Abstract
Preclinical research demonstrates that cannabinoids have differing effects in adolescent and adult animals. Whether these findings translate to humans has not yet been investigated. Here we believe we conducted the first study to compare the acute effects of cannabis in human adolescent (n=20; 16–17 years old) and adult (n=20; 24–28 years old) male cannabis users, in a placebo-controlled, double-blind cross-over design. After inhaling vaporized active or placebo cannabis, participants completed tasks assessing spatial working memory, episodic memory and response inhibition, alongside measures of blood pressure and heart rate, psychotomimetic symptoms and subjective drug effects (for example, ‘stoned', ‘want to have cannabis'). Results showed that on active cannabis, adolescents felt less stoned and reported fewer psychotomimetic symptoms than adults. Further, adults but not adolescents were more anxious and less alert during the active cannabis session (both pre- and post-drug administration). Following cannabis, cognitive impairment (reaction time on spatial working memory and prose recall following a delay) was greater in adults than adolescents. By contrast, cannabis impaired response inhibition accuracy in adolescents but not in adults. Moreover, following drug administration, the adolescents did not show satiety; instead they wanted more cannabis regardless of whether they had taken active or placebo cannabis, while the opposite was seen for adults. These contrasting profiles of adolescent resilience (blunted subjective, memory, physiological and psychotomimetic effects) and vulnerability (lack of satiety, impaired inhibitory processes) show some degree of translation from preclinical findings, and may contribute to escalated cannabis use by human adolescents.
Introduction
An estimated 13% of 15–16-year olds in Europe and 23% of 15–17-year olds in the USA have taken cannabis in the previous year.1, 2 Globally the median age of first cannabis use falls between 18–19 years old,3, 4 indicating that approximately half of all cannabis users start before reaching adulthood.
The main psychoactive ingredient of cannabis, delta-9-tetrahydrocannabinol (THC), acts on the endocannabinoid (eCB) system, primarily as a partial agonist of the cannabinoid receptor CB1R. Studies with adult cannabis users have found altered eCB levels in cerebrospinal fluid5 and downregulated cortical CB1Rs,6, 7 relative to non-using controls. Although research into adolescent development of the eCB system remains in its infancy, it appears to undergo dynamic changes throughout adolescence,8 with evidence of increasing CB1R density continuing into late adolescence9, 10 (although also see Ellgren et al.8and Moore et al.11), and changing levels of eCBs in the prefrontal cortex and nucleus accumbens throughout adolescence.8, 9 The eCB system is also thought to have an important role in neural reorganization and maturational processes occurring during adolescence,12, 13 and has recently been implicated in the maturational pruning of glutamatergic synapses14 and development of GABA-ergic systems15 in the prefrontal cortex. Disruption of the eCB system by cannabis use during adolescence may therefore interfere with brain development such that adolescents are particularly susceptible to cannabis-related harms.16
Compared with non-using controls, adolescent cannabis users have poorer cognitive and executive functioning in some domains (for example, verbal and spatial working memory, attentional processes17, 18), alongside differing task-related neural responses (for example, greater BOLD response during response inhibition19 and spatial working memory tasks20), and morphological differences in medial temporal and frontal cortices21 and white matter integrity.22, 23 However, findings are mixed, limited by cross-sectional designs and small samples, and necessarily correlational in nature.16 Epidemiological findings further suggest that younger age of cannabis use onset may be associated with increased risk of addiction,24, 25, 26, 27 cognitive impairment16, 28, 29 and psychotic illness.30, 31, 32 Again such findings are limited since individuals starting use at a younger age will also typically have more cannabis exposures over a longer period of time, making it hard to dissociate the specific effect of age.
In rodents, repeated administration studies further suggest greater vulnerability to cannabis-related harm in adolescents. Adolescent exposure led to adulthood deficits in novel object recognition and spatial working memory, but not spatial learning.9 In adolescent rhesus monkeys Verrico et al.33, 34 found that both acute and repeated doses of THC led to impaired spatial but not object working memory; further, repeated THC prevented the maturational improvement in spatial working memory typically seen at that age, but did not affect the earlier developing object working memory. However, direct comparisons between adolescent and adult chronic exposure are scarce and findings have been inconsistent.35, 36, 37, 38, 39, 40
Evidence from acute administration studies in rats of increased adolescent vulnerability to the effects of cannabis is also mixed, with some suggesting acute cannabinoid treatment has a greater impairing effect on spatial and non-spatial learning (THC)39, 40 and object recognition (WIN55, 212-2)41 in adolescent compared with adult rats. Others however report the opposite, with evidence of greater acute impairments in adult rodents—including impaired novel object recognition (WIN55, 212-2)38 and spatial learning (WIN55, 212-2).42 Further, adult rats developed conditioned place (WIN55, 212-2)43 and taste (THC)35 aversion to cannabinoid treatment while adolescents did not, and adults produced more vocalizations when handled while intoxicated, suggesting greater drug-induced aversion.35 THC has also been found to have less anxiogenic44 or even anxiolytic42 effects, alongside reduced locomotor-suppression effects,44 in adolescent rats compared with adults. Translation of these findings to humans is limited by a number of factors, including the common use of potent synthetic cannabinoids with full CB1 receptor agonism rather than THC (for example, WIN55, 212-2), and often high doses compared with typical human consumption.
Despite mixed findings, cannabinoid administration studies in adolescent rodents and non-human primates predominantly suggest that the adolescent brain is differentially sensitive to the effects of cannabis. Should these findings translate to humans, these age-related sensitivities may contribute to an increased risk of cannabis-related harms in teenagers. Indeed, it has been suggested that if adolescents are less sensitive to the acute negative effects (for example, increased anxiety) of cannabis (and other recreational substances, as has been suggested for alcohol45) then this may lead to greater drug consumption than adults.44 However, acute studies in humans have rarely explored the influence of age on drug effects. Indeed, we are aware of no controlled studies in which cannabis was administered to individuals under 18 years of age.
The present study therefore aimed to compare the acute effects of cannabis in adolescent and adult users. In adults, acute cannabis administration typically induces episodic memory impairments46, 47 and may impair working memory and response inhibition.48, 49 Acutely cannabis also increases subjective drug-related experiences (for example, feeling ‘stoned'), and psychotomimetic symptoms.50, 51 On the basis of preclinical findings, we hypothesized that adolescents would be less sensitive to the intoxicating35, 43, 44 and anxiogenic42, 44 effects of cannabis compared with adults. Further, given links between earlier onset of cannabis use and psychosis,30, 31, 32 we predicted more psychotomimetic effects of cannabis in adolescents than adults. Finally, we hypothesized greater cognitive impairment following cannabis in adolescents than adults,39, 40, 41 as indexed by spatial working memory, episodic memory and response inhibition.
Methods
Design and participants
A mixed within- and between-subjects, double-blind, cross-over design was used to compare the acute effects of active and placebo cannabis on adolescents and adults. Treatment order was counterbalanced for task version and randomized via random number generator within each age group.
We recruited 20 adolescent (aged 16–17 years) and 20 adult (24–28 years) male cannabis users, via local and online (social media) advertising and word-of-mouth. The following inclusion criteria were assessed at telephone screening: male gender (due to evidence of sex differences in onset of puberty and ontogeny of adolescent brain development); current cannabis use between 1 and 3 days per week; at least 6 months of regular (at least once per week) cannabis use; no extended period (>1 month) of daily use; score ⩽3 on the Cannabis Severity of Dependence Scale reflecting the validated adolescent cut-off for dependence;52 no other illicit drug was used more than twice per month; no current mental health problem or history (personal or immediate family) of psychosis-related disorders; healthy-range body mass index and blood pressure (BP). Participants were asked to remain abstinent from all drugs including alcohol but not cigarettes for 24 h before each testing session.
The study was approved by UCL Research Ethics Committee. All participants provided written informed consent (in the UK 16–17-year olds are able to provide informed consent without additional parental consent or assent). Participants were reimbursed for their time (£7.50 per hour) and travel expenses.
Drug administration
Medicinal-grade active (Bedrobinol; THC 12.0%) and placebo (THC <0.3%) cannabis were imported under UK Home Office license from Bedrocan (Veendan, The Netherlands). Dose was weight-adjusted as age differences in body weight were anticipated. Following previous protocols,53, 54, 55 participants received 0.89 mg kg−1 of cannabis, corresponding to ~8.0 mg THC for an individual weighing 75 kg. This dose corresponds to that contained in about a third of a typical joint.56 Similar doses have previously been shown to produce robust subjective effects via the administration method used in this study.53, 54, 55
Drug was administered via a Volcano Medic vaporizer (Storz and Bickel, Tuttlingen, Germany), operating at 210 °C. This method has been shown to be safe, producing equivalent pulmonary and plasma cannabinoid levels to those from smoked cannabis, but with lower expired carbon monoxide levels.57, 58, 59 Vapor was collected in a ‘balloon' with a non-return valve, and inhaled according to a previous timed breath-holding protocol.55 Participants inhaled, held their breath for 8 s and repeated this at their own pace until the balloon was empty. Each dose was vaporized in two sequentially administered balloons to minimize residual cannabinoids.
