Abstract
Yoga is associated with reduced stress and increased well-being, although the molecular basis for these benefits is not clear. Mounting evidence implicates the immune response, with current studies focused on protein immune markers (such as cytokines) in clinical populations. To explore the molecular impact, this pilot study uses a subsample (n=28) from a randomised waitlist control trial investigating the impact of an 8-week yoga intervention in a community population of women reporting psychological distress (N=116). We measured interleukin-6 (IL-6), tumour necrosis factor (TNF) and C-reactive protein (CRP) protein levels, and the DNA methylation of these genes and the global indicator, LINE-1. Correlations between these and psychological variables were explored, identifying moderate correlations with CRP protein levels, and methylation of IL-6, CRP and LINE-1. Many cytokine samples were below detection, however a Mann–Whitney U demonstrated a trend of moderate between-group effect for elevated IL-6 in the yoga group. Methylation analyses applied cross-sectional and non-controlled longitudinal analyses. Waist-to-height ratio and age were covaried. We demonstrated reduced methylation of the TNF region in the yoga group relative to the waitlist control group. No other genes demonstrated a significant difference. Longitudinal analysis further supported these results. This study is one of the first to explore yoga and immunological markers in a non-clinical population, and is the first study to explore DNA methylation. These findings indicate that further research into molecular impact of yoga on markers of immune function is warranted, with larger studies required.
Introduction
Yoga is an increasingly popular technique combining physical activity, meditation and breathing practices (‘moving mindfulness'1), and is often practiced as a treatment/adjunct treatment for psychiatric conditions.2 A growing body of psychological literature demonstrates that practicing yoga improves subjective well-being and positive feelings, and reduces reported levels of stress, distress and negative feelings, including clinical symptoms of depression and anxiety.3, 4, 5
Inflammation has been demonstrated to be associated with depression and exposure to stressors, specifically including the action of the inflammatory cytokines interleukin-6 (IL-6) and tumour necrosis factor (TNF6, 7, 8, 9) and the acute-phase protein C-reactive protein (CRP10, 11, 12). Further, these have been postulated to be impacted by both exercise and psychological therapies. Anti-inflammatory factors are modified by participation in moderate exercise,13 and with participation in a mindfulness-based stress reduction intervention.14 Biochemical evidence indicates practices such as yoga reduce inflammatory responses associated with stressful situations.15, 16 Our current understanding of the molecular mechanisms involved in the modulatory effect of yoga remains limited however.
Inflammation changes reported in the literature may, in part, be determined by epigenetic processes that impact gene expression, and ultimately protein expression. The epigenome regulates gene expression, and can be altered by environmental factors such as stress.17 Epigenetic changes are increasingly recognised as relevant biomarkers for mental illness, with DNA methylation the most widely studied.18, 19, 20, 21 The changes in DNA methylation have been associated with poor physical health, and high levels of inflammation.22, 23, 24, 25 As epigenetic changes are potentially reversible, they may be used for the evaluation of responses to clinical therapies.26
Emerging studies of mind-body therapies (MBTs), including yoga-based interventions, are increasingly exploring mechanisms;27, 28, 29, 30 however, most studies focus on gene-expression changes.31 Thus while a change in gene expression, and therefore a biological effect may be reported, the mechanism of this effect remains unknown. Only two epigenetic studies currently exist in the MBT literature and indicate that interventions conceptually similar to yoga may be correlated with epigenetic change. Specifically, an 8 h meditation session has been reported to rapidly alter global modification of histones, and reduce expression of histone deacetylase and pro-inflammatory genes.32 DNA methylation changes in six age-related CpG sites have also been reported in a cross-sectional study of Australian female long-term tai chi practitioners.33 However, no studies have investigated the relationship between a psychophysiological intervention, such as yoga, on indicators of genome-wide DNA methylation (which can be explored broadly utilising a repetitive element sequence as a surrogate, such as LINE-1; ref. 34), and DNA methylation patterns of immune candidate genes such as TNF, IL6 and CRP, candidates implicated in psychological distress and to be altered by mindfulness-based stress reduction and yoga practice. DNA methylation in these genes have been investigated in the context of inflammatory conditions (rheumatoid arthritis) and engagement in physical activity, age, pollution exposure and weight-related factors.34, 35, 36, 37, 38, 39, 40, 41 Although findings have been mixed, they have demonstrated that DNA methylation changes are observed in relation to physical factors and across relatively short time periods.
The objective of this pilot study is twofold: (1) to examine the epidemiological effect of a yoga intervention on markers of inflammation (IL6, TNF and CRP); and (2) to examine, for we believe the first time, whether participation in a yoga intervention (an MBT) is associated with altered levels of estimated global DNA methylation (represented by methylation of the interspersed repeat LINE-1) or changes to methylation patterns of the IL6, TNF and CRP genes. Specifically, we have conducted a longitudinal analysis on protein markers of inflammation, comparing distressed middle-aged women who have engaged in a 2-month yoga intervention with a waitlist control group. Second, we have conducted a cross-sectional analysis of between-group DNA methylation profiles comparing post-yoga intervention group with the waitlist group. Finally, we have conducted a longitudinal analysis of the waitlist group's DNA methylation profiles to corroborate the cross-sectional analysis.
Materials and methods
Participants and procedure
This study represents a subsample (n=28) of a larger clinical trial (N=116), which explores the psychophysiological effects of a yoga intervention in women reporting psychological distress (as measured by a score of 16+ on Kessler Psychological Distress Scale [K10], ref. 42) and utilises a stratified, randomised waitlist control trial design. Psychophysiological results are reported elsewhere.43 The parent study explores the psychophysiological effects of participation in an average of a 1 h yoga class per week for a period of 8 weeks. The study utilised a stratified, randomised waitlist control trial design (described in detail in ref. 44). Within the parent study, a subsample of participants were randomly allocated to provide serum samples for the analysis of cytokines (IL-6 and TNF) and high-sensitivity CRP (n=35; n=7 lost to follow-up). Women were eligible for this pilot study if they were: healthy, free from acute infection for 2 weeks before biochemical assessment, and if they had refrained from drinking alcohol in the 48 h before biochemical assessment. Additional exclusion criteria were serious physiological illnesses that would interfere with the interpretation of biochemical data (for example, anaemia, diabetes, cardiovascular diseases, blood cancers, inflammatory bowel diseases, autoimmune diseases, asthma being treated with steroids, immunodeficiency); having a body mass index >30; meeting the criteria for substance abuse or dependence; undergoing menopause; having a serious psychological illness; or, having engaged in a regular yoga practice within the previous year. Biological samples were only available for this subsample.
Selection for epigenetic analysis in this subgroup is based on participants (1) already consented to provide blood; (2) meeting the inclusion/exclusion criteria described; and (3) giving informed consent to their blood sample being used for genetic analysis before the post-treatment evaluation. The participants who fulfilled the first two criteria were identified and randomly allocated into this portion of the study using Research Randomizer.45 The mean age of participants in this subsample (M=41.21, s.d.=4.14) is younger than the parent study (M=48.14, s.d.=8.22), but participants are not appreciably different in terms of other demographic or clinical variables. This trial has been approved by the Human Research Ethics Committee of the University of Adelaide; all the participants have given informed consent. This trial is registered at the Australian New Zealand Clinical Trial Registry [ANZCTR]: ACTRN12616000612415.
