Summary
While apoptotic debris is believed to constitute the original antigenic insult in lupus (which is characterized by a time‐dependent diversification of autoreactivity), whether such debris and autoantibodies specifically recognizing its constituents mediate differential effects on innate and humoral responses in lupus‐prone mice is currently unknown. Apoptotic blebs (as opposed to cellular lysate) enhanced preferentially the maturation of dendritic cells (DCs) from bone marrow precursors drawn from lupus‐prone mice. Murine, somatically mutated, apoptotic cell‐reactive immunoglobulin (Ig)G monoclonal antibodies demonstrated enhanced recognition of DCs and also displayed a prominent lupus strain‐specific bias in mediating DC maturation. Further, immunization of such antibodies specifically in lupus‐prone mice resulted in widespread humoral autoreactivity; hypergammaglobulinaemia (a hallmark of systemic autoimmunity) was observed, accompanied by enhanced antibody titres to cellular moieties. Induced antibodies recognized antigens distinct from those recognized by the antibodies employed for immunization; in particular, nephritis‐associated anti‐double stranded (ds) DNA antibodies and neonatal lupus‐associated anti‐Ro60 antibodies were elicited by a non‐dsDNA, non‐Ro60 reactive antibody, and Sm was a favoured target. Further, only in lupus‐prone mice did such immunization enhance the kinetics of humoral anti‐self responses, resulting in the advanced onset of glomerulosclerosis. These studies reveal that preferential innate and humoral recognition of the products of cell death in a lupus milieu influence the indices associated with autoimmune pathology.
Keywords: aberrant apoptosis, apoptosis, antigen spreading, immune pathology, systemic autoreactivity, systemic lupus erthythematosus
Introduction
Systemic lupus erythematosus (SLE) is a chronic, debilitating autoimmune disease characterized by autoantibodies against more than 100 moieties, including double‐stranded DNA (dsDNA), ribonucleoproteins and phospholipids 1. In retrospective human studies, autoreactive antibodies to a restricted set of self‐moieties have been detected in sera as long as 10 years before disease onset; there then occurs a sequential expansion of antigenic targets, resulting ultimately in (or being associated with) fulminant disease 2. This phenomenon, referred to as determinant spreading, has also been observed in murine models of disease; antibody responses to new epitopes within the same antigen (intramolecular spreading) as well as to other, associated antigens (intermolecular spreading) are observed with the progression of time 3. The study of the mechanisms responsible for such diversification has obvious clinical relevance.
Aberrant apoptosis and the deficient clearance of apoptotic cells are observed frequently in lupus 4, leading to the premise that apoptotic debris acts as an immunological trigger; indeed, animals deficient in molecules that aid apoptotic cell uptake exhibit lupus‐associated pathologies 5, 6. Whether apoptotic debris, as well as the potentially pathogenic, cross‐reactive, isotyped‐switched and somatically mutated antibodies such debris engenders, affect the maturation of dendritic cells (DCs) differentially in a lupus environment remains unknown. Additionally, whether such antibodies (the ‘first humoral responders’) can affect preferentially the spectrum and kinetics of autoreactivity in lupus‐prone mice to consequently enhance the onset of glomerulosclerosis is important to determine, given previous data on the effects of stimulation of the immune network by anti‐dsDNA antibodies 7. The data indicate that differential innate and humoral immune responses, in a lupus milieu, to moieties extruded upon apoptosis may play a significant role in influencing immunopathological outcomes.
Materials and methods
Ethical approval
The study was carried out in accordance with the protocol approved by the Institutional Animal Ethics Committee (IAEC) of the National Institute of Immunology, New Delhi (IAEC number: 173/07). Blood samples were withdrawn under ketamine and xylezine anaesthesia and all efforts were made to minimize suffering. The blood withdrawal procedure from humans was approved by the Institutional Human Ethics Committee.
Mice
NZB × NZW (F1) (referred to hereafter as NZB/W F1), NZM2410 (referred to hereafter as NZM) and BALB/c mice, obtained from The Jackson Laboratory, were bred at the National Institute of Immunology, New Delhi.
Effects of apoptotic blebs on the maturation of bone marrow‐derived dendritic cells (BMDCs)
Apoptosis was induced in murine neuroblastoma CCL131 cells [American Type Culture Collection (ATCC), Manassas, VA, USA] or Jurkat cells (ATCC) by incubation with 0·5 μM staurosporine for 24 h. Cells were centrifuged at 1550 g at room temperature for 10 min to pellet apoptotic bodies. The supernatant was centrifuged further at 15 700 g for 50 min at room temperature and the pellet (comprising apoptotic blebs) resuspended in phosphate‐buffered saline (PBS). For the preparation of freeze‐thaw (FT) cellular lysate, cells were resuspended in PBS, snap‐frozen by brief incubation in liquid nitrogen and thawed immediately; the cycle was repeated three times. The lysate was centrifuged at 16 000 g for 15 min at 4°C to remove debris.
Bone marrow cells, isolated from the femur and tibia bones of 2‐month‐old NZB/W F1 or BALB/c mice, were cultured in RPMI‐1640 (Life Technologies, Paisley, UK) supplemented with 10% fetal calf serum (FCS) and 40 ng/ml granulocyte–macrophage colony‐stimulating factor (GM‐CSF) (Peprotech, Rocky Hill, NJ, USA). On day 8, cultures were incubated with varying concentrations (0·5–50 μg/ml) of apoptotic blebs or FT cellular lysate for 48 h. Supernatants were collected for cytokine analysis {interleukin (IL)−6, keratinocyte‐derived chemokine [KC (IL‐8)], IL‐10, interferon (IFN)‐γ, tumour necrosis factor (TNF)‐α; e‐Biosciences, San Diego, CA, USA}. Flow cytometric analysis for cell surface markers was carried out. Briefly, cells were incubated with biotinylated anti‐CD11c (BD Biosciences, San Jose, CA, USA) along with individual fluorescein isothiocyanate (FITC)‐labelled antibodies against murine major histocompatibility complex (MHC)‐I, MHC‐II, CD80, CD83, CD86 or CD40 (e‐Biosciences) for 1 h at 4°C. Cells were then incubated with 50 µl/well streptavidin‐phycoerythrin (PE) (BD Biosciences) for 1 h at 4°C, resuspended in 0·2% paraformaldehyde (Sigma, St Louis, MO, USA) run on a flow cytometer [BD fluorescence activated cell sorter (FACS)Calibur or BD‐LSR]. Data were analysed by FlowJo X software (Tree Star, Inc., Ashland, OR, USA). Data were expressed as ‘fold change’ in phenotypical markers: the ratio of the percentage of dual‐positive (CD11c+ positive plus MHC‐I, MHC‐II, CD80, CD83 or CD40‐positive) cells in test versus control cultures.
Assessment of reactivity of human immunoglobulin (Ig)G towards apoptotic cells and apoptotic blebs
IgG was isolated from the plasma of systemic lupus erythematosus (SLE) patients [satisfying the Systemic Lupus International Collaborating Clinics (SLICC) 2012 criteria] and the plasma of healthy donors by protein G affinity chromatography (GE Healthcare, Chicago, IL, USA). Reactivity towards healthy or apoptotic [derived upon treatment with 0·5 µM staurosporine (Sigma) for 12 h] Jurkat cells was assessed by flow cytometry. Briefly, 106 cells were incubated with the IgG preparations diluted in FACS buffer [10 mM PBS containing 1% bovine serum albumin (BSA) and 0.2% sodium azide] and incubation was carried out for 1 h at 4°C. After washes, appropriately diluted goat anti‐human Ig‐FITC (Jackson ImmunoResearch, West Grove, PA, USA) was added, followed by similar incubation. Apoptotic cells were delineated by assessing reactivity towards annexin‐V‐PHY (BD Pharmingen, San Jose, CA, USA). Analysis was carried out on a BD FACSCaliber flow cytometer. Reactivity of serum IgG towards apoptotic blebs was assessed employing standard Western blot protocols.
Generation, purification and variable region sequence analysis of murine apoptotic cell‐reactive monoclonal antibodies
Murine hybridomas were generated by fusion of splenocytes (derived from 6–8‐month‐old NZB/W F1 mice) with SP2/0 cells (ATCC) using 50% (v/v) polyethylene glycol 1500 (Sigma), following standard protocols. Culture supernatants were analysed for reactivity towards healthy, apoptotic and permeabilized cells by flow cytometry, as described below. Hybrids secreting antibodies which bound annexin‐V‐positive cells in a caspase‐dependent manner were subcloned by limiting dilution.
Monoclonal antibodies [P2B2 (IgG2aκ), 1B3 (IgG2aκ) and P2C2 (IgG2bκ)] were purified by protein G Sepharose (GE Healthcare) affinity and diethyl‐aminoethyl (DEAE) ion exchange chromatography. Consecutive rounds of purification were carried out to arrive routinely at purity levels of 98% or higher. Significant denaturation or aggregation was not observed in assays designed to assess these parameters as well as to assess purity. Such analysis also revealed an absence of all standard autoantigens, including those against which post‐immunization responses were assessed subsequently.