Measures
Baseline assessments
Premorbid verbal intelligence was assessed by the Wechsler Test of Adult Reading,60 and scores were adjusted for age. Depression and anxiety were assessed on the Beck Depression Inventory61 and Beck Anxiety Inventory.62 A validated short version of the UPPS-P Impulsive Behaviour Scale (SUPPS-P)63, 64 indexed impulsivity and the Schizotypal Personality Questionnaire65 indexed schizotypy.
Drug use
A structured interview recorded: lifetime use (yes/no); time since last use (days); duration of use (years); frequency (days/month); and amount per session (alcohol units (standard UK units of alcohol; equivalent to 8 g of pure alcohol or ~3/5ths of a NIAAA standardized drink) per typical drinking session; cigarettes/day; other illicit drugs grams/pills/tabs). Instant urine drug screens at the start of every session assessed recent use of illicit drugs (amphetamine, barbiturates, benzodiazepines, cocaine, MDMA, methamphetamine, methadone, opiates, oxycodone, phencyclidine (Supplementary Table S1).66 Problematic drug use was assessed using the Cannabis Abuse Screening Test,67 the Fagerstrom Test for Nicotine Dependence68 and the Alcohol Use Disorders Identification Test.69
Physiological measurements
Body weight, BP and heart rate were measured at baseline. BP and heart rate were monitored throughout drug administration sessions.
Subjective ratings
Participants provided ratings from 0 (not at all) to 10 (extremely) for ‘Stoned', ‘High', ‘Feel drug effect', ‘Like drug effect' ‘Alert', ‘Anxious', ‘Paranoid', ‘Dry mouth', ‘Enhanced color perception', ‘Enhanced sound perception', ‘Want to have food' and ‘Want to have cannabis', at −6 min (apart from ‘Feel drug effect' and ‘Like drug effect'), +7 min, +34 min and +77 min (drug administration started at 0 min).
Psychotic-like symptoms
Participants completed the Psychotomimetic States Inventory (PSI), a self-report questionnaire sensitive to the acute psychotomimetic effects of cannabis.70, 71
Memory tasks
Prose recall
This episodic memory task was adapted from the Rivermead Behavioural Memory Test battery.72 Participants listened to a 30 s story and then for 1 min wrote down what they remembered immediately and again after ~1 h. Each story contained 21 ‘idea units' and scoring was systematic.
Spatial N-back
A computerized spatial version of the N-back task73, 74 was used to assess spatial working memory. Stimuli appeared sequentially in one of the six possible locations on screen, around a fixation cross. Participants responded ‘yes' or ‘no' as to whether the stimulus was in the same position as the stimulus one before (low load; ‘1-back') or two before (high load; ‘2-back'). Performance was indexed by discriminability (d') and reaction time for correct trials.
Response inhibition
Stop signal
A staircase tracking version of the stop signal was used to measure response inhibition.75 Stimuli (white arrows) appeared sequentially in the center of the screen; participants responded when the white arrow pointed left or right by pressing either the left or right arrow key. On 25% of trials, the arrow became blue following a variable delay (signal trials); on these trials participants were instructed to not press either arrow key (that is, inhibit the prepotent response). Performance was assessed with stop-signal reaction time and accuracy on no-signal trials.
Procedure
Following screening, participants attended a 1-h baseline session during which they provided informed consent, completed baseline measures, drug histories, problematic use questionnaires, task training and physiological measurements.
Participants then completed two test sessions separated by at least 7 days. Participants first provided baseline subjective ratings, and BP and heart rate were measured (Time 1; T1). Active or placebo cannabis was then administered and participants again completed subjective ratings, BP and heart rate measures (Time 2; T2). Tasks and state questionnaires were then completed in the following order; prose recall (immediate), PSI, subjective ratings (Time 3; T3), spatial N-back, stop signal, prose recall (delayed), subjective ratings (Time 4; T4), BP and heart rate (T4). Test sessions finished 80 min after drug inhalation.
Power calculation
To detect a medium effect size (f=0.25) for the key interaction of interest (group × drug), with 80% power at an alpha of 5%, we required a sample size of 34. To account for drop-out and task adherence issues, we tested 40 in total.
Statistical analysis
All analyses were conducted with SPSS 21.0. Syntax and data are available from CM. Outliers and normality were assessed via diagnostic plots for all analyses. Extreme outliers (>3 times interquartile range) were winsorized within-group. Greenhouse–Geisser corrections were applied for violations of sphericity. Independent t-test, chi-squared or Mann–Whitney analyses were conducted as appropriate to compare groups (adolescent, adult) on demographic and baseline measures.
Mixed analysis of variance was conducted for all test outcomes, with the between-subjects factor of group (adolescent, adult; coded as 1, 2, respectively) and within-subjects factor of drug (placebo, cannabis; coded as 1, 2, respectively). Additional within-subjects factors were included for relevant analyses: time (T1, T2, T4; coded as 1, 2, 3, respectively) for physiological data; time (T1–T4; coded as 1, 2, 3, 4, respectively) for subjective ratings (only T2–T4 (coded as 1, 2, 3, respectively) were analyzed for stoned (due to floor effects), feel drug effect and like drug effect (as these were not collected at T1)); PSI subscale (thought distortion, perceptual distortion, cognitive disorganisation, anhedonia, manic experience; coded as 1, 2, 3, 4, 5, respectively; paranoia subscale was not included in analyses due to floor effects); N-back memory load (low, high; coded as 1, 2, respectively); prose recall delay (immediate, delayed; coded as 1, 2, respectively). Main effects and interactions with time were tested and explored via Helmert contrasts (comparing 'Pre-drug' (T1) with 'Post-drug' (mean of T2–T4)), to reduce the number of comparisons. Other interactions were explored via pairwise comparisons with local Bonferroni correction. Drug order was added as an additional between-subjects factor (placebo-first, cannabis-first; coded as 1, 2, respectively) and results were compared with reported primary analyses; unless otherwise noted results were unaffected by drug order. All statistical tests were two-tailed. Supplementary Table S2 contains descriptive data for memory and inhibition tasks.
Results
Demographics
Adolescents were younger, and had lower body weight. Groups did not differ on verbal IQ, Beck Anxiety Inventory, Beck Depression Inventory, SUPPS-P or Schizotypal Personality Questionnaire (Table 1). Adolescents currently used cannabis for more days per month than the adults, and the age of first cannabis use was younger for the adolescents compared with the adults, but overall the adults had used for longer. Groups did not differ on Cannabis Abuse Screening Test score, time since last cannabis use, or likelihood of a positive THC urine screen at baseline.
Table 1. Demographic and baseline variables for adolescents and adults.
| Adolescents (n=20) | Adults (n=20) | Test statistic | P-value | |
|---|---|---|---|---|
| Mean (s.d.) | Mean (s.d.) | |||
| Demographics | ||||
| Age (years) | 17.08 (0.44) | 25.49 (1.07) | U=400.000 | <0.001* |
| Body weight (kg) | 66.40 (10.30) | 74.96 (10.12) | U=296.000 | 0.009* |
| Cannabis weight (mg) | 58.90 (7.65) | 65.44 (6.56) | U=299.500 | 0.006* |
| Verbal IQ (n=39) | 110.20 (11.29) | 115.11 (8.70) | U=245.000 | 0.127 |
| Baseline questionnaires | ||||
| Beck Anxiety Inventory | 4.55 (4.62) | 6.45 (7.09) | U=234.500 | 0.355 |
| Beck Depression Inventory | 6.35 (4.66) | 4.55 (4.38) | U=152.000 | 0.201 |
| SUPPS-P Impulsive Behaviour Scale | 45.55 (8.00) | 45.40 (5.94) | t38=0.067 | 0.947 |
| Schizotypal Personality Questionnaire | 20.90 (10.90) | 15.21 (11.24) | U=145.000 | 0.142 |
| Cannabis use | ||||
| Age first tried cannabis (years) | 14.73 (1.25) | 17.71 (3.00) | U=338.000 | <0.001* |
| Last used cannabis (days) | 3.35 (2.52) | 4.75 (3.78) | U=259.500 | 0.108 |
| Duration of cannabis use (years) | 2.35 (1.24) | 7.78 (2.85) | U=378.500 | <0.001* |
| Cannabis use frequency (days per month) | 10.58 (4.33) | 7.94 (5.27) | U=121.000 | 0.033* |
| Positive THC urine at baseline (n=37); %(n) | 83.33 (15) | 63.16 (12) | χ21=1.908 | 0.167 |
| Cannabis Abuse Screening Test | 6.45 (2.72) | 5.60 (3.56) | t38=0.848 | 0.402 |
| Cigarette use | ||||
| Ever used cigarettes; %(n) | 95.00 (19) | 75.00 (15) | χ21=3.137 | 0.077 |
| Age first tried cigarettes (years)b | 15.06 (1.49) | 17.21 (2.61) | U=279.000 | 0.003* |
| Duration of cigarette use (years) | 1.91 (1.41) | 7.60 (3.44) | U=356.500 | <0.001* |
| Cigarette use frequency (days per month) | 19.28 (12.36) | 10.37 (11.62) | U=120.500 | 0.030* |
| Cigarettes per day | 3.74 (2.83) | 1.84 (2.06) | U=107.500 | 0.011* |
| Fagerström Test for Nicotine Dependence | 1.30 (1.03) | 0.20 (0.70) | U=81.000 | <0.001* |
| Carbon monoxide at baseline (p.p.m.; n=38) | 6.00 (4.55) | 5.68 (3.96) | U=163.000 | 0.624 |
| Alcohol use | ||||
| Ever used alcohol; %(n) | 100.00 (20) | 100.00 (20) | NA | NA |
| Age first tried alcohol (years) | 14.07 (14.07) | 14.56 (3.22) | t28=-0.611 a | 0.546 |
| Duration of alcohol use (years) | 3.01 (1.63) | 10.93 (3.71) | U=399.000 | <0.001* |
| Alcohol use frequency (days per month) | 5.80 (4.83) | 9.78 (6.00) | U=283.500 | 0.023* |
| Alcohol units per typical drinking sessionc | 9.81 (6.92) | 8.43 (2.82) | U=190.000 | 0.799 |
| Alcohol Use Disorders Identification Test | 8.95 (5.53) | 8.95 (4.82) | U=214.000 | 0.718 |
Abbreviations: NA, not applicable; THC, tetrahydrocannabinol.
