Abstract
To establish a healthy pregnancy the maternal immune system must tolerate fetal allo-antigens, yet remain competent to respond to infections. The ability of decidual NK cells (dNK) to promote migration of fetal extravillous trophoblasts (EVT) and placental growth as well as the capacity of EVT to promote immune tolerance are topics of high interest and extensive research. However, the problem of how dNK and decidual CD8+ T cells (CD8+ dT) provide immunity to infections of the placenta and the mechanisms that regulate their cytolytic function has thus far largely been ignored. Fetal EVT are the most invasive cells of the placenta and directly interact with maternal decidual immune cells at this maternal-fetal interface. Besides the expression of non-polymorphic HLA-E and HLA-G molecules that are associated with immune tolerance, EVT also express highly polymorphic HLA-C molecules that can serve as targets for maternal dNK and CD8+ dT responses. HLA-C expression by EVT has a dual role as the main molecule to which immune tolerance needs to be established and as the only molecule that can present pathogen-derived peptides and provide protective immunity when EVT are infected. The focus of this review is to address the regulation of cytotoxicity of dNK and CD8+ dT, which is essential for maternal-fetal immune tolerance as well as recent evidence that both cell types can provide immunity to infections at the maternal-fetal interface. A particular emphasis is given to the role of HLA-C expressed by EVT and its capacity to elicit dNK and CD8+ dT responses.
Keywords: Human, Pregnancy, EVT, Perforin, HCMV
Decidual NK cells
The discovery of high numbers of large granular lymphocytes (LGL) in human decidua, later identified as decidual Natural Killer cells (dNK), led to the hypothesis that fetal placental cells actively inhibit maternal dNK and avoid immunologic rejection (King et al., 1989; King et al., 1990). The characterization of dNK as poor cytotoxic lymphocytes and major cytokine and growth factor producers distinguished dNK function from that of cytotoxic peripheral blood NK cells (pNK) (Hanna et al., 2006; Koopman et al., 2003). The main role for dNK was established as cells that facilitate implantation, trophoblast invasion and vascular remodeling, processes that are of key importance for placental development and pregnancy success (Hanna et al., 2006). The role of dNK in clearance of virus infections, a main function of pNK, has been ignored until recently, Siewiera et al., 2013 demonstrated the ability of dNK to clear Human Cytomegalovirus (HCMV)-infected cells. Our lab has built upon this observation and highlighted the dual role of dNK, capable of mounting cytolytic responses during viral infections as well as both providing immune tolerance to the fetus and facilitating placental growth (Tilburgs et al., 2015b).
A dNK paradox – High levels of cytotoxic granules but low cytotoxicity
dNK form a distinct NK cell population that has many differences in gene expression, cytokine secretion and expression of cell surface receptors compared to pNK. However, dNK contain equally high levels of the cytolytic molecules perforin and granzyme B as pNK (King et al., 1993; Koopman et al., 2003). In addition, dNK express increased levels of the cytolytic molecule granulysin compared to pNK (Koopman et al., 2003). In contrast to pNK, in freshly isolated dNK, granulysin and perforin rarely co-localized (Vujaklija et al., 2013) and dNK but not pNK constitutively secrete granulysin in high levels without prior stimulation (Vujaklija et al., 2011). Granulysin is produced as an inactive 15 kDa pro-peptide that is processed in cytotoxic granules to a 9 kDa membranolytic peptide. Although the function of granulysin expression in dNK is not completely understood, the 15kDa, was shown to act as an alarmin involved in leukocyte recruitment whereas the 9kDa isoform was shown to bind and disrupt cholesterol-poor membranes, i.e. bacterial, fungal and parasite membranes and enhance clearance of these infections (Barman et al., 2006; Tewary et al., 2010; Walch et al., 2014). Despite the abundance of cytolytic granules, dNK are not able to kill Major Histocompatibility Antigen (MHC) Class I negative target cells (e. g. cell lines K652 or 721.221) efficiently as do pNK. The low cytotoxicity of dNK is due to an intrinsic block in the polarization of cytolytic granules to the immune synapse that can be overcome by incubating dNK with IL-15 (Kopcow et al., 2005; Tilburgs et al., 2015b). Thus dNK require additional activation by cytokines or activating NK receptor-ligand interactions to display their full cytotoxicity.
