Abstract
Background
Corneal neovascularization increases the risk of T cell–mediated allograft rejection. Here, we investigate whether T cells promote angiogenesis in transplantation.
Methods
Conventional effector T cells were collected from draining lymph nodes of allogeneic or syngeneic corneal transplanted BALB/c mice. T cells were either cocultured with vascular endothelial cells (VECs) to assess VEC proliferation or used in a mixed lymphocyte reaction assay. Messenger RNA (mRNA) expression of vascular endothelial growth factor (VEGF)-A, -C, and VEGF receptor 2 (VEGF-R2) in VECs was assessed by real-time PCR. VEGF-A protein expression was determined by enzyme-linked immunosorbent assay. Flow cytometry was used to analyze VEGF-R2 expression in corneal CD31+ cells, and VEGF-A and IFNγ expression in corneal CD4+ T cells.
Results
Allogeneic T cells from high-risk (HR) grafted mice induced more VEC proliferation than those from syngeneic transplant recipients (P = 0.03). Vascular endothelial growth factor-A mRNA and protein expression were higher in T cells from draining lymph nodes (P = 0.03 and P = 0.04, respectively) and cornea (protein; P = 0.04) of HR compared with low-risk (LR) grafted hosts. Vascular endothelial growth factor-A, VEGF-C, and VEGF-R2 mRNA expression were increased in VECs when cocultured with T cells from HR transplants compared with LR transplants and naive mice. In addition, IFNγ blockade in T cell/VEC coculture increased VEC proliferation and VEGF-A protein expression, whereas blocking VEGF-A significantly reduced VEC proliferation (P = 0.04).
Conclusions
Allogeneic T cells from corneal transplant hosts promote VEC proliferation, probably via VEGF-A signaling, whereas IFNγ shows an antiangiogenic effect. Our data suggest that T cells are critical mediators of angiogenesis in transplantation.
Corneal transplantation is the most common form of human solid tissue transplantation,1,2 with over 100 000 cases reported annually worldwide.3 Corneal allotransplantation does not ordinarily require systemic or permanent immunosuppression or HLA tissue matching,1,3,4 but allograft rejection causing corneal graft failure continues to be an obstacle to transplant success.5–7 When performed in nonvascularized and uninflamed host beds, termed low-risk (LR) transplantation, graft survival rates are over 90% under topical corticosteroid therapy. In contrast, graft rejection rates dramatically increase to near 50% when transplants are placed into inflamed and vascularized host beds, termed high-risk (HR) transplants, despite maximal immune suppressive therapy.1,3,4 These outcomes are worse than grafts of kidney, heart, or liver.5–7
Host bed vascularity is a principal risk factor for allograft rejection because blood vessels are critical for delivery of immune effector cells to the graft site,8 particularly T helper 1 (Th1) cells, the principal mediators of graft rejection in corneal transplantation.9 The normal cornea is devoid of blood and lymphatic vessels and actively maintains a state of “angiogenic privilege.” In LR transplantation, transient vascular engorgement or vascular sprouting in the limbus is quickly extinguished. In contrast, grafting onto HR vascularized and inflamed host beds often leads to increased angiogenesis which further increases the risk of graft rejection.10 Numerous studies have demonstrated that the innate immune system contributes to angiogenesis in corneal transplantation, particularly through the actions of macrophages.11–14 In addition, several studies have outlined the effect of T cells in inducing tumor-related angiogenesis.15 However, in transplantation, although the function of blood vessels in facilitating T cell-mediated immunity has been appreciated, very little is known whether T cells themselves can promote or regulate angiogenesis.9 Here, we hypothesized that T cells derived from inflamed HR transplant hosts disrupt angiogenic privilege through increased expression of proangiogenic factors. The vascular endothelial growth factor (VEGF) family controls angiogenesis and targeting VEGF-A in LR and HR corneal transplantation has been shown to reduce angiogenesis and improve graft survival.10 In this study, we investigated the proangiogenic effect of T cells on vascular endothelial cell (VEC) proliferation and show a direct effect of CD4+ conventional T cells (conv T cells) on VEC proliferation through increased VEGF expression.
MATERIALS AND METHODS
Animals
Male C57BL/6 and BALB/c mice 6 to 8 weeks of age were obtained from Charles River Laboratories (Wilmington, MA). Mice were housed in the Schepens Eye Research Institute animal vivarium and treated according to the guidelines set forth by the Association for Research in Vision and Ophthalmology. All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee.
Corneal Transplantation
Syngeneic (BALB/c to BALB/c) and allogeneic (C57BL/6 to BALB/c) orthotopic corneal transplantation was performed as described previously.16 Briefly, in LR transplantation, 2 mm diameter donor corneal buttons from C57BL/6 mice were affixed to 1.5 mm diameter avascular and uninflamed BALB/c host beds via 8 interrupted 11-0 nylon sutures. Inflamed and vascularized HR host beds were created by placing 3 intrastromal sutures 14 days before transplantation in BALB/c mice as described previously.16 After surgery, host eyelids were closed for 3 days via tarsorrhaphy and interrupted corneal sutures were removed 7 days after surgery. Corneal allografts were evaluated by slit lamp microscopy and graft clarity was scored according to a well-established 0 to 5+ scale, with scores of 2+ considered rejected.17 To exclude grafts undergoing primary failure, only those grafts with scores under 1 at 14 days after transplantation were used for experimentation.
T Cell Sorting
Ipsilateral draining lymph nodes were harvested from HR and LR mice. Single cell suspensions were created by homogenizing lymphoid tissue in 70-μm cell strainers. CD4+CD25− T cells were magnetically sorted using a mouse T cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany) and stimulated for 12 hours at 37°C and 5% CO2 with purified anti-mouse CD3ε antibody (1 μg/mL; Biolegend, San Diego, CA) in complete Dulbecco modified Eagle medium (DMEM).
