Abstract
Introduction
Chronic HIV/HCV co-infection carries increased risk of cirrhosis, hepatocellular carcinoma, and death. Due to anti-inflammatory properties, HMG co-A inhibitors (statins) may be useful adjunctive therapy to reduce liver disease progression.
Methods
Clinical information was extracted from the Veterans Affairs HIV and HCV Clinical Case Registries (1999–2010). HIV-related variables included combination anti-retroviral therapy (cART) era of diagnosis, CD4 cell count, and percent time with undetectable HIV viral load. Metabolic variables included diabetes, low-HDL, and hypertension. Statin use was measured as percent time with active prescription (time-updated throughout the follow-up period). Cox proportional hazards analysis was used to determine risk factors for cirrhosis (ICD-9 or APRI>2) overall and in groups stratified by alanine aminotransferase (ALT) level above and below 40 IU/L.
Results
The cohort included 5985 HIV/HCV co-infected veterans. The majority was black race, and the mean age at index date was 45 years. Statin use was significantly protective of cirrhosis for patients with ALT ≤40 IU/L; for every 30% increase in time on statin, there was a 32% decreased risk of developing cirrhosis (HR 0.68, 95% CI 0.47 −0.98). Diabetes and low-HDL were significantly associated with cirrhosis in patients with ALT > 40 IU/L (HR 1.15, p <0.04 and HR 1.3, p <0.0001).
Conclusions
Statin drug use is beneficial in mitigating the risk of liver disease progression for HIV/HCV co-infected patients without advanced liver disease. Low-HDL and diabetes in co-infected patients with abnormal ALT have greater risk of cirrhosis development.
Keywords: HIV/HCV co-infection, statin, cirrhosis, diabetes, cholesterol
Introduction
Globally, nearly 7 million people are co-infected with HIV and HCV, representing about 20% of the HIV population.[1] In the U.S. and parts of Europe, co-infection prevalence among those with HIV is nearly 30%, with higher rates seen among clusters of high-risk groups such as intravenous drug users and men who have sex with men.[2–5] Co-infection is well known to be associated with reduced HCV clearance, increased HCV viremia, rapid progression of fibrosis, and ultimately end stage liver disease (ESLD). [6–8] Several studies have demonstrated that patients with HIV/HCV co-infection who have poor immunological and virological control of HIV have increased risk of cirrhosis and hepatocellular carcinoma (HCC). [9–14]
End stage liver disease and HCC have become leading causes of mortality among the co-infected population in the era of combination antiretroviral therapy (cART).[15] One cross-sectional study from France showed that death from liver disease contributed to nearly 25% of non-AIDS-related deaths. Among those with ESLD, death due to HCC increased steadily over 10 years from 5% to 25%.[16] Compared to the general population, patients with co-infection are diagnosed at a younger age, and are more than twice as likely to die of liver cancer. [15, 17, 18] Thus, reducing the risk of liver disease progression, cirrhosis, and HCC are of the utmost importance in the HIV/HCV co-infected population.
Although successful treatment of HCV with direct acting antivirals (DAAs) plays an important role in reducing the risk of ESLD, HCC and mortality compared to those without, [19] cost and other barriers to therapy limit access to these medications for many patients. In addition, there is a residual risk for development of HCC after sustained viral response from DAAs for HCV in patients with cirrhosis. [20] Thus, utilizing other strategies to decrease the risk of ESLD and cirrhosis, including the use of medications that inhibit HMG-CoA reductase (statins) as adjuncts in care for patients with chronic liver disease and HIV are needed. Statins’ pleotropic properties including their immunomodulatory, anti-inflammatory, and antineoplastic traits are thought to be beneficial in chronic disease states beyond their primary cardio-protective utility.[21, 22]
Several studies of HCV mono-infected patients have also shown statins to be associated with reduced risk of cirrhosis and HCC.[23–26] One study in veterans with diabetes suggested that statin medication may be useful in reducing the risk of developing HCC by 46% (95%, CI 0.55–0.75). [24] To investigate the impact of statin medications and metabolic risk factors on liver progression among HIV/HCV co-infected individuals, we performed a retrospective study utilizing the Veterans Affairs (VA) HIV and HCV Clinical Case Registries (CCR).
Methods
Data Sources and Subjects
The study population consisted of HIV and HCV co-infected veterans whose health-related information was collected from the HIV and HCV VA CCR dating from January 1, 1999 to December 31, 2010. The CCRs are comprehensive databases extracted from the VA electronic medical record and includes all laboratory, outpatient, inpatient, and pharmacy data on included patients.[27] The original cohort has been previously described in detail.[12] Figure 1 shows the additional restrictions made to the original cohort, which resulted in the final cohort used for these analyses. This study was approved by the Institutional Review Board of Baylor College of Medicine and Affiliated Institutions and the Michael E. DeBakey VA Medical Center Research and Development Committee.
Figure 1.