For variable region gene analysis, total RNA was isolated from hybridoma cells. cDNA synthesis was carried out using the IMProm II Reverse Transcription System (Promega, Madison, WI, USA). Polymerase chain reaction (PCR) amplification was carried out using murine heavy and light chain antibody primers (Novagen, Masdison, WI, USA). The PCR product was ligated into pGEM‐T‐Ez (Promega) and DH5α Escherichia coli cells transformed. Selection was carried out as described previously 8. Cloned antibody gene inserts were sequenced using T7 and SP6 primers. Analysis of the immunoglobulin variable regions was carried out using online National Center for Biotechnology Information (NCBI) databases (http://www.ncbi.nlm.nih.gov/igblast). Replacement mutations per amino acid were calculated individually for the framework (FWR) and complementarity determining regions (CDR) of the heavy and light chains, essentially as described previously 8.
Binding of murine apoptotic cell‐reactive monoclonal antibodies to BMDCs and effects on BMDC maturation
Binding
Immature BMDCs on day 8 (derived from lupus‐prone and healthy mice, as described above) were incubated individually with the antibodies P2C2 and 1B3, or respective isotype control antibodies, at varying concentrations (1–50 μg/ml), diluted appropriately in FACS buffer, for 1 h at 4°C. After washes, appropriately diluted goat anti‐mouse Ig‐FITC (Jackson ImmunoResearch) and anti‐mouse CD11c biotin (eBiosciences) were added, followed by 1 h incubation. Cells were then incubated with streptavidin‐PE (BD Biosciences) and analysed by flow cytometry (FACSCalibur). Data were evaluated using FlowJo software (Tree Star, Inc.). For competition analysis, immature DCs on day 8 (derived from lupus‐prone and healthy mice) were pre‐incubated with a 50‐fold excess of respective isotype control antibodies before addition of the apoptotic cell‐reactive antibodies. On day 10, supernatants were collected for cytokine quantification.
Maturation
Immature DCs on day 8 (derived from lupus‐prone and healthy mice) were incubated with varying concentrations (1–50 μg/ml) of antibodies P2C2, 1B3 (or respective isotype control antibodies) for 48 h. On day 10, supernatants were collected for cytokine quantification and cells were processed by flow cytometric analysis for the expression of CD11c, MHC‐I, MHC‐II, CD80, CD83, CD86 and CD40 as described above.
Immunization of mice with murine apoptotic cell‐reactive monoclonal antibodies
Eight‐week‐old lupus‐prone and healthy mice were immunized with a water‐in‐oil emulsion between PBS (containing antibodies P2B2, 1B3 or P2C2) and incomplete Freund's adjuvant (Difco, Lawrence, KS, USA). Animals were administered three subcutaneous injections (100 µg/200 μl) at fortnightly intervals. Mice immunized with adjuvant alone served as controls. Blood samples were collected 1 week after each injection.
Quantification of serum antibody isotypes
Total immunoglobulins in the sera of adjuvant‐ and antibody‐immunized mice were quantified using matched capture and revealing reagents (Becton Dickinson). Briefly, isotype‐specific capture antibodies were adsorbed onto enzyme‐linked immunosorbent assay (ELISA) plates. Following the addition of diluted sera, enzyme‐labelled secondary antibodies were added, followed by the substrate. Immunoglobulin levels were quantified with reference to appropriate standards.
Autoreactivity towards cellular moieties
Healthy, permeabilized (obtained upon brief incubation in chilled methanol containing 0·001% Triton‐X‐100) and apoptotic cells served as targets to assess the specificity of monoclonal and serum‐derived antibodies. In some cases, apoptosis was induced in the presence of 25 µM Z‐VAD‐FMK (Sigma); 106 cells were incubated with primary antibody (monoclonal antibodies or antibodies in elicited antisera) diluted appropriately in FACS buffer, and incubation was carried out for 1 h at 4°C. After washes, appropriately diluted goat anti‐mouse Ig‐FITC (Jackson ImmunoResearch) was added, followed by similar incubation. Apoptotic cells were delineated by assessing reactivity towards annexin‐V‐PHY (BD Pharmingen). Analysis was carried out on a BD FACSCaliber flow cytometer.
CCL131 cells were dispensed onto glass coverslips and fixed by incubation with 4% paraformaldehyde for 30 min at room temperature. Cells were permeabilized by incubation in 10 mM PBS containing 0·1% Triton‐X‐100. Non‐specific sites were blocked by incubation for 16 h at 4°C in PBS containing 10% normal goat serum. Incubation was carried out with diluted primary antibodies (monoclonal antibodies or antibodies in elicited antisera) for 1 h at 37°C, followed by a similar incubation with goat anti‐mouse Ig‐FITC. Coverslips were mounted on glass slides using a medium containing 4',6‐diamidino‐2‐phenylindole (DAPI; Vectashield, Vector Laboratories, Burlingame, CA, USA) and digital images were acquired on a Carl Zeiss confocal microscope.
Electrophoresed moieties in cellular lysates were transferred to nitrocellulose membranes (MDI). Blots were probed with antisera generated upon antibody or adjuvant immunization. Reactive moieties were visualized on X‐ray film by enhanced chemiluminescence (ECL; Pierce ThermoFisher Scientific, Fremont, CA, USa).
Reactivity towards ribonucleoproteins, phospholipids and dsDNA
Purified ribonucleoprotein autoantigens (250 ng/well in 0·1 M carbonate buffer; Arotec Diagnostics, Wellington, New Zealand) and calf thymus DNA (1 μg/well in PBS; Sigma) were dispensed into ELISA plates. A working concentration of 200 µg/ml (Avanti Polar Lipids Ltd, Alabaster, AL, USA) was prepared in a 1 : 3 ratio of chloroform : methanol for each phospholipid except for cardiolipin, which was prepared in ethanol. Thirty µl of phospholipid solution was dispensed per well. Reactivity of monoclonal antibodies and antibodies in elicited antisera towards these moieties was assessed by standard ELISA protocols.
Histological analysis
Kidneys were isolated from lupus‐prone or healthy mice immunized with either adjuvant or apoptotic cell‐reactive antibodies. Tissues were fixed in 10% formaldehyde and embedded in paraffin. Five‐μm sections were stained with haematoxylin and eosin (H&E), silver‐methenamine (SM) or periodic acid‐Schiff (PAS).
For immunohistochemical analysis, sections were exposed to decreasing concentrations of alcohol (100, 90, 70, 50 and 30%) and then washed with saline and PBS. Antigen retrieval was carried out by heating at 95°C in 10 mM citrate‐phosphate buffer (pH 6·0) containing 0·05% v/v Tween‐20 for 20 min. Sections were then incubated in 5% normal goat serum (in PBS containing 0·25% v/v Triton‐X 100) for 1 h. After incubation in 3% v/v for 10 min, sections were incubated with horseradish peroxidase (HRP)‐conjugated goat anti‐mouse C3 antibodies (Cappel Laboratories Inc., Cochranville, PA, USA ) or with HRP‐conjugated goat anti‐mouse IgG (Jackson ImmunoResearch), diluted appropriately in PBS containing 1% BSA and 0·25% v/v Triton‐X 100 for 16 h at 4°C. After washes with PBS, freshly prepared 3,3‐diaminobenzidine/tetrahydrochloride (DAB; Vector Laboratories) was added, followed by counterstaining with haematoxylin. Sections were subjected to increasing concentrations of alcohol (50, 70, 90 and 100%), a 10‐min submersion in xylene : alcohol (1 : 1) and then a 10‐min submersion in xylene. Finally, DPX mountant (Merck, Kenilworth, NJ, USA) was added. In all cases, images were captured on an Olympus microscope and analysed by a pathologist in a blinded protocol.
Statistical analysis
Either the Shapiro–Wilk test or the Kolomogorov–Smirnov test were applied (depending on sample size) to assess for normal distribution of data; all data demonstrated normal distribution. Statistical analysis was performed using the unpaired Student's t‐test. In immunization experiments, animal survival was analysed with the Kaplan–Meier method using the log‐rank (Mantel–Cox) test.
Results
Effect of apoptotic blebs on the maturation of BMDCs
Immature BMDCs derived from NZB/W F1 or BALB/c mice were incubated with apoptotic blebs or FT cellular lysate for 48 h. Blebs induced the up‐regulation of MHC‐I, MHC‐II, CD80, CD83, CD86 and CD40 on CD11c+ DCs derived from NZB/W F1 mice to a much greater extent than on CD11c+ DCs derived from BALB/c mice; the effect was dose‐dependent. While FT cellular lysate did not induce a significant up‐regulation of these markers in BALB/c mice, its effects in NZB/W F1 animals were significantly lower than those mediated by apoptotic blebs (Fig. 1a). Upon stimulation with apoptotic blebs, BMDCs derived from NZB/W F1 mice secreted heightened levels of IL‐6, IL‐8, TNF‐α and IL‐10 in comparison with BMDCs stimulated with FT cellular lysate, as well as higher amounts of IL‐8 than similarly stimulated BMDCs from BALB/c mice. Interestingly, apoptotic blebs were also more efficient than FT cellular lysate in stimulating the secretion of TNF‐α and IL‐10 from BALB/c‐derived BMDCs (Fig. 1b). The data indicate that self‐antigens, particularly in the form of apoptotic blebs, can have differential effects on the maturation of DCs in a lupus milieu.
Figure 1.