dNK – EVT interactions result in immune tolerance
Human Leukocyte Antigen (HLA)-G+ extravillous trophoblasts (EVT) are the most invasive cells of fetal origin that migrate deeply into maternal tissues and establish direct contact with maternal dNK (Hiby et al., 2010). In vitro co-culture of primary EVT and dNK obtained from the same pregnancy sample demonstrated an abundance of contacts formed between EVT and dNK. In the contacts between dNK and EVT, perforin did not localize to the immune synapse and both dNK and pNK were unable to kill EVT, even when activated by pro-inflammatory cytokines (Tilburgs et al., 2015b). However under pro-inflammatory conditions (i.e. IL-2 hyperstimulation) dNK were able to induce apoptosis in the trophoblast cell line HTR-8/SV40neo. dNK-derived granulysin actively accumulated in the nuclei of EVTs, causing the death of EVTs due to apoptosis (Nakashima et al., 2008). Interaction of dNK with primary EVT led to the acquisition of HLA-G by dNK through trogocytosis that was followed by a cycle of internalization, degradation, and reacquisition of HLA-G (Tilburgs et al., 2015b). Cytokine activation of dNK facilitated degradation of trogocytosed HLA-G and coincided with increased cytotoxicity by dNK. Thus the HLA-G cycle may provide a direct inhibition of cytotoxicity at an individual EVT–NK synapse as well as a prolonged but temporary inhibition of the dNK cytolytic machinery during HLA-G internalization and signaling events. Signaling may involve the HLA-G receptor Killer cell mmunoglobulin-like Receptor-2DL4 (KIR2DL4) as well as the activating HLA-C receptor KIR2DS1 that were both increased on HLA-G+ dNK. The inhibition of dNK cytotoxicity as well as other mechanisms of immune regulation such as the direct induction of FOXP3+ regulatory T cells (Treg) by EVT are important facets of maternal–fetal tolerance (Tilburgs et al., 2015a). However, it raises the problem of how the pregnant female is able to respond to events that require participation of cytotoxic dNK and/or decidual effector T cells, for example during a placental HCMV infection, a common viral infection at this site.
The role of activating NK receptors in pregnancy complications and viral infections
Killer cell Ig-like Receptors (KIR) are the major MHC Class I receptors expressed by NK cells and can be inhibitory or activating depended on the presence of Immunoreceptor tyrosine-based inhibitory motifs (ITIM) or Immunoreceptor tyrosine-based activating motifs (ITAM). Both inhibitory and activating KIR have specificity for discrete groups of MHC Class I alleles (Falk et al., 1993). Several viruses, including HCMV, impair the expression of MHC molecules to avoid immune recognition by T cells. The lack of MHC ligands for inhibitory KIR expressed by NK cells allows for activation of NK cytotoxicity through missing self-recognition. On the other hand viruses can induce the expression of activating ligands (e. g. MICA and MICB) on the surface of infected cells that directly bind activating NK receptors and promote NK cytotoxicity. Whether or not an NK cell kills a target cell is the result of the balance of inhibitory and activating signals between NK and target cells (Long, 1999). While the presence of activating ligands on the infected cell with activating receptors on the NK cells will promote cytotoxicity, the presence of MHC molecules that interact with inhibitory receptors will prevent cytotoxicity (Figure 1) (Long, 1999). Of high importance for pregnancy is the activating KIR2DS1 receptor that binds HLA-C2 group allotypes of HLA-C. Both KIR2DS1 as well as inhibitory KIR for HLA-C, KIR2DL1 and KIR2DL2/3 that respectively bind HLA-C2 and HLA-C1 group allotypes, are significantly higher expressed by dNK compared to pNK. These observations suggest that there is a skewing of KIR expression by dNK towards recognition of HLA-C, and especially the potential of dNK to develop an activating response to HLA-C2 (Sharkey et al., 2008; Xiong et al., 2013). The presence of KIR2DS1 in the maternal genome was associated with a lower risk for pregnancy complications such as miscarriage, fetal growth restriction and preeclampsia (Hiby et al., 2010; Hiby et al., 2004). This was most obvious when the fetus expressed HLA-C2, the ligand for KIR2DS1. Recently, the presence of KIR2DS5 has also been associated with lower risk to develop pregnancy complications in African women that may imply a wider role for activating KIR during pregnancy (Nakimuli et al., 2015). Although the underlying molecular mechanisms that explain the genetic associations between KIR2DS1, HLA-C2 and pregnancy complications remain largely unknown, the current hypothesis suggests that dominance of the inhibitory HLA-C receptors (KIR2DL1, KIR2DL2 and KIR2DL3) reduces the activation of dNK by HLA-C expressed by EVT. Activation of KIR2DS1 on dNK was shown to enhance granulocyte-monocyte colony stimulation factor (GM-CSF) secretion, a growth factor important for trophoblast migration and placental growth (Xiong et al., 2013). The increase in GM-CSF production was observed when dNK were stimulated with anti-KIR2DS1 antibodies and classical NK target cells that expressed HLA-C2. However, we recently demonstrated that primary EVT do not elicit cytokine responses by dNK even when KIR2DS1 and HLA-C2 are present (Tilburgs et al., 2015a). Furthermore, a murine model to address inhibition and activation of dNK by MHC expressed on trophoblasts demonstrated that the parental origin of the inhibitory MHC was irrelevant for pregnancy outcome (Kieckbusch et al., 2014). Therefore, these genetic associations demand further investigation into the molecular and cellular mechanisms underlying the reduced pregnancy risk linked to activating KIR, and in particular KIR2DS1.
