (S983). Exon 14, encoding part of the juxtamembrane domain, and the putative 998-SVD-1000 caspase site (black square), the PEST sequence, the PTP-S binding site, and the TPR-MET fusion site are indicated.
We further mapped the sequence 998-SVD-1000 (Fig. 3), which is a site for caspase cleavage identified in other proteins, such as RET (5), DCC (19), and TRAF3 (13). To check whether MET is a caspase substrate, we constructed a constitutive dimerized form of MET by fusing the intracellular domain of mouse MET to the dimerization domain of LEX (LEX-MET), resulting in a 70-kDa fusion protein. This LEX-MET fusion translated in vitro was incubated with purified caspases. Caspase 3, but not caspase 8, was able to cleave intracellular MET, generating two main fragments of ∼40 and 30 kDa, which together correspond to the 70-kDa LEX-MET protein (Fig. 4). In contrast, no fragment was produced when aspartic acid of the putative caspase site was replaced by asparagine (D1000N).
FIG. 4.
Aspartic acid 1000 of MET is necessary for caspase 3 in vitro cleavage. The in vitro-translated full intracellular domain of MET WT fused to LEX sequence (LEX-MET) or mutated on aspartic acid 1000 (LEX-MET D1000-N) was incubated with or without (−) caspase 8 (0.3 μM) or caspase 3 (0.3 μM) for 2 h. Caspase 3 treatment generated p40 and p30 fragments, and their formation was impaired using the D1000N LEX-MET mutant. Two minor fragments of ∼35 and 25 kDa were also generated by caspase 3 treatment; these fragments might be generated from smaller in vitro translation products of LEX-MET. The arrowheads indicate the positions of the LEX-MET, p40, and p30 fragments.
We next investigated whether caspase cleavage of MET occurs at aspartic acid 1000 when cells are exposed to stress conditions. Experiments were performed using TRK-MET chimeric receptors, which have been shown to mediate MET-specific signals in epithelial cells in response to NGF (39, 40). These chimeras consist of the extracellular portion of the human TRK-A receptor coupled to the transmembrane and intracellular domains of mouse MET, resulting in a 150-kDa fusion protein. Transfected TRK-MET was detected by using an anti-mouse MET antibody, which does not detect endogenous canine MET. Anisomycin induced generation of the C-terminal p40 MET fragment from wild-type TRK-MET receptors. In contrast, the fragment was not generated from the TRK-MET D1000N mutant (Fig. 5). These results demonstrated that the intracellular 40-kDa MET fragment, containing the kinase domain and extending to the C-terminal tail, is generated by caspase cleavage at aspartic acid 1000 within the juxtamembrane domain.
FIG. 5.
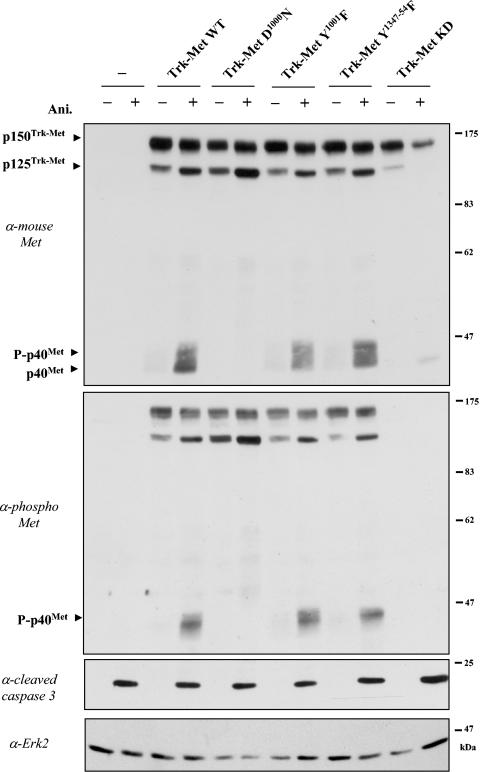
Generation of p40 MET requires aspartic acid 1000 but does not require functional docking sites. MDCK cells were transiently transfected with 1 μg of vector/ml, either empty (−) or expressing TRK-MET receptor, WT, mutated on aspartic acid 1000 (D1000N), mutated on tyrosine residues (Y1001F or Y1347-1354F), or kinase dead (TRK-MET KD). The following day, the cells were treated for 6 h with anisomycin (Ani.; 50 μM) (+) or left untreated (−). For each condition, the same amount of protein was resolved by SDS-10% PAGE and analyzed by Western blotting using an antibody directed against the C-terminal tail of mouse MET (upper panel). The filter was stripped and reprobed sequentially using an anti-phospho-MET antibody (upper middle panel) and an anti-Erk2 antibody (lower panel). The same extracts were analyzed by Western blotting using an anti-cleaved caspase 3 antibody (lower middle panel). The arrowheads indicate the positions of the p150 TRK-MET (fully glycosylated) and p125 TRK-MET (partially glycosylated) and phosphorylated (P-p40 MET) and nonphosphorylated p40 MET fragments.
