Summary
Coronary heart disease (CHD) is one of the most common types of organ lesions caused by atherosclerosis, in which CD4+CD25+forkhead box protein 3 (FoxP3+) regulatory T cells (Treg) play an atheroprotective role. However, Treg cell numbers are decreased and their functions are impaired in atherosclerosis; the underlying mechanisms remain unclear. CD31 plays an important part in T cell response and contributes to maintaining T cell tolerance. The immunomodulatory effects of CD31 are also implicated in atherosclerosis. In this study, we found that decreased frequencies of the CD31+ subpopulation in Treg cells (CD31+Tr cells) correlated positively with decreased FoxP3 expression in CHD patients. Cell culture in vitro demonstrated CD31+Tr cells maintaining stable FoxP3 expression after activation and exhibited enhanced proliferation and immunosuppression compared with the CD31− subpopulation in Treg cells (CD31−Tr cells). We also confirmed impaired secretion of transforming growth factor (TGF)‐β1 and interleukin (IL)‐10 in CD31+Tr cells of CHD patients. Further analysis revealed reduced phospho‐SHP2 (associated with CD31 activation) and phospho‐signal transducer and activator of transcription‐5 (STAT‐5) (associated with FoxP3 transcription) levels in CD31+Tr cells of CHD patients, suggesting that decreased FoxP3 expression in CD31+Tr cells might be because of attenuated SHP2 and STAT‐5 activation. These data indicate that decreased frequencies and impaired functions of the CD31+Tr subpopulation associated with decreased FoxP3 expression give rise, at least in part, to Treg cell defects in CHD patients. Our findings emphasize the important role of the CD31+Tr subpopulation in maintaining Treg cell normal function and may provide a novel explanation for impaired immunoregulation of Treg cells in CHD.
Keywords: atherosclerosis, CD31, coronary heart disease, FoxP3, Treg cell
Introduction
Coronary atherosclerotic heart disease (CHD) is one of the most common types of organ lesions caused by atherosclerosis. Atherosclerosis is a chronic inflammatory disease, in which abundant immune cells are involved 1. T cells present during all stages of the disease are essential to the development of atherosclerotic plaque 2. Among them, T helper type 1 (Th1)/Th17‐mediated proinflammatory responses aggravate atherosclerosis while regulatory T cells (Treg) play a key atheroprotective role by limiting inflammation and counterbalancing plaque formation.
Human CD4+CD25+ Treg cells can suppress activation of a variety of immune cells mediated through cell‐to‐cell contacts and/or secretion of inhibitory cytokines such as transforming growth factor (TGF)‐β1 and interleukin (IL)‐10 to prevent self‐reactive immune responses and maintain dominant self‐tolerance 3, 4, 5, 6. Forkhead box transcription factor protein 3 (FoxP3) is a molecular marker of and the cell lineage specification factor for CD4+CD25+ Treg cells 7, 8. Studies show that FoxP3 gene mutation or expression deficiency causes abnormal development and immune dysfunction of Treg cells, leading to serious autoimmune diseases 9, 10. Clinical studies in patients with coronary atherosclerosis report Treg cell functional impairments associated with an obvious decrease in their numbers, FoxP3 levels and Treg‐related cytokines (TGF‐β1 and IL‐10) 11, 12, 13. Animal experiments also confirm that increasing Treg cell numbers and improving their functions would greatly reduce atherosclerotic plaque 14, 15. However, the causes and mechanisms underlying Treg cell defects in atherosclerosis remain unclear.
CD31, also known as platelet endothelial cell adhesion molecule‐1 (PECAM‐1), a transmembrane homophilic and inhibitory receptor containing two immunoreceptor tyrosine inhibitory motifs (ITIMs) located in its cytoplasmic tails, is expressed by endothelial cells, platelets and immune cells and is regarded generally as an endothelial marker. Interestingly, recent studies reveal an important role of this molecule in the regulation of T cell responses. During the interactions of immune cells, CD31 signal transduction is induced by homophilic engagements and is mediated through recruitment and activation of tyrosine‐phosphatases, such as SH2‐containing inositol 5 phosphatase (SHIP), Src homology region 2 domain‐containing phosphatase‐1 (SHP1) and SHP2 by its ITIMs, and CD31 deficiency is associated with excessive immunoreactivity, which means uncontrolled immune response, and susceptibility to cytotoxic killing, which means decreased cell viability 16. Under immunological stress, lack of CD31 accelerates and aggravates T cell‐mediated inflammation in mice 17, 18. The immunoregulatory role of CD31 has also been implicated in atherosclerosis. CD31 gene knock‐out results in an enhanced atherosclerotic lesion formation in LDL receptor‐deficient mice 19. Decreased CD31+ T cells are correlated positively with the occurrence of atherothrombosis in mice 20 and of abdominal aortic aneurysm in patients 21.
Furthermore, over‐expression of a CD31 receptor globulin leads to the induction of T cell hyporesponsiveness and impairment of T cell activation, which indicates that CD31 signalling may contribute to the establishment and maintenance of T cell tolerance 22. It is well known that Treg cells play a key role in maintaining self‐tolerance, preventing the development of autoimmunity. Although a direct link between CD31 expression and Treg cell function has not yet been reported, CD31 signal activation has been shown to increase Treg cell proportion in vivo 23, and the tyrosine‐phosphatase SHP2 recruited by CD31 ITIMs can promote Treg cell generation mediated by the Grb‐2 associated binder–extracellular‐regulated kinase–mitogen‐activated protein kinase (Gab–Erk–MAPK) pathway 24. Studies have also suggested a relationship between CD31 expression and Treg cell function in atherosclerosis. Administration of a CD31‐derived peptide prevents atherosclerotic progression in apolipoprotein E knock‐out mice by driving enrichment of the Treg cell compartment at the expense of the effector T (Teff) cell compartment 23. Restoring the CD31 signal contributes to modulating T cell activation and improves experimental atherosclerosis via enrichment of circulating Treg cells 25. In addition, naive Treg cells contribute to maintaining FoxP3 expression of Treg cells after expansion in vitro 26. It is worth noting that CD31 expression level in naive Treg cells is clearly higher than in memory Treg cells 27. Thus, it presents the possibility that there is some correlation between CD31 and FoxP3, and CD31 may affect Treg functions on the basis of this association in the development of atherosclerosis. In this study, we investigate the role of the CD31+ subpopulation in Treg cells as well as the correlation with FoxP3 expression in patients with CHD or in healthy individuals.
Materials and methods
Patients and controls
Seventy‐one patients with CHD who were diagnosed by coronary angiography and displayed one or more coronary arteries with at least 50% stenosis were enrolled for inclusion at Xin Hua Hospital affiliated to Shanghai Jiao Tong University School of Medicine. The CHD patients were classified into stable angina pectoris (SAP) (n = 34) and acute coronary syndrome (ACS) (n = 37) subgroups. SAP was defined by the following inclusion criteria: typical effort angina symptoms associated with down‐sloping or horizontal ST‐segment depression greater than 1 mm in an exercise test. ACS was defined based on clinical presentation (chest pain within the preceding 12 h), presence of electrocardiogram (ECG) changes (ST‐segment changes and/or T‐wave inversions) and testing positive for cardiac troponins. Thirty‐five peripheral blood samples from age‐ and sex‐matched healthy controls (HC) were obtained from the medical examination center of Xin Hua Hospital. The following exclusion criteria were used: previous myocardial infarction within 6 months, previous revascularization procedures, inflammatory conditions likely to be associated with an acute phase response, neoplastic disease, advanced liver disease, autoimmune disease or current use of immunosuppressive agents, renal failure or severe heart failure (NYHA classes III–IV). The cardiovascular risk factors include the smoking, hypertension and diabetes mellitus. The laboratory tests were measured in the clinical chemistry laboratory of Xin Hua Hospital: total cholesterol, triglycerides, low‐density lipoprotein (LDL), highly sensitive C‐response protein (Hs‐CRP), troponin I (TnI) and N‐terminal pro‐brain natriuretic peptide (NT‐proBNP). This work was approved by Ethics Committee of Xin Hua Hospital affiliated to Shanghai Jiao Tong University School of Medicine (Approval no. XHEC‐D‐2016‐034) and was performed in accordance with the Declaration of Helsinki. Written informed consents were obtained from all patients and healthy donors prior to their participation.
