Abstract
During early rodent development, the parietal endoderm appears from an inner cell mass and produces large amounts of basement membrane components, such as laminin-1 and collagen IV. To elucidate the regulatory network for gene expression during these procedures, we constructed a series of short interfering RNA expression vectors targeted to various transcription factors, transfected them into F9 embryonal carcinoma cells, and evaluated the effects of the gene silencing on the induction of parietal endoderm differentiation and basement membrane component production by treating F9 cells with all trans-retinoic acid and dibutyryl cyclic AMP. Among the transcription factors tested, silencing of Sox7 or combined silencing of Gata-4 and Gata-6 resulted in suppression of cell shape changes and laminin-1 production, which are the hallmarks of parietal endoderm differentiation. In cells silenced for Sox7, induction of Gata-4 and Gata-6 by retinoic acid and cyclic AMP treatment was inhibited, while induction of Sox7 was not affected in cells silenced for Gata-4 and Gata-6, indicating that Sox7 is an upstream regulatory factor for these Gata factors. Nevertheless, silencing of Sox7 did not totally cancel the action of retinoic acid, since upregulation of coup-tf2, keratin 19, and retinoic acid receptor β2 was not abolished in Sox7-silenced F9 cells. Although overexpression of Sox7 alone was insufficient to induce parietal endoderm differentiation, overexpression of Gata-4 or Gata-6 in Sox7-silenced F9 cells restored the differentiation into parietal endoderm. Sox7 is therefore required for the induction of Gata-4 and Gata-6, and the interplay among these transcription factors plays a crucial role in parietal endoderm differentiation.
During early rodent development, extraembryonic tissues play an important role in embryonic growth and differentiation. The earlier lineage of extraembryonic tissue, the primitive endoderm, arises in the blastocyst stage and differentiates into two cell types, the visceral endoderm and the parietal endoderm. Parietal endoderm cells migrate along the inner surface of the trophectoderm, where they produce a large amount of extracellular matrix that is deposited to form Reichert's membrane, an extraordinarily thick basement membrane-like structure that separates the yolk cavity from the maternal tissues (43, 52).
Mouse F9 embryonal carcinoma cells have been used as a cell culture model to mimic early murine embryogenesis. Undifferentiated stem-like F9 cells express multiple embryonic stem (ES) cell markers, such as Oct-3/4, Rex-1, and Sox2 (51, 56). Upon treatment with retinoic acid (RA) and dibutyryl cyclic AMP (Bt2cAMP), F9 cells differentiate into parietal endoderm-like cells with coordinated expression of basement membrane components (3, 17, 24, 46). In a previous study, the authors analyzed the gene expression profiles of F9 cells during the differentiation into parietal endoderm (16). Upon differentiation, expression of the genes encoding basement membrane components, such as laminin-1 subunits (α1, β1, and γ1), collagen IV subunits (α1 and α2), nidogen-1, and heparan sulfate proteoglycan core proteins (including perlecan), was upregulated. These changes in gene expression were accompanied by coordinated upregulation of genes encoding endoplasmic reticulum machineries, such as various enzymes for posttranslational modification and molecular chaperones, suggesting that parietal endoderm cells are an optimized “factory” for synthesizing basement membrane components. The authors of the previous study also identified a number of transcription factors that possibly regulate the changes in the gene expression patterns (16). These genes were also highly expressed in the parietal endoderm in mouse embryos at embryonic day 13.5 as well as in other parietal endoderm-like cells, including the Engelbreth-Holm-Swarm tumor, the extract of which also contains large amounts of basement membrane components and is widely used as an invaluable culture material in tissue engineering (16, 19, 25, 26).
The coordinate expression of these basement membrane-specific genes during parietal endoderm differentiation suggests that a well-organized regulatory mechanism operates their transcriptional regulation, but this regulatory mechanism is only partially understood. The transcription factors upregulated during F9 differentiation include hepatocyte nuclear factor 1β (Hnf1b) (5), folkhead box A2 (Foxa2/Hnf3β) (39), GATA-binding proteins (Gata-4 and Gata-6) (2, 27), Sry-box family proteins (Sox7 and Sox17) (21, 50), chicken ovalbumin upstream promoter transcription factors (Coup-tf1 and Coup-tf2) (32, 38), CBP/p300-interacting transactivator with Glu/Asp-rich carboxy-terminal domains 1 and 2 (Cited1 and Cited2) (4, 55), activating transcription factor 3 (Atf3) (18), and endothelial PAS domain protein 1 (Epas1) (35). Among these, overexpression of Gata-4 and Gata-6 was reported to induce extraembryonic endoderm differentiation of ES cells (15). Hnf1b has been shown through analysis of Hnf1b-deficient mouse embryos to be required for visceral endoderm specification (5). On the other hand, the roles of the other factors during early extraembryonic endoderm differentiation have yet to be elucidated, although several transcription factors are regarded as early endoderm differentiation markers in vertebrates (11, 15, 49). In the early endoderm, structurally and/or functionally homologous transcription factors are often coexpressed, and such redundant expression may hamper the analysis of gene expression regulatory mechanisms. However, it is important to unveil the gene regulatory network involved in parietal endoderm differentiation, since such findings should contribute not only to an understanding of the mechanisms of cell type specification during development but also to the engineering of parietal endoderm cells for establishing a large-scale production system for basement membrane components with modified biological activities (19).
To elucidate the gene regulatory network during parietal endoderm differentiation, a loss-of-function screening system for the transcription factors was constructed using vector-based short interfering RNA (siRNA)-mediated gene silencing (29, 47). A series of siRNA expression vectors that target the candidate transcription factors upregulated during parietal endoderm differentiation were constructed, and the effect of the gene silencing was evaluated by laminin α1 gene expression in RA- and Bt2cAMP-treated F9 cells. Through these approaches, an Sry-related HMG box protein, Sox7, which plays a critical role in parietal endoderm differentiation, was identified, as were Gata-4 and Gata-6. Further analysis of the interactions among Sox7, Gata-4, and Gata-6 demonstrated that Sox7 is critical for Gata-4 and Gata-6 induction.
MATERIALS AND METHODS
Cell culture and differentiation.
Murine F9 embryonal carcinoma cells were obtained from the Health Science Research Resource Bank (http://www.jhsf.or.jp/English/index_e.html; Osaka, Japan) and cultured on gelatin-coated culture dishes (Asahi Techno Glass Corp., Chiba, Japan) in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum (FBS) at 37°C under an atmosphere of 95% air, 5% CO2, and 100% humidity. To induce differentiation into parietal endoderm-like cells, F9 cells were treated with 1 μM RA and 1 mM (RA/Bt2cAMP) for 96 h (46). Undifferentiated and parietal endoderm-differentiated F9 cells without transfection of exogenous plasmids are referred to as F9-S and F9-PE, respectively.
Construction of stem-loop siRNA expression vectors.
A mouse H1-RNA gene promoter (34) fragment (mouse H1 promoter) was generated by annealing and filling in a sense primer, 5′-GGCATGCAAATTACGCGCTGTGCTTTGTGGGAAATCACCCTAAACGTAAAATTTATTC-3′, and an antisense primer, 5′-GTGTGTCGACCGGCCGCCACTATAAGGCTCGAAAGAGGAATAAATTTTACGTTT-3′. The sense and antisense primers contained SphI and SalI sites, respectively (underlined). The resulting double-stranded DNA fragment was digested and inserted into the SphI/SalI restriction sites of pGEM-Teasy (Promega, Madison, Wisc.) to generate a control vector, pH1S. Specific hairpin-forming inserts containing the 19-mer siRNA target sequence (N19), a linker sequence (TTCAAGAGA), and five thymidines as a termination signal were generated using a pair of oligonucleotides, 5′-GCCGGTCGACACCCTAAA(N19)TTCAAGAGA-3′ including a SalI site (underlined) and 5′-CAGATGCATTTTCCAAAAA(N19)TCTCTTGAA-3′ including an NsiI site (underlined). After the annealing and filling in of the oligonucleotides, the resulting double-stranded fragments were digested with SalI and NsiI and ligated into the corresponding sites in the pH1S vector (Fig. 1). To verify silencing efficiency, four pH1RNAi vectors were tested individually or in combination, and the most efficient vector (or combination) was used for further analysis.
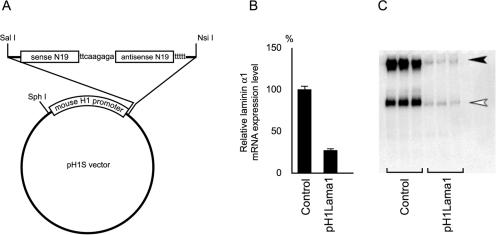
FIG. 1.
