Abstract
Bcr-Abl, activated in chronic myelogenous leukemias, is a potent cell death inhibitor. Previous reports have shown that Bcr-Abl prevents apoptosis through inhibition of mitochondrial cytochrome c release. We report here that Bcr-Abl also inhibits caspase activation after the release of cytochrome c. Bcr-Abl inhibited caspase activation by cytochrome c added to cell-free lysates and prevented apoptosis when cytochrome c was microinjected into intact cells. Bcr-Abl acted posttranslationally to prevent the cytochrome c-induced binding of Apaf-1 to procaspase 9. Although Bcr-Abl prevented interaction of endogenous Apaf-1 with the recombinant prodomain of caspase 9, it did not affect the association of endogenous caspase 9 with the isolated Apaf-1 caspase recruitment domain (CARD) or Apaf-1 lacking WD-40 repeats. These data suggest that Apaf-1 recruitment of caspase 9 is faulty in the presence of Bcr-Abl and that cytochrome c/dATP-induced exposure of the Apaf-1 CARD is likely defective. These data provide a novel locus of Bcr-Abl antiapoptotic action and suggest a distinct mechanism of apoptosomal inhibition.
Chronic myelogenous leukemia (CML) is a human malignancy marked by the presence of a distinct cytogenetic abnormality that results from a translocation between chromosomes 9 and 22, known as the Philadelphia chromosome (17). This translocation causes aberrant expression of Bcr-Abl, a constitutively active tyrosine kinase that has been directly linked to the pathogenesis of CML. Moreover, Bcr-Abl expression is sufficient to cause malignant transformation of hematopoietic cell lines (15). Bcr-Abl is thought to promote malignant transformation by altering cellular adhesion properties, stimulating mitogenic signaling pathways, and inhibiting programmed cell death (apoptosis) (5, 17, 35). The potent ability of Bcr-Abl to inhibit cell death limits the efficacy of apoptosis-inducing chemotherapeutics in the treatment of CML (4). Although STI-571, a specific inhibitor of Bcr-Abl, has shown remarkable success in the treatment of CML (45), the prevalence of STI-571-resistant leukemias has been steadily increasing. This resistance arises most often from mutations in the kinase domain of Bcr-Abl that render it insensitive to STI-571 (25) or from overexpression of the Bcr-Abl protein (31). Therefore, an understanding of the mechanisms used by Bcr-Abl to inhibit apoptotic signaling pathways is crucial to the development of alternative pharmacologic agents for the chemotherapeutic treatment of CML.
In response to cellular stress such as DNA damage induced by chemotherapeutic drugs, the cell's mitochondria are triggered to release cytochrome c (a component of the electron transport chain) into the cytosol. Once released, cytochrome c plays a critical role in the formation of a proteolytic cell death machine known as the apoptosome. The formation of the apoptosome results in the activation of a group of zymogenic cysteine proteases (caspases), which carry out the cell death program (23, 38, 57). Cytosolic cytochrome c initiates apoptosome formation by binding to the adaptor protein Apaf-1 and promoting its oligomerization into a higher-ordered structure (61). Oligomerization of Apaf-1 then allows binding of the initiator caspase 9, which results in dimerization-induced self-activation (55). Once activated, caspase 9 can cleave and activate effector caspases 3 and 7, which subsequently cleave a number of cellular substrates. This results in orderly dismantling of the cell and the hallmark features of apoptosis (60). The release of cytochrome c from the mitochondria is tightly regulated by Bcl-2 proteins, a family comprising both proapoptotic (e.g., Bax and Bak) and antiapoptotic (e.g., Bcl-2 and Bcl-XL) family members (16). These proteins act as mitochondrial gatekeepers and regulate apoptosis by governing the release of cytochrome c. Proapoptotic members such as Bak and Bax promote mitochondrial cytochrome c release, while the antiapoptotic Bcl-2 and Bcl-XL proteins maintain the integrity of the mitochondria to prevent the release of cytochrome c.
Alterations of apoptotic signaling pathways at a number of loci allow malignant cells to evade cell death, a phenomenon thought, in many cases, to be critical for tumor development (24). Although regulation of caspase activation upstream of cytochrome c release has been subject to intense scrutiny, the regulation of apoptosis downstream of mitochondrial cytochrome c release is only beginning to be understood. One mode of caspase regulation post cytochrome c release involves direct binding and inhibition of active caspases by the IAP (inhibitor of apoptosis) family of proteins (20, 47). Kinase signaling pathways have also been shown to impinge upon the proper functioning of the apoptosome. For example, both Akt and ERK, two kinases commonly active in cancer cells, can phosphorylate caspase 9 and subsequently inhibit its enzymatic activity (1, 12, 56). Furthermore, several additional proteins have been identified which can inhibit apoptosis by binding to either Apaf-1 or caspase 9 (e.g., Hsp70 and Aven) to prevent proper functioning of the apoptosome (6-8, 13, 46, 49).
Prior to cytochrome c release, Bcr-Abl can inhibit apoptosis through regulation of Bcl-2 family members (3). Specifically, Bcr-Abl increases expression of antiapoptotic Bcl-2 family members such as Bcl-2 and Bcl-XL through activation of the transcription factor STAT5 (3, 27, 48, 50). Additionally, Bcr-Abl has also been shown to prevent mitochondrial cytochrome c release through a posttranslational mechanism by signaling through the phosphatidylinositol 3-kinase (PI3K)/Akt pathway to phosphorylate and inhibit Bad (26, 36, 52). However, Bcr-Abl has recently been reported to be a more effective inhibitor of apoptosis than either Bcl-2 or Bcl-XL. As the Bcl-2 and Bcl-xL proteins can potently suppress mitochondrial cytochrome c release, these data suggested that Bcr-Abl might act at additional sites, perhaps downstream of the mitochondria (9). We report here that Bcr-Abl can act through posttranslational signaling mechanisms to prevent apoptosis downstream of mitochondrial cytochrome c release. Our data indicate that Bcr-Abl protection from cytochrome c differs from that reported for other apoptosome-inhibiting kinases (ERK and Akt) in that caspase 9 phosphorylation does not seem to underlie the protection. Rather, despite unperturbed binding to cytochrome c, Apaf-1 from Bcr-Abl-expressing cells appears to be defective in the ability to recruit the caspase 9 prodomain. Collectively, these results demonstrate a novel role for Bcr-Abl in apoptotic signaling and present a potential target for the development of apoptosis-inducing chemotherapeutics.
MATERIALS AND METHODS
Preparation of Xenopus egg extracts.
To induce egg laying, mature female frogs were injected with 100 U of pregnant mare serum gonadotropin (Calbiochem, La Jolla, Calif.), followed by injection (3 to 10 days later) with human chorionic gonadotropin (Sigma, St. Louis, Mo.). Twenty to 24 h after human chorionic gonadotropin injection, eggs were harvested for extract production. Jelly coats were removed from the eggs by incubation with 2% cysteine (pH 8.0), after which eggs were washed three times in modified Ringer solution (100 mM NaCl, 2 mM KCl, 1 mM MgSO4, 2.5 mM CaCl2, 0.5 mM HEPES [pH 7.8], 0.08 mM EDTA) and then washed in ELB (250 mM sucrose, 2.5 mM MgCl2, 50 mM KCl, 10 mM HEPES [pH 7.7]). Eggs were packed by low-speed centrifugation at 400 × g and then supplemented with aprotinin and leupeptin (final concentration, 5 μg/ml), cytochalasin B (final concentration, 5 μg/ml), and cycloheximide (50 μg/ml), after which eggs were lysed by centrifugation at 10,000 × g for 15 min. The resulting crude interphase egg extract was used to assess the spontaneous apoptosis program or further fractionated into cytosolic and membrane components. To separate mitochondrial and cytosolic components, crude interphase extract was centrifuged for 45 min at 55,000 rpm (250,000 × g) with a Beckman TLS-55 rotor (Beckman Instruments, Fullerton, Calif.). The cytosolic fraction was removed and recentrifuged at 55,000 rpm for an additional 25 min, aliquoted, frozen in liquid nitrogen, and then stored at −80°C for future use.
Cell lines and cell culture.
Vector control and Bcr-Abl-expressing 32D cells (a gift from Ann Marie Pendergast, Duke University) were maintained in RPMI medium supplemented with 10% fetal bovine serum (FBS) and 10% WEHI 3-conditioned medium. K562 and HL60 cells were maintained in Iscove's modified Dulbecco's medium supplemented with 10% FBS. Human embryonic kidney 293T cells and Rat-1 fibroblasts were maintained in high-glucose Dulbecco modified Eagle medium (DMEM) supplemented with 10% FBS. For the generation of stable green fluorescent protein (GFP)-expressing or GFP- and Bcr-Abl-expressing Rat-1 fibroblast lines, Rat-1 cells were infected with retroviruses produced from the bicistronic retroviral vectors GFP-MIGR1 and p185 Bcr-Abl MIGR1 (a gift from Ann Marie Pendergast) as previously described (39). Briefly, 293T cells were cotransfected with PSVΨ2 and MIGR1 vectors with Superfect transfection reagent (QIAGEN) in accordance with the manufacturer's instructions. Twenty-four hours after transfection, 10 mM sodium butyrate was added to the cell medium to enhance the virus yield. Two days posttransfection, Bcr-Abl-containing and control GFP-containing retroviral supernatants were collected. Viral supernatants were then incubated with 5.0 × 105 Rat-1 fibroblasts in high-glucose DMEM supplemented with 10% FBS and 4 μg of Polybrene per ml in a volume of 10 ml for 6 h. The cells were then washed with phosphate-buffered saline (PBS) and replenished with growth medium. Two days after infection, cells were sorted for GFP expression. Cells were periodically resorted to maintain >90% GFP-positive cells.
