Abstract
Basement membrane components are targets of autoimmune attack in diverse diseases that destroy kidneys, lungs, skin, mucous membranes, joints, and other organs in man. Epitopes on collagen and laminin, in particular, are targeted by autoantibodies and T cells in anti-glomerular basement membrane glomerulonephritis, Goodpasture’s disease, rheumatoid arthritis, post-lung transplant bronchiolitis obliterans syndrome, and multiple autoimmune dermatoses. This review examines major diseases linked to basement membrane autoreactivity, with a focus on investigations in patients and animal models that advance our understanding of disease pathogenesis. Autoimmunity to glomerular basement membrane type IV is discussed in depth as a prototypic organ-specific autoimmune disease yielding novel insights into the complexity of anti-basement membrane immunity and the roles of genetic and environmental susceptibility.
Keywords: basement membrane, autoimmunity, anti-glomerular basement membrane
Introduction
Autoimmunity affects up to 20% of the US population, a prevalence similar to that of heart disease and cancer. Many autoimmune diseases strike young adults and destroy vital organs and tissues, causing extensive morbidity and disability over a lifetime. Annual treatment costs are estimated in the range of $100 billion [1]. Medications commonly used to treat autoimmune disorders have devastating long-term effects, adding to the toll on patients. The root cause remains unknown, and therapies are nonspecific and fraught with serious complications. Novel less toxic treatments are urgently needed, but rational design will require better understanding of disease pathogenesis.
It is thus notable that for multiple autoimmune diseases the target antigen (Ag) is a basement membrane (BM) component. Epitopes on collagens and laminins, in particular, in kidney, lung, joints, skin, mucous membranes, and other tissues are targeted by autoantibodies and T cells. Humoral autoimmunity is prominent, and identification of autoantibodies in the circulation or tissue is key to diagnosis; elimination or suppression of autoreactive lymphocytes is a major goal of therapy. This review will examine major diseases linked to BM reactivity (Table 1), with reference to the historical context and a focus on investigations in man and animals that advance our understanding of disease pathogenesis. Special attention is paid to autoimmunity to type IV collagen in renal glomerular and pulmonary alveolar BMs, because meticulous dissection of antigenic epitopes and pathogenic mechanisms in these diseases has provided novel paradigms for induction of autoimmunity targeting BM and a blueprint for approaching less well defined diseases.
Table 1.
Major Diseases Linked to Autoimmunity to Basement Membrane Components.
| Human Diseases | Major Organ System | Key Clinical Features | BM Target |
|---|---|---|---|
| Anti-GBM GN & GP disease | Kidney & Lung & lung hemorrhage | GN with renal failure α5(IV)NC1 collagen | α3(IV)NC1 collagen |
| Bronchiolitis obliterans (post lung transplant) | Lung | Fibro-obliterative | Collagen V pulmonary failure |
| Rheumatoid arthritis | Synovial Joints | Destructive arthritis | Collagen II (native & citrullinated) |
| Autoimmune Dermatoses | |||
| Bullous pemphigoid | Skin | Epidermal-dermal blisters | Collagen XVII/BP180 |
| Epidermolysis bullosa acquisita | Skin | Epidermal-dermal blisters | Collagen VII |
| Anti-laminin γ1 pemphigoid | Skin | Epidermal-dermal blisters | Laminin γ1 chain |
| Anti-laminin-332 mucous membrane pemphigoid | Mucous membranes | Epidermal-dermal blisters | Laminin-332 (laminin-5) |
Abbreviations: BM, basement membrane; GBM, glomerular basement membrane; GN, glomerulonephritis; GP, Goodpasture’s; NCq, noncollagenous domain1.
Autoimmune anti-GBM glomerulonephritis and Goodpasture’s disease
Clinical manifestations and epidemiology
Autoimmune anti-glomerular basement membrane (GBM) glomerulonephritis (GN) and its systemic counterpart Goodpasture’s (GP) disease, the term used when clinical lung involvement is evident, are considered a prototype for organ-specific autoimmunity. Although rare, anti-GBM GN was the first human nephritis for which an intrinsic glomerular Ag target was well characterized and clinical and pathologic manifestations duplicated in animal models by transfer of patients’ IgG and by Ag immunization. Thus, anti-GBM nephritis has been considered a key disease with which to dissect mechanisms and interventions relevant to human nephritis, with anticipation that insights will be far reaching.
Anti-GBM GN and GP disease affect both sexes, with a bimodal age distribution showing peaks around age 20 and 60–70 years of age [2]. Disease occurs in children but is rare [3]. Clinical manifestations can be severe, and include rapidly progressive glomerulonephritis with irreversible renal failure and catastrophic lung hemorrhage due to involvement of the alveolar capillaries. This clinical phenotype reflects the highly restricted, tissue-specific distribution of the dominant target Ag, the non-collagenous 1 (NC1) domain of the α3 chain of type IV collagen, or α3NC1. Expression of α3(IV) collagen is limited to BM of renal glomerular capillaries and tubules, alveolar capillaries, cochlea, anterior lens capsule, Descemet’s membrane, ovary, and testis [4]. Sites other than kidney and lung appear to be protected from autoantibody attack.
Patients often present with nonspecific symptoms, including malaise, weakness, and fatigue due to anemia or renal failure, or complain of dark or blood-tinged urine, coughing up blood (hemoptysis), or difficulty breathing. Anemia is common and may be due to occult blood loss from renal or lung involvement, identified as infiltrates on chest radiograph. Signs of inflammation in the glomerulus, the filtering unit of the kidney, are typically present, including blood and protein in the urine (hematuria and proteinuria). Some patients present with rapidly progressive GN (RPGN), a clinical syndrome with rapid loss of glomerular filtration rate, often defined as 50% loss within 3 months or a rise in serum creatinine of greater than 1 mg/dL/week. Among patients presenting with RPGN, uremia, oliguria, and hypertension are common.
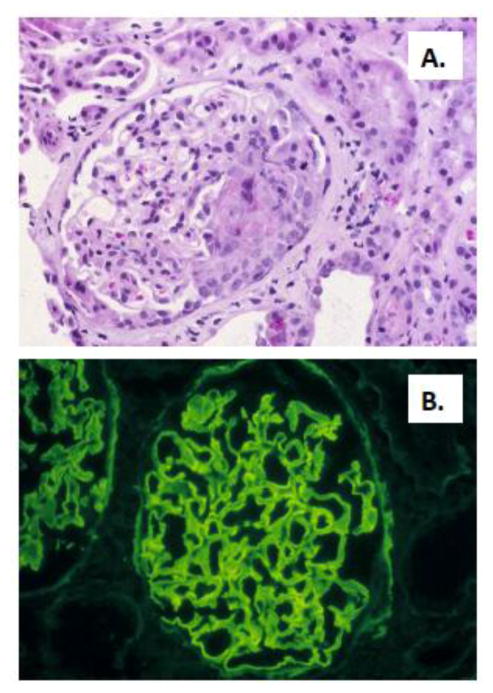
Evidence of renal insufficiency usually prompts kidney biopsy, which typically reveals focal and segmental necrotizing crescentic GN (Figure 1). Direct immunofluorescence findings are pathognomonic, with intense linear deposition of IgG and often C3 along the glomerular capillary walls. IgG1 and IgG4 subclasses predominate, though all subclasses can be seen [5–8]. Linear tubular BM IgG staining may also be detected. Electron microscopy is negative for dense deposits. Diagnosis of anti-GBM antibody-mediated GN or GP disease requires identification of anti-GBM autoantibodies, either in the circulation or deposited in a linear fashion along the glomerular or alveolar BM. Lung biopsy is uncommon, but when performed typically reveals diffuse intraalveolar hemorrhage and intraseptal hemosiderin-laden macrophages. Tissue biopsy may be critical to determine the correct diagnosis, because cases of anti-GBM GN and alveolar hemorrhage due to anti-GBM IgG but with undetectable circulating anti-GBM autoantibodies are reported [9].
Figure 1.
Renal biopsy specimen from a patient with anti-GBM GN. A. Light microscopy showing focal and segmental necrotizing crescentic GN, with a segmental cellular crescent. B. Direct immunofluorescence showing intense linear staining for IgG along the glomerular capillary walls. Photomicrographs are courtesy of David N. Howell, MD PhD, Department of Pathology, Duke University Medical Center.
Approximately 25% of anti-GBM GN or GP disease patients co-express antineutrophil cytoplasmic autoantibodies (ANCA). In some cases, renal biopsy shows histopathologic features of both anti-GBM GN (linear GBM IgG deposits) and ANCA vasculitis (extraglomerular vasculitic lesions) [10–12]. Both perinuclear (P, anti-myeloperoxidase) and cytoplasmic (C, anti-proteinase 3) ANCA have been detected, though anti-MPO ANCA may dominate among dual positive patients with crescentic GN [12]. The origins and pathogenic significance of this dual autoantibody positive disease remain unclear, as does the impact on clinical manifestations and patient prognosis.
Anti-GBM GN is usually an aggressive disease with rapid progression to renal failure, with a low likelihood of renal functional recovery once a patient becomes dialysis dependent. Urgent autoantibody removal and immunosuppressive therapy is recommended. A typical regimen consists of plasmapharesis for 2–3 weeks until serum autoantibodies are undetectable and hemoptysis resolves, if present. Patients also receive high dose steroids, tapered over 2–6 months, and oral or intravenous cyclophosphamide for 2–4 months to inhibit antibody production and T cell function. A longer course of therapy is indicated in the setting of active hemoptysis or persistent circulating anti-GBM Ig. Rarely, in specialized centers extracorporeal oxygenation is used to support patients suffering severe respiratory distress and refractory hypoxemia. Serum autoantibody levels guide treatment decisions, including the duration of plasmapheresis and the timing of transplantation [13]. Even with immunosuppressive therapy, circulating autoantibodies occasionally persist for weeks, and occasionally up to 6–9 months.
Once autoantibodies disappear, it is uncommon for anti-GBM GN or GP disease to recur, and long-term maintenance therapy is not usual. One exception is the substantial subset of patients with dual anti-GBM/ANCA-positive GN, for whom chronic maintenance therapy similar to that used for ANCA vasculitis, a chronic relapsing disease, may be needed. In anti-GBM GN patients who reach ESRD and are candidates for transplantation, general practice is to wait until circulating anti-GBM IgG disappear, because disease can recur rapidly and lead to renal allograft loss in the presence of persistent circulating anti-GBM antibodies [14]. With this protocol, allograft loss from recurrent anti-GBM GN is rare, although recurrence as late as 12 years has been reported.
Clinical variants of anti-GBM GN exist, and in many cases may be attributable to atypical GBM epitope binding. Ohlsson et al reported 4 cases of young women with life threatening lung hemorrhage mediated by IgG4 subclass anti-GBM autoantibodies [9]; all had linear GBM IgG staining on renal biopsy, 3 of 4 had crescentic or focal necrotizing GN, and 2 developed severe acute renal failure. All were ANCA negative. In 2 patients, anti-GBM IgG bound better to Ag coated in saline, compared to GP IgG that normally bind better to Ag coated with a denaturing (guanidine) solution, suggesting binding to atypical α3NC1 epitopes. Conventional anti-GBM ELISA results were false negative in each case, presumably due to poor detection of human IgG4 by the antisera [9]. Occasional mild cases of anti-GBM GN with normal renal function are also reported [15–19]. Careful dissection of serum anti-GBM IgG from one patient with mild GN and linear GBM IgG deposition but no crescents revealed restriction to anti-GBM IgG4 that bound to native α345NC1 hexamers but not to α3NC1 monomers, unlike typical GP autoantibodies [19]. Atypical BM Ag targets, including collagenase-sensitive epitopes of α1α2(IV) and NC1 domains of α5α6(IV) collagen, have also been identified in patients with a rare form of anti-GBM GN mediated by IgA autoantibodies [20–23]. Initial assay for serum anti-GBM IgG is often negative in atypical cases, and diagnosis is made only after immunofluorescent staining of renal biopsy specimens reveals linear Ig deposits, because conventional assays used to screen serum typically use only α3NC1 target Ag or detect only common IgG subclasses.
Concurrent anti-GBM GN and membranous nephropathy (MN) is also reported [24], though considerably less frequently than anti-GBM/ANCA overlap disease. MN is a common cause of nephrotic syndrome in adults; a dominant mechanism of pathogenesis involves autoantibody binding to glomerular podocyte surface Ags [25, 26]. It is notable that a MN-like pathology is induced in DBA/1 mice with certain α3NC1 immunization protocols [27], suggesting a possible pathogenetic link between the two diseases.
Gene and environmental susceptibility in anti-GBM GN
Considerable evidence suggests that both gene and environmental susceptibility contribute to induction of anti-GBM GN and to the loss of immune regulation that is fundamental to disease pathogenesis. The highly polymorphic genes of the major histocompatibility complex (MHC) are among the most potent known autoimmune susceptibility and resistance genes [28]. It is thus striking that 75–90% of anti-GBM patients express the human leukocyte Ag (HLA) class II allele DRB1*1501 [29–34]. DRB1*1501 is co-expressed with monomorphic DRA1*0101 to form class II molecule DR2. Like other class II molecules, DR2 is expressed on the surface of Ag presenting cells (APC), including B cells, monocytes, macrophages, and dendritic cells, to bind and present processed Ag peptide for recognition by CD4+ T cells, and is expressed on thymic medullary cells that direct positive selection of T cell receptors (TCR) during CD4+ T cell development. Thus, based on classical paradigms, genetic control of anti-α3NC1 autoimmune responses resides in polymorphic sites within the DR2 peptide binding groove encoded by DRB1*1501, and is exerted either in shaping T cell repertoires or activation of anti-α3NC1 T cells and B cells in the periphery, or both. It is notable, however, that Class II molecules also have non-classical Ag-independent signaling functions that modulate adaptive and innate immunity [35–38] and can be induced on non-hematopoietic parenchymal cells, including renal tubular epithelium, to control local inflammation and tolerance [39]. Thus determining the role of DRB1*1501 in anti-GBM GN could facilitate development of targeted treatments, noting that a therapeutic agent (copolymer 1, glatiramer) that binds and inhibits DR2 function is already in clinical use in autoimmune diseases [40].
DRB1*1501 is also a potent risk allele for multiple sclerosis (MS), a neurological autoimmune disease [41]. This may in part explain reports of anti-GBM GN developing in MS patients treated with alemtuzumab (previously known as Campath 1H), a pan-lymphocyte-depleting humanized mAb that binds CD52 on B and T cells [42–45]. Another autoantibody-mediated disorder, Graves disease, is found in approximately 30% of MS patients receiving alemtuzumab [43], suggesting a particular susceptibility to humoral autoimmune dysregulation with this drug. The function of CD52 is not known, but anti-CD52 mAb therapy results in prolonged lymphopenia (6–12 months), leading to speculation that it permits emergence of autoreactive B cells. Immune dysreguation due to deficiency of regulatory T cells seems less likely, based on a paradoxical relative increase in regulatory T cells reported in alemtuzumab-treated patients [43].
In addition to the potent disease susceptibility linked to DRB1*1501, associations with other Class II alleles are reported, including a protective effect linked to some HLA genes, such as DRB1*07 [30, 32]. It is also likely that non-HLA genes linked to immunoregulation and effector functions are involved [46, 47]. Copy number variation and polymorphisms in Fcgamma receptors (FcγR) have been linked to anti-GBM susceptibility in humans [48, 49]. In mice, genetic deletion of FcγRIIb, an inhibitory FcγR expressed on B cells and myeloid cells and implicated in both antibody production and IgG effector mechanisms, worsens anti-GBM GN and/or alveolar hemorrhage in some models [50, 51].