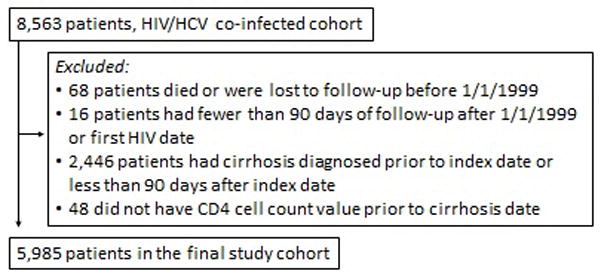
Selection criteria for the final HIV/HCV co-infected study cohort*
*Details of full cohort have previously been described. [12]
Cirrhosis was defined by ICD-9 codes for cirrhosis (571.2, 571.5, 571.6) or aspartate aminotransferase (AST) to platelet ratio index > 2 (APRI score). A previous chart review validation study of the cirrhosis ICD-9 code showed that the positive predictive value compared to chart review was 90% [40]. Female veterans were also excluded due to small numbers (<2%). Patients lacking HCV RNA, follow-up CD4 count or HIV viral load information were also excluded. Within the confirmed HIV/HCV co-infected cohort, patients with a diagnosis of cirrhosis prior to or within 90 days after the diagnosis of HIV or index date (January 1, 1999) were excluded. Patients were censored at death, at the date of their last recorded health care encounter, or at the end of the study period.
Study Variables
Metabolic risk factors were ascertained including degree of obesity, diabetes, hypertension, and low-HDL. Diabetes was defined by hemoglobin A1c measurement ≥6.5% or ICD-9 codes: 250.0–250.9, 357.2, 362.0, and 366.41. Hypertension was defined by ICD-9 code: 401.00 to 405.99. Low-HDL was defined as HDL less than 40 mg/dL. Obesity was defined as body mass index (BMI) greater than 30 kg/m2. The maximum BMI was retained throughout the follow-up period. Alcohol use was defined as documented use of alcohol designated by the following ICD-9 codes: 571.0–571.3, 291, 303.0, 303.9, 305.0, and 535.3. Statin medication use was defined as percent of follow-up time with an outpatient prescription for a statin drug prior to diagnosis of cirrhosis and was time-updated throughout the follow-up period. Prescriptions included rosuvastsatin, atorvastatin, simvastatin, pravastatin, fluvastatin, or lovastatin.
Other covariates defined at index date included age at HIV diagnosis, race, use of cART, and era of HIV diagnosis defined as pre-cART (<1996), early cART (1996–2001), and late cART (2002–2010), and HCV genotype. Other HIV-specific variables included were CD4 count (cells/μL) most recent to event or censor and percent of time with undetectable HIV viral load, both as time-updated variables throughout the study period. Co-morbidities measured by Deyo Comorbidity score (excluding HIV) were also included in the multivariate analyses. All variables were time-dependent except age, race, era of HIV diagnosis, and HCV genotype. For time varying co-variates, missing data were excluded from the analysis until it became available later in the follow-up time and then the last value was carried forward until a new value was available.
Data Analysis
Primary outcome was time to cirrhosis. Descriptive analyses with Chi-square and Fisher’s exact tests were used to characterize and compare the demographic and disease characteristics of the non-cirrhotic and cirrhotic groups. Cox proportional hazard regression analysis was used to calculate the effect of demographic, HIV-specific variables, metabolic variables, and statin drug use on time to cirrhosis. An interaction analysis between ALT and statin was performed, which led to stratification of patients based on ALT above and below 40 IU/L. Log-rank test and Kaplan-Meier method were used to compare and demonstrate cumulative incidence of cirrhosis for patients group by ALT greater or less than 40 IU/L and percent time on statin drug.
SAS version 9.4 (SAS Institute Inc, Cary, NC) was used for all analyses. Cox proportional hazards regression was performed with SAS’s PHREG procedure.
Results
The mean age among the final cohort (N = 5985) was 45 years at the time of HIV diagnosis, and the mean follow-up time was 6.2 years. Of the total HIV/HCV co-infected cohort, 2,265 developed cirrhosis by the end of follow-up, with crude incidence of cirrhosis of 6.1/100 person-years. The majority of patients in the cohort were black (66%) and were diagnosed with HIV during the fourth decade of life and in the early cART period. (Table 1) Initial APRI scores (median) were 0.6 and 0.47 (p <.0001) in the patients with and without cirrhosis, respectively. By the end of follow up, the patients with cirrhosis had significantly higher APRI scores than patients without cirrhosis (2.23 versus 0.46, respectively, p <.0001). Eighty-three percent had received cART and had CD4 values greater than 350 cells/μL (55%). Patients who developed cirrhosis were significantly less likely to receive cART (79.5%) and less likely to have CD4 >350 cells/μL (48.9%) compared to those without cirrhosis (85.7% and 59.2%, respectively). HIV viral load was suppressed in over 80% of the time; this was significantly less in the patients with cirrhosis compared to the patients without cirrhosis (19.8% v 29%, p < .0001)
Table 1.
