Abstract
An increasing literature suggests that schizophrenia is associated with a reduction in hippocampal interneuron function. Thus, we posit that stem cell-derived interneuron transplants may be an effective therapeutic strategy to reduce hippocampal hyperactivity and attenuate behavioral deficits in schizophrenia. Here we used a dual-reporter embryonic stem cell line to generate enriched populations of parvalbumin (PV)- or somatostatin (SST)-positive interneurons, which were transplanted into the ventral hippocampus of the methylazoxymethanol (MAM) rodent model of schizophrenia. These interneuron transplants integrate within the existing circuitry, reduce hippocampal hyperactivity, and normalize aberrant dopamine neuron activity. Further, interneuron transplants alleviate behaviors that model negative and cognitive symptoms, including deficits in social interaction and cognitive inflexibility. Interestingly, PV- and SST-enriched transplants produced differential effects on behavior, with PV-enriched populations effectively normalizing all the behaviors examined. These data suggest that stem cell-derived interneuron transplants may represent a novel therapeutic strategy for schizophrenia.
INTRODUCTION
Schizophrenia is a devastating psychiatric disorder characterized by positive, negative, and cognitive symptoms. One of the longest-standing hypotheses of schizophrenia posits that excessive subcortical dopamine release is responsible for the positive symptoms of the disorder. Support for this hypothesis stems from the fact that all antipsychotics are D2 receptor antagonists 1 and can reduce positive symptoms 2. Unfortunately, these drugs are not effective in all patients, possess debilitating side effects, and have little to no impact on the negative or cognitive symptom domains 2. Currently, there are no FDA-approved medications that specifically target the negative and cognitive symptoms of schizophrenia, which are associated with a dramatically reduced quality of life 3. Negative symptoms, such as blunted affect and social avoidance, and cognitive symptoms, like deficits in attention and executive function, can be severely debilitating3, 4. Even among schizophrenia patients that respond to antipsychotic medications, disability rates remain high and functional outcomes poor, highlighting the urgent need to treat these residual negative and cognitive symptoms of schizophrenia 3. Thus, new approaches targeting all three symptom domains are urgently needed.
Although the dopamine hypothesis has received wide support, there has been little observable primary pathology identified in the dopamine system of schizophrenia patients. This has led to the suggestion that the pathology may lie upstream in brain regions that regulate the dopamine system, such as the ventral hippocampus (vHipp) 5. The vHipp not only regulates the activity of the dopamine system 6, but also projects directly to the prefrontal cortex 7, making this a reasonable therapeutic target not only for the positive symptoms associated with increased dopamine system function, but also for the negative and cognitive symptoms that rely on prefrontal cortical function 8, 9. Hippocampal pathology is routinely observed in schizophrenia patients with an increased activity at rest 10 being correlated with both psychosis 11 and cognitive dysfunction 12. This hyperactivity has been attributed to a deficit of inhibitory interneuron function 13. Indeed, some schizophrenia patients display reductions in hippocampal interneurons that express the Ca2+-binding protein, parvalbumin (PV), and those that express the neuromodulator, somatostatin (SST) 14, 15. Recently, we demonstrated that a loss of PV expression is sufficient, in and of itself, to increase vHipp activity and produce downstream alterations in dopamine system function and schizophrenia-like changes in behavior 16. Therefore, we hypothesize that a loss of interneuron function leads to an increase in hippocampal activity and aberrant dopamine system function. Restoring inhibitory interneuron function in the vHipp may normalize both hippocampal and dopamine neuron activity, resulting in a resolution of the positive, negative and cognitive symptoms of schizophrenia.
We have recently tested this hypothesis using interneuron transplants derived from rat fetal tissue 17. During development, the majority of PV- and SST-positive interneurons are derived from the telencephalic structure known as the medial ganglionic eminence (MGE)18. Interneuron precursors born in the MGE have the remarkable ability to migrate tangentially across cortical regions 19. Therefore, we used fetal MGE tissue to restore interneuron function in the methylazoxymethanol (MAM) developmental model of schizophrenia 17. We found that interneuron transplants into the vHipp reduced hippocampal hyperactivity and dopamine population activity, and normalized amphetamine-induced locomotor activity, a model for the positive symptoms of schizophrenia. A subsequent paper showed similar effects of interneuron transplants on hippocampal hyperactivity and psychosis-related behaviors in a transgenic mouse model with reduced PV-positive interneurons in the hippocampus 20. While these results are exciting, this approach is not feasible in patients due to the source of the interneurons (fetal brain). Further, these studies did not examine negative and cognitive symptoms of schizophrenia, which are poorly treated by antipsychotics, and did not differentiate between interneuron subclasses. This is important since the distinct axon targeting and firing properties of interneuron subclasses is likely to differentially affect local activity after transplantation13. Moreover, different interneuron subtypes display discrete neurophysiological characteristics that may alter their therapeutic utility13. Thus, to address these issues in the current studies, we used embryonic stem cells that were differentiated into enriched populations of distinct interneuron subgroups 21. Here we explore the role of specific interneuron subclasses in treating neurophysiological and behavioral alterations in the MAM model of schizophrenia and expand our recent findings to determine if vHipp interneuron transplants can also alleviate correlates of the negative and cognitive symptoms associated with this disorder.
MATERIALS AND METHODS
Drugs and Reagents
DNQX, picrotoxin, MK-801, kynurenic acid, guanosine 5′ triphosphate sodium salt hydrate (GTP), adenosine 5′ triphosphate magnesium salt (ATP), EGTA, Pentobarbital, and chloryl hydrate were purchased from Sigma-Aldrich. K-methylsulfate was purchased from Acros Organics. Tetrodotoxin citrate (TTX) was purchased from AbCam. Neurobiotin tracer was purchased from Vector Laboratories. Cell culture media and Leukemia inhibitory factor were purchased from Life Technologies. LDN-193189, Y27632, and XAV939 were purchased from Stemgent. Fibroblast growth factor-2, Insulin-like growth factor-1, and sonic hedgehog were purchased from R&D Systems. N2 and B27 supplements were purchased from Gibco. Cyclosporin A was purchased from Fisher Scientific.
MAM Administration
Timed pregnant female Sprague-Dawley rats were administered methylazoxymethanol (MAM: 22 mg/kg i.p.) or saline on gestational day 17 as reported previously 22. Male pups were weaned on postnatal day 21 and all experiments included pups from multiple litters.
Embryonic Stem Cells
Enriched populations of parvalbumin (PV)- or somatostatin (SST)-positive interneurons were generated as published previously using the J27 mouse embryonic stem cell line containing dual reporters (Lhx6::GFP and Nkx2.1::mCherry) 21. Briefly, undifferentiated stem cells were cultured on mouse embryonic fibroblasts (GlobalStem) in embryonic stem cell medium (knock-out DMEM containing knockout serum replacement, nonessential amino acids, beta-mercaptoethanol, leukemia-inhibitory factor, and L-glutamine). To differentiate the stem cells into interneurons, cells were grown in suspension as embryoid bodies in embryonic stem cell medium containing 1% N2, the BMP inhibitor, LDN-193189 (250nM), the Rho-kinase inhibitor, Y27632 (10μM), and the WNT inhibitor, XAV939 (10μM). On differentiation day 3, embryoid bodies were dissociated and plated onto an adherent dish in the presence of Fibroblast growth factor-2 (10ng/ml), Insulin-like growth factor-1 (20ng/ml) and sonic hedgehog (50ng/ml for SST-enriched cells only). Media was changed daily until cells were sorted by flow cytometry in the presence of the viability marker, Zombie Violet. SST-enriched cells were sorted for GFP+ cells on differentiation day 12 (Figure 1a) and PV-enriched populations were sorted for mCherry+ cells on differentiation day 17 (Figure 1b).
