Abstract
SWAP-70, an unusual phosphatidylinositol-3-kinase-dependent protein that interacts with the RhoGTPase Rac, is highly expressed in mast cells. Cultured bone marrow mast cells (BMMC) from SWAP-70−/− mice are reduced in FcɛRI-triggered degranulation. This report describes the hitherto-unknown role of SWAP-70 in c-kit receptor signaling, a key proliferation and differentiation pathway in mast cells. Consistent with the role of Rac in cell motility and regulation of the actin cytoskeleton, mutant cells show abnormal actin rearrangements and are deficient in migration in vitro and in vivo. SWAP-70−/− BMMC are impaired in calcium flux, in proper translocation and activity of Akt kinase (required for mast cell activation and survival), and in translocation of Rac1 and Rac2 upon c-kit stimulation. Adhesion to fibronectin is reduced, but homotypic cell association induced through c-kit is strongly increased in SWAP-70−/− BMMC. Homotypic association requires extracellular Ca2+ and depends on the integrin αLβ2 (LFA-1). ERK is hyperactivated upon c-kit signaling in adherent and dispersed mutant cells. Together, we suggest that SWAP-70 is an important regulator of specific effector pathways in c-kit signaling, including mast cell activation, migration, and cell adhesion.
Mast cells are hematopoietic cells derived from progenitor stem cells in the bone marrow (reviewed in references 15, 37, and 40). Mast cells leave the bone marrow into the peripheral blood as mast cell-committed precursors (49) and enter peripheral tissues, mostly mucosal and connective tissue, and the peritoneal fluid. Immature mast cells can be cultivated in vitro in the presence of interleukin-3 (IL-3) from either murine blood mast cells (BMC) or bone marrow mast cells (BMMC) (32, 42, 54, 65). Such cultured, proliferating primary mast cells express the high-affinity receptor for immunoglobulin E (IgE) (FcɛRI) and the c-kit receptor, possess granules, and are capable of exocytosis.
Signaling through c-kit is essential for mast cell development, as well as for other processes in hematopoiesis, erythropoiesis, melanogenesis, and gametogenesis (reviewed in references 1, 7, 38, 50, and 58). Mice completely deficient in c-kit or its ligand stem cell factor (SCF; also named kit ligand [KL] or steel factor [SL]) die during embryogenesis or neonatally of severe anemia. The addition of SCF to cultured BMMC stimulates proliferation, maturation, secretion, cytoskeletal actin rearrangements, membrane ruffling, migration, and cell survival. The SCF receptor is a type III receptor tyrosine kinase bearing five immunoglobulin-like extracellular domains and no ITAM. Binding of SCF to c-kit causes autophosphorylation of the receptor, which then phosphorylates a range of substrates triggering specific pathways to mount the responses mentioned above. Proteins that become activated during c-kit signaling include phosphatidylinositiol 3-kinase (PI3K) and small RhoGTPases, e.g., Rac. Actin reorganization and the release of Ca2+ are triggered, and kinases such as the mitogen-activated protein kinase (MAPK) kinase MEK and its substrates ERK1 and ERK2, as well as survival factors such as Akt/PKB, are activated. Thus, a complex set of signaling pathways is activated in BMMC through c-kit stimulation, and not all factors involved in these pathways have been elucidated.
This report describes functions of the signaling protein SWAP-70 in c-kit signaling in mast cells. SWAP-70, originally isolated from the nucleus of activated, mature B lymphocytes (4), features an unusual combination of amino acid sequence motifs and domains (4, 5, 39, 53) (see Fig. S1 in the supplemental material). It carries three nuclear localization signals and a nuclear exit signal and in B cells localizes to the cytoplasm and/or the nucleus depending on the activation status of the cell. In mast cells, the protein is cytoplasmic or localizes to the cytoplasmic membrane (17). Membrane localization is consistent with the pleckstrin homology (PH) domain (36) present in the center region of SWAP-70. Through its PH domain SWAP-70 binds phosphatidylinositol-3,4,5-triphosphate (PIP3), the second messenger product generated by PI3K. Binding of PIP3 is necessary for SWAP-70 to localize to membrane actin structures called membrane ruffles (53), sites of membrane actin rearrangements that occur upon cell activation. A second key domain in SWAP-70 is its Dbl homology domain (DH), which is found in guanine nucleotide exchange factors for small Rho GTPases (24, 68). Contrasting with other known members of the Dbl family of proteins, the DH domain in SWAP-70 is positioned not N terminal but C terminal to its PH domain. Through a mechanism that is still to be fully defined, SWAP-70 promotes formation of the GTP-bound, activated form of the GTPase Rac (53), a central molecular switch in a variety of signaling pathways. Pathways regulated through Rac include signaling from immunoglobulin superfamily receptors such as the B-cell receptor and the FcɛRI, from growth factor receptors including c-kit, and from cytokine receptors (reviewed in references 13, 49, and 57). Like SWAP-70, Rac localizes to membrane ruffles, and the translocation of Rac to these sites appears to be necessary for its activation (33). SWAP-70's only close homolog is a protein called Def-6, SLAT, or IBP (20, 25, 57), which was reported to function in T-cell signaling (20, 57).
Since the only two primary cell types currently known to highly express SWAP-70 are mast cells and B cells, we started to investigate the functions of SWAP-70 in mast cells, aided by the availability of SWAP-70-deficient mice (6, 17), and cultures of IL-3-dependent BMMC or BMC from wild-type or SWAP-70−/− mice. Fluorescence-activated cell sorting (FACS) analysis of the FcɛRI, visualized by bound IgE and of c-kit showed that >95% of wild-type or mutant cells were positive for both receptors with similar intensities. Wild-type or SWAP-70−/− mast cells respond similarly to IL-3 or SCF, or combinations of both ligands, with regard to the induction of proliferation. In FcɛRI-mediated degranulation assays, SWAP-70−/− BMMC or BMC, stimulated through cross-linked IgE/FcɛRI complexes, yield a signal of only one-fifth of that of wild-type cells. Release of nonpreformed mast cell-derived mediators such as tumor necrosis factor alpha (TNF-α) or IL-6 is consistently lower in SWAP-70−/− than wild-type BMMC (17). Together, this suggests that SWAP-70 plays a specific role in mast cell signaling. The present study aimed at identifying contributions of SWAP-70 to c-kit signaling in mast cells.
MATERIALS AND METHODS
Mast cell cultures, cytokine antibodies, and inhibitors
BMMC were established as described earlier (17) with marrow from 4- to 8-week-old wild-type or SWAP−/− mice. Recombinant murine SCF was either collected from supernatant of SCF-secreting BHK cells or purchased from R&D systems (Minneapolis, Minn.). Concentrations of SCF or IL-3 in the tissue culture supernatants were measured by enzyme-linked immunosorbent assay. Antiphosphotyrosine antibody (clone 4G10) was produced from a hybridoma line and purified by protein G chromatography. Anti-p-Ser473 Akt, anti-P-Thr308 Akt, anti-Akt, and anti-LFA-1 (αLβ2; CD11a/CD18) were obtained from UBI (Lake Placid, N.Y.). Anti-phosphorylated phospholipase Cγ-1 (anti-P-PLCγ-1), anti-PLCγ1, anti-PLCγ2, anti-P-Erk (Thr183/Tyr185), and anti-ERK were purchased from Cell Signaling Technology Inc. (Beverly, Mass.). Anti-Rac1 and anti-Rac2 were obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, Calif.). Recombinant PAK-glutathione S-transferase (GST) was prepared from overexpression of the gene, kindly provided by J. Chernoff, Fox Chase Cancer Center, in Escherichia coli BL21 and purification on a glutathione resin. Cell Tracker Green (CMFDA), the Indo1 calcium binding dye, and tetramethyl rhodamine isothiocyanate (TRITO)-phalloidin were purchased from Molecular Probes (Eugene, Oreg.). PD98059, an inhibitor of ERK, was purchased from Calbiochem (San Diego, Calif.).
Isolation and analysis of subcellular protein fractions
BMMC cultures were starved overnight in medium with 2% serum without IL-3. Approximately 1.5 × 107 BMMC were stimulated by addition of SCF, followed by lysis in hypotonic lysis buffer (20 mM Tris-HCl [pH 7.4]; 5 mM EDTA; 5 mM EGTA; 5 mM dithiothreitol; 5 mM Na3VO4; 0.5 mM phenylmethylsulfonyl fluoride; 1-μg/ml [each] aprotinin, leupeptin, and pepstatin; 1 mM Na3VO4; 1 mM NaF; 1 mM glycerol phosphate) by sonication on ice (15 bursts). After centrifugation at 2,000 × g for 5 min at 4°C, supernatants were recentrifuged in an airfuge rotor (Beckman Inc.) at 100,000 × g for 5 min. Cytosolic fractions comprising the supernatants and the pellets were redissolved in lysis buffer containing 1% NP-40 and incubated on ice for 60 min. Samples were then subjected to ultracentrifugation at 100,000 × g for 10 min. Supernatants were collected as the detergent-soluble membrane fractions, and protein from the pellets were extracted into sodium dodecyl sulfate (SDS) sample buffer as the total insoluble fraction, which consists primarily of the cytoskeleton besides a detergent-resistant membrane subfraction (61). For total cellular lysates 2 × 105 cells per sample were solubilized in SDS sample buffer containing protease inhibitors (1-μg/ml each aprotinin, leupeptin, pepstatin), 1 mM Na3VO4, 1 mM FaF, and 1 mM glycerol phosphate.