Cell Culture
A mouse endothelial cell (EC) line, MILE SVEN-1 (MS-1) (a kind gift from Dr. Patricia D’Amore, Boston, MA, and originally purchased at ATCC, Manassas, VA),18 was used in our experiments. We evaluated these cells for their surface expression of VEGF-R2 by flow cytometry to assure their EC characteristics before performing experiments. Cells were cultured in DMEM (Lonza, Basel, Swiss) with 10% (v/v) fetal bovine serum (Invitrogen, Carlsbad, CA). At day 0, 1 × 104 MS-1 ECs were seeded in the lower compartment of a 96 PET transwell system (6.5 mm insert size, 1 μm pore size; Corning). At day 1, when the MS-1 cells covered approximately 30% of the lower wells, 1 × 105 CD4+CD25− anti-CD3ε stimulated T cells were placed in the upper compartment of the 96 PET transwell system and cocultured in serum-free DMEM medium with ECs. After 24 to 30 hours of coculture, EC proliferation was assessed using the bromodoxyuridine (BrdU) incorporation assay (Millipore, Billerica, MA) and a spectrophotometer microplate reader (PerkinElmer, Waltham, MA). MILE SVEN-1 cells cultured in DMEM only served as a negative control, and MS-1 cells treated with 20 ng/mL recombinant VEGF-A (Biolegend) were used as a positive control.
Mixed Lymphocyte Reaction
Purified allogeneic T cells (1 × 105) were isolated from syngeneic and allogeneic HR and LR graft recipients and MACS-sorted for CD90.2 (Miltenyi Biotec), according to the manufacturer’s instructions. Isolated T cells were cocultured with BALB/c APCs (1 × 105) and alloantigens from C57BL/6 spleen (cell lysate was generated by sonicating spleen cells from a naive C57BL/6 mouse and underwent a freeze-thaw cycle) for 72 hours in 96-well, round-bottom plates. Bromodoxyuridine reagent (Sigma-Aldrich) was added to each well, 16 hours before collecting the cells. The proliferation of T cells was measured by using the BrdU incorporation assay kit (Millipore), according to the manufacturer’s instructions.
Flow Cytometry
At day 14 after corneal transplantation, the ipsilateral cervical lymphoid tissue was collected from allogeneic and syngeneic HR and LR transplant recipients. Single cell suspensions were prepared by homogenizing lymphoid tissue in 70-μm cell strainers. Grafts and host corneal beds were collected 21 days posttransplantation and digested with DNase (0.2 mg/mL; Roche, Basel, Switzerland) and collagenase (0.4 mg/mL, Roche) to make single cell suspensions and were incubated for 4 hours at 37°C with 0.1% Golgistop (BD Biosciences, USA) to prevent cellular release of VEGF-A. All cell suspensions were incubated with an Fc-blocking agent (R&D Systems, Minneapolis, MN) before surface antibody staining. Corneal cells were stained with BV421 anti-CD4 (Biolegend) and Alexa Fluor488 anti-VEGF-A (Bioss, Woburn, MA) antibodies. To determine VEGFR2 expression on ECs in the cornea, we stained single cell suspensions with APC-conjugated anti-VEGFR2 (R&D Systems) and PE-conjugated anti-CD31 (eBiosciences). Lymph node cells were stimulated with phorbol 12-myristate 13-acetate (Sigma Aldrich, St Louis, MO) and ionomycin (Sigma Aldrich) in the presence of Golgi-stop (BD Biosciences, USA) for 5 hours at 37°C and 5% CO2, and then stained with FITC-conjugated anti-CD4 antibody (Biolegend). Cells were fixed and permeabilized overnight and stained with APC-conjugated anti-IFNγ antibody (Biolegend). MILE SVEN-1 VECs were analyzed after coculture with CD4 T cells and stained with APC conjugated anti-VEGF-R2 (R&D Systems). Data were analyzed using a BD LSR II flow cytometer (BD Biosciences) and Summit v4.3 software (DAKO Corporation, Carpinteria, CA).
RNA Isolation, Reverse Transcription PCR, and Quantitative Real-Time PCR
Anti-CD3ε stimulated conv T cells and VECs were collected and their RNA was isolated using the RNeasy micro kit (Qiagen, Valencia, CA). The RNA was then reverse-transcribed using the Quantitec reverse transcription kit (Qiagen) with a Taqman Universal PCR Master Mix (Applied Biosystems, Carlsbad, CA). Quantitative real-time PCR was performed using a Taq Man-Mastermix and preformulated primers (Life Technologies) for murine IFNγ (Mm01168134_m1), VEGF-A (Mm01281449_m1), VEGF-C (Mm00437310_m1), VEGF-R2 (Mm01222421_m1), bFGF (Mm01285715_m1), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; Mm99999915_gl). Comparative threshold (CT) values were measured using a LightCycler 480 II System (Roche, Indianapolis, IN) and target CT values were normalized to the CT value of GAPDH, which served as an endogenous control. The fold change of messenger RNA (mRNA) levels relative to control groups was then calculated.
Enzyme-linked Immunosorbent Assay
The VEGF-A protein expression was measured in the supernatants of cocultured conv T cells and VECs using an enzyme-linked immunosorbent assay (ELISA) assay according to manufacturer’s instructions (Mouse VEGF-A Platinum ELISA kit, eBioscience).
In Vitro IFNγ Stimulation
Proliferation of MS-1 VECs stimulated with 20 ng/mL VEGF-A and treated with different doses of recombinant IFNγ (1, 100, 500 ng/mL) (Prepotech, Rock Hill, NJ) or 10 ng/mL of recombinant IL-1β (Biolegend) (as a positive control) was assessed using the BrdU incorporation assay (Millipore).
In Vitro Neutralization
A neutralizing anti-IFNγ antibody (1 mg/mL; LEAF purified antimouse IFNγ antibody; Biolegend) or an anti–VEGF-A antibody (Biolegend) and their respective isotype control antibodies (anti-IFNγ: LEAF purified armenian Hamster IgG isotype ctrl antibody, Biolegend; anti-VEGF-A: LEAF purified rat IgG2a, k isotype Ctrl, Biolegend) were used for in vitro blockade of IFNγ or VEGF-A, respectively, in our culture system with T cells and VECs. After 24 hours of culture, VECs proliferation was assessed using the BrdU incorporation assay (Millipore). After anti-IFNγ treatment, we also assessed VEGF-A expression by ELISA.
