Abstract
Background
Systemic Lupus Erythematosus (SLE) is a life-threatening multisystem autoimmune disease that is more severe in patients of African ancestry and children, yet pediatric SLE (pSLE) on the African continent has been understudied. This study describes a cohort of pediatric SLE (PULSE) patients in South Africa (SA).
Methods
Patients with a diagnosis of SLE (1997 American College of Rheumatology criteria) diagnosed prior to age 19 years in Cape Town, South Africa, were enrolled in this cross-sectional study from September 2013 to December 2014. Information on clinical and serologic characteristics was extracted from medical records. Results were compared to a well-described North American pSLE cohort.
Results
Seventy-two SA patients were enrolled in the study; mean age 11.5 years, 82% female. The racial distribution was 68% Coloured, 24% Black, 5% White, and 3% Asian/Indian. Most patients presented with severe lupus nephritis (LN) documented by renal biopsy (61%). Of patients with LN, 63% presented with International Society of Nephrology/Renal Pathology Society (ISN/RPS) class III or IV. Patients in the PULSE cohort were more likely to be treated with cyclophosphamide, methotrexate, and azathioprine. The PULSE cohort had high disease activity at diagnosis (mean Systemic Lupus Erythematosus Disease Activity Index-2K [SLEDAI-2K] 20.6). The SLEDAI-2K at enrollment in the PULSE cohort (5.0) did not differ from the North American pSLE cohort (4.8). Sixty three % of PULSE cohort had end organ damage with System Lupus International Collaborating Clinic-Damage Index (SLICC-DI) score >0 (mean SLICC-DI 1.9), compared to 23% in a previously reported US cohort. Within the PULSE cohort, 9 (13%) developed ESRD with 6 (8%) requiring transplant, strikingly higher than North American peers (transplant rate <1%).
Conclusions
The PULSE cohort had highly active multiorgan disease at diagnosis and significant disease damage at enrollment in the SA registry. SA patients have severe lupus nephritis and poor renal outcomes compared to North American peers. Our study reveals a severe disease phenotype in the PULSE cohort resulting in poor outcomes in this high-risk population.
Keywords: Systemic lupus erythematosus, pediatric lupus, Africa, lupus nephritis
Introduction
Systemic lupus erythematosus (SLE) is a life-threatening multisystem autoimmune disease. In North America (NA) and Europe, SLE is more common and more severe with increased mortality risk in people of African extraction compared to Caucasians.1–4 Despite SLE’s disproportionate prevalence among individuals of African descent, literature describing the epidemiology, diagnosis and treatment of SLE among individuals living on the African continent is sparse.5–12 Adult cohort studies from South Africa (SA) show poor outcomes, with five year survival rates ranging from 69–78%, compared to 88–95% in high-income countries. The only published pediatric SLE studies in sub-Saharan Africa have been reported from South Africa.13–16 The paucity of data has led to perceptions that SLE is rare in Africa,17, 18 although more recent studies suggest under-recognition, under-diagnosis, under-reporting, and poor access to care rather than rarity of disease.19–21
Children are at higher risk for severe SLE, typically presenting with higher disease activity and continuing with higher activity over time than adults. Presentation in childhood occurs in 15–20% of all SLE patients, with median diagnosis age for pediatric SLE (pSLE) at 12–14 years.22–24 Children are more likely to have serious renal, neurologic, and hematologic involvement than their adult counterparts.25 Mortality rates are higher in pediatric SLE patients than adults, despite having fewer comorbidities.26
Lupus nephritis (LN) is a serious complication of SLE, influencing prognosis and life expectancy more than any other organ system involvement.27, 28 SLE patients with LN show a 15–24 year decrease in life expectancy compared to SLE patients without nephritis.29 In high income countries, patients of African ancestry are two to three times more likely to develop LN with a more severe renal disease course,30 and those with end-stage renal disease (ESRD) have higher mortality rates than Caucasians with ESRD.31, 32 Furthermore, lupus nephritis is common in children.33 PSLE patients have higher rates of renal damage and proceed to ESRD more frequently than their adult counterparts.25 Treatment resistance disproportionately affects African American children.34 In addition, LN is a major cause of morbidity and mortality for SLE patients in low income nations.35
Thus, pediatric SLE patients in Africa are potentially at high risk for poor outcomes based on race and age, yet strikingly little research has addressed this population.9, 20, 21, 36 To date, only one cohort study of pSLE in sub-Saharan Africa has been published and there has not been an updated report on pSLE in South Africa over the past 10 years.16
The aim of the current study is to determine if pSLE patients in SA have statistically significant and clinically important differences in disease severity, rates or severity of renal involvement, and disease damage compared to pSLE patients in a well-characterized NA cohort. We initiated a pSLE registry in SA and have performed an initial cross sectional analysis at enrollment of pediatric SLE patients presenting to care since 1988 at several hospitals in Cape Town, SA (referred to hereafter as PULSE cohort). Here, we describe this cohort, compare it to patients in the Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry, a database that includes over 900 pSLE patients from 60 sites in North America,37 and attempt to identify modifiable risk factors that could improve treatment and reduce organ damage in this population.
Methods
This retrospective study enrolled patients diagnosed with SLE prior to age 19 at two tertiary care, government-funded university hospitals (Red Cross War Memorial Children’s Hospital; Groote Schuur Hospital) and one private medical center (Winelands Rheumatology Centre) in Cape Town, SA. The study was conducted as a collaboration between Duke University, USA and the University of Cape Town, South Africa, and approved by the Duke Institutional Review Board and the University of Cape Town Ethics Committee.
Participants
To develop the PULSE cohort, we identified eligible patients by searching the computerized databases of the two hospital centers where the study was conducted using ICD-10 codes for SLE or LN from hospital admissions and clinic visits. In addition, we polled physicians on-site to generate a comprehensive list of pSLE patients. To include patients seen in private practice, we presented at national SA rheumatology meetings and sent recruitment flyers to all private rheumatology offices in the Western Cape. Inclusion criteria encompassed patients age <19 years at SLE diagnosis who met the ACR classification criteria for SLE,38 and who had received care in the Western Cape Province, SA.
