Abstract
Women have more of the stress-related behavioral profile that has been linked to cardiovascular disease than men. For example, women double the rates of stress-related mental disorders such as depression and posttraumatic stress disorder (PTSD) than men, and have higher rates of exposure to adversity early in life. This profile may increase women's long-term risk of cardiometabolic conditions linked to stress, especially coronary heart disease (CHD). In addition to having a higher prevalence of psychosocial stress-ors, women may be more vulnerable to the adverse effects of these stressors on CHD, perhaps through altered neurobiological physiology. Emerging data suggest that young women are disproportionally susceptible to the adverse effects of stress on the risk of cardiovascular disease, both in terms of initiating the disease as well as worsening the prognosis in women who have already exhibited symptoms of the disease. Women's potential vulnerability to psychosocial stress could also help explain their higher propensity toward abnormal coronary vasomotion and microvascular disease compared with men.
Keywords: Stress, women, cardiovascular disease, mental health, gender factors
1. Introduction
In spite of significant advances in the prevention and treatment of coronary heart disease (CHD), this condition still represents the leading cause of mortality and disability in women (Go et al., 2013; Mehta et al., 2016).
There are several differences between women and men in the presentation, pathophysiology, and clinical course of CHD. For women, CHD becomes clinically manifest later in life than men, and is associated with less narrowing of the coronary arteries related to atherosclerotic plaque. Despite this apparent protection from atherosclerotic plaque, women have a similar or worse morbidity and mortality for CHD than men (Vaccarino et al., 2013a). This may be related to gender differences in pathophysiology—specifically, the fact that microvascular coronary disease (a failure of small coronary arteries to dilate during stress) and endothelial dysfunction (an imbalance between vasodilating and vasoconstricting substances regulating the endothelium) may be more important factors in the etiology of myocardial ischemia than plaque burden in women compared to men (Bugiardini and Bairey Merz, 2005). Microvascular disease and endothelial dysfunction, however, have not been studied as much as atherosclerotic plaque, hindering efforts to prevent, diagnose and treat CHD related to these factors, which may especially disadvantage women (Vaccarino, 2010; Vaccarino et al., 2011).
Great strides have been made in the reduction of CHD-related mortality over the last few decades. This has primarily seen in older persons, however, and young women in particular have not shared a similar decline than men in mortality from CHD in recent decades, although they have continued to enjoy lower rates than men (Wilmot et al., 2015). Furthermore, hospitalization rates for acute myocardial infarction (MI) are on the rise in young women (i.e. ≤55 years) compared to other groups (Gupta et al., 2014; Izadnegahdar et al., 2014), and the pre-hospital case fatality of MI has also declined less in young women (Lehto et al., 2011). Notably, young women with an MI have an unexplained increase in-hospital mortality compared to men of the same age which is not explainable by differences in severity of disease or risk factors for CHD (Bangalore et al., 2012; Gupta et al., 2014; Izadnegahdar et al., 2014; Vaccarino et al., 2009a).
Although rates of traditional CHD risk factors such as obesity and diabetes are growing in the younger age groups (Geiss et al., 2014; Ladabaum et al., 2014), it is likely that exposure to emotional factors (acute and chronic stress, psychosocial factors and mental disorders) play a critical role in the risk for CHD in young populations, especially for women (Goldstein et al., 2015b; Huang et al., 2009; Shah et al., 2011; Wyman et al., 2012). A recent statement from the American Heart Association highlighted the importance of this issue (Goldstein et al., 2015a). Young women with CAD are often from disadvantaged backgrounds and have a high burden of psychosocial factors that have been linked to increased CHD risk, such as depression, early life adversities and posttraumatic stress disorder (PTSD)(Beckie et al., 2015; Low et al., 2010; Mallik et al., 2006; Shah et al., 2014; Smolderen et al., 2015; Vaccarino et al., 2014; Xu et al., 2015). Psychosocial factors are important predictors of future hospitalizations, mortality and delayed recovery in young women with early-onset CAD (Shah et al., 2014; Xu et al., 2015). In addition to having a higher prevalence of psychosocial factors, women may be more vulnerable to the adverse effects of these stressors on CHD. Even among young individuals from the general population, depression and early life stress are more powerful predictors of cardiovascular disease in women than in men (Korkeila et al., 2010; Shah et al., 2011; Wyman et al., 2012). As a whole, these data suggest a profound role of emotional factors on cardiovascular risk in women, although research in this area is limited.
In this chapter, we propose that stress plays a fundamental role in conferring vulnerability to CHD in women and placing women on a trajectory for increased risk for CHD that may not manifest until later in life. To date, considerable research has focused on CHD in women at the age around or after menopause. However, we advocate a paradigm shift toward the idea that pathophysiological processes, such as those secondary to stressful exposures, beginning before menopause lead to CHD later in life in many women. Processes that begin in the premenopausal years, a time when women are typically considered “low risk,” may be key elements, albeit neglected, of a cumulative increase in CHD risk as women age.
2. Studies of non-human primates
Animal models, particularly nonhuman primates, have provided invaluable direct experimental evidence of the adverse cardiovascular effects of psychological stress (Kaplan et al., 2009; Shively et al., 2009). These studies have shown that stress interacts with female neurohormonal systems to increase the risk of CHD. Psychosocial stress causes endothelial damage and accelerates atherosclerosis in females. Monkeys under social stress develop endothelial injury; this effect is blocked by propranolol, the beta-adrenergic receptor antagonist, indicating that norepinephrine, a hormone involved in the stress response, is implicated (Kaplan et al., 2009). Among females, these effects occur in part through stress-induced ovarian dysfunction (Kaplan and Manuck, 2008).
These studies in non-human primates showed important sex differences in the relationship between stress and atherosclerotic vascular disease. Dominant males develop more extensive atherosclerosis than subordinates when housed in unstable social groups, while in females it is the subordinates who develop accelerated atherosclerosis (Kaplan et al., 2009). Subordinate females also show increased cortisol levels, ovarian dysfunction, increased abdominal fat, and an increase in depression-like behaviors (Kaplan et al., 2002; Kaplan and Manuck, 2008). Furthermore, pre-treatment with estrogen prevents the accelerated atherosclerosis of subordinate young females, while ovariectomy eliminates the resilience to stress-induced promotion of atherosclerosis seen in dominant females (Kaplan et al., 2002; Kaplan and Manuck, 2008).
Several lines of evidence suggest that similar mechanisms may be at play in the pathogenesis of CHD in women. Stress is known to be a major cause of ovarian dysfunction in premenopausal women (Chrousos et al., 1998; Ferin, 1999). Stress-induced infertility and hypoestrogenism, known as “functional hypothalamic anovulation,” has also been linked to poor emotional, cardiovascular, skeletal, and cognitive health outcomes (Berga and Loucks, 2005; Marcus et al., 2001).
Studies of stress and cardiovascular disease using non-human primates have traditionally focused on atherosclerosis as animals can be sacrificed at the end of experiments and atherosclerotic plaque severity can be quantified. However, this animal model has also yielded a wealth of data on the effects of stress on endothelial injury and dysfunction, although typically these studies have included male animals (Skantze et al., 1998; Strawn et al., 1991; Williams et al., 1991, 1993). Due to the lack of studies of females, from these experimental studies it is unclear whether, in females, the effects of stress are more pronounced on vascular dysfunction as opposed to atherosclerotic plaque burden. Nonetheless, these processes are interrelated and both strong predictors of CHD risk.
3. Neurobiology of stress and stress-related psychiatric disorders in women in relation to CHD risk
3.1. Stress and mental health
Psychological stress and trauma, especially early in life, can have lasting effects on cognitive and neurobiological function that in turn impact physical health, including the development of cardiovascular disease (Vaccarino and Bremner, 2015). About one fifth of women in the United States are affected by childhood sexual abuse, vs. about 7% of men (“Adverse Childhood Experiences Reported by Adults—Five States, 2009, 2010; MacMillan et al., 1997; McCauley et al., 1997). Emotional and physical abuse are also common in women and can have long-lasting consequences that persist into childhood (“Adverse Childhood Experiences Reported by Adults—Five States, 2009, 2010), especially for emotional abuse (Bremner et al., 2007b; Bremner et al., 2000). Childhood sexual abuse, a common trauma in women, can lead to the development of PTSD in adulthood, which affects 9.7% of women (past year prevalence), vs. 3.6% men (National Comorbidity Survey, 2005), as well as substance abuse, depression, obesity, cardiovascular disease, premature mortality, and psychosomatic disorders (Bremner et al., 1996c; Bremner, 2002; Brown et al., 2009; Danese and McEwen, 2012; Dube et al., 2003; Kessler et al., 1995; Mayer and Bushnell, 2009). Stress-related psychiatric disorders, including PTSD, depression, borderline personality disorder (BPD) and dissociative identity disorder (DID), are at least twice as common in women as in men, while psychiatric disorders not linked to trauma are not (Kessler et al., 1995). As described later, these trauma-related psychiatric disorders share a similar neurobiology, and have considerable overlap of symptoms, which has led Bremner to formulate a model of trauma-spectrum psychiatric disorders (Bremner, 2002). Interestingly, emerging evidence suggests that these disorders share a common increased risk for cardiovascular disease, suggesting that early abuse may result in a re-setting of physiology that results in both stress-related psychiatric disorders and cardiovascular disease that may be particularly linked to stress (Vaccarino and Bremner, 2015).
Psychological trauma can lead to PTSD, which in about half of cases becomes a chronic condition. PTSD symptoms include intrusive memories of the trauma, avoidance of reminders of the trauma, emotional numbing or withdrawal, negative distorted thoughts about the self or the world, hyperarousal, poor concentration, nightmares, sleep disturbances, and increased startle responses. Although not technically part of the PTSD diagnosis, patients with PTSD often have problems with memory and concentration, as well as headaches and chronic pain.
Symptoms of PTSD are a manifestation of stress-related changes in neurobiology and the brain. Neurohormonal systems including cortisol and the hypothalamic-pituitary-adrenal (HPA) axis and the norepinephrine system, which play a critical role in the stress response, are perturbed in PTSD. Specifically, brain regions involved in learning and memory and the fear response, including the hippocampus, amygdala, and prefrontal cortex, were found to be affected in women with PTSD (Bremner, 2002; Bremner and Charney, 2010; Vermetten and Bremner, 2002a,b). It is not clear if these effects are specifically for women, since studies have not done direct comparisons between men and women with PTSD. However, other data suggest that stress may interact with gender to determine neurobiological responses. Normal men show greater cortisol responses to achievement challenges, while women have increased cortisol responses to social rejection (Stroud et al., 2002). Furthermore, brain imaging studies of healthy subjects have shown gender differences in neural correlates of emotional memory. These studies demonstrated increased activation of the posterior hippocampus and decreased medial prefrontal activation with retrieval of emotional words in women versus men (Bremner et al., 2001).
Differences in sex hormones between men and women also affect brain function and can interact with the stress response. The female sex hormone, estrogen, modulates neuronal spine density in the hippocampus (Gould et al., 1990; Woolley and McEwen, 1992, 1993, 1994) with variations shown over the course of the menstrual cycle. We have found that women with PTSD from childhood abuse have elevated estrogen levels measured in repeated plasma samples over a 24-h period (Bremner et al., 2007a). In summary, these studies demonstrate a possible interaction between stress and gender in the neurobiology of stress.
Another common consequence of stress, especially in early life, is depression. Major depressive disorder is characterized by depressed mood or loss of interest in things that last for over two weeks, with associated loss of appetite, decreased sleep, restlessness, agitation, crying spells, loss of energy, poor concentration, feelings of worthlessness and hopelessness, guilt, and sometimes suicidal thinking (American Psychiatric Association, 2013). Rates of depression are almost twice in women as in men (Alonso et al., 2004; Ayuso-Mateos et al., 2001; Kuehner, 2003), with a one-year prevalence of 8.1% in women and 5.1 in men (Substance Abuse and Mental Health Services Administration, 2014). Compared with men, women have more severe depression with an earlier age at onset and two fold increase in number of depressive episodes compared to men with depression (Sullivan et al., 2000). Increased rates of depression in women compared with men are seen in countries around the world (Weissman et al., 1996), suggesting that intrinsic factors may underlie the gender difference in depression.
Higher rates of anxiety disorders, which are 60% more common in women (Kessler et al., 2005a; Kessler et al., 2005b), are seen following psychological trauma, although their association with childhood trauma is not as strong as for depression and PTSD. Anxiety disorders include generalized anxiety disorder (GAD), panic disorder, obsessive-compulsive disorder, and phobic disorders. About 14–18% of adults develop an anxiety disorder at some time in their lives (Alonso et al., 2004; Kessler et al., 2005b). Other disorders linked to trauma include borderline personality disorder (BPD) and dissociative identity disorder (DID). BPD is more common in women and is accompanied by intense fears of abandonment, feelings of internal emptiness, swings of mood, dangerous and self-destructive behavior, and sometimes self-mutilation (Lieb et al., 2004; Schmahl & Bremner, 2006). DID is much more common in women than in men and is linked to severe early childhood trauma (Spiegel et al., 2011). It is characterized by distortion or fragmentation of identity, accompanied by gaps in memory (amnesia), distortions of the perception of one's body or feeling disconnected from the body (depersonalization), and distortions of perception (derealization).