We further asked whether tyrosine kinase activity and crucial tyrosine residues of MET are necessary for caspase cleavage. These include tyrosine residues Y1347 and Y1354 of the C-terminal region of MET, which mediate the recruitment of cytoplasmic signal transducers and most biological responses (26, 38), and tyrosine residue 1001, which is essential for ligand-induced CBL-mediated ubiquitination of MET (24, 25). As for wild-type TRK-MET, anisomycin induced the generation of p40 MET from TRK-MET receptors mutated either on C-terminal tyrosine residues (TRK-MET Y1347-1354F) or on juxtamembrane tyrosine residue 1001 (TRK-MET Y1001F) (Fig. 5). These results demonstrated that cleavage of MET does not require the known tyrosine docking sites of MET for downstream signaling proteins.
The p40 MET fragment was also generated from kinase-dead TRK-MET (K1108A), but in a less pronounced manner. It is worth noticing that anisomycin treatment caused decreased detection of kinase-dead TRK-MET, in contrast to all other TRK-MET chimeras used. This could be explained by the absence of survival signals triggered by kinase-dead TRK-MET, which could therefore not compensate for apoptosis induced by anisomycin, in contrast to other chimeras.
Furthermore, the generated p40 MET was detected as a smear for all TRK-MET and as a faint single band for the kinase-dead TRK-MET (Fig. 5, upper panel). The upper bands correspond to phosphorylated forms, as demonstrated using an anti-phosphorylated MET antibody (Fig. 5, upper middle panel). This shows that p40 MET generated from transfected TRK-MET receptor is tyrosine phosphorylated.
Kinase-active p40 MET is sufficient to induce apoptosis.
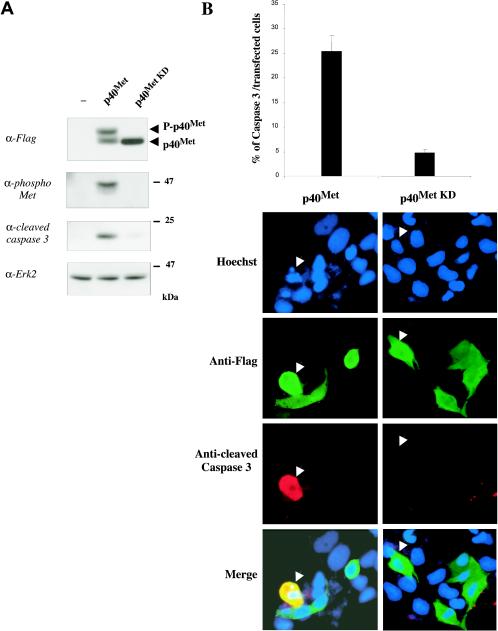
In order to evaluate the biological relevance of p40 MET, we engineered Flag-tagged versions of wild-type and kinase-dead p40 MET (p40 MET KD). Following their transfection into MDCK cells and analysis by Western blotting using an anti-Flag antibody, p40 MET was detected as a doublet, with the upper band corresponding to its phosphorylated form, and kinase-dead p40 MET was detected as an unphosphorylated singlet band (Fig. 6A). Interestingly, expression of cleaved caspase 3 was induced only by wild-type p40 MET (Fig. 6A).
FIG. 6.
p40 MET expression induces apoptosis of MDCK epithelial cells. (A) MDCK cells were transiently transfected with 1 μg of vector/ml, either empty (−) or expressing wild-type (p40 Met) or kinase-dead (p40 Met KD) p40 MET. Forty-eight hours after transfection, the proteins were resolved by SDS-10% PAGE and analyzed by Western blotting using an anti-Flag antibody (top). The filter was stripped and reprobed sequentially using an anti-phospho-MET antibody and an anti-Erk2 antibody. The same extracts were analyzed by Western blotting using an anti-active caspase 3 antibody. The arrowheads indicate the positions of phosphorylated and nonphosphorylated p40 MET. (B) MDCK cells were transfected with 1 μg of vector/ml expressing either wild-type (p40 Met) or kinase-dead (p40 Met KD) p40 MET. Twenty-four hours after transfection, nuclei were detected by using Hoechst staining (Hoechst; blue staining) and immunofluorescence assays were performed using an anti-Flag antibody (Anti-Flag; green staining) and an anti-cleaved caspase 3 antibody (Anti-cleaved Caspase 3; red staining). An overlay of the three stains is shown (Merge). Percentages of caspase 3-positive cells over p40 MET WT- or KD-transfected cells were determined (n = 3; plus standard deviation). The white arrowheads indicate representative cells expressing transfected p40 MET WT or KD.
