Summary
Several memory B‐cell subclasses with distinct functions have been described, of which the most effective is the class‐switched (CS) memory B‐cell population. We have previously shown, using virus‐like particles (VLPs), that the proliferative potential of these CS memory B cells is limited and they fail to re‐enter germinal centres (GCs). However, VLP‐specific memory B cells quickly differentiated into secondary plasma cells (PCs) with the virtue of elevated antibody production compared with primary PCs. Whereas the induction of VLP + memory B cells was strongly dependent on T helper cells, we were wondering whether re‐stimulation of VLP + memory B cells and their differentiation into secondary PCs would also require T helper cells. Global absence of T helper cells led to strongly impaired memory B cell proliferation and PC differentiation. In contrast, lack of interleukin‐21 receptor‐dependent follicular T helper cells or CD40 ligand signalling strongly affected proliferation of memory B cells, but differentiation into mature secondary PCs exhibiting increased antibody production was essentially normal. This contrasts with primary B‐cell responses, where a strong dependence on CD40 ligand but limited importance of interleukin‐21 receptor was seen. Hence, T helper cell dependence differs between primary and secondary B‐cell responses as well as between memory B‐cell proliferation and PC differentiation.
Keywords: antibodies, memory B cells, plasma cell differentiation, secondary response, T helper cell‐dependent immune response
Introduction
B cells are activated when antigens such as viral particles bind to and crosslink their B‐cell receptors (BCRs). After binding, antigen is taken up and processed for antigen presentation on MHC class II (MHC II) molecules to enable interaction with T helper (Th) cells,1, 2 which provide the concomitant second signal for integral B‐cell activation. The Th cells are provided by cell surface molecules such as CD40 ligand (CD40L)3, 4 as well as secreted cytokines such as interleukin‐4 (IL‐4), interferon‐γ or IL‐21.5, 6 With the help of these T‐cell‐derived co‐stimulatory factors, B cells efficiently expand and initiate germinal centre (GC) formation.7 Within GCs, follicular Th (Thf) cells further aid the B‐cell response, eventually leading to affinity maturation and the formation of memory B cells and plasma cells (PCs). These events represent the hallmarks of the humoral immune response and provide the means to avoid re‐infections for years or even lifelong. In the absence of Th cells, B cells fail to respond to soluble proteins. However, early B‐cell responses may occur independently of Th cells upon exposure to antigens exhibiting a repetitive structure, allowing efficient crosslinking of the BCRs8, 9, 10 and/or binding to Toll‐like receptors (TLRs).11, 12, 13 Nevertheless, antibody responses induced in the absence of Th cells are usually short‐lived, dominated by the IgM isotype, and the induction of B‐cell memory and long‐lived PCs is inefficient. Th cell‐independent B cell responses usually occur in the extra‐follicular space and can be prolonged by cytokine secretion of the tumour necrosis factor superfamily such as BLyS (also called BAFF) or APRIL14, 15 produced by Th cells as well as dendritic cells (DCs) and macrophages. Of note, although repetitive viral particles are able to induce transient Th cell‐independent B cell responses, they induce strong and long‐lived antibody responses in the presence of Th cells. Hence, viral particles are both Th cell‐independent and Th cell‐dependent antigens. In the presence of Th cells, B cells form GCs, where the interplay between B cells, Tfh cells and follicular DCs occurs. The H chains of the BCRs undergo isotype switching; mutations accumulate within the complementarity‐determining region (CDRs) followed by subsequent selection for best fit for the antigen. Hypermutation and affinity maturation are largely restricted to B cells, as evidence for a similar process happening for T cell receptors is very limited.16 The question of how BCR affinity affects the B‐cell response remains ill defined. Earlier studies suggested that the BCR affinity may affect the differentiation of an activated B‐cell into plasma blasts, GC B cells or memory B cells.17, 18, 19 On the other hand, it has been shown that the affinity of the BCR does not influence the differentiation but rather the expansion and survival of the differentiated B cells.20, 21, 22 Whereas antibodies secreted by early PCs mediate protection against primary infection, there is evidence that class‐switched (CS) memory B cells are important for mediating protection during secondary infections as they rapidly differentiate into secondary PCs secreting increased levels of antibody upon antigenic re‐exposure.23, 24, 25 In contrast to CS memory B cells, IgM+ memory B cells or naive B cells are partly recruited to GCs to generate a new memory B‐cell pool after antigen re‐exposure.23, 24, 26 Although it has been shown that a memory B cell's fate is dictated by the isotype expressed, it has been proposed that CS memory B cells can re‐enter GCs and acquire additional mutations within the variable regions of the BCR providing enhanced protection.25, 27 Recent findings even suggested that memory B cell function is dependent on the expression of certain surface markers (CD80, CD73, PD‐L2) and not on the BCR isotype.25 Although different functions of memory B cells have been described in recent years, the role of Th cells during memory B cell responses requires further investigation.
We used virus‐like particles (VLPs) derived from the bacteriophage Qβ as the model antigen, which induces strong B cell responses due to the particulate and repetitive structure as well as the packaging of RNA as a natural TLR7/8 ligand. It is known that Qβ‐VLPs can drive Th cell‐independent IgM responses followed by long‐lived Th cell‐dependent IgG responses.28, 29, 30 Here, we demonstrate that VLP‐specific memory B‐cell responses exhibit a hierarchical dependence on Th cells. Memory B cells were generated in a primary host and adoptively transferred into different secondary hosts globally deficient in Th cells (MHC II‐deficient) or specifically lacking CD40L or IL‐21 receptor (IL‐21R) (as a model for Tfh deficiency). We observed that proliferation of VLP‐specific memory B cells showed a strong Th cell dependence and furthermore required CD40L and IL‐21R signalling. In contrast, differentiation of Qβ‐specific memory B cells into PCs and fully mature secondary PCs were only strongly reduced in the global absence of Th cells, whereas CD40L and IL‐21R were dispensable to a large degree.
Materials and methods
Mice
C57BL/6 mice were purchased from Harlan (Horst, the Netherlands). Mouse strains such as Ly5.1 [B6.SJL‐Ptprc<a> Pepc<b>/BoyJ (#002014)], IgHa [B6.Cg‐Gpi1<a> Thy1<a> Igh<a>/J(#001317)], and MHC II−/− [B6.129S2‐H2<dlAb1‐Ea>/J(#003584)] mice were obtained from Jackson Laboratory (Bar Harbor, ME). We thank Prof. Manfred Kopf for the kind donation of IL‐21R−/− [B6N.129‐Il21r<tm1Kopf>/J (#019115)] and Prof. Annette Oxenius for CD40L−/− mice (B6‐CD40L<tm1Renshaw>). All mice were kept under specific pathogen‐free conditions at the BZL mouse facility at the University Hospital Zurich, Switzerland and experiments were performed in accordance with the ethical principles and guidelines of the Cantonal Veterinary Office, Zurich, Switzerland.
Antigen
The Qβ‐bacteriophage‐derived VLPs self‐assemble and enclose mRNA (ssRNA) during the production process in Escherichia coli. They reveal a particulate and repetitive structure rendering them highly immunogenic. The purification process is described elsewhere.31
Immunization
Memory B cells were generated by immunization with 50 μg of Qβ‐VLPs intravenously. Splenocytes of immunized mice were isolated and adoptively transferred into recipient mice, which were challenged with 20 μg Qβ‐VLPs intravenously. The VLPs were diluted with sterile PBS and administered intravenously in a volume of 150 μl.
Adoptive transfer of memory B cells
Congenic mice (Ly5.1 or IgHa) were immunized with Qβ‐VLPs. After at least 4 weeks, spleens were collected in Dulbecco's modified Eagle's medium supplemented with 2% fetal calf serum, antibiotics and 10 mm HEPES. A single‐cell suspension of the splenocytes was prepared. Red blood cells were lysed using ACK buffer (0·15 m ammonium chloride, 0·01 m potassium hydrogen carbonate, pH 7·2–7·4). Splenocytes were either directly transferred or negatively purified by CD4+ MACS beads and transferred into recipients that were Ly5.2‐positive. Purification by CD4+ MACS beads (Miltenyi Biotec, Bergisch Gladbach, Germany) was performed according to the manufacturer's protocol. Purities of CD4‐depleted splenocytes used for adoptive transfers of 98–100% were achieved.
