Abstract
Background
Kidneys with “high” kidney donor profile index (KDPI) are often biopsied and pumped, yet frequently discarded.
Methods
In this multicenter study, we describe the characteristics and outcomes of kidneys with KDPI ≥80 that were procured from 338 deceased donors. We excluded donors with anatomical kidney abnormalities.
Results
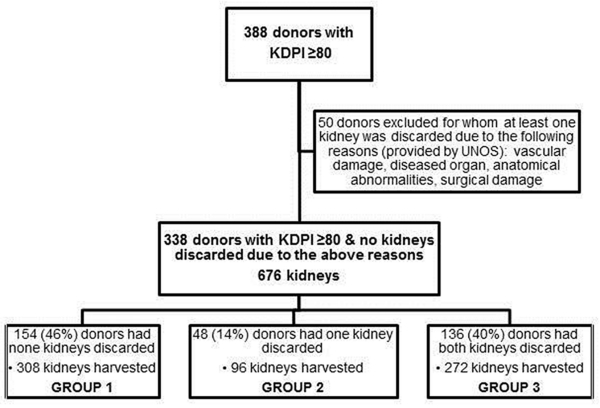
Donors were categorized by the number of kidneys discarded: 1) none (n=154, 46%), 2) 1 discarded and 1 transplanted (n=48, 14%), 3) both discarded (n=136, 40%). Donors in group 3 were older, more often white, and had higher terminal creatinine and KDPI than group 1 (all p<0.05). Biopsy was performed in 92% of all kidneys, and 47% were pumped. Discard was associated with biopsy findings and 1st hour renal resistance. Kidney injury biomarker levels (NGAL, IL-18, and KIM-1 measured from donor urine at procurement and from perfusate soon after pump perfusion) were not different between groups. There was no significant difference in 1-year estimated glomerular filtration rate (eGFR) or graft failure between groups 1 and 2 (41.5±18 vs. 41.4±22 mL/min/1.73m2; p=0.97 and 9% vs. 10%; p=0.76).
Conclusions
Kidneys with KDPI ≥80 comprise the most resource consuming fraction of our donor kidney pool and have the highest rates of discard. Our data suggest that some discarded kidneys with KDPI ≥80 are viable; however, current tools and urine- and perfusate-biomarkers to identify these viable kidneys are not satisfactory. We need better methods to assess viability of kidneys with high KDPI.
Introduction
The ever-growing disparity between demand for kidneys and their availability is a motivation to expand the pool of deceased kidney donors and reduce organ discard. As a move towards more effective organ utilization, the Organ Procurement and Transplantation Network (OPTN) modified the organ allocation system in 2014 to better match allograft and recipient longevity and to expand the offer of marginal kidneys regionally to minimize cold ischemia time and improve their viability (1). As part of this system, all kidneys from deceased donors are now given a Kidney Donor Risk Index (KDRI), which is calculated from 14 donor and transplant characteristics and represents the risk (hazard ratio, HR) of allograft failure compared with a kidney from an “average” deceased donor between 1995 and 2005 (2). The Kidney Donor Profile Index (KDPI) ranks the quality of those kidneys deemed transplantable (as a percentile from 1 to 100). KDPI is derived from a simplified KDRI (using 10 donor-only characteristics) that has been rank-ordered and normalized to the median donor-only KDRI value from the prior year. While KDPI can be considered a prognostic score, some clinicians may use it as a discriminatory tool to accept or reject kidney offers. Among the organs retrieved between 2002 and 2012, discard rates for kidneys with KDPI 80-90 and KDPI >90 are reported to be 36% and 63%, respectively (3). The discard of high-KDPI kidneys should be reevaluated, given the increase in the number of patients on the waiting list and the steady rise in the number of elderly transplant candidates who may not survive on dialysis long enough to get a kidney with lower KDPI (4). Massie et al reported that the benefit of receiving a kidney with KDPI ≥80 was far superior to the conservative approach of waiting and perhaps receiving a lower KDPI kidney transplant, especially in candidates older than 50 years and those with an expected wait time in excess of 33 months (5).
Currently, preimplant biopsy and pump parameters are also used to determine kidney quality from less desirable donors. Preimplant biopsy is obtained in over half of all deceased donors, and these biopsy results are the most commonly cited reason for discard of the kidney (6). Several studies have evaluated the correlation of biopsy findings with graft outcomes and have reported varying results. While Kasiske et al (6) reported a lack of association between procurement biopsy results and graft outcomes, a recent study by Gandolfini et al (7) showed that standardized preimplant biopsy scoring (used to allocate marginal kidneys as dual versus single kidney transplants) increased organ utilization without compromising 3-year graft survival.
Similarly, hypothermic machine perfusion has been recommended to improve graft outcomes for marginal-quality kidneys (8). While the pump parameters and their trends are often used to discern kidney quality, this practice was challenged by Guarrera et al, who demonstrated reasonably good outcomes for kidneys with sub-optimal pump parameter values (9). Measuring protein biomarker levels in urine and pump perfusion solution is yet another emerging tool to assess kidney injury and quality. We and others have shown that certain biomarkers are associated with delayed graft function and even 1-year graft outcomes (8, 10-13).
The predictive power of traditional tools to evaluate organ quality over and above clinical donor characteristics remains questionable, but findings from these tools contribute to the most common reasons for discard. Some of these tools have been assessed individually in small, single-center studies, but high-quality evidence is lacking (14, 15). Thus, we conducted a sub-study within our large multicenter cohort to describe and evaluate the performance of traditional (biopsy reports and perfusion parameters) and newer (urine and perfusate protein biomarkers) organ quality assessment tools with regard to predicting early graft and patient outcomes in recipients of kidneys with KDPI ≥80.