Levene's test for homogeneity of variance violated.
Calculated only on those who had ever used cigarettes (n=34).
Units used are standard UK units of alcohol; equivalent to 8 g of pure alcohol or ~3/5ths of a NIAAA standardized drink.
*P<0.05.
Values reflect mean (s.d.) unless otherwise stated; P-values reflect independent t-test comparing mean, Mann–Whitney U-test comparing median or chi-squared comparing frequency (as appropriate), by age group.
Physiological data
Heart rate
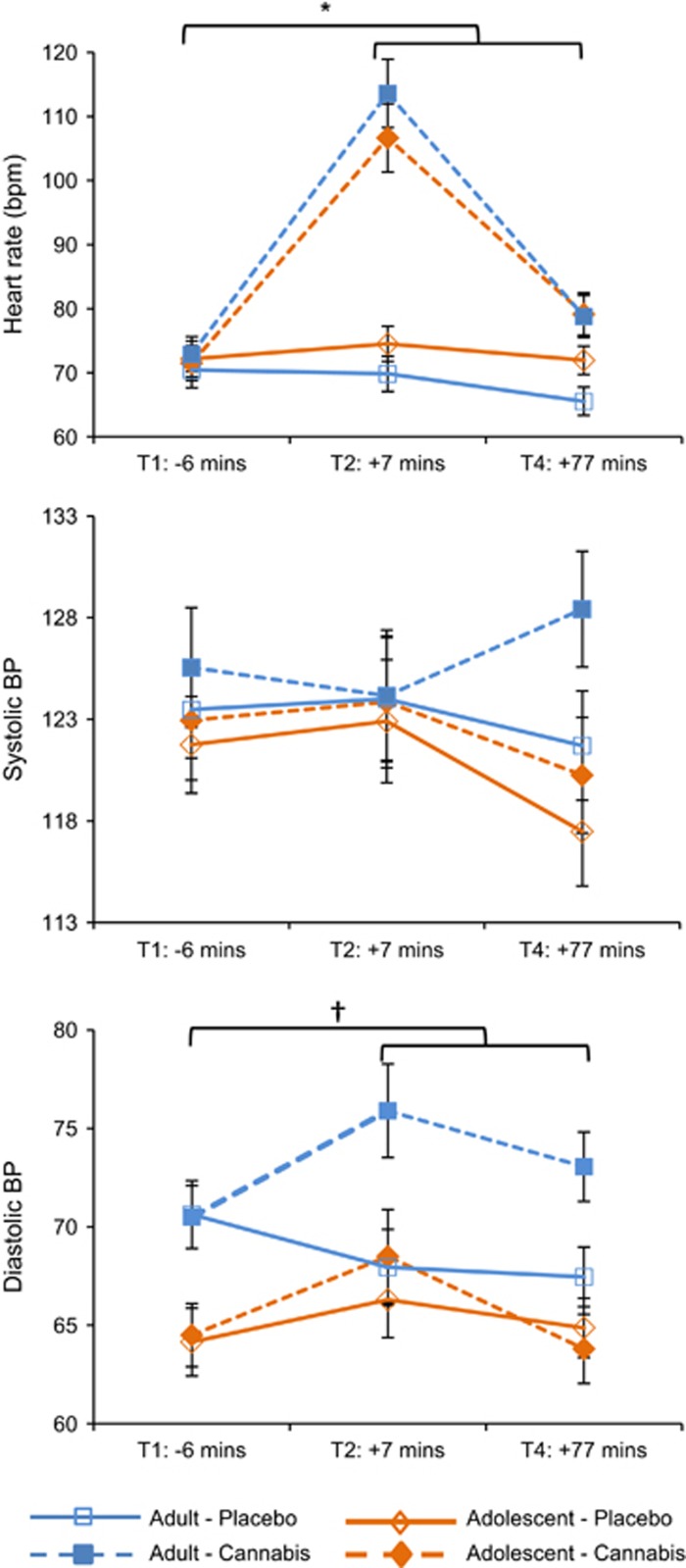
An interaction of drug × time (F1,38=82.879, P<0.001, η2p=0.69) was found, with heart rate increasing from Pre-drug to Post-drug for cannabis (P<0.001, η2p=0.65) but not placebo (P=0.449, η2p=0.01; Figure 1). Main effects of drug (F1,38=89.327, P<0.001, η2p=0.70) and time (F1,38=44.141, P<0.001, η2p=0.54) also emerged.
Figure 1.
Mean (s.e.) values for heart rate (bpm), systolic and diastolic blood pressure (BP) for adolescents and adults on cannabis and placebo. *Heart rate increased from Pre-drug to Post-drug for cannabis (P<0.001) but not placebo (P=0.449); †=for adults diastolic BP increased from Pre-drug to Post-drug on cannabis (P=0.016) but not placebo (P=0.060).
Systolic BP
No main effects or interactions were found.
Diastolic BP
Interactions of drug × group × time (F1,38=4.393, P=0.043, η2p=0.10), drug × group (F1,38=4.744, P=0.036, η2p=0.11) and drug × time (F1,38=4.977, P=0.032, η2p=0.12) emerged. For adolescents, there was no drug × time interaction (P=0.919, η2p<0.01); while for adults a drug × time interaction (P=0.010, η2p=0.30) revealed an increase in diastolic BP from Pre-drug to Post-drug for cannabis (P=0.016, η2p=0.27), but no change over time for placebo (P=0.060, η2p=0.17). Main effects of drug (F1,38=7.390, P=0.010, η2p=0.16) and group (F1,38=7.998, P=0.007, η2p=0.17) also emerged.
Subjective ratings
Stoned
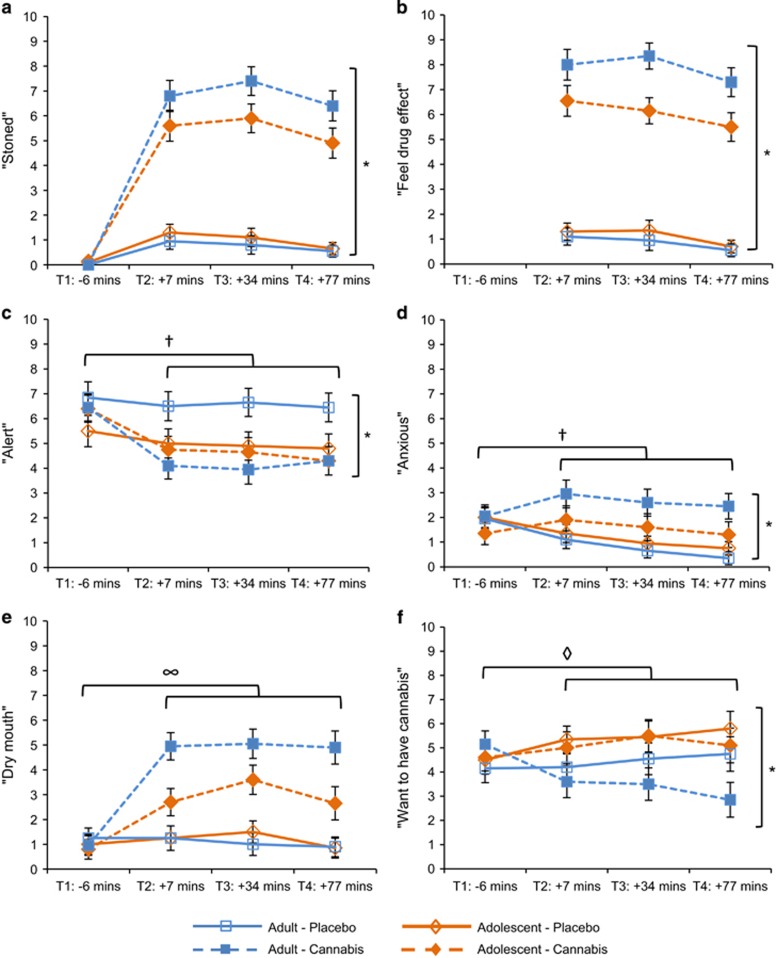
There was an interaction of drug × group (F1,38=4.893, P=0.033, η2p=0.11; Figure 2). Ratings of both adolescents (P<0.001, η2p=0.65) and adults (P<0.001, η2p=0.78) were higher after cannabis compared with placebo; however, the increase was larger in adults. Main effects of drug (F1,38=200.055, P<0.001, η2p=0.84) and time (F2,63=8.271, P=0.001, η2p=0.18) also emerged.