Once identified, living patients were approached for consent by their physician or study personnel. For patients less than 18 years old, we obtained parental consent; for patients aged 13 to 18 years, child assent was also obtained. Consent was conducted in the patient’s language of choice (Afrikaans, isiXhosa, or English). Deceased pSLE patients meeting enrollment criteria were included in review, for which a waiver of consent was obtained.
The CARRA Registry
For comparison, we used an existing registry of the Childhood Arthritis and Rheumatology Research Alliance (CARRA). The CARRA Registry is a multicenter collaborative database that collects standardized information from 60 expert centers in NA.39 Thus, we refer to the NA cohort as the Total CARRA cohort. As of July 2012, there were over 900 children with pSLE enrolled. Patients with pSLE were eligible for inclusion in the CARRA Registry if they met at least four of eleven ACR criteria for SLE classification, developed pSLE prior to age 18 years, and were less than 21 years at the time of enrollment.40
The following demographic features were obtained via chart review: age at disease diagnosis, gender, disease duration, and self-reported race.41 In SA, race was reported as one of the five racial groups employed in SA population surveys: Coloured, Black, White, Indian/Asian, and other (see Appendix 1). As most of the PULSE cohort is of African descent, we compared those patients to the African American subjects of the CARRA cohort (AA CARRA) to match more evenly on race/ethnicity between cohorts.
Clinical features
We measured presence of organ system involvement at time of enrollment. To measure disease activity, we employed a well validated SLE disease activity index measure (SLEDAI-2K).42 We describe catastrophic clinical presentation in the SA patients. This is a term we created to capture the severe illness with which the majority of the PULSE cohort presented, including stroke, seizure, blindness, pericarditis causing tamponade, or acute renal failure. There are no data available on the frequency of such presentations in CARRA patients for comparison.
Laboratory tests and renal biopsy
We captured rates of positive tests for ANA, anti-Sm and anti-dsDNA antibodies. The anti-Ro, anti-La, and anti-RNP antibodies have only recently become part of the routine assessment in SA, and were not included in this analysis.
In SA, only International Society of Nephrology and Renal Pathology Society (ISN/RPS)43 histologic classification is utilized; we therefore excluded CARRA patients with only World Health Organization (WHO) biopsy classification from this comparison (n=148).
Therapy
Inpatient and outpatient records were reviewed for current and prior therapy. All medications are reported as binary variables. The medication was scored as positive if the patient was receiving or had received the medication ever at study enrollment.
Outcomes
Through chart review, we extracted data on ESRD, dialysis, transplant, and mortality. To measure chronic disease damage, we calculated Systemic Lupus International Collaborating Clinics/ACR Damage Index for Systemic Lupus Erythematosus (SLICC-DI) scores.44
Statistical analysis
We compared age of SLE diagnosis, race, presenting features, rate and class of LN, laboratory features, SLEDAI-2K and SLICC-DI scores, treatment, and outcomes between the SA and NA patient cohorts. Characterization of the patient population was summarized using descriptive statistics with 95% confidence intervals of means and proportions for cohort comparison.
We evaluated enrollment SLEDAI-2K scores of the PULSE cohort compared to the CARRA cohort. Where appropriate, Kruskall-Wallis, Pearson, Χ2 or Fisher's exact test were used to evaluate differences in both clinical and demographic features and disease scores of the two cohorts. For better comparison of subjects of African ancestry between cohorts, we compared the PULSE cohort to the African American subjects in the CARRA cohort (AA CARRA).
As an exploratory aim, we attempted to identify risk factors for poor outcomes within the PULSE cohort. Unfortunately, the PULSE cohort was not large enough to provide sufficient power for this sub-analysis.
All calculations were performed using STATA statistical software (Stata Corp., College Station, TX, USA).
Results
Enrollment characteristics are summarized in Table 1. As expected for patients from the African continent, 92% of the patients in the PULSE cohort are of African descent, versus 35% of the CARRA cohort. A larger proportion of the CARRA cohort (64% vs. 46%) had a diagnosis of SLE for at least 2 years at time of enrollment. Seventy-three percent of the PULSE cohort had age of onset less than or equal to 13 years of age at diagnosis, compared to only 48% of the CARRA cohort.
Table 1.
Demographic Features at Enrollment.