Statistical Analysis
Statistical differences were evaluated using Prism 5.0 software (Prism 5.0 software; GraphPad Software, Inc., La Jolla, CA). Student t test was used to analyze differences between 2 groups. Data were expressed as the mean ± SEM of at least 2 independent experiments. A P value less than 0.05 was considered significant (*P < 0.05; **P < 0.01; ***P < 0.001).
RESULTS
Alloprimed T Cells From Transplanted Mice Induce VEC Proliferation
We evaluated the effect of allogeneic and syngeneic T cells isolated from naive, HR, and LR transplanted mice on inducing VEC proliferation in vitro. We compared the proangiogenic effect of conv T cells collected from HR versus LR transplant hosts using a coculture system with a VEC line (MS-1). We found that allogeneic but not syngeneic conv T cells from HR transplanted mice induced more VEC proliferation than those from LR grafted and naïve mice (HR allogeneic vs syngeneic: P = 0.03; Figure 1A). To verify that this effect depends on the alloreactivity of T cells, we performed mixed lymphocyte reaction with T cells from syngeneic and allogeneic transplanted mice. Only allogeneic T cells were able to promote T-cell proliferation but not syngeneic T cells (HR allogeneic vs syngeneic: P = 0.008; Figure 1B).
FIGURE 1.
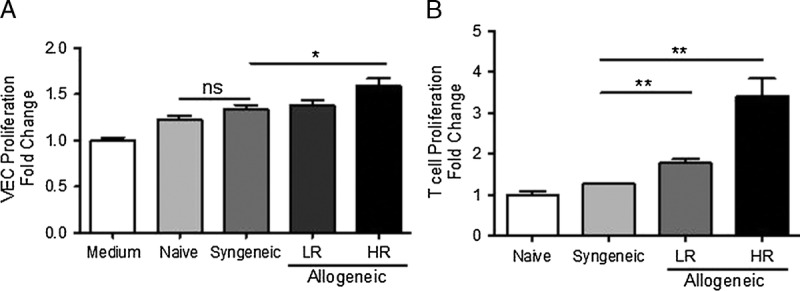
Alloprimed T cells from transplanted mice induce vascular endothelial cell proliferation. A, VECs were cultured alone (medium), or with allogeneic or syngeneic T cells collected from naive, LR, or HR transplanted mice. After 24 hours, the proliferation of VECs was assessed using the BrdU incorporation assay. B, Purified, allogeneic or syngeneic T cells were cocultured with APCs for 72 hours. BrdU reagent was added to each well 16 hours before collecting the cells, and T cell proliferation was measured. Each experiment has been performed at least 2 times, with 4 mice per group per experiment.
Alloprimed T Cells Release Proangiogenic VEGF-A
We next found that VEGF-A mRNA expression was increased in conv T cells recovered from HR recipients compared with T cells collected from LR recipients and naive mice (HR 3.4 ± 0.2 l vs LR 1.7 ± 0.43, P = 0.03; HR vs naive 1.09 ± 0.23, P = 0.003; Figure 2A). We additionally confirmed that this increase also occurs at the protein level, because VEGF-A expression was increased in the supernatant of cultured T cells from HR but not LR graft recipients or naive mice (HR 18 ± 1.7 vs LR 9.8 ± 2.0 P = 0.04; HR vs naïve 9 ± 0.9, P = 0.01; Figure 2B). Analyzing VEGF-A expression in transplanted corneas showed a significant increase in VEGF-A expression in CD4 T cells isolated from HR and LR transplanted grafts (P = 0.04; Figures 2C and D).
FIGURE 2.
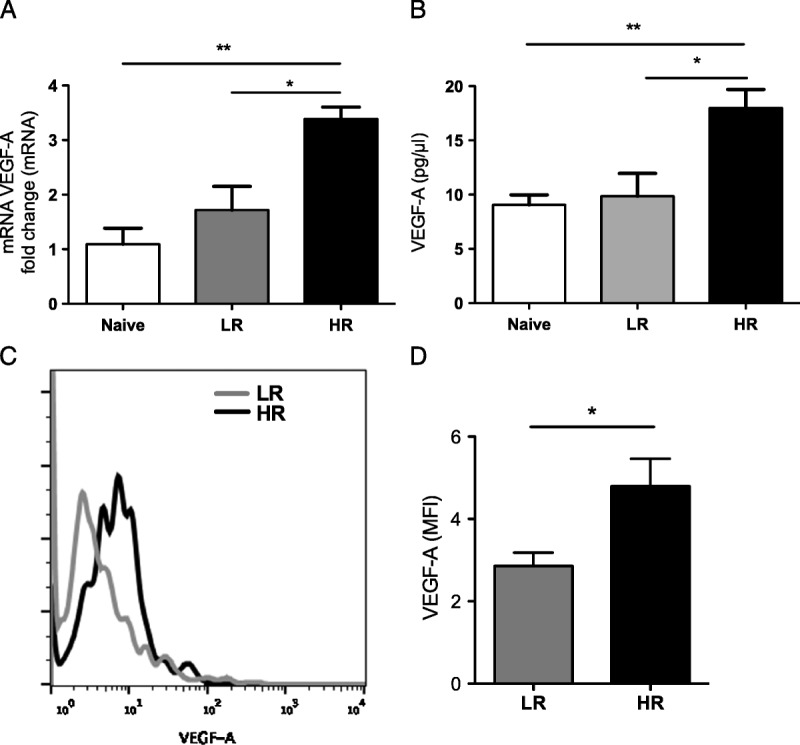
Alloprimed T cells from high-risk mice release increased proangiogenic VEGF-A. A, T cells from naïve, LR, HR transplanted mice were stimulated with an anti-CD3 antibody for 12 hours. VEGF-A mRNA expression of T cells was assessed using real-time PCR and (B) VEGF-A protein concentration was detected in the culture supernatant using ELISA. Each experiment has been performed at least 3 times, with 10 mice per group per experiment. (C and D) Protein expression (median fluorescence intensity, MFI) of VEGF-A in corneal CD4 T cells isolated from HR and LR graft recipients 21 days posttransplantation was analyzed by flow cytometry (P = 0.04; n = 4/group).
