Abstract
Current understanding of cell regulatory systems suggests a diverse array of extracellular stimuli commonly recruit a limited cadre of core signal transduction modules to drive discrete stimulus-specific responses. One such module is the Raf-MEK-extracellular signal-regulated kinase (ERK) kinase cascade. Little information exists about how this pathway can be appropriately coupled to discrete cell biological processes. Contributing factors may include regulation of the duration, amplitude, and/or subcellular compartmentalization of active ERK1/2. To define properties of ERK1/2 that may help mediate stimulus-selective signal propagation, we have examined the dynamic behavior of native ERK1/2 activation at the single-cell level. In primary human cell cultures, ERK1/2 activation is not an all-or-none response. Instead, the amount of active ERK1/2 in individual cells accumulated in proportion to the concentration of external stimulus. The variable degree of ERK1/2 activation correlated well with the degree of ERK1/2 effector activation. Therefore, the relative amplitude of ERK1/2 activation within a cell can be modulated and may contribute to the generation of stimulus-specific biological responses. Importantly, we also found that the capacity of active ERK1/2 to accumulate in the nucleus and drive immediate-early gene expression is dependent upon the nature of the inductive signal, but independent of the amplitude of ERK1/2 activation. Therefore, nuclear accumulation of active ERK1/2 is a discrete regulated step that can direct the function of the kinase in response to specific stimuli.
Activation of the extracellular signal-regulated kinase 1/2 (ERK1/2) kinase cascade has been demonstrated to engage signaling proteins controlling diverse regulatory programs, including cellular proliferation, differentiation, migration, and survival (16, 23). ERK1/2 effectors are located throughout the cell and include the nuclear transcription factors c-Fos and Elk-1, cytoplasmic protein kinases such as p90RSK and myosin light chain kinase, and other enzymes such as phospholipase A2 (8, 9, 11, 12, 17). The pleiotropic consequences of ERK1/2 activation imply that the interaction between activated ERK1/2 and its diverse substrates is selectively regulated to allow appropriate cellular responses to distinct stimuli. By analogy to other regulatory systems, potential mechanisms to selectively restrict ERK1/2 effector activation include stimulus-specific modulation of the amount and/or subcellular localization of the active kinase.
Several reported observations suggest that the relative amplitude of ERK1/2 activation can be coupled to specific biological outcomes. For example, in KSR-null mice, the amount of ERK1/2 activated in response to stimuli is reduced by half compared to that in wild-type mice. This correlates with reduced T-cell proliferation and delayed onset of polyomavirus MT-induced mammary tumors (22). In colon carcinoma cells, the degree of ERK1/2 activation correlates with expression of Fra-1 and inhibition of anoikis (26). However, from these studies it is unclear if suboptimal ERK1/2 activation reflects the degree of ERK1/2 activation in all cells or a reduction of the fraction of cells that can activate ERK1/2. Xenopus oocytes are particularly amenable to studying ERK1/2 behavior at the single-cell level due to their large size. Ferrell and colleagues demonstrated that above a specific concentration of progesterone, all the ERK in a single oocyte is activated. Below this threshold concentration, no ERK is active (6). The response of ERK1/2 in single cells to different ligand concentrations has not been examined in mammalian cells.
ERK1/2 proteins are cytoplasmic or evenly distributed throughout resting cells (4). Following activation, ERK1/2 proteins have been shown to accumulate in the nucleus, a localization pattern required for proliferation of 3T3 cells and differentiation of PC12 cells (18, 24, 25). It is currently unknown if nuclear accumulation is an intrinsic property of active ERK1/2 or if it can be regulated. As mentioned above, ERK1/2 has a number of cytoplasmic substrates that regulate processes such as motility and inflammation (14, 17). Ligand-selective regulation of active ERK1/2 compartmentalization is a mechanism that could restrict ERK1/2 effector activation by promoting activation of relevant substrates while preventing interaction with inappropriate effectors. Currently, ligand-specific localization patterns of active ERK1/2 have not been identified.