| PULSE N=72 |
Total CARRA N=982 |
P value | |
|---|---|---|---|
| Mean age of SLE diagnosis, yrs. (SD) | 11.5 (3.5) | 12.4 (3.2) | 0.027 |
| Mean disease duration, yrs. (SD) | 2.4 (3.2) | 3.5 (3.0) | 0.001 |
| % Female (n) | 82 (59) | 83 (810) | 0.873 |
| % White (n) | 6 (4) | 47 (459) | <0.001 |
| % African descent (n) | 92 (66) | 35 (339) | <0.001 |
| % other (n) | 3 (2) | 19 (184) | <0.001 |
| % age ≤13 at diagnosis (n) | 73 (53) | 48 (475) | <0.001 |
| % disease duration≥2 yrs. (n) | 46 (33) | 64 (632) | 0.003 |
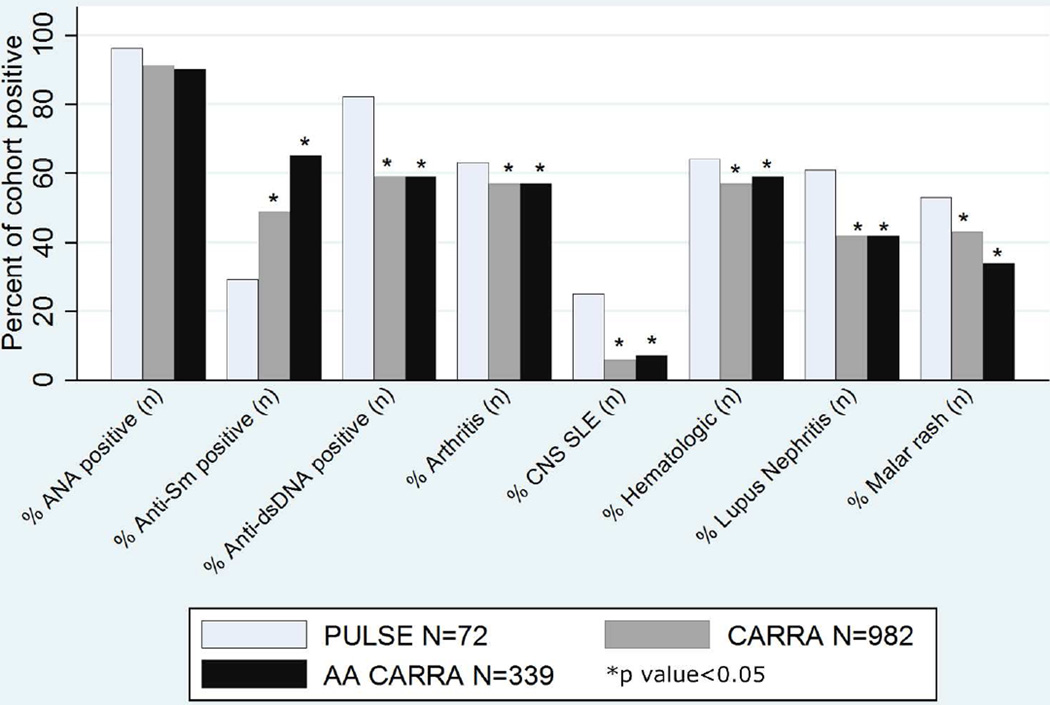
There are important differences in clinical and serological features of the cohorts (Figure 1, Table 2). Notably, a much higher percentage of the PULSE cohort had LN at enrollment compared to the AA CARRA group (61% vs. 42%, p value<0.001). The PULSE cohort demonstrated high disease activity at time of diagnosis, represented by average SLEDAI-2K of 20.5; however, the score was recorded for only 44 of 72 PULSE subjects, and must be interpreted with caution due to the large amount of missing data. The CARRA registry does not capture SLEDAI-2K at time of diagnosis. On chart review of the PULSE subjects, we noted that more than half of the patients (57%) presented with emergencies, such as acute renal failure, pericarditis causing tamponade, or stroke. There were 51 catastrophic events recorded among 72 patients. Seven patients (10%) had a severe disease manifestation in more than one category (i.e. presenting with stroke and acute renal failure). More than one third of patients in the PULSE cohort (37%) had acute kidney injury with decreased renal function at time of diagnosis, 5% stroke, 4% deep vein thrombosis, 5% pericarditis requiring intervention, and 3% seizure. At time of enrollment, average SLEDAI-2K of the PULSE cohort was not different from that of the total CARRA subjects or the AA CARRA subjects.
Figure 1.
Clinical Features at Enrollment.
Table 2.
Catastrophic Presentation and SLEDAI at Enrollment.
| PULSE N=72 |
Total CARRA N=982 |
P value | AA CARRA N=339 |
P value | |
|---|---|---|---|---|---|
| % Catastrophic presentation (n) | 57 (40)a | NRb | — | NR | — |
| Average SLEDAI-2K at diagnosisc (SD) | 20.6 (9.9) | NR | — | NR | — |
| Average SLEDAI-2K at enrollmentc(SD) | 5.0(5.2) | 4.8 (5.7) | 0.437 | 5.4 (6.5) | 0.960 |
= Catastrophic presentations include acute kidney injury, seizure, deep vein thrombosis, pericarditis requiring intervention, stroke, cytopenia requiring therapy, and blindness
NR = Not reported
= [range 0–105]
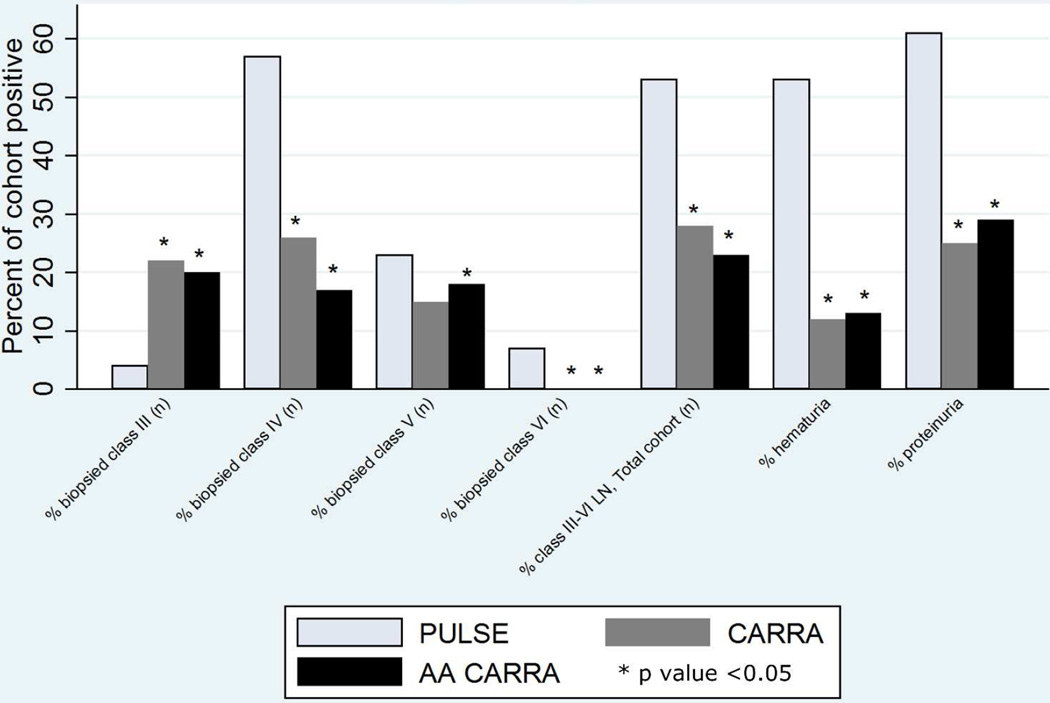
PULSE patients displayed an increased rate of severe renal disease compared to NA peers (Figures 1 and 2). The rate of biopsy proven lupus nephritis (61%) in the PULSE cohort is higher than the CARRA cohort (42%). In comparison with CARRA, nearly all biopsied patients in the PULSE cohort (93%) had ISN/RPS Class III, IV, V, or VI LN. Notably, 63% of those biopsied in the PULSE cohort had class III or IV LN, while only 48% of the CARRA cohort had these findings on biopsy. Moreover, 7% of the PULSE cohort had Class VI LN on renal biopsy at enrollment, while none of the total or AA CARRA cohort demonstrated advanced sclerosis.
