Summary
Interactions between dendritic cells (DCs) and environmental, dietary and pathogen antigens play a key role in immune homeostasis and regulation of inflammation. Dietary polyphenols such as proanthocyanidins (PAC) may reduce inflammation, and we therefore hypothesized that PAC may suppress lipopolysaccharide (LPS) ‐induced responses in human DCs and subsequent T helper type 1 (Th1) ‐type responses in naive T cells. Moreover, we proposed that, because DCs are likely to be exposed to multiple stimuli, the activity of PAC may synergise with other bioactive molecules that have anti‐inflammatory activity, e.g. soluble products from the helminth parasite Trichuris suis (TsSP). We show that PAC are endocytosed by monocyte‐derived DCs and selectively induce CD86 expression. Subsequently, PAC suppress the LPS‐induced secretion of interleukin‐6 (IL‐6) and IL‐12p70, while enhancing secretion of IL‐10. Incubation of DCs with PAC did not affect lymphocyte proliferation; however, subsequent interferon‐γ production was markedly suppressed, while IL‐4 production was unaffected. The activity of PAC was confined to oligomers (degree of polymerization ≥ 4). Co‐pulsing DCs with TsSP and PAC synergistically reduced secretion of tumour necrosis factor‐α, IL‐6 and IL‐12p70 while increasing IL‐10 secretion. Moreover, both TsSP and PAC alone induced Th2‐associated OX40L expression in DCs, and together synergized to up‐regulate OX40L. These data suggest that PAC induce an anti‐inflammatory phenotype in human DCs that selectively down‐regulates Th1 response in naive T cells, and that they also act cooperatively with TsSP. Our results indicate a novel interaction between dietary compounds and parasite products to influence immune function, and may suggest that combinations of PAC and TsSP can have therapeutic potential for inflammatory disorders.
Keywords: dendritic cells, inflammation, parasite, proanthocyanidins, Trichuris suis
Abbreviations
- COC
cocoa
- DC
dendritic cells
- ECGC
epigallocatechin gallate
- F1
fraction 1
- F2
fraction 2
- GALT
gut‐associated lymphoid tissue
- LPS
lipopolysaccharide
- PAC
proanthocyanidins
- TsSP
Trichuris suis soluble products
- WCF
white clover flowers
Introduction
Dendritic cells (DCs) are key players in immune surveillance and homeostasis in various organs, particularly those with large mucosal surfaces such as the gastrointestinal tract. A number of specialized populations of DCs reside in the lamina propria and the gut‐associated lymphoid tissue (GALT) such as the Peyer's patches. Human intestinal DCs are not well characterized, but in mice different subsets are distinguished by their expression of CD11b, CD103, CX3CR1 and CCR7, and they play an important role through antigen sampling from the intestinal lumen and subsequent presentation of pathogen antigens to T cells in the GALT.1, 2 Hence, DCs are exposed to both harmless gut flora and pathogenic intestinal microorganisms such as viruses, bacteria and parasites, as well as dietary components. They therefore play a key role in maintaining effective immune homeostasis; overt inflammatory responses by DCs such as excessive secretion of pro‐inflammatory cytokines [e.g. tumour necrosis factor‐α (TNF‐α)] may lead to the development of chronic inflammation, while appropriate cytokine secretion and T‐cell activation are also important for effective clearance of potentially harmful pathogens.3, 4, 5 Therefore, modulation of DC activity may be an effective strategy for ameliorating autoimmune diseases, as well as invoking a desirable immune response for protection against intestinal pathogens.
The cytokine profiles secreted by DCs upon activation by microbial antigens can vary markedly according to the nature of the pathogen. The established paradigm is that pathogenic, intracellular bacteria and viruses promote a vigorous inflammatory response from DCs characterized by secretion of high levels of TNF‐α, interleukin‐12 (IL‐12) and IL‐6, and subsequent induction of T helper type 1 (Th1) CD4+ T cells that produce large amounts of IL‐2 and interferon‐γ (IFN‐γ).6, 7 In contrast, multicellular helminth parasites invariably invoke a Th2 response, whereby DCs induce T cells that secrete high amounts of IL‐4, IL‐5 and IL‐13, and little IFN‐γ. Protective immunity is thought to derive in part from an IL‐4/IL‐13‐driven increase in gut motility and fluid secretion that removes parasites from their intestinal niche.8, 9, 10, 11 In addition, this Th2‐driven response results in alternatively‐activated macrophages with wound‐healing and anti‐inflammatory properties.12 Interestingly, concurrent stimulation of DCs with helminth antigens has been shown to actively down‐regulate the Th1 inflammatory response induced by Toll‐like receptor (TLR) agonists such as lipopolysaccharide (LPS).13 Helminths and/or their secreted products have therefore been proposed as novel therapy for chronic inflammatory disorders such as Crohn's disease or multiple sclerosis.14, 15
Bioactive dietary compounds also have the potential to markedly influence the immunological milieu of the body, through either absorption and subsequent systemic activity or interaction with the numerous innate immune sentinel cells that reside in the gastrointestinal mucosa. Indeed, many plant compounds have been reported to have anti‐inflammatory effects; the flavan‐3‐ol, epigallocatechin gallate (EGCG), an abundant molecule in green tea, has been shown to alleviate symptoms of autoimmune inflammation in mice,16, 17 and in vitro experiments have demonstrated that EGCG inhibits inflammatory responses in macrophages through inhibition of TLR‐dependent pathways.18 A related group of compounds are proanthocyanidins (PAC; synonym – condensed tannins), which are oligomeric and polymeric forms of flavan‐3‐ols found in dietary components such as fruits, nuts, berries and beans.
The flavan‐3‐ol monomeric units that give rise to PAC are predominantly catechin or epicatechin (comprising procyanidin‐type PAC) or gallocatechin or epigallocatechin (comprising prodelphinidin‐type PAC, which are less numerous than procyanidins but found in large amounts in, for example, blackcurrants and other berries). The major difference between these monomeric units is an extra hydroxyl group in the B‐ring of prodelphinidins (Fig. 1). Large variations are also observed in molecular weight depending on the number of linked flavan‐3‐ol units, i.e. leading to different degrees of polymerization. These molecules have strong bioactivity as they bind readily to other macromolecules such as proteins and polysaccharides, and have been extensively studied for their antioxidant19 and antiviral20 properties. In addition, a number of studies have highlighted the anti‐inflammatory properties of PAC; administration of oligomeric PAC has been shown to alleviate the symptoms of inflammatory disorders such as autoimmune arthritis21 or experimental autoimmune encephalomyelitis22 in mice. The anti‐inflammatory mechanisms of PAC have not been elucidated fully, but have been suggested to involve inhibition of TLR‐dependent signalling pathways and antigen‐presenting capacity in macrophages,22, 23 as well as down‐regulation of CD11b surface expression in monocytes.24
Figure 1.
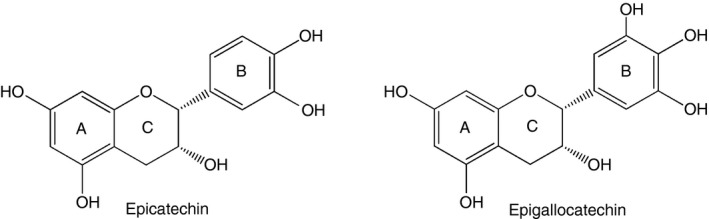
Examples of procyanidin and prodelphinidin structure. Chemical structures of epicatechin and epigallocatechin, the monomeric cis isomers giving rise to procyanidin‐type and prodelphinidin‐type polymers, respectively. Note the extra hydroxyl group in the B‐ring of gallocatechin.
Despite increased interest in the anti‐inflammatory properties of PAC, their interactions with human DCs is not yet clear. Peripheral blood monocyte‐derived DCs represent a convenient and widely used model to assess the effects of various immunomodulatory agents on human DC activity.25, 26, 27 Here, we prepared well‐characterized PAC fractions to investigate effects on human monocyte‐derived DC activity. We hypothesized that PAC would be recognized and taken up by DCs, and subsequently would inhibit LPS‐induced inflammatory responses. Moreover, we postulated that any anti‐inflammatory activity of PAC would not act in isolation, but would interact with other modulatory substances that may be found in the same environment sampled by the DCs, which in the case of dietary compounds such as PAC, would include gastrointestinal parasites. Therefore, we also determined the effects of simultaneous DC exposure to PAC and products from the helminth Trichuris suis, a pathogenic pig parasite that causes large problems in swine production, but has also shown promise in treating autoimmune diseases in humans. We reasoned that the well‐known Th2‐polarizing effects of T. suis 11, 28 may synergize with PAC to modulate DC function. We show that PAC interact directly with human DCs, and down‐regulate inflammatory cytokine responses and subsequent Th1 responses in naive T cells. Furthermore, PAC and T. suis soluble products (TsSP) synergize to suppress inflammatory responses in DCs. Our results may indicate a novel function of PAC to down‐regulate DC‐driven inflammatory processes, and suggest that dietary components and parasites can interact to modulate immune responses.
Materials and methods
Proanthocyanidins
Purified PAC were prepared from either cocoa beans (COC) or white clover flowers (WCF) to obtain exclusively procyanidin‐type or prodelphinidin‐type PAC, respectively. Two fractions were isolated from each plant extract; a first fraction (F1) that contained lower‐molecular‐weight PAC (as measured by mean degree of polymerization), and a second fraction (F2) containing higher‐molecular‐weight PAC. The extraction, purification and analysis procedures have been described before in detail.29 Briefly, to purify PAC, plant material (50 g) was extracted with acetone/water [7 : 3; volume/volume (v/v)] and PAC fractions were obtained by Sephadex LH‐20 chromatography, with F1 eluted using 3 : 7 v/v acetone/water and F2 eluted with 1 : 1 v/v acetone/water. Analysis of PAC was undertaken by HPLC‐MS to calculate purity, the molar proportions of monomeric sub‐units and mean degree of polymerization. Results of these analyses have been published previously,29, 30 and are summarized here briefly in Table 1. In addition, 5‐([4,6‐dichlorotriazin‐2‐yl]amino)fluorescein (DTAF)‐tagged PAC were obtained similarly by acetone/water extraction of Lotus corniculatus and chromatographic purification, followed by conjugation to DTAF as previously described.31 Untagged PAC were prepared in a similar fashion but omitting the DTAF conjugation step.
Table 1.
Chemical analysis of fractionated proanthocyanidins from two different plant sources
| Sample | % purity | mDP | % PC | % PD | |
|---|---|---|---|---|---|
| COC | F1 | 58·5 | 2·3 | 100 | 0 |
| F2 | 75·5 | 5·4 | 100 | 0 | |
| WCF | F1 | 11·8 | 1·8 | 1·8 | 98·2 |
| F2 | 100 | 8·6 | 1·3 | 98·7 |
Parasite material
Adult T. suis worms were collected from the caecum and colon of experimentally infected pigs and washed extensively in warm saline. The TsSP were prepared by homogenization and sonication of whole worms as previously described11.