While ligand-dependent differences in the kinetics of ERK1/2 activation clearly correlate with discrete phenotypic responses, it is unclear if selective control of the amplitude or localization of active ERK1/2 can also contribute to the interpretation of environmental cues (13, 25). The majority of published studies examining activation of the ERK1/2 kinase cascade use readouts based on the activity of cell populations rather than individual cells (6, 20). From a population-based analysis, observations of stimulus-dependent variation in the amplitude of pathway activation may be due to fractional activation amplitudes within individual cells or to different numbers of cells responding with an inflexible, all-or-none activation mechanism (6). It is unknown if the amplitude of ERK1/2 activation is tunable within a somatic cell and, if so, if this has consequences on effector activation. Here we report the characterization of the behavior of ERK1/2 activation in individual cells. We examined both the amplitude and localization of ERK1/2 in primary foreskin fibroblasts and some continuous cell lines. We find that ERK1/2 activation in mammalian cells is graded in proportion to the concentration of the activating ligand, and we describe discrete cytoplasmic and nuclear localization patterns of active ERK1/2 that occur following the stimulation of cells with specific ligands. We conclude that nuclear entry of ERK1/2 is not an obligatory step following activation, but can be regulated to target ERK1/2 to specific substrates.
MATERIALS AND METHODS
Cell culture.
HeLa cells were obtained from the American Type Culture Collection and human foreskin fibroblasts (HFFs) were obtained from human foreskin specimens (<20 population doublings). Two independent strains of fibroblasts that were derived from two subjects were used. Cell lines were maintained in Dulbecco's modified Eagles' medium (Gibco; no. 12100-061) supplemented with 10% fetal bovine serum (Gibco; no. 26140-095) and 1.0% glutamine (Invitrogen; no. 25030-081). HeLa cells were incubated in Dulbecco's modified Eagle's medium plus 0.5% serum for 6 h prior to analysis. HFFs were plated on 50-μg/ml collagen (Vitrogen; no. FXP-019) 24 h before removal of serum. HFFs were incubated in 0.5% serum overnight before analysis. Epidermal growth factor (EGF) was obtained from BD Biosciences (no. 354001), and phorbol myristate acetate (PMA) was obtained from Sigma (no. P8139). Leptomycin B (LB; Sigma no. L2913) was used for 90 min at a final concentration of 200 ng/ml prior to stimulation of cells.
Immunoblotting.
HeLa cells were lysed in buffer containing 50 mM HEPES, 150 mM NaCl, 80 mM β-glycerolphosphate, 1 mM Na3VO4, 5% Triton X-100, and protease inhibitors. HFF cells were lysed in 2× sample buffer. Lysates were separated in 10% polyacrylamide gels. Total ERK (SC-93) and c-fos (SC-7202) antibodies were obtained from Santa Cruz. Antibodies specific for dually phosphorylated ERK (M-8159) and cyclin D1 (C-5588) antibodies were obtained from Sigma, and phospho-RSKT57 (9346) was obtained from Cell Signaling Technologies. Antiserum Y691 was used for total ERK analysis in HeLa cells as previously described (2).
Immunofluorescence and fluorescence measurements.
All experiments used two coverslips per condition. HeLa cells were fixed and permeabilized for 10 min in methanol at −20°C. HFFs were fixed in 3.7% formaldehyde for 10 min at room temperature followed by methanol permeabilization at −20°C for 10 min. Additionally, HFFs were fixed and permeabilized with methanol at −20°C, and similar results were obtained. All cells were then blocked for a minimum of 30 min in PBTA (1× phosphate-buffered saline, 5% bovine serum albumin, 0.1% Tween 20). Primary antibodies were used at a 1:100 dilution in PBTA for 1 h at room temperature followed by washing and secondary antibody staining with Alexa 488 or 546 (Molecular Probes) for 30 min at 37°C. Cells were washed and mounted with Polymount. Images for HeLa cells and HFFs were acquired at magnifications of ×63 and ×40, respectively, using a Zeiss Axiocam equipped with an Orca II Hamamatsu black and white charge-coupled device camera. To quantitate fluorescence intensities, exposure settings were used that were within the linear range of sensitivity of the charge-coupled device camera. This was determined empirically with bracketed exposure times. For any given experiment, identical exposure settings (including times) were used for all images. All data were collected at the same time for individual experiments. For each cell, the perimeter was defined manually and the total fluorescence intensity of the area defined by the perimeter was calculated with Improvision Open Lab 3.1. A minimum of 50 cells were analyzed for each experimental group. Background staining was determined by measuring fluorescence of fields without any cells. The total area for individual cells was then multiplied by the average fluorescence intensity for the unstained regions. This value was subtracted from the total intensity measurements. To represent the data graphically, we binned the intensity measurements and graphed the numbers of each cell in individual bins as a histogram.