Flow cytometric analysis
Human peripheral blood mononuclear cells (PBMCs) were freshly isolated by Ficoll density gradient centrifugation and resuspended in phosphate‐buffered saline (PBS) supplemented with 2% bovine serum albumin (BSA). Fluorochrome‐labelled mouse anti‐human monoclonal antibodies targeted against CD4‐eFluor® 450 [anti‐human CD4 (OKT4)], CD25‐allophycocyanin (APC) (BC96), CD127‐peridinin chlorophyll (PerCP)‐cyanin (Cy)5.5 (eBioRDR5), CD31‐phycoerythrin (PE) (WM59) and FoxP3‐Alexa Fluor® 488 (236A/E7) were purchased from eBioscience (San Diego, CA, USA). To investigate the phenotypical characteristics of different Treg cell subsets, PBMCs from healthy individuals or CHD patients were first surface‐stained with antibodies against CD4, CD25, CD31 and CD127, then intracellular‐stained with antibodies against FoxP3 using the fixation/permeabilization kit (eBioscience) according to the manufacturer's instructions. Because CD127 expression correlated inversely with FoxP3 expression 28, 29, we used both the Treg cell markers of CD4+C25+CD127− and CD4+CD25+FoxP3+ as the phenotypes for total Treg cells in this study. A multiple‐colour flow cytometric analysis was performed with the fluorescence activated cell sorter (FACS)Canto II (BD Bioscience, San Jose, CA, USA).
For intracellular detection of cytokines, PBMCs from healthy individuals or CHD patients were incubated for 5 h and stimulated concurrently with or without cell stimulation cocktail (containing phorbol 12‐myristate 13‐acetate and ionomycin; eBioscience) at a dilution of 1 : 500, as well as protein transport inhibitor cocktail (containing brefeldin A and monensin; eBioscience) at a dilution of 1 : 500. The resulting cells were first surface‐stained with antibodies against CD4‐eFluor® 450 (OKT4), CD25‐PE‐Cy7 (BC96) and CD31‐APC‐eFluor® 780 (WM59) (eBioscience), then intracellular‐stained with antibodies against FoxP3‐Alexa Fluor® 488 (236A/E7) (eBioscience), TGF‐β1‐PerCP‐Cy™ 5.5 (TW4‐2F8) and IL‐10‐PE (JES3‐19F1) together with their isotype controls (BD Bioscience) using the fixation/permeabilization kit according to the manufacturer's instructions. Secretion levels of TGF‐β1 and IL‐10 in different cell subsets were detected using a flow cytometer.
To analyse the phosphorylation levels of SHP2, signal tranduser and activator of transcription (STAT)−5 and STAT‐3, PBMCs from healthy individuals or CHD patients were left unstimulated or were stimulated for 15 min with 5 μg/ml anti‐CD3 and 5 μg/ml anti‐CD28 antibodies (eBioscience) for SHP2, 100 ng/ml IL‐2 (R&D Systems, Minnneapolis, MN, USA) for STAT‐5 and 100 ng/ml IL‐6 (Peprotech, Rocky Hill, NJ, USA) for STAT‐3, respectively. Cells were then fixed with 1% paraformaldehyde, permeabilized with 1 ml of ice‐cold methanol for 30 min on ice and stained with antibodies against CD4‐eFluor® 450 (OKT4), CD25‐PE‐Cy7 (BC96), CD31‐APC‐eFluor® 780 (WM59) and FoxP3‐Alexa Fluor® 488 (236A/E7) (eBioscience) as well as phosphospecific antibodies against SHP2 (pY542)‐PE (L99‐921), STAT‐5 (pY694)‐Alexa Fluor® 647 (47/STAT‐5) or STAT‐3 (pY705)‐PerCP‐Cy5.5 (4/P‐STAT‐3) and their isotype controls (BD Bioscience). Phosphorylation levels of SHP2, STAT‐5 and STAT‐3 in different cell subsets were analysed by flow cytometry (FCM).
Cell sorting and functional assays
Human PBMCs were freshly isolated by Ficoll density gradient centrifugation from healthy donors. Total‐Tr (CD4+CD25+FoxP3+), CD31+Tr (CD4+CD25+FoxP3+CD31+) and CD31− Tr (CD4+CD25+FoxP3+CD31−) cells were obtained from PBMCs using the human CD4+CD25+ regulatory T cell isolation kit (Miltenyi Biotec, San Diego, CA, USA) with FACS. Cell purities and phenotypes were determined by reanalysis and stained with antibodies against CD4, CD25, FoxP3 and CD31.
FACS‐sorted total‐Tr, CD31+Tr and CD31−Tr cells (1 × 105 cells/well) were cultured, respectively, in 200 μl of complete medium containing 10% FBS in 96‐well U‐bottomed plates by stimulation with precoated 5 μg/ml anti‐CD3 and soluble 5 μg/ml anti‐CD28 antibodies for up to 7 days. To assess cell proliferation, the CellTraceTM carboxyfluorescein diacetate, succinimidyl ester (CFSE) cell proliferation kit (Molecular Probes, Eugene, OR, USA) was used according to the manufacturer's instructions and cell morphology was observed by inverted microscope.
For functional suppression assay, total‐Tr, CD31+Tr and CD31−Tr cells, respectively, were co‐cultured with FACS‐sorted CD4+CD25− T cells as Teff cells at a ratio of 1 : 1 (total 1 × 105 cells/well) under the same culture conditions described above, and Teff cells in each co‐cultured group were pretreated with CFSE staining. After 5 days, Teff cell proliferation was analysed using a flow cytometer.
Statistical analysis
Data are expressed as mean ± standard deviation (s.d.). Comparisons between groups were analysed by two‐tailed Student's t‐test or analysis of variance (anova) test when appropriate. For normally distributed data, differences between groups were evaluated using Tukey's test, and associations were assessed using Pearson's correlation coefficient. For non‐normally distributed data, differences between groups were evaluated using the non‐parametric Mann–Whitney U‐test, and associations were assessed using a Spearman's rank correlation coefficient. A value of P < 0·05 was considered statistically significant. All statistical analyses were performed using GraphPad Prism (version 5·0).