Ca2+ flux measurements.
Calcium flux was measured by using Indo-1, a calcium binding dye. A total of 106 BMMC was loaded with 3 mM Indo-1 AM (Molecular Probes) for 30 min at 37°C in Iscove modified Dulbecco medium (IMDM) containing 2% fetal calf serum (FCS). The cells were then resuspended in Tyrodes buffer (119 mM NaCl, 5 mM KCl, and 0.4 mM MgSO4 in 25 mM PIPES [pH 7.5] with 1 mM CaCl2, 5.6 mM glucose, and 0.1% bovine serum albumin [BSA]), and changes in dye fluorescence with time were determined by flow cytometry on a FACS Vantage (Becton Dickinson, Inc., Mountain View, Calif.) system after the addition of SCF to a final concentration of 100 ng/ml at 37°C or 2 μM ionomycin for 5 min. These experiments were repeated four times with either recombinant SCF or SCF-containing supernatant from an SCF-producer cell line.
Isolation and analysis of subcellular protein fractions.
Subcellular fractions were prepared according to (60) with some modifications described in the supplemental material. The subcellular fractions, adjusted for signal visibility to equivalents of 2 × 105 cells for the cytosolic, 8 × 105 for the membrane, or 105 cells for the insoluble fraction or total cellular lysate were resolved by SDS-polyacrylamide gel electrophoresis (PAGE), transferred to nitrocellulose membrane, and immunoblotted with the respective antibodies, followed by subsequent enhanced chemiluminescence readout.
Rac activation.
After stimulation of 106 BMMC with 6 ng of SCF/ml, the cells were lysed with equal volumes of 2× lysis buffer containing 50 mM Tris-HCl (pH 8.0), 1% Triton X-100, 150 mM NaCl, 10% glycerol, 2 mM dithiothreitol, and 5 mM MgCl2, protease, and phosphatase inhibitors and then centrifuged for 10 min at 15,000 rpm. The supernatant was incubated for 30 min with GST-PAK1 CRIB (Cdc42/Rac interactive binding) domain prebound to glutathione-Sepharose beads. The beads were washed, and the protein bound to the beads was analyzed by SDS-PAGE, followed by immunoblotting with anti-Rac1 or anti-Rac2 antibodies. The activation assay was repeated eight times.
The assay for Rac translocation was performed as described previously (53). Briefly, after stimulation, 107 cells were lysed in hypotonic Triton X-100 lysis buffer (20 mM Tris-HCl [pH 7.4], 3 mM MgCl2 8% sucrose, 0.5% Triton X-100, and 5 mM EGTA supplemented with protease and phosphatase inhibitors) and subjected to ultracentrifugation at 60,000 rpm for 2 h. After centrifugation the pellet was designated the Triton-insoluble fraction. Pellets were resuspended in SDS-sample buffer and boiled for 10 min, and proteins were separated by SDS-PAGE and blotted with either anti-Rac1 or anti-Rac2 antibodies. These experiments were repeated at least three times.
Akt and ERK kinase assays.
Akt and ERK activity assay kits were obtained from Calbiochem and Cell Signaling, respectively. The assays were performed according to the manufacturers' instructions. Briefly, 5 × 106 SWAP+/+ or SWAP−/− BMMC per assay were either stimulated with 6 ng of SCF/ml or left unstimulated. Stimulation was terminated by washing with ice-cold phosphate-buffered saline (PBS). For Akt assays, cells were lysed, and antibody specific for phosphorylated Akt was added to the lysate and incubated on a rotary shaker at room temperature for 45 min, followed by the addition of protein A-Sepharose and incubation for 45 min. Beads were then washed with 0.5 ml of kinase assay buffer, and kinase assays were performed in the presence of a GSK3α (66) protein-ATP mixture for 4 h at room temperature. The assays were terminated by using SDS sample buffer and analyzed by SDS-PAGE, immunoblotting, and probing with antibody specific for phosphorylated GSK-3α. The same filter was then probed with a control antibody to GST to detect the input GSK3α-GST fusion protein used as a substrate in each assay.
For ERK assays, cells were lysed and immobilized anti-phospho ERK (Thr202/Tyr204) antibody was added to the lysate, followed by incubation on a rotary shaker at 4°C for 30 min. For a positive control, active ERK was added to a lysate sample. After incubation, the beads were washed twice with lysis buffer and twice with a kinase assay buffer. Kinase assays were performed in the presence of 2 μg of Elk-1-GST fusion protein/ml and 200 μM ATP for 30 min at room temperature. The assays were terminated by using SDS sample buffer and then analyzed by SDS-PAGE and immunoblotting with antibody specific for phosphorylated Elk-1. The filter was later probed with a control antibody to GST to detect the total input Elk-1-GST fusion protein used as a substrate in each assay.
Actin measurements and imaging.
Actin polymerization was quantified by using TRITC (tetramethyl rhodamine isothiocyanate)-phalloidin staining of cells, followed by flow cytometry, performed in three independent experiments. A total of 2 × 105 BMMC were stimulated for various times with 6 ng of SCF/ml. Subsequently cells were fixed with 3.6% formaldehyde for 15 min. Total cellular filamentous actin (F-actin) was stained by using TRITC-conjugated phalloidin (10 nM) and analyzed by flow cytometry. A total of 40,000 events were acquired, and increases in cellular fluorescence relative to control samples were used to define an “F-actin high” population and its percentage within the total cell population. Imaging of F-actin in cells by TRITC-phalloidin staining, as well as of Rac1 or Rac2, was performed with a Leica fluorescence microscope by using the Openlab/Velocity software (Improvision, Inc., Lexington, Mass.).
Migration assays.
In vitro migration assays were performed by using 8-μm-pore-size transwell migration plates (Costar, Corning, N.Y.). The assay was repeated eight times to include titrations and kinetic analyses. A total of 106 BMMC in 100 μl of IMDM containing 1% FCS, either untreated or pretreated for 10 min with 10 μg of anti-LFA-1 (αLβ2, CD11a/CD18) or 5 mM EGTA/ml, were added to the upper chamber of the transwell insert. Various concentrations of SCF were added to the lower wells. The plates were incubated for 4 h at 37°C in 5% CO2. Controls without SCF were maintained in each experiment to account for passive diffusion of cells. The migration of BMMC was determined by counting cells that migrated to the lower chamber as percentage of total cells that were loaded, with the passive diffusion subtracted as background (<3% of the signal).
Haptotactic cell migration assays (59) were performed by using matrix-coated polycarbonate filters (8-μm pore size, transwell). The membrane undersurface was coated with fibronectin (10 μg/ml) in PBS at 4°C overnight or with only PBS for controls and was blocked with 3% BSA in Hanks balanced salt solution (HBSS) for 1 h at 37°C. The lower chamber was filled with 500 μl of IMDM medium with or without 6 ng of SCF/ml. Either SWAP-70+/+ or SWAP−/− BMMC were plated in the upper chamber in duplicate wells at a density of 2 × 105 in 100 μl of IMDM medium and then incubated at 37°C for 4 h. After incubation, cells in the upper compartment were removed by using a cotton wool swab, and cells that migrated to the lower chamber, as well as those adhered to fibronectin on the membrane, were counted individually by microscopy. An average of six fields per filter was obtained by cumulative frequency analysis, and the experiment was repeated thrice.
In vivo migration experiments were performed by tail vein injections of labeled BMMC and were repeated twice. Groups of three recipient mice of either wild-type or W/W-v mice, which lack endogenous mast cells (16), were injected with 106 cells derived from donors of each genotype, after labeling with 10 μM CTG (Cell Tracker Green CMFDA) at 37°C according to the manufacturer's instructions. The frequency of labeled cells that were present in the recipient peritoneum or for control those still present in the blood of the recipient was determined 24 h later by flow cytometric analysis of 100,000 cells. Recovery was calculated from the number of labeled cells injected.
Homotypic and fibronectin adhesion assays.
Mast cell aggregation assays were performed by treating 106 BMMC with or without SCF in BMMC growth medium for 2 to 24 h and then monitoring the aggregation by microscopic visualization and counting of aggregates containing more than 5 cells. In some experiments, BMMC were pretreated for 10 min with either 100 μM PD98059, an inhibitor of MEK kinase required for ERK phosphorylation, or 5 μg of anti-LFA-1 antibody/ml or 5 mM EGTA before addition of SCF. This assay was repeated six times.
Fibronectin adhesion assays were carried out as described previously (34) with slight modifications. 96-well Nunc Maxisorp plates (Nunc, Naperville, Ill.) were coated with 50 μg of bovine fibronectin/ml in PBS overnight at 4°C, washed three times with PBS, blocked with 3% BSA (Sigma) in HBSS for 1 h at 37°C, and then washed three times with HBSS-0.03% BSA (assay medium). Wild-type and SWAP70−/− BMMC were starved overnight in IMDM medium with 2% FCS, labeled with CTG dye at 3 mM at 37°C for 1 h, and resuspended to 106 cells/ml in PBS. At the desired time intervals, 50 μl of PBS with or without SCF was added to the fibronectin-coated wells prior to the addition of 50 μl of cell suspension. After we allowed adhesion to the plates for the indicated times, the nonadherent cells were removed carefully from the wells (except from respective control wells) by pipetting them into fresh wells, and any unattached cells were washed away once with PBS. Adhesion was measured as the percentage of fluorescence left in the wells compared to the total fluorescence in control wells, which were assigned as 100%. Alternatively, the adherent cells were lysed in SDS sample buffer and analyzed by SDS-PAGE, followed by Western blotting with the desired antibodies. This assay was performed four times with CTG-labeled cells and three times with unlabeled cells.