RESULTS AND DISCUSSION
The fraction of active ERK1/2 in individual cells is proportional to the concentration of stimulus.
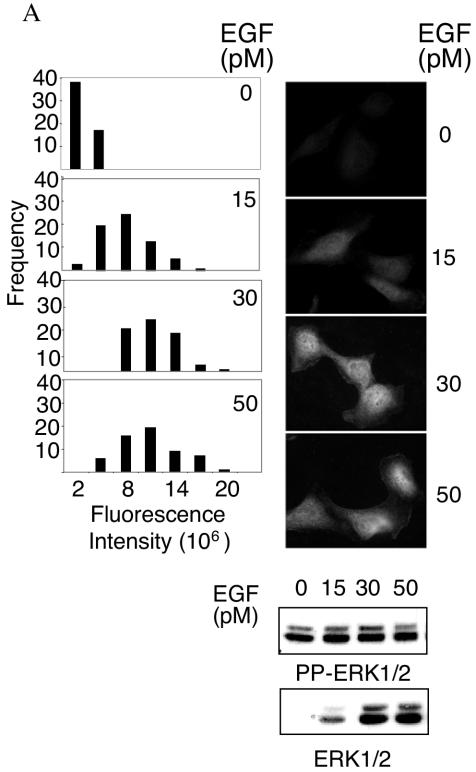
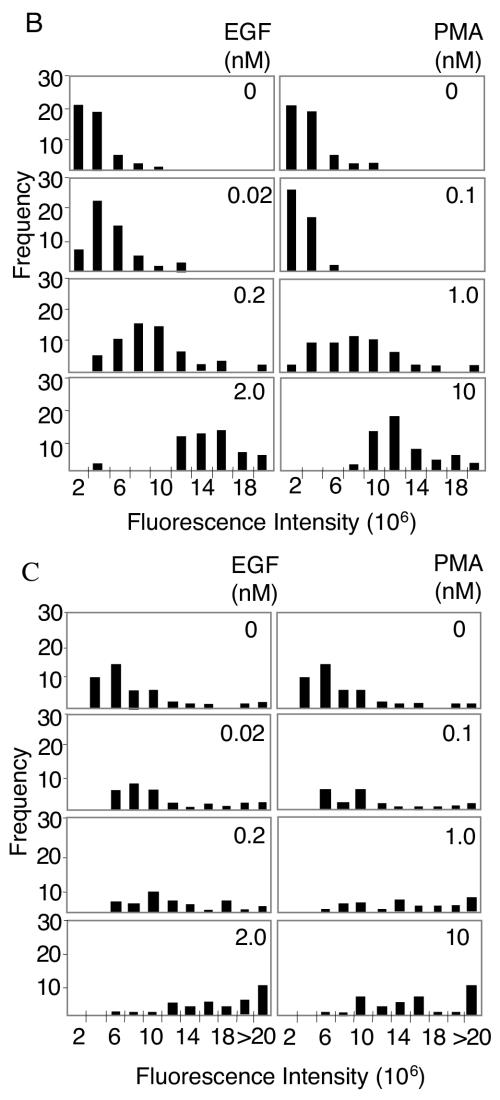
To examine ERK1/2 activation at single-cell resolution, we measured the fluorescence intensity of individual cells immunostained with antibodies specific for dually phosphorylated active ERK1/2 (pp-ERK). As shown in Fig. 1, both HeLa cells and primary HFFs responded to EGF stimulation in a dose-dependent manner. Suboptimal activation of ERK1/2 by intermediate concentrations of EGF, as assessed by immunoblotting of whole-cell lysates, corresponded to an intermediate range of signals, as assessed by immunofluorescence, in individual cells. These intermediate signals occurred in response to PMA as well as EGF in HFFs and were observed at both 5 and 45 min poststimulation (Fig. 1B to D). Studies of ERK activation in single Xenopus oocytes demonstrated that the response to progesterone is ultrasensitive, resulting in an all-or-none activation of the entire cellular population of ERK (6). This observation implies that regulation of the amplitude of ERK1/2 activation is unlikely to contribute to the specificity or selectivity of ERK1/2 effector activation. However, the observations shown here suggest that, in somatic cells, ERK1/2 activation is not an all-or-none response. Instead, the amplitude of activation is adjustable and responsive to the dose of external stimulus. Therefore, it is possible that the magnitude of ERK1/2 activation in a cell can influence the nature of the cellular response.