Demographic Information, N = 5,985 (HIV/HCV Co-infected)
| No Cirrhosis N= 3720 (%) |
Cirrhosis N=2265 (%) |
p value | |
|---|---|---|---|
|
| |||
| Age at HIV Diagnosis, years (mean) | 46 | 44 | <.0001 |
| Race | 913 (24.5) | 620 (27.3) | <.0001 |
| White/other/unknown | 2565 (68.9) | 1420 (62.7) | |
| Black | 242 (6.5) | 225 (9.9) | |
| Hispanic | |||
|
| |||
| Age at index, years | |||
| <40 | 513 (13.8) | 303 (13.4) | <.0001 |
| 40–50 | 1999 (53.7) | 1371 (60.5) | |
| >50 | 1208 (32.5) | 591 (26.1) | |
|
| |||
| Duration of follow-up, years | 8.5 | 3.5 | <.0001 |
|
| |||
| Outpatient encounters, year prior to index date (mean) | 14 | 16.5 | .0002 |
|
| |||
| Deyo Comorbidity Score* (w/o HIV) | |||
| 0 | 2760 (74.2) | 1851 (81.7) | <.0001 |
| 1 | 535 (14.4) | 255 (11.3) | |
| >2 | 425 (11.4) | 159 (7.0) | |
|
| |||
| Era of HIV Diagnosis | |||
| Pre-cART | 1035 (27.8) | 892 (39.4) | <.0001 |
| Early cART | 1353 (36.4) | 916 (40.4) | |
| Late cART | 1332 (35.8) | 457 (20.2) | |
|
| |||
| Ever cART* | 3189 (85.7) | 1802 (79.5) | <.0001 |
|
| |||
| CD4 Count * Cells/μL | |||
| <200 | 775 (20.8) | 638 (28.0) | <.0001 |
| 200–349 | 744 (20.0) | 520 (23.0) | |
| >350 | 2201 (59.2) | 1107 (48.9) | |
|
| |||
| CD4 Count Nadir* Cells/μL | |||
| ≤200 | 1943 (52.2) | 1178 (52.0) | 0.87 |
| >200 | 1777 (47.8) | 1087 (48.0) | |
|
| |||
| % Time HIV VL Undetectable* | |||
| 0–40% | 1550 (41.7) | 1373 (60.6) | <.0001 |
| 40–80% | 1091 (29.3) | 443 (19.6) | |
| >80% | 1079 (29.0) | 449 (19.8) | |
|
| |||
| HCV Genotype | |||
| 1 & 4 | 2185 (58.7) | 1136 (50.2) | <.0001 |
| 2 & 3 | 281 (7.5) | 137 (6.0) | |
| unknown | 1254 (33.7) | 992 (43.8) | |
|
| |||
| HCV Treatment* | |||
| None | 3321 (89.3) | 1940 (85.6) | <.0001 |
| No SVR | 290 (7.8) | 253 (11.2) | |
| SVR | 109 (2.9) | 72 (3.2) | |
|
| |||
| APRI Score (median) | |||
| Initial value | 0.47 | 0.6 | <.0001 |
| Most recent value | 0.46 | 2.23 | |
|
| |||
| HCC* | 22 (0.6) | 91 (4.0) | <.0001 |
|
| |||
| Max BMI*, kg/m2 | |||
| <30 | 2867 (77.1) | 1815 (80.1) | 0.005 |
| >30 | 853 (22.9) | 450 (19.9) | |
|
| |||
| ALT*, IU/L | |||
| ≤40 | 2036 (54.7) | 273 (12.0) | <.0001 |
| 41–60 | 896 (24.1) | 266 (11.7) | |
| >60 | 788 (21.2) | 1726 (76.2) | |
|
| |||
| Diabetes* | 622 (16.7) | 343 (15.1) | 0.11 |
|
| |||
| Alcohol* | 2218 (59.6) | 1319 (58.2) | 0.29 |
|
| |||
| Hypertension* | 2256 (60.6) | 1034 (45.6) | <.0001 |
|
| |||
| Low-HDL* | 2407 (64.7) | 1253 (55.3) | <.0001 |
|
| |||
| LDL (mean) | |||
| Initial value | 94 | 91 | <.0001 |
| Most recent value | 89 | 84 | |
|
| |||
| Initial LDL, mg/dL | |||
| <130 | 2688 (54.2) | 1541 (31) | <.0001 |
| 130–159 | 345 (7) | 184 (3.7) | |
| >160 | 136 (2.7) | 68 (1.4) | |
|
| |||
| Statin* | 767 (20.6) | 182 (8.0) | <.0001 |
|
| |||
| % Time on Statin at censor/cirrhosis | <.0001 | ||
| 0% | 2953 (79.4) | 2083 (92.0) | |
| >0–50% | 624 (16.8) | 147 (6.5) | |
| 51–100% | 143 (3.8) | 35 (1.5) | |
|
| |||
| Death | 892 (14.9) | 953 (15.9) | <.0001 |
Time-updated measurement
For the metabolic risk factors, over one-fifth of the total cohort was obese based on body mass index (BMI) greater than 30 kg/m2. Diabetes was present in about 16% of the total group, and was not significantly different between the patients with and without cirrhosis. Over 50% of the cohort had hypertension and low-HDL, and these features were more prevalent in the patients without cirrhosis compared to those with cirrhosis (60% and 64% compared to 45.6% and 55.6%, respectively). Initial LDL in patients with cirrhosis was 91.2 mg/dL, and at last follow-up it was 84.4 mg/dL. In patients without cirrhosis, index LDL and last-follow-up LDL were 94.4 and 89.4 mg/dL, respectively. Few patients had ever been prescribed statin medications, and prescriptions were significantly lower in the cirrhotic group compared to non-cirrhotic (8% and 20.6%, respectively). Among all patients, statins were prescribed in 12.5% and 21.2% of patients with and without cirrhosis, respectively (p = 0.0001).