RESULTS
Mast cell migration and proper F-actin polymerization depend on SWAP-70.
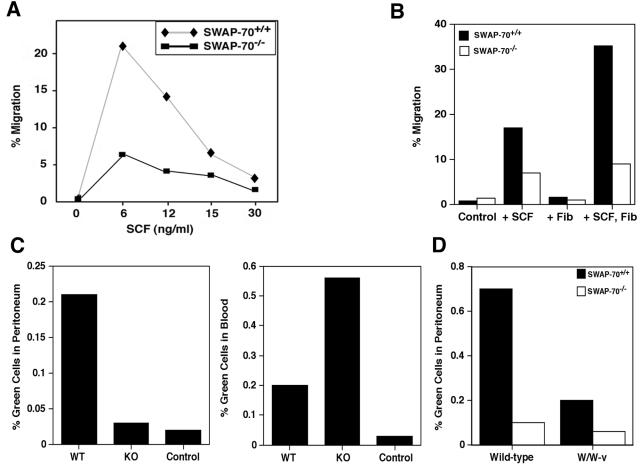
Rac is important for cell migration, and mast cells or B cells deficient in Rac2 were found to migrate approximately threefold less efficiently than wild-type cells (9, 61). SWAP-70 interacts with Rac, contributes to its activation, and also associates with specific actin structures (22, 53). Thus, SWAP-70 is likely involved in cell motility, and we sought to determine whether SCF-induced migration of BMMC depends on SWAP-70. Migration was assayed in a transwell chamber device with a pore size of 8 μm in medium containing SCF as a chemoattractant. The percentage of migrated cells was determined after 4 h (Fig. 1A). Cells migrated best at an optimal concentration of SCF (6 ng/ml). A similar concentration dependence of migration was reported for B cells (9) or mast cells (67). At this SCF concentration, SWAP-70−/− BMMC migrated with a fourfold-reduced efficiency compared to wild-type cells.
FIG. 1.
SWAP-70−/− BMMC are impaired in migration. (A) Transwell assay. Migration of wild-type or mutant BMMC toward the indicated concentrations of SCF, placed in the lower chamber, was assessed by cell counting after 4 h of incubation. The percentage of cells observed in the lower chamber is indicated. (B to D) In vivo migration of BMMC. Wild-type or mutant cells labeled with Cell Tracker Green were injected into the tail vein of 129SvEMS mice (B and C) or of wild-type or W/W-v mice (D). The percentage of green cells found after 24 h in the peritoneum (B and D) or in the blood (C) is shown. The background is given as “Control.”
To address whether the requirement for SWAP-70 is alleviated by the presence of an extracellular matrix protein, we performed a haptotactic migration assay (Fig. 1B). SCF-dependent migration of wild-type or mutant cells through fibronectin-coated membranes was assayed. After 4 h of incubation, very little migration was seen in the absence of SCF, independent of the fibronectin layer. The addition of SCF triggered efficient migration in wild-type cells, which was further increased by the presence of fibronectin. Mutant cells migrated only at less than half the wild-type efficiency without fibronectin. The fibronectin layer did not increase migration of mutant cells, resulting in only 25% migration efficiency compared to wild type. The adherence of cells to the fibronectin layer was assessed by light microscopy, and more than twice as many wild-type than mutant cells were observed (not shown).
To assess whether SWAP-70 is also required for migration of BMMC in vivo, we loaded BMMC with Cell Tracker Green dye, injected equal numbers of wild-type or mutant cells into the tail vein of mice, and counted green cells 24 h later in peritoneal fluid and blood from the animals. Approximately 10-fold less SWAP-70−/− BMMC than wild-type cells were found in the peritoneum of wild-type mice, indicating a requirement for SWAP-70 for translocation into the peritoneum (Fig. 1B). In blood, labeled SWAP-70−/− BMMC were more frequent than labeled wild-type cells, confirming the reduced translocation of mutant BMMC from the blood into the periphery (Fig. 1C). We also analyzed migration of injected BMMC in the absence of potentially competing endogenous mast cells and used c-kit-deficient W/W-v mice, which lack mast cells, as hosts (Fig. 1D) (16). For control, wild-type littermates from the same breeding of heterozygote parents were used. Migration of SWAP-70-deficient BMMC into the peritoneum was reduced threefold, and the presence of mutant cells in the blood was increased twofold (not shown) compared to wild-type BMMC. The lower overall migration of wild-type cells to the peritoneum in W/W-v mice may result from the absence of various cytokines and other attractants in these mice.
Polymerization and depolymerization of F-actin drive rearrangements of the actin cytoskeleton, which are essential in mast cells and other cells for changing cell shapes, cell migration, and other processes (45, 46, 52, 55). Since SWAP-70 not only localizes to membrane ruffles but the formation of ruffles also partially depends on SWAP-70 (53), we sought to determine whether F-actin polymerization in BMMC is affected by SWAP-70 deficiency. The total F-actin content of BMMC was measured by FACS after fixation, permeabilization, and TRITC-phalloidin staining of the cells at different time points after c-kit stimulation (Fig. 2A). We defined an F-actin high population represented by the peak of cells intensely stained with rhodamine-phalloidin and monitored changes in this population relative to control cells, i.e., nonstimulated wild-type or SWAP-70−/− BMMC. The assay revealed characteristic patterns of increase and decrease in F-actin content in the cells after stimulation. Although the observed changes are complex, the data demonstrate differences between wild-type and SWAP-70−/− cells at several time points. In wild-type cells, the increase in F-actin high cells peaked at ca. 4 min, whereas in mutant cells the accumulation of F-actin high cells increased faster and continued through at least 10 min, with >90% of the cells being F-actin high at this time point. There was no decrease in mutant F-actin high cells. This suggests altered regulation of F-actin polymerization and/or depolymerization in SWAP-70-deficient cells.
FIG. 2.
Altered F-actin dynamics in SWAP-70−/− BMMC. (A) Total F-actin content was measured by FACS after TRITC-phalloidin staining of the cells. Unstained cells served as controls, and measurements were taken immediately before and at the indicated time points after SCF (6 ng/ml) stimulation. The indicated gate shows the percentage of cells within the F-actin-positive (high) population. (B) Staining of BMMC by TRITC-phalloidin and imaging by fluorescence microscopy revealed filopodia-like protrusions on mutant BMMC, which are best visible at the 5- and 10-min time points. +/+, wild-type BMMC; −/−, SWAP-70−/− BMMC.
Consistent with abnormal F-actin content in mutant BMMC, aberrant formation of multiple elongated F-actin protrusions, which are reminiscent of filopodia, was seen by immunofluorescence all around the mutant cells (Fig. 2B). No such protrusions occurred in wild-type cells. The protrusions started to appear after 2 min of stimulation and are clearly visible at the 5- and 10-min time points. Wild-type cells also appeared generally more polarized than SWAP-70−/− BMMC.
Impaired translocation of Rac.
In a recent in vitro study, SWAP-70 was found to specifically bind Rac and to enhance its activated form (53). Since Rac is a key regulator in c-kit signaling, migration, and cytoskeletal rearrangements, we investigated the requirement for SWAP-70 in Rac activation in this pathway. Besides Rac1, hematopoietic cells express the highly homologous Rac2, which is important for mast cell exocytosis, migration, and survival (12, 19, 67). Total levels of Rac1 and Rac2 are the same in cells of the two genotypes (Fig. 3A).
FIG. 3.
Impaired Rac activation in SWAP-70−/− BMMC. (A) Total amounts of Rac1 or Rac2 in wild-type or mutant BMMC. GST-tagged Rac1 or Rac2 was loaded for controls. (B) Translocation of Rac1 or Rac2 into the detergent-resistant cytoskeletal fraction. The cytoskeletal fraction was analyzed by immunoblotting and probing with antibodies specific for either Rac protein. Actin probing served as the loading control (C) Immunofluorescence analysis of Rac translocation. Starved wild-type or SWAP-70−/− cells were stimulated by SCF (6 ng/ml) for the indicated time periods and stained for actin by rhodamine-phalloidine and for either Rac1 or Rac2 through FITC-labeled secondary antibody. (D) Rac activation measured by a pulldown method that specifically isolates activated Rac, followed by immunoblotting and probing for either Rac1 or Rac2. Rac1-positive controls are very strong, burning out the signal. +/+, wild-type BMMC; −/−, SWAP-70−/− BMMC.