FIG. 1.
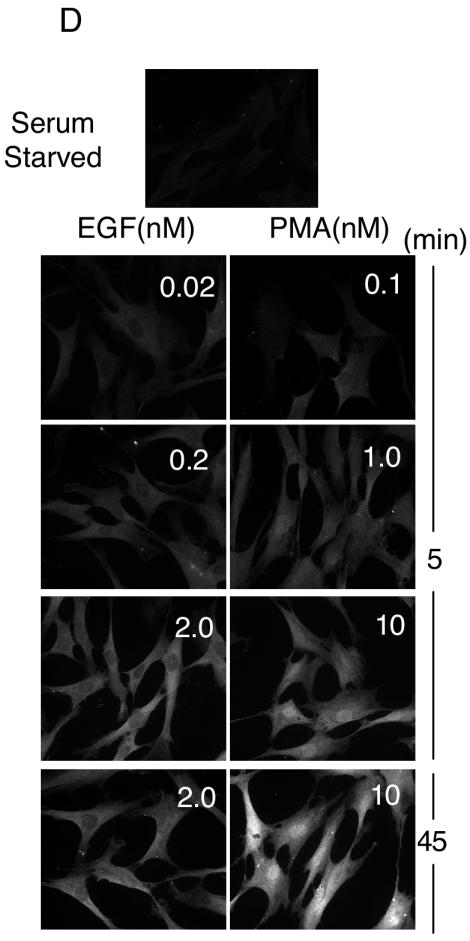
Analysis of ERK1/2 activation at single-cell resolution. (A) Following stimulation for 5 min with EGF at the indicated concentrations, HeLa cells were immunostained or immunoblotted with the dual phospho-ERK1/2 antibody as described in Materials and Methods. Phospho-ERK1/2 immunofluorescence signal intensities from individual cells were calculated as described in Materials and Methods. Histograms derived from greater than 50 cells per condition are shown for a representative experiment. Five additional independent experiments showed similar results. Representative images of phospho-ERK1/2-immunostained cells are shown on the right together with Western analysis of whole-cell lysates. PP-ERK indicates dually phosphorylated active ERK1/2; T-ERK indicates total ERK1/2. (B) The per-cell fluorescence intensity from phospho-ERK1/2-immunostained primary HFFs, stimulated for 5 min with the indicated concentrations of EGF or PMA, was analyzed as for panel A. The histograms shown are a representative experiment from four independent experiments showing similar results. (C) The per-cell fluorescence intensity from phospho-ERK1/2-immunostained primary HFFs, stimulated for 45 min with the indicated concentrations of EGF or PMA, was analyzed as in panel A. (D) Representative images of dual phospho-ERK1/2 staining in stimulated HFFs at indicated ligand concentrations and times. Similar localization patterns were observed at 2 h poststimulation (data not shown).
Stimulus-dependent nuclear accumulation of activated ERK1/2.
