Abstract
Although the notion that alcohol promotes violence is widespread, not all individuals are aggressive while intoxicated. Genetic variation could be a contributing factor to individual differences in alcohol-heightened aggression. The present study examines the effects of OPRM1C77G genotype on responses to threat in rhesus macaques under normal conditions and following alcohol administration. Prior studies have shown that a low CSF level of 5-HIAA is a trait marker for individuals prone to escalated aggression. We wanted to examine whether the predictive value for this marker on aggression was moderated by OPRM1 genotype. Animals were administered alcohol (BAC 100-200 mg%), were provoked by a human intruder, and aggressive responses were recorded. Factor analysis was performed to generate aggressive response factors, which were then used as dependent variables for ANOVA, with OPRM1 genotype and CSF 5-HIAA as independent variables. Factor analysis generated three factors (“Threatening”, “Distance Decreasing” and “High Intensity”). We found that High Intensity aggression was increased among carriers of the OPRM1 G allele, especially among individuals with low CSF levels of 5-HIAA. Aggression in the non-intoxicated state was predicted by 5-HIAA, but not by genotype. This study demonstrates that OPRM1 genotype predicts alcohol-heightened aggression in rhesus macaques with low CSF levels of 5-HIAA. Since OPRM1 variation predicts similar effects on alcohol response and behavior in humans and macaques, this study could suggest a role for OPRM1 genotype in alcohol-heightened aggression in humans. If so, it may be that compounds that block this receptor could reduce alcohol-associated violence in selected patient populations
Keywords: OPRM1, Serotonin, Alcohol, Aggression, Macaque, Genetic
Introduction
Violent crime is frequently associated with alcohol intoxication, and alcohol consumption is widely held to be a causative factor in violent or aggressive behavior (Moss and Tarter, 1993). Meta-analyses indicate that large proportions of various types of violent crimes (including homicide, assault, sexual assault, marital violence, and child abuse) are perpetuated under the influence of alcohol (Roizen, 1997). Although these associations are not causal and may reflect the association between psychopathology or certain personality traits and higher levels and frequencies of alcohol drinking, controlled studies performed in a laboratory setting consistently demonstrate a causal relationship between alcohol consumption and enhanced aggressive responding in some individuals (Bushman, 1997; Giancola and Parrott, 2008). It is also known, however, that only a minority is likely to become aggressive during periods of intoxication. Similarly, alcohol-enhanced aggression is a relatively stable individual trait characteristic of a minority of individuals of other species (Miczek et al., 1998; Miczek et al., 1992). Identifying the factors that contribute to alcohol-facilitated aggression may permit development of strategies for the prevention and treatment of alcohol-related violence, which would positively impact both affected individuals and potential victims.
Alcohol induces psychopharmacological effects that contribute to alcohol-facilitated aggression through modulation of serotonin, endogenous opioid, dopamine, and GABA systems (Couppis and Kennedy, 2008; de Almeida et al., 2005; Kramer et al., 2007). Not only are impulse control deficits instrumental in driving excessive alcohol consumption, but intoxication can further impair information processing and impulse control, and it is proposed that this may lead to misinterpretation of social cues or to escalated aggressive responding (Gilman and Hommer, 2008; Gilman et al., 2012). Because of alcohol's anxiolytic and disinhibitory effects, intoxicated individuals may also be more likely to risk engaging in aggressive encounters under conditions of threat or provocation (Kramer et al., 2007). Finally, alcohol increases levels of psychomotor stimulation, reward, and novelty seeking among some, and activation of the reward systems is known to underlie competitive or predatory forms of aggression in a variety of animal species (Fish et al., 2005). Knowledge of the effects of alcohol on these individual neurobiological and behavioral systems can potentially inform us of the roots of alcohol-mediated aggression and may provide candidate systems in which trait or genetic variation would be likely to moderate risk for alcohol-facilitated violence (Heinz et al., 2011).
Despite alcohol being widely held to induce aggressive behavior, other factors likely play moderating roles. For example, some studies demonstrate that alcohol results in heightened aggressive behavior particularly in response to provocation (Kramer et al., 2007). In addition, although studies are mixed, some have suggested that males are more prone to alcohol-heightened aggression than are females, potentially because aggression is more “socially acceptable” among males but also because females can be more sensitive to the sedating/anxiolytic effects of alcohol and less sensitive to alcohol's stimulating effects (Urban et al., 2010). It is also thought that trait-like differences in impulse control and aggression may be linked to alcohol-related aggression (Godlaski and Giancola, 2009). The latter relationship could possibly relate to the fact that impaired impulse control leads to early, uncontrolled alcohol intake, which is observed among individuals with antisocial personality disorder (ASPD) and early-onset Type II alcohol dependence; Decades ago, it was demonstrated that human subjects at risk for Type II alcoholism have lower cerebrospinal fluid concentrations of the serotonin metabolite, 5-hydroxy-indole-acetic acid (CSF 5-HIAA), suggesting that serotonin system dysfunction is a risk factor for early onset alcohol dependence (Coccaro and Kavoussi, 1996; Virkkunen and Linnoila, 1996). Low CSF levels of 5-HIAA have also been associated with risk taking and aggressive behavior and, among alcohol-dependent subjects, those who have low CSF 5-HIAA concentrations are particularly prone to violent or aggressive behavior. A link between low CSF levels of 5-HIAA, risk taking, and impulsive aggression has been demonstrated to exist in nonhuman primate species as well (Higley and Linnoila, 1997b; Higley et al., 1996b), and other studies have shown there to be translational value for the rhesus macaque in examining how environmental, genetic and neurobiological markers linked to serotonin neurotransmission and turnover also predict individual differences in aggression and psychopathology (Caspi et al., 2002; Heinz et al., 1998b; Ichise et al., 2006). Although it has been proposed that genetic factors are likely to play a role in alcohol-related violence (Cloninger, 1987; Crabbe et al., 1994; Fish et al., 2002; McBride and Li, 1998), candidate-gene based studies in this area are somewhat lacking, though there are a handful (Johansson et al., 2012). It has been noted that alcohol-heightened aggression is higher on the ascending curve of blood alcohol concentrations, the phase during which alcohol's rewarding/stimulating effects predominate over those of sedation (Hoaken and Stewart, 2003). Whether genetic factors that predispose individuals to violence under the influence of alcohol are independent of genetic or trait-like factors that contribute to aggression in the non-intoxicated state has not formally been considered. We propose that genetic variation that underlies individual differences in alcohol-induced reward and stimulation may be a factor that contributes to alcohol-facilitated aggression. In humans, there is a nonsynonymous SNP in the first exon of the OPRM1 gene (OPRM1 A118G), which encodes the ligand-binding domain the receptor and which has been associated with increased alcohol-induced stimulation and euphoria (Ray et al., 2012). There is also a functional nonsynonymous SNP (OPRM1 C77G) that exists in rhesus macaques (Miller et al., 2004), and we have previously demonstrated this variant to predict individual differences in various indices of reward sensitivity. Not only do we find that animals carrying the G allele exhibit increased sensitivity to natural rewards (Barr et al., 2008b), but they also show heightened alcohol-induced stimulation (Barr et al., 2007), suggesting that they may also be useful for modeling how genetic variation contributes to alcohol-related aggression.