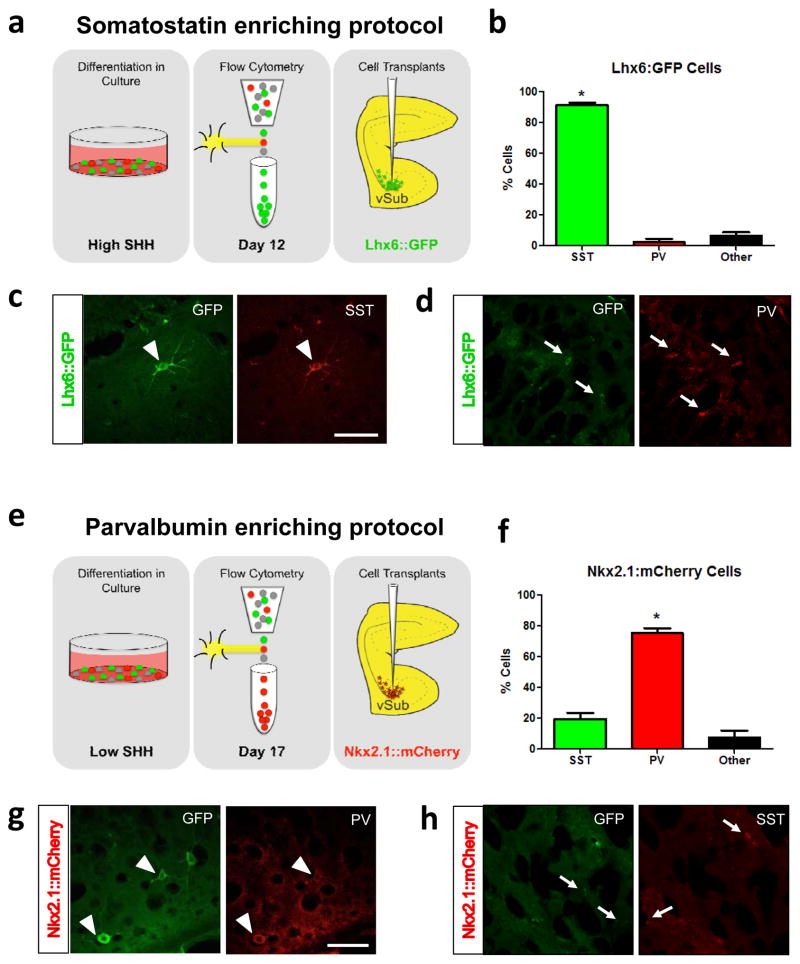
Figure 1.
Schematic depicting the protocol used to generate SST-enriched cells is shown in (a). The majority of cells grown using the SST-enriching protocol express SST. * is significantly different than the percentage of cells that express PV (b). Representative image of a transplanted Lhx6::GFP cell in which GFP and SST are co-localized (c). Representative image of a transplanted Lhx6::GFP cell in which GFP and PV are not co-localized (d). Schematic depicting the protocol used to generate PV-enriched cells is shown in (e). The majority of cells grown using the PV-enriching protocol express PV. * is significantly different than the percentage of cells that express SST (f). Representative image of a transplanted Nkx2.1::mCherry cell in which GFP and PV are co-localized (g). Representative image of a transplanted Nkx2.1::mCherry cell in which GFP and SST are not co-localized (h). Arrowheads indicate double-labeled cells. Arrows indicate cells that are not double-labeled. Scale bar is 100 microns. n = 11–31 cells per rat.
Cell Sorting
Cells were incubated in accutase containing DNAse at 37°C for 30 min, then gently triturated to form a single cell suspension. Cells were centrifuged (250 x g) and the pellet was resuspended in DMEM:F12 with 20% fetal bovine serum (FBS) and 10μM Y-27632. Cells were incubated with Zombie Violet (Biolegend) for 15 min at room temperature. After centrifugation, the pellet was resuspended in DMEM:F12 containing FBS and Y-27632, passed through a 70um filter and stored on ice until sorting began. Cells were sorted using a BD FACSAria III (70 micron nozzle, 50 psi) and analyzed using FACS Diva 6.1.3 software (See Supplementary Figure 1). After excluding dead cells and doublets, mCherry-positive or GFP-positive cells were collected in neurobasal medium containing B27, 2mM L-glutamine, and 10μM Y-27632.
Cell Transplants
On postnatal days 40–45, animals were anesthetized (sodium pentobarbital, 60mg/kg, i.p.) and placed in a stereotaxic apparatus. Bilateral cannula aimed at the vHipp (A/P +4.8, M/L ±4.8, D/V −7.0 mm from bregma) were used to inject approximately 40,000 cells into each hemisphere. Control animals received dead cell transplants, produced by repeated freezing and thawing and confirmed by trypan blue. Rats were treated with an immunosuppressant (cyclosporin A, 10mg/kg, s.c.) for 7 days to prevent rejection of mouse embryonic stem cells. After day 7, cyclosporin A was administered in the drinking water (100ug/ml) for the duration of the experiment. All electrophysiological and behavioral experiments were performed at least 30 days post-transplantation, a time point at which the cells have had sufficient time to migrate and integrate into the cortex 21.
Ex vivo electrophysiology
To confirm that the transplanted cells integrated into the existing hippocampal circuitry, we performed patch clamp recordings in brain slices from transplanted rats. Briefly, rats were anesthetized with pentobarbital (60 mg/kg, i.p.) and transcardially perfused for 60 seconds with ice cold aCSF containing (in mM): 126 NaCl, 2.5 KCl, 1.2 MgCl2, 2.4 CaCl2, 1.4 NaH2PO4, 25 NaHCO3, 11 D-glucose, and 1.25 kynurenic acid. After extraction, brains were blocked and mounted on a vibrating microtome (Leica Microsystems). Horizontal brain slices (200 μm thick) containing the vHipp were collected and maintained at 34°C in aCSF plus the NMDA receptor antagonist MK-801 (20 μM), continuously bubbled with 95% O2/5% CO2. Recordings were performed at 34°C under continual perfusion of aCSF at a flow rate of 2 ml/min. Pyramidal neurons were identified visually and whole cell patch clamp recordings were performed using pipettes of 1.5–2.2 MΩ resistance filled with an intracellular solution containing (in mM): 57.5 K-methylsulfate, 57.5 KCl, 20 NaCl, 1.5 MgCl2, 10 HEPES, 0.025 EGTA, 2 ATP, and 0.4 GTP plus 0.2% neurobiotin, pH 7.35–7.40, 267–275 mOsm/L. To isolate spontaneous and miniature GABAA receptor-mediated inhibitory postsynaptic currents (sIPSCs and mIPSCs), whole cell voltage clamp recordings were performed at a holding voltage of −70 mV in the presence of the AMPA receptor antagonist DNQX (10 μM). During these brief (< 5min) experiments, electrical access to the interior compartment of the cell was continuously monitored and recordings were discarded if series resistance exceeded 5 MΩ. In some experiments, TTX was added (1 μM) to the bath to isolate mIPSCs. Data were acquired at 50 kHz and filtered at 6 kHz using a Multiclamp 700B amplifier (Molecular Devices). Data were recorded and analyzed using Axograph X software (www.axograph.com). Spontaneous and miniature IPSCs were analyzed by an investigator that was blinded to the experimental group. For each cell, 50 sec of data were analyzed with the detection software set to the following minimum criteria: slope threshold −50 pA/ms, separation between events 5 ms, amplitude 15 pA, rise time 100 μs, event half-width 600 μs.
In vivo electrophysiology
In order to measure putative pyramidal cell activity in the vHipp and downstream changes in dopamine neuron activity, we performed in vivo extracellular recordings. Rats were anesthetized with 8% chloral hydrate (400 mg/kg−1, i.p.) and placed in a stereotaxic apparatus. Supplemental anesthesia was administered as necessary and a core body temperature of 37°C was maintained. Extracellular glass microelectrodes (impedance 6–14MOhms) were lowered into the vHipp (A/P −5.0, M/L +/−4.5, D/V −4.0—8.0 from bregma) or ventral tegmental area (VTA; A/P −5.3, M/L +/−0.6, D/V −6.5—9.0 from bregma). Putative pyramidal neurons in the vHipp (identified by a firing rate <2 Hz 23) were recorded. In the VTA, previously established electrophysiological criteria were used to identify spontaneously active dopamine neurons 24 (Figure 3c). The following parameters of dopamine neuron activity were measured: 1) population activity, 2) basal firing rate, and 3) the proportion of action potentials occurring in bursts.