To be able to directly follow the antibody response either derived from transferred memory B cells or from newly activated host B cells, we used IgHa mice to generate memory B cells and transferred them into C57BL/6 mice, which expressed the IgHb‐allotype. In general, recipient mice [Ly5.2, IgHb, CD40L knockout (KO), MHC II KO and IL‐21R KO] received 1/10 of a donor‐mouse (Ly5.1 or IgHa) ‐derived memory spleen. Approximately 1 × 107 cells of total splenocytes containing ~ 0.03‐0.08% (3 × 103 to 8 × 103) VLP‐specific memory B cells were transferred. Control mice received either no splenocytes or 1/10 of a whole spleen of naive Ly5.1 or naive IgHa mice, respectively.
ELISA
Blood was collected at the indicated time points. To determine Qβ‐VLP‐specific IgG antibodies, ELISA plates (Nunc Immuno MaxiSorp, Rochester, NY) were coated with Qβ‐VLPs (1 μg/ml) overnight. Binding of specific serum antibodies was detected by horseradish peroxidase‐labelled goat anti‐mouse IgG (Jackson ImmunoResearch, West Grove, PA). To identify allotype specific antibodies (IgHa or IgHb) pairs of IgHa‐specific [biotin ms anti‐ms IgG1[a] (10·9), biotin ms anti‐ms IgG2a[a] (8·3) from BD (Franklin Lakes, NJ)] and IgHb‐specific [biotin ms anti‐ms IgG1[b] (B68‐2), biotin ms anti‐ms IgG2a[b] (5·7) from BD, Franklin Lakes, NJ] markers were chosen. Antibody binding was determined by horseradish peroxidase‐labelled streptavidin (BD, Franklin Lakes, NJ). Absorbance readings at 450 nm of the 1,2‐phenylenediamine dihydrochloride colour reaction were analysed as OD50.
ELISPOT
Numbers of Qβ‐VLP‐specific PCs in bone marrow (BM) were detected as previously described.32 Briefly, 24‐well plates were coated with 10 μg/ml Qβ‐VLPs overnight. A single‐cell suspension of BM cells was seeded in Dulbecco's modified Eagle's medium containing 2% fetal calf serum and incubated for 5 hr at 37°C. Subsequently, the BM cells were washed off and antibodies produced by specific PCs were detected by binding to goat anti‐mouse IgG (EY Labs, San Mateo, CA), followed by alkaline phosphatase‐conjugated donkey anti‐goat IgG antibody (Jackson ImmunoResearch). ELISPOTS were identified by the development of an alkaline phosphatase colour reaction.
Flow cytometry
Splenocytes were analysed by flow cytometry analysis. Before staining, a single‐cell suspension and red blood cell lysis of samples were performed. Fc receptors were generally blocked with an anti‐CD16/32 antibody. Isotype‐switched memory B cells were stained with and identified as phycoerythrin‐Cy7‐conjugated B220+, negative for phycoerythrin‐conjugated IgD, IgM, CD4, CD8, CD11b, CD11c and Gr‐1, and positive for binding to the fluorochrome Alexa Fluor 488‐labelled Qβ‐VLPs. In addition, specific plasma cells in spleens were stained and characterized as phycoerythrin‐Cy7‐conjugated B220low and negative for phycoerythrin‐conjugated CD4, CD8, CD11b, CD11c and Gr‐1. Subsequently, cells were permeabilized using FACS lysing solution (BD) and intracellular binding of labelled Qβ‐VLPs (Alexa 488) was detected. Previous staining and fixation, and surface binding was blocked by unlabelled Qβ‐VLPs. All B cells derived from the transfer were analysed for allotype marker (Ly5.1) expression by binding to Ly5.1‐allophycocyanin antibody. VLPs were labelled by the fluorochrome Alexa Fluor 488 in accordance with the producer's instruction (Invitrogen, Carlsbad, CA). The cell acquisition was performed by the FACS Canto II (BD) and data were analysed by flow jo version 7.6.4 (Tree Star software, Ashland, OR). All antibodies were purchased from BD Biosciences (Franklin Lakes, NJ) and eBioscience (San Diego, CA).
Statistics
Statistical analysis was performed using the software graph pad prism 6 (Graphpad, San Diego, CA). Statistically significant differences for the two groups were calculated by an unpaired Student's t‐test. For the comparison of three groups, the statistical analysis was performed with a F‐test (analysis of variance). Statistical significance was defined as P < 0·05.
Results
Purification of memory splenocytes comprising memory B cells does not alter the responsiveness of memory B cells
We have previously shown that adoptively transferred unpurified splenocytes containing memory B cells from mice primed with Qβ‐VLPs into wild type (WT) recipient mice mount a secondary B cell response to VLPs upon re‐challenge. Memory B cells poorly proliferated but rapidly differentiated into PCs with an increased capacity to produce antibodies.23 To study the role of Th cells in a naive environment during the re‐call responses of memory B cells, we first wanted to address whether memory B cell proliferation and differentiation were affected by the presence of memory Th cells in the splenocyte preparations. To this end, 8‐week‐old congenic mice (Ly5.1+) were immunized with Qβ‐VLPs. After 4 weeks, spleens containing memory B cells and memory Th cells were isolated and an adoptive transfer of splenocytes that were either left untreated (‘memory transfer’) or CD4+ Th cell‐depleted by MACS purification (‘CD4‐depleted memory transfer’) into Ly5.2+ mice was performed (Fig. 1a). Mice were challenged with Qβ‐VLPs 24 hr after the adoptive transfer on day 0. WT mice (Ly5.2+) that received no adoptive transfer (‘No transfer’) served as a control and mounted a primary response. Detection of VLP‐specific CS B cells in spleen was assessed by flow cytometry (FCM) analysis within the B220+, IgM−, IgD−, CD4−, CD8−, CD11b−, CD11c− and Gr1− population (Fig. 1b, and see Supplementary material, Fig. S1a). Transferred memory B cells were identified within the specific CS B‐cell population using the allotype marker Ly5.1.
Figure 1.
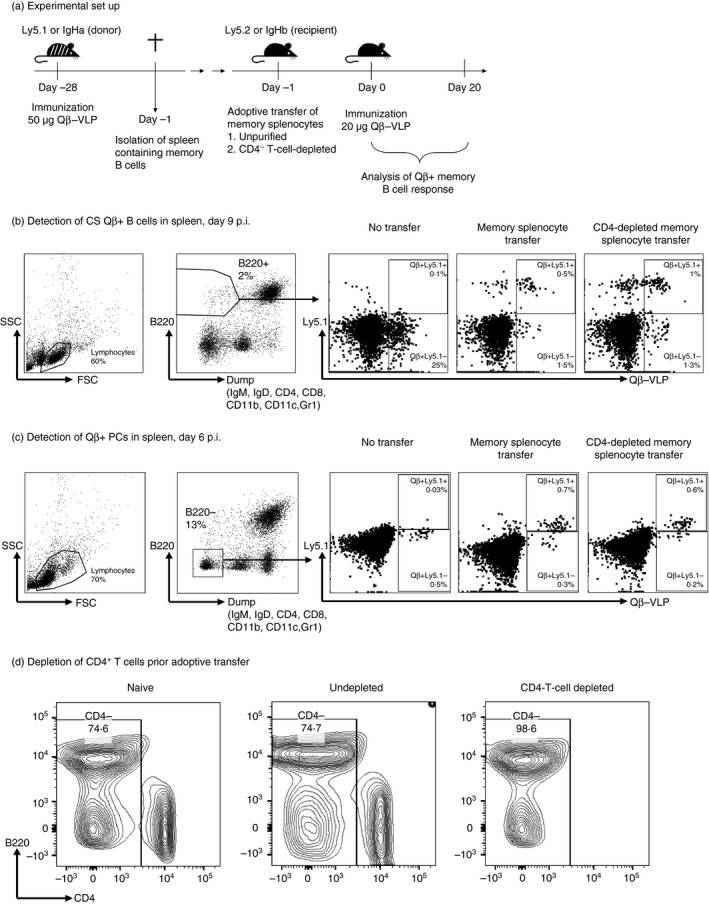
Adoptive transfer of virus‐like particle (VLP) ‐specific memory B cells and the detection of Qβ‐VLP + class‐switched (CS) B cells and plasma cells (PCs) in spleen by flow cytometry analysis. (a) Congenic mice of the Ly5.1 or IgHa strain were immunized to generate memory B cells in an unmanipulated environment. After 4 weeks of immunization, spleens containing memory B cells were isolated and either left untreated or CD4‐depleted by MACS purification. Unpurified or purified splenocytes were transferred into recipient mice (Ly5.2 or IgHb). 24 hr after the adoptive transfer, recipient mice were challenged with Qβ‐VLP. (b) Representative flow cytometry plot to identify CS Qβ‐positive B cells in the spleen of mice 9 days after Qβ‐VLP immunization. B220‐positive lymphocytes negative for IgM, IgD, CD4, CD8, CD11b, CD11c, Gr1 were analysed for Qβ‐VLP binding. Transfer‐derived specific CS B cells in spleen were identified by simultaneous binding to anti‐CD45.1 (Ly5.1) monoclonal antibody. (c) Representative flow cytometry plot to detect Qβ‐specific PCs in the spleen of mice 6 days after immunization with Qβ‐VLP. B220‐negative lymphocytes (also negative for IgM, IgD, CD4, CD8, CD11b, CD11c and Gr1) were analysed for intracellular Qβ‐VLP binding upon permeabilization of the cell membrane. Transfer‐derived PCs were identified by simultaneous binding to anti‐CD45.1 (Ly5.1) monoclonal antibody. (d) Depletion of CD4+ T cells of the splenocyte population before adoptive transfer of memory B cells. A representative example of flow cytometry analysis of murine splenocytes from immunized mice is shown that have been purified by CD4 MACS beads. The proportion of CD4+ T cells within the splenocyte population of naive mice (‘Naive’) and immunized mice (‘Undepleted’) is ~ 20–25% and of CD4‐depleted immunized mice is 1–2%. All splenocytes stained for B220 and CD4 pre‐gated on living cells are shown.