3.2. Neurobiology
The hypothalamic-pituitary-adrenal (HPA) axis and the norepinephrine system play critical roles in the stress response. The HPA axis regulates metabolic changes in response to a stressor that are critical for survival in threatening circumstances. Corticotropin-releasing factor (CRF) is released from the paraventricular nucleus of the hypothalamus, which in turn stimulates release of adrenocorticotropin hormone (ACTH) from the pituitary. ACTH, when released into the circulation, causes an increase in cortisol release from the adrenal gland. CRF also acts in the brain to stimulate fear-related behaviors (Arborelius et al., 1999) and activates the noradrenergic neurotransmitter system via the brainstem's locus coeruleus as well as other stress-responsive systems (Melia and Duman, 1991). The norepinephrine system acts like the fire alarm of the brain, increasing fear and alertness, as well as stimulating respiratory and cardiovascular responses to stress (Bremner et al., 1996a,b; Nisenbaum et al., 1991).
Stress early in life is linked to long-term sensitization of the HPA axis and increases in CRF (Coplan et al., 1996; Levine et al., 1993; Makino et al., 1995; Plotsky and Meaney, 1993; Stanton et al., 1988). Stress also results in chronic over activation of the norepinephrine system, which plays a critical role in the fight or flight response (Bremner et al., 1996a,b).
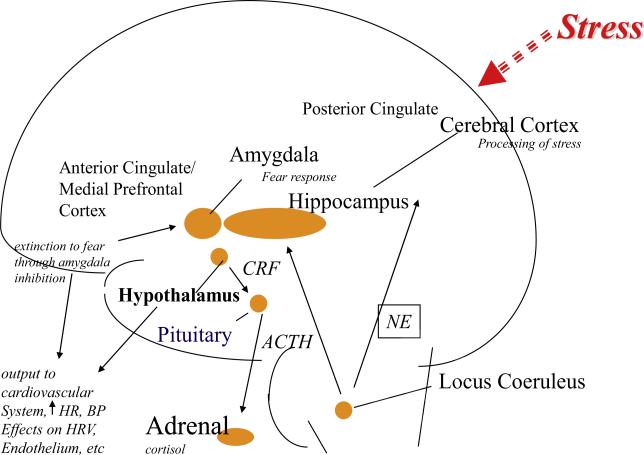
Stress affects many brain areas, including the hippocampus, medial prefrontal cortex, and amygdala (Bremner, 2005; Bremner and Charney, 2010; Bremner, 2011; Bremner and Vermetten, 2012; Rauch et al., 2006). The medial prefrontal cortex (which includes anterior cingulate and orbitofrontal cortex, and adjacent areas) is an important area involved in the link between stress and cardiovascular disease (Fig. 1). This brain area, in addition to the hypothalamus and insula, is unique in its role in the regulation of cardiovascular function through direct connections to brain stem areas (Diorio et al., 1993; Feldman et al., 1995; Frysztak and Neafsey, 1994). The medial prefrontal cortex is highly sensitive to stress (Arnsten, 2000) and plays an important role in the regulation of emotions and coping with environmental challenges (Devinsky et al., 1995; Pani et al., 2000). Fear extinction, or the inhibition of fear, is mediated by inputs from the medial prefrontal cortex to fear-learning center of the brain (Quirk et al., 2006). Animal studies show that early life stress is associated with a decrease in branching of neurons in the medial prefrontal cortex (Radley et al., 2004). No gender differences in this response have been shown. Altered function in medial prefrontal cortex affects outputs from this area which regulate physiological responses to stress in the periphery, with implications for cardiovascular risk.
Fig. 1.
Neurobiology of stress and cardiovascular function. Brain regions involved in stress and depression (amygdala, hippocampus, medial prefrontal cortex, posterior cingulate, anterior cingulate) have outputs directly (medial prefrontal cortex) or indirectly through the hypothalamus and the medial prefrontal cortex to neurohormonal systems (cortisol, norepinephrine) affected by stress and depression. These pathways mediate increased heart rate and blood pressure, decreased heart rate variability, changes in platelet aggregation and endothelial function, conferring risk of coronary heart disease. BP: blood pressure; HR: heart rate; HRV: heart rate variability; CRF: corticotropin releasing factor; ACTH: adrenocorticotropic hormone; NE: noradrenaline.
Studies in women with early life stress and stress-spectrum disorders like depression and PTSD have corroborated the changes in brain and neurobiology found in preclinical studies (Bremner, 2005; Bremner and Vaccarino, 2015). Girls with early life stress with associated PTSD and/or depression show increased cortisol levels and/or blunted ACTH response to CRF (which indicates down-regulation due to chronic hypercortisolemia) (Cicchetti and Rogosch, 2001; De Bellis et al., 1994, 1999; Gunnar et al., 2001) as well as increased cortisol response to stress (Luby et al., 2003). Notably, these changes in neurobiology persist into adulthood especially if there is PTSD (Bremner, 2005; Bremner et al., 2008; Bremner and Vaccarino, 2015). Women with PTSD secondary to childhood sexual abuse show decreased baseline cortisol based on 24-hour diurnal assessments, with flattening of the normal diurnal cortisol curve and increased pulsatility of cortisol reflecting dysregulation of CRF release (Bremner et al., 2007a). Women with PTSD also have increased cortisol response to stressors, especially trauma-specific stressors (Elzinga et al., 2003). They also show enhanced suppression of cortisol with low dose dexamethasone, suggesting increased sensitivity of glucocorticoid receptors (Stein et al., 1997), and blunted ACTH response to CRF (Heim et al., 2001).
In summary, trauma early in life results in increased hypercortisolemia, while with the transition to adulthood the HPA axis becomes dysregulated at baseline, with a hyper-responsive reaction to stress. Studies suggest that specific psychological conditions, like social rejection, lead to greater cortisol responses in women compared to men, while men may be more sensitive to stress related to achievement difficulties. Depression and PTSD are also associated with increased noradrenergic function in women, as in men (Bremner et al., 1996a,b), including increased baseline concentrations of norepinephrine and epinephrine in 24-h urine. As a whole, these findings are consistent with long-term alterations in neurobiological function in women with stress, depression and PTSD and may have implications for cardiovascular disease.
4. Emotional factors and coronary heart disease in women
4.1. Acute stress
Stressful exposures can contribute to cardiovascular morbidity and mortality even if they do not result in stress-related mental disorders. Acute stressors, such as bouts of anger, bereavement, or intense emotions like fear or extreme excitement can cause MI or sudden death (Bhattacharyya and Steptoe, 2007; Mostofsky et al., 2012, 2013; Steptoe and Kivimaki, 2013). Both enhanced and blunted cardiovascular reactivity to an acute stressor predict future cardiovascular diseases or CHD risk factors, suggesting that abnormal regulation of cardiovascular responses to stress may be an underlying mechanism (Chida and Steptoe, 2010; Phillips et al., 2013; Treiber et al., 2003).
Stress can be reproduced in the laboratory under controlled conditions using standardized methodology, such as performing arithmetic problems or giving a public speech, a procedure known as mental stress testing. In patients with CHD, mental stress can induce coronary blood flow imbalances causing a transitory perfusion deficit at the time of stress, a phenomenon known as mental stress-induced myocardial ischemia (MSIMI). MSIMI occurs in approximately one third to half of patients with CHD and correlates with myocardial ischemia in daily life (Holmes et al., 2006; Strike and Steptoe, 2003). The prognosis for patients with MSIMI is similar than that of patients with exercise or exertion induced myocardial ischemia (Wei et al., 2014b) and is not related to the severity of coronary atherosclerosis (Ramadan et al., 2013).
Although most studies of mental stress ischemia were performed predominantly in men, emerging data show that MSIMI is more common in women than in men. In a study of stable CHD patients, women had a 39% higher incidence of MSIMI assessed by ventricular wall motion using echocardiography compared with men (Samad et al., 2014). Recent studies using single-photon emission tomography (SPECT) myocardial perfusion imaging to detect ischemia during mental stress showed that young women (50 years or younger) with a recent history of MI had twice the rate of mental stress ischemia compared with age-matched men with previous MI, while older men and women showed no difference (Vaccarino et al., 2014). These results were not explained by severity of disease, traditional cardiovascular risk factors, or even behavioral or psychosocial factors, hinting to intrinsic biological differences between women and men underlying a propensity towards MSIMI.
It is not clear why younger women have higher rates of myocardial ischemia with mental stress than men of the same age or older women. The women in these studies were more likely to have psychosocial risk factors for cardiovascular disease, including lower income, minority race, history of emotional or sexual abuse in childhood, and higher number of depressive symptoms (Samad et al., 2014; Vaccarino et al., 2014; Xu et al., 2015). This psychosocial profile may result in increased physiological responsivity to acute stressors, which is particularly well captured using myocardial perfusion imaging during stress (Burg and Soufer, 2014). MSIMI in women does not appear to be related to a greater cardiovascular reactivity, however, as no differences were found blood pressure, heart rate and other hemodynamic parameters between women and men, with men even showing a tendency for higher responses with stress (Becker et al., 1996; Samad et al., 2014; Steptoe et al., 1996; Vaccarino et al., 2014; York et al., 2007). Women, however, appear to have more subjective distress and negative emotions than men with mental stress, which may explain their higher rate of MSIMI at least partially (Samad et al., 2014). They also show an increase in negative emotions with non-psychological stressors, such as an inflammatory challenge with endotoxin, even though the inflammatory response is similar (Moieni et al., 2015). The similar physiological response to an inflammatory stimulus in women compared with men in this experimental study parallels the finding that young women with a previous history of MI show the same inflammatory response to mental stress as age-matched men; however, women exhibit double the level of interleukin-6 (IL-6) than men, both at baseline and at 90 min after the stress challenge (Rooks et al., 2016). Even in absence of CHD, women exhibit higher levels of C-reactive protein, a commonly studied inflammatory marker, compared with men of similar age, starting at age 16, potentially due to the onset of menarche (Ford et al., 2003; Wener et al., 2000; Woloshin and Schwartz, 2005). Data on IL-6 and other biomarkers, such as fibrinogen, are more mixed, but still there is a tendency for young and middle-aged women of European or African descent to show higher levels than men in the same age bracket (Coe et al., 2011; Gruenewald et al., 2009), although this is not a uniform finding (Cartier et al., 2009). Thus, even though women do not exhibit a higher immune reactivity to stress compared with men, it is possible that the high absolute levels of inflammation reached during stressful events pose young women above a threshold of risk for abnormal vascular responses and future ischemic events. Alternatively, women could react differently than men to the testing environment itself.
Another possible explanation for the increase in MSIMI in women is differences in vasomotor tone. A working model, supported by emerging data, proposes that MSIMI is at least in part caused by coronary microcirculatory dysfunction, due to a failure of small coronary arteries to dilate during stress (Ghiadoni et al., 2000; Kop et al., 2001; Pepine et al., 2014; Ramadan et al., 2013). Coronary microvascular dysfunction refers to abnormal vasomotor regulation of the small coronary arterioles (<200 μm in diameter), which regulate coronary vascular resistance but are not visualized by coronary angiography. Coronary microvascular dysfunction may precede the development of clinical manifestations of CHD and bears independent prognostic value (Kaul and Ito, 2004). Peripheral endothelial dysfunction, defined as an imbalance between vasodilating and vasoconstricting mediators regulating the endothelium and influencing vasomotor tone, can also be implicated (Deanfield et al., 2005). Coronary and peripheral vascular dysfunction may play a role in MSIMI through sympathetic nervous system activation via adrenergic receptors in the vascular system. Women show a tendency towards abnormal vasomotion and microvascular dysfunction, to the point that this phenomenon has been proposed as a possible mechanism of ischemic heart disease in women (Pepine et al., 2010; von Mering et al., 2004; Wong et al., 2002). Coronary microvascular dysfunction is commonly seen in women with chest pain, even in the absence of significant coronary obstruction (Buchthal et al., 2000; Reis et al., 2001). It is also possible that peripheral vasoconstriction during stress, with an attendant rise in afterload, plays a role in MSIMI. Indeed, MSIMI is associated with an increase in systemic vascular resistance (Burg et al., 2009; Ramadan et al., 2013). Due to their propensity to vasomotor reactivity, it is possible that women exhibit more microvascular dysregulation with stress, as suggested by the fact that young women have more endothelial dysfunction with mental stress than men (Martin et al., 2008). The higher prevalence of MSIMI in women may also be secondary to the fact that women report more emotional distress and mood disturbances during daily life than men. Since these disturbances have been linked to ambulatory ischemia and MSIMI (Burg et al., 2014; Gullette et al., 1997a; Wei et al., 2014a), it is possible that repeated, cumulative distress in everyday life leads to a chronic form of microvascular diastolic dysfunction which is present at baseline and predicts MSIMI (Ersboll et al., 2014). All these potential vascular effects may be accentuated in young women given their higher baseline levels of inflammation (Rooks et al., 2016). Thus, the fact that vascular dysfunction is an important mechanism of MSIMI could explain a female predominance of this phenomenon.
4.2. Early life stress
Early life stress has been linked to an increased risk for cardiovascular disease in adulthood in women. In the Nurses’ Health Study 2, childhood abuse was associated with an increased risk of cardiovascular disease of 46% for physical abuse, and 56% for sexual abuse. This was significant after adjusting for other risk factors for cardiovascular disease (Rich-Edwards et al., 2012). A meta-analysis confirmed a link between childhood abuse and cardiovascular disease (Wegman and Stetler, 2009).