Effects of apoptotic blebs and freeze–thaw (FT) cellular lysate on the maturation of bone marrow‐derived dendritic cells (BMDCs) derived from NZB × NZW (F1) (NZB/W F1) and BALB/c mice. Immature BMDCs were incubated with apoptotic blebs or FT for 2 days and cells were assessed concurrently for CD11c and the indicated markers by flow cytometry. Cytokines in supernatants were quantified by enzyme‐linked immunosorbent assay (ELISA). (a) Fold change (over the medium control) of CD11c+ BMDCs expressing major histocompatibility complex (MHC)‐I, MHC‐II, CD80, CD83, CD86 and CD40 upon incubation with apoptotic blebs or FT cellular lysate (*P < 0·05; **P < 0·01; ***P < 0·001: apoptotic bleb‐treated NZB/W F1 BMDCs versus apoptotic bleb‐treated BALB/c BMDCs; # P < 0·05, ## P < 0·01, ### P < 0·001: apoptotic bleb‐treated NZB/W F1 BMDCs versus FT cellular lysate‐treated NZB/W F1 BMDCs). (b) Cytokine secretion from BMDCs derived from NZB/W F1 and BALB/c mice incubated with apoptotic blebs or FT cellular lysate (*P < 0·05; **P < 0·01). Data represent mean ± standard error of the mean (s.e.m.) of four experiments.
Reactivity and characteristics of IgG derived from human lupus patients and lupus‐prone mice
Apoptotic cell‐reactive human IgG
Whether or not apoptotic material extruded in the form of blebs is recognized differentially by autoreactive antibodies in SLE was assessed. IgG antibodies in the sera of seven of eight lupus patients were preferentially reactive towards apoptotic Jurkat cells; in contrast, IgG antibodies derived from eight of eight healthy individuals were non‐reactive to annexin V‐positive cells (Fig. 2a). IgG from SLE patients as well as from healthy individuals were non‐reactive towards healthy cells (Fig. 2b). Upon Western blot, IgG antibodies in the sera of SLE patients were preferentially reactive towards several moieties on apoptotic blebs derived from human cells (Supporting information, Fig. S1). As has also been reported in other studies in various other contexts, differential humoral reactivity against the products of cell death is therefore readily demonstrable in SLE patients.
Figure 2.

Reactivity of human immunoglobulin (Ig)G towards Jurkat cells. Apoptosis was induced by incubation with 0·5 µM staurosporine for 12 h. Reactivity of (a) apoptotic and (b) healthy cells to human immunoglobulin (Ig)G and annexin‐V was assessed concurrently by flow cytometry. In each case, the upper row depicts IgG purified from the sera of systemic lupus erythematosus (SLE) patients and the lower row IgG purified from sera of healthy donors. Each dot‐plot represents an individual immunoglobulin preparation, employed at 10 μg/ml. The percentage of cells in each quadrant is indicated. Data are representative of three experiments.
Apoptotic cell‐reactive murine monoclonal antibodies
Reactivity towards intracellular moieties, apoptotic cells and autoantigens
A murine lupus model was employed to study the characteristics of such apoptotic cell‐reactive humoral responses. Three murine monoclonal antibodies [designated P2B2 (IgG2aκ), 1B3 (IgG2aκ), P2C2 (IgG2bκ)], generated from ageing NZB/W F1 mice, were selected for this study. All antibodies were non‐reactive towards non‐permeabilized cells while being highly reactive towards permeabilized cells (Fig. 3a–c, left panels). Antibodies P2B2 and 1B3 recognized nuclear/perinuclear moieties on CCL131 cells, while P2C2 bound both cytoplasmic and nuclear antigens (Fig. 3a–c, right panels). In cultures containing apoptotic cells (whose membranes were confirmed impermeable), the antibodies essentially recognized only those cells which were also bound by annexin‐V. Cells that bound annexin‐V but not antibody were apparent, indicating that the antibodies demonstrated recognition of cells at a relatively late stage of apoptosis; the demonstration of antibody binding to PI‐positive cells whose membranes were confirmed impermeable by trypan blue exclusion confirmed this conclusion (data not shown). When apoptosis was induced in the presence of the pan‐caspase inhibitor Z‐VAD‐FMK, a significant reduction in the reactivity of the antibodies was observed (Fig. 3d–f). The data therefore suggest that the monoclonal antibodies recognize cellular moieties extruded during the process of apoptosis.
Figure 3.

Characterization of anti‐self‐reactivity of monoclonal antibodies generated from NZB × NZW (F1) (NZB/W F1) mice. (a–c) Flow cytometric and confocal analysis. The left panels in each case depict reactivity of antibodies on permeabilized (green profiles) and non‐permeabilized (black profiles) CCL131 cells by flow cytometry. Filled red profiles represent negative controls where only appropriate isotype control antibodies were employed. Panels on the right depict confocal images of co‐localization on CCL131 cells (bottom left) of antibody (green; top left) and 4',6‐diamidino‐2‐phenylindole (DAPI) (blue; top right) reactivity along with differential interference contrast (DIC) images (bottom right). Bars = 10 μm. (a) Antibody P2B2; (b) antibody 1B3; (c) antibody P2C2. (d–f) Reactivity of antibodies towards CCL131 cells incubated with medium (left panels), staurosporine (centre panels) or with Z‐VAD‐FMK plus staurosporine (right panels). The x‐axis represents reactivity of respective antibodies while the y‐axis represents annexin‐V binding. Experiments employed 5 μg/ml antibody. (d) Antibody P2B2; (e) antibody 1B3; (f) antibody P2C2. The percentage of cells in each quadrant is indicated.
Several autoantigens are known to cluster into surface‐associated blebs during apoptosis. While antibody P2B2 bound dsDNA, antibodies 1B3 and P2C2 demonstrated polyreactivity towards proteinaceous autoantigens, with antibody P2C2 additionally binding dsDNA (Supporting information, Fig. S2a–c, left panels). While antibody P2B2 was minimally reactive to phospholipids, antibody P2C2 exhibited a variable degree of reactivity towards all phospholipids except phosphatidylcholine, and antibody 1B3 demonstrated reactivity to cardiolipin (Supporting information, Fig. S2a–c, right panels). IgG apoptotic cell‐reactive monoclonal antibodies can therefore demonstrate frank polyreactivity.
Variable region gene sequences
Whether apoptotic cell reactivity correlated with specific variable region gene usage by the antibodies was ascertained; such analysis also permitted determination of whether the presence of somatic mutations correlated with the ability to generate hypergammaglobulinaemia and autoimmune responses upon immunization, as described below. The heavy and light chain variable region genes of antibody 1B3 have been characterized previously 8 (GenBank Accession numbers EF063585.1, EF063586.1; http://www.ncbi.nlm.nih.gov/genbank). Heavy and light chain genes of antibodies P2B2 and P2C2 were sequenced (GenBank Accession numbers JN400654.1‐JN400657.1; http://www.ncbi.nlm.nih.gov/genbank) and all sequences were analysed by Ig‐BLAST (http://www.ncbi.nlm.nih.gov/igblast). Supporting information, Fig. S3a describes the closest germline variable region segments. Interestingly, the D segment of antibody P2B2 could not be assigned. There existed no preferential use of V region gene segments of either the light or heavy chain. Analysis of CDR/FWR replacement mutation frequencies revealed the heavy chain of antibodies 1B3 and P2C2 to be mutated significantly (Supporting information, Fig. S3b). IgG antibodies reactive towards extruded apoptotic moieties can therefore exhibit varying degrees of somatic mutations in their variable region genes.
Analysis of apoptotic cell‐reactive antibody‐BMDC interaction
While autoreactive antibodies and immune complexes have been demonstrated previously to influence the phenotype and function of antigen‐presenting cells, whether the increased interaction of such moieties with the cell surface can influence such an outcome remains unclear at present. The extent of interaction of apoptotic cell‐reactive antibodies with DCs was therefore ascertained. Flow cytometry was employed to assess the binding of the monoclonal antibodies to CD11c+ BMDCs derived from both lupus‐prone and healthy mice. Results were compared with data obtained using respective isotype control antibodies. In each instance, antibodies bound CD11c+ BMDCs derived from both strains to a significantly higher extent than did respective isotype control antibodies (Supporting information, Fig. S4a). Interestingly, antibody 1B3 bound BMDCs derived from lupus‐prone mice to a greater degree than BMDCs derived from healthy mice. Competition assays were carried out to analyse the antibody–BMDC interaction further, using TNF‐α as a readout. Both antibodies stimulated the secretion of higher levels of TNF‐α by lupus‐prone BMDCs compared with BMDCs derived from healthy mice, and also compared with respective isotype control antibodies. Significantly, pre‐incubation of BMDCs with 50‐fold excess of the respective isotype control antibodies did not diminish significantly the secretion of TNF‐α upon the addition of the apoptotic cell‐reactive antibodies, indicating a lack of competition (Supporting information, Fig. S4b). The data suggest that, while apoptotic cell‐reactive antibodies react to a greater extent (compared to control antibodies) with BMDCs derived from either lupus‐prone or healthy mice, enhanced inflammatory consequences of this interaction occur in the former, via unknown mechanisms.