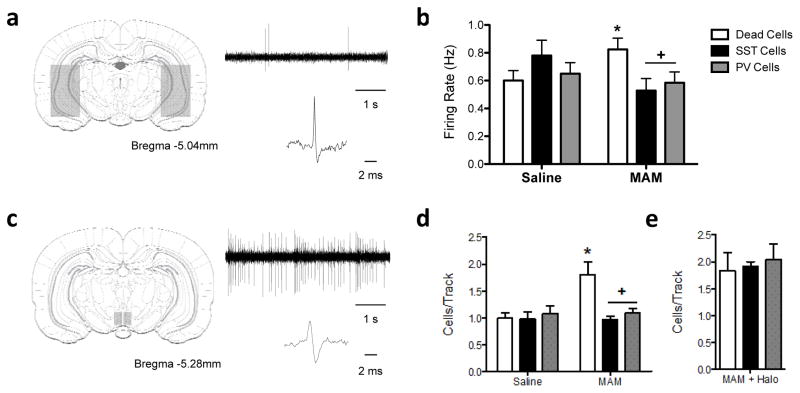
Figure 3.
Stem cell derived interneuron transplants reverse aberrant neuronal activity in the vHipp (a) and VTA (c). MAM-treated rats display an increase in vHipp pyramidal neuron activity that was normalized by both PV- and SST-positive transplants (b: n=23–48 cells per group). Similarly, PV- and SST-positive vHipp transplants were also able to normalize downstream alterations in dopamine neuron population activity in the ventral tegmental area (d: n=5–7 rats/group). The effect on interneuron transplants on dopamine activity is completely reversed when transplanted cells are optogenetically silenced (e: MAM + Halo n=2–5 per group). + denotes significant difference from MAM-treated animals that received dead cell transplants while * represents significant difference from saline controls.
Optogenetics
To inhibit the transplanted interneurons, sorted cells were resuspended in neurobasal medium containing B27, plated, and incubated overnight with adeno-associated 2 virus (AAV2) vectors driven by human the alpha-synuclein promoter expressing -HaloRhodopsin (AAV2-hSyn-eNpHR3.0-EYFP: 5×1010 vm/well, UNC Vector Core). Cells were then dissociated, washed and transplanted into the vHipp as described above. On the day of the experiment, a fiber-optic cannulae, connected to a 100mW 593nm DPSS Laser (OEM laser systems), was lowered into the vHipp and yellow light (~20mW) was delivered continuously. Simultaneously, spontaneously active dopamine cells were recorded in the VTA as described above.
Latent Inhibition
The latent inhibition test was used to model a positive symptom of schizophrenia. Briefly, rats were placed into a 30.5×25.4×30.5 cm3 square conditioning chamber with metal walls and a stainless steel grid shock floor (Coulbourn Instruments) and randomly assigned to two groups: tone pre-exposed and non-exposed. Pre-exposed rats were presented with a 20 sec tone over 16 trials with a pseudorandom intertrial interval averaging 2 min. Immediately following tone exposure, rats underwent an established fear conditioning procedure in which the 20 sec tone co-terminated with a mild (0.8 mA, 0.5 sec) footshock. The tone-shock pairing was presented 4 times with a pseudorandom intertrial interval averaging 2 min. Twenty-four hours after conditioning, rats were returned to the conditioning chamber and re-exposed to the 20 sec tone over 4 trials. Behavior was video-recorded at each stage and freezing behavior was analyzed off-line using FreezeFrame and FreezeView software (Coulbourn Instruments). Freezing was defined as behavior below a motion index threshold of 10 lasting at least 1 sec. The percentage of latent inhibition was determined using the following formula: % LI = 100 − NoTone/Tone x 100.
Social Interaction
To model a negative symptom of schizophrenia, the Social Interaction Test was performed as described previously 25. Briefly, rats were placed in a test arena (100×100×40cm) alone for 15 min per day for 2 days prior to testing. On the test day, experimental rats were placed in the arena with a weight-matched “stimulus” rat. The 10 min test was recorded by video camera for offline analysis by a blind experimenter. The dependent measure was the time that the test animal spent actively engaged in social interaction, including sniffing, climbing on, following, grooming, or wrestling with the stimulus rat.
Attentional Set-shifting Test
To measure cognitive function, we performed the AST as described 26. Briefly, rats were restricted to 12g food/day for 7 days prior to testing. Using a cheerio reward, rats were trained to dig in pots defined by cues along two stimulus dimensions: the digging medium filling the pot, and an odor applied to the inner rim of the pot. During testing, the rat was taken through a series of stages, each requiring a different discrimination, with a criterion of 6 consecutive correct trials required to proceed to the next stage. The first stage was a simple discrimination (SD), with only one stimulus dimension (odor or medium) present. Odor was the initial relevant dimension (signaling the location of the reward) for half the animals, and medium for the other half. The second stage was a compound discrimination (CD) in which the same discrimination was required and the second irrelevant dimension was introduced. The third stage was a reversal (R1) in which the same odors and media were used, and the same dimension remained relevant, but the negative cue from the previous stage became positive and the positive cue from the previous stage is now negative. Stage 4 was an intradimensional shift (ID), in which all new odors and media were introduced but the same dimension remained relevant. After performing a second reversal (R2), the last stage was an extradimensional set shift (ED). During ED all new odors and media were presented and the previously irrelevant stimulus became relevant.
Immunohistochemistry
After behavioral or electrophysiological testing, dual-fluorescence immunohistochemistry was used to determine the percentage of interneuron transplants that expressed SST or PV. Briefly, rats were transcardially perfused with saline followed by 4% paraformaldehyde. Brains were postfixed and cryoprotected in 10% sucrose in phosphate-buffered saline (PBS). Lhx6 is expressed in all PV- and SST-positive interneurons from the time cells exit the cell cycle into adulthood, therefore we used GFP, which is expressed under the control of Lhx6, to label transplanted cells. Free-floating coronal sections from the vHipp (50 μm) were washed in PBS, blocked (2% normal goat serum and 0.3% Triton X-100), then incubated with mouse anti-GFP antibody (AbCam; 1:500) at 4C overnight. AlexaFluor 488 goat anti-mouse secondary antibody (1:1000) was then used to label transplanted cells. After washing, sections were incubated with rabbit anti-PV (AbCam; 1:2000) or rabbit anti-SST (Millipore; 1:400) antibodies at 4C overnight. Sections were then incubated with AlexaFluor 594 goat anti-rabbit secondary antibody (1:1000) and mounted on slides and coverslipped using Prolong gold anti-fade reagent with DAPI. Sections were imaged using an AxioCam ICc 1 (Zeiss) camera attached to an Axio Lab.A1 microscope (Zeiss). The number GFP-positive cells that colocalized with SST- or PV-positive cells were counted on 4 GFP+ sections per animal. The representative images were acquired using an Olympus IX81 Motorized inverted confocal microscope and FV10-ASW software and enhanced using ImageJ.
Data Analysis
In all figures, data are shown as mean ± s.e.m and n is indicated in the figure legend. Data was analyzed by one- or two-way ANOVA and the Holm-Sidak post-hoc test was used when significant interactions were present. When comparing groups with unequal variances the nonparametric Kruskal-Wallis test was used followed by Dunn’s multiple comparison test. All tests were two-tailed, and significance was determined at p<0.05.
RESULTS
Embryonic stem cell-derived interneuron transplants
We have demonstrated previously that enriched populations of parvalbumin (PV)- or somatostatin (SST)-positive interneuron precursors can be generated from embryonic stem cells by recapitulating developmental programs in vitro21. After using flow cytometry to isolate the GFP- or mCherry-positive cells (Supplementary Figure 1a), we performed qPCR to confirm SST mRNA expression. We found that the GFP+ cells, which had been prepared according to the SST-enriching protocol (Figure 1a), had a significant increase in SST mRNA expression compared to negative cells (Supplementary Figure 1b). The mCherry-positive cells that had been treated according to the PV-enriching protocol did not show a significant increase in SST expression compared to negative control cells (Supplementary Figure 1b; Fold Increase: GFP+ cells = 7.15 ± 1.07; mCherry+ cells = 3.31 ± 0.83; One-way ANOVA: F = 15.80, p < 0.05), suggesting that the embryonic stem cells began to differentiate into specific interneuron subtypes in culture. To confirm that the precursor cells continued to develop into mature interneurons once they were transplanted into the rat hippocampus, we used dual fluorescence immunohistochemistry to label GFP and PV or SST. We found that when we used the SST-enriching protocol, 91% of GFP-positive cells also expressed SST (Figure 1a). The PV-enriching protocol resulted in 75% GFP-positive cells that were also PV-positive (Figure 1b). Together, these results confirm that our in vitro cell differentiation protocol produces enriched populations of SST- or PV-positive interneurons.
