Abstract
Purpose
To summarize our single‐center safety experience with the off‐label use of ferumoxytol for magnetic resonance imaging (MRI) and to compare the effects of ferumoxytol on monitored physiologic indices in patients under anesthesia with those of gadofosveset trisodium.
Materials and Methods
Consecutive patients who underwent ferumoxytol‐enhanced (FE) MRI exams were included. Adverse events (AEs) were classified according to the Common Terminology Criteria for Adverse Events v4.0. In a subgroup of patients examined under general anesthesia, recording of blood pressure, heart rate, oxygen saturation, and end‐tidal CO2 was performed. A comparable group of 23 patients who underwent gadofosveset‐enhanced (GE) MRI under anesthesia with similar monitoring was also analyzed.
Results
In all, 217 unique patients, ages 3 days to 94 years, underwent FE‐MRI. No ferumoxytol‐related severe, life‐threatening, or fatal AEs occurred acutely or at follow‐up. Two patients developed ferumoxytol‐related nausea. Between‐group (FE‐ vs. GE‐MRI) comparisons showed no statistical difference in heart rate (P = 0.69, 95% confidence interval [CI] 96–113 bpm), mean arterial blood pressure (MAP) (P = 0.74, 95% CI 44–52 mmHg), oxygen saturation (P = 0.76, 95% CI 94–98%), and end‐tidal CO2 (P = 0.73, 95% CI 31–37 mmHg). No significant change in MAP (P = 0.12, 95% CI 50–58 mmHg) or heart rate (P = 0.25, 95% CI 91–105 bpm) was noted between slow infusion of ferumoxytol (n = 113) vs. bolus injection (n = 104).
Conclusion
In our single‐center experience, no serious AEs occurred with the diagnostic use of ferumoxytol across a wide spectrum of age, renal function, and indications. Because of the limited sample size, firm conclusions cannot be drawn about the generalizability of our results. Thus, vigilance and monitoring are recommended to mitigate potential rare adverse reactions.
Level of Evidence: 2
J. Magn. Reson. Imaging 2017;45:804–812.
Keywords: ferumoxytol, USPIO, magnetic resonance imaging, contrast agent, magnetic resonance angiography
Based on data generated by the National Health and Nutrition Examination Survey1 and the 2000 US census,2 there are ∼1.4 million people who either have Stage 4 kidney disease or require renal replacement therapy. Both iodinated‐ and gadolinium‐based contrast agents (GBCAs) have limited utility in this population and the use of contrast agents typically requires careful assessment of risks to benefits. In this context, ferumoxytol (Feraheme, AMAG Pharmaceuticals, Waltham, MA) has been considered an alternative to GBCAs for magnetic resonance imaging (MRI) in patients with renal impairment. Due to its large molecular size and dextran coat, ferumoxytol has a long intravascular half‐life of ∼14–15 hours3 and thus provides a wide and stable time window for specific vascular enhancement. In addition, ferumoxytol has a high r1 relaxivity comparable to gadofosveset (∼9 mM−1s−1at 3.0T),4 the only intravascular GBCA that is approved by the US FDA (Food and Drug Administration). Because of these unique properties, ferumoxytol can support a variety of MRI solutions not possible or practical with available GBCAs and in patients with renal impairment.
Furthermore, recent concerns relating to the gadolinium deposition in brain and bone tissues5 have also added a new dimension of urgency to the quest for alternative agents. Whereas gadolinium is an accidental constituent of biological systems when administered, iron is an essential nutrient and once the carbohydrate shell of ferumoxytol is degraded, the elemental iron at its core is incorporated into the hematopoietic pathway. Although ferumoxytol is currently approved by the FDA for intravenous (IV) treatment of iron deficiency anemia in the setting of chronic kidney disease (CKD),6 it was originally developed as an MRI contrast agent more than 20 years ago.3, 7, 8 To date, ferumoxytol has been described in cardiovascular,3, 8, 9 neuro,10, 11, 12 inflammation,13, 14 and oncologic12, 15, 16, 17, 18, 19 imaging applications.
In March 2015, however, based on 79 postmarketing adverse event case reports following the therapeutic use of ferumoxytol, the FDA issued a Black Box warning highlighting the risk of rare but potentially fatal hypersensitivity reactions. The FDA statement had immediate and widespread repercussions in the scientific and clinical community, generating fear and uncertainty about the safety of ferumoxytol as a diagnostic imaging agent. Because of the increasing recognition of potential benefit available to large populations of patients through the appropriate use of ferumoxytol as a contrast agent, it is incumbent on the scientific community to explore fully the real risks and benefits associated with such use and to maximize the overall benefit‐to‐risk ratio. To date, data on the safety of ferumoxytol as an off‐label MRI contrast agent are accumulating steadily, but remain limited. In this report we summarize our single‐center safety experience on the diagnostic use of ferumoxytol across the entire age spectrum and compare its effect on objective physiological parameters to that of an FDA‐approved intravascular contrast agent, gadofosveset trisodium (Ablavar, Lantheus Medical Imaging, N. Billerica, MA) in patients imaged under anesthesia.