Anti-GBM GN and GP disease have been repeatedly associated with cigarette smoking and inhalational exposure to hydrocarbons [9, 52–55]. It is speculated that inhaled toxins promote anti-GBM autoantibody production by exposing collagen epitopes in injured alveoli. However, this view is difficult to reconcile with the absence of pathogenic anti-GBM Ig in the vast numbers of individuals who have common destructive inflammatory lung diseases. Rather, disruption of pulmonary immune regulatory circuits and/or systemic effects of proinflammatory cytokines or chemical byproducts seems likely. The recent observation that thiocyanate inhibits activity of peroxidasin, the enzyme that catalyzes crosslinks in GBM type IV collagen hexamers, raises an intriguing alternative possibility. A subset of smokers have elevated blood levels of thiocynate, which could inhibit peroxidasin and increase the abundance of non-cross-linked hexamers, thus promoting in vivo anti-GBM Ig access to pathogenic α3NC1 epitopes [56].
Autoantigens in anti-GBM GN and GP disease
A pathogenic role for anti-GBM autoantibodies in patients was confirmed by classic experiments of Lerner and colleagues in which IgG eluted from kidneys of two patients with GP disease were injected into unilaterally nephrectomized squirrel monkeys, leading to anti-GBM GN [57]. Recipients demonstrated linear staining for human IgG along the glomerular BM and developed proteinuria within 24 hours and proliferative GN with renal failure by day 6. These early experiments focused attention on the role of autoantibodies and humoral autoimmunity in mediating anti-GBM GN, and provided a rationale for therapeutic plasmapheresis to remove autoantibodies in patients with severe disease.
It was subsequently determined that patients’ circulating and kidney-bound antibodies bound to epitopes on isolated bovine and recombinant human α3NC1 domains [58–62]. The role of α3NC1 in inducing autoimmune GN in vivo was confirmed in rabbits immunized with bovine α3NC1-containing dimers and rats immunized with recombinant human α3NC1 [63–65]. A range of immunization protocols have been used to induce experimental autoimmune GN (EAG), employing a variety of species and heterologous and homologous Ag preparations, including collagenase-solubilized fractions of GBM collagen IV extracts as well as isolated and recombinant α345NC1 hexamers and α3NC1 monomers [63–74]. In rodents, anti-GBM IgG autoantibodies and GN are induced in a strain-dependent manner, with WKY rat and DBA/1 mouse inbred strains being particularly susceptible to GN [68, 73]. IgG eluted from kidneys of immunized rats with GN bind α3NC1 [75]. Strain differences in disease susceptibility likely reflect both capacity to break tolerance to generate autoantibodies of pathogenic specificity or IgG subclass, and differences in end-organ susceptibility to inflammation and immune injury [75, 76]. However, recapitulation of the robust GN observed in patients and evidence of pulmonary hemorrhage are rare in mouse EAG models.
A refined understanding of epitope specificity in anti-GBM GN patients and the complexity of epitope exposure has emerged in the past two decades. This provides some insight into the requirements for, and barriers to, anti-α3NC1 autoimmunity. It is now well established that classic GP autoantibodies bind conformational and cryptic epitopes buried in the native GBM type IV collagen molecule. Using chimeric collagen proteins with short segments of α3NC1 substituted on an α1NC1 scaffold, Netzer et al confirmed that GP autoantibodies bind conformational epitopes on α3NC1, demonstrating that protein reduction abolished autoantibody binding. These investigators also identified two immunodominant epitopes, C2 (later termed EA-α3, residues 17–31) and C6 (later, EB-α3, residues 127–141) bound by subpopulations of patients’ anti-GBM IgG [77]. Additional minor epitope specificities were observed in a subset of patients. It was further determined that α345NC1 hexamer dissociation is required for optimal IgG binding. A hallmark of GP IgG is increased reactivity towards α3NC1 monomers and dissociated, as compared to native, hexamers [78–81]. This is in part because pathogenic epitopes incorporate amino acids with hydrophobic side chains that are exposed by dissociation [81, 82], a feature that also suggest a requirement for a compatible hydrophobic interface within the antibody variable region structure. A key breakthrough came in the elegant studies of Vanacore and colleagues, who discovered that type IV collagen hexamers are reinforced by novel covalent peroxidasin-catalyzed sulfimine (S=N) bonds, with up to six bonds per hexamer interface [83–85]. GP anti-α3NC1 IgG cannot access pathogenic epitopes within highly crosslinked hexamers, whereas they not only access but even facilitate dissociation and full epitope exposure in non-crosslinked hexamers [80].
Using recombinant human collagen IV NC1 monomers, α5/α1 chimeras, and isolated NC1 hexamers, Pedchenko et al confirmed the presence of high affinity anti-α3NC1 IgG in 100% of 57 sera and 14 kidney eluates from patients with anti-GBM GN or GP disease. Unexpectedly, they also detected anti-α5NC1 IgG of lower affinity in 72% of patients’ sera and 79% of kidney eluates [81]. Competition and binding assays determined that the anti-α3NC1 and α5NC1 IgG are distinct, non-crossreactive autoantibody populations; both bind conformational, cryptic epitopes exposed on dissociated NC1 monomers, but not on native hexamers. The anti-α5NC1 IgG bind an epitope, termed EA-α5, homologous to EA-α3. Epitope specificity of kidney eluate IgG mirrors that of serum autoantibodies. Serum IgG from subsets of patients also demonstrate additional generally low level binding to NC1 domains of other type IV collagen chains; however, this broader specificity is not observed in kidney eluate IgG. No sera IgG from 18 healthy individuals bind the recombinant Ags. Based on these observations and structural modeling, the investigators propose a new paradigm for development of anti-GBM GN and GP disease: disruption of sulfilimine crosslinks permits dissociation of hexamers and NC1 conformational changes that expose buried residues and promote formation of neoepitopes. This facilitates not only access and binding by preformed GP autoantibodies, but can also induction of an anti-GBM response [81].
Discovery of sulfilimine hexamer crosslinks also suggests novel mechanisms for anti-GBM disease susceptibility. As noted earlier, elevated blood levels of thiocyanate, a potent inhibitor of peroxidasin, in cigarette smokers may promote destabilization of the collagen IV scaffold, and explains the link between smoking and anti-GBM GN [56]. Additionally, anti-peroxidasin autoantibodies capable of inhibiting peroxidasin activity were recently described in a subset of anti-GBM GN and GP patients, raising the possibility that a distinct population of autoantibodies may indirectly facilitate GP epitope exposure in some patients [86].
Origins of anti-GBM GN autoimmunity: Anti-GBM B cells and autoantibodies
Analysis of serum and kidney-eluted anti-GBM IgG from anti-GBM GN and GP disease patients indicates that they are high affinity and bind a restricted set of epitopes on α3NC1 and, to a lesser extent, α5NC1 [79, 81, 87, 88]. Yet little is known about the origins or structure of patients’ anti-GBM B cells and autoIg. GP IgG binding properties are consistent with an Ag-driven immune response. However, natural anti-GBM IgG4 and IgG2 that bind pathogenic epitopes shared with patients’ anti-GBM IgG can be identified at low titer in the serum of healthy individuals when sensitive techniques are used [89, 90]. Subclass analysis suggests that human natural anti-α3NC1 IgG are restricted to IgG4 and IgG2 [8].
Sequence analysis of patients’ anti-GBM Ig would provide critical insight into their genetics and structure, as well as structural information about cognate epitopes. Moreover, because Ig function as Ag-specific signal-transducing receptors on B cells, analysis of Ig sequences will provide insight into the type of B cell populations producing the anti-α3NC1 IgG and the structure of tolerogens that regulate cell activation. However, polyclonal serum-derived or kidney-eluted IgG are not readily amenable to sequence analysis, due to technical limitations and because no RNA template is available. Novel approaches that combine deep sequencing and proteomics to dissect polyclonal IgG mixtures, a formerly prohibitive task, have been used successfully to sequence vaccine-induced Ag-specific serum IgG [91, 92], but have not yet been applied to pathogenic IgG in anti-GBM GN. Patients’ sequence information is currently most readily obtained using monoclonal B cell lines derived by viral transformation or other techniques, but to date there are no reports of recovery of human α3NC1-specific mAbs from anti-GBM GN patients, and no probe has been described that permits capture of conformational epitope-specific anti-α3NC1 B cells that could be used for single cell analysis.
Murine anti-α3NC1 IgM mAb that recognize epitopes crossreactive with those in patients have been produced from mice immunized with bovine α3NC1 [93]. Sequence analysis of 5 anti- α3NC1 mAb derived from 3 immunized mice of two different strains revealed a shared motif in the heavy chain complementarity determining region 3 (HCDR3), a critical part of the Ag binding site and major determinant of Ag specificity. Analysis showed exclusive use of IGKV3 genes encoding the light chain variable regions. This suggests selection for certain structures critical for α3NC1 binding in mice. The mAb are unmutated, further suggesting that the parent B cells were present in the mouse preimmune repertoire. Extrapolation to origins of anti-α3NC1 IgG in man is tempered, however, by the substantial species differences in Ig gene loci and B cell repertoires [94].
Humanized mouse surrogates have provided some insight. In Xenomice, mouse Ig genes are replaced by their human counterparts. Immunization of Xenomice with α3NC1 in adjuvant induced human anti-α3NC1 IgG, linear GBM IgG deposition, and proliferative GN [95]. Several IgG mAb were recovered, one of which, an IgG2/kappa termed mAb F1.1, induced similar alterations when transferred to naïve mice. In the Hu-HSC humanized mouse model, infusion of human CD34+ hematopoietic stem cells (HSC) into conditioned immunodeficient NOD-scid-gamma (NSG) recipient mice reconstitutes a human immune system. Immunization of Hu-HSC mice with bovine α3NC1 in Freund’s adjuvant permitted recovery of human anti-α3NC1 IgM mAb from splenocytes using Epstein Barr virus transformation and fusion with a mouse/human chimeric myeloma cell line [96, 97]. Sequence analysis revealed expression of unmutated human Ig genes, and identified an Ig heavy chain variable region gene segment, termed IGHV4–4 (formerly VH4 DP-70), that is also expressed by the Xenomouse-derived mAb F1.1, suggesting bias in selection of Ig genes in the human anti-α3NC1 Ig response [96]. All of the Hu-HSC-derived human anti-α3NC1 mAb expressed an uncommon binding motif in their HCDR3s, that is characteristic of unusual Ig particularly suited for binding to recessed or “hidden” epitopes [97]. These findings collectively suggest that anti-α3NC1 B cells are not purged from the human preimmune repertoire, but that they are part of a rare B cell population that is nonetheless regulated, such that a transient breach in tolerance is necessary for their activation.
A major limitation of Hu-HSC mice, however, is their limited capacity to develop human IgG responses. In conventional Hu-HSC mice, developing human T cells necessarily select on mouse MHC within the mouse thymus. This prevents optimal HLA-mediated human APC/T cell interactions in the periphery. It is likely that next-generation humanized models, including Hu-HSC bearing transgenic HLA class II DRB1*1501, will facilitate in vivo study of human anti-α3NC1 IgG responses. Information gained from IgM anti-α3NC1 B cells and autoantibodies is useful, nonetheless, because IgG ultimately arise from IgM+ precursors that are the initial targets of tolerogens, Ags, and environmental agents. IgM are particularly likely to provide insight into events initiating autoimmune responses. Current Hu-HSC models are less useful for studying effector mechanisms, however, because of the immunodeficiency required for human HSC engraftment, absence of complement factor 5 in the NOD background, and lack of Ig access to pathogenic tissue target epitopes due to extensive hexamer crosslinking in mice [98].
It is notable that human anti-α3NC1 Ig were not recovered in serum or by EBV transformation from Hu-PBL mice generated by injection of peripheral blood cells from anti-GBM patients [96]. This is despite evidence that the infused human B cells are xenoactivated and differentiate to plasma cells. This suggests that anti-α3NC1 B cells are rare in the circulation of patients. This is not unexpected, in that considerable evidence indicates that the circulation half-life of Ag-specific B cells is brief, and that the vast majority of serum IgG derive from bone marrow plasma cells [92, 99, 100]. This dissociates circulating B cells from contemporaneously circulating IgG in patients, highlighting the need for alternative models and approaches to study this aspect of disease.
Additional insight comes from study of alloantibodies that develop in patients with Alport syndrome who develop end stage renal failure and receive kidney transplants. Alport syndrome is a heritable disorder characterized by progressive GN and sensory neural deafness. It results from abnormal assembly of type IV collagen due to mutations in genes encoding the α3, α4, or α5(IV) collagen chains. Dysfunction of any chain disrupts α345NC1 hexamer and network assembly. Thus Alport patients do not express, and do not develop tolerance to, α345NC1 epitopes. In most Alport transplant patients, introduction of native Ag in the kidney allograft induces alloantibodies reactive with the “foreign” NC1 domains [101]. However, only a small proportion (3–5%) of patients develop active Alport posttransplant nephritis (APTN) [102], perhaps in part due to posttransplant immunosuppressive therapy. Epitope specificities of Alport alloantibodies differ from those of anti-GBM GN IgG: alloantibodies preferentially bind exposed epitopes on native α345NC1 hexamers [19, 81, 103, 104]. This suggest that in a non-tolerant Alport patient (lacking the α345NC1 self-Ag), exposed (allo)epitopes are immunodominant. This is supported by observations in Col4A3-knockout mice; alloantibodies from mice immunized with native (or even acid-dissociated) hexamers recognize Alport-like epitopes, whereas only mice immunized with α3NC1 monomers develop GP-like alloantibodies reactive with the EA-α3 or EB-α3 epitopes [19].
Clinical and biochemical observations in anti-GBM GN and GP disease present a puzzling paradox that has been difficult to reconcile experimentally. The target epitopes of pathogenic autoantibodies are hidden from circulating IgG and B cells, such that anti-α3NC1 B cells should be immunologically ignorant. Yet anti-α3NC1 Ig are rare in sera of healthy individuals using conventional assays, and even aggressive lung or kidney inflammation from diverse causes does not induce pathogenic anti-α3NC1 IgG. A possible explanation is revealed from an autoantibody transgenic mouse model, constructed to express genes encoding a mouse anti-α3NC1 Ig that crossreacts with epitopes bound by GP IgG. Rather than the expected immunologic ignorance, transgene-expressing B cells in this model are regulated by deletion, receptor editing, and anergy, indicating encounter with a regulating self Ag, or tolerogen, in vivo [105]. This tolerance phenotype is not altered when the anti-α3NC1 Ig transgene is established in Col4A3-deficient mice [106]. This indicates that the tissue target Ag, α3NC1, is not the tolerogen in this model; rather, it suggests the presence of a crossreactive and as yet unidentified second self-Ag that regulates anti- α3NC1 B cells. Such stringent tolerance may help explain the rarity of anti-GBM GN.