Materials and Methods
Study design
The study cohort was selected from a prospective, multicenter, observational cohort study conducted via collaboration between 5 organ procurement organizations (OPO) and 5 academic centers. Yale University served as the sample and data coordinating center. The study was approved by the scientific review committee at each OPO and the institutional review board at each academic center. Protocols for managing deceased donors and obtaining consent for research were followed according to individual OPO guidelines. This study used data from the Organ Procurement and Transplantation Network (OPTN). The OPTN data system includes data on all donor, waitlisted candidates, and transplant recipients in the U.S. submitted by the members of the OPTN. The Health Resources and Services Administration (HRSA), U.S. Department of Health and Human Services provides oversight to the activities of the OPTN contractor.
Study population
Participating OPOs enrolled deceased kidney donors between May 2010 and December 2013. KDRI was calculated based on the following donor characteristics: age, race, height, weight, stroke as the cause of death, donation after cardiovascular determination of death (DCD) status, terminal serum creatinine (SCr), hepatitis C serostatus, and history of hypertension and diabetes. The KDRI for each donor was converted, as per convention, to obtain the KDPI based on the 2010 scaling factor (2). The revised allocation system for kidneys with KDPI ≥85 went into effect after our study enrollment had ended, and therefore, did not impact distribution of kidneys included in the study cohort. Donors with KDPI ≥80 were included in this study based on previously published discard data (3). Since this sub-study was designed to evaluate tools used to assess organ quality and their associations with graft outcomes, kidneys discarded due to anatomical/technical issues as reported by UNOS were excluded, ie, kidneys with vascular damage, diseased appearance, anatomical abnormalities, surgical damage, or not flushed properly. There were no dual kidney transplants in the current cohort. Donors were categorized into 3 groups based on the number of discarded kidneys: Group 1) none discarded, Group 2) 1 discarded and 1 transplanted, and Group 3) both discarded.
Donor management and sample collection
Donor management, kidney biopsy decisions, and machine perfusion were carried out based on individual OPO protocols. Ten mL of fresh urine was collected from all donors in the operating room just prior to organ procurement and was transported on ice to the individual OPOs. Wedge biopsies were performed in a subset of donors immediately after kidney procurement, and pathology services associated with these OPOs generated biopsy reports for review by potential transplant centers. The Life-Port Kidney Transporter (Organ Recovery Systems, Itasca, IL) and RM3 Renal Preservation System were used for individually- and en-bloc-perfused kidneys, respectively. All kidneys were pumped using pulsatile flow with 1L of Kidney Preservation Solution-1. Perfusate samples were collected immediately after placing on pump and then just before leaving the OPO, referred to as ‘base’ and ‘post’ samples, respectively. Both the urine and perfusate samples were temporarily stored at the OPO and shipped at least monthly to the sample coordinating center, where they were stored at −80°C until biomarker measurement. Additional details regarding collection of biopsy and perfusate samples can be obtained from our previous publications (10, 16).
Data collection
Donor characteristics and transplant details were abstracted by study staff from OPO donor charts and from the United Network for Organ Sharing (UNOS, the current OPTN contractor) database. Donor acute kidney injury (AKI) was defined according to AKI Network criteria based on admission to terminal SCr (irrespective of time between measurements and urine output cut offs) as follows: Stage 1, increase in SCr by ≥0.3 mg/dL or 1.5 to <2-fold increase; Stage 2, 2 to <3-fold increase; and Stage 3, ≥3-fold increase, or terminal SCr ≥4.0 mg/dL after a rise of at least 0.5 mg/dL (no donors were dialyzed) (17). OPO biopsy reports were retrospectively categorized via standardized adjudication for the presence of acute tubular necrosis (ATN), arteriosclerosis, and interstitial fibrosis. The data on glomerulosclerosis for each biopsied kidney was retrieved from the OPTN/UNOS database. Each finding was graded as ‘mild’, ‘moderate’, or ‘severe’ if tissue involvement was noted to be <25%, 26-50% or >50%, respectively. The histological finding was considered absent only if the report specifically indicated it as absent or indeterminate. Perfusion parameters (pump duration, resistance, and flow) were abstracted from OPO perfusion records that were sent to the data coordinating center.
Measurements of biomarkers in urine and perfusate samples
Neutrophil gelatinase-associated lipocalin (NGAL) measurement was performed with the Architect platform (Abbott Diagnostics). Kidney injury molecule-1 (KIM-1) and Interleukin-18 (IL-18) were measured using the Meso Scale Discovery platform (Meso Scale diagnostics, Gaithersburg, MD, USA), which employs electrochemiluminescence detection combined with patterned arrays. Liver-type fatty acid binding protein (L-FABP) was measured using latex-enhanced immunoturbidimetry with anti-human L-FABP mouse monoclonal antibodies (Sekisui Medial Co. Ltd).
Supplemental Digital Content (SDC), Materials and Methods provides further information about assays for biomarker measurement.
Recipient Outcomes
Recipient outcomes up to 2 years were ascertained from the OPTN/UNOS database and were compared between study donor groups. Delayed graft function (DGF) was defined as any dialysis in the first week after transplantation. Graft failure was defined as return to chronic dialysis or a repeat transplant and was censored for patient death. Primary nonfunction (PNF) was defined as the need for continued/permanent dialysis beyond 90 days posttransplant and was considered a graft failure event. Estimated glomerular filtration rate (eGFR) was calculated from SCr reported in UNOS follow-up forms at 1- and 2-years using the Chronic Kidney Disease Epidemiology Collaboration equation (18). For recipients with graft failure, we imputed eGFR as 10 ml/min/1.73m2. For recipients who died with a functioning graft, we carried forward the last available SCr to calculate eGFR. Due to incomplete reporting on acute rejection rates beyond 6 months and SCr at 2 years in the OPTN/UNOS database, analyses were not performed for these individual outcomes.
Effect of transplant center volume on utilization and outcomes of these kidneys was also studied. The 235 adult kidney transplant centers in United States were classified as high, medium, and low volume based on quartile of transplants performed during the study duration (>75%, 75-25% & <25% respectively).