Reward may underlie aggressive responding across species, but upon activation of the reward systems, genetic factors that moderate reward sensitivity may contribute to a greater extent to individual difference in aggression. The present study aims to examine the effects of OPRM1 C77G genotype on responses to threatening stimuli in rhesus macaques under normal conditions and following administration of alcohol. Prior studies performed in rhesus macaques in the field and in the laboratory have shown that a low CSF level of 5-HIAA is a trait marker for individuals prone to impaired impulse control and escalated aggression (Higley and Linnoila, 1997a; Mehlman et al., 1994; Shannon et al., 2005). We, therefore, wanted to examine whether the predictive value for this marker on aggressive responding would be moderated by OPRM1 genotype. Finally, because sex differences have been shown to play a role in human studies and because males exhibit higher levels of psychomotor stimulation following consumption of alcohol (Giancola et al., 2002; Giancola et al., 2009), we wanted to examine the effects of sex on aggressive responding.
Methods and Materials
Provocation using an unfamiliar conspecific- Intruder Challenge Test
Like humans, many rhesus monkeys will only exhibit escalated aggressive behavior when presented with a threatening stimulus. One means by which aggressive responses can be provoked in rhesus monkeys is with the use of an “intruder”. We performed an Intruder Challenge Test (Barr et al., 2008a) using as the intruder an unfamiliar, age and sex matched conspecific. Behavioral responses to an unfamiliar intruder were recorded in adolescent/adult rhesus macaques (ages, 2-9 years of age, N = 62). Intruder animals were selected based on the age and sex of the test subjects. Intruders were also selected to match the size of the test subjects as closely as possible. All intruder animals were completely unfamiliar to the test subjects. Prior to the intruder challenge test, the intruder animal was placed into an individual transfer cage, measuring 0.76 m wide X 0.63 m deep X 0.91 m high, for a 30-minute acclimation period. All subjects were tested three at a time in the home run. For the test, three randomly grouped animals from their age-matched cohort were locked into the outdoor portion of their home run, an enclosure measuring 2.64 m wide X 3 m long X 2.44 m high. After ten minutes, the intruder animal's cage was placed directly at the front of the enclosure and behavioral scoring of the test subjects was initiated. One observer was assigned to each test subject, which was observed for 30 minutes using focal animal continuous recording. Behaviors were recorded as previously described (Schwandt et al., 2010). Behaviors were generally scored in seconds in duration. Vocalization, aggression, and approach intruder were scored in frequency. Inter-observer reliability was established at greater than r = 0.85 (Cohen's kappa) for all behaviors.
Measurement of Alcohol-Heightened Aggression
In order to assess alcohol-heightened aggression in response to provocation, animals (ages, 2-4 years of age, N = 81) were administered alcohol, and aggressive responses were provoked using a human intruder (Kalin and Shelton, 2003). Ethyl alcohol (ethanol) (16.8% (v/v) USP) was administered intravenously under restraint, as described previously (2.2 grams/kg or 2.0 grams/kg for males and females, respectively) (Barr et al., 2007). The rationale for administering a higher dose to males is that, as in humans, rhesus males have less body fat than females and have been demonstrated to require more alcohol/kg to produce identical blood alcohol concentrations (Baraona et al., 2001). At 10 minutes following initiation of the infusion, blood samples were obtained from the femoral vein for assessment of blood alcohol concentration (BAC). Blood alcohol concentrations were quantified enzymatically using a commercial kit (Sigma™, St. Louis, MO).
Following the IV ethanol infusions, animals were placed in a padded testing room. After 30 min, incidents of provoked aggressive behavior directed toward an experimenter were recorded for 5 minutes for each monkey. To obtain these scores, an investigator wearing capture gloves entered the room, stood in the opposite corner, and maintained eye contact with the subject for 2.5 minutes, a procedure shown to elicit mild aggression in macaques. During the last 2.5 minutes, the investigator maintained eye contact with the subject, while pulling down his protective mask and imitating a macaque open mouth threat (open mouth with teeth showing, a gesture typically eliciting an aggressive response from rhesus macaques once every 30 seconds) (Barr et al., 2003). During the 5-minute period, the three observers recorded the frequencies of lunges, open-mouth threats, stares, head-bobs, and barks. Head bobs, lunges, and barks were recorded as frequency, whereas stares and open mouth threats were recorded as seconds in duration. During a series of vocalizations, each bark was given a score of “1.” Each animal went through a two separate alcohol infusion and behavioral testing sessions, and measures were averaged across the two sessions.
CSF Sample Collection
One to three months prior to assessments, animals were anesthetized using ketamine hydrochloride (15 mg/kg dosage), and a cisternal CSF sample was drawn into a 5 ml syringe using a 1-inch, 22-gauge needle. Samples were taken from all animals within 30 minutes of ketamine administration. CSF was assayed for concentrations of the serotonin metabolite 5-hydroxyindoleacetic acid (5-HIAA) with gas chromatography-mass spectrometry (GCMS) using deuterated internal standards (Higley et al., 1996a). Inter- and intra-assay variabilities for all assays were less than 10%. Values are reported in picomoles per milliliter.
Animal studies were performed prior to 2007, and all protocols were reviewed and approved by the NICHD and NIAAA Animal Care and Use Committees.