Isolation of monocytes and DC culture
Buffy coats were collected (Copenhagen University Hospital, Copenhagen, Denmark) from healthy, anonymous volunteers following written, informed consent. Peripheral blood mononuclear cells were isolated on histopaque (Sigma‐Aldrich, St Louis, MO) and monocytes were purified by anti‐CD14 microbeads and magnetic separation (MACS, Miltenyi Biotech, Bergisch Gladbach, Germany). Monocytes were cultured at 37° in 5% CO2 in complete RPMI media (RPMI‐1640 containing 10% inactivated fetal bovine serum, 2 mm l‐glutamine, 100 μg/ml streptomycin and 100 U/ml penicillin). Monocytes were differentiated into immature DCs in the presence of 12·5 ng/ml each of IL‐4 and granulocyte–macrophage colony‐stimulating factor (R&D Systems, Abingdon, UK) and routinely used at day 4. Immature DCs were then pre‐treated for 1 hr with PAC or PBS as a control. Concentrations of fractions were adjusted to ensure an equal concentration of PAC between the different samples – hence, all concentrations refer to concentration [weight (w)/v] of PAC. After optimization, 10 μg/ml of COC PAC and 20 μg/ml of WCF PAC (w/v) were found to be optimal concentrations; higher concentrations resulted in significant cytotoxicity as judged by 7AAD staining (see Supplementary material, Fig. S1). Where indicated, TsSP were added for the final 30 min. Lipopolysaccharide (LPS; 10 ng/ml) was then added and the cells were cultured for a further 24 hr. For blocking experiments, 10 μg/ml either anti‐CD11b (Clone ICRF44, BD Pharmingen, San Diego, CA) or anti‐67LR (Clone MLuC5, Abcam, Cambridge, UK), or appropriate isotype controls were added 15 min before the addition of PAC and incubated at 37°. In some experiments, PAC fractions were pre‐incubated in polyvinylpolypyrrolidone (PVPP) overnight (10 : 1 PVPP : PAC; w/w) at 4°, followed by centrifugation at 3000 g for 10 min, and the supernatant was retained and used to stimulate DCs. Controls consisted of media alone incubated with PVPP, and PAC with no PVPP incubated overnight in an identical fashion.
Mixed lymphocyte reactions
Immature DCs were incubated with either LPS or LPS with 20 μg/ml WCF F2 for 48 hr, then washed and counted. For preparation of responder cells, allogenic peripheral blood mononuclear cells were depleted of monocytes by removal of CD14+ cells by MACS separation as described above. The responding lymphocytes were then labelled with CFSE (1 μm; Sigma‐Aldrich) and then added at a 1 : 10 (DC : responder cell) ratio and the cells were cultured for 6 days, after which fluorescence was analysed by flow cytometry.
Flow cytometry
After 24 hr, DCs were harvested, washed and stained with either anti CD80‐phycoerythrin (Clone L307.4), CD86‐allophycocyanin (Clone FUN‐1), MHC‐II‐FITC (Clone Tu39), OX40L‐phycoerythrin (Clone Ik‐1) CD11c‐FITC (Clone B‐ly6), CD11b‐allophycocyanin (Clone ICRF44), CD103‐FITC (clone Ber‐ACT8) or CX3CR1‐phycoerythrin (clone2A9‐1; all from BD Pharmingen). Cells were acquired on an Accuri C6 flow cytometer. Mean fluorescence intensities were calculated after gating on viable cells. Data were analysed using fcs version 5 (De Novo Software, Glendale, CA).
ELISA
Supernatants from DC cultures were harvested after 24 hr and the levels of TNF‐α, IL‐6 and IL‐10 were measured using the appropriate cytosets (Thermo Fisher, Copenhagen, Denmark) according to the manufacturer's instructions. For IL‐12p70, plates were coated with anti‐IL‐12p70 (eBioscience, San Diego, CA) and detected with biotinylated anti‐IL‐12p40/70 (BD Pharmingen), followed by streptavidin–horseradish peroxidase (Life Technologies) and 3,3',5,5'‐Tetramethylbenzidine (TMB) substrate (Sigma‐Aldrich, Schnelldorf, Germany).
Fluorescence microscopy
Immature DCs were stimulated for 1 or 2 hr at 37° or 1 hr at 4° with either media only, DTAF‐tagged PAC (50 μg/ml) or an equivalent concentration of untagged PAC. Cells were then washed in PBS, fixed in 4% paraformaldehyde, settled onto poly‐l‐lysine‐coated coverslips and blocked for 1 hr at room temperature with 2% BSA in PBS. Cells were then stained with Alexa Fluor‐594 anti‐human CD31 (Clone WM59; BioLegend, San Diego, CA) or made permeable with 0·1% saponin followed by staining with anti‐human CD107b (Clone H4B4; BioLegend) and Alexa Fluor 594 goat‐anti mouse conjugate (Thermo Fisher, Copenhagen, Denmark). Cells were mounted in Vectashield with DAPI (Vector Labs, Carlsbad, CA), and then examined microscopically at ×100 magnification on a Leica HMR DC fluorescence microscope. Images were processed using imagej software(National Institutes of Health, Bethesda, MD, USA).
TLR4‐reporter cells
Human embryonic kidney 293 (HEK 293) cells stably expressing TLR432 were cultured in Dulbecco's modified Eagle's medium supplemented with 10% heat inactivated bovine serum, 2 mm l‐glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin. Cells were stimulated with 20 μg/ml WCF or COC F2 fraction and/or 10 ng/ml LPS. After 24 hr, supernatant was harvested and IL‐8 production was measured by ELISA.
Th1/Th2 skewing assay
Immature DCs were stimulated for 48 hr with either LPS alone, or in the presence of PAC and/or TsSP. Naive, allogenic CD45RA+ CD4+ T cells were isolated using the naive T‐cell isolation kit (MACS, Miltenyi Biotech). After 48 hr, DCs were extensively washed in PBS and then added to naive T cells at a ratio of 1 : 10 DC : T cells. Cells were then cultured for 12 days in complete RPMI medium supplemented with 50 U/ml IL‐2 (Life Technologies), with the media changed every 2–3 days for fresh media containing IL‐2. Cells were then washed and stimulated for 5 hr with a mixture of ionomycin (Sigma‐Aldrich; 1 μg/ml), phorbol 12‐myristate 13‐acetate (Sigma‐Aldrich; 30 ng/ml) and brefeldin A (Sigma‐Aldrich; 10 μg/ml). Cells were then fixed and permeabilized using the cytofix/cytoperm kit (BD Pharmingen), and intracellular cytokine staining was carried out by flow cytometry using anti IL‐4‐allophycocyanin (Clone 8D4‐8) and IFN‐γ‐FITC (Clone 4S.B3; both from BD Pharmingen). Background responses from unstimulated cells were subtracted from the stimulated responses.
Data analysis and statistics
Where indicated, anova analyses with Bonferroni post‐hoc testing or paired t‐tests were carried out using graphpad prism (v6.00, GraphPad Software, La Jolla, CA; www.graphpad.com). Normality of data was assessed using Shapiro–Wilk tests, and where data did not conform to a normal distribution logarithmic transformation was carried out before analysis. Statistical analyses were performed on either raw cytokine concentrations (ELISA) or mean fluorescence intensities/percentage of positive cells (flow cytometry); howeve, for ease of interpretation, the data are presented in most instances as a percentage of the response of cells to LPS, and means ± SEM. of untransformed data are presented.
Results
Structurally diverse PAC induce CD86 expression in DCs
To determine if PAC are recognized by DCs, we first asked whether DCs incubated with PAC respond by up‐regulating classical cell‐surface markers of DC maturation, and whether structural features of PAC may affect any such response. To this end, we used purified PAC fractions that consisted exclusively of either procyanidins (COC) or prodelphinidins (WCF). Monocyte‐derived DCs were then exposed to the various PAC fractions. After 24 hr, incubation with PAC did not affect expression of CD80 or MHC‐II, in contrast to the strong up‐regulation induced by the TLR4 agonist LPS (Fig. 2a). However, incubation with F2 fractions from both COC and WCF induced up‐regulation of CD86 expression (P < 0·01), with both PAC types inducing a similar level of up‐regulation, though the expression was not as profound as that induced by LPS (Fig. 2a).
Figure 2.
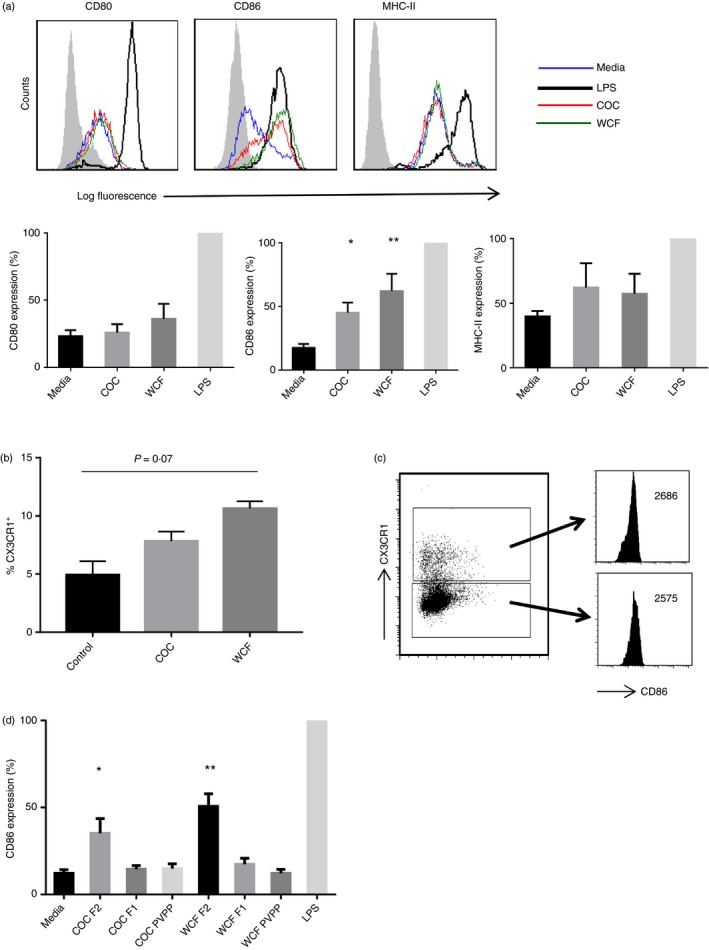
Oligomeric proanthocyanidins induce CD86 expression in human dendritic cells (DCs). (a) Induction of CD80, CD86 and MHC‐II expression in DCs after 24 hr incubation with cocoa (COC) fraction 2 (F2; 10 μg/ml) or white clover flower (WCF) F2 (20 μg/ml). Shown are representative FACS plots from five different experiments, each performed with cells from different donors, and comparison of expression of each DC surface molecule to that induced by lipopolysaccharide (LPS), which was set at 100%. Data are shown as mean ± SEM. *P < 0·05; **P < 0·01 by one‐way analysis of variance when compared with medium alone. Grey shaded area represents isotype control. (b) Percentage of CX3CR1‐positive cells in unstimulated DCs or DCs incubated for 24 hr with COC F2 (10 μg/ml) or WCF F2 (20 μg/ml). Experiments were performed three times with cells from different donors, and the mean and inter‐donor SEM are shown. (c) CD86 expression (mean fluorescence intensities) in DCs treated for 24 hr with WC F2 (20 μg/ml) and gated as being either CX3CR1+ or CX3CR1−. Similar results were obtained with COC F2 (10 μg/ml). The experiments were performed twice with cells from different donors, with similar results. (d) Induction of CD86 expression in DCs incubated for 24 hr with either LPS, 10 μg/ml COC F1, F2 or F2 pre‐incubated with polyvinylpolypyrrolidone (PVPP), or 20 μg/ml WCF F1, F2 or F2 pre‐incubated with PVPP. CD86 expression was assessed by mean fluorescence intensity relative to LPS alone, which was set at 100%. Experiments were performed three times with cells from different donors, and the mean and inter‐donor SEM are shown. *P < 0·05; **P < 0·01 by one‐way analysis of variance when compared with medium alone.