Yoga intervention
The yoga intervention comprised 8 weeks of twice-weekly, hour-long yoga classes (the total number of classes offered was 16). Per-protocol completion was considered attendance at eight classes, as weekly practice reflects the average community practitioners' engagement.2, 46 For further details, see Harkess et al.44
Study design
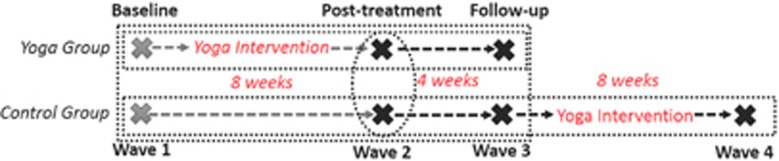
The study analyses involve two parts. The first utilises a randomised trial design to compare protein markers of inflammation of the participants who completed the yoga intervention to those of the control group (IL-6, TNF and CRP). The second utilises a cross-sectional trial design to compare DNA methylation patterns of participants who completed the yoga intervention to those of the control group at the post-treatment assessment. This is due to consent and ethics for genetic analysis being granted after initiation of the study, but before post-treatment data and sample collection. DNA methylation patterns are also explored longitudinally in a non-controlled trial design, with the waitlist control group examined from post-treatment and follow-up time points, until after the completion of the second round of yoga classes utilising our standardised protocol. To avoid confusion, we will refer to these as ‘waves' (see Figure 1).
Figure 1.
A visual depiction of the parent study and the current sub-study to explicate the analyses conducted. Grey ‘X' markings depict where only serum samples were available for the analysis (inflammatory markers), and black ‘X' markings indicate that both serum and whole bloods were available for the analysis (inflammatory markers and DNA methylation). The perforated rectangles indicate the longitudinal analyses conducted (where possible), and the perforated oval indicates the conduct of cross-sectional analysis.
Sample collection
Assessment included completing online surveys including demographic and psychological variables (detailed below), which participants completed before an in-person assessment. The in-person assessment involved physiological tests (that is, waist and height measurements) and collection of blood samples through routine venepuncture at baseline (wave 1), post-test (wave 2), 1-month follow-up (wave 3) and waitlist control intervention post test (wave 4). The participants were requested to abstain from stimulants, such as coffee, on the day of testing. At wave 1, the phlebotomist drew 21 ml intravenous blood sample from each participant. Each sample provided 3 ml for a complete blood picture analysis (to screen samples for abnormalities), 9 ml for cytokine analysis and 9 ml for hsCRP analysis. At waves 2, 3 and 4, the phlebotomist drew a total of 30 ml, with 9 ml extra to allow for genetic analysis. VACUETTE Plastic K3EDTA tubes (purple top) were used for complete blood picture and genetic analysis of samples, and VACUETTE Z Serum Sep Clot Activator (white top) were used for cytokines and hsCRP. The complete blood picture was analysed on the day of testing. To avoid problems with assay drift and interassay variability, samples for hsCRP and cytokines were centrifuged as per manufacturer's protocol, and the serum was frozen at −80 °C until the study was completed (post wave 4). For DNA analysis, whole blood samples were aliquoted into seven to eight eppendorfs, each containing 1 ml volume of whole blood and stored at −80 °C for DNA extraction and analysis as required. The remaining 1 ml was stored in RNAlater (Life Biosciences, Thermo Fisher, Vilnius, Lithuania) and is stored at −20 °C for future gene-expression analysis.
Mental health variables
As a set of secondary analyses, we also explored associations between biochemical outcomes (protein and DNA methylation inflammatory candidate markers) and psychological variables that have already demonstrated between-group effects in this population (Harkess et al., in press). Specifically, the study explores outcome scores at post-test on the (a) Kessler Psychological Distress Scale (K10), which gives a global measure of psychological distress based on questions about anxiety and depression symptoms;42 and the (b) Perceived Stress Scale, which measures the degree to which situations in one's life are appraised as stressful;47 and Positive Affect of the Positive and Negative Affect Schedule, which is a mood scale that measures people's positive affect.48
Protein analysis
Biological analyses were conducted blind of treatment/control groupings, with individuals across groups included in any batch to prevent group-specific batch effects. The study determined hsCRP serum concentration using the Beckman Coulter AU2700 analyser (Olympus, Hamburg, Germany; Beckman Coulter, Krefeld, Germany) and the Beckman Coulter CRP Latex method (immune-turbidimetric test), following the manufacturer's recommended protocol. A highly sensitive application that has a dynamic range of 0.08 to 80 mg l−1 was used. The samples from all the four waves were run by one individual in a batch of 20–30 over 2 days. The calibration was performed as required and quality control samples were run in accordance with SA Pathology protocols (internal quality controls were reported to be between 7–9% at the time of analysis).
Cytokine (IL-6 and TNF) serum concentrations were measured by cytokine capturing beads, using the BD cytometric Bead Array Human Enhanced Sensitivity Master Buffer kit and following the manufacturer's recommended protocols. Sensitivity of this kit is reported between the range of 0.27 to 200 pg. The samples were analysed by flow cytometry on the BD Canto1 flow cytometer. Quality control was performed daily, using Cytometer Setup and Tracking beads and an assay utilising the reported kit to determine whether proper cytokine readings were taken. A number of samples demonstrated levels below the 0.274 pg threshold for detection (IL-6: wave 1=11; wave 2=10; wave 3=10; wave 4=6; and TNF: wave 1=16; wave 2=16; wave 3=15; wave 4=7).
Methylation analysis
Biological analyses were conducted blind of treatment/control groupings, with individuals across groups included in any batch to prevent group-specific batch effects. Methylation assays were designed with Epidesigner software (www.epidesigner.com) and covered key regions found to be differentially methylated in previous studies investigating other exposures or disease outcomes: TNF;37, 40, 41, 49, 50 IL6;35, 36, 38, 39, 40 previously reported LINE-1 primers;51 and, the CRP assay was designed to target the CpG sites in the promoter region. (See Supplementary Table 6 for the assay designs) Cleavage patterns were determined using the Bioconductor MassArray package in R (www.bioconductor.org). DNA was extracted using the QIAamp DNA Mini Kit (QIAGEN, Hilden, Germany), and bisulphite converted using the MethylEasy Xceed Kit (Genetic Signatures, Darlinghurst, NSW, Australia). The samples were PCR amplified and assayed in triplicate. DNA methylation was quantified using the SEQUENOM MassARRAY (San Diego, CA, USA) and methylation ratios calculated using EpiTyper software (v.1.2; SEQUENOM). Further PCR protocol details and conditions are included in Supplementary Materials (Supplementary Tables 7).
The mean methylation from three technical replicates for each sample was determined; outlying values (deviation of ±10% methylation from the median) were discarded. Any individual with only one methylation datapoint following outlier identification was excluded from cross-sectional analyses. In longitudinal analysis, discarding these individuals limited the sample size with multiple datapoints (that is, sample was <6), so we retained single methylation datapoint individuals for the purpose of this pilot study (n=10), with sensitivity analyses excluding these individuals in the Supplementary Data (Supplementary Tables 1).
Statistical analysis
Statistical analyses were conducted using SPSS for Windows, version 21, software (SPSS, Chicago, IL, USA). The non-normal IL-6 and TNF distributions were dealt with by utilising non-parametric statistical tests.
IL-6 and TNF protein marker analysis
With no non-parametric equivalent to a two-way analysis of vairance (ANOVA), we used two Friedman Tests for longitudinal analyses to investigate change over time within each group (yoga and waitlist control groups, separately), which allowed use of all the available data within the study: to investigate change over time in waves 1, 2 and 3 in the yoga group (analyses 1); to investigate change over time in waves 1, 2, 3 and 4 in the waitlist control group (yoga was engaged in with the waitlist control group between waves 3 and 4; analyses 2). To compare the between-group differences post intervention at wave 2 on IL-6 and TNF protein levels, a cross-sectional analysis was applied: Mann–Whitney U-tests were used (analyses 3).
hsCRP analysis
(Analyses 4) A mixed between–within subjects ANOVA was conducted to assess the impact of the yoga intervention on hsCRP levels. This included data from waves 1, 2 and 3 for both yoga and waitlist control groups. (Analyses 5) A one-way repeated-measures ANOVA was used to investigate whether change over time was observed for hsCRP in the waitlist control group following yoga exposure; this included three waves before yoga (1, 2 and 3) with the final wave post yoga (wave 4).