Preparation of cell extracts.
Cell extracts were prepared from 32D, K562, HL60, or Rat-1 cells containing control GFP or Bcr-Abl. Briefly, cells were harvested, washed once with ice-cold PBS, and pelleted. Cell pellets were then resuspended in twice the pellet volume of hypotonic lysis buffer (20 mM HEPES [pH 7.4], 10 mM KCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 1 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 5 μg of leupeptin per ml, 5 μg of aprotinin per ml, 1 mM NaVO4, 1 μM okadaic acid [Alexis Biochemicals]) and rocked at 4°C for 20 min. The cells were then lysed by passage through a 27-gauge needle 10 times and subsequently centrifuged at 14,000 rpm (Eppendorf 5415C) for 30 min at 4°C. Alternatively, for some experiments, cells were collected, washed in ice-cold PBS, pelleted, and then resuspended in an equal volume of ice-cold cell extraction buffer [50 mM piperazine-N,N′-bis(2-ethanesulfonic acid) (PIPES; pH 7.4), 50 mM KCl, 5 mM EGTA, 2 mM MgCl2, 1 mM DTT, 1 mM PMSF, 5 μg of leupeptin per ml, 5 μg of aprotinin per ml, 1 mM NaVO4, 1 μM okadaic acid]. Cells were lysed by freeze-thawing three times (11) and centrifuged for 30 min at 14,000 rpm (Eppendorf 5415C) at 4°C. The supernatants were collected, assayed for protein content with the Bradford assay (Bio-Rad), and either used immediately or snap-frozen in liquid nitrogen and stored in aliquots at −80°C.
Production of purified Bcr-Abl proteins.
Wild-type (WT) and kinase-dead (K671R) p185 Bcr-Abl constructs in the MIGR1 vector were kindly provided by Ann Marie Pendergast. The p185 inserts were cut out of the MIGR1 vector with EcoRI and ligated into the pFastBac plasmid vector (Gibco). Recombinant baculoviruses were produced in accordance with the Gibco Bac-to-bac protocol. Sf9 cells were infected with Bcr-Abl (WT) or (K671R) baculovirus for 54 h, and recombinant Bcr-Abl proteins were purified by a two-step purification strategy as previously described (40).
Measurement of in vitro caspase activity.
To activate endogenous caspases in vitro, egg extract that had been incubated with WT or K671R Bcr-Abl (1 to 2 μg/50 μl of extract) or cell lysates (equivalent of 250 to 300 μg of protein) were incubated with recombinant horse heart cytochrome c (0.6 to 10 ng/μl; Sigma) and/or 1 mM dATP at 37°C for various times. For some experiments, 2 μl of in vitro-translated, [35S]methionine-labeled human procaspase 9 or catalytically inactive human procaspase 3 (a gift from Yigong Shi, Princeton University) was added. Protein aliquots (30 to 50 μg) were removed at various times for assessment of caspase processing by immunoblot analysis, sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and autoradiography to visualize cleavage of radiolabeled caspases or measurement of DEVDase activity as follows. Three microliters of egg extract or 5 μl of mammalian cell lysate was incubated with 10 μl of the colorimetric substrate Ac-DEVD-pNA (Biomol) in assay buffer {50 mM HEPES (pH 7.7), 100 mM NaCl, 1 mM EDTA, 10% glycerol, 0.1% 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS), 10 mM DTT } for 1 h at 37°C. The reaction was read at 405 nm with a Labsystems Multiscan Plus plate reader (Fisher Scientific, Pittsburgh, Pa.).
Assessment of Abl immunoprecipitates.
Anti-Abl antibody (BD Biosciences Pharmingen) was used to immunoprecipitate Bcr-Abl from Bcr-Abl-expressing Rat-1 fibroblasts. Briefly, 1 mg of cell extract was precleared with protein G beads for 15 min at 4°C. The lysates were then incubated with 2 μg of Abl antibody and rocked at 4°C for 2 h, after which protein G beads were added, The lysates were subsequently rocked for 1 h at 4°C, after which the beads were washed three times in cell extraction buffer (described above). The immunoprecipitates were then incubated with control GFP-expressing Rat-1 cell lysates in the presence of 20 μM unlabeled ATP for 30 min at 30°C. The cell lysates were then removed from the immunoprecipitates and assessed for cytochrome c-induced caspase activity as described above.
Western blot analysis.
Cytosolic egg extract (80 μg) or Rat-1 cell lysates (100 μg) were subjected to SDS-PAGE analysis, and proteins were transferred to PVDF membranes (Immobilon P). Membranes were subsequently immunoblotted with antibodies directed against phospho-ERK (Cell Signaling Technologies), rat Apaf-1 (clone 131FF; Alexis Biochemicals), and rat caspase 9 (Cell Signaling Technologies). For some experiments, a polyclonal antibody directed against caspase 9 (Neomarkers) was used for immunoblot analysis.
Apaf-1 and caspase 9 binding assays.
The caspase 9 prodomain, Apaf-1 caspase recruitment domain (CARD), and Apaf-1 (1-543) cDNAs were generated from full-length human caspase 9 and full-length human Apaf-1 (gift from Xiaodong Wang, University of Texas Southwestern) and subsequently cloned into the pGEX-KG expression plasmid. Recombinant GST fusion proteins were produced in BL21 bacteria by inducing protein expression with 0.2 mM isopropyl-β-d-thiogalactopyranoside (IPTG) at 18°C overnight. GST fusion proteins were purified with glutathione beads by a standard protocol (21). To assess the ability of Apaf-1 and caspase 9 to bind to cytochrome c, cell lysates from Rat-1 control GFP- or Bcr-Abl-expressing cells (500 μg of total protein in a 100-μl total volume) were incubated with cytochrome c-Sepharose (Sigma) in the presence of 1 mM dATP for 30 min at room temperature and then incubated for an additional 60 min at 4°C. The cytochrome c-Sepharose was subsequently washed three times with cell extraction buffer containing 300 mM NaCl, and bound proteins were then assessed via SDS-PAGE and Western blot analysis. To assess the ability of Apaf-1 or caspase 9 to bind to the Apaf-1 CARD, Apaf-1 (1-543), or the caspase 9 prodomain, the GST fusion proteins were incubated with cell lysates from control GFP- or Bcr-Abl-expressing Rat-1 cells in the presence of 1 mM dATP for 30 min at room temperature. The GST fusion proteins were then recaptured on glutathione beads. The bead-bound protein complexes were washed three times with cell extraction buffer containing 300 mM NaCl and then subjected to SDS-PAGE and immunoblot analysis.
In vivo labeling of Rat-1 fibroblasts.
control GFP- and Bcr-Abl-expressing Rat-1 fibroblasts were plated at a density of 5.0 × 105 per 100-mm-diameter dish. Twenty-four hours later, the growth medium was replaced with phosphate-free, high-glucose DMEM supplemented with 10% dialyzed FBS and the cells were returned to the incubator for 2 h. Five millicuries of 32Pi was then added, and the cells were incubated for an additional 5 h. The cells were then washed three times with warm, phosphate-free DMEM and subsequently lysed in 1 ml of radioimmunoprecipitation assay buffer (50 mM Tris-HCl [pH 7.4]; 1% NP-40; 0.25% Na deoxycholate; 150 mM NaCl; 1 mM EDTA; 1 mM PMSF; 1 μg each of aprotinin, leupeptin, and pepstatin per ml; 1 mM Na3VO4; 1 mM NaF) containing 30 mM Na pyrophosphate. The cell lysates were then incubated on ice for 15 min and then centrifuged at 14,000 rpm (Eppendorf 5415C) for 20 min at 4°C. Total protein was assessed with the Bradford assay (Bio-Rad). Because there are no antibodies available that immunoprecipitate rat caspase 9, endogenous rat caspase 9 was captured from the radiolabeled cell lysates with the GST fusion protein containing the CARD of Apaf-1, as described above. The bound proteins were then subjected to SDS-PAGE and autoradiography. In parallel, unlabeled control GFP- and Bcr-Abl-expressing Rat-1 cells were subjected to the same cell lysis protocol and immunoblot analysis was performed with the rat-specific caspase 9 antibody described above.
Kinase assays and phosphotyrosine assessment.