Results
Clinical characteristics of CHD patients and healthy individuals
A cohort of 71 patients with CHD was included in this study and divided into SAP and ACS subgroups, according to the clinical diagnosis. The baseline characteristics of enrolled patients were well‐matched among groups and are summarized in Table 1. Among the groups, no significant differences were observed with regard to the demographic and risk factor information, such as gender, age, smoking, hypertension and diabetes mellitus, as well as the biochemical parameters, including total cholesterol, triglycerides and LDL. However, the other biochemical parameters which contribute to reflect disease activity and severity, such as Hs‐CRP, TnI and NT‐proBNP, were significantly higher in the ACS group than in the SAP and HC groups. In addition, the differences of baseline characteristics between the overall disease group (CHD = SAP + ACS) and the HC group are compared in Table 1 in order to help further statistical analysis for the following experimental data. No significant differences were observed with regard to the demographic and risk factor information as well as all the biochemical parameters between the two groups.
Table 1.
Clinical characteristics of the study groups
| HC (n = 35) | SAP (n = 34) | ACS (n = 37) | CHD (=SAP+ACS) (n = 71) | |
|---|---|---|---|---|
| Gender (male/female) | 21/14 | 21/13 | 24/13 | 45/26 |
| Age (mean ± s.d. years) | 58 ± 7 | 61 ± 7·5 | 63 ± 9 | 61 ± 8 |
| Smoking | 11 (31%) | 11 (32%) | 12 (32%) | 23 (32%) |
| Hypertension | 7 (20%) | 11 (32%) | 17 (46%) | 28 (39%) |
| Diabetes mellitus | 4 (11%) | 4 (12%) | 10 (27%) | 14 (20%) |
| Total cholesterol (mmol/l) | 4·44 ± 1·22 | 3·99 ± 0·87 | 4·47 ± 1·23 | 4·22 ± 1·11 |
| Triglycerides (mmol/l) | 2·20 ± 0·95 | 1·93 ± 0·96 | 2·15 ± 1·05 | 2·09 ± 0·98 |
| LDL (mmol/l) | 2·32 ± 0·73 | 2·20 ± 0·58 | 2·53 ± 0·91 | 2·41 ± 0·79 |
| Hs‐CRP (ng/ml) | 1·27 ± 0·44 | 1·31 ± 0·50 | 1·86 ± 0·76* | 1·69 ± 0·78 |
| TnI (ng/ml) | 0·003 ± 0·0018 | 0·009 ± 0·0033 | 0·112 ± 0·0527* | 0·066 ± 0·0312 |
| NT‐proBNP (pg/ml) | 74·8 ± 49·5 | 83·5 ± 53·9 | 150·9 ± 82·1* | 118·3 ± 73·2 |
*P < 0·05, one‐way analysis of variance (anova) test for comparison among HC, SAP and ACS groups. Two‐tailed Student's t‐test for comparison between HC and CHD groups. HC = healthy controls; SAP = stable angina pectoris; ACS = acute coronary syndrome; CHD = coronary heart disease = SAP + ACS; LDL = low‐density lipoprotein; CRP = C‐reactive protein; s.d. = standard deviation; NT‐proBNP = N‐terminal pro‐brain natriuretic peptide.
Decreased frequencies of CD31+Tr cells associated with decreased FoxP3 expression in CHD patients
To investigate the variation of different Treg cell subsets in CHD patients, we first measured the proportion of total‐Tr (CD4+CD25+CD127−), CD31+Tr (CD4+CD25+CD127−CD31+) and CD31−Tr (CD4+CD25+CD127−CD31−) cells, respectively, from the HC, SAP and ACS groups 28, 30. As shown in Fig. 1a, the frequencies of total‐Tr cells were decreased significantly in ACS patients (3·7 ± 1·0%) compared with in SAP patients (4·8 ± 1·4%, P < 0·05) and the HC group (5·6 ± 1.5%, P < 0·05), both SAP and HC groups had comparable amounts of total‐Tr cells (P > 0·05). This result was consistent with our previous findings 31, 32. As shown in Fig. 1b,c, the frequencies of CD31+Tr cells were markedly higher in the HC group (33·4 ± 6·8%) than in SAP (24·3 ± 6·4%, P < 0·05) and ACS patients (20·1 ± 5·7%, P < 0·05); in contrast, the frequencies of CD31−Tr cells were clearly lower in the HC group (63·.4 ± 7·5%) than in SAP (74·6 ± 6·9%, P < 0·05) and ACS patients (77·6 ± 6·3%, P < 0·05), while both the SAP and ACS groups had comparable amounts of CD31+Tr or CD31−Tr cells (P > 0·05).
Figure 1.
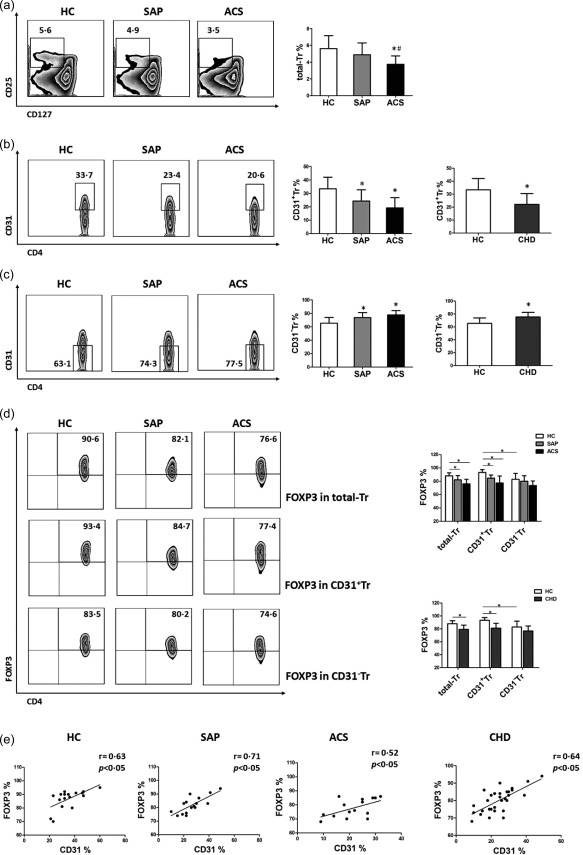
Decreased frequencies of CD31+Tr cells associated with decreased forkhead box protein 3 (FoxP3) expression in coronary heart disease (CHD) patients. (a–c) The percentages of CD4+CD25+CD127− regulatory T cells (Treg) (total‐Tr) (a), CD31+ subpopulation in total‐Tr cells (CD31+Tr) (b) and CD31− subpopulation in total‐Tr cells (CD31−Tr) (c) derived from peripheral blood mononuclear cells (PBMCs) of stable angina pectoris (SAP) patients, acute coronary syndrome (ACS) patients and healthy controls (HC) were determined by flow cytometry (FCM). Comparisons between HC and CHD groups are shown in the right‐hand histograms (CHD = SAP + ACS). Representative FCM Zebra plots are shown. Data are mean ± standard deviation (s.d.), n = 20. *P < 0·05 versus HC, # P < 0·05 versus SAP, one‐way analysis of variance (anova) test for comparison among the HC, SAP and ACS groups, two‐tailed Student's t‐test for comparison between the HC and CHD groups. (d) FoxP3 expression levels in total‐Tr, CD31+Tr and CD31−Tr cells, respectively, from the HC, SAP and ACS groups. Comparison between the HC and CHD groups is shown in the histogram (below right). Representative FCM Zebra plots are shown. Data are mean ± standard deviation (s.d.), n = 20. *P < 0·05, two‐way anova test. (e) Correlation of CD31 and FoxP3 levels in total‐Tr cells from HC, SAP, ACS and CHD groups. The r‐value represents the calculated correlation coefficient; P < 0·05 is considered statistically significant.
