Abstract
Researchers have sought to distinguish between individuals whose alcohol use disorder (AUD) is maintained by drinking to relieve negative affect (“relief drinkers”) and those whose AUD is maintained by the rewarding effects of alcohol (“reward drinkers”). As an opioid receptor antagonist, naltrexone may be particularly effective for reward drinkers. Acamprosate, which has been shown to down-regulate the glutamatergic system, may be particularly effective for relief drinkers. This study sought to replicate and extend prior work (PREDICT study; Glöckner-Rist et al. 2013) by examining dimensions of reward and relief temptation to drink and subtypes of individuals with distinct patterns of reward/relief temptation. We utilized data from two randomized clinical trials for AUD (Project MATCH, n=1726 and COMBINE study, n=1383). We also tested whether classes of reward/relief temptation would predict differential response to naltrexone and acamprosate in COMBINE. Results replicated prior work by identifying reward and relief temptation factors, which had excellent reliability and construct validity. Using factor mixture modeling, we identified 5 distinct classes of reward/relief temptation that replicated across studies. In COMBINE, we found a significant class-by-acamprosate interaction effect. Among those most likely classified in the high relief/moderate reward temptation class, individuals had better drinking outcomes if assigned to acamprosate versus placebo. We did not find a significant class-by-naltrexone interaction effect. Our study questions the orthogonal classification of drinkers into only two types (reward or relief drinkers) and adds to the body of research on moderators of acamprosate, which may inform clinical decision making in the treatment of AUD.
Keywords: acamprosate, naltrexone, alcohol use disorder, temptation
Introduction
Across various models of alcohol use disorder (AUD), different processes are implicated in the development and progression of AUD (Skinner & Aubin 2010). The allostatic model of addiction (Koob 2003; Koob & Volkow 2010), which builds on the opponent process theory (Solomon & Corbit 1974), posits that early stages of addiction are primarily driven by positive reinforcement and later and more severe stages of addiction are driven by negative reinforcement. The incentive-sensitization theory (Robinson & Berridge 1993) highlights that repeated alcohol use leads to hypersensitization (progressively increasing responses) of the dopamine neural system, which enhances the incentive salience of alcohol, thereby increasing subjective “wanting” for alcohol. Relatedly, Fields and Cox (2008) proposed that sensitization of the dopamine system drives alcohol attentional biases for alcohol-related cues, which increases alcohol-seeking behavior. Finally, the motivational model of AUD (Cox & Klinger 1988) claims that individuals make conscious choices to consume alcohol when they are motivated by a desire to improve their affective state through alcohol consumption.
There may be distinct types of individuals with AUD whose alcohol use is differentially driven by these processes. In fact, research has focused on how different AUD treatments may differentially impact AUD outcomes depending on what underlying processes are maintaining drinking behaviors (Heilig, Goldman, Berrettini, & O’Brien 2011; Mann et al. 2009; Ray 2012). Verheul et al. (1999) proposed that “reward cravers,” individuals whose drinking is maintained through the rewarding effects of alcohol, may respond better to naltrexone and “relief cravers,” individuals whose drinking is maintained through consuming alcohol to relieve negative affective states, may respond better to acamprosate. Researchers have elaborated on hypotheses regarding reward and relief craving as potential moderators of naltrexone and acamprosate, respectively, for AUD (Addolorato, Abenavoli, Leggio, & Gasbarrini 2005; Heilig et al. 2011; Mann, Kiefer, Spanagel, & Littleton 2008; Mann et al. 2009; Ooteman 2005). Specifically, because naltrexone is an opioid receptor antagonist that may reduce the positive rewarding effects of alcohol (Myrick et al. 2008), it may be particularly effective for reward cravers who primarily crave alcohol in contexts associated with rewarding effects of alcohol (Worley et al. 2015). Because acamprosate has been shown to down-regulate the glutamatergic system (Spanagel & Zieglgänsberger 1997; Spanagel & Kiefer 2008), it may be particularly effective for relief cravers who primarily crave alcohol in contexts associated with relieving effects of alcohol.
Mann and the PREDICT study team (2014) examined whether naltrexone was most efficacious among individuals with elevated pre-treatment cue-induced ventral striatum (VS) activation, an indication of elevated sensitivity to the rewarding effects of alcohol. As hypothesized, they found that naltrexone, but not acamprosate, predicted better alcohol treatment outcomes among individuals with elevated cue-induced VS activation.
Measuring Reward and Relief Types of AUD Clients
Neuroimaging may be a powerful method to identify functional client factors (Mann et al. 2014), yet neuroimaging is not a practical approach for treatment matching in clinical practice. To move closer to precision medicine in AUD treatment, we must also develop valid, reliable, and practical tools (e.g., self-report measures) for measuring functional client treatment matching factors, such as reward and relief drinking tendencies. Researchers are beginning to examine these types of tools. There are preliminary studies on the Amsterdam Motives for Drinking Scale (Ooteman, Koeter, Verheul, Schippers, & van Den Brink 2006), the Craving Typology Questionnaire (Martinotti et al. 2013), the Reasons for Heavy Drinking Questionnaire (Adams, Schacht, Randall, & Anton, 2016), the Alcohol Abstinence Self-Efficacy Scale (AASE; Glöckner-Rist et al. 2013), and the Reward-Relief Drinking Scale (RRDS; Mann & Nakovics under review) as self-report methods for identifying reward/relief drinking tendencies. More research is needed to replicate these initial findings and clarify which methods are most effective for identifying reward and relief tendencies.
Current Study
We focused on replicating and extending findings from analyses of PREDICT data (Glöckner-Rist et al. 2013), which examined the Temptation scale of the Alcohol Abstinence Self Efficacy Scale (AASE; DiClemente et al. 1994) to assess relief and reward temptation. Reward temptation is the degree to which one feels compelled to drink in contexts (i.e., positive affect and social situations) associated with rewarding effects of alcohol. Relief temptation is the degree to which one feels compelled to drink in contexts (i.e., negative affect) associated with relieving effects of alcohol. Glöckner-Rist et al. (2013) found initial support for using 10 items from the AASE to assess continuous dimensions of relief and reward temptation. Glöckner-Rist et al. (2013) also examined subgroups based on these dimensions and found four subgroups: a subgroup with high relief tendencies, a subgroup with high reward tendencies, a subgroup that was high on both dimensions, and a subgroup that was low on both dimensions.
In this study we utilized data from two alcohol treatment studies: COMBINE (Anton et al. 2006) and Project MATCH (Project MATCH Research Group 1997). Our first aim was to provide further support for using 10 items from the Temptation Scale of the AASE as a valid and reliable assessment of relief and reward temptation. We examined the same factor model presented by PREDICT (Glöckner-Rist et al. 2013) and examined the construct validity of these constructs by evaluating their associations with age, dependence severity, depressive symptoms, and percentage of drinkers in one’s social network. Furthermore, we conducted analyses to identify distinct subgroups of individuals characterized by varying patterns of reward and relief temptation.
