Abstract
Background
Pneumonia is a common and potentially serious disease, with an incidence of ca. 300 per 100 000 persons per year. Until now, there have been only a few population-based studies of risk factors for pneumonia.
Methods
From 2000 to 2002, nearly 10 000 persons aged 50 to 75 were recruited into the prospective ESTHER cohort study while visiting their family physician for a check-up. The mean duration of follow-up was 10.6 years. Data on newly diagnosed pneumonia were acquired from the participants and their physicians by means of standardized questionnaires. Potential associations with various predictors were studied in survival-time regression models.
Results
435 participants had pneumonia at least once during follow-up. The cumulative 10-year-incidence was 4.5% (95% confidence interval [4.0; 4.9]). Multiple regression revealed that age (relative risk [RR]: 1.43 [1.22; 1.67] per 10 years), current cigarette smoking (RR: 1.56 [1.19; 2.05], compared with never having smoked), and known congestive heart failure (RR: 1.65 [1.24; 2.20]) were independently associated with an elevated risk of pneumonia. The risk was insignificantly elevated in persons with diabetes mellitus (RR: 1.29 [0.98; 1.68]). Alcohol consumption, obesity, stroke, and cancer were not associated with an elevated risk of pneumonia in age- and sex-adjusted analyses.
Conclusion
Pneumonia plays an important role in the medical care of non-institutionalized older people. With the aid of the predictors identified in this study, primary care physicians can identify patients at risk, smokers can gain additional motivation to quit, treatment compliance can be increased, and patients may become more willing to be vaccinated as recommended in the current guidelines.
Pneumonia is a severe, potentially fatal disease (1). In Germany, there are now ca. 300 hospitalizations for community-acquired pneumonia per 100 000 persons per year; demographic trends imply that the incidence is likely to rise dramatically (2, 3). Pneumonia elevates mortality both during acute hospitalization and after discharge, with an in-hospital mortality of 14% (2) and a 10-year mortality 65% higher than that of control patients without pneumonia (4). The potentially ensuing long-term decline in independence and the ability to care for oneself adversely affect both the individual and society at large (5).
An extensive analysis of routine insurance data in hospitalized patients (6) and multiple research projects in the setting of the CAPNETZ initiative (7, 8) have recently yielded detailed information on community-acquired pneumonia. Nonetheless, there have been very few population-based, long-term studies on the epidemiology of pneumonia in the general population (i.e., including persons who were not hospitalized). Most studies, including those conducted outside Germany, have centered on cases treated in the hospital (9– 12). We report a study of the incidence of pneumonia in the non-institutionalized, older general population over a follow-up period of 11 years, based on the ESTHER cohort of persons in the German federal state of Saarland (13– 16). The focus of this initial analysis lies on selected important risk factors, in order to yield information on pneumonia that will be of practical relevance in the primary care setting.
Methods
Study design and baseline data acquisition
The larger epidemiological study from which the participants in this study were drawn (the ESTHER study) is a representative, prospective cohort study of persons in Saarland, aged 50 to 75 at the time of recruitment, which concerns potential opportunities for the prevention, early detection, and optimized treatment of chronic diseases in this age group. 9949 participants were recruited from July 2000 to December 2002 (13). The treating physicians invited their patients to enroll in the study during routine outpatient check-ups. 420 community-based physicians recruited a mean of 24 participants each. Baseline data were obtained from the record of the check-up at the time of enrollment, and by means of standardized questionnaires regarding health-related behavior and the past medical history.
The following items of information were obtained at the check-up or from the questionnaires at the time of enrollment and used for further analysis in this study:
age (categorized by the median for descriptive purposes; treated as a continuous variable in regression models)
sex
smoking (non-, ex-, or current smoker)
alcohol consumption (>10 g/day in women or >20 g/day in men, vs. lesser consumption)
obesity (body mass index [BMI] = 30 kg/m²)
current diabetes mellitus (physician’s diagnosis, antidiabetic medication, HbA1c = 6.5%)
fasting blood sugar = 126 mg/dL
glucose = 200 mg/dL
history of stroke, congestive heart failure, and/or cancer.
Any abnormal auscultatory finding of the lungs or past or present lung disease was noted at the time of check-up and designated in the baseline data as a positive history of lung disease.
The ESTHER study (13) conforms to the ethical standards of the Helsinki Declaration and was approved by the ethics committees of the University of Heidelberg Medical Faculty and the Saarland Medical Association. Written informed consent was a precondition for inclusion.
Follow-up and the detection of pneumonia
Follow-up data were obtained from the participants and their physicians by means of standardized questionnaires two, five, eight, and eleven years after enrollment. The questionnaires included a question about pneumonia during the interval since the last questionnaire. Episodes of pneumonia reported either by the participants or their physicians were included in the main analysis; an analysis restricted to the episodes reported by the physicians was carried out in a sensitivity model. As a rule, only the first episode of pneumonia during the follow-up was considered.
The follow-up with respect to mortality (up to April 2013) was based on data from the relevant governmental authorities and health departments.
Statistical analysis
The participants were described with respect to the features listed above, including the frequency of pneumonia in each group. Cumulative pneumonia incidence curves and 10-year-incidences were calculated, stratified by participant features. Next, age- and sex-adjusted associations with the incidence of pneumonia were studied in survival-time regression models. The analytical methods that were used considered dropping out of the study because of death as a competing event (ebox) (17). Features found to have a significant (p <0.05) age- and sex-adjusted association with the incidence of pneumonia were included as predictors in a multiple regression model. The preconditions for the model were checked by an inspection of Schoenfeld residuals. Rate advancement periods (RAPs) were calculated for all statistically significant predictors of incident pneumonia (ebox) (18). RAP estimators are used to characterize risk factors for diseases whose incidence increases with age: for any given age x, the RAP is the number of years for which persons of age x who are exposed to the risk factor have the same incidence as unexposed persons of age x + RAP (18).
eBOX. Further description of statistical methods.