Materials and Methods
This is a retrospective analysis of a prospective study that was approved by our Institutional Review Board and is compliant with the Health Insurance and Portability and Accountability Act (HIPAA). All patients or their legal representative(s) provided written informed consent for participation in one of two specific studies and for inclusion in our institutional research database. We evaluated safety data obtained from our database of ferumoxytol‐enhanced MRI (FE‐MRI) exams performed between June 2013 and March 2016. Briefly, study data were collected and managed using REDCap20 electronic data capture tools hosted at our institution. REDCap (Research Electronic Data Capture) is a secure, web‐based application designed to support data capture for research studies by providing 1) an intuitive user interface for validated data entry; 2) audit trails for tracking data manipulation and export procedures; 3) automated export procedures for data downloads to common statistical packages; and 4) procedures for importing data from external sources. Preliminary phenotypic data fields currently include general demographics, clinical indication, laboratory data, imaging sequence type, contrast type and administration, hemodynamic measurements, and limited outcome parameters. Data summarized in the current study consists of internal, single‐center data from the authors' institution.
Clinical eligibility criteria for FE‐MRI included patients of all ages, gender, ethnicity, and renal function without a history of allergic reaction to iron agents or laboratory work concerning iron disorders. Those with routine clinical contraindications for MRI (metallic foreign objects, deep brain stimulators, shrapnel, aneurysm clips, cochlear implants, dental implants) were not eligible. Those with implanted cardiac devices were included; device and institutional‐specific safety protocols were followed according to previously published work.21 All consecutive FE‐MRI exams logged in our database were included. To compare the effects of ferumoxytol on objective measures of blood pressure, heart rate, oxygen saturation, and end‐tidal CO2 to those of gadofosveset, we identified an age‐ and gender‐matched group of patients who had undergone gadofosveset enhanced (GE) and FE cardiovascular MRI exams and whose detailed physiologic documentation were available for review. Gadofosveset was chosen as a comparator because, as an FDA‐approved GBCA, it has the longest intravascular half‐life and its relaxivity properties most closely resemble those of ferumoxytol.
Adverse Events (AEs)
AEs and their severity were defined according to the Common Terminology Criteria for Adverse Events v4.0 (CTCAE) developed by the National Cancer Institute22: AEs are “any unfavorable and unintended signs, symptoms, or disease temporally associated” with the administration of ferumoxytol or gadofosveset. Note that this definition does not require that the AE be causally related to the agent, only to its temporal administration. An attending physician assessed the patient's baseline condition and was present throughout the MRI exams. AEs were graded as: mild (grade 1), moderate (grade 2), severe (grade 3), life‐threatening (grade 4), or fatal (grade 5) (Table 1A). The causal relationship of AEs to ferumoxytol administration was also rated by the attending physician as definitely, probably, possibly, or unrelated (Table 1B) and confirmed by consensus discussion with referring physicians. All patients were observed for at least 30 minutes following ferumoxytol or gadofosveset injection. All MRI scans were performed in facilities where resuscitation equipment was readily available and with close proximity to an emergency room or acute care facility. Two investigators retrospectively reviewed the electronic medical charts for follow‐up information.
Table 1.
Classification of Adverse Events and Their Relationship to Ferumoxytol Administration
| A. Classification of Adverse Events (AE)a | |
| Severity | Definition |
| Mild (grade 1) | Experience resulting in transient or mild discomfort; no limitation in activity; no medical intervention or therapy required. The patient may be aware of the sign or symptom but tolerates it reasonably well. |
| Moderate (grade 2) | Experience resulting in mild to moderate limitation in age‐appropriate instrumental activity of daily living (ADLs)b; noninvasive or minimal medical intervention/therapy required. |
| Severe (grade 3) | Experience resulting in marked limitation in age‐appropriate self‐care ADLsb but not life‐threatening, medical intervention/therapy required, hospitalizations or prolongation of hospitalizations possible. |
| Life‐threatening (grade 4) | Experience resulting in risk of death due to the adverse experience as it occurred; urgent intervention required. |
| Fatal (grade 5) | Experience resulting in death related to AE, persistent or significant disability/incapacity, and congenital anomaly/birth defect. |
| B. Relationship of AE to Ferumoxytol Administration | |
| Classification | Definition |
| Definitely | Previously known toxicity of agent; or an event that follows a reasonable temporal sequence from administration of the drug; that follows a known or expected response pattern to the suspected drug; that is confirmed by stopping or reducing the dosage of the drug; and that is not explained by any other reasonable hypothesis. |
| Probably | An event that follows a reasonable temporal sequence from administration of the drug; that follows a known or expected response pattern to the suspected drug; that is confirmed by stopping or reducing the dosage of the drug; and that is unlikely to be explained by the known characteristics of the subject's clinical state or by other interventions. |
| Possibly | An event that follows a reasonable temporal sequence from administration of the drug; that follows a known or expected response pattern to that suspected drug; but that could readily have been produced by a number of other factors. |
| Unrelated | An event that can be determined with certainty to have no relationship to the study drug. |
Based on the Common Terminology Criteria for Adverse Events (CTCAE) v4.0 developed by the National Cancer Institute(22). Adverse events (AE) are defined as any unfavorable and unintended signs, symptoms, or disease temporally associated' with the administration of ferumoxytol regardless of the causal relationship.
Activities of daily living (ADLs). Definitions are as defined in CTCAE v4.0: Instrumental ADLs refer to preparing meals, shopping for groceries or clothes, using the telephone, managing money, etc. Self‐care ADLs refer to bathing, dressing and undressing, feeding self, using the toilet, taking medications, and not bedridden.
