Summary
The current view of type 1 diabetes (T1D) is that it is an immune‐mediated disease where lymphocytes infiltrate the pancreatic islets, promote killing of beta cells and cause overt diabetes. Although tissue resident immune cells have been demonstrated in several organs, the composition of lymphocytes in human healthy pancreatic islets have been scarcely studied. Here we aimed to investigate the phenotype of immune cells associated with human islets of non‐diabetic organ donors. A flow cytometry analysis of isolated islets from perfused pancreases (n = 38) was employed to identify alpha, beta, T, natural killer (NK) and B cells. Moreover, the expression of insulin and glucagon transcripts was evaluated by RNA sequencing. Up to 80% of the lymphocytes were CD3+ T cells with a remarkable bias towards CD8+ cells. Central memory and effector memory phenotypes dominated within the CD8+ and CD4+ T cells and most CD8+ T cells were positive for CD69 and up to 50–70% for CD103, both markers of resident memory cells. The frequency of B and NK cells was low in most islet preparations (12 and 3% of CD45+ cells, respectively), and the frequency of alpha and beta cells varied between donors and correlated clearly with insulin and glucagon mRNA expression. In conclusion, we demonstrated the predominance of canonical tissue resident memory CD8+ T cells associated with human islets. We believe that these results are important to understand more clearly the immunobiology of human islets and the disease‐related phenotypes observed in diabetes.
Keywords: diabetes, human, memory, pancreas, T cells
Introduction
Type 1 diabetes (T1D) is generally considered a cell‐mediated autoimmune disease, which results in pancreatic beta cell loss/dysfunction and leads to insulin deficiency 1. In humans the immunological mechanisms behind the disease have not yet been understood fully. Insulitis has been indicated as a hallmark of new‐onset T1D and is defined as inflammatory lesions of the islets of Langerhans, with lymphocytes being the predominant population of cells, directly surrounding the islets (peri‐insulitis) or throughout the islets parenchyma (intra‐insulitis) 2. The current consensus for markers of insulitis is considered an immunohistochemical positivity for no fewer than 15 CD45+ cells within the islets 2. Due to the paucity of clear pathological inflammatory lesions found in the islets of several T1D patients, the histopathology of the disease has been challenged recently 3, 4, 5, 6.
Based on histological examination, the dominant type of lymphocytes in most cases of human T1D were CD8+ T cells and B cells at the more advanced stage of insulitis 3, 7, 8. Histopathology of the pancreas in autoantibody‐positive (aAb+) non‐diabetic individuals has also been investigated, showing the presence of T cells in non‐diabetic pancreases and no clear differences in comparison to aab‐negative (aAb–) subjects 9, 10, 11. Intriguingly, an increase of CD45+ cells in the pancreatic exocrine tissue has been observed in aAb+ non‐diabetic organ donors 11. In line with these findings, another study confirmed the rarity of insulitis in aAb+ non‐diabetic subjects and found only two cases with insulitis in the pancreas of 62 aAb+ and none in 62 control subjects 12.
Although immunohistological assessment allows deeper understanding of the pancreas as an organ and defines the tissue‐specific cell interactions, it poses many limitations for a more comprehensive characterization of the composition and phenotype of immune cells in healthy and diabetic subjects. More recently, Butcher et al. used flow cytometry to characterize leucocytes present in human islet preparation of organ donors with and without type 2 diabetes (T2D) and demonstrated the presence of T and B lymphocytes in all donors and a higher frequency of CD45+ cells in T2D islets 13. However, the differentiation profile of pancreatic islet‐associated T cells has been investigated poorly in humans.
Two main subsets of memory T cells, named central memory (Tcm) and effector memory (Tem) cells, have been defined by their phenotype and function 14. Studies on parenchymal lymphocytes performed on mice ex vivo have revealed a new subset of memory T cells, which are localized stably within the organ 15, 16. These T cells, which are usually termed canonical resident memory T cells (Trm), are able to migrate into several non‐lymphoid tissues and differentiate into organ‐specific non‐recirculating memory T cells by maintaining stable expression of CD103 (αE integrin) and CD69 17, 18, 19, 20, 21, 22.
Studies in mice suggest an important role for Trm in protective immunity against tissue‐specific pathogens 17, 22 but, in contrast to some studies suggesting that recruitment and maintenance of Trm in the tissue is an antigen‐dependent process 23, it has been demonstrated that Trm can also be generated in the absence of antigens, and it seems that signals from the local microenvironment are crucial for their differentiation and/or survival 24, 25. The specific role of Trm in protective responses is not understood fully, but memory CD8+ T cells positive for CD103 have been described to participate in the regulation of murine ileitis 15, indicating a possible role in inflammatory responses. While tissue CD8+CD122+ T cells with phenotypical markers related to central memory have been described as regulatory cells in mice, the human counterpart has not yet been identified 15.
In this study we have investigated the frequency and phenotype of resident lymphocytes present in human pancreatic islet preparation of 38 non‐diabetic aAb‐islet donors. Our main finding is the presence of CD4+ and CD8+ T cells with memory and effector phenotype, representing the major subset of lymphoid cells in pancreatic islets.