ERK1/2 effectors are present in a variety of cell compartments. It is well established in a number of model systems that, following activation, ERK1/2 accumulates in the nucleus and that this nuclear localization is correlated with proliferation and differentiation (3, 4, 10). Differences in nuclear localization of ERK1/2 have been attributed to cell-type-specific expression of proteins, such as PEA-15 and calponin, that restrict their distribution (7, 19). However, in HFFs, ERK1/2 immunostaining revealed strikingly different localization patterns of pp-ERK1/2 in response to EGF compared to PMA (Fig. 1C). At ligand concentrations causing both intermediate and maximal stimulation of ERK1/2, pp-ERK1/2 activated by EGF remained predominantly cytoplasmic while pp-ERK1/2 activated by PMA clearly accumulated in the nucleus. The stimulus-specific subcellular compartmentalization of pp-ERK1/2 was independent of either the amplitude or the duration of ERK1/2 activation. Immunostaining of total ERK1/2 (unphosphorylated and phosphorylated) revealed a similar stimulus-dependent compartmentalization pattern (see Fig. 3A). These results suggest that nuclear accumulation is not an intrinsic property of active ERK1/2, but rather a property conferred on ERK1/2 by the signal inducing ERK1/2 activation. Thus, stimuli can selectively influence both the amount and the localization of activated ERK1/2 in the same cell type.
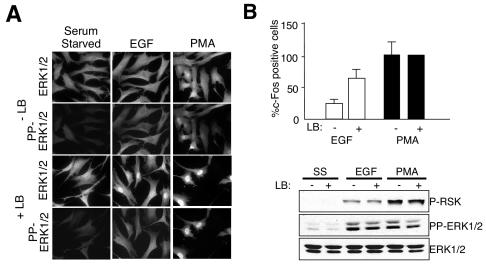
FIG. 3.
Effect of LB on active ERK1/2 localization and accumulation of c-Fos. (A) Following pretreatment with 200-ng/ml LB (+LB) or without LB (−LB), serum-starved HFFs were stimulated with 2 nM EGF or 10 nM PMA for 45 min. Fixed cells were stained with the indicated antibodies. (B) Quantitation of c-Fos-expressing cells treated as in panel A. The percentage of c-Fos-expressing cells in the population is shown normalized to that observed upon PMA stimulation in the absence of LB (arbitrarily set to 100). Error bars represent the standard deviation from the mean from three independent experiments. (C) Whole-cell lysates from HFFs treated as in panel A were immunoblotted with the indicated antibodies.
Effect of pp-ERK1/2 amplitude and localization on effector activation.
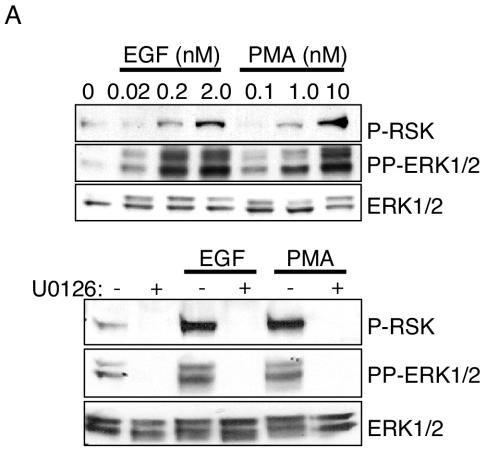
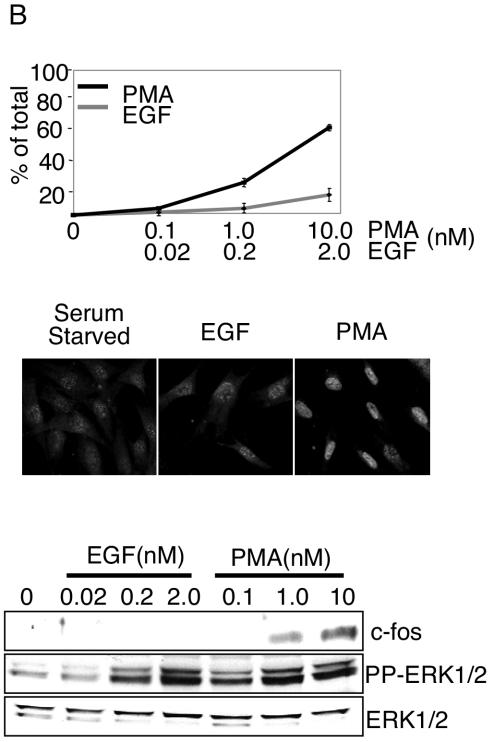
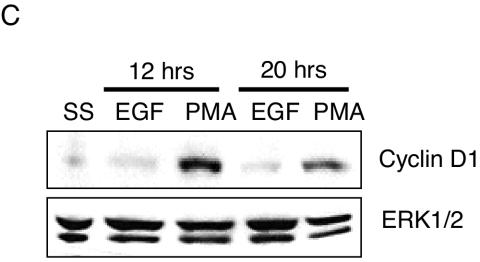
