Abstract
Disrupted social behavior is a core symptom of multiple psychiatric and neurodevelopmental disorders. Many of these disorders are exacerbated by adverse infant experiences, including maltreatment and abuse, which negatively affect amygdala development. Although a link between impaired social behavior, abnormal amygdala function and depressive-like behavior following early adversity has been demonstrated in humans and animal models, the developmental emergence of maltreatment-related social deficits and associated amygdala neural activity are unknown. We used a naturalistic rodent model of maternal maltreatment during a sensitive period, postnatal days 8–12 (PN8–12), which produces social behavior deficits that precede adolescent depressive-like behavior and amygdala dysfunction, to examine social behavior in infancy, periweaning and adolescence. Neural activity in response to the social behavior test was assessed via c-Fos immunohistochemistry at these ages. A separate group of animals was tested for adult depressive-like behavior in the forced swim test. Maltreatment spared infant (PN16–18) social behavior but disrupted periweaning (PN20–22) and adolescent (PN42–48) social behavior. Maltreated rats exhibited blunted neural activation in the amygdala and other areas implicated in social functioning, including the medial prefrontal cortex and nucleus accumbens, at these ages and increased adult depressive-like behavior. These findings may suggest corticolimbic involvement in the emergence of maltreatment-induced social deficits that are linked to adult depressive-like behavior, thereby highlighting potential targets for therapeutic intervention. Understanding how infant experiences influence social behavior and age-specific expression across development may provide insights into basic neural mechanisms of social behaviors and disease-relevant social dysfunction exacerbated by early-life stress.
Introduction
Social behavior deficits are a hallmark feature of psychiatric and neurodevelopmental disorders, including depression, anxiety, autism and schizophrenia,1, 2, 3, 4 and are associated with abnormal amygdala structure and function.5, 6, 7, 8 Animal models suggest a causal link between social deficits and the amygdala.9 Amygdala involvement in social behavior has been demonstrated by (i) lesion studies in rodents10 and nonhuman primates,11, 12 (ii) socially evoked changes in neuronal firing activity within the basolateral amygdala (BLA),13 (iii) bidirectional modulation of social behavior via optogenetic manipulation of BLA fibers14, 15 and (iv) neuroimaging studies of social cognition in humans.16 Here we extend this work to include social behavior deficits within an animal model of depressive-like behavior induced by early-life experience with a maltreating mother.
Importantly, many psychiatric and neurodevelopmental disorders have origins in early life and are exacerbated by stressful infant experiences including early-life abuse and maltreatment, which alter the brain development and increase the risk for later-life psychopathologies such as depression.17, 18, 19, 20, 21, 22 Adverse early-life experiences involving the caregiver negatively affect the development of the amygdala23, 24 a critical brain area for emotion and social behavior25, 26 in humans27, 28, 29, 30, 31 and other mammals.24, 32, 33, 34, 35 Furthermore, maltreatment and abuse lead to social impairments,36, 37, 38, 39 which typically precede the onset of later-life psychopathology and serve as a predictive marker for later-life symptoms related to psychopathology.40, 41 Similar findings have been obtained using naturalistic rodent models of early-life stress that mimic maternal maltreatment.42
The amygdala has a key role in social behavior,25 the long-term effects of childhood abuse/maltreatment17, 21, 27 and the pathophysiology of depression,43, 44 a common outcome of early-life abuse.18, 20, 43, 44, 45 A link between deficient social behavior, abnormal amygdala function and depressive-like behavior following early-life adversity has been demonstrated in humans46, 47 and rodent models.42, 46, 47, 48 Despite these findings, the developmental emergence of social behavior deficits and related neural activity following maltreatment are unknown. To this end, we used a rodent model of maternal maltreatment that consists of creating a low resource environment (that is, insufficient bedding for nest building) for the dam from postnatal (PN) days 8–12, which stresses the mother and increases the frequency of negative maternal behaviors that are painful to the pups, although pups maintain normal weight gain.34, 49 This model closely reflects clinical literature indicating that abused and/or maltreated children exhibit social behavior dysfunction and are at increased risk for developing later-life depression,37, 40, 41 as we have previously shown that maltreatment-induced social deficits serve as a predictive marker for adolescent depressive-like behavior and amygdala dysfunction.42 Our lab has characterized PN8–12 as a sensitive period in the amygdala development during which alterations in the maternal behavior owing to low-bedding stress result in long-lasting social behavior deficits and later-life depressive-like behavior mediated by the amygdala.22, 23, 24, 42 However, the ontogeny of social behavior deficits, as well as brain regions associated with disrupted social behavior, has not been explored.
To this end, we assessed the emergence of maltreatment-induced social behavior deficits and associated neural activity patterns through immunohistochemical detection of c-Fos protein expression across early development (that is, infancy, periweaning, adolescence) in rats. Neural activity in response to the social behavior test was examined within the amygdala as well as in other brain areas sensitive to early-life stress and implicated in the neurobiology of social behavior and depression,4, 50, 51, 52, 53, 54 such as the medial prefrontal cortex (mPFC)55, 56, 57, 58 and the nucleus accumbens (NA).53, 59, 60 We focused on the lateral, basal and central amygdala because previous work suggests that these subnuclei are selectively affected by maltreatment at the ages explored here (that is, periweaning, adolescence).42 Briefly, the lateral amygdala is the major site receiving inputs from sensory systems and generally viewed as the gatekeeper: the basal amygdala receives inputs from the lateral amygdala and connects with the central amygdala as well as other striatal areas involved in the control of instrumental behaviors, while the central nucleus is an important output region for the expression of emotional responses and associated physiological responses.26, 61 Finally, since childhood maltreatment/abuse is a risk factor for adult depression in humans,17, 18, 45 we tested adult depressive-like behavior in the forced swim test (FST)—a measure of behavioral despair in rodents.62
Materials and methods
Animals
Male and female Long–Evans rats born and bred in our colony were housed in polypropylene cages (34 × 29 × 17 cm) with an abundant amount of wood shavings for nest building, and kept in a 20±1 °C environment with a 12:12 light–dark cycle. Food and water were available ad libitum. The day of birth was considered PN0, litters were culled to 12 pups (six males, six females) on PN1 and the rats were weaned on PN23. To avoid possible confounding of litter effects with variables of interest, no more than one male and female animal from a given litter was assigned to an experimental condition and at least four different litters per infant condition (ran over separate cohorts) were used in all the experimental procedures. The sample sizes were chosen on the basis of previously reported findings.42 All animal care and experimental procedures were approved by the Institutional Animal Care and Use Committee, which follow the guidelines from the National Institute of Health.