Hemodynamic and Respiratory Monitoring
For those examined under general anesthesia, cardiovascular anesthesiologists performed continuous monitoring of vital signs including extremity pulse oximetry waveforms and ventilatory parameters.23 Hemodynamic and respiratory parameters including heart rate, blood pressure (noninvasive and/or direct arterial), pulse oximetry, and end‐tidal CO2 were recorded in the electronic medical record and encompassed a 30‐minute time window following ferumoxytol or gadofosveset administration. Although the stability of physiologic parameters and the absence or presence of AEs were documented in the electronic medical records for all patients in our study, detailed data on the specifics of physiological parameters (heart rate, blood pressure, pulse oximetry) were entered into the imaging database only for those patients who underwent imaging under anesthesia. Our hemodynamic and respiratory monitoring and recording procedures were independent of whether ferumoxytol was administered as a bolus or slow infusion.
Contrast Infusion and Image Acquisition
Technical details for FE‐MRI have been previously described.4, 8, 9, 24 Images were acquired at both 1.5T (n = 19) and 3.0T (n = 198). Each stock 17‐mL ferumoxytol vial containing 510 mg elemental iron (30 mg Fe/mL) and was diluted 8–10× based on the patient's size. While the adult therapeutic dose calls for two 510‐mg injections given 3–8 days apart, the dose for diagnostic purposes is generally 4 mg/kg (∼280 mg for an average adult). Prior to March 2015, we administered ferumoxytol as an IV bolus up to 2 mg/kg for first‐pass imaging and steady‐state images were subsequently acquired (at a total dose of 4 mg Fe/kg). Following the FDA's warning of rare hypersensitivity reactions and recommendation for slow IV infusion, we administered dilute ferumoxytol only as a slow infusion over 10 minutes and only steady‐state imaging was performed. For gadofosveset enhanced MRI (GE‐MRI), gadofosveset trisodium was administered at 0.06 mmol/kg as a single bolus and technical details have also been previously described.23
Statistics
Statistical analyses were performed using MedCalc 12.0.1.0 (Mariakerke, Belgium). Data were tested for normality using the D'Agostino‐Pearson test and are reported as mean ± standard deviation or median and interquartile range (IQR). Categorical data are reported as absolute or relative frequencies. Analysis of variance (ANOVA) was used to compare between group (FE‐MRI vs. GE‐MRI) and within‐group variations in physiologic indices immediately preinjection, immediately postinjection, and 30 minutes postinjection. Hemodynamic variations between bolus vs. slow infusion of ferumoxytol were also compared using ANOVA. Bonferroni correction was used to adjust for multiple comparisons. A two‐sided P‐value <0.05 was considered statistically significant.
Results
Characteristics of Study Population
Table 2 outlines the characteristics of our patient population who underwent FE‐MRI. A total of 217 unique consecutive patients (n = 91 pediatric; n = 126 adults), ages 3 days to 94 years, had 237 injections for FE‐MRI over a 2.75‐year period at our institution. Ninety‐four patients underwent FE‐MRI under anesthesia, of whom 71 had detailed hemodynamic and physiologic data available in our database. Of the 217 patients, five were pregnant and three had pacemakers. The pregnancies were all considered high‐risk and FE‐MRI examinations were performed to exclude placenta accreta (n = 4) and renal vein thrombosis (n = 1) in the setting of acute renal failure. There was a total of 237 ferumoxytol injections: 124 injections without anesthesia, 108 injections under anesthesia, and five injections under mild oral sedation. In all, 104 patients received bolus injections, while 113 received slow infusions of ferumoxytol. Figure 1 summarizes the clinical indications, while Fig. 2 characterizes the age spectrum of the clinical population that underwent FE‐MRI. A group of 23 patients with congenital heart disease (ages 2 days to 12.5 years, 43% female, weighing 11 [3.6–17.8] kg, creatinine 26.5 [24.8–44.2] μmol/L, estimated glomerular filtration rate [eGFR] 21 to >60 mL/min/1.73m2), had GE‐MRI under general anesthesia.
Table 2.
Characteristics of Patients Undergoing Ferumoxytol‐Enhanced MRI
| Entire cohort (n = 217 unique cases) | Anesthesia only (n = 94) | Nonanesthesia cases (n = 123) | Bolus infusions (n = 104) | Slow infusions (n = 113) | |
|---|---|---|---|---|---|
| Age (range) | 3d–94y | 3d–86y | 12y–94y | 3d–94y | 3d–89y |
| Gender, % female | 43% (n = 93) | 45% (n = 42) | 41% (n = 51) | 40% (n = 42) | 45% (n = 51) |
| Weight, kg | 50.1 (9.9–73.4) | 12.6 (3.6–23.0) | 68.5 (52.2–81.4) | 59.4 (16.5–76.9) | 37.3 (3.2–70.4) |
| Creatinine (μmol/L) | 168.0 (61.9–282.9) | 53.0 (35.4–265.2) | 194.5 (132.6–291.7) | 168.0 (70.7–265.2) | 168.0 (53.0–282.9) |
| eGFR (mL/min/1.73m2) | 31 (18–56) | 41 (19 to ≥ 60) | 29 (18–43) | 32 (19–51) | 28 (17–58) |
Data are reported as median (interquartile range). For adults (age ≥ 18 years), the eGFR was derived from the MDRD (Modification of Diet in Renal Disease) equation (Levey AS et al. Ann Intern Med. 2009;150:604–612). For children (<18 years), the Bedside Schwartz equation was used to calculate eGFR (Schwartz GJ et al. J Am Soc Nephrol. 2009;4:1832–1843). d, days; eGFR, estimated glomerular filtration rate; y, years.
Figure 1.