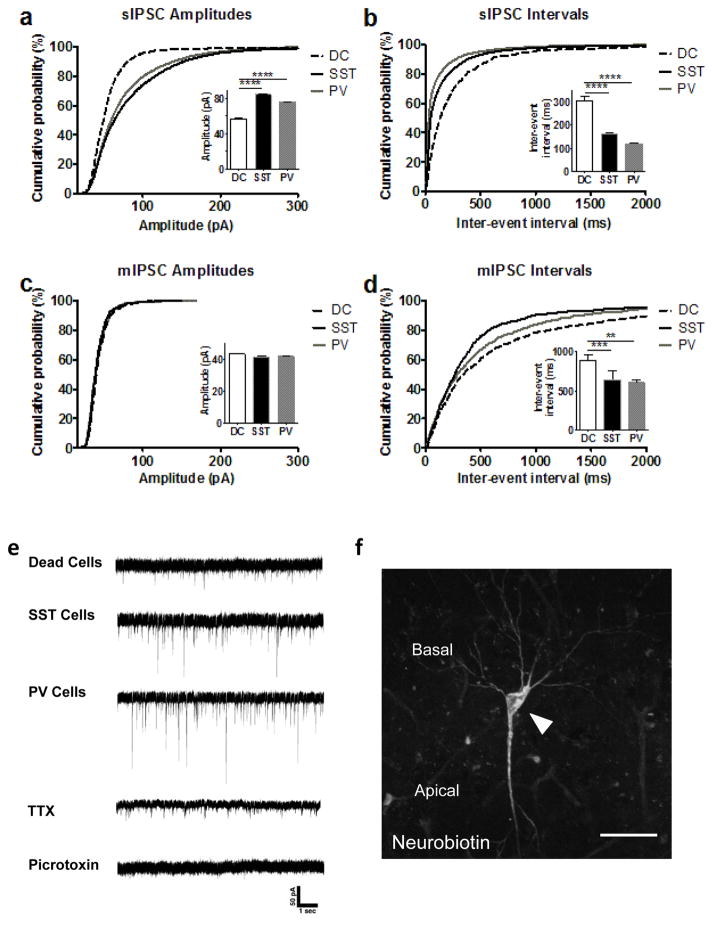
To demonstrate that the transplanted interneurons integrate into the existing circuitry, we first used dual fluorescence immunohistochemistry to show that the transplanted interneurons form synapses on endogenous pyramidal cells in the vHipp (Supplementary Figure 2). Next, we used ex vivo patch clamp electrophysiology to measure spontaneous inhibitory postsynaptic currents (sIPSCs) in endogenous pyramidal cells throughout the vHipp. We found that the MAM animals that received PV- and SST- transplants show significant higher sIPSC amplitudes (Figure 2a; sIPSC Amplitudes: MAM/Dead cells = 56.38 ± 1.05 pA, MAM/SST cells = 84.32 ± 0.91 pA, MAM/PV cells = 75.64 ± 0.63 pA; Kruskal-Wallis: K = 292.5, p<0.0001) and significant shorter inter-event intervals (Figure 2b; sIPSC Intervals: MAM/Dead cells = 303.9 ± 19.8 ms, MAM/SST cells = 160.9 ± 4.28 ms, MAM/PV cells = 118.1 ± 3.25 ms; n=13–34 cells from 2–3 rats/group; Kruskal-Wallis: K = 844.7, p<0.0001) than the dead cell group, suggesting that the transplanted interneurons indeed integrated into the hippocampal circuitry. In the presence of the sodium channel blocker TTX there was no longer a difference in amplitudes (Figure 2c; mIPSC Amplitude: MAM/Dead cells = 43.14 ± 0.55 pA, MAM/SST cells = 41.42 ± 0.69 pA, MAM/PV cells = 41.80 ± 0.50 pA) but the event intervals remained significantly shorter (Figure 2d; mIPSC Intervals: MAM/Dead cells = 884.6 ± 73.5 ms, MAM/SST cells = 644.0 ± 111 ms, MAM/PV cells = 601.3 ± 36.8 ms; n=13–17 cells from 2–3 rats/group; Kruskal-Wallis: K = 18.36, p<0.0001). In all animal groups, sIPSCs and mIPSCs were eliminated by the GABAA receptor antagonist picrotoxin (100 μM). Representative traces are depicted in Figure 2e and a representative pyramidal cell is shown in Figure 2f.
Figure 2.
Stem cell derived interneuron transplants functionally integrate within the existing circuitry. Ex vivo patch clamp electrophysiology (holding voltage: −70 mV) of endogenous pyramidal neurons demonstrated significant higher sIPSC amplitudes (a) and shorter inter-event intervals (b) compared to dead cell controls. When TTX was added and mIPSCs were analyzed, the difference in amplitudes between groups was eliminated (c), but inter-event intervals remained shorter (d) compared to dead cell controls. Example traces are depicted in (e). A representative pyramidal cell, labeled with neurobiotin is shown in (f). Scale bar is 50 microns. n = 13–34 cells from 2–3 rats/group.
To further demonstrate that the interneuron transplants regulate vHipp activity, we performed in vivo electrophysiological recordings of putative pyramidal cells in chloral hydrate anesthetized rats (Fig 3a). As we have shown previously17, MAM-treated rats show increased pyramidal cell firing in the vHipp compared to saline controls (Figure 3b; Saline/Dead cells = 0.60 ± 0.07 Hz, MAM/Dead cells = 0.82 ± 0.08 Hz; Two-way ANOVA: Interaction F2,239=3.995, p=0.02; Holm-Sidak: t=2.034, p=0.043). This is consistent with our hypothesis that the increased vHipp activity is due to a loss of GABAergic interneuron function 5. In line with this, we now demonstrate that both SST- and PV-enriched transplants can reverse aberrant pyramidal cell firing in MAM rats to control levels (Figure 4b; MAM/SST cells = 0.528 ± 0.9 Hz, MAM/PV cells = 0.58 ± 0.08; Holm-Sidak: MAM/Dead vs. MAM/SST t=2.564, p=0.033; MAM/Dead vs. MAM/PV t=2.269, p=0.048).
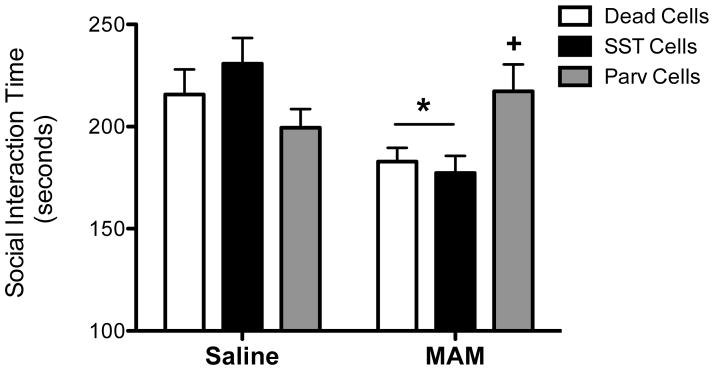
Figure 4.
PV-positive transplants, but not SST-positive transplants, into the ventral hippocampus reverse deficits in social interaction in MAM-treated rats. * demonstrates significant difference from saline-treated controls while + represents significant difference from MAM-treated animals that received dead cell transplants. n=9–14 per group.