Figure 2.
Lupus Nephritis at Enrollment.
We compared the medical management of patients in each cohort (Table 3). Notably, a much larger number of patients in the CARRA cohort had been treated with an antimalarial and mycophenolate mofetil (MMF) at the time of enrollment. The PULSE cohort had a higher percentage of patients treated with azathioprine and methotrexate. The PULSE cohort also had a higher percentage of patients treated with intravenous immunoglobulin (IVIg).
Table 3.
Treatment History at Enrollment.
| PULSE N=72 |
Total CARRA N=982 |
P value | AA CARRA N=339 |
P value | |
|---|---|---|---|---|---|
| % treated with steroids (n) | 92 (66) | 94 (917) | 0.725 | 94 (320) | 0.538 |
| % treated with antimalarial (n) | 75 (54) | 87 (853) | 0.001 | 88 (297) | <0.001 |
| % treated with cyclophosphamide (n) | 42 (30) | 27 (267) | 0.022 | 30 (101) | 0.061 |
| % treated with mycophenolate mofetil (n) | 38 (28) | 57 (556) | 0.004 | 64 (205) | <0.001 |
| % treated with azathioprine (n) | 56 (41) | 15 (146) | 0.001 | 14 (47) | <0.001 |
| % treated with methotrexate (n) | 26 (19) | 19 (184) | 0.019 | 17 (59) | 0.082 |
| % treated with rituximab (n) | 6 (4) | 10 (99) | — | 14 (49) | — |
| % treated with IVIg (n) | 11 (8) | 2 (17) | — | 6 (21) | — |
We identified a high rate of irreversible damage in the PULSE cohort (Table 4), with an average SLICC-DI score of 1.9. The PULSE cohort demonstrates poor renal outcomes with 15% requiring dialysis as renal replacement therapy (RRT), versus 1 % in the CARRA cohort, and 1.5% in the AA CARRA cohort. SA pSLE patients with LN also had much higher rates of renal transplant than both the total and AA CARRA subjects.
Table 4.
Measures of disease damage and renal outcomes.
| PULSE N=72 |
Total CARRA N=982 |
P value | AA CARRA N=339 |
P value | |
|---|---|---|---|---|---|
| % ESRD (n) | 13 (9) | NRa | — | NR | — |
| % requiring dialysis (n) | 15 (11) | 1 (11) | <0.001 | 1.5 (5) | <0.001 |
| % requiring transplant (n) | 8 (6) | 0.7 (7) | <0.001 | 0.3 (1) | <0.001 |
| SLICC score, median | 1.9 | NR | — | NR | — |
NR=Not reported
Discussion
This study describes a cohort of 72 pSLE patients diagnosed before the age of 19 years in two medical centers and one office practice in Cape Town, SA (PULSE). This is the largest cohort of pSLE patients described in Africa to date. The only previous study of pSLE from sub-Saharan Africa was a retrospective cohort of 36 patients who presented to a Johannesburg hospital between 1974–2000.16 The previous report had a much higher proportion of White patients (42%) than the current PULSE cohort, likely reflecting shifts in recognition, diagnosis, and access to subspecialty care for Black and Coloured patients in SA since the end of apartheid in 1994.45 The PULSE cohort reflects the racial composition of Western Cape Province (32% Black, 49% Coloured, 16% White, 1% Indian/Asian, 2% Other) but different from South Africa as a whole (80% Black, 9% Coloured, 2.5% Indian, 8.5% White).46
The PULSE cohort is similar to the CARRA cohort in gender distribution, but differs in important measures of race, age, specific antibody profile and disease duration. Previous work suggests that young age and black race37 predispose to increased disease activity in pSLE patients, and may contribute to the severe disease phenotype seen in our PULSE cohort. We compared the PULSE cohort to the AA patients in the CARRA cohort for a comparison based on self-reported race. Although the CARRA registry uses the eligibility criterion of SLE diagnosed prior to age 18, the present study includes all SA patients diagnosed prior to age 19 years. We extended the cut-off age to include those diagnosed at age 18 to accommodate SA physician opinion that diagnosis was often delayed (Scott, C; June 2013; personal communication).
We observed increased clinical severity at presentation, higher rates of LN, and worse outcomes, as measured by SLICC-DI and need for renal dialysis and transplant, amongst pSLE patients in SA. The PULSE cohort had higher rates of every disease manifestation examined, but the largest differences were seen in rates of renal and CNS involvement, which have the most impact on patient health and prognosis,34, 47, 48 and heavily influence treatment choice in pSLE.49, 50 The high SLEDAI-2K score at diagnosis in the PULSE cohort indicates high disease activity at time of presentation. The CARRA cohort study does not capture SLEDAI-2K at presentation, and data on SLEDAI-2K at presentation amongst diverse races/ethnicities are limited. One previous study of a well-described cohort of NA patients reports a mean SLEDAI-2K of 13.8 in Caucasian patients and 12.6 in non-Caucasians at diagnosis, although black patients comprised only 15% of patients in that study.51 The high SLEDAI-2K score and increased rates of all disease manifestations suggest a more severe phenotype in the PULSE cohort. Notably, the majority of SA patients had a catastrophic presentation, presenting with severe life-threatening manifestations such as acute renal failure requiring dialysis, blindness, or hemorrhagic stroke. These findings add to the evidence that SA patients have severe active SLE at diagnosis. It is not possible to determine if barriers to diagnosis and care influence the severity of disease on presentation in this cross-sectional cohort study; however in depth interviews with patient families suggest that this is likely a contributing factor (unpublished data). The SLEDAI-2K score at diagnosis was missing for more than half of PULSE subjects. For those in SA who had an enrollment SLEDAI recorded, it did not differ from that of the CARRA cohort. Although we cannot infer causality in this retrospective study, this finding suggests that PULSE patients respond to therapy despite high activity at presentation.