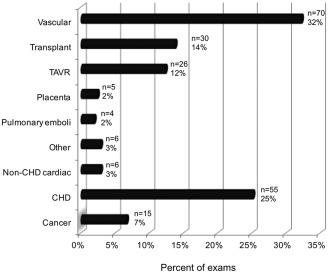
Clinical indications of FE‐MRI exams (n = 217 unique exams). CHD, congenital heart disease; TAVR, transcatheter aortic valve replacement.
Figure 2.
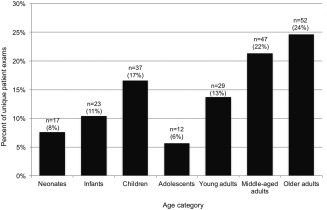
Age spectrum of unique patients (n = 217, 91 pediatric, 126 adults) who had FE‐MRI. Patients of a wide age range including those with immature (pediatrics) or impaired renal function (elderly) underwent FE‐MRI. Age definitions: Neonates ≤1 month; Infants >1 month to ≤2 years; Children >2 years to ≤12 years; Adolescents >12 years to ≤16 years; Young adults >16 years to ≤39 years; Middle‐aged adults >39 years to ≤65 years; Older adults >65 years.
Adverse Events
No severe, life‐threatening, or fatal AEs occurred acutely or at follow‐up for either FE‐MRI or GE‐MRI. Average follow‐up time was 14.9 ± 8.4 months for FE‐MRI and 45.3 ± 5.7 months for GE‐MRI. Two ferumoxytol‐related mild AEs occurred acutely. Patient 1 was a 20‐year‐old female with CKD and an upper extremity thrombus who underwent FE‐MRI to exclude thrombi in the central veins. Prior to undergoing the FE‐MRI, she felt nauseated and claustrophobic, but decided to proceed with FE‐MRI and had a recurrent bout of nausea immediately after a 0.8‐mL timing test injection of ferumoxytol. The study was terminated and she returned to her room with stable vital signs. The following day, ferumoxytol was administered while the patient was in her room; she was transported to the MRI suite and had successful imaging under anesthesia. Her nausea was possibly related to ferumoxytol injection, but may have been coincidental, since she was felt to have uremic gastritis. Patient 2 was a 19‐year‐old male with oxalosis and CKD on dialysis who underwent FE‐MRI without anesthesia to exclude the presence of a thrombus at the tip of the dialysis catheter. The patient was on opioid medications for chronic pain and was in mild distress prior to the MRI exam. Within several minutes of ferumoxytol injection, he felt nauseated, but did not vomit. Vital signs were stable. He was able to complete the study without requiring treatment. His nausea was felt definitely related to ferumoxytol injection, possibly with potentiation by opiates. Two other mild AEs were noted (one case of nausea and vomiting and one case with hypoglycemic symptoms), but these were felt to be secondary to the patients' comorbidities and unrelated to ferumoxytol administration. Tryptase levels (marker of mast cell release) were not obtained. On review of medical records at follow‐up, there were 10 deaths, all of which were secondary to progression of underlying disease and unrelated to ferumoxytol injection.
Hemodynamic and Respiratory Parameters
Distribution and transient temporal variations of physiologic indices for patients undergoing FE‐MRI and GE‐MRI under general anesthesia are presented in Fig. 3. Across the entire FE‐MRI cohort, there were no statistically significant changes in the physiological parameters between preinjection and up to 30 minutes postinjection (P = 0.12, 95% confidence interval [CI] 93–106 bpm for heart rate, P = 0.92, 95% CI 49–57 for mean arterial blood pressure, P = 0.68, 95% CI 95–98 mmHg for oxygen saturation, and P = 0.86, 95% CI 32–35 mmHg for end‐tidal CO2). Between‐group (FE‐MRI vs. GE‐MRI) variations among age‐ and gender‐matched groups also showed no statistical difference in heart rate (P = 0.69, 95% CI 96–113 bpm), mean arterial blood pressure (P = 0.74, 95% CI 44–52 mmHg), oxygen saturation (P = 0.76, 95% CI 94–98%), and end‐tidal CO2 (P = 0.73, 95% CI 31–37 mmHg). Comparison of hemodynamic parameters between those who received bolus injections vs. slow infusions of ferumoxytol also showed no significant difference in mean arterial blood pressure (P = 0.12, 95% CI 50–58 mmHg) or heart rate (P = 0.25, 95% CI = 91–105 bpm).
Figure 3.
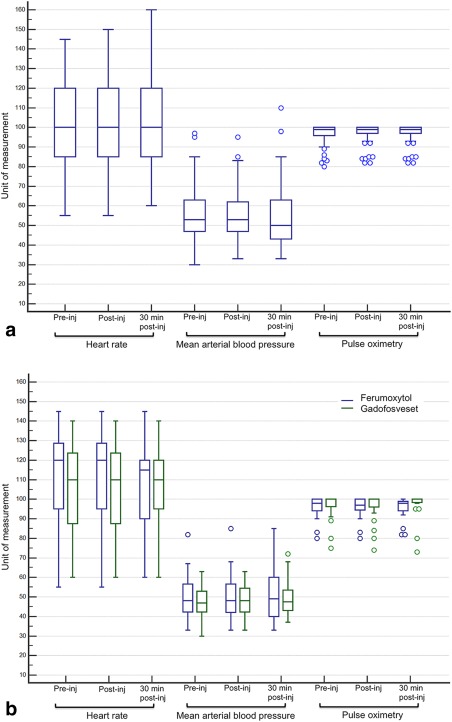
Distribution of physiologic indices in patients who had FE‐MRI exams and GE‐MRI exams. Data reflect values immediately preinjection (pre‐inj), immediately postinjection (post‐inj), and 30‐minute postinjection of ferumoxytol or gadofosveset. Whiskers represent data within the lower and upper 1.5 IQR. The bottom and top of the box represent the first and third quartile, while the band within the box represents the median. A: The HR (bpm), mean arterial blood pressure (MAP, mmHg), and pulse oximetry (%) distribution of all patients (n = 94, ages 3 days to 86 years, 36% female) undergoing FE‐MRI. Variations in HR (P = 0.12, 95% CI 93–106 bpm), MAP (P = 0.92, 95% CI 49–57 mmHg), and pulse oximetry (P = 0.68, 95% CI 95–98%) were not statistically significant. B: The HR, MAP, and pulse oximetry distribution between a comparable group of patients undergoing FE‐MRI (n = 23, ages 3 days to 13 years, 43% female) and GE‐MRI (n = 23, ages 2 days to 12.6 years, 43% female) under general anesthesia. Between‐group variations were not statistically significant (HR [P = 0.69, 95% CI 96–113 bpm], MAP [P = 0.74, 95% CI 44–52 mmHg], pulse oximetry [P = 0.76, 95% CI 94–98%]). All patients were examined under general anesthesia. bpm, beats per minute; HR, heart rate; MAP, mean arterial blood pressure.