Survival-time analyses
The cumulative pneumonia incidence curves and the 10-year incidences of pneumonia were calculated with stratification by participant characteristics, and the raw associations were studied with Gray’s test. Dropping out of the study because of death was considered a competing event (e1). Next, age- and sex-adjusted associations with the incidence of pneumonia were studied with Fine-Gray regression models. The Fine-Gray method is suitable for survival-time analyses that take account of competing events (e1). Traditional Cox models were computed as well.
The relative risks (RR) presented in the main findings are the estimators derived from the Fine-Gray models (subdistribution relative hazard [sdRH] according to Lau et al. [e1]).
In the Results section, the competing event is sometimes simply designated as mortality. This designation for dropping out of the study because of death is not precise because of the definition of survival time, but it seemed appropriate for the purpose of readability.
Rate advancement periods
The rate advancement periods (RAP) and corresponding 95% confidence intervals were calculated by application of the general RAP equations (e2) to the regression coefficients and covariance matrix of the adjusted Fine-Gray regression model.
Survival-time definitions
For the survival-time analyses, the person-times were defined as follows:
time from study enrollment to the first pneumonia (reported either by the participant or by a physician) (main endpoint),
time from study enrollment to the last filled-out participant or physician questionnaire before the patient’s death, if the patient died (secondary endpoint; the period of observation ended with the last questionnaire, as any incident pneumonia between the last questionnaire and the patient’s death would not have been registered),
time from study enrollment to the last filled-out participant or physician questionnaire in patients who did not die thereafter (regular censoring due to restricted duration of follow-up).
Additional comment on findings
Findings of Cox models for pneumonia incidence
The relative risks estimated in Fine-Gray models (sdRH) were moderately lower than those estimated in traditional Cox models, i.e., so-called cause-specific relative hazards (csRH) (eTables 1 and eTables 2). This was what we had expected for predictors that were positively associated with both the main outcome event and the competing event (e1).
The residuals of the Cox models suggested a mild violation of hazard proportionality for the association of congestive heart failure with pneumonia, as well as for that of age with the competing event. In both cases, there was a tendency toward stronger associations with increasing time of observation.
Details on the definition of person-times for survival-time analyses are provided in the eBox. In the main analysis, five deaths from pneumonia were considered as competing events, in order to enable an unambiguous definition of person-times. In a sensitivity analysis, these events were included among the main endpoints; the end of the person-times of deceased participants (main and competing events) was defined as the date of death. Moreover, in a sensitivity analysis, pneumonia was considered to have occurred only if reported or confirmed by the physicians caring for the participants, while, in a further sensitivity analysis, a regression model simultaneously including all characteristics of the participants was used for an extended, adjusted analysis. The main model was also studied with adjustment for prior lung disease, as this might affect the associations of risk factors with the risk of pneumonia.
Observations for which the values of covariables were missing were excluded from the analyses only if the variable in question was necessary. The criterion for statistical significance was p <0.05 (two-tailed). The statistical analyses were performed with SAS 9.3 (SAS Institute Inc., Cary, NC, USA, 2002–2010) and R 3.0.2 (R Foundation for Statistical Computing, Vienna, Austria, 2013), with various additional functions (19– 21).
Results
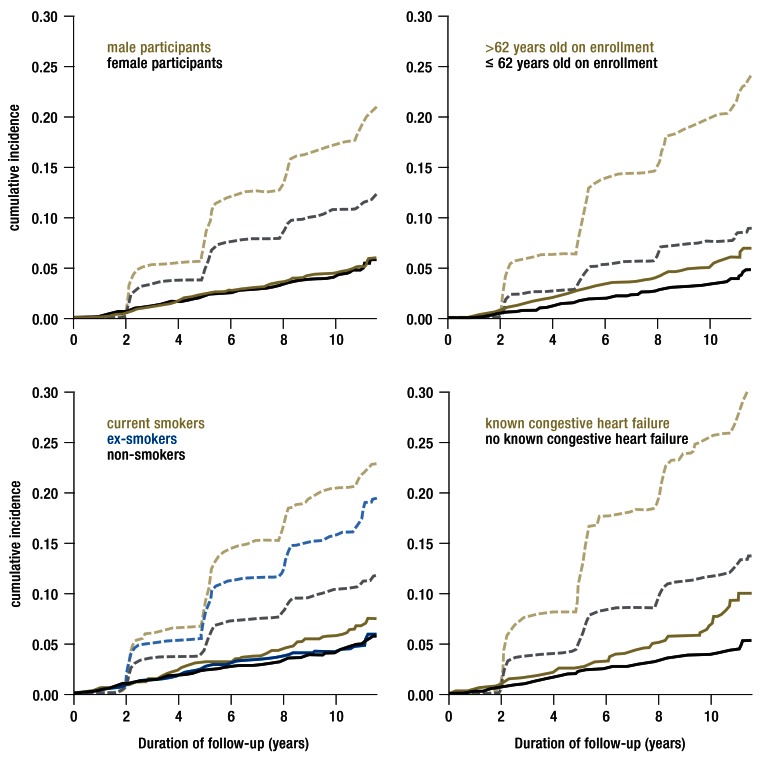
Follow-up data with adequate information on incident pneumonias were available for 9419 (95%) of the 9949 participants. The general return rates of the questionnaires at two, five, eight, and eleven years were 96%, 88%, 81%, and 70%. With a median follow-up duration of 10.6 years (interquartile range: 5.7–10.9 years), 435 first pneumonias and 1229 deaths were recorded, yielding a 10-year pneumonia incidence of 4.5% (95% confidence interval: [4.0; 4.9]) and a 10-year mortality of 13.8% [13.1; 14.0]. Of the 435 cases of pneumonia, 128 were reported only by the participant, 131 only by the physician, and 176 by both. The participants’ main features and 10-year incidences are summarized in Table 1. All of the features studied were clearly associated with mortality; associations with the incidence of pneumonia were found only for age, smoking, diabetes mellitus, and congestive heart failure. Selected cumulative incidence curves are shown in the Figure.