Material and methods
Human pancreatic islets
Human pancreatic islets of 38 non‐diabetic brain‐dead organ donors (15 female and 23 male) were obtained from The Nordic Network for Islet Transplantation, Uppsala University, through the Human Tissue Laboratory at Lund University Diabetes Center, Malmö, Sweden. Islets were isolated as described previously 26. Briefly, a clamp was used to compress the pancreatic duct at the head of the pancreas, and the tissue adjacent to the clamp was taken as a biopsy and stored in formalin for later immunohistochemistry studies. Quality tests were performed on homogenized isolated islets using the Gyrolab workstation (Gyros, Uppsala, Sweden) and purity was determined by dithizone staining 27. The average donor age was 59 ± 11·27 years, and the body mass index (BMI) averaged 26·4 ± 4·12 kg/m2 (Table 1). Due to ethical reasons, we were not able to retrieve any information concerning the cause of death and the time or the treatment organ donors received in the intensive care unit (ICU) before the organs were explanted.
Table 1.
Demographic data of islets donors included in the study; BMI = body mass index
| No. of donors | 38 |
| Age (years) | 59 ± 11·27 |
| Males | 23 |
| Females | 15 |
| BMI | 26·4 ± 4·12 |
| HbA1c% | 5·92 ± 0·43 |
The Regional Ethics Committee in Lund, Sweden approved the study according to the Act Concerning the Ethical Review of Research Involving Humans. Subjects were considered for inclusion if consent to donate organs to research was obtained by the donor's physician from the potential donor or from the relatives of the deceased donor.
Measurement of diabetes‐associated autoantibodies
Autoantibodies against diabetes‐associated antigens, glutamic acid decarboxylase (GAD)65 and islet antigen (IA)−2A, were measured in the serum of all islets donors using enzyme‐linked immunosorbent assay (ELISA) (Elisa GADAb and IA‐2Ab; RSR Limited, Cardiff, UK). GADA levels exceeding 5 IU and IA‐2A levels exceeding 8 IU were considered positive, in line with clinical practice in Sweden. None of the donors resulted positive.
Analysis of pancreatic islet cells
The islets were cultured in CMRL1066 (ICN Biomedicals, Irvine, CA, USA) supplemented with 10 mM HEPES, 2 mM L‐glutamine, 50 µg/ml gentamicin, 0·25 µg/ml Fungizone (Gibco, Carlsbad, CA, USA), 20 µg/ml ciprofloxacin (Bayer Healthcare, Berlin, Germany) and 10 mM nicotinamide at 37°C (5% CO2) prior to RNA and single‐cell suspension preparation.
Flow cytometric analysis of human dispersed islets
Between 1500 and 10 000 islet equivalent (IEQ) human islets were dissociated to single‐cell suspension using Accutase (Becton Dickinson, Franklin Lakes, NJ, USA) (between 8 × 104 and 3 × 106 total cells) dissociated islet cells were maintained during the analysis process in CMRL 1066. Flow cytometric analysis was performed according to standard procedures and antibodies used were: CD3 fluorescein isothiocyanate (FITC) [mouse anti‐human immunoglobulin (Ig)G1,k clone UCHT1], CD56 phycoerythrin‐cyanin 7 (PE‐Cy7) (mouse anti‐human IgG1,k clone B159), CD19 allophycocyanin (APC) mouse anti‐human IgG1,k clone SJ25C1 (BD Biosciences, San Jose, CA, USA), CD8 APC‐Cy7 mouse anti‐human IgG1,k clone SK1 (BD Pharmingen, San Diego, CA, USA), CD4 PacBlue mouse anti‐human IgG1,k clone RPA‐T4 (BD Pharmingen), CD25 FITC mouse anti‐human, IgG1,k clone M‐A251 (BD Pharmingen), CD45RO PE‐Cy7, mouse anti‐human IgG2a clone UCHL‐1 (BD Biosciences), CD69 mouse anti‐human IgG1,k clone FN50 (eBioscience, San Diego, CA, USA), CD103 PE‐Cy7 mouse anti‐human IgG1,k clone Ber‐ACT8 (Biolegend, San Diego, CA, USA), CD27 APC mouse anti‐human IgG1,k clone O323 (eBioscience), CD16 PE mouse anti‐human IgG2a clone 5D2 (Immuno Tools, Friesoythe, Germany), CD45 peridinin chlorophyll (PerCP) mouse anti‐human IgG1,k clone HI30 (Biolegend), flow cytometry intracellular analysis of insulin and glucagon content in the islet cells were performed using insulin rabbit anti‐human IgG (Cell Signaling Technology, Danvers, MA, USA) and glucagon mouse anti‐human, IgG2a clone 181402 (R&D Systems, Minneapolis, MN, USA) conjugated with PE and APC, respectively, using Lightning‐Link technology (Innova Bioscience, Cambridge, UK). All antibodies were tested previously for appropriate dilution and for specificity using isotype control antibodies. Flow cytometry data were acquired on a CyAN ADP (Beckman Coulter, Brea, CA, USA) and analysed using FlowJo software version 7.6.5 (TreeStar, Ashland, OR, USA).
Electronic compensation of fluorescence spillover was performed using the fluorescence‐minus‐one (FMO) approach for each antibody panel 28.