Characterization of the monocyte‐derived DCs showed that most of the DCs were CD11c+, CD11b+ and CD103−. Most of the DCs were CX3CR1−, but a small population (~5%) of the DCs were CX3CR1+ (see Supplementary material, Fig. S2). Interestingly, PAC seemed to induce CX3CR1 expression with higher proportions of CX3CR1+ cells present following PAC exposure (Fig. 2b). Given that PAC probably exert their activity locally in the intestinal mucosa, and in mice CX3CR1 has been shown to be important for allowing DCs to sample antigens from the intestinal lumen,33 we examined PAC‐induced CD86 up‐regulation in CX3CR1− and CX3CR1+ DCs. CD86 expression induced by PAC was identical in these two populations (Fig. 2c).
The effect of PAC was clearly dependent on the degree of polymerization, as F1 from COC and WCF containing an equal amount (w/w) of low mean degree of polymerization (≤ 2·3) PAC did not induce CD86 expression (P > 0·05; Fig. 2c). No interactions between LPS and PAC were evident; PAC did not inhibit LPS‐induced expression of any cell‐surface activation marker, nor did they additively increase the expression of CD86 (data not shown). To confirm the role of PAC, the F2 fractions were pre‐incubated with PVPP to selectively neutralize PAC. CD86 expression was subsequently abolished in these PVPP‐treated samples (Fig. 2c). These data suggest that structurally diverse PAC are able to selectively induce CD86 expression in DCs, which is dependent on their degree of polymerization.
Proanthocyanidins inhibit LPS‐induced pro‐inflammatory cytokine secretion in DCs
We next investigated whether PAC induced cytokine secretion in DCs. Incubation of DCs with PAC alone did not result in any measurable cytokine production (data not shown). However, incubation of DCs with LPS and both COC or WCF F2 resulted in a reduction (P < 0·001) in the LPS‐induced secretion of the Th1‐type cytokines IL‐6 and IL‐12p70, while TNF‐α secretion was not affected (Fig. 3a). In contrast, PAC increased the LPS‐induced secretion of the Th2/regulatory type cytokine IL‐10 (Fig. 3a,b). The dynamic range of PAC activity was low, with concentrations of 5 μg/ml of both COC and WCF still inhibiting IL‐6 and IL‐12p70 secretion, but no inhibition was evident at 2·5 μg/ml (see Supplementary material, Fig. S3a).
Figure 3.
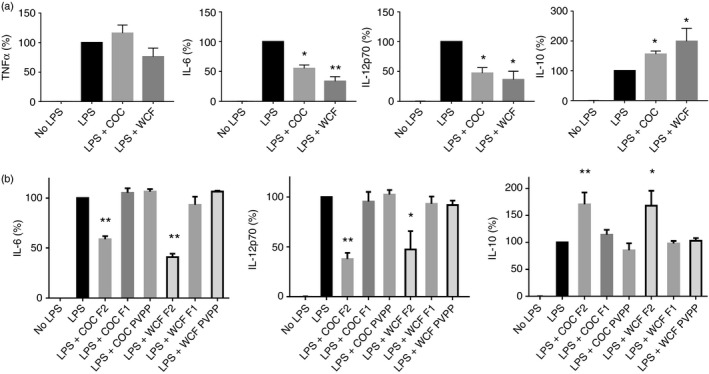
Oligomeric proanthocyanidins (PAC) modulate cytokine secretion in lipopolysaccharide (LPS) ‐activated dendritic cells (DCs). (a) Suppression of LPS‐induced cytokine secretion in DCs by addition of 10 μg/ml cocoa (COC) fraction 2 (F2) or 20 μg/ml white clover flower (WCF) F2. Cytokine secretion in PAC‐treated cells is expressed as a percentage of the LPS‐induced secretion for each cytokine, where 100% corresponds to 32 ± 3·1 ng/ml for tumour necrosis factor‐α (TNF‐α), 16 ± 4·4 ng/ml for interleukin‐6 (IL‐6), 3 ± 0·5 ng/ml for IL‐12p70 and 3 ± 1·9 ng/ml for IL‐10. The experiments have been performed at least five times with cells from different donors. The mean and inter‐donor SEM are shown. *P < 0·05; ***P < 0·001 by one‐way analysis of variance compared with LPS only. (b) Suppression of LPS‐induced cytokine secretion in DCs by addition of either COC or WCF F2, COC or WCF F1, or COC or WCF F2 pre‐incubated with polyvinylpolypyrrolidone; 10 μg/ml PAC was used for COC fractions and 20 μg/ml PAC was used for WCF fractions. Cytokine secretion in PAC‐treated cells is expressed as a percentage of the Toll‐like receptor (TLR) ‐induced secretion for each cytokine as described above. The experiments have been performed at least three times with cells from different donors. Mean with inter‐donor SEM is shown. *P < 0·05; **P < 0·01; ***P < 0·001 by one‐way analysis of variance compared with LPS only.
The WCF PAC appeared to more strongly inhibit IL‐6 and IL‐12p70 production and augment IL‐10 production than COC PAC, suggesting that PAC comprised of prodelphinidins are stronger regulators of DC activity than those comprised of procyanidins. A higher concentration of WCF could be used because of lower cytotoxicity issues, but we also observed that when equal concentrations of WCF and COC F2 were used, suppression of IL‐12p70 secretion was greater with WCF than with COC (P < 0·05), whereas suppression of IL‐6 showed no significant difference (see Supplementary material, Fig. S3b).
Similar to the CD86 expression data (Fig. 2), effects on cytokine secretion were dependent on polymerization, with COC and WCF F1 proving inefficient at modulating cytokine secretion, and the activity of F2 was abolished by pre‐incubation in PVPP (Fig. 3b). Overall, these data suggest that oligomeric PAC induce a regulatory phenotype in DCs that may inhibit inflammatory responses.
Proanthocyanidins are internalized by DCs and localize to lysosomes
Having established that PAC modulate DC activity, we next investigated whether PAC were internalized by DCs or interacted only with the cell periphery. To accomplish this, we used PAC purified from L. corniculatus and tagged with the fluorophore DTAF. These PAC had a mean degree of polymerization of 9·5 (as determine by thiolysis), indicating a similar molecular weight to the biologically active F2 fractions from COC and WCF. We also confirmed that these DTAF‐tagged PAC induced similar functional activity in DCs as COC and WCF PAC, as evidenced by significant inhibition of IL‐6 and IL‐12p70 secretion in LPS‐activated DCs (P < 0·05; see Supplementary material, Fig. S4), indicating that the DTAF‐tagging procedure does not alter the functional activity of PAC. We then exposed monocyte‐derived DCs to either DTAF‐tagged PAC or controls consisting of untagged PAC or medium only. Single‐cell imaging of DCs demonstrated that the DTAF‐tagged PAC were clearly visible within the cell after 1 hr of exposure (Fig. 4a). No fluorescence was observed in DCs exposed to untagged PAC (Fig. 4a) or medium only (data not shown). To confirm that PAC are internalized by DCs, we performed dual labelling experiments with antibodies against the DC surface protein CD31, which clearly showed that PAC were located in the interior of the cell (Fig. 4b).
Figure 4.
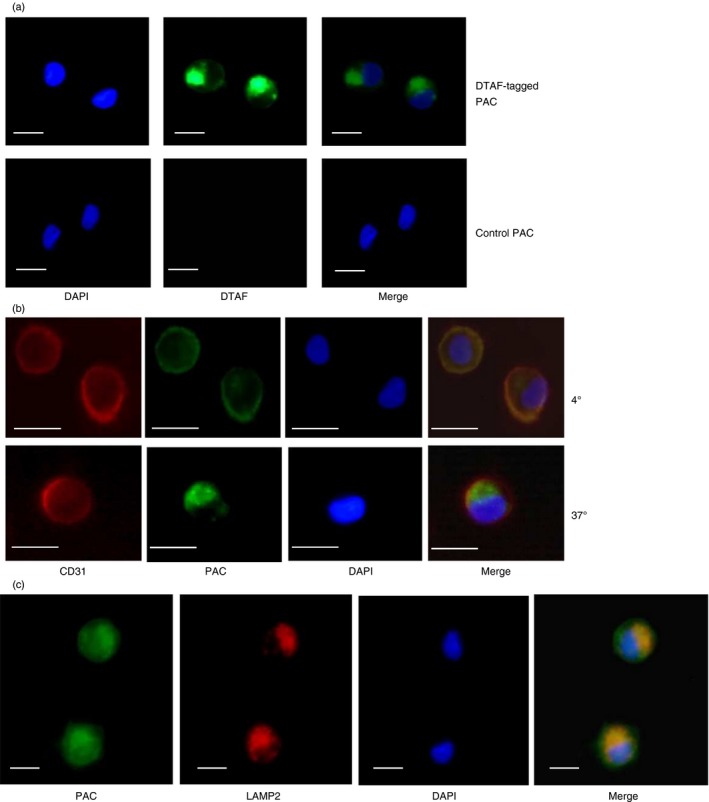
Endocytosis of proanthocyanidins (PAC) by dendritic cells (DCs). (a) DCs were incubated for 1 hr with either DTAF‐tagged PAC or equivalent PAC lacking the DTAF tag (‘Control PAC’). DAPI staining was used to visualize nuclei. Representative results from five different experiments with cells from different donors. (b) DCs were incubated with DTAF‐tagged PAC at either 4° or 37° and stained with DAPI to visualize nuclei and Alexa‐594‐conjugated anti‐CD31 to visualize DC surface. Representative results from three different experiments with cells from different donors. (c) DCs were incubated with DTAF‐tagged PAC at 37° for 2 hr and stained with DAPI to visualize nuclei and Alexa‐594‐conjugated anti‐LAMP2 to visualize lysosomes. Representative results from three different experiments with cells from different donors. For all images scale bar indicates 5 μm.
