Summary
Ageing of the innate and adaptive immune system, collectively termed immune senescence, is a complex process. One method to understand the components of ageing involves dissociating the effects of ageing on the cells of the immune system, on the microenvironment in lymphoid organs and tissues where immune cells reside and on the circulating factors that interact with both immune cells and their microenvironment. Heterochronic parabiosis, a surgical union of two organisms of disparate ages, is ideal for this type of study, as it has the power to dissociate the age of the cell and the age of the microenvironment into which the cell resides or is migrating. So far, however, it has been used sparingly to study immune ageing. Here we review the limited literature on homeostatic innate immune cell trafficking in ageing in the absence of chronic inflammation. We also review our own recent data on trafficking of innate immune subsets between primary and secondary lymphoid organs in heterochronic parabiosis. We found no systemic bias in retention or acceptance of neutrophils, macrophages, dendritic cells or natural killer cells with ageing in primary and secondary lymphoid organs. We conclude that these four innate immune cell types migrate to and populate lymphoid organs (peripheral lymph nodes, spleen and bone marrow), regardless of their own age and of the age of lymphoid organs.
Keywords: ageing, cell trafficking, dendritic cells, immunosenescence, macrophage, natural killer cells, spleen and lymph nodes
OTHER ARTICLES PUBLISHED IN THIS REVIEW SERIES
Immunosenescence: the importance of considering age in health and disease. Clinical and Experimental Immunology 2017, 187: 1–3.
The convergence of senescence and nutrient sensing during lymphocyte ageing. Clinical and Experimental Immunology 2017, 187: 4–5.
Immune senescence: significance of the stromal microenvironment. Clinical and Experimental Immunology 2017, 187: 6–15.
Innate immune responses in the ageing lung. Clinical and Experimental Immunology 2017, 187: 16–25.
Age‐related alterations in immune responses to West Nile virus infection. Clinical and Experimental Immunology 2017, 187: 26–34.
Intracellular signalling pathways: targets to reverse immunosenescence. Clinical and Experimental Immunology 2017, 187: 35–43.
Ageing and inflammation in patients with HIV infection. Clinical and Experimental Immunology 2017, 187: 44–52.
Considerations for successful cancer immunotherapy in aged hosts. Clinical and Experimental Immunology 2017, 187: 53–63.
Ageing and obesity similarly impair antibody responses. Clinical and Experimental Immunology 2017, 187: 64–70.
The life cycle of a T cell after vaccination – where does immune ageing strike? Clinical and Experimental Immunology 2017, 187: 71–81.
Herpes zoster and the search for an effective vaccine. Clinical and Experimental Immunology 2017, 187: 82–92.
Adult vaccination against tetanus and diphtheria: the European perspective. Clinical and Experimental Immunology 2017, 187: 93–99.
Introduction – outstanding questions in innate cellular migration during immune ageing
Ageing leads to numerous changes in the innate and adaptive immune system, collectively termed immune senescence. The most prominent whole‐organism effect of these changes manifests itself in an increased morbidity and mortality of older organisms from infectious diseases. While the understanding of immune senescence has progressed steadily over the past several decades, we still do not understand fully the interplay of age‐related changes of different components of the immune system. Gaining such knowledge is essential to ameliorate, delay or prevent age‐related immune decline.
One of the most critical questions in gerontology is how ageing affects critical components of the organism. Taking that question to the level of the immune system, we can distinguish three main components: various immune cells, lymphoid organs in which the cells function (with their stromal elements that provide dedicated microenvironments for development, maintenance and/or function of immune cells) and circulating factors (chemokines, cytokines, inflammatory molecules) that surround the immune cells and act within, as well as outside, the lymphoid organs. With that in mind, specific questions can be formulated. Specifically, which of the components are most affected by ageing, and which may still be relatively intact? Of those that are affected, are the changes intrinsic to that component or to the environment in which that component operates? Obtaining mechanistic answers to these questions is essential to craft interventions that may improve function of the ageing immune system. While there is a substantial body of evidence examining migration of certain innate immune subsets during the course of infection, this is not a topic of this text. This review is focused specifically on the migratory properties of innate immune cells in the course of ageing in the absence of overt infection, as it relates to circulation between blood, primary (thymus and bone marrow) and secondary (spleen and peripheral lymph nodes) lymphoid organs. We will address specifically the question of whether ageing of either the migrating cells or of lymphoid organs presents a barrier to steady‐state circulation of frequent innate immune cell types in the course of normal ageing.