As observed before23 upon transfer of memory splenocytes and subsequent challenge, the capacity of specific CS B cells of host (Fig. 1b and see Supplementary material, Fig. S1a) and transferred B cells (Fig. 2a) to proliferate was limited (Figs 1b and 2a). No difference between CD4‐depleted and non‐depleted memory splenocyte transfer could be observed, indicating that the co‐transferred memory Th cells had no influence on the proliferation of specific B cells. In comparison, Qβ + CS B cells of control mice (‘No transfer’) immunized with Qβ‐VLPs proliferated more extensively and mounted a strong primary VLP‐specific B‐cell response at day 20 after immunization (see Supplementary material, Fig. S1a).
Figure 2.
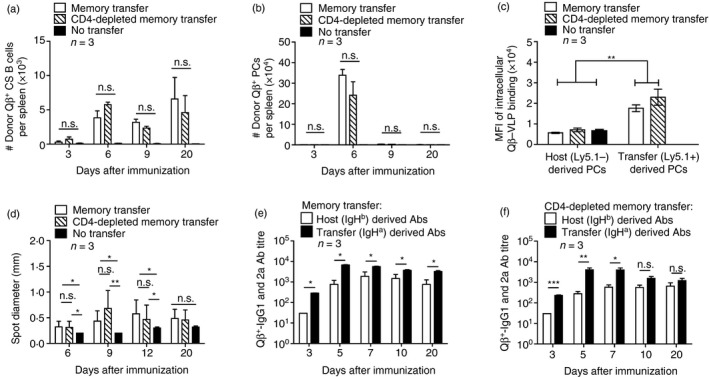
Comparison of adoptive transfers of unpurified or CD4‐depleted memory splenocytes containing memory B cells in wild type (WT) mice after Qβ‐VLP challenge. Congenic mice (Ly5.1 or IgHa) were immunized with Qβ‐VLP to generate memory B cells in an unmanipulated environment. After 4 weeks of immunization, spleens containing memory B cells were isolated and transferred into WT recipient mice (Ly5.2 or IgHb). Splenocytes were either directly and non‐purified transferred as ‘Memory transfer’ or CD4‐negatively purified (MACS beads) and transferred as ‘CD4‐depleted memory transfer’. Mice were challenged with Qβ‐VLP 24 hr after the transfer on day 0. WT control mice (Ly5.2) received ‘No transfer’ and were only immunized on day 0. (a) Detection of donor (Ly5.1+) Qβ‐specific B cells within the class‐switched (CS) B‐cell population (B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen by flow cytometry. Mean with SEM. P‐values represent significances and were obtained by an unpaired Student's t‐test. The control group ‘No transfer’ did not receive Ly5.1+ splenocytes from donor mice and was therefore not considered for statistical analysis. (b) Detection of donor (Ly5.1+) Qβ‐specific plasma cells (PCs) in spleen within B220‐negative lymphocyte population characterized as B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1− by flow cytometry. Mean with SEM. P‐values were obtained by an unpaired Student's t‐test. The control group ‘No transfer’ did not receive Ly5.1+ splenocytes from donor mice and was therefore not considered for statistical analysis. (c) Detection of the mean fluorescence intensity (MFI) of intracellular binding of Qβ‐VLP of host (Ly5.1−) or transfer‐derived (Ly5.1+) PCs in spleen by flow cytometry. P‐value was obtained by an unpaired Student's t‐test. (d) Analysis of spot diameter of bone marrow (BM) ‐derived PCs specific for Qβ‐VLPs in ELISPOT. P values were obtained by an unpaired Student's t‐test. (e) Qβ‐VLP‐specific IgG1 and IgG2a antibody ELISA titre analysed in serum originated from allotype Ha (derived from transferred memory B cells) or Hb (derived from newly activated host B cells) upon transfer of unpurified memory splenocytes. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (f) Qβ‐VLP‐specific IgG1 and IgG2a antibody ELISA titre analysed in serum originated from allotype Ha (derived from transferred memory B cells) or Hb (derived from newly activated host B cells) upon transfer of CD4‐depleted memory splenocytes. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. *P < 0·05, **P < 0·01, ***P < 0·001. Mice per group n = 3. Data are representative of at least three independent experiments.
However, transferred memory B cells quickly differentiated into Qβ‐specific PCs characterized as Qβ + (intracellular), B220−, IgM−, IgD−, CD4−, CD8−, CD11b−, CD11c− and Gr1− in spleen at day 6 after challenge compared with control mice (‘No transfer’) (Fig. 1c, and see Supplementary material, Fig. S1b). Of note, most of the Qβ‐specific PCs in mice that had received an adoptive memory splenocyte transfer were positive for Ly5.1 and therefore donor derived (Fig. 2b). Compared with control mice (‘No transfer’) we did not only detect an increased number of VLP‐specific PCs in spleen by FCM but also in BM by ELISPOT. The increased PC populations were only found at a very early time point (day 6) upon transfer of memory splenocytes containing memory B cells indicating homing of PCs to the BM (see Supplementary material, Fig. S1c). This observation is in contrast to the primary response, in which a significant specific PC population in the BM is usually only detected at later time points. The increased donor‐derived Qβ‐VLP‐specific PC populations in spleen and BM caused an elevated antibody level detected in serum at early time points (day 4 and day 6) compared with immunized control mice (‘No transfer’) (see Supplementary material, Fig. S1d). In comparison, upon transfer of CD4‐depleted memory splenocytes containing memory B cells, the differentiation of memory B cells into PCs (Figs 1c and 2b), the kinetics and magnitude (see Supplementary material, Fig. S1b–d) of specific IgG responses were similar to those in the presence of memory CD4+ Th cells.
As described before,23 memory B‐cell‐derived secondary PCs exhibited an enhanced capacity to produce antibodies. In comparison to non‐memory‐derived primary PCs, the mean fluorescence intensity of intracellular Qβ‐VLP binding in secondary PCs in spleen and the ELISPOT sizes of secondary PCs in BM were increased, indicating increased antibody production. Consistent with this previous finding we observed increased capacity for antibody production in secondary PCs derived from both transferred CD4‐depleted and unpurified memory splenocytes (Fig. 2c, d) compared with mice, which did not receive an adoptive transfer (‘No transfer’).
To assess the induction of secondary PCs, we quantified the production of host versus donor‐derived antibodies by using congenic mice expressing allotype a (IgHa) instead of b (IgHb) for adoptive transfer (Fig. 1a). As for Ly5.1 congenic mice, PCs of the allotype a, which had differentiated from transferred memory B cells, produced more antibodies, especially at early time‐points, than PCs that were derived from newly activated host B cells of the allotype b (Fig. 2c, d). Transfer of either unpurified (Fig. 2e) or CD4‐depleted (Fig. 2f) memory splenocytes containing memory B cells from immunized congenic IgHa into IgHb mice could directly show that most antibodies were secreted by donor‐derived PCs.
Altogether our findings showed that induction of secondary PCs was not affected by the absence of CD4+ memory T cells, indicating a limited influence of co‐transferred memory Th cells on the VLP‐specific memory B cell response. Hence, the purification of memory splenocytes by CD4+ MACS beads treatment did not affect the responsiveness of VLP‐specific memory B cells. Therefore, the transfer of CD4‐depleted memory splenocytes containing memory B cells without Th cell correlates into different knockout strains is a suitable means to investigate the role of Th cells in VLP‐specific memory B‐cell responses.