Immunofluorescence analysis revealed that wild-type and kinase-dead p40 MET are expressed in both the cytoplasm and the nucleus. p40 MET-expressing cells were compact, and Hoechst staining showed characteristic signs of apoptotis such as condensed DNA or a strong decrease in DNA content. About 25% of p40 MET-transfected cells were positive for cleaved caspase 3 staining (Fig. 6B, left). In contrast, kinase-dead p40 MET-expressing cells display normal morphology, with ∼5% cleaved caspase 3-positive cells (Fig. 6B, right).
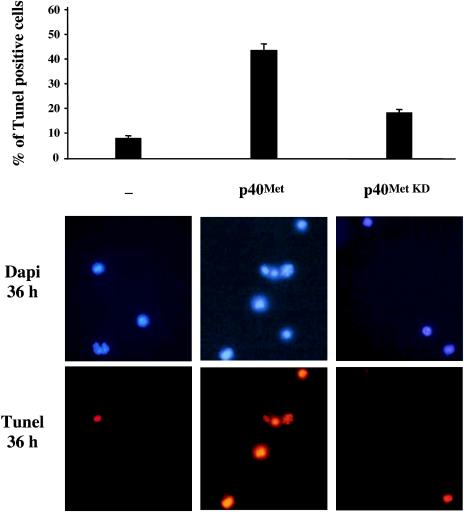
We next asked whether proapoptotic activity of p40 MET would also occur in primary-culture cells. Mouse embryonic cortical neurons were electroporated with empty vector or wild-type or kinase-dead p40 MET. Thirty-six hours after electroporation, apoptosis was assessed by DNA fragmentation using both DAPI staining and TUNEL reaction. Immunostaining with an anti-Flag antibody showed that an average of 70% of cortical neurons were indeed electroporated (data not shown). p40 MET-transfected cells display a high percentage of TUNEL-positive cells (45%) compared to levels of apoptosis in cortical neurons transfected with either empty vector or kinase-dead p40 MET (Fig. 7). As a control, comparable levels of apoptotic cells (55%) were obtained by electroporation of Forkhead, a known apoptosis inducer (data not shown).
FIG. 7.
p40 MET expression induces apoptosis of embryonic cortical neurons. After dissociation, mouse embryonic cortical neurons were electroporated with either empty vector or wild-type (p40 Met) or kinase-dead (p40 Met KD) p40 MET. Thirty-six hours after transfection, TUNEL staining was performed and nuclei were detected using DAPI staining. Percentages of TUNEL-positive cells over the total number of cells were determined (n = 3; plus standard deviation). Representative DAPI and TUNEL staining is shown. The arrows indicate representative DNA condensation in apoptotic cells.
These results show that expression of p40 MET is sufficient to cause apoptosis of either MDCK epithelial cells or embryonic cortical neurons, even in the absence of stress stimuli. In addition, this effect is an active process, since the proapoptotic function of p40 MET requires its intact tyrosine kinase activity.
HGF/SF treatment does not favor generation of p40 MET.
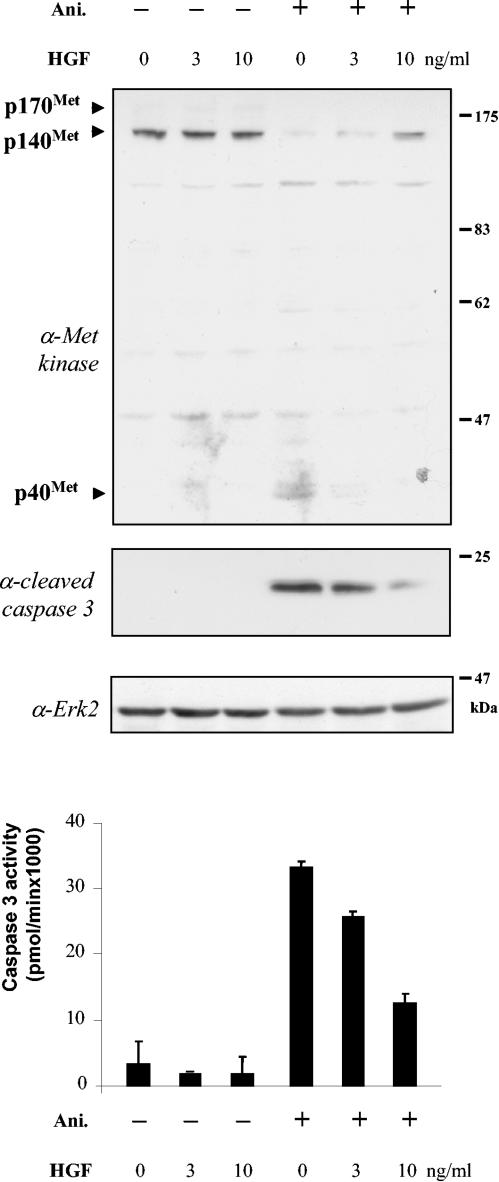
The proapoptotic function of p40 MET contrasts with the well-known survival function of the MET receptor against stress stimuli. To unravel these opposite functions of MET kinase, we asked whether HGF/SF stimulation could prevent the generation of p40 MET, thus preventing its proapoptotic activity. MDCK cells were treated with anisomycin in the presence or absence of HGF/SF or left untreated. Stimulation by HGF/SF prevented the generation of anisomycin-mediated p40 MET in a dose-dependent manner (Fig. 8). Consistently, this event was associated with reduced caspase 3 activation. Similar results were obtained by exploring the effects of HGF/SF at doses below 1 ng/ml or above 10 ng/ml (data not shown). Protection was also found using MDCK cells stably expressing TRK-MET receptors and stimulated by NGF (data not shown). This provided evidence of a balance between the actions of HGF/SF and stress stimuli, with HGF/SF acting through the MET receptor and mediating an antiapoptotic input, whereas stress conditions generated p40 MET, which by itself mediates a proapoptotic input.