Structure of the pH1RNAi vector and efficiency of gene silencing. (A) Structure of the pH1RNAi vector. The 19-mer siRNA target sequence is shown as N19. The sequence of the linker (ttcaagaga) and five thymidines (t) signaling termination are also shown. The transcribed RNA molecules self anneal to form hairpin-shaped short interfering RNA. (B) Silencing effect of the pH1Lama1 vector on laminin α1 expression at the mRNA level. The expression level of laminin α1 mRNA estimated by qPCR in cells transfected with the pH1Lama1 vector (pH1Lama1) is shown as a percentage relative to that in control cells transfected with the pH1S vector (control). The means ± SD of results from triplicate transfections are shown. (C) Silencing effect of the pH1Lama1 vector on laminin α1 expression at the protein level. Western blotting for laminin-1 proteins in conditioned medium from control cells or cells transfected with pH1Lama1 is shown. The solid arrowhead indicates laminin-1 (heterotrimer of α1, β1, and γ1 subunits), and the open arrowhead indicates either the α1 monomer or a heterodimer of β1 and γ1.
Gene silencing by pH1RNAi vectors in F9 cells.
Undifferentiated F9 cells were transiently transfected with pH1RNAi vectors by using Lipofectamine 2000 (Invitrogen, Carlsbad, Calif.). In brief, 4 μg of vectors was diluted in 100 μl of Opti-MEM I (Invitrogen), combined with 4 μl of Lipofectamine 2000 reagent diluted in 100 μl of Opti-MEM I, and incubated for 20 min at room temperature. They were then applied to cells plated in 24-well plates at a density of 5 × 104 cells/well. Subsequently, 0.5 ml of Dulbecco's modified Eagle's medium containing 10% FBS and RA/Bt2cAMP was added to the cells to induce differentiation. The medium was replaced every 24 h with fresh medium containing 10% FBS and RA/Bt2cAMP. Cells were harvested 96 h after transfection and differentiation for RNA extraction or preparation of a nuclear extract. Occasionally, enhanced green fluorescent protein (EGFP) expression plasmids were cotransfected to monitor the efficiency of transfection, which typically ranged from 80 to 90%. To generate stable transfectants, F9 cells were cotransfected with a vector containing the puromycin resistance expression cassette and a pH1RNAi vector. Cells were selected with puromycin, and resistant clones were isolated. A fraction of each clone was treated with RA/Bt2cAMP for 96 h, and the gene expression levels were analyzed by quantitative PCR (qPCR) as described below. Clones in which the target gene expression levels were most efficiently silenced were used for further analysis.
RNA extraction, reverse transcription, and real-time monitored qPCR.
Gene expression levels were estimated by real-time monitored qPCR. RNA extraction, reverse transcription, and qPCR were carried out as described previously (16). For qPCR, an ABI PRISM 7000 Sequence Detection System (Applied Biosystems, Foster City, Calif.) and SYBR Green PCR Master Mix (Applied Biosystems) were used according to the manufacturer's protocol. The relative expression levels from triplicate experiments were averaged and expressed as the mean ± standard deviation (SD). The sequences of the primers used for qPCR are available on request.
Preparation of nuclear extracts and Western blotting.
To prepare crude nuclear extracts, cells harvested from 100-mm-diameter culture dishes were disrupted by adding 1 ml of lysis buffer (10 mM HEPES [pH 7.9], 10 mM KCl, 0.1 mM EDTA, 1.5 mM MgCl2, 0.2% [vol/vol] Nonidet P-40, 1 mM dithiothreitol) and incubated for 5 min on ice with intermittent vortexing. The nuclear pellets were obtained by centrifugation at 3,000 rpm for 5 min. The pellets were resuspended in 1 packed nuclear volume of extract buffer (20 mM HEPES [pH 7.9], 420 mM NaCl, 0.1 mM EDTA, 1.5 mM MgCl2, 25% [vol/vol] glycerol, 1 mM dithiothreitol) and incubated for 10 min on ice with intermittent gentle vortexing. After the nuclei were pelleted by centrifugation, the supernatants were collected as crude nuclear extracts. One hundred micrograms of protein from the nuclear extracts and 5 μl of F9 conditioned media were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing and nonreducing conditions, respectively. Following transfer onto an Immobilon-P membrane (Millipore, Bedford, Mass.), the proteins were detected using antibodies against Gata-4 (sc-9053; Santa Cruz), Gata-6 (sc-7244; Santa Cruz), and EHS-laminin (Sanbio BV, Uden, The Netherlands).
Construction of expression vectors.
Fragments of mouse Sox7, Gata-4, and Gata-6 cDNAs were amplified by reverse transcription-PCR using total RNA extracted from differentiated F9 cells and inserted into pCMV-Tag2a (Stratagene, La Jolla, Calif.). The expression vectors were transfected into HEK293T cells, and protein expression was confirmed by Western blotting using an anti-FLAG M2 monoclonal antibody (Sigma-Aldrich, St. Louis, Mo.) and polyclonal antibodies against Gata-4 and Gata-6 as described above (data not shown).
RESULTS
Evaluation of gene silencing efficiency and specificity by short hairpin RNA-expressing vectors in F9 cells.
A vector system for expressing short hairpin RNAs under the control of a mouse H1 gene promoter driven by RNA polymerase III (pH1RNAi) was constructed (Fig. 1A; see Materials and Methods for details). To evaluate the silencing efficiency and specificity, a vector targeting laminin α1 mRNA (pH1Lama1) or a control vector without the hairpin-forming insert (pH1S) was transfected into undifferentiated F9 cells, and the transfection protocol typically yielded 80 to 90% transfection efficiency (see Materials and Methods). The cells were cultured for 96 h in the presence of RA/Bt2cAMP to induce differentiation and analyzed for laminin α1 expression. The laminin α1 mRNA level in cells transfected with pH1Lama1 was 31.2% ± 6.2% of that in control cells transfected with pH1S (Fig. 1B). The expression levels of the mRNAs for the laminin β1 and γ1 subunits were not affected by pH1Lama1 transfection (data not shown). The laminin α1 subunit forms a heterotrimer with the β1 and γ1 subunits to constitute the laminin-1 molecule, and this subunit has been reported to be the limiting factor for laminin-1 secretion (57). As shown in Fig. 1C, the secretion of laminin-1 into the culture medium was markedly inhibited under laminin α1 gene silencing. These results indicate that the gene silencing effect of the pH1RNAi vector system is efficient, specific, and sustainable for at least 96 h after transfection.
Silencing of the transcription factors and the effect on laminin α1 expression.
By utilizing the pH1RNAi vectors, loss-of-function screening of the transcription factors was carried out to elucidate their roles during F9 cell differentiation. The transcription factors upregulated during the parietal endoderm differentiation of F9 cells are thought to be candidates for regulating the overproduction of basement membrane components. pH1RNAi vectors targeting such transcription factors were constructed and transfected individually into undifferentiated F9 cells, and the target mRNA expression levels were estimated by qPCR after 96 h of RA/Bt2cAMP treatment. The 19-mer siRNA target sequences, nucleotide positions of the target sequences, and expression levels of the target mRNAs relative to those in control cells are summarized in Table 1. As shown in the table, the target mRNA expression levels were mostly reduced to one-third the levels in control cells. pH1RNAi vectors that efficiently silence Sox17, Foxa2/Hnf3b, or Coup-tf1 could not be obtained, and therefore, these transcription factors were excluded from the following experiments.
TABLE 1.