Effect of apoptotic cell‐reactive monoclonal autoantibodies on the maturation of BMDCs
Whether such spontaneously arising, isotype‐switched, somatically mutated antibodies could induce differentially the phenotypical maturation of DCs derived from lupus‐prone mice was then assessed. Immature BMDCs derived from NZB/W F1 and BALB/c mice were incubated with antibodies P2C2 and 1B3 for 48 h and the surface expression of cell surface markers was analysed. Both antibodies induced significantly higher up‐regulation of MHC‐I, MHC‐II, CD80, CD83 and CD40 on CD11c+ BMDCs derived from NZB/W F1 mice than on such cells derived from BALB/c mice. Isotype control antibodies induced negligible up‐regulation of these markers in both murine strains (Fig. 4a, c). Antibody P2C2 induced the heightened secretion of IL‐6 and IL‐8 and antibody 1B3 the heightened secretion of IL‐6 and IL‐8 and TNF‐α (over respective isotype control antibodies), from BMDCs derived from NZB/W F1 mice. Further, levels of secreted IL‐6 and IL‐8 induced by antibody P2C2 were enhanced over those in supernatants of similarly stimulated BALB/c‐derived BMDCs. Similarly, antibody 1B3 induced the heightened secretion of IL‐8 from BMDCs derived from NZB/W F1 mice compared to BMDCs derived from BALB/c mice. Interestingly, antibody P2C2 also induced increased levels of IL‐8 and antibody 1B3 increased levels of IL‐6 and IL‐8 (over respective isotype control antibodies) when added to BALB/c‐derived BMDCs (Fig. 4b, d). IgG apoptotic cell‐reactive autoantibodies can therefore influence preferentially the phenotype of antigen‐presenting cells from lupus‐prone mice, as well as induce the heightened secretion of inflammatory cytokines from such cells; further, such antibodies can also cause (albeit significantly diminished) effects in antigen‐presenting cells from healthy mice. To conclude, while both antibody specificity and cellular genotype appear to contribute, apoptotic cell‐reactive antibodies mediate the enhanced maturation of lupus‐derived DCs, based on two independent criteria.
Figure 4.

Effects of apoptotic cell‐reactive antibodies on the maturation of bone marrow‐derived dendritic cells (BMDCs) derived from NZB × NZW (F1) (NZB/W F1) and BALB/c mice. Immature BMDCs were incubated with antibodies P2C2 or 1B3 for 48 h, after which cells were assessed concurrently for CD11c and the indicated markers by flow cytometry. Cytokines in supernatants were quantified by enzyme‐linked immunosorbent assay (ELISA). Effects of (a,b) antibody P2C2 and (c,d) antibody 1B3 on (a,c) cell surface phenotype and (b,d) cytokine secretion. (a,c) Fold change (over the medium control) of CD11c+ BMDCs expressing major histocompatibility complex (MHC)‐I, MHC‐II, CD80, CD83, CD86 and CD40 upon incubation with (a) antibody P2C2 or an immunoglobulin (Ig)G2bκ isotype control antibody and (c) autoantibody 1B3 or an IgG2aκ isotype control antibody. (b,d) Quantification of cytokines in culture supernatants of BMDCs incubated with (b) antibody P2C2 or an IgG2bκ isotype control antibody and (d) autoantibody 1B3 or an IgG2aκ isotype control antibody (*P < 0·05; **P < 0·01; ***P < 0·001). Data represent mean ± standard error of the mean (s.e.m.) of four experiments.
Effects of autoantibody immunization in lupus‐prone mice
Whether or not the apoptotic cell‐reactive antibodies, which are differentially inflammatory on antigen presenting cells derived from lupus‐prone mice, are also immunogenic in such mice was assessed.
Hypergammaglobulinaemia
Immunization of the apoptotic cell‐reactive antibodies into lupus‐prone mice resulted in hypergammaglobulinaemia. Compared with both pre‐immune and adjuvant‐immunized animals, while immunization with antibody P2C2 enhanced IgG1, IgG2a and IgG2b levels (Fig. 5a), immunization with antibody 1B3 enhanced IgG1 levels (Fig. 5b). Immunization with antibody P2B2 enhanced IgG1, IgG2a and IgG2b levels (Supporting information, Fig. S5a). In all cases, IgG3 and IgM levels remained essentially unaltered.
Figure 5.

Characterization of humoral anti‐self responses elicited upon immunization of apoptotic cell‐reactive antibodies in NZB × NZW (F1) (NZB/W F1) mice. Eight‐week‐old NZB/W F1 mice were administered subcutaneous injections of 100 µg of antibodies P2C2 or 1B3 [or incomplete Freund's adjuvant (IFA)], and antisera analysed for total immunoglobulin levels and anti‐self reactivity. (a,b) Total immunoglobulin isotypes in sera were estimated in mice immunized with (a) antibody P2C2 (*P < 0·01; **P < 0·00005; ***P < 0·00002) and (b) antibody 1B3 (*P < 0·0001). Bars represent mean ± standard error of the mean (s.e.m.) of four experiments. (c,d) Flow cytometric analysis of humoral anti‐self responses elicited upon immunization of mice with (c) antibody P2C2 or (d) antibody 1B3. Permeabilized CCL131 cells were used as targets. The filled grey profile represents the negative control where only the secondary antibody was employed. Pre‐immune sera (derived both from mice later administered IFA and from mice later administered the respective antibodies) and sera from animals immunized with adjuvant IFA alone were included as controls, as indicated. In each case, sera pooled from individual animals (n = 6) were employed at a dilution of 1 : 500. (e) Flow cytometric analysis of the reactivity of antibodies in sera of mice immunized with antibody 2C2 or antibody 1B3 towards healthy and post‐apoptotic (secondary) necrotic cells (with membranes rendered permeable), as indicated. Cells were stained concurrently with annexin‐V. Reactivity of antibodies in pooled sera from adjuvant IFA‐immunized animals is also shown. In all experiments, sera were employed at a dilution of 1 : 500. Data for day 34 post‐immunization are shown. The percentage of cells in each quadrant is indicated.
Autoreactivity towards cellular antigens, ribonucleoproteins, lipids and dsDNA
Antibodies in sera from animals immunized with antibody 2C2 (Fig. 5c) or antibody 1B3 (Fig. 5d), depicted enhanced reactivity towards intracellular antigens in comparison with antibodies in sera from animals immunized with adjuvant alone. While these results suggest that the hypergammaglobulinaemia elicited upon immunization with apoptotic cell‐reactive antibodies in lupus‐prone mice can be associated with increased autoreactivity, the correlation was imperfect; antibodies in sera from animals immunized with antibody P2B2 did not exhibit such anti‐self reactivity (Supporting information, Fig. S5b). Antibodies in the sera of antibody P2C2 and antibody 1B3‐immunized animals were non‐reactive towards healthy cells, but demonstrated reactivity towards cells undergoing post‐apoptotic secondary necrosis (which rendered the membrane permeable), unlike antibodies in adjuvant‐induced antisera (Fig. 5e). Humoral autoimmune cascades initiated upon the recognition of apoptotic debris may therefore serve to amplify responses to cellular antigens.
Antibodies in the sera of antibody P2C2‐immunized animals demonstrated reactivity towards cytoplasmic moieties (Fig. 6b); antibodies in sera from incomplete Freund's adjuvant (IFA)‐immunized mice were non‐reactive (Fig. 6a). On Western blot analysis on cellular lysate, antibodies in the sera of antibody P2C2‐immunized animals bound a large number of moieties migrating between ≅ 15 kDa and ≅ 60 kDa. While antibody P2C2 principally bound La and dsDNA (Supporting information, Fig. S2), antibodies in sera generated upon immunization with the antibody bound Sm to a significant extent. dsDNA was also recognized predominantly by antibodies elicited upon the immunization of antibody P2C2, in contrast to antibodies generated upon adjuvant immunization. In addition, enhanced recognition of La, Ro60 and Ro52 was observed by antibodies elicited upon antibody P2C2 immunization (Fig. 6e). Of interest was the fact that antibodies generated upon antibody immunization also demonstrated recognition of several lipids, while adjuvant immunization did not elicit such responses (Fig. 6f). Antibodies generated upon immunization with antibody 1B3 demonstrated reactivity towards cytoplasmic moieties, along with reactivity to discrete nuclear components (Fig. 6c), compared to exclusive nuclear recognition by antibody 1B3 (Fig. 3b). Upon Western blot analysis, antibodies in antisera induced upon antibody 1B3 immunization specifically recognized several antigens between ≅ 17 kDa and ≅ 45 kDa (Fig. 6d). Interestingly, even though antibody 1B3 was non‐reactive towards dsDNA and Ro60 (Supporting information, Fig. S2), antisera induced upon its immunization contained antibodies which bound both moieties. Antisera also contained antibodies which bound Sm to a significant extent, and reactivity to La was induced upon antibody but not adjuvant immunization (Fig. 6e). Autoantibodies induced upon immunization with antibody 1B3, unlike those induced upon antibody P2C2, were non‐reactive towards lipids (Fig. 6f). Further analysis revealed the longevity and uniformity of such responses; significantly enhanced anti‐self‐reactivity was observed for 133 days (day 77 post‐immunization) in all animals (data not shown). The data indicate that immunization of apoptotic cell‐reactive antibodies in lupus‐prone mice results in the earlier appearance of humoral anti‐self responses, and that such response exhibit enhanced titres as well as an expanded repertoire.
Figure 6.