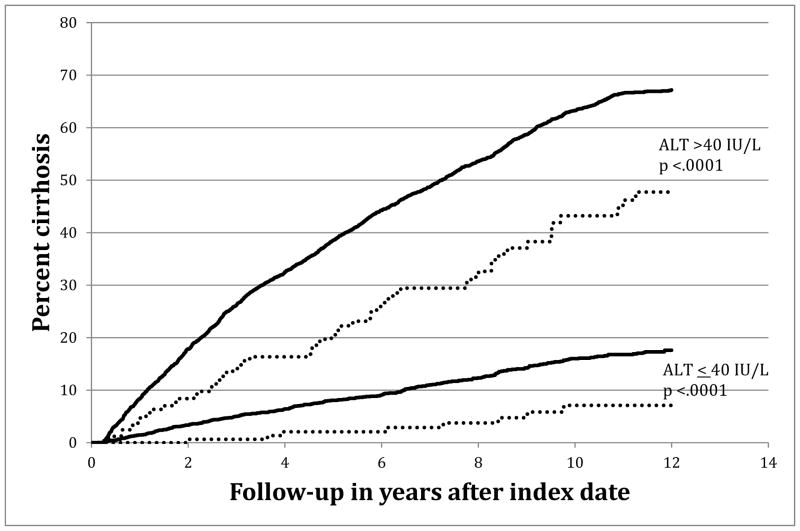
Figure 2 demonstrates patient groups stratified by ALT value and percent time on statin. For patients with ALT <40 IU/L, statin use greater or equal to 30% time was significantly associated with reduced risk of development of cirrhosis compared to those with less time on statin drugs. Likewise, for patients with ALT >40 IU/L patients with more than 30% time on statin was also significantly protective for cirrhosis development compared to those with less time on statin drugs.
Figure 2.
Kaplan-Meier Cumulative Cirrhosis Incidence among HIV/HCV Co-infected patients as stratified by ALT groups and between those with more or less than 30% time with statin medication
Legend: ——— Statin <30% time
..……… Statin ≥30% time
In preliminary multivariate analyses, an interaction between ALT and statin use was statistically significant (Supplemental table 1). Thus, further multivariate analyses were stratified by ALT level to minimize the effect of modification bias of ALT on statin use in the multivariate analyses. In the ALT <40 IU/L group, every 1% time on statin was associated with a decreased risk of cirrhosis (aHR 0.98, 95% CI 0.97–0.99). This translates to a 32% (aHR 0.68, 95% CI 0.47–0.98) reduction in progression to cirrhosis for individuals with ≥30% time on statins. (Table 2) For patients with ALT >40 IU/L levels, each 1% of time on statins was associated with a non-significant decreased risk of cirrhosis (aHR 0.95, 95% CI 0.9–1). In order to adjust for the competing risk of death, we performed a sensitivity analysis using multivariate hazard regression models excluding patients who died before the diagnosis of cirrhosis or censor. After excluding patients who died before cirrhosis or censor date, we found that every 30% of time on statin was still associated with a significant 35% reduction in cirrhosis progression (95% CI: 0.45–0.95).
Table 2.
Multivariate Survival Analysis to Cirrhosis Among HIV/HCV co-infected veterans N 5,985
| ALT ≤ 40 IU/L | P-value | ALT >40 IU/L | P-value | |
|---|---|---|---|---|
| N = 273 | N = 1992 | |||
| HR (CI) | HR (CI) | |||
|
| ||||
| Race | ||||
| White/other/unknown | reference | |||
| Black | 0.64 (0.48–0.84) | 0.001 | 0.67 (0.61–0.74) | <.0001 |
|
| ||||
| Age at index, years | ||||
| <40 | reference | |||
| 40–50 | 1.84 (1.16–2.92) | 0.009 | 1.14 (1.00–1.30) | 0.047 |
| >50 | 2.54 (1.54–4.18) | 0.0002 | 1.28 (1.09–1.50) | 0.002 |
|
| ||||
| Deyo Comorbidity Score (without HIV) | ||||
| 0 | reference | |||
| 1 | 1.2 (0.84–1.75) | 0.29 | 1.1 (0.96–1.27) | 0.17 |
| ≥2 | 3.23 (2.31–4.50) | <.0001 | 1.5 (1.28–1.93) | <.0001 |
|
| ||||
| Era of HIV Diagnosis | ||||
| Pre-cART | reference | |||
| Early cART | 0.83 (0.64–1.10) | 0.19 | 0.93 (0.85–1.03) | 0.19 |
| Late cART | 0.87 (0.61–1.26) | 0.47 | 0.96 (0.84–1.1) | 0.59 |
|
| ||||
| CD4 Count, cells/μL | ||||
| <200 | 2.4 (1.80–3.19) | <.0001 | 1.9 (1.71–2.12) | <.0001 |
| 201–349 | 1.2 (0.91–1.73) | 0.15 | 1.19 (1.07–1.34) | 0.001 |
| >350 | reference | |||
|
| ||||
| Percent Undetectable | ||||
| 0–40% | reference | |||
| 40–80% | 0.68 (0.49–0.94) | 0.02 | 0.76 (0.68–0.86) | <.0001 |
| >80% | 0.72 (0.51–1.00) | 0.05 | 0.72 (0.64–0.8) | <.0001 |
|
| ||||
| Genotype | ||||
| 2 & 3 or unknown | reference | |||
| 1 & 4 | 1.53 (1.18–1.99) | 0.001 | 1.23 (1.12–1.36) | <.0001 |
|
| ||||
| Maximum BMI, > 30 kg/m2 | 0.84 (0.60–1.16) | 0.28 | 0.85 (0.76–0.95) | 0.005 |
|
| ||||
| Diabetes | 1.24 (0.88–1.75) | 0.20 | 1.15 (1.01–1.31) | 0.04 |
|
| ||||
| Alcohol | 1.04 (0.81–1.34) | 0.75 | 1.15 (1.04–1.26) | 0.004 |
|
| ||||
| Hypertension | 1.07 (0.82–1.41) | 0.61 | 1.09 (0.98–1.2) | 0.096 |
|
| ||||
| Low-HDL | 1.26 (0.97–1.64) | 0.08 | 1.3 (1.2–1.44) | <.0001 |
|
| ||||
| Percent Statin (per 1%) | 0.98 (0.97–0.99) | 0.04 | 0.99(0.99–1.00) | 0.43 |
|
| ||||
| Statin by 30% increment* (Point estimate) | 0.68 (0.47–0.98) | - | 0.95 (0.83–1.01) | - |
|
| ||||
| Deaths stratified by statin (at time of death or last follow-up) | ||||
| >30% statin use | 33 (0.55) | <.0001 | 26 (0.4) | <.0001 |
| ≤30% statin use | 974 (16.3) | 812 (13.6) | ||
The 30% point-estimate is a re-calculation of the 1% analysis to show a larger scale.