Target genes and silencing efficiencies of the pH1RNAi vectors
| Target gene | siRNA target sequence (N19) | Nucleotide position from ATG | Mean target gene expression ± SD (% of control level) |
|---|---|---|---|
| Laminin αI | TACCAGAAGGGAGATTGAC | 1968 | 31.2 ± 6.2 |
| Hnf1β | GCAQTCAGGATCAGCTGCT | 590 | 31.5 ± 4.2 |
| Gata4 | GGCAGAGAGTGTGTCAATT | 637 | 42.1 ± 1.9 |
| Gata6 | TGCGTTGCAGCAATCAGTG | 1993 | 18.5 ± 0.9 |
| Sox7 | GATCTACCCTGGTTCGGAA | 1988 | 33.7 ± 3.2 |
| Cited1 | GGATCGCAAGGCAGTGACT | 111 | 32.9 ± 3.3 |
| Cited2 | GTGCTTATGTCCTTAGTGA | 508 | 15.9 ± 4.7 |
| Coup-tf2a | GCTGTACAGAGAGGCAGGA | 442 | 46.1 ± 2.1 |
| GTGGAGAAGCTCAAGGCAC | 895 | ||
| GAGTACGTTAGGAGCCAGT | 1039 | ||
| GGATGTTACAAGTTTGCTA | 1343 | ||
| Epas1 | GCCAGAACTTCGATGAACC | 1253 | 36.2 ± 6.7 |
| Atf3 | GAGCTGAGATTCGCCATCC | 130 | 32.2 ± 1.1 |
| Sox17 | GTCTTGGAAGGCGTTGACC | 312 | 68.8 ± 0.62 |
| Foxa2/Hnf3β | GACATACCGACGCAGCTAC | 447 | 76.0 ± 4.4 |
| Coup-tf1 | CTTACACATGCCGTGCCAA | 353 | 88.3 ± 25.5 |
A mixture of four vectors harboring different N19 sequences was used for transfection.
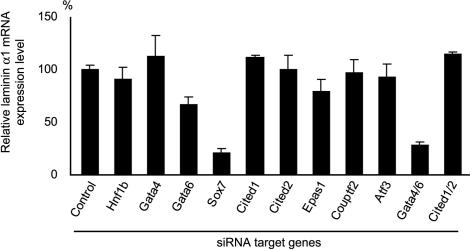
To investigate the roles of these transcription factors in parietal endoderm differentiation and/or basement membrane component production, the expression level of laminin α1 mRNA was examined in the RA/Bt2cAMP-treated F9 cells silenced for each transcription factor (Fig. 2). Laminin α1 expression was also examined in F9 cells silenced for both Gata-4 and Gata-6 (Gata-4/6), and for both Cited1 and Cited2 (Cited1/2), since these pairs of transcription factors were expected to have redundant functions. The laminin α1 expression levels were significantly reduced in cells silenced for Sox7, Gata-6, or Gata-4/6 compared to those in control cells, indicating that either Sox7 or Gata-6 is indispensable for the induction of laminin α1 expression in F9 cells treated with RA/Bt2cAMP (Fig. 2). Silencing of both Gata-4 and Gata-6 resulted in a reduction in the laminin α1 expression level that was more efficient than the silencing of Gata-6 alone, while silencing of Gata-4 alone did not affect the laminin α1 expression level. These results indicate that Gata-4 and Gata-6 have redundant functions for laminin α1 expression. Collectively, Sox7, as well as Gata-4 and Gata-6, play critical roles in the induction of laminin α1 expression during parietal endoderm differentiation of F9 cells.
FIG. 2.
Laminin α1 expression in cells silenced for various transcription factors. The siRNA target genes are indicated at the bottom. In Gata4/6 and Cited1/2, both Gata-4 and Gata-6 and both Cited-1 and Cited-2 were silenced, respectively. The laminin α1 mRNA levels estimated by qPCR are presented relative to that in control cells transfected with pH1S, a control vector without a hairpin-forming insert, and treated with RA/Bt2cAMP for 96 h after transfection. The means ± SD of results from triplicate experiments are shown.
Silencing of either Gata-4/6 or Sox7 inhibits the parietal endoderm differentiation of F9 cells.
To further estimate the effects caused by Gata-4/6 or Sox7 silencing on the parietal endoderm differentiation of F9 cells, the cell morphology and expression of genes encoding basement membrane components and other transcription factors were examined. After treatment with RA/Bt2cAMP, the control cells became rounded with a scattered distribution, which is typical of parietal endoderm-like cells (Fig. 3A). On the other hand, most of the cells silenced for Gata-4/6 or Sox7 remained flat with an epithelium-like shape and resembled undifferentiated F9 cells even after RA/Bt2cAMP treatment (Fig. 3A). As shown in Fig. 3B, the mRNA expression levels of basement membrane component genes, namely laminin-1 subunits (Lamb1 and Lamc1) and type IV collagen (Col4a1), were reduced in the cells silenced for Gata-4/6 or Sox7, indicating that the upregulation of genes encoding basement membrane components was inhibited by silencing either Gata-4/6 or Sox7. The amounts of laminin-1 protein secreted into the culture medium also decreased in accordance with the mRNA expression levels (Fig. 3C). The silencing of Gata-6 alone resulted in a weaker effect than the combined silencing of Gata-4/6, while the silencing of Gata-4 did not affect the expression levels of the basement membrane component genes. The expression levels of the transcription factors upregulated during parietal endoderm differentiation, namely Sox17, Hnf1b, and Foxa2/Hnf3b, were also reduced by silencing Gata-4/6 or Sox7 (Fig. 3D), suggesting that expression changes were suppressed for a broad spectrum of genes, including not only the direct target genes of Sox7 and the Gata factors but also those genes indirectly regulated through other transcription factors during differentiation, such as Sox17 and hepatocyte nuclear factors. These results indicate that both Gata-4/6 and Sox7 play central roles in the parietal endoderm differentiation of F9 cells.
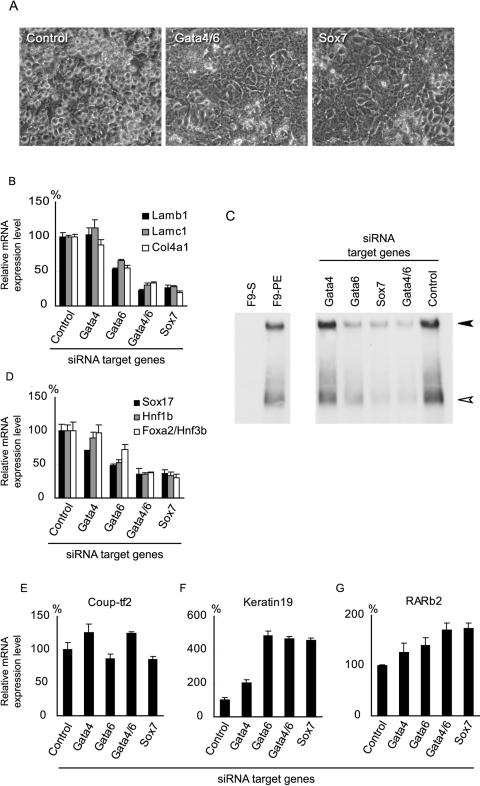
FIG. 3.
Effects of Gata-4, Gata-6, or Sox7 silencing on the morphological changes and gene expression induced by RA/Bt2cAMP treatment in F9 cells. (A) Morphology of cells treated with RA/Bt2cAMP for 96 h after transfection. Note that the cells transfected with pH1S (control) are round with a scattered distribution, whereas those silenced for both Gata-4 and Gata-6 (Gata4/6) or Sox7 show a flat epithelium-like shape. (B) Expression levels of the mRNAs for basement membrane components (Lamb1, laminin β1; Lamc1, laminin γ1; Col4a1, type IV collagen α1) in F9 cells silenced for Gata-4, Gata-6, Gata-4/6, or Sox7 after RA/Bt2cAMP treatment. (C) Secretion of laminin-1 into conditioned media. The solid arrowhead indicates laminin-1 (heterotrimer of α1, β1, and γ1 subunits), and the open arrowhead indicates either the α1 monomer or a heterodimer of β1 and γ1. Conditioned media from F9 cells silenced for the indicated genes and treated with RA/Bt2cAMP for 96 h were subjected to Western blotting under nonreducing conditions. Control cells were transfected with the pH1S vector and treated with RA/Bt2cAMP. Conditioned media from undifferentiated F9 cells and F9 cells treated with RA/Bt2cAMP for 96 h without transfection of exogenous plasmids (F9-S and F9-PE, respectively) were also subjected to the analysis for reference. (D) Expression levels of the mRNAs for the transcription factors (Sox17, Hnf1b, and Foxa2/Hnf3b) upregulated during parietal endoderm differentiation. (E to G) Expression levels of the mRNAs for Coup-tf2 (E), keratin 19 (F), and RARb2 (G). Cells were treated with RA/Bt2cAMP for 96 h after transfection with pH1RNAi vectors targeting the indicated genes. Control indicates F9 cells transfected with the pH1S vector and treated with RA/Bt2cAMP. The expression levels relative to those in the control cells are shown as the means ± SD of results from triplicate experiments.