Table 1. Distribution of the predictors studied*1.
| Overall cohort | Pneumonia cases*2 | 10-year incidence(%, 95% CI) | ||
| Predictor | (n, %) | (n, %) | Pneumonia*2 | Mortality*2 |
| Sex | ||||
| female | 5217 (55.4) | 232 (53.3) | 4.3 [3.7; 4.9] | 10.8 [10.0; 11.8] |
| male | 4202 (44.6) | 203 (46.7) | 4.7 [4.0; 5.4] | 17.4 [16.2; 18.6] |
| p = 0.54 | p <0.0001 | |||
| Age | ||||
| ≤ 62 years | 4731 (50.2) | 181 (41.6) | 3.6 [3.1; 4.2] | 7.8 [7.0; 8.6] |
| >62 years | 4688 (49.8) | 254 (58.4) | 5.3 [4.7; 6.1] | 20.1 [18.9; 21.4] |
| p <0.0001 | p <0.0001 | |||
| Smoking | ||||
| non-smoker | 4607 (50.3) | 198 (47.1) | 4.1 [3.6; 4.8] | 10.2 [9.3; 11.2] |
| ex-smoker | 3046 (33.2) | 134 (31.9) | 4.1 [3.5; 4.9] | 15.6 [14.3; 17.0] |
| current smoker | 1510 (16.5) | 88 (21.0) | 5.7 [4.6; 7.1] | 20.2 [18.1; 22.5] |
| p = 0.028 | p <0.0001 | |||
| Alcohol consumption | ||||
| ≤ 10 resp. 20 g/day (♀/♂) | 6647 (77.9) | 318 (80.5) | 4.6 [4.1; 5.2] | 14.1 [13.2; 15.0] |
| > 10 resp. 20 g/day (♀/♂) | 1890 (22.1) | 77 (19.5) | 3.8 [3.0; 4.8] | 11.1 [9.7; 12.7] |
| p = 0.11 | p = 0.0021 | |||
| Body weight | ||||
| BMI <30 kg/m² | 7009 (74.5) | 324 (74.7) | 4.5 [4.0; 5.1] | 12.8 [12.0; 13.7] |
| BMI ≥ 30 kg/m² | 2396 (25.5) | 110 (25.3) | 4.3 [3.5; 5.2] | 16.7 [15.2; 18.4] |
| p = 0.99 | p <0.0001 | |||
| Diabetes mellitus | ||||
| no | 7872 (84.6) | 346 (79.9) | 4.2 [3.8; 4.7] | 11.8 [11.0; 12.6] |
| yes | 1432 (15.4) | 87 (20.1) | 5.9 [4.7; 7.3] | 25.1 [22.7; 27.6] |
| p = 0.0034 | p <0.0001 | |||
| Stroke | ||||
| no | 8830 (96.9) | 398 (95.7) | 4.4 [3.9; 4.8] | 12.8 [12.0; 13.5] |
| yes | 287 (3.1) | 18 (4.3) | 6.0 [3.5; 9.3] | 35.0 [29.1; 41.0] |
| p = 0.13 | p <0.0001 | |||
| Congestive heart failure | ||||
| no | 7685 (90.5) | 320 (83.8) | 4.0 [3.6; 4.5] | 11.7 [11.0; 12.5] |
| yes | 807 (9.5) | 62 (16.2) | 7.1 [5.3; 9.2] | 25.7 [22.5; 29.0] |
| p <0.0001 | p <0.0001 | |||
| Cancer | ||||
| no | 8545 (93.5) | 386 (92.6) | 4.4 [3.9; 4.9] | 12.9 [12.2; 13.7] |
| yes | 598 (6.5) | 31 (7.4) | 4.7 [3.1; 6.7] | 22.9 [19.4; 26.5] |
| p = 0.50 | p <0.0001 | |||
*1 among the 9419 analyzed ESTHER participants and the 435 participants with incident pneumonia up to 11 years of follow-up, and hereafter stratified cumulative 10-year incidences with 95% CI; missing data on smoking (256), alcohol (882), BMI (14), diabetes mellitus (115), stroke (302), congestive heart failure (927), and cancer (276)
*2 first pneumonia during prospective follow-up; dropping out of the study because of death
BMI, Body-mass index; ESTHER, an epidemiologic study on potential opportunities for the prevention, early detection, and improved treatement of chronic diseases among older persons in Germany; CI, confidence interval; n, number
Figure 1.
Cumulative incidence curves stratified for selected predictors, for pneumonia (solid lines: main endpoint) and dropping out of the study because of death (dashed lines: competing event) in the prospective ESTHER cohort study.
ESTHER, an epidemiologic study on potential opportunities for the prevention, early detection, and improved treatement of chronic diseases among older persons in Germany.
The age- and sex-adjusted regression models are shown in Table 2. In these models, advanced age, current smoking, prevalent diabetes mellitus, and congestive heart failure were associated with an increased risk. The inspection of residuals and the corresponding smoothing curves yielded no evidence of model violations. The relative risk (RR) was nearly unchanged when all of the significant predictors were included in a multiple regression model, but the diabetes-associated RR was no longer significant (table 3). The RAP estimator for current smoking as a risk factor was 12.5 years [3.5; 21.5], while that for congestive heart failure as a risk factor was 14.2 years [3.1; 25.3].