Figure 2.
Mean (s.e.) values for subjective ratings (0–10) for ‘stoned', ‘feel drug effect', ‘alert', ‘anxious', ‘dry mouth', ‘want to have cannabis', for adolescents and adults on placebo and cannabis. *Drug × group interaction (P⩽0.046); †drug × time interaction (P⩽0.003); ∞drug × group × time interaction (P=0.004); ◊group × time interaction (P=0.004).
Feel drug effect
There was an interaction of drug × group (F1,38=8.877, P=0.005, η2p=0.19), with adolescents feeling the drug effect less than adults after cannabis (P=0.017, η2p=0.14), but not after placebo (P=0.565, η2p=0.01). Main effects of drug (F1,38=297.629, P<0.001, η2p=0.89) and time (F2,65=9.629, P<0.001, η2p=0.20) also emerged.
Alert
There was an interaction of drug × group (F1,38=9.123, P=0.004, η2p=0.19), with adolescents rating no difference in alertness on cannabis compared with placebo (P=0.955, η2p<0.01), whereas adults rated lower alertness on cannabis compared with placebo (P<0.001, η2p=0.33). There was also an interaction of drug × time (F1,38=42.844, P<0.001, η2p=0.53); with alertness decreasing from Pre-drug to Post-drug in both sessions, though the decrease was larger for cannabis (P<0.001, η2p=0.65) than for placebo (P=0.005, η2p=0.19). Main effects of drug (F1,38=9.613, P=0.004, η2p=0.20) and time (F1,38=60.071, P<0.001, η2p=0.61) also emerged.
Anxious
There was an interaction of drug × group (F1,38=4.272, P=0.046, η2p=0.10), with adolescents reporting no difference in anxiety between drugs (P=0.516, η2p=0.01), but adults reporting more anxiety on cannabis compared with placebo (P=0.001, η2p=0.25). There was also an interaction of drug × time (F1,38=9.914, P=0.003, η2p=0.21); with no change over time in anxiety for cannabis (P=0.275, η2p=0.03) and a decrease in anxiety from Pre-drug to Post-drug for placebo (P<0.001, η2p=0.39). A main effect of drug (F1,38=8.969, P=0.005, η2p=0.19) also emerged.
Dry mouth
There were interactions of drug × group × time (F1,38=9.417, P=0.004, η2p=0.20), drug × group (F1,38=6.436, P=0.015, η2p=0.15) and drug × time (F1,38=72.572, P<0.001, η2p=0.66). Both adolescents (P<0.001, η2p=0.52) and adults (P<0.001, η2p=0.72) reported an increase in dry mouth from Pre-drug to Post-drug on cannabis, though the increase was greater for adults. On placebo there was no change in dry mouth over time for adolescents (P=0.495, η2p=0.03) or adults (P=0.244, η2p=0.07). Main effects of drug (F1,38=44.682, P<0.001, η2p=0.54) and time (F1,38=46.168, P<0.001, η2p=0.55) also emerged.
Want to have cannabis
There was an interaction of group × time (F1,38=9.661, P=0.004, η2p=0.20). From Pre-drug to Post-drug, wanting of cannabis increased in the adolescents (P=0.048, η2p=0.19) and decreased in the adults (P=0.031, η2p=0.22). There was also an interaction of drug × time (F1,38=5.933, P=0.020, η2p=0.14); wanting of cannabis increased after taking placebo (P=0.037, η2p=0.11), but did not change after taking cannabis (P=0.177, η2p=0.05).
Other subjective ratings
Comparable analyses revealed that compared with placebo, cannabis increased subjective ratings for ‘paranoid', ‘mentally impaired', ‘high', ‘like drug effect', ‘want to have food', ‘enhanced color perception' and ‘enhanced sound perception' (all P's <0.05). However, there were no group-related differences or interactions for any of these ratings (all p's >0.05).
Psychotomimetic effects
PSI
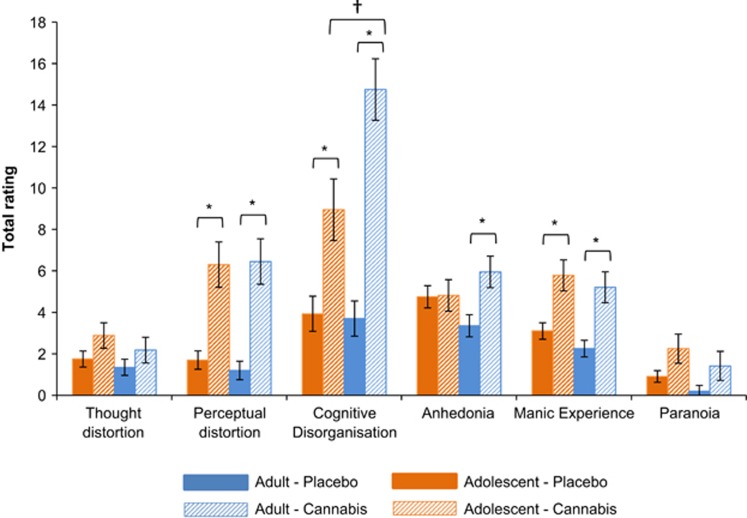
There were interactions of drug × subscale × group (F4,152=6.241, P<0.001, η2p=0.14), subscale × group (F4,152=5.111, P=0.001, η2p=0.12), drug × subscale (F3,116=32.032, P<0.001, η2p=0.46), and drug × group (F1,38=4.281, P=0.045, η2p=0.10; Figure 3). Neither group had increased thought distortion following cannabis compared to placebo (all P's⩾0.076, all η2p⩽0.08). Both groups had higher perceptual distortion, manic experience and cognitive disorganization ratings on cannabis compared with placebo (all P's⩽0.001, all η2p⩾0.27). On cannabis adults reported higher cognitive disorganization than adolescents (P=0.009, η2p=0.17). Lastly, cannabis increased anhedonia in adults (P=0.001, η2p=0.25) but not adolescents (P=0.925, η2p< 0.01). Main effects of drug (F1,38=66.453, P<0.001, η2p=0.64) and subscale (F3,102=43.544, P<.001, η2p=0.53) also emerged.
Figure 3.
Mean (s.e.) values for total ratings of each subscale of the Psychotomimetic States Inventory (PSI), for adolescents and adults on placebo and cannabis. *Ratings on cannabis were higher than on placebo (P⩽0.001); †ratings on cannabis were higher for adults than adolescents (P=0.009).
Cognitive tasks
Spatial N-back
Five participants were excluded (three adults, two adolescents) due to <50% accuracy.
Discriminability
Main effects of drug (F1,33=30.495, P<0.001, η2p=0.48) and load (F1,33=26.054, P<0.001, η2p=0.44) were found. Discriminability was poorer on cannabis (M=2.47, s.e.=0.12) than placebo (M=3.09, s.e.=0.10) and on high load (M=2.49, SE=0.13) than low load (M=3.07, s.e.=0.09).
Reaction time (correct trials)
Initial analyses demonstrated main effects of drug (F1,33=12.221, P=0.001, η2p=0.27) and load (F1,33=44.430, P<0.001, η2p=0.57), with no interactions. Reaction times were longer on cannabis than placebo and on high load (M=706.77, s.e.=25.58) than low load (M=566.95, s.e.=16.87). However, after adding drug order to the model, an interaction of drug × group (F1,31=4.447, P=0.043, η2p=0.13) also emerged. For adolescents there was no difference in reaction times between cannabis (M=632.63, s.e.=30.74) and placebo (M=589.75, s.e.=23.83; P=0.076, η2p=0.10), while for adults reaction times were longer after cannabis (M=720.40, s.e.=32.14) than placebo (M=606.31, s.e.=24.92; P<0.001, η2p=0.41).