We then evaluated whether reward/relief subgroups would predict differential response to naltrexone and acamprosate in the COMBINE study. Primary analyses from COMBINE found that naltrexone produced improved outcomes relative to placebo, but acamprosate did not produce improved outcomes relative to placebo (Anton et al. 2006). Based on theoretical work (Mann et al. 2008; Verheul et al. 1999), we hypothesized that individuals characterized by higher relief temptation, relative to reward temptation, would have better drinking outcomes if randomly assigned to active acamprosate and individuals with higher reward temptation, relative to relief temptation, would have a better treatment response if randomly assigned to active naltrexone.
Materials and Method
Participants and Procedure
We utilized data from two randomized clinical trials on treatments for AUD: the COMBINE study (Anton et al. 2006) and Project MATCH (Project MATCH Research Group 1997). COMBINE was a multi-site randomized clinical trial evaluating combinations of medications and outpatient-based psychosocial interventions for AUD. Individuals in eight of the treatment groups were randomly assigned to receive 1) either active naltrexone (100mg/day) or placebo naltrexone, 2) either active acamprosate (3mg/day) or placebo acamprosate, and 3) either medication management with a combined behavioral intervention (CBI) or medication management alone. A ninth group received only CBI. For more information on the CBI see Miller et al. (2004). All medication and psychosocial interventions were delivered over the course of 16 weeks and participants were followed up with for up to a year after treatment. Demographic data among the full COMBINE sample (n = 1,383) were: male (68.8%), mean age = 44.43 (SD = 10.19), non-Hispanic white (76.7%), Black/African American (7.9%), Asian (0.3%), Hispanic (11.2%), American-Indian/Alaskan Native (1.3%), multi-racial (1.3%), other race (1.2%). For further details on the design of COMBINE see COMBINE Study Research Group (2003).
Project MATCH was a multi-site randomized clinical trial comparing three psychosocial treatments for AUD: Cognitive Behavioral Therapy, Motivational Enhancement Therapy, or Twelve-Step Facilitation. Project MATCH involved an outpatient arm (n = 952) and an aftercare arm (n = 774). Treatment was delivered over the course of 12 weeks and participants were followed up with for up to a year after treatment. Demographic data among the full sample (n = 1,726) were: male (75.7 %), mean age = 40.23 (SD = 10.99), non-Hispanic white (80.0 %), Black/African American (9.8%), Asian (0.1%), Hispanic (8.2%), American-Indian/Alaskan Native (1.4%), other race (0.5%). For further details on the design of Project MATCH see Project MATCH Research Group (1993).
Measures
Reward and relief temptation
In COMBINE and MATCH, 10 items from the Temptation subscale of the Alcohol Abstinence Self-Efficacy Scale (AASE; DiClemente et al. 1994) were used to assess reward and relief temptation at baseline. The original Temptation subscale of the AASE includes 20 items and asks participants to indicate the degree to which they are tempted to drink in various situations on a scale ranging from 1 (not at all tempting) to 5 (extremely tempting). The 10 items were selected based on work by PREDICT (Glöckner-Rist et al. 2013). The internal consistency reliability for both factors was high: relief temptation (COMBINE: α = .88; MATCH: α = .88) and reward temptation (COMBINE: α = .92; MATCH: α = .90).
Baseline covariates
The number of alcohol dependence criteria endorsed on the Structured Clinical Interview for DSM (version IV in COMBINE and version III- R in MATCH; Spitzer et al. 1992; First et al. 1997) was used to assess dependence severity, and the Important People and Activities instrument (IPA; Clifford & Longabaugh 1991) was used to assess percentage of drinkers in one’s social network. In MATCH, the Beck Depression Inventory (BDI; Beck, Steer, & Garbin 1988) was used to assess depressive symptoms. Reliability of the BDI was high (α = .89). In COMBINE, the depression subscale of the Brief Symptom Inventory (BSI; Derogatis 1993) was used to assess depressive symptoms. Reliability of the depression subscale of the BSI was high (α = .87). In COMBINE, data from the physical exam was used to assess body mass index (BMI).
Alcohol use outcomes
In COMBINE, alcohol consumption was measured using calendar-based methods: the Form-90 (Miller 1996) and the Timeline Follow-back Interview (TLFB; Sobell & Sobell 1992). We examined two indices of alcohol consumption: percent drinking days (PDD) and percent heavy drinking days (PHD; with heavy drinking defined as 4+/5+ drinks for women/men). We examined PDD and PHD during the 30 days prior to the baseline assessment, the end-of-treatment assessment (week 16), and the first post-treatment follow-up assessment (week 26).
Statistical Analyses
Missing data
In COMBINE, only 7 participants out of the full sample (n = 1383) had no available AASE data. In MATCH, only 38 participants out of the full sample (n = 1726) had no available AASE data. For all models we used all available data and parameters were estimated with full information maximum likelihood (Witkiewitz et al. 2014).
Confirmatory factor analyses
Mplus Version 7.2 (Muthén & Muthén 2012) was used to conduct all latent variable models. In COMBINE and MATCH, we used confirmatory factor analysis (CFA) to test a latent variable model in which a reward temptation latent factor predicted scores on five items of the AASE (items 4, 8, 15, 17, 20), and a relief temptation latent factor predicted scores on five items of the AASE (items 3, 6, 12, 16, 18). Model fit was evaluated by χ2 values, the Root Mean Square Error of Approximation (RMSEA), and the Comparative Fit Index (CFI). Models with RMSEA < 0.05, and CFI > 0.95 were considered a good fit to the observed data (Hu & Bentler 1999). Models with RMSEA < 0.08 and CFI > 0.90 were considered a reasonable fit.
Construct validity analyses
We assessed the construct validity of the temptation factors by conducting structural regression models in which the temptation factors were examined as associated with age, dependence severity, depressive symptoms, and percentage of drinkers in one’s social network.
Factor Mixture Models
We utilized factor mixture modeling (FMM) to identify subgroups of individuals with varying patterns of reward and relief temptation. FMM combines factor analysis and latent class analysis (Clark et al. 2013). In this study, the FMM included the continuous reward and relief factors, as well as a single categorical latent class variable, which in turn allowed for the classification of individuals into distinct subgroups. Consistent with recommendations (Collins & Lanza 2010), we considered a variety of factors to determine the optimal class solution: 1) the Lo-Mendell-Rubin Adjusted Likelihood Ratio Test (Lo et al. 2001), which compares whether a k class solution fits better than a k – 1 class solution, 2) Akaike’s Information Criterion (AIC), Bayesian Information Criterion (BIC), and sample size adjusted BIC (aBIC), with lower values indicating better fit, 3) entropy values, with higher value indicating better classification precision, and, 4) the parsimony and theoretical utility of the class solution. Parameters of interest were: latent class prevalences and the probability of each response for the reward and relief items given expected classification in a particular latent class. We conducted a series of FMMs in COMBINE and determined the optimal class solution. Then we conducted FMMs in MATCH to determine whether the class solution could be replicated in a separate sample.
Moderation analyses in COMBINE
We examined whether latent class interacted with medication assignment in the prediction of drinking outcomes, utilizing the inclusive classify-analyze approach, as described by Bray, Lanza, and Tan (2015). In line with this approach, we included the outcome variable of interest as a covariate in the FMM, which in turn was used to derive posterior probabilities of class membership. Individuals were then assigned class membership based on these posterior probabilities. A series of dummy-coded variables (e.g., 1 = classified to class x, 0 = not classified to class x) were created to be used in the subsequent moderation analyses.