Human procaspase 9 was immunoprecipitated from human embryonic kidney 293T cells with polyclonal caspase 9 antiserum (2 μg of antibody per mg of total protein; BD Biosciences Pharmingen). The caspase 9 or immunoglobulin G control precipitates were then used as substrates in an in vitro kinase assay with control GFP- or Bcr-Abl-expressing Rat-1 cell lysates. Briefly, 500 μg of control GFP- or Bcr-Abl-expressing Rat-1 cell lysate (5 mg/ml) was supplemented with 20 μM unlabeled ATP, 15 mM MgCl2, and 1 μCi of [γ-32P]ATP and then incubated for 30 min at 30°C. The precipitates were washed three times with cell extraction buffer (described above) containing 300 mM NaCl and then subjected to SDS-PAGE and autoradiography. Recombinant ERK (Upstate Biotechnology) was incubated in GFP-expressing control cell lysate to serve as a positive control for caspase 9 phosphorylation (1). The Apaf-1 (1-543)-GST fusion protein also served as a substrate for in vitro kinase assays with control GFP- and Bcr-Abl-expressing Rat-1 cell lysates. For these experiments, GST-Apaf-1 (1-543), GST-Crk, or GST alone bound to glutathione beads was incubated in control GFP- or Bcr-Abl-expressing Rat-1 lysate (5 mg/ml) supplemented with 20 μM unlabeled ATP, 15 mM MgCl2, and 1 μCi of [γ-32P]ATP for 30 min at 30°C. The beads were then washed three times with cell extraction buffer containing 300 mM NaCl and subjected to SDS-PAGE and autoradiography. To assess whether Bcr-Abl phosphorylates Apaf-1 directly, endogenous Apaf-1 was immunoprecipitated from control GFP- or Rat-1-expressing cell lysates with a rat-specific Apaf-1 antibody (clone 131FF; Alexxa). Briefly, 1 μg of Apaf-1 antibody was precoupled to 30 μl of protein G-Sepharose (Sigma) in PBS for 1 h by rocking at 4°C. Control GFP- or Bcr-Abl-expressing cell lysates (1 mg) were then incubated with the bead-bound antibody for 2 h at 4°C. The immunoprecipitates were subsequently washed five times with cell extraction buffer containing 300 mM NaCl and then immunoblotted with phosphotyrosine (Upstate Biotechnologies) and rat Apaf-1 antibodies (clone 131FF; Alexis Biochemicals).
Microinjection studies.
Rat-1 fibroblasts were microinjected with soluble cytochrome c as previously described (42). The concentration of bovine heart cytochrome c (Sigma) injected was 1 μg/μl. In order to mark the microinjected cells, the microinjection solution (100 mM KCl, 10 mM KPi [pH 7.4]) contained 4 mg of rhodamine dextran per ml. Cell viability was determined by counting the rhodamine-positive cells that had intact, phase-bright cell bodies immediately after injection and at various times postinjection. Cell survival is expressed as a percentage of the original number of cells microinjected.
RESULTS
Bcr-Abl kinase activity prevents cytochrome c-induced caspase activity in cytosolic Xenopus egg extracts.
Extracts prepared from Xenopus eggs have proven to be a powerful tool for the faithful in vitro reconstitution of many of the biochemical events of apoptosis (37). When egg extracts are incubated at room temperature for an extended period of time, mitochondrial cytochrome c is released, leading to caspase activation, cleavage of cellular substrates, and nuclear fragmentation. As the egg extracts used for apoptosis assays are transcriptionally and translationally inactive, we used this system to determine if Bcr-Abl-mediated inhibition of apoptosis could occur through an entirely posttranslational mechanism, independently of altered gene expression. For this purpose, crude egg extract was incubated with WT Bcr-Abl, enzymatically inactive (K671R) Bcr-Abl, or buffer alone and caspase 3 activity was monitored over time with a synthetic peptide substrate (DEVD-pNA). Interestingly, we found that treatment of extracts with WT recombinant Bcr-Abl completely inhibited the spontaneous apoptotic program in the egg extract (Fig. 1A). We did observe a slight delay in apoptosis with the K671R Bcr-Abl protein, suggesting that some kinase-independent actions of Bcr-Abl may contribute to its potent antiapoptotic effects.
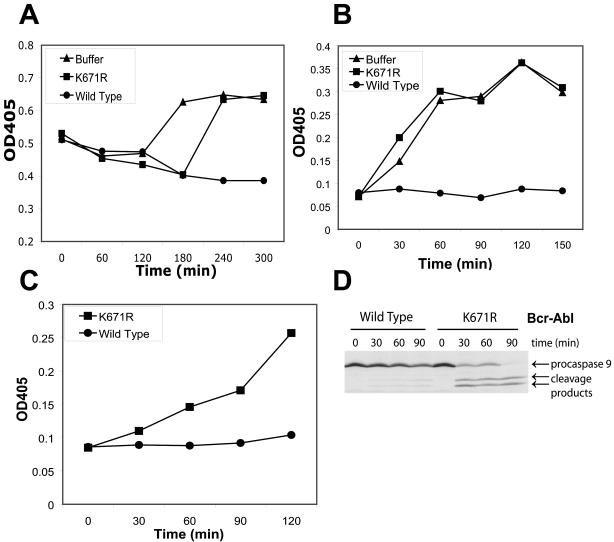
FIG. 1.
Post-cytochrome c protection by Bcr-Abl in Xenopus egg extracts. (A) Crude egg extract was incubated with buffer alone, WT Bcr-Abl, or kinase-dead (K671R) Bcr-Abl, and caspase 3 activity was measured at various time points. Caspase 3 activity was measured spectrophotometrically via cleavage of the colorimetric caspase substrate DEVD-pNA. (B) Cytosolic egg extract was pretreated for 30 min with WT Bcr-Abl, K671R Bcr-Abl, or buffer alone and then incubated with 0.6 ng of cytochrome c per μl. Caspase 3 activity was measured by cleavage of DEVD-pNA over time. (C) Cytosolic egg extract was pretreated for 30 min with WT or K671R mutant Bcr-Abl and then incubated with 0.7 ng of cytochrome c per μl for various times. Caspase 9 activity was measured spectrophotometrically by cleavage of the colorimetric caspase 9 substrate LEHD-pNA. (D) In vitro-translated, 35S-labeled procaspase 9 and soluble cytochrome c (0.6 ng/μl) were added to cytosolic egg extracts that were pretreated with WT or K671R mutant Bcr-Abl. Cleavage of procaspase 9 was assessed via autoradiography. In panels A to C, the graphs are representative of at least three independent experiments. OD405, absorbance reading at 405 nm.
As the egg apoptotic program is entirely dependent upon mitochondrial cytochrome c release, a process previously reported to be inhibited by Bcr-Abl, these results were perhaps not surprising. However, when we prepared isolated egg cytosol devoid of mitochondria, we found that WT Bcr-Abl, but not its inactive counterpart, could also prevent the robust caspase activation resulting from addition of purified exogenous cytochrome c (which, as in mammalian cells, nucleates apoptosome formation (30, 56) (Fig. 1B). Moreover, treatment of extracts with WT but not kinase-inactive Bcr-Abl was able to prevent the cleavage of in vitro-translated, 35S-labeled procaspase 9, as well as the development of caspase 9 activity as measured by LEHDase activity (Fig. 1C and D). Although cleavage is not required for caspase 9 activity, its cleavage can serve as an indicator that it has been activated. Thus, taken together, our results strongly suggest that Bcr-Abl can inhibit cytochrome c-induced apoptosome activation in the absence of de novo transcription or translation. Moreover, although K671R Bcr-Abl had a minor inhibitory effect on the spontaneous apoptotic program in crude egg extracts (containing mitochondria; Fig. 1A), this protein was unable to prevent cytochrome c-induced apoptosome activation, suggesting that any kinase-independent effects of Bcr-Abl are probably exerted upstream of the mitochondria and that the post-cytochrome c effects of Bcr-Abl are kinase dependent.
Bcr-Abl expression prevents cytochrome c-induced caspase activity in cell extracts.
Given that Bcr-Abl was able to inhibit apoptosome activity and caspase activation in Xenopus egg extracts, we wished to extend these results to mammalian cells. In mammalian cell extracts, addition of dATP and/or cytochrome c has been shown to initiate formation of the apoptosome, caspase activation, and caspase-mediated cleavage of substrates (presumably, dATP allows apoptosome formation nucleated by cytochrome c that has leaked into the cytosol during extract preparation) (34). Hence, we assessed cytochrome c-induced caspase activity in lysates prepared from the human acute myelocytic leukemia cell line HL60, which does not express Bcr-Abl, and the K562 cell line, which is derived from a patient with CML and thus has abundant levels of Bcr-Abl protein. Addition of cytochrome c and dATP to HL60 cell lysates provoked marked caspase 3 activity, while lysates from the K562 cells were notably resistant to cytochrome c, failing to exhibit substantial levels of cytochrome c-induced caspase 3 activity (Fig. 2A). These data suggested that Bcr-Abl could exert its effects downstream of cytochrome c release in human CML cells. However, these cell lines do not provide an ideal system for studying post-cytochrome c protection from apoptosis in that they are not isogenic and thus do not allow assessment of Bcr-Abl-specific effects. Therefore, we obtained mouse 32D lymphoblasts stably expressing either GFP alone or GFP and Bcr-Abl from a bicistronic construct. We also generated Rat-1 fibroblasts stably expressing either GFP alone or both GFP and Bcr-Abl. Cell lysates prepared from these cells were then tested for the ability to develop caspase activity upon addition of cytochrome c and dATP. Consistent with the results obtained with K562 cells, we found that there was a marked inhibition of cytochrome c-induced caspase activation in extracts prepared from either mouse or rat cells expressing Bcr-Abl (Fig. 2B, C, and D). These data corroborated our observations on Xenopus egg extracts demonstrating that Bcr-Abl can protect from cytochrome c-induced caspase activation.