Prose recall
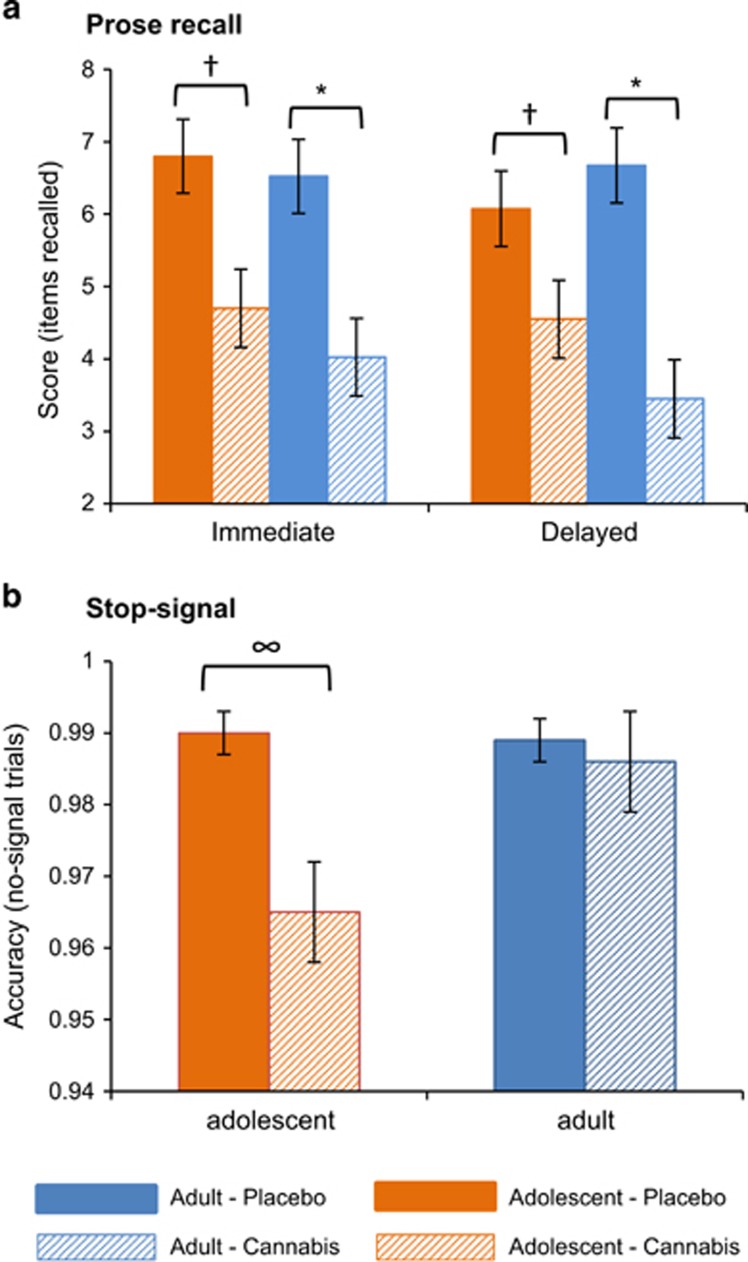
There was an interaction of drug × delay × group (F1,38=5.518, P=0.024, η2p=0.13), with adolescents recalling fewer items after cannabis than placebo, both immediately (P=0.002, η2p=0.22) and after the delay (P=0.038, η2p=0.11; Figure 4a). Adults also recalled fewer items after cannabis than placebo, both immediately (P<0.001, η2p=0.28) and after the delay (P<0.001, η2p=0.35); however, the reduction in items recalled after cannabis compared with placebo for delayed recall was twice as large in adults than adolescents. A main effect of drug (F1,38=25.869, P<0.001, η2p=0.41) also emerged.
Figure 4.
Mean (s.e.) values for (a) prose recall score (number of items recalled, out of a total of 21) and (b) stop-signal accuracy (proportion of no-signal trials with a correct response), for adolescents and adults on placebo and cannabis. *Adult scores after taking cannabis were lower than after taking placebo (P<0.001); †adolescent scores after taking cannabis were lower than after taking placebo (P⩽0.038); ∞adolescents were less accurate after taking cannabis than placebo (P=0.001).
Stop-signal
Two participants (one adult, one adolescent) had missing data due to technical issues; one adult was excluded due to an improbable stop-signal reaction time (<50 ms[ref. 76]).
Stop-signal reaction time
No main effects or interactions were found.
Accuracy on no-signal trials
There was an interaction of drug × group (F1,35=4.906, P=0.033, η2p=0.12), with adolescents being less accurate on cannabis compared with placebo (P=0.001, η2p=0.28), whereas drug did not affect adults' accuracy (P=0.644, η2p=0.01; Figure 4b). A main effect of drug (F1,35=8.306, P=0.007, η2p=0.19) also emerged.
Correlations
Within-group correlations were conducted between all cannabis session outcomes in which we found group main effects or interactions, and variables showing baseline group differences (at P<0.10; Table 1), including administered cannabis weight. Cannabis weight was not found to correlate with any outcome in either group. None were found to correlate (at P<0.10) with any outcome measure in both the adolescent and adult groups, and so were not entered into models.
Discussion
In what we believe is the first study to examine the causal effects of acute cannabis administration in human adolescence and adulthood, we found two differing profiles of effects. Compared with adults, adolescents experienced blunted subjective, physiological and psychotomimetic effects of cannabis, while cannabis impaired inhibitory processes in adolescents but not adults. Specifically, on cannabis adolescents reported feeling less stoned, feeling less effect of the drug, less dry mouth and less cognitive disorganization than adults. The adults were also markedly more anxious and less alert during the cannabis session than the placebo session, while no session difference was found for the adolescents (however, since these group differences did not differ over time, these may be session effects rather than effects of cannabis). Indeed, there was no subjective rating on which adolescents reported greater drug effect than adults. Further, adults' but not adolescents' diastolic BP rose after cannabis.
Intriguingly, we found opposing effects between age groups on wanting of cannabis following drug administration. The adolescents did not show a typical satiety effect, wanting more cannabis post drug regardless of whether they had taken cannabis or placebo. Meanwhile the adults wanted less cannabis post drug, an effect that appears to be driven by a decrease in wanting following cannabis but not after placebo (although this putative interpretation remains tentative in the absence of a group × drug × time interaction).
In terms of cognitive effects, when intoxicated with cannabis adults showed greater impaired recall of prose following a delay than adolescents. After adjusting for drug order, the adults also had longer response times on the spatial working memory task following cannabis, while the adolescents were not affected. Although neither group was impaired at inhibiting a pre-potent response following cannabis, the adolescents but not adults were less accurate on the inhibition task after cannabis.
These results are in line with our first hypothesis that adolescents would be less sensitive to physiological, intoxication and anxiogenic effects compared with adults. These findings accord with the preclinical evidence that shows reduced anxiogenic, aversive and locomotor effects in adolescent rodents.35, 42, 43, 44 Further, while our second hypothesis predicted a greater degree of psychotomimetic effects following cannabis in the adolescents compared with the adults, we instead found the opposite: cognitive disorganization was especially elevated in adults compared with adolescents after cannabis. This unexpected finding is however in agreement with our first hypothesis of lesser intoxication effects in adolescents, perhaps suggesting a common mechanism by which adolescents are resilient to the acute negative effects of cannabis. It may also reflect an awareness in adults of the greater cognitive impairments they were experiencing, rather than amplified psychotic-like effects of cannabis per se. We also found that cannabis increased anhedonia symptoms in adults but not in adolescents; interestingly however, on placebo the adolescents had (non-significantly) higher levels of anhedonia than the adults.
Lastly, partial support for our third hypothesis, that we would see greater cognitive impairment following cannabis in adolescents than adults, was seen in greater impairment of response inhibition accuracy following cannabis in the adolescents compared with adults. However, contrary to expectations we did not see greater cannabis-related memory impairment in the adolescents, instead finding evidence of greater impairment in adults. Preclinical evidence for greater adolescent sensitivity to acute memory-impairing effects of cannabis is however inconsistent.77 In adult humans cannabis appears to selectively impair episodic and working memory domains,78 leaving other memory domains intact, while rodents typically become impaired on a wide range of memory tasks across domains including object recognition and spatial learning, implying that preclinical findings for cannabis and memory may be somewhat limited in translation.