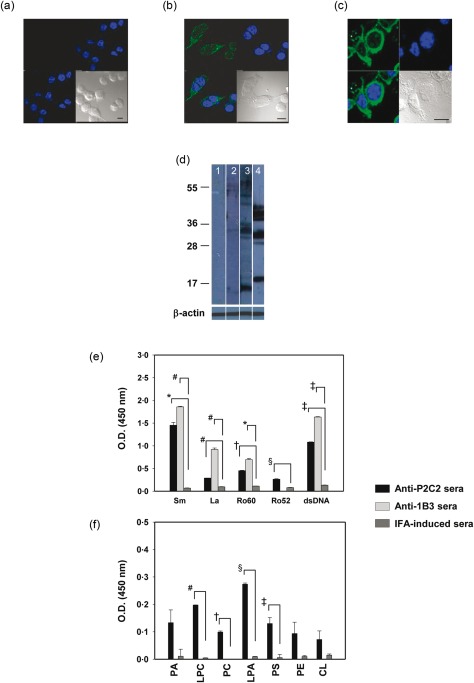
Characterization of humoral anti‐self responses elicited upon immunization of apoptotic cell‐reactive antibodies in NZB × NZW (F1) (NZB/W F1) mice. (a–c) Confocal microscopy on CCL131 cells depicting cellular reactivity of antibodies in antisera generated upon immunization with (a) incomplete Freund's adjuvant (IFA), (b) antibody P2C2 and (c) antibody 1B3. In each case, co‐localization (bottom left) of antibody (green; top left) and 4',6‐diamidino‐2‐phenylindole (DAPI) (blue; top right) reactivity are depicted, along with differential interference contrast (DIC) images (bottom right). Bars = 10 μm. (d) Western blot analysis of the reactivity of antibodies in pre‐immune sera (lane 1), in sera generated upon IFA immunization (lane 2), in sera generated upon antibody P2C2 immunization (lane 3) and in sera generated upon antibody 1B3 immunization (lane 4) towards cellular lysate. Molecular weights are indicated in kDa. β‐actin was employed as the loading control. (e) Reactivity of antibodies in sera generated upon immunization with antibody P2C2, antibody 1B3 or IFA towards ribonucleoproteins and dsDNA (*P < 0·003, # P < 0·002, † P < 0·0008, § P < 0·004, ‡ P < 0·0002). (f) Reactivity of antibodies in sera generated upon immunization with antibody P2C2, antibody 1B3 or IFA towards phospholipids (# P < 0·0002, † P < 0·003, § P < 0·0004, ‡ P < 0·04) (PA = phosphatidic acid; LPC = lysophosphatidylcholine; PC = phosphatidylcholine; LPA = lysophosphatidic acid; PS = phosphatidylserine; PE = phosphatidylethanolamine). In each case, sera pooled from individual animals (n = 6) were employed at a dilution of 1 : 500. Data for day 34 post‐immunization are shown. Data in (d–f) represent mean ± standard error of the mean (s.e.m.) of four experiments.
Whether or not immunization of non‐lupus prone BALB/c mice with antibody P2C2 would also elicit similar responses was assessed; no increases in anti‐self responses were observed on flow cytometric (Supporting information, Fig S6a) or Western blot (Supporting information, Fig. S6b) analysis. Therefore, antibodies recognizing apoptotic cells appear to drive the generation of anti‐self responses preferentially only in an autoimmune milieu.
Kidney pathology and animal survival
As immunization with apoptotic cell‐reactive antibodies in lupus‐prone mice led to hypergammaglobulinaemia and, in some instances, the early appearance of autoantibodies, including antibodies against dsDNA (the presence of which has been associated with kidney disorder both in lupus‐prone mice and in SLE patients 9), whether such immunization is also associated with pathological changes in the kidneys was assessed. Antibody P2C2 immunization in lupus‐prone mice led to severe glomerulosclerosis and increases in the mesangial matrix in the kidneys, relative to animals immunized with adjuvant. PAS and SM staining also provided evidence for severe thickening of the basement membrane, significant increase in the mesangial matrix and sclerotic glomeruli. BALB/c mice immunized with antibody P2C2 demonstrated normal glomeruli with no increase in the mesangium as well as unaltered basement membranes, relative to animals immunized with IFA. Pronounced C3 deposition and moderate IgG antibody deposition were observed in the glomeruli of kidneys derived from antibody P2C2‐immunized lupus‐prone mice; in contrast, IFA‐immunized lupus‐prone mice demonstrated only mild deposition of C3 and IgG in glomeruli. Antibody P2C2‐immunized BALB/c mice demonstrated moderate deposition, and IFA‐immunized mice mild deposition, of C3 in glomeruli. Deposition of IgG was not observed in glomeruli of either antibody P2C2‐ or IFA‐immunized BALB/c mice (Fig. 7a, b). These findings suggest strongly that anti‐self responses that arise preferentially and selectively in lupus‐prone mice upon immunization with apoptotic cell‐reactive antibodies drive enhanced degenerative changes in the kidneys and are associated with lupus pathology. Such deleterious effects were accompanied by a significant curtailment in the survival of lupus‐prone mice immunized with antibody P2C2 compared with mice immunized with IFA; the latter exhibited the expected rate of age‐related demise (Fig. 7c)
Figure 7.

Histological analysis of kidney sections upon immunization of antibody P2C2 in (a) lupus‐prone and (b) healthy mice. Images include haematoxylin and eosin (H&E)‐, periodic acid–Schiff (PAS)‐ and silver‐methenamine (SM)‐stained sections as well as immunohistochemical localization of C3 and IgG (×400) from antibody‐ and incomplete Freund's adjuvant (IFA)‐immunized mice. G, G1, G2 = glomeruli, T = tubules. (c) Kaplan–Meier analysis of the effects of immunization of lupus‐prone mice (n = 6) with antibody P2C2 or only the adjuvant (IFA) on survival. P < 0·0118: IFA‐immunized mice versus antibody P2C2‐immunized mice [log‐rank (Mantel–Cox) test].
Discussion
The present study was constructed based upon two well‐documented facets about lupus. The first facet is the association of lupus with excessive apoptosis, along with the deficient clearance of apoptotic cells 4, and the several lines of evidence which suggest that apoptotic debris provides the initial impetus for anti‐self immune responses 5, 6, 10, 11. Whether such debris, as well as early immune responses directed against its components, affect antigen‐presenting cells derived preferentially from lupus‐prone animals is therefore worthy of investigation. The second facet is the observation that, during systemic autoimmune responses, a gradual diversification of immune targets is observed in human subjects 2, 12 and in animal models of disease 3, 13, 14. Several targeted antigens exist as macro‐molecular complexes, and it is believed that breakage of T cell tolerance to epitope(s) on such a complex can result in ‘help’ to B cells of different specificities 15, a theory which is proving inadequate in its ability to explain all related events; alternative mechanisms that explain antibody diversity would provide additional insights into disease processes.
Inherent aberrances in DC phenotype and function of monocyte‐derived DCs have been described in lupus 16. Late‐stage apoptotic cells have been shown to induce the maturation of immature monocyte‐derived DCs 17. Nucleosomes 18, DNA 19 and RNA 20 are also reported to trigger DC maturation. Apoptotic blebs, in contrast to apoptotic bodies, have been shown to induce the phenotypical maturation of mouse DCs and heighten the secretion of IL‐6 and TNF‐α, possibly driving the production of IL‐17 21. Microparticles (considered increasingly a potential source of autoantigens 11) derived from lupus patients were shown to enhance the expression of co‐stimulatory markers on pDCs and mDCs derived from blood of healthy subjects while stimulating the release of TNF‐α, IL‐6 and IFN‐α. Interestingly, microparticles isolated from healthy subjects did not mediate equivalent effects 22; apoptotic microvesicles sourced from lupus‐prone mice, compared with such microvesicles sourced from healthy mice, also mediate differential effects on BMDC maturation 23. A few reports have presented a comparative analysis delineating the specific influence of an autoimmune genotype. A study employing ‘apoptotic‐cell‐derived membrane vesicles’ demonstrated increases in maturation markers on monocyte‐derived DCs derived from both healthy donors and SLE patients, with one exception: the down‐regulation of MHC‐II, seen in the former, is absent in the latter 24. Opsonization of human apoptotic cells with iC3b greatly enhanced uptake by DCs derived from healthy humans and was associated with decreased levels of MHC‐II and CD86 on the cell surface 25. Conversely, while DCs derived from lupus patients were less efficient in the uptake of iC3b‐opsonized apoptotic cells, levels of MHC‐II and CD86 were enhanced 26. These observations, while similar in some respects with ours, differ in others, due possibly to species and cell lineage differences; in the present study, apoptotic blebs (as opposed to cellular lysate) preferentially induced up‐regulation of markers on BMDCs derived from lupus‐prone mice, while levels (including of MHC‐II) on BMDCs derived from healthy mice remained minimally affected. In addition, BMDCs derived from lupus‐prone mice secreted significantly higher amounts of IL‐6, IL‐8 and TNF‐α, cytokines implicated in lupus pathology when incubated with apoptotic blebs. These results suggest that apoptotic blebs (more so than cellular lysate) can induce the maturation of DCs derived from lupus‐prone mice, more so than of DCs derived from healthy mice.