DNA methylation
For each immune candidate (IL6, TNF, CRP), a mean percentage of methylation was calculated across all the CpG sites in each region assayed. Two sets of analyses were conducted: (analyses 6) an analysis of covariance model was used to evaluate cross-sectional outcome measures for DNA methylation data, with yoga as the predictor (no covariates). A second analysis of covariance was run to control for potential confounders (age and waist-to-height ratio at wave 2). We conducted two analyses due to the small sample size (N=28) and the exploratory nature of this study. Utilising the two analyses allows examination of the impact of additional covariates on the F-value, which is sensitive to degrees of freedom. (Analyses 7) To evaluate change across time following yoga intervention, we were restricted to utilising the waitlist control group only for longitudinal analysis as we did not have DNA methylation data for wave 1; a t-test was conducted, with the mean of the two pre-intervention results (wave 2 and wave 3) compared with post-intervention methylation (wave 4).
Secondary exploratory analyses of measures of mental health
(Analyses 8) We were restricted to correlational analyses owing to insufficient numbers to enable regression-based analyses.52 The protein biomarkers, IL-6 and TNF, exhibit non-normal distributions, thus non-parametric correlational analyses were applied. A Spearman rank-order correlation was performed to explore post-test associations of inflammatory protein markers, DNA methylation and mental health outcome variables.
Effect size and significance
As recommended by Perneger,53 we discuss the results in regard to both statistical significance and effect size (where possible), specifically Spearman's r (small=0.10, medium=0.30, large=0.50), partial eta squared (ηρ2; small=0.01, medium=0.06, large=0.138) and Cohen's d (small=0.02, medium=0.50 and large=0.80).54 Each hypothesis has been considered individually.53
Code availability
Computer code used to analyse the data can be made available by email upon request.
Results
Characteristics of the participants
As displayed in Table 1, the main characteristic of the participants did not differ significantly between the yoga and control groups, including in energy expenditure (METs), indicating equal engagement in physical activity. All the participants who participated in the blood sampling were Caucasian; therefore, we did not control for ethnicity.
Table 1. Baseline participant characteristics.
|
Overall (N=26) |
No. | % |
Control (N=15) |
No. | % |
Yoga (N=11) |
No. | % | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | s.d. | Mean | s.d. | Mean | s.d. | |||||||
| Age (years) | 41.12 | 4.28 | 40.80 | 4.36 | 41.55 | 4.34 | ||||||
| Waist-to-height | 0.50 | 0.07 | 0.48 | 0.08 | 0.52 | 0.07 | ||||||
| BMI | 24.78 | 4.96 | 24.20 | 5.38 | 25.57 | 4.46 | ||||||
| METS | 3162.00 | 7987.60 | 4080.40 | 10 515.93 | 1909.64 | 1302.00 | ||||||
| K10 | 23.69 | 5.22 | 24.20 | 5.45 | 23.00 | 5.06 | ||||||
| WBCCa | 6.29 | 1.59 | 6.28 | 1.04 | 6.32 | 2.19 | ||||||
| hsCRPa | 1.43 | 1.23 | 1.73 | 1.45 | 1.02 | 0.73 | ||||||
| Marital status | ||||||||||||
| Single | 4 | 15.4 | 2 | 13.3 | 2 | 18.2 | ||||||
| Common-law/married | 17 | 69.2 | 10 | 66.7 | 8 | 72.7 | ||||||
| Separated/divorced | 3 | 11.5 | 2 | 13.3 | 1 | 9.1 | ||||||
| Declined to answer | 1 | 3.8 | 1 | 6.7 | ||||||||
| Parous | 16 | 61.5 | 7 | 46.7 | 8 | 72.7 | ||||||
| Education level | ||||||||||||
| High school or less | 4 | 15.3 | 1 | 6.7 | 3 | 27.3 | ||||||
| Vocational school | 4 | 15.4 | 2 | 13.3 | 2 | 18.2 | ||||||
| University graduate | 14 | 53.8 | 9 | 60 | 5 | 45.5 | ||||||
| Postgraduate | 4 | 15.4 | 3 | 20 | 1 | 9.1 | ||||||
Abbreviations: BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; K10, psychological distress; METS, metabolic equivalent; WBCC, white blood cell count.
Levels are reported in untransformed units.
Analysis of inflammatory markers
Analyses 1
The Friedman Test indicated there was no evidence of a longitudinal difference in IL-6 or TNF across the three time points (X2 (2, n=11)=2.34, P=0.310; X2 (2, n=11)=0.50, P=0.779). Analyses 2: the Friedman Test indicated there was no evidence of a difference in IL-6 or TNF across the four time points (X2 (3, n=9)=0.57, P=0.904; X2 (3, n=9)=2.10, P=0.551). Analyses 3: a Mann–Whitney U-test revealed a nonsignificant, but moderate effect size suggesting that at post-test IL-6 levels were higher in the intervention group (Md=1.33, n=11) than in the control group (Md=0.00 n=15; U=49.0, z=−1.79, P=0.073, r=0.35). There was no evidence for differences in TNF levels (non-detectable intervention: Md=0.00, n=11; control: Md=0.00, n=15; U=78.0, z=−0.27, P=0.790, r=0.05).
Analysis 4
A mixed between–within subjects ANOVA demonstrated nonsignificant effect, but good effect size for differences in CRP over time, Wilks' Lambda=0.75, F(2,19)=3.17, P=0.065, ηρ2=0.25; though there was no evidence of a group by time interaction, Wilks' Lambda=0.91, F(2,19)=0.91, P=0.421, ηρ2=0.09. The means and standard deviations are presented in Table 2. Analysis 5: a one-way repeated-measures ANOVA (analysis 5) indicated there was no effect for time, Wilks' Lambda=0.54, F(3,5)=1.42, P=0.342, ηρ2=0.46. The means and standard deviations are presented in Table 3.
Table 2. Descriptive statistics for hsCRP for varying time points.
|
Yoga intervention group |
Waitlist control group |
Total/combined |
||||
|---|---|---|---|---|---|---|
| N | Mean (s.d.) | N | Mean (s.d.) | N | Mean (s.d.) | |
| Baseline (wave 1) | 10 | 1.00 (0.76) | 12 | 1.49 (1.38) | 22 | 1.27 (1.14) |
| Post-test (wave 2) | 10 | 1.05 (0.81) | 12 | 0.99 (0.49) | 22 | 1.02 (0.64) |
| Follow-up (wave 3) | 10 | 1.79 (1.59) | 12 | 1.50 (1.24) | 22 | 1.63 (1.38) |
Abbreviation: hsCRP, high-sensitivity C-reactive protein.
Table 3. Descriptive statistics for hsCRP for pre-intervention time points and post-test.
| Time period | N | Mean | s.d. |
|---|---|---|---|
| Pre-test (wave 1) | 8 | 1.56 | 1.67 |
| Pre-test (wave 2) | 8 | 0.84 | 0.29 |
| Pre-test (wave 3) | 8 | 1.45 | 1.49 |
| Post-test (wave 4) | 8 | 1.00 | 0.96 |
Abbreviation: hsCRP, high-sensitivity C-reactive protein.
Analysis of DNA methylation
Please see Table 4 for depiction of the cross-sectional analysis (wave 2; analysis 6), described below.