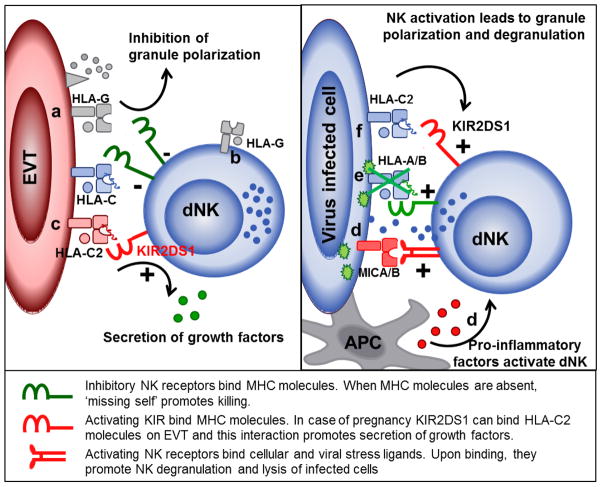
Figure 1. Three functions of dNK include support of trophoblast invasion and placental growth, maintenance of maternal-fetal tolerance and to provide immunity to infections.
a) HLA-G provides direct inhibition of cytotoxicity at an individual EVT–NK synapse and b) prolonged but temporary inhibition of the dNK cytolytic machinery after HLA-G trogocytosis; c) Interaction of KIR2DS1 and HLA-C2 may activate dNK to secrete cytokines and growth factors that benefit trophoblast migration and placental growth; d) Virus-induced stress ligands and pro-inflammatory factors activate dNK cytotoxicity; e) virus mediated down regulation of MHC activates NK cytotoxicity through inhibitory KIR and ‘missing self’ and f) activating KIR may provide additional NK activation and increase clearance of virus-infected cells.
A hint may lie in the observation that individuals who carry the KIR-B haplotype with more activating KIR also have a significantly improved outcome after viral infections such as HCMV, HIV and HPV (Bonagura et al., 2010; Martin et al., 2007; Stern et al., 2008). Of particular interest is that activating KIR were shown to play a role in NK mediated clearance of HCMV infection following hematopoietic stem cell (HSC) or solid organ transplantation (Cook et al., 2006; Stern et al., 2008; Stern et al., 2011). Primary HCMV infection or reactivation of latent HCMV infection can cause major problems during pregnancy. HCMV causes placental thickening and insufficiency, leading to fetal growth restriction (Pereira et al., 2014; Revello et al., 2006; Warner et al., 2012). Placental HCMV infection interferes with trophoblast invasion and placental development and can give rise to placental pathology and congenital syndromes. HCMV sero-positive woman also have a 1.5 fold increased risk to develop preeclampsia (Xie et al., 2010). CD8+ T cells represent only 2–7% of CD45+ lymphocytes in decidual tissue in 1st trimester pregnancy and thus dNK are the prime candidates to respond to viral infections at this site (Tilburgs et al., 2010; Tilburgs and Strominger, 2013). Siewiera et al., provided the first evidence that dNK are able to clear HCMV-infected decidual stromal cells (Siewiera et al., 2013). We recently demonstrated that dNK can clear HCMV infection of maternal decidual stromal cells but that HCMV-infected EVT cannot be cleared (Tilburgs et al., 2015b). The failure of dNK to kill EVT, even when infected with HCMV, may reduce the risk of immune rejection of EVT and placental tissue (Crespo et al. submitted for publication). However inefficient clearance of virus-infected placental cells may result in virus-induced placental pathology and development of complications later in pregnancy. Therefore investigation of the benefits of activating KIR/MHC combinations on the ability of dNK to clear HCMV in placental tissue will be of high interest to identify factors that can reduce the risks of placental HCMV infection and associated immunopathology in the placenta. Furthermore, understanding the mechanisms that regulate the switch between immune tolerance and immunity in dNK will contribute to the development of novel strategies to limit virus-induced placental pathology and congenital infection.
Regulation of cytolytic activity of CD8+ dT
Besides dNK, decidual CD8+ T cells (CD8+ dT) are key cytolytic effector cells present at the maternal-fetal interface. CD8+ dT form a minority of leukocytes present in first trimester decidua (~2–7% of CD45+ cells) but their proportion increases to ~30% in term pregnancy decidua (Bulmer et al., 1991). CD8+ dT have a predominant effector-memory (EM) phenotype (Saito et al., 1994; Tilburgs et al., 2010) but have reduced expression of perforin and granzyme B proteins in comparison with peripheral blood CD8+ T cells (CD8+ pT) (Tilburgs et al., 2009a; Tilburgs et al., 2010). Interestingly, CD8+ dT had increased levels of perforin and granzyme B mRNA content. This suggests post-translational modifications that block translation of perforin and granzyme B mRNA into functional proteins that may be mediated by miRNAs (Tilburgs et al., 2010; Trifari et al., 2013). Interestingly, similar to dNK, CD8+ dT express increased levels of granulysin suggesting they are locally activated and may play a protective role against different extracellular or intracellular pathogens (Tilburgs et al., 2010; Vujaklija et al., AJRI 2011; Nakashima et al., 2008). Despite the low levels of cytolytic granules, upon stimulation with PMA, first trimester CD8+ dT were shown to be cytolytic and produce cytokines that affect invasion of trophoblasts (Scaife et al., 2006). We recently demonstrated that CD8+ dT are fully functional and upon activation in ex-vivo cultures, upregulate expression of perforin and granzyme B proteins and degranulate in levels equal to CD8+ pT. Thus the cytolytic activity of CD8+ EM dT seems regulated by a blockade in the translation of mRNA in to cytolytic proteins, possibly to prevent detrimental responses to foreign fetal and placental cells. However this blockade can be overcome by addition of pro-inflammatory cytokines and T cell receptor (TCR) stimulation that may allow clearance of infected cells (Figure 2). This is in contrast to dNK that have an abundance of pre-stored intracellular granules containing cytolytic molecules and fail to polarize these granules to the immune synapse in the absence of pro-inflammatory signals. Further elucidation of the molecular mechanisms that regulate the expression of cytolytic molecules by CD8+ EM dT will be key to understanding how these cells provide immunity to infection yet maintain immune tolerance to fetal and placental cells.