Immunohistochemistry
Sections of 6 µm formalin‐fixed and paraffin‐embedded pancreatic biopsies were processed and labelled using a standard immunoperoxidase technique for paraffin sections. A mouse monoclonal antibody (clone C8‐144B; Dako, Glostrup, Denmark) was used at 1 : 2000 dilution to label CD8+ cells according to the manufacturer's instructions and detection was performed using the EnVision1 system‐horseradish peroxidase (HRP) and alkaline phosphatase anti‐alkaline phosphatase (APAAP). All sections were counterstained with Mayer's haematoxylin (HistoLab, Gothenburg, Sweden). Human spleen was used as positive control, as described previously 11. Sections were visualized using an Olympus X70 microscope and contrast phase images were taken using U‐PHOTO Universal Photo System (Olympus Corporation, Center Valley, PA, USA).
RNA sequencing
Total RNA was extracted using the Qiagen AllPrep DNA/RNA kit (Qiagen, Valencia, CA, USA) from nine islet donors and RNA quality and concentration were measured using an Agilent 2100 bioanalyser (Bio‐Rad, Hercules, CA, USA) and a Nanodrop ND‐1000 (NanoDrop Technologies, Wilmington, DE, USA). RNA sequencing and analysis of gene expression was performed as described previously 29. Briefly, RNA‐seq libraries were generated using the TruSeq RNA sample preparation kit (Illumina, San Diego, CA, USA) and sequenced on an Illumina HiSeq 2000 using paired‐end chemistry and 100‐base pairs (bp) cycles to an average depth of 32 M read pairs/sample. Reads were aligned to the reference transcriptome (hg19) with Bowtie2 (http://www.genomebiology.com/2009/10/3/R25,http://www.nature.com/nmeth/journal/v9/n4/full/nmeth.1923.html) and gene expression was estimated as fragments per kilobase of transcript per million mapped reads (FPKM) using Rsem software (http://www.ncbi.nlm.nih.gov/pubmed/21816040).
Statistical analysis
Statistical analysis was performed using Prism version 6 (Graphpad Software, San Diego, CA, USA) and spss version 20 for Windows (SPSS, Inc., Chicago, IL, USA). Linear regression analysis was used to analyse an impact of purity as covariate on P‐values. Correlations were analysed using the parametric Pearson's correlation test and the non‐parametric Spearman's correlation test. A P‐value of less than 0·05 was considered significant. All the data are presented as mean ± standard deviation (s.d.).
Results
Lymphoid cell composition in pancreatic islets
To examine the lymphoid composition of human pancreatic islets, we analysed the frequencies and phenotypes of CD45+ cells present in dispersed pancreatic islets from 38 non‐diabetic and diabetes‐associated aAb‐(GADA and IA‐2A) human organ donors by multi‐colour flow cytometry. CD3+ T cells were found to be the major lymphocyte population in human pancreatic islets of non‐diabetic donors (Fig. 1a). The frequency of CD3+ T cells among the lymphocyte population was consistent between all donors (79 ± 23·14%). The frequency of the total NK cell population (CD3–CD16+CD56+) was very low for all donors (3·16 ± 3·89%) (Fig. 1b). Because we used anti‐CD56 and anti‐CD16 conjugated to the same fluorochrome and analysed on the same flow cytometry channel, we were not able to identify the two major NK cell subpopulations CD56brightCD16dim/− and CD56dimCD16+. However, in a pilot experiment performed in few donors from whom we received a larger number of islets, we separated the analysis of CD56 and CD16 and could not observe a measurable CD3–CD56bright cell population, and the frequency of the CD3–CD56–CD16+ population was extremely low, if not absent (data not shown). The frequency of B cells, defined as CD3–CD19+, was generally low (12·50 ± 22·23%), except for seven donors where the percentage of B cells was > 20% (Fig. 1b).
Figure 1.
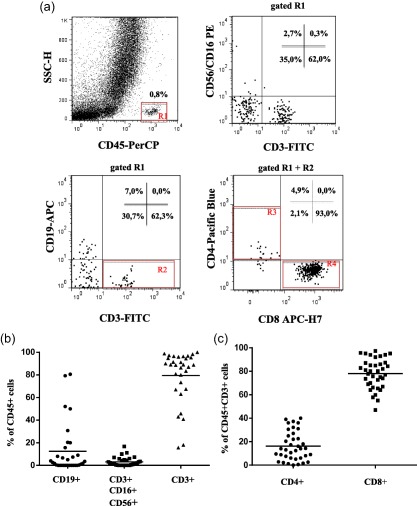
Ex‐vivo flow cytometry analysis of B, natural killer (NK) and T lymphocytes in dissociated pancreatic islets of non‐diabetic donors. (a) Representative flow cytometry dot plots showing the gating strategy used for the identification of total lymphocyte (CD45+), total T cells (CD3+), NK cells (CD3–CD56+CD16+), B cells (CD19+) as well as CD4+ and CD8+ T cells in dissociated human pancreatic islets (b) Cumulative analysis of CD4+ and (c) CD8+ T cells within the T cell compartment in the dissociated pancreatic islets from 38 organ donors. The data are shown as the mean percentage and standard deviation. [Colour figure can be viewed at wileyonlinelibrary.com]
Within the T cell population, CD8+ T cells were dominant in most of the donors (78·05 ±12·79%) compared to the lower frequencies of CD4+ T cells (16·20 ± 12·34%), as shown in Fig. 1c. The expression of CD25 on the surface of CD4+ and CD8+ T cells was very low, if not undetectable, and a cumulative and statistical analysis could not be performed. An example of flow cytometry analysis from a representative donor showing detectable CD4+CD25+ T cells is shown in Supporting information, Fig. S1.