Table 2. Age- and sex-adjusted relative risks of pneumonia and mortality in the?prospective ESTHER study.
| RR (95% CI) | ||||
| Predictor | Pneumonia | Mortality | ||
| Sex | ||||
| female | 1 (ref) | 1 (ref) | ||
| male | 1.05 [0.87; 1.27] | 1.71 [1.53; 1.91] | ||
| p = 0.59 | p <0.0001 | |||
| Age | ||||
| per 10 years | 1.46 [1.26; 1.69] | 2.65 [2.40; 2.92] | ||
| p <0.0001 | p <0.0001 | |||
| Smoking | ||||
| non-smoker | 1 (ref) | 1 (ref) | ||
| ex-smoker | 1.01 [0.80; 1.27] | 1.45 [1.26; 1.67] | ||
| current smoker | 1.55 [1.20; 1.99] | 2.64 [2.26; 3.08] | ||
| p = 0.0014 | p <0.0001 | |||
| Alcohol consumption | ||||
| ≤ 10 resp. 20 g/day (♀/♂) | 1 (ref) | 1 (ref) | ||
| > 10 resp. 20 g/day(♀/♂) | 0.82 [0.64; 1.05] | 0.76 [0.66; 0.89] | ||
| p = 0.11 | p = 0.00048 | |||
| Body weight | ||||
| BMI <30 kg/m² | 1 (ref) | 1 (ref) | ||
| BMI ≥ 30 kg/m² | 1.01 [0.82; 1.26] | 1.37 [1.21; 1.55] | ||
| p = 0.90 | p <0.0001 | |||
| Diabetes mellitus | ||||
| no | 1 (ref) | 1 (ref) | ||
| yes | 1.32 [1.04; 1.67] | 1.84 [1.62; 2.10] | ||
| p = 0.022 | p <0.0001 | |||
| Stroke | ||||
| no | 1 (ref) | 1 (ref) | ||
| yes | 1.26 [0.78; 2.03] | 2.25 [1.82; 2.78] | ||
| p = 0.35 | p <0.0001 | |||
| Congestive heart failure | ||||
| no | 1 (ref) | 1 (ref) | ||
| yes | 1.68 [1.28; 2.21] | 1.73 [1.48; 2.02] | ||
| p = 0.00021 | p <0.0001 | |||
| Cancer | ||||
| no | 1 (ref) | 1 (ref) | ||
| yes | 1.06 [0.73; 1.53] | 1.61 [1.34; 1.94] | ||
| p = 0.76 | p <0.0001 | |||
BMI, body-mass index; CI, confidence interval; ESTHER, an epidemiologic study on potential opportunities for the prevention, early detection, and improved treatement of chronic diseases among older persons in Germany; ref, reference category; RR, relative risk
Table 3. Adjusted*1 relative risks (95% confidence interval) for pneumonia in the ?prospective ESTHER study*2.
| Prädiktor | Main model | Completely adjusted | Reported by physician |
| Sex | |||
| female | 1 (ref) | 1 (ref) | 1 (ref) |
| male | 1.07 [0.86; 1.33] | 1.06 [0.84; 1.34] | 1.07 [0.83; 1.37] |
| p = 0.53 | p = 0.61 | p = 0.62 | |
| Age | |||
| per 10 years | 1.43 [1.22; 1.67] | 1.41 [1.19; 1.66] | 1.60 [1.32; 1.93] |
| p <0.0001 | p <0.0001 | p <0.0001 | |
| Smoking | |||
| non-smoker | 1 (ref) | 1 (ref) | 1 (ref) |
| ex-smoker | 1.01 [0.79; 1.29] | 1.04 [0.80; 1.35] | 1.04 [0.77; 1.39] |
| current smoker | 1.56 [1.19; 2.05] | 1.65 [1.24; 2.20] | 1.57 [1.13; 2.17] |
| p = 0.0026 | p = 0.0012 | p = 0.017 | |
| Alcohol consumption | |||
| ≤ 10 resp. 20 g/day (♀/♂) | – | 1 (ref) | – |
| >10 resp. 20 g/day(♀/♂) | – | 0.89 [0.68; 1.15] | – |
| – | p = 0.37 | – | |
| Body weight | |||
| BMI <30 kg/m² | – | 1 (ref) | – |
| BMI ≥ 30 kg/m² | – | 1.01 [0.78; 1.30] | – |
| – | p = 0.94 | – | |
| Diabetes mellitus | |||
| no | 1 (ref) | 1 (ref) | 1 (ref) |
| yes | 1.29 [0.98; 1.68] | 1.34 [1.01; 1.78] | 1.28 [0.93; 1.75] |
| p = 0.065 | p = 0.041 | p = 0.12 | |
| Stroke | |||
| no | – | 1 (ref) | – |
| yes | – | 0.94 [0.50; 1.75] | – |
| – | p = 0.84 | – | |
| Congestive heart failure | |||
| no | 1 (ref) | 1 (ref) | 1 (ref) |
| yes | 1.65 [1.24; 2.20] | 1.61 [1.18; 2.20] | 1.52 [1.08; 2.13] |
| p = 0.00057 | p = 0.0027 | p = 0.017 | |
| Cancer | |||
| no | – | 1 (ref) | – |
| yes | – | 1.22 [0.83; 1.79] | – |
| – | p = 0.32 | – | |
*1 The models were adjusted for all variables whose relative risk (RR) is given; N = 8196 (main model and sensitivity model with pneumonia reported by physician) and N = 7450 (completely adjusted model) because of missing values of covariables
*2 main evaluation and sensitivity models
BMI, body-mass index; ESTHER, an epidemiologic study on potential opportunities for the prevention, early detection, and improved treatement of chronic diseases among older persons in Germany; ref, reference category
The estimated RRs were moderately lower than those found in commonly used Cox models, which do not take competing events into account (eTables 1 and 2).
eTable 1. Age- and sex-adjusted relative risks (with 95% confidence intervals) for pneumonia and mortality in the prospective ?ESTHER study*1.