Early trauma is a stronger predictor of cardiovascular disease in younger women than it is in similarly aged men (Batten et al., 2004; Korkeila et al., 2010). Sexual abuse, in particular, was associated with a fivefold increase in self-reported cardiovascular events in the previous 12 months among adult women younger than 55 years (Goodwin and Stein, 2004), while no association was found among men. In a Finnish community sample of 23,916 individuals less than 55 years of age at baseline, there was a progressive increase in heart disease risk among women (but not among men) with successive higher numbers of childhood traumatic events (Korkeila et al., 2010). Among women, having three or more events was associated with a threefold increased risk, after adjusting for demographic and behavioral factors.
Studies suggest that a partial explanation for the association between early trauma and cardiovascular disease lies in behavioral and lifestyle factors associated with this exposure (Rich-Edwards et al., 2012). For example, childhood abuse is associated with an increase in smoking rates, even in the absence of psychiatric disorders (Roberts et al., 2008). Early trauma may lead to these behavioral profiles through persistent changes in the brain and physiological systems (Danese and McEwen, 2012).
In summary, there is emerging evidence that early trauma increases cardiovascular risk in adulthood and this association may be especially pronounced for younger (i.e. pre-menopausal) women.
4.3. Depression
As mentioned above, depression is twice as common in women as in men in the general population. Although cardiac patients as a whole have a 2–3 fold increase in prevalence of depression compared to the general population, rates are still doubled in younger female cardiac patients compared to men (Mallik et al., 2006; Vaccarino et al., 2014). Fifty percent of younger women (age 50 years and younger) with a history of MI suffer from for major depression compared to 30% of similarly aged men (Vaccarino et al., 2014). Depression is an important risk factor for CHD in women, increasing a woman's risk by at least 50% (Mehta et al., 2016), and a recognized prognostic factor among CHD patients (Lichtman et al., 2014).
There are several possible explanations for the higher prevalence of depression in young women with CHD. Young women with severe medical illnesses may be more vulnerable to the development of depression. For example, young women with a recent MI have a higher level of inflammation than men of similar age or older patients (Rooks et al., 2016), which in turn may predispose them towards developing depression. The high prevalence of depression might also be explained by the fact that depression is a strong risk factor for CHD in young/middle aged women (as compared to men and older populations). Although the literature on gender differences in depression as a risk factor for CHD is mixed, when young/middle-aged populations were studied, a larger effect of depression in women than men was usually observed. In the Third National Health and Nutrition Examination Survey (NHANES III), women under age 40 with either major depression or attempted suicide had more than threefold the adjusted risk of cardiovas cular death (a hazards ratio [HR] of 3.2) and almost 15-fold the adjusted risk of CHD death (HR 14.6), while for men these risks were increased respectively by 2.4 and 3.5 (Shah et al., 2011). The proportion of risk for CHD from depression in these women was 65% compared to 13% for men, and higher than the attributable risk for traditional cardiovascular risk factors. In the prospective Community Mental Health Epidemiology Study of Washington County, MD, depression increased cardiovascular risk in women younger than 40 years more than six fold, while no association was found among men (Wyman et al., 2012). Other studies showed a 2-fold risk of carotid plaques assessed with ultrasound in younger women with recurrent depression (Jones et al., 2003). Depression in adolescence and young adulthood is also a predictor of type II diabetes in women only (Suglia et al., 2016). As a whole, these data suggest that depression is a powerful predictor of cardiometabolic risk in young women, and potentially a stronger predictor than in men of similar age.
Depression is also a strong predictor of poor prognosis in women with CHD. Women in the Women's Ischemia Syndrome Evaluation (WISE) study with depression requiring treatment had a three-fold higher risk of death and cardiac events relative to women without depression (Rutledge et al., 2006). Another study showed a 3.3 fold increased cardiac mortality in women with depression (Frasure-Smith and Lesperance, 2008). However, available data indicate that men and women with CHD, all ages combined, are equally susceptible to the adverse effects of depression on prognosis (Frasure-Smith et al., 1999b; Parashar et al., 2009). Yet it is possible that depression is an especially strong prognostic factor for young women with CHD, who show disproportionately high mortality compared with men of the same age after a MI (Mehta et al., 2016). This needs further study.
4.4. Anxiety disorders
Data have been inconsistent regarding the association between anxiety (either anxiety symptom scales or anxiety disorders) and CHD. About half of the studies have failed to find a significant association. A meta-analysis pooled the results of approximately 20 longitudinal studies and found a modest (26%) increased risk of incident CHD and a 48% increased risk of cardiac death for persons with elevated anxiety symptoms or the diagnosis of an anxiety disorder, even after controlling for risk factors for heart disease (Roest et al., 2010). Although data are limited and the findings from available studies show considerable heterogeneity, there does appear to be a pattern of a stronger link between anxiety symptoms and CHD risk in in men than in women, both for incident CHD (Eaker et al., 2005; Ringback Weitoft and Rosen, 2005), and for recurrent CHD events (Frasure-Smith et al., 1999a; Frasure-Smith and Lesperance, 2008). Among women, however, recent studies have linked panic attacks (Smoller et al., 2007) and phobic anxiety (Albert et al., 2005) with incident CHD and sudden cardiac death. Notably, there is not as strong a relationship between exposure to psychological trauma, especially in childhood, and anxiety disorders as there is for PTSD and depression. This may explain why the link between anxiety disorders and CHD risk in women is less strong than for depression and PTSD. Furthermore, the overall divergence of results may be due to the fact anxiety disorders represent a heterogeneous group, which may differ in their biological substrate, and thus in their relationship with CHD, in women and men.
4.5. Post-traumatic stress disorder
The prospective association between PTSD and CHD has been examined in only five studies. Four of these investigations included predominantly male veterans and found a significant association between PTSD symptoms or a PTSD diagnosis with fatal or nonfatal CHD events (Ahmadi et al., 2011; Boscarino, 2008; Kubzansky et al., 2007; Vaccarino et al., 2013b), as well as objective indicators of coronary heart disease such as coronary artery calcium (Ahmadi et al., 2011), myocardial perfusion defects (Vaccarino et al., 2013b), or other indicators of myocardial ischemia (Turner et al., 2013). Only two prospective studies have examined women. Among 1059 women free of CHD at baseline in the Baltimore cohort of the Epidemiologic Catchment Area study, those with five or more symptoms of PTSD had over threefold higher risk of CHD compared with those without PTSD symptoms, even after controlling for risks factors, depression and anxiety (Kubzansky et al., 2009). In the Nurses’ Health Study II, women who endorsed ≥4 PTSD symptoms had a 60% higher risk of cardiovascular events after adjusting for demographic factors and family history (hazard ratio, 1.60; 95% confidence interval, 1.20–2.13). However, women exposed to trauma and endorsing no PTSD symptoms also showed an elevated cardiovascular disease risk (hazard ratio, 1.45; 95% confidence interval, 1.15–1.83), while those who were exposed to trauma and endorsing 1 to 3 PTSD symptoms were not (Sumner et al., 2015). These studies suggest that exposure to psychological trauma in women increases the risk of heart disease, possibly even in the absence of PTSD, although the presence of the disorder likely increases the risk additively to trauma exposure. Interestingly, the results suggest that resilience in the face of trauma could be protective for the development of heart disease in women.
5. Potential mechanisms
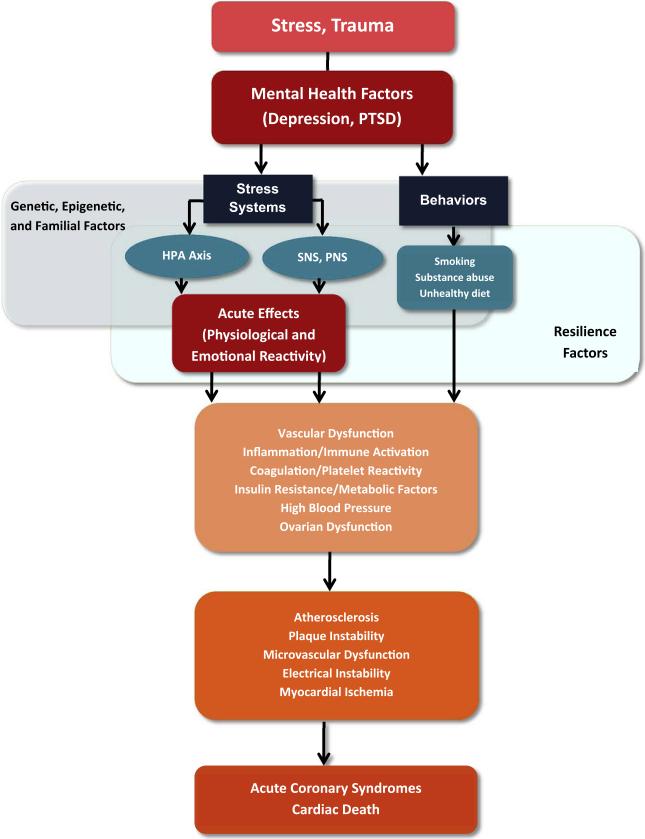
There are multiple possible mechanisms linking stress, emotional factors, and stress-related mental disorders with increased CHD risk in women (Fig. 2). Behavioral and lifestyle factors are strongly related with stress-related psychopathology and with CHD risk, but do not appear to explain entirely the connection between stress/stress-related disorders and CHD. Depression, childhood trauma, and PTSD are all linked to unhealthy behaviors such as smoking, sedentary lifestyle, delay in seeking treatment and lower adherence to treatment and prevention recommendations (Breslau et al., 2003; Carney et al., 1995; Whooley et al., 2008). Childhood sexual abuse in women is specifically associated with obesity in adulthood, even after controlling for behavioral risk factors linked to obesity.
Fig. 2.
Diagram summarizing mechanisms linking behavioral and emotional factors to coronary heart disease in women. Neurobiological pathways affected by stressful exposures and stress-related mental disorders have downstream vascular, immune, metabolic and reproductive effects that, in turn, affect cardiovascular risk pathways. There is also the influence of health behaviors and the cumulative effects of acute stressful exposures resulting in repeated physiological and emotional reactivity. Genetic/epigenetic factors and individual resilience factors modulate these responses. HPA: Hypothalamic-Pituitary-Adrenal; SNS: Sympathetic Nervous System; PNS: Parasympathetic Nervous System.
Alterations in neurohormonal stress response systems following exposure to chronic stress, as described above, represent plausible mechanisms by which stress is linked to CHD. One model states that repeated activation of the sympathetic nervous system and parasympathetic nervous system withdrawal with stress leads to long-term dysregulation of these systems contributing to elevations in blood pressure, heart rate, plasma glucose, insulin resistance and dyslipidemia.
Increased inflammation, endothelial dysfunction and hypercoagulability may also play a role (McEwen, 2008). Inflammatory processes are linked to a hypercoagulable state and to endothelial dysfunction, which are mechanisms for CHD. Animal studies demonstrate a causal link between stress and inflammation. Stress-induced norepinephrine release activates the transcription factor NF-kB in circulating monocytes, initiating the inflammatory cascade (Bierhaus et al., 2003), with potentially long lasting abnormalities in immune function. These findings appear to apply to human populations as well. For example, in a prospective study, maltreated children showed higher inflammation 20 years later, which persisted after accounting for other childhood exposures and health behaviors (Danese et al., 2007). Depression and PTSD have also been associated with higher levels of inflammatory biomarkers and evidence of immune dysregulation (Empana et al., 2005; Hoge et al., 2009; Plantinga et al., 2013; Spitzer et al., 2010; Uddin et al., 2010; Vaccarino et al., 2007; von Känel et al., 2007).
Enhanced platelet activity has long been proposed as a potential link between depression and cardiac events, but data are limited and results mixed (von Kanel, 2004). Endothelial dysfunction in depression has also been described (Sherwood et al., 2005), and limited evidence links PTSD symptom severity to endothelium-derived circulating proteins and blood clotting factors (Plantinga et al., 2013; von Kanel et al., 2006, 2008).
Growing evidence suggests that some psychosocial factors, particularly depression, may share genetic pathways with CHD (Mulle and Vaccarino, 2013). This implies that depression and CHD could be different phenotypic expressions of the same genetic substrate (de Geus, 2006; Kendler et al., 2009; Vaccarino et al., 2009b), an effect that may be more true for women than men (Kendler et al., 2009). In the Swedish twin registry, onset of cardiovascular disease predicted future depression risk, and, in turn, onset of depression predicted future cardiovascular disease risk, supporting a model of common factors contributing to the risk for both disorders. However, for males, shared genetic factors contributed to the depression-cardiovascular disease comorbidity in younger age groups only. In contrast, among women common genetic factors contributed to this comorbidity across all age groups (Kendler et al., 2009). Notably, genetic influences on depression are stronger in females compared to males; the heritability of depression has been estimated at 42% in women but only 29% in men (Kendler et al., 2001, 2006). Thus, as a whole, the above data suggest that the link between depression and CHD in women is more often attributable to genetic factors than in men. Genes related to inflammation, platelet aggregation, and the serotonin system may be especially relevant to the comorbidity of depression and cardiovascular disease. Consistent with this, an haplotype in the leukotriene A4 hydrolase gene, encoding the potent inflammatory mediators leukotrienes, showed pleiotropy for depression and coronary artery disease severity in women only (Zhao et al., 2009). Clearly, more studies are needed in this area.