Rodent model of maternal maltreatment
The mother and her pups were housed in a cage with limited (100 ml) nesting/bedding material (that is, alpine shavings, Northeastern Product, Warrensburg, NY, USA) from PN8 to PN12. This bedding manipulation alters the maternal behavior and increases negative behaviors painful to the pup such as stepping, dragging and rough handling, which involves improper transport of pups (that is, picking it up and moving it (anywhere but the nest)) as well as the frequency of audible pup vocalizations (Supplementary Table 1).24, 34, 42, 48, 49 Notably, this procedure mimics the effects of a stressful rearing environment (that is, resource depletion) as a risk factor for potentiating infant abuse.24, 49 This paradigm is similar to the more stressful low-bedding manipulation developed in the Baram laboratory,63 where more stressors (that is, grid floor, unchanged bedding) and significant reduced pup weight gain could model much greater adversity.63, 64 Control mothers and pups were housed in a cage with abundant nesting/bedding material (~4500 ml) from PN8 to PN12, which allows the mother to build an adequate nest and spend most of her time caring for pups.42, 48, 63, 64
Behavioral studies
Social behavior test
The social approach behavior was tested as previously described,42 during infancy (PN16–18; n=7 control, n=5 maltreated), periweaning (PN20–22; n=6 control, n=6 maltreated) and adolescence (PN42–48; n=7 control, n=7 maltreated). The animals from both infant conditions were tested and scored blind on the same day. Briefly, each animal received a 5-min acclimation period in the testing apparatus. After habituation, the rat was removed from the testing apparatus and a younger same sex (that is, social stimulus) animal was placed inside one of a metal cube, which allows for olfactory, auditory and tactile communication but prevents aggressive or sexual interactions.65 The test animal was placed in the control chamber and the number of chamber crossings and time spent in the social stimulus chamber was recorded and scored for 10 min. Social behavior was measured as the total time spent in the social stimulus chamber, as previously reported by our laboratory42, 48, 66 and total number of chamber crossings was used as an index of general locomotor activity.42 Decreased time spent in the social compartment compared with the non-social compartment is defined as social avoidance and thought to reflect a reduction in social motivation.67, 68
Forced swim test
The FST is a measure of behavioral despair in which rodents are forced to swim under inescapable conditions and the duration of immobility behavior is recorded.66, 69 Rats (n=7 control, n=7 maltreated) were tested for depressive-like behavior in the FST during adulthood (>PN75) using a transparent acrylic cylinder (36.8 × 36.8 × 47 cm) filled with clean water (25±1 °C; depth prevented escape and tail touching bottom) for each animal and without knowledge of the experimental condition. The animals underwent two swim sessions on two consecutive days. Day 1 consisted of a 15-min pretest swim to habituate the rats to the test situation, thereby providing a stable, high level of immobility during the 5-min test on the following day (day 2).62, 70 Two parameters of depressive-like behavior were recorded and scored blindly: time spent immobile, defined as passive floating without struggling, slightly hunched but upright position with minor movements to maintain head above water,42, 48, 70 as well as the latency to immobility—the first time at which the animal initiated a stationary posture that did not reflect attempts to escape/struggle. This passive posture had to last 5 s or longer to be scored as an immobility bout. The rats were gently dried, placed on a heated chamber and returned to the home cage after both sessions.
Neural assessment
We used c-Fos protein expression as a metabolic marker of cell activation.71 Although resting-state levels of c-Fos are typically low, physiological or psychosocial challenges induce the expression of c-Fos protein, which serves as an indirect marker for neuronal activity.71, 72 The animals were decapitated 90 minutes following the end of the social behavior test because peak expression of c-Fos occurs around this time.71, 72 The brains were removed, frozen and stored in a −80 °C freezer until sectioning in a Leica CM3050S cryostat (20 μm) at −20 °C. The brains were cut in two series: every fourth section was collected for c-Fos immunohistochemistry and the next section was collected for cresyl violet staining so that the distance between each fos-stained section is 80 μm. The sections received a 15 min post-fix in 4% paraformaldehyde/0.1 m phosphate-buffered saline (PBS, pH 7.4). Following fixation, the sections were rinsed in PBS three times. To eliminate peroxidase activity, the sections were incubated in 3% H2O2 and 97% methanol for 15 min. Following four PBS rinses, the slides (Fisherbrand, Fisher Scientific, Pittsburgh, PA, USA) were incubated in a blocking solution containing 1% normal goat serum (Jackson ImmunoResearch Laboratories, West Grove, PA, USA; Catalog No. 005-000-121) and 1% albumin for 30 min. The slides were then treated overnight at room temperature with the primary antibody (anti-c-Fos (Ab5) (4-17) Rabbit pAb, Calbiochem, San Diego, CA, USA; Product No. PC38-100UL) diluted 1:1000 in blocking solution. Afterwards, they were rinsed in three PBS washes and incubated in the secondary antibody (goat anti-rabbit IgG, Vector Labs, Burlingame, CA, USA; Catalog No. BA-1000) diluted 1:200 in 50% blocking solution for 30 min at room temperature followed by additional PBS rinses. The sections were treated for 30 min in avidin–biotin–peroxidase complex solution (ABC Elite kit, Catalog No. PK-6101, Vector Labs) and the slides were then rinsed three times in PBS and treated with a solution containing Vector VIP (VIP), H2O2 and nickel (Vector VIP peroxidase kit, Catalog No SK-4600; Vector Labs) for 5 min, rinsed in PBS, subsequently dehydrated in alcohol and xylene, and coverslipped for microscope examination.
The c-Fos-positive cells were counted bilaterally and the brain areas were outlined using a stereotaxic rat brain atlas.73 All the c-Fos-positive cells were distinguished from the background by density of staining, shape and size of cells and were counted without knowledge of the experimental condition. The mean bilateral count of the number of cells containing c-Fos for an animal was determined by averaging the counts from three sections per brain area, as described previously.34 The brain areas examined included the basal and lateral amygdala nuclei, the PFC cingulate, prelimbic (PL) and infralimbic (IL) cortices, and the NA core and shell.
Statistical analysis
The social behavior/chamber crossing data were analyzed with two-way analysis of variance followed by post hoc Fisher tests. The c-Fos data and adult FST data were analyzed by t-tests. The data were expressed as mean (±s.e.m.) and in all the cases, differences were considered significant when P<0.05.
Results
Maternal maltreatment disrupts social behavior during periweaning and adolescence, but not during infancy
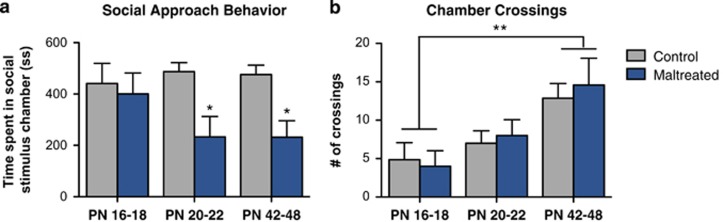
The exposure to caregiver maltreatment from PN8 to PN12 spared infant social behavior but impaired periweaning and adolescent sociability (Figure 1), confirming a developmental delay for the emergence of social behavior deficits as the pups approach weaning.42 Although infant (that is, PN16–18) rats reared with an maltreating mother exhibited social behavior that did not differ from controls, periweaning (that is, PN20–22) and adolescent rats exposed to infant maltreatment spent significantly less time in the social chamber than rats reared with a normal mother (F(1,31)=9.996, P<0.05; Figure 1a), which is thought to reflect social avoidance.68 Significant effects of age were found for chamber crossings (F(2,32)=12.27, P<0.01; Figure 1b). Maltreated animals did not differ from control animals in the number of chamber crossings at any age, although adolescent (PN42–48) rats of both groups (control, maltreated) exhibited a higher amount of chamber crossings than infant (PN16–18) rats. These findings suggest that although there is an age-related increase in locomotor activity, the observed effects of maltreatment at periweaning and adolescent are not owing to between-group differences in locomotion.
Figure 1.
Developmental emergence of social behavior deficits following maternal maltreatment. (a) Maltreated rats show normal social behavior during infancy (that is, postnatal day (PN)16–18). Periweaning rats (that is, PN20–22) and adolescent (that is, PN42–48) experiencing maternal maltreatment exhibit reduced social approach behavior compared with controls. (b) Maltreated animals do not differ from control animals in the number of chamber crossings at any age, although adolescent rats of both groups (control, maltreated) exhibited a higher amount of chamber crossings; *P<0.05, ** P<0.01. Error bars represent s.e.m. (n=5–7 per group).