FIG. 8.
HGF/SF stimulation of the MET receptor does not favor p40 MET generation. MDCK cells were pretreated for 1 h with HGF/SF at the concentration indicated or not pretreated (0) and then were treated for 8 h with anisomycin (Ani.; 50 μM) (+) or left untreated (−). For each condition, caspase 3 activity was determined from the same amount of proteins (n = 3; plus standard deviation). Caspase activity is expressed as pmol/min of degraded substrate. A representative extract was resolved by SDS-10% PAGE and analyzed by Western blotting using an anti-MET antibody directed against the kinase domain. The filter was stripped and reprobed sequentially using an anti-active caspase 3 antibody and an anti-Erk2 antibody.
DISCUSSION
Activation of the MET tyrosine kinase receptor by HGF/SF is classically associated with cell survival against various stress stimuli. Nonetheless, we demonstrated that stress stimuli can also lead to caspase-dependent cleavage that converts MET into a proapoptotic factor.
The caspase cleavage site is located in the juxtamembrane region of MET, an essential negative regulatory region (Fig. 3). Initial evidence for the role of this region, came from the identification of MET proteins lacking exon 14, including the TPR-MET oncogenic version of MET. TPR-MET comes from a chromosomal rearrangement, which creates a fusion protein between TPR (providing two leucine zipper domains) and intracellular MET, resulting in a constitutive dimerized and therefore activated MET kinase (22). In this TPR-MET fusion, the intracellular portion of the MET receptor is conserved, with the notable exception of exon 14, encoding part of the juxtamembrane region. The transforming activity of TPR-MET was lost when exon 14 was reintroduced into the TPR-MET sequence (35). In mouse tissue, this exon 14 is also absent in an alternative spliced form of MET, which causes aberrant MET signaling (12). More direct evidence for the relevance of the juxtamembrane region came from the identification of several negative regulatory sites. These included a protein kinase C phosphorylation site (Ser 983), which negatively regulates MET kinase activity (9); a PEST sequence (amino acids 986 to 1001), which can confer susceptibility to intracellular proteolysis (amino acids 986 to 1001) (6); a sequence (amino acids 993 to 1007) associated with phosphatase activity (36); and a c-CBL binding site (Tyr-1001) responsible for MET ubiquitination and proteasomal degradation (24).
The caspase cleavage site (Asp-1000) identified here is an additional novel negative regulatory site within the juxtamembrane region of MET. Indeed, the cleavage releases a p40 fragment containing the kinase domain, which becomes ligand independent and acquires proapoptotic activity. Its putative counterpart, p100 MET, which includes the extracellular region and part of the juxtamembrane region, was not detected in canine MDCK cells by using currently available antibodies. Nonetheless, both p40 and p100 MET could be detected in human epithelial cells exposed to stress conditions (unpublished observations), confirming the possibility that caspase cleavage splits the receptor. The biological relevance of p100 MET is being investigated. More importantly, we have provided evidence that this caspase cleavage site operates in situations and/or through mechanisms distinct from those of all other sites previously described. In particular, the caspase cleavage site is effective in the absence of the ligand, whereas all others sites are involved in down-regulation and/or degradation of the receptor following activation by the ligand. Accordingly, HGF/SF induced activation, ubiquitination, and proteasomal degradation of the receptor with a strict requirement for intact tyrosine kinase activity (10, 31). In contrast, stress stimuli induced both cleavage and degradation of MET with tyrosine kinase activity being dispensable, since kinase-dead MET receptors were still cleaved. Finally, Asp-1000 is adjacent to Tyr-1001, which is involved in the recruitment of c-CBL, leading to degradation of activated MET receptor (24). It was found that mutations of Tyr-1001 and surrounding amino acids, including Asp-1000, cause similar constitutive epithelial-cell scattering (40), probably through lack of degradation of activated MET receptor. These results suggest that Asp-1000 belongs to the binding site of c-CBL. However, our present data showing that mutation of Asp-1000, but not mutation of Tyr-1001, impairs the generation of p40 MET indicate that caspase-dependent cleavage of MET can occur independently of c-CBL recruitment.