Statistical analyses
Descriptive statistics were reported as mean (standard deviation) or median [interquartile range] for continuous variables and as frequency (percentage) for categorical variables. Donor characteristics, kidney biopsy findings, pump parameters, and urine and perfusate biomarkers were compared among the 3 donor study groups. We compared recipient outcomes between Group 1 (ie, the sister kidney was transplanted) and Group 2 (ie, the sister kidney was discarded). Pairwise comparisons were made using Wilcoxon rank sum tests for continuous variables and Pearson’s chi-square test for categorical variables. Generalized Estimating Equations for continuous outcomes; logistic model with Firth adjustment for rare events for categorical outcomes were used for adjusted analyses, accounting for both recipient factors (age, gender, race, duration of dialysis) and transplant center volume. Kaplan-Meier survival curves were constructed to compare composite outcomes of death or graft loss censored for death between the 2 groups. Cox proportional hazards models of time to death or graft loss censored for death were constructed and adjusted for differences in recipient age, gender, race, and duration of dialysis.
We generated a forest plot to compare 1-year eGFR between recipient groups categorized by the different quality assessment tools such as acceptance of the mate kidney for transplant, clinical AKI in the donor, biopsy findings, pump parameters, and KDPI. SAS 9.3 statistical software for Windows (SAS Institute, Cary, NC) was used for statistical testing, and all statistical tests and confidence intervals were 2-sided with a significance level of 0.05.
Results
Description of study cohort
Figure 1 shows the flowchart for deceased kidney donor inclusion into the present study. The characteristics of deceased donors included in our study were similar to that in UNOS during the same period (Table 1). Of the 676 kidneys included in the study cohort, 320 (47%) kidneys were discarded. Reasons for discard reported to UNOS were biopsy findings (59%), no recipient located (18%), poor organ function (8%), long cold time/warm time (4%), and unclear reasons (11%). The donors were grouped based on number of kidneys that were discarded: Group 1 (none discarded) consisted of 154 (46%) donors, Group 2 (1 kidney discarded and the other transplanted) had 48 (14%) donors, and Group 3 (both kidneys discarded) had 136 (40%) donors. Donor characteristics by study group are shown in Table 2. KDPI increased across the groups. Compared with donors in Group 1, those in Group 3 were older, frequently white, less likely to be diabetic, and had higher terminal SCr (all p-values <0.001). The presence of clinical AKI in the donor was not statistically different between groups.
Figure 1. Enrollment of deceased kidney donors into the study cohort.
Table 1. Comparison of donor characteristics between study cohort and all deceased donor kidneys with KDPI ≥80 reported to UNOS from same study period.
| Study Cohort (ndonor=338) |
UNOS (Ndonor=6844) |
||
|---|---|---|---|
| KDPI | 90 (6) | 91 (6) | |
| Age, years | 60.9 (10.32) | 57.5 (15.89) | |
| Male | 156 (46%) | 3304 (48%) | |
| Black race | 104 (31%) | 2096 (31%) | |
| DCD | 21 (6%) | 345 (5%) | |
| Hypertension | 257 (76%) | 5341 (78%) | |
| Diabetes | 63 (19%) | 2300 (34%) | |
| Height, cm | 166 (13) | 163 (25) | |
| Weight, kg | 81.6 (23) | 79.1 (28) | |
| Cause of death |
Head trauma |
35 (11%) | 716 (10%) |
| Anoxia | 63 (19%) | 1419 (21%) | |
| Stroke | 228 (70%) | 4608 (67%) | |
| Other | 2 (1%) | 101 (1%) | |
| Terminal serum creatinine, mg/dL |
1.3 (0.8) | 1.8 (1.9) | |
KDPI, kidney donor profile index; DCD, donation after cardiovascular determination of death Values are mean (SD) or n (%).
Table 2. Donor characteristics by discard groups.
| ALL (ndonor=338) |
GROUP 1 None discarded (ndonor=154) |
GROUP 2 One discarded (ndonor=48) |
GROUP 3 Both discarded (ndonor=136) |
P (GRP 1 vs. GRP 2) |
P (GRP 1 vs. GRP 3) |
||
|---|---|---|---|---|---|---|---|
| KDPI | 90 (6) | 88.1 (6) | 89.8 (5) | 92.3 (6) | 0.052 | <.001 | |
| Age, years | 60.9 (10.32) | 58.6 (8.9) | 59.3 (6.4) | 64.1 (12.1) | 0.958 | <.001 | |
| Male | 156 (46%) | 66 (43%) | 22 (46%) | 68 (50%) | 0.717 | 0.223 | |
| Black race | 104 (31%) | 55 (36%) | 16 (33%) | 33 (24%) | 0.763 | 0.034 | |
| DCD | 21 (6%) | 10 (6%) | 4 (8%) | 7 (5%) | 0.661 | 0.626 | |
| Hypertension | 257 (76%) | 118 (77%) | 33 (69%) | 106 (78%) | 0.273 | 0.789 | |
| Diabetes | 63 (19%) | 49 (32%) | 14 (29%) | 0 (0%) | 0.729 | <.001 | |
| Cause of death |
Head Trauma | 35 (11%) | 12 (8%) | 9 (20%) | 14 (11%) | 0.015 | 0.573 |
| Anoxia | 63 (19%) | 27 (18%) | 11 (24%) | 25 (19%) | |||
| Stroke | 228 (70%) | 112 (74%) | 25 (54%) | 91 (69%) | |||
| Other | 2 (1%) | 1 (2%) | 1 (1%) | ||||
| Terminal serum creatinine, mg/dL |
1.28 (0.82) | 1.07 (0.45) | 1.27 (0.64) | 1.51 (1.1) | 0.072 | <.001 | |
| Acute Kidney Injury |
No | 219 (66%) | 105 (69%) | 31 (66%) | 83 (63%) | 0.731 | 0.307 |
| Stage 1 or higher |
113 (34%) | 48 (31%) | 16 (34%) | 49 (37%) | |||
Values are mean (SD) or n (%).
P-values were obtained by Wilcoxon rank sum test for continuous variables and Chi-square test for categorical variables.