To determine if this was an active endocytotic process, DCs were incubated with DTAF‐tagged PAC at either 37° or 4°, which demonstrated that internalization of PAC occurred only at 37°, whereas at 4° PAC bound to the cell membrane but remained on the exterior of the cell, co‐localizing with CD31 (Fig. 4b). These data suggest that DCs recognize and actively internalize PAC. Moreover, PAC appeared to localize to a lysosome‐associated membrane protein 2 (LAMP2)‐bright compartment within the DC, suggesting that PAC traffic to lysosomes following internalization (Fig. 4c).
Proanthocyanidins do not inhibit binding of LPS to TLR4 and do not signal through 67LR or CD11b
To determine if PAC inhibited LPS‐induced cytokine secretion by either binding to and neutralizing TLR4, or else binding to LPS and preventing it being bound by TLR4, we used TLR4 reporter cells that secrete IL‐8 following TLR4 ligation. As shown in Fig. 5(a), neither COC nor WCF F2 induced IL‐8 secretion in these cells, indicating that they do not interact directly with TLR4. Furthermore, IL‐8 secretion was unchanged after the addition of LPS in the presence of PAC, demonstrating that PAC do not inhibit the interaction of LPS with TLR4.
Figure 5.
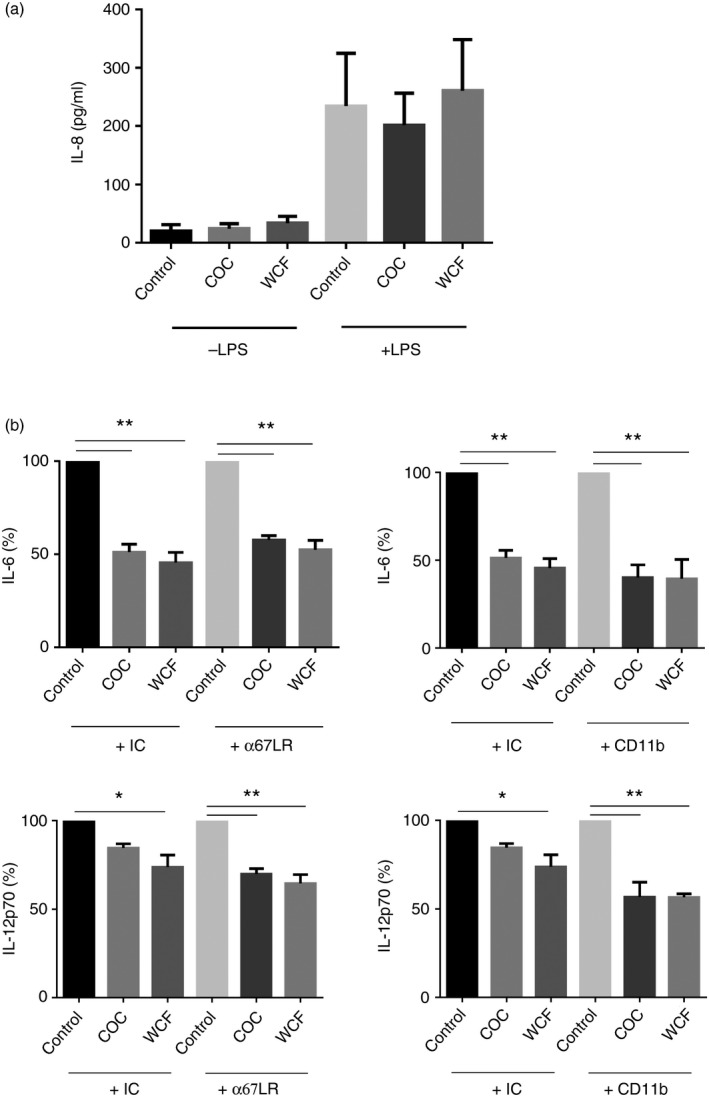
Proanthocyanidins (PAC) do not inhibit binding of lipopolysaccharide (LPS) to Toll‐like receptor 4 (TLR4) and do not bind 67LR or CD11b on dendritic cells. (a) TLR4 reporter cells were incubated with 20 μg/ml of either cocoa (COC) fraction 2 (F2) or white clover flower (WCF) F2 for 24 hr, and stimulated with 10 ng/ml LPS or vehicle control. TLR4 activation was assessed by measuring the production of interleukin‐8 (IL‐8). Results are the mean ± SEM. of three independent experiments. (b) Dendritic cells were incubated with anti‐67LR, anti‐CD11b or isotype controls (IC) for 15 min before the addition of COC F2 or WCF F2 and then LPS. IL‐6 and IL‐12p70 secretion was quantified after 24 hr. Cytokine secretion in PAC‐treated cells incubated with either isotype controls or anti‐67LR or CD11b is expressed as a percentage of the secretion induced by LPS alone + isotype control, or LPS alone + anti67LR or CD11b. Mean secretion in isotype‐control treated cells activated with LPS alone was 44 ± 8·6 ng/ml for IL‐6 and 4 ± 1·7 ng/ml for IL‐12p70. Experiments were performed three times with cells from different donors, and presented as mean ± inter‐donor SEM. *P < 0·05; **P < 0·001 as assessed by one‐way analysis of variance.
Given that PAC were not bound by TLR4, we asked whether there could be another cognate receptor that interacts with PAC. Previous studies have demonstrated that EGCG interacts specifically with 67LR on mouse DCs,34 while oligomeric PAC have been shown to interact with CD11b on bovine monocytes.24 We therefore tested whether blocking either or both these receptors would ablate the effects of PAC on LPS‐stimulated DCs. However, anti‐67LR or anti‐CD11b treatment (Fig. 5b), or treatment with both monoclonal antibodies (data not shown), did not reverse the inhibition of LPS‐induced IL‐6 and IL‐2p70 secretion in PAC‐treated DCs.
Proanthocyanidin‐primed DCs do not inhibit proliferation but inhibit Th1 responses in naive CD4+ T cells
We next assessed whether PAC‐primed DCs inhibit lymphocyte proliferation and/or skew naive CD4+ cells to a Th1 or Th2 phenotype. The DCs were activated with either LPS or LPS in the presence of WCF PAC, as these were most effective at modulating cytokine secretion (Fig. 2a). Proliferation of lymphocytes (monocyte‐depleted peripheral blood mononuclear cells) cultured with LPS‐matured DCs was not inhibited by co‐pulsing DCs with PAC (Fig. 6a). T cells activated by LPS‐primed DCs produced both IFN‐γ and IL‐4, consistent with a mixed Th1/Th2 type response that is known to be induced by this antigen (Fig. 6b). In contrast, T cells activated by DCs that had been primed by LPS in the presence of PAC produced very little IFN‐γ, and IL‐4 production was unchanged (Fig. 6b). Hence, the IL‐4/IFN‐γ ratio was far higher for T cells activated by PAC‐treated DCs (5·3 versus 1·9; P < 0·01 by paired t‐test; Fig. 6b), hence suggesting that PAC‐primed DCs do not inhibit T‐cell proliferation but subsequently drive a Th2 phenotype in naive T cells by selective down‐regulation of Th1 responses.
Figure 6.
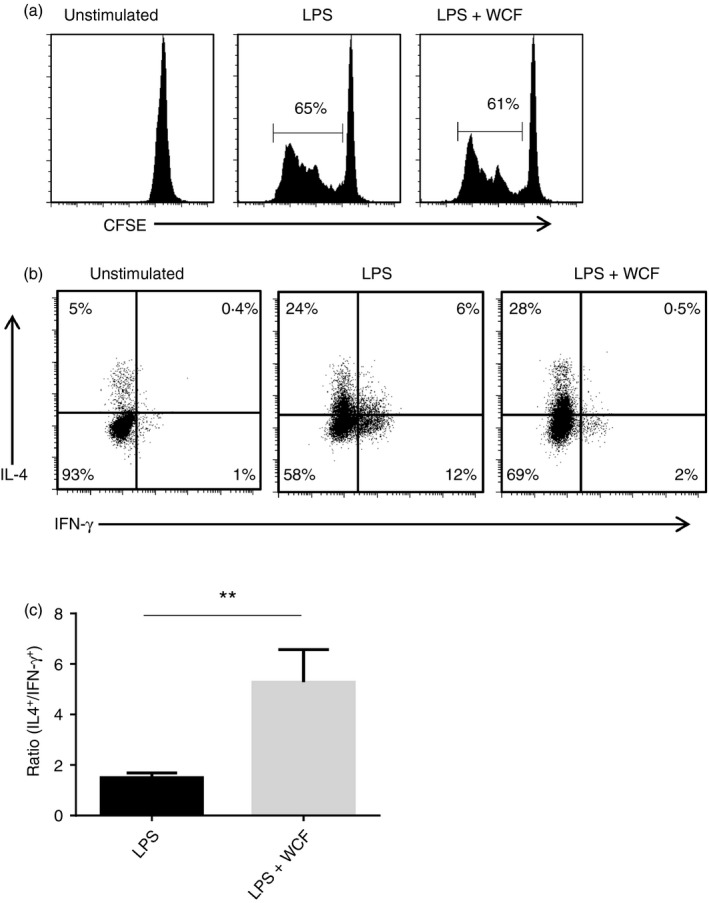
Proanthocyanidin (PAC) ‐exposed dendritic cells (DCs) do not reduce lymphocyte proliferation but inhibit interferon‐γ (IFN‐γ) production in CD4+ T cells. (a) Representative data plot from one of three experiments showing allogenic lymphocyte proliferation following stimulation with DCs matured with lipopolysaccharide (LPS) or LPS + 20 μg/ml WCF F2. (b) Representative data plot from one of four experiments showing interleukin‐4 (IL‐4) (vertical axis) and IFN‐γ (horizontal axis) production in CD4+ T cells. Allogenic T cells were activated by DCs previously incubated for 48 hr with either LPS or LPS + 20 μg/ml white clover flower (WCF) fraction 2 (F2). T cells were cultured for 12 days and then stimulated for 5 hr with ionomycin and PMA to measure cytokine production. Unstimulated cells are shown as a control. (c) Ratio of IL‐4‐positive cells to IFN‐γ‐positive cells in CD4+ T cells activated by DCs previously incubated with LPS or LPS + WCF F2 as described above. Results are mean ± SEM of four independent experiments with DCs from different donors. **P < 0·01 by paired t‐test.