We have previously shown that the MAM model of schizophrenia has an increase in the number of spontaneously active dopamine cells in the ventral tegmental area (VTA)27. Further, we have also demonstrated that this increase in dopamine population activity is directly attributable to a pathologically enhanced drive from the vHipp 5, 28, 29. In the current experiments, we now show that in MAM-treated animals, both SST- and PV-enriched transplants reduce the number of spontaneously active dopamine cells to control levels (Figure 3c, d; Saline/SST cells = 0.98 ± 0.128 cells/track, Saline/PV cells = 1.08 ± 0.14 cells/track, MAM/SST cells = 0.97 ± 0.06 cells/track, MAM/PV cells = 1.09 ± 0.08 cells/track; Holm-Sidak: MAM/Dead vs. MAM/SST t=4.229, p<0.001; MAM/Dead vs. MAM/PV t=3.618, p=0.003). Further, we used optogenetics in a subset of animals to demonstrate that this effect was dependent on the activity of the stem cell derived interneuron transplants (Figure 3). When HaloRhodopsin and a 593nm laser were used to inhibit the activity of the transplanted interneurons, the beneficial effect on dopamine population activity was completely abolished (Figure 3e; Dead cells/Halo = 1.84 ± 0.24 cells/track, SST cells/Halo = 1.92 ± 0.24 cells/track; PV cells/Halo = 2.04 ± .24 cells/track; Holm-Sidak: SST cells/Halo vs. MAM/Dead cells t=0.24, p=0.817; PV cells/Halo vs. MAM/Dead cells t=0.60, p=0.91). Taken together, these data demonstrate that stem cell-derived interneuron transplants functionally integrate within the existing circuitry to normalize aberrant vHipp pyramidal cell activity and reverse downstream changes in dopamine system function.
Effect of cell transplants on behavioral correlates of positive, negative and cognitive symptoms
We have previously demonstrated that fetal-derived MGE transplants can reverse the augmented amphetamine-induced locomotor activity in MAM-treated rats17. To examine an additional behavioral correlate of positive symptoms we examined whether stem cell derived interneurons can reverse deficits in latent inhibition. Consistent with our previous observations, MAM-treated rats show a trend toward impaired latent inhibition (Supplementary Figure 3b, c; Saline/Dead cells = 29.81 ± 28.266% freezing, MAM/Dead cells = −14.64 ± 28.27% freezing). The Saline- and treated rats that received SST-positive interneuron transplants also show deficits in latent inhibition (Supplementary Figure 3b, c; Holm-Sidak, t = 2.52, p = 0.03; Saline/SST cells = −70.70 ± 28.27, −38.29 ± 30.53% freezing). The PV-positive interneuron transplants show a trend toward improvement in latent inhibition in the MAM-treated rats, although this effect did not reach significance (Supplementary Figure 3b, c; Saline/PV cells = 41.17 ± 30.53% freezing, MAM/PV cells = 9.32 ± 33.45% freezing).
Currently prescribed antipsychotic medications are poorly effective in treating negative symptoms of schizophrenia2. Therefore, we used the social interaction test to model the negative symptoms of schizophrenia to determine if interneuron transplants can improve performance in this symptom domain. Consistent with observations in schizophrenia patients, MAM rodents spend less time engaged in social interaction compared to saline controls (Figure 4; Two-way ANOVA, Interaction F2,63=5.676, p=0.006; Holm-Sidak, t=2.396, p=0.02; Saline/Dead cells = 215.64 ± 12.291 sec, MAM/Dead cells = 182.86 ± 6.76 sec). The SST-enriched transplants had no effect on social interaction time in either the saline- or MAM-treated rats (Holm-Sidak, Saline/dead cells vs. Saline/SST cells, t=1.022, p=0.311; Holm-Sidak, MAM/dead vs. MAM/SST cells, t=0.395, p=0.694; Saline/SST cells = 230.8 ± 12.517 sec, MAM/SST cells = 177.3 ± 8.37 sec). In contrast, PV-enriched transplants abolished the schizophrenia-like deficit in social interaction without altering social interaction in control animals (Holm-Sidak, Saline/dead vs. Saline/PV cells, t=1.061, p=0.5; Holm-Sidak, MAM/dead vs. MAM/PV cells, t=2.443, p=0.035; Saline/PV cells = 199.44 ± 9.09 sec, MAM/PV cells = 217.2 ± 13.15 sec), suggesting that PV-positive interneuron transplants, derived from embryonic stem cells, may be effective in reducing negative symptoms of schizophrenia.
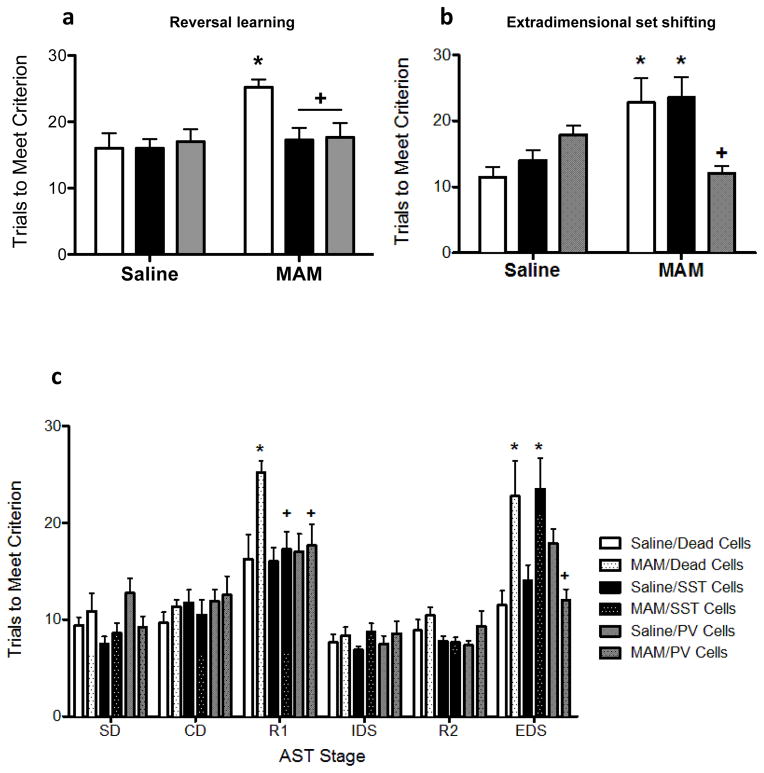
Cognitive functions are also poorly treated by currently available antipsychotic medications2. Therefore, we used the attentional set-shifting test to measure two forms of cognitive flexibility that are impaired in schizophrenia patients 30. Consistent with previous reports 31, MAM-treated rodents show deficits in reversal learning (Figure 5a; Two-way ANOVA, Interaction F2,50=3.461, p=0.04; Holm-Sidak, t=3.641, p<0.001; Saline/Dead cells = 16.0 ± 2.28 trials, MAM/Dead cells = 25.22 ± 1.16 trials). Both SST- and PV-enriched transplants completely abolish this deficit, returning trials to meet criterion to control levels (Holm-Sidak, MAM/dead vs. MAM/SST, t=3.054, p=0.011; Holm-Sidak, MAM/dead vs. MAM/PV, t=2.983, p=0.009; MAM/SST cells = 17.25 ± 1.84, MAM/PV cells = 17.67 ± 2.13). In contrast to the effects on reversal learning, PV and SST interneuron transplants produced disparate results on extradimensional set shifting (Figure 5b). Thus, while SST-positive interneuron transplants had no effect (Holm-Sidak, t=0.217, p=0.829; MAM/SST cells = 23.5 ± 3.21 trials), the PV-enriched transplants completely abolished the cognitive deficit observed in MAM rats (Holm-Sidak, t=3.244, p=0.005; MAM/PV cells = 12.0 ± 1.15 trials). There were no effects of prenatal treatment or cell transplants on any other stage of the test (Figure 5c).
Figure 5.
PV- and SST- interneuron transplants differentially affect cognitive flexibility as determined by the attentional set shifting test. MAM-treated rats display deficits in reversal learning (a) and extradimensional set shifting (b). Both PV- and SST-positive vHipp transplants abolish reversal learning deficits in MAM-treated rats. However, only PV- enriched transplants also reverse extra-dimensional set shifting deficits. Neither prenatal treatment nor cell transplants influenced the other stages of the attentional set shifting test (c). SD is simple discrimination. CD is compound discrimination. R1 is the first reversal. ID is intradimensional set shift. R2 is the second reversal. ED is extradimensional set shift. * demonstrates significant difference from saline-treated controls while + represents significant difference from MAM-treated animals that received dead cell transplants. n=8–9 per group.