These findings have important implications for public health, especially given the current changes in legislation that are making cannabis more available and may influence adolescent use in several parts of the globe. If adolescents do not feel satiated after an acute dose of the drug while also experiencing fewer negative effects, they may well use more cannabis in a smoking session than adults,44 potentially contributing to the increased risk of long-term harms associated with younger age of use, including addiction.16 In turn, adults' experience of more negative effects of cannabis may limit their use and reduce their risk of harms, which would concur with the declining prevalence of cannabis use seen from early adulthood.4 A clear next step from these findings is therefore replication (importantly with females as well as males) and then assessment of naturalistic use of cannabis in different age groups, using measures that clearly record weight and potency of cannabis smoked,79 topography of inhalation,56 alongside ratings of subjective negative and positive intoxication effects. Tracking these participants longitudinally would be important in determining how these age-related sensitivities may impact in the long term on cannabis use patterns and mental and physical health outcomes.
Our study has several critical strengths. Importantly our groups were well matched on baseline measures including premorbid IQ and levels of anxiety, depression, impulsivity and schizotypy. This increases our confidence that participants in the two age groups were drawn from similar populations, and maximizes comparability between groups. Further the use of cannabis plant material, rather than extracted or synthetic cannabinoids, via an ecologically valid administration procedure (that is, inhalation) enhances the relevance of our findings to the real world use of this drug. Administering a known THC dosage that closely corresponds to that contained in about a third of a typical joint,56 which was weight adjusted to allow for weight differences in adolescents and adults, are both strengths of this controlled study.
The study is not without limitations. First, we cannot speak to mechanism of the reported age-related sensitivities. Although the findings may represent age-related neural sensitivities to cannabis, there are a number of alternative explanations. Adolescents have a higher basal metabolism than adults,80, 81 alongside lower percentage body fat,82, 83 potentially affecting the speed of THC metabolism between the groups. Should THC and its by-products be metabolized more quickly in adolescents than adults, this could potentially result in the reduced subjective and episodic memory effects seen in adolescents; however, if drug metabolism in the adolescents was faster, a quicker decline of drug effects would be expected, which does not appear to be the case. Further, this would not explain the adolescent's impaired inhibition accuracy when the adults were unaffected. Group differences in the effect of cannabis on diastolic BP are also intriguing, though adolescents' diastolic BP was lower on both sessions at baseline, consistent with normative data.84 This finding should also be viewed alongside a lack of a group difference in the more robust effect of cannabis increasing heart rate. Relatedly, participants were given a weight-adjusted dose, meaning that because adolescents typically weigh less than adults,85 on average they received a lower dose. We cannot therefore rule out the possibility that the blunted effects seen in the adolescents are due to the reduced dose; however, again this would not explain the overall pattern of results including the adolescents' (but not adults') impaired response inhibition accuracy. Moreover, critically the weight of cannabis administered did not correlate with any outcome in either group. Groups could potentially be matched for body weight in future research, however this would result in biased samples that do not reflect the population as a whole. An important goal now is to investigate the mechanisms by which these apparent group differences occur, for instance, a first step would be to repeat key components of our protocol using an fMRI paradigm.
Second, all our participants were necessarily regular cannabis users, raising the possibility that our findings may be affected by group differences in past cannabis use. Although the groups were matched for cannabis abuse symptomology and days since last use, the adolescents did report more days of cannabis use per month than the adults (11 days versus 8 days); further while the adults had been using for more years, they had started using from an older age. Tolerance to some cannabis effects following frequent use has been reported (including for spatial working memory and episodic memory78), however findings are inconsistent86 and little is known about the development of cannabis tolerance and how different usage patterns affect this. As such it is possible that differing cannabis use histories and patterns may explain group differences in outcomes. Importantly however, none of our measures of cannabis use correlated with outcomes in both the adolescent and adult groups. Relatedly, the adolescents were more frequent and heavier cigarette smokers, with higher nicotine-dependence scores, and they had started tobacco smoking from a younger age than the adults. The groups were well matched for age of first alcohol use, but the adolescents were less frequent alcohol drinkers. It is possible that cross-tolerance to cannabis from previous alcohol or tobacco use may occur, though we are not aware of evidence demonstrating such an effect. A recent ecological momentary assessment study suggested that acutely tobacco use may offset acute impairment of working memory from cannabis,87 though this has yet to be replicated in a controlled study. It is possible therefore that the age group differences in alcohol and cigarette use may be contributing to our findings.
Third, we recruited only males, due to differing age of puberty onset and potentially differing brain development trajectories between sexes, thus precluding generalization of findings to teenage girls. Samples in cannabis research are often predominantly male and gender effects have rarely been assessed, with inconsistent findings.78 Some have shown heightened subjective88 and working memory89 effects in women compared with men, though others found no differences.90 Recently it was found that younger age of cannabis use onset predicted poorer episodic memory in women but not men,91 suggesting that there may be age-dependent sex differences in the cognitive effects of cannabis. Given such findings, there is a clear evidence gap regarding the effects of cannabis in young women and girls and future research should assess whether our findings generalize to females.
Finally, since this was a novel study, with multiple statistical comparisons and limited or mixed evidence on which to base our prior hypotheses, it is important to treat these findings with caution. Replications with larger sample sizes (which can now be determined according to effect sizes reported in this paper) are required before strong conclusions can be drawn.
In conclusion, compared with adults, adolescent cannabis users experienced blunted subjective, physiological and memory impairing effects of cannabis. Further, adolescents were not satiated by cannabis and the drug impaired their inhibitory processes while leaving those of adults intact. To our knowledge, this is the first study to administer cannabis in a controlled setting to humans under 18, and it therefore represents a significant step forward in the translation of preclinical developmental psychopharmacology. In agreement with preclinical cannabinoid administration studies, we found evidence to suggest that human adolescents and adults are differentially sensitive to the acute effects of cannabis. Longitudinal research is now needed to determine the degree to which age-related sensitivities are indeed contributing to escalated use and increased risk of cannabis-related harms in adolescent cannabis users.
Acknowledgments
We thank Frederick Verbruggen for providing the stop-signal program. We also thank Storz and Bickel GmbH & Co., Germany, for providing the Volcano Medic. This research was funded by an MRC Studentship awarded to the first author. Funders had no role in the study design, analysis or interpretation of the data or in the writing of the report.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- Hibell B, Guttormsson U, Ahlstrom S, Balakireva O, Bjarnason T, Kokkevi A et al. The 2011 ESPAD report. Substance use among students in 36 European countries. The Swedish Council for information on Alcohol and Other Drugs, Stockholm, Sweden, 2012. Available at http://www.espad.org (accessed September 2012)..