Proanthocyanidins synergize with Trichuris suis products to modulate cytokine secretion and induce OX40L expression in DCs
Given the anti‐inflammatory properties of PAC, we next assessed whether PAC would interact with TsSP, which we have previously shown to markedly inhibit inflammatory responses in DCs. Consistent with our previous work,11, 13 we observed here significant decreases in LPS‐induced TNF‐α, IL‐6 and IL‐12p70 secretion after pre‐incubation of DCs with a concentration of 40 μg/ml TsSP (mean reductions of 64%, 43% and 57%, respectively; Fig. 7a). Incubation of DCs with higher concentrations of TsSP (up to 160 μg/ml) failed to result in increased inhibition of secretion of TNF‐α 13 and IL‐6 and IL‐12p70 (see Supplementary material, Fig. S5), indicating a saturating effect of TsSP at this concentration. However, addition of either COC or WCF F2 further decreased secretion of TNF‐α, IL‐6 and IL‐12p70, indicating that PAC and TsSP synergized to reduce secretion of these cytokines (mean reductions of 74%, 69% and 86%, respectively, for COC and 84%, 79% and 97%, respectively, for WCF; Fig. 7a). Furthermore, an enhancement of IL‐10 secretion was observed when TsSP and PAC were combined. Cells exposed to TsSP secreted more IL‐10 than DCs pulsed only with LPS, and this effect was boosted by co‐incubation with PAC (Fig. 7a), although large variations between experiments with cells from different blood donors prevented statistically significant differences. These data suggest that reductions in Th1 cytokine secretion, and a corresponding enhancement of IL‐10 secretion, are co‐operatively achieved by PAC and TsSP in a combined effect that is above and beyond the effect achieved by a saturating concentration of either treatment in isolation.
Figure 7.
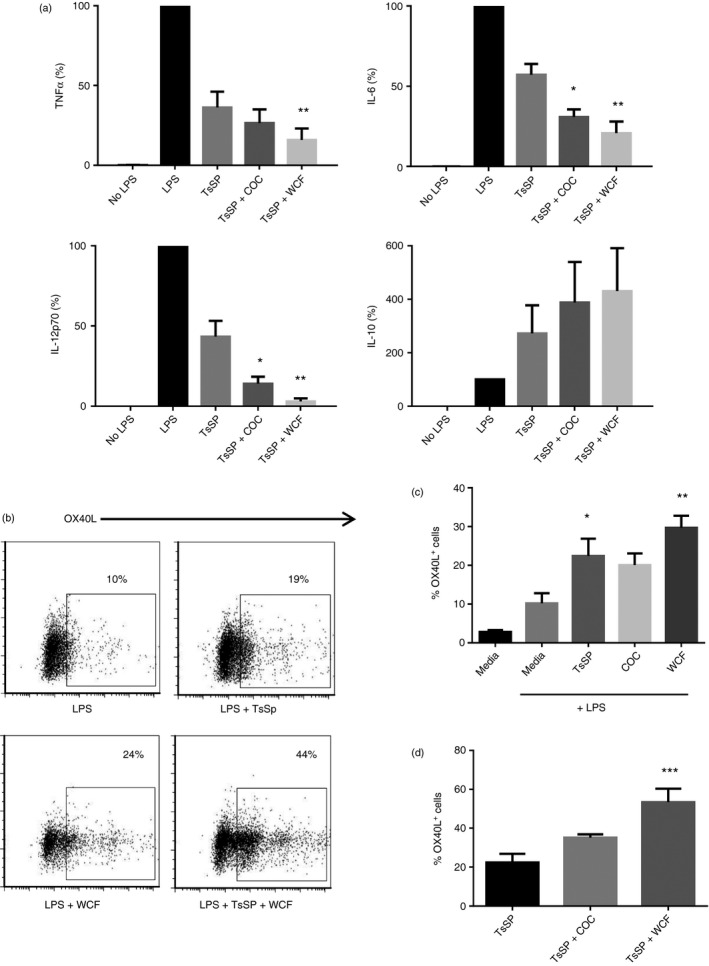
Proanthocyanidins (PAC) and soluble products from Trichuris suis co‐operatively inhibit inflammatory cytokine secretion and induce OX40L expression in lipopolysaccharide (LPS) ‐activated dendritic cells (DCs). (a) Suppression of LPS‐induced cytokine secretion in DCs by addition of 40 μg/ml Trichuris suis soluble products (TsSP) or TsSP combined with 10 μg/ml cocoa (COC) fraction 2 (F2) or 20 μg/ml white clover flower (WCF) F2. Cytokine secretion in treated cells is expressed as a percentage of the LPS‐induced secretion for each cytokine, where 100% corresponds to 36 ± 4·2 ng/ml for tumour necrosis factor‐α (TNF‐α), 25 ± 3·9 ng/ml for interleukin‐6 (IL‐6), 5 ± 0·7 ng/ml for IL‐12p70 and 2 ± 1·2 ng/ml for IL‐10. The experiments have been performed at least four times with cells from different donors and the mean and inter‐donor SEM are shown. *P < 0·05; ***P < 0·001 by one‐way analysis of variance compared with TsSP only. (b) Representative FACS plots from one of four independent experiments showing OX40+ DCs after 24 hr incubation with media only, LPS, or LPS combined with either TsSP, COC F2 or WCF F2, and also TsSP + either COC F2 or WCF F2. (c) Percentages of OX40+ DCs after 24 hr incubation with LPS only or LPS combined with either TsSP, COC F2 or WCF F2. Experiments were performed four times with cells from different donors. Mean ± inter‐donor SEM are shown. *P < 0·05, **P < 0·01 by one‐way analysis of variance compared with LPS only. (d) Percentages of OX40+ DCs after 24 hr incubation with LPS combined with either TsSP, or TsSP + COC F2 or WCF F2. Experiments were performed four times with cells from different donors. The mean ± inter‐donor SEM are shown. **P < 0·001 by one‐way analysis of variance compared with TsSP only.
Given that we have also previously observed that TsSP up‐regulates OX40L expression in DCs11 which is important in driving Th2 responses,35 we also assessed whether TsSP and PAC would synergize to up‐regulate OX40L expression. Incubation of PAC or TsSP with immature DCs did not result in increased OX40L expression (data not shown). As expected, TsSP significantly induced OX40L expression in LPS‐stimulated DCs (P < 0·05; Fig. 7b,c). Similarly, addition of both COC and WCF F2 to LPS‐pulsed DCs resulted in increases in OX40L expression, although only statistically significant for WCF (Fig. 7b,c). Strikingly, co‐incubation of LPS‐stimulated DCs with TsSP and PAC, particularly WCF F2, resulted in increased expression of OX40L compared with TsSP alone (P < 0·001 for WCF; Fig. 7b,d), indicating augmentation of the helminth‐induced Th2 polarization by PAC. Hence, PAC and TsSP interact to influence both cytokine secretion and OX40L up‐regulation in DCs.
Finally, we assessed whether pulsing DCs with a combination of WCF F2 and TsSP would result in a lower production of IFN‐γ in naive CD4+ T cells, compared with that observed with WCF F2 alone (Fig. 6). Similar to the data with WCF F2, T cells activated by DCs primed by TsSP produced only low amounts of IFN‐γ (Fig. 8a); however, IFN‐γ production in T cells activated by DCs exposed to both WCF F2 and TsSP was not further reduced from the already low levels observed with each treatment in isolation, whereas IL‐4 production was again unaffected. Hence, IL‐4 : IFN‐γ ratios were similar (P = 0·8) in T cells activated by DCs treated with either WCF F2 alone, TsSP alone, or WCF and TsSP in combination (Fig. 8b).
Figure 8.
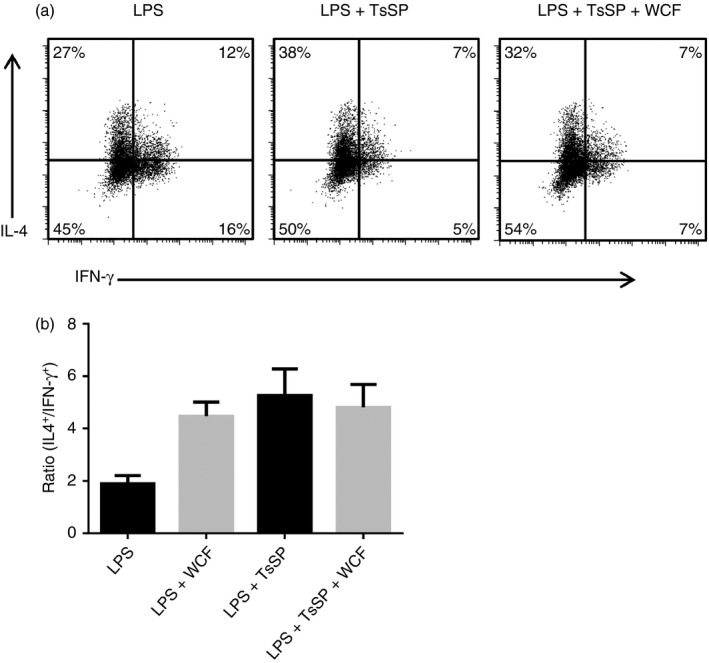
Interferon‐γ (IFN‐γ) production in CD4+ T cells activated with dendritic cells (DCs) exposed to Trichuris suis soluble products (TsSP) and/or proanthocyanidins (PAC). (a) Representative data plot from one of four experiments showing interleukin‐4 (IL‐4) (vertical axis) and IFN‐γ (horizontal axis) production in CD4+ T cells. Allogenic T cells were activated by DCs previously incubated for 48 hr with either lipopolysaccharide (LPS), LPS + 40 μg/ml TsSP or LPS + 20 μg/ml white clover flower (WCF) fraction 2 (F2)/40 μg/ml TsSP. T cells were cultured for 12 days and then stimulated for 5 hr with ionomycin and PMA to measure cytokine production. (b) Ratio of IL‐4‐positive cells to IFN‐γ‐positive cells in CD4+ T cells activated by DCs previously incubated with LPS, LPS + WCF F2, LPS + TsSP or LPS + WCF F2/TsSP as described above. Results are mean ± SEM of four independent experiments with DCs from different donors.
Discussion
The objective of this work was to test the hypothesis that PAC would modulate LPS‐induced responses in human DCs, and would also interact with TsSP, which are already known to possess such inhibitory activity.