Brief overview of homeostatic circulation and migration of immune cells
All immune cells originate from the bone marrow progenitor, specifically from the haematopoietic stem cells. Moreover, other than T cells, that develop in the thymus, all other immune cells remain in the bone marrow throughout the process of progressive differentiation and lineage specification. A general paradigm has emerged whereby, once the immune cells mature sufficiently, they down‐regulate those molecules that hold them in primary immune organs [e.g. sphingosine‐1‐phosphate (S1P1) for T cells], and up‐regulate molecules that will guide them to enter secondary lymphoid organs such as the lymph nodes (CD62L, CCR7) 1, 2, 3, 4. Innate cells follow the same general paradigm, although there are mobilizing factors, such as granulocyte–colony‐stimulating factor (G‐CSF), granulocyte–macrophage CSF (GM–CSF) and fibromyalgia syndrome (FMS)‐like tyrosine kinase 3 ligand (Flt‐3L) [for dendritic cells (DC)], that facilitate the release of specific innate cell types from bone marrow and mobilize them into blood by binding to specific receptors on these cells. Following the initiation of primary immune responses, different integrins and addressins will be up‐regulated at the cell surface to facilitate migration of innate immune cells into tissues. Moreover, such molecules may be up‐regulated over longer periods of time in situations of protracted inflammation, including chronic inflammatory diseases. However, neither of these two situations (immune responses or chronic inflammation) are a topic of this review.
Heterochronic parabiosis as means to study ageing and (immune) cell migration
Studies of immune cell migration typically rely upon in‐situ labelling of cells of interest (e.g. by reporter dyes, reporter‐labelled antibodies or by genetic means to express reporters in a given cell population); or on isolation, labelling (reporter dyes or congenic markers) and injection of cells into adoptive hosts; or on parabiosis of two organisms where the cells of one organism are distinguishable from the other, again usually using congenic allelic cell surface markers. Of these, the first approach, in‐situ labelling, cannot be used to assess the relative impacts of immune cell and lymphoid organ ageing. With regard to the other two methods, isolation of immune cells has the potential to activate them and/or change their homing capacities. Moreover, intravenous transfer has the disadvantage that only a minority of cells (< 10%) will engraft into circulation and lymphoid organs, whereas the vast majority will be removed in the liver or lung 5. Therefore, in the ageing setting, parabiosis is the preferred approach where, following initial surgical trauma to the joined skin, there is gentle and physiological intermixing of circulation and circulating cells of the two organisms. Other than surgical complications (reviewed in 6) described by other authors, one has to be careful with selection of appropriate co‐isogenic genotypes for cell tracking studies, due to the risk of graft‐versus‐host disease, but genetic and animal resource tools are available from approved vendors to circumvent this issue, and were used in experiments shown below.
Heterochronic parabiosis has been used increasingly during the last decade to investigate the presence of anti‐geronic and pro‐geronic molecules, the manipulation of which could afford extension of the health span 6. While it is possible that a single molecule may possess wide‐ranging anti‐geronic effects, it is more likely that a combination of factors will be required to repel different aspects of ageing simultaneously. This is where heterochronic parabiosis, the surgical union between an adult and an old organism, can be used as a powerful platform to both isolate individual pro‐ and anti‐geronic factors and to understand the complexity of ageing. The anti‐geronic effects of heterochronic parabiosis have been demonstrated thoroughly in skeletal muscle 7, central nervous system 8, 9, 10, 11, pancreatic β cell proliferation 12, cardiac tissue 13, bone repair 14 and systemic cholesterol turnover 15. In some cases, single molecules or pathways were identified as playing a significant pro‐ or anti‐geronic role in the aetiology of ageing in the reported tissue. On the anti‐geronic side, increasing Notch signalling was found to improve skeletal muscle regeneration 7, whereas increasing growth differentiation factor 11 (GDF11) levels helped skeletal muscle 16, cardiac 13 and neurovascular 10 rejuvenation and remodelling.
In other cases, heterochronic parabiosis failed to rejuvenate the aged phenotype, e.g. in the case of impaired Schwann cell function 17, impaired T cell subset distribution 18, function 19 and thymic involution 18, 20, and in the first two examples pro‐geronic effects were noted in the adult animal. Regarding individual pro‐geronic molecules, increased levels of CCL11 and β2m with age are associated with decreased brain functions 8, 11 and increased β‐catenin signalling was implicated in defects in bone repair 14.
With regard to ageing of the immune system, Pishel et al. published observations of accelerated ageing of the immune system as determined by the failure to restore thymic mass of the old animal and an increase of CD44+ memory T cells in the young animal and diminished T cell proliferation and T cell receptor (TCR) signalling upon in‐vitro stimulation 18, 19. These studies did not use congenic markers to distinguish between adult and old T cells in critical experiments, and conducted bulk T cell assays that do not account for the different proportions of T cell subsets with age. However, more recently, in agreement with Pishel and our unpublished data, Kim et al. published that heterochronic parabiosis fails to restore thymic function in the old animal and the finding was attributed primarily to defects in the thymic stroma 20. These studies with heterochronic parabiosis did not examine peripheral innate immune cell trafficking between parabionts in the ageing environment.