In parallel, we analysed FoxP3 expression levels in total‐Tr, CD31+Tr and CD31−Tr cells, respectively, from the HC, SAP and ACS groups. As shown in Fig. 1d, horizontal comparison found that FoxP3 expression levels of total‐Tr cells were higher in the HC group (91·0 ± 2·6%) than in SAP (82·3 ± 6·5%, P < 0·05) and ACS patients (76·1 ± 6·9%, P < 0·05). FoxP3 expression levels of CD31+ Tr cells were also higher in the HC group (92·8 ± 4·1%) than in SAP (83·3 ± 4·8%, P < 0·05) and ACS patients (77·8 ± 9·5%, P < 0·05), while no significant differences in FoxP3 expression levels of CD31−Tr cells among the HC, SAP and ACS groups were observed (P > 0·05). Vertical comparison found that FoxP3 expression levels of CD31+Tr cells (92·8 ± 4·1%) were comparable to those of total‐Tr cells (91·0 ± 2·6%, P > 0·05) but were clearly higher than those of CD31−Tr cells (82·9 ± 6·8%, P < 0·05) in the HC group; however, no significant differences in FoxP3 expression levels of total‐Tr, CD31+Tr and CD31−Tr cells were observed in the SAP or ACS groups (P > 0·05).
We further analysed the correlation between CD31 and FoxP3 expression levels in total‐Tr cells from the HC, SAP and ACS groups. It was demonstrated that CD31 was correlated positively with FoxP3 whether in the HC (r = 0·63, P = 0·0053), SAP (r = 0·71, P = 0·0023) or ACS groups (r = 0·52, P = 0·0246) (Fig. 1e).
As there were no significant differences between the SAP and ACS groups with respect to the frequencies of CD31+Tr or CD31−Tr cells and FoxP3 expression levels of three different Treg cell subsets, we merged SAP and ACS into the CHD group in order to elucidate concisely the differences between the healthy population and the whole CHD population, as shown in the CHD histograms of Fig. 1b–e. We then used the CHD group as disease controls for the subsequent experiments. Overall, these results confirmed the decreased frequencies of CD31+Tr cells associated with decreased FoxP3 expression present in CHD patients.
Stable FoxP3 expression in CD31+Tr cells and decreased FoxP3 expression in CD31−Tr cells after activation in vitro
We next investigated the phenotypes of total‐Tr, CD31+Tr and CD31−Tr cells before and after activation in vitro. Total‐Tr cells (CD4+CD25+FoxP3+) were first isolated from PBMCs of healthy donors and their purities (CD4+CD25+ from 2·53 ± 0·39% to 92·2 ± 4·3%) and phenotypes (FoxP3+ averaged more than 92%) were identified, as shown in Fig. 2a. Then, CD31+Tr (CD4+CD25+FoxP3+CD31+) and CD31−Tr (CD4+CD25+FoxP3+CD31−) cells were isolated further from total‐Tr cells and their purities were also identified, as shown in Fig. 2b (CD31+Tr from 32·0 ± 4·2% to 92·7 ± 4·6%) and in Fig. 2c (CD31− Tr from 67·5 ± 5·3% to 94·6 ± 3·8%).
Figure 2.
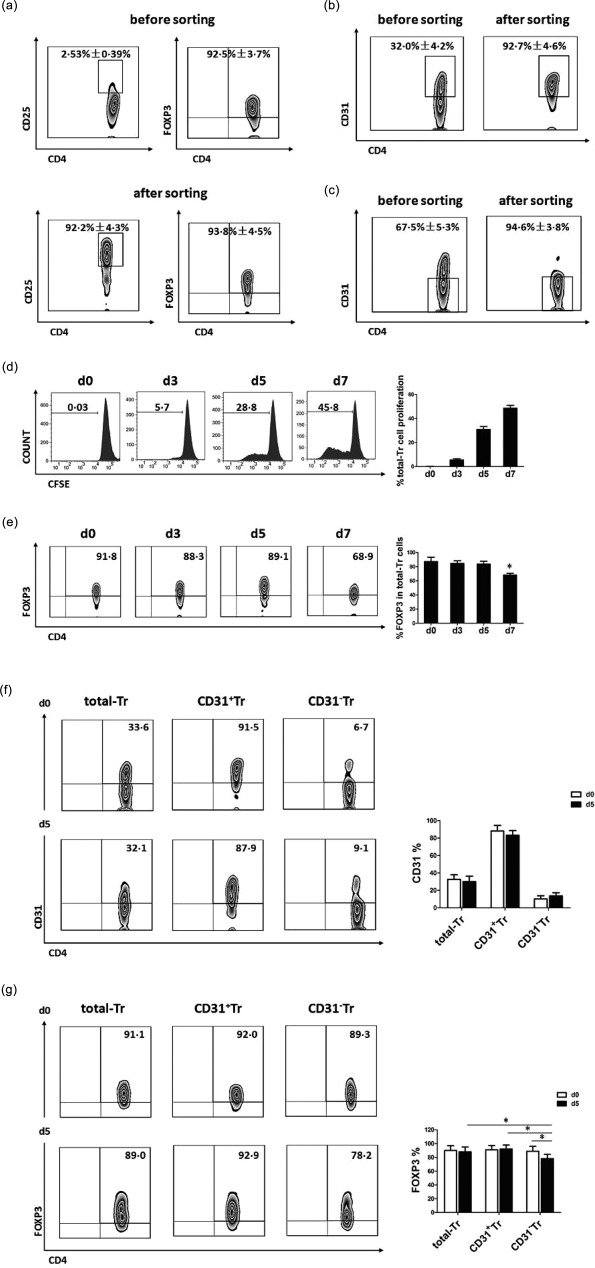
Stable forkhead box protein 3 (FoxP3) expression in CD31+Tr cells while decreased FoxP3 expression in CD31−Tr cells after activation in vitro. (a) Total‐Tr cells (CD4+CD25+FoxP3+) were isolated from peripheral blood mononuclear cells (PBMCs) of healthy donors using the human CD4+CD25+ regulatory T cell isolation kit and their purities (CD4+CD25+ %) and phenotypes (FoxP3+ %) were identified before and after sorting. (b,c) CD31+Tr cells (CD4+CD25+FoxP3+ CD31+) (b) and CD31−Tr cells (CD4+CD25+FoxP3+CD31−) (c) were isolated further from total‐Tr cells and their purities were identified before and after sorting. (d,e) Total‐Tr cells were cultured in vitro by stimulation with precoated anti‐CD3 and soluble anti‐CD28 antibodies for 7 days; cell proliferation (d) was assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) assay and FoxP3 expression level (e) was determined by flow cytometry (FCM). (f,g) Total‐Tr, CD31+Tr and CD31−Tr cells were cultured in vitro by stimulation with precoated anti‐CD3 and soluble anti‐CD28 antibodies for 5 days, respectively, in which the expression levels of CD31 (f) and FoxP3 (g) were determined before (day 0) and after (day 5) activation. Representative FCM Zebra plots are shown. Data are mean ± standard deviation (s.d.), n = 5. *P < 0·05 versus day 0, one‐way analysis of variance (anova) test used in (e). *P < 0·05, two‐way anova test used in (f) and (g).
Total‐Tr cells were cultured and activated with anti‐CD3 and anti‐CD28 antibodies for 7 days and showed an increasing trend of cell proliferation (Fig. 2d). FoxP3 expression levels were maintained stably for 5 days, but decreased on day 7 (Fig. 2e). The optimal activation time of 5 days was used, and also for the following experiments in vitro, in order to ensure stable phenotypes of Treg cells.