DISCUSSION
In the current experiments we demonstrate that interneuron transplants, derived from embryonic stem cells, may be a viable treatment strategy to target negative and cognitive symptoms of schizophrenia, which are poorly treated by currently prescribed antipsychotic medications2. Previously, we have shown that interneuron transplants (derived from fetal tissue) can improve positive symptoms in the MAM model of schizophrenia17. This is consistent with data from a distinct model of schizophrenia, the cyclin D2 knock-out mouse, where Moore and colleagues also report that interneuron transplants, derived from fetal tissue, attenuate schizophrenia like deficits in amphetamine-induced locomotor activity20, suggesting that the beneficial effect of interneuron transplants is not model-specific. In the current experiments, we have expanded on these findings to show that embryonic stem cells, differentiated into specific interneuron populations, can improve function in the MAM model of schizophrenia. Importantly, the interneuron transplants improve function in behavioral correlates of negative and cognitive symptoms, two symptom domains that are currently poorly treated4.
The medial ganglionic eminence (MGE) gives rise to the majority of cortical PV- and SST-positive interneurons and the specification of MGE cortical interneurons has been well established (for review see18). The dorsal region of the MGE is enriched for SST-positive interneurons while more PV-positive interneurons are found in the ventral regions of MGE32, 33. This effect is thought to be due to sonic hedgehog (Shh) signaling, which occurs in a gradient in the MGE, with higher levels of Shh in the dorsal versus ventral areas of the MGE32. Sonic hedgehog (Shh) signaling is required for both the induction and maintenance of expression of the transcription factor, Nkx2.134. We have shown previously that higher levels of Shh can promote differentiation of SST versus PV interneurons21, 35. Therefore, in the current experiments, we used high versus low Shh in culture to promote differentiation of SST or PV interneurons, respectively. In addition to Shh signaling, the fate determination of cortical interneurons also depends on the time at which the neurons are born21, 33. SST interneurons are generally born earlier and occupy deeper layers of the cortex while PV interneurons originate later in neurogenesis and are found in more superficial cortical layers36. Because interneurons in the MGE go through a stereotyped developmental program in which Nkx2.1 is expressed at high levels in dividing progenitor cells until the time of cell cycle exit, at which point Nkx2.1 decreases and Lhx6 expression begins to increase and remains high, we used Nkx2.1 and Lhx6 expression to identify early versus late born interneurons37. Therefore, in the current experiments, we used a protocol to mimic the stereotyped pattern of SST- and PV-positive interneuron development to generate enriched populations of SST or PV interneurons.
Interestingly, we found that SST- and PV-enriched transplants had differential effects on behaviors related to schizophrenia. Given that both PV and SST interneuron transplants reversed vHipp hyperactivity, the differential behavioral effects of these transplants on extradimensional set shifting are likely associated with the differences in the unique anatomical, biochemical, and physiological characteristics of these two interneuron subtypes 38. For example, PV- and SST- positive interneurons use laminar targeting to synapse on distinct subcellular compartments of post-synaptic cells13. SST-positive cells primarily target dendrites, allowing this interneuron subtype to regulate synaptic plasticity and integrate synaptic inputs38. Conversely, PV-positive cells can regulate the timing and generation of action potentials by targeting the cell soma or axon initial segment 38. Further, PV- and SST-positive cells can be also distinguished by their firing patterns. PV-positive interneurons display a fast firing, non-adapting pattern 39 while SST cells show a non-fast firing, accommodating pattern 40. Indeed, we have shown previously that transplanted PV- and SST-positive interneurons, derived from embryonic stem cells, display firing patterns that resemble endogenous PV- and SST-positive interneurons 21.
Another way in which SST- and PV-positive interneuron transplants may differentially influence schizophrenia-like behaviors is via the regulation of unique neural pathways from the vHipp. The classic dopamine hypothesis of schizophrenia posits that hyperactivity in the mesolimbic dopamine system underlies the positive symptoms of the disorder41. In the current experiments, we demonstrate that both SST- and PV-positive interneuron transplants into the vHipp can reduce dopamine population activity in MAM-treated rats, which is likely a result of normalization of the characterized polysynaptic vHipp-VTA pathway5.
Reversal learning, a form of cognitive flexibility, is disrupted in schizophrenia patients 42. Reversal learning is thought to involve the orbitofrontal cortex 43, a brain region that is not robustly targeted by the vHipp. However, an association between dopamine system function and reversal learning has been previously demonstrated. For example, reversal learning performance is correlated with striatal dopamine synthesis capacity 44 and striatal D2 receptor availability 45. Although OFC-specific dopamine depletion has no effect 46, dopamine depletion at the level of the caudate nucleus impairs reversal learning 47. Further, both pharmacological agonism 48 and antagonism 49 of D2/D3 receptors can disrupt reversal learning, suggesting that this type of cognitive flexibility requires tightly controlled dopamine signaling. Thus, similar to the effects of interneuron transplants on positive symptoms17, 20, the beneficial effect of PV and SST transplants on reversal learning may be secondary to restoration of normal dopamine neuron activity.
Although both PV- and SST-positive transplants effectively normalized dopamine system function, latent inhibition, and reversal learning, only PV-positive transplants were also able to abolish deficits in social interaction and extradimensional set shifting. Interestingly, both social interaction and extradimensional set-shifting have been associated with the medial prefrontal cortex (mPFC), a brain region that receives direct projections from the vHipp 7 and that is dysregulated in schizophrenia patients 50. For example, elevating the balance between excitation and inhibition in the mPFC can impair social interaction, an effect that is normalized by restoring inhibitory signaling 9. In the current experiments, restoration of parvalbumin interneuron function in vHipp was also able to increase social interaction in MAM rats, suggesting that this interneuron type may normalize signaling between the vHipp and mPFC.
Extradimensional set-shifting is another form of cognitive flexibility that is disrupted in schizophrenia 51, 52. While reversal learning is associated with orbitofrontal activity and subcortical dopamine function, extradimensional set shifting, like social interaction, is more dependent on the mPFC 8. Indeed, mPFC lesions cause specific deficits in extradimensional set-shifting without influencing other forms of cognitive flexibility, such as reversal learning 8. In the current experiments, we demonstrate that only PV-positive interneuron transplants were able to reverse deficits in extradimensional set-shifting, which we posit is due to modulation of pyramidal cells that project directly to the mPFC 53. Together, our results demonstrate that PV- and SST-positive cells in the vHipp differentially influence schizophrenia-like behaviors and suggest that specifically targeting PV-positive interneurons in the vHipp may be a more effective treatment strategy to alleviate multiple symptom domains of schizophrenia.
In the current experiments, we have demonstrated that interneuron transplants may be an effective treatment strategy for schizophrenia. However, before this strategy can move into the clinic, it will be important to consider the timing of the transplant surgeries. In the current studies, cells were transplanted between postnatal day (PND) 40–45, a time point that has been determined to be post-puberty based on balano-preputial separation and circulating androgen levels 54. Using the MAM rodent model of schizophrenia, we have demonstrated previously that rats begin to show schizophrenia-like changes in behavior and physiology as early as PND 4055. Specifically, we found that PND 40 MAM rats show increased locomotor activity in response to amphetamine, a stable reduction of PV expression in the vHipp, and an increase in the number of spontaneously active dopamine cells in the VTA55. Studies in humans have demonstrated that poor prognosis is correlated with the time that schizophrenia is left untreated 56; therefore, we believe that to be most effective, stem cells should be transplanted as early in the course of the disease as possible. However, this early intervention strategy raises the question of long-term outcomes. Although we demonstrated that interneuron transplants attenuated schizophrenia-like changes in behavior and physiology when animals were tested >30 days post-transplantation, it remains unclear whether the cells would continue to have beneficial effects throughout the animal’s lifetime. A long-lasting therapeutic effect would depend first on the survival of the cells. In our experiments, we observed GFP+ interneurons up to 6 months after transplantation (data not shown). Further, in Parkinson’s Disease patients, ventral midbrain grafts derived from fetal tissue have been shown to survive in the brain for up to 14 years57. Although these results suggest that transplanted cells can survive in the brain for extended periods of time, future experiments will be required to determine if the transplanted interneurons remain viable throughout the lifetime of the rat and whether these surviving cells continue to produce therapeutic changes.