We also assessed bFGF mRNA expression in T cells, IL-1β concentration in the supernatant of cultured T cells (using ELISA), and IL-17 expression in T cells (using flow cytometry) from HR and LR recipients and found no difference between both groups (Fig. S1, SDC, http://links.lww.com/TP/B320).
VEGF-A, VEGF-C, and VEGF-R2 Are Increased in VECs
We next analyzed the mRNA expression of VEGF-A, VEGF-C, and VEGF-R2 in VECs after culturing them with T cells sorted from HR and LR grafted mice. T cells from HR and LR recipient mice induced VEGF-A, VEGF-C, and VEGF-R2 expression in VECs compared with VECs cultured in medium alone (VEGF-A: HR 6.3 ± 1.13 vs medium 1.1 ± 0.2, P = 0.047; LR 2.47 ± 0.05 vs medium P = 0.047; VEGF-C: HR 67.96 ± 23.35 vs medium 1.8 ± 1.5, P = 0.048; LR 12.74 ± 6.57 vs medium P = 0.18; VEGF-R2: HR 3.3 ± 0.73 vs medium 0.88 ± 0.03, P = 0.01; LR 1.36 ± 0.09 vs medium, P = 0.0006; Figures 3A-C). The VEGF-A, VEGF-C, and VEGF-R2 mRNA expression in VECs was highest in VECs cocultured with HR T cells compared with LR cells (VEGF-A, HR 6.3 ± 1.13 vs LR 2.47 ± 0.5 P = 0.008; VEGF-C: HR 67.9 ± 23.3, LR 12.7 ± 6.5, P = 0.08; VEGFR-2: HR 3.3 ± 0.73; LR 1.3 ± 0.09, P = 0.049). Frequencies of VEGF-R2hi VECs were similar after culture with T cells from HR vs. LR grafted hosts (data not shown). When we analyzed VEGFR2 protein expression levels on CD31+ ECs in the cornea posttransplantation, we found a statistically significant increase in VEGFR2 expression in ECs (CD31+) in HR compared with LR transplanted corneas (P < 0.0001; Figures 3D and E).
FIGURE 3.
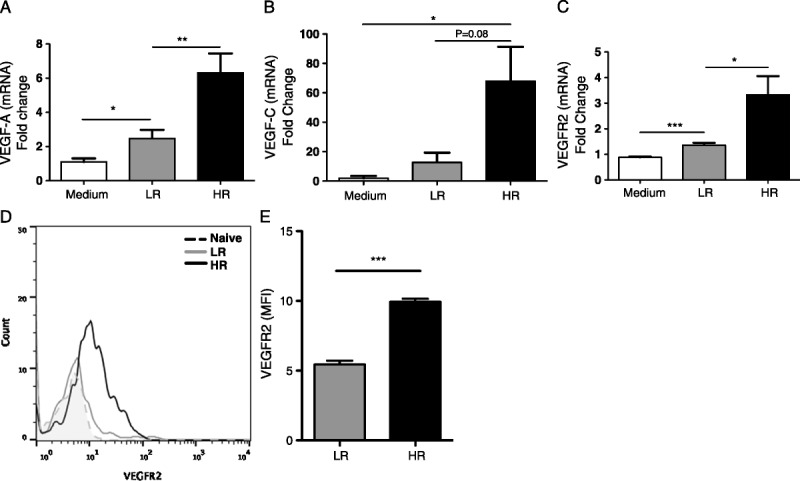
VEGF-A, VEGF-C, and VEGF-R2 are increased in vascular endothelial cells. VECs were cultured alone (medium), or with T cells collected from LR or HR transplanted mice. mRNA expression of (A) VEGF-A, (B) VEGF-C, and (C) VEGF-Receptor 2 (VEGF-R2) was determined in VECs 24 hours after coculture. Each real-time analysis has been performed at least 3 times, with 10 mice per group per experiment. D and E, Flow cytometry analysis showing VEGF-R2 expression by CD31+ endothelial cells in the cornea of HR and LR transplanted mice 21 days postsurgery (P < 0.0001; n = 4/group).
HR Transplantation Promotes Th1 Cells and IFNY Expression in the Lymph Nodes
T helper 1 cells are the principal mediators of acute corneal graft rejection; thus, we assessed their frequencies in draining lymph nodes and their expression of IFNγ before and 14 days posttransplantation. The frequencies of CD4+IFNγ+ T cells were increased in HR compared with LR transplanted mice (P = 0.008; Figure 4A). Moreover, the expression level (median fluorescence intensity) of IFNγ in conv T cells was increased in HR compared with LR transplant recipients and naive mice (P = 0.03; Figure 4B).
FIGURE 4.
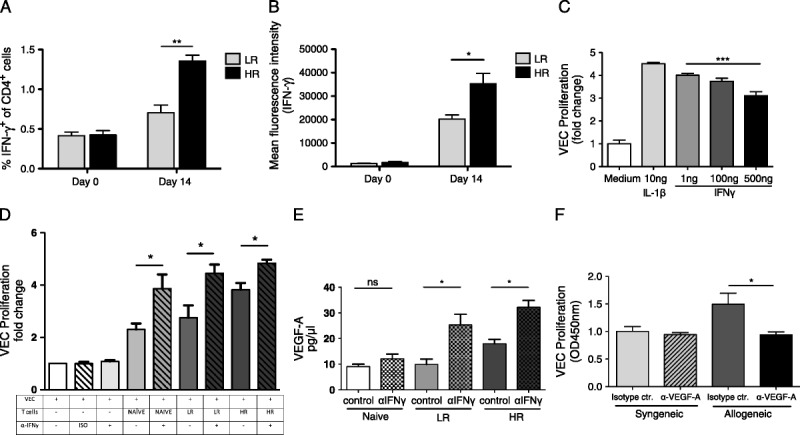
Blocking IFNγ increases vascular endothelial cell proliferation. A and B, Before surgery (day 0) and 14 days after LR and HR corneal transplantation, draining lymph nodes were isolated and analyzed for (A) frequencies of IFNγ-producing T cells and (B) protein expression (MFI) of IFNγ in CD4 T cells. C, VECs were cultured in DMEM and stimulated with VEGF-A (20 ng/mL). Different concentrations of IFNγ (1, 100, 500 ng/mL) and IL-1β (10 ng/mL) were added for 24 hours. VEC proliferation was assessed using the BrdU incorporation assay. D, VECs were cultured in DMEM only, or with T cells from naïve, LR, or HR mice and treated with an anti-IFNγ or isotype control antibody as indicated. 24 hours later VEC proliferation was assessed using the BrdU incorporation assay. E, VEGF-A protein expression was assessed in the supernatant after culture using ELISA. F, Syngeneic and allogeneic T cells were cocultured with VECs and treated with anti-VEGF-A or isotype control for 24 hours. VEC proliferation was assessed using the BrdU incorporation assay. Each experiment has been performed at least 3 times, with 10 mice per group per experiment.