The reported rate of lupus nephritis in children with SLE is high (ranging from 50–67%) and patients of African descent in high income nations are two to three times more likely to develop lupus, more likely to have severe disease, and more likely to progress to end stage renal disease (ESRD) than Caucasian counterparts.31, 32, 47 We found the rate of biopsy proven LN to be 61% in the PULSE cohort. The majority of biopsied patients in the PULSE cohort had Class III-IV lupus nephritis on biopsy at enrollment, while less than half of biopsied CARRA patients had Class III or IV lupus nephritis. Also, a percentage of biopsied patients in the PULSE cohort had advanced sclerosis, while no one in the CARRA cohort demonstrated this at time of enrollment. One third of the PULSE cohort had acute kidney injury (AKI) at diagnosis. There is no information from the CARRA cohort on AKI for comparison.
The severity of lupus nephritis in the PULSE cohort directly translates into poor outcomes. The PULSE cohort had high rates of ESRD, dialysis use, and renal transplant at enrollment, in stark contrast to the much lower rate requiring dialysis and transplant in the AA CARRA cohort. Higher rates of dialysis and transplant in the PULSE cohort are not surprising given the higher rates of lupus nephritis. However, the South African medical system is resource-limited with renal replacement and transplant therapy tightly rationed, requiring application and approval.52 In context of the hurdles for access to these treatments, the increased rate in the PULSE cohort is especially striking.
Disease management differs between the PULSE and CARRA cohorts. Although antimalarial therapy is standard care worldwide, the PULSE cohort demonstrated lower use than the AA CARRA cohort. Antimalarial therapy is associated with higher rates of remission, fewer relapses, and reduced organ damage.53 It is unclear if this difference is due to lack of provider knowledge, drug cost, or adherence. The PULSE cohort was more frequently treated with cyclophosphamide, methotrexate, and azathioprine, and less often with MMF than the NA comparators. Treatment differences are impacted by medication rationing practices in SA. Therefore, the increased use of IVIg is surprising. However, 20% of the PULSE cohort initially presented with severe idiopathic thrombocytopenic purpura which is commonly managed with IVIg in both NA and SA. It is not possible to determine if medication management differences resulted in differences in disease damage or outcomes in this cross-sectional study.
The PULSE cohort had significant organ damage, as reflected in SLICC-DI scores. The SLICC-DI was not recorded in the CARRA database, so direct comparison between the two cohorts is not possible. Instead, we compared the PULSE SLICC-DI to those published in the Atherosclerosis Prevention in Pediatric Lupus Erythematosus (APPLE) cohort study, in which 25% of the 221 patients had a SLICC-DI score greater than zero.54 This is vastly less than the 63% of the PULSE cohort with a SLICC-DI score greater than zero at enrollment. Higher SLICC-DI scores correlate with mortality in populations in high and low income nations.55 Thus, high SLICC-DI scores indicate high morbidity and predict high mortality risk in the PULSE cohort.
The mortality rate in the PULSE cohort was 9.7%. Five year survival for pSLE patients has been reported as >95%,33 however, reports from other lower income nations show a poorer prognosis.56, 57 Most deaths (6/7) in the PULSE cohort were related to renal disease, whereas in the US, SLE deaths are most commonly due to infections.58 This contrast reinforces the importance of LN and poor access to RRT and transplant on prognosis in SA.
This study is limited by the relatively small number of patients enrolled in the PULSE cohort. Although it is the largest cohort in South Africa to date, the small total number of patients does not provide statistical power to look for meaningful subgroup differences within the cohort. The study is cross-sectional and limited by the documented medical chart. In addition, the study is restricted to one city in SA, and most patients were recruited from two major academic medical centers. This may limit generalizability, although the majority of CARRA participants are recruited from academic centers as well. The study uses standard local definitions of self-reported race; however, race is a social, not a biologic construct, and patients are not readily separated into distinct categories. Our study included patients of African ancestry from two continents. The admixture of Africans, Caucasians, and patients of other ancestry is arbitrary and inexact,59 therefore comparison between populations is difficult. The specific racial contributions of South African Black and Coloured patients are different from African Americans. The South African Black population is derived from Bantu tribes and the Coloured population has indigenous Khoisan roots with an admixture of Dutch, British, and Asian ancestry.60 The majority of African Americans have ancestral links to West Africa61 and the genetic diversity of Africa is reportedly vast.62 Nevertheless, SLE research has shown race to be a risk factor for severe disease.3, 4, 32, 37, 47 The only pediatric rheumatologists in sub-Saharan Africa are in South Africa; therefore we rely on this data for the first glimpse into pediatric SLE in the larger region.20
In summary, the PULSE cohort demonstrates high disease activity at diagnosis and increased rates of lupus nephritis, end stage renal disease, and organ damage at enrollment compared to NA cohorts. The high percentage of patients presenting with AKI suggests delayed diagnosis may play a role in the poorer outcomes seen in the PULSE cohort. The differences in outcome may be due to racial/ethnic predisposition to severe disease, late presentation due to barriers to health care access, or a combination of these, and other factors. These differences were apparent even when compared to a NA cohort of African descent. Improving early recognition, diagnosis, and access to treatment for pSLE patients in SA may lead to reduced organ damage. Additionally, early aggressive therapy of LN may lower rates of ESRD requiring either RRT or renal transplant. Given the limited resource of dialysis and transplant, preventing primary damage in this cohort is especially important.
Further prospective research is necessary to gain better understanding of the burden of pSLE in SA. A larger, prospective study is necessary to increase the cohort of patients with pSLE, determine management, and identify risk factors for poor outcomes. Translational research investigating biomarkers of lupus severity or genetic markers for increased disease severity may help to identify patients at high risk for severe disease in both low and high income nations. Future qualitative studies examining this population could help determine the role of barriers to care, which may in part explain disease severity at onset. Identifying risk factors and barriers to care is necessary to inform interventions to improve outcomes in this high-risk pediatric population.