Discussion
The results of our study showed no severe, life‐threatening, or fatal AEs caused by 237 ferumoxytol injections in 217 unique patients across a wide range of ages, renal function, and clinical indications. Based on defined criteria set forth in CTCAE v4.0, there were two mild AEs, which were related or possibly related to ferumoxytol injection. No AEs occurred in any patients examined under anesthesia in either the FE‐MRI group or the GE‐MRI group. Between‐group (FE‐MRI vs. GE‐MRI) and within‐group variations in heart rate, blood pressure, pulse oximetry, and end‐tidal CO2 were not statistically different. Blood pressure and heart rate changes between those receiving bolus ferumoxytol injections vs. slow infusions were also not statistically significant.
Compared to other IV iron supplements, ferumoxytol was developed to have lower free‐iron release,25 decreased immunologic allergic reaction, and improved safety profile.25, 26 The safety of ferumoxytol as a therapeutic agent was assessed in three randomized, open‐label, controlled, premarketing clinical trials (n = 1726).27 Overall, the general consensus was that true hypersensitivity reactions associated with newer IV iron products are rare and that the therapeutic benefits outweigh the risks.28
However, based on 79 AEs during the postmarketing surveillance period, the FDA issued a Black Box warning regarding the rare, but potential for fatal hypersensitivity reactions. To date, “anaphylactic” reactions29 have been at the forefront of these safety concerns. Although an immune‐mediated anaphylaxis mechanism has been demonstrated for older, high‐molecular weight iron dextran formulations,30 an IgE‐mediated pathway for new IV iron products has not been elucidated.31 While all IV iron products are associated with hypersensitivity reactions, the risk of serious AEs (anaphylaxis) is rare.28, 32 The FDA's boxed warning in March 2015 reiterated a serious hypersensitivity risk of 0.2% (3/1726) in those with kidney disease and a risk of 2.6% (16/1014) in those without kidney disease.29 A pooled analysis of postmarketing safety trials33, 34, 35, 36, 37 related to therapeutic use of ferumoxytol support an aggregate hypersensitivity risk of 0.03% (3/9820), with a range of 0.02–1.3% (2/8666 to 1/80, respectively)35, 36 of serious AEs. In two recent observational, head‐to‐head studies comparing outcomes and AEs relating to the therapeutic use of IV iron formulations including ferumoxytol,32, 38 no increased cardiovascular mortality was identified in dialysis‐dependent patients receiving ferumoxytol (n = 3752, mean age 65 [IQR 54–75 years], HR 0.99; 95% CI 0.83–1.19).38 The anaphylaxis rate for ferumoxytol was 0.03% (28/82117).32 Although low, these rates are higher than the reported rate of anaphylaxis for all GBCAs (0.002%39 to 0.008%40. Regarding gadofosveset use in children and young adults, however, the risk of severe AEs has been reported as 0.28% (2 of 711 injections) postmarketing,41 which is comparable to the serious hypersensitivity risk of ferumoxytol in patients with impaired renal function as cited by the FDA.
The second concern raised for ferumoxytol is hypotension, which was reported in the March 2015 FDA communication as having a risk of 1.7%.29 The mechanism may be related to release of labile free iron,42 potentially leading to hypotension and cardiorespiratory compromise.32 Free iron is known to be reactive and effects have been observed with other IV iron formulations.43, 44 As a result, the FDA has recommended dilution and slow administration. In our study, fluctuations in blood pressure and heart rate were not statistically significant in between‐group (FE‐MRI vs. GE‐MRI) or within‐group comparisons. When we compared the variations in blood pressure and heart rate between those receiving bolus vs. slow infusions, we also did not find a statistically significant difference. Whereas it is possible that our more moderate diagnostic bolus infusion rates impose a lower labile iron challenge than the therapy infusion rate, our sample size is insufficient to draw any conclusion about mechanism. In our study, at all levels of blood pressure and heart rate fluctuations, blood oxygen saturation and the extremity perfusion waveforms remained completely stable, suggesting that peripheral perfusion, and therefore whole body perfusion, was unaffected.