Central memory and effector memory T cells are enriched in the pancreatic islets
In order to understand the differentiation stage of T cells present in the islets of non‐diabetic donors, we used markers to distinguish the T cell subset of the memory compartment. We defined the different stages of memory differentiation based on the expression of CD45RO and CD27 (Fig. 2). The majority of CD4+ and CD8+ T cells displayed a central memory (Tcm, CD45RO+CD27+) and effector memory (Tem, CD45RO+CD27–) phenotype, while terminal effector (Tte, CD45RO–CD27–) and naive T cells (Tn, CD45RO–CD27+) were generally low (Fig. 2a). Of the CD4+ T cells, 57 ± 28·71% were Tcm, 28·80 ±27·90% Tem, 11·18 ± 15·21% Tn and 3·32 ± 6·74% Tte (Fig. 2b). The CD8+ T cell compartment consisted of 52·96 ± 25·72% Tcm, 30·87 ± 25·42% Tem, 10·32 ± 16·11% Tn and 5·26 ± 7·18% Tte (Fig. 2c).
Figure 2.
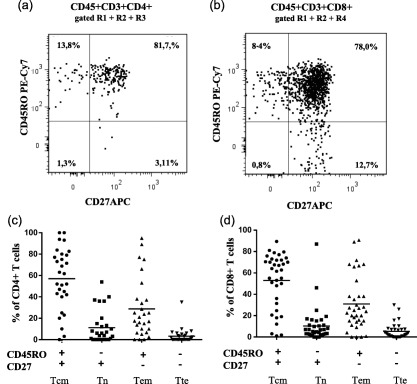
Naive and memory CD4+ and CD8+ T cell subpopulations in dissociated pancreatic islets of non‐diabetic donors. Representative flow cytometry dot‐plots showing naive and memory subpopulations of (a) CD4+ and (b) CD8+ T cells from one representative organ donor. (c) Cumulative analysis of naive and memory subpopulation of CD4+ (n = 29) and D) CD8+ T cells (n = 35) cells from non‐diabetic donors. The data are shown as the mean percentage and standard deviation. Tcm = central memory T cells, Tem = effector memory T cells, Tte = terminal effector T cells, Tn = naive T cells.
Immunohistochemistry analysis of CD8+ T cells
In order to verify the presence and localization of CD8+ T cells within and around the islets, we performed immunohistochemistry analysis of paraffin‐embedded pancreas specimens from the biopsies of the organ before islets isolation. The presence of CD8+ T cells could be observed in the exocrine tissue and surrounding the islets and, in some cases, also within the islets (Fig. 3a).
Figure 3.
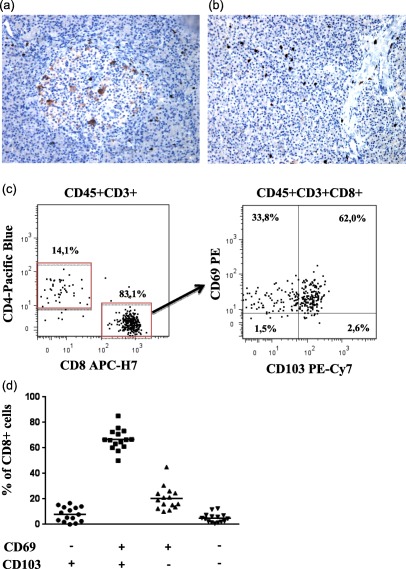
Characterization of resident CD8+ T cells in dissociated pancreatic islets of non‐diabetic donors. (a) Immunohistochemical analysis of CD8+ T cells in pancreatic biopsy of a representative donor depicting the presence of CD8+ T cells within and in close proximity of one islet and in the surrounding exocrine tissue and (b) several CD8+ T cells spread within the exocrine tissue. Both images were taken with ×20 magnification. (c) Representative flow cytometric dot‐plots showing the expression of CD69 and CD103 on CD8+ T cells in islets cell suspensions of one representative organ donor. (d) Cumulative analysis of CD4+ and CD8+ T cells expressing the CD69 and CD103 from 15 organ donors. The data are shown as the mean percentage and standard deviation. [Colour figure can be viewed at wileyonlinelibrary.com]
CD8+ T cells have phenotypical characteristics of canonical memory tissue resident T cells
To understand more clearly the nature of islets‐associated memory CD8+ T cells, we investigated whether these cells expressed markers of tissue‐specific resident memory cells, CD69 and CD103. We analysed an additional 15 non‐diabetic donors and demonstrated that most CD8+ T cells were CD69+CD103+ (66·62 ± 11·48%), while CD69+CD103– accounted for 20·42 ± 10·48%. Conversely, CD8+CD6–CD103– (4·96 ± 0·63%) and CD69–CD103+ (3·23 ± 3·19%) were detected at very low frequencies (Fig. 3b,c).