Qβ‐VLP‐induced memory B cell responses are strongly reduced in the absence of CD4+ T cell help
Whether CD4+ Th cells are required at all for memory B cell responses against VLPs was addressed next. To this end, we performed adoptive transfer experiments of memory B cells into MHC II‐deficient mice that have virtually no CD4+ T cells;33 therefore no T cell help is provided. In MHC II KO mice B cells still develop normally from BM progenitors and home to the spleen forming B cell follicles.
Congenic (Ly5.1+) mice were immunized with Qβ‐VLPs and after 4–6 weeks spleens were isolated. To deplete specific donor Th cells, splenocytes of immunized mice were negatively purified by CD4+ MACS beads and transferred into WT as well as MHC II KO mice followed by immunization with Qβ‐VLPs after 24 hr. To observe differences of primary versus memory B cell responses, control WT and MHC II KO mice did not receive an adoptive transfer and were only immunized.
As described above Qβ + CS B cells and Qβ + PCs in spleen were analysed by FCM and identified as Qβ + lymphocytes (Fig. 1b,c) at representative time points (combined data are shown in Fig. 3; first experiment analysed on days 5 and 15; second experiment analysed on days 5 and 18).
Figure 3.
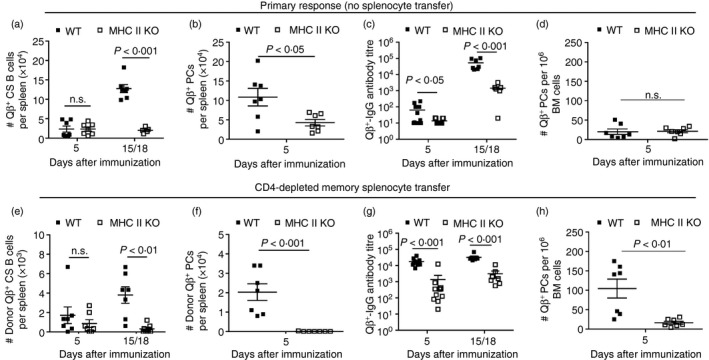
Primary and memory B‐cell responses to Qβ‐VLP in wild type (WT) and MHC II knockout (KO) mice. WT and MHC II KO mice received no adoptive transfer and were only immunized with Qβ‐VLPs on day 0. The primary response of those mice is shown in (a)–(d). Splenocytes of Qβ‐VLP‐immunized congenic (Ly5.1+) mice were isolated and negatively purified by CD4+ MACS beads. Purified splenocytes containing memory B cells were transferred into WT and MHC II KO mice and challenged with Qβ‐VLPs 24 hr after the adoptive transfer on day 0. The secondary response of the transferred memory B cell is shown in (e)–(h). Primary response (No splenocyte transfer). (a) Detection of specific B cells within the B220‐positive lymphocyte population (numbers of Qβ +, B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and MHC II KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (b) Detection of specific plasma cells (PCs) within the B220‐negative lymphocyte population (numbers of Qβ +, B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and MHC II KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (c) Measurement of Qβ‐VLPs total IgG antibody ELISA titre in sera of WT and MHCII KO mice. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (d) ELISPOT analysis of bone marrow (BM) ‐derived PCs specific for Qβ‐VLPs. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. Memory response (CD4‐depleted memory splenocyte transfer). (e) Detection of memory B‐cell transfer‐derived (Donor, Ly5.1+) specific class‐switched (CS) B cells within the B220‐positive lymphocyte population (numbers of Ly5.1+, Qβ +, B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and MHC II KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (f) Detection of memory B‐cell transfer‐derived (Donor, Ly5.1+) ‐specific PCs within the B220‐negative lymphocyte population (numbers of Ly5.1+, Qβ +, B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and MHC II KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (g) Measurement of Qβ‐VLP total IgG antibody ELISA titre in sera of WT and MHC II KO mice. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (h) ELISPOT analysis of BM‐derived PCs specific for Qβ‐VLP. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. Combined data of two independent representative experiments. First experiment shows data from days 5 and 15 and second experiment shows data from days 5 and 18. Mice per group first experiment n = 3 and second experiment n = 4 (or n = 8 on day 5 in c and g).
During the primary response, the Qβ‐specific CS B cell and PC populations in spleen were strongly reduced upon Qβ‐VLP immunization in MHC II KO mice compared with WT mice (Fig. 3a, b). A few specific PCs were detected on day 5 in the BM by ELISPOT analysis in WT and MHC II KO mice (Fig. 3d). Along with the reduced cellular response,34 the IgG antibody production in mice lacking the MHC II molecule was predominantly impaired at a late time point (day 15/18) when compared with WT mice (Fig. 3c). Transferred CD4‐depleted memory splenocytes containing memory B cells proliferated in WT mice, whereas the proliferation of memory B cells in MHC II KO mice was strongly impaired (Fig. 3e). The differentiation of transferred memory B cells into PCs found in spleen (Fig. 3f) and BM (Fig. 3h) was also significantly reduced in MHC II KO mice compared with WT mice. The reduced specific B cell response in MHC II KO mice upon adoptive transfer of memory B cells was accompanied by a strongly reduced IgG antibody response at the analysed time points (day 5 and day 15/18) (Fig. 3g).
As observed before,28, 29 Qβ‐VLP immunization of WT mice leads to a strong cellular activation reflected by rapid and strong CS B cell and PC populations in spleen as well as humoral response during a primary response. When memory B cells were transferred into WT mice, they proliferated poorly, as the number of transfer‐derived Qβ + CS B cells was overall strongly reduced (Fig. 3e, f) compared with the Qβ + CS B‐cell population in a primary response (Fig. 3a, b). However, the PC population, particularly in the BM, was increased (Fig. 3h) and led to an increased Qβ + IgG antibody level, especially at an early time point. In the absence of Th cells, Qβ‐VLP immunization of MHC II KO mice led to a poor cellular activation of Qβ + CS B cells and PCs in spleen (Fig. 3a, b) and low Qβ + IgG antibody titre (Fig. 3c) during the primary response when compared with WT mice. Transferred memory B cells poorly proliferated and failed to differentiate into PCs in spleen and BM in MHC II KO mice (Fig. 3e, f, h). Nevertheless, IgG humoral responses in MHC II KO mice were elevated in the presence of memory B cells (Fig. 3g) compared with the primary response in MHC II KO mice (Fig. 3c), indicating that some differentiation of memory B cells into PCs occurred in the complete absence of Th cells.
In summary, the induction of Qβ‐specific B cell responses was strongly impaired in MHC II KO mice upon primary injection of Qβ‐VLPs. Specific memory B cell responses, however, were even more affected as almost no proliferation nor differentiation was observed in the absence of Th cells during VLP re‐challenge.
Memory B cells can efficiently differentiate into PCs and produce vast amounts of antibodies independent of CD40L signalling
The most important molecule for cognate Th cells is CD40L, which triggers CD40 on B cells.35 To address the role of CD40L on memory B cell responses, we performed the same set of adoptive transfer experiments as described above for MHC II‐deficient mice using CD40L‐deficient recipient mice. Briefly, memory B cells were generated in congenic mice (Ly5.1+) in an unmanipulated environment in the presence of Th cells. Isolated splenocytes were CD4+ T cell‐depleted before transfer into WT or CD40L KO mice. These mice were immunized with Qβ‐VLPs 24 hr after the transfer. To be able to compare memory B cell responses with primary B cell responses, control WT and CD40L KO mice were immunized with Qβ‐VLPs without an adoptive transfer of memory splenocytes.
Qβ + B lymphocytes of primary and memory B cell responses were analysed by FCM (Fig. 1b, c) at representative time points (combined data are shown in Fig. 4; first experiment analysed on days 5 and 15; second experiment analysed on days 5 and 18). BM‐derived specific PCs were assessed in ELISPOT analysis.
Figure 4.