T cells in induction of anti-GBM GN
A role for CD4+ T cells in anti-GBM GN disease initiation is expected, based on paradigms of B-T cell interaction necessary for induction of high affinity, IgG isotype switched antibodies. It is also supported by the strong association with HLA class II alleles [32]. CD4+, as well as CD8+, T cells that react with α3NC1 can be isolated from blood of anti-GBM patients, as well as from healthy individuals [107–111]. Using limiting dilution analysis, Salama et al observed an increased frequency of circulating anti-α3NC1 CD4+ T cells in patients during acute disease [110]. The mechanisms by which anti-α3NC1 T cells are normally held in check, and how regulation is abrogated in anti-GBM disease, is unknown. The Ag is expressed in normal human thymus [110, 112], which would predict deletion of high affinity α3NC1-reactive T cells under normal circumstances. Two types of anti-α3NC1 regulatory T cells have been described in convalescing patients, including CD25+ CD4+ regulatory T cells and an IL-10-producing CD4+ subset [109, 113]. The collective evidence is consistent with the notion that a transient breach in tolerance is involved in disease initiation.
Multiple investigators have searched for a common immunodominant T cell epitope that might serve as a target for a peptide-based intervention [114]. Cairns and colleagues observed HLA DR-restricted responses to α3NC1 among blood cells of six GP patients, and identified two dominant CD4 T cell epitopes, peptides α371–90 and α3131–150, shared by all 6 patients tested [109]. A murine CD4+ T cell epitope, peptide α3136–146, that overlaps with one of the patients’ peptides was identified using B6 mice bearing an HLA DR2 (DRB1*1501) transgene [115]. A single shared epitope has not been identified across all EAG models.
Experimental evidence suggests that anti-α3NC1 T cells have a role in induction of anti-GBM GN independent of their support for anti-α3NC1 autoantibody production. T cells reactive with α3NC1 can be isolated from rat and murine EAG models. In immunized WKY rats in particular, there is evidence that immunization with T cell epitopes or transfer of α3NC1-specific T cell lines can induce a pauciimmune crescentic GN independent of autoantibodies, in some cases associated with a secondary anti-GBM autoantibody response [71, 72, 74, 116–118]. Murine IFNγ-secreting CD4 T cell clones specific for the α3136–146 peptide transfer an apparent pauciimmune crescentic GN to naïve HLA-DR2+ mouse recipients [115]. This is reminiscent of the pauciimmune GN that develops in bursectomized chickens immunized with heterologous GBM, and that can be transferred to naïve recipients by T lymphocytes [67, 119]. The applicability of the pauciimmune pathology to conventional human anti-GBM GN and GP disease is unclear, because identification of either circulating or linear GBM-deposited Ig is mandatory for diagnosis in patients. Nonetheless, these observations may provide insight into disease pathogenesis in a subset of patients with anti-GBM/ANCA+ overlap disease.
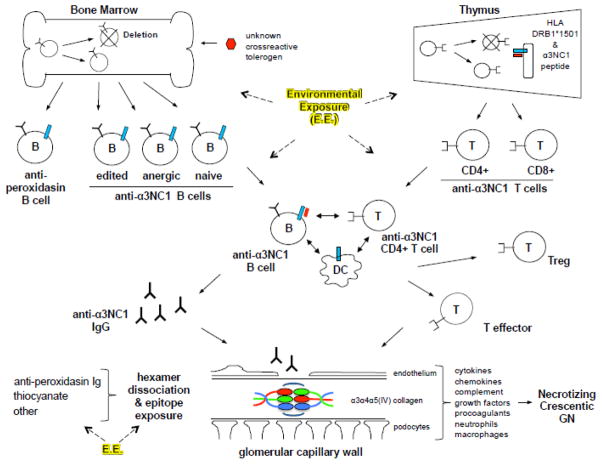
Collectively, studies in patients and animal models suggest that control of anti-GBM autoimmunity, and breaches in regulation that lead to disease, occur at multiple levels (Figure 2). The evidence indicates that anti-α3NC1 B cells and T cells are exposed to tolerizing Ag in central lymphoid organs (bone marrow and thymus), but that at least a subset of anti-α3NC1 lymphocytes escape deletion to reach the periphery. Tolerance mechanisms including anergy induction and regulatory T cells (Treg) can prevent cell activation in the spleen, lymph nodes, and local tissues. In the event that anti-α3NC1 B cells are activated and produce autoantibodies, sequestration of the target epitopes within crosslinked hexamers in BM may prevent Ig binding. HLA Class II molecules, and DR2 (DRB1*1501) in particular, facilitate autoreactivity, likely through selection of potentially pathogenic T cell repertoires and permitting interactions between pathogenic anti-α3NC1 B and T cells. Environmental exposures appear to be critical for disease initiation, although little is known regarding how exposures influence the anti-α3NC1 response or effector mechanisms. Distinct environmental exposures may act together or serially, via different mechanisms and at different or multiple steps: release of circulating agents or chemicals that deplete tolerogens or regulatory leukocytes, alter tolerance pathways, function as molecular mimics, promote inflammatory microenvironments and release of inflammatory cytokines, alter autoantibody glycosylation, or disrupt hexamer crosslinks, to name a few. Understanding the roles and interaction of genetic and environmental susceptibility factors will be key to understanding the origins of anti-GBM GN and GP disease.
Figure 2.
Schematic illustrating potential checkpoints in the production and control of anti-α3NC1 autoimmunity. This includes putative levels at which breach in regulation may occur. B cells and T cells are labeled B and T; DC, dendritic cells; Treg and Teffector indicate differentiated regulatory or effector T cells, respectively; blue bars on B cells, DC, and thymic epithelium indicate HLA Class II DR2 (DRB1*1501) molecules; short red bars represent α3NC1 peptide. The anatomic sites at which anti-α3NC1 B and T cells interact, shown in the middle of the schematic, are unknown, and may include secondary lymphoid organs (spleen and lymph nodes) and tertiary (ectopic) lymphoid tissues induced in affected organs. Also unknown is the site at which anti-α3NC1 autoantibody-producing plasma cells reside. Environmental exposures, indicated as E.E., may influence the anti-α3NC1 immune response and pathogenicity at multiple levels.
Effector mechanisms in anti-GBM GN
Linear GBM IgG deposits, often dominated by IgG1 and IgG4, are the hallmark of human anti-GBM GN and GP disease, suggesting a critical role for these isotypes in pathogenesis. Mechanisms are presumed to involve Fc mediated functions, in part, including engagement of FcγR on myeloid and intrinsic renal cells and activation of complement. Increased numbers of CD4 and CD8 T cells are also observed in glomeruli of patients with anti-GBM GN [120]. This supports a direct role for cellular immunity in development of crescentic GN, as suggested for EAG in the WKY rat (see above). In murine EAG, GN is typically mild compared to aggressive GN seen in patients. Nonetheless, Th1 and Th17 cells accumulate in mouse kidneys with late necrotizing GN induced with human α3NC1 [73, 121], and TNFα-, IFNγ-, or IL17A-producing CD4+ T cells reactive with mouse α3NC1 can be isolated from spleens and kidneys of DBA/1 mice immunized with homologous Ag [122].
In contrast to most EAG models, more severe and reproducible crescentic GN develops in the nephrotoxic serum nephritis (NSN) models of anti-GBM GN. NSN is a non-autoimmune, passive transfer, anti-GBM antibody-mediated disease that has been particularly useful in studying effector mechanisms of crescentic GN. In NSN, heterologous serum or IgG from a donor (typically goat or rabbit) immunized with GBM is injected into a recipient, where it binds to GBM and functions as a planted foreign Ag. In a frequently used accelerated version, the recipient is first immunized with donor species IgG. Although the Ags and epitopes recognized by anti-GBM IgG in NSN are generally not well defined, the model has revealed multiple disease mediators recruited by deposited anti-GBM IgG, including proinflammatory cytokines, chemokines, growth factors, complement, procoagulants, neutrophils, macrophages, and GBM-specific T cells (reviewed in [123, 124]. Comparison of GN histopathologic features with those in patients suggests that similar mechanisms are involved.
An unexpected finding in murine EAG induced in DBA/1 mice using recombinant human α3NC1 is the development of a membranous nephropathy (MN) phenotype, rather than necrotizing crescentic GN, in mice subjected to a primary immunization in Freund’s adjuvant followed by one boost [27]. Mice develop heavy albuminuria, hypoalbuminemia, and edema, similar to nephrotic syndrome classically seen in human MN. Histopathologic examination of kidneys shows only rare crescents or inflammation, and rather reveals BM “spikes” using silver stain, with granular capillary loop IgG and complement C3 and C5–9 deposits detected by immunofluorescence, and thickened BM, subepithelial electron dense deposits, and foot process effacement by electron microscopy. Hopfer and colleagues report similar histopathology after two immunizations with either human or mouse recombinant Ag [73, 122]. The mechanism by which anti-α3NC1 induces MN lesions is not understood. Exogenous human Ag colocalizes with IgG in glomerular capillary walls, prompting speculation that anti-α3NC1 IgG bind heterologous α3NC1 planted in the capillary wall by binding either specific podocyte receptors [125, 126] or anionic sites in the glomerular filter, analogous to mechanisms underlying idiopathic MN in man and mouse [27]. With more aggressive immunization in DBA/1 mice (3 boosts), renal disease evolves to crescentic GN [122].
It was also recently revealed that Ag accessibility may be a critical disease determinant in experimental anti-GBM GN. Luo et al demonstrated that infusion of patients’ pathogenic IgG into mice does not produce linear GBM Ig deposits [98]. In vitro studies clearly demonstrate that crossreactive pathogenic epitopes are present in mouse GBM, but they can only be accessed after epitopes are unmasked using denaturing agents [98]. These observations contrast starkly with the prominent linear GBM staining reported when patients’ IgG are infused into squirrel monkeys [57]. This difference is attributed to species differences in extent of hexamer crosslinking; in highly crosslinked hexamers of mouse GBM, pathogenic epitopes are not accessible even to high affinity GP IgG. These findings raise questions regarding what GBM Ag and epitopes are targeted by IgG that form linear GBM deposits in the various EAG models, including whether the epitopes are similar to autoepitopes bound by patients’ pathogenic IgG or more similar to alloepitopes targeted in APTN.
Bronchiolitis obliterans syndrome (BOS) after lung transplant
Chronic rejection after lung transplant is common, and manifests clinically as BOS, a progressive fibro-obliterative lung injury that leads to pulmonary functional decline and is a major cause of death after lung transplant. BOS is reported in 49% of lung transplant recipients by 5 years, and 75% by 10 years [127]. Considerable evidence from human, rat, and mouse studies of BOS implicates humoral and cellular autoimmune responses to two lung autoantigens, collagen V (Col-V) and K-alpha-1-tublin (Kα1T). Peripheral blood cells from lung transplant recipients frequently react to Col-V, and reactivity correlates with incidence and severity of BOS [128]. Autoantibodies to Col V and Kα1T are common in recipients developing BOS, and often precede development of fibrosis [129–131]. Preemptive depletion of anti-Col-V and anti-Kα1T autoantibodies and anti-HLA alloantibodies significantly lowers the risk of BOS [132]. Preexisting Col-V sensitization may increase the risk of BOS; Bobadilla et al detected prior sensitization to Col-V in 58.8% of patients with idiopathic pulmonary fibrosis on the wait list for lung transplant, and demonstrated moderate to severe lung injury in rats immunized with Col-V prior to lung isografting [133]. Other risk factors for BOS include acute rejection, pulmonary viral infections, alloimmunity to donor HLA class I antigens, and receipt of an HLA-DR15+ donor lung [134].
Col-V is a regulatory fibril-forming collagen broadly distributed as the heterotrimer α1(V)2α2(V) isoform in the extracellular matrix (ECM) in connective tissues. In healthy lung, Col-V is a minor collagen component, expressed in intralveolar septa and walls of bronchi, terminal bronchioles, and blood vessels. Col-V is co-expressed with collagen I (Col-I), with which it co-assembles into heterotypic Col-I/V fibrils [135]; both are markedly increased in fibrotic lungs. Kα1T is produced in airway epithelial cells. It is proposed that Col-V and Kα1T are normally sequestered in lung: Col-V within the Col-I/V fibril in the ECM, and Kα1T within intracellular microtubules. Damage to the lung during the transplantation process, particularly during ischemic reperfusion injury in the setting of preexisting MHC class I alloimmunity, is thought to play a role in unmasking the self Ags [129, 136]. Instillation of anti-MHC Class I antibodies into native mouse lungs induces anti-Col-V and anti-Kα1T autoimmunity and Th17-dependent bronchiolitis and fibrosis [137]; autoimmunity and bronchiolitis are B cell-dependent in this model [138].
The nature of the immunological link between the two self-Ags, Col-V and anti-Kα1T, is unknown. Subramaniam et al found that weekly injection of rabbit IgG reactive with either Col-V or Kα1T into B6 mice after syngeneic left lung transplant induced lung fibrosis in the isograft, but not in the native right lung [139]. Fibrosis was preceded by rapid development of mouse autoantibodies (detectable within 7 days) and autoreactive Th1, Th2, and Th17 T cell responses. The hosts developed autoimmune responses to both the targeted and non-targeted self-Ag: regardless of which xenoantibody was infused, the induced autoantibodies targeted both Col-Vα1 and Kα1T. The response was restricted, because no autoantibodies bound to Col-Vα2 or collagen II. The studies suggest that alloimmunity is not necessary for development of autoimmunity and fibrosis in this model. The involvement of only the isograft, and not the native R lung, is consistent with a requirement for local transplant-related injury to induce self-Ag exposure. Injury did not develop in mice receiving nonspecific rabbit IgG, indicating that Ag binding by administered xenoantibody was critical to maintain self-Ag exposure and permit development of the autoimmune response. T cells were also critical, as neither autoantibodies nor fibrosis developed in T cell-deficient recipients.
In animal models, induced Col-V tolerance can regulate lung rejection and fibrosis. Administration of Col-V in a non-immunogenic manner by intravenous, nebulized, or oral routes reduces lung rejection and BOS in experimental lung transplantation [140] and decreases fibrosis in experimental bleomycin-induced lung injury [141, 142]. In the bleomycin model, the Col-V tolerizing protocol also attenuates anti-Col-V autoantibody production, T cell proliferative responses, local lung and systemic inflammation, and profibrotic pathways [142]. The data provide a rationale for developing Col-V tolerizing protocols to prevent BOS in patients.
Rheumatoid arthritis and experimental collagen-induced arthritis (CIA)
Rheumatoid arthritis (RA) is a common systemic autoimmune disease, affecting approximately 1% of the world’s population, and is the most common type of autoimume arthritis. It frequently targets the wrist and small joints of the hand, though any synovial joint can be affected. Inflammation of the synovial membranes causes pain and stiffness and leads to cartilage and bony erosion and chronic joint deformities. Skin, heart, lung, and other tissues can also be affected. Onset often occurs in middle age, with women affected more frequently than men. Disease is linked to both HLA and non-HLA genes [143], with the strongest genetic risk associated with certain HLA-DRB1 Class II alleles, including DRB1*0401. These HLA alleles express a 5 amino acid sequence motif, termed the “shared epitope”, in the cusp region adjacent to the peptide binding site that mediates peptide-independent signal transduction functions [37, 144]. Cigarette smoking poses the greatest known environmental risk in RA [145],
Autoantibodies and autoreactive T cells are implicated in pathogenesis of RA. Citrullinated proteins and peptides, generated by protein arginine deiminase-catalyzed conversion of arginine to citrulline, are thought to be major arthritogenic antigens in RA. Patients’ T cells recognize citrullinated peptides, and anti-citrullinated protein autoantibodies (ACPA), including ACPA that bind citrullinated fibrinogen, fibronectin, histone, and other targets, are detected in approximately two-thirds of RA patients. ACPA that recognize citrullinated epitopes on Col-II, a major component of articular cartilage, are detected in 21% of RA patients, and bind to RA cartilage in vitro [146]. The major risk factors for RA may directly influence ACPA autoimmunity: HLA shared epitope alleles influence ACPA levels, and smoking induces arginine deiminase and protein citrullination [147, 148]. ACPA often appear years before clinical disease, and together with anti-IgG rheumatoid factors are key to diagnosis of RA [149]. ACPA are enriched in synovial fluid, and can promote complement activation, macrophage TNFα production, and osteoclastogenesis (reviewed in [150]. Immunization with citrullinated proteins can induce arthritis in HLA DR4+ transgenic, but not wildtype, mice [151]. A subset of RA patients also produce autoantibodies and synovial infiltrating T cells that react with native Col-II [152–155].