Other hypersensitivity reactions such as pruritus, rash, urticaria, back pain, chest pain, nausea, and vomiting have also been postulated to be related to free iron release.42 These manifestations are felt to be nonimmunologic reactions either due to free iron or mediated by complement activation and mast cell release,45 but can be misinterpreted as anaphylaxis and result in the inappropriate escalation of care. In our study, the two cases of nausea were self‐limiting and required no therapy. Those who use ferumoxytol as a therapeutic agent have advocated for a period of “watchful waiting” when mild reactions do occur.46 While premedication with diphenhydramine and H1 blockers have previously been used in the context of hypersensitivity reactions, it has recently been suggested that their effect may in fact be deleterious. Further, the sedative component can confound the patient's mental status and potentiate hemodynamic fluctuations such as tachycardia and hypotension.45
To date, two published studies47, 48 have specifically evaluated the safety of FE‐MRI in children and young adults. One study47 reported a transient 6–10 mmHg fluctuation in blood pressure for a cohort of 86 subjects (age 1 day to 34 years). No AEs were reported. The second study48 was performed under an FDA‐approved investigational new drug (IND) protocol. Vital signs and laboratory data in 68 patients, ages 5 to 25 years, were assessed and no significant changes in vital signs or relevant laboratory values occurred. There were four mild AEs (two cases of transient hypotension, one case of nausea, one case of erythema and warmth at the injection site), which were all self‐limited. In all studies that have specifically assessed the safety of ferumoxytol as an MRI contrast agent to date, no severe, life‐threatening, or fatal AEs have been reported. Further, while transient variations in blood pressure have been observed in both FE‐MRI and GE‐MRI groups in our study, definitive conclusions are challenging, particularly when there is wide variability in blood pressure for both adults49, 50 and children.51, 52 Regardless, standard procedures should be in place to manage complications should they arise.
Compared to prior safety reports of ferumoxytol as an MRI contrast agent, our work was performed in a larger patient cohort of all ages, renal function, and wider range of clinical indications. We also provide a comparison using a current FDA‐approved intravascular MRI contrast agent (gadofosveset trisodium). While not conducted under an FDA‐approved IND protocol, our findings are consistent with and expand on already published experiences47, 48 with ferumoxytol. More important, the safety experience reported in this study reflects postmarketing safety findings in a clinical population. Since completing this analysis, we have obtained an FDA‐approved IND protocol to use ferumoxytol as an MRI contrast agent in children with congenital heart disease (IND# 129441; ClinicalTrials.gov identifier NCT02752191).
While the American College of Radiology, the Canadian Association of Radiologists, and the European Medicine Agency caution the use of GBCAs in the setting of acute renal failure and/or an eGFR <30 ml/min/1.73m2, it is instructive to note, however, that 79% (55/70 survey respondents) of pediatric imaging specialists (radiologists and cardiologists) in an international survey currently use GBCAs in neonates, a population where the physiologic eGFR is impaired (26 ml/min/1.73m2) due to immature renal function.53 While the standard of care may vary from one institution to another and occasionally deviate from guidelines, the risk‐to‐benefit ratio is always considered in daily clinical practice. In this context, where other agents or tests may be suboptimal in the setting of renal dysfunction or immature renal function, the risk‐to‐benefit ratio of ferumoxytol as an MRI contrast agent should also be weighed.
Our study has several limitations. First, selection bias relating to the retrospective nature of the study design, the clinical patient population, and follow‐up duration limit the overall generalizability of the study. Because the study was not designed as a randomized clinical trial, patients referred for ferumoxytol MRI frequently had complex medical conditions, were acutely sick, and had limited alternative cardiovascular imaging options. While there is frequent and regular contact between imaging investigators and referring physicians, specific follow‐up timepoints were not prospectively determined, which may have been an additional source of bias. Second, because of the low frequency of serious hypersensitivity reactions, our study is not sufficiently powered to reach definitive conclusions regarding the rate of adverse reactions associated with diagnostic use of ferumoxytol or to detect differences in the AE rates between ferumoxytol and conventional GBCA. Our report should be viewed as a single‐center interim safety experience of ferumoxytol as an off‐label MRI contrast agent in a spectrum of patients with varying age and clinical indications.
In conclusion, based on our experience, the diagnostic off‐label use of ferumoxytol for MRI is well‐tolerated with few AEs. Given ferumoxytol's dual potential as a therapeutic drug and an MRI contrast agent, prospective and systematic investigations will be helpful to better define the mechanisms involved in the mediation of AEs and the diagnostic effectiveness of FE‐MRI. A multicenter safety registry and related clinical research trials are needed to help maximize the benefit and mitigate risks associated with the use of ferumoxytol as an MRI contrast agent.
Conflict of Interest
The authors declare no conflicts of interest.
Acknowledgments
Contract grant sponsor: National Heart, Lung, and Blood Institute; contract grant number: R01HL127153; Contract grant sponsor: REDCap hosting by the NIH/National Center for Advancing Translational Science (NCATS) CTSI; contract grant number: UL1TR000124.
References
- 1. Coresh J, Selvin E, Stevens LA, et al. Prevalence of chronic kidney disease in the United States. JAMA 2007;298:2038–2047. [DOI] [PubMed] [Google Scholar]
- 2. US Census Bureau . Census 2000 Gateway. Accessed March 1, 2016. http://www.census.gov/main/www.cen2000.html
- 3. Li W, Tutton S, Vu AT, et al. First‐pass contrast‐enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)‐based blood pool agent. J Magn Reson Imaging 2005;21:46–52. [DOI] [PubMed] [Google Scholar]
- 4. Han F, Rapacchi S, Khan S, et al. Four‐dimensional, multiphase, steady‐state imaging with contrast enhancement (MUSIC) in the heart: A feasibility study in children. Magn Reson Med 2015;74:1042–1049. [DOI] [PubMed] [Google Scholar]
- 5. Kanal E, Tweedle MF. Residual or retained gadolinium: practical implications for radiologists and our patients. Radiology 2015;275:630–634. [DOI] [PubMed] [Google Scholar]
- 6. AMAG Pharmaceuticals . Feraheme Drug Label. Volume April 2015; 2014. (revised March 2015).