We conducted a series of moderated regression analyses (Aiken & West 1991) to examine the interaction of latent class and medication assignment in the prediction of drinking outcomes. We created dummy variables for naltrexone (0 = placebo naltrexone, 1 = active naltrexone) and acamprosate (0 = placebo acamprosate, 1 = active acamprosate). Then we created interaction terms by multiplying the latent classes with the dummy treatment variables. For each model, we included the interaction terms, the latent class dummy variables, the medication assignment dummy variables, as well as a number of covariates as predictors of each of the four drinking outcomes (PDD and PHD at week 16 and 26). Covariates included: baseline alcohol use, age, gender, marital status (married vs. not married), race (white vs. non-white), alcohol dependence severity, body mass index (BMI), and a dummy variable to control for whether one received the combined behavioral intervention (CBI). We chose the abovementioned covariates based on variables that have been shown to be related to drinking outcomes in prior studies utilizing COMBINE data (Anton et al. 2006; Gueorguieva et al. 2015; Witkiewitz 2011). Because we were examining the moderators of medication, we excluded participants in the CBI only (no pills) condition (n = 157) from moderation analyses. Only significant omnibus interaction effects (p < .05) were probed in subsequent analyses, which entailed using t-tests to examine differences in medication effects by latent class.
Results
Confirmatory Factor Analyses in COMBINE and MATCH
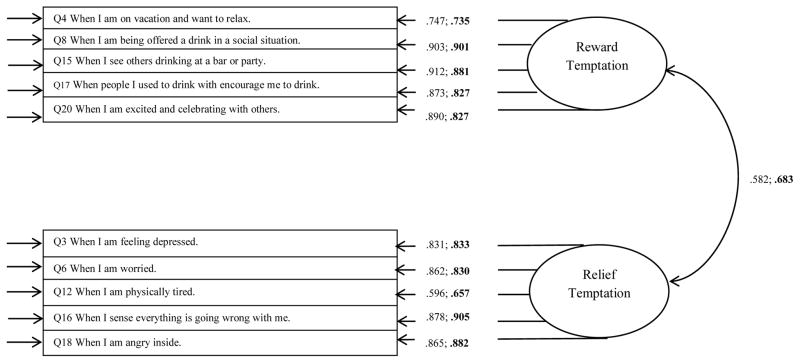
Results from the CFA models indicated that the model of the reward and relief temptation factors provided an adequate fit to the observed data (COMBINE: χ2 (34) = 245.105, p < 0.001; RMSEA = 0.067 (90% CI [0.059, 0.075]; CFI = 0.993; MATCH: χ2 (34) = 399.435, p < 0.001; RMSEA = 0.08 (90% CI [0.073, 0.087]; CFI = 0.989). Figure 1 shows the standardized factor loadings for the CFA, which were nearly all above 0.7.
Figure 1.
Standardized factor loadings for confirmatory factor analysis models of AASE temptation items. AASE = Alcohol Abstinence Self-Efficacy Scale. Arrows on far left of figure represent the residual errors. Unbolded parameters are for the COMBINE study sample. Bolded parameters are for MATCH study sample.
Construct Validity Analyses in COMBINE and MATCH
A structural regression model with the latent factors of reward and relief temptation associated with the observed covariates of depressive symptoms, age, dependence severity, and percentage of drinkers in one’s social network provided a reasonable fit to the data (COMBINE: χ2 (78) = 515.609, p < 0.001; RMSEA = 0.064 (90% CI [0.059, 0.069]; CFI = 0.988; MATCH: χ2 (78) = 1146.443, p < 0.001; RMSEA = 0.089 (90% CI [0.085, 0.094]; CFI = 0.975). Table 1 presents a summary of the associations found in these models. In both samples, reward temptation was positively associated with percentage of drinker’s in one’s social network and negatively associated with depressive symptoms, age, and dependence severity. Relief temptation was positively associated with depressive symptoms, age, and dependence severity in both samples, and negatively associated with percentage of drinker’s in one’s social network in MATCH, but not significantly associated with percentage of drinker’s in one’s social network in COMBINE.
Table 1.
Associations between temptation factors and covariates in construct validity models in COMBINE (bolded) and MATCH (unbolded)
| Reward Temptation | ||||
|---|---|---|---|---|
|
| ||||
| Percentage of Drinkers in Social Network | Depressive Symptoms | Dependence Severity | Age | |
| COMBINE | β = 0.13; B (SE) = 0.05 (0.01)** | β = − 0.14, B (SE)= − 2.04 (0.48)** | β = − 0.10, B (SE) = − 0.17(0.06)** | β= − 0.22, B (SE)= − 2.93 (0.512)** |
| MATCH | β = 0.25, B (SE) = 0.09 (0.01)** | β = − 0.22, B (SE) = −2.50 (0.39)** | β = − 0.04, B (SE) = −0.18 (0.08)* | β = − 0.34, B (SE) = −5.18 (0.56)** |
| Relief Temptation | ||||
|---|---|---|---|---|
|
| ||||
| Percentage of Drinkers in Social Network | Depressive Symptoms | Dependence Severity | Age | |
| COMBINE | β = − 0.07, B (SE) = −0.02 (0.01) | β = 0.44, B (SE) = 5.63 (0.46)** | β = 0.33, B (SE) = 0.50 (0.06)** | β = 0.09, B (SE) = 1.09 (0.44)** |
| MATCH | β = −0.24, B (SE) = −0.07 (0.01)** | β = 0.56, B (SE) = 5.47(0.33)** | β = 0.18, B (SE) = 0.67 (0.05)** | β = 0.18, B (SE) = 2.36 (0.46)** |
Note.
p < 0.05
p < 0.01;
B = unstandardized regression coefficient; SE = standard error; β = standardized regression coefficient.
Factor Mixture Models in COMBINE and MATCH
First, we conducted a series of factor mixture models (FMMs) in COMBINE. Based on the AIC, BIC, aBIC, the LRT test, the entropy (each shown in Table 2), and the substantively interpretable pattern of item response probabilities (shown in Table 3), we chose a 5-class solution in COMBINE. Entropy of 0.865 indicated excellent classification precision. Next, we sought to replicate these results in MATCH. Based on the FMMs in MATCH, a 5-class solution provided an adequate fit to the observed data, had excellent classification precision, and there was a highly similar pattern of item response probabilities for each class (see Table 3). Based on the pattern of item response probabilities shown in Table 2, we labeled class 1 as “low reward/low relief”, class 2 as the “moderate reward/moderate relief,” class 3 as “high reward/moderate relief,” class 4 as “high relief/moderate reward,” and class 5 as “high reward/high relief.”
Table 2.