To determine if the amplitude and/or localization of active ERK1/2 impacts activation of its effectors, we monitored EGF- and PMA-induced p90RSK phosphorylation and c-Fos expression. p90RSK is phosphorylated by ERK1/2 in the cytoplasm and subsequently translocates to the nucleus. Threonine 573 of p90RSK is an ERK1/2-specific phosphorylation site (5). Therefore, antibodies that selectively recognize phosphorylated T573 were used to monitor ERK1/2-dependent p90RSK activation. Both EGF and PMA stimulated p90RSK(T573) phosphorylation, which was blocked by pretreatment with 10 μM MEK1/2/5 selective inhibitor U0126 (Fig. 2A, bottom panel). The amplitude of p90RSK phosphorylation at both early (5 min) and later (45 min) time points poststimulation correlated with the dose of the stimulus and degree of ERK1/2 activation (Fig. 2A) (data not shown). Therefore, submaximal amounts of cellular pp-ERK1/2 translate to p90RSK phosphorylation, but we were unable to unequivocally determine by single-cell analysis whether this phosphorylation proceeds in a graded or all-or-none manner. We next examined a nuclear target of ERK1/2, c-Fos. ERK1/2 induces c-Fos accumulation by both promoter activation and phosphorylation-dependent inhibition of c-Fos protein turnover (21). c-Fos accumulation was examined by immunostaining and immunoblotting 45 min poststimulation (Fig. 2B). Despite obvious and comparable levels of ERK1/2 activation by both EGF and PMA, and in contrast to the relatively similar effects of PMA and EGF on p90RSK phosphorylation, only PMA effectively induced c-Fos protein accumulation. This response was blocked by U0126. The quantity of c-Fos protein was proportional to the quantity of active ERK1/2 at different concentrations of PMA. The gene coding for Prad1/cyclin D1 is a target gene regulated by c-Fos (15). Consistent with restricted biological activity of EGF- versus PMA-activated ERK1/2 on c-Fos, cyclin D1 accumulation was observed only in response to PMA (Fig. 2C).
FIG. 2.
Differential responses of ERK1/2 effectors to EGF and PMA stimulation. (A) Whole-cell lysates from serum-starved HFFs stimulated for 5 min with 2.0 nM EGF or 10 nM PMA were immunoblotted with the indicated antibodies. U0126 was used at 10 μM for 45 min prior to stimulation. (B) Forty-five minutes following stimulation of HFFs with the indicated concentrations of EGF or PMA, c-Fos expression was evaluated by immunostaining of fixed cells (top panel) or immunoblotting of whole-cell lysates (bottom panel). The percentage of c-Fos-expressing cells from three independent experiments is shown. Error bars represent the standard deviation from the mean. Representative images from EGF- and PMA-stimulated cells are shown beneath the graph. (C) Cyclin D1 and total ERK1/2 immunoblots from whole-cell lysates of serum-starved (SS) HFFs stimulated for the indicated times with 2 nM EGF or 10 nM PMA.