Activated Rac translocates to a detergent-resistant fraction that consists primarily of the cytoskeleton (9, 12, 19, 67). Isolating that fraction and measuring its Rac content serves as an assay for relocalization of activated Rac. Comparing the presence of Rac1 or Rac2 in the detergent-resistant fraction of SWAP-70+/+ or SWAP-70−/− BMMC up to 15 min after SCF stimulation showed efficient translocation in wild-type cells, whereas Rac1 and Rac2 translocated poorly in SWAP-70−/− cells (Fig. 3B). At later time points, Rac accumulates in the cytoskeletal fraction (not shown), pointing to a kinetic impairment in the absence of SWAP-70. The inability of SWAP-70-deficient cells to properly translocate Rac was confirmed in immunofluorescence experiments (Fig. 3C), which showed that Rac1 and Rac2 remain scattered in dots throughout the mutant cells, whereas in wild-type cells Rac localizes to the periphery, overlapping with F-actin.
To determine levels of activated Rac, age-matched wild-type or mutant BMMC were stimulated with SCF, and Rac activation was measured at different time points thereafter by lysing the cells and performing a pull-down assay, in which only activated Rac-GTP is precipitated by PAK1-GST beads, and then analyzed by Western blotting (53). The assay was performed numerous times with cells that had either been starved in the absence of FCS or in the presence of 2% FCS. Equal amounts of PAK1-GST beads loaded on the gel were controlled by anti-GST blotting (not shown). Under none of the starvation conditions did SCF induce a strong increase of activated Rac. The rather low levels of activated Rac that were obtained differed mildly between wild-type and mutant cells (Fig. 3D). At every time point, however, the levels of activated Rac1 and Rac2 were lower in SWAP-70−/− cells than in wild-type cells.
SWAP-70 is required for efficient Ca2+ release.
The release of Ca2+ from intracellular stores and the subsequent influx of Ca2+ from the extracellular environment is a hallmark of c-kit signaling (64) and is negatively affected by F-actin polymerization (44). We assayed the increase of free Ca2+ in wild-type or mutant BMMC upon SCF treatment (Fig. 4A). After the addition of SCF to 100 ng/ml, wild-type cells showed a rapid surge of free Ca2+ within seconds, which then slowly increased further to reach a plateau at ca. 2 min after induction. SWAP-70−/− cells, however, failed to show the initial surge and subsequent increase. After more than 4 min, Ca2+ release was barely above background. At lower SCF doses (30 ng/ml), we did not observe significant Ca2+ release. Ca2+ release per se is not absent in SWAP-70−/− BMMC, since 2 μM ionomycin triggered efficient release, albeit at lower levels than in wild-type BMMC, which indicates a general impairment (Fig. 4B). However, at higher concentrations of ionomycin (10 μM), Ca2+ flux was similar in wild-type and mutant cells (not shown), indicating that only under limiting conditions is ionomycin-triggered Ca2+ release less efficient in the absence of SWAP-70.
FIG. 4.
Impaired Ca2+ flux in SWAP-70−/− BMMC. (A) Ca2+ flux measured by Indo-1 binding upon SCF stimulation. SCF was added at the 50-s time point. (B) Indo-1 binding of Ca2+ triggered by ionomycin (2 μM). The wavelengths 405/485 reflect the ratio of bound to free Ca2+.
Aberrant activation of Akt and ERK.
A number of other signaling factors are instrumental in c-kit signaling and are required either for cell survival, proliferation, or cell-cell interaction, among them Akt (PKB) and ERK. Activation of Akt, triggered by c-kit signaling (3), supports mast cell survival and is partially dependent on Rac (67). Analysis of the total cellular lysates by immunoblotting with phospho-specific anti-Akt antibodies—either for P-Ser 473 or P-Thr 308—and an antibody to total Akt as control revealed no differences between wild-type and SWAP-70−/− BMMC upon SCF stimulation (Fig. 5A and B). However, analyses of the cytoplasmic or membrane fractions showed differences in Akt translocation between cells of the two genotypes (Fig. 5C and D). Some of the Akt protein translocates into a detergent-soluble membrane fraction. In wild-type cells SCF-triggered membrane localization of P-Ser Akt is seen only at the 1-min time point, where total levels of Akt are also highest in the membrane fraction. In mutant cells, phosphorylated Akt peaks later at 4 min and continues to be present. The amounts of total Akt in the membrane fraction remain high in mutant cells. This difference between wild-type and mutant cells may be even more pronounced considering the slightly more intense moesin loading control signal in wild-type lanes. In the cytoplasmic fraction we see similar amounts of total Akt, although slightly reduced at 4 and 8 min in wild-type cells. Phosphorylated Akt parallels this pattern, with mildly higher levels in mutant cells. Thus, the most significant effect of SWAP-70 is on translocation into the membrane fraction. No Akt was found in the insoluble, cytoskeletal protein fraction prepared as described for Rac (not shown). SWAP-70 also translocated within the first minute from the soluble pool to the membrane upon SCF stimulation, indicating that translocation of SWAP-70 and Akt correlate (not shown). Quantification of these data is shown in Fig. S3 in the supplemental material.
FIG. 5.
Activation and translocation of Akt in wild-type or SWAP-70−/− BMMC. (A and B) Generation of Akt phosphorylated at Thr-308 (A) or at Ser-473 (B) at different time points after SCF stimulation, as assessed by immunoblotting of total cell lysates and probing with the respective antibodies. (C and D) Presence of Akt or P-Ser-Akt either in the soluble cytoplasmic fraction (C) or in the NP-40 solubilized membrane fraction (D). (E) Akt kinase activity assay with GSK3α as substrate. The lower image shows the substrate loading control. The kinase activity was quantified by the ImageQuant analysis software (Amersham Biotech) as indicated below the images.
To determine whether SWAP-70 is also required for functionality of Akt as a kinase, we measured the activity of Akt in kinase assays with GSK3α as substrate (Fig. 5E). The activity of Akt is strongly reduced in SWAP-70-deficient BMMC. Thus, SWAP-70 directly or indirectly links translocation and activation of Akt in mast cells.
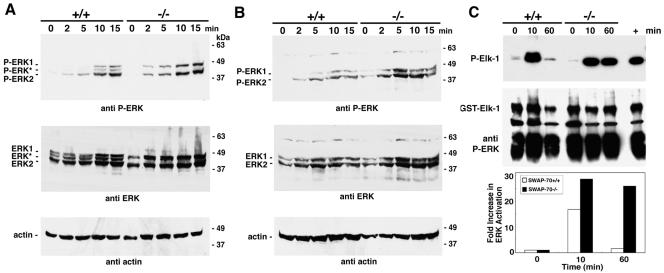
The extracellular signal-regulated kinase 1 (ERK1) and ERK2 constitute a subfamily of MAPKs, which become activated through stimulation of receptor tyrosine kinases such as c-kit. MEK kinase phosphorylates and activates ERK, which is involved in the control of cell proliferation, cell shape, cell mobility, and cell-cell interactions (for reviews, see references 26 and 29). Since signaling through c-kit stimulates ERK (28) and since Rac and its effector PAK have not only been reported to act in parallel to but also upstream of ERK (11, 14, 29), we sought to determine whether ERK activation may be affected by SWAP-70 deficiency. We used Western blotting to test total amounts of ERK1 and ERK2 and to analyze the phosphorylation of ERK1 and ERK2 with a phospho-specific antibody (Fig. 6). Loading was controlled by anti-actin probing of the same blots. In BMMC kept at low 2% serum before or after SCF treatment, total levels of ERK2 are very similar in wild-type and mutant cells, but ERK1 is expressed at slightly higher levels in SWAP-70-deficient BMMC. In wild-type but not mutant BMMC we consistently observed not only weaker bands for ERK1 but also an additional antibody-reactive band migrating between ERK1 and ERK2. If considered a proteolytic form of ERK1, the total levels of ERK1 in wild-type cells may approach those in mutant cells. The intermediate band is not present in nonstarved cells. Upon stimulation of cells in low serum by c-kit, we found phosphorylation of both ERKs to be upregulated in wild-type and mutant cells. In wild-type cells, this includes the intermediate band. However, upregulation became visible at later time points in wild-type cells and appeared somewhat lower than in the mutant (Fig. 6A). If cells were kept at 2% FCS in the presence of IL-3, the intermediate band was absent, and mutant cells appeared to express slightly higher levels of ERK (Fig. 6B). Phosphorylation of both ERKs is significantly lower in wild-type cells. For quantification, see Fig. S4 in the supplemental material. At late time points, e.g., after 1 h of SCF treatment of cells in low serum, phosphorylation is still lower in wild-type than in mutant cells (see Fig. 7D).
FIG. 6.
Activation of ERK in wild-type or SWAP-70−/− BMMC. Total cell lysates from wild-type or mutant BMMC, stimulated by SCF for the indicated times, were immunoblotted and probed with antibodies specific for phosphorylated ERK1/ERK2 or for total ERK1/ERK2 as indicated. Probing for actin served as loading control. Cells were starved in 2% FCS (A) or cells were kept in 2% FCS with IL-3 for 16 h before the experiment (B). (C) ERK kinase activity assay with Elk-1 as substrate and P-ERK pulled down from BMMC lysates (starved without FCS). The lower panel shows for control the input substrate (Elk-1-GST) and the amount of anti-P-ERK antibody used. The kinase activity was quantified by the ImageQuant analysis software as indicated below the images.
FIG. 7.