Insulin and glucagon expression in human pancreatic islets
In order to verify and validate the flow cytometry assessment of alpha and beta cells, we correlated the percentage of insulin‐ and glucagon‐positive cells analysed by flow cytometry (Fig. 4a,b) with the mRNA expression of insulin and glucagon obtained through RNA sequencing (RNAseq) of 19 islet preparations. We found a positive correlation for insulin (r = 0·706; P = 0·04) and glucagon (r = 0·904; P = 0·02) between the two different methods analysing the protein and mRNA levels, validating the assessment of beta and alpha cell proportion by flow cytometry (Fig. 4c,d).
Figure 4.
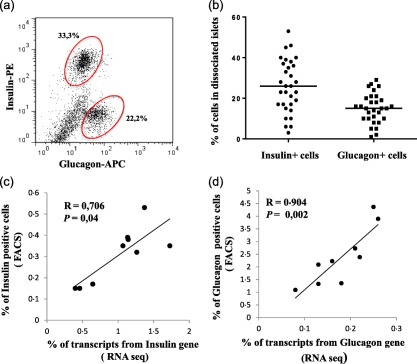
Percentages of pancreatic insulin and glucagon positive cell populations of non‐diabetic donors and their correlation with RNA expression for insulin and glucagon. (a) Representative flow cytometry analysis of islets cell suspension depicting the percentage of insulin and glucagon positive cells and (b) cumulative analysis of 31 non‐diabetic donors are shown as the mean percentage and standard deviation. Spearman's correlation test between (c) insulin and (d) glucagon gene expression level determined by RNA sequencing in nine non‐diabetic donors and the percentage of insulin and glucagon cells assessed by flow cytometry. [Colour figure can be viewed at wileyonlinelibrary.com]
Correlation between the frequency of insulin‐ and glucagon‐positive cells and different lymphocyte populations
Next, we wanted to evaluate whether there was a statistical correlation between the frequency and phenotype of pancreatic resident lymphocytes and the frequency of alpha and beta cells in isolated islets. We therefore performed a linear regression analysis with purity as covariate and found that purity had no impact upon P‐values. Naive CD4+ T cells correlated negatively with alpha cells (P = 0·02; r = −0·469) and frequency of CD4+ Tte showed a negative correlation with insulin‐positive cells (P = 0·05; r = −0·387). Within the CD8+ T cell population, CD8+ Tcm correlated negatively with glucagon‐positive cells (P = 0·015; r = −0·435), whereas Tem correlated positively with both glucagon‐ and insulin‐positive cells (P = 0·0084; r = 0·469 and P = 0·049; r = 0·360, respectively) (Supporting information, Table S1).
Discussion
The nature and phenotype of immune cells associated with pancreatic islets of non‐diabetic subjects still remains largely uncharacterized. In this study, we performed a flow cytometry analysis of dissociated human pancreatic islets, obtained from perfused pancreases of living organ donors, in order to evaluate the presence, phenotype and frequency of immune cells (T, B and NK cells). We found that the vast majority of lymphoid cells in these islets were T cells, with a clear predominance of CD8+ versus CD4+ T cells. We continued our analysis and interrogated these T cells regarding their memory differentiation status. First, we found that the majority of CD4+ and CD8+ T cells have a pronounced central memory phenotype (CD45RO+CD27+) and, interestingly, CD8+ T cells comprised the majority of the lymphocyte population compared to a low proportion of CD4+ T cells, NK and B cells. Using immunohistochemistry, we confirmed the presence of CD8+ T cells in the pancreatic tissue surrounding the islets and most importantly also within the islets (Fig. 2a), as also observed previously 11. The predominance of memory CD8+ T cells within the islets‐associated lymphoid cells in non‐diabetic organ donors raises several questions, such as the origin of these cells, what is their function in the pancreatic islets, their antigen specificities and eventually their role in the development of T1D and T2D. Interestingly, several studies have shown a higher frequency of CD8+ T cells in the inflammatory lesions in T1D patients, and autoreactive CD8+ T cells have been visualized in the islets 8, 30, 31.
A more recent study has characterized immune cells present in pancreatic islets of non‐diabetic and T2D donors using flow cytometry, and similarly to our results have also found the presence of T cells and B cells in the islets of non‐diabetic donors 13. However, our study is the first to show a deeper phenotypical characterization of islet‐associated T cells using markers for memory compartment (CD45RO and CD27), as well as for canonical tissue resident T cells (CD69 and CD103).
When T cells become activated, they change their pattern of migration 14. By definition, Tcm migrate between the spleen, blood and lymph nodes while Tem cells migrate between the spleen, blood and peripheral tissues 14, 32. Recent investigations indicate that CD8+ T memory cells can reside permanently in peripheral tissues such as kidney, skin, intestines, lungs and brain and they do not re‐enter into circulation 16, 19, 22, 24, whereas CD4+ T cells that reside in tissues have not been well defined. Also, aside from differences in localization, it is currently a matter of debate whether the profile of these cells differs from tissue to tissue. It was demonstrated recently, for example, that CD8+ central memory T cells situated in the lung are crucial for lung allograft acceptance and that they have regulatory properties, and that Trm differs between the gut mucosa and other lymphoid tissue 16, 33.