To examine if the absence of a c-Fos response to EGF may be a consequence of a restricted compartmentalization of pp-ERK1/2, we attempted to drive nuclear accumulation of pp-ERK1/2 artificially in EGF-stimulated cells. Treatment of cells with the Crm1 inhibitor, LB, results in aberrant nuclear accumulation of ERK1/2 (1). In HFFs, LB treatment enhanced both basal and stimulus-induced nuclear accumulation of ERK1/2. In addition, active ERK1/2 accumulated in the nucleus in response to both EGF and PMA in the presence of LB. As shown in Fig. 3B, EGF increased c-Fos expression in the presence of LB compared to that in cells treated with EGF alone, while c-Fos accumulation in response to PMA was unchanged by LB (Fig. 3B). LB treatment did not alter ERK1/2 or p90RSK activation in response to EGF or PMA (Fig. 3C). Therefore, disabling Crm1-dependent nuclear export machinery is sufficient to allow nuclear accumulation of active ERK and c-Fos expression in response to EGF. The cellular responses to Crm1 inhibition are likely to be complex. However, the observed correlation is consistent with the hypothesis that stimulus-dependent compartmentalization of p-ERK has consequences for the specificity of ERK1/2 effector activation.
Here we have shown that stimuli can induce differences in the extent of activation as well as the compartmentalization of ERK1/2 in individual cells. This flexibility contributes to generation of discrete phenotypic responses to distinct environmental stimuli that recruit a common core signal transduction module.
Acknowledgments
This work was supported by grants DK34128 (M.H.C.) and CA71443 (M.A.W.) from the National Institutes of Health and grants I1243 (M.H.C.) and I-1414 (M.A.W.) from the Welch Foundation. A.W.W. was supported by an NIGMS Pharmacological Sciences training grant.
We thank Fred Grinnell for generously providing HFFs, Bing-e Xu for comments about the manuscript, and Dionne Ware for administrative assistance.
REFERENCES
- 1.Adachi, M., M. Fukuda, and E. Nishida. 2000. Nuclear export of MAP kinase (ERK) involves a MAP kinase kinase (MEK)-dependent active transport mechanism. J. Cell Biol. 148:849-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boulton, T. G., and M. H. Cobb. 1991. Identification of multiple extracellular signal-regulated kinases (ERKs) with antipeptide antibodies. Cell Regul. 2:357-371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brunet, A., D. Roux, P. Lenormand, S. Dowd, S. Keyse, and J. Pouyssegur. 1999. Nuclear translocation of p42/p44 mitogen-activated protein kinase is required for growth factor-induced gene expression and cell cycle entry. EMBO J. 18:664-674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen, R.-H., C. Sarnecki, and J. Blenis. 1992. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol. Cell. Biol. 12:915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalby, K. N., N. Morrice, F. B. Caudwell, J. Avruch, and P. Cohen. 1998. Identification of regulatory phosphorylation sites in mitogen-activated protein kinase (MAPK)-activated protein kinase-1a/p90rsk that are inducible by MAPK. J. Biol. Chem. 273:1496-1505. [DOI] [PubMed] [Google Scholar]
- 6.Ferrell, J. E., Jr., and E. M. Machleder. 1998. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science 280:895-898. [DOI] [PubMed] [Google Scholar]
- 7.Formstecher, E., J. W. Ramos, M. Fauquest, D. A. Calderwood, J. C. Hseih, B. Canton, X. T. Nguyen, J. V. Barnier, J. Camonis, M. H. Ginsberg, and H. Chneiweiss. 2001. PEA-15 mediates cytoplasmic sequestration of ERK MAP kinase. Dev. Cell 1:239-250. [DOI] [PubMed] [Google Scholar]
- 8.Gavin, A.-C., and A. R. Nebreda. 1999. A MAP kinase docking site is required for phosphorylation and activation of p90rsk/MAPKAP kinase-1. Curr. Biol. 9:281-284. [DOI] [PubMed] [Google Scholar]