In the ALT ≤40 IU/L group, other metabolic risk factors including diabetes, low-HDL, and hypertension were harmful, but did not significantly impact progression to cirrhosis. Within the ALT >40 IU/L group, diabetes and low-HDL were consistently associated with an increased risk of cirrhosis (aHR 1.15, 95% CI 1.01–1.31 and aHR 1.3, 95% CI 1.2–1.44), respectively. Hypertension was associated with a greater risk of cirrhosis in both groups, but it was not significant. Obesity was found to have a protective effect in both groups, but was only significant in those with ALT >40 IU/L (aHR 0.85, 95% CI 0.76–0.95).
Several demographic and HIV-specific co-variates were significantly related to cirrhosis incidence. Patients over 50 years old with ALT ≤40 IU/L group were at higher risk for cirrhosis (aHR 2.5, 95% CI 1.54–4.18). Patients with Deyo co-morbidity score of greater than 1 in the ALT ≤40 IU/L group also increased the risk cirrhosis (aHR 3.23, 95% CI 2.3–4.5). Alcohol use was significantly associated with liver disease progression only in patients with ALT >40 IU/L (aHR 1.15, 95% CI 1.04–1.26). Poor immunological control with CD4 count less than 200 cells/μL conferred greatest risk of cirrhosis in both ALT ≤40 IU/L and >40 IU/L groups (aHR 2.4, 95% CI 1.8–3.2 and aHR 1.9, 95% 1.71–2.12), respectively. Those patients with > 80% time with an undetectable HIV viral load in both ALT ≤40 IU/L and >40 IU/L had a significantly lower risk of cirrhosis (aHR 0.72, CI 0.5–1.0, aHR 0.72, CI 0.64–0.8), respectively.
Discussion
To our knowledge, this is the largest study of HIV/HCV co-infected individuals to examine the effect of statin drugs on cirrhosis development. Furthermore, in contrast to previous studies, the current study specifically adjusts for confounding by indication of non-statin receipt due to elevated ALT during follow-up years. We have demonstrated that statin drug use in patients with HIV/HCV co-infection and normal liver function decreased the risk of cirrhosis, particularly among those with ALT ≤40 IU/L. Among patients with ALT >40 IU/L metabolic risk factors including low-HDL and diabetes also were significantly associated with development of cirrhosis.
Statin therapy in HCV mono-infection has been shown in two recent studies from the U.S. (small, prospective) and Taiwan (large, retrospective) to reduce the cirrhosis risk by 69% and 87%, respectively. [25, 26] Although the study conducted in Taiwan did not adjust for ALT variation within the study participants, it demonstrated both dose and time-dependent effects in the development of cirrhosis. This may be due in part to better regulation of the pro-inflammatory mechanisms and oxidative stress seen in chronic HCV. Alternately, statins may have a more direct role interfering with the ability of HCV to harness the host lipid metabolism in its replication mechanisms. [28]
Other metabolic risk factors demonstrated significant associations with the development of cirrhosis for patients with elevated ALT in our study. The development of metabolic derangements is common in patients with chronic HCV and can be seen in over 50% of this population, making this population at high risk for a pro-inflammatory and high oxidative stress environment. [29] In addition, many of these metabolic risk factors such as diabetes and lipid derangements with liver fibrosis are associated with NAFLD progression, another growing problem in the HIV infected population. [14–19] Although we did not find a protective effect of statins on those with ALT >40 IU/L, statin medications may improve low HDL, a manifestation of lipid dysregulation in chronic HCV, as well as improve anti-inflammatory and anti-proliferative effects.
Diabetes has been shown to increase the risk of HCC in HCV mono-infected individuals.[30] Diabetes control is also a modifiable risk factor and can be targeted as a therapeutic strategy to reduce oxidative stress and decrease the risk of fibrosis progression. Only few studies have examined the role of anti-diabetic agents in NAFLD; however, there is substantial promise for these agents, especially the thializodinediones, in mitigating risk of liver fibrosis.[31] The benefit of anti-diabetes medications in HCV/HIV co-infection is less clearly understood and represents an area of further research.