Biopsy was performed in 619 (92%) of all kidneys, and the proportion biopsied did not significantly vary between study groups (Table 3). Kidneys from donor Group 3 had the highest proportion with moderate/severe arteriosclerosis, fibrosis, and glomerulosclerosis (Table 3). Of the kidneys that were biopsied and then discarded (292/619, 47%), 131 (45%) had absent or mild biopsy findings, and 161 (55%) had moderate/severe biopsy findings across any of the compartments. Sixty-four (40%) of the latter had isolated findings of glomerulosclerosis >20%, 34 (21%) had isolated moderate/severe arteriosclerosis, 6 (4%) had isolated moderate/severe fibrosis, and 57 (35%) had 2 or more abnormalities.
Table 3. Kidney biopsy findings and pump parameters by discard group.
| ALL (Nkidney=676) |
GROUP 1 None discarded (Nkidney=308) |
GROUP 2 One discarded (Nkidney=96) |
GROUP 3 Both discarded (Nkidney=272) |
P (GRP 1 vs. GRP 2) |
P (GRP 1 vs. GRP 3) |
||
|---|---|---|---|---|---|---|---|
| Kidney and Biopsy Characteristics | |||||||
| Kidney Biopsy Taken | 619 (92%) | 283 (92%) | 88 (92%) | 248 (91%) | 0.946 | 0.760 | |
| ATN** | Absent | 308 (78%) | 144 (80%) | 40 (77%) | 124 (75%) | 0.013 | 0.407 |
| Mild | 60 (15%) | 27 (15%) | 4 (8%) | 29 (18%) | |||
| Moderate/Severe | 28 (7%) | 8 (4%) | 8 (15%) | 12 (7%) | |||
| Arterioscle rosis |
Absent | 220 (37%) | 119 (44%) | 36 (42%) | 65 (27%) | 0.537 | <.001 |
| Mild | 279 (47%) | 138 (51%) | 42 (49%) | 99 (41%) | |||
| Moderate/Severe | 99 (17%) | 16 (6%) | 8 (9%) | 75 (31%) | |||
| Fibrosis | Absent | 252 (42%) | 137 (50%) | 34 (40%) | 81 (34%) | 0.004 | <.001 |
| Mild | 300 (50%) | 133 (49%) | 46 (53%) | 121 (51%) | |||
| Moderate/Severe | 46 (8%) | 3 (1%) | 6 (7%) | 37 (15%) | |||
| Glomerulo sclerosis |
Indeterminate or less than 10% |
378 (61%) | 237 (84%) | 63 (72%) | 78 (31%) | 0.020 | <.001 |
| 11%-20% | 114 (18%) | 38 (13%) | 18 (20%) | 58 (23%) | |||
| More than 20% | 127 (21%) | 8 (3%) | 7 (8%) | 112 (45%) | |||
| Pump Parameters | |||||||
| Pumped | 320 (47%) | 172 (56%) | 46 (48%) | 102 (38%) | 0.174 | <.001 | |
| Pump duration | 9.5 [6.3, 12.9] | 10.3 [5.9, 14.2] N=167 |
9.8 [7.0, 13.5] N=43 |
8.8 [6.7, 11.3] N=101 |
0.756 | 0.098 | |
| Renal resistance, mmHg/mL/min (hour 1) |
0.3 [0.24, 0.41] |
0.3 [0.24, 0.38] |
0.29 [0.22, 0.41] |
0.36 [0.27, 0.5] | 0.932 | <.001 | |
| Pump flow, mL/min (hour 1) | 89 [68, 109] | 93 [72, 111] | 103 [74, 113] | 77.5 [55.5, 102] |
0.536 | <.001 | |
| Perfusate collection time | 8.25 [5.43, 11.42] |
7.63 [5, 10.72] |
9.13 [6.45, 11.92] |
8.83 [7.5, 11.42] |
0.114 | 0.011 | |
Values are median [interquartile range] or n(%). “Absent” biopsy status also includes indeterminate findings.
ATN reporting was described only in 64% of all biopsies. P-values were obtained by Wilcoxon rank sum test for continuous variables and Chi-square test for categorical variables.
As shown in Table 3, 320 (47%) kidneys were placed on pump for a median of 8.3 [5.4-11.4] hours. Kidneys from Group 3 donors were less likely to have been placed on pump than kidneys from Group 1 donors (38% versus 56%, p<0.001). Median 1-hour renal resistance was highest and pump flow was lowest in kidneys from Group 3 donors. Of the kidneys that were placed on pump and then discarded (125/320, 39%), 60 (48%) had 1-hour renal resistance >0.35 mm Hg/mL/min. Of the 320 kidneys that were discarded from the study cohort, 123 (38%) were both biopsied and pumped.
Urine samples were available from 335 donors (99%) for biomarker assays. As shown in Table 4A, there was no significant difference in urinary concentrations between study groups for NGAL, IL-18, KIM-1 and L-FABP. Of the 320 kidneys placed on pump, 28 were pumped en-bloc and were excluded from perfusate biomarker analyses. Perfusate samples were available for 292 kidneys (91% of those placed on pump) for the same biomarker assays. As shown in Table 4B, median base perfusate L-FABP was higher in pumped kidneys from Group 2 than Group 1 (18.7 [10, 35.6] versus 9.75 [2.8, 26.4] ng/ml, p=0.04). Otherwise, perfusate biomarker levels did not differ significantly between study groups.