Homeostatic migration of immune cells in the course of ageing and in heterochronic parabiosis
Neutrophils
The heterogeneity and lack of precise identification of various subsets of myeloid cells that could be identified as neutrophils complicate the study of neutrophils, even outside the context of ageing 21, 22. Neutrophils are produced at rates up to 2 × 1011 per day and were thought to live for 1·5–8 h, but have now been shown to live at least until 12·5 h, and in inflammation even up to several days (reviewed in 22). Most authors believe that neutrophil numbers remain constant with ageing, although some authors reported reduced numbers 23. With ageing, neutrophils have been implicated in the pathogenesis of Alzheimer's disease, atherosclerosis, cancer and autoimmune diseases 23, and a role of the microbiome has been implicated recently in neutrophil ageing, with a demonstration that neutrophil proinflammatory function in vivo correlates positively with age 24. While GM‐CSF promotes the chemotaxis of neutrophils in an adult animal, this is not the case in old neutrophils because of defects in the Janus kinase/signal transducer and activator of transcription (Jak/STAT) pathway 25, 26. Furthermore, diminished neutrophil chemotaxis and infiltration were found to be involved with delayed wound healing in aged mice 27. Moreover, altered chemokine expression [such as an increase in the CXC‐chemokine receptor 4 (CXCR4)] was described in aged neutrophils, potentially directing them back to the bone marrow, where they are then eliminated 22. (This theme of altered cytokine and chemokine receptor expression with ageing extends to other innate cells described below.) Ageing also reduces the phagocytic and bactericidal activity in numerous models 28, 29, 30. Heterochronic parabiosis has not been applied so far to the studies of neutrophil migration in old and adult tissues.
For our studies of parabiosis discussed here, for all cell types examined, we have used control adult (3–4 months, 24–27 g body weight) and old male mice (18 months, 29–34 g body weight). In our hands there was a trend of an age‐related increase in neutrophils in every tissue besides the thymus; however, the data were significant only in the bone marrow (not shown). This trend extended to a proportional increase of neutrophils in all aged tissues, including the thymus, achieving significance in the blood and bone marrow; we found no impact of the CD45 allelic genetic differences on the neutrophil numbers and representation (not shown). In isochronic parabiosis, we found a trend for host preference of neutrophils in every tissue besides the thymus (Fig. 1a,b). However, the only significant differences were found in blood and bone marrow, and that trend was similar to the one seen with other innate cells examined. We speculate that the preference for bone marrow occurs because that is the site of neutrophil formation. Results in blood are more difficult to explain, unless initial dissemination from bone marrow also affords some advantage to these cells in the host's peripheral blood.
Figure 1.
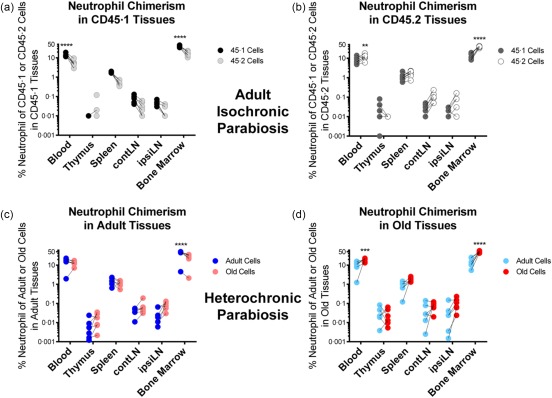
Neutrophil distribution in isochronic and heterochronic parabiosis. For this and all subsequent studies, for all cell types examined, we have used control adult (3–4 months, 24–27 g body weight) and old male mice (18 months, 29–34 g body weight). Parabiosis was performed following 1 week of co‐habitation of parabionts, precisely as described in references 6, 7. Shown are frequencies of neutrophils, defined as CD11b+granulocyte marker 1 (Gr1hi) cells within the CD45·1 (adult) or CD45·2 (adult or old) compartment, respectively, from the indicated tissue. Dead cells were gated out using a vital dye. (a) Analysis of CD45·1 or CD45·2 neutrophils in isochronic adult CD45·1 tissues. (b) Analysis of CD45·1 or CD45·2 neutrophil chimerism in isochronic adult CD45·2 tissues. (c) Analysis of the frequency of adult or old neutrophils in heterochronic adult tissues. (d) Analysis of the frequency of adult or old neutrophils in heterochronic old tissues. All panels include data from three to four experiments, n = 6. Significance was determined by repeated‐measures two‐way analysis of variance (anova) with Sidak's post‐test. *P < 0·05; **P < 0·01; ***P < 0·001; ****P < 0·0001.