Collagen-induced arthritis (CIA) induced by immunization of various species with Col-II remains one of the best and most widely used experimental models of RA. Clinical, immunological, and histopathological features are similar to the human disease, including development of proliferative synovitis with T cell and inflammatory cell infiltrates [156–158]. Rodent anti-Col-II autoantibodies bind epitopes on the triple helical portion of Col-II that are shared with patients’ anti-Col-II autoantibodies [159, 160]. Disease in rodents is linked to the MHC and is strain dependent, with DBA/1 mice being particularly susceptible. Notably, DBA/1 mice immunized with bovine Col-II also develop ACPAs [161], and inhibition of arginine deiminase partially alleviates CIA [162].
A pathogenic role for anti-Col-II autoantibodies is clearly documented. Severe arthritis similar to that in human RA is recapitulated by passive transfer of mouse immune serum IgG or anti-collagen II mAb to naïve recipients [159, 163, 164]. Injections of combinations of mAbs that bind different epitopes induces more severe arthritis than that induced by individual mAb [165]. Co-administration of ACPAs with subarthritogenic doses of anti-Col-II mAb produces greater joint injury than seen with either autoantibody alone [161]. Sequence analysis revealed mutations in many murine arthritogenic anti-Coll-II mAb; however, molecular modeling and mutation analyses indicate that Col-II binding is present in Ig encoded by the unmutated Ig genes [166, 167]. This implies that pathogenic anti-Col-II specificities are present in the preimmune B cell repertoire. This is supported by observations in a novel mouse strain genetically manipulated such that B cells express a Col-II specific Ig heavy chain: anti-Col-II B cells are not tolerized, but rather are spontaneously activated in vivo [168]. Peripheral tolerance may be critical to control of anti-Col-II humoral immunity. CIA in rodents can be prevented by induction of regulatory T cells or by administering oral Col-II (reviewed in [169]. Effector mechanisms engaged by anti-Col-II autoantibodies are dependent on glycosylation and FcγR functions. FcγR deficiency and highly sialylated IgG abrogate capacity of the anti-Col-II IgG to induce arthritis [170–172].
Autoimmune Dermatoses
In several acquired autoimmune bullous skin diseases, the adaptive immune system targets self Ags in the skin BM zone that mediate epidermal-dermal adherence (Table 1) (reviewed in [173]. These disorders are generally diagnosed in older adults, and have a prominent autoantibody component that assists in diagnosis and contributes to pathogenesis. IgG or IgA deposition in the BM zone promotes local inflammation, destruction of hemidesmosomes, and separation of the epidermis from the dermis, with fluid accumulation creating subepidermal bullae or blisters.
Autoimmune bullous pemphigoid (BP) is the most common of these disorders, and is characterized by IgG autoantibodies that bind to BP180, also known as collagen XVII (Col-17), and less commonly to BP230, in the hemidesomosome that connects the epidermal keratinocyte to the dermis. Col-17 is a transmembrane protein involved in assembly of hemidesmisomes. Diagnosis involves demonstration of linear IgG and/or complement C3 staining at the epidermal-dermal junction. The vast majority of patients have autoantibodies against epitopes on the extracellular noncollagenous 16A (NC16A) domain of Col-17 [174]. A subgroup of patients suffer from chronic pruritus and develop urticarial plaques, anti-Col-17 IgE autoantibodies, elevated IgE levels, and lesional eosinophilic infiltrates [175, 176]. Patients’ autoantibodies do not bind mouse Col-17 due to cross-species differences in Col-17 sequence; however, passive transfer of rabbit anti-mouse BP180 Ig induces BP-like skin lesions in wildtype mice [177], and patients’ IgG autoantibodies induce blisters in Col-17-humanized mice [178], confirming a critical role for autoantibodies in pathogenesis. Several Ig effector pathways are implicated in blister formation, including Fc dependent (complement and FcγR binding) and independent pathways, mast cell degranulation, neutrophil recruitment, and antibody-mediated local Col-17 depletion (reviewed in [179]. Anti-Col-17 autoantibodies can be induced in multiple mouse strains by repetitive immunization with a peptide derived from a homologous noncollagenous domain of murine Col-17; however, blisters and eosinophilic infiltrates develop only in the SJL/J strain [180].
It was recently determined that a subset of BP autoantibodies target epitopes, including neoepitopes, on the collagenous and noncollagenous regions of the soluble ectodomain of Col-17 that is shed by proteolysis [181]. Serum IgG or IgA autoantibodies from patients with several rarer autoimmune dermatoses, including cicatricial pemphigoid, linear IgA dermatosis, and chronic bullous dermatosis of childhood, also bind to shed Col-17 ectodomains.
Epidermolysis bullosa acquisita (EBA) is a blistering disease of skin and mucous membranes characterized by autoantibodies that target epitopes in the noncollagenous NC1 and NC2 domains of collagen VII (Col-7) [182–185]. Col-7 is a major constituent of anchoring fibrils in the lamina densa at the epidermal-dermal junction [186]. Whereas early attempts to establish a mouse model by passive transfer of patient IgG were unsuccessful, lesions similar to those in patients were induced in mice by transfer of rabbit anti-mouse Col-7 IgG to nude and wildtype B6 and BALB/c recipients [187]. Failure of transferred F(ab’)2 fragments or C5-deficient recipients to develop blisters implicates Fc- and complement-dependent mechanisms in local injury in this disease. Autoimmune EBA can be induced in mice, particularly the SJL-1 strain, by active immunization with murine Col-7 [188]. Multiple pathogenic epitopes distributed across the Col-7 NC1, but not NC2, domain have been identified in mice [189]. Anti-Col-7 autoantibodies are also implicated in blistering skin manifestations in SLE, termed bullous SLE or BSLE.
Autoimmunity against laminin is implicated in several dermatoses. Anti-laminin-332 mucous membrane pemphigoid (MMP) is a rare blistering disorder that primarily affects the mucous membranes of the oral cavity, pharynx, and larynx [190, 191]. MMP is characterized by autoantibodies to laminin- α3β3γ2 (previously laminin 5, or epiligrin), a component of anchoring filaments in the epidermal BM. Most patients’ IgG are reactive with the α subunit [192], and do not recognize mouse BM. Passive transfer of rabbit anti-laminin5 antibodies induces subepidermal skin and mucous membrane blisters in neonatal mice [193], and transfer of patients’ α-subunit-specific anti-laminin IgG induces subepidermal blisters in human skin grafts on SCID mice [194], confirming in vivo pathogenicity.
In anti-laminin γ1 pemphigoid, previously known as anti-p200 pemphigoid, subepidermal blisters are induced by anti-laminin γ1 IgG [195]. Laminin γ1 is found in most laminin isoforms and is ubiquitous in BM; it is found in several laminin isoforms in skin. It is unclear why disease is restricted to skin in patients with circulating anti-laminin γ1 autoantibodies and blisters. Autoantibodies to laminin γ1 have also been associated with skin lesions in cutaneous and systemic lupus erythematosus and scleroderma [196–198]. Rabbit anti-mouse laminin γ1 IgG have been demonstrated to disrupt adhesion and initiate Fc-mediated effector functions in vitro, suggesting a direct role in pathogenicity [198].
BM autoimmunity in additional clinical and experimental settings
Autoantibodies to BM components have been implicated in several additional settings. Anti-laminin antibodies are detected in murine and human SLE, including among a subset of crossreactive anti-DNA Ig, and may mediate BM binding [199–202]. Anti-laminin autoantibodies have been associated with Raynaud’s phenomenon, cardiomyopathy, pregnancy loss, and psoriasis in man [203–205]. Pathogenic potential of anti-laminin IgG is suggested by induction of proteinuria and glomerular BM alterations in rats after passive transfer of anti-laminin Ig [206], and by the ability of laminin peptides to suppress lupus nephritis in MRL/lpr mice [207]. Glomerular and alveolar BM changes with immune deposits, myocarditis, and pregnancy loss have been reported in rodents immunized with laminin [208–210]. The limited and variable capacity to induce pathogenic anti-laminin responses may be related in part to stringent tolerance mechanisms controlling anti-laminin B cells. In an autoantibody transgenic model generated with a prototypic murine lupus-derived antibody reactive with laminin1, anti-laminin B cells are stringently regulated by deletion, receptor editing, and anergy in nonautoimmune B6 mice [211, 212]. Altered tolerance phenotypes are observed when the Ig transgene is expressed on different lupus backgrounds; however, additional stimuli, such as endotoxin exposure, are generally required to induce anti-laminin autoantibodies [213].
Anti-collagen V autoimmunity was recently implicated in atherosclerosis. Collagens are major components of atherosclerotic plaques, as are infiltrating CD4+ T cells and macrophages. Dart et al demonstrated that peripheral blood cells from patients with severe coronary artery disease exhibit specific cellular immunity to the α1 chain of Col-V in a transvivo delayed-type hypersensitivity assay [214]. Cell depletion or the presence of neutralizing antibodies indicate that the anti-Col-V response is dependent on CD4+ T cells, HLA-DR, CD14+ monocytes, and Th1 and Th17 cytokines. Patients’ cells do not react with α2(V), Col-I, or Col-II, and elevated serum levels of anti-Col-V autoantibodies are not detected in patients. A similar collagen-type-specific T cell response was identified in ApoE-deficient mice fed a high fat diet, a murine model of atherosclerosis [214]. Unlike patients, atherosclerotic mice also developed elevated levels of anti-Col-V autoantibodies. Whereas these responses were not seen in unmanipulated ApoE-deficient mice fed regular chow, immunization with Col-V induced anti-Col-V cellular immunity, anti-Col-V autoantibodies, aortic wall C3d deposits, and severe atherosclerotic lesions containing IL-17+ T cells and macrophages, supporting a causal role for Col-V autoimmunity.
Summary
BM components are targets of autoimmune attack that leads to destruction of kidneys, lungs, joints, skin, and other organs in man. Major antigenic targets include the α3 and α5 noncollagenous domains of type IV collagen in anti-GBM GN and GP disease, Col-V in BOS that is a major cause of death in lung transplant recipients, native and citrullinated Col-II in RA, and Col-17, Col-7, laminin332, and lamininγ1 in autoimmune dermatoses. Animal models based on passive transfer and active immunization have demonstrated a key role for autoantibodies in pathogenesis in each disease, and provided insight into Fc dependent and independent effector mechanisms that destroy tissues. Novel humanized models that eliminate critical cross-species differences have aided this effort. Rigorous dissection of antigenic epitopes and mechanisms engaged in autoimmunity to type IV collagen in kidney glomerular BM has provided insight into the complexity of autoimmunity targeting BM, and the likely requirement for disruption of regulatory controls at multiple levels for full expression of disease. Additional models and approaches will be needed to dissect the origins of spontaneous anti-basement membrane autoimmunity and to fully understand the roles of environmental factors in disease onset and propagation.