Developmental emergence of social behavior deficit following maternal maltreatment is associated with blunted cellular activation within corticolimbic structures
Amygdala
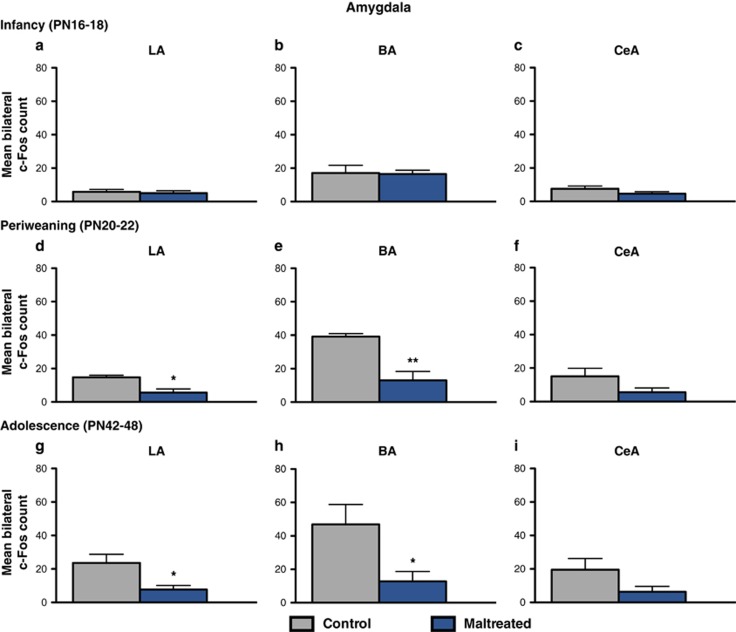
Infant maltreatment attenuated amygdala activation within the lateral and basal amygdala nuclei in response to social behavior testing during periweaning and adolescence, but not during infancy (Figure 2). Periweaning animals reared with a maltreating mother from PN8 to 12 exhibited a reduction of c-Fos positive cells in the lateral (t=3.318, df=7; P<0.05, Cohen's d=2.324, effect size r=0.758) and basal (t=4.197, df=7; P<0.01, Cohen's d=2.981, effect size r=0.830) amygdala nuclei compared with controls (Figures 2d and e). No difference was found between the control and maltreated rats in the central amygdala nuclei (P=0.1074; Figure 2f). A similar pattern was found in maltreated adolescent animals, which also had lower counts of c-Fos positive cells in the lateral (t=2.795, df=10; P<0.05, Cohen's d=1.613, effect size r=0.628) and basal (t=2.555, df=10; P<0.05, Cohen's d=1.475, effect size r=0.594) amygdala nuclei, but not the central (P=0.1053) compared with control animals reared by a normal mother (Figures 2g–i).
Figure 2.
Amygdala neural activity in response to the social behavior test at infancy, periweaning and adolescence. (a–c) At postnatal day (PN)16–18, maltreated rats showed no significant difference in c-Fos expression in the lateral, basal or central amygdala nuclei compared with control animals. (d–f) Periweaning (PN20–22) and adolescent (PN42–48) rats exposed to maltreatment exhibited a significant reduction in c-Fos expression in the lateral (d and g) and basal (e and h) amygdala nuclei in response to the social behavior test compared with control animals (n=4–6 per group; P<0.05); *P<0.05, **P<0.01. Bars represent the number (mean±s.e.m.) of c-Fos positive cells counted bilaterally in the lateral and basal amygdala nuclei. BA, basal amygdala; CeA, central amygdala; LA, lateral amygdala.
Prefrontal cortex
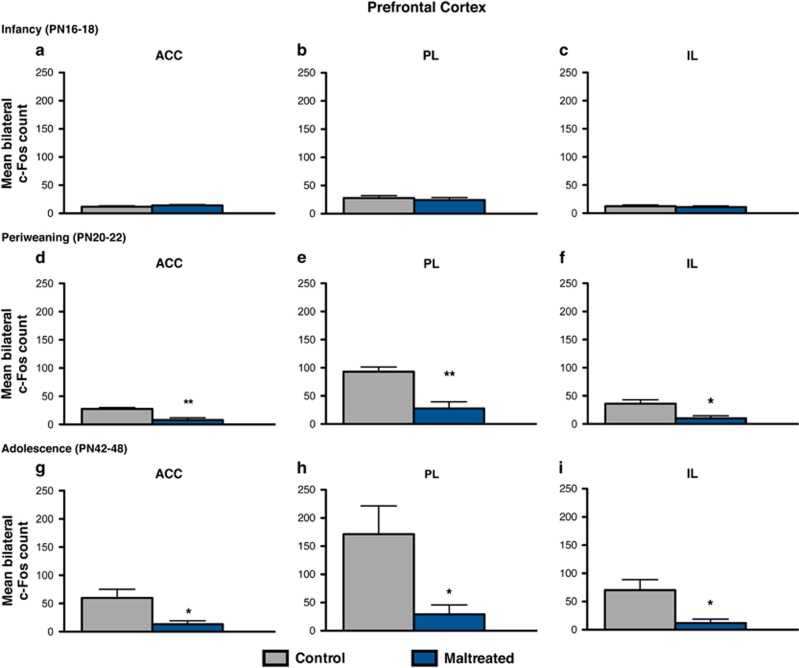
Maternal maltreatment dampened c-Fos protein expression in the mPFC of periweaning and adolescent animals, but not infant animals (Figure 3). The assessment of neural activity in the mPFC, including the anterior cingulate (ACC), PL and IL cortices, in response to the social behavior test revealed a widespread reduction of c-Fos immunoreactivity in each of these subdivisions during the periweaning (Figures 3d–f) and adolescent (Figures 3g–i) periods following early-life abuse. Periweaning animals exposed to maltreatment exhibited decreased c-Fos expression in the ACC (t=3.375, df=7; P<0.01, Cohen's d=2.613, effect size r=0.794), PL (t=4.216, df=7; P<0.01, Cohen's d=2.916, effect size r=0.825) and IL (t=3.342, df=7; P<0.05, Cohen's d=2.189, effect size r=0.738) compared with control animals (Figures 3d–f). Adolescent animals experiencing infant maltreatment also showed a similar reduction in the ACC (t=2.610, df=9; P<0.05, Cohen's d=1.648, effect size r=0.636), PL (t=2.483, df=9; P<0.05, Cohen's d=1.571, effect size r=0.618) and IL (t=2.731, df=9; P<0.05, Cohen's d=1.723, effect size r=0.653) compared with control animals (Figures 3g–i).
Figure 3.
Maltreatment effects on cellular activation in response to social behavior testing within the mPFC during infancy, periweaning and adolescence. (a–c) At postnatal day (PN)16–18, no significant differences were found in any of the medial prefrontal cortices in response to the social behavior test between maltreated and control animals. (d–f) Periweaning animals that were maltreated from PN8 to PN12 exhibited decreased cellular activation in the mPFC, as indicated by lower counts of c-Fos expression, in the cingulate (d), prelimbic (e) and infralimbic (f) cortices compared with control animals (n=4–5 per group; P<0.05). (g–i) A similar pattern was observed in maltreated adolescent animals, which also showed attenuated c-Fos expression in the cingulate (g), prelimbic (h) and infralimbic (i) cortices compared with control animals (n=5–6 per group; P<0.05); *P<0.05; **P<0.01. Bars represent the number (mean±s.e.m.) of c-Fos positive cells counted bilaterally in each nuclei. ACC, anterior cingulate cortex; IL, infralimbic cortex; mPFC, medial prefrontal cortex; PL, prelimbic cortex.