Genotyping
Using standard extraction methods, DNA was isolated from whole blood, collected from the femoral vein under ketamine anesthesia (15 mg/kg, IM). Genotyping was performed using the procedure modified from Miller et al., 2004 (Miller et al., 2004; Schwandt et al., 2011). A portion of OPRM1 exon 1 was amplified from 25 ng of genomic DNA with flanking oligonucleotide primers museekf1 (5’-TCA GTA CCA TGG ACA GCA GCG CTG TCC CCA CGA A- 3’) and museekr1 (5’-GTC GGA CAG GTT GCC ATC TAA GTG-3’) in 15 μl reactions using AmpliTaq Gold® and 2.5 mM MgCl2 according to the manufacturers instructions (Invitrogen, Carlsbad, CA, USA). Amplifications were performed on a PerkinElmer thermocycler (9700) with one cycle at 96°C followed by 30 cycles of 94°C/15 sec, 56°C/15 sec, 72°C/30 sec, and a final 3-minute extension at 72°C. Restriction digest by Fnu4HI (New England Biolabs, Beverly, MA, USA) was then performed using 0.5μl of PCR product in a total volume of 20 μl for 2 h at 37°C. Samples were separated by electrophoresis on 10% polyacrylamide gels, and the C and G alleles were identified by direct visualization following ethidium bromide staining.
Statistical Analyses
In order to identify different aggressive response dimensions, we performed factor analysis on behaviors collected during both the Intruder Challenge Test and the IV Alcohol testing sessions. In each instance, principal component extraction, followed by standard orthogonal (Varimax) normalized rotation, was performed, and factor scores were generated for each individual. Behavioral dimensions were labeled by investigators with expertise in primate behavior. Factor analysis of behavioral data collected in response to provocation in the form of an unfamiliar conspecific (ICT) demonstrated there to be two factors relating to aggressive behavior, which together accounted for 63% of the variance. These were labeled “Aggression toward Intruder” (positive loading of both contact and non-contact aggression directed towards the intruder) and “Within-Group Aggression” (positive loading of contact and non-contact aggression toward other members of the social group) (Table 1). Factor analysis of aggressive behaviors exhibited toward the investigator in response to provocation following alcohol infusion yielded 3 factors, which accounted for 70% of the variance. These were labeled “Threatening” (positive loading for stare and head bob), “Distance Decreasing” (positive loading for bark and yawn), and “High Intensity” (positive loading for open mouth threat and lunge) aggression (Table 1).
Table 1.
Aggressive response dimensions generated using factor analysis of behaviors collected following IV-alcohol infusion and provocation by an intruder. Orthogonal factors were generated using principal component extraction followed by varimax rotation.
| Aggression Type- IV Alcohol/Human Intruder | Eigen Value | Loading | |
|---|---|---|---|
| “Threatening” | 2.1 | Stare Head-bob |
.826 .933 |
| “Distance- Decreasing” | 1.1 | Bark Yawn |
.734 .756 |
| “High Intensity” | 1 | OM Threat Lunge |
.827 .735 |
| Aggression Type-Intruder Challenge Test | Eigen Value | Loading | |
|---|---|---|---|
| “Within Group Aggression” | 1.3 | Contact (physical) Non-Contact |
.811 .810 |
| “Aggression Toward Intruder” | 1 | Contact (physical) Non-Contact |
.769 .685 |
Using factor scores as the dependent variables, we examined whether aggressive responses to provocation were moderated by OPRM1 genotype. We performed ANCOVA with genotype (C/C vs. G allele carrier) and sex (male vs. female) as independent nominal variables and CSF levels of 5-HIAA as a continuous co-variable. It has been shown that CSF levels of 5-HIAA are stable across time and situation, but since 5-HIAA levels change as a function of age and because macaques under the age of 2 years rarely exhibit highly aggressive behavior, we limited our analyses to individuals that were at least two years of age. Analyses were performed with rearing condition as a co-independent variable, but since there were no main effects or interactions and because of addition of this variable did not reduce the residual variance, it was removed from the analyses. High Intensity aggression factor scores were inversely correlated with blood alcohol concentrations (r2 = −0.2, P < 0.03). Although there was no relationship between CSF levels of 5-HIAA or genotype with BAC, there was a trend for males to have higher BAC concentrations, as they received a slightly higher dose of alcohol (males = 0.239 ± 0.03 vs. females = 0.233 ± 0.03, P = 0.15). Therefore, analyses were also repeated with BAC included as an additional co-variable in the analyses.
Allele frequencies were in agreement with Hardy-Weinberg. Since there were few animals with the G/G genotype among these datasets, G/G subjects were collapsed with G allele carriers for the purpose of this analysis. Analyses were performed using Statview 5.01 statistical software. Criterion for significance was set at P ≤ 0.05.
Results
Aggression Provoked by an Unfamiliar Conspecific in the Absence of Alcohol
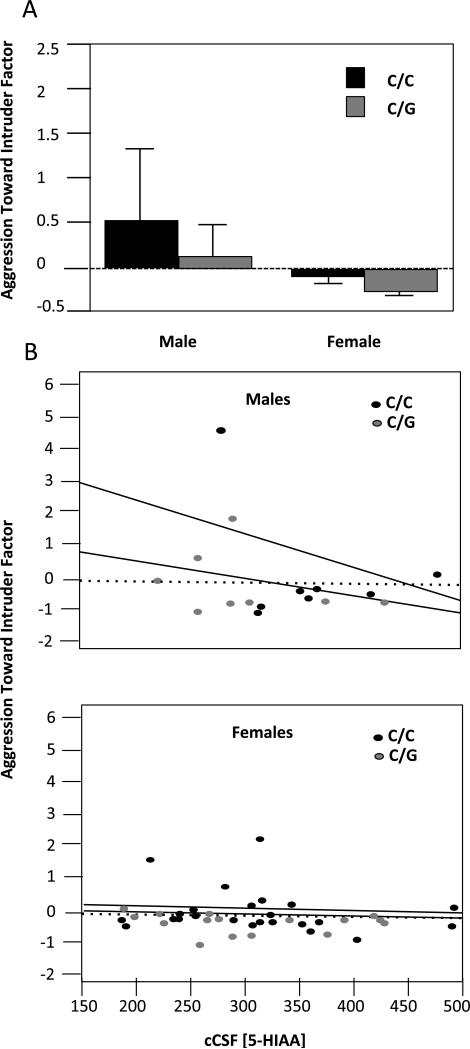
There were no significant effects of genotype, sex, or CSF 5-HIAA on Within-Group Aggression. There were main effects of sex (Fig 1A, F(1,54)= 7.21, P = 0.009) and CSF levels of 5-HIAA (Figure 1B, F(1,54)= 5.44, P < 0.03) and an interaction between the two variables on levels of Aggression Toward Intruder (Fig 1B, F(1,54)= 4.2, P < 0.05). Males were more likely to exhibit aggressive behavior toward an unfamiliar intruder male, especially if they had low CSF levels of 5-HIAA (Fig 1B). For both males and females, there was no effect of genotype (F(1,54) = 2.07, P = 0.16) on aggression exhibited toward the intruder.