Acknowledgments
The author has received recent support from the NIDDK (R01DK088904, P30DK096493), the NIEHS (R21ES024451), the Institute for Medical Research, and the Durham VA Medical and Research Services. The author thanks the many pioneers and investigators in this field whose critical contributions could not be acknowledged due to space limitations.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American autoimmune related diseases association (AARDA) Autoimmune Statistics. 2016 http://www.Aarda.Org/autoimmune-information/autoimmune-statistics/
- 2.Savage CO, Pusey CD, Bowman C, Rees AJ, Lockwood CM. Antiglomerular basement membrane antibody mediated disease in the British Isles 1980–4. Br Med J (Clin Res Ed) 1986;292:301–4. doi: 10.1136/bmj.292.6516.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bayat A, Kamperis K, Herlin T. Characteristics and outcome of Goodpasture’s disease in children. Clin Rheumatol. 2012;31:1745–51. doi: 10.1007/s10067-012-2062-9. [DOI] [PubMed] [Google Scholar]
- 4.Hudson B, Reeders S, Tryggvason K. Type iv collagen: Structure, gene organization, and role in human diseases. Molecular basis of Goodpasture and Alport syndromes and diffuse leiomyomatosis. J Biol Chem. 1993;268:26033–26036. [PubMed] [Google Scholar]
- 5.Bowman C, Ambrus K, Lockwood CM. Restriction of human IgG subclass expression in the population of auto-antibodies to glomerular basement membrane. Clin Exp Immunol. 1987;69:341–9. [PMC free article] [PubMed] [Google Scholar]
- 6.Segelmark M, Butkowski R, Weislander J. Antigen restriction and IgG subclass among anti-GBM autoantibodies. Nephrol Dial Transplant. 1990;5:991–996. doi: 10.1093/ndt/5.12.991. [DOI] [PubMed] [Google Scholar]
- 7.Noel L, Aucouturier P, Monteiro R, Preud’homme J, Lesavre P. Glomerular and serum immunoglobulin G subclasses in membranous and anti-glomerular basement membrane nephritis. Clin Immunol Immunopath. 1988;46:186–194. doi: 10.1016/0090-1229(88)90181-x. [DOI] [PubMed] [Google Scholar]
- 8.Zhao J, Yan Y, Cui Z, Yang R, Zhao MH. The immunoglobulin G subclass distribution of anti-GBM autoantibodies against rhalpha3(IV)NC1 is associated with disease severity. Hum Immunol. 2009;70:425–9. doi: 10.1016/j.humimm.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 9.Ohlsson S, Herlitz H, Lundberg S, Selga D, Molne J, Wieslander J, et al. Circulating anti-glomerular basement membrane antibodies with predominance of subclass IgG4 and false-negative immunoassay test results in anti-glomerular basement membrane disease. Am J Kidney Dis. 2014;63:289–93. doi: 10.1053/j.ajkd.2013.08.032. [DOI] [PubMed] [Google Scholar]
- 10.O’Donoghue DJ, Short CD, Brenchley PE, Lawler W, Ballardie FW. Sequential development of systemic vasculitis with anti-neutrophil cytoplasmic antibodies complicating anti-glomerular basement membrane disease. Clin Neph. 1989;32:251–5. [PubMed] [Google Scholar]
- 11.Jayne DR, Marshall PD, Jones SJ, Lockwood CM. Autoantibodies to GBM and neutrophil cytoplasm in rapidly progressive glomerulonephritis. Kidney Int. 1990;37:965–70. doi: 10.1038/ki.1990.72. [DOI] [PubMed] [Google Scholar]
- 12.Rutgers A, Slot M, van Paassen P, van Breda Vriesman P, Heeringa P, Tervaert JW. Coexistence of anti-glomerular basement membrane antibodies and myeloperoxidase-ancas in crescentic glomerulonephritis. Am J Kidney Dis. 2005;46:253–62. doi: 10.1053/j.ajkd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 13.Hellmark T, Segelmark M. Diagnosis and classification of Goodpasture’s disease (anti-GBM) J Autoimmunity. 2014;48–49:108–12. doi: 10.1016/j.jaut.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Wilson C, Dixon F. Anti-glomerular basement membrane antidbdy-induced glomeulonephritis. Kidney Int. 1973;3:74–89. doi: 10.1038/ki.1973.14. [DOI] [PubMed] [Google Scholar]
- 15.Knoll G, Rabin E, Burns BF. Antiglomerular basement membrane antibody-mediated nephritis with normal pulmonary and renal function. A case report and review of the literature. Am J Nephrol. 1993;13:494–6. doi: 10.1159/000168670. [DOI] [PubMed] [Google Scholar]
- 16.Ang C, Savige J, Dawborn J, Miach P, Heale W, Clarke B, et al. Anti-glomerular basement membrane (GBM)-antibody-mediated disease with normal renal function. Nephrol Dial Transplant. 1998;13:935–9. doi: 10.1093/ndt/13.4.935. [DOI] [PubMed] [Google Scholar]
- 17.Cui Z, Zhao MH, Singh AK, Wang HY. Antiglomerular basement membrane disease with normal renal function. Kidney Int. 2007;72:1403–8. doi: 10.1038/sj.ki.5002525. [DOI] [PubMed] [Google Scholar]
- 18.Sethi S, Lewin M, Lopez L, Lager D. Linear anti-glomerular basement membrane IgG but no glomerular disease: Goodpasture’s syndrome restricted to the lung. Nephrol Dial Transplant. 2007;22:1233–5. doi: 10.1093/ndt/gfl841. [DOI] [PubMed] [Google Scholar]
- 19.Olaru F, Wang XP, Luo W, Ge L, Miner JH, Kleinau S, et al. Proteolysis breaks tolerance toward intact alpha345(IV) collagen, eliciting novel anti-glomerular basement membrane autoantibodies specific for alpha345NC1 hexamers. J Immunol. 2013;190:1424–32. doi: 10.4049/jimmunol.1202204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaer AJ, Stewart LR, Cheek DE, Hurray D, Self SE. IgA antiglomerular basement membrane nephritis associated with Crohn’s disease: A case report and review of glomerulonephritis in inflammatory bowel disease. Am J Kidney Dis. 2003;41:1097–109. doi: 10.1016/s0272-6386(03)00208-7. [DOI] [PubMed] [Google Scholar]
- 21.Ghohestani RF, Rotunda SL, Hudson B, Gaughan WJ, Farber JL, Webster G, et al. Crescentic glomerulonephritis and subepidermal blisters with autoantibodies to alpha5 and alpha6 chains of type IV collagen. Lab Invest. 2003;83:605–11. doi: 10.1097/01.lab.0000067497.86646.4d. [DOI] [PubMed] [Google Scholar]
- 22.Borza DB, Chedid MF, Colon S, Lager DJ, Leung N, Fervenza FC. Recurrent Goodpasture’s disease secondary to a monoclonal IgA-kappa antibody autoreactive with the alpha1/alpha2 chains of type IV collagen. Am J Kidney Dis. 2005;45:397–406. doi: 10.1053/j.ajkd.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 23.Ho J, Gibson IW, Zacharias J, Fervenza F, Colon S, Borza DB. Antigenic heterogeneity of IgA anti-GBM disease: New renal targets of IgA autoantibodies. Am J Kidney Dis. 2008;52:761–5. doi: 10.1053/j.ajkd.2008.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Troxell ML, Saxena AB, Kambham N. Concurrent anti-glomerular basement membrane disease and membranous glomerulonephritis: A case report and literature review. Clin Nephrol. 2006;66:120–7. doi: 10.5414/cnp66120. [DOI] [PubMed] [Google Scholar]
- 25.Beck LH, Jr, Bonegio RG, Lambeau G, Beck DM, Powell DW, Cummins TD, et al. M-type phospholipase A2 receptor as target antigen in idiopathic membranous nephropathy. N Engl J Med. 2009;361:11–21. doi: 10.1056/NEJMoa0810457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomas NM, Beck LH, Jr, Meyer-Schwesinger C, Seitz-Polski B, Ma H, Zahner G, et al. Thrombospondin type-1 domain-containing 7a in idiopathic membranous nephropathy. N Engl J Med. 2014;371:2277–87. doi: 10.1056/NEJMoa1409354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JJ, Malekpour M, Luo W, Ge L, Olaru F, Wang XP, et al. Murine membranous nephropathy: Immunization with alpha3(IV) collagen fragment induces subepithelial immune complexes and FcgammaR-independent nephrotic syndrome. J Immunol. 2012;188:3268–77. doi: 10.4049/jimmunol.1103368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsai S, Santamaria P. MHC class II polymorphisms, autoreactive T-cells, and autoimmunity. Front Immunol. 2013;4:321. doi: 10.3389/fimmu.2013.00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rees AJ, Peters DK, Compston DA, Batchelor JR. Strong association between HLA-DRW2 and antibody-mediated Goodpasture’s syndrome. Lancet. 1978;1:966–8. doi: 10.1016/s0140-6736(78)90252-0. [DOI] [PubMed] [Google Scholar]
- 30.Huey B, McCormick K, Capper J, Ratliff C, Colombe B, Garovoy M, et al. Associations of HLA-DR and HLA-DQ types with anti-GBM nephritis by sequence-specific oligonucleotide probe hybridization. Kidney Int. 1993;44:307–312. doi: 10.1038/ki.1993.245. [DOI] [PubMed] [Google Scholar]
- 31.Fisher M, Pusey CD, Vaughan RW, Rees AJ. Susceptibility to anti-glomerular basement membrane disease is strongly associated with HLA-DRB1 genes. Kidney Int. 1997;51:222–9. doi: 10.1038/ki.1997.27. [DOI] [PubMed] [Google Scholar]
- 32.Phelps RG, Rees AJ. The HLA complex in Goodpasture’s disease: A model for analyzing susceptibility to autoimmunity. Kidney Int. 1999;56:1638–53. doi: 10.1046/j.1523-1755.1999.00720.x. [DOI] [PubMed] [Google Scholar]
- 33.Kitagawa W, Imai H, Komatsuda A, Maki N, Wakui H, Hiki Y, et al. The HLA-DRB1*1501 allele is prevalent among Japanese patients with anti-glomerular basement membrane antibody-mediated disease. Nephrol Dial Transplant. 2008;23:3126–9. doi: 10.1093/ndt/gfn179. [DOI] [PubMed] [Google Scholar]
- 34.Yang R, Cui Z, Zhao J, Zhao MH. The role of HLA-DRB1 alleles on susceptibility of Chinese patients with anti-GBM disease. Clin Immunol. 2009;133:245–50. doi: 10.1016/j.clim.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Guo W, Castaigne JG, Mooney N, Charron D, Al-Daccak R. Signaling through HLA-DR induces PKC beta-dependent B cell death outside rafts. Eur J Immunol. 2003;33:928–38. doi: 10.1002/eji.200323351. [DOI] [PubMed] [Google Scholar]
- 36.Al-Daccak R, Mooney N, Charron D. MHC class II signaling in antigen-presenting cells. Curr Opin Immunol. 2004;16:108–13. doi: 10.1016/j.coi.2003.11.006. [DOI] [PubMed] [Google Scholar]
- 37.de Almeida DE, Holoshitz J. MHC molecules in health and disease: At the cusp of a paradigm shift. Self/nonself. 2011;2:43–48. doi: 10.4161/self.2.1.15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holoshitz J, Liu Y, Fu J, Joseph J, Ling S, Colletta A, et al. An HLA-DRB1-coded signal transduction ligand facilitates inflammatory arthritis: A new mechanism of autoimmunity. J Immunol. 2013;190:48–57. doi: 10.4049/jimmunol.1202150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin-Kelley VE, Jevnikar AM. Antigen presentation by renal tubular epithelial cells. J Am Soc Nephrol. 1991;2:13–26. doi: 10.1681/ASN.V2113. [DOI] [PubMed] [Google Scholar]
- 40.Fusco C, Andreone V, Coppola G, Luongo V, Guerini F, Pace E, et al. HLA-DRB1*1501 and response to copolymer-1 therapy in relapsing-remitting multiple sclerosis. Neurology. 2001;57:1976–9. doi: 10.1212/wnl.57.11.1976. [DOI] [PubMed] [Google Scholar]
- 41.Svejgaard A. The immunogenetics of multiple sclerosis. Immunogenetics. 2008;60:275–86. doi: 10.1007/s00251-008-0295-1. [DOI] [PubMed] [Google Scholar]
- 42.Henderson RD, Saltissi D, Pender MP. Goodpasture’s syndrome associated with multiple sclerosis. Acta Neurologica Scandinavica. 1998;98:134–5. doi: 10.1111/j.1600-0404.1998.tb01733.x. [DOI] [PubMed] [Google Scholar]
- 43.Coles AJ, Cox A, Le Page E, Jones J, Trip SA, Deans J, et al. The window of therapeutic opportunity in multiple sclerosis: Evidence from monoclonal antibody therapy. J Neurology. 2006;253:98–108. doi: 10.1007/s00415-005-0934-5. [DOI] [PubMed] [Google Scholar]
- 44.Clatworthy MR, Wallin EF, Jayne DR. Anti-glomerular basement membrane disease after alemtuzumab. N Engl J Med. 2008;359:768–9. doi: 10.1056/NEJMc0800484. [DOI] [PubMed] [Google Scholar]
- 45.Meyer D, Coles A, Oyuela P, Purvis A, Margolin DH. Case report of anti-glomerular basement membrane disease following alemtuzumab treatment of relapsing-remitting multiple sclerosis. Multiple sclerosis and related disorders. 2013;2:60–3. doi: 10.1016/j.msard.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 46.Rees A, Demaine A, Welsh K. Association of immunoglobulin gm allotypes with antiglomerular basement membrane antibodies and their titer. Hum Immunol. 1984;10:213–220. doi: 10.1016/0198-8859(84)90087-9. [DOI] [PubMed] [Google Scholar]
- 47.Rees A. Immunogenetics of renal disease. In: Neilson EG, Couser WG, editors. Immunologoy Renal Disease. Lippioncott-Raven; Phil: 1997. pp. 99–125. [Google Scholar]
- 48.Zhou XJ, Lv JC, Bu DF, Yu L, Yang YR, Zhao J, et al. Copy number variation of FcgR3a rather than FcgR3b and FcgR2b is associated with susceptibility to anti-GBM disease. Int Immunol. 2010;22:45–51. doi: 10.1093/intimm/dxp113. [DOI] [PubMed] [Google Scholar]
- 49.Zhou XJ, Lv JC, Yu L, Cui Z, Zhao J, Yang R, et al. FcgR2b gene polymorphism rather than FcgR2a, FcgR3a and FcgR3b is associated with anti-GBM disease in Chinese. Nephrol Dial Transplant. 2010;25:97–101. doi: 10.1093/ndt/gfp374. [DOI] [PubMed] [Google Scholar]
- 50.Nakamura A, Yuasa T, Ujike A, Ono M, Nukiwa T, Ravetch JV, et al. Fcgamma receptor IIb-deficient mice develop Goodpasture’s syndrome upon immunization with type IV collagen: A novel murine model for autoimmune glomerular basement membrane disease. J Exp Med. 2000;191:899–906. doi: 10.1084/jem.191.5.899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharp PE, Martin-Ramirez J, Boross P, Mangsbo SM, Reynolds J, Moss J, et al. Increased incidence of anti-GBM disease in Fcgamma receptor 2b deficient mice, but not mice with conditional deletion of FcgR2b on either B cells or myeloid cells alone. Mol Immunol. 2012;50:49–56. doi: 10.1016/j.molimm.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 52.Donaghy M, Rees A. Cigarette smoking and lung hemorrhage in glomerulonephritis caused by antibodies to glomerular basement membrane. Lancet. 1983;2:1390–1392. doi: 10.1016/s0140-6736(83)90923-6. [DOI] [PubMed] [Google Scholar]
- 53.Kelly P, Haponik E. Goodpasture’s syndrome: Molecular and clinical advances. Medicine. 1994;73:171–185. doi: 10.1097/00005792-199407000-00001. [DOI] [PubMed] [Google Scholar]
- 54.Lazor R, Bigay-Game L, Cottin V, Cadranel J, Decaux O, Fellrath JM, et al. Alveolar hemorrhage in anti-basement membrane antibody disease: A series of 28 cases. Medicine. 2007;86:181–93. doi: 10.1097/md.0b013e318067da56. [DOI] [PubMed] [Google Scholar]
- 55.Williamson SR, Phillips CL, Andreoli SP, Nailescu C. A 25-year experience with pediatric anti-glomerular basement membrane disease. Pediatric Nephrol. 2011;26:85–91. doi: 10.1007/s00467-010-1663-2. [DOI] [PubMed] [Google Scholar]