Mouse monoclonal anti‐dsDNA antibodies, complexed with DNA, have been shown to induce the secretion of inflammatory cytokines from mononuclear cells 27. Immune complexes in serum stimulate the production of IFN‐α, IFN‐β and IL‐6 in an FcγR‐ and Toll‐like receptor (TLR)−7‐ dependent manner and up‐regulate co‐stimulatory molecules on mouse DCs 28. Immune complexes of lupus IgG with apoptotic cells 29 or with nucleic acids 30, 31, 32, 33 induce secretion of IFN‐α and/or IL‐6 from plasmacytoid DCs. U1‐snRNP containing immune complexes appear to activate the Nod‐like receptor (NLR)‐P3 inflammasome via TLR‐7/8 34. Few previous studies have compared the effects of autoantibodies/immune complexes on DCs derived from healthy and lupus‐prone mice; the current work provides some insights in this regard. Antibodies P2C2 and 1B3 induced significant up‐regulation of several phenotypical markers on CD11c+ BMDCs derived from lupus‐prone mice, and elicited the enhanced secretion of lupus‐associated inflammatory cytokines; effects on BALB/c‐derived BMDCs were much more modest. That said, the fact that the lupus antibodies also heightened the secretion of inflammatory cytokines from healthy DCs (to a much lower extent) was indicative of the contribution of both genotype and antibody specificity to a maximal inflammatory outcome. Studies investigating the binding of the antibodies to BMDCs added an additional perspective. The antibodies bound the surface of BMDCs derived from lupus‐prone and healthy mice to a significantly greater extent than did respective isotype control antibodies. As an excess of isotype control antibodies did not result in competitive inhibition, the possibility that autoimmune antibodies assume a distinct conformation (possibly upon complexation with autoantigen(s) released during the course of culture), thereby gaining the ability to bind putative novel cellular binding sites, can be considered.
Autoantibody‐mediated pathology continues to be an area of intense study in lupus, and the fact that disease onset coincides with the presence of multiple autoantibody specificities 2, and that specific autoantibodies have been associated with specific disease phenotypes 9, 35, 36, 37, 38, 39, make studies reported in this paper clinically relevant. To take just a few examples, the association of anti‐dsDNA antibodies with glomerulonephritis has been known for some time 9, and more recent studies have demonstrated the cross‐reactivity of such antibodies with NMDA receptors in the brain 35, elucidating their potential role in neurological dysfunction. Antibodies to Ro, linked to neonatal lupus 36, may contribute to congenital heart block by preventing the physiological clearance of apoptotic fetal cardiomyocytes 37. Anti‐phospholipid antibodies have been associated with thrombosis and early pregnancy loss 38, 39.
Although reports suggest that perturbation of the immune system by some self‐reactive immunoglobulins can result in immune pathology 7, 40, 41, the specific contributions of antibodies that recognize dying cells (comprising the earliest autoantibody responses in lupus) to antigenic diversification and lupus pathology have not been explored in any detail. In this study, hypergammaglobulinaemia was observed upon the isogeneic immunization with apoptotic cell‐reactive antibodies; elicited autoantibodies bound to distinct moieties. Elicited antibodies bound dsDNA, irrespective of whether the immunizing antibody bound dsDNA (antibody P2C2) or not (antibody 1B3). The aetiology of anti‐dsDNA antibodies has been a matter of some debate, with some studies suggesting a requirement for a break of T cell tolerance to histones 42. Data presented in the current work potentially implicates an alternate mechanism for the generation of an autoantibody specificity associated with the onset of glomerulonephritis. Antibody 1B3, although non‐reactive to Ro60, elicited the production of anti‐Ro60 antibodies upon immunization, again a finding of possible clinical significance, given the discussion above. Of interest was also the fact that only the phospholipid‐reactive antibody (antibody P2C2) generated antibodies that also recognized a wide spectrum of phospholipids upon immunization. Such a process of amplification could serve to precipitate the thrombotic events associated with antibodies of this specificity. This study further reveals that apoptotic cell‐directed humoral immune responses, in addition to inducing determinant spreading, also enhanced the kinetics of autoreactivity, culminating in early onset glomerulosclerosis.
Significantly, anti‐self responses and consequent pathological effects on the kidneys were not observed upon the immunization of healthy mice with an antibody that induced pathology in lupus‐prone mice. Further, substituting the adjuvant IFA with alum abolished the effects of antibody immunization in lupus‐prone mice (data not shown). These results lend support a ‘two‐hit’ mechanism (autoantigenic material alongside strong adjuvantic stimulation) for the generation of sustained autoimmune responses in a conducive milieu, while also demonstrating an absolute requirement for such a milieu. Evidence for such requirements also exists in the literature. Administration to lupus‐prone mice of DCs that have phagocytosed apoptotic cells induced persistent autoreactive responses, immunopathology and renal failure, as did apoptotic cells when injected along with the adjuvant IFA; control DCs, apoptotic cells or IFA injected alone did not induce similar effects 43. In contrast, administration of apoptotic cell‐pulsed DCs to healthy mice induced only transient autoreactive responses, without associated organ dysfunction 44. Cumulatively, these findings suggest that both apoptotic debris, as well as the some of the antibodies it stimulates, exhibit similar requirements for the breakage of immune tolerance.
It is perhaps significant that, even in lupus‐prone mice, all apoptotic cell‐reactive antibodies were unable to mediate diversification of autoreactive responses upon immunization. The fact that antigenic spreading was only observed upon immunization with antibodies that exhibited somatically mutated heavy chains in the present study may be pertinent, as somatic mutations arising within antibody variable regions may give rise to T cell epitopes against which tolerance may not exist 45. The possibility that this indeed occurs in the current model is strengthened by previous reports indicating a breakage of T cell tolerance to an increasing number of anti‐dsDNA antibody variable region epitopes as lupus‐prone mice age 46. Recent evidence suggests further that T cell specific to V region peptides on anti‐dsDNA antibodies can co‐operate with anti‐dsDNA‐specific B cells to perpetuate pathology 47. Molecular mimicry between variable region peptides derived from antibodies of differing specificities may then contribute to further diversification 46, 48. Induction of tolerance to such epitopes has been shown to provide prophylactic benefit 49, an indication of their relevance to autoimmune pathology. That said, the rules that govern apoptotic cell‐reactive antibody‐driven epitope spreading are far from clear; recent work from our laboratory suggests efficient amplification of autoreactive responses by non‐mutated, apoptotic cell‐reactive IgM antibodies 50, ascribing a new role for an antibody isotype and specificity considered traditionally to be contribute to immune homeostasis and tolerance. Additionally, the current work raises the possibility that the extent of inflammation an apoptotic cell‐reactive antibody induces may also have a bearing on downstream events.
The present study, in consonance with previous work 8, 51, 52, 53, suggests that immunological cross‐reactivity amongt lupus‐related antigens is quite common, a fact that adds an additional layer of complexity in attempts to delineate fully the phenomenon of autoantibody‐induced determinant spreading. It is apparent, however, that it is possibly not simply the mere presence of excessive apoptotic debris that triggers immunological aberrance in systemic autoimmunity; both the innate and adaptive immune systems in lupus‐prone mice appear uniquely sensitive towards such debris and towards the humoral immune responses it engenders. The influence lupus‐associated apoptotic cell‐reactive antibodies exert on the phagocytic clearance of dying cells 8, 53 can contribute further to self‐sustaining cascades of autoreactivity. A deeper appreciation of such processes may lead to newer therapies for this chronically debilitating disease.
Disclosures
The authors declare no financial/commercial interests.
Supporting information
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Figure 1: Reactivity of IgG antibodies in the sera of SLE patients and healthy controls towards apoptotic blebs derived from Jurkat cells. Lanes 1‐5: SLE IgG; Lane 6: Secondary antibody control; Lanes 7‐11: Normal human IgG.
Supplementary Figure 2: Reactivity of monoclonal antibodies generated from NZB/W F1 mice towards auto‐antigens. Left panels: Reactivity to proteinaceous antigens and dsDNA. Right panels: Reactivity to phospholipids (PA: Phosphatidic acid; LPC: Lysophosphatidylcholine; PC: Phosphatidylcholine; LPA: Lysophosphatidic acid; PS: Phosphatidylserine; PE: Phosphatidylethanolamine; CL: Cardiolipin). Experiments employed 5 mg/ml of (a) Antibody P2B2, (b) Antibody 1B3 and (c) Antibody P2C2. Data represents mean + SEM of four experiments.
Supplementary Figure 3: Immunoglobulin variable region segment analysis. (a) Closest variable region gene segments for heavy and light chains of Antibody P2B2, Antibody 1B3 and Antibody P2C2. “?” indicates that the D region of Antibody P2B2 could not be determined as the intervening sequence (GAGCGAAAAACGTCCGG) between the V and J regions could not be assigned to any known D region sequence in the NCBI IgBLAST database. (b) Ratio of Replacement Mutation Frequencies (replacement mutations per amino acid) in the Complementarity Determining Regions (CDR) over the Frame Work Regions (FWR) of the heavy and light chains of Antibody P2B2, Antibody 1B3 and Antibody P2C2.
Supplementary Figure 4: Binding and competition analysis of autoantibodies on CD11c+ BMDCs derived from lupus‐prone and healthy mice. (a) Flow cytometric analysis of the binding of Antibodies P2C2 and 1B3. The binding of relevant isotype control (I.C.) antibodies is also shown. *p < 0.05; **p < 0.01; ***p < 0.001. (b) Estimation of TNF‐a in culture supernatants of BMDCs upon incubation with Antibodies P2C2 or 1B3, or with relevant isotype control (I.C.) antibodies. The effects of pre‐incubation with a 50‐fold excess of the isotype control antibody (“I.C. block”) are also shown. *p < 0.05 vs medium; #@p < 0.05; ^p < 0.01; NS: Not Significant.
Supplementary Figure 5: Characterization of humoral anti‐self responses elicited upon immunization of Antibody P2B2 or IFA in NZB/W F1 mice. Eight week‐old NZB/W F1 mice were administered subcutaneous injections of 100 μg of the Antibody (or IFA), and anti‐sera analyzed for total immunoglobulin levels and anti‐self reactivity. (a) Total immunoglobulin isotypes (*p < 0.004, * p < 0.007). Bars represent mean ± SEM of four experiments. (b) Flow cytometric analysis of humoral anti‐self responses. Permeabilized CCL131 cells were used as targets. The filled gray profile represents the negative control where only the secondary antibody was employed. Pre‐immune sera and sera from animals immunized with IFA were included as controls, as indicated. In each case, sera pooled from individual animals (n = 6) were employed at a dilution of 1:500.