We found no significant differences in CD31 expression levels of total‐Tr, CD31+Tr or CD31−Tr cells before (day 0) and after (day 5) cell activation (Fig. 2f), which indicated that CD31 phenotypes in the three groups of cells had not changed during cell activation. However, FoxP3 expression levels of CD31−Tr cells on day 5 were lower than on day 0 and had decreased significantly compared with those of total‐Tr and CD31+Tr cells on day 5 (Fig. 2g).
Collectively, these data demonstrated stable FoxP3 expression in CD31+Tr cells with decreased FoxP3 expression in CD31−Tr cells after activation in vitro, which indicated the CD31+ subpopulation in activated CD4+CD25+FoxP3+ Treg cells contributing to maintaining the phenotypical characteristics of Treg cells.
Cell proliferation and immunosuppression enhanced in CD31+Tr cells but attenuated in CD31−Tr cells in vitro
We also investigated the functional characteristics of total‐Tr, CD31+Tr and CD31−Tr cells in vitro. As shown in Fig. 3a, total‐Tr, CD31+Tr and CD31−Tr cells were cultured and activated with anti‐CD3 and anti‐CD28 antibodies for 5 days. We found that cell proliferative activities increased in CD31+Tr cells and decreased in CD31−Tr cells by cell morphological observation and CFSE assay. Moreover, mixed lymphocyte reaction confirmed that all three groups of cells could inhibit Teff cell proliferation and the most effective suppression was observed in the group CD31+Tr : Teff (Fig. 3b). These results suggest that the CD31+Tr subpopulation played a major role on maintaining normal functional activities of Treg cells.
Figure 3.
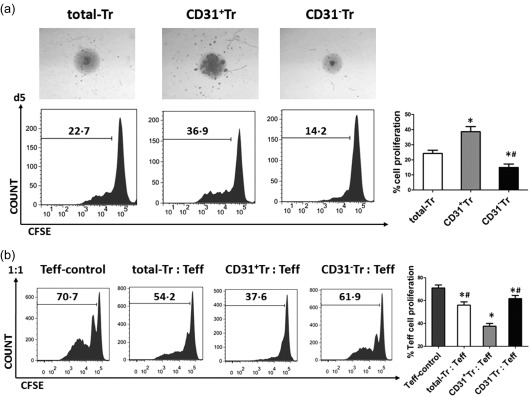
Cell proliferation and immunosuppression enhanced in CD31+Tr cells but attenuated in CD31−Tr cells in vitro. (a) Total‐Tr, CD31+Tr and CD31−Tr cells isolated from peripheral blood mononuclear cells (PBMCs) of healthy donors were cultured in vitro by stimulation with precoated anti‐CD3 and soluble anti‐CD28 antibodies for 5 days. Cell morphology was observed by inverted microscope (magnification ×40) and cell proliferation was assessed by carboxyfluorescein diacetate succinimidyl ester (CFSE) assay. Representative morphological images are shown. Data are mean ± standard deviation (s.d.), n = 5. *P < 0·05 versus total‐Tr, # P < 0·05 versus CD31+Tr, one‐way analysis of variance (anova) test. (b) Total‐Tr, CD31+Tr and CD31−Tr cells were co‐cultured, respectively, with effector T cells (Teff) (CD4+CD25−) at a ratio of 1 : 1 and Teff cells were cultured alone as controls in vitro by stimulation with precoated anti‐CD3 and soluble anti‐CD28 antibodies for 5 days. Teff cell proliferation in each group was determined by CFSE assay. Data are mean ± s.d., n = 5. *P < 0·05 versus Teff‐control, # P < 0·05 versus CD31+ Tr : Teff, one‐way analysis of variance (anova) test.
Decreased secretion levels of TGF‐β1 and IL‐10 in CD31+Tr cells of patients with CHD
It is known that secretion of inhibitory cytokines is one of the important immunoregulatory modes of action for Treg cells 5, 6. Therefore, we analysed the intracellular TGF‐β1 and IL‐10 secretion profiles of CD31+Tr or CD31−Tr cells from patients with CHD or healthy controls (HC). As shown in Fig. 4a (upper right), whether stimulated with (ST) or without (US) cell stimulation cocktail, decreased TGF‐β1 secretion levels in CD31+Tr cells were observed in CHD patients compared with the HC group, while no significant differences in TGF‐β1 secretion levels in CD31−Tr cells were observed between the CHD and HC groups. We also analysed the differences in TGF‐β1 secretion levels between CD31+Tr and CD31−Tr cells in the CHD or HC groups, as shown in Fig. 4a (below right). In the HC group, higher TGF‐β1 secretion levels were observed in CD31+Tr cells than in CD31−Tr cells. However, in CHD group, the differences between the two groups of cells were especially attenuated by US treatment.
Figure 4.
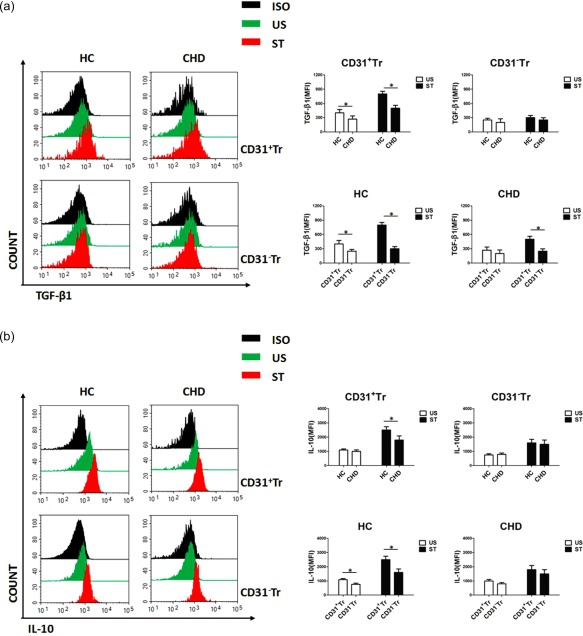
Decreased secretion levels of transforming growth factor (TGF)‐β1 and interleukin (IL)−10 in CD31+Tr cells of patients with coronary heart disease (CHD). (a,b) CD31+Tr or CD31−Tr cells derived from peripheral blood mononuclear cells (PBMCs) of healthy controls (HC) or CHD patients were analysed for cytokine secretion using flow cytometry (FCM). TGF‐β1 (a) and IL‐10 (b) secretion levels were detected by stimulation (ST) with or without (US) cell stimulation cocktail for 5 h. Data are mean ± standard deviation (s.d.), n = 20. MFI = mean fluorescent intensity; ISO = isotype; US = unstimulation; ST = stimulation. *P < 0·05, two‐way analysis of variance (anova) test. [Colour figure can be viewed at wileyonlinelibrary.com]
Furthermore, similar results were also obtained with detection of IL‐10 secretion levels in the same conditions. That is, reduced IL‐10 secretion levels of CD31+Tr cells were observed by ST treatment in CHD patients compared with the HC group (Fig. 4b, upper right); comparison of the secretion levels of IL‐10 between CD31+Tr and CD31−Tr cells showed statistical differences in the HC group, but not in the CHD group (Fig. 4b, below right).
In brief, we confirmed enhanced secretion of TGF‐β1 and IL‐10 in CD31+Tr cells compared with CD31−Tr cells, and impaired secretion of these cytokines in CD31+Tr cells in CHD patients compared with healthy individuals.