- 9.Gille, H., M. Kortenjann, O. Thomae, C. Moomaw, C. Slaughter, M. H. Cobb, and P. E. Shaw. 1995. ERK phosphorylation potentiates Elk-1-mediated ternary complex formation and transactivation. EMBO J. 14:951-962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gupta, S., and R. J. Davis. 1994. MAP kinase binds to the NH2-terminal activation domain of c-Myc. FEBS Lett. 353:281-285. [DOI] [PubMed] [Google Scholar]
- 11.Hsiao, K., S. Chou, S. Shih, and J. Ferrell, Jr. 1994. Evidence that inactive p42 mitogen-activated protein kinase and inactive Rsk exist as a heterodimer in vivo. Proc. Natl. Acad. Sci. USA 91:5480-5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Janknecht, R., W. Ernst, V. Pingoud, and A. Nordheim. 1993. Activation of ternary complex factor Elk-1 by MAP kinases. EMBO J. 12:5097-5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kao, S., R. K. Jaiswal, W. Kolch, and G. E. Landreth. 2001. Identification of the mechanisms regulating the differential activation of the mapk cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 276:18169-18177. [DOI] [PubMed] [Google Scholar]
- 14.Klemke, R. L., S. Cai, A. L. Giannini, P. J. Gallagher, P. de Lanerolle, and D. A. Cheresh. 1997. Regulation of cell motility by mitogen-activated protein kinase. J. Cell Biol. 137:481-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lavoie, J. N., G. L'Allemain, A. Brunet, R. Muller, and J. Pouyssegur. 1996. Cyclin D1 expression is regulated positively by the p42/p44MAPK and negatively by the p38/HOGMAPK pathway. J. Biol. Chem. 271:20608-20616. [DOI] [PubMed] [Google Scholar]
- 16.Lewis, T. S., P. S. Shapiro, and N. G. Ahn. 1998. Signal transduction through MAP kinase cascades. Adv. Cancer Res. 74:49-139. [DOI] [PubMed] [Google Scholar]
- 17.Lin, L. L., M. Wartmann, A. Y. Lin, J. L. Knopf, A. Seth, and R. J. Davis. 1993. cPLA2 is phosphorylated and activated by MAP kinase. Cell 72:269-278. [DOI] [PubMed] [Google Scholar]
- 18.Marshall, C. J. 1995. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell 80:179-185. [DOI] [PubMed] [Google Scholar]
- 19.Menice, C. B., J. Hulvershorn, L. P. Adam, C.-L. A. Wang, and K. Morgan. 1997. Calponin and mitogen-activated protein kinase signaling in differentiated vascular smooth muscle. J. Biol. Chem. 272:25157-25161. [DOI] [PubMed] [Google Scholar]
- 20.Murphy, L. O., J. P. MacKeigan, and J. Blenis. 2004. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Mol. Cell. Biol. 24:144-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murphy, L. O., S. Smith, R. H. Chen, D. C. Fingar, and J. Blenis. 2002. Molecular interpretation of ERK signal duration by immediate early gene products. Nat. Cell Biol. 4:556-564. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen, A., W. R. Burack, J. L. Stock, R. Kortum, O. V. Chaika, M. Afkarian, W. J. Muller, K. M. Murphy, D. K. Morrison, R. E. Lewis, J. McNeish, and A. S. Shaw. 2002. Kinase suppressor of Ras (KSR) is a scaffold which facilitates mitogen-activated protein kinase activation in vivo. Mol. Cell. Biol. 22:3035-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pearson, G., F. Robinson, T. Beers Gibson, B. E. Xu, M. Karandikar, K. Berman, and M. H. Cobb. 2001. Mitogen-activated protein (MAP) kinase pathways: regulation and physiological functions. Endocr. Rev. 22:153-183. [DOI] [PubMed] [Google Scholar]
- 24.Robinson, M. J., S. A. Stippec, E. Goldsmith, M. A. White, and M. H. Cobb. 1998. A constitutively active and nuclear form of the MAP kinase ERK2 is sufficient for neurite outgrowth and cell transformation. Curr. Biol. 8:1141-1150. [DOI] [PubMed] [Google Scholar]
- 25.Traverse, S., N. Gomez, H. Paterson, C. Marshall, and P. Cohen. 1992. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem. J. 288:351-355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vial, E., and C. J. Marshall. 2003. Elevated ERK-MAP kinase activity protects the FOS family member FRA-1 against proteasomal degradation in colon carcinoma cells. J. Cell Sci. 116:4957-4963. [DOI] [PubMed] [Google Scholar]