While most pharmacological interventions only target symptoms, cell-based therapies hold considerable promise for the cure of neurological disorders such as Parkinson’s Disease and Stroke 58. To date, very few groups have explored the potential of cell transplants in schizophrenia17, 20. Those groups that have tested the therapeutic benefit of interneuron transplants in schizophrenia have used interneuron precursors derived from fetal tissue17, 20. Here we provide the first preclinical evidence that PV-positive interneuron transplants, derived from embryonic stem cells, may effectively reduce not only positive but also negative and cognitive symptoms in an animal model of schizophrenia. Further, most literature focuses on positive symptoms of schizophrenia, which are already the target of currently prescribed antipsychotic medication2. Here, we demonstrate that interneuron transplants into the vHipp alleviate not only positive, but also negative and cognitive symptoms in the MAM rodent model. Together, our results provide insight into the role of PV- and SST-positive interneurons in the pathology of schizophrenia and offer an exciting new treatment strategy to target all symptom domains of this debilitating disease.
Supplementary Material
Acknowledgments
This work was supported by the Owens Foundation and by R01 MH090067 (DL) and R01 MH066912 (SA), and R01 DA032701 (MB) from the NIH. Cell sorting was performed by the Flow Cytometry Shared Resource Facility, supported by UTHSCSA, NIH-NCI P30 CA054174-20 (CTRC at UTHSCSA) and UL1 TR001120 (CTSA grant). We would like to acknowledge Jordan Thomas for his technical assistance.
Footnotes
CONFLICTS OF INTEREST
The authors have no conflicts of interest to disclose.
AUTHOR CONTRIBUTIONS JD and DL participated in research design. JD and SB conducted experiments. JT and SA contributed new reagents. JD, SB, MB, and DL performed the data analysis. All authors wrote or contributed to the writing of the manuscript.
Supplementary information is available at Molecular Psychiatry’s website.
References
- 1.Seeman P, Chau-Wong M, Tedesco J, Wong K. Brain receptors for antipsychotic drugs and dopamine: direct binding assays. Proceedings of the National Academy of Sciences of the United States of America. 1975;72(11):4376–4380. doi: 10.1073/pnas.72.11.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Leucht S, Cipriani A, Spineli L, Mavridis D, Örey D, Richter F, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. The Lancet. 2013;382(9896):951–962. doi: 10.1016/S0140-6736(13)60733-3. [DOI] [PubMed] [Google Scholar]
- 3.Strassnig MT, Raykov T, O’Gorman C, Bowie CR, Sabbag S, Durand D, et al. Determinants of different aspects of everyday outcome in schizophrenia: The roles of negative symptoms, cognition, and functional capacity. Schizophrenia research. 2015 doi: 10.1016/j.schres.2015.03.033. (0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Citrome L. Unmet needs in the treatment of schizophrenia: new targets to help different symptom domains. The Journal of clinical psychiatry. 2014;75(Suppl 1):21–26. doi: 10.4088/JCP.13049su1c.04. [DOI] [PubMed] [Google Scholar]
- 5.Lodge DJ, Grace AA. Hippocampal dysregulation of dopamine system function and the pathophysiology of schizophrenia. Trends Pharmacol Sci. 2011;32(9):507–513. doi: 10.1016/j.tips.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Legault M, Wise RA. Injections of N-methyl-D-aspartate into the ventral hippocampus increase extracellular dopamine in the ventral tegmental area and nucleus accumbens. Synapse. 1999;31(4):241–249. doi: 10.1002/(SICI)1098-2396(19990315)31:4<241::AID-SYN1>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 7.Rosene DL, Hoesen GWV. Hippocampal efferents reach widespread areas of cerebral cortex and amygdala in the rhesus monkey. Science. 1977;198(4314):315–317. doi: 10.1126/science.410102. [DOI] [PubMed] [Google Scholar]
- 8.Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000;20(11):4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O/’Shea DJ, et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature. 2011;477(7363):171–178. doi: 10.1038/nature10360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malaspina D, Storer S, Furman V, Esser P, Printz D, Berman A, et al. SPECT study of visual fixation in schizophrenia and comparison subjects. Biol Psychiatry. 1999;46(1):89–93. doi: 10.1016/s0006-3223(98)00306-0. [DOI] [PubMed] [Google Scholar]
- 11.Schobel SA, Lewandowski NM, Corcoran CM, et al. Differential targeting of the ca1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch Gen Psychiatry. 2009;66(9):938–946. doi: 10.1001/archgenpsychiatry.2009.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heckers S, Konradi C. GABAergic mechanisms of hippocampal hyperactivity in schizophrenia. Schizophrenia research. 2014 doi: 10.1016/j.schres.2014.09.041. (0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lewis DA, Hashimoto T, Volk DW. Cortical inhibitory neurons and schizophrenia. Nat Rev Neurosci. 2005;6(4):312–324. doi: 10.1038/nrn1648. [DOI] [PubMed] [Google Scholar]
- 14.Zhang ZJ, Reynolds GP. A selective decrease in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia. Schizophrenia research. 2002;55(1–2):1–10. doi: 10.1016/s0920-9964(01)00188-8. [DOI] [PubMed] [Google Scholar]
- 15.Konradi C, Yang CK, Zimmerman EI, Lohmann KM, Gresch P, Pantazopoulos H, et al. Hippocampal interneurons are abnormal in schizophrenia. Schizophrenia research. 2011;131(1–3):165–173. doi: 10.1016/j.schres.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Boley AM, Perez SM, Lodge DJ. A fundamental role for hippocampal parvalbumin in the dopamine hyperfunction associated with schizophrenia. Schizophrenia research. 2014;157(1–3):238–243. doi: 10.1016/j.schres.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perez SM, Lodge DJ. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol Psychiatry. 2013;18(11):1193–1198. doi: 10.1038/mp.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nat Rev Neurosci. 2006;7(9):687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- 19.Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128(19):3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- 20.Gilani AI, Chohan MO, Inan M, Schobel SA, Chaudhury NH, Paskewitz S, et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proceedings of the National Academy of Sciences. 2014;111(20):7450–7455. doi: 10.1073/pnas.1316488111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tyson JA, Goldberg EM, Maroof AM, Xu Q, Petros TJ, Anderson SA. Duration of culture and sonic hedgehog signaling differentially specify PV versus SST cortical interneuron fates from embryonic stem cells. Development. 2015;142(7):1267–1278. doi: 10.1242/dev.111526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lodge DJ, Grace AA. Gestational methylazoxymethanol acetate administration: a developmental disruption model of schizophrenia. Behavioral Brain Research. 2009;204(2):306–312. doi: 10.1016/j.bbr.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ranck JBJ. Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41(2):461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 24.Grace AA, Bunney BS. Intracellular and extracellular electrophysiology of nigral dopaminergic neurons—1. Identification and characterization. Neuroscience. 1983;10(2):301–315. doi: 10.1016/0306-4522(83)90135-5. [DOI] [PubMed] [Google Scholar]
- 25.Cecchi M, Khoshbouei H, Morilak DA. Modulatory effects of norepinephrine, acting on alpha1 receptors in the central nucleus of the amygdala, on behavioral and neuroendocrine responses to acute immobilization stress. Neuropharmacology. 2002;43(7):1139–1147. doi: 10.1016/s0028-3908(02)00292-7. [DOI] [PubMed] [Google Scholar]
- 26.Lapiz MD, Morilak DA. Noradrenergic modulation of cognitive function in rat medial prefrontal cortex as measured by attentional set shifting capability. Neuroscience. 2006;137(3):1039–1049. doi: 10.1016/j.neuroscience.2005.09.031. [DOI] [PubMed] [Google Scholar]