As well as the data indicating that CD8+ Trm cells can proliferate in situ upon antigen challenge 34, 35, there is evidence that these cells can also be maintained by the local tissue environment 24, 36. In order to distinguish between resident and circulating memory T cells, we analysed the expression of CD69 and CD103 makers for canonical Trm 16 in 15 non‐diabetic donors. We demonstrated that the vast majority of CD8+ cells expressed these markers and could be defined as canonical Trm cells. The predominance of CD8+ T cells in the pancreatic tissue (exocrine and endocrine) has been reported recently in both autoantibody‐positive and ‐negative non‐diabetic islets donors which, although indirectly, support the presence of canonical resident CD8+ T cells in pancreatic islets in non‐diabetic individuals 11. We were not able to analyse the expression of CD69 and CD103 on CD4+ T cells because of the generally very low frequency of these cells in most of the islet preparations.
Unfortunately, due also to the low numbers of islet‐associated immune cells, it was not possible at this stage to determine the antigen specificities of the CD4+ and CD8+ T cells applying the most established T cell assays. Of course, understanding whether islets and pancreas resident T cells recognize self‐antigens, recall antigens or reflect simply the polyclonality of peripheral T cells remains a very relevant and unresolved question. We hope that investigations using new technologies, as RNAseq of sorted single cells and mass spectrometry based‐flow cytometry including MHC class I and class II tetramers, will allow us in the near future to address some of these questions and increase our understanding of the function of these cells and, most importantly, their role in the development of T1D.
The presence of canonical CD4+CD25hi regulatory T cells (Tregs) within the T cell pool was also investigated. Surprisingly, the expression of CD25 was very low, if not absent, and in the few individuals with detectable expression of CD25 on the surface of both CD4+ and CD8+ T cells, a cell population with high expression of CD25 could not be defined clearly (Supporting information, Fig. S1). These data raise the question of whether Tregs circulate in the pancreatic tissue and within the islets in physiological condition or whether they are recruited only in case of inflammatory injury. It could also be that their frequency is too low to be detected unless large amounts of islets are collected. Future experiments aimed, for example, at investigating specific gene expression and epigenetic signatures of these cell populations at the single‐cell level will hopefully answer some of these questions.
We also confirmed the presence of B cells, albeit at low frequency, in pancreatic islets and in some donors even higher than T cells. A recent immunohistological study performed on post‐mortem pancreas samples of recently diagnosed T1D described two different entities of insulitis, one with higher numbers of B cells CD20+, considered as a more aggressive form of autoimmune process, compared to the other type of insulitis with low B cell numbers 30. We speculate that B cells present in healthy pancreas may also be involved in promoting tissue‐specific memory immune responses, and could eventually influence autoreactive T cell activation and proliferation through chronic antigen exposure.
In line with published studies showing the presence of resident NK cells with anti‐viral and anti‐tumour activity in several tissues 37, we found also a low frequency of NK cells in human isolated islets, which might be important to maintain islets immune‐surveillance activity in situ.
We next examined whether different subsets of pancreatic resident immune cells have any impact upon islet cells. Importantly, CD4+ Tte showed a negative correlation with beta cells and CD8+ Tcm correlated negatively with alpha cells, whereas Tem correlated positively with both alpha and beta cells. Thus, it appears that whereas CD8+ Tem would have a positive impact in maintaining endocrine cell numbers, CD4+ Tte and CD8+ Tcm would have the opposite effect. Also, B cells have shown negative and NK cell‐positive correlation with frequency of beta cells. Although these results should be taken with caution, it is tempting to speculate that resident T cells might play a role in maintaining islet cell homeostasis, most probably through the secretion of soluble factors. More direct biological evidence of this process needs to be gathered to understand the significance and relevance of these observations.
It is worth noting that the organ donors included in this study were aged between 40 and 60 years. This raises questions of whether resident T cells accumulate during age and, if so, whether they are relevant in the context of T1D, which is known to develop mainly during childhood. However, although there are only few studies addressing age‐related changes in human tissue resident lymphocytes, a study by Thome et al. has demonstrated that tissue‐specific Trm cells are maintained stably over life in mucosal tissues, while in lymphoid sites they undergo age‐associated changes 16. It remains to be determined whether Trm also populate the pancreas at early ages and whether these cells are important for the establishment of immunological tolerance after birth.
In order to ensure that our flow cytometry analysis was not biased by the proportion of alpha and beta cells due to the preparation of islet cell suspension, we performed a correlation study between the percentage of insulin‐ and glucagon‐positive cells measured by flow cytometry with RNA expression from non‐dissociated islets by RNA sequencing. We were able to demonstrate a significant linear correlation showing homogeneity of islet composition.
Taken together, our results suggest that the majority of T cells found associated with human pancreatic islets of non‐diabetic donors are resident memory T cells predominantly of central memory phenotype. Further work will be required to determine whether accumulation of these cells in the pancreas is antigen‐driven, as well as the function of these cells in the pancreas and the maintenance of immunological tolerance to islet antigens. In any case, it appears clear that the presence of lymphocytes in pancreatic islets is not driven per se by autoimmunity, and careful comparison between T1D and healthy pancreases should be necessary to understand fully the interplay between the immune system and islet cells in immunopathology.