Nucleus accumbens
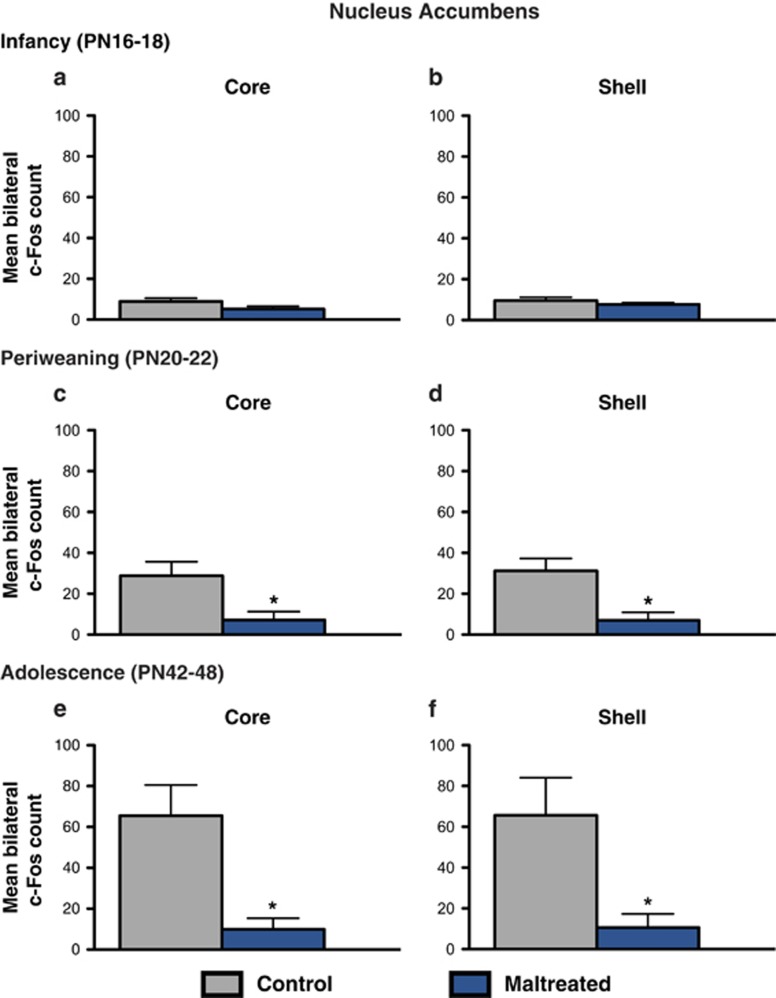
Similar to the amygdala and the mPFC, maternal maltreatment resulted in blunted c-Fos expression following social behavior testing that was specific to the periweaning and adolescent periods (Figure 4). Periweaning animals receiving infant maltreatment had diminished c-Fos counts in both the NA core (t=2.656, df=6; P<0.05, Cohen's d=1.878, effect size r=0.685) and NA shell (t=3.322, df=6; P<0.05, Cohen's d=2.350, effect size r=0.761) compared with control animals (Figures 4c and d). This decline in the number of c-Fos immunoreactive cells was also observable in the NA core (t=3.202, df=9; P<0.05, Cohen's d=2.024, effect size r=0.711) and shell (t=2.589, df=9; P<0.05, Cohen's d=1.636, effect size r=0.633) of adolescent animals experiencing maternal maltreatment (Figures 4e and f).
Figure 4.
Activity in the nucleus accumbens in response to the social behavior test across development. (a and b) During infancy (postnatal day (PN)16–18), no differences were found in the NA core or shell of maltreated and control animals. (c and d) Periweaning (PN20–22) animals exposed to maternal maltreatment had reduced c-Fos expression in both the NA core and shell compared with control animals (n=4 per group; P<0.05). (e and f) Previously maltreated adolescent animals also had reduced activation (that is, lower number of c-Fos positive cells) in the NA core and shell (n=5–6 per group; P<0.05). *P<0.05. Bars represent the number (mean±s.e.m.) of c-Fos positive cells counted bilaterally in each nuclei. NA, nucleus accumbens.
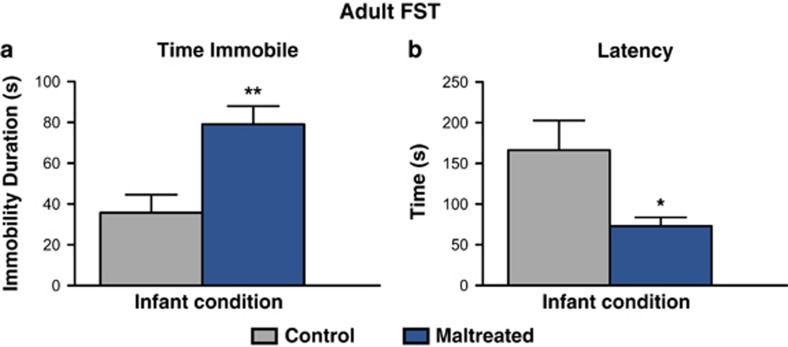
Maternal maltreatment increases immobility duration and decreases latency to immobility in the FST during adulthood
Infant maltreatment induced adult depressive-like behavior in the FST across two parameters: time spent immobile and latency to immobility (Figure 5). Maltreated rats reared displayed increased immobility duration (that is, passive floating) during the FST compared with controls (n=7 per group; t=3.462, df=12; P<0.01, Cohen's d=1.850, effect size r=0.679; variance, P=0.9851; Figure 5a), which was accompanied by a reduction in the latency to immobility (n=7 per group; t=2.468, df=12; P<0.05, Cohen's d=1.319, effect size r=0.550; variance, P=0.0080) compared with controls (Figure 5b). Collectively, these findings suggest that early life abuse, as modeled by maternal maltreatment, increases behavioral despair in response to the adult FST.
Figure 5.
Maternal maltreatment programs adult depressive-like behavior in the FST. (a) Maltreated adult rats displayed increased immobility duration (n=7 per group; P<0.01) in the FST compared with control animals reared with a normal mother from postnatal day (PN)8 to PN12. (b) Maltreated animals also exhibited a reduced latency to immobility (n=7 per group; P<0.05) compared with controls; *P<0.05, **P<0.01. Error bars represent s.e.m. FST, forced swim test.