Epigenetic mechanisms may also be implicated, since variations in DNA methylation are involved in the etiology of various mental disorders, including depression (Fuchikami et al., 2010) and PTSD (Uddin et al., 2010), but also play a role in the pathophysiology cardiovascular disease (Baccarelli et al., 2010). DNA methylation provides a potential biological basis for gene–environment interactions relevant to stress, mental health and chronic diseases, for example, interactions between genes and stress exposure (Fraga et al., 2005; McGowan et al., 2009; Mehta et al., 2013; Unternaehrer et al., 2012; Wong et al., 2010). Epigenetics may also underlie modifications in gene expression that may affect multiple phenotypes, such as mental health and cardiovascular disease.
Psychosocial factors may increase CHD risk through acute effects by inducing silent ischemia, triggering acute coronary syndromes, or heightening cardiovascular reactivity in daily life. For example, depressive mood can trigger ischemia detected by electrocardiographic ambulatory monitoring in patients with CHD (Gullette et al., 1997b), and acute bereavement or bursts of anger can trigger acute MI (Mostofsky et al., 2012, 2013). These effects may be secondary to a surge in sympathetic nervous system activation, with sudden increase in myocardial oxygen demand or abnormal coronary vasomotion which may result in myocardial ischemia, cardiac arrhythmias, or coronary death.
There is also evidence that certain psychological traits are associated with myocardial ischemia induced by mental stress. Emotional reactivity in everyday life, as well as anxiety and depression, have been associated with myocardial ischemia during mental stress but, in general, not with exercise-induced ischemia (Boyle et al., 2013; Burg et al., 2014; Carels et al., 1999; Wei et al., 2014a). Aggressive responding, hostile affect, and trait and state anger also have been associated with MSIMI (Burg et al., 1993; Pimple et al., 2015).
Finally, reproductive function may represent an additional mechanism by which stress increases CHD risk in women. Over 20% of women have some form of ovarian disruption during their reproductive years, which often goes unrecognized, and stress is one of the most common causes of this (Berga, 2008). Epidemiologic data in young women, similar to experimental data in non-human primates, support the notion that even mild ovarian insufficiency exacerbates coronary atherosclerosis and CHD (Kaplan and Manuck, 2008). Thus, women under severe stress or with stress-related psychopathology during their reproductive years may enter a trajectory of higher cardiovascular risk due, to some extent, to ovarian dysfunction. The fact that several psychosocial factors are most predictive of CHD in younger women and that MSIMI is more common in this group suggest that the specific milieu of premenopausal women could play a role (Vaccarino et al., 2014).
Stress-induced early menarche (before age 12) represents yet another mechanism by which stress may increase risk for cardiovascular disease in women (Campbell and Udry, 1995). Early menarche is also associated with increased risk for metabolic syndrome and other cardiovascular disease risk factors in girls and young women, and with cardiovascular disease later in life (Lakshman et al., 2009). The mechanisms are not clear, and early menarche could just be a marker of childhood obesity, an important correlate of early puberty. Late menarche (after age 14) is also associated with increased cardiovascular risk (Lakshman et al., 2009).
6. Summary and Conclusions
The work reviewed in this chapter shows important gender differences in both the frequency of psychosocial risk factors and their relationship with the risk of CHD. Women have double the rates of stress-related psychiatric disorders, including PTSD and depression, compared to men. These disorders are clearly associated with CHD risk in women. In contrast, anxiety disorders, which are not as closely linked to stress, do not show a consistent relationship with cardiovascular disease risk in women.
The evidence for a link between stress, especially early trauma, mental disorders and risk of CHD is especially strong among young women. Thus, psychosocial risk factors, in particular depression, early life stress and PTSD, may be key in defining a path of vulnerability for CHD early in women's lives. Future studies should examine women in earlier developmental epochs in order to better understand mechanisms linking stress to CHD risk in women and help develop better intervention and prevention strategies.
If heart disease in younger women shows more of a pattern of endothelial dysfunction and altered vasomotor reactivity due to stress, as emerging data suggest, then different approaches to treatment should be tailored for them. Most psychosocial interventions or drug treatment trials for depression have not shown a benefit for improving CHD outcomes, and this lack of efficacy was especially true for women (Baumeister et al., 2011; Linden et al., 2007). The Enhancing Recovery in Coronary Heart Disease (ENRICHD) randomized trial, for example, which tested the efficacy of cognitive behavioral therapy for depression (supplemented by antidepressant medications) and lack of social support in reducing recurrent cardiac events in patients with coronary heart disease, showed a tendency for the intervention to benefit men but a tendency to harm women (Berkman et al., 2003). These results are reminiscent of the earlier M-Hart trial, where a behavioral intervention focusing on psychological stress after MI did not have any effect among men but worsened mortality among women (Frasure-Smith et al., 1997). In the more recent Bypassing the Blues clinical trial, a telephone-delivered collaborative care intervention for depression after bypass surgery significantly improved mental and physical health outcomes in men but was null among women (Rollman et al., 2009). Thus, at least among patients with heart disease, there appears to be a differential response to behavioral interventions for women compared with men in terms of physical health benefits. Hence, attention should be given to psychosocial pathways specific for women in order to address CHD risk in this group. Psychosocial interventions specifically designed to address women's stressors could be the most helpful approach in the prevention and treatment of CHD in women (Orth-Gomer, 2012).
Footnotes
The work presented in this review was supported by grants from the National Institutes of Health (K24 HL077506, K24 MH076955, R01 HL68630, R01 AG026255, R21 HL093665, R01 HL109413, R01 MH056120, R01 HL088726, and P01 HL 101398) and by the National Center for Advancing Translational Sciences funded by the NIH (UL1TR000454).
References
- Adverse Childhood Experiences Reported by Adults—Five States, 2009. MMWR. Morb. Mortal. Wkly. Rep. 2010;59(49):1609–1613. [PubMed] [Google Scholar]
- Ahmadi N, Hajsadeghi F, Mirshkarlo HB, Budoff M, Yehuda R, Ebrahimi R. Post-traumatic stress disorder, coronary atherosclerosis, and mortality. Am. J. Cardiol. 2011;108(1):29–33. doi: 10.1016/j.amjcard.2011.02.340. [DOI] [PubMed] [Google Scholar]
- Albert CM, Chae CU, Rexrode KM, Manson JE, Kawachi I. Phobic anxiety and risk of coronary heart disease and sudden cardiac death among women. Circulation. 2005;111(4):480–487. doi: 10.1161/01.CIR.0000153813.64165.5D. [DOI] [PubMed] [Google Scholar]
- Alonso J, Angermeyer MC, Bernert S, Bruffaerts R, Brugha TS, Bryson H, de Girolamo G, Graaf R, Demyttenaere K, Gasquet I, Haro JM, Katz SJ, Kessler RC, Kovess V, Lepine JP, Ormel J, Polidori G, Russo LJ, Vilagut G, Almansa J, Arbabzadeh-Bouchez S, Autonell J, Bernal M, Buist-Bouwman MA, Codony M, Domingo-Salvany A, Ferrer M, Joo SS, Martinez-Alonso M, Matschinger H, Mazzi F, Morgan Z, Morosini P, Palacin C, Romera B, Taub N, Vollebergh WA, EsemeD/Mhedea Investigators, European Study of the Epidemiology of Mental Disorders Project Prevalence of mental disorders in Europe: results from the European study of the epidemiology of mental disorders (ESEMeD) project. Acta Psychiatr. Scand. Suppl. 2004;420:21–27. doi: 10.1111/j.1600-0047.2004.00327.x. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 5th ed. American Psychiatric Association; Arlington, VA: 2013. [Google Scholar]
- Arborelius L, Owens MJ, Plotsky PM, Nemeroff CB. The role of corticotropin-releasing factor in depression and anxiety disorders. J. Endocrinol. 1999;160:1–12. doi: 10.1677/joe.0.1600001. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Stress impairs prefrontal cortical function in rats and monkeys: role of dopamine D1 and norepinephrine alpha-1 receptor mechanisms. Prog. Brain Res. 2000;126:183–192. doi: 10.1016/S0079-6123(00)26014-7. [DOI] [PubMed] [Google Scholar]
- Ayuso-Mateos JL, Vazquez-Barquero JL, Dowrick C, Lehtinen V, Dalgard OS, Casey P, Wilkinson C, Lasa L, Page H, Dunn G, Wilkinson G, Group, Odin. Depressive disorders in Europe: prevalence figures from the ODIN study. Br. J. Psychiatry. 2001;179:308–316. doi: 10.1192/bjp.179.4.308. [DOI] [PubMed] [Google Scholar]
- Baccarelli A, Rienstra M, Benjamin EJ. Cardiovascular epigenetics: basic concepts and results from animal and human studies. Circ. Cardiovasc. Genet. 2010;3(6):567–573. doi: 10.1161/CIRCGENETICS.110.958744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bangalore S, Fonarow GC, Peterson ED, Hellkamp AS, Hernandez AF, Laskey W, Peacock WF, Cannon CP, Schwamm LH, Bhatt DL, Get with the Guidelines Steering, Committee, & Investigators Age and gender differences in quality of care and outcomes for patients with ST-segment elevation myocardial infarction. Am. J. Med. 2012;125(10):1000–1009. doi: 10.1016/j.amjmed.2011.11.016. [DOI] [PubMed] [Google Scholar]
- Batten SV, Aslan M, Maciejewski PK, Mazure CM. Childhood maltreatment as a risk factor for adult cardiovascular disease and depression. J. Clin. Psychiatry. 2004;65:249–254. doi: 10.4088/jcp.v65n0217. [DOI] [PubMed] [Google Scholar]
- Baumeister H, Hutter N, Bengel J. Psychological and pharmacological interventions for depression in patients with coronary artery disease. Cochrane Database Syst. Rev. 2011;9:CD008012. doi: 10.1002/14651858.CD008012.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker LC, Pepine CJ, Bonsall R, Cohen JD, Goldberg AD, Coghlan C, Stone PH, Forman S, Knatterud G, Sheps DS, Kaufmann PG. Left ventricular, peripheral vascular, and neurohumoral responses to mental stress in normal middle-aged men and women. Reference group for the psychophysiological investigations of myocardial ischemia (PIMI) study. Circulation. 1996;94(11):2768–2777. doi: 10.1161/01.cir.94.11.2768. [DOI] [PubMed] [Google Scholar]
- Beckie TM, Fletcher G, Groer MW, Kip KE, Ji M. Biopsychosocial health disparities among young women enrolled in cardiac rehabilitation. J. Cardiopulm. Rehabil. Prev. 2015;35(2):103–113. doi: 10.1097/HCR.0000000000000095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berga SL, Loucks TL. The diagnosis and treatment of stress-induced anovulation. Minerva Ginecol. 2005;57(1):45–54. [PubMed] [Google Scholar]
- Berga SL. Stress and reprodution: a tale of false dichotomy? Endocrinology. 2008;149(3):867–868. doi: 10.1210/en.2008-0004. [DOI] [PubMed] [Google Scholar]
- Berkman LF, Blumenthal J, Burg M, Carney RM, Catellier D, Cowan MJ, Czajkowski SM, DeBusk R, Hosking J, Jaffe A, Kaufmann PG, Mitchell P, Norman J, Powell LH, Raczynski JM, Schneiderman N, Enhancing Recovery in Coronary Heart Disease Patients, Investigators Effects of treating depression and low perceived social support on clinical events after myocardial infarction: the enhancing recovery in coronary heart disease patients (ENRICHD) randomized trial. JAMA. 2003;289(23):3106–3116. doi: 10.1001/jama.289.23.3106. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya MR, Steptoe A. Emotional triggers of acute coronary syndromes: strength of evidence, biological processes, and clinical implications. Prog. Cardiovasc. Dis. 2007;49(5):353–365. doi: 10.1016/j.pcad.2006.11.002. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc. Natl. Acad. Sci. U. S. A. 2003;100(4):1920–1925. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boscarino JA. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom. Med. 2008;70(6):668–676. doi: 10.1097/PSY.0b013e31817bccaf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle SH, Samad Z, Becker RC, Williams R, Kuhn C, Ortel TL, Kuchibhatla M, Prybol K, Rogers J, O'Connor C, Velazquez EJ, Jiang W. Depressive symptoms and mental stress-induced myocardial ischemia in patients with coronary heart disease. Psychosom. Med. 2013;75(9):822–831. doi: 10.1097/PSY.0b013e3182a893ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner D, Vermetten E, Kelley ME. Cortisol, dehydroepiandrosterone, and estradiol measured over 24 hours in women with childhood sexual abuse-related posttraumatic stress disorder. J. Nerv. Ment. Dis. 2007a;195(11):919–927. doi: 10.1097/NMD.0b013e3181594ca0. [DOI] [PubMed] [Google Scholar]
- Bremner J, Bolus R, Mayer E. Psychometric properties of the early trauma inventory-Self report. J. Nerv. Ment. Dis. 2007b;195(3):211–218. doi: 10.1097/01.nmd.0000243824.84651.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD. The neurobiology of childhood sexual abuse in women with posttraumatic stress disorder. In: Kendall-Tackett KA, editor. Handbook of Women, Stress and Trauma. Brunner-Routledge; New York: 2005. pp. 181–206. [Google Scholar]