VEC Proliferation Is Increased with IFNY Blockade and Decreased With VEGF Blockade
To investigate the antiproliferative effect of IFNγ on VECs, we treated them with different doses of IFNγ and added IL-1β as a positive control. Addition of IL-1β significantly upregulated VEC proliferation as shown before (P = 0.003; Figure 4C).19 High IFNγ concentrations significantly reduced VEC proliferation compared with low IFNγ concentrations (P = 0.0006; Figure 4C). Next, we cultured VECs with T cells from HR versus LR grafted mice with or without blocking IFNγ. We found that VEC proliferation further increased when we blocked IFNγ (naive 1 ± 0.04 naive + αIFNγ 2.3 ± 0.2, P = 0.038; LR 2.7 ± 0.46, LR + αIFNγ 4.4 ± 0.33 P = 0.04; HR 3.8 ± 0.26, HR + αIFNγ 4.8 ± 0.1 P = 0.03; Figure 4D). Next, we measured VEGF-A protein expression in our coculture of conv T cells and VECs and found a significant increase in VEGF-A in LR and HR groups treated with anti-IFNγ antibody compared to untreated groups (naive: 12.17 ± 1.7 vs 9.066 ± 0.09, P = 0.2; LR: 25.32 ± 4.1 vs 9.861 ± 2.093, P = 0.03; HR: 32.22 ± 2.6 vs 17.99 ± 1.7, P = 0.01; Figure 4E). Finally, we blocked VEGF-A signaling in our T and VEC coculture and found that VEC proliferation was inhibited after treatment with anti-VEGF-A (P = 0.04; Figure 4F).
DISCUSSION
Angiogenesis represents a key process in transplant rejection in the liver,20 kidney,21,22 lung,23 and cornea24 and serves as a key risk factor for allorejection.6 Although the contribution of innate immunity to angiogenesis has been well established,3,5,7,11,25,26 little is known about the role of T cell–mediated adaptive immunity in regulating the development of neovessels. A recent study has reported that a decrease in antiangiogenic signals in the cornea is mediated by graft-infiltrating allospecific T cells through reduction of corneal endostatin, a broad inhibitor of angiogenic factors.9 In the current study, we report that alloreactive T cells from transplanted mice directly promote the proliferation of VECs in vitro, and that these T cells express proangiogenic VEGF-A, the main mediator of VEC proliferation and migration. Further, our data suggest that alloprimed T cells also promote angiogenesis by inducing VEC expression of VEGF-A and C.
Transplantation into an inflamed, or “high risk” host bed increases the chance of graft rejection.27 Corneal neovascularization induced after chemical burns and posttrauma is primarily mediated by the innate immune system, and this angiogenic response facilitates healing and regeneration, and later spontaneously regresses.28,29 When corneal transplantation is performed onto an inflamed bed, this early phase of neovascularization is followed by a later and more invasive neovascularization which promotes allograft rejection. Because alloreactive T cells produce VEGF-A and significantly increase VEC proliferation, we hypothesize that this second graft-threatening phase of neovascularization is mediated, at least in part by alloreactive T cells.
Here, we show that performing transplantation in an inflamed host bed leads to increased VEGF-A secretion by lymphoid (Figure 2B) and corneal T cells (Figures 2C and D), and these “high-risk” T cells promote VEC proliferation and enhanced VEGF signaling in a coculture system. VECs cocultured with alloreactive T cells, especially from HR graft recipients show increased mRNA VEGF-R2 expression compared to VECs cocultured with T cells from LR transplant recipients (Figure 3C). However, flow cytometry analysis shows only a modest increase in the frequencies of VEGFR2+ VECs cultured with T cells from HR transplants (data not shown). Analyzing VEGFR2 expression in corneal ECs after transplantation shows significantly increased VEGFR2 expression in HR versus LR transplanted corneas (Figures 3D and E). In addition, blockade of VEGF-A in our culture system reduces VEC proliferation to a level seen in VECs cultured with syngeneic T cells (Figure 4F), suggesting a crucial role for VEGF-A in T cell–mediated angiogenesis. Autocrine VEGF-A secretion and signaling via VEGF-R2 on VECs is crucial for their vascular homeostasis and survival.30,31 It has also been shown that ECs upregulate VEGF-A production under stress, such as hypoxia or inflammation. These findings suggest that an autocrine pathway may amplify the paracrine effects of VEGF-A in inducing angiogenesis.32 Thus, in the aggregate, our data suggest that alloprimed T cells from HR grafted hosts can mediate neovascularization through increased VEGF-A expression, which is a critical factor in the mechanisms of allograft rejection; indeed, increased graft survival has been reported after in vivo neutralization of VEGF.10,19,33
Angiogenesis is a complex process requiring numerous interactions between proangiogenic and antiangiogenic signals. Although most scientists agree that VEGF-A is the dominant member of the family, other members expressed by T cells may also promote angiogenesis. For example, fibroblast growth factor (FGF) was first investigated for its role in angiogenesis in the 1980s,34 and FGF-2 (also named bFGF) has been shown to have a proangiogenic role in the corneal stroma of mice.35,36 In addition, in a previous study, we have shown that IL-1 induces expression of VEGF-A in corneal stromal fibroblasts, and thus promotes angiogenesis in the cornea.37 Finally, it has been reported that IL-17 can promote migration and tube formation in an in vitro model of age related macular degeneration, as well as in cancer-related angiogenesis.38,39 However, FGF-2, IL-1, and IL-17 expression was not increased in T cells isolated from HR versus LR transplanted recipients (Fig. S1, SDC, http://links.lww.com/TP/B320).