- 27.Lodge DJ, Grace AA. Aberrant Hippocampal Activity Underlies the Dopamine Dysregulation in an Animal Model of Schizophrenia. The Journal of Neuroscience. 2007;27(42):11424–11430. doi: 10.1523/JNEUROSCI.2847-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lodge DJ, Grace AA. The Hippocampus Modulates Dopamine Neuron Responsivity by Regulating the Intensity of Phasic Neuron Activation. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31(7):1356–1361. doi: 10.1038/sj.npp.1300963. [DOI] [PubMed] [Google Scholar]
- 29.Floresco SB, Todd CL, Grace AA. Glutamatergic Afferents from the Hippocampus to the Nucleus Accumbens Regulate Activity of Ventral Tegmental Area Dopamine Neurons. The Journal of Neuroscience. 2001;21(13):4915–4922. doi: 10.1523/JNEUROSCI.21-13-04915.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pantelis C, Barber FZ, Barnes TRE, Nelson HE, Owen AM, Robbins TW. Comparison of set-shifting ability in patients with chronic schizophrenia and frontal lobe damage. Schizophrenia research. 1999;37(3):251–270. doi: 10.1016/s0920-9964(98)00156-x. [DOI] [PubMed] [Google Scholar]
- 31.Gastambide F, Cotel M-C, Gilmour G, O’Neill MJ, Robbins TW, Tricklebank MD. Selective Remediation of Reversal Learning Deficits in the Neurodevelopmental MAM Model of Schizophrenia by a Novel mGlu5 Positive Allosteric Modulator. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2012;37(4):1057–1066. doi: 10.1038/npp.2011.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wonders CP, Welagen J, Taylor L, Mbata IC, Xiang JZ, Anderson SA. A spatial bias for the origins of interneuron subgroups within the medial ganglionic eminence. Dev Biol. 2008;314(1):127–136. doi: 10.1016/j.ydbio.2007.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Inan M, Welagen J, Anderson SA. Spatial and Temporal Bias in the Mitotic Origins of Somatostatin- and Parvalbumin-Expressing Interneuron Subgroups and the Chandelier Subtype in the Medial Ganglionic Eminence. Cereb Cortex. 2012;22(4):820–827. doi: 10.1093/cercor/bhr148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Q, Wonders CP, Anderson SA. Sonic hedgehog maintains the identity of cortical interneuron progenitors in the ventral telencephalon. Development. 2005;132(22):4987–4998. doi: 10.1242/dev.02090. [DOI] [PubMed] [Google Scholar]
- 35.Xu Q, Guo L, Moore H, Waclaw RR, Campbell K, Anderson SA. Sonic Hedgehog Signaling Confers Ventral Telencephalic Progenitors with Distinct Cortical Interneuron Fates. Neuron. 2010;65(3):328–340. doi: 10.1016/j.neuron.2010.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.DeBoer EM, Anderson SA. Fate determination of cerebral cortical GABAergic interneurons and their derivation from stem cells. Brain Res. doi: 10.1016/j.brainres.2015.12.031. [DOI] [PubMed] [Google Scholar]
- 37.Tyson JA, Anderson SA. The protracted maturation of human ESC-derived interneurons. Cell cycle (Georgetown, Tex) 2013;12(19):3129–3130. doi: 10.4161/cc.26351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5(10):793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- 39.Kawaguchi Y. Physiological subgroups of nonpyramidal cells with specific morphological characteristics in layer II/III of rat frontal cortex. The Journal of Neuroscience. 1995;15(4):2638–2655. doi: 10.1523/JNEUROSCI.15-04-02638.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kawaguchi Y, Kubota Y. Physiological and morphological identification of somatostatin- or vasoactive intestinal polypeptide-containing cells among GABAergic cell subtypes in rat frontal cortex. The Journal of Neuroscience. 1996;16(8):2701–2715. doi: 10.1523/JNEUROSCI.16-08-02701.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Davis KL, Kahn RS, Ko G, Davidson M. Dopamine in schizophrenia: a review and reconceptualization. Am J Psychiatry. 1991;148(11):1474–1486. doi: 10.1176/ajp.148.11.1474. [DOI] [PubMed] [Google Scholar]
- 42.Kehagia AA, Murray GK, Robbins TW. Learning and cognitive flexibility: frontostriatal function and monoaminergic modulation. Curr Opin Neurobiol. 2010;20(2):199–204. doi: 10.1016/j.conb.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 43.Dias R, Robbins TW, Roberts AC. Dissociation in prefrontal cortex of affective and attentional shifts. Nature. 1996;380(6569):69–72. doi: 10.1038/380069a0. [DOI] [PubMed] [Google Scholar]
- 44.Cools R, Frank MJ, Gibbs SE, Miyakawa A, Jagust W, D’Esposito M. Striatal dopamine predicts outcome-specific reversal learning and its sensitivity to dopaminergic drug administration. The Journal of Neuroscience. 2009;29(5):1538–1543. doi: 10.1523/JNEUROSCI.4467-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jocham G, Klein TA, Neumann J, von Cramon DY, Reuter M, Ullsperger M. Dopamine DRD2 polymorphism alters reversal learning and associated neural activity. The Journal of Neuroscience. 2009;29(12):3695–3704. doi: 10.1523/JNEUROSCI.5195-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clarke HF, Walker SC, Dalley JW, Robbins TW, Roberts AC. Cognitive inflexibility after prefrontal serotonin depletion is behaviorally and neurochemically specific. Cereb Cortex. 2007;17(1):18–27. doi: 10.1093/cercor/bhj120. [DOI] [PubMed] [Google Scholar]
- 47.Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. The Journal of Neuroscience. 2011;31(11):4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boulougouris V, Castañé A, Robbins T. Dopamine D2/D3 receptor agonist quinpirole impairs spatial reversal learning in rats: investigation of D3 receptor involvement in persistent behavior. Psychopharmacology. 2009;202(4):611–620. doi: 10.1007/s00213-008-1341-2. [DOI] [PubMed] [Google Scholar]
- 49.Lee B, Groman S, London ED, Jentsch JD. Dopamine D2/D3 receptors play a specific role in the reversal of a learned visual discrimination in monkeys. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2007;32(10):2125–2134. doi: 10.1038/sj.npp.1301337. [DOI] [PubMed] [Google Scholar]
- 50.Bunney WE, Bunney BG. Evidence for a compromised dorsolateral prefrontal cortical parallel circuit in schizophrenia. Brain Res Rev. 2000;31(2–3):138–146. doi: 10.1016/s0165-0173(99)00031-4. [DOI] [PubMed] [Google Scholar]
- 51.Tyson PJ, Laws KR, Roberts KH, Mortimer AM. Stability of set-shifting and planning abilities in patients with schizophrenia. Psychiatry research. 2004;129(3):229–239. doi: 10.1016/j.psychres.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 52.Leeson VC, Robbins TW, Matheson E, Hutton SB, Ron MA, Barnes TRE, et al. Discrimination learning, reversal, and set-shifting in first-episode schizophrenia: Stability over six years and specific associations with medication type and disorganization syndrome. Biol Psychiatry. 2009;66(6):586–593. doi: 10.1016/j.biopsych.2009.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jay TM, Witter MP. Distribution of hippocampal CA1 and subicular efferents in the prefrontal cortex of the rat studied by means of anterograde transport of Phaseolus vulgaris-leucoagglutinin. J Comp Neurol. 1991;313(4):574–586. doi: 10.1002/cne.903130404. [DOI] [PubMed] [Google Scholar]
- 54.Korenbrot CC, Huhtaniemi IT, Weiner RI. Preputial Separation as an External Sign of Pubertal Development in the Male Rat. Biol Reprod. 1977;17(2):298–303. doi: 10.1095/biolreprod17.2.298. [DOI] [PubMed] [Google Scholar]
- 55.Chen L, Perez SM, Lodge DJ. An augmented dopamine system function is present prior to puberty in the methylazoxymethanol acetate rodent model of schizophrenia. Developmental Neurobiology. 2014;74(9):907–917. doi: 10.1002/dneu.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perkins DO, Gu H, Boteva K, Lieberman JA. Relationship Between Duration of Untreated Psychosis and Outcome in First-Episode Schizophrenia: A Critical Review and Meta-Analysis. Am J Psychiatry. 2005;162(10):1785–1804. doi: 10.1176/appi.ajp.162.10.1785. [DOI] [PubMed] [Google Scholar]
- 57.Mendez I, Vinuela A, Astradsson A, Mukhida K, Hallett P, Robertson H, et al. Dopamine neurons implanted into people with Parkinson’s disease survive without pathology for 14 years. Nat Med. 2008;14(5):507–509. doi: 10.1038/nm1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Steinbeck Julius A, Studer L. Moving Stem Cells to the Clinic: Potential and Limitations for Brain Repair. Neuron. 2015;86(1):187–206. doi: 10.1016/j.neuron.2015.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.