Table 4A. Donor Urine biomarkers by discard group.
| ALL (Ndonor=338) |
GROUP 1 None discarded (Ndonor=154) |
GROUP 2 One discarded (Ndonor=48) |
GROUP 3 Both discarded (Ndonor=136) |
P (GRP 1 vs. GRP 2) |
P (GRP 1 vs. GRP 3) |
|
|---|---|---|---|---|---|---|
| At least 1 urine biomarker sampled |
335 (99%) | 152 (99%) | 47 (98%) | 136 (100%) | 0.695 | 0.182 |
| NGAL, ng/mL | 60 [17.5, 199.6] |
52.1 [17, 164.85] |
61.3 [19.1, 123.9] |
81.35 [17.45, 239.1] |
0.934 | 0.116 |
| IL-18, pg/mL | 53.38 [22.66, 113.37] |
49.16 [19.57, 109.39] |
45.64 [21.26, 99.25] |
62.61 [28.83, 125] |
0.575 | 0.166 |
| KIM-1, pg/mL | 1411.28 [635.59, 3315.15] |
1312.83 [644.57, 3305.89] |
1374.13 [591.21, 3110.94] |
1499.7 [635.36, 3472.53] |
0.825 | 0.634 |
| L-FABP, ng/mL | 15.4 [5.2, 60] | 12.8 [4.4, 56.4] |
11.2 [4.8, 35.6] |
19.8 [6.4, 71.2] |
0.538 | 0.122 |
Values are median [interquartile range].
P-values were obtained by Wilcoxon rank sum.
Table 4B. Perfusate biomarkers by discard group.
| ALL (Nkidney=320 ) |
GROUP 1 None discarded (Nkidney=172 ) |
GROUP 2 One kidney discarded (Nkidney=46) |
GROUP 3 Both discarded (Nkidneyr=102) |
P (GRP 1 vs. GRP 2) |
P (GRP 1 vs. GRP 3) |
|
|---|---|---|---|---|---|---|
| At least 1 base perfusate biomarker sampled |
274 (86%) | 144 (84%) | 23 (50%) | 93 (91%) | 0.005 | 0.081 |
| At least 1 post perfusate biomarker sampled |
215 (67%) | 124 (72%) | 23 (50%) | 68 (67%) | 0.868 | 0.343 |
| Base NGAL, ng/mL | 3.65 [0.3, 9.2] |
4.15 [1.3, 9.15] |
7.8 [4.4, 17.9] |
3 [0, 8.4] | 0.868 | 0.160 |
| Post NGAL, ng/mL | 9 [5, 16.6] | 9 [4.6, 17.6] | 3.38 [2.58, 7.44] |
9.6 [6.65, 14.4] |
0.969 | 0.497 |
| Base IL-18, pg/mL | 4.08 [2.58, 8.13] |
4.34 [2.58, 8.24] |
10.76 [7.65, 30.07] |
4.27 [2.58, 7.75] |
0.513 | 0.666 |
| Post IL-18, pg/mL | 12.18 [7.4, 18.85] |
12.1 [6.22, 18.75] |
58.96 [58.96, 58.96] |
12.85 [8.47, 18.45] |
0.717 | 0.367 |
| Base KIM-1, ng/mL | 58.96 [58.96, 58.96] |
58.96 [58.96, 58.96] |
61.53 [58.96, 96.69] |
58.96 [58.96, 58.96] |
0.102 | 0.285 |
| Post KIM-1, ng/mL | 58.96 [58.96, 110.76] |
58.96 [58.96, 119.78] |
6.6 [1.3, 12.4] |
58.96 [58.96, 103.74] |
0.710 | 0.923 |
| Base L-FABP, ng/mL | 8.4 [2.5, 23.5] |
9.75 [2.8, 26.4] |
18.7 [10, 35.6] |
7.6 [1.4, 21.5] |
0.042 | 0.123 |
| Post L-FABP, ng/mL | 3.65 [0.3, 9.2] |
4.15 [1.3, 9.15] |
7.8 [4.4, 17.9] |
3 [0, 8.4] | 0.203 | 0.578 |
Values are median [interquartile range].
P-values were obtained by Wilcoxon rank sum test for continuous variables.
Recipient Characteristics and Outcomes
Table 5 shows recipient characteristics for study groups 1 and 2. Recipients of kidneys from Group 2 were more frequently black race (p=0.019) and had shorter dialysis vintage (p=0.035). There was a trend in increased utilization of kidneys from Group 2 by high volume transplant centers. The overall incidence of DGF and PNF was 39% and 3%, respectively, and 6-month acute rejection rate was 8%. At 1 year, 33 (9%) patients had graft failure and 26 (7%) died with a functioning graft. Overall mean 1-year eGFR was 41.5 (19) mL/min/1.73m2. Notably, there were no significant differences between study groups 1 and 2 for any of these early or 1- or 2-year recipient outcomes, even after adjusting for transplant volume and recipient factors.
Table 5. Recipient characteristics and outcomes by discard group.
| ALL (Nkidney=356) |
GROUP 1 None discarded (Nkidney=308) |
GROUP 2 One kidney discarded (Nkidney=48) |
P* | P – adjusted #1 |
P – adjusted #2 |
||
|---|---|---|---|---|---|---|---|
| Age | 62.54 (9.57) | 62.35 (9.64) | 63.77 (9.12) | 0.556 | … | ||
| Male | 236 (66%) | 208 (68%) | 28 (58%) | 0.210 | … | ||
| Black race | 145 (41%) | 118 (38%) | 27 (56%) | 0.019 | … | ||
| Duration of dialysis, months | 50.65 (28.67) | 52.02 (28.93) | 42.11 (25.68) | 0.035 | … | ||
| Cause of ESRD |
Diabetes | 120 (34%) | 102 (33%) | 18 (38%) | 0.787 | ||
| Hypertension | 126 (35%) | 109 (35%) | 17 (35%) | ||||
| Other/unknown | 30 (8%) | 28 (9%) | 2 (4%) | ||||
| Glomerulonephritis | 19 (5%) | 16 (5%) | 3 (6%) | ||||
| Graft failure | 61 (17%) | 53 (17%) | 8 (17%) | ||||
| Transplant center volume |
Low | 23 (6%) | 21 (7%) | 2 (4%) | 0.079 | ||
| Medium | 104 (29%) | 96 (31%) | 8 (17%) | ||||
| High | 229 (64%) | 191 (62%) | 38 (79%) | ||||
| DGF | 139 (39%) | 118 (38%) | 21 (44%) | 0.477 | 0.471 | 0.463 | |
| PNF | 12 (3%) | 11 (4%) | 1 (2%) | 0.605 | 0.657 | 0.764 | |
| 6-month acute rejection | 28 (8%) | 25 (8%) | 3 (7%) | 0.686 | 0.734 | 0.838 | |
| 1-year death censored graft failure | 33 (9%) | 28 (9%) | 5 (10%) | 0.769 | 0.788 | 0.598 | |
| 1-year recipient death | 26 (7%) | 21 (7%) | 5 (10%) | 0.382 | 0.343 | 0.313 | |
| 1-year composite outcome (death or graft failure) |
47 (7%) | 38 (12%) | 9 (9%) | 0.194 | 0.211 | ||
| 2-year death-censored graft failure | 47 (15%) | 41 (13%) | 6 (13%) | 0.877 | 0.924 | 0.938 | |
| 2-year recipient death | 36 (11%) | 30 (10%) | 6 (13%) | 0.560 | 0.531 | 0.388 | |
| 2-year composite outcome (death or graft failure) |
69 (24%) | 59 (19%) | 10 (21%) | 0.786 | 0.746 | 0.613 | |
| 1-year eGFR, mL/min/1.73m2 | 41.5 (19) | 41.5 (18) | 41.4 (22) | 0.977 | 0.981 | 0.978 | |
ESRD: end stage kidney disease, DGF: delayed graft function, PNF: primary non-function; Values are mean (SD) or n (%).