Fit Statistics for Class Solutions 1 through 6 for Factor Mixture Models
| COMBINE
| ||||||
|---|---|---|---|---|---|---|
| Number of Classes | ||||||
| Fit Statistics | 1 | 2 | 3 | 4 | 5 | 6 |
| AIC | 41301.06 | 37430.043 | 35727.305 | 34962.992 | 34225.759 | 33720.942 |
| BIC | 41551.96 | 37696.617 | 36009.559 | 35260.927 | 34539.375 | 34050.239 |
| Adjusted BIC | 41399.48 | 37534.610 | 35838.023 | 35079.861 | 34348.779 | 33850.113 |
| Lo-Mendell-Rubin test | 3828.856 | 1633.400 | 736.350 | 674.584 | 488.295 | |
| ----- | p < 0.01 | p < 0.01 | p < 0.01 | p < 0.01 | p = 0.316 | |
| Entropy | ----- | 0.867 | 0.893 | 0.858 | 0.865 | 0.864 |
|
| ||||||
| MATCH
| ||||||
| AIC | 50545.849 | 45471.103 | 43451.756 | 42598.433 | 41938.743 | 41507.283 |
| BIC | 50820.716 | 45762.139 | 43758.961 | 42921.806 | 42278.286 | 41862.994 |
| Adjusted BIC | 50658.699 | 45590.591 | 43577.882 | 42731.197 | 42078.146 | 41653.324 |
| Lo-Mendell-Rubin test | 5019.000 | 1937.930 | 822.233 | 636.957 | 418.578 | |
| ----- | p < 0.01 | p < 0.01 | p < 0.05 | p = 0.256 | p = 0.130 | |
| Entropy | ----- | 0.889 | 0.876 | 0.860 | 0.846 | 0.846 |
Note. Akaike’s Information Criterion (AIC), the Bayesian Information Criterion (BIC), sample size adjusted BIC (adjusted BIC). Lower values of AIC, BIC and Adjusted BIC indicate a better fitting model.
Table 3.
Latent class item response probabilities for Reward and Relief items in COMBINE (bolded) and MATCH (unbolded)
| Class 1 (low, low) | Class 2 (moderate, moderate) | Class 3 (high reward, moderate relief) | Class 4 (high relief, moderate reward) | Class 5 (high, high) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Response | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 | 1 | 2 | 3 | 4 | 5 |
| Reward Temptation Items | |||||||||||||||||||||||||
| 4. When I am on vacation and want to relax. | .603 | .246 | .124 | .024 | .003 | .095 | .184 | .431 | .246 | .044 | .007 | .020 | .123 | .463 | .387 | .035 | .083 | .340 | .426 | .117 | .002 | .006 | .042 | .271 | .678 |
| .613 | .217 | .132 | .033 | .005 | .147 | .200 | .388 | .220 | .046 | .025 | .049 | .220 | .464 | .242 | .130 | .185 | .391 | .242 | .052 | .007 | .015 | .084 | .366 | .528 | |
| 8. When I am being offered a drink in a social situation. | .783 | .179 | .033 | .005 | .000 | .070 | .275 | .463 | .180 | .012 | .002 | .010 | .075 | .565 | .349 | .016 | .086 | .373 | .473 | .053 | .000 | .002 | .014 | .230 | .753 |
| .832 | .131 | .032 | .004 | .000 | .133 | .315 | .423 | .115 | .014 | .008 | .033 | .220 | .523 | .216 | .110 | .285 | .449 | .138 | .018 | .001 | .005 | .041 | .290 | .663 | |
| 15. When I see others drinking at a bar or a party. | .791 | .177 | .028 | .004 | .000 | .066 | .297 | .462 | .162 | .012 | .001 | .010 | .076 | .540 | .373 | .014 | .09 | .387 | .453 | .055 | .000 | .002 | .014 | .203 | .781 |
| .858 | .114 | .024 | .004 | .000 | .134 | .335 | .393 | .123 | .014 | .007 | .031 | .180 | .538 | .244 | .110 | .302 | .421 | .150 | .018 | .001 | .004 | .029 | .248 | .718 | |
| 17. When people I used to drink with encourage me to drink. | .813 | .152 | .030 | .005 | .000 | .115 | .336 | .392 | .142 | .015 | .004 | .021 | .120 | .527 | .327 | .031 | .137 | .402 | .372 | .059 | .001 | .004 | .028 | .263 | .703 |
| .885 | .087 | .022 | .005 | .001 | .284 | .353 | .255 | .091 | .017 | .031 | .093 | .275 | .424 | .178 | .247 | .345 | .280 | .107 | .021 | .006 | .02 | .085 | .353 | .536 | |
| 20. When I am excited or celebrating with others. | .686 | .271 | .038 | .005 | .000 | .036 | .237 | .494 | .219 | .014 | .001 | .006 | .049 | .508 | .436 | .007 | .061 | .324 | .541 | .067 | .000 | .001 | .008 | .161 | .830 |
| .723 | .208 | .057 | .011 | .001 | .088 | .088 | .088 | .088 | .088 | .006 | .023 | .126 | .514 | .331 | .073 | .216 | .423 | .253 | .036 | .001 | .004 | .023 | .211 | .762 | |
| Relief Temptation Items | |||||||||||||||||||||||||
| 3. When I am feeling depressed. | .406 | .378 | .191 | .022 | .002 | .123 | .304 | .464 | .098 | .011 | .075 | .226 | .524 | .157 | .018 | .008 | .033 | .276 | .525 | .158 | .001 | .006 | .070 | .413 | .510 |
| .651 | .237 | .098 | .013 | .002 | .185 | .305 | .401 | .097 | .013 | .032 | .092 | .421 | .372 | .083 | .010 | .031 | .225 | .503 | .230 | .002 | .006 | .052 | .309 | .631 | |
| 6. When I am worried. | .469 | .374 | .147 | .010 | .001 | .143 | .360 | .443 | .048 | .006 | .086 | .276 | .546 | .082 | .011 | .008 | .039 | .413 | .430 | .110 | .001 | .007 | .115 | .448 | .429 |
| .689 | .221 | .075 | .014 | .002 | .217 | .340 | .333 | .098 | .012 | .040 | .120 | .392 | .373 | .074 | .013 | .043 | .220 | .518 | .206 | .002 | .008 | .054 | .344 | .592 | |
| 12. When I am physically tired. | .445 | .371 | .145 | .030 | .009 | .252 | .399 | .260 | .068 | .021 | .200 | .380 | .304 | .088 | .028 | .066 | .214 | .402 | .226 | .092 | .027 | .106 | .324 | .338 | .206 |
| .750 | .173 | .053 | .017 | .004 | .461 | .316 | .150 | .058 | .014 | .213 | .312 | .278 | .154 | .042 | .117 | .234 | .316 | .25 | .083 | .044 | .115 | .253 | .382 | .206 | |
| 16. When I sense everything is going wrong for me. | .416 | .383 | .169 | .030 | .002 | .105 | .29 | .438 | .155 | .013 | .059 | .199 | .468 | .250 | .024 | .004 | .020 | .133 | .583 | .259 | .001 | .003 | .022 | .262 | .712 |
| .708 | .222 | .053 | .012 | .001 | .165 | .367 | .329 | .125 | .013 | .020 | .083 | .283 | .500 | .114 | .005 | .022 | .104 | .519 | .350 | .001 | .003 | .015 | .168 | .814 | |
| 18. When I am feeling angry inside. | .467 | .359 | .152 | .021 | .002 | .131 | .317 | .434 | .108 | .011 | .075 | .230 | .496 | .179 | .019 | .006 | .026 | .201 | .558 | .209 | .001 | .004 | .038 | .317 | .640 |
| .713 | .202 | .067 | .012 | .001 | .197 | .328 | .354 | .107 | .014 | .029 | .089 | .349 | .425 | .108 | .008 | .027 | .155 | .498 | .312 | .001 | .004 | .028 | .209 | .758 | |
Note. 1 = Not at all tempted, 2 = Not very tempted, 3 = Moderately tempted, 4 = Very tempted, 5 = Extremely tempted.