- Bremner JD, Elzinga B, Schmahl C, Vermetten E. Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog. Brain Res. 2008;167:171–186. doi: 10.1016/S0079-6123(07)67012-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: I. Preclinical studies. Synapse. 1996a;23:28–38. doi: 10.1002/(SICI)1098-2396(199605)23:1<28::AID-SYN4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, Charney DS. Noradrenergic mechanisms in stress and anxiety: II. Clinical studies. Synapse. 1996b;23:39–51. doi: 10.1002/(SICI)1098-2396(199605)23:1<39::AID-SYN5>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Southwick SM, Darnell A, Charney DS. Chronic PTSD in Vietnam combat veterans: course of illness and substance abuse. Am. J. Psychiatry. 1996c;153:369–375. doi: 10.1176/ajp.153.3.369. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Vermetten E, Mazure CM. Development and preliminary psychometric properties of an instrument for the measurement of childhood trauma: the early trauma inventory. Depress. Anxiety. 2000;12:1–12. doi: 10.1002/1520-6394(2000)12:1<1::AID-DA1>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bremner JD, Soufer R, McCarthy G, Delaney RC, Staib LH, Duncan JS, Charney DS. Gender differences in cognitive and neural correlates of remembrance of emotional words. Psychopharmacol. Bull. 2001;35:55–87. [PubMed] [Google Scholar]
- Bremner JD. Does Stress Damage the Brain? Understanding Trauma-related Disorders from a Mind-Body Perspective. W.W. Norton; New York: 2002. [Google Scholar]
- Bremner JD, Charney DS. Neural circuits in fear and anxiety. In: Stein DJ, Hollander E, Rothbaum BO, editors. Textbook of Anxiety Disorders. 2 ed. American Psychiatric Publishing; Arlington, VA: 2010. pp. 55–71. [Google Scholar]
- Bremner JD. Stress and human neuroimaging studies. In: Conrad CD, editor. The Handbook of Stress: Neuropsychological Effects on the Brain. Blackwell Press; 2011. [Google Scholar]
- Bremner JD, Vermetten E. The hippocampus and post-traumatic stress disorders. In: Bartsch T, editor. The Clinical Neurobiology of the Hippocampus: An Integrative View. Oxford University Press; 2012. pp. 262–272. [Google Scholar]
- Bremner JD, Vaccarino V. Neurobiology of early life stress in women. In: Orth-Gomér K, Schneiderman N, Vaccarino V, Hans-Christian D, editors. Psychosocial Stress and Cardiovascular Disease in Women: Concepts, Findings, Future Perspectives. Springer International Publishing; 2015. pp. 161–168. [Google Scholar]
- Breslau N, Davis GC, Schultz LR. Posttraumatic stress disorder and the incidence of nicotine, alcohol, and other drug disorders in persons who have experienced trauma. Arch. Gen. Psychiatry. 2003;60(3):289–294. doi: 10.1001/archpsyc.60.3.289. [DOI] [PubMed] [Google Scholar]
- Brown DW, Anda RF, Tiemeier H, Felitti VJ, Edwards VJ, Croft JB, Giles WH. Adverse childhood experiences and the risk of premature mortality. Am. J. Prev. Med. 2009;37(5):389–396. doi: 10.1016/j.amepre.2009.06.021. [DOI] [PubMed] [Google Scholar]
- Buchthal SD, den Hollander JA, Merz CN, Rogers WJ, Pepine CJ, Reichek N, Sharaf BL, Reis S, Kelsey SF, Pohost GM. Abnormal myocardial phosphorus-31 nuclear magnetic resonance spectroscopy in women with chest pain but normal coronary angiograms. N. Engl. J. Med. 2000;342(12):829–835. doi: 10.1056/NEJM200003233421201. [DOI] [PubMed] [Google Scholar]
- Bugiardini, Raffaele, Merz Bairey, Noel C. Angina with normal coronary arteries: a changing philosophy. JAMA. 2005;293(4):477–484. doi: 10.1001/jama.293.4.477. [DOI] [PubMed] [Google Scholar]
- Burg MM, Jain D, Soufer R, Kerns RD, Zaret BL. Role of behavioral and psychological factors in mental stress-induced silent left ventricular dysfunction in coronary artery disease. J. Am. Coll. Cardiol. 1993;22(2):440–448. doi: 10.1016/0735-1097(93)90048-6. [DOI] [PubMed] [Google Scholar]
- Burg MM, Graeber B, Vashist A, Collins D, Earley C, Liu J, Lampert R, Soufer R. Noninvasive detection of risk for emotion-provoked myocardial ischemia. Psychosom. Med. 2009;71(1):14–20. doi: 10.1097/PSY.0b013e318187c035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MM, Meadows J, Shimbo D, Davidson KW, Schwartz JE, Soufer R. Confluence of depression and acute psychological stress among patients with stable coronary heart disease: effects on myocardial perfusion. J. Am. Heart Assoc. 2014;3(6) doi: 10.1161/JAHA.114.000898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg MM, Soufer R. Psychological stress and induced ischemic syndromes. Curr. Cardiovasc. Risk Rep. 2014;8(4):377. doi: 10.1007/s12170-014-0377-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell BC, Udry JR. Stress and age at menarche of mothers and daughters. J. Biosoc. Sci. 1995;27(2):127–134. doi: 10.1017/s0021932000022641. [DOI] [PubMed] [Google Scholar]
- Carels RA, Sherwood A, Babyak M, Gullette EC, Coleman RE, Waugh R, Jiang W, Blumenthal JA. Emotional responsivity and transient myocardial ischemia. J. Consult. Clin. Psychol. 1999;67(4):605–610. doi: 10.1037//0022-006x.67.4.605. [DOI] [PubMed] [Google Scholar]
- Carney RM, Freedland KE, Eisen SA, Rich MW, Jaffe AS. Major depression and medication adherence in elderly patients with coronary artery disease. Health Psychol. 1995;14:88–90. doi: 10.1037//0278-6133.14.1.88. [DOI] [PubMed] [Google Scholar]
- Cartier A, Cote M, Lemieux I, Perusse L, Tremblay A, Bouchard C, Despres JP. Sex differences in inflammatory markers: what is the contribution of visceral adiposity? Am. J. Clin. Nutr. 2009;89(5):1307–1314. doi: 10.3945/ajcn.2008.27030. [DOI] [PubMed] [Google Scholar]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension. 2010;55(4):1026–1032. doi: 10.1161/HYPERTENSIONAHA.109.146621. [DOI] [PubMed] [Google Scholar]
- Chrousos GP, Torpy DJ, Gold PW. Interactions between the hypothalamic-pituitary-adrenal axis and the female reproductive system: clinical implications. Ann. Intern. Med. 1998;129(3):229–240. doi: 10.7326/0003-4819-129-3-199808010-00012. [DOI] [PubMed] [Google Scholar]
- Cicchetti D, Rogosch FA. The impact of child maltreatment and psychopathology on neuroendocrine functioning. Dev. Psychopathol. 2001;13:783–804. [PubMed] [Google Scholar]
- Coe CL, Love GD, Karasawa M, Kawakami N, Kitayama S, Markus HR, Tracy RP, Ryff CD. Population differences in proinflammatory biology: Japanese have healthier profiles than Americans. Brain. Behav. Immun. 2011;25(3):494–502. doi: 10.1016/j.bbi.2010.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coplan JD, Andrews MW, Rosenblum LA, Owens MJ, Friedman S, Gorman JM, Nemeroff CB. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc. Natl. Acad. Sci. U. S. A. 1996;93:1619–1623. doi: 10.1073/pnas.93.4.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc. Natl. Acad. Sci. U. S. A. 2007;104(4):1319–1324. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol. Behav. 2012;106(1):29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Chrousos GP, Dorn LD, Burke L, Helmers K, Kling MA, Trickett PK, Putnam FW. Hypothalamic pituitary adrenal dysregulation in sexually abused girls. J. Clin. Endocrinol. Metab. 1994;78:249–255. doi: 10.1210/jcem.78.2.8106608. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Baum AS, Keshavan MS, Eccard CH, Boring AM, Jenkins FJ, Ryan ND. A.E. Bennett research award: developmental traumatology: part I: biological stress systems. Biol. Psychiatry. 1999;45:1259–1270. doi: 10.1016/s0006-3223(99)00044-x. [DOI] [PubMed] [Google Scholar]
- de Geus EJ. Genetic pleiotropy in depression and coronary artery disease. Psychosom. Med. 2006;68(2):185–186. doi: 10.1097/01.psy.0000208628.90274.bc. [DOI] [PubMed] [Google Scholar]
- Deanfield J, Donald A, Ferri C, Giannattasio C, Halcox J, Halligan S, Lerman A, Mancia G, Oliver JJ, Pessina AC, Rizzoni D, Rossi GP, Salvetti A, Schiffrin EL, Taddei S, Webb DJ. Endothelial function and dysfunction. Part I: methodological issues for assessment in the different vascular beds: a statement by the working group on endothelin and endothelial factors of the European society of hypertension. J. Hypertens. 2005;23(1):7–17. doi: 10.1097/00004872-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate to behavior. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J. Neurosci. 1993;13(9):3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube SR, Felitti VJ, Dong M, Giles WH, Anda RF. The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev. Med. 2003;37:268–277. doi: 10.1016/s0091-7435(03)00123-3. [DOI] [PubMed] [Google Scholar]
- Eaker ED, Sullivan LM, Kelly-Hayes M, D'Agostino RB, Sr., Benjamin EJ. Tension and anxiety and the prediction of the 10-year incidence of coronary heart disease, atrial fibrillation, and total mortality: the Framingham offspring study. Psychosom. Med. 2005;67(5):692–696. doi: 10.1097/01.psy.0000174050.87193.96. [DOI] [PubMed] [Google Scholar]
- Elzinga BM, Schmahl CS, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology. 2003;28(9):1656–1665. doi: 10.1038/sj.npp.1300226. [DOI] [PubMed] [Google Scholar]
- Empana JP, Sykes DH, Luc G, Juhan-Vague I, Arveiler D, Ferrieres J, Amouyel P, Bingham A, Montaye M, Ruidavets JB, Haas B, Evans A, Jouven X, Ducimetiere P, Prime Study Group Contributions of depressive mood and circulating inflammatory markers to coronary heart disease in healthy European men: the prospective epidemiological study of myocardial infarction (PRIME). Circulation. 2005;111(18):2299–2305. doi: 10.1161/01.CIR.0000164203.54111.AE. [DOI] [PubMed] [Google Scholar]
- Ersboll M, Al Enezi F, Samad Z, Sedberry B, Boyle SH, O'Connor C, Jiang W, Velazquez EJ, Remit Investigators Impaired resting myocardial annular velocities are independently associated with mental stress-induced ischemia in coronary heart disease. J. Am. Coll. Cardiol. Imaging. 2014;7(4):351–361. doi: 10.1016/j.jcmg.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman S, Conforti N, Weidenfeld J. Limbic pathways and hypothalamic neurotransmitters mediating adrenocortical responses to neural stimuli. Neurosci. Biobehav. Rev. 1995;19(2):235–240. doi: 10.1016/0149-7634(94)00062-6. [DOI] [PubMed] [Google Scholar]
- Ferin M. Clinical review 105: stress and the reproductive cycle. J. Clin. Endocrinol. Metab. 1999;84(6):1768–1774. doi: 10.1210/jcem.84.6.5367. [DOI] [PubMed] [Google Scholar]
- Ford ES, Giles WH, Myers GL, Rifai N, Ridker PM, Mannino DM. C-reactive protein concentration distribution among US children and young adults: findings from the national health and nutrition examination survey, 1999–2000. Clin. Chem. 2003;49(8):1353–1357. doi: 10.1373/49.8.1353. [DOI] [PubMed] [Google Scholar]
- Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. U. S. A. 2005;102(30):10604–10609. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression, and one-year prognosis after myocardial infarction. Psychosom. Med. 1999a;61(1):26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F, Juneau M, Talajic M, Bourassa MG. Gender, depression and one-year prognosis after myocardial infarction. Psychosom. Med. 1999b;61(1):26–37. doi: 10.1097/00006842-199901000-00006. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith N, Lesperance F. Depression and anxiety as predictors of 2-year cardiac events in patients with stable coronary artery disease. Arch. Gen. Psychiatry. 2008;65(1):62–71. doi: 10.1001/archgenpsychiatry.2007.4. [DOI] [PubMed] [Google Scholar]
- Frasure-Smith, Nancy, Lespérance, Francois, Prince, Raymond H, Verrier, Pierre, Garber, Rachel A, Juneau, Martin, Wolfson, Christina, Bourassa, Martial G. Randomised trial of home-based psychosocial nursing intervention for patients recovering from myocardial infarction. Lancet. 1997;350(9076):473–479. doi: 10.1016/S0140-6736(97)02142-9. [DOI] [PubMed] [Google Scholar]
- Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Yamamoto S, Morinobu S, Takei S, Yamawaki S. Epigenetic regulation of BDNF gene in response to stress. Psychiatry Invest. 2010;7(4):251–256. doi: 10.4306/pi.2010.7.4.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiss LS, Wang J, Cheng YJ, Thompson TJ, Barker L, Li Y, Albright AL, Gregg EW. Prevalence and incidence trends for diagnosed diabetes among adults aged 20–79 years, United States, 1980–2012. JAMA. 2014;312(12):1218–1226. doi: 10.1001/jama.2014.11494. [DOI] [PubMed] [Google Scholar]
- Ghiadoni, Lorenzo, Donald, Ann E, Cropley, Mark, Mullen, Michael J, Oakley, Gillian, Taylor, Mia, O'Connor, Georgina, Betteridge, John, Klein, Nigel, Steptoe, Andrew, Deanfield, John E. Mental stress induces transient endothelial dysfunction in humans. Circulation. 2000;102(20):2473–2478. doi: 10.1161/01.cir.102.20.2473. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Magid D, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, Moy CS, Mussolino ME, Nichol G, Paynter NP, Schreiner PJ, Sorlie PD, Stein J, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. American Heart Association Statistics, Committee, & Stroke Statistics, Subcommittee. Heart Dis. Stroke. 2013;127(1):e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein BI, Carnethon MR, Matthews KA, McIntyre RS, Miller GE, Raghuveer G, Stoney CM, Wasiak H, McCrindle BW. Major depressive disorder and bipolar disorder predispose youth to accelerated atherosclerosis and early cardiovascular disease: a scientific statement from the american heart association. Circulation. 2015a doi: 10.1161/CIR.0000000000000229. [DOI] [PubMed] [Google Scholar]
- Goldstein BI, Schaffer A, Wang S, Blanco C. Excessive and premature new-onset cardiovascular disease among adults with bipolar disorder in the US NESARC cohort. J. Clin. Psychiatry. 2015b;76(2):163–169. doi: 10.4088/JCP.14m09300. [DOI] [PubMed] [Google Scholar]