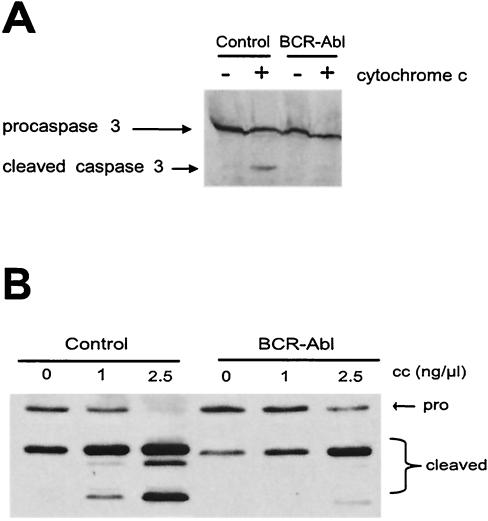
FIG. 2.
Post-cytochrome c protection by Bcr-Abl in mammalian cell lysates. Cell lysates were prepared from human (A), mouse (B), or rat (C) cells as described in Materials and Methods. The lysates were incubated with 5 ng of cytochrome c per μl and 1 mM dATP, and caspase 3 activity was then assayed by measuring the cleavage of DEVD-pNA over time. (D) DEVDase activity was measured in the control GFP (C)- and Bcr-Abl (B)-expressing Rat-1 lysates following the addition of 1 mM dATP and various doses of soluble cytochrome c. The results shown for panels A to D are representative of at least three independent experiments. OD405, absorbance reading at 405 nm.
The failure of cytochrome c to elicit DEVDase activity in the Bcr-Abl-expressing cell lysates implied that the effector caspase, caspase 3, could not be activated by cleavage in the presence of active Bcr-Abl. To address this issue, we supplemented the cell-free Rat-1 lysates with in vitro-translated, 35S-radiolabeled, catalytically inactive caspase 3 and then activated the lysates with cytochrome c and dATP. As shown in Fig. 3A, processing of radiolabeled, catalytically inactive caspase 3 (which yields only the caspase 9-induced cleavage product) was greatly dampened in lysates containing Bcr-Abl compared with that in lysates expressing GFP alone. Furthermore, we found that cleavage of endogenous rat caspase 9 was also largely inhibited in these lysates following cytochrome c addition (Fig. 3B). Note that all of the extracts tested were supplemented with dATP, most likely accounting for some processing in the absence of exogenous cytochrome c. Taken together, these results are very similar to those shown in Fig. 1 for Bcr-Abl-treated egg extracts, although the inhibition of caspase processing was not as complete, most likely owing to the lower level of Bcr-Abl expression in the Rat-1 cells. Although, as mentioned above, caspase 9 processing is not required for its enzymatic activity, the inhibition of cleavage did imply a dampening of both caspase 3 and caspase 9 activities in these extracts as both contribute to the cleavage of caspase 9. Therefore, these data pointed to some component of the apoptosome as the target of Bcr-Abl action.
FIG. 3.
Bcr-Abl prevents processing of caspase 3 and caspase 9. (A) In vitro-translated, 35S-radiolabeled, catalytically inactive procaspase 3 was added to control GFP- or Bcr-Abl-expressing Rat-1 lysates in the presence of soluble cytochrome c (1 ng/μl). The processing of caspase 3 was observed via autoradiography. (B) Cell lysates from control GFP- or Bcr-Abl-expressing Rat-1 fibroblasts were incubated with 1 mM dATP and various concentrations of soluble cytochrome c. The processing of endogenous caspase 9 was assayed by Western analysis. Note that the fragment derived from caspase 9-mediated caspase 9 cleavage (the middle of the three cleaved bands indicative that the apoptosome has been activated) is barely visible in the presence of Bcr-Abl. The small amount of (unsuppressed) residual caspase 9 activity in this experiment most likely led to caspase 3 activation, resulting in amplification of caspase 9 processing through caspase 3-mediated caspase 9 cleavage (the upper band of the triplet).
Posttranslational inhibition of apoptosome function by Bcr-Abl in mammalian cell lysates.
Bcr-Abl is known to regulate the transcription of multiple genes and to activate a number of signaling pathways (e.g., mitogen-activated protein kinase and PI3 kinase pathways) (14, 41, 52, 53). Because the cell extract experiments described above had been carried out with lysates prepared from cells stably expressing Bcr-Abl, it was possible that proteins up (or down)-regulated by Bcr-Abl in the intact cells were instrumental in promoting protection from apoptosis. To rule out this possibility, we introduced Abl immunoprecipitates from cells expressing GFP alone or Bcr-Abl into naive cell lysates prepared from Rat-1 cells. Lysates treated with Abl precipitates from Bcr-Abl-expressing cells, but not precipitates from control cells, were also protected from the action of cytochrome c. This suggested that Bcr-Abl-mediated modification of a cytosolic factor renders the lysate refractory to cytochrome c (Fig. 4A). These results confirm our Xenopus data presented above, demonstrating that Bcr-Abl can protect from cytochrome c-induced apoptosis without inducing changes in transcription-translation.
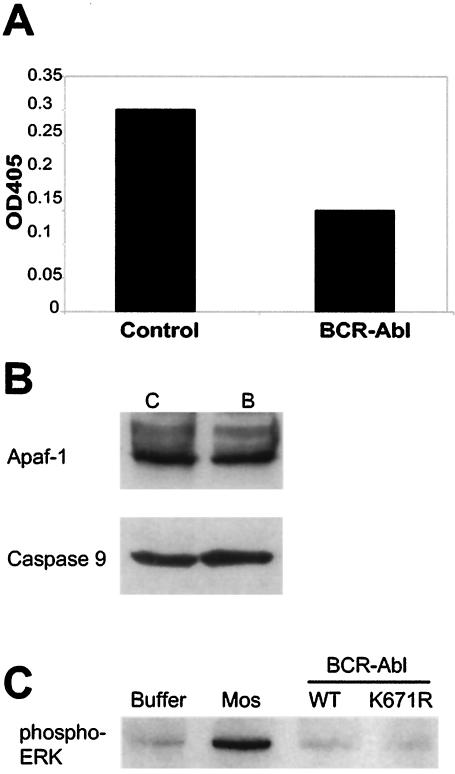
FIG. 4.
Bcr-Abl post-cytochrome c protection in cell lysates occurs by a posttranslational mechanism. (A) Control GFP-expressing cell lysates were preincubated with Abl immunoprecipitates from control GFP- or Bcr-Abl-expressing Rat-1 cells, after which caspase activity was assessed following the addition of 1 ng of soluble cytochrome c per μl. Caspase 3 activity was measured by assessing the cleavage of the colorimetric DEVD-pNA substrate. The results shown are representative of three independent experiments. (B) Control GFP (lane C)- and Bcr-Abl (lane B)-expressing Rat-1 lysates were immunoblotted for the Apaf-1 and caspase 9 proteins. (C) Cytosolic Xenopus egg extracts were pretreated with Mos, WT Bcr-Abl (WT), or kinase-dead Bcr-Abl (K671R). ERK activation was assessed by Western immunoblot analysis with an antibody directed against phospho-ERK. OD405, absorbance reading at 405 nm.
In some systems, regulation of apoptosis after the release of cytochrome c has been reported to be regulated by changes in the protein levels of the core apoptosomal proteins Apaf-1 and caspase 9 (29). Indeed, in some leukemic cell lines, Apaf-1 is underexpressed, potentially accounting for the observed protection from cytochrome c-induced caspase activation. To address this issue, we examined Apaf-1 and caspase 9 levels in both control GFP- and Bcr-Abl-expressing rat fibroblasts, which had been markedly different in their responsiveness to cytochrome c. We found that the expression of the Apaf-1 and caspase 9 proteins was equivalent in these cells (Fig. 4B), strongly suggesting that Bcr-Abl could confer protection from cytochrome c without altering the concentration of the core apoptosomal components.