GEE model for continuous outcomes; logistic model with Firth adjustment for rare events for categorical outcomes
adjusted #1 – adjusted for transplant center volume (low/medium/high)
adjusted #2 – adjusted for recipient factors (age, male, race, duration of dialysis) and recipient transplant center volume (low/med/high)
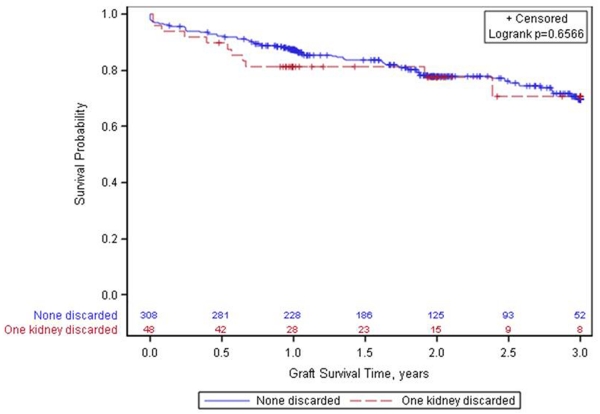
Figure 2 shows there was no significant difference in the composite outcome of death or graft loss censored for death between the 2 groups (p=0.46). There appears to be an early increased risk of graft loss in the group where 1 kidney is discarded, but thereafter, the graft attrition rates appear similar between the 2 groups. There was no significant difference in time to death-censored graft loss between the groups; unadjusted HR 1.25 (0.69-2.25) and HR adjusted for recipient age, gender, race, and duration of dialysis 1.30 (0.72-2.37).
Figure 2. Kaplan-Meier graphs to assess differences in composite outcomes of death and graft loss censored for death.
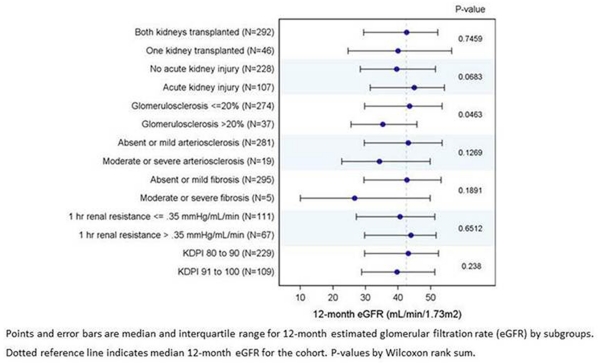
Figure 3 depicts median 1-year recipient eGFR for recipient groups based on different quality-assessment measures. Only glomerulosclerosis >20% was associated with worse allograft function at 1 year, p=0.046.
Figure 3. 12-month graft function in subgroups of kidneys with KDPI ≥80.
Discussion
This is the largest prospective, multicenter cohort study to evaluate simultaneously associations between several allograft quality assessment tools and outcomes (including discard) of deceased-donor kidneys with KDPI ≥80. Importantly, our overall cohort is reflective of the national kidney pool, with approximately 23% of donors having a KDPI ≥80 (19). Our results indicate that nearly half of potential kidney transplants with KDPI ≥80 are discarded. We have also shown that these kidneys almost universally undergo procurement biopsy (over 90%). The most common reason reported to OPTN/UNOS for kidney discard was “biopsy findings” (59% of those discarded), though 45% of the discarded kidneys in the current cohort had no more than minimal biopsy findings with regard to arteriosclerosis, interstitial fibrosis, and glomerulosclerosis. For kidneys with minimal biopsy findings and yet discarded, common reasons for discard reported to UNOS were ‘recipient not located’ and ‘poor organ function’. While biopsy information clearly plays an important role, our data support the idea that multiple factors likely influence the clinical decision to accept or refuse a kidney offer from a “high-KDPI” donor.
In addition, we found that nearly half of these “high-KDPI” kidneys were placed on pump (likely for therapeutic and quality assessment purposes as supported by long pump duration), yet more than one-third of the pumped kidneys were discarded. Of those kidneys that were pumped and discarded, only half had moderately elevated renal resistance >0.35 mm Hg/mL/min at 1 hour. This observation also provides evidence that clinicians do not predominantly consider pump parameters in isolation. As widely known, accepting or refusing a kidney offer for transplant is a multifactorial decision-making process.
We measured urinary and perfusate biomarkers for ischemic injury in nearly all of the donors and pumped kidneys in the current cohort. Levels of these protein biomarkers were not significantly different between donor groups, indicating that the degree of ischemic kidney injury does not appear to vary based on current organ acceptance patterns.