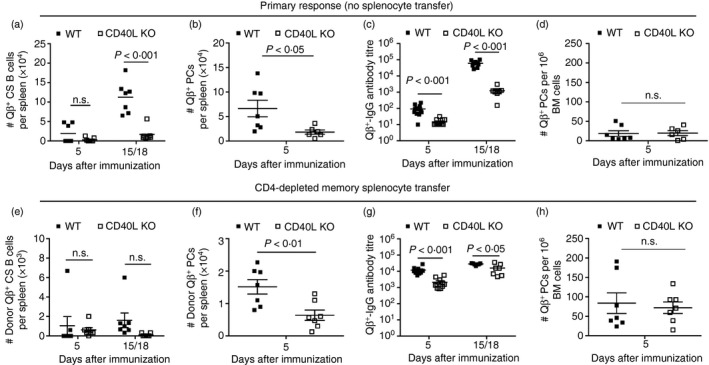
Primary and memory B‐cell responses to Qβ‐VLP in wild type (WT) and CD40L KO mice. WT and CD40L knockout (KO) mice received no adoptive transfer and were only immunized with Qβ‐VLPs on day 0. The primary response of those mice is shown in (a)–(d). Splenocytes of Qβ‐VLP‐immunized congenic (Ly5.1+) mice were isolated and CD4 T cell‐depleted (by MACS beads). CD4‐depleted memory splenocytes containing memory B cells were transferred into WT and CD40L KO mice and challenged with Qβ‐VLPs 24 hr after the adoptive transfer on day 0. The secondary response of the transferred memory B cells is shown in (e)–(h). Primary response (No splenocyte transfer). (a) Detection of specific B cells within the B220‐positive lymphocyte population (numbers of Qβ +, B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and CD40L KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (b) Detection of specific plasma cells (PCs) within the B220‐negative lymphocyte population (numbers of Qβ +, B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and CD40L KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (c) Measurement of Qβ‐VLP total IgG antibody ELISA titre in sera of WT and CD40L KO mice. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (d) ELISPOT analysis of bone marrow (BM) ‐derived PCs specific for Qβ‐VLPs. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. Memory response (CD4‐depleted memory splenocyte transfer). (e) Detection of memory B‐cell transfer‐derived (Donor, Ly5.1+) specific class‐switched (CS) B cells within the B220‐positive lymphocyte population (numbers of Ly5.1+, Qβ +, B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and CD40L KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (f) Detection of memory B‐cell‐transfer‐derived (Donor, Ly5.1+) specific PCs within the B220‐negative lymphocyte population (numbers of Ly5.1+, Qβ +, B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and CD40L KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (g) Measurement of Qβ‐VLP total IgG antibody ELISA titre in sera of WT and CD40L KO mice. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (h) ELISPOT analysis of BM‐derived PCs specific for Qβ‐VLPs. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. Combined data of two independent representative experiments. First experiment shows data from days 5 and 15 and second experiment shows data from days 5 and 18. Mice per group first experiment n = 3 and second experiment n = 4 (or n = 8 on day 5 in c and g).
The induction of Qβ‐VLP‐specific CS B cells (Fig. 4a) and PCs (Fig. 4b) in spleen as well as IgG antibodies in blood (Fig. 4c) were strongly reduced during primary responses in mice lacking CD40L compared with WT mice. In CD40L KO mice, transferred CD4‐depleted memory B cells proliferated to a low extent detectable on days 5 and 15/18 (Fig. 4e) and differentiated into memory B cell‐derived PCs (Fig. 4f) analysed on day 5 in spleen. The number of specific PCs in BM on day 5 was comparable in WT and CD40L KO mice upon transfer of memory B cells (Fig. 4h). Although the Qβ‐specific B cell populations were reduced in spleen (Fig. 4e, f), the BM‐derived PC population was similar in KO and WT recipient mice (Fig. 4h) resulting in only slightly reduced VLP‐specific IgG responses in sera at an early time point (day 5) and almost comparable levels at a later time point (day 15/18) when compared with WT mice (Fig. 4g).
Taken together, the primary response to Qβ‐VLPs is strongly reduced in CD40L KO mice compared with WT mice. In contrast, only the proliferation of VLP‐specific memory B cells is highly impaired in the absence of CD40L but differentiation into PCs found in spleen and BM and IgG antibody responses were almost normal in the absence of CD40L signalling.
The influence of IL‐21R signalling on memory B‐cell responses
To address whether Tfh cells play a role in memory B cell responses we performed memory B cell adoptive transfer experiments as described above in IL‐21R KO mice. IL‐21R KO mice exhibit strongly reduced levels of functional Tfh cells as IL‐21 signalling is essential for their differentiation and localization in B cell follicles.36, 37 They also show reduced granulocyte numbers and activity, reduced proliferation of lymphocytes as well as decreased antibody levels especially of the IgA and IgG1 isotypes.38 The primary immune response induced by VLP vaccination is, however, only marginally affected as granulocytes are not involved in the response and TLR7 engagement by the packaged RNA largely overcomes IL‐21R/IL‐21 signalling dependence.39, 40 The IL‐21/IL‐21R signalling has a strong influence on the formation of GCs in B cell follicles during the primary B cell response. In particular, IL‐21 has an impact on the differentiation and proliferation of B cells and promotes the interaction of GC B cells with Tfh cells as well as the formation of PCs.41 In our recent study, we found that VLP‐specific memory B cells differentiated into PCs and did not form or re‐enter GCs.23 Hence, the influence of IL‐21R signalling in GC reactions mediated by Qβ + memory B cells can be neglected in our setting, as it does not happen.
Congenic (Ly5.1+) WT mice were immunized to develop memory B cells in an immunocompetent environment. Spleens were collected 4–6 weeks after immunization. CD4‐depleted splenocytes were subsequently adoptively transferred into WT and IL‐21R KO mice. Animals were challenged with Qβ‐VLPs 24 hr later. Control WT and IL‐21R KO mice were only immunized (‘Primary response’). The analysis of specific B cell responses was performed as described above (Fig. 1b, c). As shown previously, during the primary response, the generation of specific CS B cells was unaffected or even increased (Fig. 5a), whereas differentiation into PCs found in spleen (Fig. 5b) as well as Qβ + IgG antibodies (Fig. 5c) were slightly reduced in IL‐21R‐deficient mice when compared with WT mice.40 No difference in the early specific PC population in BM was observed (Fig. 5d).
Figure 5.
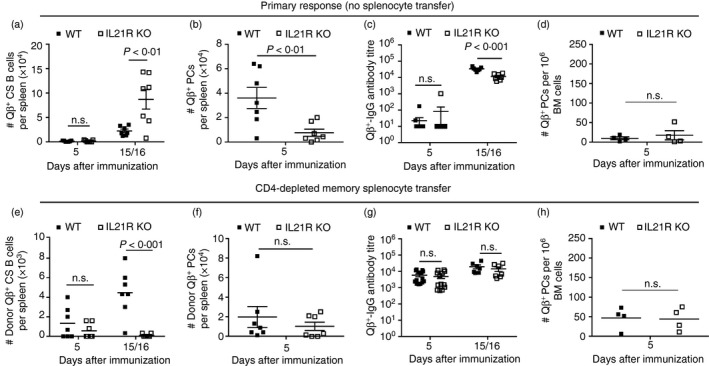
Primary and memory B‐cell responses to Qβ‐VLPs in wild type (WT) and interleukin‐21 receptor (IL‐21R) knockout (KO) mice. WT and IL‐21R KO mice received no adoptive transfer and were only immunized with Qβ‐VLPs on day 0. The primary response of those mice is shown in (a)–(d). Splenocytes of Qβ‐VLP‐immunized congenic (Ly5.1+) mice were isolated and CD4‐depleted (by MACS beads). CD4‐depleted memory splenocytes containing memory B cells were transferred into WT and IL‐21R KO mice and challenged with Qβ‐VLPs 24 hr after the adoptive transfer on day 0. The secondary response of the transferred memory B cells is shown in (e)–(h). Primary response (No splenocyte transfer). (a) Detection of specific B cells within the B220‐positive lymphocyte population (numbers of Qβ +, B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and IL‐21R KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (b) Detection of specific plasma cells (PCs) within the B220‐negative lymphocyte population (numbers of Qβ +, B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and IL‐21R KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (c) Measurement of Qβ‐VLPs total IgG antibody ELISA titre in sera of WT and IL‐21R KO mice. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (d) ELISPOT analysis of bone marrow (BM) ‐derived PCs specific for Qβ‐VLPs. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. Memory response (CD4‐depleted memory splenocyte transfer). (e) Detection of memory B‐cell transfer derived (Donor, Ly5.1+) specific class‐switched (CS) B cells within the B220‐positive lymphocyte population (numbers of Ly5.1+, Qβ +, B220+, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and IL‐21R KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (f) Detection of memory B‐cell transfer‐derived (Donor, Ly5.1+) specific PCs within the B220‐negative lymphocyte population (numbers of Ly5.1+, Qβ +, B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1−) in spleen of WT and IL‐21R KO mice by flow cytometry. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (g) Measurement of Qβ‐VLP total IgG antibody ELISA titre in sera of WT and IL‐21R KO mice. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. (h) ELISPOT analysis of BM‐derived PCs specific for Qβ‐VLP. Mean with SEM. P‐values were calculated by an unpaired Student's t‐test. Combined data of two independent representative experiments. First experiment shows data from days 5 and 15 (a–c, e–g) and second experiment shows data from day 5 and 16 (a–h). Mice per group first experiment n = 3 and second experiment n = 4 (or 8 on day 5 in c and g, in d and h only n = 4).