Nevertheless, the expression levels of several other genes were not decreased by Sox7 or Gata-4/6 silencing. As shown in Fig. 3E, the expression level of Coup-tf2 increased slightly when Gata-4 or Gata-4/6 was silenced. The induction of keratin 19 expression, a marker for parietal endoderm differentiation (33), was markedly enhanced in cells silenced for Sox7, Gata-4, Gata-6, or Gata-4/6 (Fig. 3F). In addition, the upregulation of a well-documented retinoic acid-responsive gene, encoding retinoic acid receptor β2 (RARb2) (13), was also slightly enhanced in the silenced cells (Fig. 3G). These results indicate that Sox7 or Gata-4/6 silencing does not totally cancel the effect of RA/Bt2cAMP treatment, despite the central roles of Gata-4/6 and Sox7 in the morphological cell changes and transcriptional activation of basement membrane components associated with parietal endoderm differentiation.
Sox7 is required for the induction of Gata-4 and Gata-6 during parietal endoderm differentiation.
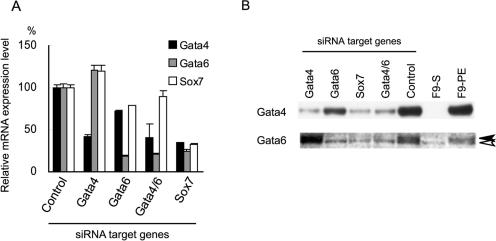
Since Sox7 silencing exerts effects similar to those of Gata-4/6 silencing, we next examined whether these transcription factors regulate the expression of each other (Fig. 4A). Notably, the silencing of Sox7 resulted in reduced expression levels of Gata-4 and Gata-6, whereas Gata-4/6 silencing only marginally affected the Sox7 expression level, indicating that Sox7 is required for the upregulation of Gata-4 and Gata-6, while the Gata factors are not essential for Sox7 induction. The expression levels of the Gata-4 and Gata-6 proteins were confirmed by Western blotting (Fig. 4B). The Gata-4 protein was not detectable in undifferentiated F9 cells (Fig. 4B, F9-S) but was induced during parietal endoderm differentiation (Fig. 4B, F9-PE). The Gata-6 protein (Fig. 4B) was also barely detected in F9-S but markedly induced in F9-PE. The faint lower molecular mass band also detected in F9-S was considered to be nonspecific, since the expression level of Gata-6 mRNA in F9-S was less than 1% of that in F9-PE (see Fig. 7) (16). Consistent with the mRNA expression level, the silencing of Sox7 resulted in decreased amounts of both Gata-4 and Gata-6 proteins, as observed during Gata-4/6 silencing.
FIG. 4.
Regulatory network among Gata-4, Gata-6, and Sox7. (A) Expression of Gata-4, Gata-6, and Sox7 mRNAs. F9 cells were transfected with pH1RNAi vectors targeting the genes indicated at the bottom and treated with RA/Bt2cAMP for 96 h. Control indicates F9 cells transfected with the pH1S vector and treated with RA/Bt2cAMP. The expression levels relative to those in the control cells are shown as the means ± SD of triplicate experiments. (B) Expression of Gata-4 and Gata-6 proteins. F9 cells were transfected with pH1RNAi vectors targeting the genes indicated at the top and treated with RA/Bt2cAMP for 96 h. Nuclear extracts were subjected to Western blotting using polyclonal antibodies against Gata-4 and Gata-6. Nuclear extracts from undifferentiated and RA/Bt2cAMP-treated F9 cells without transfection (F9-S and F9-PE, respectively) were also analyzed. The lower molecular mass band indicated by the open arrowhead in the Gata-6 blot is considered to be a nonspecific signal, and the solid arrowhead indicates the Gata-6 protein.
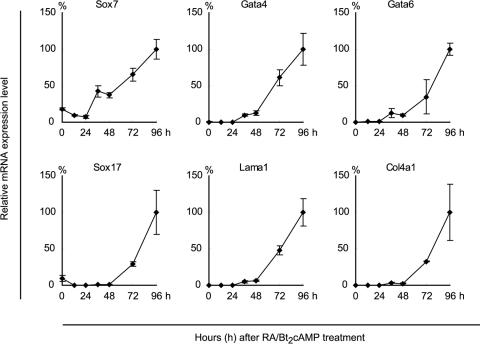
FIG. 7.
Time course analyses of gene expression patterns during F9 differentiation. Cells were harvested every 12 h until 48 h and then every 24 h until 96 h after RA/Bt2cAMP treatment, and the mRNA expression levels were estimated by qPCR. The analyzed genes are indicated at the top of each panel. The mRNA expression levels are expressed as the percentages relative to those at 96 h, and the means ± SD of results from triplicate experiments are shown. Lama1, laminin α1.
Gata-6 silencing resulted in moderate decreases in the Gata-4 and Sox7 mRNA levels, suggesting that Gata-6 positively regulates these transcription factors. On the other hand, Gata-4 silencing appeared to result in moderate upregulation of Gata-6 and Sox7. However, whether or not Gata-4 regulates the expression of Gata-6 and Sox7 remains ambiguous, since the silencing efficiency of Gata-4 was rather low (Table 1).
Exogenous Gata-4 and Gata-6, but not Sox7, induce parietal endoderm differentiation.
To further address the role of the Gata factors and Sox7 in parietal endoderm differentiation, the expression vectors for these transcription factors were introduced into undifferentiated F9 cells, and the expression levels of laminin α1 and Hnf1b were analyzed as differentiation markers. As shown in Fig. 5A, the expression of laminin α1 mRNA was slightly, but significantly, upregulated by the exogenous expression of either Gata-4 or Gata-6. However, no significant induction of laminin α1 mRNA was observed upon overexpression of Sox7, suggesting that Sox7 alone is insufficient for modulating parietal endoderm-associated gene expression in undifferentiated F9 cells. This observation is further supported by the expression level of Hnf1b mRNA, which was also moderately upregulated by Gata-4 or Gata-6 but not by Sox7 (Fig. 5B).
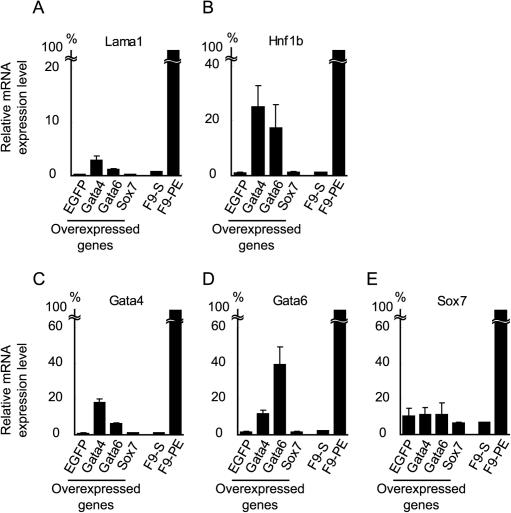
FIG. 5.
Effects of Gata-4, Gata-6, or Sox7 overexpression on undifferentiated F9 cells. F9 cells were transfected with the expression vectors indicated at the bottom of each panel and cultured for 96 h without RA/Bt2cAMP treatment. The expression levels of laminin α1 (Lama1) (A), Hnf1b (B), endogenous Gata-4 (C), endogenous Gata-6 (D), and endogenous Sox7 (E) were estimated by qPCR. The primers used were designed to selectively amplify the endogenous, but not the transfected, gene products. The mRNA expression levels in undifferentiated and RA/Bt2cAMP-treated F9 cells without transfection (F9-S and F9-PE, respectively) are also shown for reference. The expression levels relative to those in F9-PE cells are shown as the means ± SD of results from triplicate experiments.
The expression levels of endogenous Gata-4, Gata-6, and Sox7 mRNAs were also examined in these cells (Fig. 5C to E). The primers used for the qPCR of these genes were designed to selectively amplify the endogenous, but not the transfected, gene products. Endogenous Gata-4 expression was induced by overexpression of Gata-4 as well as Gata-6. Similarly, endogenous Gata-6 expression was induced by overexpression of either Gata-4 or Gata-6 (Fig. 5C and D), indicating that these Gata factors regulate themselves through positive feedback loops. This finding is in agreement with a previous report that overexpression of either Gata-4 or Gata-6 in ES cells was sufficient to induce the expression of the endogenous Gata factors and the differentiation of extraembryonic lineages (15). Endogenous Sox7 expression was not induced by the Gata factors (Fig. 5E), consistent with the observation that the Gata factors were not essential for Sox7 induction (Fig. 4A). These results suggest that Sox7 is upstream of the Gata factors in the regulatory pathway for parietal endoderm differentiation. However, overexpression of Sox7 did not induce either Gata-4 or Gata-6, suggesting that induction of Gata-4 and Gata-6 requires other factors that are potentiated by the RA/Bt2cAMP treatment of F9 cells.