Decreased phosphorylation levels of SHP2 and STAT‐5 in CD31+Tr cells of patients with CHD
SHP2 is one of the tyrosine‐phosphatases recruited and phosphorylated directly by the activated CD31 ITIMs 16, and thus its phosphorylation level may help to reflect the activation status of CD31. Studies have shown that STAT‐5‐dependent Treg cells enhance FoxP3 expression and inhibit inflammatory responses 33, 34, while hyperactivation of STAT‐3 destabilizes Treg cells by suppressing FoxP3 expression and promotes inflammatory responses 35, 36, meaning that activation of STAT‐5 or STAT‐3 can regulate FoxP3 expression positively or negatively. Having shown that the decreased frequencies of CD31+Tr cells correlated with decreased FoxP3 expression in patients with CHD and FoxP3 stable expression in CD31+Tr cells from healthy donors after activation in vitro, we wanted to know whether FoxP3 expression in CD31+Tr cells from patients with CHD or healthy individuals may be affected, related partly to different activation of SHP2, STAT‐5 or STAT‐3.
As shown in Fig. 5a, significant changes in phospho‐SHP2 (p‐SHP2) levels were observed only in CD31+Tr cells but not in CD31−Tr cells, whether stimulated with (ST) or without (US) anti‐CD3 and anti‐CD28 antibodies, as well as clearly higher p‐SHP2 levels in CD31+Tr cells than in CD31−Tr cells, and decreased p‐SHP2 levels of CD31+Tr cells were found in CHD patients compared with the HC group. These data suggest a close correlation between p‐SHP2 and CD31, and impaired CD31 activation was likely to be present in Treg cells of CHD patients.
Figure 5.
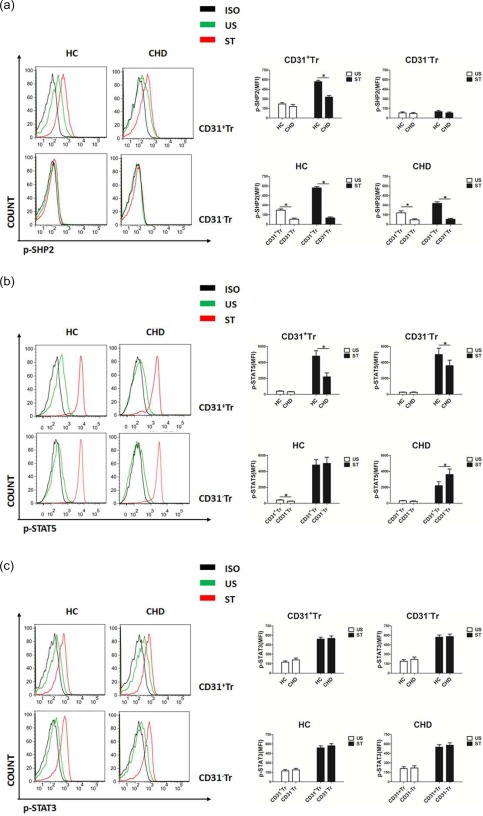
Decreased phosphorylation levels of Src homology region 2 domain‐containing phosphatase (SHP2) and signal transducer and activation of transcription‐5 (STAT‐5) in CD31+Tr cells of patients with coronary heart disease (CHD). (a–c) CD31+Tr or CD31−Tr cells derived from peripheral blood mononuclear cells (PBMCs) of healthy controls (HC) or CHD patients were analysed for protein phosphorylation using flow cytometry (FCM). SHP2 (a), STAT‐5 (b) and STAT‐3 (c) phosphorylation levels were measured by stimulation with (ST) or without (US) anti‐CD3/anti‐CD28 antibodies, interleukin (IL)−2 and IL‐6 for 15 min, respectively. Data are mean ± standard deviation (s.d.), n = 20. MFI = mean fluorescent intensity; ISO = isotype; US = unstimulation; ST = stimulation. *P < 0·05, two‐way analysis of variance (anova) test. [Colour figure can be viewed at wileyonlinelibrary.com]
With IL‐2 stimulation (ST), phospho‐STAT‐5 (p‐STAT‐5) levels in both CD31+Tr and CD31−Tr cells were attenuated in the CHD group compared with the HC group and greater decreased p‐STAT‐5 levels were observed in CD31+Tr cells than in CD31−Tr cells (Fig. 5b, upper right). As shown in Fig. 5b (below right), in the HC group, p‐STAT‐5 levels in CD31+Tr cells were higher than in CD31−Tr cells without IL‐2 stimulation (US) or were comparable to CD31−Tr cells by ST treatment; in the CHD group, no significant differences were seen in p‐STAT‐5 levels between CD31+Tr and CD31−Tr cells by US treatment, while significant differences between them by ST treatment were observed. These results indicate that the existence of decreased p‐STAT‐5 levels might lead to decreased FoxP3 expression, especially in CD31+Tr cells in patients with CHD.
However, we found no significant differences in phospho‐STAT‐3 (p‐STAT‐3) levels between CD31+Tr and CD31−Tr cells whether with (ST) or without (US) IL‐6 stimulation and whether in CHD patients or in the HC group (Fig. 5c), which implied no close relationship between p‐STAT‐3 and CD31 in Treg cells.
Discussion
In this study, we analysed and compared the frequencies and functions of three different subsets of Treg cells; that is, total‐Tr, CD31+Tr and CD31− Tr cells isolated from PBMCs from healthy individuals or patients with CHD. We also preliminarily investigated changes in the phosphorylation levels of SHP2 (associated with CD31 activation) and STAT‐5/3 (associated with FoxP3 transcription) in different cell groups. Our study demonstrated that the decreased frequencies and impaired functions of CD31+Tr cells were associated with decreased FoxP3 expression in CHD patients, in which SHP2 and STAT‐5 activations were attenuated.
CHD is one of the most common diseases seriously harmful to human health, and one of the leading causes of death in China in recent years 37. Atherosclerosis is the pathophysiological basis of CHD, and prevention and treatment of atherosclerosis are basic strategies for CHD therapy. In atherosclerotic plaques, Th1 and Th17 cells aggregate and produce high levels of inflammatory cytokines such as IFN‐γ and IL‐17 38, 39, while Treg cells play an anti‐inflammatory role through cell‐to‐cell contacts and/or secretion of inhibitory cytokines such as TGF‐β1 and IL‐10 3, 4, 5, 6. Furthermore, studies have found that Th17/Treg imbalance occurs in atherosclerosis and its clinical complications such as ACS 31, 32, 40, 41. The imbalance between immune inflammation and immune regulation contributes to the development of atherosclerosis. Treg cell numbers are decreased and their functions are impaired in atherosclerosis; however, the underlying mechanisms remain unclear. Recently, CD31 has been found to be involved in T cell responses and contribute to T cell tolerance, and the immunoregulatory role of CD31 has been implicated in atherosclerosis. However, there are few studies concerning the direct link between CD31 expression and Treg cell function in atherosclerosis and its vascular complications such as CHD. Thus, in this study we investigated the role of the CD31+ subpopulation in Treg cells as well as the correlation with FoxP3 expression in patients with CHD or in healthy individuals. Our main purpose was to observe the changes in the quantity and quality of CD31+ subpopulation in Treg cells under physiological or pathological conditions and conduct a brief investigation on the molecular mechanisms underlying these changes in order to elucidate the importance of CD31+ subpopulation in Treg cells.