The proliferation of transferred Qβ‐specific memory B cells was highly reduced in IL‐21R KO mice compared with WT mice (Fig. 5e). In contrast, memory B cell‐derived PCs found in spleen (Fig. 5f) and BM (Fig. 5h) on day 5 after VLP re‐challenge were comparable in IL‐21R KO and WT mice. In line with this, Qβ‐specific IgG antibody responses were similar in IL‐21R KO mice compared with WT mice at the analysed time points (day 5 and day 15/16) (Fig. 5g).
Taken together, primary B‐cell responses to Qβ‐VLPs upon immunization in IL‐21R KO mice compared with WT mice were only partly reduced, as shown for the PC population in spleen and antibody levels. These observations go in line with B cell responses described by Zotos et al.41 In comparison, absence of Tfh cells caused a similar phenotype to that of CD40L‐deficiency for memory B cell responses, as memory B cell proliferation was reduced while PC differentiation and IgG production were normal. Hence, CD40L and IL‐21R signalling are more important during primary than secondary Qβ‐specific responses.
Generation of fully mature secondary PCs is Th cell‐dependent
We recently found that VLP‐specific memory B cells adoptively transferred into WT recipient mice differentiated into PCs upon cognate antigen re‐encounter, exhibiting the unique feature of producing more antibodies than primary PCs generated in a primary response. Whether the generation of fully mature secondary PCs was dependent on Th cells was addressed next. Secondary PCs derived from CD4‐depleted memory splenocyte transfer containing memory B cells were compared with primary PCs derived from non‐memory host B cells in recipient mice in the spleen for their intracellular binding of Qβ‐VLPs. The increased mean fluorescence intensity reflects the increased amount of antibodies within these PCs.
To this end, memory B cells were generated in congenic (Ly5.1+) mice upon Qβ‐VLP immunization. After 4–6 weeks spleens were isolated and CD4 was depleted by MACS bead purification before transfer into WT, MHC II KO, CD40L KO and IL‐21R KO mice. The animals were challenged with Qβ‐VLPs 24 hr after the transfer. On days 5 and/or 6, spleens of mice that had received the adoptive CD4‐depleted memory splenocyte transfer were analysed. Specific PCs in spleens were identified as Qβ + B220− lymphocytes (being negative for IgM, IgD, CD4, CD8, Gr1, CD11b, CD11c) (Fig. 1b). Memory B cell‐derived PCs were distinguished from host‐derived PCs by the expression of the Ly5.1 congenic cell marker and could be directly compared in one host. In WT mice, memory B cell‐derived PCs (Ly5.1+) showed an increased binding for intracellular Qβ‐VLP compared with host (Ly5.1−) ‐derived PCs (Fig. 6a–c, WT panels) reflecting the generation of secondary PCs from memory B cells with an enhanced capacity to produce antibodies. In MHC II KO mice almost none of the memory B cell‐derived PCs (Ly5.1+, only in two out of seven mice) (Fig. 3f) nor host‐derived PCs (Ly5.1−) (data not shown) were detected because of the complete failure of PC generation in the absence of global Th cells. Although general Th cells were critical for generation of secondary PCs, absence of CD40L and IL‐21R signalling was dispensable for the generation of secondary PCs. Memory B cell‐derived PCs in CD40L KO and IL‐21R KO mice showed increased intracellular Qβ‐VLP binding compared with their primary counterparts (host Ly5.1−) (Fig. 6b, c).
Figure 6.
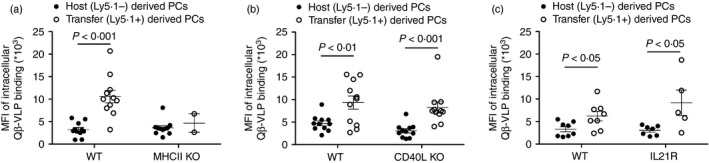
Comparison of intracellular binding of Qβ‐VLPs of host derived (Ly5.1−) versus memory B cell transfer‐derived (Ly5.1+) plasma cells. Congenic mice (Ly5.1+) were immunized with Qβ‐VLPs and their splenocytes were isolated after 4–6 weeks. Isolated splenocytes containing memory B cells were CD4‐depleted by MACS purification and transferred into Ly5.2+ recipient mice [wild type (WT), MHC II knockout (KO), CD40L KO and IL21R KO]. Mice were challenged with Qβ‐VLPs after 24 hr upon adoptive transfer on day 0. Mean fluorescence intensity of intracellular binding of Qβ‐VLPs of host‐derived (Ly5.1−) and memory B cell transfer‐derived (Ly5.1+) plasma cells (PCs) were measured by flow cytometry characterized as B220−, IgM−, IgD−, CD4−, CD8−, CD11c−, CD11b−, Gr‐1− in WT and MHC II KO mice (a), in WT and CD40L KO mice (b) and in WT and IL21R KO mice (c) on days 5 and 6 post‐challenge with Qβ‐VLP. Mean with SEM. P‐values were obtained by an unpaired Student's t‐test. Combined data of two independent representative experiments for MHC II KO (a) and CD40L KO (b); first experiment n = 4, days 5 and 6; second experiment n = 3, day 5. Results from two experiments for IL‐21R KO; first experiment n = 4, day 5; second experiment n = 3, day 5.
In summary, proliferation of memory B cells and differentiation into PCs were highly dependent on Th cells. However, CD40L and IL‐21R signalling were not essential for the differentiation of Qβ + memory B cells into secondary PCs exhibiting increased capacity for antibody production.
Discussion
Primary B cell responses are generally dependent on Th cells. While a transient IgM response may be induced by T cell‐independent antigens, long‐lived IgG responses are usually dependent on CD4+ Th cells and, moreover on Tfh cells in GC reactions and the concomitant interaction of CD40L with CD40. We used three different mouse KO strains to study the role of T cell help: MHC II KO for absence of all Th cells and CD40L KO to investigate the role of one of the key signalling interactions between B and T cells. IL‐21R KO mice were considered to serve as a model of Tfh cell deficiency as Tfh cells are dependent on IL‐21 signalling in a number of systems. Th cell‐dependence may vary among antigens, as very high antigen load upon viral infection may enable the Th cell dependence of IgG responses to be overcome,42 and antigens linked to TLR ligands are able to induce almost normal IgG responses in the absence of Tfh cells.40 In this study, we used Qβ‐VLPs to investigate the Th dependence of memory B cell responses. This antigen induces a Th cell‐independent IgM response, followed by a Th cell‐dependent IgG response.43 During primary responses, global absence of Th cells as well as CD40/CD40L strongly reduces specific IgG responses. In contrast, the absence of Tfh cells leaves IgG responses largely unaffected, as the VLPs are loaded with bacterial RNA, a TLR7/8 ligand.