- Goodwin RD, Stein MB. Association between childhood trauma and physical disorders among adults in the United States. Psychol. Med. 2004;34(3):509–520. doi: 10.1017/s003329170300134x. [DOI] [PubMed] [Google Scholar]
- Gould E, Woolley CS, Frankfurt M, McEwen BS. Gonadal steroids regulate dendritic spine density in hippocampal pyramidal cells in adulthood. J. Neurosci. 1990;10(4):1286–1291. doi: 10.1523/JNEUROSCI.10-04-01286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruenewald TL, Cohen S, Matthews KA, Tracy R, Seeman TE. Association of socioeconomic status with inflammation markers in black and white men and women in the coronary artery risk development in young adults (CARDIA) study. Soc. Sci. Med. 2009;69(3):451–459. doi: 10.1016/j.socscimed.2009.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gullette EC, Blumenthal JA, Babyak M, Jiang W, Waugh RA, Frid DJ, O'Connor CM, Morris JJ, Krantz DS. Effects of mental stress on myocardial ischemia during daily life. JAMA. 1997a;277(19):1521–1526. [PubMed] [Google Scholar]
- Gullette EC, Blumenthal JA, Babyak M, Jiang W, Waugh RA, Frid DJ, O'Connor CM, Morris JJ, Krantz DS. Effect of mental stress on myocardial ischemia during daily life. JAMA. 1997b;277:1521–1526. [PubMed] [Google Scholar]
- Gunnar MR, Morison SJ, Chisolm K, Schuder M. Salivary cortisol levels in children adopted from Romanian orphanages. Dev. Psychopathol. 2001;13:611–628. doi: 10.1017/s095457940100311x. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Spertus JA, Geda M, Lorenze N, Nkonde-Price C, D'Onofrio G, Lichtman JH, Krumholz HM. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001–2010. J. Am. Coll. Cardiol. 2014;64(4):337–345. doi: 10.1016/j.jacc.2014.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim C, Newport DJ, Bonsall R, Miller AH, Nemeroff CB. Altered pituitary-adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress. Anxiety. 2009;26(5):447–455. doi: 10.1002/da.20564. [DOI] [PubMed] [Google Scholar]
- Holmes, Sari D, Krantz, David S, Rogers, Heather, Gottdiener, John, Contrada, Richard J. Mental stress and coronary artery disease: a multidisciplinary guide. Prog. Cardiovasc. Dis. 2006;49(2):106–122. doi: 10.1016/j.pcad.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Huang KL, Su TP, Chen TJ, Chou YH, Bai YM. Comorbidity of cardiovascular diseases with mood and anxiety disorder: a population based 4-year study. Psychiatry Clin. Neurosci. 2009;63(3):401–409. doi: 10.1111/j.1440-1819.2009.01974.x. [DOI] [PubMed] [Google Scholar]
- Izadnegahdar M, Singer J, Lee MK, Gao M, Thompson CR, Kopec J, Humphries KH. Do younger women fare worse? Sex differences in acute myocardial infarction hospitalization and early mortality rates over ten years. J. Womens Health (Larchmt) 2014;23(1):10–17. doi: 10.1089/jwh.2013.4507. [DOI] [PubMed] [Google Scholar]
- Jones DJ, Bromberger JT, Sutton-Tyrrell K, Matthews KA. Lifetime history of depression and carotid atherosclerosis in middle-aged women. Arch. Gen. Psychiatry. 2003;60:153–160. doi: 10.1001/archpsyc.60.2.153. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB, Anthony MS, Clarkson TB. Premenopausal social status and hormone exposure predict postmenopausal atherosclerosis in female monkeys. Obstet. Gynecol. 2002;99(3):381–388. doi: 10.1016/s0029-7844(01)01659-3. [DOI] [PubMed] [Google Scholar]
- Kaplan JR, Manuck SB. Ovarian dysfunction and the premenopausal origins of coronary heart disease. Menopause. 2008;15(4 Pt 1):768–776. doi: 10.1097/gme.0b013e31815eb18e. [DOI] [PubMed] [Google Scholar]
- Kaplan, Jay R, Chen, Haiying, Manuck, Stephen B. The relationship between social status and atherosclerosis in male and female monkeys as revealed by meta-analysis. Am. J. Primatol. 2009;71(9):732–741. doi: 10.1002/ajp.20707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul, Sanjiv, Ito, Hiroshi Microvasculature in acute myocardial ischemia: part I: evolving concepts in pathophysiology, diagnosis, and treatment. Circulation. 2004;109(2):146–149. doi: 10.1161/01.CIR.0000111582.02736.CD. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Neale MC, Prescott CA. Genetic risk factors for major depression in men and women: similar or different heritabilities and same or partly distinct genes? Psychol. Med. 2001;31(4):605–616. doi: 10.1017/s0033291701003907. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gatz M, Gardner CO, Pedersen NL. A Swedish national twin study of lifetime major depression. Am. J. Psychiatry. 2006;163(1):109–114. doi: 10.1176/appi.ajp.163.1.109. [DOI] [PubMed] [Google Scholar]
- Kendler, Kenneth S, Gardner, Charles O, Fiske, Amy, Margaret M. Major depression and coronary artery disease in the Swedish Twin Registry: phenotypic, genetic, and environmental sources of comorbidity. Arch. Gen. Psychiatry. 2009;66(8):857–863. doi: 10.1001/archgenpsychiatry.2009.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005a;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the national comorbidity survey replication. Arch. Gen. Psychiatry. 2005b;62(6):617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the national comorbidity survey. Arch. Gen. Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kop WJ, Krantz DS, Howell RH, Ferguson MA, Papademetriou V, Lu D, Popma JJ, Quigley JF, Vernalis M, Gottdiener JS. Effects of mental stress on coronary epicardial vasomotion and flow velocity in coronary artery disease: relationship with hemodynamic stress responses. J. Am. Coll. Cardiol. 2001;37(5):1359–1366. doi: 10.1016/s0735-1097(01)01136-6. [DOI] [PubMed] [Google Scholar]
- Korkeila J, Vahtera J, Korkeila K, Kivimaki M, Sumanen M, Koskenvuo K, Koskenvuo M. Childhood adversities as predictors of incident coronary heart disease and cerebrovascular disease. Heart. 2010;96(4):298–303. doi: 10.1136/hrt.2009.188250. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Spiro A, 3rd, Vokonas PS, Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the normative aging study. Arch. Gen. Psychiatry. 2007;64(1):109–116. doi: 10.1001/archpsyc.64.1.109. [DOI] [PubMed] [Google Scholar]
- Kubzansky LD, Koenen KC, Jones C, Eaton WW. A prospective study of posttraumatic stress disorder symptoms and coronary heart disease in women. Health Psychol. 2009;28(1):125–130. doi: 10.1037/0278-6133.28.1.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehner C. Gender differences in unipolar depression: an update of epidemiological findings and possible explanations. Acta Psychiatr. Scand. 2003;108(3):163–174. doi: 10.1034/j.1600-0447.2003.00204.x. [DOI] [PubMed] [Google Scholar]
- Ladabaum U, Mannalithara A, Myer PA, Singh G. Obesity, abdominal obesity, physical activity, and caloric intake in US adults: 1988–2010. Am. J. Med. 2014;127(8):717–727. e712. doi: 10.1016/j.amjmed.2014.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshman R, Forouhi NG, Sharp SJ, Luben R, Bingham SA, Khaw KT, Wareham NJ, Ong KK. Early age at menarche associated with cardiovascular disease and mortality. J. Clin. Endocrinol. Metab. 2009;94(12):4953–4960. doi: 10.1210/jc.2009-1789. [DOI] [PubMed] [Google Scholar]
- Lehto HR, Lehto S, Havulinna AS, Ketonen M, Lehtonen A, Kesaniemi YA, Airaksinen KJ, Salomaa V. Sex differences in short- and long-term case-fatality of myocardial infarction. Eur. J. Epidemiol. 2011;26(11):851–861. doi: 10.1007/s10654-011-9601-6. [DOI] [PubMed] [Google Scholar]
- Levine S, Weiner SG, Coe CL. Temporal and social factors influencing behavioral and hormonal responses to separation in mother and infant squirrel monkeys. Psychoneuroendocrinology. 1993;4:297–306. doi: 10.1016/0306-4530(93)90026-h. [DOI] [PubMed] [Google Scholar]
- Lichtman JH, Froelicher ES, Blumenthal JA, Carney RM, Doering LV, Frasure-Smith N, Freedland KE, Jaffe AS, Leifheit-Limson EC, Sheps DS, Vaccarino V, Wulsin L, American Heart Association Statistics Committee of the Council on Epidemiology and Prevention and the Council on Cardiovascular and Stroke Nursing Depression as a risk factor for poor prognosis among patients with acute coronary syndrome: systematic review and recommendations: a scientific statement from the American heart association. Circulation. 2014;129(12):1350–1369. doi: 10.1161/CIR.0000000000000019. [DOI] [PubMed] [Google Scholar]
- Lieb K, Zanarini M, Schmahl C, Linehan M, Bohus M. Borderline personality disorder. Lancet. 2004;364:453–461. doi: 10.1016/S0140-6736(04)16770-6. [DOI] [PubMed] [Google Scholar]
- Linden, Wolfgang, Phillips, Jayne Melanie, Leclerc, Jocelyne Psychological treatment of cardiac patients: a meta-analysis. Eur. Heart J. 2007;28(24):2972–2984. doi: 10.1093/eurheartj/ehm504. [DOI] [PubMed] [Google Scholar]
- Low CA, Thurston RC, Matthews KA. Psychosocial factors in the development of heart disease in women: current research and future directions. Psychosom. Med. 2010;72(9):842–854. doi: 10.1097/PSY.0b013e3181f6934f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby JL, Heffelfinger A, Mrakotsky C, Brown K, Hessler M, Spitznagel E. Alterations in stress cortisol reactivity in depressed preschoolers relative to psychiatric and no-disorder comparison groups. Arch. Gen. Psychiatry. 2003;60(12):1248–1255. doi: 10.1001/archpsyc.60.12.1248. [DOI] [PubMed] [Google Scholar]
- MacMillan HL, Fleming JE, Trocme N, Boyle MH, Wong M, Racine YA, Beardslee WR, Offord DR. Prevalence of child physical and sexual abuse in the community: results from the Ontario health supplement. J. Am. Med. Assoc. 1997;278:131–135. [PubMed] [Google Scholar]
- Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger-ribonucleic acid (messenger RNA) in the hypothalamic paraventricular nucleus during repeated stress-association with reduction in glucocorticoid messenger-RNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- Mallik S, Spertus JA, Reid KJ, Krumholz HM, Rumsfeld JS, Weintraub WS, Agarwal P, Santra M, Bidyasar S, Lichtman JH, Wenger NK, Vaccarino V. Depressive symptoms after acute myocardial infarction: evidence for highest rates in younger women. Arch. Intern. Med. 2006;166(8):876–883. doi: 10.1001/archinte.166.8.876. [DOI] [PubMed] [Google Scholar]
- Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil. Steril. 2001;76(2):310–316. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- Martin EA, Tan SL, MacBride LR, Lavi S, Lerman LO, Lerman A. Sex differences in vascular and endothelial responses to acute mental stress. Clin. Auton. Res. 2008;18(6):339–345. doi: 10.1007/s10286-008-0497-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Bushnell MC, editors. IASP Press; Seattle, WA: 2009. [Google Scholar]
- McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, Ryden J, Derogatis LR, Bass EG. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. J. Am. Med. Assoc. 1997;277:1362–1368. [PubMed] [Google Scholar]
- McEwen, Bruce S. Central effects of stress hormones in health and disease: understanding the protective and damaging effects of stress and stress mediators. Eur. J. Pharmacol. 2008;583(2–3):174–185. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonte B, Szyf M, Turecki G, Meaney MJ. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12(3):342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, Pace TW, Rex-Haffner M, Loeschner A, Gonik M, Mercer KB, Bradley B, Muller-Myhsok B, Ressler KJ, Binder EB. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl. Acad. Sci. U. S. A. 2013 doi: 10.1073/pnas.1217750110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta LS, Beckie TM, DeVon HA, Grines CL, Krumholz HM, Johnson MN, Lindley KJ, Vaccarino V, Wang TY, Watson KE, Wenger NK. Acute myocardial infarction in women: a scientific statement from the american heart association. Circulation. 2016 doi: 10.1161/CIR.0000000000000351. [DOI] [PubMed] [Google Scholar]
- Melia KR, Duman RS. Involvement of corticotropin-releasing factor in chronic stress regulation of the brain noradrenergic system. Proc. Natl. Acad. Sci. U. S. A. 1991;88:8382–8386. doi: 10.1073/pnas.88.19.8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moieni M, Irwin MR, Jevtic I, Olmstead R, Breen EC, Eisenberger NI. Sex differences in depressive and socioemotional responses to an inflammatory challenge: implications for sex differences in depression. Neuropsychopharmacology. 2015;40(7):1709–1716. doi: 10.1038/npp.2015.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E, Maclure M, Sherwood JB, Tofler GH, Muller JE, Mittleman MA. Risk of acute myocardial infarction after the death of a significant person in one's life: the determinants of myocardial infarction onset study. Circulation. 2012;125(3):491–496. doi: 10.1161/CIRCULATIONAHA.111.061770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostofsky E, Maclure M, Tofler GH, Muller JE, Mittleman MA. Relation of outbursts of anger and risk of acute myocardial infarction. Am. J. Cardiol. 2013;112(3):343–348. doi: 10.1016/j.amjcard.2013.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulle JG, Vaccarino V. Cardiovascular disease, psychosocial factors, and genetics: the case of depression. Prog. Cardiovasc. Dis. 2013;55(6):557–562. doi: 10.1016/j.pcad.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Comorbidity Survey NCS-R Appendix tables: Table 1. Lifetime prevalence of DSM-IV/WMH-CIDI disorders by sex and cohort. Table 2. Twelve-month prevalence of DSM-IV/WMH-CIDI disorders by sex and cohort. 2005 Accessed at: http://www.hcp.med.harvard.edu/ncs/publications.php.