Increased homotypic association of SWAP-70−/− BMMC. (A) Inhibition of homotypic association induced by 6 ng of SCF/ml (S) by either 5 mM EGTA (E) or by 5 μg of anti-LFA-1 (αLβ2) antibody (αL)/ml. (B) Inhibition of MEK kinase activity by PD98059 (100 μM) or EGTA (5 mM). BMMC, kept at 5% FCS with IL-3, were stimulated with 6 ng of SCF/ml for 2 h, and lysates were prepared and analyzed by immunoblotting and probing with either anti-P-ERK or anti-ERK antibody. +/+, wild-type BMMC; −/−, SWAP-70−/− BMMC. (C) Adhesion to fibronectin of wild-type (open symbols) or mutant cells (solid symbols) in the presence or absence of SCF, measured at different time points as indicated. (D) ERK phosphorylation of cells (grown in 2% FCS) adherent to fibronectin after 1 h in the presence or absence of SCF. For control nonadherent cells, unstimulated or stimulated are also shown.
The activity of ERK was tested in kinase assays that use immunoprecipitated phosphorylated ERK and the transcription factor Elk-1 (GST tagged) as substrate (Fig. 6C). After SCF treatment, serum-starved wild-type and mutant cells initially (10 min and also at 5 min [not shown]) show a similar strong increase in ERK activity. However, ERK remains highly active only in mutant cells at the 60-min time point. Thus, both phosphorylation and kinase activity of ERK are lower in the presence of SWAP-70.
Cell adhesion is regulated by SWAP-70.
Rac1 and Rac2 are required for efficient cell adhesion (18). Therefore, we analyzed homotypic cell adhesion in BMMC cultures stimulated by SCF. Aggregation of BMMC after 24 h in different concentrations of SCF was assessed microscopically. SWAP-70-deficient BMMC show markedly increased formation of homotypic cell aggregates that contain up to ca. 50 cells (Fig. S2A in the supplemental material). Only very few such aggregates were observed in wild-type cell cultures. Quantitation revealed an app. 20-fold increase of aggregates in mutant BMMC. Extracellular Ca2+ is known to be required for signaling by various integrins, which are metalloproteins that bind Ca2+ (35, 47). Hyperaggregation of SWAP-70−/− BMMC depends on extracellular Ca2+ since the addition of EGTA to 5 mM completely abolishes cell aggregation (Fig. 7A). The lymphocyte function-associated antigen 1 (LFA-1; αLβ2), a member of the CD11/CD18 family of integrins (21), is required for mast cell homotypic and heterotypic interactions (27, 63). We sought to determine whether the hyperaggregation seen in mutant BMMC depends on LFA-1 (Fig. 7A). Surface expression of this integrin is not upregulated but rather mildly reduced on SWAP-70−/− BMMC compared to wild-type cells (47.1% for the mutant versus 59.6% for the wild type). We added anti-LFA-1 antibody known to disrupt LFA-1-mediated adhesion to the SCF-stimulated cultures. Very few aggregates of SWAP-70−/− cells were seen after 24 h, suggesting that LFA-1 is crucial in SWAP-70 regulated cell-cell interaction. Other integrins that are expressed on murine mast cells include VLA-4 (27). Surface expression levels for VLA-4 are very similar in wild-type or mutant cells (ca. 75% VLA-4 positive for each genotype) and did not change after SCF treatment. Anti-VLA4 antibodies had no effect on cell-cell associations (not shown).
We report above reduced migration of SWAP-70−/− BMMC in the transwell assay (Fig. 1). The results in this assay may be influenced by enhanced aggregation of BMMC, since large aggregates may not migrate as well through the 8-μm pores. However, cells in the upper chamber were not directly exposed to SCF; that is, they migrate along a gradient, and we saw aggregation only with at least 3 ng of SCF/ml. To further exclude aggregation, we performed the migration assay also in the presence of anti-LFA-1 antibody, which blocks aggregation (Fig. S2B in the supplemental material). This treatment did not significantly affect the efficiency of migration of SWAP-70-deficient cells, and their migration was still clearly reduced compared to wild type. The addition of EGTA completely blocked migration.
Treatment of the cells with the MEK-specific inhibitor PD98059 had no effect on hyperaggregation of SWAP-70−/− BMMC (not shown). Blocking MEK-mediated ERK phosphorylation by PD98059 reduced phosphorylation in wild-type BMMC but was inefficient in SWAP-70−/− BMMC (Fig. 7B), suggesting that activation of ERK is MEK independent in the absence of SWAP-70. Phosphorylation of ERK2 was less affected than that of ERK1, probably since MEK2 is less sensitive to the inhibitor than MEK1. Also, starting amounts of phosphorylated ERK2 are higher than those of ERK1. ERK phosphorylation was dependent on Ca2+, since EGTA abolished it in both cell types.
Recruitment of mast cells from their hematopoietic precursors in the bone marrow to the peripheral tissues through circulation not only requires migration but also integrin-mediated adhesion to tissue components (45). Since we saw aberrant, integrin-dependent homotypic association, we next investigated whether SWAP-70 is also required for adhesion to fibronectin, a well-described constituent of the extracellular matrix in connective tissue (10, 34). Adhesion was measured at a series of time points either in the absence or presence of SCF (Fig. 7C). SCF stimulated adhesion for wild-type and mutant cells to the same relative extent. However, at every time point SWAP-70−/− BMMC adhered to fibronectin with lower efficiency (on average, 1.4- to 2.2-fold lower) than wild-type cells, independent of the addition of SCF. These results fit with the lack of fibronectin-mediated stimulation of migration in the haptotactic migration assay and the lower number of mutant cells observed to adhere to the fibronectin-coated membrane (Fig. 1B). As reported earlier for adhesion of BMMC to fibronectin (31, 34), this adhesion was transient and declined in parallel in wild-type and mutant cells.
There were no homotypic associations of cells adherent to fibronectin, i.e., the adherent cells were all singular. It was noted previously that ERK activation is not required for SCF-mediated adhesion of BMMC to fibronectin (34), and we wondered whether ERK would be aberrantly upregulated in adherent mutant cells. We analyzed the status of ERK phosphorylation in those cells (Fig. 7D). After 1 h of incubation, nonstimulated adherent wild-type cells did not show phosphorylated ERK and, after stimulation by SCF, a rather weak signal was observed. Nonadherent cells more strongly activated ERK, and our data suggest downregulation of SCF-induced ERK phosphorylation in fibronectin-adherent cells. In SCF-stimulated SWAP-70−/− BMMC, adherent to fibronectin, phosphorylation of ERK was much greater than in the corresponding wild-type cells, even if the slightly higher amounts of total ERK in the mutant adherent cells are taken into account. Without SCF, no phosphorylation was seen. Considering the total levels of ERK, ERK phosphorylation of adherent mutant cells was even somewhat higher than that of nonadherent mutant cells. Similar to Fig. 6, SCF-induced phosphorylation was higher in nonadherent mutant cells than in nonadherent wild-type cells. Together, these findings suggest that, unlike in wild-type BMMC, adhesion to fibronectin does not abolish SCF-induced hyperactivation of ERK in SWAP-70−/− BMMC and again illustrates upregulation of ERK phosphorylation in the absence of SWAP-70.
DISCUSSION
Signaling in mast cells through the c-kit receptor is important for the regulation of migration, chemotaxis, survival, cell adhesion, and enhancement of degranulation (1, 38, 50, 59). In this report we identify a new and important component of the c-kit signaling pathway, SWAP-70. Deficiency in SWAP-70 results in impaired c-kit signaling. However, signaling from the c-kit receptor per se is not defective in SWAP-70−/− BMMC. SCF treatment induces proliferation as in wild-type cells and induces tyrosine phosphorylation of a range of proteins similar to the wild-type (not shown), and the radioprotective effect known to be generated through c-kit signaling (43) also occurs normally in the mutant cells (17). The present study rather suggests that a subset of specific c-kit signaling pathways and functions are regulated by SWAP-70.
Translocation of Rac is essential for actin cytoskeletal rearrangements, which are crucial for changes in cell shape, cell motility, cell adhesion, and cytokinesis, and is also involved in phagocytosis, the regulation of secretory vesicle transport in exocytosis, mast cell degranulation, and the regulation of gene expression (reviewed in references 2, 51, and 56). Rac1 and Rac2 are activated to a lesser degree in the absence of SWAP-70, and translocation of the Rac proteins into the detergent-resistant, largely cytoskeletal fraction is strongly impaired. Also, localization of Rac1 and Rac2 to peripheral F-actin is deficient in the absence of SWAP-70. Thus, we hypothesize that SWAP-70 links activated Rac to the actin cytoskeleton. Phenotypes of SWAP-70−/− mast cells that are linked to actin reorganization are likely to be caused by an impairment of this function. Mast cell degranulation, which is reduced in SWAP-70−/− BMMC (17), is accompanied by extensive reshaping of the actin cytoskeleton, reflected by complex changes in polymerization and depolymerization of F-actin (61). SWAP-70 itself has been reported to colocalize with actin structures such as membrane ruffles (53), or with a transient subset of actin filaments (22). The dynamic F-actin profiles in SWAP-70−/− BMMC differ from those in wild-type cells. FACS analysis revealed an increased population of cells high in F-actin shortly after the addition of SCF. The F-actin-positive population is maintained longer than in wild-type cells, suggesting that depolymerization of F-actin rather than its initial synthesis is negatively affected by the absence of SWAP-70. That depolymerization is important for degranulation may explain the impairment of FcɛRI-triggered degranulation in SWAP-70-deficient cells (44). Also, filopodia-like protrusions emerge from the surface of mutant cells, reflecting the disturbed F-actin dynamics in these cells. The accumulation of F-actin and the disturbed balance between RhoGTPases may cause such structures. Since filopodia formation is supported by the Cdc42 Rho GTPase, whereas ruffling and lamellipodia formation is regulated by Rac, we speculate that besides increased F-actin, an impaired Rac function causes a shift in balance between these two GTPases, leading to enhanced filopodia formation. In Cdc42 activation assays we did not see altered Cdc42 activation in SWAP-70-deficient BMMC (not shown), and we therefore hypothesize that disturbance of the balance between Cdc42 and Rac by absence of SWAP-70 may lead to an “overweight” of Cdc42 and thus to increased filopodia formation, even without the need for additional upregulation of Cdc42.