Our data clearly show that the exposure of DCs to PAC induces an anti‐inflammatory phenotype, with marked reductions in secretion of inflammatory cytokines as well as a significant impairment in IFN‐γ production by naive CD4+ T cells activated by PAC‐pulsed DCs. The DC phenotype induced by PAC was remarkably similar to that induced by TsSP, as well as what has been observed previously with products from other helminths such as Schistosoma mansoni or Trichinella spiralis.11 Notably, exposure of DCs to both PAC and TsSP resulted in an augmentation of this phenotype that was above and beyond that achieved by either single stimulus, perhaps suggesting that PAC and TsSP modulate DC activity through independent pathways that co‐operatively inhibit pro‐inflammatory cytokine production. Therefore, our results may shed light on a novel interaction between diet and an intestinal pathogen that may be important for regulation of inflammation and immunity.
Proanthocyanidins are clearly recognized by DCs, with our experiments showing that DCs actively endocytose PAC, which appear to be trafficked to the lysosomes within 2 hr, consistent with the putative intracellular trafficking of EGCG and tannin–lysozyme complexes by mouse macrophages.36, 37 DCs then respond by selectively up‐regulating CD86 (and possibly CX3CR1, to a lesser extent), suggesting at least a partial maturation of the DCs. However, no cytokine secretion was evident in the absence of TLR ligation, indicating that PAC do not fully induce functional DC activity and, instead, selectively modulate TLR4‐driven responses. The presence in the lysosome raises the possibility that PAC may exert activity through a modulation of antigen processing, but, clearly, further studies will be necessary to determine the mechanistic aspects of how PAC are trafficked within DCs, and the signalling pathways that are modulated by PAC in LPS‐stimulated cells. Ligation of TLR4 primarily triggers two signalling pathways: the MyD88‐dependent pathway, which leads to nuclear factor‐κB translocation and inflammatory gene expression, and the TIR‐domain‐containing adapter‐inducing interferon‐β (TRIF)‐dependent pathway, which results in type I interferon transcription38. The influence of PAC on these two pathways is clearly an area requiring investigation. In addition to a number of reports from murine models that dietary PAC may alleviate inflammatory disorders such as colitis,39, 40 in vitro studies, working mainly with model murine cell lines, have demonstrated that polymeric PAC can have specific anti‐inflammatory activity such as a decrease in nitric oxide production or a reduction in nuclear factor‐κB activation and IL‐1β secretion.41, 42 Functional consequences of this modulation of DC activity may be a marked change in naive T helper cell function, as evidenced by our data showing significantly impaired IFN‐γ production in CD4+ T cells activated by PAC‐pulsed DCs, consistent with previous work showing that dietary PAC can selectively down‐regulate Th1‐type inflammatory responses in mice in vivo.43 The initial contact and process(es) involved in recognition of PAC by DCs will also require further clarification. Given the strong protein‐binding properties of PAC,44 it is perhaps unlikely that PAC are bound by a cognate receptor and instead are more likely to interact with a variety of cell surface molecules, and perhaps also lipid rafts in the cell membrane, as has been shown for the interaction between COC PAC and human enterocytes45.
Similarly to bioactive dietary compounds such as PAC, helminths (or their secreted and/or somatic products) can be said to possess anti‐inflammatory properties; indeed, controlled helminth infection has been shown to alleviate signs of auto‐inflammatory disorders in humans.15 We therefore hypothesized that the human DC response to helminths and PAC may share certain similarities. In support of our hypothesis, we observed a remarkably similar phenotype whereby LPS‐stimulated DCs pulsed either with TsSP or PAC secreted significantly less IL‐6 and IL‐12p70, and more IL‐10, than control cells treated only with LPS, and also resulted in a significantly increased expression of OX40L, which has been shown to be important in driving Th2 responses in the context of helminth infections.46 Importantly, we showed that these effects could be synergistic, in the sense that responses significantly greater than either treatment in isolation could be achieved by their combination. The effective concentrations of PAC were saturated by cytotoxicity at higher concentrations, whereas the effective range of concentrations of TsSP was also shown to be saturable at concentrations > 40 μg/ml13, perhaps through a lack of availability of surface receptors needed to bind the active components of TsSP on DC function. However, the addition of PAC significantly enhances the effects of this saturating concentration of TsSP, suggesting that PAC operate through a mechanism distinct from that of TsSP to influence DC activity, with a similar end‐result in terms of cytokine secretion and OX40L expression. Although we did not observe a co‐operative inhibition of IFN‐γ production in T cells activated by DCs exposed to both PAC and TsSP, this can probably be attributed to the low levels of IFN‐γ that were produced by each treatment in isolation, so lowering the scope to observe synergistic effects. Overall, our data may indicate that the immune‐modulating activity of T. suis can be augmented by the presence of PAC. Interestingly, Zhong et al. have recently noted a similar phenomenon in sheep leucocyte preparations exposed in vitro to Haemonchus contortus antigens, whereby enhanced IL‐10 production and reduced IL‐12 production were evident after co‐incubation of the cells with another type of tannin, tannic acid (a mixture of hydrolysable tannins that contain glucose as a central core that is surrounded by six or more galloyl groups)47, suggesting possibly a conserved interaction between helminth antigens and oligomeric polyphenols.
This interaction between pathogens and these dietary compounds raises a number of possibilities. In the in vivo situation, gastrointestinal pathogens such as helminths and bioactive food compounds are likely to be found in close proximity within the gut and associated with the intestinal mucosa, suggesting that the co‐operative effects we have observed in vitro may also be relevant in vivo. The concentrations of PAC used in our studies are likely to represent physiological concentrations expected to be present in the digesta following consumption of PAC‐containing foods,40, 48, 49 although precise measurements of local PAC concentrations at the mucosal surface are limited by methodological difficulties.50 Oligomeric PAC are known to be poorly absorbed by enterocytes,51 and are thought to be largely stable through transit of the monogastric gastrointestinal tract (e.g. that of humans, pigs or rats).49, 50 This suggests that the immune‐modulating activity of dietary PAC is probably mediated by sentinel cells in the gut mucosa, and subsequent modulation of T‐cell activity in the GALT. Dendritic cells are key candidates to be the cells that mediate these effects of PAC, because of their ability to directly interact with the contents of the intestinal digesta52.
Intestinal DCs may acquire luminal antigens in two ways; antigens may be delivered to follicular or lamina propria‐resident DCs by M cells in the epithelium that capture antigen from the intestinal lumen, or DCs may sample the luminal contents directly, by either extending dendrites through the epithelial barrier and transporting antigens back to the sub‐epithelial zone, or by actively migrating into the lumen where they interact with the intestinal digesta.52, 53, 54, 55 If and how DCs interact with PAC in vivo is a key question of interest: do DCs directly sample PAC from the intestinal lumen, or are PAC recognized by M cells and delivered by transcytosis to DCs residing in the sub‐epithelial region of the gastrointestinal mucosa? In mice, intestinal DCs appear to be a heterogeneous population, with differential functions based on the expression of surface markers. In the steady state, both CD103+ and CX3CR1+ reside in the lamina propria and lymphoid tissue, and appear to have distinct functions. CX3CR1 expression has been shown to be crucial in the ability to sample luminal antigens,56 whereas others have shown that CX3CR1+ DCs seem to be a non‐migratory population within the lamina propria. Instead, CX3CR1− CD103+ cells can uptake antigen in the epithelium and are better equipped for subsequent migration to the lymph nodes and activation of T cells, and are important in the induction of regulatory T cells and intestinal homeostasis.53, 57, 58, 59 In contrast, murine CD103− DCs have also been shown to have migratory and T‐cell activation ability but instead induce pro‐inflammatory Th1 and Th17 responses.58, 60 Moreover, during infection and inflammation, monocytes migrate from the blood into the mucosa and can differentiate into DCs with a strong inflammatory phenotype,61, 62 so probably resemble monocyte‐derived DCs as used in our current experiments (consisting of CD103− DCs). We also observed that both CX3CR1− cells and a small population of CX3CR1+ cells seemed to equally recognize PAC (as judged by CD86 expression), suggestive of a conserved response across distinct populations of DCs. However whether or not different populations of intestinal DCs respond identically to PAC is a question that warrants further attention.
It is also notable that T. suis infection has been observed to decrease barrier function in the intestinal mucosa at the site of the infection,63, 64 and Hiemstra et al. have shown that excretory/secretory products from T. suis can disrupt the tight barrier junctions in epithelial cells in vitro, enhancing direct contact of antigens to immune cells residing in sub‐epithelial locations.65 Hence, an intriguing possibility is that interactions between PAC and the GALT could be amplified during helminth infection due to the ‘leaky’ epithelial barriers allowing the passage of oligomeric PAC molecules that under normal circumstances may be confined to the gut lumen. The in vivo interactions between PAC and the gut mucosa, both alone and in the context of helminth (or other pathogen) infection is clearly an area where much work is required.
This putative interaction between a common class of bioactive dietary compounds and a gut pathogen may have far‐reaching implications. PAC‐rich feed supplements are becoming popular in livestock production because of their antioxidant and anti‐inflammatory properties,19, 66 and may have beneficial effects in helminth‐infected animals such as enhancement of Th2‐type protective immune mechanisms that enhance worm expulsion, or control of excessive gut inflammation. Paradoxically, T. suis has also been investigated as a therapeutic treatment in humans suffering from autoimmune disease, in part due to the strong modulatory effects observed on DC and macrophage function.67, 68 However, clinical trials to investigate effects on diseases such as colitis and multiple sclerosis have not been equivocal, with some reported successes but also a number of negative results.14, 69, 70 Our current data may suggest that the efficacy of helminth therapy could be augmented/enhanced by concurrent nutritional treatment with PAC, either in the form of PAC‐rich dietary sources or administration of purified, concentrated PAC. Although it is possible, but expensive, to synthesize oligomeric PAC, large‐scale extraction and purification procedures exist to obtain PAC from a number of inexpensive plant sources, including some agricultural waste products such as berry pomace.71 Moreover, as discussed above, the physiological concentrations of PAC needed to exert activity may be achievable through a PAC‐rich diet rather than targeted treatment with purified PAC. Therefore, ‘combination therapy’ with helminth products and PAC may be a novel future avenue for autoimmune treatment.
In conclusion, this study provides the first evidence that oligomeric PAC can modulate human DC activity, and that the potential for synergism exists with the modulatory activity of gastrointestinal parasites that may be present within the intestine. The activity of PAC was confined to oligomers with a mean degree of polymerization of at least four, and appeared to be more closely associated with prodelphinidins rather than procyanidins. Further investigation of the in vivo interactions between dietary PAC and intestinal parasites on immune function is highly warranted.
Disclosure
The authors declare no conflict of interest.
Supporting information
Figure S1. Viability of human dendritic cells (as judged by 7AAD staining) after exposure for 24 hr to proanthocyanidins (PAC) derived from cocoa (COC F2) or white clover flowers (WCF F2).
Figure S2. Characterization of monocyte‐derived human dendritic cells.