Acknowledgments
Funding: LL was funded by the Lupus Foundation of America Early Career Award, T32 Training grant 5T32 AI0007217-31, Duke Global Health Institute Fieldwork grant, and the Fogarty International Center of the NIH R25TW009337. The CARRA Registry was supported by grants from NIAMS RC2AR058934, Friends of CARRA, and the Arthritis Foundation.
The authors thank Melissa Watt, PhD and Coleen Cunningham, MD for their thoughtful review of this work. Larry Park, PhD and Joseph Egger, PhD of Duke Global Health Institute assisted with statistical analysis. CARRA provided access to the North American SLE database. We would like to acknowledge the research team at Red Cross War Memorial Children’s Hospital, including Dr. Kate Webb, Dr. Lawrence O’kongo, Dr. Nicola Brice, Sr. Dorothy Brown, Sr. Caroline, and Zodwa Sam. Bradley Otterson, MS, Biomedical Librarian/Informationist, NIH Library at National Institutes of Health provided critical reading of this manuscript.
This article was prepared while Laura B. Lewandowski was employed at Duke University Medical Center. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States government.
Appendix 1
In South Africa, the term Coloured refers to a racially heterogeneous ethnic group who possess ancestry from Europe, Asia, Malaysia, and various Khoisan and Bantu tribes of Southern Africa. Black refers to the population with Southern African Bantu tribal ancestry. White refers to people of European descent. Indian/Asian refers to those of Indian or Asian ancestry; and other is anyone who does not identify with one of the described races.63
Footnotes
Conflict of Interest:
The authors have no conflicts of interest to declare.
References
- 1.Uribe AG, McGwin G, Jr, Reveille JD, Alarcon GS. What have we learned from a 10-year experience with the LUMINA (Lupus in Minorities; Nature vs. nurture) cohort? Where are we heading? Autoimmunity reviews. 2004;3:321–329. doi: 10.1016/j.autrev.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Cervera R, Doria A, Amoura Z, et al. Patterns of systemic lupus erythematosus expression in Europe. Autoimmunity reviews. 2014;13:621–629. doi: 10.1016/j.autrev.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 3.Danchenko N, Satia JA, Anthony MS. Epidemiology of systemic lupus erythematosus: a comparison of worldwide disease burden. Lupus. 2006;15:308–318. doi: 10.1191/0961203306lu2305xx. [DOI] [PubMed] [Google Scholar]
- 4.Gomez-Puerta JA, Barbhaiya M, Guan H, Feldman CH, Alarcon GS, Costenbader KH. Racial/Ethnic variation in all-cause mortality among United States medicaid recipients with systemic lupus erythematosus: a Hispanic and asian paradox. Arthritis Rheumatol. 2015;67:752–760. doi: 10.1002/art.38981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Taylor H, Stein C. Systemic lupus erythematosus in Zimbabwe. Annals of the rheumatic diseases. 1986;45:645–648. doi: 10.1136/ard.45.8.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Adelowo OO, Oguntona SA. Pattern of systemic lupus erythematosus among Nigerians. Clinical Rheumatology. 2009;28:699–703. doi: 10.1007/s10067-009-1139-6. [DOI] [PubMed] [Google Scholar]
- 7.Okpechi IG, Ayodele OE, Jones ES, Duffield M, Swanepoel CR. Outcome of patients with membranous lupus nephritis in Cape Town South Africa. Nephrology Dialysis Transplantation. 2012;27:3509–3515. doi: 10.1093/ndt/gfs122. [DOI] [PubMed] [Google Scholar]
- 8.Ka MM, Diallo S, Kane A, et al. Systemic lupus erythematosus and lupus syndromes in Senegal. A retrospective study of 30 patients seen over 10 years. Rev Rhum Engl Ed. 1998;65:471–476. [PubMed] [Google Scholar]
- 9.el-Garf A, Salah S. Juvenile systemic lupus erythematosus among Egyptian children. J Rheumatol. 1990;17:1168–1170. [PubMed] [Google Scholar]
- 10.Bakr A. Epidemiology treatment and outcome of childhood systemic lupus erythematosus in Egypt. Pediatr Nephrol. 2005;20:1081–1086. doi: 10.1007/s00467-005-1900-2. [DOI] [PubMed] [Google Scholar]
- 11.Salah S, Lotfy HM, Sabry SM, El Hamshary A, Taher H. Systemic lupus erythematosus in Egyptian children. Rheumatol Int. 2009;29:1463–1468. doi: 10.1007/s00296-009-0888-5. [DOI] [PubMed] [Google Scholar]
- 12.Abdel-Hafez MA, Abdel-Nabi H. Juvenile systemic lupus erythematosus: onset patterns and short-term outcome in Egyptian children, a single-center experience. Lupus. 2015;24:1455–1461. doi: 10.1177/0961203315598016. [DOI] [PubMed] [Google Scholar]
- 13.Ransome OJ, Thomson PD. Systemic lupus erythematosus with nephritis in children. A report of 6 cases. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1986;69:629–633. [PubMed] [Google Scholar]
- 14.Rovers MJ, Coovadia HM. Systemic lupus erythematosus in children. A report of 3 cases. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1981;60:711–713. [PubMed] [Google Scholar]
- 15.Coovadia HM, Hussain A, Mwelase LH. Systemic lupus erythematosus in a black South African child. First documented case report. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 1991;79:101–103. [PubMed] [Google Scholar]
- 16.Faller G, Thomson PD, Kala UK, Hahn D. Demographics and presenting clinical features of childhood systemic lupus erythematosus. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2005;95:424–427. [PubMed] [Google Scholar]
- 17.Kumar K, Chambers S, Gordon C. Challenges of ethnicity in SLE. Best Practice & Research Clinical Rheumatology. 2009;23:549–561. doi: 10.1016/j.berh.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 18.Clark IA, al-Yaman FM, Cowden WB, Rockett KA. Does malarial tolerance, through nitric oxide, explain the low incidence of autoimmune disease in tropical Africa? Lancet. 1996;348:1492–1494. doi: 10.1016/s0140-6736(96)07342-4. [DOI] [PubMed] [Google Scholar]
- 19.Bae SC, Fraser P, Liang MH. The epidemiology of systemic lupus erythematosus in populations of African ancestry: a critical review of the “prevalence gradient hypothesis”. Arthritis & Rheumatism. 1998;41:2091–2099. doi: 10.1002/1529-0131(199812)41:12<2091::AID-ART2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 20.Tiffin N, Hodkinson B, Okpechi I. Lupus in Africa: can we dispel the myths and face the challenges? Lupus. 2013;0:1–10. doi: 10.1177/0961203313509296. [DOI] [PubMed] [Google Scholar]