We first confirmed decreased frequencies of CD31+ Tr cells in CHD patients and observed a positive correlation between CD31 and FoxP3 expression of total‐Tr cells, not only in CHD patients but also in healthy individuals. Interestingly, although decreased total‐Tr cell frequencies occurred mainly in patients with ACS, significantly decreased CD31+ Tr cell frequencies began in SAP patients accompanied by a decline of FoxP3 expression, meaning that CD31+Tr cells may show abnormal activities from the early stages of CHD.
We then demonstrated stable FoxP3 expression in CD31+Tr cells in healthy individuals with enhanced proliferation and immunosuppression and decreased FoxP3 expression in CD31−Tr cells with attenuated proliferation and immunosuppression after activation in vitro. Put another way, FoxP3 stable expression and increased proliferation observed in CD31+Tr cells may also contribute to enhance their immunosuppression in vitro. Having known that increasing CD31 expression helps to improve Treg cell survival 16, 23, 24, this may account partly for good proliferation of CD31+Tr cells in vitro. Unfortunately, we are unable to repeat the above‐mentioned cell culture and functional assays in vitro in CHD patients due to the lack of sufficient blood samples.
Because Treg cells regulate Teff cell proliferation not only via cellular direct contacts, but also via secretion of inhibitory cytokines, we analysed the secretion levels of TGF‐β1 and IL‐10 in different subsets of Treg cells from healthy individuals or CHD patients. We demonstrated elevated secretion levels of TGF‐β1 and IL‐10 in CD31+Tr cells compared with CD31−Tr cells with impaired secretion of these cytokines in CD31+Tr cells present in CHD patients. Results from intracellular cytokine detection revealed that CD31+Tr cells had functional impairment in addition to their lower frequencies in patients with CHD.
The key role of FoxP3 in regulating the suppressor function of Treg cells has been well documented 7, 9, 42. In this study, we observed decreased FoxP3 expression as well as decreased TGF‐β1 and IL‐10 secretion in CD31+ Tr cells of CHD patients, and considered that the former possibly accounted for the latter based on the findings of others, that attenuated FoxP3 expression leads to impaired secretion of inhibitory cytokines and subsequent suppressor function of Treg cells 43, 44. Further analysis revealed that the levels of p‐SHP2 and p‐STAT‐5 were both reduced in CD31+Tr cells of CHD patients, suggesting that decreased FoxP3 expression in CD31+Tr cells might be because of attenuated SHP2 and STAT‐5 activation. Because SHP2 phosphorylation is likely to reflect CD31 activation, this result indicates that the attenuated CD31 signal is also present in CD31+Tr cells of CHD patients. However, no close relation between p‐STAT‐3 and CD31 in Treg cells was found in this study. Thus, we considered that the CD31 signal might affect FoxP3 expression associated with activation of SHP2 and STAT‐5 in CD31+Tr cells.
In summary, these data indicate that disorders of the CD31+ subpopulation in CD4+CD25+FoxP3+ Treg cells lead, at least in part, to Treg cell defects in patients with CHD. This work also has limitations. We mainly compared the clinical data of patients with different types of CHD, including their comorbidities, such as hypertension, diabetes and dyslipidaemia (Table 1). However, we did not analyse specific drug use by different patients, in view of the majority of them taking similar anti‐CHD medications including anti‐platelet drugs, statins, angiotensin‐converting enzyme inhibitors and β‐blockers. To our knowledge, and beyond the disease context of CHD, our findings emphasize the important role of the CD31+ subpopulation in maintaining normal Treg cell function, and propose a novel explanation for impaired immunoregulation of Treg cells in CHD.
Disclosure
The authors declare no disclosures.
Author contributions
L. H., Y. Z. and L. S. conceived and designed the experiments. L. H., Y. Z., X. Y., Y. M., G. X., W. W., H. C. and L. S. performed the experiments. L. H., Y. Z., X. Y., Y. M., G. X., W. W., H. C. and L. S. analysed the data. L. S. contributed reagents/materials/analysis tools. L. H., Y. Z. and L. S. wrote the paper.
Acknowledgements
We thank the patients and healthy individuals for kindly providing samples for this study. This work was supported by grants from the National Natural Science Foundation of China no. 81372641, 81202351 and 81571525.
References
- 1. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med 2005; 352:1685–95. [DOI] [PubMed] [Google Scholar]
- 2. Ketelhuth DF, Hansson GK. Cellular immunity, low‐density lipoprotein and atherosclerosis: break of tolerance in the artery wall. Thromb Haemost 2011; 106:779–86. [DOI] [PubMed] [Google Scholar]
- 3. Miyara M, Sakaguchi S. Natural regulatory T cells: mechanisms of suppression. Trends Mol Med 2007; 13:108–16. [DOI] [PubMed] [Google Scholar]
- 4. Sakaguchi S, Setoguchi R, Yagi H, Nomura T. Naturally arising Foxp3‐expressing CD25+CD4+ regulatory T cells in self‐tolerance and autoimmune disease. Curr Top Microbiol Immunol 2006; 305:51–66. [DOI] [PubMed] [Google Scholar]
- 5. Sun L, Yi S, O'Connell PJ. IL‐10 is required for human CD4+CD25+ regulatory T cell‐mediated suppression of xenogeneic proliferation. Immunol Cell Biol 2010; 88:477–85. [DOI] [PubMed] [Google Scholar]
- 6. Joetham A, Takeda K, Taube C et al Naturally occurring lung CD4(+)CD25(+) T cell regulation of airway allergic responses depends on IL‐10 induction of TGF‐beta. J Immunol 2007; 178:1433–42. [DOI] [PubMed] [Google Scholar]
- 7. Miyara M, Sakaguchi S. Human FoxP3(+)CD4(+) regulatory T cells: their knowns and unknowns. Immunol Cell Biol 2011; 89:346–51. [DOI] [PubMed] [Google Scholar]
- 8. Zorn E, Nelson EA, Mohseni M et al IL‐2 regulates FOXP3 expression in human CD4+CD25+ regulatory T cells through a STAT‐dependent mechanism and induces the expansion of these cells in vivo. Blood 2006; 108:1571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for scurfin in CD4+CD25+ T regulatory cells. Nat Immunol 2003; 4:337–42. [DOI] [PubMed] [Google Scholar]
- 10. Torgerson T, Ochs HD. Immune dysregulation, polyendoerinopathy, enteropathy, X‐linked: forkhead box protein 3 mutations and lack of regulatory T cells. J Allergy Clin Immunol 2007; 120:744–50. [DOI] [PubMed] [Google Scholar]
- 11. Li N, Bian H, Zhang J, Li X, Ji X, Zhang Y. The Th17/Treg imbalance exists in patients with heart failure with normal ejection fraction and heart failure with reduced ejection fraction. Clin Chim Acta 2010; 411:1963–8. [DOI] [PubMed] [Google Scholar]
- 12. Cheng X, Yu X, Ding YJ et al The Th17/Treg imbalance in patients with acute coronary syndrome. Clin Immunol 2008; 127:89–97. [DOI] [PubMed] [Google Scholar]