| Cox regression | Fine-Gray regression | |||
| Predictor | csRH (pneumonia) | csRH (mortality) | sdRH (pneumonia) | sdRH (mortality) |
| Sex | ||||
| female | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| male | 1.10 [0.91; 1.33] | 1.72 [1.54; 1.93] | 1.05 [0.87; 1.27] | 1.71 [1.53; 1.91] |
| p = 0.32 | p <0.0001 | p = 0.59 | p <0.0001 | |
| Age | ||||
| per 10 years | 1.59 [1.37; 1.85] | 2.70 [2.46; 2.97] | 1.46 [1.26; 1.69] | 2.65 [2.40; 2.92] |
| p <0.0001 | p <0.0001 | p <0.0001 | p <0.0001 | |
| Smoking | ||||
| non-smoker | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| ex-smoker | 1.04 [0.82; 1.32] | 1.46 [1.27; 1.68] | 1.01 [0.80; 1.27] | 1.45 [1.26; 1.67] |
| current smoker | 1.72 [1.32; 2.23] | 2.70 [2.31; 3.15] | 1.55 [1.20; 1.99] | 2.64 [2.26; 3.08] |
| p = 0.00011 | p <0.0001 | p = 0.0014 | p <0.0001 | |
| Alcohol consumption | ||||
| ≤ 10 resp. 20 g/day (♀/♂) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| >10 resp. 20 g/day (♀/♂) | 0.80 [0.62; 1.02] | 0.75 [0.65; 0.87] | 0.82 [0.64; 1.05] | 0.76 [0.66; 0.89] |
| p = 0.076 | p = 0.00022 | p = 0.11 | p = 0.00048 | |
| Weight | ||||
| BMI <30 kg/m² | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| BMI ≥ 30 kg/m² | 1.05 [0.84; 1.30] | 1.38 [1.22; 1.56] | 1.01 [0.82; 1.26] | 1.37 [1.21; 1.55] |
| p = 0.68 | p <0.0001 | p = 0.90 | p <0.0001 | |
| Diabetes mellitus | ||||
| no | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| yes | 1.42 [1.12; 1.81] | 1.89 [1.66; 2.14] | 1.32 [1.04; 1.67] | 1.84 [1.62; 2.10] |
| p = 0.0035 | p <0.0001 | p = 0.022 | p <0.0001 | |
| Stroke | ||||
| no | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| yes | 1.47 [0.91; 2.36] | 2.32 [1.87; 2.87] | 1.26 [0.78; 2.03] | 2.25 [1.82; 2.78] |
| p = 0.11 | p <0.0001 | p = 0.35 | p <0.0001 | |
| Congestive heart failure | ||||
| no | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| yes | 1.80 [1.36; 2.37] | 1.76 [1.50; 2.06] | 1.68 [1.28; 2.21] | 1.73 [1.48; 2.02] |
| p <0.0001 | p <0.0001 | p = 0.00021 | p <0.0001 | |
| Cancer | ||||
| no | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) |
| yes | 1.12 [0.78; 1.62] | 1.63 [1.36; 1.96] | 1.06 [0.73; 1.53] | 1.61 [1.34; 1.94] |
| p = 0.55 | p <0.0001 | p = 0.76 | p <0.0001 | |
*1 Comparison of results of Cox regression (csRH) and Fine-Gray models (sdRH).
BMI, body-mass index; ref, reference category; ESTHER, an epidemiologic study on potential opportunities for the prevention, early detection, and improved treatement of chronic diseases among older persons in Germany; csRH, cause-specific relative hazard; sdRH, subdistribution relative hazard (nomenclature of Lau et al.) (e1)
eTable 2. Adjusted relative risks*1 of pneumonia in the prospective ESTHER study.
| Predictor | csRH (95% CI) | sdRH (95% CI) |
| Sex | ||
| female | 1 (ref) | 1 (ref) |
| male | 1.11 [0.89; 1.38] | 1.07 [0.86; 1.33] |
| p = 0.36 | p = 0.53 | |
| Age | ||
| per 10 years | 1.56 [1.32; 1.84] | 1.43 [1.22; 1.67] |
| p <0.0001 | p <0.0001 | |
| Smoking | ||
| non-smoker | 1 (ref) | 1 (ref) |
| ex-smoker | 1.04 [0.81; 1.34] | 1.01 [0.79; 1.29] |
| current smoker | 1.71 [1.30; 2.26] | 1.56 [1.19; 2.05] |
| p = 0.00030 | p = 0.0026 | |
| Diabetes mellitus | ||
| no | 1 (ref) | 1 (ref) |
| yes | 1.37 [1.05; 1.78] | 1.29 [0.98; 1.68] |
| p = 0.019 | p = 0.065 | |
| Congestive heart failure | ||
| no | 1 (ref) | 1 (ref) |
| yes | 1.76 [1.32; 2.33] | 1.65 [1.24; 2.20] |
| p <0.0001 | p = 0.00057 | |
*1 Comparison of findings of Cox regression (csRH) and Fine-Gray models (sdRH); both models adjusted for all variables in the table; N = 8196 because of missing data
CI, confidence interval; ESTHER, an epidemiologic study on potential opportunities for the prevention, early detection, and improved treatement of chronic diseases among older persons in Germany; csRH, cause-specific relative hazard; sdRH, subdistribution relative hazard (nomenclature of Lau et al.) (e1)
The findings of the main analysis did not differ in any relevant way from those of the sensitivity model that included all of the participants’ features. There was, however, a statistically significant association of diabetes mellitus with pneumonia (table 3). There was little change in the findings when the main model was restricted to pneumonias reported by a physician (table 3). Moreover, the findings were robust with respect to the alternative definition of an event, in which, for deceased participants, the person-time was considered as terminating at death, and deaths from pneumonia were counted as a main event. When an additional control for the effect of prior lung disease (RR: 1.75 [1.35; 2.28]) was introduced into the main model, there was a drop only in the calculated association with current smoking (RR: 1.46 [1.11; 1.93]).