Allogeneic T cells mediate acute alloimmune response by releasing IFNγ.5,40–43 In this study, we show that T cells from HR hosts, despite their increased production of VEGF-A and proangiogenic role, express significantly higher levels of IFNγ compared to T cells from LR transplants (Figures 4A and B). IFNγ is known to stimulate proliferation of CD4+ T cells via Signal Transducer and Activator of Transcription 1 in an autocrine and paracrine way, sustaining the immune response against alloantigens.44,45 IFN-γ further exerts proinflammatory effects via upregulating IFNγ induced protein 10, which attracts Th1 cells, and thus initiates and amplifies the host alloresponse that may lead to acute rejection. Besides its proinflammatory function, however, previous studies have shown that IFN-γ blockade can inhibit neovascularization in the cornea.46 IFNγ can suppress angiogenesis via several mechanisms: (1) It can suppress VEC proliferation and migration by activating the antiangiogenic factor, induced protein 10, in VECs.46–50 (2) IFNγ can indirectly suppress angiogenesis by suppressing the Signal Transducer and Activator of Transcription 3 pathway in T cells, which induces VEGF-A release by immune cells.47,51–53 (3) IFNγ can also downregulate the expression of metalloproteinases, which are required for the breakdown of the extracellular matrix to allow new capillaries to sprout, and thus may affect angiogenesis. (4) It has been shown that IFNγ inhibits VEGF-A secretion47 and EC proliferation in human cell lines.54 Here, we demonstrate that IFNγ reduces VEC proliferation in a dose-dependent manner. High amounts of IFNγ reduce VEC proliferation by 30% compared with low doses of IFNγ (Figure 4C). Blocking IFNγ in the T cell/VEC coculture promotes VEC proliferation and enhances VEGF-A expression in vitro (Figures 4D and E). Thus, T cells and their expressed cytokines may be critical regulators of angiogenesis in transplantation. We speculate that there is a balance between T cell–secreted proangiogenic factors, principally VEGF-A, and antiangiogenic mediators, specifically IFNγ. Thus, T cells and their expressed cytokines may be critical regulators of angiogenesis in transplantation. Our data suggest that in the context of transplantation, performed in the inflamed microenvironment of the HR host bed, the net result of these 2 opposing T cell-derived mechanisms is proangiogenic (Figure 5). Thus, in this context, the antiangiogenic and proinflammatory functions of IFN-γ are not mutually exclusive.
FIGURE 5.
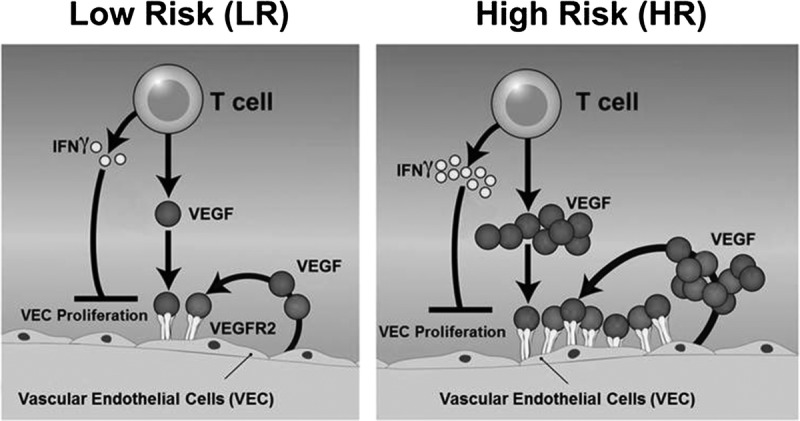
Schematic diagram of T cell mediated angiogenesis. In our vitro model, T cells promote angiogenesis in 3 ways: (1) conv T cells induce VEC proliferation by releasing the angiogenic factor VEGF-A. T cells collected from HR recipients (right) are more proangiogenic than T cells obtained from LR grafted mice (left); (2) VEC autocrine secretion of VEGF-A and VEGF-C promote VEC proliferation; and (3) VECs upregulate expression of VEGF-R2, which is the receptor for VEGF-A and VEGF-C. The release of these ligands and increase in expression of VEGF-R are more pronounced in the HR setting than LR setting. Our data also show an antiangiogenic effect of IFNγ; however, the balance between T cell–induced VEGF-mediated proangiogenic, and IFNγ antiangiogenic, mechanisms appears to lie in favor of the proangiogenic effects.
In conclusion, our study provides new insights into the role of adaptive immunity and transplantation-associated angiogenesis. We show for the first time that alloreactive host T cells promote angiogenesis after transplantation, largely through expression of VEGF-A. Accordingly, we propose that the angiogenic process after corneal transplantation develops in 2 phases: an early phase related to the postoperative healing process mediated by innate immune cells, as described elsewhere,55–57 and a late phase mediated by alloreactive T cells responsible for graft rejection.
ACKNOWLEDGMENTS
The authors would like to thank Dr. Susanne Eiglmeier (Schepens Eye Research Institute) for her editorial assistance and helpful discussions.
Footnotes
This study was funded by the National Institutes of Health (NIH R01 EY12963) and the NIH National Eye Institute core grant P30EY003790.
The authors declare no conflicts of interest.
A.D.Z. and M.T. contributed equally to this work.
A.D.Z. participated in the performance of the research, data analysis, and writing the article. M.T. participated in the performance of the research and data analysis. B.S. participated in the performance of the research and data analysis. J.Y. participated in the performance of the research. T.H.D. participated in the performance of the research. T.I. participated in the performance of the research. A.M. participated in the performance of the research and writing the article. S.K.C. and R.D. participated in the research design and writing the article.
Correspondence: Reza Dana, MD, MPH, MSc, Schepens Eye Research Institute, Massachusetts Eye & Ear Infirmary, 20 Staniford Street, Boston, MA, 02114. (Reza_Dana@meei.harvard.edu).
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantjournal.com).