Migration of SWAP-70−/− BMMC toward SCF in a transwell apparatus was reduced up to fourfold in mutant BMMC. This deficiency may be a direct consequence of failing Ca2+ release and may also be caused by aberrant rearrangements of the actin cytoskeleton. In vivo SWAP-70-deficient cells also translocated with reduced efficiency into the peritoneum. The increased retainment of mutant cells in blood excludes the possibility of loss due to cell death, and the equal proliferation and survival rates seen in vitro for mutant and wild-type BMMC or BMC suggest the same (17). Although over time near-normal numbers of mast cells may accumulate in the periphery of SWAP-70-deficient mice, the reduced numbers of peripheral mast cells found in the skin and the peritoneum of mutant mice (17) may indicate a failure in replenishment but may also be explained otherwise, e.g., by a developmental deficiency. The translocation phenotype is likely caused by migration and/or adhesion deficiencies of the mutant cells. The fourfold reduction in chemotaxis toward SCF is similar to the reduced chemotaxis seen in Rac2−/− mast cells (67). This is not the only similarity between SWAP-70−/− and Rac2−/− mast cells: both mutants are impaired in degranulation, Akt activation, or translocation, and the respective mice have decreased numbers of mature, peripheral mast cells (17, 67). However, differences exist, for example, in phosphorylation of Akt, which is entirely absent in Rac2−/− cells but not impaired in SWAP-70−/− cells. In some regards, Rac2 deficiency appears to affect mast cells more severely than the absence of SWAP-70, perhaps since various activators of Rac may (partially) complement SWAP-70's role in Rac activation. There are many more proteins that contain DH domains than there are Rho family GTPases (24, 68). Thus, it is likely that functional differentiation has occurred so that individual Dbl family proteins may act on a given Rho GTPase only in certain cell types and/or upon specific types of cell activation. In addition, coordinated action of several Dbl proteins on one GTPase may be required for full, multieffectorial activation of that GTPase.
Akt kinase acts in multiple processes, including apoptosis and cell survival, proliferation, cell motility, gene expression, and angiogenesis (for a review, see 41). Akt is activated in signaling by several receptors, including c-kit, and depends among others on PI3K and Rac (67). Akt binds to PIP3 in the cytoplasmic membrane via its PH domain and two residues, Ser-473 and Thr-308, become phosphorylated for activation. Subsequently, Akt dissociates from the membrane to act on various substrates. Thus, Akt is present in the cytoplasm either in its inactive or in its fully activated form and resides in between at the cytoplasmic membrane. Here, it can be isolated in detergent-soluble extract fractions. We observed, upon c-kit stimulation, an altered distribution of Akt in SWAP-70−/− BMMC and found significant accumulation of Akt in the membrane fraction at later time intervals than in wild-type cells. Since phosphorylation appears normal, we hypothesize that dissociation of the active form into the cytoplasm is impaired in the absence of SWAP-70. It is possible that reduced Rac activity affects Akt translocation. Consistent with the impaired translocation, we found reduced Akt kinase activity upon c-kit stimulation in SWAP-70-deficient BMMC.
Generally, integrins such as VLA-4 (α4β1) or LFA-1 (αLβ2) are tightly regulated in hematopoietic cells to avoid inappropriate or even dangerous interactions in vivo. The increase in homotypic cell aggregation of SWAP-70−/− BMMC reported here suggests that SWAP-70 is important in negatively regulating integrin-mediated cell-cell adhesion. There is evidence from erythroid progenitor cells that signaling from c-kit affects integrin activity and that this pathway involves ERK (30). Furthermore, VLA-4 or ICAM-1/LFA-1-mediated cell adhesion, at least in certain cell types such as B cells or monocytes, depends on activation of the small GTPase Rho (21, 23, 48, 62), and Rac1 and Rac2 are required for matrix adhesion of neutrophils (18). Our data show that SWAP-70 negatively regulates LFA-1-mediated cell aggregation. Thus, SWAP-70 may through its interaction with Rac balance Rho-mediated activation of cell-cell adhesion. In this respect, SWAP-70 may mediate cross talk between c-kit and integrins and would be involved in regulating inside-out signaling of integrins. SWAP-70 deficiency has a negative, SCF-independent effect on fibronectin adhesion. This suggests that SWAP-70 positively regulates matrix adhesion and thus may balance homotypic with matrix associations.
The reduced levels of adhesion to fibronectin seen with SWAP-70−/− BMMC correspond to a similar reduction of Rac2−/− mast cells (67). In contrast to FcɛRI-promoted adhesion, SCF-mediated fibronectin adhesion has been reported in mast cells to be independent of ERK phosphorylation (34). In erythroid progenitor cells, however, α5β1 (but not α4β1)-mediated adhesion stimulated by SCF results in enhanced and sustained ERK activation (30). Since SWAP-70−/− mast cells, stimulated by SCF and adherent to fibronectin, show enhanced ERK phosphorylation, future analysis of the precise role of SWAP-70 in signaling from these two (and additional) integrins will be warranted.
SWAP-70-deficient BMMC are unable to efficiently release Ca2+ upon c-kit signaling. Although not specifically shown for mast cells, at least two c-kit-dependent pathways exist that cause release of Ca2+. One is the Ras-p38 MAPK pathway; the other involves PI3K (3). Since we observed very little Ca2+ release, it is likely that an upstream step, to be defined in future work, common to the various Ca2+ release signaling chains requires SWAP-70. It has been reported that MAPK-dependent Ca2+ influx activates ERK in B cells or fibroblasts (3). However, the impairment of efficient Ca2+ release observed in SWAP-70-deficient BMMC did not abolish or significantly decrease ERK activation, which, to the contrary, is elevated in these cells. Thus, either the Ca2+ requirement in mast cells for ERK activation is generally low or reduced Ca2+-flux promotes inactivation of a phosphatase that normally would dephosphorylate ERK. Similarly, in mouse embryonic fibroblasts, reduced Ca2+ flux causes prolonged ERK phosphorylation (8). However, Ca2+ depletion by EGTA treatment substantially reduced ERK phosphorylation in wild-type and mutant cells, indicating that a putative phosphatase in these cells does not require Ca2+. MEK kinase inhibition experiments indicate that ERK activation in SWAP-70-deficient cells is not as dependent on this kinase as it is in wild-type cells. Possibly, an alternative kinase is hyperactive, a phosphatase is downregulated, or the phosphorylated protein is more stable in the absence of SWAP-70. The results also suggest that SWAP-70 is involved in linking MEK to ERK.
Together, there are several signaling deficiencies in SWAP-70-deficient mast cells, including FcɛRI-triggered degranulation, and as reported here in c-kit signaling. We speculate that many, if not all, of these deficiencies are caused by a failure in the key interactions SWAP-70 takes part in, that is, the interaction with Rac and F-actin. Impaired Rac function and aberrant turnover of F-actin may cause migration, adhesion, and degranulation deficiencies and failure to activate downstream signaling molecules.
Obviously, the individual functions of SWAP-70 in c-kit signaling may not be limited to those shown and certainly need to be further elucidated. With its newly revealed dual role in FcɛRI (17) and in c-kit signaling in mast cells, SWAP-70 not only contributes important functions to mast cell biology but may also be considered important for the understanding of mast cell differentiation and of mast cell-dependent allergic reactions, in which both pathways come to bear.
Supplementary Material
Acknowledgments
We thank Shuang Fu and Svetlana Kupershtokh for technical help and Glen Pearce for critically reading the manuscript. We also thank Yasuhisa Fukui and D. Illenberger for the Rac1-GST and Rac2-GST constructs, respectively.
This study was supported by grants from the NIH (AI49282) and from Hoffmann LaRoche, Inc., of Switzerland.
Footnotes
Supplemental material for this article may be found at http://mcb.asm.org/.