Discussion
In this prospective observational study of the older general population in Saarland, age was the most important independent predictor for incident pneumonia. Current smoking, an important modifiable risk factor, was also associated with a markedly elevate risk. In the adjusted regression, the association with prevalent diabetes mellitus was not robust, but there remained an independent association with a history of congestive heart failure at baseline. These associations can help the primary care physician determine which patients are at greater risk of pneumonia, therefore possibly needing earlier and more intensive diagnostic efforts and treatment if the clinical suspicion of pneumonia arises. Moreover, the demonstrated elevation of the risk of pneumonia due to smoking and congestive heart failure may help motivate persons in these two groups to quit smoking and to comply better with medical treatment. It can be said, on the basis of the calculated RAPs, that a person who smokes has the same risk of pneumonia as a nonsmoker who is about 12.5 years older, and the comparable figure for congestive heart failure as a risk factor is 14.2 years; this information, too, may help motivate patients with these risk factors to adhere to medical advice more closely (18, 22). Overall, the findings enable a general communication of the risk, particularly when multiple risk factors are present at once. The development of a detailed prognosis score would have been beyond the scope of this study.
The associations revealed by this study are biologically plausible. Smoking is a major risk factor for structural lung disease, particularly chronic obstructive pulmonary disease (COPD), and also impairs the immune system. Both of these problems can raise the patient’s susceptibility to pneumonia (23). In congestive heart failure, pulmonary congestion with fluid presumably impairs both the self-cleansing mechanisms of the lungs and the physiological immune reaction. As a result, persons with congestive heart failure are more likely to develop pneumonia, and they recover from it less well (11, 24). Diabetes was not robustly associated with a higher incidence of pneumonia in this study, but it is considered a risk factor for infection in general, including pneumonia. The epidemiologic data are inconsistent, however, and the mechanisms are not yet fully understood (25).
The association of smoking with pneumonia that is documented in the present study is entirely in line with the findings of earlier studies. Two major studies in the USA—the Health Professionals Follow-Up Study and the Nurses’ Health Study II—yielded a relative risk of incident pneumonia of 1.46 for male and 1.55 for female smokers. Persons with pre-existing diseases were excluded from the analysis (26). Another study showed that smoking elevated the risk of hospitalization for pneumonia by 61%, but no information was given on adjusted analyses (12). The same study showed a somewhat more marked association with congestive heart failure (RR: 2.15). In two further studies that documented an association of congestive heart failure, and heart disease in general, with both the incidence of pneumonia and the rate of hospitalization for it (RR: 1.8–1.9), no information about smoking was available for analysis (11, 27). In the somewhat older National Health and Nutrition Examination Survey I, adjusted analyses revealed a doubling of the risk of hospitalization for pneumonia in men who smoked or had congestive heart failure; in women, it was only diabetes mellitus that significantly increased the risk (9). Mor et al. (11) pointed out that studies based on hospitalizations may be biased, as pre-existing illnesses would tend to increase the likelihood that a patient with pneumonia will be referred and admitted to the hospital. Thus, any study of that type might overestimate the association of any pre-existing illness with pneumonia. We cannot be certain whether the higher risk estimators found in some of the earlier studies are best explained by this mechanism, by different types of adjustment (especially for smoking behavior), or perhaps by statistical methods that did not take account of competing events.
In this study, the consumption of more than 20 grams of alcohol per day in men, or more than 10 grams of alcohol per day in women, was associated with lower rates of pneumonia, but only as a trend that did not reach statistical significance. This finding contrasts with those of multiple earlier studies, in which alcohol consumption or abuse was found to increase the risk of pneumonia, very markedly in some studies (10, 27). Our statistically insignificant findings on this question should not be overinterpreted. Some studies failed to reveal any association of alcohol use with the risk of pneumonia (9, 12, 26). One may speculate that intercultural differences in drinking behavior may have accounted, in part, for the variation in findings across studies.
Nor did the present study reveal any association of obesity with the risk of pneumonia. Earlier findings on this question were inconsistent. There is evidence of sex-specific risk elevations due to overweight on the one hand (26) and very low BMI on the other (9); these might be studied in the future with detailed dose-effect analyses.
Using somewhat simplistic assumptions, we can calculate that the 10-year incidence of pneumonia found in the ESTHER study corresponds to an incidence of 4.6 cases [4.1; 5.1] per 1000 persons per year. This value is near the lower end of the range for pneumonia incidence (3.7 to 10.1 cases per 1000 persons per year) that was obtained in a study comparing the findings of multiple approaches for estimating the incidence of pneumonia in an urban area in Germany (28). The present study ignored any further incident episodes of pneumonia after the first one; this may have biased the calculated incidence rate downward. Moreover, for patients who died, the official cause of death was the underlying illness that led to death. Although pneumonia may also have been marked as a prior cause on some patients’ death certificates, this information was not available for the study and could not be used in the analysis. This is probably the reason for the negligibly small number of deaths attributable to pneumonia in this study, which certainly does not reflect the overall mortality of pneumonia. At the same time, the case definition of pneumonia was broad, leading perhaps to an overestimation of its incidence.