In the course of heterochronic parabiosis, neutrophils exhibited a similar behaviour compared to isochronic parabiosis, with a strong preference towards self‐bone marrow (Fig. 1c,d). However, the propensity of neutrophils towards its own (host) blood was not found in the adult animal during heterochronic parabiosis (Fig. 1c,d). In both adult and old tissues, old neutrophils appeared more prominent, but these differences were often not significant. Overall, the proportion and distribution of neutrophils did not change greatly, if at all, in the course of heterochronic parabiosis relative to isochronic pairs. We therefore conclude that old neutrophils, defined as above, exhibit no migration or retention defects due to their age or the age of primary or secondary tissue.
Macrophages
Heterogeneity among macrophages and their subsets is plentiful and difficult to discern 31, 32. Attempts to determine changes in macrophage function have yielded inconsistent results with respect to cytokine production, phagocytosis and migration during healthy ageing 33. While some phagocytic activity is retained with age, the ability of macrophages to phagocytose apoptotic cells is generally reduced with age in mice and humans alike 34, 35. Toll‐like receptor (TLR) signalling and cytokine production defects were also reported to occur in macrophages with ageing across species 28, 36. Other studies found that macrophages, as well as the myeloid‐derived suppressor cells (MDSC), changed prevalence with ageing, with more M2 and MDSC cells relative to younger mice and co‐expressed M1 and M2 markers such as CD40 and CX3CR1, that are usually expressed in a mutually exclusive manner in young animals 37, Functionally, these cells expressed more transforming growth factor (TGF)‐β and interleukin (IL)−4, suggesting a more suppressive phenotype 37.
In unmanipulated adult and old mice, we found no numerical differences in macrophages, as defined by the CD11b++F4/80++ phenotype, across primary and secondary lymphoid tissues (not shown). The higher percentage of CD11b++F4/80++ in the blood of aged animals also corresponds to a trend towards higher numbers, although the difference was not statistically significant. Overall, we found no remarkable differences in macrophages across strains or age of mice when analysed at this level. In the course of isochronic parabiosis, similar to neutrophils, the major finding was an abundance of host macrophages/monocytes in the bone marrow and blood (Fig. 2a,b). We found no limitations to chimerism and retention of congenic adult macrophages in the adult spleen and lymph nodes (Fig. 2a,b).
Figure 2.
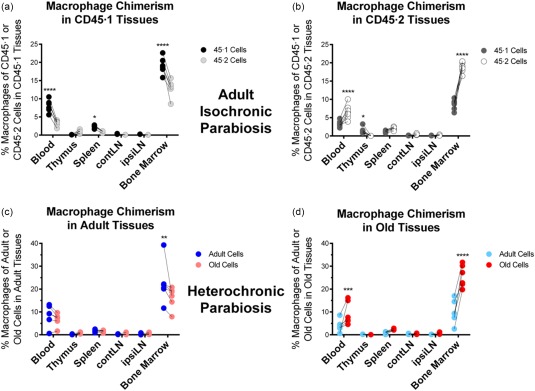
Macrophage chimerism in adult isochronic and heterochronic parabiosis. Data represent the frequency of macrophages (CD11bhiF4/80+) within the CD45·1 (adult) or CD45·2 (adult or old) compartment, respectively, from the indicated tissue. (a) Analysis of CD45·1 or CD45·2 macrophages in isochronic adult CD45·1 tissues. (b) Analysis of CD45·1 or CD45·2 macrophages in isochronic adult CD45·2 tissues. (c) Analysis of the frequency of adult or old macrophages in heterochronic adult tissues. (d) Analysis of the frequency of adult or old macrophages in heterochronic old tissues. Numbers of experiments, animals and statistical treatment and levels are as in Fig. 1.
Similar to isochronic pairs, there were no remarkable findings with regard to monocyte/macrophage chimerism or engraftment during heterochronic parabiosis, at least using the phenotypical definition specified above (Fig. 2c,d). A few differences noted in the isochronic parabiosis in the thymus, and to a lesser extent the blood, were not significant during heterochronic parabiosis. Therefore, macrophages do not seem to be restricted in tissue residence due to either the age of the macrophage or age of the tissue (Fig. 2). One should note, however, that: (i) we did not examine function of macrophages (or any other cell type discussed here) in heterochronic parabiosis (an issue that certainly warrants attention); (ii) that non‐lymphoid macrophages are involved in many aspects of ageing and were not examined here; and (iii) that, as mentioned both above and below, our results are limited by the markers used to define cell types globally. Finally, the important role of the monocyte/macrophage lineage in age‐related inflammatory diseases also remains to be elucidated with ageing.