Figure S3. (a) Inhibition of lipopolysaccharide (LPS) ‐induced interleukin‐6 (IL‐6) and IL‐12p70 secretion in human dendritic cells by different concentrations of cocoa (COC) and white clover flower (WCF) fraction 2 (F2). Inhibition is relative to LPS only, which was set at 100%. Results are the mean of four independent experiments, each performed with cells from different donors. Mean ± inter‐donor SEM are shown. *P < 0·05, **P < 0·01, ***P < 0·001 relative to LPS only by one‐way analysis of variance. (b) Inhibition of LPS‐induced IL‐6 and IL‐12p70 secretion in human dendritic cells by 10 µg/ml of COC and WCF F2. Inhibition is relative to LPS only, which was set at 100%. Results are the mean of four independent experiments, each performed with cells from different donors. Mean ± inter‐donor SEM are shown.
Figure S4. Inhibition of lipopolysaccharide (LPS) ‐induced interleukin‐6 (IL‐6) and IL‐12p70 secretion in human dendritic cells by 25 µg/ml of DTAF‐tagged proanthocyanidin (PAC).
Figure S5. Inhibition of lipopolysaccharide‐induced interleukin‐6 and IL‐12p70 secretion in human dendritic cells by varying concentrations of Trichuris suis soluble products.
Acknowledgements
This work was supported by the Danish Council for Independent Research (Grant 12‐126630), the Lundbeck Foundation (Grant 14‐3670A) and the European Commission (PITN‐GA‐2011‐289377, ‘LegumePlus’ project). Chris Drake (University of Reading) is gratefully acknowledged for assistance with the thiolysis procedures. ARW, IVD and SMT conceived and designed the study. ARW, EJK, LCL and RD performed the dendritic cell experiments. AR, CF, JDR and IMH prepared PAC samples and performed chemical analyses. HK and SS contributed essential reagents and/or materials. ARW wrote the manuscript, with input from all other authors.
References
- 1. Ko H‐J, Chang S‐Y. Regulation of intestinal immune system by dendritic cells. Immune Netw 2015; 15:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rescigno M. Chapter 4 – Intestinal dendritic cells In: Sidonia F, Andrea C, eds. Advances in Immunology: Cambridge, MA: Academic Press, 2010: 109–38. [DOI] [PubMed] [Google Scholar]
- 3. Zozulya A, Clarkson B, Ortler S, Fabry Z, Wiendl H. The role of dendritic cells in CNS autoimmunity. J Mol Med 2010; 88:535–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Radford KJ, Caminschi I. New generation of dendritic cell vaccines. Hum Vaccin Immunother 2013; 9:259–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dissanayake D, Hall H, Berg‐Brown N, Elford AR, Hamilton SR, Murakami K et al Nuclear factor‐κB1 controls the functional maturation of dendritic cells and prevents the activation of autoreactive T cells. Nat Med 2011; 17:1663–7. [DOI] [PubMed] [Google Scholar]
- 6. Prendergast KA, Kirman JR. Dendritic cell subsets in mycobacterial infection: control of bacterial growth and T‐cell responses. Tuberculosis 2013; 93:115–22. [DOI] [PubMed] [Google Scholar]
- 7. Schulte BM, Gielen PR, Kers‐Rebel ED, Schreibelt G, van Kuppeveld FJM, Adema GJ. Enterovirus‐infected β‐cells induce distinct response patterns in BDCA1+ and BDCA3+ human dendritic cells. PLoS ONE 2015; 10:e0121670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Carvalho L, Sun J, Kane C, Marshall F, Krawczyk C, Pearce EJ. Review series on helminths, immune modulation and the hygiene hypothesis: mechanisms underlying helminth modulation of dendritic cell function. Immunology 2009; 126:28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Notari L, Riera DC, Sun R, Bohl JA, McLean LP, Madden KB et al Role of macrophages in the altered epithelial function during a type 2 immune response induced by enteric nematode infection. PLoS ONE 2014; 9:e84763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Smith A, Madden KB, Yeung KJA, Zhao A, Elfrey J, Finkelman F et al Deficiencies in selenium and/or Vitamin E lower the resistance of mice to Heligmosomoides polygyrus infections. J Nutr 2005; 135:830–6. [DOI] [PubMed] [Google Scholar]
- 11. Kuijk LM, Klaver EJ, Kooij G, van der Pol SMA, Heijnen P, Bruijns SCM et al Soluble helminth products suppress clinical signs in murine experimental autoimmune encephalomyelitis and differentially modulate human dendritic cell activation. Mol Immunol 2012; 51:210–8. [DOI] [PubMed] [Google Scholar]
- 12. Chen F, Liu Z, Wu W, Rozo C, Bowdridge S, Millman A et al An essential role for TH2‐type responses in limiting acute tissue damage during experimental helminth infection. Nat Med 2012; 18:260–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Klaver EJ, Kuijk LM, Laan LC, Kringel H, van Vliet SJ, Bouma G et al Trichuris suis‐induced modulation of human dendritic cell function is glycan‐mediated. Int J Parasitol 2013; 43:191–200. [DOI] [PubMed] [Google Scholar]
- 14. Fleming J. Helminth therapy and mutiple sclerosis. Int J Parasitol 2013; 43:259–74. [DOI] [PubMed] [Google Scholar]
- 15. Summers RW, Elliott DE, Urban JF, Thompson R, Weinstock JV. Trichuris suis therapy in Crohn's disease. Gut 2005; 54:87–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Oz HS, Chen T, de Villiers WJ. Green tea polyphenols and sulfasalazine have parallel anti‐inflammatory properties in colitis models. Front Immunol 2013; 4:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Yang E‐J, Lee J, Lee S‐Y, Kim E‐K, Moon Y‐M, Jung YO et al EGCG attenuates autoimmune arthritis by inhibition of STAT3 and HIF‐1α with Th17/Treg control. PLoS ONE 2014; 9:e86062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hong Byun E, Fujimura Y, Yamada K, Tachibana H. TLR4 signaling inhibitory pathway induced by green tea polyphenol epigallocatechin‐3‐Gallate through 67‐kDa laminin receptor. J Immunol 2010; 185:33–45. [DOI] [PubMed] [Google Scholar]
- 19. Gessner DK, Fiesel A, Most E, Dinges J, Wen G, Ringseis R et al Supplementation of a grape seed and grape marc meal extract decreases activities of the oxidative stress‐responsive transcription factors NF‐κB and Nrf2 in the duodenal mucosa of pigs. Acta Vet Scand 2013; 55:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Martinez‐Micaelo N, González‐Abuín N, Ardèvol A, Pinent M, Blay MT. Procyanidins and inflammation: molecular targets and health implications. BioFactors 2012; 38:257–65. [DOI] [PubMed] [Google Scholar]
- 21. Ahmad SF, Zoheir KMA, Abdel‐Hamied HE, Ashour AE, Bakheet SA, Attia SM et al Grape seed proanthocyanidin extract has potent anti‐arthritic effects on collagen‐induced arthritis by modifying the T cell balance. Int Immunopharmacol 2013; 17:79–87. [DOI] [PubMed] [Google Scholar]
- 22. Miyake M, Sasaki K, Ide K, Matsukura Y, Shijima K, Fujiwara D. Highly oligomeric procyanidins ameliorate experimental autoimmune encephalomyelitis via suppression of Th1 immunity. J Immunol 2006; 176:5797–804. [DOI] [PubMed] [Google Scholar]
- 23. Sung N‐Y, Yang M‐S, Song D‐S, Kim J‐K, Park J‐H, Song B‐S et al Procyanidin dimer B2‐mediated IRAK‐M induction negatively regulates TLR4 signaling in macrophages. Biochem Biophys Res Commun 2013; 438:122–8. [DOI] [PubMed] [Google Scholar]
- 24. Graff JC, Jutila MA. Differential regulation of CD11b on γδ T cells and monocytes in response to unripe apple polyphenols. J Leukoc Biol 2007; 82:603–7. [DOI] [PubMed] [Google Scholar]
- 25. Tosh KW, Mittereder L, Bonne‐Annee S, Hieny S, Nutman TB, Singer SM et al The IL‐12 response of primary human dendritic cells and monocytes to Toxoplasma gondii is stimulated by phagocytosis of live parasites rather than host cell invasion. J Immunol 2016; 196:345–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Collin M, McGovern N, Haniffa M. Human dendritic cell subsets. Immunology 2013; 140:22–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schinnerling K, García‐González P, Aguillón JC. Gene expression profiling of human monocyte‐derived dendritic cells – searching for molecular regulators of tolerogenicity. Front Immunol 2015; 6:528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kringel H, Iburg T, Dawson H, Aasted B, Roepstorff A. A time course study of immunological responses in Trichuris suis infected pigs demonstrates induction of a local type 2 response associated with worm burden. Int J Parasitol 2006; 36:915–24. [DOI] [PubMed] [Google Scholar]
- 29. Williams AR, Fryganas C, Ramsay A, Mueller‐Harvey I, Thamsborg SM. Direct anthelmintic effects of condensed tannins from diverse plant sources against Ascaris suum . PLoS ONE 2014; 9:e97053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Williams AR, Ramsay A, Hansen TVA, Ropiak HM, Mejer H, Nejsum P et al Anthelmintic activity of trans‐cinnamaldehyde and A‐ and B‐type proanthocyanidins derived from cinnamon (Cinnamomum verum). Sci Rep 2015; 5:14791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Feliciano RP, Heintz JA, Krueger CG, Vestling MM, Reed JD. Fluorescent labeling of cranberry proanthocyanidins with 5‐([4,6‐dichlorotriazin‐2‐yl]amino)fluorescein (DTAF). Food Chem 2015; 166:337–45. [DOI] [PubMed] [Google Scholar]
- 32. van Stijn CMW, Meyer S, van den Broek M, Bruijns SCM, van Kooyk Y, Geyer R et al Schistosoma mansoni worm glycolipids induce an inflammatory phenotype in human dendritic cells by cooperation of TLR4 and DC‐SIGN. Mol Immunol 2010; 47:1544–52. [DOI] [PubMed] [Google Scholar]
- 33. Sprong T, Brandtzaeg P, Fung M, Pharo AM, Høiby EA, Michaelsen TE et al Inhibition of C5a‐induced inflammation with preserved C5b‐9‐mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood 2003; 102:3702. [DOI] [PubMed] [Google Scholar]
- 34. Byun E, Choi H, Sung N, Byun E. Green tea polyphenol epigallocatechin‐3‐gallate inhibits TLR4 signaling through the 67‐kDa laminin receptor on lipopolysaccharide‐stimulated dendritic cells. Biochem Biophys Res Commun 2012; 426:480–5. [DOI] [PubMed] [Google Scholar]