Exogenous Sox7, as well as Gata-4 and Gata-6, rescues the differentiation of F9 cells stably silenced for Sox7.
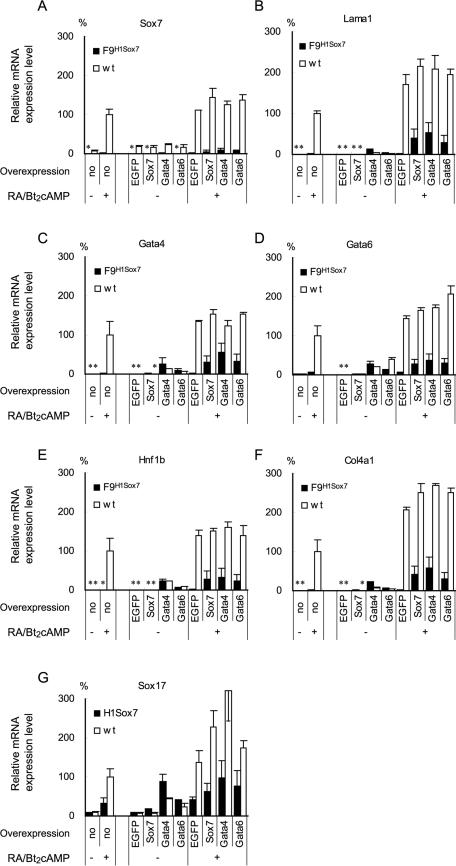
To further address the role of the Gata factors and Sox7 in parietal endoderm differentiation, we tested whether exogenous Sox7 or the Gata factors could rescue the effect of Sox7 silencing. For this purpose, we established three F9 clones in which Sox7 gene expression was stably silenced (F9H1Sox7). As shown in Fig. 6A, the expression of Sox7 mRNA with or without RA/Bt2cAMP treatment was almost inhibited in F9H1Sox7 cells, in contrast to that in wild-type F9 cells. Consistent with the results of transient Sox7 silencing (Fig. 3 and 4), the expression of genes encoding laminin α1, the Gata factors, and other parietal endoderm differentiation markers, such as Hnf1b and Col4a1, was markedly attenuated in F9H1Sox7 cells, while the induction of Sox17 was also attenuated, although the effect was less pronounced (Fig. 6B to G, bars marked “no overexpression”). Without RA/Bt2cAMP treatment, overexpression of EGFP or Sox7 showed marginal effects on the gene expression patterns in either F9H1Sox7 or wild-type F9 cells. It should be noted that exogenous Sox7 expression was not affected by the silencing, since the siRNA target sequence for Sox7 was located in the 3′-untranslated region of Sox7 mRNA, which is not present in the transcript derived from the Sox7 expression vector. Upon RA/Bt2cAMP treatment, overexpression of Sox7, but not EGFP, substantially restored the induction of the genes in F9H1Sox7 cells, suggesting that the capability of F9H1Sox7 cells for parietal endoderm differentiation is not severely altered, except for the Sox7 expression. Overexpression of Gata-4 or Gata-6 also substantially restored RA/Bt2cAMP-induced expression of the genes in F9H1Sox7 cells. Notably, overexpression of Gata-4 or Gata-6 induced expression of the genes in both F9H1Sox7 and wild-type F9 cells even in the absence of RA/Bt2cAMP treatment, suggesting that Gata-4 or Gata-6 can induce parietal endoderm differentiation even when Sox7 expression is silenced.
FIG. 6.
Rescue of F9 cells stably silenced for Sox7 by exogenous expression of Sox7 or the Gata factors. Three independent clones of the F9 cells stably transfected with the pH1RNAi vector targeting Sox7 (F9H1Sox7; solid bars) and wild-type F9 (wt; open bars) were analyzed for their mRNA expression levels by qPCR. Cells were transfected with expression vectors and cultured for 96 h with or without RA/Bt2cAMP treatment (+ or −, respectively). The panels show the endogenous gene expression levels of Sox7 (A), laminin α1 (B), Gata-4 (C), Gata-6 (D), Hnf1b (E), Col4a1 (F), and Sox17 (G). The overexpressed genes are indicated at the bottom of each panel. The expression levels are presented relative to those in the nontransfected wild-type F9 cells (indicated as “no”) treated with RA/Bt2cAMP. The means ± SD of results from triplicate experiments for three clones of F9H1Sox7 cells and wild-type F9 cells are shown. Note that some bars (indicated by asterisks) are hardly visible due to the extremely low levels of expression (less than 2%).
Sequential upregulation of Sox7, Gata-4, Gata-6, and other genes during parietal endoderm differentiation of F9 cells.
The present results suggested that the regulation of transcriptional activation is stepwise, i.e., that Sox7 is indispensable for inducing Gata-4 and Gata-6 gene expression, which in turn is required for the upregulation of parietal endoderm differentiation marker genes, including the laminin α1 gene. To examine the plausibility of such stepwise gene activation, we analyzed the time course of the induction of genes associated with parietal endoderm differentiation of wild-type F9 cells (Fig. 7). To focus on the timing of the gene upregulation, cells were harvested every 12 h until 48 h and then every 24 h until 96 h after the RA/Bt2cAMP treatment. The mRNA expression levels were calculated relative to those at 96 h after the RA/Bt2cAMP treatment. The Sox7 gene expression levels were 7 to 18% until 24 h after the initiation of RA/Bt2cAMP treatment, whereas the expression levels of the other genes examined were less than 1%, indicating that the basal mRNA level of Sox7 is higher than those of the other genes. The Sox7 mRNA level increased up to ∼40% at 48 h. At this time point, the mRNA levels of Gata-4 and Gata-6 increased up to around 10%, while the mRNA levels of laminin α1, Sox17, and Col4a1 were only marginally increased. After 48 h, the mRNA levels of all of the genes increased linearly until 96 h. Thus, the upregulation of Sox7 expression preceded that of the other genes, supporting our notion that Sox7 is a factor located upstream of Gata-4 and Gata-6 in the gene expression regulatory network during parietal endoderm differentiation of F9 cells. Furthermore, the increases in the Gata-4 and Gata-6 mRNA levels appeared to be synchronized with, if not to precede, the upregulation of Sox17, laminin α1, and Col4a1. Taken together with the results of the gene silencing and overexpression experiments, we concluded that the sequential induction of Sox7 and the Gata factors plays a pivotal role in the induction of the later genes.
DISCUSSION
The present study aimed to elucidate the regulatory network of gene expression during the parietal endoderm differentiation of F9 cells through loss-of-function screening using siRNA-mediated gene silencing. The silencing of Sox7 was shown to inhibit the induction of Gata-4 and Gata-6, which play critical roles in the changes in the gene expression patterns and cell shape that occur during parietal endoderm differentiation. Figure 8 schematically summarizes a hypothetical regulatory network for the parietal endoderm differentiation of F9 cells. RA/Bt2cAMP treatment induces changes in the expression levels of a broad spectrum of genes, including the Sox7 gene and an unidentified factor(s) (factor X). Retinoic acid receptors, especially RARb2, which is also upregulated by retinoic acid treatment, are considered to play an important role in the changes in gene expression that occur in F9 cells (42). Targeted disruption of RARb2 in F9 cells resulted in a loss of the retinoic acid-dependent regulation of a broad spectrum of genes, including the genes encoding laminin β1 and Gata-6 (14, 58), while retinoic acid-dependent morphological changes were inhibited in RARb2−/− F9 cells (13). In this study, the similarities in phenotype between the RARb2−/− F9 cells and the F9 cells silenced for Sox7 or Gata-4/6 (Fig. 3) indicates that RARb2 action is mostly mediated through the upregulation of Sox7 and these Gata factors during parietal endoderm differentiation. Sox7 is required for the induction of Gata-6, although an additional factor, factor X, is also required, given that the exogenous expression of Sox7 alone was insufficient for induction (Fig. 5 and 6). Gata-6 positively regulates Gata-4, and these functionally redundant Gata factors also induce themselves through positive feedback loops. Once the two Gata factors are induced, these factors activate other genes, including those encoding transcription factors, such as hepatocyte nuclear factors and Sox17, and basement membrane components. The expression patterns of RARb2, Coup-tf2, and keratin 19 indicate that other regulatory pathways are also evoked by RA/Bt2cAMP treatment. Interestingly, silencing of either the Gata factors or Sox7 markedly enhanced the expression levels of keratin 19, suggesting that these transcription factors can suppress keratin 19 expression (Fig. 8). Nevertheless, the hallmarks of parietal endoderm differentiation, i.e., morphological cell changes and upregulation of basement membrane component genes, are dependent on the Gata-Sox regulatory network.