Table 4. Results of DNA methylation cross-sectional ANCOVA analyses.
| Promoter region |
Control |
Yoga |
Main effect |
Effect with covariates
(age and WtHR) |
|||
|---|---|---|---|---|---|---|---|
| Mean (s.d.) | n | Mean (s.d.) | n | Yoga vs control | Yoga vs control | Covariates | |
| IL61 | |||||||
| CpG 1 | 0.898 (0.024) | 13 | 0.921 (0.021) | 9 | F(1,20)=5.29, P=0.032*, ηρ2=0.21 | F(3,18)=4.30, P=0.053†, ηρ2=0.19 | Age ηρ2=0.11, WtHR ηρ2=0.02 |
| CpG 2/3 | 0.931 (0.013) | 10 | 0.924 (0.019) | 8 | F(1,16)=0.768, P=0.394, ηρ2=0.05 | F(3,14)=1.12, P=0.307, ηρ2=0.07 | Age ηρ2=0.07, WtHR ηρ2=0.04 |
| CpG 4/5/6 | 0.928 (0.016) | 14 | 0.933 (0.009) | 9 | F(1,21)=0.59, P=0.452, ηρ2=0.03 | F(3,19)=0.27, P=0.609, ηρ2=0.01 | Age ηρ2=0.02, WtHR ηρ2=0.11 |
| Mean | 0.853 (0.246) | 14 | 0.926 (0.011) | 9 | F(1,21)=0.78, P=0.387, ηρ2=0.04 | F(3,19)=0.72, P=0.406, ηρ2=0.04 | Age ηρ2=0.10, WtHR ηρ2=0.00 |
| IL62 | |||||||
| CpG 1 | 0.035 (0.009) | 15 | 0.037 (0.010) | 11 | F(1,24)=0.24, P=0.626, ηρ2=0.01 | F(3,22)=0.14, P=0.717, ηρ2=0.00 | Age ηρ2=0.02, WtHR ηρ2=0.04 |
| CpG 2 | 0.006 (0.009) | 15 | 0.003 (0.005) | 11 | F(1,24)=0.91, P=0.349, ηρ2=0.04 | F(3,22)=1.22, P=0.281, ηρ2=0.05 | Age ηρ2=0.05, WtHR ηρ2=0.04 |
| CpG 4/5/6 | 0.033(0.011) | 15 | 0.034 (0.009) | 11 | F(1,24)=0.04, P=0.852, ηρ2=0.00 | F(3,22)=0.01, P=0.931, ηρ2=0.00 | Age ηρ2=0.04, WtHR ηρ2=0.00 |
| CpG 7/8 | 0.078(0.020) | 15 | 0.084 (0.015) | 11 | F(1,24)=0.56, P=0.463, ηρ2=0.02 | F(3,22)=0.44, P=0.515, ηρ2=0.02 | Age ηρ2=0.00, WtHR ηρ2=0.01 |
| Mean | 0.038 (0.008) | 15 | 0.040 (0.007) | 11 | F(1,24)=0.13, P=0.720, ηρ2=0.01 | F(1,22)=0.05, P=0.824, ηρ2=0.00 | Age ηρ2=0.04, WtHR ηρ2=0.03 |
| TNF | |||||||
| CpG 1 | 0.829 (0.079) | 13 | 0.748 (0.108) | 6 | F(1,17)=3.45, P=0.081†, ηρ2=0.17 | F(3,15)=2.32, P=0.148, ηρ2=0.13 | Age ηρ2=0.04, WtHR ηρ2=0.00 |
| CpG 2 | 0.814 (0.079) | 13 | 0.738 (0.086) | 8 | F(1,19)=4.30, P=0.052†, ηρ2=0.19 | F(3,17)=4.56, P=0.049*, ηρ2=0.21 | Age ηρ2=0.00, WtHR ηρ2=0.04 |
| CpG 4/5/6 | 0.142 (0.074) | 13 | 0.106 (0.043) | 9 | F(1,20)=1.69, P=0.208, ηρ2=0.09 | F(3,18)=2.51, P=0.131, ηρ2=0.12 | Age ηρ2=0.00, WtHR ηρ2=0.20* |
| CpG 8 | 0.231 (0.096) | 14 | 0.210 (0.091) | 10 | F(1,22)=0.28, P=0.599, ηρ2=0.01 | F(3,20)=0.33, P=0.573, ηρ2=0.02 | Age ηρ2=0.02, WtHR ηρ2=0.02 |
| CpG 9 | 0.089 (0.050) | 13 | 0.075 (0.030) | 10 | F(1,21)=0.63, P=0.437, ηρ2=0.03 | F(3,19)=0.57, P=0.458, ηρ2=0.03 | Age ηρ2=0.01, WtHR ηρ2=0.00 |
| CpG 12 | 0.087 (0.060) | 13 | 0.073 (0.031) | 8 | F(1,19)=0.39, P=0.537, ηρ2=0.02 | F(3,17)=0.49, P=0.495, ηρ2=0.03 | Age ηρ2=0.17, WtHR ηρ2=0.05 |
| Mean | 0.367 (0.048) | 15 | 0.322 (0.046) | 11 | F(1,24) =5.68, P=0.025*, ηρ2=0.19 | F(3,22)=6.16, P=0.021*, ηρ2=0.22 | Age ηρ2=0.00, WtHR ηρ2=0.09 |
| CRP | |||||||
| CpG 1 | 0.875 (0.048) | 12 | 0.885 (0.031) | 10 | F(1,20)=0.32, P=0.579, ηρ2=0.02 | F(3,18)=0.18, P=0.675, ηρ2=0.01 | Age ηρ2=0.12, WtHR ηρ2=0.00 |
| CpG 2 | 0.733 (0.064) | 12 | 0.740 (0.058) | 10 | F(1,20)=0.06, P=0.803, ηρ2=0.00 | F(3,18)=0.01, P=0.937, ηρ2=0.00 | Age ηρ2=0.33, WtHR ηρ2=0.22 |
| CpG 4 | 0.726 (0.074) | 12 | 0.715 (0.051) | 10 | F(1,20)=0.15, P=0.701, ηρ2=0.01 | F(3,18)=0.39, P=0.539, ηρ2=0.02 | Age ηρ2=0.14, WtHR ηρ2=0.07 |
| Mean | 0.717 (0.220) | 13 | 0.709 (0.237) | 11 | F(1,22)=0.01, P=0.934, ηρ2=0.00 | F(3,20)=0.01, P=0.908, ηρ2=0.00 | Age ηρ2=0.01, WtHR ηρ2=0.02 |
| LINE-1 | |||||||
| CpG 1 | 0.693 (0.020) | 15 | 0.692 (0.017) | 11 | F(1,24)=0.00, P=0.958, ηρ2=0.00 | F(3,22)=0.00, P=0.974, ηρ2=0.00 | Age ηρ2=0.06, WtHR ηρ2=0.07 |
| CpG 2 | 0.721 (0.014) | 15 | 0.724 (0.009) | 11 | F(1,24)=0.32, P=0.577, ηρ2=0.01 | F(3,22)=0.25, P=0.626, ηρ2=0.01 | Age ηρ2=0.00, WtHR ηρ2=0.04 |
| CpG 3 | 0.607 (0.012) | 15 | 0.605 (0.015) | 11 | F(1,24)=0.20, P=0.662, ηρ2=0.01 | F(3,22)=0.24, P=0.632, ηρ2=0.01 | Age ηρ2=0.00, WtHR ηρ2=0.04 |
| Mean | 0.674 (0.014) | 15 | 0.674 (0.012) | 11 | F(1,24)=0.00, P=0.997, ηρ2=0.00 | F(1,22)=0.00, P=0.982, ηρ2=0.00 | Age ηρ2=0.02, WtHR ηρ2=0.07 |
Abbreviations: ANCOVA, analysis of covariance; CRP, C-reactive protein; IL-6, interleukin-6; TNF, tumour necrosis factor.
*P<0.05; †P<0.10 (two-tailed tests). ηρ2=partial eta squared.