In conclusion, we have characterized successfully the phenotype of pancreatic islet‐associated resident lymphocytes in non‐diabetic organ donors and demonstrated the striking predominance of CD8+ T cells with a canonical Trm phenotype, highlighting their possible role in islet immune‐regulation and maintenance of immune‐surveillance in situ. These results are important to understand the interplay between the immune system and the pancreatic islets and will be instrumental when investigating the histopathological lesions in human forms of diabetes to shed light into the pathogenesis of these diseases.
Author contributions
This study was designed by C. M. C. Human islets were prepared at Uppsala University and provided by O. K. and S. O., who also carried out the immunohistochemistry on pancreas biopsies. R. M., U. K. and A. J. collected the data. M. R., J. A. and B. P.‐A. contributed to the handling of the islets and FACS analysis. S P. V. and V. P. performed RNA sequencing and analysed the data. R. M. and U. T. performed the statistical analysis. R. M., U. K., S. L. and C. M. C. revised and interpreted the results. M. R. wrote and C. M. C., U. K., S. L. and S. O. reviewed and edited the manuscript. All the authors have read and edited the manuscript and approved the version to be published. Human islets were obtained through The Nordic Network for Clinical Islet Transplantation and the Tissue Laboratory of the Lund University Diabetes Center supported by the Swedish national strategic research initiative EXODIAB (Excellence of Diabetes Research in Sweden).
Disclosure
The authors have no competing interests to report.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Flow cytometry analysis of CD25 expression on the surface of CD4+ and CD8+ T cells in dissociated pancreatic islets of non‐diabetic donors. CD45+CD3+ T cells were gated on CD4+ and CD8+ cells to evaluate the expression of CD25. The frequency of CD25 on both T cells population was extremely low, if not totally undetectable, in all donors analysed (n = 10) and it was not possible to distinguish between CD25hi and CD25low cell populations.
Table S1. Correlation between the percent of lymphocyte subsets and the percentage of glucagon‐ and insulin‐positive cells determined by flow cytometry. Statistical significance are shown as P‐values and they were calculated using Pearson's correlation test. The numbers in bold type are significant.
Acknowledgements
This work was supported by the Swedish Research Council, Swedish Diabetes Foundation, Barndiabetesfonden, Skåne University Hospital Foundations and Novo Nordisk Research Foundation to C. M C.
References
- 1. Atkinson MA, von Herrath M, Powers AC, Clare‐Salzler M. Current concepts on the pathogenesis of type 1 diabetes–considerations for attempts to prevent and reverse the disease. Diabetes Care 2015; 38:979–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Campbell‐Thompson ML, Atkinson MA, Butler AE et al The diagnosis of insulitis in human type 1 diabetes. Diabetologia 2013; 56:2541–3. [DOI] [PubMed] [Google Scholar]
- 3. Gianani R, Campbell‐Thompson M, Sarkar SA et al Dimorphic histopathology of long‐standing childhood‐onset diabetes. Diabetologia 2010; 53:690–8. [DOI] [PubMed] [Google Scholar]
- 4. In't Veld P. Insulitis in human type 1 diabetes: the quest for an elusive lesion. Islets 2011; 3:131–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krogvold L, Wiberg A, Edwin B et al Insulitis and characterisation of infiltrating T cells in surgical pancreatic tail resections from patients at onset of type 1 diabetes. Diabetologia 2016; 59:492–501. [DOI] [PubMed] [Google Scholar]
- 6. Reddy S, Zeng N, Al‐Diery H et al Analysis of peri‐islet CD45‐positive leucocytic infiltrates in long‐standing type 1 diabetic patients. Diabetologia 2015; 58:1024–35. [DOI] [PubMed] [Google Scholar]
- 7. Campbell‐Thompson M, Fu A, Kaddis JS et al Insulitis and beta‐cell mass in the natural history of type 1 diabetes. Diabetes 2016; 65:719–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Willcox A, Richardson SJ, Bone AJ, Foulis AK, Morgan NG. Analysis of islet inflammation in human type 1 diabetes. Clin Exp Immunol 2009; 155:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gianani R, Putnam A, Still T et al Initial results of screening of nondiabetic organ donors for expression of islet autoantibodies. J Clin Endocrinol Metab 2006; 91:1855–61. [DOI] [PubMed] [Google Scholar]
- 10. Wagner R, McNally JM, Bonifacio E et al Lack of immunohistological changes in the islets of nondiabetic, autoimmune, polyendocrine patients with beta‐selective GAD‐specific islet cell antibodies. Diabetes 1994; 43:851–6. [DOI] [PubMed] [Google Scholar]
- 11. Wiberg A, Granstam A, Ingvast S et al Characterization of human organ donors testing positive for type 1 diabetes‐associated autoantibodies. Clin Exp Immunol 2015; 182:278–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. In't Veld P, Lievens D, De Grijse J et al Screening for insulitis in adult autoantibody‐positive organ donors. Diabetes 2007; 56:2400–4. [DOI] [PubMed] [Google Scholar]
- 13. Butcher MJ, Hallinger D, Garcia E et al Association of proinflammatory cytokines and islet resident leucocytes with islet dysfunction in type 2 diabetes. Diabetologia 2014; 57:491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999; 401:708–12. [DOI] [PubMed] [Google Scholar]