FIG. 8.
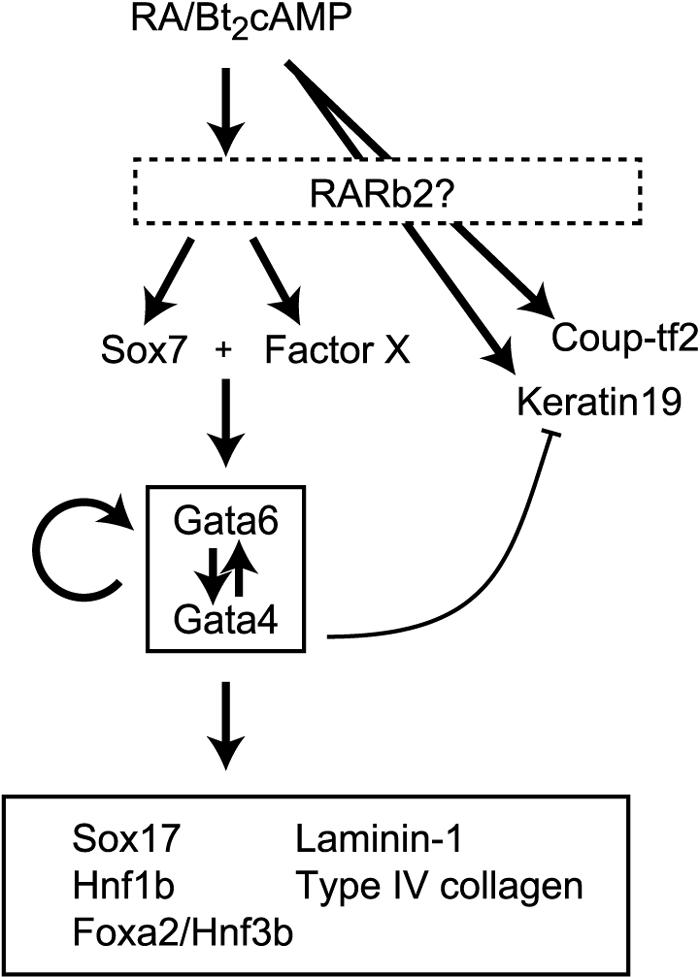
Schematic model of the regulatory network operating during the parietal endoderm differentiation of F9 cells. Arrows indicate positive regulation of the gene expression. Sox7 and an unidentified factor, factor X, function synergistically to induce Gata-4 and Gata-6 expression. Gata-4 and Gata-6 constitute a functional unit with mutual positive regulation and functional redundancy. The curved line with the flattened arrowhead indicates suppressive regulation.
Sox family transcription factors have been implicated in multiple developmental processes (7, 53). The Sox7 gene belongs to subgroup F of the Sox family (7, 48, 50), which also includes Sox17 (21) and Sox18 (12). Sox17 null mice showed severe developmental defects in the posterior gut endoderm, resulting in prenatal lethality (22). Although Sox17 is also expressed in the extraembryonic and anterior definitive endoderm, no apparent defects were observed in these tissues, suggesting functional compensation by Sox7 (22). The expression of Sox17 in F9 cells is also induced by either RA/Bt2cAMP treatment (16) or exogenous expression of Gata-4 or Gata-6 (Fig. 6). Even under silenced Sox7 expression, exogenous expression of the Gata factors combined with RA/Bt2cAMP treatment induced Sox17 expression and substantially restored parietal endoderm differentiation (Fig. 6). Under such conditions, Sox17 possibly compensates for the function of Sox7 to induce parietal endoderm-associated gene expression changes. On the other hand, the expression of Sox18 was not induced during the parietal endoderm differentiation of F9 cells (unpublished observation), and Sox18 null mice showed a mild phenotype with no prenatal lethality (36, 37), suggesting a small, if any, contribution of Sox18 to the parietal endoderm differentiation of F9 cells.
The gene regulatory networks of early endoderm formation in vertebrates have been extensively studied in zebra fish, in which Sox17 has been identified as a key molecule for endoderm differentiation (1, 44). Another Sox protein belonging to subgroup F, Casanova (Cas/Sox32), has also been identified as essential for endoderm differentiation and Sox17 expression in zebra fish (10, 23). Although the mammalian orthologue of Cas/Sox32 has not yet been identified, the results presented here indicate that Sox7 is a functional equivalent of zebra fish Cas/Sox32 during the differentiation of extraembryonic lineages in mice.
The overexpression experiments conducted in the present study showed that Sox7 expression alone was insufficient for inducing the expression of Gata-4 and Gata-6, or parietal endoderm differentiation. These observations suggest that an additional factor(s) (factor X [Fig. 8]) must be involved in the induction of expression of the Gata factor. Sox7 may function synergistically with factor X, which is induced by RA/Bt2cAMP independently of Sox7 or the Gata factors. It has been documented that transcription factors of the Sox family interact with other transcription factors or cofactors that regulate their transcriptional activities and specificities for target genes (8, 54). For example, Sox2 associated with a POU domain transcription factor, Oct-3/4, on specific enhancer elements to form a ternary HMG/POU/DNA complex (41, 56). Recently, another POU domain protein, Spg (Pou2/Oct4), was shown to be essential for endoderm formation in zebra fish by acting synergistically with Cas/Sox32 (40). Since the molecular mechanisms for early endoderm formation are evolutionarily well conserved, it is likely that Sox7 directly associates with a mammalian counterpart of the POU domain protein to induce the Gata factors and parietal endoderm differentiation.
Another possibility is that posttranslational modification induced by RA/Bt2cAMP treatment is required for Sox7 to modulate its target gene expression. Several studies have shown that the DNA binding or transcriptional activity of SRY-related HMG box proteins can be regulated by phosphorylation. For example, the DNA binding activity of human SRY protein was shown to be modified by phosphorylation mediated by cyclic AMP-dependent protein kinase (PKA) both in vitro and in vivo (9). Sox9 was also phosphorylated by PKA on its two consensus PKA phosphorylation sites, and the phosphorylation enhanced its transcriptional and DNA binding activities (20). While the consensus phosphorylation sites for PKA are not present in the amino acid sequence of Sox7, those for protein kinase C and casein kinase II are found inside the HMG domain and in the region C terminal to the HMG domain, respectively (data not shown), suggesting the possibility that Sox7 transcriptional activity is regulated through phosphorylation events mediated by factor X.
In contrast to Sox7, exogenous expression of Gata-4 or Gata-6 induced the expression of laminin α1 and Hnf1b in undifferentiated F9 and F9H1Sox7 cells without RA/Bt2cAMP treatment (Fig. 5 and 6). Furthermore, silencing of Gata-6 or combined silencing of Gata-4 and Gata-6 attenuated parietal endoderm differentiation without affecting the induction of Sox7 (Fig. 3 and 4). These observations indicate that Sox7 plays a permissive role in the induction of the Gata factors, and that these Gata factors, once induced, no longer require Sox7 to induce parietal endoderm differentiation. Significant roles for the Gata factors during embryogenesis have been demonstrated by targeted mutagenesis in mice. Gata-6 null mice are known to die around the primitive streak stage (27, 31). Gata-6-deficient ES cells cannot form a visceral endoderm layer in vitro, and Gata-4 expression is not induced in these cells (31). Targeted mutagenesis of the Gata-4 gene in mice also resulted in early-developmental-stage lethality due to the disruption of endoderm formation and cardiac development (28, 30). Embryonic stem cells derived from Gata-4 null ES cells failed to form a visceral endoderm layer in vitro; however, differentiation of these cells into extraembryonic lineages was restored by retinoic acid treatment, probably owing to the enhanced expression of Gata-6 (6, 45). These findings indicate that Gata-4 and especially Gata-6 play a critical role in extraembryonic endoderm differentiation and harbor a redundant function. The report by Fujikura et al., showing that overexpression of either Gata-4 or Gata-6 in ES cells is sufficient for inducing differentiation towards early endoderm lineages (15), further confirms the pivotal roles of these Gata factors in extraembryonic differentiation.
The present study screened for transcription factors that play critical roles during parietal endoderm differentiation and basement membrane component production using siRNA-based gene silencing combined with overexpression studies. Through these approaches, Sox7 was successfully characterized as a critical factor residing upstream of Gata-4 and Gata-6 in the gene regulatory network for the differentiation of F9 cells towards parietal endoderm-like cells. Since siRNA-based strategies are relatively easy and offer higher throughput than studies using targeted gene disruption, it will be possible to clarify the gene regulatory network involved in parietal endoderm differentiation in the near future.