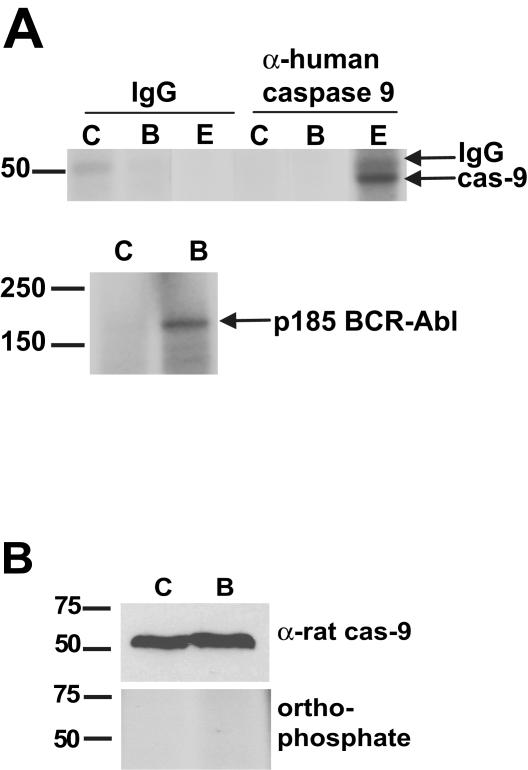
Bcr-Abl has been reported to activate ERK and Akt in some cell types, and both of these kinases have been shown to phosphorylate and inactivate caspase 9. However, we found no increase in ERK activation (as measured by immunoblotting against phospho-ERK) by Bcr-Abl in cytosolic egg extracts, where cytochrome c-induced caspase activity was inhibited, although activated ERK was readily detected in extracts treated with the mitogen-activated protein kinase kinase kinase Mos (Fig. 4C). As these extracts were devoid of membrane components, a requirement for PI3K-mediated Akt activation, it seems unlikely that Akt could mediate the post-cytochrome c protection by Bcr-Abl in the egg extract. Moreover, a strong inhibition of cytochrome c-induced caspase activity was observed in rat and mouse cells expressing Bcr-Abl, and caspase 9 from these species does not contain an Akt phosphorylation site (44). Furthermore, in the Rat-1 cells used in our cytochrome c protection experiments, Bcr-Abl expression did not enhance the activation of either ERK or Akt as assessed by the presence of phospho-ERK and phospho-Akt levels in Rat-1 cells (Z. T. Schafer, P. B. Deming, and S. Kornbluth, unpublished data). Finally, we looked to see if caspase 9 could be phosphorylated in lysates from Bcr-Abl-expressing cells. As shown in Fig. 5A, immunoprecipitated human procaspase 9 (containing the reported Akt phosphorylation site) was not radiolabeled after incubation in lysates with [γ-32P]ATP, either in the presence or in the absence of Bcr-Abl under conditions that allowed both autophosphorylation of Bcr-Abl and ERK-mediated phosphorylation of caspase 9. Moreover, there was no detectable phosphorylation of caspase 9 precipitated from Bcr-Abl-expressing Rat-1 cells metabolically labeled with orthophosphate (Fig. 5B). Taken together, these data suggest that ERK and Akt are unlikely to play a role in Bcr-Abl-mediated inhibition of apoptosis post cytochrome c release.
FIG. 5.
Bcr-Abl signaling does not induce phosphorylation of caspase 9. (A) Endogenous caspase 9 (cas-9) was immunoprecipitated from 293T cells, and in vitro kinase assays were performed with control GFP (lanes C)- or Bcr-Abl (lanes B)-expressing cell lysates supplemented with [γ-32P]ATP under conditions in which Bcr-Abl was autophosphorylated (bottom). Lysates were treated with recombinant active ERK (lanes E) as a control for caspase 9 phosphorylation. IgG, immunoglobulin G. (B) control GFP (lane C)- and Bcr-Abl (lane B)-expressing Rat-1 fibroblasts were radiolabeled with orthophosphate. Endogenous caspase 9 was captured with a GST fusion protein containing the CARD of Apaf-1. Caspase 9 bound to the Apaf-1 CARD was then subjected to SDS-PAGE and autoradiography or immunoblot analysis with an antibody directed against rat caspase 9. The values on the left are molecular size markers in kilodaltons.
Bcr-Abl inhibits cytochrome c-induced apoptosis in intact cells.
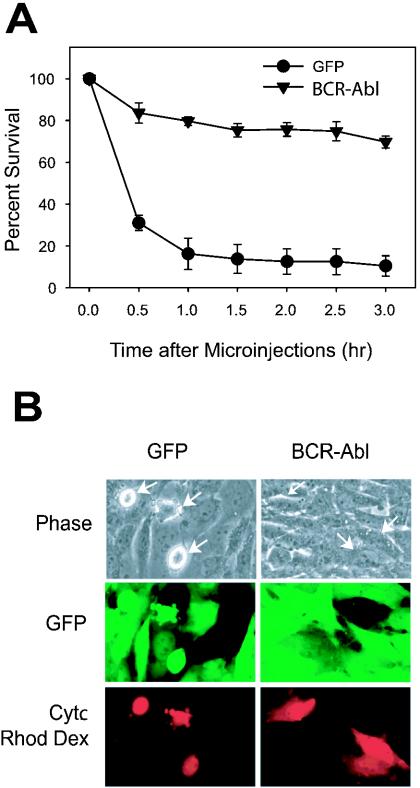
Although our accumulated data obtained with cell lysates indicated that Bcr-Abl could inhibit proper apoptosomal function in vitro, we wished to validate these findings by demonstrating that cytochrome c introduced directly into the milieu of an intact Bcr-Abl-positive cell cytoplasm would be effectively prevented from activating caspases. Fibroblast cell lines have been demonstrated to undergo rapid, caspase-dependent cell death following cytosolic microinjection of cytochrome c (10, 32), thus circumventing the mitochondria in an intact cellular setting. Thus, we microinjected Rat-1 fibroblasts expressing either GFP alone or Bcr-Abl with cytochrome c and examined these cells for apoptotic death. Cells expressing only GFP died rapidly after microinjection with 1 μg of cytochrome c per μl, while cells expressing Bcr-Abl were highly resistant to apoptosis (Fig. 6A and B). The effect was readily evident as microinjected cells expressing GFP alone began to bleb, round up, and lift off the plate while microinjected cells expressing Bcr-Abl remained intact and adherent. Indeed, within 1 h of microinjection, greater than 80% of injected control cells had died while less than 20% of the Bcr-Abl-expressing cells had done so. These data confirm our in vitro findings demonstrating that Bcr-Abl expression renders cells refractory to the apoptosis-inducing activity of cytoplasmic cytochrome c.
FIG. 6.
Bcr-Abl prevents apoptosis induced by microinjection of cytochrome c. (A) Rat-1 fibroblasts expressing either a GFP control or Bcr-Abl were microinjected with 1 μg of cytochrome c (Cytc) per μl and with rhodamine dextran (Rhod Dex; to visualize injected cells). Representative pictures are shown in panel B.
Bcr-Abl inhibits the interaction of caspase 9 with Apaf-1.
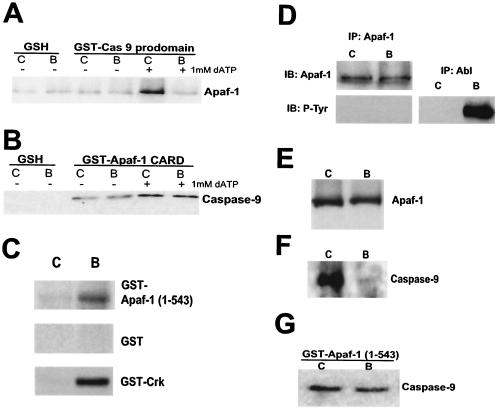
As our results (obtained with both lysates and intact cells) suggested a likely role for Bcr-Abl in regulating the apoptosome, we sought to determine if interactions between apoptosomal components occur normally in cells expressing Bcr-Abl. To address this question, we took advantage of the ability of the isolated prodomain of caspase 9, which contains the Apaf-1 binding determinants (the CARD), to retrieve fully nucleated apoptosomes from crude cell lysates (58). The soluble GST-tagged caspase 9 prodomain was added to lysates from control GFP- or Bcr-Abl-expressing cells, and apoptosomes were then precipitated with glutathione-Sepharose. These precipitates were resolved by SDS-PAGE and immunoblotted with anti-Apaf-1 antibody. It is important to note that the binding of Apaf-1 to the caspase 9 prodomain should occur only after Apaf-1 “activation” by the addition of dATP. Accordingly, in control cell lysates from GFP-expressing cells, Apaf-1 was bound to the caspase 9 prodomain only in samples supplemented with dATP (Fig. 7A). However, the ability of Apaf-1 to bind to the caspase 9 prodomain in activated lysates was severely compromised in the presence of Bcr-Abl (Fig. 7A). These data suggested that recruitment of the caspase 9 prodomain by Apaf-1 might be faulty in the presence of Bcr-Abl.
FIG. 7.
Bcr-Abl inhibits caspase 9 recruitment to the apoptosome. GST fusion proteins containing the prodomain of caspase 9 (A), the CARD of Apaf-1 (B), or Apaf-1 (1-543) (G) were incubated with control GFP (lanes C)- or Bcr-Abl (lanes B)-expressing lysates in the presence of 1 mM dATP. GST proteins were then rebound to glutathione beads (GSH), and the binding ability of Apaf-1 (A) or caspase 9 (B and G) was examined via immunoblot analysis. (C) GST-Apaf-1 (1-543), GST, or GST-Crk protein was incubated with [γ-32P]ATP in either control GFP (lane C)- or Bcr-Abl (lane B)-expressing cell lysates, and radiolabeling was assessed via autoradiography. (D) Apaf-1 was immunoprecipitated from either control GFP (lane C)- or Bcr-Abl (lane B)-expressing lysates, and precipitates were immunoblotted (IB) for Apaf-1 or phosphotyrosine (P-Tyr). Anti-Abl immunoprecipitates (IP) were also immunoblotted for P-Tyr as a positive control for the phosphotyrosine Western analysis. (E and F) Control GFP (lane C)- or Bcr-Abl (lane B)-expressing lysates were incubated with cytochrome c-Sepharose and 1 mM dATP. The binding ability of Apaf-1 (E) and caspase 9 (F) was then examined by immunoblot analysis.