Natural killer (NK) cells
General defects of important NK cell functions, such as cytotoxicity and cytokine production, have been documented with ageing 28. A consensus is that there is a decrease in the immature, cytokine‐producing (CD56+) NK cells and a proportional increase in the mature, cytotoxic NK cells; however, both their functions are diminished with age. Lastly, there is evidence of the inability of aged NK cells to migrate to the inflamed sites during infection and reports of altered NK cell repertoire 28, 38, 39, 40, 41. Overall, the vital role of NK cells in preventing oncogenesis and viral pathogenesis seems to deteriorate with age.
In unmanipulated old mice, we found NK cells to increase in numbers in the blood (2·7 × 106 ± 6·4 × 105) relative to the CD45·1 and CD45·2 adult mice (1·7 × 106 ± 1·7 × 105 and 1·8 × 106 ± 3·8 × 105, respectively), but this was not found in other tissues. Other data from the non‐surgical control mice illustrate a massive increase in NK cells in the bone marrow of CD45.1 mice 5·8 × 106 ± 6·9 × 105 cells, relative to 2·1 × 106 ± 2·8 × 105 and 2·1 × 106 ± 1·2 × 105 cells in the marrow of adult and old CD45.2 mice, respectively), suggesting an allelic effect. While there were no numerical differences of NK cells in the lymph nodes, they were proportionally over‐represented with age, due probably to the decrease in naive T cell cellularity.
Despite a marked increase in NK cell numbers and proportion in the bone marrow of CD45.1 adult mice, there were no remarkable differences during isochronic parabiosis (Fig. 3a,b). The increased NK cells in host bone marrow were not surprising, both because this is the tissue where these cells are produced and because a similar trend was found in neutrophils and macrophages. This situation was paralleled strictly by the chimerism or engraftment of NK cells during heterochronic parabiosis (Fig. 3c,d), with the trends of increased host NK cells in the blood and bone marrow.
Figure 3.
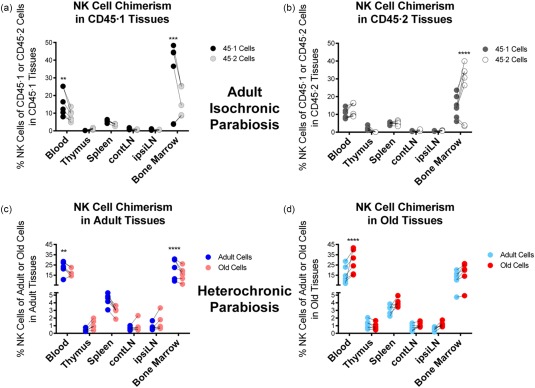
Natural killer (NK) cell chimerism in adult isochronic and heterochronic parabiosis. Data represent the frequency of NK cells (DX5+CD3–CD19–) within the CD45·1 (adult) or CD45·2 (adult or old) compartment, respectively, from the indicated tissue. (a) Analysis of CD45·1 or CD45·2 NK cells in isochronic adult CD45·1 tissues. (b) Analysis of CD45·1 or CD45·2 NK cells in isochronic adult CD45·2 tissues. (c) Analysis of the frequency of adult or old NK cells in heterochronic adult tissues. (d) Analysis of the frequency of adult or old NK cells in heterochronic old tissues. Numbers of experiments, animals and statistical treatment and levels are as in Fig. 1.
DCs
DCs represent a heterogeneous population of cells that are distributed throughout the body with a primary task to serve as sentinels that sense, ingest, process and present microbial antigens. The literature regarding the precise definition of DC subsets and their respective functions has only recently begun to be resolved in the adult immune system. The literature regarding aged DCs is even more controversial, with almost every discovery, other than migratory defects, being contested 23, 42, 43, 44. Many of these controversies arose from differential utilization of in‐vitro protocols to generate DCs. These protocols involve 6–7 days of culture, with inherent expansion and selection of the best responders to differentiation cytokines, which has the potential to obscure completely all age‐related differences at the onset of culture. However, as with other innate cells, defects in DC migration have been described and in some cases have been ascribed to altered production of cytokines, chemokines or other mediators. This has been shown for increased local production of prostaglandin D2 in the lung and the consequent reduction of CCR7 on DC with ageing 44, as well as for production of the vasoactive intestinal peptide (VIP) that up‐regulates CCR1 and down‐regulates CCR7 45.