Acknowledgments
We express our sincere gratitude to Nobuo Kato, President of Aichi Medical University, and Koji Kimata, Director of the Institute for Molecular Science of Medicine, Aichi Medical University, for their enthusiastic encouragement and kind provision of research facilities.
REFERENCES
- 1.Alexander, J., and D. Y. Stainier. 1999. A molecular pathway leading to endoderm formation in zebrafish. Curr. Biol. 9:1147-1157. [DOI] [PubMed] [Google Scholar]
- 2.Arceci, R. J., A. A. King, M. C. Simon, S. H. Orkin, and D. B. Wilson. 1993. Mouse GATA-4: a retinoic acid-inducible GATA-binding transcription factor expressed in endodermally derived tissues and heart. Mol. Cell. Biol. 13:2235-2246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baldwin, C. T., J. E. Silbert, D. E. Humphries, J. N. Cogburn, and B. D. Smith. 1989. Increased proteoglycan synthesis following the differentiation of F9 embryonal carcinoma cells: formation of a differentiation-specific proteoheparan sulfate. Matrix 9:389-396. [DOI] [PubMed] [Google Scholar]
- 4.Bamforth, S. D., J. Braganca, J. J. Eloranta, J. N. Murdoch, F. I. Marques, K. R. Kranc, H. Farza, D. J. Henderson, H. C. Hurst, and S. Bhattacharya. 2001. Cardiac malformations, adrenal agenesis, neural crest defects and exencephaly in mice lacking Cited2, a new Tfap2 co-activator. Nat. Genet. 29:469-474. [DOI] [PubMed] [Google Scholar]
- 5.Barbacci, E., M. Reber, M. O. Ott, C. Breillat, F. Huetz, and S. Cereghini. 1999. Variant hepatocyte nuclear factor 1 is required for visceral endoderm specification. Development 126:4795-4805. [DOI] [PubMed] [Google Scholar]
- 6.Bielinska, M., and D. B. Wilson. 1997. Induction of yolk sac endoderm in GATA-4-deficient embryoid bodies by retinoic acid. Mech. Dev. 65:43-54. [DOI] [PubMed] [Google Scholar]
- 7.Bowles, J., G. Schepers, and P. Koopman. 2000. Phylogeny of the SOX family of developmental transcription factors based on sequence and structural indicators. Dev. Biol. 227:239-255. [DOI] [PubMed] [Google Scholar]
- 8.Dailey, L., and C. Basilico. 2001. Coevolution of HMG domains and homeodomains and the generation of transcriptional regulation by Sox/POU complexes. J. Cell. Physiol. 186:315-328. [DOI] [PubMed] [Google Scholar]
- 9.Desclozeaux, M., F. Poulat, B. P. de Santa, J. P. Capony, P. Turowski, P. Jay, C. Mejean, B. Moniot, B. Boizet, and P. Berta. 1998. Phosphorylation of an N-terminal motif enhances DNA-binding activity of the human SRY protein. J. Biol. Chem. 273:7988-7995. [DOI] [PubMed] [Google Scholar]
- 10.Dickmeis, T., P. Mourrain, L. Saint-Etienne, N. Fischer, P. Aanstad, M. Clark, U. Strahle, and F. Rosa. 2001. A crucial component of the endoderm formation pathway, CASANOVA, is encoded by a novel sox-related gene. Genes Dev. 15:1487-1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duncan, S. A., M. A. Navas, D. Dufort, J. Rossant, and M. Stoffel. 1998. Regulation of a transcription factor network required for differentiation and metabolism. Science 281:692-695. [DOI] [PubMed] [Google Scholar]
- 12.Dunn, T. L., L. Mynett-Johnson, E. M. Wright, B. M. Hosking, P. A. Koopman, and G. E. Muscat. 1995. Sequence and expression of Sox-18 encoding a new HMG-box transcription factor. Gene 161:223-225. [DOI] [PubMed] [Google Scholar]
- 13.Faria, T. N., C. Mendelsohn, P. Chambon, and L. J. Gudas. 1999. The targeted disruption of both alleles of RARbeta(2) in F9 cells results in the loss of retinoic acid-associated growth arrest. J. Biol. Chem. 274:26783-26788. [DOI] [PubMed] [Google Scholar]
- 14.Farina, A. R., A. Tiberio, A. Tacconelli, L. Cappabianca, A. Gulino, and A. R. Mackay. 1996. Identification of plasminogen in Matrigel and its activation by reconstitution of this basement membrane extract. BioTechniques 21:904-909. [DOI] [PubMed] [Google Scholar]
- 15.Fujikura, J., E. Yamato, S. Yonemura, K. Hosoda, S. Masui, K. Nakao, J. J. Miyazaki, and H. Niwa. 2002. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 16:784-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Futaki, S., Y. Hayashi, M. Yamashita, K. Yagi, H. Bono, Y. Hayashizaki, Y. Okazaki, and K. Sekiguchi. 2003. Molecular basis of constitutive production of basement membrane components. Gene expression profiles of Engelbreth-Holm-Swarm tumor and F9 embryonal carcinoma cells. J. Biol. Chem. 278:50691-50701. [DOI] [PubMed] [Google Scholar]
- 17.Gudas, L. J., J. F. Grippo, K. W. Kim, G. J. Larosa, and C. M. Stoner. 1990. The regulation of the expression of genes encoding basement membrane proteins during the retinoic acid-associated differentiation of murine teratocarcinoma cells. Ann. N. Y. Acad. Sci. 580:245-251. [DOI] [PubMed] [Google Scholar]
- 18.Hai, T., and M. G. Hartman. 2001. The molecular biology and nomenclature of the activating transcription factor/cAMP responsive element binding family of transcription factors: activating transcription factor proteins and homeostasis. Gene 273:1-11. [DOI] [PubMed] [Google Scholar]
- 19.Hayashi, Y., T. Emoto, S. Futaki, and K. Sekiguchi. 2004. Establishment and characterization of a parietal endoderm-like cell line derived from Engelbreth-Holm-Swarm tumor (EHSPEL), a possible resource for an engineered basement membrane matrix. Matrix Biol. 23:47-62. [DOI] [PubMed] [Google Scholar]
- 20.Huang, W., X. Zhou, V. Lefebvre, and B. de Crombrugghe. 2000. Phosphorylation of SOX9 by cyclic AMP-dependent protein kinase A enhances SOX9's ability to transactivate a Col2a1 chondrocyte-specific enhancer. Mol. Cell. Biol. 20:4149-4158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kanai, Y., M. Kanai-Azuma, T. Noce, T. C. Saido, T. Shiroishi, Y. Hayashi, and K. Yazaki. 1996. Identification of two Sox17 messenger RNA isoforms, with and without the high mobility group box region, and their differential expression in mouse spermatogenesis. J. Cell Biol. 133:667-681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kanai-Azuma, M., Y. Kanai, J. M. Gad, Y. Tajima, C. Taya, M. Kurohmaru, Y. Sanai, H. Yonekawa, K. Yazaki, P. P. Tam, and Y. Hayashi. 2002. Depletion of definitive gut endoderm in Sox17-null mutant mice. Development 129:2367-2379. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi, Y., A. Agathon, J. Alexander, C. Thisse, S. Waldron, D. Yelon, B. Thisse, and D. Y. Stainier. 2001. casanova encodes a novel Sox-related protein necessary and sufficient for early endoderm formation in zebrafish. Genes Dev. 15:1493-1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kleinman, H. K., I. Ebihara, P. D. Killen, M. Sasaki, F. B. Cannon, Y. Yamada, and G. R. Martin. 1987. Genes for basement membrane proteins are coordinately expressed in differentiating F9 cells but not in normal adult murine tissues. Dev. Biol. 122:373-378. [DOI] [PubMed] [Google Scholar]
- 25.Kleinman, H. K., M. L. McGarvey, J. R. Hassell, V. L. Star, F. B. Cannon, G. W. Laurie, and G. R. Martin. 1986. Basement membrane complexes with biological activity. Biochemistry 25:312-318. [DOI] [PubMed] [Google Scholar]
- 26.Kleinman, H. K., M. L. McGarvey, L. A. Liotta, P. G. Robey, K. Tryggvason, and G. R. Martin. 1982. Isolation and characterization of type IV procollagen, laminin, and heparan sulfate proteoglycan from the EHS sarcoma. Biochemistry 21:6188-6193. [DOI] [PubMed] [Google Scholar]