Discussion
Adverse social experiences during early life are associated with marked dysfunction in social functioning across the lifetime.67 Here we demonstrate that maternal maltreatment, as modeled by rearing PN8–12 pups with dam provided with insufficient bedding for nest building, induced long-lasting changes in sociability that were characterized by a decrease in social approach behavior (that is, social avoidance; Figure 1), thought to reflect a reduction in social motivation.67, 68 These data corroborate prior findings showing that early-life caregiver maltreatment results in atypical social behavior during periweaning (PN20–22) and adolescence (PN42–47; Figure 1),42 but broadens these results to younger ages and expands on brain areas closely associated with affect and social behavior (Figures 2, 3, 4).50, 69, 74 The inclusion of a younger age group (that is, PN16–18) indicates that social behavior deficits emerge later in development, as pups approach independence, and are accompanied by neural alterations in the amygdala, NA and PFC (Figures 2, 3, 4)—all of which are part of the social motivation network in humans and rodents.4, 25, 50, 67 Recent work from our laboratory has shown that aberrant social behavior following infant maltreatment persists into adulthood,48 which is consistent with preclinical reports that adult sociability is disrupted by prenatal, neonatal and juvenile stress exposure, all of which reduce social motivation and inhibit social interactions.67 Furthermore, these findings are in accordance with clinical studies indicating that childhood maltreatment is linked to impaired social skills46, 47 and is a strong predictor of later-life social behavior problems, including adolescent social withdrawal and adult antisocial behavior.37, 38, 75
Identification of neural substrates implicated in the developmental disruption of social behavior by caregiver maltreatment is an important translational goal, as it may provide insight into potential therapeutic targets for correcting social behavior dysfunction present in depression and other affective disorders exacerbated by early-life adversity. Here we examined the long-term effects of early-life stress, as modeled by caregiver maltreatment, on neural activation in a subset of brain regions implicated in the social brain, which refers to brain areas activated in humans in social cognition tasks,73 following social behavior testing. Notably, the social brain network in other mammals overlaps with the human social brain, including the amygdala, prefrontal cortex and the ventral striatum.25, 50, 67, 73 Infant maltreatment dramatically dampened neural activation in these corticolimbic structures in response to the social behavior test, as indicated by a widespread reduction of c-Fos immunoreactivity in the BLA, mPFC and NA (Figures 2, 3, 4). Moreover, these effects were specific to infant condition and age and were only observable in relation to social behavior deficits. These findings are in agreement with previous reports demonstrating blunted neural activity within these structures in adolescent rats subjected to postweaning social isolation stress. Previous studies have identified changes in c-Fos expression in rats younger than those included in this study (that is, <PN15), suggesting that the lack of neural activity changes during infancy (that is, PN16–18) is not due to a methodological floor effect.
Maternal maltreatment attenuated amygdala activity in the lateral and basal nuclei, but not the central nuclei, in response to the social behavior test at periweaning and adolescence (Figure 2). This is consistent with preclinical findings of dampened socioemotional behavior and reduced basolateral amygdala neuronal excitability following prenatal stress78 and with clinical reports of dysfunctional social approach behavior in depressed patients, which is correlated with a strong decrease in amygdala activation.7 The amygdala's role in social behavior is complex, as it has extensive connections to other subcortical and cortical structures whose function it modulates.25, 50, 79 For example, the amygdala connects with prefrontal and striatal areas implicated in guiding social affiliation or avoidance, such as the mPFC and NA.80, 81, 82 Indeed, social play behavior in rats increases neural activity (that is, enhances c-Fos immunoreactivity) in the amygdala, mPFC and NA, and correlations between social play behavior and cellular activation in cortico–amygdala and amygdalo–striatal connections have been suggested.83
Social interactions, including social approach behavior and social play, activate neurons in the mPFC,83, 84 and pharmacological inactivation of the mPFC reduces social interaction.85 Consistent with these data, infant maltreatment reduced social behavior (Figure 1) and blunted neural activity in the PFCs (that is, cingulate, PL, IL) at both periweaning and adolescence (Figure 3). This is of clinical relevance because adults with a history of childhood maltreatment exhibit hypoactive mPFC function.57 Moreover, the mPFC, which shares reciprocal connections with the BLA,86, 87 exhibits profound alterations in a variety of neurodevelopmental and psychiatric disorders involving impaired social cognition and dysregulated affect, including depression.52, 88 Furthermore, early-life adversity such as childhood maltreatment and/or maternal deprivation alters amygdala–PFC connectivity56 and results in structural abnormalities in both structures.29, 58 Such changes are also frequently found in individuals with abnormal social behavior.67 In rodents, the BLA–mPFC pathway has a causal role in the bidirectional modulation of social behavior.14
A similar decline in neural activation in response to social behavior testing was also observed in the NA core and shell (Figure 4), which receive projections from both the mPFC and the amygdala that are involved in the motivational aspects of behavior.80, 81, 89 Although the NA had previously been implicated in social play behavior,83 a causal role for the NA in social approach/interaction behavior has recently been revealed. In female rats, increases in the activity of ventral tegmental area dopamine neurons and the ventral tegmental area–NA pathway encode and predict key features of social behavior through a dopamine D1-receptor mechanism.90 The NA has also been implicated in both the pursuit of social reward and the avoidance of social punishment in humans.91
The alterations in the connections between the amygdala, the mPFC and the NA have been implicated in social inhibition,92, 93 depression7, 94 and the neurobiological sequelae of early-life stress.33, 53, 56 For example, functional changes in the mPFC can cause prominent changes in social behavior in both humans and other mammals owing to its projections to subcortical limbic structures involved in initiating behaviors related to the motivational significance of sensory stimuli, like the amygdala and the NA.89, 95 In humans, high levels of social inhibition—the tendency to withdraw from new people and avoid social situations—are associated with reduced connectivity within limbic, striatal and prefrontal regions.92 In rodents, early social stressors, postweaning social isolation and chronic adult stressors reduce activation in most areas of the social brain when animals are exposed to other conspecifics76, 77, 78, 96 (Figures 2, 3, 4), which is consistent with general impairment of social behaviors induced by such stressors.
Intriguingly, many brain areas particularly vulnerable to early-life stress, such as the amygdala, mPFC, NA, are characterized by protracted postnatal development, high density of glucocorticoid receptors and exhibit functional and/or structural alterations in individuals with abnormal social behavior.21, 24, 54, 97 Collectively, these findings suggest that the developmental trajectory of these structures is sensitive to early-life adversity, which programs later-life social behavior deficits by altering the way cortical and limbic structures respond to social encounters, which may enhance susceptibility towards developing additional symptoms relating to psychopathology. However, it is important to note that the effects of early-life stress are ubiquitous throughout the brain and there are additional areas not examined here, such as the hippocampus and hypothalamic areas, that may be affected by maltreatment and contribute to the social dysfunction and increased risk for psychopathology associated with early adversity.73, 98
As we integrate these findings into previous work from our lab, it becomes clear that infant maltreatment produces task-specific changes in brain activity patterns. For example, maltreated adolescents have attenuated amygdala responses in the social behavior test but exhibit a hyperactive response to an inescapable, uncontrollable stressor (that is, FST) during the same developmental period.42 Generalized statements about early-life experiences attenuating or potentiating neural activity should include task-specific information, which likely use different circuits within a brain area. Furthermore, the amygdala's contribution to social behavior is not rigid and universal,4 but context-dependent and susceptible to individual differences.50 Effects of early adversity on social behavior changes and associated neural structures may reflect a change in the way context-dependent situations (stimuli in the context of an emotionally significant or socially significant setting) modulate motivated behavior. Given that the social experience (that is, time spent in social chamber) was lower for maltreated animals, an alternative explanation may be that the behavioral differences between each group induced reduced social stimuli exposure, which induced the Fos difference. In this case, our Fos results may reflect differences in stimulus exposure owing to individual-initiated activity differences in these areas after maternal maltreatment, which may lead to a change in their developmental trajectories. Indeed, in the human literature, reduced social interaction is thought to initiate a developmental cascade that can potentiate the effects of early-life adversity.36, 99, 100 However, even when stimulus exposure is controlled, previous work from our lab suggests neural differences between maltreated and control animals. Specifically, weaning-aged pups show significant differences in amygdala and PFC in response to other social odors (maternal and adult male odors) even when exposure time is controlled in weaning-aged pups.101 Furthermore, maltreated animals without stimulus exposure exhibit alterations in resting-state functional connectivity between the ACC/mPFC and amygdala, as well as between the ACC/mPFC and the striatum compared with control animals, and some of these connectivity patterns change from adolescence to early adulthood.102 Together, it is likely that adversity-induced brain changes are further modified by behavior differences induced by the adversity.