Fig 1.
CSF levels of 5-HIAA and Sex Interact to Predict Aggression Toward an Intruder Macaque. Rhesus macaques were exposed in the home run to an age- and sex-matched intruder. Effects of OPRM1 genotype (C/C vs. G allele carrier) on aggressive response factors were assessed using ANOVA, with 5-HIAA and sex as a co-variables. A. Males exhibited higher levels of aggression, but there were no effects of genotype or genotype × sex interactions. B. Males with low 5-HIAA exhibited higher levels of aggression toward an unfamiliar conspecific.
Aggression Provoked following Alcohol Administration
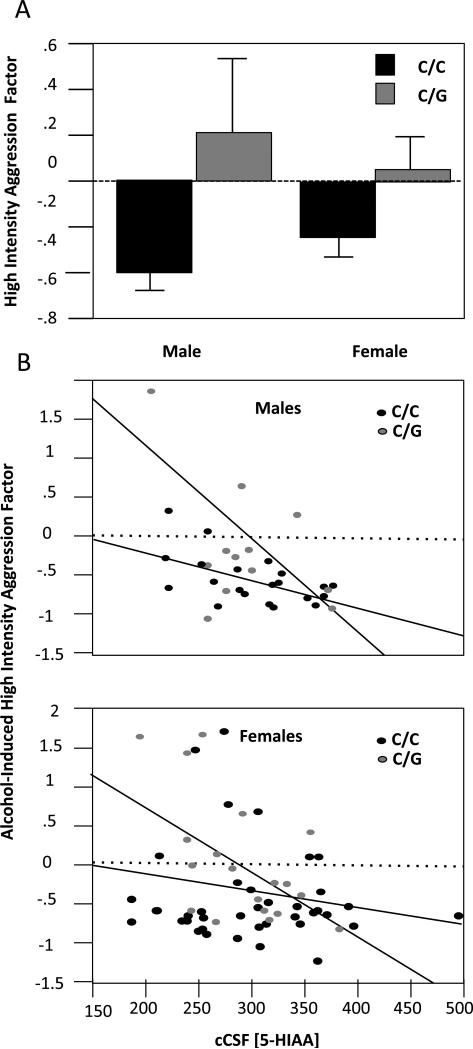
There were main effects of genotype (Fig 2A, F(1,73) =7.06, P =0.009) and CSF 5-HIAA (Fig 2B, (1,73) =13.947, P= 0.0004) on High Intensity Aggression following IV alcohol infusion. There was also an interaction between genotype and CSF levels of 5-HIAA (F(1,73)-4.2, P=0.04). Independent of sex, individuals with low 5-HIAA levels exhibited higher levels of High Intensity aggression, particularly if they were carriers of the OPRM1 77G allele (Figure 2B). When we repeated our analyses with blood alcohol concentrations included as another covariable, results remained the same. G allele carriers exhibited higher levels of High Intensity Aggression, even at low blood alcohol concentrations (data not shown).
Fig 2.
OPRM1 Genotype Interacts with CSF levels of 5-HIAA to Predict High Intensity Aggression Following Alcohol Administration. Rhesus macaques were administered a binge dose of alcohol and provoked using a human intruder challenge test. Effects of OPRM1 genotype (C/C vs. G allele carrier) on aggressive response factors were assessed using ANOVA, with 5-HIAA and sex as a co-variables. A. There were effects of genotype on Escalated physical aggression, with G allele carriers exhibiting heightened aggression. B. Individuals with low 5-HIAA exhibited higher levels of High Intensity aggression if they were carriers of the 77G allele. There were no effects of sex or any genotype × sex or 5-HIAA × sex interactions.
There were no effects of sex on High Intensity Aggression. The less intense forms of aggression (Threatening or Distance Decreasing) were not predicted by any of the variables tested (data not shown).
Discussion
Across species, aggression is important for the protection of self and offspring and in the defense and/or acquisition of rank, territory or resources; It can also be exhibited in response to fear or pain or in order to execute control over other individuals (reviewed in Barr and Driscoll, 2014). Among humans, while potentially rooted in their adaptive origins, excessive and inappropriate aggressive behavior is a feature of many psychiatric disorders, such as borderline personality disorder, antisocial personality disorder, post-traumatic stress disorder, and depression (American Psychiatric Association, 2013). It is also a trait observed among alcohol-dependent individuals, and aggressive tendencies can escalate under the influence of some drugs of abuse, including alcohol.
While it is known that alcohol intoxication can promote violent behavior, because of ethical constraints, highly aggressive behavior or violence cannot be elicited and studied in human subjects being tested in the laboratory. Various laboratory paradigms have been used to assess individual differences in alcohol-facilitated aggression using computer-simulations or questionnaires. However, these are limiting, since most of these studies are performed at relatively low blood alcohol concentrations and because conditioned effects of alcohol may also play a role (Duke et al., 2011; Giancola, 2006; Levinson et al., 2011). Moreover, physical arousal and violence cannot be as readily assessed using these paradigms. Genetic factors that impact aggressivity in rhesus macaques, and the mechanisms by which these may promote or moderate aggressive behavior, have been shown to be quite informative and may be predictive of the human condition (Barr and Driscoll, 2014). One system that is activated in response to both natural and artificial rewards is the endogenous opioid system. In multiple primate species, there are non-synonymous single-nucleotide polymorphisms (SNPs) in the first exon of the OPRM1 gene, which produce amino acid changes in the N-terminal domain of the receptor (Bond et al., 1998; Miller et al., 2004). Among these are the A118G SNP in humans and the C77G SNP in rhesus macaques. Studies examining intermediate phenotypes likely to be under the control of this receptor (for example, alcohol response or HPA axis activity) suggest gain-of function roles for these polymorphisms (Barr et al., 2007; Chong et al., 2006; Ray and Hutchison, 2004; Schwandt et al., 2011). Prior studies performed in rhesus macaques have shown that this SNP is associated with un-provoked, home-cage aggressive behaviors (Miller et al., 2004). Here, we used an approach that involved using an intruder to provoke aggressive responses and then performed factor analyses to delineate specific aggression response dimensions (“Threatening,” “Distance-Decreasing” and “High Intensity”). We show that rhesus macaques carrying the 77G allele exhibit High Intensity aggression when provoked by an intruder during the intoxicated, but not the non-intoxicated, state.