The only age‐related difference found in DCs prior to parabiosis was a decrease in numbers in the spleen accompanying age, from 2·1 × 106 ± 0·6 × 105 in CD45·2 adults to 1·7 × 106 ± 1·6 × 105 in old spleens; no changes were noted in other lymphoid organs. Isochronic control data indicated that DCs do not form equal chimerism in lymphoid organs, particularly the spleen and lymph nodes (LNs), with the host component dominating its own tissues (Fig. 4a,b). The only exception was that CD45.1 DCs homed more efficiently to the CD45.2 thymus than the CD45.1 thymus, and were more prominent there than the CD45.2 DCs. It is unclear at present what factors may govern DC homing to the thymic medulla, and therefore the resolution of mechanisms behind this phenomenon will have to be provided at a later date. Interestingly, the bias of host DCs for its own spleen and LNs seen in isochronic pairs was not observed during heterochronic parabiosis (Fig. 4c,d). Therefore, there were no remarkable differences in migration and retention of adult and old DCs in adult and old tissues, suggesting that normal ageing may not critically modulate their mobility.
Figure 4.
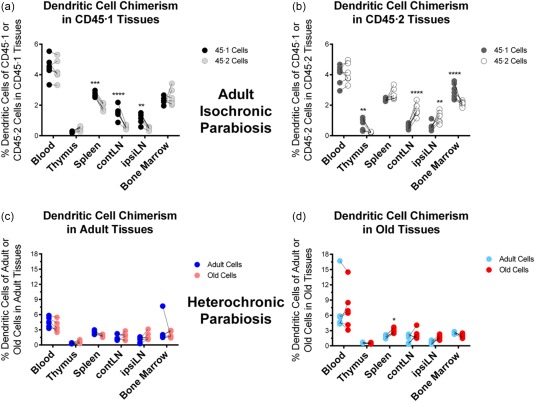
Dendritic cell chimerism in adult isochronic and heterochronic parabiosis. Data represent the frequency of dendritic cells (CD11c+Gr1mid/loF4/80–) within the CD45·1 (adult) or CD45·2 (old) compartment, respectively, from the indicated tissue. (a) Analysis of CD45·1 or CD45·2 DCs in isochronic adult CD45·1 tissues. (b) Analysis of CD45·1 or CD45·2 DCs in isochronic adult CD45·2 tissues. (c) Analysis of the frequency of adult or old DCs in heterochronic adult tissues. (d) Analysis of the frequency of adult or old DCs in heterochronic old tissues. Numbers of experiments, animals and statistical treatment and levels are as in Fig. 1.
Conclusions
So far, trafficking of the numerically major types of innate immune cells – neutrophils, macrophages, NK cells and DCs – has not been reviewed as a function of age outside infection/inflammation models. We reviewed this issue and have added our own recent results examining this issue using parabiosis of old and adult mice in the absence of inflammation or infection, and in the context of healthy ageing. In providing original data, we have followed phenotypical definitions of innate immune cell types that could be debated (e.g. CD11b++F4/80++ gating of macrophages could encompass some myeloid DC 46), but we feel that they were sufficiently descriptive to allow us to draw our main conclusions.
Unlike our findings in T cells, that will be reported separately (Davies et al., Heterochronic parabiosis reveals age‐related changes in migration and maintenance of murine T cell subsets in lymphoid organs; submitted for publication), we found no numerical defects in the four innate cell types across strains or age, with proportional differences exhibited in the blood and bone marrow after isochronic parabiosis. These differences followed a pattern that favours the host component, and were seen with all innate cells. In general, the differences were less prominent following heterochronic parabiosis, suggesting that innate immune subsets cells move freely throughout adult and old tissues regardless of their age. Within the limits of the present phenotypical analysis, we conclude that ageing does not present a barrier to homeostatic trafficking of innate cells examined herein, either with regard to the age of the migrating cell or the age of the primary or secondary lymphoid organ into which the cells are migrating.
Disclosure
The authors declare no conflicts of interest. J. N.‐Z. is an unpaid Scientific Advisory Board member of Organic Vaccines, Inc., an entity that had no input into any part of studies reported here.
Acknowledgements
The authors wish to acknowledge Dr Vesna Pulko for help with the experiments, Drs Karuna Patil, Trenton Bryfogle and Paula Johnson from the UA Central Animal Services for helps with establishing the parabiosis model and the members of the Nikolich laboratory for helpful discussion. All animal studies were approved by the University of Arizona Animal Care and Use Committee. This study was supported by the NIAID contract N01‐AI‐000017 from the National Institutes of health.