ECs express more when VEGF-A, VEGF-C and VEGF-R2 mRNA when cocultured with T cells from high-risk inflamed transplants compared to low-risk transplants and naı¨ve mice. ECs proliferate in response to VEGF-A when cultured with allogeneic T cells from corneal transplant while IFNg shows an antiangiogenic effect. Supplemental digital content is available in the text.
REFERENCES
- 1.Dana MR, Qian Y, Hamrah P. Twenty-five-year panorama of corneal immunology: emerging concepts in the immunopathogenesis of microbial keratitis, peripheral ulcerative keratitis, and corneal transplant rejection Cornea 2000. 19625–643 [DOI] [PubMed] [Google Scholar]
- 2.Hamrah P. High-risk penetrating keratoplasty Arch Soc Esp Oftalmol 2005. 805–7 [PubMed] [Google Scholar]
- 3.Lam H, Dana MR. Corneal graft rejection Int Ophthalmol Clin 2009. 4931–41 [DOI] [PubMed] [Google Scholar]
- 4.Zhu SN, Yamada J, Streilein JW. ICAM-1 deficiency suppresses host allosensitization and rejection of MHC-disparate corneal transplants Transplantation 2000. 691008–1013 [DOI] [PubMed] [Google Scholar]
- 5.Qazi Y, Hamrah P. Corneal allograft rejection: immunopathogenesis to therapeutics. J Clin Cell Immunol. 2013;2013(Suppl 9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Australian Corneal Graft Registry. 1990 to 1992 report. Aust N Z J Ophthalmol. 1993;21(2 Suppl):1–48. [PubMed] [Google Scholar]
- 7.Williams KA, Roder D, Esterman A. Factors predictive of corneal graft survival. Report from the Australian Corneal Graft Registry Ophthalmology 1992. 99403–414 [DOI] [PubMed] [Google Scholar]
- 8.Cursiefen C, Cao J, Chen L. Inhibition of hemangiogenesis and lymphangiogenesis after normal-risk corneal transplantation by neutralizing VEGF promotes graft survival Invest Ophthalmol Vis Sci 2004. 452666–2673 [DOI] [PubMed] [Google Scholar]
- 9.Tan Y, Cruz-Guilloty F, Medina-Mendez CA. Immunological disruption of antiangiogenic signals by recruited allospecific T cells leads to corneal allograft rejection J Immunol 2012. 1885962–5969 [DOI] [PubMed] [Google Scholar]
- 10.Stevenson W, Cheng SF, Dastjerdi MH. Corneal neovascularization and the utility of topical VEGF inhibition: ranibizumab (Lucentis) vs bevacizumab (Avastin) Ocul Surf 2012. 1067–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao S, Hata Y, Miura M. Dexamethasone inhibits interleukin-1beta-induced corneal neovascularization: role of nuclear factor-kappaB-activated stromal cells in inflammatory angiogenesis Am J Pathol 2007. 1711058–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung ES, Chauhan SK, Jin Y. Contribution of macrophages to angiogenesis induced by vascular endothelial growth factor receptor-3-specific ligands Am J Pathol 2009. 1751984–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jin Y, Arita M, Zhang Q. Anti-angiogenesis effect of the novel anti-inflammatory and pro-resolving lipid mediators Invest Ophthalmol Vis Sci 2009. 504743–4752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cursiefen C, Chen L, Borges LP. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment J Clin Invest 2004. 1131040–1050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freeman MR, Schneck FX, Gagnon ML. Peripheral blood T lymphocytes and lymphocytes infiltrating human cancers express vascular endothelial growth factor: a potential role for T cells in angiogenesis Cancer Res 1995. 554140–4145 [PubMed] [Google Scholar]
- 16.Dana MR, Yamada J, Streilein JW. Topical interleukin 1 receptor antagonist promotes corneal transplant survival Transplantation 1997. 631501–1507 [DOI] [PubMed] [Google Scholar]
- 17.Sonoda Y, Streilein JW. Orthotopic corneal transplantation in mice—evidence that the immunogenetic rules of rejection do not apply Transplantation 1992. 54694–704 [DOI] [PubMed] [Google Scholar]
- 18.Lee HK, Chauhan SK, Kay E. Flt-1 regulates vascular endothelial cell migration via a protein tyrosine kinase-7-dependent pathway Blood 2011. 1175762–5771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voronov E, Carmi Y, Apte RN. The role IL-1 in tumor-mediated angiogenesis. Front Physiol. 2014;5:114. doi: 10.3389/fphys.2014.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhou TB, Yang GS. Roles of vascular endothelial growth factor in acute rejection reaction following liver transplantation Transpl Immunol 2011. 25207–209 [DOI] [PubMed] [Google Scholar]
- 21.Ozdemir BH, Ozdemir FN, Gungen Y. Role of macrophages and lymphocytes in the induction of neovascularization in renal allograft rejection Am J Kidney Dis 2002. 39347–353 [DOI] [PubMed] [Google Scholar]
- 22.Reinders ME, Rabelink TJ, Briscoe DM. Angiogenesis and endothelial cell repair in renal disease and allograft rejection J Am Soc Nephrol 2006. 17932–942 [DOI] [PubMed] [Google Scholar]
- 23.Dashkevich A, Heilmann C, Kayser G. Lymph angiogenesis after lung transplantation and relation to acute organ rejection in humans Ann Thorac Surg 2010. 90406–411 [DOI] [PubMed] [Google Scholar]
- 24.Dastjerdi MH, Saban DR, Okanobo A. Effects of topical and subconjunctival bevacizumab in high-risk corneal transplant survival Invest Ophthalmol Vis Sci 2010. 512411–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maruyama K, Ii M, Cursiefen C. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages J Clin Invest 2005. 1152363–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harvey NL, Gordon EJ. Deciphering the roles of macrophages in developmental and inflammation stimulated lymphangiogenesis. Vasc Cell. 2012;4:15. doi: 10.1186/2045-824X-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chauhan SK, Dohlman TH, Dana R. Corneal lymphatics: role in ocular inflammation as inducer and responder of adaptive immunity. J Clin Cell Immunol. 2014:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh JY, Choi H, Lee RH. Identification of the HSPB4/TLR2/NF-kappaB axis in macrophage as a therapeutic target for sterile inflammation of the cornea EMBO Mol Med 2012. 4435–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wagoner MD. Chemical injuries of the eye: current concepts in pathophysiology and therapy Surv Ophthalmol 1997. 41275–313 [DOI] [PubMed] [Google Scholar]