Though there are studies and anecdotal evidence demonstrating a link between alcohol-heightened violence and aggressive personality traits (reviewed in Moss and Tarter, 1993), it is also recognized that individuals who are generally irritable or particularly aggressive do not necessarily exhibit violent behavior during periods of intoxication. In this study, we performed Intruder Challenge Tests in animals both in the absence and presence of an intoxicating dose of alcohol (BACs between 0.2-0.3) and examined the effects of OPRM1 genotype, sex and CSF levels of 5-HIAA on aggressive responding. Human studies have repeatedly demonstrated that CSF 5-HIAA levels are negatively correlated with impulsive behavior and aggression, an association found across species and one which has been proposed to be a trait-like, rather than a state-like, biological marker (Higley and Linnoila, 1997b; Higley et al., 1996b; Shannon et al., 2005). Here, we report that aggression exhibited toward an intruder is inversely correlated with CSF levels of 5-HIAA across testing conditions, suggesting that impaired inhibitory control plays a role in driving aggressive responses to threat in both the non-intoxicated and the intoxicated states. It may also be that, if individuals with low CSF 5-HIAA are more anxious (Higley and Linnoila, 1997b), they also rate higher on threat perception, a factor also known to conteribute to individual differences in alcohol-heightened aggression (Stewart and Hoaken, 2003). We have previously shown that animals with low levels of 5-HIAA consume higher levels of alcohol (Higley et al., 1996b), and higher levels of alcohol intake have also been demonstrated among carriers of the OPRM1 77G allele (Barr et al., 2007). If our findings translate to the human condition, it may be that these two factors (low CSF 5-HIAA and OPRM1 A118G genotype) simultaneously increase risk for high levels of alcohol use and for alcohol-associated aggression or violence.
We also found that following alcohol administration, female and male rhesus macaques did not differ significantly in their levels of aggressive responding to provocation. Human laboratory studies have shown that females can be aggressive in response to provocation, and that this is not moderated by alcohol intoxication. Our study shows the opposite- that females only exhibit aggressive responses that approach those of males during periods of intoxication. A limitation of the current study is that we used an unfamiliar conspecific as the stimulus for animals tested in the non-intoxicated state. This is in contrast to the aggression provoked following alcohol administration, during which all subjects were provoked with a controlled suite of aggression-provoking behaviors, exhibited by a single investigator. Regardless of this limitation, we were able to show that females and males did not differ significantly in terms of their aggressive responses during periods of intoxication. More importantly, we were able to demonstrate that both females and males were more likely to exhibit aggression as a function of OPRM1 genotype and low CSF concentrations of 5-HIAA. In both sexes, the form of aggression affected was high in intensity. Many studies demonstrate that males are more likely than females to exhibit alcohol-potentiated aggression in the laboratory (Giancola et al., 2002). Our findings in macaques demonstrate that both male and female G allele carriers with low 5-HIAA exhibit high intensity aggression during intoxication. This could potentially suggest that sex differences in social acceptance of aggression may be a confounding factor in human alcohol-mediated aggression laboratory studies and is in keeping with the fact that a high proportion of violent crimes perpetuated by females are done so under the influence of alcohol.
Studies performed in humans and in nonhuman primates have demonstrated that early adversity can be a predictor for long-term serotonin system dysfunction, aggression and psychopathology. The first G × E studies to be performed in humans, and later replicated in rhesus macaques, examined functional genetic variation at the MAOA and SLC6A4 genes, both of which influence serotonin reuptake and turnover at the synapse (Caspi et al., 2002; Caspi et al., 2003; reviewed in Barr and Driscoll, 2014). For both loci and in both species, interactive effects between genotype and early adversity were demonstrated. Of note, in the present study, we did not observe any rearing effects (neither main nor interactive) on aggressive behavior. It could be that any potential effects of rearing condition are better explained with the use of CSF 5-HIAA, a continuous variable known to be modulated by early rearing history (Heinz et al., 1998a; Ichise et al., 2006; Shannon et al., 2005).
Identification of factors that contribute to alcohol-heightened violence may aid in personalized prevention and treatment. Alcoholism has been sub-classified on the bases of behavioral phenotypes, age of onset, and presumptive etiology (Cloninger, 1987). Whereas Type I alcohol-dependent individuals begin consuming large quantities of alcohol at a later age, largely for its anxiolytic properties, Type II alcoholics begin drinking early in life and commonly exhibit impulsive behaviors and unprovoked aggression. Type II alcoholism also has a high rate of co-morbidity with antisocial personality disorder (ASPD) (Moeller and Dougherty, 2001). Historically, this type of alcoholism has been attributed to serotonin system dysfunction, suggested by diminished cerebrospinal fluid concentrations of 5-HIAA. Low CSF 5-HIAA has been associated with impaired impulse control and aggressive behavior both in humans and in animal models (Godlaski and Giancola, 2009; Van den Bergh et al., 2006). Results from the present study suggest the following: 1. That individuals who carry genetic variants that make them experience more alcohol-induced stimulation are more likely react aggressively to provocation while intoxicated. 2. That subjects that exhibit trait-like aggression may be particularly prone to aggression during periods of intoxication if they are also more stimulated as a result of genetic predisposition. By extension, these data suggest that the same factors that give rise to early, uncontrolled alcohol intake (impaired impulse control and increased alcohol-mediated reward) may also be risk factors for alcohol-facilitated aggressive responding. If our findings translate to the human condition, it may be that OPRM1 A118G genotype may simultaneously increase risk for high levels of alcohol use and for alcohol-associated aggression or violence.