In addition to social behavior deficits and neural alterations, maltreatment induced adult depressive-like behavior, as indexed by increased immobility duration and reduced latency to immobility in the FST (Figure 5). We have previously shown that depressive-like behavior in the FST emerges during adolescence and is associated with enhanced amygdala activity in the lateral, basal and central amygdala nuclei.42 Amygdala hyperactivity is causal in the expression of depressive-like behavior, as pharmacological inactivation of the amygdala via muscimol infusion before the FST rescues depressive-like behavior.42 Thus, our rodent model of early-life abuse recapitulates findings in humans indicating that early stress stemming from childhood adversity is a predisposing risk factor for the development of social deficits and adult depression.103
In summary, here we used a naturalistic rodent model of chronic early-life stress to demonstrate long-term effects of early-life adversity on social behavior. This paradigm recapitulates the neurobehavioral sequelae of abused children, including social behavior deficits that are frequently comorbid with depression and usually precede the expression of depression-related symptoms.46, 47 This is exemplified by abuse-induced social behavior deficits that emerge at periweaning and persevere throughout the lifetime,42, 48 although depressive-like behavior in the FST emerges in adolescence42 and persists into adulthood. This alteration in social behavior is associated with blunted activation of corticolimbic regions that comprise the social brain, including the BLA, the mPFC and the NA, and are critically involved in the neurobiology of mood and psychiatric disorders, including depression.54, 88, 97 Thus, these data support human and preclinical research indicating that adverse early-life events involving the caregiver negatively affect the developmental trajectory and function of cortical and limbic regions implicated in decision-making, emotion and social behavior.23, 31, 33, 56 Finally, our findings provide insight into the mechanisms by which early-life stress affects brain structures implicated in learning, reward processing, motivation and sociability51 and the involvement of these areas in the onset of disease-relevant social dysfunction exacerbated by early-life stress.
Acknowledgments
This research was supported by the National Science Foundation (NSF) Graduate Research Fellowship Program under Grant No. DGE-1137475 to MR-C and National Institute of Health (NIH)-MH091451, NIH-DC009910, NIH-HD083217 to RMS.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- American Psychiatric AssociationDiagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association: Arlington, VA, USA, 2013. [Google Scholar]
- Derntl B, Habel U. Deficits in social cognition: a marker for psychiatric disorders? Eur Arch Psychiatry Clin Neurosci 2011; 261(Suppl 2): S145–S149. [DOI] [PubMed] [Google Scholar]
- Hoertnagl CM, Hofer A. Social cognition in serious mental illness. Curr Opin Psychiatry 2014; 27: 197–202. [DOI] [PubMed] [Google Scholar]
- Kennedy DP, Adolphs R. The social brain in psychiatric and neurological disorders. Trends Cogn Sci 2012; 16: 559–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anand A, Shekhar A. Brain imaging studies in mood and anxiety disorders: special emphasis on the amygdala. Ann N Y Acad Sci 2003; 985: 370–388. [DOI] [PubMed] [Google Scholar]
- Brunet-Gouet E, Decety J. Social brain dysfunctions in schizophrenia: a review of neuroimaging studies. Psychiatry Res 2006; 148: 75–92. [DOI] [PubMed] [Google Scholar]
- Derntl B, Seidel EM, Eickhoff SB, Kellermann T, Gur RC, Schneider F et al. Neural correlates of social approach and withdrawal in patients with major depression. Soc Neurosci 2011; 6: 482–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann CM, Bauman MD, Amaral DG. Abnormal structure or function of the amygdala is a common component of neurodevelopmental disorders. Neuropsychologia 2011; 49: 745–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allsop SA, Vander Weele CM, Wichmann R, Tye KM. Optogenetic insights on the relationship between anxiety-related behaviors and social deficits. Front Behav Neurosci 2014; 8: 241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daenen EW, Wolterink G, Gerrits MA, Van Ree JM. The effects of neonatal lesions in the amygdala or ventral hippocampus on social behaviour later in life. Behav Brain Res 2002; 136: 571–582. [DOI] [PubMed] [Google Scholar]
- Bliss-Moreau E, Moadab G, Bauman MD, Amaral DG. The impact of early amygdala damage on juvenile rhesus macaque social behavior. J Cogn Neurosci 2013; 25: 2124–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raper J, Stephens SB, Sanchez M, Bachevalier J, Wallen K. Neonatal amygdala lesions alter mother-infant interactions in rhesus monkeys living in a species-typical social environment. Dev Psychobiol 2014; 56: 1711–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsana AJ, Li N, Brown TH. Positive and negative ultrasonic social signals elicit opposing firing patterns in rat amygdala. Behav Brain Res 2012; 226: 77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Burgos-Robles A, Bhagat ND, Leppla CA, Tye KM. Bidirectional modulation of anxiety-related and social behaviors by amygdala projections to the medial prefrontal cortex. Neuroscience 2015; 321: 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felix-Ortiz AC, Tye KM. Amygdala inputs to the ventral hippocampus bidirectionally modulate social behavior. J Neurosci 2014; 34: 586–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio AR. The human amygdala in social judgment. Nature 1998; 393: 470–474. [DOI] [PubMed] [Google Scholar]
- McCrory E, De Brito SA, Viding E. The link between child abuse and psychopathology: a review of neurobiological and genetic research. J R Soc Med 2012; 105: 151–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanni V, Uher R, Danese A. Childhood maltreatment predicts unfavorable course of illness and treatment outcome in depression: a meta-analysis. Am J Psychiatry 2012; 169: 141–151. [DOI] [PubMed] [Google Scholar]
- Neigh GN, Gillespie CF, Nemeroff CB. The neurobiological toll of child abuse and neglect. Trauma Violence Abuse 2009; 10: 389–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman RE, Byambaa M, De R, Butchart A, Scott J, Vos T. The long-term health consequences of child physical abuse, emotional abuse, and neglect: a systematic review and meta-analysis. PLoS Med 2012; 9: e1001349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teicher MH, Samson JA. Annual research review: enduring neurobiological effects of childhood abuse and neglect. J Child Psychol Psychiatry 2016; 57: 241–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Casey BJ. The impact of developmental timing for stress and recovery. Neurobiol Stress 2015; 1: 184–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drury SS, Sanchez MM, Gonzalez A. When mothering goes awry: challenges and opportunities for utilizing evidence across rodent, nonhuman primate and human studies to better define the biological consequences of negative early caregiving. Horm Behav 2016; 77: 182–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rincon-Cortes M, Sullivan RM. Early life trauma and attachment: immediate and enduring effects on neurobehavioral and stress axis development. Front Endocrinol 2014; 5: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickart KC, Dickerson BC, Barrett LF. The amygdala as a hub in brain networks that support social life. Neuropsychologia 2014; 63: 235–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia-Filho AA, Londero RG, Achaval M. Functional activities of the amygdala: an overview. J Psychiatry Neurosci 2000; 25: 14–23. [PMC free article] [PubMed] [Google Scholar]