- 15. Masopust D, Picker LJ. Hidden memories: frontline memory T cells and early pathogen interception. J Immunol 2012; 188:5811–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Thome JJ, Yudanin N, Ohmura Y et al Spatial map of human T cell compartmentalization and maintenance over decades of life. Cell 2014; 159:814–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol 2009; 10:524–30. [DOI] [PubMed] [Google Scholar]
- 18. Hofmann M, Pircher H. E‐cadherin promotes accumulation of a unique memory CD8 T‐cell population in murine salivary glands. Proc Natl Acad Sci USA 2011; 108:16741–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang XD, Clark RA, Liu L, Wagers AJ, Fuhlbrigge RC, Kupper TS. Skin infection generates non‐migratory memory CD8(+) T‐RM cells providing global skin immunity. Nature 2012; 483:227−31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Masopust D, Choo D, Vezys V et al Dynamic T cell migration program provides resident memory within intestinal epithelium. J Exp Med 2010; 207:553–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wakim LM, Woodward‐Davis A, Bevan MJ. Memory T cells persisting within the brain after local infection show functional adaptations to their tissue of residence. Proc Natl Acad Sci USA 2010; 107:17872–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Teijaro JR, Turner D, Pham Q, Wherry EJ, Lefrançois L, Farber DL. Cutting edge: tissue‐retentive lung memory CD4 T cells mediate optimal protection to respiratory virus infection. J Immunol 2011; 187:5510–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lee YT, Suarez‐Ramirez JE, Wu T et al Environmental and antigen receptor‐derived signals support sustained surveillance of the lungs by pathogen‐specific cytotoxic T lymphocytes. J Virol 2011; 85:4085–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casey KA, Fraser KA, Schenkel JM et al Antigen‐independent differentiation and maintenance of effector‐like resident memory T cells in tissues. J Immunol 2012; 188:4866–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohlmeier JE, Miller SC, Woodland DL. Cutting edge: antigen is not required for the activation and maintenance of virus‐specific memory CD8+ T cells in the lung airways. J Immunol 2007; 178:4721–5. [DOI] [PubMed] [Google Scholar]
- 26. Goto M, Eich TM, Felldin M et al Refinement of the automated method for human islet isolation and presentation of a closed system for in vitro islet culture. Transplantation 2004; 78:1367–75. [DOI] [PubMed] [Google Scholar]
- 27. Lind K, Richardson SJ, Leete P, Morgan NG, Korsgren O, Flodström‐Tullberg M. Induction of an antiviral state and attenuated coxsackievirus replication in type III interferon‐treated primary human pancreatic islets. J Virol 2013; 87:7646–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Roederer M. Spectral compensation for flow cytometry: visualization artifacts, limitations, and caveats. Cytometry 2001; 45:194–205. [DOI] [PubMed] [Google Scholar]
- 29. Fadista J, Vikman P, Laakso EO et al Global genomic and transcriptomic analysis of human pancreatic islets reveals novel genes influencing glucose metabolism. Proc Natl Acad Sci USA 2014; 111:13924–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Arif S, Leete P, Nguyen V et al Blood and islet phenotypes indicate immunological heterogeneity in type 1 diabetes. Diabetes 2014; 63:3835–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Coppieters KT, Dotta F, Amirian N et al Demonstration of islet‐autoreactive CD8 T cells in insulitic lesions from recent onset and long‐term type 1 diabetes patients. J Exp Med 2012; 209:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. von Andrian UH, Mackay CR. T‐cell function and migration. Two sides of the same coin. N Engl J Med 2000; 343:1020–34. [DOI] [PubMed] [Google Scholar]
- 33. Krupnick AS, Lin X, Li W et al Central memory CD8+ T lymphocytes mediate lung allograft acceptance. J Clin Invest 2014; 124:1130–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cuburu N, Graham BS, Buck CB et al Intravaginal immunization with HPV vectors induces tissue‐resident CD8+ T cell responses. J Clin Invest 2012; 122:4606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wakim LM, Gebhardt T, Heath WR, Carbone FR. Cutting edge: local recall responses by memory T cells newly recruited to peripheral nonlymphoid tissues. J Immunol 2008; 181:5837–41. [DOI] [PubMed] [Google Scholar]
- 36. Masopust D, Vezys V, Wherry EJ, Barber DL, Ahmed R. Cutting edge: gut microenvironment promotes differentiation of a unique memory CD8 T cell population. J Immunol 2006; 176:2079–83. [DOI] [PubMed] [Google Scholar]
- 37. Sojka DK, Tian Z, Yokoyama WM. Tissue‐resident natural killer cells and their potential diversity. Semin Immunol 2014; 26:127–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web‐site:
Fig. S1. Flow cytometry analysis of CD25 expression on the surface of CD4+ and CD8+ T cells in dissociated pancreatic islets of non‐diabetic donors. CD45+CD3+ T cells were gated on CD4+ and CD8+ cells to evaluate the expression of CD25. The frequency of CD25 on both T cells population was extremely low, if not totally undetectable, in all donors analysed (n = 10) and it was not possible to distinguish between CD25hi and CD25low cell populations.
Table S1. Correlation between the percent of lymphocyte subsets and the percentage of glucagon‐ and insulin‐positive cells determined by flow cytometry. Statistical significance are shown as P‐values and they were calculated using Pearson's correlation test. The numbers in bold type are significant.