The latent class prevalences (proportions of individuals expected to be classified within each class) were: low reward/low relief (COMBINE = 0.07; MATCH = 0.11), moderate reward/moderate relief (COMBINE = 0.27; MATCH = 0.23), high reward/moderate relief (COMBINE = 0.19; MATCH = 0.33), high relief/moderate reward (COMBINE = 0.28; MATCH = 0.16), high reward/high relief (COMBINE =0.17; MATCH = 0.16). Overall, the discrete patterns identified by the FMMs indicated that many individuals (those most likely classified in classes 1, 2, and 5; approximately 51% of the COMBINE sample and 50% of the MATCH sample) had similar temptation scores for the reward and relief factors (either low, moderate, or high, which corresponded to classes 1, 2, and 5, respectively). A smaller number of individuals (those most likely classified in class 3, approximately 19% of the COMBINE sample and 33% of the MATCH sample) were higher in reward temptation than relief temptation. Finally, there was a group of individuals (those most likely classified in class 4, approximately 28% of the COMBINE sample and 16% of the MATCH sample) who were higher in relief temptation than reward temptation.
Interaction Effects of Latent Class and Medication Predicting Outcomes in COMBINE
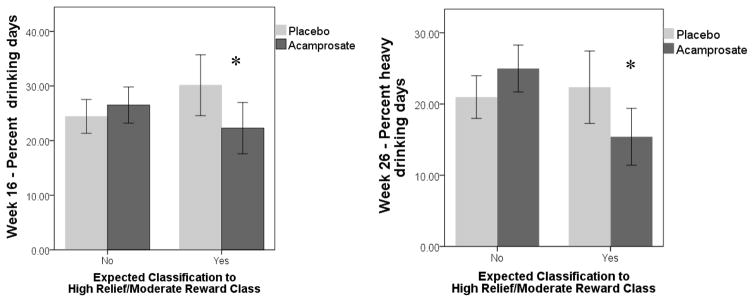
Results of primary interest were the interaction of latent class and medication assignment in predicting drinking outcomes (PDD and PHD at weeks 16 and 26). For all models, the low reward/low relief class (class 1) was the reference class. As seen in Table 4, the interaction of high relief/moderate reward class by acamprosate assignment significantly predicted week 16 PDD, week 26 PDD, and week 26 PHD (all p’s < .05). Additionally, the interaction of high reward/moderate relief (as compared to the low reward/low relief reference class) by acamprosate assignment significantly predicted week 26 PDD. Significant interaction effects were probed with t-tests to determine the robustness of the effects for making clinical inferences regarding potential treatment assignments. Among only individuals with expected classification to the high relief/moderate reward class, those who received active acamprosate versus placebo acamprosate reported significantly lower week 16 PDD (active M (SD) = 22.28 (31.60) vs. placebo M (SD) = 30.14 (34.51); Cohen’s d = 0.24, p = 0.034) and significantly lower week 26 PHD (active M (SD) = 15.40 (26.74) vs. placebo M (SD) = 22.37 (31.44); Cohen’s d = 0.24, p = 0.032), and non-significantly lower week 26 PDD (active M (SD) = 27.80 (33.53) vs. placebo M (SD) = 34.00 (35.85), Cohen’s d = 0.17; p = 0.110). Figure 2 displays the mean differences in week 16 PDD and week 26 PHD by acamprosate condition, first among the group of individuals with expected classification to high relief/moderate reward class, and then among the group of individuals with expected classification to the other 4 classes. Among only individuals with expected classification to the high reward/moderate relief class, those who received active acamprosate versus placebo acamprosate were not significantly different on week 26 PDD (active M (SD) = 36.39 (35.44) vs. placebo M (SD) = 37.95 (36.48), Cohen’s d = 0.04; p = 0.743). There were no other signification interaction effects between latent class and medication assignment. Notably, there were no significant interaction effects between naltrexone assignment and latent class in the prediction of drinking outcomes.
Table 4.
Summary of results for latent class X treatment condition predicting drinking outcomes
| Predictor | Week 16 PDD B (SE) |
Week 26 PDD B (SE) |
Week 16 PHD B (SE) |
Week 26 PHD B (SE) |
|---|---|---|---|---|
| Main Effects | ||||
| Naltrexone assignment vs. placebo | − 8.25 (7.24) | 1.29 (7.55) | − 12. 28 (6.24)* | − 2.27 (6.86) |
| Acamprosate assignment vs. placebo | 12.73 (7.22) | 18.27 (7.52)** | 4.16 (6.24) | 12.15 (6.84) |
| Moderate/Moderate class | − 5.79 (4.07) | − 10.35 (4.25)* | − 2.98 (3.52) | − 10.68 (3.87)** |
| High Reward/Moderate Relief class | 2.19 (4.21) | − 0.141 (4.40) | − 1.07 (3.64) | − 6.27 (4.0) |
| High Relief/Moderate Reward class | − 1.29 (4.03) | − 6.45 (4.22) | − 2.98 (3.52) | − 11.02 (3.83)** |
| High/High class | − 0.08 (4.30) | − 0.517 (4.51) | 0.23 (3.72) | − 3.27 (4.10) |
| Interaction Effects | ||||
| Moderate/Moderate X Acamprosate | − 3.28 (8.15) | − 8.87 (8.52) | 3.60 (7.04) | − 3.02 (7.74) |
| High Reward/Moderate Relief X Acamprosate | − 14.84 (8.37) | − 18.87 (8.75)* | − 3.49 (7.23) | − 8.36 (7.95) |
| High Relief/Moderate Reward X Acamprosate | − 20.41 (8.09)* | − 24.43 (8.46)** | − 8.35 (7.00) | − 18.07 (7.69)* |
| High/High X Acamprosate | − 16.69 (8.55) | − 17.43 (8.96) | − 7.00 (7.39) | − 14.61 (8.15) |
| Moderate/Moderate X Naltrexone | 3.86 (8.17) | − 5.99 (8.54) | 6.21 (7.04) | − 2.05 (7.76) |
| High Reward/Moderate Relief X Naltrexone | 8.58 (8.42) | − 0.026 (8.80) | 7.13 (7.25) | 0.73 (7.99) |
| High Relief/Moderate Reward X Naltrexone | 2.92 (8.11) | − 4.08 (8.47) | 9.18 (6.98) | 0.44 (7.70) |
| High/High X Naltrexone | 3.43 (8.57) | −7.27 (8.99) | 9.91 (7.38) | − 6.20 (8.17) |
Note.
p < 0.05
p < 0.01;
PHD = Percent heavy drinking days; PDD = Percent drinking days. Models controlled for the following: CBI condition, dependence severity, baseline drinking, age, gender, body mass index, marital status (married vs. not married), and race (white vs. non-white). The low/low class was the reference class for each predictor.
Figure 2.
Interaction of acamprosate and latent class in predicting drinking outcomes. * = mean difference is significant at p < .05. Error bars are 95% confidence intervals.