- Dannlowski U, Stuhrmann A, Beutelmann V, Zwanzger P, Lenzen T, Grotegerd D et al. Limbic scars: long-term consequences of childhood maltreatment revealed by functional and structural magnetic resonance imaging. Biol Psychiatry 2012; 71: 286–293. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T et al. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Dev Sci 2010; 13: 46–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim L, Radua J, Rubia K. Gray matter abnormalities in childhood maltreatment: a voxel-wise meta-analysis. Am J Psychiatry 2014; 171: 854–863. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Lyons-Ruth K, Anderson CM, Teicher MH. Sensitive periods of amygdala development: the role of maltreatment in preadolescence. Neuroimage 2014; 97: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Nacewicz BM, Sutterer MJ, Cayo AA, Schaefer SM, Rudolph KD et al. Behavioral problems after early life stress: contributions of the hippocampus and amygdala. Biol Psychiatry 2015; 77: 314–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell BR, Grand AP, McCormack KM, Shi Y, LaPrarie JL, Maestripieri D et al. Early adverse experience increases emotional reactivity in juvenile rhesus macaques: relation to amygdala volume. Dev Psychobiol 2014; 56: 1735–1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malter Cohen M, Jing D, Yang RR, Tottenham N, Lee FS, Casey BJ. Early-life stress has persistent effects on amygdala function and development in mice and humans. Proc Natl Acad Sci USA 2013; 110: 18274–18278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Moriceau S, Sullivan RM. Developing a neurobehavioral animal model of infant attachment to an abusive caregiver. Biol Psychiatry 2010; 67: 1137–1145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Matt S, Chen K, Blaze J. Bdnf DNA methylation modifications in the hippocampus and amygdala of male and female rats exposed to different caregiving environments outside the homecage. Dev Psychobiol 2014; 56: 1755–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alink LR, Cicchetti D, Kim J, Rogosch FA. Longitudinal associations among child maltreatment, social functioning, and cortisol regulation. Dev Psychol 2012; 48: 224–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansford JE, Dodge KA, Pettit GS, Bates JE, Crozier J, Kaplow J. A 12-year prospective study of the long-term effects of early child physical maltreatment on psychological, behavioral, and academic problems in adolescence. Arch Pediatr Adolesc Med 2002; 156: 824–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ometto M, de Oliveira PA, Milioni AL, Dos Santos B, Scivoletto S, Busatto GF et al. Social skills and psychopathic traits in maltreated adolescents. Eur Child Adolesc Psychiatry 2015; 25: 397–405. [DOI] [PubMed] [Google Scholar]
- Shields AM, Cicchetti D, Ryan RM. The development of emotional and behavioral self-regulation and social competence among maltreated school-age children. Dev Psychopathol 1994; 6: 57–75. [DOI] [PubMed] [Google Scholar]
- Mason WA, Kosterman R, Hawkins JD, Herrenkohl TI, Lengua LJ, McCauley E. Predicting depression, social phobia, and violence in early adulthood from childhood behavior problems. J Am Acad Child Adolesc Psychiatry 2004; 43: 307–315. [DOI] [PubMed] [Google Scholar]
- Mazza JJ, Fleming CB, Abbott RD, Haggerty KP, Catalano RF. Identifying trajectories of adolescents' depressive phenomena: an examination of early risk factors. J Youth Adolesc 2010; 39: 579–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Cortes MR, Belnoue L, Sullivan RM. Effects of early-life abuse differ across development: infant social behavior deficits are followed by adolescent depressive-like behaviors mediated by the amygdala. J Neurosci 2012; 32: 7758–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC. Neuroimaging abnormalities in the amygdala in mood disorders. Ann N Y Acad Sci 2003; 985: 420–444. [DOI] [PubMed] [Google Scholar]
- Sibille E, Wang Y, Joeyen-Waldorf J, Gaiteri C, Surget A, Oh S et al. A molecular signature of depression in the amygdala. Am J Psychiatry 2009; 166: 1011–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibb BE, Butler AC, Beck JS. Childhood abuse, depression, and anxiety in adult psychiatric outpatients. Depress Anxiety 2003; 17: 226–228. [DOI] [PubMed] [Google Scholar]
- Huh HJ, Kim SY, Yu JJ, Chae JH. Childhood trauma and adult interpersonal relationship problems in patients with depression and anxiety disorders. Ann Gen Psychiatry 2014; 13: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pechtel P, Pizzagalli DA. Effects of early life stress on cognitive and affective function: an integrated review of human literature. Psychopharmacology 2011; 214: 55–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raineki C, Sarro E, Rincon-Cortes M, Perry R, Boggs J, Holman CJ et al. Paradoxical neurobehavioral rescue by memories of early-life abuse: the safety signal value of odors learned during abusive attachment. Neuropsychopharmacology 2015; 40: 906–914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth TL, Sullivan RM. Memory of early maltreatment: neonatal behavioral and neural correlates of maternal maltreatment within the context of classical conditioning. Biol Psychiatry 2005; 57: 823–831. [DOI] [PubMed] [Google Scholar]
- Adolphs R. What does the amygdala contribute to social cognition? Ann N Y Acad Sci 2010; 1191: 42–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev 2002; 26: 321–352. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213: 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Gee DG, Telzer EH, Humphreys KL, Gabard-Durnam L, Flannery J et al. Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience 2013; 249: 129–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Morrison JH. The brain on stress: vulnerability and plasticity of the prefrontal cortex over the life course. Neuron 2013; 79: 16–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaze J, Asok A, Roth TL. Long-term effects of early-life caregiving experiences on brain-derived neurotrophic factor histone acetylation in the adult rat mPFC. Stress 2015; 18: 607–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH et al. Early developmental emergence of human amygdala-prefrontal connectivity after maternal deprivation. Proc Natl Acad Sci USA 2013; 110: 15638–15643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, Dalgleish T, van der Wee NJ, Veltman DJ, Aleman A et al. Hypoactive medial prefrontal cortex functioning in adults reporting childhood emotional maltreatment. Soc Cogn Affect Neurosci 2014; 9: 2026–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen AL, van Tol MJ, van der Wee NJ, Veltman DJ, Aleman A, Spinhoven P et al. Reduced medial prefrontal cortex volume in adults reporting childhood emotional maltreatment. Biol Psychiatry 2010; 68: 832–838. [DOI] [PubMed] [Google Scholar]
- Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci 2008; 31: 183–191. [DOI] [PubMed] [Google Scholar]
- Hanson JL, Hariri AR, Williamson DE. Blunted ventral striatum development in adolescence reflects emotional neglect and predicts depressive symptoms. Biol Psychiatry 2015; 78: 598–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux J. The amygdala. Curr Biol 2007; 17: R868–R874. [DOI] [PubMed] [Google Scholar]
- Porsolt RD, Brossard G, Hautbois C, Roux S. Rodent models of depression: forced swimming and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 2001; Chapter 8: Unit 8.10A. [DOI] [PubMed] [Google Scholar]
- Gilles EE, Schultz L, Baram TZ. Abnormal corticosterone regulation in an immature rat model of continuous chronic stress. Pediatr Neurol 1996; 15: 114–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivy AS, Brunson KL, Sandman C, Baram TZ. Dysfunctional nurturing behavior in rat dams with limited access to nesting material: a clinically relevant model for early-life stress. Neuroscience 2008; 154: 1132–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricceri L, Moles A, Crawley J. Behavioral phenotyping of mouse models of neurodevelopmental disorders: relevant social behavior patterns across the life span. Behav Brain Res 2007; 176: 40–52. [DOI] [PubMed] [Google Scholar]