- 21.Li BZ, Pan HF, Ye DQ. A bibliometric study of literature on SLE research in PubMed (2002–2011) Lupus. 2013;22:772–777. doi: 10.1177/0961203313491850. [DOI] [PubMed] [Google Scholar]
- 22.Brunner HI, Huggins J, Klein-Gitelman MS. Pediatric SLE--towards a comprehensive management plan. Nat Rev Rheumatol. 2011;7:225–233. doi: 10.1038/nrrheum.2011.15. [DOI] [PubMed] [Google Scholar]
- 23.Watson L, Leone V, Pilkington C, et al. Disease activity, severity, and damage in the UK Juvenile-Onset Systemic Lupus Erythematosus Cohort. Arthritis and rheumatism. 2012;64:2356–2365. doi: 10.1002/art.34410. [DOI] [PubMed] [Google Scholar]
- 24.Papadimitraki ED, Isenberg DA. Childhood- and adult-onset lupus: an update of similarities and differences. Expert review of clinical immunology. 2009;5:391–403. doi: 10.1586/eci.09.29. [DOI] [PubMed] [Google Scholar]
- 25.Mina R, Brunner HI. Pediatric lupus--are there differences in presentation, genetics, response to therapy, and damage accrual compared with adult lupus? Rheumatic diseases clinics of North America. 2010;36:53–80. vii–viii. doi: 10.1016/j.rdc.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ardoin SP, Schanberg LE. Paediatric rheumatic disease: lessons from SLE: children are not little adults. Nat Rev Rheumatol. 2012;8:444–445. doi: 10.1038/nrrheum.2012.109. [DOI] [PubMed] [Google Scholar]
- 27.Maroz N, Segal MS. Lupus nephritis and end-stage kidney disease. The American journal of the medical sciences. 2013;346:319–323. doi: 10.1097/MAJ.0b013e31827f4ee3. [DOI] [PubMed] [Google Scholar]
- 28.Inda-Filho A, Neugarten J, Putterman C, Broder A. Improving outcomes in patients with lupus and end-stage renal disease. Seminars in dialysis. 2013;26:590–596. doi: 10.1111/sdi.12122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis and rheumatism. 2013;65:2154–2160. doi: 10.1002/art.38006. [DOI] [PubMed] [Google Scholar]
- 30.Ward MM. Recent clinical trials in lupus nephritis. Rheum Dis Clin North Am. 2014;40:519–535. ix. doi: 10.1016/j.rdc.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sule S, Fivush B, Neu A, Furth S. Increased risk of death in African American patients with end-stage renal disease secondary to lupus. Clinical Kidney Journal. 2014;7:40–44. doi: 10.1093/ckj/sft157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nee R, Martinez-Osorio J, Yuan CM, et al. Survival disparity ofAfrican American Versus non-African American patients With ESRD due to SLE. Am J Kidney Dis. 2015;66:630–637. doi: 10.1053/j.ajkd.2015.04.011. [DOI] [PubMed] [Google Scholar]
- 33.Hiraki LT, Benseler SM, Tyrrell PN, Hebert D, Harvey E, Silverman ED. Clinical and laboratory characteristics and long-term outcome of pediatric systemic lupus erythematosus: a longitudinal study. The Journal of pediatrics. 2008;152:550–556. doi: 10.1016/j.jpeds.2007.09.019. [DOI] [PubMed] [Google Scholar]
- 34.Gibson KL, Gipson DS, Massengill SA, et al. Predictors of relapse and end stage kidney disease in proliferative lupus nephritis: focus on children, adolescents, and young adults. Clinical journal of the American Society of Nephrology : CJASN. 2009;4:1962–1967. doi: 10.2215/CJN.00490109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tikly M, V. Navarra S. Lupus in the developing world – is it any different? Best Practice & Research Clinical Rheumatology. 2008;22:643–655. doi: 10.1016/j.berh.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Borchers AT, Naguwa SM, Shoenfeld Y, Gershwin ME. The geoepidemiology of systemic lupus erythematosus. Autoimmunity reviews. 2010;9:A277–A287. doi: 10.1016/j.autrev.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 37.Rullo OJ, McKurdy DM, Yadin O, et al. Race, Ethnicity, and Gender Affect the Severity of Renal Outcomes in Patients with Pediatric Systemic Lupus Erythematosus: An Analysis of the CARRAnet Data At Baseline Visit [abstract] American College of Rheumatology/ARHP Scientific Meeting. Chicago, IL: Arthritis and Rheumatology. 2011;63:S781–S782. [Google Scholar]
- 38.Hochberg MC. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997;40:1725. doi: 10.1002/art.1780400928. [DOI] [PubMed] [Google Scholar]
- 39.Natter MD, Winsor J, Fox K, et al. American College of Rheumatology/AHRP Scientific Meeting. Chicago, IL: Arthritis Rheum; 2011. The Childhood Arthritis & Rheumatology Research Alliance Network Registry: Demographics and Characteristics of the Initial One Year Cohort [abstract] [Google Scholar]
- 40.Boneparth A, Ilowite NT Investigators CR. Comparison of renal response parameters for juvenile membranous plus proliferative lupus nephritis versus isolated proliferative lupus nephritis: a cross-sectional analysis of the CARRA Registry. Lupus. 2014;23:898–904. doi: 10.1177/0961203314531841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin SS, Kelsey JL. Use of Race and Ethnicity in Epidemiologic Research: Concepts, Methodological Issues, and Suggestions for Research. Epidemiologic Reviews. 2000;22:187–202. doi: 10.1093/oxfordjournals.epirev.a018032. [DOI] [PubMed] [Google Scholar]
- 42.Hawker G, Gabriel S, Bombardier C, Goldsmith C, Caron D, Gladman D. A reliability study of SLEDAI: a disease activity index for systemic lupus erythematosus. The Journal of rheumatology. 1993;20:657–660. [PubMed] [Google Scholar]
- 43.Markowitz GS, D'Agati VD. The ISN//RPS 2003 classification of lupus nephritis: An assessment at 3 years. Kidney Int. 2007;71:491–495. doi: 10.1038/sj.ki.5002118. [DOI] [PubMed] [Google Scholar]
- 44.Stoll T, Seifert B, Isenberg DA. SLICC/ACR Damage Index is valid, and renal and pulmonary organ scores are predictors of severe outcome in patients with systemic lupus erythematosus. British journal of rheumatology. 1996;35:248–254. doi: 10.1093/rheumatology/35.3.248. [DOI] [PubMed] [Google Scholar]
- 45.Lewandowski L, Scott C. Apartheid and health care sccess for pediatric systemic lupus erythematosus patients in South Africa. South Afr J Child Health. 2015;9:36. [Google Scholar]
- 46.Statistics South Africa. www.statssa.gov.za/publications/P0302/P03022014.pdf; Mid-year Population Estimates 2014. 2014 Statistical release P0302.