Discussion
The first aim of this study was to replicate findings from the PREDICT study (Glöckner-Rist et al. 2013) to provide further support for using 10 items from the AASE to measure reward and relief temptation among individuals with AUD. We replicated the substantive findings from PREDICT (Glöckner-Rist et al. 2013) using data drawn from two large clinical trials of treatments for AUD: the COMBINE study and Project MATCH. In both samples, confirmatory factor analyses supported the two factor structure model found by Glöckner-Rist et al. (2013). Construct validity testing across both samples indicated that the temptation factors were associated with covariates in the expected directions. Reward temptation was positively related to percentage of drinkers in one’s network and relief temptation was positively related to depressive symptoms, age, and dependence severity.
We also examined whether there were distinct classes of individuals characterized by particular patterns of reward and relief temptation. Using factor mixture modeling, we identified 5 reward/relief classes at baseline in both COMBINE and MATCH: a low reward/low relief class characterized by low temptation overall, a moderate reward/moderate relief class characterized by moderate temptation overall, a high reward/high relief class characterized by high temptation overall, a high reward/moderate relief class characterized by high reward temptation and moderate relief temptation, and a high relief/moderate reward class characterized by high relief temptation and moderate reward temptation. These findings are very similar to findings from Glöckner-Rist et al. (2013) regarding subtypes of reward/relief temptation, with the exception that this prior study did not identify a moderate/moderate class. The identification of classes of individuals who reported similar levels of reward and relief temptation in the current study and Glöckner-Rist et al. (2013) calls into question the orthogonal classification of individuals as either reward drinkers or relief drinkers. Rather, the current study seems to indicate that many individuals have temptation on both the reward and relief dimensions, which supports the notion that for many individuals drinking behavior is likely maintained by histories of both positive and negative reinforcement for drinking (Koob, 2013).
Another primary aim was to evaluate whether the latent reward/relief temptation classes moderated response to acamprosate and naltrexone in COMBINE. As hypothesized, we found a significant interaction effect between the high relief/moderate reward class and acamprosate in the prediction of PDD at the end-of-treatment (week 16) and PHD 10 weeks post-treatment (week 26). Among individuals with expected classification to the high relief/moderate reward class, those who received active acamprosate had significantly lower PDD at week 16 and lower PHD at week 26 compared to those who received placebo acamprosate. This finding is consistent with hypotheses regarding the mechanism of action of acamprosate in the treatment of AUD (Mann et al. 2009; Mason 2005; Spanagel & Kiefer 2008; Witkiewitz et al. 2012). Of note, we found two other significant omnibus interactions between latent class and acamprosate (high relief/moderate reward class x acamprosate and high reward/moderate relief class x acamprosate in the prediction of PDD week 26). However, follow-up analyses revealed that the differential effects of acamprosate within each class were not statistically significant and that the interaction effects were not substantively meaningful. Overall, the key results from the moderation analyses are that acamprosate seems particularly effective in reducing drinking frequency during active medication treatment and drinking intensity following active medication treatment among individuals who exhibit high relief temptation and moderate reward temptation prior to treatment.
Interestingly, the high relief/moderate reward class was not characterized by the highest absolute level of relief temptation among the classes. Rather, the high relief/high reward temptation class was characterized by the highest relief temptation scores. These findings suggest that acamprosate may be most effective for individuals who have higher relief temptation relative to reward temptation, rather than individuals with the highest levels of relief temptation and equally high levels of reward temptation. To our knowledge, our study is the first study to use latent class moderation to identify a moderator of acamprosate response. Our findings suggest that latent class moderation may be a promising analytic approach for identifying subgroups of individuals who exhibit unique patterns of reward and relief tendencies and may respond differentially to AUD treatments.
Counter to hypotheses, we did not find a significant interaction effect between the high reward/moderate relief class and naltrexone in predicting any drinking outcomes. One explanation for this null finding is that naltrexone was generally more effective for most patients in COMBINE with a significant main effect of medication (Anton et al. 2006). It is also possible that items from the AASE are more useful for identifying relief drinkers rather than reward drinkers. For example, Glöckner-Rist et al. (2013) note that several reward items from the AASE ask about temptation in general social situations and it is not clear that positive reinforcement mechanisms are driving reward temptation to drink, as assessed by the AASE (e.g., item 8: When I am being offered a drink in a social situation, item 15: When I see others drinking at a bar or party, and item 17: When people I used to drink with encourage me to drink). Other self-report measures may be better suited to measure reward drinking tendencies. For example, in PREDICT, Mann and Nakovics (under review) measured reward drinking tendencies using an adapted version of the Inventory of Drinking Situations (Annis, Davis, Graham, & Ontario 1987) called the Reward-Relief Drinking Scale (RRDS). They found that reward drinkers, as assessed by the RRDS, were more likely to respond to naltrexone than relief drinkers.
Limitations
One limitation of the current study was the use of self-report measures of alcohol use and temptation. Biochemical verification and collateral reports were used to verify self-reported drinking in COMBINE and MATCH, respectively, however, self-reported temptation could have been influenced by recall biases. The current analyses did not examine genetic and neurobiological factors, which may also play a role in affecting reward and relief drinking tendencies and why some individuals may respond better to acamprosate and naltrexone. A major limitation of the AASE is that it relies on patient’s being subjectively aware of their own reward and relief temptation tendencies. However, certain processes related to the rewarding effects of alcohol, such as incentive sensitization and alcohol attentional biases (Fields & Cox 2008; Robinson & Berridge 1993), may operate outside of patients’ awareness. The lack of a significant reward x naltrexone effect in this study indicates that the AASE may not be well-suited to identify naltrexone responders. We focused on the AASE because it was available in both COMBINE and MATCH, but there are other measures that could identify reward and relief drinkers. There is evidence to support identification of reward and relief drinkers among AUD patients using the Amsterdam Motives for Drinking Scale (Ooteman et al. 2006), the Craving Typology Questionnaire (Martinotti et al. 2013), the Reasons for Heavy Drinking Questionnaire (Adams et al. 2016), and the Reward-Relief Drinking Scale (RRDS; Mann & Nakovics under review).
Conclusions and Future Directions
Results provide further support for the utility of 10 items from AASE in measuring continuous reward and relief dimensions of temptation to drink. Moreover, results suggest that the reward and relief temptation scales of the 10-item AASE can be used to identify subtypes of individuals with distinct pre-treatment patterns of reward/relief temptation. In particular, the 10-item AASE may be useful in identifying AUD clients who respond best to acamprosate. In accordance with theory, we found that acamprosate was particularly effective among individuals with high relief and moderate reward temptation (Mann et al. 2009; Verheul et al. 1999). Pending replication of this finding, clinicians could administer the brief 10-item AASE to clients before treatment in order to identify those clients in which acamprosate may be a suitable pharmacotherapy option. Although retrospective self-report measures, such as the AASE, have limitations (e.g., recall bias), brief self-report measures are also highly practical tools to implement in clinical practice for identifying subgroups of patients who respond best to particular treatments. Our results indicate that further research on the 10-item AASE is warranted in order to better understand whether this measure is a valid, reliable and effective tool for identifying acamprosate responders. Future research could examine how the 10-item AASE relates to other self-report measure of reward and relief tendencies, as well as physiological, neurological, and genetic measures of relevant constructs (e.g., alcohol cue reactivity).