- Rincon-Cortes M, Barr GA, Mouly AM, Shionoya K, Nunez BS, Sullivan RM. Enduring good memories of infant trauma: rescue of adult neurobehavioral deficits via amygdala serotonin and corticosterone interaction. Proc Natl Acad Sci USA 2015; 112: 881–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandi C, Haller J. Stress and the social brain: behavioural effects and neurobiological mechanisms. Nat Rev Neurosci 2015; 16: 290–304. [DOI] [PubMed] [Google Scholar]
- Toth I, Neumann ID. Animal models of social avoidance and social fear. Cell Tissue Res 2013; 354: 107–118. [DOI] [PubMed] [Google Scholar]
- Aupperle RL, Paulus MP. Neural systems underlying approach and avoidance in anxiety disorders. Dialogues Clin Neurosci 2010; 12: 517–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castagne V, Moser P, Roux S, Porsolt RD. Rodent models of depression: forced swim and tail suspension behavioral despair tests in rats and mice. Curr Protoc Neurosci 2011; Chapter 8: Unit 8 10A. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Verbalis JG. c-Fos and related immediate early gene products as markers of activity in neuroendocrine systems. Front Neuroendocrinol 1993; 14: 173–213. [DOI] [PubMed] [Google Scholar]
- Kovacs KJ. Measurement of immediate-early gene activation- c-fos and beyond. J Neuroendocrinol 2008; 20: 665–672. [DOI] [PubMed] [Google Scholar]
- Insel TR, Fernald RD. How the brain processes social information: searching for the social brain. Ann Rev Neurosci 2004; 27: 697–722. [DOI] [PubMed] [Google Scholar]
- Skuse D, Morris J, Lawrence K. The amygdala and development of the social brain. Ann N Y Acad Sci 2003; 1008: 91–101. [DOI] [PubMed] [Google Scholar]
- Shaffer A, Yates TM, Egeland BR. The relation of emotional maltreatment to early adolescent competence: developmental processes in a prospective study. Child Abuse Neglect 2009; 33: 36–44. [DOI] [PubMed] [Google Scholar]
- Ahern M, Goodell DJ, Adams J, Bland ST. Brain regional differences in social encounter-induced Fos expression in male and female rats after post-weaning social isolation. Brain Res 2016; 1630: 120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams T, Rosenkranz JA. Social Isolation during postweaning development causes hypoactivity of neurons in the medial nucleus of the male rat amygdala. Neuropsychopharmacology 2016; 41: 1929–1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich DE, Rainnie DG. Prenatal stress alters the development of socioemotional behavior and amygdala neuron excitability in rats. Neuropsychopharmacology 2015; 40: 2135–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Loucks RA, Palmer AL, Brown AC, Solomon KM, Marchante AN et al. The structural and functional connectivity of the amygdala: from normal emotion to pathological anxiety. Behav Brain Res 2011; 223: 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley AE, Domesick VB, Nauta WJ. The amygdalostriatal projection in the rat—an anatomical study by anterograde and retrograde tracing methods. Neuroscience 1982; 7: 615–630. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Topographical organization of amygdaloid projections to the caudatoputamen, nucleus accumbens, and related striatal-like areas of the rat brain. Neuroscience 1991; 44: 15–33. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the prefrontal cortex and associated striatum in the rat. Neuroscience 1991; 44: 1–14. [DOI] [PubMed] [Google Scholar]
- van Kerkhof LW, Trezza V, Mulder T, Gao P, Voorn P, Vanderschuren LJ. Cellular activation in limbic brain systems during social play behaviour in rats. Brain Struct Funct 2014; 219: 1181–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E, Rhim I, Lee JW, Ghim JW, Lee S, Kim E et al. Enhanced neuronal activity in the medial prefrontal cortex during social approach behavior. J Neurosci 2016; 36: 6926–6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Walton ME, Millette BH, Shirley E, Rushworth MF, Bannerman DM. Distinct contributions of frontal areas to emotion and social behaviour in the rat. Eur J Neurosci 2007; 26: 2315–2326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell MD, Wright DJ. Topography of projections from the medial prefrontal cortex to the amygdala in the rat. Brain Res Bull 1986; 17: 321–333. [DOI] [PubMed] [Google Scholar]
- Hoover WB, Vertes RP. Anatomical analysis of afferent projections to the medial prefrontal cortex in the rat. Brain Struct Funct 2007; 212: 149–179. [DOI] [PubMed] [Google Scholar]
- Malykhin NV, Carter R, Hegadoren KM, Seres P, Coupland NJ. Fronto-limbic volumetric changes in major depressive disorder. J Affect Disord 2012; 136: 1104–1113. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain 1995; 118Pt 1279–306. [DOI] [PubMed] [Google Scholar]
- Gunaydin LA, Grosenick L, Finkelstein JC, Kauvar IV, Fenno LE, Adhikari A et al. Natural neural projection dynamics underlying social behavior. Cell 2014; 157: 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohls G, Perino MT, Taylor JM, Madva EN, Cayless SJ, Troiani V et al. The nucleus accumbens is involved in both the pursuit of social reward and the avoidance of social punishment. Neuropsychologia 2013; 51: 2062–2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackford JU, Clauss JA, Avery SN, Cowan RL, Benningfield MM, VanDerKlok RM. Amygdala-cingulate intrinsic connectivity is associated with degree of social inhibition. Biol Psychol 2014; 99: 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn A, Stein P, Windischberger C, Weissenbacher A, Spindelegger C, Moser E et al. Reduced resting-state functional connectivity between amygdala and orbitofrontal cortex in social anxiety disorder. Neuroimage 2011; 56: 881–889. [DOI] [PubMed] [Google Scholar]
- Wang L, Paul N, Stanton SJ, Greeson JM, Smoski MJ. Loss of sustained activity in the ventromedial prefrontal cortex in response to repeated stress in individuals with early-life emotional abuse: implications for depression vulnerability. Front Psychol 2013; 4: 320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck PH, Bannerman DM, Rushworth MF. The contribution of distinct subregions of the ventromedial frontal cortex to emotion, social behavior, and decision making. Cogn Affect Behav Neurosci 2008; 8: 485–497. [DOI] [PubMed] [Google Scholar]
- Wall VL, Fischer EK, Bland ST. Isolation rearing attenuates social interaction-induced expression of immediate early gene protein products in the medial prefrontal cortex of male and female rats. Physiol Behav 2012; 107: 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goff B, Tottenham N. Early-life adversity and adolescent depression: mechanisms involving the ventral striatum. CNS Spectr 2015; 20: 337–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Baram TZ. Toward understanding how early-life stress reprograms cognitive and emotional brain networks. Neuropsychopharmacology 2015; 41: 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez MM, Pollak SD. Socio-emotional development following early abuse and neglect. Challenges and insights from translational research. In: dH M, Gunnar MR (eds). Handbook of Developmental Neuroscience. Guilford Press: New York, NY, USA, 2009, pp 497–520. [Google Scholar]
- Howell BR, Sanchez MM. Understanding behavioral effects of early life stress using the reactive scope and allostatic load models. Dev Psychopathol 2011; 23: 1001–1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry RE, Al Ain S, Raineki C, Sullivan RM, Wilson DA. Development of odor hedonics: experience-dependent ontogeny of circuits supporting maternal and predator odor responses in rats. J Neurosci 2016; 36: 6634–6650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan C-G, Rincon-Cortes M, Raineki C, Sarro E, Colcombe S, Guilfoyle D et al. Aberrant development of intrinsic brain activity in a rat model of caregiver maltreatment of offspring (submitted). [DOI] [PMC free article] [PubMed]
- Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord 2004; 82: 217–225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.