- 56.McCall AS, Cummings CF, Bhave G, Vanacore R, Page-McCaw A, Hudson BG. Bromine is an essential trace element for assembly of collagen IV scaffolds in tissue development and architecture. Cell. 2014;157:1380–92. doi: 10.1016/j.cell.2014.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lerner RA, Glassock RJ, Dixon FJ. The role of anti-glomerular basement membrane antibody in the pathogenesis of human glomerulonephritis. J Exp Med. 1967;126:989–1004. doi: 10.1084/jem.126.6.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Butkowski R, Langeveld J, Weislander J, Hamilton J, Hudson B. Localization of the Goodpasture epitope to a novel chain of basement membrane collagen. J Biol Chem. 1987;262:7874–7877. [PubMed] [Google Scholar]
- 59.Saus J, Wieslander J, Langeveld J, Quinones S, Hudson B. Identification of the Goodpasture antigen as the alpha 3(IV) chain of collagen IV. J Biol Chem. 1988;263:13374–80. [PubMed] [Google Scholar]
- 60.Turner N, Mason PJ, Brown R, Fox M, Povey S, Rees A, et al. Molecular cloning of the human Goodpasture antigen demonstrates it to be the alpha 3 chain of type IV collagen. J Clin Invest. 1992;89:592–601. doi: 10.1172/JCI115625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Neilson E, Kalluri R, Sun M, Gunwa rS, Danoff T, Mariyama M, et al. Specificity of Goodpasture autoantibodies for the recombinant noncollagenous domains of human type IV collagen. J Biol Chem. 1993;268:8402–5. [PubMed] [Google Scholar]
- 62.Kalluri R, Wilson CB, Weber M, Gunwar S, Chonko AM, Neilson EG, et al. Identification of the alpha3(IV) chain of type IV collagen as the common autoantigen in anti-basement membrane disease and Goodpasture syndrome. J Am Soc Nephrol. 1995;6:1178–1185. doi: 10.1681/ASN.V641178. [DOI] [PubMed] [Google Scholar]
- 63.Kalluri R, Gattone VJ, Noelken M, Hudson B. The alpha3(IV) chain of type IV collagen induces autoimmune Goodpasture’s syndrome. Proc Natl Acad Sci USA. 1994;91:6201–6205. doi: 10.1073/pnas.91.13.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sado Y, Boutaud A, Kagawa M, Naito I, Ninomiya Y, Hudson B. Induction of anti-GBM nephritis in rats by recombinant alpha 3(IV)NC1 and alpha 4(IV)NC1 of type IV collagen. Kidney Int. 1998;53:664–71. doi: 10.1046/j.1523-1755.1998.00795.x. [DOI] [PubMed] [Google Scholar]
- 65.Abbate M, Kalluri R, Corna D, Yamaguchi N, McCluskey R, Hudson B, et al. Experimental Goodpasture’s syndrome in Wistar-Kyoto rats immunized with alpha3 chain of type IV collagen. Kidney Int. 1998;54:1550–61. doi: 10.1046/j.1523-1755.1998.00153.x. [DOI] [PubMed] [Google Scholar]
- 66.Steblay R. Glomerulonephritis induced in sheep by injections of heterologous glomerular basement membrane and Freund’s complete adjuvant. J Exp Med. 1962;116:253–272. doi: 10.1084/jem.116.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bolton WK, Tucker FL, Sturgill BC. New avian model of experimental glomerulonephritis consistent with mediation by cellular immunity. J Clin Invest. 1984;73:1263–1276. doi: 10.1172/JCI111328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sado Y, Naito I, Akita M, Okigaki T. Strain specific responses of inbred rats on the severity of experimental autoimmune glomerulonephritis. J Clin Lab Immunol. 1986;19:193–9. [PubMed] [Google Scholar]
- 69.Kalluri R, Danoff TM, Okada H, Neilson EG. Susceptibility to anti-glomerular basement membrane disease and goodpasture syndrome is linked to MHC class II genes and the emergence of T cell-mediated immunity in mice. J Clin Invest. 1997;100:2263–2275. doi: 10.1172/JCI119764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ryan JJ, Reynolds J, Norgan VA, Pusey CD. Expression and characterization of recombinant rat alpha 3(IV)NC1 and its use in induction of experimental autoimmune glomerulonephritis. Nephrol Dial Transplant. 2001;16:253–61. doi: 10.1093/ndt/16.2.253. [DOI] [PubMed] [Google Scholar]
- 71.Wu J, Hicks J, Ou C, Singleton D, Borillo J, Lou YH. Glomerulonephritis induced by recombinant collagen IV alpha 3 chain noncollagen domain 1 is not associated with glomerular basement membrane antibody: A potential T cell-mediated mechanism. J Immunol. 2001;167:2388–95. doi: 10.4049/jimmunol.167.4.2388. [DOI] [PubMed] [Google Scholar]
- 72.Wu J, Hicks J, Borillo J, Glass WF, II, Lou Y-H. CD4+ T cells specific to a glomerular basement membrane antigen mediate glomerulonephritis. J Clin Invest. 2002;109:517–524. doi: 10.1172/JCI13876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hopfer H, Maron R, Butzmann U, Helmchen U, Weiner HL, Kalluri R. The importance of cell-mediated immunity in the course and severity of autoimmune anti-glomerular basement membrane disease in mice. Faseb J. 2003;17:860–8. doi: 10.1096/fj.02-0746com. [DOI] [PubMed] [Google Scholar]
- 74.Bolton WK, Chen L, Hellmark T, Wieslander J, Fox JW. Epitope spreading and autoimmune glomerulonephritis in rats induced by a T cell epitope of Goodpasture’s antigen. J Am Soc Nephrol. 2005;16:2657–2666. doi: 10.1681/ASN.2004100823. [DOI] [PubMed] [Google Scholar]
- 75.Reynolds J, Albouainain A, Duda MA, Evans DJ, Pusey CD. Strain susceptibility to active induction and passive transfer of experimental autoimmune glomerulonephritis in the rat. Nephrol Dial Transplant. 2006;21:3398–408. doi: 10.1093/ndt/gfl523. [DOI] [PubMed] [Google Scholar]
- 76.Xie C, Sharma R, Wang H, Zhou XJ, Mohan C. Strain distribution pattern of susceptibility to immune-mediated nephritis. J Immunol. 2004;172:5047–55. doi: 10.4049/jimmunol.172.8.5047. [DOI] [PubMed] [Google Scholar]
- 77.Netzer KO, Leinonen A, Boutaud A, Borza DB, Todd P, Gunwar S, et al. The Goodpasture autoantigen. Mapping the major conformational epitope(s) of alpha3(IV) collagen to residues 17–31 and 127–141 of the NC1 domain. J Biol Chem. 1999;274:11267–74. doi: 10.1074/jbc.274.16.11267. [DOI] [PubMed] [Google Scholar]
- 78.Wieslander J, Langeveld J, Butkowski R, Jodlowski M, Noelken M, Hudson BG. Physical and immunochemical studies of the globular domain of type IV collagen: Cryptic properties of the Goodpasture antigen. J Biol Chem. 1985;260:8564–8570. [PubMed] [Google Scholar]
- 79.Borza DB, Netzer KO, Leinonen A, Todd P, Cervera J, Saus J, et al. The Goodpasture autoantigen. Identification of multiple cryptic epitopes on the NC1 domain of the alpha3(IV) collagen chain. J Biol Chem. 2000;275:6030–7. doi: 10.1074/jbc.275.8.6030. [DOI] [PubMed] [Google Scholar]
- 80.Borza DB, Bondar O, Colon S, Todd P, Sado Y, Neilson EG, et al. Goodpasture autoantibodies unmask cryptic epitopes by selectively dissociating autoantigen complexes lacking structural reinforcement: Novel mechanisms for immune privilege and autoimmune pathogenesis. J Biol Chem. 2005;280:27147–54. doi: 10.1074/jbc.M504050200. [DOI] [PubMed] [Google Scholar]
- 81.Pedchenko V, Bondar O, Fogo A, vanacore R, Voziyan P, Kitching AR, et al. Molecular architecture of the Goodpasture autoantigen in anti-GBM nephritis. N Engl J Med. 2010;363:343–354. doi: 10.1056/NEJMoa0910500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Borza DB, Hudson BG. Molecular characterization of the target antigens of anti-glomerular basement membrane antibody disease. Springer Semin Immunopathol. 2003;24:345–61. doi: 10.1007/s00281-002-0103-1. [DOI] [PubMed] [Google Scholar]
- 83.Vanacore RM, Ham AJ, Cartailler JP, Sundaramoorthy M, Todd P, Pedchenko V, et al. A role for collagen IV cross-links in conferring immune privilege to the Goodpasture autoantigen: Structural basis for the crypticity of B cell epitopes. J Biol Chem. 2008;283:22737–48. doi: 10.1074/jbc.M803451200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vanacore R, Ham AJ, Voehler M, Sanders CR, Conrads TP, Veenstra TD, et al. A sulfilimine bond identified in collagen IV. Science. 2009;325:1230–4. doi: 10.1126/science.1176811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Bhave G, Cummings CF, Vanacore RM, Kumagai-Cresse C, Ero-Tolliver IA, Rafi M, et al. Peroxidasin forms sulfilimine chemical bonds using hypohalous acids in tissue genesis. Nat Chem Biol. 2012;8:784–90. doi: 10.1038/nchembio.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McCall AS, Bhave GB, Pedchenko V, Fogo AB, Little DJ, Baker TP, et al. Novel anti-peroxidasin antibodies are part of the autoimmune milieu in preclinical and clinical Goodpasture’s disease. J Am Soc Nephrol. 2015;26:47A. [Google Scholar]
- 87.Rutgers A, Meyers KE, Canziani G, Kalluri R, Lin J, Madaio MP. High affinity of anti-GBM antibodies from Goodpasture and transplanted Alport patients to alpha3(IV)NC1 collagen. Kidney Int. 2000;58:115–22. doi: 10.1046/j.1523-1755.2000.00146.x. [DOI] [PubMed] [Google Scholar]
- 88.Dougan T, Levy JB, Salama A, George AJ, Pusey CD. Characterization of autoantibodies from patients with Goodpasture’s disease using a resonant mirror biosensor. Clin Exp Immunol. 2002;128:555–61. doi: 10.1046/j.1365-2249.2002.01867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Cui Z, Wang H, Zhao M. Natural autoantibodies against glomerular basement membrane exist in normal human sera. Kidney Int. 2006;69:894–9. doi: 10.1038/sj.ki.5000135. [DOI] [PubMed] [Google Scholar]
- 90.Cui Z, Zhao MH, Segelmark M, Hellmark T. Natural autoantibodies to myeloperoxidase, proteinase 3, and the glomerular basement membrane are present in normal individuals. Kidney Int. 2010;78:590–7. doi: 10.1038/ki.2010.198. [DOI] [PubMed] [Google Scholar]
- 91.Wine Y, Boutz DR, Lavinder JJ, Miklos AE, Hughes RA, Hoi KH, et al. Molecular deconvolution of the monoclonal antibodies that comprise the polyclonal serum response. Proc Natl Acad Sci U S A. 2013;110:2993–8. doi: 10.1073/pnas.1213737110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lavinder JJ, Wine Y, Giesecke C, Ippolito GC, Horton AP, Lungu OI, et al. Identification and characterization of the constituent human serum antibodies elicited by vaccination. Proc Natl Acad Sci U S A. 2014;111:2259–64. doi: 10.1073/pnas.1317793111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Sackey FN, Congdon KL, Brady GF, Hopfer H, Zhang Y, Mackin KM, Clark AG, Foster MH. Shared variable domain elements among anti-collagen antibodies reactive with Goodpasture epitopes. In: Vogel FL, Zimmermann LF, editors. Autoimmunity: Role, Regulation and Disorder. Nova Science Publishers, Inc; Hauppauge, NY: 2008. pp. 269–281. [Google Scholar]
- 94.de Bono B, Madera M, Chothia C. Vh gene segments in the mouse and human genomes. J Mol Biol. 2004;342:131–43. doi: 10.1016/j.jmb.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 95.Meyers KE, Christensen M, Madaio MP. Modeling of human anti-GBM antibody-alpha3(IV)NC1 interactions predicts antigenic cross-linking through contact of both heavy chains with repeating epitopes on alpha3(IV)NC1. Am J Nephrol. 2009;30:474–80. doi: 10.1159/000242476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Worni-Schudel IM, Clark AG, Chien T, Hwang KK, Chen BJ, Foster MH. Recovery of a human natural antibody against the noncollagenous-1 domain of type IV collagen using humanized models. J Transl Med. 2015;13:185–196. doi: 10.1186/s12967-015-0539-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Foster MH, Buckley ES, Clark AG. Uncommon features in antigen binding sites of human anti-glomerular basement membrane autoantibodies. J Am Soc Nephrol. 2015;26:588A. [Google Scholar]
- 98.Luo W, Wang XP, Kashtan CE, Borza DB. Alport alloantibodies but not Goodpasture autoantibodies induce murine glomerulonephritis: Protection by quinary crosslinks locking cryptic alpha3(IV) collagen autoepitopes in vivo. J Immunol. 2010;185:3520–8. doi: 10.4049/jimmunol.1001152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stevens RH, Macy E, Morrow C, Saxon A. Characterization of a circulating subpopulation of spontaneous antitetanus toxoid antibody producing B cells following in vivo booster immunization. J Immunol. 1979;122:2498–504. [PubMed] [Google Scholar]
- 100.Reddy ST, Ge X, Miklos AE, Hughes RA, Kang SH, Hoi KH, et al. Monoclonal antibodies isolated without screening by analyzing the variable-gene repertoire of plasma cells. Nat Biotech. 2010;28:965–9. doi: 10.1038/nbt.1673. [DOI] [PubMed] [Google Scholar]
- 101.Kalluri R, Torre A, Shield CF, 3rd, Zamborsky ED, Werner MC, Suchin E, et al. Identification of alpha3, alpha4, and alpha5 chains of type IV collagen as alloantigens for Alport posttransplant anti-glomerular basement membrane antibodies. Transplantation. 2000;69:679–83. doi: 10.1097/00007890-200002270-00038. [DOI] [PubMed] [Google Scholar]
- 102.Kashtan CE. Renal transplantation in patients with Alport syndrome. Pediatr Transplant. 2006;10:651–7. doi: 10.1111/j.1399-3046.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 103.Wang XP, Fogo AB, Colon S, Giannico G, Abul-Ezz SR, Miner JH, et al. Distinct epitopes for anti-glomerular basement membrane Alport alloantibodies and Goodpasture autoantibodies within the noncollagenous domain of alpha3(IV) collagen: A janus-faced antigen. J Am Soc Nephrol. 2005;16:3563–71. doi: 10.1681/ASN.2005060670. [DOI] [PubMed] [Google Scholar]
- 104.Kang JS, Kashtan CE, Turner AN, Heidet L, Hudson BG, Borza DB. The alloantigenic sites of alpha3alpha4alpha5(IV) collagen: Pathogenic X-linked Alport alloantibodies target two accessible conformational epitopes in the alpha5NC1 domain. J Biol Chem. 2007;282:10670–7. doi: 10.1074/jbc.M611892200. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Su SC, Hecox DB, Brady GF, Mackin KM, Clark AG, et al. Central tolerance regulates B cells reactive with Goodpasture antigen alpha3(IV)NC1 collagen. J Immunol. 2008;181:6092–100. doi: 10.4049/jimmunol.181.9.6092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Clark AG, Mackin KM, Foster MH. Genetic elimination of alpha3(IV) collagen fails to rescue anti-collagen B cells. Immunol letters. 2011;141:134–9. doi: 10.1016/j.imlet.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Derry CJ, Ross CN, Lombardi G, Mason PD, Rees AJ, Lechler RI, et al. Analysis of T-cell responses to the autoantigen in Goodpasture’s disease. Clin Exp Immunol. 1995;100:262–268. doi: 10.1111/j.1365-2249.1995.tb03663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Merkel F, Kalluri R, Marx M, Enders U, Stevanovic S, Giegerich G, et al. Autoreactive T-cells in Goodpasture’s syndrome recognize the N-terminal NCi domain on a3 type IV collagen. Kidney Int. 1996;49:1127–1133. doi: 10.1038/ki.1996.163. [DOI] [PubMed] [Google Scholar]