In this study, we focused on examining a self-report measure of two particular theory-derived functional constructs, reward and relief temptation tendencies. Undoubtedly, further research is needed that examines different tools and different constructs that may be useful for identifying subgroups of treatment responders. Ideally, future research on various self-report, physiological, neurobiological, and genetic measures of key client matching factors could ultimately be integrated to identify an optimal battery of pre-treatment measures and methods to identify subgroups of AUD patients who respond best to particular treatments. The current study also points to the possibility, which we have observed clinically, that many individuals are tempted by both reward and relief reasons. There are also some individuals who are differentially driven by reward temptation and those who are differentially driven by relief temptation. Future work could consider the potential for non-orthogonal reinforcement histories for drinking behavior (i.e., relief and reward) and, similar to the current study, perform analyses that can handle these overlapping continua regarding reasons for drinking.
Importantly, the current study findings contribute to the very limited body of research on moderators of acamprosate and shed light on potential promising future research directions in this area of research. In order to move closer to precision medicine in AUD treatment, more work is needed to identify consistent moderators of both naltrexone and acamprosate (Garbutt et al. 2014; Gueorguieva et al. 2015; Maisel et al. 2014).
Acknowledgments
This research was supported in part by a grant from the National Institute on Alcohol Abuse and Alcoholism: R01 AA022328, Witkiewitz, PI (CRR and KW).
Footnotes
The content is solely the responsibility of the authors and does not necessarily reflect the views of NIH.
Author Contribution
CRR, KW, and KM designed the study concept. CRR and KW wrote the majority of the manuscript. CRR conducted the statistical analyses and KW supervised these analyses. KM contributed to variable selection for analyses, interpretation of the results, and literature review. All authors have contributed to and approved the final manuscript.
Contributor Information
Corey R. Roos, Department of Psychology, University of New Mexico
Karl Mann, Central Institute of Mental Health, Medical Faculty Mannheim, University of Heidelberg, Germany.
Katie Witkiewitz, Department of Psychology, Center on Alcoholism, Substance Abuse, and Addictions, University of New Mexico.
References
- Adams ZW, Schacht JP, Randall P, Anton RF. The Reasons for Heavy Drinking Questionnaire: Factor structure and validity in alcohol dependent adults involved in clinical trials. J Stud Alcohol Drugs. 2016;77:354–361. doi: 10.15288/jsad.2016.77.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addolorato G, Abenavoli L, Leggio L, Gasbarrini G. How many cravings? Pharmacological aspects of craving treatment in alcohol addiction: a review. Neuropsychobiology. 2005;51:59–66. doi: 10.1159/000084161. [DOI] [PubMed] [Google Scholar]
- Aiken LS, West SG. Multiple regression: Testing and interpreting interactions. Thousand Oaks: Sage; 1991. [Google Scholar]
- Annis H, Davis CS, Graham JM, Ontario . Inventory of drinking situations (IDS): User’s guide. Addiction Research Foundation; 1987. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, … Longabaugh R. Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clin Psychol Rev. 1988;8:77–100. [Google Scholar]
- Bray BC, Lanza ST, Tan X. Eliminating bias in classify-analyze approaches for latent class analysis. Struct equ modeling. 2015;22:1–11. doi: 10.1080/10705511.2014.935265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark SL, Muthén B, Kaprio J, D’Onofrio BM, Viken R, Rose RJ. Models and strategies for factor mixture analysis: An example concerning the structure underlying psychological disorders. Struct equ modeling. 2013;20:681–703. doi: 10.1080/10705511.2013.824786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford PR, Longabaugh R. Manual for the administration of the important people and activities instrument. Adapted for use by Project MATCH for NIAAA. 1991;5 R01AA06698-05. [Google Scholar]
- Collins LM, Lanza ST. Latent class and latent transition analysis: With applications in the social, behavioral, and health sciences. Vol. 718. John Wiley & Sons; 2010. [Google Scholar]
- COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: rationale and methods. Alcohol Clin Exp Res. 2003;27:1107–1122. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Cooper ML. Motivations for alcohol use among adolescents: Development and validation of a four-factor model. Psychol Assess. 1994;6:117–128. [Google Scholar]
- Cooper ML, Frone MR, Russell M, Mudar P. Drinking to regulate positive and negative emotions: a motivational model of alcohol use. J Pers Soc Psychol. 1995;69:990–1005. doi: 10.1037//0022-3514.69.5.990. [DOI] [PubMed] [Google Scholar]
- Cox WM, Klinger E. A motivational model of alcohol use. J Abnorm Psychol. 1988;97:168–180. doi: 10.1037//0021-843x.97.2.168. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. Brief Symptom Inventory: administration, scoring & procedures manual. National Computer Systems; 1993. [Google Scholar]
- DiClemente CC, Carbonari JP, Montgomery RPG, Hughes SO. The alcohol abstinence self-efficacy scale. J Stud Alcohol. 1994;55:141–148. doi: 10.15288/jsa.1994.55.141. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug and alcohol dependence. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. User’s guide for the Structured clinical interview for DSM-IV axis I disorders SCID-I: clinician version. American Psychiatric Pub; 1997. [Google Scholar]
- Garbutt JC, Greenblatt AM, West SL, Morgan LC, Kampov-Polevoy A, Jordan HS, Bobashev GV. Clinical and biological moderators of response to naltrexone in alcohol dependence: a systematic review of the evidence. Addiction. 2014;109:1274–1284. doi: 10.1111/add.12557. [DOI] [PubMed] [Google Scholar]
- Glöckner-Rist A, Lémenager T, Mann K PREDICT Study Research Group. Reward and relief craving tendencies in patients with alcohol use disorders: Results from the PREDICT study. Addict Behav. 2013;38:1532–1540. doi: 10.1016/j.addbeh.2012.06.018. [DOI] [PubMed] [Google Scholar]
- Gueorguieva R, Wu R, Tsai WM, O’Connor PG, Fucito L, Zhang H, O’Malley SS. An analysis of moderators in the COMBINE study: Identifying subgroups of patients who benefit from acamprosate. Eur Neuropsychopharmacol. 2015;25:1586–1599. doi: 10.1016/j.euroneuro.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarberg A, Jayaram-Lindstrom N, Beck O, Franck J, Reid MS. The effects of acamprosate on alcohol cue reactivity and alcohol priming in dependent patients: A randomized controlled trial. Pyschopharmacology. 2009;205:53–62. doi: 10.1007/s00213-009-1515-6. [DOI] [PubMed] [Google Scholar]
- Heilig M, Goldman D, Berrettini W, O’Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat Rev Neurosci. 2011;12:670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu LT, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6:1–55. [Google Scholar]
- Koob GF. Alcoholism: allostasis and beyond. Alcohol Clin Exp Res. 2003;27:232–243. doi: 10.1097/01.ALC.0000057122.36127.C2. [DOI] [PubMed] [Google Scholar]