- 7. Anzai Y, Prince MR, Chenevert TL, et al. MR angiography with an ultrasmall superparamagnetic iron oxide blood pool agent. J Magn Reson Imaging 1997;7:209–214. [DOI] [PubMed] [Google Scholar]
- 8. Prince MR, Zhang HL, Chabra SG, Jacobs P, Wang Y. A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J X‐ray Sci Technol 2003;11:231–240. [PubMed] [Google Scholar]
- 9. Nayak AB, Luhar A, Hanudel M, et al. High‐resolution, whole‐body vascular imaging with ferumoxytol as an alternative to gadolinium agents in a pediatric chronic kidney disease cohort. Pediatr Nephrol (Berlin, Germany) 2015;30:515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hamilton BE, Nesbit GM, Dosa E, et al. Comparative analysis of ferumoxytol and gadoteridol enhancement using T1‐ and T2‐weighted MRI in neuroimaging. AJR Am J Roentgenol 2011;197:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nakamoto BK, Shikuma CM, Ogata‐Arakaki D, et al. Feasibility and potential role of ferumoxytol‐enhanced neuroimaging in HIV‐associated neurocognitive disorder. J Neurovirol 2013;19:601–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Varallyay CG, Nesbit E, Fu R, et al. High‐resolution steady‐state cerebral blood volume maps in patients with central nervous system neoplasms using ferumoxytol, a superparamagnetic iron oxide nanoparticle. J Cereb Blood Flow Metab 2013;33:780–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Neuwelt A, Sidhu N, Hu CA, Mlady G, Eberhardt SC, Sillerud LO. Iron‐based superparamagnetic nanoparticle contrast agents for MRI of infection and inflammation. AJR Am J Roentgenol 2015;204:W302–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hasan DM, Amans M, Tihan T, et al. Ferumoxytol‐enhanced MRI to Image Inflammation within Human Brain Arteriovenous Malformations: A Pilot Investigation. Transl Stroke Res 2012;3(Suppl 1):166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Turkbey B, Agarwal HK, Shih J, et al. A phase I dosing study of ferumoxytol for MR lymphography at 3 T in patients with prostate cancer. AJR Am J Roentgenol 2015;205:64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gahramanov S, Raslan AM, Muldoon LL, et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility‐weighted contrast‐enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys 2011;79:514–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nasseri M, Gahramanov S, Netto JP, et al. Evaluation of pseudoprogression in patients with glioblastoma multiforme using dynamic magnetic resonance imaging with ferumoxytol calls RANO criteria into question. Neuro Oncol 2014;16:1146–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dosa E, Guillaume DJ, Haluska M, et al. Magnetic resonance imaging of intracranial tumors: intra‐patient comparison of gadoteridol and ferumoxytol. Neuro Oncol 2011;13:251–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gahramanov S, Muldoon LL, Varallyay CG, et al. Pseudoprogression of glioblastoma after chemo‐ and radiation therapy: diagnosis by using dynamic susceptibility‐weighted contrast‐enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology 2013;266:842–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology 2014;270:269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. National Cancer Institute . Common Terminology Criteria for Adverse Events v4.0. May 2009. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 23. Nguyen KL, Khan SN, Moriarty JM, et al. High‐field MR imaging in pediatric congenital heart disease: Initial results. Pediatr Radiol 2015;45:42–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vasanawala SS, Nguyen KL, Hope MD, et al. Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 2016;75:2107–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Balakrishnan VS, Rao M, Kausz AT, et al. Physicochemical properties of ferumoxytol, a new intravenous iron preparation. Eur J Clin Invest 2009;39:489–496. [DOI] [PubMed] [Google Scholar]
- 26. Provenzano R, Schiller B, Rao M, Coyne D, Brenner L, Pereira BJ. Ferumoxytol as an intravenous iron replacement therapy in hemodialysis patients. Clin J Am Soc Nephrol 2009;4:386–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lu M, Cohen MH, Rieves D, Pazdur R. FDA report: Ferumoxytol for intravenous iron therapy in adult patients with chronic kidney disease. Am J Hematol 2010;85:315–319. [DOI] [PubMed] [Google Scholar]
- 28. Chertow GM, Winkelmayer WC. On the relative safety of intravenous iron formulations: new answers, new questions. Am J Hematol 2010;85:643–644. [DOI] [PubMed] [Google Scholar]
- 29. US FDA . FDA Drug Safety Communication: FDA strengthens warnings and changes prescribing instructions to decrease the risk of serious allergic reactions with anemia drug Feraheme (ferumoxytol). Accessed March 30, 2015. http://www.fda.gov/Drugs/DrugSafety/ucm440138.htm
- 30. Kraft D, Hedin H, Richter W, Scheiner O, Rumpold H, Devey ME. Immunoglobulin class and subclass distribution of dextran‐reactive antibodies in human reactors and non reactors to clinical dextran. Allergy 1982;37:481–489. [DOI] [PubMed] [Google Scholar]