While not initially considered, it is possible that the findings of the current study may have translational value outside of the area of research on alcohol-heighted aggression. Recent studies in human subjects indicate that a low serotonin state predicts not only impaired impulse control, but decreased harm aversion (an aversive reaction to observing or participating in harm being done to others) (Crockett et al., 2010). Even modest depletion of brain serotonin in humans abolishes aversively motivated inhibition, and it is at this intersection that low serotonin states have recently been proposed to drive human aggression (Siegel and Crockett, 2013). We find that rhesus macaques with decreased serotonin system functioning and that are more sensitive to the rewarding properties of alcohol due to OPRM1 gene variation are more likely to exhibit heightened aggression in the presence of alcohol. Although we do not find an interaction between 5-HIAA and OPRM1 genotype in the non-intoxicated state, this could theoretically inform us of genetic or trait variables that increase risk for predatory violence and killing in human subjects (Birkley et al., 2013). Affective aggression in humans is easily modeled in macaques and are likely similar in etiology. However, goal-directed and predatory killing, as seen with sociopathy/psychopathy, are less easily modeled in the nonhuman primates. This type of aggression may have its basis in reward. For human subjects with impaired central serotonin function and/or among those deficient in empathy, the combination of experiencing heightened reward during predatory killing combined with diminished harm aversion and an impaired ability to control predatory impulses could, theoretically, increase risk for psychopathic behavior. Of relevance to this and to the current study, the only candidate gene in which polymorphism has been shown to increase risk for alcohol-heightened aggression in humans is the oxytocin receptor (OXTR) gene, which predicts individual differences in both reward processing and social cognition (Johansson et al., 2012).
“Detrimental consequences (of alcohol-associated violence) exist not only for the minority of alcoholics and heavy drinkers, but also for society as a whole“ (WHO, 2004). However, despite the fact that alcohol-related violence is a problem for many societies, successful treatment or prevention programs remain somewhat limited (Cook and Moore, 1993). Moreover, while there are only a handful of compounds labeled for the treatment of alcohol dependence and related problems, several studies have shown that alcohol-dependent subjects who are carriers of a functional variant in the OPRM1 gene are more likely to respond to treatment with the mu-opioid receptor antagonist, naltrexone (Oslin et al., 2015). Whether naltrexone treatment could help to prevent alcohol-facilitated aggression in subjects that continue to consume alcohol during treatment or, further, whether naltrexone specifically decreases aggression in a genotype-dependent manner may be factors that could be considered for treatment of affected patient populations.
Acknowledgements
We would like to thank the NIAAA, NICHD, and NIHAC support staff. This study was supported by the NIAAA and NICHD intramural programs. These data were presented in a symposium at the 2010 Research Society on Alcoholism meeting.
Footnotes
Financial Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
References
- American Psychiatric Association, DSM Task Force Diagnostic and statistical manual of mental disorders : DSM-5. (Fifth edition) 2013 [Google Scholar]
- Baraona E, Abittan CS, Dohmen K, Moretti M, Pozzato G, Chayes ZW, Schaefer C, Lieber CS. Gender differences in pharmacokinetics of alcohol. Alcoholism, clinical and experimental research. 2001;25:502–507. [PubMed] [Google Scholar]
- Barr C, Becker ML, Suomi SJ, Higley D. Relationships among CSF monoamine metabolite levels, alcohol sensitivity and alcohol-related aggression in rhesus macaques. Aggressive Behavior. 2003;29:288–301. [Google Scholar]
- Barr CS, Driscoll C. Neurogenetics of aggressive behavior: studies in primates. Current topics in behavioral neurosciences. 2014;17:45–71. doi: 10.1007/7854_2013_267. [DOI] [PubMed] [Google Scholar]
- Barr CS, Dvoskin RL, Yuan Q, Lipsky RH, Gupte M, Hu X, Zhou Z, Schwandt ML, Lindell SG, McKee M, Becker ML, Kling MA, Gold PW, Higley D, Heilig M, Suomi SJ, Goldman D. CRH haplotype as a factor influencing cerebrospinal fluid levels of corticotropin-releasing hormone, hypothalamic-pituitary-adrenal axis activity, temperament, and alcohol consumption in rhesus macaques. Archives of general psychiatry. 2008a;65:934–944. doi: 10.1001/archpsyc.65.8.934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr CS, Schwandt M, Lindell SG, Chen SA, Goldman D, Suomi SJ, Higley JD, Heilig M. Association of a functional polymorphism in the mu-opioid receptor gene with alcohol response and consumption in male rhesus macaques. Archives of general psychiatry. 2007;64:369–376. doi: 10.1001/archpsyc.64.3.369. [DOI] [PubMed] [Google Scholar]
- Barr CS, Schwandt ML, Lindell SG, Higley JD, Maestripieri D, Goldman D, Suomi SJ, Heilig M. Variation at the mu-opioid receptor gene (OPRM1) influences attachment behavior in infant primates. Proceedings of the National Academy of Sciences of the United States of America. 2008b;105:5277–5281. doi: 10.1073/pnas.0710225105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birkley EL, Giancola PR, Lance CE. Psychopathy and the prediction of alcoholrelated physical aggression: the roles of impulsive antisociality and fearless dominance. Drug Alcohol Depend. 2013;128:58–63. doi: 10.1016/j.drugalcdep.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong JA, Leal SM, Tischfield JA, Kreek MJ, Yu L. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bushman BJ. Effects of alcohol on human aggression: Validity of proposed explanations. In: Galanter M, editor. Recent Developments in Alcoholism. Plenum Press; New York: 1997. pp. 227–243. [DOI] [PubMed] [Google Scholar]
- Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R. Role of genotype in the cycle of violence in maltreated children. Science. 2002;297:851–854. doi: 10.1126/science.1072290. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–389. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chong RY, Oswald L, Yang X, Uhart M, Lin PI, Wand GS. The mu-opioid receptor polymorphism A118G predicts cortisol responses to naloxone and stress. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2006;31:204–211. doi: 10.1038/sj.npp.1300856. [DOI] [PubMed] [Google Scholar]
- Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ. Neurotransmitter correlates of impulsive aggression. In: Stoff DM, Cairns RB, editors. Aggression and Violence. Lawrence Erlbaum; Mahwah: 1996. pp. 67–86. [Google Scholar]
- Cook PJ, Moore MJ. Economic perspectives on reducing alcohol-related violence. In: Martin SE, editor. Alcohol and Interpersonal Violence. NIAAA; Rockville, MD: 1993. pp. 193–212. [Google Scholar]
- Couppis MH, Kennedy CH. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology. 2008;197:449–456. doi: 10.1007/s00213-007-1054-y. [DOI] [PubMed] [Google Scholar]
- Crabbe JC, Belknap JK, Buck KJ. Genetic animal models of alcohol and drug abuse. Science. 1994;264:1715–1723. doi: 10.1126/science.8209252. [DOI] [PubMed] [Google Scholar]
- Crockett MJ, Clark L, Hauser MD, Robbins TW. Serotonin selectively influences moral judgment and behavior through effects on harm aversion. Proceedings of the National Academy of Sciences. 2010;107:17433–17438. doi: 10.1073/pnas.1009396107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida RM, Ferrari PF, Parmigiani S, Miczek KA. Escalated aggressive behavior: dopamine, serotonin and GABA. European journal of pharmacology. 2005;526:51–64. doi: 10.1016/j.ejphar.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Duke AA, Giancola PR, Morris DH, Holt JC, Gunn RL. Alcohol dose and aggression: another reason why drinking more is a bad idea. J Stud Alcohol Drugs. 2011;72:34–43. doi: 10.15288/jsad.2011.72.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, DeBold JF, Miczek KA. Repeated alcohol: behavioral sensitization and alcohol-heightened aggression in mice. Psychopharmacology. 2002;160:39–48. doi: 10.1007/s00213-001-0934-9. [DOI] [PubMed] [Google Scholar]
- Fish EW, DeBold JF, Miczek KA. Escalated aggression as a reward: corticosterone and GABA(A) receptor positive modulators in mice. Psychopharmacology. 2005;182:116–127. doi: 10.1007/s00213-005-0064-x. [DOI] [PubMed] [Google Scholar]
- Giancola PR. Influence of subjective intoxication, breath alcohol concentration, and expectancies on the alcohol-aggression relation. Alcoholism, clinical and experimental research. 2006;30:844–850. doi: 10.1111/j.1530-0277.2006.00099.x. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Helton EL, Osborne AB, Terry MK, Fuss AM, Westerfield JA. The effects of alcohol and provocation on aggressive behavior in men and women. J Stud Alcohol. 2002;63:64–73. [PubMed] [Google Scholar]
- Giancola PR, Levinson CA, Corman MD, Godlaski AJ, Morris DH, Phillips JP, Holt JC. Men and women, alcohol and aggression. Experimental and clinical psychopharmacology. 2009;17:154–164. doi: 10.1037/a0016385. [DOI] [PubMed] [Google Scholar]
- Giancola PR, Parrott DJ. Further evidence for the validity of the Taylor Aggression Paradigm. Aggress Behav. 2008;34:214–229. doi: 10.1002/ab.20235. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Hommer DW. Modulation of brain response to emotional images by alcohol cues in alcohol-dependent patients. Addiction biology. 2008;13:423–434. doi: 10.1111/j.1369-1600.2008.00111.x. [DOI] [PubMed] [Google Scholar]
- Gilman JM, Smith AR, Ramchandani VA, Momenan R, Hommer DW. The effect of intravenous alcohol on the neural correlates of risky decision making in healthy social drinkers. Addiction biology. 2012;17:465–478. doi: 10.1111/j.1369-1600.2011.00383.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godlaski AJ, Giancola PR. Executive functioning, irritability, and alcoholrelated aggression. Psychol Addict Behav. 2009;23:391–403. doi: 10.1037/a0016582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz A, Higley JD, Gorey JG, Saunders RC, Jones DW, Hommer D, Zajicek K, Suomi SJ, Lesch KP, Weinberger DR, Linnoila M. In vivo association between alcohol intoxication, aggression, and serotonin transporter availability in nonhuman primates. Am J Psychiatry. 1998a;155:1023–1028. doi: 10.1176/ajp.155.8.1023. [DOI] [PubMed] [Google Scholar]
- Heinz A, Ragan P, Jones DW, Hommer D, Williams W, Knable MB, Gorey JG, Doty L, Geyer C, Lee KS, Coppola R, Weinberger DR, Linnoila M. Reduced central serotonin transporters in alcoholism. Am J Psychiatry. 1998b;155:1544–1549. doi: 10.1176/ajp.155.11.1544. [DOI] [PubMed] [Google Scholar]
- Heinz AJ, Beck A, Meyer-Lindenberg A, Sterzer P, Heinz A. Cognitive and neurobiological mechanisms of alcohol-related aggression. Nat Rev Neurosci. 2011;12:400–413. doi: 10.1038/nrn3042. [DOI] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. Low central nervous system serotonergic activity is traitlike and correlates with impulsive behavior. A nonhuman primate model investigating genetic and environmental influences on neurotransmission. Annals of the New York Academy of Sciences. 1997a;836:39–56. doi: 10.1111/j.1749-6632.1997.tb52354.x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Linnoila M. A nonhuman primate model of excessive alcohol intake. Personality and neurobiological parallels of type I- and type II-like alcoholism. Recent Dev Alcohol. 1997b;13:191–219. [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Poland RE, Taub DM, Vickers J, Suomi SJ, Linnoila M. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biological psychiatry. 1996a;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A nonhuman primate model of type II excessive alcohol consumption? Part 1. Low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations and diminished social competence correlate with excessive alcohol consumption. Alcoholism, clinical and experimental research. 1996b;20:629–642. doi: 10.1111/j.1530-0277.1996.tb01665.x. [DOI] [PubMed] [Google Scholar]
- Hoaken PN, Stewart SH. Drugs of abuse and the elicitation of human aggressive behavior. Addict Behav. 2003;28:1533–1554. doi: 10.1016/j.addbeh.2003.08.033. [DOI] [PubMed] [Google Scholar]