- 13. Mor A, Luboshits G, Planer D, Keren G, George J. Altered status of CD4+CD25+ regulatory T cells in patients with acute coronary syndromes. Eur Heart J 2006; 27:2530–7. [DOI] [PubMed] [Google Scholar]
- 14. Ait‐Oufella H, Salomon BL, Potteaux S et al Natural regulatory T cells control the development of atherosclerosis in mice. Nat Med 2006; 12:178–80. [DOI] [PubMed] [Google Scholar]
- 15. Mor A, Planer D, Luboshits G et al Role of naturally occurring CD4+CD25+ regulatory T cells in experimental atherosclerosis. Arterioscler Thromb Vasc Biol 2007; 27:893–900. [DOI] [PubMed] [Google Scholar]
- 16. Marelli‐Berg FM, Clement M, Mauro C, Caligiuri G. An immunologist's guide to CD31 function in T‐cells. J Cell Sci 2013; 126:2343–52. [DOI] [PubMed] [Google Scholar]
- 17. Graesser D, Solowiej A, Bruckner M et al Altered vascular permeability and early onset of experimental autoimmune encephalomyelitis in PECAM‐1‐deficient mice. J Clin Invest 2002; 109:383–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wong MX, Hayball JD, Hogarth PM, Jackson DE. The inhibitory co‐receptor PECAM‐1 provides a protective effect in suppression of collagen‐induced arthritis. J Clin Immunol 2005; 25:19–28. [DOI] [PubMed] [Google Scholar]
- 19. Goel R, Schrank BR, Arora S et al Site‐specific effects of PECAM‐1 on atherosclerosis in LDL receptor‐deficient mice. Arterioscler Thromb Vasc Biol 2008; 28:1996–2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Caligiuri G, Groyer E, Khallou‐Laschet J et al Reduced immunoregulatory CD31+ T cells in the blood of atherosclerotic mice with plaque thrombosis. Arterioscler Thromb Vasc Biol 2005; 25:1659–64. [DOI] [PubMed] [Google Scholar]
- 21. Caligiuri G, Rossignol P, Julia P et al Reduced immunoregulatory CD31+ T cells in patients with atherosclerotic abdominal aortic aneurysm. Arterioscler Thromb Vasc Biol 2006; 26:618–23. [DOI] [PubMed] [Google Scholar]
- 22. Prager E, Staffler G, Majdic O et al Induction of hyporesponsiveness and impaired T lymphocyte activation by the CD31 receptor:ligand pathway in T cells. J Immunol 2001; 166:2364–71. [DOI] [PubMed] [Google Scholar]
- 23. Fornasa G, Clement M, Groyer E et al A CD31‐derived peptide prevents angiotensin II‐induced atherosclerosis progression and aneurysm formation. Cardiovasc Res 2012; 94:30–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nishihara M, Ogura H, Ueda N et al IL‐6‐gp130‐STAT3 in T cells directs the development of IL‐17+ Th with a minimum effect on that of Treg in the steady state. Int Immunol 2007; 19:695–702. [DOI] [PubMed] [Google Scholar]
- 25. Groyer E, Nicoletti A, Ait‐Oufella H et al Atheroprotective effect of CD31 receptor globulin through enrichment of circulating regulatory T‐cells. J Am Coll Cardiol 2007; 50:344–50. [DOI] [PubMed] [Google Scholar]
- 26. Hoffmann P, Eder R, Boeld TJ et al Only the CD45RA+ subpopulation of CD4+CD25high T cells gives rise to homogeneous regulatory T‐cell lines upon in vitro expansion. Blood 2006; 108:4260–7. [DOI] [PubMed] [Google Scholar]
- 27. Haas J, Fritzsching B, Trubswetter P et al Prevalence of newly generated naïve regulatory T cells (Treg) is critical for Treg suppressive function and determines Treg dysfunction in multiple sclerosis. J Immunol 2007; 179:1322–30. [DOI] [PubMed] [Google Scholar]
- 28. Shen LS, Wang J, Shen DF et al CD4(+)CD25(+)CD127(low/–) regulatory T cells express Foxp3 and suppress effector T cell proliferation and contribute to gastric cancers progression. Clin Immunol 2009; 131:109–18. [DOI] [PubMed] [Google Scholar]
- 29. Liu W, Putnam AL, Xu‐Yu Z et al CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ Treg cells. J Exp Med 2006; 203:1701–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Yuan XL, Chen L, Li MX et al Elevated expression of Foxp3 in tumor‐infiltrating Treg cells suppresses T‐cell proliferation and contributes to gastric cancer progression in a COX‐2‐dependent manner. Clin Immunol 2010; 134:277–88. [DOI] [PubMed] [Google Scholar]
- 31. Ma Y, Yuan X, Deng L et al Imbalanced frequencies of Th17 and Treg cells in acute coronary syndromes are mediated by IL‐6‐STAT3 signaling. PLOS ONE 2013; 8:e72804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zheng Y, Wang Z, Deng L et al Osteopontin promotes inflammation in patients with acute coronary syndrome through its activity on IL‐17 producing cells. Eur J Immunol 2012; 42:2803–14. [DOI] [PubMed] [Google Scholar]
- 33. Murawski MR, Litherland SA, Clare‐Salzler MJ, Davoodi‐Semiromi A. Upregulation of Foxp3 expression in mouse and human Treg is IL‐2/STAT5 dependent: implications for the NOD STAT5B mutation in diabetes pathogenesis. Ann NY Acad Sci 2006; 1079:198–204. [DOI] [PubMed] [Google Scholar]
- 34. Yao Z, Kanno Y, Kerenyi M et al Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 2007; 109:4368–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laurence A, Amarnath S, Mariotti J et al STAT3 transcription factor promotes instability of nTreg cells and limits generation of iTreg cells during acute murine graft‐versus‐host disease. Immunity 2012; 37:209–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yang XO, Panopoulos AD, Nurieva R et al STAT3 regulates cytokine‐mediated generation of inflammatory helper T cells. J Biol Chem 2007; 282:9358–63. [DOI] [PubMed] [Google Scholar]
- 37. Yang G, Wang Y, Zeng Y et al Rapid health transition in China, 1990–2010: findings from the Global Burden of Disease Study 2010. Lancet 2013; 381:1987–2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Laurat E, Poirier B, Tupin E et al In vivo downregulation of T helper cell 1 immune responses reduces atherogenesis in apolipoprotein E‐knockout mice. Circulation 2001; 104:197–202. [DOI] [PubMed] [Google Scholar]
- 39. Harrington LE, Hatton RD, Mangan PR et al Interleukin 17‐producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol 2005; 6:1123–32. [DOI] [PubMed] [Google Scholar]
- 40. Xie JJ, Wang J, Tang TT et al The Th17/Treg functional imbalance during atherogenesis in ApoE(–/–) mice. Cytokine 2010; 49:185–93. [DOI] [PubMed] [Google Scholar]
- 41. Liu Z, Lu F, Pan H et al Correlation of peripheral Th17 cells and Th17‐associated cytokines to the severity of carotid artery plaque and its clinical implication. Atherosclerosis 2012; 221:232–41. [DOI] [PubMed] [Google Scholar]
- 42. Wan YY, Flavell RA. Regulatory T‐cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature 2007; 445:766–70. [DOI] [PubMed] [Google Scholar]
- 43. Chauhan SK, Saban DR, Lee HK, Dana R. Levels of Foxp3 in regulatory T cells reflect their functional status in transplantation. J Immunol 2009; 182:148–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sun L, Yi S, O'Connell PJ. Foxp3 regulates human natural CD4+CD25+ regulatory T‐cell‐mediated suppression of xenogeneic response. Xenotransplantation 2010; 17:121–30. [DOI] [PubMed] [Google Scholar]