In contrast to embryonic hepatocytes and cortical neurons, we found that stress stimuli induced a strong decrease in MET expression accompanying the generation of endogenous p40 MET in an MDCK epithelial cell line. In these cells, both cleavage and degradation of MET may be mediated by apoptotic proteases. However, a MET sequence mutated on the caspase site or cell treatment with the zVAD-FMK caspase inhibitor abolished p40 MET generation but only partially restored MET expression. These results indicate that additional mechanisms degrade MET in MDCK epithelial cells under stress conditions, including proteolytic degradation through caspase-independent mechanisms. Furthermore, we found that the kinase-dead TRK-MET is more degraded than the other TRK-MET chimeras, with, however, a reduction in p40 MET production compared to the other chimeras. This suggests that other mechanisms of degradation can compete with the caspase-dependent cleavage of MET in epithelial-cell lines.
We cannot exclude the possibility that other proteases than caspase 3 can cleave MET under stress conditions. Nonetheless, we provide evidence for an amplification loop between generation of p40 MET and active caspase 3. Indeed, active caspase 3 generated p40 MET from MET in vitro, and transfected p40 MET induced the formation of active caspase 3, at least in epithelial cells. Furthermore, in all cell types examined, we found strict correlation between the level of p40 MET generation and caspase 3 activation. Altogether, these findings suggest the existence of a novel mechanism that makes use of MET signaling to accelerate apoptosis in cells exposed to stress conditions in the absence of HGF/SF.
By investigating the biological relevance of the p40 MET fragment, we found that p40 MET, but not its kinase-dead version, was able to induce apoptosis, demonstrating that tyrosine kinase activity is crucial for this process. This apoptotic capacity of a kinase-active form of p40 MET is further supported by phosphorylation of p40 MET, both endogenously generated from a transfected TRK-MET chimera and upon transfection of p40 MET. Because of their common intracellular sequences, it is reasonable to ask how active p40 MET can induce apoptosis whereas active full-length MET is known to favor survival. It is possible that p40 MET as a non-membrane-bound kinase does not signal like MET, and our preliminary results support this hypothesis. Indeed, we found that p40 MET does not activate ERK MAP kinase, a conventional target of survival pathways activated by MET (unpublished observations), but it may act on distinct apoptotic effectors which still need to be identified. We therefore speculate that p40 MET does not signal like MET but rather redirects the MET catalytic activity toward apoptosis, according to the cellular context of proapoptotic conditions.
The consequences of caspase cleavage are different depending on the substrates. In most cases, caspase cleavage is known to inactivate survival substrates. For instance, the tyrosine kinase receptors EGFR and ErbB2 are cleaved and inactivated by caspases during apoptosis (2, 32). Nonetheless, some substrates are activated by caspase cleavage, such as the MEKK1 serine-threonine kinase, which releases a C-terminal fragment containing the kinase domain that causes apoptosis (41). In line with such data, the MET survival receptor is converted to a proapoptotic p40 MET fragment.
Our results showing cleavage of MET by caspases generating a proapoptotic fragment and negative regulation of this process by its ligand are similar to previously reported data for the RET tyrosine kinase receptor and the DCC netrin-1 receptor and their respective ligands (5, 19). RET and DCC are further classified as dependence receptors. The overall concept of dependence receptors is that, in the absence of ligand, such receptors are cleaved by caspases or other proteases and release or expose a proapoptotic domain that in turn leads to an increase of caspase activity and then more receptor cleavage (5, 15, 19). Thus, MET may behave like a dependence receptor. Nonetheless, current data do not show a proapoptotic effect of MET upon ectopic expression. Rather, we and others have found that ectopic MET expression induces positive downstream signaling involved in scattering, morphogenesis, and survival (8, 33) that masks a potential proapoptotic effect of cleaved MET.
In conclusion, we have provided evidence for a balance between HGF/SF leading to activation of the MET tyrosine kinase receptor and stress stimuli leading to the generation of p40 MET. Indeed, HGF/SF treatment counteracts caspase 3 activation in MDCK cells. This was accompanied by a decrease in p40 MET generation and restoration of full MET expression, suggesting an antiapoptotic function of ligand-activated MET that prevents its own cleavage by switching off caspase activation. Balance between apoptosis and survival is known to be crucial for a number of physiological and pathological processes. Accordingly, genetic invalidation of either MET or HGF/SF caused liver reduction with massive apoptosis (4, 28), while overexpression of HGF/SF in transgenic mice induced abnormal development and tumor formation in the liver (27). A critical balance between the antiapoptotic input of HGF/SF bound to MET and the proapoptotic input generated by MET caspase-dependent cleavage might be crucial for homeostasis triggered by HGF/SF and MET.