- Grucza RA, Agrawal A, Krauss MJ, Bongu J, Plunk AD, Cavazos-Rehg PA et al. Declining prevalence of marijuana use disorders among adolescents in the United States, 2002 to 2013. J Am Acad Child Adolesc Psychiatry 2016; 55: 487–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Chiu W-T, Sampson N, Kessler RC, Anthony JC, Angermeyer M et al. Toward a global view of alcohol, tobacco, cannabis, and cocaine use: findings from the WHO World Mental Health Surveys. PLoS Med 2008; 5: e141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degenhardt L, Stockings E, Patton G, Hall WD, Lynskey M. The increasing global health priority of substance use in young people. Lancet Psychiatry 2016; 3: 251–264. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Page E, Schaefer C, Chatten K, Manocha A, Gulati S et al. Cerebrospinal fluid anandamide levels, cannabis use and psychotic-like symptoms. Br J Psychiatry 2013; 202: 381–382. [DOI] [PubMed] [Google Scholar]
- Hirvonen J, Goodwin R, Li C-T, Terry G, Zoghbi S, Morse C et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry 2012; 17: 642–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Souza DC, Cortes-Briones JA, Ranganathan M, Thurnauer H, Creatura G, Surti T et al. Rapid changes in cannabinoid 1 receptor availability in cannabis-dependent male subjects after abstinence from cannabis. Biol Psychiatry Cogn Neurosci Neuroimaging 2016; 1: 60–67. [DOI] [PubMed] [Google Scholar]
- Ellgren M, Artmann A, Tkalych O, Gupta A, Hansen HS, Hansen S et al. Dynamic changes of the endogenous cannabinoid and opioid mesocorticolimbic systems during adolescence: THC effects. Eur Neuropsychopharmacol 2008; 18: 826–834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Parolaro D. The impact of exposure to cannabinoids in adolescence: insights from animal models. Biol Psychiatry 2016; 79: 578–585. [DOI] [PubMed] [Google Scholar]
- Verdurand M, Nguyen V, Stark D, Zahra D, Gregoire M-C, Greguric I et al. Comparison of cannabinoid CB1 receptor binding in adolescent and adult rats: a positron emission tomography study using [18 F] MK-9470. Int J Mol Imaging 2011; 2011: 548123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore NL, Greenleaf AL, Acheson SK, Wilson WA, Swartzwelder HS, Kuhn CM. Role of cannabinoid receptor type 1 desensitization in greater tetrahydrocannabinol impairment of memory in adolescent rats. J Pharmacol Exp Ther 2010; 335: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol 2010; 92: 370–385. [DOI] [PubMed] [Google Scholar]
- Lubman DI, Cheetham A, Yücel M. Cannabis and adolescent brain development. Pharmacol Ther 2015; 148: 1–16. [DOI] [PubMed] [Google Scholar]
- Rubino T, Prini P, Piscitelli F, Zamberletti E, Trusel M, Melis M et al. Adolescent exposure to THC in female rats disrupts developmental changes in the prefrontal cortex. Neurobiol Dis 2015; 73: 60–69. [DOI] [PubMed] [Google Scholar]
- Cass DK, Flores-Barrera E, Thomases DR, Vital WF, Caballero A, Tseng KY. CB1 cannabinoid receptor stimulation during adolescence impairs the maturation of GABA function in the adult rat prefrontal cortex. Mol Psychiatry 2014; 19: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran HV, Freeman TP, Mokrysz C, Lewis DA, Morgan CJ, Parsons LH. Keep off the grass? Cannabis, cognition and addiction. Nat Rev Neurosci 2016; 17: 293–306. [DOI] [PubMed] [Google Scholar]
- Hanson KL, Winward JL, Schweinsburg AD, Medina KL, Brown SA, Tapert SF. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav 2010; 35: 970–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey MA, Harvey MA, Sellman JD, Harvey MA, Sellman JD, Porter RJ et al. The relationship between non-acute adolescent cannabis use and cognition. Drug Alcohol Rev 2007; 26: 309–319. [DOI] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Drummond SP, Paulus MP, Brown SA, Yang TT et al. Functional MRI of inhibitory processing in abstinent adolescent marijuana users. Psychopharmacology 2007; 194: 173–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweinsburg AD, Nagel BJ, Schweinsburg BC, Park A, Theilmann RJ, Tapert SF. Abstinent adolescent marijuana users show altered fMRI response during spatial working memory. Psychiatry Res 2008; 163: 40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batalla A, Bhattacharyya S, Yücel M, Fusar-Poli P, Crippa JA, Nogué S et al. Structural and functional imaging studies in chronic cannabis users: a systematic review of adolescent and adult findings. PloS One 2013; 8: e55821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Dahlgren MK, Sagar KA, Gönenç A, Lukas SE. Worth the wait: effects of age of onset of marijuana use on white matter and impulsivity. Psychopharmacology 2014; 231: 1455–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein KA, Kumra S. White matter fractional anisotropy over two time points in early onset schizophrenia and adolescent cannabis use disorder: a naturalistic diffusion tensor imaging study. Psychiatry Res 2015; 232: 34–41. [DOI] [PubMed] [Google Scholar]
- von Sydow K, Lieb R, Pfister H, Höfler M, Wittchen H-U. What predicts incident use of cannabis and progression to abuse and dependence?: A 4-year prospective examination of risk factors in a community sample of adolescents and young adults. Drug Alcohol Depend 2002; 68: 49–64. [DOI] [PubMed] [Google Scholar]
- Chen CY, Anthony JC. Possible age-associated bias in reporting of clinical features of drug dependence: epidemiological evidence on adolescent-onset marijuana use. Addiction 2003; 98: 71–82. [DOI] [PubMed] [Google Scholar]
- Chen C-Y, O'Brien MS, Anthony JC. Who becomes cannabis dependent soon after onset of use? Epidemiological evidence from the United States: 2000–2001. Drug Alcohol Depend 2005; 79: 11–22. [DOI] [PubMed] [Google Scholar]
- Hines LA, Morley KI, Strang J, Agrawal A, Nelson EC, Statham D et al. The association between speed of transition from initiation to subsequent use of cannabis and later problematic cannabis use, abuse and dependence. Addiction 2015; 110: 1311–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS et al. Persistent cannabis users show neuropsychological decline from childhood to midlife. Proc Natl Acad Sci USA 2012; 109: E2657–E2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber SA, Sagar KA, Dahlgren MK, Racine M, Lukas SE. Age of onset of marijuana use and executive function. Psychol Addict Behav 2012; 26: 496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arseneault L, Cannon M, Poulton R, Murray R, Caspi A, Moffitt TE. Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 2002; 325: 1212–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore TH, Zammit S, Lingford-Hughes A, Barnes TR, Jones PB, Burke M et al. Cannabis use and risk of psychotic or affective mental health outcomes: a systematic review. Lancet 2007; 370: 319–328. [DOI] [PubMed] [Google Scholar]
- Di Forti M, Sallis H, Allegri F, Trotta A, Ferraro L, Stilo SA et al. Daily use, especially of high-potency cannabis, drives the earlier onset of psychosis in cannabis users. Schizophr Bull 2014; 40: 1509–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Gu H, Peterson ML, Sampson AR, Lewis DA. Repeated Δ9-tetrahydrocannabinol exposure in adolescent monkeys: persistent effects selective for spatial working memory. Am J Psychiatry 2014; 171: 416–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrico CD, Liu S, Bitler EJ, Gu H, Sampson AR, Bradberry CW et al. Delay-and dose-dependent effects of Δ9-tetrahydrocannabinol administration on spatial and object working memory tasks in adolescent rhesus monkeys. Neuropsychopharmacology 2012; 37: 1357–1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinn HR, Matsumoto I, Callaghan PD, Long LE, Arnold JC, Gunasekaran N et al. Adolescent rats find repeated Δ9-THC less aversive than adult rats but display greater residual cognitive deficits and changes in hippocampal protein expression following exposure. Neuropsychopharmacology 2008; 33: 1113–1126. [DOI] [PubMed] [Google Scholar]
- Schneider M, Koch M. Chronic pubertal, but not adult chronic cannabinoid treatment impairs sensorimotor gating, recognition memory, and the performance in a progressive ratio task in adult rats. Neuropsychopharmacology 2003; 28: 1760–1769. [DOI] [PubMed] [Google Scholar]
- O'Shea M, Singh ME, McGregor IS, Mallet PE. Chronic cannabinoid exposure produces lasting memory impairment and increased anxiety in adolescent but not adult rats. J Psychopharmacol 2004; 18: 502–508. [DOI] [PubMed] [Google Scholar]
- Fox KM, Sterling RC, Van Bockstaele EJ. Cannabinoids and novelty investigation: influence of age and duration of exposure. Behav Brain Res 2009; 196: 248–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha YM, White AM, Kuhn CM, Wilson WA, Swartzwelder H. Differential effects of delta 9-THC on learning in adolescent and adult rats. Pharmacol Biochem Behav 2006; 83: 448–455. [DOI] [PubMed] [Google Scholar]
- Cha YM, Jones KH, Kuhn CM, Wilson WA, Swartzwelder HS. Sex differences in the effects of Δ9-tetrahydrocannabinol on spatial learning in adolescent and adult rats. Behav Pharmacol 2007; 18: 563–569. [DOI] [PubMed] [Google Scholar]
- Schneider M, Schömig E, Leweke FM. PRECLINICAL STUDY: acute and chronic cannabinoid treatment differentially affects recognition memory and social behavior in pubertal and adult rats. Addict Biol 2008; 13: 345–357. [DOI] [PubMed] [Google Scholar]