- 31. Novey HS, Pahl M, Haydik I, Vaziri ND. Immunologic studies of anaphylaxis to iron dextran in patients on renal dialysis. Ann Allergy 1994;72:224–228. [PubMed] [Google Scholar]
- 32. Wang C, Graham DJ, Kane RC, et al. Comparative risk of anaphylactic reactions associated with intravenous iron products. JAMA 2015;314:2062–2068. [DOI] [PubMed] [Google Scholar]
- 33. Hetzel D, Strauss W, Bernard K, Li Z, Urboniene A, Allen LF. A Phase III, randomized, open‐label trial of ferumoxytol compared with iron sucrose for the treatment of iron deficiency anemia in patients with a history of unsatisfactory oral iron therapy. Am J Hematol 2014;89:646–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vadhan‐Raj S, Strauss W, Ford D, et al. Efficacy and safety of IV ferumoxytol for adults with iron deficiency anemia previously unresponsive to or unable to tolerate oral iron. Am J Hematol 2014;89:7–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Macdougall IC, Strauss WE, McLaughlin J, Li Z, Dellanna F, Hertel J. A randomized comparison of ferumoxytol and iron sucrose for treating iron deficiency anemia in patients with CKD. Clin J Am Soc Nephrol 2014;9:705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schiller B, Bhat P, Sharma A. Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the United States over a 12‐month period. Clin Ther 2014;36:70–83. [DOI] [PubMed] [Google Scholar]
- 37. Auerbach M, Strauss W, Auerbach S, Rineer S, Bahrain H. Safety and efficacy of total dose infusion of 1,020 mg of ferumoxytol administered over 15 min. Am J Hematol 2013;88:944–947. [DOI] [PubMed] [Google Scholar]
- 38. Airy M, Mandayam S, Mitani AA, et al. Comparative outcomes of predominant facility‐level use of ferumoxytol versus other intravenous iron formulations in incident hemodialysis patients. Nephrol Dial Transplant 2015;30:2068–2075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Prince MR, Zhang H, Zou Z, Staron RB, Brill PW. Incidence of immediate gadolinium contrast media reactions. AJR Am J Roentgenol 2011;196:W138–143. [DOI] [PubMed] [Google Scholar]
- 40. Jung JW, Kang HR, Kim MH, et al. Immediate hypersensitivity reaction to gadolinium‐based MR contrast media. Radiology 2012;264:414–422. [DOI] [PubMed] [Google Scholar]
- 41. Rigsby CK, Popescu AR, Nelson P, et al. Safety of blood pool contrast agent administration in children and young adults. AJR Am J Roentgenol 2015;205:1114–1120. [DOI] [PubMed] [Google Scholar]
- 42. Van Wyck DB. Labile iron: manifestations and clinical implications. J Am Soc Nephrol 2004;15(Suppl 2):S107–111. [DOI] [PubMed] [Google Scholar]
- 43. Koskenkorva‐Frank TS, Weiss G, Koppenol WH, Burckhardt S. The complex interplay of iron metabolism, reactive oxygen species, and reactive nitrogen species: insights into the potential of various iron therapies to induce oxidative and nitrosative stress. Free Radic Biol Med 2013;65:1174–1194. [DOI] [PubMed] [Google Scholar]
- 44. Neiser S, Rentsch D, Dippon U, et al. Physico‐chemical properties of the new generation IV iron preparations ferumoxytol, iron isomaltoside 1000 and ferric carboxymaltose. Biometals 2015;28:615–635. [DOI] [PubMed] [Google Scholar]
- 45. Bircher AJ, Auerbach M. Hypersensitivity from intravenous iron products. Immunol Allergy Clin North Am 2014;34:707–723, x–xi. [DOI] [PubMed] [Google Scholar]
- 46. Rampton D, Folkersen J, Fishbane S, et al. Hypersensitivity reactions to intravenous iron: guidance for risk minimization and management. Haematologica 2014;99:1671–1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Ning P, Zucker EJ, Wong P, Vasanawala SS. Hemodynamic safety and efficacy of ferumoxytol as an intravenous contrast agents in pediatric patients and young adults. Magn Reson Imaging 2016;34:152–158. [DOI] [PubMed] [Google Scholar]
- 48. Muehe AM, Feng D, von Eyben R, et al. Safety report of ferumoxytol for magnetic resonance imaging in children and young adults. Invest Radiol 2016;51:221–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. O'Brien E, Murphy J, Tyndall A, et al. Twenty‐four‐hour ambulatory blood pressure in men and women aged 17 to 80 years: the Allied Irish Bank Study. J Hypertens 1991;9:355–360. [DOI] [PubMed] [Google Scholar]
- 50. Loimaala A, Turjanmaa V, Vuori I, Oja P, Pasanen M, Uusitalo A. Variation of ambulatory blood pressure in healthy middle‐aged men. J Hum Hypertens 1997;11:227–231. [DOI] [PubMed] [Google Scholar]
- 51. Varda NM, Gregoric A. Twenty‐four‐hour ambulatory blood pressure monitoring in infants and toddlers. Pediatr Nephrol (Berlin, Germany) 2005;20:798–802. [DOI] [PubMed] [Google Scholar]
- 52. Wuhl E, Witte K, Soergel M, Mehls O, Schaefer F. Distribution of 24‐h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 2002;20:1995–2007. [DOI] [PubMed] [Google Scholar]
- 53. Meng H, Grosse‐Wortmann L. Gadolinium in pediatric cardiovascular magnetic resonance: what we know and how we practice. J Cardiovasc Magn Reson 2012;14:56. [DOI] [PMC free article] [PubMed] [Google Scholar]