The findings of this study should be interpreted in the light of its limitations. In particular, predictors based on self-reported data on smoking and alcohol consumption will always be affected by some degree of misclassification, which, in turn, may weaken the estimated associations. As for congestive heart failure, a more detailed clinical and echocardiographic characterization of the condition might have yielded useful additional information but was not practically possible in the setting of this epidemiological study. The primary definition of the main endpoint was relatively soft, including cases in which the diagnosis was not confirmed by a physician, yet sensitivity analyses in which only physician-confirmed pneumonias were considered left the findings essentially unchanged. Another limitation of the study is that the diagnosis of pneumonia requires, as a rule, a radiological correlate to the clinical findings, but this requirement is not always met in the primary-care setting; some of the events in this study may thus have been bronchitis, rather than pneumonia. There is a certain resulting vagueness in the definition of the end point in the present study, which, however, reflects everyday diagnostic reality. The estimated associations were otherwise plausible and robust with respect to alternative definitions of person-time. Finally, the evaluation in the present study was intentionally conservative with respect to the selection of risk factors to be considered, and it emphasized the descriptive aspect. Some of the associations pointed out here merit more detailed characterization in the future. For example, an analysis including consideration of the duration of diabetes and the results of treatment might shed light on the question of a possibly higher incidence of pneumonia in some subgroups of patients with diabetes mellitus (29).
An adequate analysis of many other potential risk factors for pneumonia in older people would have been beyond the scope of this study. Such factors should be investigated separately in individually dedicated studies. The effect of pneumonia on mortality should also be studied in future, as there seems to be a substantial rise in mortality after community-acquired pneumonia (4). Reliable figures on these matters, in combination with the risk elevations revealed in the present study, could help motivate patients to be vaccinated against influenza and pneumococcus as recommended in the current guidelines (30).
Overview
These findings document the high epidemiological importance of pneumonia and are broadly complementary to the findings of other studies on the causes and prognosis of community-acquired pneumonia leading to hospitalization (31). They may thus be of practical use not only for risk stratification in routine clinical practice, but also as a means of improving treatment compliance and patients’ willingness to be vaccinated.
Key Messages.
Pneumonia is a a common disease that can permanently impair health, particularly in older persons. Yet representative, population-based longitudinal studies on the frequency of pneumonia and its risk factors have been scarce to date, both in Germany and abroad.
In the ESTHER study, the cumulative 10-year incidence of pneumonia was 4.5% [4.0; 4.9] in a representative sample of the older general population in the German federal state of Saarland.
In the present study, multiple regression models showed that advanced age, current smoking, and known congestive heart failure independently increase the risk of pneumonia. A possible association of diabetes mellitus with the risk of pneumonia was less clear.
These findings can help physicians identify patients who may need to be followed with greater vigilance and for whom diagnostic and therapeutic measures should be intensified promptly if pneumonia is suspected.
These findings may also be of use in the prevention of pneumonia. Knowledge of the risk factors for pneumonia may help motivate smokers to quit and may lead to greater treatment compliance and greater willingness to be immunized against influenza and pneumococcus, as recommended in current guidelines, among patients with conditions that put them at risk.
Acknowledgments
Acknowledgement
The ESTHER study would not have been possible without the great patience and motivation of our participants. Thanks are also due to all of our community-based colleagues in primary care, whose continuing support was essential to the performance of this research project.
Footnotes
Conflict of interest statement
The authors state that they have no conflicts of interest.
Manuscript submitted on 15 March 2016, revised version accepted a on 9 June 2016.
Translated from the original German by Ethan Taub, M.D.
References
- 1.Wesemann T, Nüllmann H, Pflug MA, Heppner HJ, Pientka L, Thiem U. Pneumonia severity, comorbidity and 1-year mortality in predominantly older adults with community-acquired pneumonia: a cohort study. BMC Infect Dis. 2015;15 doi: 10.1186/s12879-014-0730-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Welte T. Community-acquired pneumonia: a disease of the elderly. Z Gerontol Geriatr. 2011;44:221–228. doi: 10.1007/s00391-011-0183-4. [DOI] [PubMed] [Google Scholar]
- 3.Pletz MW, Ewig S, Lange C, Welte T, Höffken G. [Update pneumonia 2012] Dtsch Med Wochenschr. 2012;137:2265–2280. doi: 10.1055/s-0032-1305297. [DOI] [PubMed] [Google Scholar]
- 4.Eurich DT, Marrie TJ, Minhas-Sandhu JK, Majumdar SR. Ten-year mortality after community-acquired pneumonia A prospective cohort. Am J Respir Crit Care Med. 2015;192:597–604. doi: 10.1164/rccm.201501-0140OC. [DOI] [PubMed] [Google Scholar]
- 5.El Solh A, Pineda L, Bouquin P, Mankowski C. Determinants of short and long term functional recovery after hospitalization for community-acquired pneumonia in the elderly: role of inflammatory markers. BMC Geriatr. 2006;6 doi: 10.1186/1471-2318-6-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Strauß R, Ewig S, Richter K, König T, Heller G, Bauer TT. The prognostic significance of respiratory rate in patients with pneumonia: a retrospective analysis of data from 705,928 hospitalized patients in Germany from 2010-2012. Dtsch Arztebl Int. 2014;111:503–508. doi: 10.3238/arztebl.2014.0503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Welte T, Marre R, Suttorp N. CAPNetz - Kompetenznetzwerk ambulant erworbene Pneumonie: Strukturen und Ziele. Pneumologie. 2003;57:34–41. doi: 10.1055/s-2003-36635. [DOI] [PubMed] [Google Scholar]