- 35. Jenkins SJ, Perona‐Wright G, Worsley AGF, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal th2 priming and memory induction in vivo . J Immunol 2007; 179:3515–23. [DOI] [PubMed] [Google Scholar]
- 36. Li W, Zhu S, Li J, Assa A, Jundoria A, Xu J et al EGCG stimulates autophagy and reduces cytoplasmic HMGB1 levels in endotoxin‐stimulated macrophages. Biochem Pharmacol 2011; 81:1152–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Madrigal‐Carballo S, Haas L, Vestling M, Krueger CG, Reed JD. Non‐covalent pomegranate (Punica granatum) hydrolyzable tannin‐protein complexes modulate antigen uptake, processing and presentation by a T‐cell hybridoma line co‐cultured with murine peritoneal macrophages. Int J Food Sci Nutr 2016; 67:960–8. [DOI] [PubMed] [Google Scholar]
- 38. Pålsson‐McDermott EM, O'Neill LAJ. Signal transduction by the lipopolysaccharide receptor, Toll‐like receptor‐4. Immunology 2004; 113:153–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Skyberg JA, Robison A, Golden S, Rollins MF, Callis G, Huarte E et al Apple polyphenols require T cells to ameliorate dextran sulfate sodium‐induced colitis and dampen proinflammatory cytokine expression. J Leukoc Biol 2011; 90:1043–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Yoshioka Y, Akiyama H, Nakano M, Shoji T, Kanda T, Ohtake Y et al Orally administered apple procyanidins protect against experimental inflammatory bowel disease in mice. Int Immunopharmacol 2008; 8:1802–7. [DOI] [PubMed] [Google Scholar]
- 41. Gentile C, Allegra M, Angileri F, Pintaudi AM, Livrea MA, Tesoriere L. Polymeric proanthocyanidins from Sicilian pistachio (Pistacia vera L.) nut extract inhibit lipopolysaccharide‐induced inflammatory response in RAW 264.7 cells. Eur J Nutr 2012; 51:353–63. [DOI] [PubMed] [Google Scholar]
- 42. Bak M‐J, Truong VL, Kang H‐S, Jun M, Jeong W‐S. Anti‐inflammatory effect of procyanidins from wild grape (Vitis amurensis) seeds in LPS‐induced RAW 264.7 cells. Oxid Med Cell Longev 2013; 2013:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ahmad S, Zoheir KA, Abdel‐Hamied H, Attia S, Bakheet S, Ashour A et al Grape seed proanthocyanidin extract protects against carrageenan‐induced lung inflammation in mice through reduction of pro‐inflammatory markers and chemokine expressions. Inflammation 2014; 37:500–11. [DOI] [PubMed] [Google Scholar]
- 44. Xu Z, Du P, Mesier P, Jacob C. Proanthocyanidins: oligomeric structures with unique biochemical properties and great therapeutic promise. Nat Prod Commun 2012; 7:381–8. [PubMed] [Google Scholar]
- 45. Verstraeten SV, Jaggers GK, Fraga CG, Oteiza PI. Procyanidins can interact with Caco‐2 cell membrane lipid rafts: involvement of cholesterol. Biochim Biophys Acta 2013; 1828:2646–53. [DOI] [PubMed] [Google Scholar]
- 46. Ekkens MJ, Liu Z, Liu Q, Whitmire J, Xiao S, Foster A et al The role of OX40 ligand interactions in the development of the Th2 response to the gastrointestinal nematode parasite Heligmosomoides polygyrus . J Immunol 2003; 170:384–93. [DOI] [PubMed] [Google Scholar]
- 47. Zhong RZ, Sun HX, Liu HW, Zhou DW. Effects of tannic acid on Haemonchus contortus larvae viability and immune responses of sheep white blood cells in vitro . Parasite Immunol 2014; 36:100–6. [DOI] [PubMed] [Google Scholar]
- 48. Mueller‐Harvey I. Unravelling the conundrum of tannins in animal nutrition and health. J Sci Food Agric 2006; 86:2010–37. [Google Scholar]
- 49. Rios LY, Bennett RN, Lazarus SA, Rémésy C, Scalbert A, Williamson G. Cocoa procyanidins are stable during gastric transit in humans. Am J Clin Nutr 2002; 76:1106–10. [DOI] [PubMed] [Google Scholar]
- 50. Reed JD. Nutritional toxicology of tannins and related polyphenols in forage legumes. J Anim Sci 1995; 73:1516–28. [DOI] [PubMed] [Google Scholar]
- 51. Deprez S, Mila I, Huneau J‐F, Tome D, Scalbert A. Transport of proanthocyanidin dimer, trimer, and polymer across monolayers of human intestinal epithelial Caco‐2 cells. Antioxid Redox Signal 2001; 3:957–67. [DOI] [PubMed] [Google Scholar]
- 52. Schulz O, Pabst O. Antigen sampling in the small intestine. Trends Immunol 2013; 34:155–61. [DOI] [PubMed] [Google Scholar]
- 53. Farache J, Koren I, Milo I, Gurevich I, Kim K‐W, Zigmond E et al Luminal bacteria recruit CD103+ dendritic cells into the intestinal epithelium to sample bacterial antigens for presentation. Immunity 2013; 38:581–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Mucosal Iwasaki A. Mucosal dendritic cells. Annu Rev Immunol 2007; 25:381–418. [DOI] [PubMed] [Google Scholar]
- 55. Chang S‐Y, Ko H‐J, Kweon M‐N. Mucosal dendritic cells shape mucosal immunity. Exp Mol Med 2014; 46:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Niess JH, Brand S, Gu X, Landsman L, Jung S, McCormick BA et al CX3CR1‐mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005; 307:254. [DOI] [PubMed] [Google Scholar]
- 57. Schulz O, Jaensson E, Persson EK, Liu X, Worbs T, Agace WW et al Intestinal CD103+, but not CX3CR1+, antigen sampling cells migrate in lymph and serve classical dendritic cell functions. J Exp Med 2009; 206:3101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ruane DT, Lavelle EC. The role of CD103+ dendritic cells in the intestinal mucosal immune system. Front Immunol 2011; 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Persson EK, Scott CL, Mowat AM, Agace WW. Dendritic cell subsets in the intestinal lamina propria: ontogeny and function. Eur J Immunol 2013; 43:3098–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Cerovic V, Houston SA, Scott CL, Aumeunier A, Yrlid U, Mowat AM et al Intestinal CD103– dendritic cells migrate in lymph and prime effector T cells. Mucosal Immunol 2013; 6:104–13. [DOI] [PubMed] [Google Scholar]
- 61. Coombes JL, Powrie F. Dendritic cells in intestinal immune regulation. Nat Rev Immunol 2008; 8:435–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Varol C, Landsman L, Fogg DK, Greenshtein L, Gildor B, Margalit R et al Monocytes give rise to mucosal, but not splenic, conventional dendritic cells. J Exp Med 2007; 204:171–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Abner SR, Hill DE, Turner JR, Black ED, Bartlett P, Urban JF et al Responsee of intestinal epithlial cells to Trichuris suis excretory–secretory products and the influence on Campylobacter jejuni invasion under in vitro conditions. J Parasitol 2002; 88:738–45. [DOI] [PubMed] [Google Scholar]
- 64. Mansfield LS, Urban JF Jr. The pathogenesis of necrotic proliferative colitis in swine is linked to whipworm induced suppression of mucosal immunity to resident bacteria. Vet Immunol Immunopathol 1996; 50:1–17. [DOI] [PubMed] [Google Scholar]
- 65. Hiemstra IH, Klaver EJ, Vrijland K, Kringel H, Andreasen A, Bouma G et al Excreted/secreted Trichuris suis products reduce barrier function and suppress inflammatory cytokine production of intestinal epithelial cells. Mol Immunol 2014; 60:1–7. [DOI] [PubMed] [Google Scholar]
- 66. Sehm J, Lindermayer H, Dummer C, Treutter D, Pfaffl MW. The influence of polyphenol rich apple pomace or red‐wine pomace diet on the gut morphology in weaning piglets. J Anim Physiol Anim Nutr 2007; 91:289–96. [DOI] [PubMed] [Google Scholar]
- 67. Ottow MK, Klaver EJ, van der Pouw Kraan TCTM, Heijnen PD, Laan LC, Kringel H et al The helminth Trichuris suis suppresses TLR4‐induced inflammatory responses in human macrophages. Genes Immun 2014; 15:477–86. [DOI] [PubMed] [Google Scholar]
- 68. Klaver EJ, van der Pouw Kraan TCTM, Laan LC, Kringel H, Cummings RD, Bouma G et al Trichuris suis soluble products induce Rab7b expression and limit TLR4 responses in human dendritic cells. Genes Immun 2015; 16:378–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Fleming J, Isaak A, Lee J, Luzzio C, Carrithers M, Cook T et al Probiotic helminth administration in relapsing–remitting multiple sclerosis: a phase 1 study. Mult Scler 2011; 17:743–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Voldsgaard A, Bager P, Garde E, Åkeson P, Leffers A, Madsen C et al Trichuris suis ova therapy in relapsing multiple sclerosis is safe but without signals of beneficial effect. Mult Scler 2015; 21:1723–9. [DOI] [PubMed] [Google Scholar]
- 71. Molina‐Alcaide E, Moumen A, Martín‐García AI. By‐products from viticulture and the wine industry: potential as sources of nutrients for ruminants. J Sci Food Agric 2008; 88:597–604. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Viability of human dendritic cells (as judged by 7AAD staining) after exposure for 24 hr to proanthocyanidins (PAC) derived from cocoa (COC F2) or white clover flowers (WCF F2).
Figure S2. Characterization of monocyte‐derived human dendritic cells.
Figure S3. (a) Inhibition of lipopolysaccharide (LPS) ‐induced interleukin‐6 (IL‐6) and IL‐12p70 secretion in human dendritic cells by different concentrations of cocoa (COC) and white clover flower (WCF) fraction 2 (F2). Inhibition is relative to LPS only, which was set at 100%. Results are the mean of four independent experiments, each performed with cells from different donors. Mean ± inter‐donor SEM are shown. *P < 0·05, **P < 0·01, ***P < 0·001 relative to LPS only by one‐way analysis of variance. (b) Inhibition of LPS‐induced IL‐6 and IL‐12p70 secretion in human dendritic cells by 10 µg/ml of COC and WCF F2. Inhibition is relative to LPS only, which was set at 100%. Results are the mean of four independent experiments, each performed with cells from different donors. Mean ± inter‐donor SEM are shown.
Figure S4. Inhibition of lipopolysaccharide (LPS) ‐induced interleukin‐6 (IL‐6) and IL‐12p70 secretion in human dendritic cells by 25 µg/ml of DTAF‐tagged proanthocyanidin (PAC).
Figure S5. Inhibition of lipopolysaccharide‐induced interleukin‐6 and IL‐12p70 secretion in human dendritic cells by varying concentrations of Trichuris suis soluble products.