Acknowledgments
This work was supported by the Institut Pasteur de Lille, the CNRS, and INSERM, and by grants from the Association Régionale pour l'Enseignement et la Recherche Scientifique et Technologique, the Groupement des Entreprises Françaises dans la Lutte contre le Cancer, the Fondation de France, the Ligue Nationale contre le Cancer, the Association Française contre le Myopathies, the Fondation pour la Recherche Médicale, the Association pour la Recherche sur le Cancer, the Shlumberger Fundation, and NIH. A.M was supported by a Ligue contre le Cancer fellowship.
REFERENCES
- 1.Arakaki, N., J. A. Kazi, T. Kazihara, T. Ohnishi, and Y. Daikuhara. 1998. Hepatocyte growth factor/scatter factor activates the apoptosis signaling pathway by increasing caspase-3 activity in sarcoma 180 cells. Biochem. Biophys. Res. Commun. 245:211-215. [DOI] [PubMed] [Google Scholar]
- 2.Bae, S. S., J. H. Choi, Y. S. Oh, D. K. Perry, S. H. Ryu, and P. G. Suh. 2001. Proteolytic cleavage of epidermal growth factor receptor by caspases. FEBS Lett. 491:16-20. [DOI] [PubMed] [Google Scholar]
- 3.Birchmeier, C., W. Birchmeier, E. Gherardi, and G. F. Vande Woude. 2003. Met, metastasis, motility and more. Nat. Rev. Mol. Cell. Biol. 4:915-925. [DOI] [PubMed] [Google Scholar]
- 4.Bladt, F., D. Riethmacher, S. Isenmann, A. Aguzzi, and C. Birchmeier. 1995. Essential role for the c-met receptor in the migration of myogenic precursor cells into the limb bud. Nature 376:768-771. [DOI] [PubMed] [Google Scholar]
- 5.Bordeaux, M. C., C. Forcet, L. Granger, V. Corset, C. Bidaud, M. Billaud, D. E. Bredesen, P. Edery, and P. Mehlen. 2000. The RET proto-oncogene induces apoptosis: a novel mechanism for Hirschsprung disease. EMBO J. 19:4056-4063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Crepaldi, T., M. Prat, S. Giordano, E. Medico, and P. M. Comoglio. 1994. Generation of a truncated hepatocyte growth factor receptor in the endoplasmic reticulum. J. Biol. Chem. 269:1750-1755. [PubMed] [Google Scholar]
- 7.Di Renzo, M. F., M. Olivero, T. Martone, A. Maffe, P. Maggiora, A. D. Stefani, G. Valente, S. Giordano, G. Cortesina, and P. M. Comoglio. 2000. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene 19:1547-1555. [DOI] [PubMed] [Google Scholar]
- 8.Furge, K. A., Y. W. Zhang, and G. F. Vande Woude. 2000. Met receptor tyrosine kinase: enhanced signaling through adapter proteins. Oncogene 19:5582-5589. [DOI] [PubMed] [Google Scholar]
- 9.Gandino, L., P. Longati, E. Medico, M. Prat, and P. M. Comoglio. 1994. Phosphorylation of serine 985 negatively regulates the hepatocyte growth factor receptor kinase. J. Biol. Chem. 269:1815-1820. [PubMed] [Google Scholar]
- 10.Jeffers, M., G. A. Taylor, K. M. Weidner, S. Omura, and G. F. Vande-Woude. 1997. Degradation of the Met tyrosine kinase receptor by the ubiquitin-proteasome pathway. Mol. Cell. Biol. 17:799-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosai, K., K. Matsumoto, S. Nagata, Y. Tsujimoto, and T. Nakamura. 1998. Abrogation of Fas-induced fulminant hepatic failure in mice by hepatocyte growth factor. Biochem. Biophys. Res. Commun. 244:683-690. [DOI] [PubMed] [Google Scholar]
- 12.Lee, C. C., and K. M. Yamada. 1995. Alternatively spliced juxtamembrane domain of a tyrosine kinase receptor is a multifunctional regulatory site. Deletion alters cellular tyrosine phosphorylation pattern and facilitates binding of phosphatidylinositol-3-OH kinase to the hepatocyte growth factor receptor. J. Biol. Chem. 270:507-510. [DOI] [PubMed] [Google Scholar]
- 13.Lee, Z. H., S. E. Lee, K. Kwack, W. Yeo, T. H. Lee, S. S. Bae, P. G. Suh, and H. H. Kim. 2001. Caspase-mediated cleavage of TRAF3 in FasL-stimulated Jurkat-T cells. J. Leukoc. Biol. 69:490-496. [PubMed] [Google Scholar]