- 8.Pletz MW, Rohde G, Schütte H, Bals R, von Baum H, Welte T. für die CAPNETZ-Studiengruppe: [Epidemiology and aetiology of community-acquired pneumonia (CAP)] Dtsch Med Wochenschr. 2011;136:775–780. doi: 10.1055/s-0031-1275806. [DOI] [PubMed] [Google Scholar]
- 9.LaCroix AZ, Lipson S, Miles TP, White L. Prospective study of pneumonia hospitalizations and mortality of US. older people: the role of chronic conditions, health behaviors, and nutritional status. Public Health Rep. 1989;104:350–360. [PMC free article] [PubMed] [Google Scholar]
- 10.Kornum JB, Due KM, Norgaard M, et al. Alcohol drinking and risk of subsequent hospitalisation with pneumonia. Eur Respir J. 2012;39:149–155. doi: 10.1183/09031936.00000611. [DOI] [PubMed] [Google Scholar]
- 11.Mor A, Thomsen RW, Ulrichsen SP, Sorensen HT. Chronic heart failure and risk of hospitalization with pneumonia: a population-based study. Eur J Intern Med. 2013;24:349–353. doi: 10.1016/j.ejim.2013.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Yende S, Alvarez K, Loehr L, et al. Epidemiology and long-term clinical and biologic risk factors for pneumonia in community-dwelling older Americans: analysis of three cohorts. Chest. 2013;144:1008–1017. doi: 10.1378/chest.12-2818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Löw M, Stegmaier C, Ziegler H, Rothenbacher D, Brenner H. [Epidemiological investigations of the chances of preventing, recognizing early and optimally treating chronic diseases in an elderly population (ESTHER study)] Dtsch Med Wochenschr. 2004;129:2643–2647. doi: 10.1055/s-2004-836089. [DOI] [PubMed] [Google Scholar]
- 14.Breitling LP, Rothenbacher D, Stegmaier C, Raum E, Brenner H. Older smokers’ motivation and attempts to quit smoking: epidemiological insight into the question of lifestyle versus addiction. Dtsch Arztebl Int. 2009;106:451–455. doi: 10.3238/arztebl.2009.0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schöttker B, Haug U, Schomburg L, et al. Strong associations of 25-hydroxyvitamin D concentrations with all-cause, cardiovascular, cancer, and respiratory disease mortality in a large cohort study. Am J Clin Nutr. 2013;97:782–793. doi: 10.3945/ajcn.112.047712. [DOI] [PubMed] [Google Scholar]
- 16.Schöttker B, Herder C, Rothenbacher D, et al. Proinflammatory cytokines, adiponectin, and increased risk of primary cardiovascular events in diabetic patients with or without renal dysfunction: results from the ESTHER study. Diabetes Care. 2013;36:1703–1711. doi: 10.2337/dc12-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brenner H, Gefeller O, Greenland S. Risk and rate advancement periods as measures of exposure impact on the occurrence of chronic diseases. Epidemiology. 1993;4:229–236. doi: 10.1097/00001648-199305000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Scrucca L, Santucci A, Aversa F. Competing risk analysis using R: an easy guide for clinicians. Bone Marrow Transplant. 2007;40:381–387. doi: 10.1038/sj.bmt.1705727. [DOI] [PubMed] [Google Scholar]
- 20.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone Marrow Transplant. 2010;45:1388–1395. doi: 10.1038/bmt.2009.359. [DOI] [PubMed] [Google Scholar]
- 21.Gray B. Cmprsk: subdistribution analysis of competing risks R package version 2.2-7. CRAN.R-project.org/package=cmprsk (last accessed on 15 February 2016) 2014 [Google Scholar]
- 22.Liese AD, Hense HW, Brenner H, Löwel H, Keil U. Assessing the impact of classical risk factors on myocardial infarction by rate advancement periods. Am J Epidemiol. 2000;152:884–888. doi: 10.1093/aje/152.9.884. [DOI] [PubMed] [Google Scholar]
- 23.Rom O, Avezov K, Aizenbud D, Reznick AZ. Cigarette smoking and inflammation revisited. Respir Physiol Neurobiol. 2013;187:5–10. doi: 10.1016/j.resp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 24.Thomsen RW, Kasatpibal N, Riis A, Norgaard M, Sorensen HT. The impact of pre-existing heart failure on pneumonia prognosis: population-based cohort study. J Gen Intern Med. 2008;23:1407–1413. doi: 10.1007/s11606-008-0672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Knapp S. Diabetes and infection: is there a link? A mini-review. Gerontology. 2013;59:99–104. doi: 10.1159/000345107. [DOI] [PubMed] [Google Scholar]
- 26.Baik I, Curhan GC, Rimm EB, Bendich A, Willett WC, Fawzi WW. A prospective study of age and lifestyle factors in relation to community-acquired pneumonia in US men and women. Arch Intern Med. 2000;160:3082–3088. doi: 10.1001/archinte.160.20.3082. [DOI] [PubMed] [Google Scholar]
- 27.Koivula I, Sten M, Mäkelä PH. Risk factors for pneumonia in the elderly. Am J Med. 1994;96:313–320. doi: 10.1016/0002-9343(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 28.Schnoor M, Hedicke J, Dalhoff K, Raspe H, Schäfer T. CAPNETZ-Studiengruppe: Approaches to estimate the population-based incidence of community acquired pneumonia. J Infect. 2007;55:233–239. doi: 10.1016/j.jinf.2007.04.355. [DOI] [PubMed] [Google Scholar]
- 29.Breitling LP. Evidence of non-linearity in the association of glycemic control with influenza/pneumonia mortality: a study of 19000 adults from the US general population. Diabetes Metab Res Rev. 2016:;32:111–120. doi: 10.1002/dmrr.2681. [DOI] [PubMed] [Google Scholar]
- 30.Ewig S, Höffken G, Kern WV, et al. Behandlung von erwachsenen Patienten mit ambulant erworbener Pneumonie und Prävention - Update 2016. Pneumologie. 2016;70:151–200. doi: 10.1055/s-0042-101873. [DOI] [PubMed] [Google Scholar]
- 31.Kolditz M, Ewig S, Klapdor B, et al. [Community-acquired pneumonia as medical emergency: predictors of early deterioration] Thorax. 2015;70:551–558. doi: 10.1136/thoraxjnl-2014-206744. [DOI] [PubMed] [Google Scholar]
- E1.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. doi: 10.1093/aje/kwp107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- E2.Brenner H, Gefeller O, Greenland S. Risk and rate advancement periods as measures of exposure impact on the occurrence of chronic diseases. Epidemiology. 1993;4:229–236. doi: 10.1097/00001648-199305000-00006. [DOI] [PubMed] [Google Scholar]