Figure 2. Two functions of CD8+ dT include maintenance of maternal-fetal immune tolerance and to provide for anti-viral immunity.

a) The presence of HLA-G, anti-inflammatory cytokines and high levels of Treg inhibit CD8+ dT cytotoxicity and promote immune tolerance; b) The presence of allogeneic HLA-C and c) fetal mHag provide targets for maternal effector T cell responses; d) Virus-infected EVT can present viral peptides in HLA-C to HLA-C-restricted CD8+ CTL; e) Virus-induced pro-inflammatory factors can enhance cytolytic activity of CD8+ dT.
Antigen specificity of CD8+ dT
The existence of highly differentiated CD8+ EM dT indicates the presence of antigens at the maternal-fetal interface that attract an antigen-specific CD8+ dT response. The antigen-specificity of CD8+ dT cells may include MHC molecules of paternal origin (HLA-C expressed by EVT), minor histocompatibility antigens (mHags) or pathogen-derived antigens (recently reviewed in (Tilburgs and Strominger, 2013)). Although a large proportion of women generate antibodies and cytotoxic T lymphocytes (CTL) specific for paternal MHC and paternal mHags that can be expressed by fetal and placental tissues, there is no evidence that these immune responses compromise pregnancy outcome in humans or mice (Bouma et al., 1996; Erlebacher et al., 2007; Holland et al., 2012; Lissauer et al., 2012; Tafuri et al., 1995; Verdijk et al., 2004). There are many possibilities as to why these fetus-specific T cells do not cause rejection during pregnancy; i) Incomplete activation of T cells during pregnancy may result in a lack of effector function (Erlebacher et al., 2007; Tilburgs et al., 2010), ii) High levels of Treg suppress CD8+ T cell responses in decidua (Tilburgs et al., 2008; Tilburgs et al., 2009b), iii) A diminished influx of effector cells due to silencing of key T cell attracting chemokines (Nancy et al., 2012) iv) High levels of anti-inflammatory cytokines such as TGF-β may increase the T cell activation threshold in decidua (Yao et al., 2015).
Anti-viral and bacterial immunity by CD8 T cells in pregnancy
Viral infections were shown to alter the dynamics of peripheral blood CD8+ T cell (CD8+ pT) responses during pregnancy (Lissauer et al., 2011). However, very few studies have addressed the role of decidual CD8+ EM T cells and their ability to clear placental infections. A murine study demonstrated that antigen-specific CD8+ T cells in pregnant and non-pregnant mice during acute LCMV infection have similar function and proliferative capacity (Constantin et al., 2007). However, despite the significant expansion and infiltration of antigen-specific CD8+ T cells into the uterus and placental tissues, viral infection persisted in the uterus and placenta of LCMV-infected mice. The virus was cleared in every other tissue (e. g. Serum, Spleen, Liver and Lung) assayed (Constantin et al., 2007). The authors suggested that the lack of MHC class I expression on murine trophoblasts could prevent efficient clearance of the virus by MHC-restricted T cells (Constantin et al., 2007). Another study demonstrated that fetal wastage in mice triggered by prenatal Listeria monocytogenes infection was the result of placental recruitment of CXCL9-producing inflammatory neutrophils and macrophages that subsequently promote infiltration of maternal fetal-specific CD8+ T cells. Upregulation of CXCR3 expression by maternal CD8+ T cells with fetal specificity was responsible for influx of these T cells to the decidual tissue (Chaturvedi et al., 2015). During infection with an OVA-expressing Listeria monocytogenes (LM-OVA), OVA- specific CD8+ T cells were shown to accumulate in higher proportions in the decidua compared to the spleen when mice were inoculated with LM-OVA prior to pregnancy (Clark et al., 2014). Despite the influx of T cells to decidua, bacteria were not efficiently cleared from placental tissue, confirming the study by Constantin et al. Furthermore, both studies suggest that the despite chemokine gene silencing to prevent influx of T cells to decidual tissue in healthy murine gestation (Erlebacher et al., 2007), incomplete protection against T-cell recruitment to the maternal–fetal interface occurs when T cells are primed by infection (Clark et al., 2014).