As in previous studies of HIV/HCV co-infected individuals, we found that host-related factors, such as older age at diagnosis of HIV, greater co-morbidities, and low CD4 count were significantly linked to liver fibrosis development in our cohort for ALT groups below and above 40 IU/L. [8, 32, 33] We also found that patients who had a higher percent time with undetectable viral load during follow-up decreased risk of cirrhosis development. [13, 34] HIV treatment with cART and adherence to therapy are known to play critical roles in reducing risk of liver disease progression and ultimately decompensation. Patients with cirrhosis received less cART, which may have multifactorial reasons including adherence, poor-social support, as well as provider-hesitation. Our results, however, demonstrate that above and beyond other known factors related to greater immunologic control and viral suppression known to be associated with reduced risk of liver fibrosis and slower progression in HIV/HCV co-infection, statin use additionally decreases cirrhosis risk. [13, 35, 36]
Unsurprisingly, we found that our cohort of chronic liver disease patients received few statin prescriptions, particularly among patients with ALT >40 IU/L. This phenomenon is likely due to provider hesitation in prescribing medications with potential hepatotoxicity.[37] Statin medication use in the presence of liver disease or enzyme elevation is generally safe and well tolerated, [38, 39] and if followed closely, patients with slightly elevated ALT should be able to receive statins. Our finding that statins are beneficial in reducing progression to cirrhosis adds to the growing body of evidence of the beneficial use of statins beyond its utility in cardiovascular disease, and they should be considered in patients even with chronic liver disease.
Study limitations include the following: the study was a retrospective cohort study using data extracted by ICD-9 codes, lab values, and pharmacy records from medical care records, however, many of these variables have been validated in other VA studies. [40] Specifically, alcohol utilization may not be completely captured with ICD-9 codes. Outside claims data including Medicare and fee-based sources were not included in the analyses because they are not included in the CCR. In addition, prescribing practices among different providers and varying cART regimens may have affected frequency and dosage of statin drug use. However, we attempted to address this limitation by carefully identifying the cohort and adjusting for cART use and stratifying by ALT to minimize confounding. Because we had so few patients on statins in the ALT >40 patients, our analyses of individuals with more advanced cirrhosis and high ALT are limited and should be interpreted with caution. In addition, patients with prolonged chronic hepatitis may have had abnormal lipid profiles with low total cholesterol and low-LDL,[28] which may have led to less statin receipt for hyperlipidemia, however we adjusted for lipid values in the multivariable analyses. In addition, although we were unable to conduct classic competing risk analyses for death due to use of time-dependent variables in the Cox model, in our sensitivity analysis of excluding individuals who died prior to the cirrhosis diagnosis or censor from the cohort, we continued to find a significant protective effect from statin use. Finally, generalizability may also be limited as our study included only male patients, and the majority were over 40 years old at time of HIV diagnosis. In conclusion, this study demonstrated that statin use in an HIV/HCV co-infected population with minimal liver dysfunction was associated with reduced risk of progression to advanced liver disease. Until effective therapies for treating HCV become widely available, statin use may be an important, cost-effective adjunct in the care of HIV/HCV co-infected patients. We also found that metabolic risk factors such as low-HDL and diabetes are associated with development of cirrhosis. It is possible that treatment of these co-morbidities could also help mitigate hepatic decompensation. Further prospective studies are needed to better understand the safety and efficacy of statin use in the prevention of cirrhosis and HCC.
Supplementary Material
Keypoints.
Statin drugs may be a useful adjunct in mitigating risk of cirrhosis in patients with chronic liver disease, but normal liver function. Metabolic risk factors including low-HDL and diabetes are also associated with liver disease progression. Older age, more co-morbidities, and poorer HIV control are associated with cirrhosis development.
Acknowledgments
Nora T. Oliver was the primary author of the manuscript. Christine M. Hartman performed the statistical analyses and contributed to revisions of the manuscript. Jennifer R. Kramer contributed to the content and revisions of the paper. Elizabeth Y. Chiao was the principal investigator and contributed content and revisions to the manuscript.
Funding Support: Funding. Drs. Elizabeth Chiao and Jennifer Kramer received research funding for this project through a 2011 developmental grant from the Baylor-UTHouston Center for AIDS Research (CFAR), a NIH-funded program (NIH P30 CA125123). This work was also supported in part by the Center for Innovations in Quality, Effectiveness and Safety (#CIN 13-413).
Footnotes
Disclaimer: The views expressed in this article are those of the author(s) and do not necessarily represent the views of the Department of Veterans Affairs.
Conflicts of Interest: No authors have conflicts of interest to declare.