Memory B cell responses differ from primary B cell responses in various aspects. But both of them take place in B cell follicles of secondary lymphoid organs44 and strictly require the presence of specific antigen.45, 46 Activated Th cells or TLR ligands alone are not able to drive a memory B cell response in the absence of an antigen.45 An important difference between primary and secondary B cell responses is that the latter occurs with accelerated kinetics16 compared with primary responses. A critical reason for the increased speed of memory B cell responses is the fact that memory B cells remain present in the host at increased frequencies and rapidly differentiate into PCs upon antigenic re‐encounter.17, 23, 47 Although it has been shown that memory B cell populations are maintained in the absence of Th cells and antigen,48 the role of Th cells during memory B cell responses is less clear. The majority of model antigens used to study B cell responses are soluble proteins or cells24, 26 which are Th cell‐dependent antigens and do not cross‐link BCRs as efficiently as VLPs do and are therefore unable to efficiently activate B cells in the absence of Th cells. In addition, these antigens are not linked to TLR ligands. VLPs are able to induce strong T cell‐independent primary IgM responses in the complete absence of Th cells and primary IgG responses occur almost normally in the absence of Tfh cells. Therefore, it may be expected that VLPs drive memory B cell responses in a more Th cell‐independent fashion than other soluble antigens. In line with this, we have previously shown that adoptively transferred memory B cells mounted partially T cell‐independent IgG responses in irradiated recipient mice upon immunization with viral particles but not with recombinant protein.49 In another study, adoptive transfer experiments were performed using RAG1‐deficient mice to compare memory B cell responses to intact virus and soluble viral protein.50 The authors reported that the transferred memory B cells mediated a humoral response to the viral particle which was independent of cognate as well as bystander Th cells. To assess the immune response of memory B cells transferred into RAG1 KO mice may be difficult, as B cells are transferred into a non‐physiological environment free of lymphocytes and secondary lymphoid organs with appropriate structures.51 To avoid this issue, we generated VLP‐specific memory B cells in unmanipulated WT mice and transferred them into hosts deficient of T cell help, which allows us to study their responses in an environment containing B cell follicles in secondary lymphoid structures. Another study by Weisel et al.44 from the same group revealed that virus‐specific memory B cells may differentiate into PCs in the absence of Th cells, as indicated by the detection of antibodies after VLP challenge. This study, however, failed to assess memory B cell and PC responses in parallel. Nevertheless, these observations made by Weisel et al.44 are partly in line with our findings. We also detected IgG production in the absence of CD4+ Th cells; however, these responses were markedly reduced. In addition, PC formation and expansion of CS memory B cells were strongly dependent on the presence of CD4+ T cells during Qβ + memory B cell responses. Antibody responses and PC generation, however, were apparently not dependent on cognate Th cells, as they occurred rather efficiently in the absence of CD40L/CD40 as well as IL‐21R signalling. In contrast, expansion of specific CS memory B cells was almost completely abrogated in the absence of CD40L or IL‐21R. This demonstrates that cognate Th cells are required for expansion of memory B cells but not for their differentiation into PCs. Hence interaction with antigen alone in an environment containing CD4+ Th cells is sufficient for differentiation of VLP‐specific memory B cells into PCs. In contrast, a direct interaction of memory B cells with cognate Th cells is required for their proliferation. These findings are consistent with the observation that GC B cells preferentially differentiate into PCs in the absence of CD40L in vitro.17
Interestingly, CD40L and Tfh cells were not required for differentiation of VLP‐specific memory B cells into secondary PCs producing increased amounts of antibodies. In contrast, secondary PCs were not generated in the global absence of Th cells. It will be interesting to determine whether the few PCs generated in the absence of Th cells mount more short‐lived responses compared with fully differentiated secondary PCs. The observation that secondary PC responses depended strongly on Th cells but were rather independent of CD40L and IL‐21R signalling indicates that either non‐cognate functions of Th cells can induce secondary PCs from memory B cells or that a cognate function of Th cells other than CD40L or ILl21R is involved. Therefore it will be interesting to investigate the role of other co‐stimulatory interactions such as CD28/CD80 or inducible T cell co‐stimulator (ICOS)/ICOS ligand or other cytokines (e.g. IL‐4, interferon‐γ) for the generation of secondary PCs. Although IL‐21R signalling was absent in IL‐21R KO recipient mice, the transferred memory B cells were still capable of conducting IL‐21 signalling. It will be interesting to investigate whether IL‐21 signalling in B cells was key for the differentiation of transferred memory B cells into mature secondary PCs.
Failure to differentiate into fully mature secondary PCs in the absence of Th cells may be interpreted as a safety measure as this step is not only controlled by the presence of antigen but also by the presence of Th cells. As Th cell tolerance is usually stricter than B cell tolerance, in particular after B cells have undergone rounds of hypermutation, control by Th cells may avoid the generation of self‐specific secondary PCs producing maximal amounts of antibodies. Nevertheless, differentiation of memory B cells to IgG‐producing PCs is less strictly controlled than the generation of IgG‐producing PCs during primary responses. This may reflect the fact that memory B cells are present at increased frequencies compared with ‘specific’ naive cells. Therefore, less proliferation is required to generate measurable frequencies of specific PCs from memory B cells. In addition, generation of memory B cells has been cross‐checked previously by the presence of specific Th cells during the primary response.
As shown before, Qβ‐VLPs induced largely normal primary B cell responses in the absence of Tfh cells at both the humoral and cellular levels. It is therefore rather unexpected that the expansion of CS memory B cells was strongly dependent on Tfh cells. This may be a way by which the immune system restricts proliferation of memory B cells to allow naive B cells to enter the memory B cell pool during secondary B cell responses.
Taken together, this study's findings reveal distinct novel mechanisms regulating primary versus secondary B cell responses as well as memory B cell proliferation versus PC differentiation.
Disclosures
The authors have no financial conflicts of interest.
Supporting information
Figure S1. Tracking of transferred memory splenocytes containing memory B cell in wild type (WT) mice after Qβ‐bacteriophage‐derived virus‐like particle (Qβ‐VLP) challenge.
Acknowledgements
This project was supported by funding of the Swiss National Science Foundation (SNF grant 31003A_149925 to Prof. Dr Martin Bachmann). FZ planned, performed and analysed the experiments, interpreted the data and wrote the manuscript. AF and PJ interpreted data and contributed to the discussions. MV, TMK and MFB interpreted the data and contributed to writing the manuscript and discussions.
References
- 1. Noelle RJ, Snow EC. T helper cell‐dependent B cell activation. FASEB J 1991; 5:2770–6. [DOI] [PubMed] [Google Scholar]
- 2. Parker DC. T cell‐dependent B cell activation. Annu Rev Immunol 1993; 11:331–60. [DOI] [PubMed] [Google Scholar]
- 3. Klaus SJ, Berberich I, Shu G, Clark EA. CD40 and its ligand in the regulation of humoral immunity. Semin Immunol 1994; 6:279–86. [DOI] [PubMed] [Google Scholar]
- 4. Klaus SJ, Pinchuk LM, Ochs HD, Law CL, Fanslow WC, Armitage RJ et al Costimulation through CD28 enhances T cell‐dependent B cell activation via CD40–CD40L interaction. J Immunol 1994; 152:5643–52. [PubMed] [Google Scholar]
- 5. Parrish‐Novak J, Dillon SR, Nelson A, Hammond A, Sprecher C, Gross JA et al Interleukin 21 and its receptor are involved in NK cell expansion and regulation of lymphocyte function. Nature 2000; 408:57–63. [DOI] [PubMed] [Google Scholar]
- 6. Rudge EU, Cutler AJ, Pritchard NR, Smith KG. Interleukin 4 reduces expression of inhibitory receptors on B cells and abolishes CD22 and FcγRII‐mediated B cell suppression. J Exp Med 2002; 195:1079–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. MacLennan IC. Germinal centers. Annu Rev Immunol 1994; 12:117–39. [DOI] [PubMed] [Google Scholar]
- 8. Perelson AS, Wiegel FW. Theoretical considerations of the role of antigen structure in B cell activation. Fed Proc 1981; 40:1479–83. [PubMed] [Google Scholar]
- 9. Dintzis RZ, Middleton MH, Dintzis HM. Studies on the immunogenicity and tolerogenicity of T‐independent antigens. J Immunol 1983; 131:2196–203. [PubMed] [Google Scholar]