- Ichise M, Vines DC, Gura T, Anderson GM, Suomi SJ, Higley JD, Innis RB. Effects of early life stress on [11C]DASB positron emission tomography imaging of serotonin transporters in adolescent peer- and mother-reared rhesus monkeys. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:4638–4643. doi: 10.1523/JNEUROSCI.5199-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson A, Bergman H, Corander J, Waldman ID, Karrani N, Salo B, Jern P, Algars M, Sandnabba K, Santtila P, Westberg L. Alcohol and aggressive behavior in men--moderating effects of oxytocin receptor gene (OXTR) polymorphisms. Genes, brain, and behavior. 2012;11:214–221. doi: 10.1111/j.1601-183X.2011.00744.x. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Nonhuman primate models to study anxiety, emotion regulation, and psychopathology. Annals of the New York Academy of Sciences. 2003;1008:189–200. doi: 10.1196/annals.1301.021. [DOI] [PubMed] [Google Scholar]
- Kramer UM, Jansma H, Tempelmann C, Munte TF. Tit-for-tat: the neural basis of reactive aggression. NeuroImage. 2007;38:203–211. doi: 10.1016/j.neuroimage.2007.07.029. [DOI] [PubMed] [Google Scholar]
- Levinson CA, Giancola PR, Parrott DJ. Beliefs about aggression moderate alcohol's effects on aggression. Experimental and clinical psychopharmacology. 2011;19:64–74. doi: 10.1037/a0022113. [DOI] [PubMed] [Google Scholar]
- McBride WJ, Li TK. Animal models of alcoholism: neurobiology of high alcohol-drinking behavior in rodents. Crit Rev Neurobiol. 1998;12:339–369. doi: 10.1615/critrevneurobiol.v12.i4.40. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, Suomi SJ, Linnoila M. Low CSF 5-HIAA concentrations and severe aggression and impaired impulse control in nonhuman primates. Am J Psychiatry. 1994;151:1485–1491. doi: 10.1176/ajp.151.10.1485. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Barros HM, Sakoda L, Weerts EM. Alcohol and heightened aggression in individual mice. Alcoholism, clinical and experimental research. 1998;22:1698–1705. [PubMed] [Google Scholar]
- Miczek KA, Weerts EM, Tornatzky W, DeBold JF, Vatne TM. Alcohol and “bursts” of aggressive behavior: ethological analysis of individual differences in rats. Psychopharmacology. 1992;107:551–563. doi: 10.1007/BF02245270. [DOI] [PubMed] [Google Scholar]
- Miller GM, Bendor J, Tiefenbacher S, Yang H, Novak MA, Madras BK. A muopioid receptor single nucleotide polymorphism in rhesus monkey: association with stress response and aggression. Mol Psychiatry. 2004;9:99–108. doi: 10.1038/sj.mp.4001378. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM. Antisocial personality disorder, alcohol, and aggression. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25:5–11. [PMC free article] [PubMed] [Google Scholar]
- Moss HB, Tarter RE. Substance abuse, agression, and violence: What are the connections?: Clinical update. American Jouranl of Addiction. 1993;2:149–160. [Google Scholar]
- Oslin DW, Leong SH, Lynch KG, Berrettini W, O'Brien CP, Gordon AJ, Rukstalis M. Naltrexone vs Placebo for the Treatment of Alcohol Dependence: A Randomized Clinical Trial. JAMA Psychiatry. 2015;72:430–437. doi: 10.1001/jamapsychiatry.2014.3053. [DOI] [PubMed] [Google Scholar]
- Ray LA, Barr CS, Blendy JA, Oslin D, Goldman D, Anton RF. The role of the Asn40Asp polymorphism of the mu opioid receptor gene (OPRM1) on alcoholism etiology and treatment: a critical review. Alcoholism, clinical and experimental research. 2012;36:385–394. doi: 10.1111/j.1530-0277.2011.01633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray LA, Hutchison KE. A polymorphism of the mu-opioid receptor gene (OPRM1) and sensitivity to the effects of alcohol in humans. Alcoholism, clinical and experimental research. 2004;28:1789–1795. doi: 10.1097/01.alc.0000148114.34000.b9. [DOI] [PubMed] [Google Scholar]
- Roizen J. Epidemiological issues in alcohol-related violence. In: Galanter M, editor. Recent Developments in Alcoholism. Plenum Press; New York: 1997. pp. 7–40. [DOI] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Higley JD, Suomi SJ, Heilig M, Barr CS. OPRM1 gene variation influences hypothalamic-pituitary-adrenal axis function in response to a variety of stressors in rhesus macaques. Psychoneuroendocrinology. 2011;36:1303–1311. doi: 10.1016/j.psyneuen.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwandt ML, Lindell SG, Sjoberg RL, Chisholm KL, Higley JD, Suomi SJ, Heilig M, Barr CS. Gene-environment interactions and response to social intrusion in male and female rhesus macaques. Biological psychiatry. 2010;67:323–330. doi: 10.1016/j.biopsych.2009.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon C, Schwandt ML, Champoux M, Shoaf SE, Suomi SJ, Linnoila M, Higley JD. Maternal absence and stability of individual differences in CSF 5-HIAA concentrations in rhesus monkey infants. Am J Psychiatry. 2005;162:1658–1664. doi: 10.1176/appi.ajp.162.9.1658. [DOI] [PubMed] [Google Scholar]
- Siegel JZ, Crockett MJ. How serotonin shapes moral judgment and behavior. Annals of the New York Academy of Sciences. 2013;1299:42–51. doi: 10.1111/nyas.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban NB, Kegeles LS, Slifstein M, Xu X, Martinez D, Sakr E, Castillo F, Moadel T, O'Malley SS, Krystal JH, Abi-Dargham A. Sex differences in striatal dopamine release in young adults after oral alcohol challenge: a positron emission tomography imaging study with [(1)(1)C]raclopride. Biological psychiatry. 2010;68:689–696. doi: 10.1016/j.biopsych.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Bergh F, Spronk M, Ferreira L, Bloemarts E, Groenink L, Olivier B, Oosting R. Relationship of delay aversion and response inhibition to extinction learning, aggression, and sexual behaviour. Behavioural brain research. 2006;175:75–81. doi: 10.1016/j.bbr.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Virkkunen M, Linnoila M. Serotonin and glucose metabolism in impulsively violent alcoholic offenders. In: Stoff DM, Cairns RB, editors. Aggression and Violence. Lawrence Erlbaum; Mahwah, N.J.: 1996. pp. 87–100. [Google Scholar]
- WHO . In: Global status report on alcohol 2004. Department SA, editor. World Health Organization; Geneva: 2004. p. 88. [Google Scholar]