Human EVT can be infected by intracellular pathogens such as HCMV and Listeria monocytogenes (Pereira and Maidji, 2008; Zeldovich et al., 2011). Thus when EVT are infected, HLA-C is the only molecule that can present pathogen-derived peptides to antigen-specific CD8+ T cells. During HIV and HCMV infection HLA-C-restricted CTL responses were shown to comprise as much as 54% of the total response in peripheral blood. Moreover, HLA-C-restricted CTL were shown to be functionally and phenotypically identical to HLA-A- and HLA-B-restricted CTL (Ameres et al., 2013; Makadzange et al., 2010). In humans, HCMV sero-positivity profoundly influenced the peripheral blood T cell repertoire (Lissauer et al., 2011). HCMV sero-positive women demonstrated higher levels of CCR7−CD45RA+ effector cells as well as CCR7−CD45RA−CD28− effector-memory cells in peripheral blood during late pregnancy compared to sero-negative pregnant women. Besides the studies of virus-specific CD8+ T cells in maternal blood, a recent study demonstrated increased percentages of HCMV and Epstein Bar virus (EBV)-specific CD8+ T cells in decidual tissue compared with peripheral blood after uncomplicated pregnancy. These virus-specific CD8+ memory T cells were able to produce IFNγ and were restricted to recognize viral peptides in HLA-A or HLA-B molecules (van Egmond et al., 2016). Thus, these CD8+ dT may provide cellular immunity for infected maternal cells that express HLA-A and HLA-B. A crucial challenge is to investigate whether virus-specific and HLA-C-restricted CTL are present at the maternal-fetal interface and function to provide immunity when EVT are infected.
Besides directly eliciting maternal CD8+ T cell responses, viral infections have been shown to facilitate ascending bacterial infections and lead to fetal wastage (Racicot et al., 2013; Racicot et al., 2016). Uncontrolled placental viral (and bacterial) infections also provide a pro-inflammatory milieu that can alter the stability and function of Treg and the activation status of dNK and CD8+ EM dT (Chong and Alegre, 2014). Infections can result in enhanced alloreactivity, resistance to tolerance induction and destabilization of established tolerance. Similarly, infections in transplant recipients have been associated with failure to induce transplant tolerance and allograft rejection even after long periods of transplant tolerance (Chong and Alegre, 2014; Wang et al., 2010).
Conclusion
To prevent pathogen-induced placental pathology and transmission of infections from mother to fetus, it is essential for the maternal immune system to establish protective immunity. Whereas many studies focus on the establishment of maternal-fetal immune tolerance to promote implantation and establish a healthy pregnancy, the role of cytotoxic dNK and virus-specific CD8+ T cells during placental infections has been an underexplored research area. A particular focus should be given to the molecular mechanisms that regulate cytotoxicity of dNK and CD8+ dT cells so that they promote maternal-fetal tolerance but remain able to respond to events that require participation of cytotoxic dNK and/or CD8+ dT. Viruses, bacteria and parasites take advantage of the immune privileged status of the placenta and the presence of many immune suppressive mechanisms. Investigation of the mechanisms by which infections are controlled in the placenta as well as the effects of infection and inflammation on Treg function, effector T cell activity and EVT migration, will provide a deeper understanding of the development of pregnancy complications that are associated with infections. Developing strategies to enhance maternal immunity to common infections may prevent development of pregnancy complications and diminish the risk of transmission of infections to the fetus that may lead to severe congenital syndromes.
Highlights.
Cytotoxicity of dNK and CD8+ dT cells is required to provide immunity to placental infections
Regulation of cytotoxicity of dNK and CD8+ dT is essential for maternal-fetal immune tolerance
HLA-C expression by EVT has a dual role in immune tolerance and anti-viral immunity
Placental infections affect dNK and effector T cell activity, Treg function and EVT migration
Acknowledgments
We wish all past and current lab members for their helpful discussions. This work was supported by National Institutes of Health Grant AI053330 and the March of Dimes grant 6-FY14-453. Angela Crespo was supported by the Portuguese Foundation for Science and Technology – FCT (SFRH/BD/33885/2009). J.L.S. is a consultant for King Abdulaziz University, Jeddah, Saudi Arabia.