Regions of methylation and yoga
No between-group differences in mean methylation across CRP or the IL6 regions were observed in either analysis of covariance model (no covariates, or with age and WtHR as covariates). A significant main effect of group was found for the mean methylation of TNF, which explained 19% of the variance. Women in the yoga group demonstrated a 4.5% lower level of methylation relative to the waitlist control group (see Table 4). The main effect of the group on TNF remained (yoga group with lower methylation) when covariates age and waist-to-height ratio were included in the analysis.
Individual CpG units and yoga
No significant differences in methylation at individual CpG units were demonstrated for CRP and IL62;, differences in mean methylation for IL61 CpG site 1 were observed between groups (2.3% higher methylation in the yoga group), but this association was reduced with the inclusion of age and WtHR in the model. There appeared to be some differences between groups at individual TNF sites, but this varied depending on the inclusion of covariates. Only one covariate, WtHR, demonstrated a close to significant association with the TNF CpG site 4/5/6 (P=0.050; 20% of variance explained).
Global DNA marker LINE-1 methylation
No evidence for differences in methylation at individual LINE-1 CpG units, nor the overall mean, was demonstrated. Covariates were also not associated with differences in LINE-1 methylation.
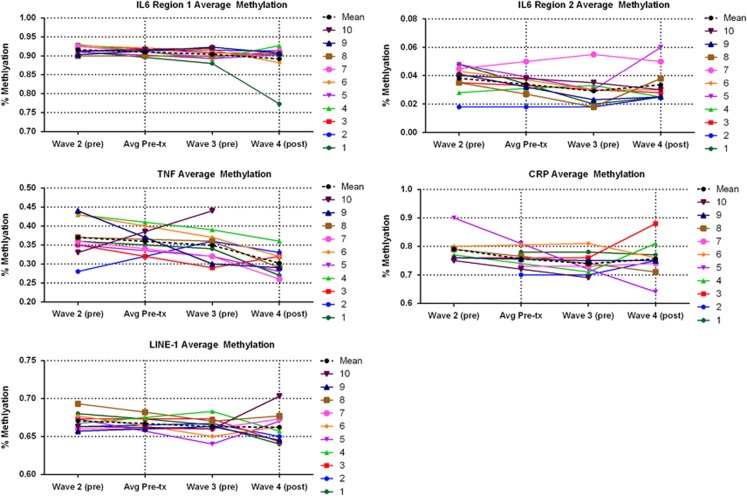
Please see Figure 2 for the depiction of longitudinal analysis of the waitlist control group (analysis 7). The sample sizes for the longitudinal analyses are small (ranging from 10 to 11). As some longitudinal techniques used to compare the groups are unreliable in the small sample sizes, we conducted paired sample t-tests to explore pre- to post-intervention (average of wave 2 and 3 to wave 4) effects to ascertain whether findings corroborated cross-sectional analyses already presented. The results of all the analyses and descriptive statistics are presented in the Supplementary Material (reference Supplementary Tables 1–5), and we present psychological outcomes that demonstrated medium or large effects of change following yoga.54
Figure 2.
Longitudinal IL-6 (regions 1 and 2), TNF, CRP and global DNA marker LINE-1 methylation patterns of the control group. Each participant is shown individually (numbered 1–10), with the mean shown as a black perforated line. (Avg Pre-tx indicates average methylation pre-yoga intervention at waves 2 and 3; pre indicates pre-yoga intervention; post indicates post-yoga intervention). CRP, C-reactive protein; IL-6, interleukin-6; TNF, tumour necrosis factor.
Regions of methylation
The yoga intervention was associated with a reduction in TNF methylation (Cohen's d=1.68) and decreased IL61 methylation (Cohen's d=0.53), although this did not reach significance.
Individual CpG units
Significant associations indicating decreased methylation at the post-yoga time point was demonstrated for TNF CpG site 1 (Cohen's d=1.11) and 4/5/6 (Cohen's d=1.00).
Global DNA marker LINE-1 methylation
No evidence of a difference for time was seen at individual LINE-1 CpG sites or for the mean.
Exploratory analyses of measures of mental health
Analyses 8
As shown in Table 5, there is a strong correlation between perceived stress and psychological distress. Moderate correlations are demonstrated between subjective well-being and perceived stress and psychological distress. A moderate and significant correlation between global DNA marker LINE-1 methylation and perceived stress is reported. A number of other moderate size correlations are observed, however, significance was not achieved, possibly due to limited power.
Table 5. Results of Spearman's rank-order correlation.
| K10 | PSS | SWB | PA | hsCRP | IL6 | TNF | IL61 | IL62 | TNF | CRP | LINE-1 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Questionnaires | ||||||||||||
| 1. K10 | — | |||||||||||
| 2. PSS | 0.654** | — | ||||||||||
| 3. SWB | −0.445* | −0.128 | — | |||||||||
| 4. PA | −0.097 | −0.273 | −0.025 | — | ||||||||
| Proteins | ||||||||||||
| 5. hsCRP | 0.089 | 0.337† | 0.113 | 0.199 | — | |||||||
| 6. IL6 | −0.061 | 0.040 | −0.188 | 0.001 | −0.003 | — | ||||||
| 7. TNF | −0.034 | 0.245 | −0.028 | −0.149 | 0.059 | 0.608** | — | |||||
| DNA methylation | ||||||||||||
| 8. IL61 | −0.031 | 0.096 | 0.400† | −0.099 | −0.119 | 0.069 | 0.312 | — | ||||
| 9. IL62 | 0.006 | 0.082 | 0.290 | −0.093 | 0.121 | −0.219 | −0.107 | −0.020 | — | |||
| 10. TNF | 0.242 | 0.154 | 0.046 | −0.011 | 0.001 | −0.222 | 0.017 | 0.129 | 0.038 | — | ||
| 11. CRP | −0.237 | −0.053 | 0.364† | −0.398† | −0.031 | −0.214 | 0.021 | 0.126 | 0.323† | 0.211 | — | |
| 12. LINE-1 | 0.126 | 0.409* | −0.125 | −0.382† | 0.202 | −0.114 | −0.057 | −0.008 | 0.074 | 0.053 | 0.384† | — |
Abbreviations: hsCRP, high-sensitivity C-reactive protein; IL, interleukin; K10, psychological distress; PA, positive affect; PSS, Perceived Stress Scale; SWB, subjective well-being; TNF, tumour necrosis factor.
**P<0.01; *P<0.05; †P<0.10 (two-tailed tests).
Discussion
This prospective pilot trial explored the relationship between yoga, psychophysiological health indicators, and inflammatory protein and methylation markers in a stressed female community population. This study was unique in exploring DNA methylation, and correlations between methylation and inflammatory markers with potential to indicate a functional relationship. The DNA methylation component was, however, included retrospectively, meaning longitudinal analysis was only possible with the waitlist participants pre- and post-yoga intervention. Overall, the study found that an 8-week yoga intervention, requiring at least weekly practice, is associated with some changes in immune protein and DNA methylation biomarkers. The yoga group demonstrated lower DNA methylation of the TNF region as a whole, and at specific sites, in cross-sectional analysis relative to the control group. This was further supported by decreased methylation seen post yoga in the longitudinal analysis of the waitlist control group that later participated in the yoga intervention. Meaningful effects sizes in both protein and methylation analysis were demonstrated, as were associations between psychological variables and biochemical measures; however, these were not found to be significant. Lack of significance may be attributed to limited statistical power of the study. Nonetheless, these results indicate that participation in an 8-week yoga intervention may have differential impacts on the methylation responses of the immune candidate genes investigated, and that further investigation in better-powered samples is important.