One simple explanation for the above data is that the caspase 9 prodomain was modified in some way or bound to an inhibitory factor, thus blocking recruitment to the Apaf-1 CARD. If this were the case, endogenous caspase 9 would be expected to be defective in recruitment to the isolated Apaf-1 CARD. To test this, we generated the isolated CARD derived from Apaf-1 as a GST fusion protein and examined the ability of this recombinant protein to bind caspase 9 from cell lysates. Under normal circumstances, dATP would drive the oligomerization of full-length Apaf-1 in a fashion that exposes the CARD, making it available to bind caspase 9. By using the CARD alone, we circumvented this step, enabling us to observe recruitment of caspase 9 in the absence of dATP. Surprisingly, we found that endogenous caspase 9 bound to the exposed Apaf-1 CARD equivalently in lysates from control GFP- and Bcr-Abl-expressing cells (Fig. 7B). This suggests that there is no inherent defect in caspase 9 derived from Bcr-Abl-containing cell lysates that would prevent its recruitment to Apaf-1. These findings are consistent with the data presented above that demonstrate no detectable phosphorylation of caspase 9 in the presence of Bcr-Abl. However, these data raise the possibility that Apaf-1 function was altered in some manner (e.g., phosphorylation) that precluded its effective recruitment of caspase 9. In fact, the Apaf-1 (1-543) protein was well phosphorylated in the presence of Bcr-Abl (Fig. 7C). This phosphorylation did not appear to be mediated directly by Bcr-Abl as there was no evidence of tyrosine phosphorylation when full-length Apaf-1 was immunoprecipitated from Bcr-Abl-expressing Rat-1 cells (Fig. 7D) or when Apaf-1 (1-543) was incubated in the presence of Bcr-Abl (P. B. Deming, Z. T. Schafer, and S. Kornbluth, unpublished data). While Apaf-1 is not known to be regulated by phosphorylation, these data raise the intriguing possibility that post-cytochrome c protection from apoptosis by Bcr-Abl involves inhibitory phosphorylation of Apaf-1.
One potential explanation for apoptosome inhibition in the presence of Bcr-Abl might be a compromised ability of Apaf-1 to interact with cytochrome c. To address this possibility, we examined the binding of endogenous Apaf-1 to cytochrome c immobilized on Sepharose. As shown in Fig. 7E, binding of Apaf-1 by this resin was equivalent in the presence and absence of Bcr-Abl. Therefore, it appeared unlikely that Bcr-Abl-exposed Apaf-1 had a markedly lowered affinity for cytochrome c. However, taking advantage of the fact that immobilized cytochrome c could very efficiently bind Apaf-1 from the lysates, we looked to see if these beads could, in effect, nucleate Apaf-1/caspase 9 complex-containing apoptosomes. We suspected that this might be the case as the immobilized cytochrome c beads had been found to activate caspases effectively in Xenopus egg extracts and mammalian cell lysates (J. S. Tashker, P. B. Deming, and S. Kornbluth, unpublished data). In performing these binding experiments with lysates from control GFP- and Bcr-Abl-expressing cells, we found that Apaf-1/cytochrome c complexes were markedly defective in the ability to recruit caspase 9 in the presence of Bcr-Abl (Fig. 7F). These data are fully consistent with our previously mentioned results and suggest that Apaf-1, rather than caspase 9, might be targeted by Bcr-Abl to prevent caspase 9 binding. Interestingly, this defect was eliminated by removal of the C-terminal WD-40 repeats of Apaf-1 (Fig. 7G). Specifically, Apaf-1 lacking the WD-40 repeats [Apaf-1 (1-543)] was, like the isolated CARD, able to recruit caspase 9 equally well in the presence and absence of Bcr-Abl. Given these results, it is attractive to speculate that cytochrome c-induced exposure of the CARD is defective in Bcr-Abl-expressing cells (possibly because of the phosphorylation of Apaf-1), rendering full-length, but not truncated, Apaf-1 unable to recruit caspase 9.
DISCUSSION
The development of leukemias may result not only from inappropriate cell proliferation but also from a failure of cells to undergo timely cell death. In addition, strong inhibition of apoptotic cell death is widely believed to underlie the resistance of leukemic cells to conventional chemotherapeutics (35). Although the clinical use of the kinase inhibitor STI-571 has been of remarkable utility in the treatment of CML, the emergence of STI-571-resistant Bcr-Abl mutants makes the search for alternative chemotherapeutics imperative (22). It is well documented that Bcr-Abl can very potently inhibit apoptotic induction by conventional chemotherapeutics (51). This resistance has been attributed to a block in the promotion of mitochondrial cytochrome c release, through both transcriptional and posttranslational regulation of Bcl-2 family members (3, 26, 27, 36, 48, 50, 52). We report here that Bcr-Abl also has the ability to robustly inhibit the apoptotic signaling cascade downstream of the mitochondria, at the level of the apoptosome. This novel function for Bcr-Abl occurs through a posttranslational mechanism that inhibits the cytochrome c-triggered recruitment of caspase 9 by Apaf-1. These data reveal a novel effect of Bcr-Abl that could potentially be exploited to increase the efficacy of CML-directed chemotherapies.
Post-cytochrome c protection from apoptosis.
The existence of cellular mechanisms to prevent caspase activation by cytoplasmic cytochrome c has only recently been appreciated. In several instances, resistance to cytochrome c has been shown to reflect a direct down-regulation of apoptosomal components. For example, a strong correlation between chemoresistance and decreased Apaf-1 protein levels has been reported for some leukemic cells, as well as bladder and skin cancers (28, 29, 33, 54). Moreover, downregulation of caspase 3 protein has been correlated with inhibition of cell death in some breast cancer cell lines and tissues (19). In addition to pathological blocks to cytochrome c-induced caspase activation, neuronal cells with limited proliferative capacity have also been shown to exhibit resistance to apoptosis following mitochondrial cytochrome c release (18). Interestingly, this feature of neuronal development can be recapitulated in PC-12 cells as they differentiate into neurons (59).
Experiments reported here, as well as in other published reports, suggest that posttranslational mechanisms to abort apoptosome function can also contribute significantly to cytochrome c resistance (or, more generally, chemoresistance). Although some leukemic cells reportedly have less Apaf-1 than their normal counterparts do, we did not observe this in our cells expressing Bcr-Abl. Rather, simple addition of Bcr-Abl to either mammalian or Xenopus cytoplasm was sufficient to confer cytochrome c resistance, highlighting the existence of purely posttranslational mechanisms of apoptosomal inhibition. In this regard, it is interesting that ovarian cancer cells have also been reported to have an improperly formed apoptosome, (33), although the mechanism for this is not known.
Inhibition of the apoptosome by Bcr-Abl.
With regard to posttranslational mechanisms for inhibiting the apoptosome, both Akt and activated ERK kinases have been reported to act through phosphorylation of caspase 9 (1, 12, 56). Despite reports that Bcr-Abl can activate Akt and ERK, we note that Bcr-Abl was clearly able to protect from apoptosis in the absence of any detectable ERK activation. Furthermore, the reported Akt phosphorylation site on human caspase 9 is not conserved in rodents (i.e., Rat-1 cells) and recombinant human caspase 9 mutated at the reported Akt site failed to override the Bcr-Abl-induced protection from cytochrome c in Xenopus egg extracts (J. S. Tashker and S. Kornbluth, unpublished). Finally, it is also of interest that previously published data have indicated that activation of PI3K (which leads to activation of Akt) does not detectably contribute to the antiapoptotic action of Bcr-Abl (2). For neither Akt nor ERK has it been determined whether caspase 9 phosphorylation results in a defect in proper apoptosomal assembly rather than direct inhibition of caspase 9 enzymatic activity.
Phosphorylation of caspase 9 is unlikely to be relevant to this Bcr-Abl-mediated action in that we were unable to detect in vitro phosphorylation of caspase 9 in lysates from Bcr-Abl-expressing cells or in vivo phosphorylation of caspase 9 captured from orthophosphate-labeled Bcr-Abl-expressing cells. Our data indicate that Bcr-Abl kinase activity inhibited the activation of caspases 3 and 9 by interfering with the binding of the prodomain of caspase 9 to the Apaf-1 CARD. Such a failure of recruitment would be expected to prevent dimerization-induced activation. Because the isolated CARD of Apaf-1 was unperturbed in its ability to recruit endogenous caspase 9 from Bcr-Abl cell lysates, it seems quite unlikely that the caspase 9 from those cells is intrinsically unable to incorporate itself into the apoptosome. Rather, these data suggested that Apaf-1 might be the target of inhibition. In support of this hypothesis, the isolated prodomain of caspase 9 could not efficiently retrieve endogenous Apaf-1 from the Bcr-Abl lysates. Again, were modification of the caspase 9 prodomain (or binding of an inhibitory factor) responsible for the apoptosomal defect, it seems likely that the prodomain of endogenous caspase 9 would have been subject to the same regulation, precluding its recruitment to the Apaf-1 CARD resin.