References
- 1.Soriano V, Vispo E, Labarga P, Medrano J, Barreiro P. Viral hepatitis and HIV co-infection. Antiviral Res. 2010;85(1):303–15. doi: 10.1016/j.antiviral.2009.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Sherman KE, Rouster SD, Chung RT, Rajicic N. Hepatitis C Virus prevalence among patients infected with Human Immunodeficiency Virus: a cross-sectional analysis of the US adult AIDS Clinical Trials Group. Clin Infect Dis. 2002;34(6):831–7. doi: 10.1086/339042. [DOI] [PubMed] [Google Scholar]
- 3.Sulkowski MS, Mast EE, Seeff LB, Thomas DL. Hepatitis C virus infection as an opportunistic disease in persons infected with human immunodeficiency virus. Clin Infect Dis. 2000;30(Suppl 1):S77–84. doi: 10.1086/313842. [DOI] [PubMed] [Google Scholar]
- 4.Garten RJ, Lai S, Zhang J, et al. Rapid transmission of hepatitis C virus among young injecting heroin users in Southern China. Int J Epidemiol. 2004;33(1):182–8. doi: 10.1093/ije/dyh019. [DOI] [PubMed] [Google Scholar]
- 5.Rauch A, Rickenbach M, Weber R, et al. Unsafe sex and increased incidence of hepatitis C virus infection among HIV-infected men who have sex with men: the Swiss HIV Cohort Study. Clin Infect Dis. 2005;41(3):395–402. doi: 10.1086/431486. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DL, Astemborski J, Rai RM, et al. The natural history of hepatitis C virus infection: host, viral, and environmental factors. JAMA. 2000;284(4):450–6. doi: 10.1001/jama.284.4.450. [DOI] [PubMed] [Google Scholar]
- 7.Thein HH, Yi Q, Dore GJ, Krahn MD. Natural history of hepatitis C virus infection in HIV-infected individuals and the impact of HIV in the era of highly active antiretroviral therapy: a meta-analysis. AIDS. 2008;22(15):1979–91. doi: 10.1097/QAD.0b013e32830e6d51. [DOI] [PubMed] [Google Scholar]
- 8.Eyster ME, Fried MW, Di Bisceqlie AM, Goedert JJ. Increasing hepatitis C virus RNA levels in hemophiliacs: relationship to human immunodeficiency virus infection and liver disease. Multicenter Hemophilia Cohort Study. Blood. 1994;84(4):1020–3. [PubMed] [Google Scholar]
- 9.Graham CS, Baden LR, Yu E, et al. Influence of human immunodeficiency virus infection on the course of hepatitis C virus infection: a meta-analysis. Clin Infect Dis. 2001;33(4):562–9. doi: 10.1086/321909. [DOI] [PubMed] [Google Scholar]
- 10.Benhamou Y, Bochet M, Di Martino V, et al. Liver fibrosis progression in human immunodeficiency virus and hepatitis C virus coinfected patients. The Multivirc Group. Hepatology. 1999;30(4):1054–8. doi: 10.1002/hep.510300409. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou Y, Di Martino V, Bochet M, et al. Factors affecting liver fibrosis in human immunodeficiency virus-and hepatitis C virus-coinfected patients: impact of protease inhibitor therapy. Hepatology. 2001;34(2):283–7. doi: 10.1053/jhep.2001.26517. [DOI] [PubMed] [Google Scholar]
- 12.Kramer JR, Kowalkowski MA, Duan Z, Chiao EY. The Effect of HIV Viral Control on the Incidence of Hepatocellular Carcinoma in Veterans With Hepatitis C and HIV Coinfection. J Acquir Immune Defic Syndr. 2015;68(4):456–62. doi: 10.1097/QAI.0000000000000494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bräu N, Salvatore M, Rios-Bedoya CF, et al. Slower fibrosis progression in HIV/HCV-coinfected patients with successful HIV suppression using antiretroviral therapy. J Hepatol. 2006;44(1):47–55. doi: 10.1016/j.jhep.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 14.Verma S, Wang CH, Govindarajan S, et al. Do type and duration of antiretroviral therapy attenuate liver fibrosis in HIV-hepatitis C virus-coinfected patients? Clin Infect Dis. 2006;42(2):262–70. doi: 10.1086/499055. [DOI] [PubMed] [Google Scholar]
- 15.Bourcier V, Winnock M, Ait Ahmed M, et al. Primary liver cancer is more aggressive in HIV-HCV coinfection than in HCV infection. A prospective study (ANRS CO13 Hepavih and CO12 Cirvir) Clin Res Hepatol Gastroenterol. 2012;36(3):214–21. doi: 10.1016/j.clinre.2011.11.002. [DOI] [PubMed] [Google Scholar]
- 16.Rosenthal E, Salmon-Ceron D, Lewden C, et al. Liver-related deaths in HIV-infected patients between 1995 and 2005 in the French GERMIVIC Joint Study Group Network (Mortavic 2005 study in collaboration with the Mortalité 2005 survey, ANRS EN19) HIV Med. 2009;10(5):282–9. doi: 10.1111/j.1468-1293.2008.00686.x. [DOI] [PubMed] [Google Scholar]
- 17.Pinchoff J, Drobnik A, Bornschlegel K, et al. Deaths among people with hepatitis C in New York City, 2000–2011. Clin Infect Dis. 2014;58(8):1047–54. doi: 10.1093/cid/ciu075. [DOI] [PubMed] [Google Scholar]
- 18.Pineda JA, Romero-Gomez M, Diaz-Garcia JA, et al. HIV coinfection shortens the survival of patients with hepatitis C virus-related decompensated cirrhosis. Hepatology. 2005;41(4):779–89. doi: 10.1002/hep.20626. [DOI] [PubMed] [Google Scholar]
- 19.Berenguer J, Rodriguez E, Miralles P, et al. Sustained virological response to interferon plus ribavirin reduces non-liver-related mortality in patients coinfected with HIV and Hepatitis C virus. Clin Infect Dis. 2012;55(5):728–36. doi: 10.1093/cid/cis500. [DOI] [PubMed] [Google Scholar]