- Nisenbaum LK, Zigmond MJ, Sved AF, Abercrombie ED. Prior exposure to chronic stress results in enhanced synthesis and release of hippocampal norepinephrine in response to a novel stressor. J. Neurosci. 1991;11:1478–1484. doi: 10.1523/JNEUROSCI.11-05-01478.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orth-Gomer K. Behavioral interventions for coronary heart disease patients. Biopsychosoc. Med. 2012;6(1):5. doi: 10.1186/1751-0759-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L, Porcella A, Gessa GL. The role of stress in the pathophysiology of the dopaminergic system. Mol. Psychiatry. 2000;5:14–21. doi: 10.1038/sj.mp.4000589. [DOI] [PubMed] [Google Scholar]
- Parashar S, Rumsfeld JS, Reid KJ, Buchanan D, Dawood N, Khizer S, Lichtman JH, Vaccarino V. Impact of depression on sex differences in outcome after myocardial infarction. Circ. Cardiovasc. Qual. Outcomes. 2009;2(1):33–40. doi: 10.1161/CIRCOUTCOMES.108.818500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepine CJ, Petersen JW, Bairey Merz CN. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. J. Am. Coll. Cardiol. Img. 2014;7(4):362–365. doi: 10.1016/j.jcmg.2013.11.009. [DOI] [PubMed] [Google Scholar]
- Pepine, Carl J, Anderson, David R, Sharaf, Barry L, Reis, Steven E, Smith, Karen M, Handberg, Eileen M, Johnson, Delia B, Sopko, George, Merz Bairey, Noel C. Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia: results from the National Heart, Lung and Blood Institute WISE (Women's Ischemia Syndrome Evaluation) study. J. Am. Coll. Cardiol. 2010;55(25):2825–2832. doi: 10.1016/j.jacc.2010.01.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips AC, Ginty AT, Hughes BM. The other side of the coin: blunted cardiovascular and cortisol reactivity are associated with negative health outcomes. Int. J. Psychophysiol. 2013;90(1):1–7. doi: 10.1016/j.ijpsycho.2013.02.002. [DOI] [PubMed] [Google Scholar]
- Pimple P, Shah A, Rooks C, Bremner JD, Nye J, Ibeanu I, Murrah N, Shallenberger L, Kelley M, Raggi P, Vaccarino V. Association between anger and mental stress-induced myocardial ischemia. Am. Heart J. 2015;169(1):115–121. e112. doi: 10.1016/j.ahj.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantinga L, Bremner JD, Miller AH, Jones DP, Veledar E, Goldberg J, Vaccarino V. Association between posttraumatic stress disorder and inflammation: a twin study. Brain. Behav. Immun. 2013;30:125–132. doi: 10.1016/j.bbi.2013.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotsky PM, Meaney MJ. Early, postnatal experience alters hypothalamic corticotropin-releasing factor (CRF) mRNA, median eminence CRF content and stress-induced release in adult rats. Mol. Brain Res. 1993;18(3):195–200. doi: 10.1016/0169-328x(93)90189-v. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol. Psychiatry. 2006;60(4):337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125(1):1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Ramadan R, Sheps D, Esteves F, Zafari AM, Bremner JD, Vaccarino V, Quyyumi AA. Myocardial ischemia during mental stress: role of coronary artery disease burden and vasomotion. J. Am. Heart Assoc. 2013;2(5):e000321. doi: 10.1161/JAHA.113.000321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauch SL, Shin LM, Phelps EA. Neurocircuitry models of posttraumatic stress disorder and extinction: human neuroimaging research-past, present, and future. Biol. Psychiatry. 2006;60(4):376–382. doi: 10.1016/j.biopsych.2006.06.004. [DOI] [PubMed] [Google Scholar]
- Reis, Steven E, Holubkov, Richard, Smith, Conrad AJ, Kelsey, Sheryl F, Sharaf, Barry L, Reichek, Nathaniel, Rogers, William J, Merz, Noel C, Sopko, George, Pepine, Carl J. Coronary microvascular dysfunction is highly prevalent in women with chest pain in the absence of coronary artery disease: results from the NHLBI WISE study. Am. Heart J. 2001;141(5):735–741. doi: 10.1067/mhj.2001.114198. [DOI] [PubMed] [Google Scholar]
- Rich-Edwards JW, Mason S, Rexrode K, Spiegelman D, Hibert E, Kawachi I, Jun HJ, Wright RJ. Physical and sexual abuse in childhood as predictors of early-onset cardiovascular events in women. Circulation. 2012;126(8):920–927. doi: 10.1161/CIRCULATIONAHA.111.076877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringback Weitoft G, Rosen M. Is perceived nervousness and anxiety a predictor of premature mortality and severe morbidity? A longitudinal follow up of the Swedish survey of living conditions. J. Epidemiol. Community Health. 2005;59(9):794–798. doi: 10.1136/jech.2005.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ME, Fuemmeler BF, McClernon J, Beckham JC. Association between trauma exposure and smoking in a population-based sample of young adults. J. Adolesc. Health. 2008;42(3):266–274. doi: 10.1016/j.jadohealth.2007.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roest, Annelieke M, Martens, Elisabeth J, de Jonge, Peter, Denollet, Johan Anxiety and risk of incident coronary heart disease: a meta-analysis. J. Am. Coll. Cardiol. 2010;56(1):38–46. doi: 10.1016/j.jacc.2010.03.034. [DOI] [PubMed] [Google Scholar]
- Rollman BL, Belnap BH, LeMenager MS, Mazumdar S, Houck PR, Counihan PJ, Kapoor WN, Schulberg HC, Reynolds CF., 3rd. Telephone-delivered collaborative care for treating post-CABG depression: a randomized controlled trial. JAMA. 2009;302(19):2095–2103. doi: 10.1001/jama.2009.1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooks CR, Ibeanu I, Shah A, Pimple P, Murrah N, Shallenberger L, Pace T, Douglas Bremner J, Raggi P, Vaccarino V. Young women post-MI have higher plasma concentrations of interleukin-6 before and after stress testing. Brain. Behav. Immun. 2016;51:92–98. doi: 10.1016/j.bbi.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge T, Reis SE, Olson MB, Owens J, Kelsey SF, Pepine CJ, Mankad S, Rogers WJ, Merz CN, Sopko G, Cornell CE, Sharaf B, Matthews KA, Vaccarino V. Depression symptom severity and reported treatment history in the prediction of cardiac risk in women with suspected myocardial ischemia: the NHLBI-sponsored WISE study. Arch. Gen. Psychiatry. 2006;63(8):874–880. doi: 10.1001/archpsyc.63.8.874. [DOI] [PubMed] [Google Scholar]
- Samad Z, Boyle S, Ersboll M, Vora AN, Zhang Y, Becker RC, Williams R, Kuhn C, Ortel TL, Rogers JG, O'Connor CM, Velazquez EJ, Jiang W, Remit Investigators Sex differences in platelet reactivity and cardiovascular and psychological response to mental stress in patients with stable ischemic heart disease: insights from the REMIT study. J. Am. Coll. Cardiol. 2014;64(16):1669–1678. doi: 10.1016/j.jacc.2014.04.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmahl CG, Bremner JD. Neuroimaging in borderline personality disorder. J. Psychiatr. Res. 2006;40(5):419–427. doi: 10.1016/j.jpsychires.2005.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AJ, Veledar E, Hong Y, Bremner JD, Vaccarino V. Depression and history of attempted suicide as risk factors for heart disease mortality in young individuals. Arch. Gen. Psychiatry. 2011;68(11):1135–1142. doi: 10.1001/archgenpsychiatry.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah AJ, Ghasemzadeh N, Zaragoza-Macias E, Patel R, Eapen DJ, Neeland IJ, Pimple PM, Zafari AM, Quyyumi AA, Vaccarino V. Sex and age differences in the association of depression with obstructive coronary artery disease and adverse cardiovascular events. J. Am. Heart Assoc. 2014;3(3):e000741. doi: 10.1161/JAHA.113.000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwood A, Hinderliter AL, Watkins LL, Waugh RA, Blumenthal JA. Impaired endothelial function in coronary heart disease patients with depressive symptomatology. J. Am. Coll. Cardiol. 2005;46(4):656–659. doi: 10.1016/j.jacc.2005.05.041. [DOI] [PubMed] [Google Scholar]
- Shively CA, Register TC, Clarkson TB. Social stress, visceral obesity, and coronary artery atherosclerosis: product of a primate adaptation. Am. J. Primatol. 2009;71(9):742–751. doi: 10.1002/ajp.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skantze, Bjork Harriet, Kaplan, Jay, Pettersson, Knut, Manuck, Stephen, Blomqvist, Nils, Kyes, Randall, Williams, Koudy, Bondjers, Goran Psychosocial stress causes endothelial injury in cynomolgus monkeys via [beta]1-adrenoceptor activation. Atherosclerosis. 1998;136(1):153–161. doi: 10.1016/s0021-9150(97)00202-5. [DOI] [PubMed] [Google Scholar]
- Smolderen KG, Strait KM, Dreyer RP, D'Onofrio G, Zhou S, Lichtman JH, Geda M, Bueno H, Beltrame J, Safdar B, Krumholz HM, Spertus JA. Depressive symptoms in younger women and men with acute myocardial infarction: insights from the VIRGO study. J. Am. Heart Assoc. 2015;4(4) doi: 10.1161/JAHA.114.001424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smoller JW, Pollack MH, Wassertheil-Smoller S, Jackson RD, Oberman A, Wong ND, Sheps D. Panic attacks and risk of incident cardiovascular events among postmenopausal women in the women's health initiative observational study. Arch. Gen. Psychiatry. 2007;64(10):1153–1160. doi: 10.1001/archpsyc.64.10.1153. [DOI] [PubMed] [Google Scholar]
- Spiegel D, Loewenstein RJ, Lewis-Fernandez R, Sar V, Simeon D, Vermetten E, Cardena E, Dell PF. Dissociative disorders in DSM-5. Depress. Anxiety. 2011;28(9):824–852. doi: 10.1002/da.20874. [DOI] [PubMed] [Google Scholar]
- Spitzer C, Barnow S, Volzke H, Wallaschofski H, John U, Freyberger HJ, Lowe B, Grabe HJ. Association of posttraumatic stress disorder with low-grade elevation of C-reactive protein: evidence from the general population. J. Psychiatr. Res. 2010;44(1):15–21. doi: 10.1016/j.jpsychires.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Stanton ME, Gutierrez YR, Levine S. Maternal deprivation potentiates pituitary-adrenal stress responses in infant rats. Behav. Neurosci. 1988;102:692–700. doi: 10.1037//0735-7044.102.5.692. [DOI] [PubMed] [Google Scholar]
- Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexamethasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol. Psychiatry. 1997;42:680–686. doi: 10.1016/s0006-3223(96)00489-1. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Fieldman G, Evans O, Perry L. Cardiovascular risk and responsivity to mental stress: the influence of age, gender and risk factors. J. Cardiovasc. Risk. 1996;3(1):83–93. [PubMed] [Google Scholar]
- Steptoe A, Kivimaki M. Stress and cardiovascular disease: an update on current knowledge. Annu. Rev. Public Health. 2013;34:337–354. doi: 10.1146/annurev-publhealth-031912-114452. [DOI] [PubMed] [Google Scholar]
- Strawn WB, Bondjers G, Kaplan JR, Manuck SB, Schwenke DC, Hansson GK, Shively CA, Clarkson TB. Endothelial dysfunction in response to psychosocial stress in monkeys. Circ. Res. 1991;68(5):1270–1279. doi: 10.1161/01.res.68.5.1270. [DOI] [PubMed] [Google Scholar]
- Strike PC, Steptoe A. Systematic review of mental stress-induced myocardial ischaemia. Eur. Heart J. 2003;24(8):690–703. doi: 10.1016/s0195-668x(02)00615-2. [DOI] [PubMed] [Google Scholar]
- Stroud LR, Salovey P, Epel ES. Sex differences in stress responses: social rejection versus achievement stress. Biol. Psychiatry. 2002;52(4):318–327. doi: 10.1016/s0006-3223(02)01333-1. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2013 National Survey on Drug Use and Health: Mental Health Finding (Vol. NSDUH Series H-49, HHS Publication No. (SMA) 14-4887) Rockville, MD: 2014. [Google Scholar]