We demonstrated that kidneys from Group 2 donors (where 1 was transplanted and the other discarded) had higher KDPI values and inferior biopsy findings, yet their 1-year outcomes were similar to kidneys from Group 1 donors (where both were transplanted) (SDC Figure S1). We acknowledge that 1-year graft failure rates and death rates were higher and 1-year eGFRs are lower with use of high-KDPI kidneys (SDC Table S1), but this has to be balanced against the mortality of remaining on dialysis. Massie et al (5) reported that receipt of a high-KDPI kidney was associated with a transient period of increased posttransplant mortality, which was followed by several years of decreased mortality compared with remaining on dialysis awaiting transplant with a lower KDPI kidney. SDC Table S2 shows the biopsy pump findings were similar between kidneys in donors from Group 2 where 1 was transplanted and the mate was discarded. Barring recipient factors, it is likely that the outcome of the discarded mate kidney in Group 2 would be similar to the 1 transplanted.
Comparison of 1-year eGFR between recipient groups stratified by widely used quality-assessment indicators such as acceptance of the mate kidney for transplant, clinical AKI in the donor, biopsy findings, pump parameters, and KDPI suggested that none of the tools (with the possible exception of biopsy findings) are particularly useful on their own. Glomerulosclerosis >20% was associated with lower eGFR at 1-year in this cohort. There was also a trend towards lower 1-year eGFR among kidneys with biopsy findings of moderate to severe arteriosclerosis or fibrosis at organ procurement; however, the groups are too small to draw meaningful conclusions.
Strengths of the current study include detailed data collection from 5 OPOs for donor, biopsy, and pump information that is not available in the OPTN/UNOS database. We also examined a near complete set of donor urine and pump perfusate samples for novel biomarker measurements. As for limitations, despite this being the largest study of its kind to date, our focus on “high-KDPI” kidneys resulted in a relatively modest sample size. There was lack in uniformity in obtaining, processing, interpreting, and reporting biopsy findings as well as in managing machine perfusion. Each OPO followed their own protocol for these procedures, which could explain their lack of correlation with graft outcomes. While 2 types of pumps were used for perfusion, 1 was exclusively used for en-bloc kidneys which were excluded from analyses on pump parameters and perfusate biomarkers. Biopsy reports were generated by multiple on-call pathologists at the individual donor hospitals, likely introducing some degree of inter-observer variability. While abnormal biopsy findings were reported to be the most common cause of discard, we do not have precise pathological reasons for declining the kidneys. The recipient outcomes are dependent on the accuracy and reliability of the OPTN/UNOS database. While we demonstrate that in Group 2 the mate and discarded kidneys were treated similarly and had similar quality (biopsy and pump parameters), the outcomes of the transplants also rely on recipient characteristics (eg, recipient demographics, medical history and comorbidities, socio-economic issues, compliance, cold ischemia time, antigen mismatch, etc.) and transplant center, which we cannot account for. There may be significant practice heterogeneity between participating transplant centers with regard to organ acceptance/refusal decisions (eg, recipient health status, prior kidney offers, number of recent poor outcomes in high-risk kidneys at a given transplant center) which are challenging to account for.
In summary, kidneys with KDPI ≥80 comprise the most resource consuming fraction of our donor kidney pool (ie, more often biopsied and/or pumped) and have the highest rates of discard. Consistent with findings of prior studies, our study confirms that current tools for quality indicators, ie, biopsy and pump perfusion, are not reliable in predicting graft outcomes. Biopsies and machine perfusion can prolong the cold ischemia time, which may be detrimental to the quality of kidneys that are already marginal. We need to carefully reconsider the use and interpretation of biopsy and machine perfusion results, especially when they have not been proven to impact graft outcomes. The newer tools to assess organ quality, ie, urinary and perfusate biomarkers, were also not discriminatory. While we show that certain kidneys with KDPI ≥80 may be used cautiously, we are unable to guide on which 1 to use. Our data suggest that kidneys with moderate to severe glomerulosclerosis (ie, >20%) should be considered cautiously due its association with lower eGFR. We acknowledge that in the current era of strict scrutiny by regulatory agencies, the increased graft failure rates for high-KDPI kidneys may not be permissive for all transplant centers. Nevertheless, given the current organ shortage and growing demand, certain kidneys with KDPI ≥80 may be beneficial cautiously, some possibly as dual kidney transplants, in order to expand the deceased-donor pool. The potential benefits of declining a kidney offer with a high KDPI in hopes of a future lower KDPI kidney should be balanced against the cumulative risk of dying while waiting for the next offer.
Supplementary Material
Acknowledgments
Dr. Parikh had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr. Parikh also affirms that he has listed everyone who made substantial contributions to this work.
The authors wish to thank Isabel Butrymowicz and Rowena Kemp for their assistance with data and sample coordination for this multicenter study. We appreciate the assistance of Dr. Eoin Cotter and Dr. Peter Doran in the UCD CRC Biomarker Laboratory, for performance of NGAL assays.
We are tremendously grateful for the study participation of the following OPOs: Gift of Life Philadelphia, the New York Organ Donor Network, the Michigan Organ and Tissue Donation Program, the New Jersey Sharing Network, and the New England Organ Bank. We also thank the study coordinators at the following transplant centers: University of Pennsylvania Transplant Institute, Barnabas Health Renal and Pancreas Transplant Division, Mount Sinai’s Recanati/Miller Transplantation Institute, Harper Hospital Transplant Program, and Yale-New Haven Transplantation Center.
The data reported here have been supplied by the United Network for Organ Sharing (UNOS) as the contractor for the Organ Procurement and Transplantation Network (OPTN). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the OPTN or the U.S. Government.
Funding:
This work was supported by the National Institutes of Health grant R01DK-93770 to Dr. Parikh and the Health Resources and Services Administration contract 234-2005-37011C.