- 109.Cairns LS, Phelps RG, Bowie L, Hall AM, Saweirs WW, Rees AJ, et al. The fine specificity and cytokine profile of T-helper cells responsive to the alpha3 chain of type IV collagen in Goodpasture’s disease. J Am Soc Nephrol. 2003;14:2801–12. doi: 10.1097/01.asn.0000091588.80007.0e. [DOI] [PubMed] [Google Scholar]
- 110.Salama AD, Chaudhry AN, Ryan JJ, Eren E, Levy JB, Pusey CD, et al. In Goodpasture’s disease, CD4(+) T cells escape thymic deletion and are reactive with the autoantigen alpha3(IV)NC1. J Am Soc Nephrol. 2001;12:1908–15. doi: 10.1681/ASN.V1291908. [DOI] [PubMed] [Google Scholar]
- 111.Zou J, Hannier S, Cairns LS, Barker RN, Rees AJ, Turner AN, et al. Healthy individuals have Goodpasture autoantigen-reactive T cells. J Am Soc Nephrol. 2008;19:396–404. doi: 10.1681/ASN.2007050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wong D, Phelps RG, Turner AN. The Goodpasture antigen is expressed in the human thymus. Kidney Int. 2001;60:1777–83. doi: 10.1046/j.1523-1755.2001.00014.x. [DOI] [PubMed] [Google Scholar]
- 113.Salama AD, Chaudhry AN, Holthaus KA, Mosley K, Kalluri R, Sayegh MH, et al. Regulation by CD25+ lymphocytes of autoantigen-specific T-cell responses in Goodpasture’s (anti-GBM) disease. Kidney Int. 2003;64:1685–94. doi: 10.1046/j.1523-1755.2003.00259.x. [DOI] [PubMed] [Google Scholar]
- 114.Fridkis-Hareli M. Design of peptide immunotherapies for MHC class-II-associated autoimmune disorders. Clin Dev Immunol. 2013;2013:826191. doi: 10.1155/2013/826191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ooi JD, Chang J, O’Sullivan KM, Pedchenko V, Hudson BG, Vandenbark AA, et al. The HLA-DRB1*15:01-restricted Goodpasture’s T cell epitope induces GN. J Am Soc Nephrol. 2013;24:419–31. doi: 10.1681/ASN.2012070705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wu J, Arends J, Borillo J, Zhou C, Merszei J, McMahon J, et al. A self T cell epitope induces autoantibody response: Mechanism for production of antibodies to diverse glomerular basement membrane antigens. J Immunol. 2004;172:4567–4574. doi: 10.4049/jimmunol.172.7.4567. [DOI] [PubMed] [Google Scholar]
- 117.Robertson J, Wu J, Arends J, Zhou C, McMahon J, Torres L, et al. Activation of glomerular basement membrane-specific B cells in the renal draining lymph node after T cell-mediated glomerular injury. J Am Soc Nephrol. 2005;16:3256–63. doi: 10.1681/ASN.2005040421. [DOI] [PubMed] [Google Scholar]
- 118.Chen L, Hellmark T, Pedchenko V, Hudson BG, Pusey CD, Fox JW, et al. A nephritogenic peptide induces intermolecular epitope spreading on collagen IV in experimental autoimmune glomerulonephritis. J Am Soc Nephrol. 2006;17:3076–81. doi: 10.1681/ASN.2006070688. [DOI] [PubMed] [Google Scholar]
- 119.Bolton W, Chandra M, Tyson T, Kirkpatrick P, Sadovonic M, Sturgill B. Transfer of experimental glomerulonephritis in chickens by mononuclear cells. Kidney Int. 1988;45:598–610. doi: 10.1038/ki.1988.224. [DOI] [PubMed] [Google Scholar]
- 120.Neale TJ, Tipping PG, Carson SD, Holdsworth SR. Participation of cell-mediated immunity in deposition of fibrin in glomerulonephritis. Lancet. 1988;2:421–4. doi: 10.1016/s0140-6736(88)90413-8. [DOI] [PubMed] [Google Scholar]
- 121.Hopfer H, Holzer J, Hunemorder S, Paust HJ, Sachs M, Meyer-Schwesinger C, et al. Characterization of the renal CD4+ Tcell response in experimental autoimmune glomerulonephritis. Kidney Int. 2012;82:60–71. doi: 10.1038/ki.2012.73. [DOI] [PubMed] [Google Scholar]
- 122.Hopfer H, Hunemorder S, Treder J, Turner JE, Paust HJ, Meyer-Schwesinger C, et al. Glomerulopathy induced by immunization with a peptide derived from the Goodpasture antigen alpha3IV-NC1. J Immunol. 2015;194:3646–55. doi: 10.4049/jimmunol.1401267. [DOI] [PubMed] [Google Scholar]
- 123.Tipping PG, Holdsworth SR. T cells in crescentic glomerulonephritis. J Am Soc Nephrol. 2006;17:1253–63. doi: 10.1681/ASN.2005091013. [DOI] [PubMed] [Google Scholar]
- 124.Henique C, Papista C, Guyonnet L, Lenoir O, Tharaux PL. Update on crescentic glomerulonephritis. Semin Immunopathol. 2014;36:479–90. doi: 10.1007/s00281-014-0435-7. [DOI] [PubMed] [Google Scholar]
- 125.Borza CM, Pozzi A, Borza DB, Pedchenko V, Hellmark T, Hudson BG, et al. Integrin alpha3beta1, a novel receptor for alpha3(IV) noncollagenous domain and a trans-dominant inhibitor for integrin alphavbeta3. J Biol Chem. 2006;281:20932–9. doi: 10.1074/jbc.M601147200. [DOI] [PubMed] [Google Scholar]
- 126.Borza CM, Borza DB, Pedchenko V, Saleem MA, Mathieson PW, Sado Y, et al. Human podocytes adhere to the KRGDS motif of the alpha3alpha4alpha5 collagen IV network. J Am Soc Nephrol. 2008;19:677–84. doi: 10.1681/ASN.2007070793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Christie JD, Edwards LB, Kucheryavaya AY, Benden C, Dobbels F, Kirk R, et al. The registry of the international society for heart and lung transplantation: Twenty-eighth adult lung and heart-lung transplant report--2011. J Heart Lung Transplant. 2011;30:1104–22. doi: 10.1016/j.healun.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 128.Burlingham WJ, Love RB, Jankowska-Gan E, Haynes LD, Xu Q, Bobadilla JL, et al. Il-17-dependent cellular immunity to collagen type V predisposes to obliterative bronchiolitis in human lung transplants. J Clin Invest. 2007;117:3498–506. doi: 10.1172/JCI28031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Saini D, Weber J, Ramachandran S, Phelan D, Tiriveedhi V, Liu M, et al. Alloimmunity-induced autoimmunity as a potential mechanism in the pathogenesis of chronic rejection of human lung allografts. J Heart Lung Transplant. 2011;30:624–31. doi: 10.1016/j.healun.2011.01.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bharat A, Saini D, Steward N, Hachem R, Trulock EP, Patterson GA, et al. Antibodies to self-antigens predispose to primary lung allograft dysfunction and chronic rejection. Ann Thorac Surg. 2010;90:1094–101. doi: 10.1016/j.athoracsur.2010.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Goers TA, Ramachandran S, Aloush A, Trulock E, Patterson GA, Mohanakumar T. De novo production of k-alpha1 tubulin-specific antibodies: Role in chronic lung allograft rejection. J Immunol. 2008;180:4487–94. doi: 10.4049/jimmunol.180.7.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Hachem RR, Tiriveedhi V, Patterson GA, Aloush A, Trulock EP, Mohanakumar T. Antibodies to k-alpha 1 tubulin and collagen V are associated with chronic rejection after lung transplantation. Am J Transplant. 2012;12:2164–71. doi: 10.1111/j.1600-6143.2012.04079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Bobadilla JL, Love RB, Jankowska-Gan E, Xu Q, Haynes LD, Braun RK, et al. Th-17, monokines, collagen type V, and primary graft dysfunction in lung transplantation. Am J Resp Crit Care Med. 2008;177:660–8. doi: 10.1164/rccm.200612-1901OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Keller MR, Haynes LD, Jankowska-Gan E, Sullivan JA, Agashe VV, Burlingham SR, et al. Epitope analysis of the collagen type V-specific T cell response in lung transplantation reveals an HLA-DRB1*15 bias in both recipient and donor. PloS one. 2013;8:e79601. doi: 10.1371/journal.pone.0079601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mak KM, Png CY, Lee DJ. Type V collagen in health, disease, and fibrosis. Anatomical Record. 2016;299:613–29. doi: 10.1002/ar.23330. [DOI] [PubMed] [Google Scholar]
- 136.Yoshida S, Haque A, Mizobuchi T, Iwata T, Chiyo M, Webb TJ, et al. Anti-type V collagen lymphocytes that express IL-17 and IL-23 induce rejection pathology in fresh and well-healed lung transplants. Am J Transplant. 2006;6:724–35. doi: 10.1111/j.1600-6143.2006.01236.x. [DOI] [PubMed] [Google Scholar]
- 137.Fukami N, Ramachandran S, Saini D, Walter M, Chapman W, Patterson GA, et al. Antibodies to MHC class I induce autoimmunity: Role in the pathogenesis of chronic rejection. J Immunol. 2009;182:309–18. doi: 10.4049/jimmunol.182.1.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Fukami N, Ramachandran S, Takenaka M, Weber J, Subramanian V, Mohanakumar T. An obligatory role for lung infiltrating B cells in the immunopathogenesis of obliterative airway disease induced by antibodies to MHC class I molecules. Am J Transplant. 2012;12:867–76. doi: 10.1111/j.1600-6143.2011.03917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Subramanian V, Ramachandran S, Banan B, Bharat A, Wang X, Benshoff N, et al. Immune response to tissue-restricted self-antigens induces airway inflammation and fibrosis following murine lung transplantation. Am J Transplant. 2014;14:2359–66. doi: 10.1111/ajt.12908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Yasufuku K, Heidler KM, Woods KA, Smith GN, Jr, Cummings OW, Fujisawa T, et al. Prevention of bronchiolitis obliterans in rat lung allografts by type V collagen-induced oral tolerance. Transplantation. 2002;73:500–5. doi: 10.1097/00007890-200202270-00002. [DOI] [PubMed] [Google Scholar]
- 141.Braun RK, Martin A, Shah S, Iwashima M, Medina M, Byrne K, et al. Inhibition of bleomycin-induced pulmonary fibrosis through pre-treatment with collagen type V. J Heart Lung Transplant. 2010;29:873–80. doi: 10.1016/j.healun.2010.03.012. [DOI] [PubMed] [Google Scholar]
- 142.Vittal R, Mickler EA, Fisher AJ, Zhang C, Rothhaar K, Gu H, et al. Type V collagen induced tolerance suppresses collagen deposition, TGF-beta and associated transcripts in pulmonary fibrosis. PloS one. 2013;8:e76451. doi: 10.1371/journal.pone.0076451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Gregersen PK, Silver J, Winchester RJ. The shared epitope hypothesis. An approach to understanding the molecular genetics of susceptibility to rheumatoid arthritis. Arthritis Rheum. 1987;30:1205–13. doi: 10.1002/art.1780301102. [DOI] [PubMed] [Google Scholar]
- 144.Ling S, Pi X, Holoshitz J. The rheumatoid arthritis shared epitope triggers innate immune signaling via cell surface calreticulin. J Immunol. 2007;179:6359–67. doi: 10.4049/jimmunol.179.9.6359. [DOI] [PubMed] [Google Scholar]
- 145.Padyukov L, Silva C, Stolt P, Alfredsson L, Klareskog L. A gene-environment interaction between smoking and shared epitope genes in HLA-DR provides a high risk of seropositive rheumatoid arthritis. Arthritis Rheum. 2004;50:3085–92. doi: 10.1002/art.20553. [DOI] [PubMed] [Google Scholar]
- 146.Haag S, Schneider N, Mason DE, Tuncel J, Andersson IE, Peters EC, et al. Identification of new citrulline-specific autoantibodies, which bind to human arthritic cartilage, by mass spectrometric analysis of citrullinated type II collagen. Arth Rheum. 2014;66:1440–9. doi: 10.1002/art.38383. [DOI] [PubMed] [Google Scholar]
- 147.van der Helm-van Mil AH, Verpoort KN, Breedveld FC, Huizinga TW, Toes RE, de Vries RR. The HLA-DRB1 shared epitope alleles are primarily a risk factor for anti-cyclic citrullinated peptide antibodies and are not an independent risk factor for development of rheumatoid arthritis. Arthritis Rheum. 2006;54:1117–21. doi: 10.1002/art.21739. [DOI] [PubMed] [Google Scholar]
- 148.Linn-Rasker SP, van der Helm-van Mil AH, van Gaalen FA, Kloppenburg M, de Vries RR, le Cessie S, et al. Smoking is a risk factor for anti-CCP antibodies only in rheumatoid arthritis patients who carry HLA-DRB1 shared epitope alleles. Ann Rheum Dis. 2006;65:366–71. doi: 10.1136/ard.2005.041079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Klareskog L, Ronnelid J, Lundberg K, Padyukov L, Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu Rev Immunol. 2008;26:651–75. doi: 10.1146/annurev.immunol.26.021607.090244. [DOI] [PubMed] [Google Scholar]
- 150.Sakkas LI, Bogdanos DP, Katsiari C, Platsoucas CD. Anti-citrullinated peptides as autoantigens in rheumatoid arthritis-relevance to treatment. Autoimmun Rev. 2014;13:1114–20. doi: 10.1016/j.autrev.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 151.Hill JA, Bell DA, Brintnell W, Yue D, Wehrli B, Jevnikar AM, et al. Arthritis induced by posttranslationally modified (citrullinated) fibrinogen in DR4-IE transgenic mice. J Exp Med. 2008;205:967–79. doi: 10.1084/jem.20072051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Clague RB, Shaw MJ, Holt PJ. Incidence and correlation between serum IgG and IgM antibodies to native type II collagen in patients with chronic inflammatory arthritis. Ann Rheum Dis. 1981;40:6–10. doi: 10.1136/ard.40.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mullazehi M, Mathsson L, Lampa J, Ronnelid J. High anti-collagen type-II antibody levels and induction of proinflammatory cytokines by anti-collagen antibody-containing immune complexes in vitro characterise a distinct rheumatoid arthritis phenotype associated with acute inflammation at the time of disease onset. Ann Rheum Dis. 2007;66:537–41. doi: 10.1136/ard.2006.064782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Londei M, Savill CM, Verhoef A, Brennan F, Leech ZA, Duance V, et al. Persistence of collagen type II-specific T-cell clones in the synovial membrane of a patient with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1989;86:636–40. doi: 10.1073/pnas.86.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Kim WU, Cho ML, Jung YO, Min SY, Park SW, Min DJ, et al. Type II collagen autoimmunity in rheumatoid arthritis. Am J Med Sci. 2004;327:202–11. doi: 10.1097/00000441-200404000-00006. [DOI] [PubMed] [Google Scholar]
- 156.Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–68. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schurgers E, Billiau A, Matthys P. Collagen-induced arthritis as an animal model for rheumatoid arthritis: Focus on interferon-gamma. J interferon Cytokine Res. 2011;31:917–26. doi: 10.1089/jir.2011.0056. [DOI] [PubMed] [Google Scholar]
- 158.Stuart JM, Townes AS, Kang AH. Collagen autoimmune arthritis. Annu Rev Immunol. 1984;2:199–218. doi: 10.1146/annurev.iy.02.040184.001215. [DOI] [PubMed] [Google Scholar]
- 159.Burkhardt H, Koller T, Engstrom A, Nandakumar KS, Turnay J, Kraetsch HG, et al. Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse. Arthritis Rheum. 2002;46:2339–48. doi: 10.1002/art.10472. [DOI] [PubMed] [Google Scholar]