- 10. Bachmann MF, Rohrer UH, Kundig TM, Burki K, Hengartner H, Zinkernagel RM. The influence of antigen organization on B cell responsiveness. Science 1993; 262:1448–51. [DOI] [PubMed] [Google Scholar]
- 11. Krieg AM, Yi AK, Matson S, Waldschmidt TJ, Bishop GA, Teasdale R et al CpG motifs in bacterial DNA trigger direct B cell activation. Nature 1995; 374:546–9. [DOI] [PubMed] [Google Scholar]
- 12. Mond JJ, Lees A, Snapper CM. T cell‐independent antigens type 2. Annu Rev Immunol 1995; 13:655–92. [DOI] [PubMed] [Google Scholar]
- 13. Vos Q, Lees A, Wu ZQ, Snapper CM, Mond JJ. B cell activation by T‐cell‐independent type 2 antigens as an integral part of the humoral immune response to pathogenic microorganisms. Immunol Rev 2000; 176:154–70. [DOI] [PubMed] [Google Scholar]
- 14. Litinskiy MB, Nardelli B, Hilbert DM, He B, Schaffer A, Casali P et al DCs induce CD40‐independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol 2002; 3:822–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Macpherson AJ, Lamarre A. BLySsful interactions between DCs and B cells. Nat Immunol 2002; 3:798–800. [DOI] [PubMed] [Google Scholar]
- 16. Dutton RW, Bradley LM, Swain SL. T cell memory. Annu Rev Immunol 1998; 16:201–23. [DOI] [PubMed] [Google Scholar]
- 17. Arpin C, Dechanet J, Van Kooten C, Merville P, Grouard G, Briere F et al Generation of memory B cells and plasma cells in vitro . Science 1995; 268:720–2. [DOI] [PubMed] [Google Scholar]
- 18. Phan TG, Paus D, Chan TD, Turner ML, Nutt SL, Basten A et al High affinity germinal center B cells are actively selected into the plasma cell compartment. J Exp Med 2006; 203:2419–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Paus D, Phan TG, Chan TD, Gardam S, Basten A, Brink R. Antigen recognition strength regulates the choice between extrafollicular plasma cell and germinal center B cell differentiation. J Exp Med 2006; 203:1081–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chan TD, Gatto D, Wood K, Camidge T, Basten A, Brink R. Antigen affinity controls rapid T‐dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol 2009; 183:3139–49. [DOI] [PubMed] [Google Scholar]
- 21. Dal Porto JM, Haberman AM, Kelsoe G, Shlomchik MJ. Very low affinity B cells form germinal centers, become memory B cells, and participate in secondary immune responses when higher affinity competition is reduced. J Exp Med 2002; 195:1215–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Anderson SM, Khalil A, Uduman M, Hershberg U, Louzoun Y, Haberman AM et al Taking advantage: high‐affinity B cells in the germinal center have lower death rates, but similar rates of division, compared to low‐affinity cells. J Immunol 2009; 183:7314–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zabel F, Mohanan D, Bessa J, Link A, Fettelschoss A, Saudan P et al Viral particles drive rapid differentiation of memory B cells into secondary plasma cells producing increased levels of antibodies. J Immunol 2014; 192:5499–508. [DOI] [PubMed] [Google Scholar]
- 24. Dogan I, Bertocci B, Vilmont V, Delbos F, Megret J, Storck S et al Multiple layers of B cell memory with different effector functions. Nat Immunol 2009; 10:1292–9. [DOI] [PubMed] [Google Scholar]
- 25. Zuccarino‐Catania GV, Sadanand S, Weisel FJ, Tomayko MM, Meng H, Kleinstein SH et al CD80 and PD‐L2 define functionally distinct memory B cell subsets that are independent of antibody isotype. Nat Immunol 2014; 15:631–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pape KA, Taylor JJ, Maul RW, Gearhart PJ, Jenkins MK. Different B cell populations mediate early and late memory during an endogenous immune response. Science 2011; 331:1203–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McHeyzer‐Williams LJ, Milpied PJ, Okitsu SL, McHeyzer‐Williams MG. Class‐switched memory B cells remodel BCRs within secondary germinal centers. Nat Immunol 2015; 16:296–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jegerlehner A, Maurer P, Bessa J, Hinton HJ, Kopf M, Bachmann MF. TLR9 signaling in B cells determines class switch recombination to IgG2a. J Immunol 2007; 178:2415–20. [DOI] [PubMed] [Google Scholar]
- 29. Jegerlehner A, Storni T, Lipowsky G, Schmid M, Pumpens P, Bachmann MF. Regulation of IgG antibody responses by epitope density and CD21‐mediated costimulation. Eur J Immunol 2002; 32:3305–14. [DOI] [PubMed] [Google Scholar]
- 30. Gatto D, Pfister T, Jegerlehner A, Martin SW, Kopf M, Bachmann MF. Complement receptors regulate differentiation of bone marrow plasma cell precursors expressing transcription factors Blimp‐1 and XBP‐1. J Exp Med 2005; 201:993–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cielens I, Ose V, Petrovskis I, Strelnikova A, Renhofa R, Kozlovska T et al Mutilation of RNA phage Qβ virus‐like particles: from icosahedrons to rods. FEBS Lett 2000; 482:261–4. [DOI] [PubMed] [Google Scholar]
- 32. Bessa J, Schmitz N, Hinton HJ, Schwarz K, Jegerlehner A, Bachmann MF. Efficient induction of mucosal and systemic immune responses by virus‐like particles administered intranasally: implications for vaccine design. Eur J Immunol 2008; 38:114–26. [DOI] [PubMed] [Google Scholar]
- 33. Madsen L, Labrecque N, Engberg J, Dierich A, Svejgaard A, Benoist C et al Mice lacking all conventional MHC class II genes. Proc Natl Acad Sci USA 1999; 96:10338–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Gatto D, Ruedl C, Odermatt B, Bachmann MF. Rapid response of marginal zone B cells to viral particles. J Immunol 2004; 173:4308–16. [DOI] [PubMed] [Google Scholar]
- 35. Banchereau J, Bazan F, Blanchard D, Briere F, Galizzi JP, van Kooten C et al The CD40 antigen and its ligand. Annu Rev Immunol 1994; 12:881–922. [DOI] [PubMed] [Google Scholar]
- 36. Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L et al Generation of T follicular helper cells is mediated by interleukin‐21 but independent of T helper 1, 2, or 17 cell lineages. Immunity 2008; 29:138–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin‐21 in the generation of T follicular helper cells. Immunity 2008; 29:127–37. [DOI] [PubMed] [Google Scholar]
- 38. Frohlich A, Marsland BJ, Sonderegger I, Kurrer M, Hodge MR, Harris NL et al IL‐21 receptor signaling is integral to the development of Th2 effector responses in vivo . Blood 2007; 109:2023–31. [DOI] [PubMed] [Google Scholar]
- 39. Bessa J, Jegerlehner A, Hinton HJ, Pumpens P, Saudan P, Schneider P et al Alveolar macrophages and lung dendritic cells sense RNA and drive mucosal IgA responses. J Immunol 2009; 183:3788–99. [DOI] [PubMed] [Google Scholar]
- 40. Bessa J, Kopf M, Bachmann MF. Cutting edge: IL‐21 and TLR signaling regulate germinal center responses in a B cell‐intrinsic manner. J Immunol 2010; 184:4615–9. [DOI] [PubMed] [Google Scholar]
- 41. Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A et al IL‐21 regulates germinal center B cell differentiation and proliferation through a B cell‐intrinsic mechanism. J Exp Med 2010; 207:365–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Szomolanyi‐Tsuda E, Le QP, Garcea RL, Welsh RM. T‐cell‐independent immunoglobulin G responses in vivo are elicited by live‐virus infection but not by immunization with viral proteins or virus‐like particles. J Virol 1998; 72:6665–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kundig T et al A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine 2002; 20:3104–12. [DOI] [PubMed] [Google Scholar]
- 44. Weisel FJ, Appelt UK, Schneider AM, Horlitz JU, van Rooijen N, Korner H et al Unique requirements for reactivation of virus‐specific memory B lymphocytes. J Immunol 2010; 185:4011–21. [DOI] [PubMed] [Google Scholar]
- 45. Benson MJ, Elgueta R, Schpero W, Molloy M, Zhang W, Usherwood E et al Distinction of the memory B cell response to cognate antigen versus bystander inflammatory signals. J Exp Med 2009; 206:2013–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Maruyama M, Lam KP, Rajewsky K. Memory B cell persistence is independent of persisting immunizing antigen. Nature 2000; 407:636–42. [DOI] [PubMed] [Google Scholar]
- 47. Good‐Jacobson KL, Shlomchik MJ. Plasticity and heterogeneity in the generation of memory B cells and long‐lived plasma cells: the influence of germinal center interactions and dynamics. J Immunol 2010; 185:3117–25. [DOI] [PubMed] [Google Scholar]
- 48. Vieira P, Rajewsky K. Persistence of memory B cells in mice deprived of T cell help. Int Immunol 1990; 2:487–94. [DOI] [PubMed] [Google Scholar]
- 49. Bachmann MF, Hengartner H, Zinkernagel RM. T helper cell‐independent neutralizing B cell response against vesicular stomatitis virus: role of antigen patterns in B cell induction? Eur J Immunol 1995; 25:3445–51. [DOI] [PubMed] [Google Scholar]
- 50. Hebeis BJ, Klenovsek K, Rohwer P, Ritter U, Schneider A, Mach M et al Activation of virus‐specific memory B cells in the absence of T cell help. J Exp Med 2004; 199:593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, Papaioannou VE. RAG‐1‐deficient mice have no mature B and T lymphocytes. Cell 1992; 68:869–77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Tracking of transferred memory splenocytes containing memory B cell in wild type (WT) mice after Qβ‐bacteriophage‐derived virus‐like particle (Qβ‐VLP) challenge.