- 27.Koutsourakis, M., A. Langeveld, R. Patient, R. Beddington, and F. Grosveld. 1999. The transcription factor GATA6 is essential for early extraembryonic development. Development 126:723-732. [PubMed] [Google Scholar]
- 28.Kuo, C. T., E. E. Morrisey, R. Anandappa, K. Sigrist, M. M. Lu, M. S. Parmacek, C. Soudais, and J. M. Leiden. 1997. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 11:1048-1060. [DOI] [PubMed] [Google Scholar]
- 29.Miyagishi, M., and K. Taira. 2004. RNAi expression vectors in mammalian cells. Methods Mol. Biol. 252:483-492. [DOI] [PubMed] [Google Scholar]
- 30.Molkentin, J. D., Q. Lin, S. A. Duncan, and E. N. Olson. 1997. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 11:1061-1072. [DOI] [PubMed] [Google Scholar]
- 31.Morrisey, E. E., Z. Tang, K. Sigrist, M. M. Lu, F. Jiang, H. S. Ip, and M. S. Parmacek. 1998. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 12:3579-3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murray, P., and D. Edgar. 2001. Regulation of laminin and COUP-TF expression in extraembryonic endodermal cells. Mech. Dev. 101:213-215. [DOI] [PubMed] [Google Scholar]
- 33.Murray, P., and D. Edgar. 2001. Regulation of the differentiation and behaviour of extra-embryonic endodermal cells by basement membranes. J. Cell Sci. 114:931-939. [DOI] [PubMed] [Google Scholar]
- 34.Myslinski, E., J. C. Ame, A. Krol, and P. Carbon. 2001. An unusually compact external promoter for RNA polymerase III transcription of the human H1RNA gene. Nucleic Acids Res. 29:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peng, J., L. Zhang, L. Drysdale, and G. H. Fong. 2000. The transcription factor EPAS-1/hypoxia-inducible factor 2alpha plays an important role in vascular remodeling. Proc. Natl. Acad. Sci. USA 97:8386-8391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pennisi, D., J. Bowles, A. Nagy, G. Muscat, and P. Koopman. 2000. Mice null for Sox18 are viable and display a mild coat defect. Mol. Cell. Biol. 20:9331-9336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pennisi, D., J. Gardner, D. Chambers, B. Hosking, J. Peters, G. Muscat, C. Abbott, and P. Koopman. 2000. Mutations in Sox18 underlie cardiovascular and hair follicle defects in ragged mice. Nat. Genet. 24:434-437. [DOI] [PubMed] [Google Scholar]
- 38.Power, S. C., and S. Cereghini. 1996. Positive regulation of the vHNF1 promoter by the orphan receptors COUP-TF1/Ear3 and COUP-TFII/Arp1. Mol. Cell. Biol. 16:778-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Reichel, R. R., S. Budhiraja, and A. Jacob. 1994. Delayed activation of HNF-3 beta upon retinoic acid-induced teratocarcinoma cell differentiation. Exp. Cell Res. 214:634-641. [DOI] [PubMed] [Google Scholar]
- 40.Reim, G., T. Mizoguchi, D. Y. Stainier, Y. Kikuchi, and M. Brand. 2004. The POU domain protein spg (pou2/Oct4) is essential for endoderm formation in cooperation with the HMG domain protein casanova. Dev. Cell 6:91-101. [DOI] [PubMed] [Google Scholar]
- 41.Remenyi, A., K. Lins, L. J. Nissen, R. Reinbold, H. R. Scholer, and M. Wilmanns. 2003. Crystal structure of a POU/HMG/DNA ternary complex suggests differential assembly of Oct4 and Sox2 on two enhancers. Genes Dev. 17:2048-2059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rochette-Egly, C., J. L. Plassat, R. Taneja, and P. Chambon. 2000. The AF-1 and AF-2 activating domains of retinoic acid receptor-alpha (RARalpha) and their phosphorylation are differentially involved in parietal endodermal differentiation of F9 cells and retinoid-induced expression of target genes. Mol. Endocrinol. 14:1398-1410. [DOI] [PubMed] [Google Scholar]
- 43.Salamat, M., N. Miosge, and R. Herken. 1995. Development of Reichert's membrane in the early mouse embryo. Anat. Embryol. 192:275-281. [DOI] [PubMed] [Google Scholar]
- 44.Shivdasani, R. A. 2002. Molecular regulation of vertebrate early endoderm development. Dev. Biol. 249:191-203. [DOI] [PubMed] [Google Scholar]
- 45.Soudais, C., M. Bielinska, M. Heikinheimo, C. A. MacArthur, N. Narita, J. E. Saffitz, M. C. Simon, J. M. Leiden, and D. B. Wilson. 1995. Targeted mutagenesis of the transcription factor GATA-4 gene in mouse embryonic stem cells disrupts visceral endoderm differentiation in vitro. Development 121:3877-3888. [DOI] [PubMed] [Google Scholar]
- 46.Strickland, S., K. K. Smith, and K. R. Marotti. 1980. Hormonal induction of differentiation in teratocarcinoma stem cells: generation of parietal endoderm by retinoic acid and dibutyryl cAMP. Cell 21:347-355. [DOI] [PubMed] [Google Scholar]
- 47.Sui, G., C. Soohoo, E. B. Affar, F. Gay, Y. Shi, W. C. Forrester, and Y. Shi. 2002. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc. Natl. Acad. Sci. USA 99:5515-5520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Takash, W., J. Canizares, N. Bonneaud, F. Poulat, M. G. Mattei, P. Jay, and P. Berta. 2001. SOX7 transcription factor: sequence, chromosomal localisation, expression, transactivation and interference with Wnt signalling. Nucleic Acids Res. 29:4274-4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tam, P. P., M. Kanai-Azuma, and Y. Kanai. 2003. Early endoderm development in vertebrates: lineage differentiation and morphogenetic function. Curr. Opin. Genet. Dev. 13:393-400. [DOI] [PubMed] [Google Scholar]
- 50.Taniguchi, K., Y. Hiraoka, M. Ogawa, Y. Sakai, S. Kido, and S. Aiso. 1999. Isolation and characterization of a mouse SRY-related cDNA, mSox7. Biochim. Biophys. Acta 1445:225-231. [DOI] [PubMed] [Google Scholar]
- 51.Thompson, J. R., and L. J. Gudas. 2002. Retinoic acid induces parietal endoderm but not primitive endoderm and visceral endoderm differentiation in F9 teratocarcinoma stem cells with a targeted deletion of the Rex-1 (Zfp-42) gene. Mol. Cell. Endocrinol. 195:119-133. [DOI] [PubMed] [Google Scholar]
- 52.Verheijen, M. H., and L. H. Defize. 1999. Signals governing extraembryonic endoderm formation in the mouse: involvement of the type 1 parathyroid hormone-related peptide (PTHrP) receptor, p21Ras and cell adhesion molecules. Int. J. Dev. Biol. 43:711-721. [PubMed] [Google Scholar]
- 53.Wegner, M. 1999. From head to toes: the multiple facets of Sox proteins. Nucleic Acids Res. 27:1409-1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wilson, M., and P. Koopman. 2002. Matching SOX: partner proteins and co-factors of the SOX family of transcriptional regulators. Curr. Opin. Genet. Dev. 12:441-446. [DOI] [PubMed] [Google Scholar]
- 55.Yahata, T., W. Shao, H. Endoh, J. Hur, K. R. Coser, H. Sun, Y. Ueda, S. Kato, K. J. Isselbacher, M. Brown, and T. Shioda. 2001. Selective coactivation of estrogen-dependent transcription by CITED1 CBP/p300-binding protein. Genes Dev. 15:2598-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yuan, H., N. Corbi, C. Basilico, and L. Dailey. 1995. Developmental-specific activity of the FGF-4 enhancer requires the synergistic action of Sox2 and Oct-3. Genes Dev. 9:2635-2645. [DOI] [PubMed] [Google Scholar]
- 57.Yurchenco, P. D., Y. Quan, H. Colognato, T. Mathus, D. Harrison, Y. Yamada, and J. J. O'Rear. 1997. The alpha chain of laminin-1 is independently secreted and drives secretion of its beta- and gamma-chain partners. Proc. Natl. Acad. Sci. USA 94:10189-10194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhuang, Y., T. N. Faria, P. Chambon, and L. J. Gudas. 2003. Identification and characterization of retinoic acid receptor beta2 target genes in F9 teratocarcinoma cells. Mol. Cancer Res. 1:619-630. [PubMed] [Google Scholar]