- 160.Lindh I, Snir O, Lonnblom E, Uysal H, Andersson I, Nandakumar KS, et al. Type II collagen antibody response is enriched in the synovial fluid of rheumatoid joints and directed to the same major epitopes as in collagen induced arthritis in primates and mice. Arthritis Res Ther. 2014;16:R143. doi: 10.1186/ar4605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kuhn KA, Kulik L, Tomooka B, Braschler KJ, Arend WP, Robinson WH, et al. Antibodies against citrullinated proteins enhance tissue injury in experimental autoimmune arthritis. J Clin Invest. 2006;116:961–73. doi: 10.1172/JCI25422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Willis VC, Gizinski AM, Banda NK, Causey CP, Knuckley B, Cordova KN, et al. N-alpha-benzoyl-n5-(2-chloro-1-iminoethyl)-l-ornithine amide, a protein arginine deiminase inhibitor, reduces the severity of murine collagen-induced arthritis. J Immunol. 2011;186:4396–404. doi: 10.4049/jimmunol.1001620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Holmdahl R, Jansson L, Larsson A, Jonsson R. Arthritis in DBA/1 mice induced with passively transferred type II collagen immune serum. Immunohistopathology and serum levels of anti-type II collagen auto-antibodies. Scand J Immunol. 1990;31:147–57. doi: 10.1111/j.1365-3083.1990.tb02754.x. [DOI] [PubMed] [Google Scholar]
- 164.Nandakumar KS, Svensson L, Holmdahl R. Collagen type II-specific monoclonal antibody-induced arthritis in mice: Description of the disease and the influence of age, sex, and genes. Am J Pathol. 2003;163:1827–37. doi: 10.1016/S0002-9440(10)63542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Nandakumar KS, Holmdahl R. Efficient promotion of collagen antibody induced arthritis (CAIA) using four monoclonal antibodies specific for the major epitopes recognized in both collagen induced arthritis and rheumatoid arthritis. J Immunol Methods. 2005;304:126–36. doi: 10.1016/j.jim.2005.06.017. [DOI] [PubMed] [Google Scholar]
- 166.Mo JA, Holmdahl R. The b cell response to autologous type II collagen: Biased V gene repertoire with V gene sharing and epitope shift. J Immunol. 1996;157:2440–8. [PubMed] [Google Scholar]
- 167.Boiers U, Lanig H, Sehnert B, Holmdahl R, Burkhardt H. Collagen type II is recognized by a pathogenic antibody through germline encoded structures. Eur J Immunol. 2008;38:2784–95. doi: 10.1002/eji.200838238. [DOI] [PubMed] [Google Scholar]
- 168.Cao D, Khmaladze I, Jia H, Bajtner E, Nandakumar KS, Blom T, et al. Pathogenic autoreactive B cells are not negatively selected toward matrix protein collagen II. J Immunol. 2011;187:4451–8. doi: 10.4049/jimmunol.1101378. [DOI] [PubMed] [Google Scholar]
- 169.Park KS, Park MJ, Cho ML, Kwok SK, Ju JH, Ko HJ, et al. Type II collagen oral tolerance; mechanism and role in collagen-induced arthritis and rheumatoid arthritis. Mod Rheumatol. 2009;19:581–9. doi: 10.1007/s10165-009-0210-0. [DOI] [PubMed] [Google Scholar]
- 170.Nandakumar KS, Andren M, Martinsson P, Bajtner E, Hellstrom S, Holmdahl R, et al. Induction of arthritis by single monoclonal IgG anti-collagen type II antibodies and enhancement of arthritis in mice lacking inhibitory FcgammaRIIb. Eur J Immunol. 2003;33:2269–77. doi: 10.1002/eji.200323810. [DOI] [PubMed] [Google Scholar]
- 171.Nandakumar KS, Collin M, Olsen A, Nimmerjahn F, Blom AM, Ravetch JV, et al. Endoglycosidase treatment abrogates IgG arthritogenicity: Importance of IgG glycosylation in arthritis. Eur J Immunol. 2007;37:2973–82. doi: 10.1002/eji.200737581. [DOI] [PubMed] [Google Scholar]
- 172.Ohmi Y, Ise W, Harazono A, Takakura D, Fukuyama H, Baba Y, et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat Commun. 2016;7:11205. doi: 10.1038/ncomms11205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mustafa MB, Porter SR, Smoller BR, Sitaru C. Oral mucosal manifestations of autoimmune skin diseases. Autoimmun Rev. 2015;14:930–51. doi: 10.1016/j.autrev.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 174.Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol. 1993;151:5742–50. [PubMed] [Google Scholar]
- 175.Messingham KN, Srikantha R, DeGueme AM, Fairley JA. FcR-independent effects of IgE and IgG autoantibodies in bullous pemphigoid. J Immunol. 2011;187:553–60. doi: 10.4049/jimmunol.1001753. [DOI] [PubMed] [Google Scholar]
- 176.Moriuchi R, Nishie W, Ujiie H, Natsuga K, Shimizu H. In vivo analysis of IgE autoantibodies in bullous pemphigoid: A study of 100 cases. J Derm Sci. 2015;78:21–5. doi: 10.1016/j.jdermsci.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 177.Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, et al. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest. 1993;92:2480–8. doi: 10.1172/JCI116856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, et al. Humanization of autoantigen. Nat Med. 2007;13:378–83. doi: 10.1038/nm1496. [DOI] [PubMed] [Google Scholar]
- 179.Nishie W. Update on the pathogenesis of bullous pemphigoid: An autoantibody-mediated blistering disease targeting collagen XVII. J Derm Sci. 2014;73:179–86. doi: 10.1016/j.jdermsci.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 180.Hirose M, Recke A, Beckmann T, Shimizu A, Ishiko A, Bieber K, et al. Repetitive immunization breaks tolerance to type XVII collagen and leads to bullous pemphigoid in mice. J Immunol. 2011;187:1176–83. doi: 10.4049/jimmunol.1100596. [DOI] [PubMed] [Google Scholar]
- 181.Schumann H, Baetge J, Tasanen K, Wojnarowska F, Schacke H, Zillikens D, et al. The shed ectodomain of collagen XVII/BP180 is targeted by autoantibodies in different blistering skin diseases. Am J Pathol. 2000;156:685–95. doi: 10.1016/S0002-9440(10)64772-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ishii N, Yoshida M, Hisamatsu Y, Ishida-Yamamoto A, Nakane H, Iizuka H, et al. Epidermolysis bullosa acquisita sera react with distinct epitopes on the NC1 and NC2 domains of type VII collagen: Study using immunoblotting of domain-specific recombinant proteins and postembedding immunoelectron microscopy. Br J Dermatol. 2004;150:843–51. doi: 10.1111/j.1365-2133.2004.05933.x. [DOI] [PubMed] [Google Scholar]
- 183.Nagase T, Ishikawa K, Nakajima D, Ohira M, Seki N, Miyajima N, et al. Prediction of the coding sequences of unidentified human genes. Vii. The complete sequences of 100 new cDNA clones from brain which can code for large proteins in vitro. DNA Res. 1997;4:141–50. doi: 10.1093/dnares/4.2.141. [DOI] [PubMed] [Google Scholar]
- 184.Saleh MA, Ishii K, Kim YJ, Murakami A, Ishii N, Hashimoto T, et al. Development of NC1 and NC2 domains of type VII collagen ELISA for the diagnosis and analysis of the time course of epidermolysis bullosa acquisita patients. J Derm Sci. 2011;62:169–75. doi: 10.1016/j.jdermsci.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 185.Tanaka T, Furukawa F, Imamura S. Epitope mapping for epidermolysis bullosa acquisita autoantibody by molecularly cloned cdna for type VII collagen. J Invest derm. 1994;102:706–9. doi: 10.1111/1523-1747.ep12374333. [DOI] [PubMed] [Google Scholar]
- 186.Woodley DT, Briggaman RA, O’Keefe EJ, Inman AO, Queen LL, Gammon WR. Identification of the skin basement-membrane autoantigen in epidermolysis bullosa acquisita. N Engl J Med. 1984;310:1007–13. doi: 10.1056/NEJM198404193101602. [DOI] [PubMed] [Google Scholar]
- 187.Sitaru C, Mihai S, Otto C, Chiriac MT, Hausser I, Dotterweich B, et al. Induction of dermal-epidermal separation in mice by passive transfer of antibodies specific to type VII collagen. J Clin Invest. 2005;115:870–8. doi: 10.1172/JCI21386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Sitaru C, Chiriac MT, Mihai S, Buning J, Gebert A, Ishiko A, et al. Induction of complement-fixing autoantibodies against type VII collagen results in subepidermal blistering in mice. J Immunol. 2006;177:3461–8. doi: 10.4049/jimmunol.177.5.3461. [DOI] [PubMed] [Google Scholar]
- 189.Csorba K, Chiriac MT, Florea F, Ghinia MG, Licarete E, Rados A, et al. Blister-inducing antibodies target multiple epitopes on collagen VII in mice. J Cell Mol Med. 2014;18:1727–39. doi: 10.1111/jcmm.12338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Domloge-Hultsch N, Anhalt GJ, Gammon WR, Lazarova Z, Briggaman R, Welch M, et al. Antiepiligrin cicatricial pemphigoid. A subepithelial bullous disorder. Arch Dermatol. 1994;130:1521–9. [PubMed] [Google Scholar]
- 191.Egan CA, Lazarova Z, Darling TN, Yee C, Yancey KB. Anti-epiligrin cicatricial pemphigoid: Clinical findings, immunopathogenesis, and significant associations. Medicine. 2003;82:177–86. doi: 10.1097/01.md.0000076003.64510.00. [DOI] [PubMed] [Google Scholar]
- 192.Kirtschig G, Marinkovich MP, Burgeson RE, Yancey KB. Anti-basement membrane autoantibodies in patients with anti-epiligrin cicatricial pemphigoid bind the alpha subunit of laminin 5. J Invest Derm. 1995;105:543–8. doi: 10.1111/1523-1747.ep12323431. [DOI] [PubMed] [Google Scholar]
- 193.Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest. 1996;98:1509–18. doi: 10.1172/JCI118942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Lazarova Z, Hsu R, Yee C, Yancey KB. Human anti-laminin 5 autoantibodies induce subepidermal blisters in an experimental human skin graft model. J Invest Derm. 2000;114:178–84. doi: 10.1046/j.1523-1747.2000.00829.x. [DOI] [PubMed] [Google Scholar]
- 195.Dainichi T, Koga H, Tsuji T, Ishii N, Ohyama B, Ueda A, et al. From anti-p200 pemphigoid to anti-laminin gamma1 pemphigoid. J Derm. 2010;37:231–8. doi: 10.1111/j.1346-8138.2009.00793.x. [DOI] [PubMed] [Google Scholar]
- 196.Caproni M, Antiga E, Cardinali C, Del Bianco E, Fabbri P. Antilaminin-1 antibodies in cutaneous lupus erythematosus patients. Lupus. 2009;18:858. doi: 10.1177/0961203308101957. [DOI] [PubMed] [Google Scholar]
- 197.Groth S, Vafia K, Recke A, Dahnrich C, Zillikens D, Stocker W, et al. Antibodies to the C-terminus of laminin gamma1 are present in a distinct subgroup of patients with systemic and cutaneous lupus erythematosus. Lupus. 2012;21:1482–3. doi: 10.1177/0961203312460113. [DOI] [PubMed] [Google Scholar]
- 198.Florea F, Bernards C, Caproni M, Kleindienst J, Hashimoto T, Koch M, et al. Ex vivo pathogenicity of anti-laminin gamma1 autoantibodies. Am J Pathol. 2014;184:494–506. doi: 10.1016/j.ajpath.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 199.Sabbaga J, Line SRP, Potocnjak P, Madaio MP. A murine nephritogenic monoclonal anti-DNA autoantibody binds directly to mouse laminin, the major non-collagenous protein component of the glomerular basement membrane. Eur J Immunol. 1989;19:137–143. doi: 10.1002/eji.1830190122. [DOI] [PubMed] [Google Scholar]
- 200.Foster MH, Sabbaga J, Line SRP, Thompson KS, Barrett KJ, Madaio MP. Molecular analysis of nephrotropic anti-laminin antibodies from an MRL/lpr autoimmune mouse. J Immunol. 1993;151:814–824. [PubMed] [Google Scholar]
- 201.Ben-Yehuda A, Rasooly L, Bar-Tana R, Breuer G, Tadmor B, Ulmansky R, et al. The urine of SLE patients contains antibodies that bind to the laminin component of the extracellular matrix. J Autoimmunity. 1995;8:279–91. doi: 10.1006/jaut.1995.0021. [DOI] [PubMed] [Google Scholar]
- 202.Amital H, Heilweil-Harel M, Ulmansky R, Harlev M, Toubi E, Hershko A, et al. Antibodies against the VRT101 laminin epitope correlate with human SLE disease activity and can be removed by extracorporeal immunoadsorption. Rheumatology (Oxford) 2007;46:1433–7. doi: 10.1093/rheumatology/kem181. [DOI] [PubMed] [Google Scholar]
- 203.Inagaki J, Kondo A, Lopez LR, Shoenfeld Y, Matsuura E. Anti-laminin-1 autoantibodies, pregnancy loss and endometriosis. Clinl Dev Immunol. 2004;11:261–6. doi: 10.1080/17402520400001678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wolff PG, Kuhl U, Schultheiss HP. Laminin distribution and autoantibodies to laminin in dilated cardiomyopathy and myocarditis. Am Heart J. 1989;117:1303–9. doi: 10.1016/0002-8703(89)90410-9. [DOI] [PubMed] [Google Scholar]
- 205.Gabrielli A, Montroni M, Rupoli S, Caniglia ML, DeLustro F, Danieli G. A retrospective study of antibodies against basement membrane antigens (type IV collagen and laminin) in patients with primary and secondary Raynaud’s phenomenon. Arthritis Rheum. 1988;31:1432–6. doi: 10.1002/art.1780311114. [DOI] [PubMed] [Google Scholar]
- 206.Abrahamson DR, Caulfield JP. Proteinuria and structural alterations in rat glomerular basement membranes induced by intravenously injected anti-laminin immunoglobulin G. J Exp Med. 1982;156:128–45. doi: 10.1084/jem.156.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Amital H, Heilweil M, Ulmansky R, Szafer F, Bar-Tana R, Morel L, et al. Treatment with a laminin-derived peptide suppresses lupus nephritis. J Immunol. 2005;175:5516–23. doi: 10.4049/jimmunol.175.8.5516. [DOI] [PubMed] [Google Scholar]
- 208.Murphy-Ullrich JE, Oberley TD. Immune-mediated injury to basement membranes in mice immunized with murine laminin. Clin Immunol Immunopathol. 1984;31:33–43. doi: 10.1016/0090-1229(84)90187-9. [DOI] [PubMed] [Google Scholar]
- 209.Nakano J, Yoshimura T, Okita M, Motomura M, Kamei S, Matsuo H, et al. Laminin-induced autoimmune myositis in rats. J Neuropathol Exp Neurol. 2005;64:790–6. doi: 10.1097/01.jnen.0000178851.76056.0b. [DOI] [PubMed] [Google Scholar]
- 210.Matalon ST, Blank M, Matsuura E, Inagaki J, Nomizu M, Levi Y, et al. Immunization of naive mice with mouse laminin-1 affected pregnancy outcome in a mouse model. Am J Reprod Immunol. 2003;50:159–65. doi: 10.1034/j.1600-0897.2003.00068.x. [DOI] [PubMed] [Google Scholar]
- 211.Rudolph EH, Congdon KL, Sackey FN, Fitzsimons MM, Foster MH. Humoral autoimmunity to basement membrane antigens is regulated in C57Bl/6 and MRL/MPJ mice transgenic for anti-laminin Ig receptors. J Immunol. 2002;168:5943–5953. doi: 10.4049/jimmunol.168.11.5943. [DOI] [PubMed] [Google Scholar]
- 212.Brady GF, Congdon KL, Clark AG, Sackey FN, Rudolph EH, Radic MZ, et al. Kappa editing rescues autoreactive B cells destined for deletion in mice transgenic for a dual specific anti-laminin Ig. J Immunol. 2004;172:5313–21. doi: 10.4049/jimmunol.172.9.5313. [DOI] [PubMed] [Google Scholar]
- 213.Clark AG, Fan Q, Brady GF, Mackin KM, Coffman ED, Weston ML, et al. Regulation of basement membrane-reactive B cells in BXSB, (NZBxNZW)F1, NZB, and MRL/lpr lupus mice. Autoimmunity. 2013;46:188–204. doi: 10.3109/08916934.2012.746671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Dart ML, Jankowska-Gan E, Huang G, Roenneburg DA, Keller MR, Torrealba JR, et al. Interleukin-17-dependent autoimmunity to collagen type V in atherosclerosis. Circ Res. 2010;107:1106–16. doi: 10.1161/CIRCRESAHA.110.221069. [DOI] [PMC free article] [PubMed] [Google Scholar]