Contributor Information
J. Nikolich‐Žugich, nikolich@email.arizona.edu
J. S. Davies, Email: john.davies@nih.gov
References
- 1. Gunn MD, Tangemann K, Tam C, Cyster JG, Rosen SD, Williams LT. A chemokine expressed in lymphoid high endothelial venules promotes the adhesion and chemotaxis of naive T lymphocytes. Proc Natl Acad Sci USA 1998; 95:258–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brinkmann V, Cyster JG, Hla T. FTY720: sphingosine 1‐phosphate receptor‐1 in the control of lymphocyte egress and endothelial barrier function. Am J Transplant 2004; 4:1019–25. [DOI] [PubMed] [Google Scholar]
- 3. Pham THM, Okada T, Matloubian M, Lo CG, Cyster JG. S1P1 receptor signaling overrides retention mediated by G alpha I‐coupled receptors to promote T cell egress. Immunity 2008; 28:122–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swan DJ, Kirby JA, Ali S. Vascular biology: the role of sphingosine 1‐phosphate in both the resting state and inflammation. J Cell Mol Med 2010; 14:2211–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Moon JJ, Chu HH, Hataye J et al Tracking epitope‐specific T cells. Nat Protoc 2009; 4:565–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Conboy MJ, Conboy IM, Rando TA. Heterochronic parabiosis: historical perspective and methodological considerations for studies of aging and longevity. Aging Cell 2013; 12:525–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005; 433:760–4. [DOI] [PubMed] [Google Scholar]
- 8. Villeda SA, Luo J, Mosher KI et al The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011; 477:90–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ruckh JM, Zhao J‐W, Shadrach JL et al Rejuvenation of regeneration in the aging central nervous system. Cell Stem Cell 2012; 10:96–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Katsimpardi L, Litterman NK, Schein PA et al Vascular and neurogenic rejuvenation of the aging mouse brain by young systemic factors. Science 2014; 344:630–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Smith LK, He Y, Park J‐S et al β2‐microglobulin is a systemic pro‐aging factor that impairs cognitive function and neurogenesis. Nat Med 2015; 21:932–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Salpeter SJ, Khalaileh A, Weinberg‐Corem N, Ziv O, Glaser B, Dor Y. Systemic regulation of the age‐related decline of pancreatic β‐cell replication. Diabetes 2013; 62:2843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Loffredo FS, Steinhauser ML, Jay SM et al Growth differentiation factor 11 is a circulating factor that reverses age‐related cardiac hypertrophy. Cell 2013; 153:828–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Baht GS, Silkstone D, Vi L et al Exposure to a youthful circulation rejuvenates bone repair through modulation of β‐catenin. Nat Commun 2015; 6:7131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hrůza Z. Increase of cholesterol turnover of old rats connected by parabiosis with young rats. Exp Gerontol 1971; 6:103–7. [DOI] [PubMed] [Google Scholar]
- 16. Sinha M, Jang YC, Oh J et al Restoring systemic gdf11 levels reverses age‐related dysfunction in mouse skeletal muscle. Science 2014; 344:649–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Painter MW, Brosius Lutz A, Cheng Y‐C et al Diminished Schwann cell repair responses underlie age‐associated impaired axonal regeneration. Neuron 2014; 83:331–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pishel I, Shytikov D, Orlova T, Peregudov A, Artyuhov I, Butenko G. Accelerated aging versus rejuvenation of the immune system in heterochronic parabiosis. Rejuvenation Res 2012; 15:239–48. [DOI] [PubMed] [Google Scholar]
- 19. Shytikov DW, Shkumat MS, Yankova TM, Peregudov AG, Artyuhov IV, Pishel IM. Splenic niche cells from young heterochronic parabionts have decreased capability to amplify T‐cell proliferation in vitro . Am J Biosci 2015; 3:46–54. [Google Scholar]
- 20. Kim M‐J, Miller CM, Shadrach JL, Wagers AJ, Serwold T. Young, proliferative thymic epithelial cells engraft and function in aging thymuses. J. Immunol 2015; 194:4784–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gabrilovich DI, Ostrand‐Rosenberg S, Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol 2012; 12:253–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol 2013; 13:159–75. [DOI] [PubMed] [Google Scholar]
- 23. Desai A, Grolleau‐Julius A, Yung R. Leukocyte function in the aging immune system. J Leukoc Biol 2010; 87:1001–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhang D, Chen G, Manwani D et al Neutrophil ageing is regulated by the microbiome. Nature 2015; 525:528–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fulop T, Larbi A, Douziech N et al Signal transduction and functional changes in neutrophils with aging. Aging Cell 2004; 3:217–26. [DOI] [PubMed] [Google Scholar]