- 14.Liu, Y., A. M. Sun, and L. D. Dworkin. 1998. Hepatocyte growth factor protects renal epithelial cells from apoptotic cell death. Biochem. Biophys. Res. Commun. 246:821-826. [DOI] [PubMed] [Google Scholar]
- 15.Llambi, F., F. Causeret, E. Bloch-Gallego, and P. Mehlen. 2001. Netrin-1 acts as a survival factor via its receptors UNC5H and DCC. EMBO J. 20:2715-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lorenzato, A., M. Olivero, S. Patane, E. Rosso, A. Oliaro, P. M. Comoglio, and M. F. Di Renzo. 2002. Novel somatic mutations of the MET oncogene in human carcinoma metastases activating cell motility and invasion. Cancer Res. 62:7025-7030. [PubMed] [Google Scholar]
- 17.Maina, F., M. C. Hilton, C. Ponzetto, A. M. Davies, and R. Klein. 1997. Met receptor signaling is required for sensory nerve development and HGF promotes axonal growth and survival of sensory neurons. Genes Dev. 11:3341-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Maina, F., G. Pante, F. Helmbacher, R. Andres, A. Porthin, A. M. Davies, C. Ponzetto, and R. Klein. 2001. Coupling Met to specific pathways results in distinct developmental outcomes. Mol. Cell 7:1293-1306. [DOI] [PubMed] [Google Scholar]
- 19.Mehlen, P., S. Rabizadeh, S. J. Snipas, N. Assa-Munt, G. S. Salvesen, and D. E. Bredesen. 1998. The DCC gene product induces apoptosis by a mechanism requiring receptor proteolysis. Nature 395:801-804. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura, T. 1991. Structure and function of hepatocyte growth factor. Prog. Growth Factor Res. 3:67-85. [DOI] [PubMed] [Google Scholar]
- 21.Naldini, L., K. M. Weidner, E. Vigna, G. K. Gaudino, A. Bardelli, C. Ponzetto, R. P. Narsimhan, G. Hartmann, R. Zarnegar, G. Michalopoulos, W. Birchmeier, and P. M. Comoglio. 1991. Scatter factor and hepatocyte growth factor are undistinguishable ligands for the MET receptor. EMBO J. 10:2867-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park, M., M. Dean, K. Kaul, M. J. Braun, M. A. Gonda, and G. Vande Woude. 1987. Sequence of MET protooncogene cDNA has features characteristic of the tyrosine kinase family of growth-factor receptors. Proc. Natl. Acad. Sci. USA 84:6379-6383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Paumelle, R., D. Tulasne, Z. Kherrouche, S. Plaza, C. Leroy, S. Reveneau, B. Vandenbunder, and V. Fafeur. 2002. Hepatocyte growth factor/scatter factor activates the ETS1 transcription factor by a RAS-RAF-MEK-ERK signaling pathway. Oncogene 21:2309-2319. [DOI] [PubMed] [Google Scholar]
- 24.Peschard, P., T. M. Fournier, L. Lamorte, M. A. Naujokas, H. Band, W. Y. Langdon, and M. Park. 2001. Mutation of the c-Cbl TKB domain binding site on the Met receptor tyrosine kinase converts it into a transforming protein. Mol. Cell 8:995-1004. [DOI] [PubMed] [Google Scholar]
- 25.Petrelli, A., G. F. Gilestro, S. Lanzardo, P. M. Comoglio, N. Migone, and S. Giordano. 2002. The endophilin-CIN85-Cbl complex mediates ligand-dependent downregulation of c-Met. Nature 416:187-190. [DOI] [PubMed] [Google Scholar]
- 26.Ponzetto, C., A. Bardelli, Z. Zhen, F. Maina, P. dalla Zonca, S. Giordano, A. Graaziani, G. Panayotou, and P. M. Comoglio. 1994. A multifunctional docking site mediates signaling and transformation by the hepatocyte growth factor/scatter factor receptor family. Cell 77:261-271. [DOI] [PubMed] [Google Scholar]
- 27.Sakata, H., H. Takayama, R. Sharp, J. S. Rubin, G. Merlino, and W. J. LaRochelle. 1996. Hepatocyte growth factor/scatter factor overexpression induces growth, abnormal development, and tumor formation in transgenic mouse livers. Cell Growth Differ. 7:1513-1523. [PubMed] [Google Scholar]
- 28.Schmidt, C., F. Bladt, S. Goedecke, V. Brinkmann, W. Zschiesche, M. Sharpe, E. Gherardi, and C. Birchmeier. 1995. Scatter factor/hepatocyte growth factor is essential for liver development. Nature 373:699-702. [DOI] [PubMed] [Google Scholar]