- Suglia SF, Demmer RT, Wahi R, Keyes KM, Koenen KC. Depressive symptoms during adolescence and young adulthood and the development of type 2 diabetes mellitus. Am. J. Epidemiol. 2016;183(4):269–276. doi: 10.1093/aje/kwv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am. J. Psychiatry. 2000;157(10):1552–1562. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- Sumner JA, Kubzansky LD, Elkind MS, Roberts AL, Agnew-Blais J, Chen Q, Cerda M, Rexrode KM, Rich-Edwards JW, Spiegelman D, Suglia SF, Rimm EB, Koenen KC. Trauma exposure and posttraumatic stress disorder symptoms predict onset of cardiovascular events in women. Circulation. 2015;132(4):251–259. doi: 10.1161/CIRCULATIONAHA.114.014492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom. Med. 2003;65(1):46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Turner JH, Neylan TC, Schiller NB, Li Y, Cohen BE. Objective evidence of myocardial ischemia in patients with posttraumatic stress disorder. Biol. Psychiatry. 2013 doi: 10.1016/j.biopsych.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M, Aiello AE, Wildman DE, Koenen KC, Pawelec G, de Los Santos R, Goldmann E, Galea S. Epigenetic and immune function profiles associated with posttraumatic stress disorder. Proc. Natl. Acad. Sci. U. S. A. 2010;107(20):9470–9475. doi: 10.1073/pnas.0910794107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S, Lieb R, Hellhammer DH, Meinlschmidt G. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl. Psychiatry. 2012;2:e150. doi: 10.1038/tp.2012.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Johnson BD, Sheps DS, Reis SE, Kelsey SF, Bittner V, Rutledge T, Shaw LJ, Sopko G, Bairey Merz CN. Depression, inflammation, and incident cardiovascular disease in women with suspected coronary ischemia: the National Heart, Lung, and Blood Institute-sponsored WISE study. J. Am. Coll. Cardiol. 2007;50(21):2044–2050. doi: 10.1016/j.jacc.2007.07.069. [DOI] [PubMed] [Google Scholar]
- Vaccarino V, Parsons L, Peterson ED, Rogers WJ, Kiefe CI, Canto J. Sex differences in mortality after acute myocardial infarction: changes from 1994 to 2006. Arch. Intern. Med. 2009a;169(19):1767–1774. doi: 10.1001/archinternmed.2009.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Votaw J, Faber T, Veledar E, Murrah NV, Jones LR, Zhao J, Su S, Goldberg J, Raggi JP, Quyyumi AA, Sheps DS, Bremner JD. Major depression and coronary flow reserve detected by positron emission tomography. Arch. Intern. Med. 2009b;169(18):1668–1676. doi: 10.1001/archinternmed.2009.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V. Ischemic heart disease in women: many questions, few facts. Circ. Cardiovasc. Qual. Outcomes. 2010;3(2):111–115. doi: 10.1161/CIRCOUTCOMES.109.925313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Hall A, Koller A, Marzilli M, Pries A, Bugiardini R, Working Group on Coronary, Pathophysiology, & Microcirculation Ischaemic heart disease in women: are there sex differences in pathophysiology and risk factors? Position paper from the working group on coronary pathophysiology and microcirculation of the European Society of Cardiology. Cardiovasc. Res. 2011;90(1):9–17. doi: 10.1093/cvr/cvq394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Badimon L, Corti R, de Wit C, Dorobantu M, Manfrini O, Koller A, Pries A, Cenko E, Bugiardini R. Presentation, management, and outcomes of ischaemic heart disease in women. Nat. Rev. Cardiol. 2013a;10(9):508–518. doi: 10.1038/nrcardio.2013.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Goldberg J, Rooks C, Shah AJ, Veledar E, Faber TL, Votaw JR, Forsberg CW, Bremner JD. Post-traumatic stress disorder and incidence of coronary heart disease: a twin study. J. Am. Coll. Cardiol. 2013b;62(11):970–978. doi: 10.1016/j.jacc.2013.04.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Shah AJ, Rooks C, Ibeanu I, Nye JA, Pimple P, Salerno A, D'Marco L, Karohl C, Bremner JD, Raggi P. Sex differences in mental stress-induced myocardial ischemia in young survivors of an acute myocardial infarction. Psychosom. Med. 2014;76(3):171–180. doi: 10.1097/PSY.0000000000000045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaccarino V, Bremner JD. Psychiatric and behavioral aspects of cardiovascular disease. In: Mann DL, Zipes DP, Libby P, Bonow RO, editors. Braunwald's Heart Disease—A Textbook of Cardiovascular Medicine. 10th ed. Elsevier-Saunders; Philadelphia, PA: 2015. [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress: I. Preclinical studies. Depress. Anxiety. 2002a;15:126–147. doi: 10.1002/da.10016. [DOI] [PubMed] [Google Scholar]
- Vermetten E, Bremner JD. Circuits and systems in stress: II. Applications to neurobiology and treatment of PTSD. Depress. Anxiety. 2002b;16:14–38. doi: 10.1002/da.10017. [DOI] [PubMed] [Google Scholar]
- von Kanel R. Platelet hyperactivity in clinical depression and the beneficial effect of antidepressant drug treatment: how strong is the evidence? Acta Psychiatr. Scand. 2004;110(3):163–177. doi: 10.1111/j.1600-0447.2004.00308.x. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Buddeberg C, Keel M, Mica L, Aschbacher K, Schnyder U. Altered blood coagulation in patients with posttraumatic stress disorder. Psychosom. Med. 2006;68(4):598–604. doi: 10.1097/01.psy.0000221229.43272.9d. [DOI] [PubMed] [Google Scholar]
- von Kanel R, Hepp U, Traber R, Kraemer B, Mica L, Keel M, Mausbach BT, Schnyder U. Measures of endothelial dysfunction in plasma of patients with posttraumatic stress disorder. Psychiatry Res. 2008;158(3):363–373. doi: 10.1016/j.psychres.2006.12.003. [DOI] [PubMed] [Google Scholar]
- von Känel, Roland, Hepp, Urs, Kraemer, Bernd, Traber, Rafael, Keel, Marius, Mica, Ladislav, Schnyder, Ulrich. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J. Psychiatr. Res. 2007;41(9):744–752. doi: 10.1016/j.jpsychires.2006.06.009. [DOI] [PubMed] [Google Scholar]
- von Mering GO, Arant CB, Wessel TR, McGorray SP, Bairey Merz CN, Sharaf BL, Smith KM, Olson MB, Johnson BD, Sopko G, Handberg E, Pepine CJ, Kerensky RA. Abnormal coronary vasomotion as a prognostic indicator of cardiovascular events in women: results from the National Heart, Lung, and Blood Institute-Sponsored Women's Ischemia Syndrome Evaluation (WISE). Circulation. 2004;109(6):722–725. doi: 10.1161/01.CIR.0000115525.92645.16. [DOI] [PubMed] [Google Scholar]
- Wegman, Holly L, Stetler, Cinnamon A meta-analytic review of the effects of childhood abuse on medical outcomes in adulthood. Psychosom. Med. 2009;71(8):805–812. doi: 10.1097/PSY.0b013e3181bb2b46. [DOI] [PubMed] [Google Scholar]
- Wei J, Pimple P, Shah AJ, Rooks C, Bremner JD, Nye JA, Ibeanu I, Murrah N, Shallenberger L, Raggi P, Vaccarino V. Depressive symptoms are associated with mental stress-induced myocardial ischemia after acute myocardial infarction. PloS One. 2014a;9(7):e102986. doi: 10.1371/journal.pone.0102986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Rooks C, Ramadan R, Shah AJ, Bremner JD, Quyyumi AA, Kutner M, Vaccarino V. Meta-analysis of mental stress-induced myocardial ischemia and subsequent cardiac events in patients with coronary artery disease. Am. J. Cardiol. 2014b;114(2):187–192. doi: 10.1016/j.amjcard.2014.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK. Cross-national epidemiology of major depression and bipolar disorder. 1996;276:293–299. [PubMed] [Google Scholar]
- Wener MH, Daum PR, McQuillan GM. The influence of age, sex, and race on the upper reference limit of serum C-reactive protein concentration. J. Rheumatol. 2000;27(10):2351–2359. [PubMed] [Google Scholar]
- Whooley, Mary A, de Jonge, Peter, Vittinghoff, Eric, Otte, Christian, Moos, Rudolf, Carney, Robert M, Ali, Sadia, Dowray, Sunaina, Na, Beeya, Feldman, Mitchell D, Schiller, Nelson B, Browner, Warren S. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JK, Vita JA, Manuck SB, Selwyn AP, Kaplan JR. Psychosocial factors impair vascular responses of coronary arteries. Circulation. 1991;84:2146–2153. doi: 10.1161/01.cir.84.5.2146. [DOI] [PubMed] [Google Scholar]
- Williams JK, Kaplan JR, Manuck SB. Effects of psychosocial stress on endothelium-mediated dilation of atherosclerotic arteries in cynomolgus monkeys. J. Clin. Invest. 1993;92:1819–1823. doi: 10.1172/JCI116772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmot KA, O'Flaherty M, Capewell S, Ford ES, Vaccarino V. Coronary heart disease mortality declines in the United States from 1979 through 2011: evidence for stagnation in young adults, especially women. Circulation. 2015;132(11):997–1002. doi: 10.1161/CIRCULATIONAHA.115.015293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woloshin S, Schwartz LM. Distribution of C-reactive protein values in the United States. N. Engl. J. Med. 2005;352(15):1611–1613. doi: 10.1056/NEJM200504143521525. [DOI] [PubMed] [Google Scholar]
- Wong CC, Caspi A, Williams B, Craig IW, Houts R, Ambler A, Moffitt TE, Mill J. A longitudinal study of epigenetic variation in twins. Epigenetics. 2010;5(6):516–526. doi: 10.4161/epi.5.6.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong TY, Klein R, Sharrett AR, Duncan BB, Couper DJ, Tielsch JM, Klein BE, Hubbard LD. Retinal arteriolar narrowing and risk of coronary heart disease in men and women. The atherosclerosis risk in communities study. JAMA. 2002;287(9):1153–1159. doi: 10.1001/jama.287.9.1153. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol mediates naturally-occurring fluctuation in adult hippocampal synapse density. J. Neurosci. 1992;12:2549–2554. doi: 10.1523/JNEUROSCI.12-07-02549.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Roles of estradiol and progesterone in regulation of hippocampal dendritic spine density during the estrous cycle in the rat. J. Comp. Neurol. 1993;336:293–306. doi: 10.1002/cne.903360210. [DOI] [PubMed] [Google Scholar]
- Woolley CS, McEwen BS. Estradiol regulates hippocampal dendritic spine density via an N-methyl-D-aspartate receptor-dependent mechanism. J. Neurosci. 1994;14(12):7680–7687. doi: 10.1523/JNEUROSCI.14-12-07680.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyman L, Crum RM, Celentano D. Depressed mood and cause-specific mortality: a 40-year general community assessment. Ann. Epidemiol. 2012;22(9):638–643. doi: 10.1016/j.annepidem.2012.06.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Bao H, Strait K, Spertus JA, Lichtman JH, D'Onofrio G, Spatz E, Bucholz EM, Geda M, Lorenze NP, Bueno H, Beltrame JF, Krumholz HM. Sex differences in perceived stress and early recovery in young and middle-aged patients with acute myocardial infarction. Circulation. 2015;131(7):614–623. doi: 10.1161/CIRCULATIONAHA.114.012826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- York KM, Hassan M, Li Q, Li H, Fillingim RB, Lucey D, Bestland M, Sheps DS. Do men and women differ on measures of mental stress-induced ischemia? Psychosom. Med. 2007;69(9):918–922. doi: 10.1097/PSY.0b013e31815a9245. [DOI] [PubMed] [Google Scholar]
- Zhao J, Quyyumi AA, Patel R, Zafari AM, Veledar E, Onufrak S, Shallenberger LH, Jones L, Vaccarino V. Sex-specific association of depression and a haplotype in leukotriene A4 hydrolase gene. Psychosom. Med. 2009;71(7):691–696. doi: 10.1097/PSY.0b013e3181b05c57. [DOI] [PMC free article] [PubMed] [Google Scholar]