- Acheson SK, Moore NL, Kuhn CM, Wilson WA, Swartzwelder HS. The synthetic cannabinoid WIN 55212-2 differentially modulates thigmotaxis but not spatial learning in adolescent and adult animals. Neurosci Lett 2011; 487: 411–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho A, Reyes B, Ramalhosa F, Sousa N, Van Bockstaele E. Repeated administration of a synthetic cannabinoid receptor agonist differentially affects cortical and accumbal neuronal morphology in adolescent and adult rats. Brain Struct Funct 2016; 221: 407–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm-Sapyta NL, Cha YM, Chaudhry S, Wilson WA, Swartzwelder HS, Kuhn CM. Differential anxiogenic, aversive, and locomotor effects of THC in adolescent and adult rats. Psychopharmacology 2007; 191: 867–877. [DOI] [PubMed] [Google Scholar]
- Spear LP. Alcohol consumption in adolescence: a translational perspective. Curr Addict Rep 2016; 3: 50–61. [Google Scholar]
- Curran VH, Brignell C, Fletcher S, Middleton P, Henry J. Cognitive and subjective dose-response effects of acute oral Δ9-tetrahydrocannabinol (THC) in infrequent cannabis users. Psychopharmacology 2002; 164: 61–70. [DOI] [PubMed] [Google Scholar]
- Ranganathan M, D'souza DC. The acute effects of cannabinoids on memory in humans: a review. Psychopharmacology 2006; 188: 425–444. [DOI] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Fusar-Poli P, Gonzalez R. Effects of cannabis on neurocognitive functioning: recent advances, neurodevelopmental influences, and sex differences. Neuropsychol Rev 2013; 23: 117–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez R. Acute and non-acute effects of cannabis on brain functioning and neuropsychological performance. Neuropsychol Rev 2007; 17: 347–361. [DOI] [PubMed] [Google Scholar]
- Sherif M, Radhakrishnan R, D'Souza DC, Ranganathan M. Human laboratory studies on cannabinoids and psychosis. Biol Psychiatry 2016; 79: 526–538. [DOI] [PubMed] [Google Scholar]
- D'Souza DC, Perry E, MacDougall L, Ammerman Y, Cooper T, Wu Y-t et al. The psychotomimetic effects of intravenous delta-9-tetrahydrocannabinol in healthy individuals: implications for psychosis. Neuropsychopharmacology 2004; 29: 1558–1572. [DOI] [PubMed] [Google Scholar]
- Martin G, Copeland J, Gates P, Gilmour S. The Severity of Dependence Scale (SDS) in an adolescent population of cannabis users: reliability, validity and diagnostic cut-off. Drug Alcohol Depend 2006; 83: 90–93. [DOI] [PubMed] [Google Scholar]
- Bossong MG, van Berckel BN, Boellaard R, Zuurman L, Schuit RC, Windhorst AD et al. Δ9-tetrahydrocannabinol induces dopamine release in the human striatum. Neuropsychopharmacology 2009; 34: 759–766. [DOI] [PubMed] [Google Scholar]
- Hindocha C, Freeman TP, Schafer G, Gardener C, Das RK, Morgan CJ et al. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: a randomised, double-blind, placebo-controlled study in cannabis users. Eur Neuropsychopharmacol 2014; 25: 325–334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawn W, Freeman T, Pope R, Joye A, Harvey L, Hindocha C et al. Acute and chronic effects of cannabinoids on effort-related decision-making and reward learning: an evaluation of the cannabis ‘amotivational' hypotheses. Psychopharmacology 2016; 233: 3537–3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Pol P, Liebregts N, Brunt T, Amsterdam J, Graaf R, Korf DJ et al. Cross-sectional and prospective relation of cannabis potency, dosing and smoking behaviour with cannabis dependence: an ecological study. Addiction 2014; 109: 1101–1109. [DOI] [PubMed] [Google Scholar]
- Lanz C, Mattsson J, Soydaner U, Brenneisen R. Medicinal Cannabis: in vitro validation of vaporizers for the smoke-free inhalation of cannabis. PloS One 2016; 11: e0147286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazekamp A, Ruhaak R, Zuurman L, van Gerven J, Verpoorte R. Evaluation of a vaporizing device (Volcano®) for the pulmonary administration of tetrahydrocannabinol. J Pharm Sci 2006; 95: 1308–1317. [DOI] [PubMed] [Google Scholar]
- Abrams DI, Vizoso HP, Shade SB, Jay C, Kelly ME, Benowitz NL. Vaporization as a smokeless cannabis delivery system: a pilot study. Clin Pharmacol Ther 2007; 82: 572–578. [DOI] [PubMed] [Google Scholar]
- Holdnack H. Wechsler Test of Adult Reading: WTAR. The Psychological Corporation: San Antonio, TX, 2001. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Beck Depression Inventory-II. Psychological Corporation: San Antonio, TX, 1996; b9. [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol 1988; 56: 893. [DOI] [PubMed] [Google Scholar]
- Lynam D. Development of a short form of the UPPS-P Impulsive Behavior Scale. Unpublished Technical Report, 2013..
- Cyders MA, Littlefield AK, Coffey S, Karyadi KA. Examination of a short English version of the UPPS-P Impulsive Behavior Scale. Addict Behav 2014; 39: 1372–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull 1991; 17: 555. [DOI] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol 1992; 16: 276–282. [DOI] [PubMed] [Google Scholar]
- Legleye S, Karila L, Beck F, Reynaud M. Validation of the CAST, a general population Cannabis Abuse Screening Test. J Subst Use 2007; 12: 233–242. [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerström test for nicotine dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict 1991; 86: 1119–1127. [DOI] [PubMed] [Google Scholar]
- Babor TF, DeLaFuentes JR, Saunders J, Grant M. AUDIT: The Alcohol Use Disorders Identification Test: guidelines for use in primaryhealth care. World Health Organisation: PSA/92.4, pp 1–301992.
- Mason OJ, Morgan CJ, Stefanovic A, Curran HV. The psychotomimetic states inventory (PSI): measuring psychotic-type experiences from ketamine and cannabis. Schizophr Res 2008; 103: 138–142. [DOI] [PubMed] [Google Scholar]
- Morgan CJ, Schafer G, Freeman TP, Curran HV. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: naturalistic study. Br J Psychiatry 2010; 197: 285–290. [DOI] [PubMed] [Google Scholar]
- Wilson B, Cockburn J, Baddeley A, Hiorns R. The development and validation of a test battery for detecting and monitoring everyday memory problems. J Clin Exp Neuropsychol 1989; 11: 855–870. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD, Nystrom LE, Jonides J, Smith EE, Noll DC. A parametric study of prefrontal cortex involvement in human working memory. Neuroimage 1997; 5: 49–62. [DOI] [PubMed] [Google Scholar]
- Freeman TP, Morgan CJ, Vaughn-Jones J, Hussain N, Karimi K, Curran HV. Cognitive and subjective effects of mephedrone and factors influencing use of a ‘new legal high'. Addiction 2012; 107: 792–800. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Logan GD, Stevens MA. STOP-IT: Windows executable software for the stop-signal paradigm. Behav Res Methods 2008; 40: 479–483. [DOI] [PubMed] [Google Scholar]
- Congdon E, Mumford JA, Cohen JR, Galvan A, Canli T, Poldrack RA. Measurement and reliability of response inhibition. Front Psychol 2012; 3: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Realini N, Rubino T, Parolaro D. Neurobiological alterations at adult age triggered by adolescent exposure to cannabinoids. Pharmacol Res 2009; 60: 132–138. [DOI] [PubMed] [Google Scholar]
- Broyd SJ, van Hell HH, Beale C, Yücel M, Solowij N. Acute and chronic effects of cannabinoids on human cognition–a systematic review. Biol Psychiatry 2015; 79: 557–567. [DOI] [PubMed] [Google Scholar]
- Freeman TP, Morgan CJ, Hindocha C, Schafer G, Das RK, Curran HV. Just say ‘know': how do cannabinoid concentrations influence users' estimates of cannabis potency and the amount they roll in joints? Addiction 2014; 109: 1686–1694. [DOI] [PubMed] [Google Scholar]
- Manini TM. Energy expenditure and aging. Ageing Res Rev 2010; 9: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black AE, Coward WA, Cole TJ, Prentice AM. Human energy expenditure in affluent societies: an analysis of 574 doubly-labelled water measurements. Eur J Clin Nutr 1996; 50: 72. [PubMed] [Google Scholar]
- Forbes GB, Reina JC. Adult lean body mass declines with age: some longitudinal observations. Metabolism 1970; 19: 653–663. [DOI] [PubMed] [Google Scholar]
- Guo SS, Chumlea WC, Roche AF, Siervogel RM. Age-and maturity-related changes in body composition during adolescence into adulthood: the Fels Longitudinal Study. Appl Radiat Isot 1998; 49: 581–585. [DOI] [PubMed] [Google Scholar]
- Beckett LA, Rosner B, Roche AF, Guo S. Serial changes in blood pressure from adolescence into adulthood. Am J Epidemiol 1992; 135: 1166–1177. [DOI] [PubMed] [Google Scholar]
- Sutton R. Adult anthropometric measures, overweight and obesity. Health Survey for England–2011. Health, Social Care and Lifestyles 2012; 325–362.
- Ramaekers JG, van Wel JH, Spronk DB, Toennes SW, Kuypers KPC, Theunissen EL, Verkes RJ. Cannabis and tolerance: acute drug impairment as a function of cannabis use history. Sci Rep 2016; 6: 26843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster RM, Mermelstein RJ, Hedeker D. Ecological momentary assessment of working memory under conditions of simultaneous marijuana and tobacco use. Addiction 2016; 111: 1466–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper ZD, Haney M. Investigation of sex-dependent effects of cannabis in daily cannabis smokers. Drug Alcohol Depend 2014; 136: 85–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makela P, Wakeley J, Gijsman H, Robson PJ, Bhagwagar Z, Rogers RD. Low doses of Δ-9 tetrahydrocannabinol (THC) have divergent effects on short-term spatial memory in young, healthy adults. Neuropsychopharmacology 2006; 31: 462–470. [DOI] [PubMed] [Google Scholar]
- Anderson BM, Rizzo M, Block RI, Pearlson GD, O'Leary DS. Sex, drugs, and cognition: effects of marijuana. J Psychoactive Drugs 2010; 42: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crane NA, Schuster RM, Mermelstein RJ, Gonzalez R. Neuropsychological sex differences associated with age of initiated use among young adult cannabis users. J Clin Exp Neuropsychol 2015; 37: 389–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.