- 30.Lee S, Chen TT, Barber CL. Autocrine VEGF signaling is required for vascular homeostasis Cell 2007. 130691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Segarra M, Ohnuki H, Maric D. Semaphorin 6A regulates angiogenesis by modulating VEGF signaling Blood 2012. 1204104–4115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Namiki A, Brogi E, Kearney M. Hypoxia induces vascular endothelial growth factor in cultured human endothelial cells J Biol Chem 1995. 27031189–31195 [DOI] [PubMed] [Google Scholar]
- 33.Bachmann BO, Bock F, Wiegand SJ. Promotion of graft survival by vascular endothelial growth factor a neutralization after high-risk corneal transplantation Arch Ophthalmol 2008. 12671–77 [DOI] [PubMed] [Google Scholar]
- 34.Presta M, Dell’Era P, Mitola S. Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis Cytokine Growth Factor Rev 2005. 16159–178 [DOI] [PubMed] [Google Scholar]
- 35.Lee P, Wang CC, Adamis AP. Ocular neovascularization: an epidemiologic review Surv Ophthalmol 1998. 43245–269 [DOI] [PubMed] [Google Scholar]
- 36.Peoples GE, Blotnick S, Takahashi K. T lymphocytes that infiltrate tumors and atherosclerotic plaques produce heparin-binding epidermal growth factor-like growth factor and basic fibroblast growth factor: a potential pathologic role Proc Natl Acad Sci U S A 1995. 926547–6551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dana MR, Zhu SN, Yamada J. Topical modulation of interleukin-1 activity in corneal neovascularization Cornea 1998. 17403–409 [DOI] [PubMed] [Google Scholar]
- 38.Du JW, Xu KY, Fang LY. Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer Mol Med Rep 2012. 61099–1102 [DOI] [PubMed] [Google Scholar]
- 39.Clements JL, Dana R. Inflammatory corneal neovascularization: etiopathogenesis Semin Ophthalmol 2011. 26235–245 [DOI] [PubMed] [Google Scholar]
- 40.Streilein JW. Immunobiology and immunopathology of corneal transplantation Chem Immunol 1999. 73186–206 [DOI] [PubMed] [Google Scholar]
- 41.Yamagami S, Amano S. Role of resident corneal leukocytes and draining cervical lymph nodes in corneal allograft rejection Cornea 2003. 22S61–S65 [DOI] [PubMed] [Google Scholar]
- 42.Yamagami S, Dana MR, Tsuru T. Draining lymph nodes play an essential role in alloimmunity generated in response to high-risk corneal transplantation Cornea 2002. 21405–409 [DOI] [PubMed] [Google Scholar]
- 43.Hegde S, Beauregard C, Mayhew E. CD4(+) T-cell-mediated mechanisms of corneal allograft rejection: role of Fas-induced apoptosis Transplantation 2005. 7923–31 [DOI] [PubMed] [Google Scholar]
- 44.Robinson DS, O’Garra A. Further checkpoints in Th1 development Immunity 2002. 16755–758 [DOI] [PubMed] [Google Scholar]
- 45.Reed JM, Branigan PJ, Bamezai A. Interferon gamma enhances clonal expansion and survival of CD4+ T cells J Interferon Cytokine Res 2008. 28611–622 [DOI] [PubMed] [Google Scholar]
- 46.Voest EE, Kenyon BM, O’Reilly MS. Inhibition of angiogenesis in vivo by interleukin 12 J Natl Cancer Inst 1995. 87581–586 [DOI] [PubMed] [Google Scholar]
- 47.Kommineni VK, Nagineni CN, William A. IFN-gamma acts as anti-angiogenic cytokine in the human cornea by regulating the expression of VEGF-A and sVEGF-R1 Biochem Biophys Res Commun 2008. 374479–484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Voloshyna I, Littlefield MJ, Reiss AB. Atherosclerosis and interferon-gamma: new insights and therapeutic targets Trends Cardiovasc Med 2014. 2445–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sato N, Nariuchi H, Tsuruoka N. Actions of TNF and IFN-gamma on angiogenesis in vitro J Invest Dermatol 1990. 9585S–89S [DOI] [PubMed] [Google Scholar]
- 50.Luster AD, Unkeless JC, Ravetch JV. Gamma-interferon transcriptionally regulates an early-response gene containing homology to platelet proteins Nature 1985. 315672–676 [DOI] [PubMed] [Google Scholar]
- 51.Sidky YA, Borden EC. Inhibition of angiogenesis by interferons: effects on tumor- and lymphocyte-induced vascular responses Cancer Res 1987. 475155–5161 [PubMed] [Google Scholar]
- 52.Darnell JE. STATs and gene regulation Science 1997. 2771630–1635 [DOI] [PubMed] [Google Scholar]
- 53.Schindler C, Levy DE, Decker T. JAK-STAT signaling: from interferons to cytokines J Biol Chem 2007. 28220059–20063 [DOI] [PubMed] [Google Scholar]
- 54.Friesel R, Komoriya A, Maciag T. Inhibition of endothelial cell proliferation by gamma-interferon J Cell Biol 1987. 104689–696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Davis GE, Senger DR. Endothelial extracellular matrix: biosynthesis, remodeling, and functions during vascular morphogenesis and neovessel stabilization Circ Res 2005. 971093–1107 [DOI] [PubMed] [Google Scholar]
- 56.Yamanaka O, Sumioka T, Saika S. The role of extracellular matrix in corneal wound healing. Cornea 2013;32(suppl): s43-s45-retracted. Cornea. 2014;33:100. doi: 10.1097/01.ico.0000441180.54146.15. [DOI] [PubMed] [Google Scholar]
- 57.Lee H, Schlereth SL, Park EY. A novel pro-angiogenic function for interferon-gamma-secreting natural killer cells Invest Ophthalmol Vis Sci 2014. 552885–2892 [DOI] [PMC free article] [PubMed] [Google Scholar]