- 26. Fortin CF, Larbi A, Dupuis G, Lesur O, Fülöp T Jr. GM‐CSF activates the Jak/STAT pathway to rescue polymorphonuclear neutrophils from spontaneous apoptosis in young but not elderly individuals. Biogerontology 2006; 8:173–87. [DOI] [PubMed] [Google Scholar]
- 27. Brubaker AL, Rendon JL, Ramirez L, Choudhry MA, Kovacs EJ. Reduced neutrophil chemotaxis and infiltration contributes to delayed resolution of cutaneous wound infection with advanced age. J Immunol 2013; 190:1746–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaw AC, Goldstein DR, Montgomery RR. Age‐dependent dysregulation of innate immunity. Nat Rev Immunol 2013; 13:875–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Simell B, Vuorela A, Ekström N et al Aging reduces the functionality of anti‐pneumococcal antibodies and the killing of Streptococcus pneumoniae by neutrophil phagocytosis. Vaccine 2011; 29:1929–34. [DOI] [PubMed] [Google Scholar]
- 30. Tseng CW, Kyme PA, Arruda A, Ramanujan VK, Tawackoli W, Liu GY. Innate immune dysfunctions in aged mice facilitate the systemic dissemination of methicillin‐resistant S. aureus . PLOS ONE 2012; 7:e41454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Klapproth K, Lasitschka F, Rodewald H‐R. Multilayered ancestry of arterial macrophages. Nat Immunol 2016; 17:117–8. [DOI] [PubMed] [Google Scholar]
- 32. Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol 2015; 15:731–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gomez CR, Nomellini V, Faunce DE, Kovacs EJ. Innate immunity and aging. Exp Gerontol 2008; 43:718–28.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agrawal A, Agrawal S, Cao J‐N, Su H, Osann K, Gupta S. Altered innate immune functioning of dendritic cells in elderly humans: a role of phosphoinositide 3‐kinase‐signaling pathway. J Immunol 2007; 178:6912–22. [DOI] [PubMed] [Google Scholar]
- 35. Aprahamian T, Takemura Y, Goukassian D, Walsh K. Ageing is associated with diminished apoptotic cell clearance in vivo . Clin Exp Immunol 2008; 152:448–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Metcalf TU, Cubas RA, Ghneim K et al Global analyses revealed age‐related alterations in innate immune responses after stimulation of pathogen recognition receptors. Aging Cell 2015; 14:421–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Jackaman C, Radley‐Crabb HG, Soffe Z, Shavlakadze T, Grounds MD, Nelson DJ. Targeting macrophages rescues age‐related immune deficiencies in C57BL/6J geriatric mice. Aging Cell 2013; 12:345–57. [DOI] [PubMed] [Google Scholar]
- 38. Nogusa S, Ritz BW, Kassim SH, Jennings SR, Gardner EM. Characterization of age‐related changes in natural killer cells during primary influenza infection in mice. Mech Ageing Dev 2008; 129:223–30. [DOI] [PubMed] [Google Scholar]
- 39. Beli E, Clinthorne JF, Duriancik DM, Hwang I, Kim S, Gardner EM. Natural killer cell function is altered during the primary response of aged mice to influenza infection. Mech Ageing Dev 2011; 132:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Almeida‐Oliveira A, Smith‐Carvalho M, Porto LC et al Age‐related changes in natural killer cell receptors from childhood through old age. Hum Immunol 2011; 72:319–29. [DOI] [PubMed] [Google Scholar]
- 41. Manser AR, Uhrberg M. Age‐related changes in natural killer cell repertoires: impact on NK cell function and immune surveillance. Cancer Immunol Immunother 2016; 65:417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Grolleau‐Julius A, Abernathy L, Harning E, Yung RL. Mechanisms of murine dendritic cell antitumor dysfunction in aging. Cancer Immunol Immunother 2008; 58:1935–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Cumberbatch M, Dearman RJ, Kimber I. Influence of ageing on Langerhans cell migration in mice: identification of a putative deficiency of epidermal interleukin‐1β. Immunology 2002; 105:466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhao J, Zhao J, Legge K, Perlman S. Age‐related increases in PGD(2) expression impair respiratory DC migration, resulting in diminished T cell responses upon respiratory virus infection in mice. J Clin Invest 2011; 121:4921–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Weng Y, Sun J, Wu Q, Pan J. Regulatory effects of vasoactive intestinal peptide on the migration of mature dendritic cells. J Neuroimmunol 2007; 182:48–54. [DOI] [PubMed] [Google Scholar]
- 46. Murray PJ, Wynn TA. Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 2011; 11:723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]