List of Abbreviations
- CD8+ dT
decidual CD8+ T cells
- CD8+ pT
peripheral blood CD8+ T cells
- CTL
Cytotoxic T Lymphocytes
- dNK
decidual NK cells
- EBV
Epstein Bar virus
- EM
Effector Memory
- EVT
Extravillous Trophoblasts
- HCMV
Human Cytomegalovirus
- HLA
Human Leukocyte Antigen
- KIR
Killer cell Immunoglobulin-like Receptor
- mHag
Minor Histocompatibility Antigen
- MHC
Major Histocompatibility Antigen
- pNK
peripheral blood NK cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ameres S, et al. Presentation of an Immunodominant Immediate-Early CD8+ T Cell Epitope Resists Human Cytomegalovirus Immunoevasion. PLoS Pathog. 2013;9:e1003383. doi: 10.1371/journal.ppat.1003383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barman H, et al. Cholesterol in negatively charged lipid bilayers modulates the effect of the antimicrobial protein granulysin. J Membr Biol. 2006;212:29–39. doi: 10.1007/s00232-006-0040-3. [DOI] [PubMed] [Google Scholar]
- Bonagura VR, et al. Recurrent respiratory papillomatosis: a complex defect in immune responsiveness to human papillomavirus-6 and -11. APMIS. 2010;118:455–470. doi: 10.1111/j.1600-0463.2010.02617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouma GJ, et al. Pregnancy can induce priming of cytotoxic T lymphocytes specific for paternal HLA antigens that is associated with antibody formation. Transplantation. 1996;62:672–678. doi: 10.1097/00007890-199609150-00023. [DOI] [PubMed] [Google Scholar]
- Bulmer JN, Morrison L, Longfellow M, Ritson A, Pace D. Granulated lymphocytes in human endometrium: histochemical and immunohistochemical studies. Hum Reprod. 1991;6:791–798. doi: 10.1093/oxfordjournals.humrep.a137430. [DOI] [PubMed] [Google Scholar]
- Chaturvedi V, et al. CXCR3 blockade protects against Listeria monocytogenes infection-induced fetal wastage. J Clin Invest. 2015;125:1713–1725. doi: 10.1172/JCI78578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong AS, Alegre ML. Transplantation tolerance and its outcome during infections and inflammation. Immunol Rev. 2014;258:80–101. doi: 10.1111/imr.12147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DR, et al. Perinatal Listeria monocytogenes susceptibility despite preconceptual priming and maintenance of pathogen-specific CD8(+) T cells during pregnancy. Cell Mol Immunol. 2014;11:595–605. doi: 10.1038/cmi.2014.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantin CM, et al. Normal establishment of virus-specific memory CD8 T cell pool following primary infection during pregnancy. J Immunol. 2007;179:4383–4389. doi: 10.4049/jimmunol.179.7.4383. [DOI] [PubMed] [Google Scholar]
- Cook M, et al. Donor KIR genotype has a major influence on the rate of cytomegalovirus reactivation following T-cell replete stem cell transplantation. Blood. 2006;107:1230–1232. doi: 10.1182/blood-2005-03-1039. [DOI] [PubMed] [Google Scholar]
- Erlebacher A, Vencato D, Price KA, Zhang D, Glimcher LH. Constraints in antigen presentation severely restrict T cell recognition of the allogeneic fetus. J Clin Invest. 2007;117:1399–1411. doi: 10.1172/JCI28214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk K, et al. Allele-specific peptide ligand motifs of HLA-C molecules: Proc. Natl Acad Sci U S A. 1993;90:12005–12009. doi: 10.1073/pnas.90.24.12005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hiby SE, et al. Maternal activating KIRs protect against human reproductive failure mediated by fetal HLA-C2. J Clin Invest. 2010;120:4102–4110. doi: 10.1172/JCI43998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiby SE, et al. Combinations of maternal KIR and fetal HLA-C genes influence the risk of preeclampsia and reproductive success. J Exp Med. 2004;200:957–965. doi: 10.1084/jem.20041214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland OJ, et al. Minor histocompatibility antigens are expressed in syncytiotrophoblast and trophoblast debris: implications for maternal alloreactivity to the fetus. Am J Pathol. 2012;180:256–266. doi: 10.1016/j.ajpath.2011.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kieckbusch J, Gaynor LM, Moffett A, Colucci F. MHC-dependent inhibition of uterine NK cells impedes fetal growth and decidual vascular remodeling. Nat Commun. 2014;5:3359. doi: 10.1038/ncomms4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King A, Kalra P, Loke YW. Human trophoblast cell resistance to decidual NK lysis is due to lack of NK target structure. Cell Immunol. 1990;127:230–237. doi: 10.1016/0008-8749(90)90128-e. [DOI] [PubMed] [Google Scholar]
- King A, Wellings V, Gardner L, Loke YW. Immunocytochemical characterization of the unusual large granular lymphocytes in human endometrium throughout the menstrual cycle. Hum Immunol. 1989;24:195–205. doi: 10.1016/0198-8859(89)90060-8. [DOI] [PubMed] [Google Scholar]
- King A, Wooding P, Gardner L, Loke YW. Expression of perforin, granzyme A and TIA-1 by human uterine CD56+ NK cells implies they are activated and capable of effector functions: Hum. Reprod. 1993;8:2061–2067. doi: 10.1093/oxfordjournals.humrep.a137982. [DOI] [PubMed] [Google Scholar]