Abbreviations
- AKI
acute kidney injury
- ATN
acute tubular necrosis
- CV
coefficient of variance
- DCD
donation after cardiovascular determination of death
- DGF
delayed graft function
- eGFR
estimated glomerular filtration rate
- IL-18
Interleukin-18
- KDRI
kidney donor risk index
- KDPI
kidney donor profile index
- KIM-1
kidney injury molecule-1
- L-FABP
liver-type fatty acid binding protein
- NGAL
neutrophil gelatinase-associated lipocalin
- SCr
serum creatinine
- OPO
organ procurement organization
- OPTN
Organ Procurement and Transplantation Network
- PNF
primary nonfunction
- UNOS
United Network for Organ Sharing
Footnotes
Author’s contributions:
MDD: concept and design of work, drafted and edited the manuscript
PPR: design and interpretation of data and critical evaluation of manuscript
IEH: design and interpretation of data and critical evaluation of manuscript
BS: design and interpretation of data and critical evaluation of manuscript
JF: analysis and interpretation of data
RNF: design and interpretation of data and critical evaluation of manuscript
FLW: design and interpretation of data and critical evaluation of manuscript
RDH: critical evaluation of manuscript
HTP: design and interpretation of data and critical evaluation of manuscript
CP: design, acquisition, analysis and interpretation of data, critical evaluation of manuscript and final approval
Disclosure:
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Tso PL. Access to renal transplantation for the elderly in the face of new allocation policy: a review of contemporary perspectives on “older” issues. Transplant Rev (Orlando) 2014;28(1):6–14. doi: 10.1016/j.trre.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 2.Rao PS, Schaubel DE, Guidinger MK, et al. A comprehensive risk quantification score for deceased donor kidneys: the kidney donor risk index. Transplantation. 2009;88(2):231–6. doi: 10.1097/TP.0b013e3181ac620b. 27. [DOI] [PubMed] [Google Scholar]
- 3.Tanriover B, Mohan S, Cohen DJ, et al. Kidneys at higher risk of discard: expanding the role of dual kidney transplantation. Am J Transplant. 2014 Feb;14(2):404–15. doi: 10.1111/ajt.12553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2012 Annual Data Report: kidney. Am J Transplant. 2014;14(Suppl 1):11–44. doi: 10.1111/ajt.12579. [DOI] [PubMed] [Google Scholar]
- 5.Massie AB, Luo X, Chow EK, Alejo JL, Desai NM, Segev DL. Survival benefit of primary deceased donor transplantation with high-KDPI kidneys. Am J Transplant. 2014;14(10):2310–6. doi: 10.1111/ajt.12830. [DOI] [PubMed] [Google Scholar]
- 6.Kasiske BL, Stewart DE, Bista BR, et al. The role of procurement biopsies in acceptance decisions for kidneys retrieved for transplant. Clinical journal of the American Society of Nephrology : CJASN. 2014;9(3):562–71. doi: 10.2215/CJN.07610713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandolfini I, Buzio C, Zanelli P, et al. The Kidney Donor Profile Index (KDPI) of marginal donors allocated by standardized pretransplant donor biopsy assessment: distribution and association with graft outcomes. Am J Transplant. 2014;14(11):2515–25. doi: 10.1111/ajt.12928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moers C, Smits JM, Maathuis MH, et al. Machine perfusion or cold storage in deceased-donor kidney transplantation. The New England journal of medicine. 2009 Jan 1;360(1):7–19. doi: 10.1056/NEJMoa0802289. [DOI] [PubMed] [Google Scholar]
- 9.Guarrera JV, Goldstein MJ, Samstein B, et al. ‘When good kidneys pump badly’: outcomes of deceased donor renal allografts with poor pulsatile perfusion characteristics. Transplant Int. 2010;23(4):444–6. doi: 10.1111/j.1432-2277.2009.00970.x. [DOI] [PubMed] [Google Scholar]
- 10.Hall IE, Bhangoo RS, Reese PP, et al. Glutathione S-transferase iso-enzymes in perfusate from pumped kidneys are associated with delayed graft function. Am J Transplant. 2014 Apr;14(4):886–96. doi: 10.1111/ajt.12635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney transplantation. J Am Soc Nephrol. 2010;21(1):189–97. doi: 10.1681/ASN.2009030264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hall IE, Doshi MD, Reese PP, Marcus RJ, Thiessen-Philbrook H, Parikh CR. Association between peritransplant kidney injury biomarkers and 1-year allograft outcomes. Clin J Am Soc Nephrol. 2012;7(8):1224–33. doi: 10.2215/CJN.00310112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hollmen ME, Kyllonen LE, Inkinen KA, Lalla ML, Merenmies J, Salmela KT. Deceased donor neutrophil gelatinase-associated lipocalin and delayed graft function after kidney transplantation: a prospective study. Crit Care. 2011;15(3):R121. doi: 10.1186/cc10220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dare AJ, Pettigrew GJ. Saeb-Parsy K. Preoperative assessment of the deceased-donor kidney: from macroscopic appearance to molecular biomarkers. Transplantation. 2014;97(8):797–807. doi: 10.1097/01.TP.0000441361.34103.53. [DOI] [PubMed] [Google Scholar]
- 15.Shapiro R, Halloran PF, Delmonico FL, Bromberg JS. The ‘two, one, zero’ decision: what to do with suboptimal deceased donor kidneys. Am J Transplant. 2010;10(9):1959–60. doi: 10.1111/j.1600-6143.2010.03204.x. [DOI] [PubMed] [Google Scholar]
- 16.Hall IE, Reese PP, Weng FL, et al. Preimplant histologic acute tubular necrosis and allograft outcomes. Clin J Am Soc Nephrol. 2014;9(3):573–82. doi: 10.2215/CJN.08270813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mehta RL, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care. 2007;11(2):R31. doi: 10.1186/cc5713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA. Estimating GFR using the CKD Epidemiology Collaboration (CKD-EPI) creatinine equation: more accurate GFR estimates, lower CKD prevalence estimates, and better risk predictions. Am J Kidney Dis. 2010;55(4):622–7. doi: 10.1053/j.ajkd.2010.02.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matas AJ, Smith JM, Skeans MA, et al. OPTN/SRTR 2013 Annual Data Report: Kidney. Am J Transplant. 2015;15(Suppl 2):1–34. doi: 10.1111/ajt.13195. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.