Of note, we did not find evidence of associations between yoga and serum measures of inflammation. Similarly, a large-scale trial did not demonstrate an association between anxiety and biomarkers of inflammation in females,55 which contrasts the associations reported in depression.56, 57 To this end, it should be considered that this is a non-clinical community population in which biomarkers of inflammation were generally low, reflected by the ‘bottoming out' of inflammatory cytokines. Nonetheless, the moderate effect indicating higher IL-6 levels in the yoga group is interesting insofar as it has a well-known role in the pro-inflammatory processes, but is increasingly recognised in healing and regeneration activities.58 For instance, Eyre et al. reports that increased IL-6 has a role in the neuroprotective effect of exercise on mood.59 A large effect for time on overall levels of hsCRP was demonstrated, though we did not find evidence of meaningful between-group difference. The general decrease at post-test may be reflecting a sample bias we have discussed elsewhere.43 Namely at baseline, women were reporting chronic stress and moderate-to-high levels of distress (potentially indicated by high levels of acute-phase proteins), however, they self-selected for this study, which indicates motivation to change. This was supported by the overall decrease in stress and distress at post-test,43 and could account for a change in hsCRP over time independent of participation in yoga. In addition, at post-test, perceived stress was found to be positively associated with global DNA marker LINE-1 methylation. Although this is not consistent with some literature, indicating elevated methylation correlates with positive health outcomes,60, 61 it is consistent with literature demonstrating hypermethylation in stressed populations.62
This study reports a robust association between engagement with an 8-week yoga intervention and reduction in mean methylation of TNF (5.5%); however, there is no evidence for sizeable correlations between the TNF methylation and serum, or psychological measures making it difficult to infer causal relationships. We do, however, report a moderate association with WtHR, parsimonious with previous reports of methylation of TNF being associated with leanness/weight-loss previously.40, 41 This could potentially account for our reported hypomethylation of TNF in our yoga group and following yoga intervention. However, including WtHR as a covariate did not alter the reported association; in fact, it strengthened it suggesting that the reduction observed in TNF methylation is not simply attributable to change in body composition in our sample following yoga. Yoga may be associated with a positive alteration on the inflammatory system that is not detectable immediately in serum analysis, and not directly responsible for reported positive psychological effects of yoga.
This sample was relatively homogeneous in variables that have been reported as risk factors for differential global DNA marker of methylation (as measured by LINE-1; for example, see refs 60,61,63,64,65,66,67,68,69). Namely, it comprised Caucasian females, aged between 35 and 50 years, with body mass indexes <30, with no reported substance abuse problems, and comparable between-group physical activity levels (no between-group differences in METS discussed in greater detail elsewhere; for example, ref. 43). The yoga and control group were overarchingly well matched for WtHR and age. Inclusion of WtHR, which was associated with TNF methylation at CpG 4/5/6, as a covariate improved detection of differences in TNF methylation following our yoga intervention. Thus, although the lack of statistical correlation and between-group effects could be attributable to the limited statistical power in our sample, the medium to large effect sizes reported are plausibly attributable to the yoga intervention, and not to previously implicated lifestyle factors due to a lack of variability of these factors in our groups (that is, ethnicity, WtHR, age). This study presents strengths for investigation of biological biomarkers, in the selection of an homogenous population, longitudinal sampling and the first study to investigate DNA methylation in context of a yoga intervention. However, there are limitations.
Limitations
There are a number of limitations to this study, which should be considered. The first is that it was a pilot study with only limited statistical power. As a result, this limited statistical analyses that could be undertaken (for example, we did not meet the sample size assumption required to conduct a regression, nor exploration of mediation/moderation), in addition to having low power when measuring the group differences (for example, ANOVA cell sizes of 30 are required for 80% power70). Second, there was no baseline DNA methylation measure, which means we cannot draw causal conclusions about between-group differences. DNA methylation investigated was from DNA extracted from peripheral whole blood, which is in keeping with our simultaneous exploration of serum markers of inflammation. However, we cannot draw inferences about the effects on specific tissues, including the brain. We only explored a limited number of immune candidate genes (two regions of IL6 and one region of TNF and CRP each), and, as demonstrated by IL6, different regions may indicate different trends. The lack of association between DNA methylation markers and serum markers of inflammation makes it difficult to interpret functional impact, although this could be due to statistical power and the possibility that methylation impacts less immediately in serum protein expression levels. It is notable that a number of cytokine samples were below the detection limit in protein analyses, which is likely due to the non-clinical nature of this sample as well as a technical limitation of the sensitivity of currently available assays. We did not use an active control group, and while between-group METs were equivalent, we cannot rule out the attentional effects of engagement with the yoga teacher and the class environment. To address some of these concerns, we focused on the presentation of effect sizes where available and also analysed the data from two different perspectives to examine the reliability of the findings (cross-sectional and longitudinal).
As levels of stress are reported to be increasing in community populations,52 an increased prevalence of stress-related disease is likely to follow.6, 71, 72, 73 Therefore, future prospective studies should continue to explore the relationship of stress and biomarkers of inflammation in community populations. We recommend replicating our study in a much larger sample and including analysis of DNA methylation profiles at baseline. A variety of active controls would also be beneficial to assist in disentangling the potentially different effects of different styles of yoga, exercise and meditation. In addition, we recommend exploring other candidate genes that may demonstrate involvement in the inflammatory response that has been associated with maladaptive psychological states and/or epigenomic methods that enable network analyses. Finally, we would recommend an experimental design that could differentiate more clearly between regressions to the mean (that is, entering the study when distress levels are maximal and a natural decrease with time as opposed to intervention) and an experimental effect would be one that took a number of pre-intervention measures.
Conclusions
Alongside the increased levels of stress and prevalence of stress-related disease reported, there has been increased engagement in MBTs, of which yoga is the most utilised.74 Although gene-expression studies in the MBT literature suggests a relationship with the immune system,75 further research into the underlying mechanisms, including possible epigenetic mechanisms, has been called for.31 To the best of our knowledge, this is the first study to investigate the role of yoga on epigenetic change, and the first MBT to investigate DNA methylation in immune candidates' methylation (IL6, TNF and CRP). Although this pilot study is small and exploratory, it nevertheless indicates that in a non-clinical chronically stressed community population, practicing a minimum of a once-weekly, hour-long yoga class, is associated with differential methylation patterns despite the waitlist control group reporting similar energy expenditure to the yoga group. This suggests that these changes may not be related to energy expenditure, but some aspect of the yoga engagement. However, it is notable that the control group shows larger variance in energy expenditure relative to the intervention group, and more definitive conclusions cannot be made without an active control group in future studies. Specifically, we report that engaging in a yoga intervention may affect the female participants' serum levels of IL-6 and their epigenetic profile of immune candidates, specifically TNF. These findings warrant further large-scale research and contribute to the growing literature seeking to explore underlying epigenetic mechanisms and the relationship between MBT, the immune system.31 In addition, they contribute to the growing body of literature seeking to explore biomarkers of inflammation in the clinical and non-clinical conditions of distress.76, 77, 78