In that the Apaf-1 (1-543) fragment recruited endogenous caspase 9 equally well from control GFP- and Bcr-Abl-expressing cells, but endogenous cytochrome c-bound Apaf-1 recruited endogenous caspase 9 differentially in these cells, it seems reasonable to speculate that cytochrome c/dATP-induced exposure of the Apaf-1 CARD was impaired by Bcr-Abl signaling. Although Apaf-1 does not appear to be tyrosine phosphorylated (Fig. 7D) and thus is not likely to be a direct Bcr-Abl substrate, we have shown increased total phosphorylation of the Apaf-1 (1-543) fragment in the presence of Bcr-Abl (Fig. 7C). Therefore, it is plausible that phosphorylation of Apaf-1 serves either to prevent the requisite cytochrome c-dATP-induced conformational change of Apaf-1 to expose the CARD or that phosphorylated residues serve as docking sites for factors that might occlude subsequent binding of procaspase 9. Although definitive proof of this hypothesis will await identification of de novo phosphorylation sites on endogenous Apaf-1, our data largely preclude other known mechanisms of apoptosomal inhibition. Indeed, we have assayed the reported apoptosomal inhibitors Aven, Hsp70, and XIAP for differential binding to Apaf-1 and/or caspase 9 in the presence and absence of Bcr-Abl and have observed no significant differences (Z. T. Schafer, P. B. Deming, and S. Kornbluth, unpublished data). Moreover, although Bcr-Abl has been demonstrated to inhibit paclitaxel-induced apoptosis through transcriptional upregulation of Hsp70 (43), we found no change in Hsp70 expression levels due to the expression of Bcr-Abl in Rat-1 fibroblasts (Z. T. Schafer and S. Kornbluth, unpublished data).
Taken together, our data derived from the use of Xenopus egg extracts, mammalian cell lysates, and the cytosolic microinjection of cytochrome c demonstrate that a posttranslational mechanism inhibits apoptosis downstream of cytochrome c release both in vitro and in intact cells. These findings are consistent with a recent report that Bcr-Abl is a more potent inhibitor of apoptosis than Bcl-XL, as would be expected if Bcr-Abl had additional loci of action, acting not only to prevent cytochrome c release but also to prevent downstream effects of cytochrome c (9). Although prevention of cytochrome c release might be sufficient to inhibit apoptosis under some circumstances, such protective mechanisms are not absolute. Indeed, it is likely that large amounts of cellular damage, as might be incurred following treatment with chemotherapeutics, could lead to some transit of cytochrome c to the cytoplasm. Moreover, mitochondrial damage in the course of a cell's lifetime may lead to inadvertent leakage of cytochrome c. In the presence of Bcr-Abl, any small amounts of cytochrome c released would be rendered impotent, resulting in a failure to activate caspase 9 and, in turn, caspase 3.
Acknowledgments
We thank Christopher Freel for aid in manuscript preparation and Jeffrey Rathmell for critical reading of the manuscript. Additionally, we thank Kevin Wright, Seth Margolis, and Jennifer Perry for helpful comments and discussion. We also thank Ann Marie Pendergast (Duke University) for the Bcr-Abl constructs and 32D cells, Yigong Shi (Princeton University) for the catalytically inactive caspase 3 construct, and Xiaodong Wang (University of Texas Southwestern) for the full-length Apaf-1 and procaspase 9 cDNAs.
This work was supported by NIH RO1 CA102702 to S.K. and NIH RO1 NS42197 to M.D. P.B.D. is a Ruth L. Kirschstein NRSA fellow. Z.T.S. is a predoctoral fellow of the Breast Cancer Research Program of the USARMC.
REFERENCES
- 1.Allan, L. A., N. Morrice, S. Brady, G. Magee, S. Pathak, and P. R. Clarke. 2003. Inhibition of caspase 9 through phosphorylation at Thr 125 by ERK MAPK. Nat. Cell Biol. 5:647-654. [DOI] [PubMed] [Google Scholar]
- 2.Amarante-Mendes, G. P., T. Jascur, W. K. Nishioka, T. Mustelin, and D. R. Green. 1997. Bcr-Abl-mediated resistance to apoptosis is independent of PI 3-kinase activity. Cell Death Differ. 4:548-554. [DOI] [PubMed] [Google Scholar]
- 3.Amarante-Mendes, G. P., A. J. McGahon, W. K. Nishioka, D. E. Afar, O. N. Witte, and D. R. Green. 1998. Bcl-2-independent Bcr-Abl-mediated resistance to apoptosis: protection is correlated with up regulation of Bcl-xL. Oncogene 16:1383-1390. [DOI] [PubMed] [Google Scholar]
- 4.Bedi, A., J. P. Barber, G. C. Bedi, W. S. el-Deiry, D. Sidransky, M. S. Vala, A. J. Akhtar, J. Hilton, and R. J. Jones. 1995. BCR-ABL-mediated inhibition of apoptosis with delay of G2/M transition after DNA damage: a mechanism of resistance to multiple anticancer agents. Blood 86:1148-1158. [PubMed] [Google Scholar]
- 5.Bedi, A., B. A. Zehnbauer, J. P. Barber, S. J. Sharkis, and R. J. Jones. 1994. Inhibition of apoptosis by BCR-ABL in chronic myeloid leukemia. Blood 83:2038-2044. [PubMed] [Google Scholar]
- 6.Beere, H. M., B. B. Wolf, K. Cain, D. D. Mosser, A. Mahboubi, T. Kuwana, P. Tailor, R. I. Morimoto, G. M. Cohen, and D. R. Green. 2000. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase 9 to the Apaf-1 apoptosome. Nat. Cell Biol. 2:469-475. [DOI] [PubMed] [Google Scholar]
- 7.Bratton, S. B., J. Lewis, M. Butterworth, C. S. Duckett, and G. M. Cohen. 2002. XIAP inhibition of caspase-3 preserves its association with the Apaf-1 apoptosome and prevents CD95- and Bax-induced apoptosis. Cell Death Differ. 9:881-892. [DOI] [PubMed] [Google Scholar]
- 8.Bratton, S. B., G. Walker, S. M. Srinivasula, X. M. Sun, M. Butterworth, E. S. Alnemri, and G. M. Cohen. 2001. Recruitment, activation and retention of caspases-9 and -3 by Apaf-1 apoptosome and associated XIAP complexes. EMBO J. 20:998-1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brumatti, G., R. Weinlich, C. F. Chehab, M. Yon, and G. P. Amarante-Mendes. 2003. Comparison of the anti-apoptotic effects of Bcr-Abl, Bcl-2 and Bcl-x(L) following diverse apoptogenic stimuli. FEBS Lett. 541:57-63. [DOI] [PubMed] [Google Scholar]
- 10.Brustugun, O. T., K. E. Fladmark, S. O. Doskeland, S. Orrenius, and B. Zhivotovsky. 1998. Apoptosis induced by microinjection of cytochrome c is caspase-dependent and is inhibited by Bcl-2. Cell Death Differ. 5:660-668. [DOI] [PubMed] [Google Scholar]
- 11.Cain, K., D. G. Brown, C. Langlais, and G. M. Cohen. 1999. Caspase activation involves the formation of the aposome, a large (approximately 700 kDa) caspase-activating complex. J. Biol. Chem. 274:22686-22692. [DOI] [PubMed] [Google Scholar]
- 12.Cardone, M. H., N. Roy, H. R. Stennicke, G. S. Salvesen, T. F. Franke, E. Stanbridge, S. Frisch, and J. C. Reed. 1998. Regulation of cell death protease caspase 9 by phosphorylation. Science 282:1318-1321. [DOI] [PubMed] [Google Scholar]
- 13.Chau, B. N., E. H. Cheng, D. A. Kerr, and J. M. Hardwick. 2000. Aven, a novel inhibitor of caspase activation, binds Bcl-xL and Apaf-1. Mol. Cell 6:31-40. [PubMed] [Google Scholar]
- 14.Cortez, D., G. Reuther, and A. M. Pendergast. 1997. The Bcr-Abl tyrosine kinase activates mitogenic signaling pathways and stimulates G1-to-S phase transition in hematopoietic cells. Oncogene 15:2333-2342. [DOI] [PubMed] [Google Scholar]
- 15.Daley, G. Q., and D. Baltimore. 1988. Transformation of an interleukin 3-dependent hematopoietic cell line by the chronic myelogenous leukemia-specific P210 bcr/abl protein. Proc. Natl. Acad. Sci. USA 85:9312-9316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danial, N. N., and S. J. Korsmeyer. 2004. Cell death: critical control points. Cell 116:205-219. [DOI] [PubMed] [Google Scholar]