- Koopman, et al. Human decidual natural killer cells are a unique NK cell subset with immunomodulatory potential. J Exp Med. 2003;198:1201–1212. doi: 10.1084/jem.20030305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopcow HD, et al. Human decidual NK cells form immature activating synapses and are not cytotoxic. Proc Natl Acad Sci U S A. 2005;102:15563–15568. doi: 10.1073/pnas.0507835102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissauer D, Choudhary M, Pachnio A, Goodyear O, Moss PA, Kilby MD. Cytomegalovirus sero positivity dramatically alters the maternal CD8+ T cell repertoire and leads to the accumulation of highly differentiated memory cells during human pregnancy. Hum Reprod. 2011;12:3355–3365. doi: 10.1093/humrep/der327. [DOI] [PubMed] [Google Scholar]
- Lissauer D, Piper K, Goodyear O, Kilby MD, Moss PA. Fetal-specific CD8+ cytotoxic T cell responses develop during normal human pregnancy and exhibit broad functional capacity. J Immunol. 2012;189:1072–1080. doi: 10.4049/jimmunol.1200544. [DOI] [PubMed] [Google Scholar]
- Long EO. Regulation of immune responses through inhibitory receptors. Annu Rev Immunol. 1999;17:875–904. doi: 10.1146/annurev.immunol.17.1.875. [DOI] [PubMed] [Google Scholar]
- Makadzange AT, et al. Characterization of an HLA-C-restricted CTL response in chronic HIV infection. Eur J Immunol. 2010;40:1036–1041. doi: 10.1002/eji.200939634. [DOI] [PubMed] [Google Scholar]
- Martin MP, et al. Innate partnership of HLA-B and KIR3DL1 subtypes against HIV-1. Nat Genet. 2007;39:733–740. doi: 10.1038/ng2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima A, et al. Granulysin produced by uterine natural killer cells induces apoptosis of extravillous trophoblasts in spontaneous abortion. Am JPathol. 2008;173:653–64. doi: 10.2353/ajpath.2008.071169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakimuli A, et al. A KIR B centromeric region present in Africans but not Europeans protects pregnant women from pre-eclampsia. Proc Natl Acad Sci U S A. 2015;112:845–850. doi: 10.1073/pnas.1413453112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nancy P, Tagliani E, Tay CS, Asp P, Levy DE, Erlebacher A. Chemokine gene silencing in decidual stromal cells limits T cell access to the maternal-fetal interface. Science. 2012;336:1317–1321. doi: 10.1126/science.1220030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira L, Maidji E. Cytomegalovirus infection in the human placenta: maternal immunity and developmentally regulated receptors on trophoblasts converge. Curr Top Microbiol Immunol. 2008;325:383–395. doi: 10.1007/978-3-540-77349-8_21. [DOI] [PubMed] [Google Scholar]
- Pereira L, et al. Intrauterine growth restriction caused by underlying congenital cytomegalovirus infection. J Infect Dis. 2014;209:1573–1584. doi: 10.1093/infdis/jiu019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot K, et al. Viral infection of the pregnant cervix predisposes to ascending bacterial infection. J Immunol. 2013;191:934–941. doi: 10.4049/jimmunol.1300661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racicot K, Kwon JY, Aldo P, Abrahams V, El-Guindy A, Romero R, Mor G. Type I Interferon Regulates the Placental Inflammatory Response to Bacteria and is Targeted by Virus: Mechanism of Polymicrobial Infection-Induced Preterm Birth. Am J Reprod Immunol. 2016;75:451–460. doi: 10.1111/aji.12501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saini Reena V, et al. J Immunol. 2011 [Google Scholar]
- Revello MG, et al. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. J Infect Dis. 2006;193:269–276. doi: 10.1086/498872. [DOI] [PubMed] [Google Scholar]
- Saito S, et al. A study of CD45RO, CD45RA and CD29 antigen expression on human decidual T cells in an early stage of pregnancy. Immunol Lett. 1994;40:193–197. doi: 10.1016/0165-2478(93)00019-a. [DOI] [PubMed] [Google Scholar]
- Scaife PJ, Bulmer JN, Robson SC, Innes BA, Searle RF. Effector activity of decidual CD8+ T lymphocytes in early human pregnancy. Biol Reprod. 2006;75:562–567. doi: 10.1095/biolreprod.106.052654. [DOI] [PubMed] [Google Scholar]
- Sharkey AM, et al. Killer Ig-like receptor expression in uterine NK cells is biased toward recognition of HLA-C and alters with gestational age. J Immunol. 2008;181:39–46. doi: 10.4049/jimmunol.181.1.39. [DOI] [PubMed] [Google Scholar]
- Siewiera J, et al. Human cytomegalovirus infection elicits new decidual natural killer cell effector functions: PLoS. Pathog. 2013;9:e1003257. doi: 10.1371/journal.ppat.1003257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern M, Elsasser H, Honger G, Steiger J, Schaub S, Hess C. The number of activating KIR genes inversely correlates with the rate of CMV infection/reactivation in kidney transplant recipients. Am J Transplant. 2008;8:1312–1317. doi: 10.1111/j.1600-6143.2008.02242.x. [DOI] [PubMed] [Google Scholar]
- Stern M, et al. Telomeric rather than centromeric activating KIR genes protect from cytomegalovirus infection after kidney transplantation. Am J Transplant. 2011;11:1302–1307. doi: 10.1111/j.1600-6143.2011.03516.x. [DOI] [PubMed] [Google Scholar]