- Koob GF. Theoretical frameworks and mechanistic aspects of alcohol addiction: Alcohol addiction as a reward deficit disorder. Curr Top Behav Neurosci. 2013;13:3–30. doi: 10.1007/7854_2011_129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuntsche E, Knibbe R, Gmel G, Engels R. Why do young people drink? A review of drinking motives. Clin Psychol Rev. 2005;25:841–861. doi: 10.1016/j.cpr.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Lo Y, Mendell N, Rubin D. Testing the number of components in a normal mixture. Biometrika. 2001;88:767–778. [Google Scholar]
- Maisel NC, Blodgett JC, Wilbourne PL, Humphreys K, Finney JW. Meta-analysis of naltrexone and acamprosate for treating alcohol use disorders: when are these medications most helpful? Addiction. 2013;108:275–293. doi: 10.1111/j.1360-0443.2012.04054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann K, Nakovics H. The reward-relief drinking scale predicts naltrexone response in alcohol dependent patients. In: Witkiewitz K, Mann K, editors. Identifying drinker phenotypes: A translational perspective on reward and relief types of drinkers; Symposium submitted for the 39th Annual Meeting of the Research Society on Alcoholism; New Orleans, LA. under review. (Chairs) [Google Scholar]
- Mann K, Kiefer F, Smolka M, Gann H, Wellek S, Heinz A. Searching for responders to acamprosate and naltrexone in alcoholism treatment: rationale and design of the PREDICT study. Alcohol Clin Exp Res. 2009;33:674–683. doi: 10.1111/j.1530-0277.2008.00884.x. [DOI] [PubMed] [Google Scholar]
- Mann K, Kiefer F, Spanagel R, Littleton J. Acamprosate: recent findings and future research directions. Alcohol Clin Exp Res. 2008;32:1105–1110. doi: 10.1111/j.1530-0277.2008.00690.x. [DOI] [PubMed] [Google Scholar]
- Mann K, Lemenager T, Hoffmann S, Reinhard I, Hermann D, Batra A, … Zimmermann US. Results of a double-blind, placebo-controlled pharmacotherapy trial in alcoholism conducted in Germany and comparison with the US COMBINE study. Addict Biol. 2013;18:937–946. doi: 10.1111/adb.12012. [DOI] [PubMed] [Google Scholar]
- Mann K, Vollstädt-Klein S, Reinhard I, Leménager T, Fauth-Bühler M, Hermann D, … Smolka MN. Predicting naltrexone response in alcohol-dependent patients: the contribution of functional magnetic resonance imaging. Alcohol Clin Exp Res. 2014;38:2754–2762. doi: 10.1111/acer.12546. [DOI] [PubMed] [Google Scholar]
- Martinotti G, Di Nicola M, Tedeschi D, Callea A, Di Giannantonio M, Janiri L Craving Study Group. Craving Typology Questionnaire (CTQ): a scale for alcohol craving in normal controls and alcoholics. Compr Psychiatry. 2013;54:925–932. doi: 10.1016/j.comppsych.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Mason BJ. Acamprosate in the treatment of alcohol dependence. Exp Opin Pharmacother. 2005;6:2103–2115. doi: 10.1517/14656566.6.12.2103. [DOI] [PubMed] [Google Scholar]
- Miller WR, Arciniega LT, Arroyo J, Barrett D, Brief D, Carty K. Combined behavioral intervention manual: A clinical research guide for therapists treating people with alcohol abuse and dependence. Vol. 1. Bethesda, MD: National Institute on Alcohol Abuse and Alcoholism; 2004. [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s Guide. 7 1998–2012. [Google Scholar]
- Myrick H, Anton RF, Li X, Henderson S, Randall PK, Voronin K. Effect of naltrexone and ondansetron on alcohol cue–induced activation of the ventral striatum in alcohol-dependent people. Arch Gen Psychiatry. 2008;65:466–475. doi: 10.1001/archpsyc.65.4.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ooteman W, Koeter M, Verheul R, Schippers G, Van Den Brink W. Development and validation of the Amsterdam Motives for Drinking Scale (AMDS): an attempt to distinguish relief and reward drinkers. Alcohol and Alcoholism. 2006;41:284–292. doi: 10.1093/alcalc/agl012. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter M, Verheul R, Schippers G, Van Den Brink W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system, and neuroendocrine reactions to alcohol-related cues in alcoholics. Eur Neuropsychopharmacol. 2007;17:558–566. doi: 10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Verheul R, Naassila M, Daoust M, Schippers GM, Koeter MW, Van Den Brink W. Patient-treatment matching with anti-craving medications in alcohol-dependent patients: A review on phenotypic, endophenotypic and genetic indicators. J Subst Use. 2005;10:75–96. [Google Scholar]
- Project MATCH Research Group. Matching alcoholism treatments to client heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol. 1997;58:7–29. [PubMed] [Google Scholar]
- Ray LA. Clinical neuroscience of addiction: applications to psychological science and practice. Clin Psychol-Sci Pr. 2012;19:154–166. [Google Scholar]
- Robinson TE, Berridge KC. The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Rev. 1993;18:247–291. doi: 10.1016/0165-0173(93)90013-p. [DOI] [PubMed] [Google Scholar]
- Skinner MD, Aubin HJ. Craving’s place in addiction theory: contributions of the major models. Neurosci Biobehav Rev. 2010;34:606–623. doi: 10.1016/j.neubiorev.2009.11.024. [DOI] [PubMed] [Google Scholar]
- Solomon RL, Corbit JD. An opponent-process theory of motivation: I. Temporal dynamics of affect. Psychol Rev. 1974;81:119–145. doi: 10.1037/h0036128. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Zieglgänsberger W. Anti-craving compounds for ethanol: new pharmacological tools to study addictive processes. Trends Pharmacol Sci. 1997;18:54–59. [PubMed] [Google Scholar]
- Spanagel R, Kiefer F. Drugs for relapse prevention of alcoholism: ten years of progress. Trends Pharmacol Sci. 2008;29:109–115. doi: 10.1016/j.tips.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. The structured clinical interview for DSM-III-R (SCID): I: history, rationale, and description. Arch Gen Psychiatry. 1992;49:624–629. doi: 10.1001/archpsyc.1992.01820080032005. [DOI] [PubMed] [Google Scholar]
- Verheul R, van den Brink W, Geerlings P. A three-pathway psychobiological model of craving for alcohol. Alcohol and Alcoholism. 1999;34:197–222. doi: 10.1093/alcalc/34.2.197. [DOI] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Donovan DM. Moderating effects of a craving intervention on the relation between negative mood and heavy drinking following treatment for alcohol dependence. J Consult Clin Psychol. 2011;79:54–63. doi: 10.1037/a0022282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Falk DE, Kranzler HR, Litten RZ, Hallgren KA, O’Malley SS, Anton RF. Methods to analyze treatment effects in the presence of missing data for a continuous heavy drinking outcome measure when participants drop out from treatment in alcohol clinical trials. Alcohol Clin Exp Res. 2014;38:2826–2834. doi: 10.1111/acer.12543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Saville K, Hamreus K. Acamprosate for the treatment of alcohol dependence: A review of mechanisms, efficacy, and clinical utility. Ther Clin Risk Manag. 2012;8:45–53. doi: 10.2147/TCRM.S23184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worley MJ, Witkiewitz K, Brown SA, Kivlahan DR, Longabaugh R. Social network moderators of naltrexone and behavioral treatment effects on heavy drinking in the COMBINE study. Alcohol Clin Exp Res. 2015;39:93–10. doi: 10.1111/acer.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]