REFERENCES
- 1.Ashman, L. K. 1999. The biology of stem cell factor and its receptor c-kit. Int. J. Biochem. Cell. Biol. 31:1037-1051. [DOI] [PubMed] [Google Scholar]
- 2.Bar-Sagi, D., and A. Hall. 2000. Ras and Rho GTPases: a family reunion. Cell 103:227-238. [DOI] [PubMed] [Google Scholar]
- 3.Blume-Jensen, P., R. Janknecht, and T. Hunter. 1998. The kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 8:779-782. [DOI] [PubMed] [Google Scholar]
- 4.Borggrefe, T., M. Wabl, A. T. Akhmedov, and R. Jessberger. 1998. A B-cell specific DNA recombination complex. J. Biol. Chem. 273:17025-17035. [DOI] [PubMed] [Google Scholar]
- 5.Borggrefe, T., L. Masat, M. Wabl, B. Riwar, G. Cattoretti, and R. Jessberger. 1999. Cellular, intracellular, and developmental expression patterns of murine SWAP-70. Eur. J. Immunol. 29:1812-1822. [DOI] [PubMed] [Google Scholar]
- 6.Borggrefe, T., T. Keshavarzi, B. Gross, M. Wabl, and R. Jessberger. 2001. Impaired IgE response in SWAP-70 deficient mice. Eur. J. Immunol. 31:2467-2475. [DOI] [PubMed] [Google Scholar]
- 7.Broudy, V. C. 1997. Stem cell factor and hematopoiesis. Blood 90:1345-1364. [PubMed] [Google Scholar]
- 8.Coleman, M. L., and C. J. Marshall. 2001. A family outing: small GTPases cyclin through G1. Nat. Cell. Biol. 3:E250-251. [DOI] [PubMed] [Google Scholar]
- 9.Croker, B. A., D. M. Tarlinton, L. A. Cluse, A. J. Tuxen, A. Light, F. C. Yang, D. A. Williams, and A. W. Roberts. 2002. The Rac2 guanosine triphosphatase regulates B lymphocyte antigen receptor responses and chemotaxis and is required for establishment of B-1a and marginal zone B lymphocytes. J. Immunol. 168:3376-3386. [DOI] [PubMed] [Google Scholar]
- 10.Dastych, J., and D. D. Metcalfe. 1994. Stem cell factor induces mast cell adhesion to fibronectin. J. Immunol. 152:213-219. [PubMed] [Google Scholar]
- 11.Eblen, S. T., J. K. Slack, M. J. Weber, and A. D. Catling. 2002. Rac-PAK signaling stimulates extracellular signal-regulated kinase (ERK) activation by regulating formation of MEK1-ERK complexes. Mol. Cell. Biol. 22:6023-6033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.el Benna, J., J. M. Ruedi, and B, M. Babior. 1994. Cytosolic guanine nucleotide-binding protein Rac2 operates in vivo as a component of the neutrophil respiratory burst oxidase: transfer of Rac2 and the cytosolic oxidase components p47phox and p67phox to the submembranous actin cytoskeleton during oxidase activation. J. Biol. Chem. 269:6729-6734. [PubMed] [Google Scholar]
- 13.Etienne-Manneville, S., and A. Hall. 2002. Rho GTPases in cell biology. Nature 420:629-635. [DOI] [PubMed] [Google Scholar]
- 14.Frost, J. A., H. Steen, P. Shapiro. T. Lewis, N. Ahn, P. E. Shaw, and M. H. Cobb. 1997. Cross-cascade activation of ERKs and ternary complex factors by Rho family proteins. EMBO J. 16:6426-6438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Galli, S. J. 2000. Mast cells and basophils. Curr. Opin. Hematol. 7:32-39. [DOI] [PubMed] [Google Scholar]
- 16.Galli, S. J., and Y. Kitamura. 1987. Animal model of human disease. Genetically mast cell-deficient W/Wv and Sl/Sld mice: their value for the analysis of the roles of mast cells in biological responses in vivo. Am. J. Pathol. 127:191-198. [PMC free article] [PubMed] [Google Scholar]
- 17.Gross, B., T. Borggrefe, M. Wabl, R. R. Sivalenka, M. Bennett, A. B. Rossi, and R. Jessberger. 2002. SWAP-70 deficient immature mast cells are blocked in IgE-mediated degranulation. Eur. J. Immunol. 32:1121-1128. [DOI] [PubMed] [Google Scholar]
- 18.Gu, Y., M. D. Filippi, J. A. Cancels, J. E. Siefring, E. P. Williams, A. C. Jasti, C. E. Harris, A. W. Lee, R. Prabhakar, S. J. Atkinson, D. J. Kwiatkowski, and D. A. Williams. 2003. Hematopoietic cell regulation by Rac1 and Rac2 guanosine triphosphatases. Science 302:445-449. [DOI] [PubMed] [Google Scholar]
- 19.Gulbins, E., K. M. Coggeshall, B. Brenner, K. Schlottmann. O. Linderkamp, and F. Lang. 1996. Fas-induced apoptosis is mediated by activation of a Ras and Rac protein-regulated signaling pathway. J. Biol. Chem. 271:26389-26394. [DOI] [PubMed] [Google Scholar]
- 20.Gupta, S., A. Lee, C. Hu, J. Fanzo, I. Goldberg, G. Cattoretti, and A. B. Pernis. 2003. Molecular cloning of IBP, a SWAP-70 homologous GEF, which is highly expressed in the immune system. Hum. Immunol. 64:389-401. [DOI] [PubMed] [Google Scholar]
- 21.Harris, E. S., T. M. McIntyre, S. M. Prescott, and G. A. Zimmerman. 2000. The leukocyte integrins. J. Biol. Chem. 275:23409-23412. [DOI] [PubMed] [Google Scholar]
- 22.Hilpela, P., P. Oberbanscheidt, P. Hahne, M. Hund, G. Kalhammer, J. V. Small, and M. Bähler. 2003. SWAP-70 identifies a transitional subset of actin filaments in motile cells. Mol. Biol. Cell 14:3242-3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hmama, Z., K. L. Knutson, P. Herrera-Velit, D. Nandan, and N. E. Reiner. 1999. Monocyte adherence induced by lipopolysaccharide involves CD14, LFA-1, and cytohesin-1: regulation by Rho and phosphatidylinositol 3-kinase. J. Biol. Chem. 274:1050-1057. [DOI] [PubMed] [Google Scholar]
- 24.Hoffmann, G. R., and R. A. Cerione. 2002. Signaling to the Rho GTPases: networking with the DH domain. FEBS Lett. 513:85-91. [DOI] [PubMed] [Google Scholar]
- 25.Hotfilder, M., S. Baxendale, M. A. Cross, and F. Sablitzky. 1999. Def-2, -3, -6, and -8, novel mouse genes differentially expressed in the haemopoietic system. Br. J. Haematol. 106:335-344. [DOI] [PubMed] [Google Scholar]
- 26.Howe, A., A. E. Aplin, and R. L. Juliano. 2002. Anchorage-dependent ERK signaling: mechanisms and consequences. Curr. Opin. Genetics Dev. 12:30-35. [DOI] [PubMed] [Google Scholar]
- 27.Inamura, N., Y. A. Mekori, S. P. Bhattacharyya, P. J. Bianchine, and D. D. Metcalfe. 1998. Induction and enhancement of FcεRI-dependent mast cell degranulation following coculture with activated T cells: dependency on ICAM-1- and leukocyte function-associated antigen (LFA)-1-mediated heterotypic aggregation. J. Immunol. 160:4026-4033. [PubMed] [Google Scholar]
- 28.Ishizuka, T., K. Chayama, K. Takeda. E. Hamelmann, N. Terada, G. M. Keller, G. L. Johnson, and G. M. Gelfand. 1999. Mitogen-activated protein kinase activation through Fc epsilon receptor I and stem cell factor receptor is differentially regulated by phosphatidylinositol 3-kinase and calcineurin in mouse bone marrow-derived mast cells. J. Immunol. 162:2087-2094. [PubMed] [Google Scholar]
- 29.Johnson, G. L., and R. Lapadat. 2002. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 298:1911-1912. [DOI] [PubMed] [Google Scholar]
- 30.Kapur, R., R. Cooper, L. Zhang, and D. A. Williams. 2001. Cross-talk between α4β1/α5β1 and c-Kit results in opposing effect on growth and survival of hematopoietic cells via the activation of focal adhesion kinase, mitogen-activated protein kinase, and Akt signaling pathways. Blood 97:1975-1981. [DOI] [PubMed] [Google Scholar]
- 31.Kinashi, T., and T. A. Springer. 1994. Steel factor and c-kit regulate cell-matrix adhesion. Blood 83:1033-1038. [PubMed] [Google Scholar]
- 32.Kitamura, Y., K. Hatanaka, M. Murakami, and H. Shibata. 1979. Presence of mast cell precursors in peripheral blood of mice demonstrated by parabiosis. Blood 53:1085-1088. [PubMed] [Google Scholar]
- 33.Kraynov, V. S., C. Chamberlain, G. M. Bokoch, M. A. Schwartz, S. Slabaugh, and K. M. Hahn. 2000. Localized Rac activation dynamics visualized in living cells. Science 290:333-337. [DOI] [PubMed] [Google Scholar]
- 34.Lam, V., J. Kalesnikoff, C. W. Lee, V. Hernandez-Hansen, B. S. Wilson, J. M. Oliver, and G. Krystal. 2003. IgE alone stimulates mast cell adhesion to fibronectin via pathways similar to those used by IgE + antigen but distinct from those used by steel factor. Blood 102:1405-1413. [DOI] [PubMed] [Google Scholar]