- Tafuri A, Alferink J, Moller P, Hammerling GJ, Arnold B. T cell awareness of paternal alloantigens during pregnancy. Science. 1995;270:630–633. doi: 10.1126/science.270.5236.630. [DOI] [PubMed] [Google Scholar]
- Tewary P, et al. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116:3465–74. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T, et al. Human HLA-G+ extravillous trophoblasts: Immune-activating cells that interact with decidual leukocytes. Proc Natl Acad Sci U S A. 2015a;112:7219–7224. doi: 10.1073/pnas.1507977112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T, Evans JE, Crespo AC, Strominger JL. The HLA-G cycle provides for both NK tolerance and immunity at the maternal-fetal interface. Proc Natl Acad Sci USA. 2015b;112:13312–17. doi: 10.1073/pnas.1517724112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilburgs T, et al. Evidence for a Selective Migration of Fetus-Specific CD4+CD25bright Regulatory T Cells from the Peripheral Blood to the Decidua in Human Pregnancy. J Immunol. 2008;180:5737–5745. doi: 10.4049/jimmunol.180.8.5737. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Scherjon SA, Roelen DL, Claas FH. Decidual CD8+CD28- T cells express CD103 but not perforin. Hum Immunol. 2009a;70:96–100. doi: 10.1016/j.humimm.2008.12.006. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, et al. Fetal-maternal HLA-C mismatch is associated with decidual T cell activation and induction of functional T regulatory cells. J Reprod Immunol. 2009b;82:148–157. doi: 10.1016/j.jri.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, et al. Human decidual tissue contains differentiated CD8+ effector-memory T cells with unique properties. J Immunol. 2010;185:4470–4477. doi: 10.4049/jimmunol.0903597. [DOI] [PubMed] [Google Scholar]
- Tilburgs T, Strominger JL. CD8+ effector T cells at the fetal-maternal interface, balancing fetal tolerance and antiviral immunity. Am J Reprod Immunol. 2013;69:395–407. doi: 10.1111/aji.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifari S, et al. MicroRNA-directed program of cytotoxic CD8+ T-cell differentiation. Proc Natl Acad Sci U S A. 2013;110:18608–18613. doi: 10.1073/pnas.1317191110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Egmond EA, van der Keur C, Swings GM, Scherjon SA, Claas FH. The possible role of virus-specific CD8(+) memory T cells in decidual tissue. J Reprod Immunol. 2016;113:1–8. doi: 10.1016/j.jri.2015.09.073. [DOI] [PubMed] [Google Scholar]
- Verdijk RM, et al. Pregnancy induces minor histocompatibility antigen-specific cytotoxic T cells: implications for stem cell transplantation and immunotherapy. Blood. 2004;103:1961–1964. doi: 10.1182/blood-2003-05-1625. [DOI] [PubMed] [Google Scholar]
- Vujaklija DV, et al. First Trimester Pregnancy Decidual Natural Killer Cells Contain and Spontaneously Release High Quantities of Granulysin. Am J Reprod Immunol. 2011;66:363–372. doi: 10.1111/j.1600-0897.2011.01015.x. [DOI] [PubMed] [Google Scholar]
- Vujaklija DV, et al. Granulysin expression and the interplay of granulysin and perforin at the maternal–fetal interface. J Reprod Immunol. 2013;97:186–196. doi: 10.1016/j.jri.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Walch M, et al. Cytotoxic cells kill intracellular bacteria through granulysin-mediated delivery of granzymes. Cell. 2014;157:1309–1323. doi: 10.1016/j.cell.2014.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, et al. Infection with the intracellular bacterium, Listeria monocytogenes, overrides established tolerance in a mouse cardiac allograft model. Am J Transplant. 2010;10:1524–1533. doi: 10.1111/j.1600-6143.2010.03066.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JA, Zwezdaryk KJ, Day B, Sullivan DE, Pridjian G, Morris CA. Human cytomegalovirus infection inhibits CXCL12-mediated migration and invasion of human extravillous cytotrophoblasts. J Virol. 2012;9:255. doi: 10.1186/1743-422X-9-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie F, et al. An association between cytomegalovirus infection and pre-eclampsia: a case-control study and data synthesis. Acta Obstet Gynecol Scand. 2010;89:1162–1167. doi: 10.3109/00016349.2010.499449. [DOI] [PubMed] [Google Scholar]
- Xiong S, et al. Maternal uterine NK cell-activating receptor KIR2DS1 enhances placentation. J Clin Invest. 2013;123:4264–4272. doi: 10.1172/JCI68991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao Y, Song J, Wang W, Liu N. Decidual vascular endothelial cells promote maternal-fetal immune tolerance by inducing regulatory T cells through canonical Notch1 signaling. Immunol Cell Biol. 2015:119. doi: 10.1038/icb.2015.119. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Zeldovich VB, Robbins JR, Kapidzic M, Lauer P, Bakardjiev AI. Invasive extravillous trophoblasts restrict intracellular growth and spread of Listeria monocytogenes. PLoS Pathog. 2011;7:e1002005. doi: 10.1371/journal.ppat.1002005. [DOI] [PMC free article] [PubMed] [Google Scholar]