Supplementary Figure 6: Characterization of humoral anti‐self responses elicited upon immunization of Antibody P2C2 or IFA in BALB/c mice. Eight week‐old BALB/c mice were administered subcutaneous injections of 100 μg of the antibody (or IFA), and anti‐sera analyzed for anti‐self reactivity. (a) Flow cytometric analysis depicting reactivity of antibodies in pre‐immune sera, in sera generated upon Antibody P2C2 immunization and in sera generated upon IFA immunization towards permeabilized CCL131 cells. (b) Western blot analysis on CCL131 cellular lysate depicting reactivity of antibodies in pre‐immune sera (Lane 1), in sera generated upon Antibody P2C2 immunization (Lane 2) and in sera generated upon IFA immunization (Lane 3). Lane 4 indicates the secondary antibody control. Molecular weights are indicated in KDa. b‐actin was employed as the loading control.
Acknowledgements
P. A., M. M., R. S., L. S. and J. D. performed the experiments, V. G. and R. P. designed the study, P. A., M. M. and R. P. wrote the paper. This work was funded by core and extramural (BT/PR9417/Med/30/26/2007) grants to R. P. from the Department of Biotechnology, Government of India. P. A. received research fellowships from the Council of Scientific and Industrial Research, Government of India. M. M. received a research fellowship from the Department of Science and Technology, Government of India.
References
- 1. Yaniv G, Twig G, Shor DB et al A volcanic explosion of autoantibodies in systemic lupus erythematosus: a diversity of 180 different antibodies found in SLE patients. Autoimmun Rev 2015; 14:75–9. [DOI] [PubMed] [Google Scholar]
- 2. Arbuckle MR, McClain MT, Rubertone MV et al Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med 2003; 349:1526–33. [DOI] [PubMed] [Google Scholar]
- 3. Deshmukh US, Bagavant H, Lewis J, Gaskin F, Fu SM. Epitope spreading within lupus‐associated ribonucleoprotein antigens. Clin Immunol 2005; 117:112–20. [DOI] [PubMed] [Google Scholar]
- 4. Shao WH, Cohen PL. Disturbances of apoptotic cell clearance in systemic lupus erythematosus. Arthritis Res Ther 2011; 13:202.] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanayama R, Tanaka M, Miyasaka K et al Autoimmune disease and impaired uptake of apoptotic cells in MFG‐E8‐deficient mice. Science 2004; 304:1147–50. [DOI] [PubMed] [Google Scholar]
- 6. Crampton SP, Morawski PA, Bolland S. Linking susceptibility genes and pathogenesis mechanisms using mouse models of systemic lupus erythematosus. Dis Model Mech 2014; 7:1033–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mendlovic S, Brocke S, Shoenfeld Y et al Induction of a systemic lupus erythematosus‐like disease in mice by a common human anti‐DNA idiotype. Proc Natl Acad Sci USA 1988; 85:2260–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Das J, Arora P, Gracias D et al Endogenous humoral autoreactive immune responses to apoptotic cells: effects on phagocytic uptake, chemotactic migration and antigenic spread. Eur J Immunol 2008; 38:3561–74. [DOI] [PubMed] [Google Scholar]
- 9. Yung S, Chan TM. Anti‐DNA antibodies in the pathogenesis of lupus nephritis – the emerging mechanisms. Autoimmun Rev 2008; 7:317–21. [DOI] [PubMed] [Google Scholar]
- 10. Casciola‐Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med 1994; 179:1317–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pisetsky DS. Microparticles as autoantigens: making immune complexes big. Arthritis Rheum 2012; 64:958–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heinlen LD, McClain MT, Ritterhouse LL et al 60 kD Ro and nRNP A frequently initiate human lupus autoimmunity. PLOS ONE 2010; 5:e9599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Topfer F, Gordon T, McCluskey J. Intra‐ and intermolecular spreading of autoimmunity involving the nuclear self‐antigens La (SS‐B) and Ro (SS‐A). Proc Natl Acad Sci USA 1995; 92:875–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. James JA, Gross T, Scofield RH, Harley JB. Immunoglobulin epitope spreading and autoimmune disease after peptide immunization: Sm B/B'‐derived PPPGMRPP and PPPGIRGP induce spliceosome autoimmunity. J Exp Med 1995; 181:453–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Monneaux F, Muller S. Epitope spreading in systemic lupus erythematosus: identification of triggering peptide sequences. Arthritis Rheum 2002; 46:1430–8. [DOI] [PubMed] [Google Scholar]
- 16. Ding D, Mehta H, McCune WJ, Kaplan MJ. Aberrant phenotype and function of myeloid dendritic cells in systemic lupus erythematosus. J Immunol 2006; 177:5878–89. [DOI] [PubMed] [Google Scholar]
- 17. Ip WK, Lau YL. Distinct maturation of, but not migration between, human monocyte‐derived dendritic cells upon ingestion of apoptotic cells of early or late phases. J Immunol 2004; 173:189–96. [DOI] [PubMed] [Google Scholar]
- 18. Decker P, Singh‐Jasuja H, Haager S, Kötter I, Rammensee HG. Nucleosome, the main autoantigen in systemic lupus erythematosus, induces direct dendritic cell activation via a MyD88‐independent pathway: consequences on inflammation. J Immunol 2005; 174:3326–34. [DOI] [PubMed] [Google Scholar]
- 19. Martin DA, Elkon KB. Intracellular mammalian DNA stimulates myeloid dendritic cells to produce type I interferons predominantly through a Toll‐like receptor 9‐independent pathway. Arthritis Rheum 2006; 54:951–62. [DOI] [PubMed] [Google Scholar]
- 20. Kelly KM, Zhuang H, Nacionales DC et al ‘Endogenous adjuvant’ activity of the RNA components of lupus autoantigens Sm/RNP and Ro 60. Arthritis Rheum 2006; 54:1557–67. [DOI] [PubMed] [Google Scholar]
- 21. Fransen JH, Hilbrands LB, Ruben J et al Mouse dendritic cells matured by ingestion of apoptotic blebs induce T cells to produce interleukin‐17. Arthritis Rheum 2009; 60:2304–13. [DOI] [PubMed] [Google Scholar]
- 22. Dieker J, Tel J, Pieterse E et al Circulating apoptotic microparticles in SLE patients drive the activation of DC subsets and prime neutrophils for NETosis. Arthritis Rheumatol 2016; 68:462–72. [DOI] [PubMed] [Google Scholar]
- 23. Dieker J, Hilbrands L, Thielen A, Dijkman H, Berden JH, van der Vlag J. Enhanced activation of dendritic cells by autologous apoptotic microvesicles in MRL/lpr mice. Arthritis Res Ther 2015; 17:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Fehr EM, Spoerl S, Heyder P et al Apoptotic‐cell‐derived membrane vesicles induce an alternative maturation of human dendritic cells which is disturbed in SLE. J Autoimmun 2013; 40:86–95. [DOI] [PubMed] [Google Scholar]
- 25. Verbovetski I, Bychkov H, Trahtemberg U et al Opsonization of apoptotic cells by autologous iC3b facilitates clearance by immature dendritic cells, down‐regulates DR and CD86, and up‐regulates CC chemokine receptor 7. J Exp Med 2002; 196:1553–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berkun Y, Verbovetski I, Ben‐Ami A et al Altered dendritic cells with tolerizing phenotype in patients with systemic lupus erythematosus. Eur J Immunol 2008; 38:2896–904. [DOI] [PubMed] [Google Scholar]
- 27. Vallin H, Perers A, Alm GV, Rönnblom L. Anti‐double‐stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN‐alpha inducer in systemic lupus erythematosus. J Immunol 1999; 163:6306–13. [PubMed] [Google Scholar]
- 28. Yasuda K, Richez C, Maciaszek JW et al Murine dendritic cell type I IFN production induced by human IgG‐RNA immune complexes is IFN regulatory factor (IRF)5 and IRF7 dependent and is required for IL‐6 production. J Immunol 2007; 178:6876–85. [DOI] [PubMed] [Google Scholar]
- 29. Båve U, Magnusson M, Eloranta ML, Perers A, Alm GV, Rönnblom L. Fc gamma RIIa is expressed on natural IFN‐alpha‐producing cells (plasmacytoid dendritic cells) and is required for the IFN‐alpha production induced by apoptotic cells combined with lupus IgG. J Immunol 2003; 171:3296–302. [DOI] [PubMed] [Google Scholar]
- 30. Means TK, Latz E, Hayashi F, Murali MR, Golenbock DT, Luster AD. Human lupus autoantibody‐DNA complexes activate DCs through cooperation of CD32 and TLR9. J Clin Invest 2005; 115:407–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Savarese E, Chae OW, Trowitzsch S et al U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood 2006; 107:3229–34. [DOI] [PubMed] [Google Scholar]
- 32. Lövgren T, Eloranta ML, Kastner B, Wahren‐Herlenius M, Alm GV, Rönnblom L. Induction of interferon‐alpha by immune complexes or liposomes containing systemic lupus erythematosus autoantigen‐ and Sjogren's syndrome autoantigen‐associated RNA. Arthritis Rheum 2006; 54:1917–27. [DOI] [PubMed] [Google Scholar]