- 20.El-Serag HB, Kanwal F, Richardson P, Kramer J. Risk of Hepatocellular Carcinoma after Sustained Virologic Response in Veterans with HCV-infection. Hepatology. 2016 doi: 10.1002/hep.28535. doi:10.1002. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jain MK, Ridker PM. Anti-inflammatory effects of statins: clinical evidence and basic mechanisms. Nat Rev Drug Discov. 2005;4(12):977–87. doi: 10.1038/nrd1901. [DOI] [PubMed] [Google Scholar]
- 22.Singh S, et al. Chemopreventive strategies in hepatocellular carcinoma. Nat Rev Gastroenterol Hepatol. 2014;11(1):45–54. doi: 10.1038/nrgastro.2013.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singh S, Singh PP, Roberts LR, Sanchez W. Statins are associated with a reduced risk of hepatocellular cancer: a systematic review and meta-analysis. Gastroenterology. 2013;144(2):323–32. doi: 10.1053/j.gastro.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Johnson ML, Hachem C, Morgan RO. Statins are associated with a reduced risk of hepatocellular carcinoma in a large cohort of patients with diabetes. Gastroenterology. 2009;136(5):1601–8. doi: 10.1053/j.gastro.2009.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Simon TG, King LY, Zheng H, Chung RT. Statin use is associated with a reduced risk of fibrosis progression in chronic hepatitis C. J Hepatol. 2015;62(1):18–23. doi: 10.1016/j.jhep.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang YH, Chen WC, Tsan YT, et al. Statin use and the risk of cirrhosis development in patients with hepatitis C virus infection. J Hepatol. 2015;63(5):111–17. doi: 10.1016/j.jhep.2015.07.006. [DOI] [PubMed] [Google Scholar]
- 27.Backus LI, Gavrilov S, Loomis TP, et al. Clinical Case Registries: simultaneous local and national disease registries for population quality management. J Am Med Inform Assoc. 2009;16(6):775–83. doi: 10.1197/jamia.M3203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Simon TG, Butt AA. Lipid dysregulation in hepatitis C virus, and impact of statin therapy upon clinical outcomes. World J Gastroenterol. 2015;21(27):8293–303. doi: 10.3748/wjg.v21.i27.8293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Modaresi Esfeh J, Ansari-Gilani K. Steatosis and hepatitis C. Gastroenterol Rep (Oxf) 2015 doi: 10.1093/gastro/gov040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Davila JA, Morgan RO, Shaib Y, McGlynn KA, El-Serag HB. Diabetes increases the risk of hepatocellular carcinoma in the United States: a population based case control study. Gut. 2005;54(4):533–9. doi: 10.1136/gut.2004.052167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boettcher E, Csako G, Pucino F, Wesley R, Loomba R. Meta-analysis: pioglitazone improves liver histology and fibrosis in patients with non-alcoholic steatohepatitis. Aliment Pharmacol Ther. 2012;35(1):66–75. doi: 10.1111/j.1365-2036.2011.04912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohsen AH, Easterbrook PJ, Taylor C, et al. Impact of human immunodeficiency virus (HIV) infection on the progression of liver fibrosis in hepatitis C virus infected patients. Gut. 2003;52(7):1035–40. doi: 10.1136/gut.52.7.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín-Carbonero L, Benhamou Y, Puoti M, et al. Incidence and predictors of severe liver fibrosis in human immunodeficiency virus-infected patients with chronic hepatitis C: a European collaborative study. Clin Infect Dis. 2004;38(1):128–33. doi: 10.1086/380130. [DOI] [PubMed] [Google Scholar]
- 34.Cooper C, Rollet-Kurhajec KC, Young J, et al. HIV virological rebounds but not blips predict liver fibrosis progression in antiretroviral-treated HIV/hepatitis C virus-coinfected patients. HIV Med. 2015;16(1):24–31. doi: 10.1111/hiv.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macías J, Berenguer J, Japon MA, et al. Fast fibrosis progression between repeated liver biopsies in patients coinfected with human immunodeficiency virus/hepatitis C virus. Hepatology. 2009;50(4):1056–63. doi: 10.1002/hep.23136. [DOI] [PubMed] [Google Scholar]
- 36.Mariné-Barjoan E, Saint-Paul MC, Pradier C, et al. Impact of antiretroviral treatment on progression of hepatic fibrosis in HIV/hepatitis C virus co-infected patients. AIDS. 2004;18(16):2163–70. doi: 10.1097/00002030-200411050-00008. [DOI] [PubMed] [Google Scholar]
- 37.Rzouq FS, Volk ML, Hatoum HH. Hepatotoxicity fears contribute to underutilization of statin medications by primary care physicians. Am J Med Sci. 2010;340(2):89–93. doi: 10.1097/MAJ.0b013e3181e15da8. [DOI] [PubMed] [Google Scholar]
- 38.Bader T. Yes! Statins can be given to liver patients. J Hepatol. 2012;56(2):305–7. doi: 10.1016/j.jhep.2011.08.016. [DOI] [PubMed] [Google Scholar]
- 39.Feinstein MJ, Achenbach CJ, Stone NJ, Lloyd-Jones DM. A Systematic Review of the Usefulness of Statin Therapy in HIV-Infected Patients. Am J Cardiol. 2015;115(12):1760–6. doi: 10.1016/j.amjcard.2015.03.025. [DOI] [PubMed] [Google Scholar]
- 40.Kramer JR, Davila JA, Miller ED, et al. The validity of viral hepatitis and chronic liver disease diagnoses in Veterans Affairs administrative databases. Aliment Pharmacol Ther. 2008;27(3):274–82. doi: 10.1111/j.1365-2036.2007.03572.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.