Acknowledgments
We thank all the participants of the study for their generous contribution. We also thank our phlebotomists Amy Rutten and Dana Aldwin. KNH was supported by an Ian Wilson Liberal Scholarship and Australian Postgraduate Award. JR was supported by an Early Career National Health and Medical Research Fellowship, Australia. SC-W was supported by a Matthew Flinders Fellowship, Flinders University, South Australia, Australia.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on the Translational Psychiatry website (http://www.nature.com/tp)
Supplementary Material
References
- La Forge R. Aligning mind and body: exploring the disciplines of mindful exercise. ACSMs Health Fitness J 2005; 9: 7–14. [Google Scholar]
- Birdee GS, Legedza AT, Saper RB, Bertisch SM, Eisenberg DM, Phillips RS. Characteristics of yoga users: results of a national survey. J Gen Intern Med 2008; 23: 1653–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer H, Lauche R, Langhorst J, Dobos G. Yoga for depression: a systematic review and meta-analysis. Depress Anxiety 2013; 30: 1068–1083. [DOI] [PubMed] [Google Scholar]
- Li AW, Goldsmith CA. The effects of yoga on anxiety and stress. Altern Med Rev 2012; 17: 21–35. [PubMed] [Google Scholar]
- Patel NK, Newstead AH, Ferrer RL. The effects of yoga on physical functioning and health related quality of life in older adults: a systematic review and meta-analysis. J Altern Complement Med 2012; 18: 902–917. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialog Clin Neurosci 2006; 8: 367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. Psychological and metabolic stress: a recipe for accelerated cellular aging? Hormones (Athens, Greece) 2009; 8: 7–22. [DOI] [PubMed] [Google Scholar]
- Hickie I, Lloyd A. Are cytokines associated with neuropsychiatric syndromes in humans? Int J Immunopharmacol 1995; 17: 677–683. [DOI] [PubMed] [Google Scholar]
- Juster RP, McEwen BS, Lupien SJ. Allostatic load biomarkers of chronic stress and impact on health and cognition. Neurosci Biobehav Rev 2010; 35: 2–16. [DOI] [PubMed] [Google Scholar]
- Gimeno D, Kivimäki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A et al. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychol Med 2009; 39: 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren MB, Lamkin DM, Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 2009; 71: 171–186. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Yaffe K, Newman AB, Simonsick EM, Rubin S et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry 2003; 54: 566–572. [DOI] [PubMed] [Google Scholar]
- Horsburgh S, Robson-Ansley P, Adams R, Smith C. Exercise and inflammation-related epigenetic modifications: focus on DNA methylation. Exerc Immunol Rev 2015; 21: 26–41. [PubMed] [Google Scholar]
- Carlson LE, Speca M, Patel KD, Goodey E. Mindfulness-based stress reduction in relation to quality of life, mood, symptoms of stress, and immune parameters in breast and prostate cancer outpatients. Psychosom Med 2003; 65: 571–581. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB et al. Yoga's impact on inflammation, mood, and fatigue in breast cancer survivors: a randomized controlled trial. J Clin Oncol 2014; 32: 1040–1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Christian L, Preston H, Houts CR, Malarkey WB, Emery CF et al. Stress, inflammation, and yoga practice. Psychosom Med 2010; 72: 113–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsankova N, Renthal W, Kumar A, Nestler EJ. Epigenetic regulation in psychiatric disorders. Nat Rev Neurosci 2007; 8: 355–367. [DOI] [PubMed] [Google Scholar]
- Docherty S, Mill J. Epigenetic mechanisms as mediators of environmental risks for psychiatric disorders. Psychiatry (GBR) 2008; 7: 500–506. [Google Scholar]
- Sananbenesi F, Fischer A. The epigenetic bottleneck of neurodegenerative and psychiatric diseases. Biol Chem 2009; 390: 1145–1153. [DOI] [PubMed] [Google Scholar]
- Toyokawa S, Uddin M, Koenen KC, Galea S. How does the social environment 'get into the mind'? Epigenetics at the intersection of social and psychiatric epidemiology. Soc Sci Med 2012; 74: 67–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unternaehrer E, Luers P, Mill J, Dempster E, Meyer AH, Staehli S et al. Dynamic changes in DNA methylation of stress-associated genes (OXTR, BDNF) after acute psychosocial stress. Transl Psychiatry 2012; 2: e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayarsaihan D. Epigenetic mechanisms in inflammation. J Dent Res 2011; 90: 9–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F, Allavena P, Sica A, Garlanda C, Mantovani A. Cancer-related inflammation, the seventh hallmark of cancer: links to genetic instability. Carcinogenesis 2009; 30: 1073–1081. [DOI] [PubMed] [Google Scholar]
- Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-kappaB, Lin28, Let-7 MicroRNA, and IL6 links inflammation to cell transformation. Cell 2009; 139: 693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perwez Hussain S, Harris CC. Inflammation and cancer: an ancient link with novel potentials. Int J Cancer 2007; 121: 2373–2380. [DOI] [PubMed] [Google Scholar]
- Levenson VV. DNA methylation as a universal biomarker. Expert Rev Mol Diagn 2010; 10: 481–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee B, Vadiraj HS, Ram A, Rao R, Jayapal M, Gopinath KS et al. Effects of an integrated yoga program in modulating psychological stress and radiation-induced genotoxic stress in breast cancer patients undergoing radiotherapy. Integr Cancer Ther 2007; 6: 242–250. [DOI] [PubMed] [Google Scholar]
- Black DS, Cole SW, Irwin MR, Breen ESt, Cyr NM, Nazarian N et al. Yogic meditation reverses NF-kappaB and IRF-related transcriptome dynamics in leukocytes of family dementia caregivers in a randomized controlled trial. Psychoneuroendocrinology 2013; 38: 348–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Greendale G, Crosswell AD, Garet D, Sternlieb B, Ganz PA et al. Yoga reduces inflammatory signaling in fatigued breast cancer survivors: a randomized controlled trial. Psychoneuroendocrinology 2014; 43: 20–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavretsky H, Epel ES, Siddarth P, Nazarian N, Cyr NS, Khalsa DS et al. A pilot study of yogic meditation for family dementia caregivers with depressive symptoms: effects on mental health, cognition, and telomerase activity. Int J Geriatr Psychiatry 2013; 28: 57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niles H, Mehta DH, Corrigan AA, Bhasin MK, Denninger JW. Functional genomics in the study of mind-body therapies. Ochsner J 2014; 14: 681–695. [PMC free article] [PubMed] [Google Scholar]
- Kaliman P, Alvarez-Lopez MJ, Cosin-Tomas M, Rosenkranz MA, Lutz A, Davidson RJ. Rapid changes in histone deacetylases and inflammatory gene expression in expert meditators. Psychoneuroendocrinology 2014; 40: 96–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren H, Collins V, Clarke SJ, Han J-S, Lam P, Clay F et al. Epigenetic changes in response to Tai Chi practice: a pilot investigation of DNA methylation marks. Evid Based Complement Altern Med 2012; 2012: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordaux R, Batzer MA. The impact of retrotransposons on human genome evolution. Nat Rev Genet 2009; 10: 691–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FF, Santella RM, Wolff M, Kappil MA, Markowitz SB, Morabia A. White blood cell global methylation and IL-6 promoter methylation in association with diet and lifestyle risk factors in a cancer-free population. Epigenetics 2012; 7: 606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefani FA, Viana MB, Dupim AC, Brito JA, Gomez RS, da Costa JE et al. Expression, polymorphism and methylation pattern of interleukin-6 in periodontal tissues. Immunobiology 2013; 218: 1012–1017. [DOI] [PubMed] [Google Scholar]
- Plant D, Wilson AG, Barton A. Genetic and epigenetic predictors of responsiveness to treatment in RA. Nat Rev Rheumatol 2014; 10: 329–337. [DOI] [PubMed] [Google Scholar]
- Nile CJ, Read RC, Akil M, Duff GW, Wilson AG. Methylation status of a single CpG site in the IL6 promoter is related to IL6 messenger RNA levels and rheumatoid arthritis. Arthritis Rheum 2008; 58: 2686–2693. [DOI] [PubMed] [Google Scholar]
- Morabia A, Zhang FF, Kappil MA, Flory J, Mirer FE, Santella RM et al. Biologic and epigenetic impact of commuting to work by car or using public transportation: a case-control study. Prev Med 2012; 54: 229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchner H, Nylen C, Laber S, Barres R, Yan J, Krook A et al. Altered promoter methylation of PDK4, IL1 B, IL6, and TNF after Roux-en Y gastric bypass. Surg Obes Relat Dis 2014; 10: 671–678. [DOI] [PubMed] [Google Scholar]
- Campión J, Milagro FI, Goyenechea E, Martínez JA. TNF-α promoter methylation as a predictive biomarker for weight-loss response. Obesity 2009; 17: 1293–1297. [DOI] [PubMed] [Google Scholar]
- Kessler R, Mroczek D. Final versions of our non-specific psychological distress scale. Memo dated 3 October 1994. Population Research and Outcome Studies. BRIEF REPORTS Number: 2002-14. The Kessler Psychological Distress Scale (K10).