- 47.Hagelberg S, Lee Y, Bargman J, et al. Longterm followup of childhood lupus nephritis. J Rheumatol. 2002;29:2635–2642. [PubMed] [Google Scholar]
- 48.Zirkzee EJ, Huizinga TW, Bollen EL, et al. Mortality in neuropsychiatric systemic lupus erythematosus (NPSLE) Lupus. 2014;23:31–38. doi: 10.1177/0961203313512540. [DOI] [PubMed] [Google Scholar]
- 49.Mina R, von Scheven E, Ardoin SP, et al. Consensus treatment plans for induction therapy of newly diagnosed proliferative lupus nephritis in juvenile systemic lupus erythematosus. Arthritis Care Res (Hoboken) 2012;64:375–383. doi: 10.1002/acr.21558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hanly JG. Diagnosis and management of neuropsychiatric SLE. Nat Rev Rheumatol. 2014;10:338–347. doi: 10.1038/nrrheum.2014.15. [DOI] [PubMed] [Google Scholar]
- 51.Hiraki LT, Benseler SM, Tyrrell PN, Harvey E, Hebert D, Silverman ED. Ethnic differences in pediatric systemic lupus erythematosus. Journal of Rheumatology. 2009;36:2539–2546. doi: 10.3899/jrheum.081141. [DOI] [PubMed] [Google Scholar]
- 52.Moosa MR, Kidd M. The dangers of rationing dialysis treatment: the dilemma facing a developing country. Kidney Int. 2006;70:1107–1114. doi: 10.1038/sj.ki.5001750. [DOI] [PubMed] [Google Scholar]
- 53.Ruiz-Irastorza G, Khamashta MA. Hydroxychloroquine: the cornerstone of lupus therapy. Lupus. 2008;17:271–273. doi: 10.1177/0961203307086643. [DOI] [PubMed] [Google Scholar]
- 54.Ardoin SP, Schanberg LE, Sandborg C, et al. Laboratory markers of cardiovascular risk in pediatric SLE: the APPLE baseline cohort. Lupus. 2010;19:1315–1325. doi: 10.1177/0961203310373937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nived O, Jonsen A, Bengtsson AA, Bengtsson C, Sturfelt G. High predictive value of the Systemic Lupus International Collaborating Clinics/American College of Rheumatology damage index for survival in systemic lupus erythematosus. J Rheumatol. 2002;29:1398–1400. [PubMed] [Google Scholar]
- 56.Singh S, Devidayal, Kumar L, Joshi K. Mortality patterns in childhood lupus-10 years' experience in a developing country. Clinical rheumatology. 2002;21:462–465. doi: 10.1007/s100670200116. [DOI] [PubMed] [Google Scholar]
- 57.Appenzeller S, Marini R, Costallat LT. Damage did not independently influence mortality in childhood systemic lupus erythematosus. Rheumatology international. 2005;25:619–624. doi: 10.1007/s00296-004-0552-z. [DOI] [PubMed] [Google Scholar]
- 58.Ravelli A, Ruperto N, Martini A. Outcome in juvenile onset systemic lupus erythematosus. Curr Opin Rheumatol. 2005;17:568–573. doi: 10.1097/01.bor.0000169364.69066.1e. [DOI] [PubMed] [Google Scholar]
- 59.Osborne NG, Feit MD. The use of race in medical research. JAMA. 1992;267:275–279. [PubMed] [Google Scholar]
- 60.Wit E, Delport W, Rugamika CE, et al. Genome-wide analysis of the structure of the South African Coloured Population in the Western Cape. Human genetics. 2010;128:145–153. doi: 10.1007/s00439-010-0836-1. [DOI] [PubMed] [Google Scholar]
- 61.Yaeger R, Avila-Bront A, Abdul K, et al. Comparing Genetic Ancestry and Self-Described Race in African Americans Born in the United States and in Africa. Cancer Epidemiology Biomarkers & Prevention. 2008;17:1329–1338. doi: 10.1158/1055-9965.EPI-07-2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tishkoff SA, Williams SM. Genetic analysis of African populations: human evolution and complex disease. Nat Rev Genet. 2002;3:611–621. doi: 10.1038/nrg865. [DOI] [PubMed] [Google Scholar]
- 63.Posel D. Race as Common Sense: Racial Classification in Twentieth-Century South Africa. African Studies Review. 2001;44:87–113. [Google Scholar]