- 33. Yasuda K, Richez C, Uccellini MB et al Requirement for DNA CpG content in TLR9‐dependent dendritic cell activation induced by DNA‐containing immune complexes. J Immunol 2009; 183:3109–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Shin MS, Kang Y, Lee N et al U1‐small nuclear ribonucleoprotein activates the NLRP3 inflammasome in human monocytes. J Immunol 2012; 188:4769–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kowal C, Degiorgio LA, Lee JY et al Human lupus autoantibodies against NMDA receptors mediate cognitive impairment. Proc Natl Acad Sci USA 2006; 103:19854–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Brucato A, Cimaz R, Caporali R, Ramoni V, Buyon J. Pregnancy outcomes in patients with autoimmune diseases and anti‐Ro/SSA antibodies. Clin Rev Allergy Immunol 2011; 40:27–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Clancy RM, Neufing PJ, Zheng P et al Impaired clearance of apoptotic cardiocytes is linked to anti‐SSA/Ro and ‐SSB/La antibodies in the pathogenesis of congenital heart block. J Clin Invest 2006; 116:2413–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Meroni PL, Borghi MO, Raschi E, Tedesco F. Pathogenesis of antiphospholipid syndrome: understanding the antibodies. Nat Rev Rheumatol 2011; 7:330–9. [DOI] [PubMed] [Google Scholar]
- 39. Ruiz‐Irastorza G, Crowther M, Branch W, Khamashta MA. Antiphospholipid syndrome. Lancet 2010; 376:1498–509. [DOI] [PubMed] [Google Scholar]
- 40. Shoenfeld Y. Idiotypic induction of autoimmunity: a new aspect of the idiotypic network. FASEB 1994; J8:1296–301. [DOI] [PubMed] [Google Scholar]
- 41. Krause I, Blank M, Levi Y, Koike T, Barak V, Shoenfeld Y. Anti‐idiotype immunomodulation of experimental anti‐phospholipid syndrome via effect on Th1/Th2 expression. Clin Exp Immunol 1999; 117:190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu L, Kaliyaperumal A, Boumpas DT, Datta SK. Major peptide autoepitopes for nucleosome‐specific T cells of human lupus. J Clin Invest 1999; 104:345–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Bondanza A, Zimmermann VS, Del'Antonio G et al Requirement of dying cells and environmental adjuvants for the induction of autoimmunity. Arthritis Rheum 2004; 50:1549–60. [DOI] [PubMed] [Google Scholar]
- 44. Bondanza A, Zimmermann VS, Dell'Antonio G et al Cutting edge: dissociation between autoimmune response and clinical disease after vaccination with dendritic cells. J Immunol 2003; 170:24–7. [DOI] [PubMed] [Google Scholar]
- 45. Wysocki LJ, Zhang X, Smith DS, Snyder CM, Bonorino C. Somatic origin of T‐cell epitopes within antibody variable regions: significance to monoclonal therapy and genesis of systemic autoimmune disease. Immunol Rev 1998; 162:233–46. [DOI] [PubMed] [Google Scholar]
- 46. Singh RR, Hahn BH, Tsao BP, Ebling FM. Evidence for multiple mechanisms of polyclonal T cell activation in murine lupus. J Clin Invest 1998; 102:1841–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Aas‐Hanssen K, Funderud A, Thompson KM, Bogen B, Munthe LA. Idiotype‐specific Th cells support oligoclonal expansion of anti‐dsDNA B cells in mice with lupus. J Immunol 2014; 193:2691–8. [DOI] [PubMed] [Google Scholar]
- 48. Singh RR. Prevention and control of reciprocal T‐B cell diversification: implications for lupus‐like autoimmunity. Mol Immunol 2004; 40:1137–45. [DOI] [PubMed] [Google Scholar]
- 49. Eilat E, Zinger H, Nyska A, Mozes E. Prevention of systemic lupus erythematosus‐like disease in (NZBxNZW) F1 mice by treating with CDR1‐ and CDR3‐based peptides of a pathogenic autoantibody. J Clin Immunol 2000; 20:268–78. [DOI] [PubMed] [Google Scholar]
- 50. Malik M, Arora P, Sachdeva R, Sharma L, Ramachandran VG, Pal R. Elucidation of the potential disease‐promoting influence of IgM apoptotic cell‐reactive antibodies in lupus. Lupus 2016; 25:684–98. [DOI] [PubMed] [Google Scholar]
- 51. Deshmukh US, Kannapell CC, Fu SM. Immune responses to small nuclear ribonucleoproteins: antigen‐dependent distinct B cell epitope spreading patterns in mice immunized with recombinant polypeptides of small nuclear ribonucleoproteins. J Immunol 2002; 168:5326–32. [DOI] [PubMed] [Google Scholar]
- 52. Pal R, Deshmukh US, Ohyama Y et al Evidence for multiple shared antigenic determinants within Ro60 and other lupus‐related ribonucleoprotein autoantigens in human autoimmune responses. J Immunol 2005; 175:7669–77. [DOI] [PubMed] [Google Scholar]
- 53. Gandhi R, Hussain E, Das J, Handa R, Pal R. Anti‐idiotype‐mediated epitope spreading and diminished phagocytosis by a human monoclonal antibody recognizing late‐stage apoptotic cells. Cell Death Differ 2006; 13:1715–26. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional supporting information may be found in the online version of this article at the publisher's web‐site.
Supplementary Figure 1: Reactivity of IgG antibodies in the sera of SLE patients and healthy controls towards apoptotic blebs derived from Jurkat cells. Lanes 1‐5: SLE IgG; Lane 6: Secondary antibody control; Lanes 7‐11: Normal human IgG.
Supplementary Figure 2: Reactivity of monoclonal antibodies generated from NZB/W F1 mice towards auto‐antigens. Left panels: Reactivity to proteinaceous antigens and dsDNA. Right panels: Reactivity to phospholipids (PA: Phosphatidic acid; LPC: Lysophosphatidylcholine; PC: Phosphatidylcholine; LPA: Lysophosphatidic acid; PS: Phosphatidylserine; PE: Phosphatidylethanolamine; CL: Cardiolipin). Experiments employed 5 mg/ml of (a) Antibody P2B2, (b) Antibody 1B3 and (c) Antibody P2C2. Data represents mean + SEM of four experiments.
Supplementary Figure 3: Immunoglobulin variable region segment analysis. (a) Closest variable region gene segments for heavy and light chains of Antibody P2B2, Antibody 1B3 and Antibody P2C2. “?” indicates that the D region of Antibody P2B2 could not be determined as the intervening sequence (GAGCGAAAAACGTCCGG) between the V and J regions could not be assigned to any known D region sequence in the NCBI IgBLAST database. (b) Ratio of Replacement Mutation Frequencies (replacement mutations per amino acid) in the Complementarity Determining Regions (CDR) over the Frame Work Regions (FWR) of the heavy and light chains of Antibody P2B2, Antibody 1B3 and Antibody P2C2.
Supplementary Figure 4: Binding and competition analysis of autoantibodies on CD11c+ BMDCs derived from lupus‐prone and healthy mice. (a) Flow cytometric analysis of the binding of Antibodies P2C2 and 1B3. The binding of relevant isotype control (I.C.) antibodies is also shown. *p < 0.05; **p < 0.01; ***p < 0.001. (b) Estimation of TNF‐a in culture supernatants of BMDCs upon incubation with Antibodies P2C2 or 1B3, or with relevant isotype control (I.C.) antibodies. The effects of pre‐incubation with a 50‐fold excess of the isotype control antibody (“I.C. block”) are also shown. *p < 0.05 vs medium; #@p < 0.05; ^p < 0.01; NS: Not Significant.
Supplementary Figure 5: Characterization of humoral anti‐self responses elicited upon immunization of Antibody P2B2 or IFA in NZB/W F1 mice. Eight week‐old NZB/W F1 mice were administered subcutaneous injections of 100 μg of the Antibody (or IFA), and anti‐sera analyzed for total immunoglobulin levels and anti‐self reactivity. (a) Total immunoglobulin isotypes (*p < 0.004, * p < 0.007). Bars represent mean ± SEM of four experiments. (b) Flow cytometric analysis of humoral anti‐self responses. Permeabilized CCL131 cells were used as targets. The filled gray profile represents the negative control where only the secondary antibody was employed. Pre‐immune sera and sera from animals immunized with IFA were included as controls, as indicated. In each case, sera pooled from individual animals (n = 6) were employed at a dilution of 1:500.
Supplementary Figure 6: Characterization of humoral anti‐self responses elicited upon immunization of Antibody P2C2 or IFA in BALB/c mice. Eight week‐old BALB/c mice were administered subcutaneous injections of 100 μg of the antibody (or IFA), and anti‐sera analyzed for anti‐self reactivity. (a) Flow cytometric analysis depicting reactivity of antibodies in pre‐immune sera, in sera generated upon Antibody P2C2 immunization and in sera generated upon IFA immunization towards permeabilized CCL131 cells. (b) Western blot analysis on CCL131 cellular lysate depicting reactivity of antibodies in pre‐immune sera (Lane 1), in sera generated upon Antibody P2C2 immunization (Lane 2) and in sera generated upon IFA immunization (Lane 3). Lane 4 indicates the secondary antibody control. Molecular weights are indicated in KDa. b‐actin was employed as the loading control.