- Harkess KN, Delfabbro P, Mortimer J, Hannaford Z, Cohen-Woods S. Brief report on the psychophysiological effects of a yoga intervention for chronic stress. J Psychophysiol; advance online publication 27 July 2016; doi:10.1027/0269-8803/a000169.
- Harkess KN, Delfabbro P, Curtis E, Cohen-Woods S. Process evaluation of a secular yoga intervention with clinical reductions of participant's reported distress (Submitted).
- Urbaniak GC, Plous S. Research Randomizer (Version 4.0) [Computer software]. 2013 [cited 10 April 2013]. Available from http://www.randomizer.org/.
- Penman S, Cohen M, Stevens P, Jackson S. Yoga in Australia: results of a national survey. Int J Yoga 2012; 5: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983; 24: 385–396. [PubMed] [Google Scholar]
- Watson D, Clark LA. Measurement and mismeasurement of mood: recurrent and emergent issues. J Pers Assess 1997; 68: 267–296. [DOI] [PubMed] [Google Scholar]
- Gowers IR, Walters K, Kiss-Toth E, Read RC, Duff GW, Wilson AG. Age-related loss of CpG methylation in the tumour necrosis factor promoter. Cytokine 2011; 56: 792–797. [DOI] [PubMed] [Google Scholar]
- Cordero JB, Macagno JP, Stefanatos RK, Strathdee KE, Cagan RL, Vidal M. Oncogenic Ras diverts a host TNF tumor suppressor activity into tumor promoter. Dev Cell 2010; 18: 999–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flotho C, Claus R, Batz C, Schneider M, Sandrock I, Ihde S et al. The DNA methyltransferase inhibitors azacitidine, decitabine and zebularine exert differential effects on cancer gene expression in acute myeloid leukemia cells. Leukemia 2009; 23: 1019–1028. [DOI] [PubMed] [Google Scholar]
- Cassey L, Ling RP-T. Stress and wellbeing in Australia survey 2014. Australian Psychological Society 2014; https://www.psychology.org.au/Assets/.../2014-APS-NPW-Survey-WEB-reduced.pdf.
- Perneger TV. What's wrong with Bonferroni adjustments. BMJ 1998; 316: 1236–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd edn. Hillsdale, New Jersey: Erlbaum, 1988.
- Vogelzangs N, Beekman ATF, De Jonge P, Penninx BWJH. Anxiety disorders and inflammation in a large adult cohort. Transl Psychiatry 2013; 3: e249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard B, Maes M. Mechanistic explanations how cell-mediated immune activation, inflammation and oxidative and nitrosative stress pathways and their sequels and concomitants play a role in the pathophysiology of unipolar depression. Neurosci Biobehav Rev 2012; 36: 764–785. [DOI] [PubMed] [Google Scholar]
- Loftis JM, Huckans M, Ruimy S, Hinrichs DJ, Hauser P. Depressive symptoms in patients with chronic hepatitis C are correlated with elevated plasma levels of interleukin-1β and tumor necrosis factor-α. Neurosci Lett 2008; 430: 264–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta 2011; 1813: 878–888. [DOI] [PubMed] [Google Scholar]
- Eyre HA, Papps E, Baune BT. Treating depression and depression-like behavior with physical activity: an immune perspective. Front Psychiatry 2013; 4: 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm CS, Kelsey KT, Butler R, Plaza S, Gagne L, Zens MS et al. Implications of LINE1 methylation for bladder cancer risk in women. Clin Cancer Res 2010; 16: 1682–1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AJ, Sandler DP, Bolick SC, Xu Z, Taylor JA, DeRoo LA. Recreational and household physical activity at different time points and DNA global methylation. Eur J Cancer 2013; 49: 2199–2206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusiecki JA, Chen L, Srikantan V, Zhang L, Yan L, Polin ML et al. DNA methylation in repetitive elements and post-traumatic stress disorder: a case-control study of US military service members. Epigenomics 2012; 4: 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu ZZ, Hou L, Bollati V, Tarantini L, Marinelli B, Cantone L et al. Predictors of global methylation levels in blood DNA of healthy subjects: a combined analysis. Int J Epidemiol 2012; 41: 126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang FF, Cardarelli R, Carroll J, Fulda KG, Kaur M, Gonzalez K et al. Significant differences in global genomic DNA methylation by gender and race/ethnicity in peripheral blood. Epigenetics 2011; 6: 623–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KY, Kim DS, Lee SK, Lee IK, Kang JH, Chang YS et al. Association of low-dose exposure to persistent organic pollutants with global DNA hypomethylation in healthy Koreans. Environ Health Perspect 2010; 118: 370–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiung DT, Marsit CJ, Houseman EA, Eddy K, Furniss CS, McClean MD et al. Global DNA methylation level in whole blood as a biomarker in head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 2007; 16: 108–114. [DOI] [PubMed] [Google Scholar]
- El-Maarri O, Walier M, Behne F, van Uum J, Singer H, Diaz-Lacava A et al. Methylation at global LINE-1 repeats in human blood are affected by gender but not by age or natural hormone cycles. PLoS One 2011; 6: e16252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Maarri O, Becker T, Junen J, Manzoor SS, Diaz-Lacava A, Schwaab R et al. Gender specific differences in levels of DNA methylation at selected loci from human total blood: a tendency toward higher methylation levels in males. Hum Genet 2007; 122: 505–514. [DOI] [PubMed] [Google Scholar]
- Chalitchagorn K, Shuangshoti S, Hourpai N, Kongruttanachok N, Tangkijvanich P, Thong-ngam D et al. Distinctive pattern of LINE-1 methylation level in normal tissues and the association with carcinogenesis. Oncogene 2004; 23: 8841–8846. [DOI] [PubMed] [Google Scholar]
- VanVoorhis CRW, Morgan BL. Understanding power and rules of thumb for determining sample sizes. Tutor Quant Methods Psychol 2007; 3: 43–50. [Google Scholar]
- Cohen S, Janicki-Deverts D, Miller GE. Psychological stress and disease. JAMA 2007; 298: 1685–1687. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Stellar E. Stress and the individual: mechanisms leading to disease. Arch Intern Med 1993; 153: 2093–2101. [PubMed] [Google Scholar]
- Segerstrom SC, Miller GE. Psychological stress and the human immune system: a meta-analytic study of 30 years of inquiry. Psychol Bull 2004; 130: 601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke TC, Black LI, Stussman BJ, Barnes PM, Nahin RL. Trends in the use of complementary health approaches among adults: United States, 2002–2012. National Health Statistics Report 2015. [PMC free article] [PubMed]
- Saatcioglu F. Regulation of gene expression by yoga, meditation and related practices: a review of recent studies. Asian J Psychiatr 2013; 6: 74–77. [DOI] [PubMed] [Google Scholar]
- DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol 1988; 54: 486–495. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, McGuire L, Robles TF, Glaser R. Emotions, morbidity, and mortality: new perspectives from psychoneuroimmunology. Annu Rev Psychol 2002; 53: 83–107. [DOI] [PubMed] [Google Scholar]
- Terry MB, Delgado-Cruzata L, Vin-Raviv N, Wu HC, Santella RM. DNA methylation in white blood cells: association with risk factors in epidemiologic studies. Epigenetics 2011; 6: 828–837. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.