- 35.Leitinger, B., A. McDowall, P. Stanle, and N. Hoog. 2000. The regulation of integrin function by Ca2+. Biochim. Biophys. Acta 1498:91-98. [DOI] [PubMed] [Google Scholar]
- 36.Lemmon, M. A., K. M. Ferguson, and C. S. Abrams. 2002. Pleckstrin homology domains and the cytoskeleton. FEBS Lett. 513:71-76. [DOI] [PubMed] [Google Scholar]
- 37.Li, L., and S. A. Krilis. 1999. Mast-cell growth and differentiation. Allergy 54:306-312. [DOI] [PubMed] [Google Scholar]
- 38.Linnekin, D. 1999. Early signaling pathways activated by c-Kit in hematopoietic cells. Int. J. Biochem. Cell. Biol. 31:1035-1074. [DOI] [PubMed] [Google Scholar]
- 39.Masat, L., J. Caldwell, R. Armstrong, H. Khoshnevisan, R. Jessberger, B. Herndier, M. Wabl, and D. Ferrick. 2000. Association of SWAP-70 with the B-cell antigen receptor complex. Proc. Natl. Acad. Sci. USA 97:2180-2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Metcalfe, D. D., D. Baram, and Y. A. Mekori. 1997. Mast cells. Physiol. Rev. 77:1033-1079. [DOI] [PubMed] [Google Scholar]
- 41.Michael, P., J. Scheid, and R. Woodgett. 2003. Unraveling the activation mechanisms of protein kinase B Akt. FEBS Lett. 546:108-112. [DOI] [PubMed] [Google Scholar]
- 42.Mitsui, H., T. Furitsu, A. M. Dvorak, A. M. Irani, L. B. Schwartz, N. Inagaki, M. Takei, K. Ishizaka, K. M. Zsebo, S. Gillis, et al. 1993. Development of human mast cells from umbilical cord blood cells by recombinant human and murine c-kit ligand. Proc. Natl. Acad. Sci. USA 90:735-739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nelson, S. Y., I. Paek, and P. Besmer. 1994. Role of kit-ligand in proliferation and suppression of apoptosis in mast cells: basis for radiosensitivity of white spotting and steel mutant mice. J. Exp. Med. 179:1777-1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oka, T., K. Sato, M. Hori, H. Ozaki, and H. Karaki. 2002. FcɛRI cross-linking-induced actin assembly mediates calcium signalling in RBL-2H3 mast cells. Br. J. Pharm. 136:837-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Okayama, Y. 2000. Mast cell matrix interaction. Clin. Exp. Allergy 30:455-457. [DOI] [PubMed] [Google Scholar]
- 46.Pfeiffer, J. R., J. C. Seagrave, B. H. Davis, G. G. Deanin, and J. M. Oliver. 1985. Membrane and cytoskeletal changes associated with IgE-mediated serotonin release from rat basophilic leukemia cells. J. Cell Biol. 101:2145-2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Plow, E. F., T. A. Haas, L. Zhang, J. Loftus, and J. W. Smith. 2000. Ligand binding to integrins. J. Biol. Chem. 275:21758-21788. [DOI] [PubMed] [Google Scholar]
- 48.Ridley, A. J. 2001. Rho family proteins: coordinating cell responses. Trends Cell. Biol. 11:471-477. [DOI] [PubMed] [Google Scholar]
- 49.Rodewald, H. R., M. Dessing, A. M. Dvorak, and S. J. Galli. 1996. Identification of a committed precursor for the mast cell lineage. Science 271:818-822. [DOI] [PubMed] [Google Scholar]
- 50.Rossi, P., C. Sette, S. Dolci, and R. Geremia. 2000. Role of c-kit in mammalian spermatogenesis. J. Endocrinol. Investig. 23:609-615. [DOI] [PubMed] [Google Scholar]
- 51.Schmitz, A. A., E. E. Govek, B. Bottner, and L. van Aelst. 2000. Rho GTPases: signaling, migration, and invasion. Exp. Cell Res. 261:1-12. [DOI] [PubMed] [Google Scholar]
- 52.Schoenenberger, C. A., N. Bischler, B. Fahrenkrog, and U. Aebi. 2002. Actin's propensity for dynamic filament patterning. FEBS Lett. 529:27-33. [DOI] [PubMed] [Google Scholar]
- 53.Shinohara, M., Y. Terada, A. Iwamatsu, A. Shinohara, N. Mochizuki, S. Ihara, S. Nagata, H. Itoh, Y. Fukui, and R. Jessberger. 2002. SWAP-70 is a guanine nucleotide exchange factor that mediates signaling of membrane ruffling. Nature 416:759-763. [DOI] [PubMed] [Google Scholar]
- 54.Sonoda, T., T. Ohno, and Y. Kitamura. 1982. Concentration of mast-cell progenitors in bone marrow, spleen, and blood of mice determined by limiting dilution analysis. J. Cell Physiol. 112:136-140. [DOI] [PubMed] [Google Scholar]
- 55.Sullivan, R., M. Burnham, K. Török, and A. Koffer. 2000. Calmodulin regulates the disassembly of cortical F-actin in mast cells but is not required for secretion. Cell Calcium 28:33-46. [DOI] [PubMed] [Google Scholar]
- 56.Takai, Y., T. Sasaki, and T. Matozaki. 2001. Small GTP-binding proteins. Physiol. Rev. 81:153-189. [DOI] [PubMed] [Google Scholar]
- 57.Tanaka, Y., K. Bi, R. Kitamura, S. Hong, Y. Altman, A. Matsumoto, T. Tabata, S. Lebedeva, P. J. Bushway, and A. Altman. 2003. SWAP-70-like adapter of T cells, an adapter protein that regulates early TCR-initiated signaling in Th2 lineage cells. Immunity 18:403-414. [DOI] [PubMed] [Google Scholar]
- 58.Taylor, M. L., and D. D. Metcalfe. 2000. Kit signal transduction. Hematol. Oncol. Clin. N. Am. 14:517-535. [DOI] [PubMed] [Google Scholar]
- 59.Thomas, G. J., M. P. Lewis, S. A. Whawell, A. Russell, D. Sheppard, I. R. Hart, P. M. Speight, and J. F. Marshall. 2001. Expression of αVβ6 integrin promotes migration and invasion in squamous carcinoma cells. J. Investig. Dermatol. 117:67-73. [DOI] [PubMed] [Google Scholar]
- 60.Timokhina, I., H. Kissel, G. Stella, and P. Besmer. 1998. Kit signaling through PI 3-kinase and Src kinase pathways: an essential role for Rac1 and JNK activation in mast cell proliferation. EMBO J. 17:6250-6262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tolarova, H., L. Draberova, P. Heneberg, and P. Draber. 2004. Involvement of filamentous actin in setting the threshold for degranulation in mast cells. Eur. J. Immunol. 34:1627-1636. [DOI] [PubMed] [Google Scholar]
- 62.Tominaga, T., K. Sugie, M. Hirata, N. Morii, J. Fukata, A. Uchida, H. Imura, and S. Narumiya. 1993. Inhibition of PMA-induced, LFA-1-dependent lymphocyte aggregation by ADP-ribosylation of the small molecular weight GTP binding protein, Rho. J. Cell Biol. 120:1529-1537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Toru, H., T. Kinashi, C. Ra, S. Nonoyama, J. Yata, and T. Nakahata. 1997. Interleukin-4 induces homotypic aggregation of human mast cells by promoting LFA-1/ICAM-1 adhesion molecules. Blood 89:3296-3302. [PubMed] [Google Scholar]
- 64.Ueda, S., M. Mizuki, H. Ikeda, T. Tsujimura, I. Matsumura, K. Nakano, H. Daino, Z. Z. Honda, J. Sonoyama, H. Shibayama, H. Sugahara, T. Machii, and Y. Kanakura. 2002. Critical roles of c-Kit tyrosine residues 567 and 719 in stem cell factor-induced chemotaxis: contribution of src family kinase and PI3-kinase on calcium mobilization and cell migration. Blood 99:3342-3349. [DOI] [PubMed] [Google Scholar]
- 65.Valent, P., E. Spanblochl, W. R. Sperr, C. Sillaber, K. M. Zsebo, H. Agis, H. Strobl, K. Geissler, P. Bettelheim, and K. Lechner. 1992. Induction of differentiation of human mast cells from bone marrow and peripheral blood mononuclear cells by recombinant human stem cell factor/kit-ligand in long-term culture. Blood 80:2237-2245. [PubMed] [Google Scholar]
- 66.van Weeren, P. C., K. M. de Bruyn, A. M. de Vries-Smits, J. van Lint, and B. M. Burgering. 1998. Essential role for protein kinase B (PKB) in insulin-induced glycogen synthase kinase 3 inactivation: characterization of dominant-negative mutant of PKB. J. Biol. Chem. 273:13150-13156. [DOI] [PubMed] [Google Scholar]
- 67.Yang, F. C., R. Kapur, A. J. King, W. Tao, C. Kim, J. Borneo, R. Breese, M. Marshall, M. C. Dinaur, and D. A. Williams. 2000. Rac2 stimulates Akt activation affecting BAD/Bcl-XL expression while mediating survival and actin function in primary mast cells. Immunity 12:557-568. [DOI] [PubMed] [Google Scholar]
- 68.Zheng. Y. 2001. Dbl family guanine nucleotide exchange factors. Trends Biochem. Sci. 26:724-732. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.