- 29.Schmidt, L., F. M. Duh, F. Chen, T. Kishida, G. Glenn, P. Choyke, S. W. Scherer, Z. Zhuang, I. Lubensky, M. Dean, R. Allikmets, A. Chidambaram, U. R. Bergerheim, J. T. Feltis, C. Casadevall, A. Zamarron, M. Bernues, S. Richard, C. J. Lips, M. M. Walther, L. C. Tsui, L. Geil, M. L. Orcutt, T. Stackhouse, B. Zbar, et al. 1997. Germline and somatic mutations in the tyrosine kinase domain of the MET proto-oncogene in papillary renal carcinomas. Nat. Genet. 16:68-73. [DOI] [PubMed] [Google Scholar]
- 30.Stoker, M., and M. Perryman. 1985. An epithelial scatter factor released by embryo fibroblasts. J. Cell Sci. 77:209-223. [DOI] [PubMed] [Google Scholar]
- 31.Taher, T. E., E. P. Tjin, E. A. Beuling, J. Borst, M. Spaargaren, and S. T. Pals. 2002. c-Cbl is involved in Met signaling in B cells and mediates hepatocyte growth factor-induced receptor ubiquitination. J. Immunol. 169:3793-3800. [DOI] [PubMed] [Google Scholar]
- 32.Tikhomirov, O., and G. Carpenter. 2001. Caspase-dependent cleavage of ErbB-2 by geldanamycin and staurosporin. J. Biol. Chem. 276:33675-33680. [DOI] [PubMed] [Google Scholar]
- 33.Tulasne, D., R. Paumelle, K. M. Weidner, B. Vandenbunder, and V. Fafeur. 1999. The multisubstrate docking site of the MET receptor is dispensable for MET-mediated RAS signaling and cell scattering. Mol. Biol. Cell 10:551-565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Uehara, Y., O. Minowa, C. Mori, K. Shlota, J. Kuno, T. Noda, and N. Kitamura. 1995. Placental defect and embryonic lethality in mice lacking hepatocyte growth factor/scatter factor. Nature 373:702-705. [DOI] [PubMed] [Google Scholar]
- 35.Vigna, E., D. Gramaglia, P. Longati, A. Bardelli, and P. M. Comoglio. 1999. Loss of the exon encoding the juxtamembrane domain is essential for the oncogenic activation of TPR-MET. Oncogene 18:4275-4281. [DOI] [PubMed] [Google Scholar]
- 36.Villa-Moruzzi, E., F. Puntoni, A. Bardelli, E. Vigna, S. De Rosa, and P. M. Comoglio. 1998. Protein tyrosine phosphatase PTP-S binds to the juxtamembrane region of the hepatocyte growth factor receptor Met. Biochem. J. 336:235-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., M. C. DeFrances, Y. Dai, P. Pediaditakis, C. Johnson, A. Bell, G. K. Michalopoulos, and R. Zarnegar. 2002. A mechanism of cell survival: sequestration of Fas by the HGF receptor Met. Mol. Cell 9:411-421. [DOI] [PubMed] [Google Scholar]
- 38.Weidner, K. M., S. Dicesare, M. Sachs, V. Brinkmann, J. Behrens, and W. Birchmeier. 1996. Interaction between gab1 and the c-Met receptor tyrosine kinase is responsible for epithelial morphogenesis. Nature 384:173-176. [DOI] [PubMed] [Google Scholar]
- 39.Weidner, K. M., G. Hartmann, L. Naldini, P. M. Comoglio, M. Sachs, C. Fonatsch, H. Rieder, and W. Birchmeier. 1993. Molecular characteristics of HGF-SF and its role in cell motility and invasion. EXS 65:311-328. [PubMed] [Google Scholar]
- 40.Weidner, K. M., M. Sachs, and W. Birchmeier. 1995. Mutation of juxtamembrane tyrosine residue 1001 suppresses loss-of-function mutations of the met receptor in epithelial cells. Proc. Natl. Acad. Sci. USA 92:2597-2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Widmann, C., P. Gerwins, N. L. Johnson, M. B. Jarpe, and G. L. Johnson. 1998. MEK kinase 1, a substrate for DEVD-directed caspases, is involved in genotoxin-induced apoptosis. Mol. Cell. Biol. 18:2416-2429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yo, Y., R. Morishita, S. Nakamura, N. Tomita, K. Yamamoto, A. Moriguchi, K. Matsumoto, T. Nakamura, J. Higaki, and T. Ogihara. 1998. Potential role of hepatocyte growth factor in the maintenance of renal structure: anti-apoptotic action of HGF on epithelial cells. Kidney Int. 54:1128-1138. [DOI] [PubMed] [Google Scholar]