- 17.Deininger, M. W., J. M. Goldman, and J. V. Melo. 2000. The molecular biology of chronic myeloid leukemia. Blood 96:3343-3356. [PubMed] [Google Scholar]
- 18.Deshmukh, M., and E. M. Johnson, Jr. 1998. Evidence of a novel event during neuronal death: development of competence-to-die in response to cytoplasmic cytochrome c. Neuron 21:695-705. [DOI] [PubMed] [Google Scholar]
- 19.Devarajan, E., A. A. Sahin, J. S. Chen, R. R. Krishnamurthy, N. Aggarwal, A. M. Brun, A. Sapino, F. Zhang, D. Sharma, X. H. Yang, A. D. Tora, and K. Mehta. 2002. Down-regulation of caspase-3 in breast cancer: a possible mechanism for chemoresistance. Oncogene 21:8843-8851. [DOI] [PubMed] [Google Scholar]
- 20.Deveraux, Q. L., and J. C. Reed. 1999. IAP family proteins—suppressors of apoptosis. Genes Dev. 13:239-252. [DOI] [PubMed] [Google Scholar]
- 21.Evans, E. K., W. Lu, S. L. Strum, B. J. Mayer, and S. Kornbluth. 1997. Crk is required for apoptosis in Xenopus egg extracts. EMBO J. 16:230-241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gorre, M. E., M. Mohammed, K. Ellwood, N. Hsu, R. Paquette, P. N. Rao, and C. L. Sawyers. 2001. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science 293:876-880. [DOI] [PubMed] [Google Scholar]
- 23.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan, D., and R. A. Weinberg. 2000. The hallmarks of cancer. Cell 100:57-70. [DOI] [PubMed] [Google Scholar]
- 25.Hochhaus, A., S. Kreil, A. Corbin, P. La Rosee, T. Lahaye, U. Berger, N. C. Cross, W. Linkesch, B. J. Druker, R. Hehlmann, C. Gambacorti-Passerini, G. Corneo, and M. D'Incalci. 2001. Roots of clinical resistance to STI-571 cancer therapy. Science 293:2163. [PubMed] [Google Scholar]
- 26.Hoover, R. R., M. J. Gerlach, E. Y. Koh, and G. Q. Daley. 2001. Cooperative and redundant effects of STAT5 and Ras signaling in BCR/ABL transformed hematopoietic cells. Oncogene 20:5826-5835. [DOI] [PubMed] [Google Scholar]
- 27.Horita, M., E. J. Andreu, A. Benito, C. Arbona, C. Sanz, I. Benet, F. Prosper, and J. L. Fernandez-Luna. 2000. Blockade of the Bcr-Abl kinase activity induces apoptosis of chronic myelogenous leukemia cells by suppressing signal transducer and activator of transcription 5-dependent expression of Bcl-xL. J. Exp. Med. 191:977-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jia, L., Y. Patwari, S. M. Kelsey, S. M. Srinivasula, S. G. Agrawal, E. S. Alnemri, and A. C. Newland. 2003. Role of Smac in human leukaemic cell apoptosis and proliferation. Oncogene 22:1589-1599. [DOI] [PubMed] [Google Scholar]
- 29.Jia, L., S. M. Srinivasula, F. T. Liu, A. C. Newland, T. Fernandes-Alnemri, E. S. Alnemri, and S. M. Kelsey. 2001. Apaf-1 protein deficiency confers resistance to cytochrome c-dependent apoptosis in human leukemic cells. Blood 98:414-421. [DOI] [PubMed] [Google Scholar]
- 30.Kluck, R. M., S. J. Martin, B. M. Hoffman, J. S. Zhou, D. R. Green, and D. D. Newmeyer. 1997. Cytochrome c activation of CPP32-like proteolysis plays a critical role in a Xenopus cell-free apoptosis system. EMBO J. 16:4639-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.le Coutre, P., E. Tassi, M. Varella-Garcia, R. Barni, L. Mologni, G. Cabrita, E. Marchesi, R. Supino, and C. Gambacorti-Passerini. 2000. Induction of resistance to the Abelson inhibitor STI571 in human leukemic cells through gene amplification. Blood 95:1758-1766. [PubMed] [Google Scholar]
- 32.Li, F., A. Srinivasan, Y. Wang, R. C. Armstrong, K. J. Tomaselli, and L. C. Fritz. 1997. Cell-specific induction of apoptosis by microinjection of cytochrome c. Bcl-xL has activity independent of cytochrome c release. J. Biol. Chem. 272:30299-30305. [DOI] [PubMed] [Google Scholar]
- 33.Liu, J. R., A. W. Opipari, L. Tan, Y. Jiang, Y. Zhang, H. Tang, and G. Nunez. 2002. Dysfunctional apoptosome activation in ovarian cancer: implications for chemoresistance. Cancer Res. 62:924-931. [PubMed] [Google Scholar]
- 34.Liu, X., C. N. Kim, J. Yang, R. Jemmerson, and X. Wang. 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86:147-157. [DOI] [PubMed] [Google Scholar]
- 35.McGahon, A., R. Bissonnette, M. Schmitt, K. M. Cotter, D. R. Green, and T. G. Cotter. 1994. BCR-ABL maintains resistance of chronic myelogenous leukemia cells to apoptotic cell death. Blood 83:1179-1187. [PubMed] [Google Scholar]
- 36.Neshat, M. S., A. B. Raitano, H. G. Wang, J. C. Reed, and C. L. Sawyers. 2000. The survival function of the Bcr-Abl oncogene is mediated by Bad-dependent and -independent pathways: roles for phosphatidylinositol 3-kinase and Raf. Mol. Cell. Biol. 20:1179-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Newmeyer, D. D., D. M. Farschon, and J. C. Reed. 1994. Cell-free apoptosis in Xenopus egg extracts: inhibition by Bcl-2 and requirement for an organelle fraction enriched in mitochondria. Cell 79:353-364. [DOI] [PubMed] [Google Scholar]
- 38.Olson, M., and S. Kornbluth. 2001. Mitochondria in apoptosis and human disease. Curr. Mol. Med. 1:91-122. [DOI] [PubMed] [Google Scholar]
- 39.Pear, W. S., J. P. Miller, L. Xu, J. C. Pui, B. Soffer, R. C. Quackenbush, A. M. Pendergast, R. Bronson, J. C. Aster, M. L. Scott, and D. Baltimore. 1998. Efficient and rapid induction of a chronic myelogenous leukemia-like myeloproliferative disease in mice receiving P210 bcr/abl-transduced bone marrow. Blood 92:3780-3792. [PubMed] [Google Scholar]
- 40.Pendergast, A. M., R. Clark, E. S. Kawasaki, F. P. McCormick, and O. N. Witte. 1989. Baculovirus expression of functional P210 BCR-ABL oncogene product. Oncogene 4:759-766. [PubMed] [Google Scholar]
- 41.Pendergast, A. M., L. A. Quilliam, L. D. Cripe, C. H. Bassing, Z. Dai, N. Li, A. Batzer, K. M. Rabun, C. J. Der, J. Schlessinger, et al. 1993. BCR-ABL-induced oncogenesis is mediated by direct interaction with the SH2 domain of the GRB-2 adaptor protein. Cell 75:175-185. [PubMed] [Google Scholar]
- 42.Potts, P. R., S. Singh, M. Knezek, C. B. Thompson, and M. Deshmukh. 2003. Critical function of endogenous XIAP in regulating caspase activation during sympathetic neuronal apoptosis. J. Cell Biol. 163:789-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ray, S., Y. Lu, S. H. Kaufmann, W. C. Gustafson, J. E. Karp, I. Boldogh, A. P. Fields, and A. R. Brasier. 2004. Genomic mechanisms of p210BCR-ABL signaling: induction of heat shock protein 70 through the GATA response element confers resistance to paclitaxel-induced apoptosis. J. Biol. Chem. 279:35604-35615. [DOI] [PubMed] [Google Scholar]
- 44.Rodriguez, J., H. Chen, S. Lin, and Y. Lazebnik. 2000. Caspase phosphorylation, cell death, and species variability. Science 287:1363a. [Google Scholar]
- 45.Roskoski, R., Jr. 2003. STI-571: an anticancer protein-tyrosine kinase inhibitor. Biochem. Biophys. Res. Commun. 309:709-717. [DOI] [PubMed] [Google Scholar]
- 46.Saleh, A., S. M. Srinivasula, L. Balkir, P. D. Robbins, and E. S. Alnemri. 2000. Negative regulation of the Apaf-1 apoptosome by Hsp70. Nat. Cell Biol. 2:476-483. [DOI] [PubMed] [Google Scholar]
- 47.Salvesen, G. S., and C. S. Duckett. 2002. IAP proteins: blocking the road to death's door. Nat. Rev. Mol. Cell. Biol. 3:401-410. [DOI] [PubMed] [Google Scholar]
- 48.Sanchez-Garcia, I., and G. Grutz. 1995. Tumorigenic activity of the BCR-ABL oncogenes is mediated by BCL2. Proc. Natl. Acad. Sci. USA 92:5287-5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shiozaki, E. N., J. Chai, D. J. Rigotti, S. J. Riedl, P. Li, S. M. Srinivasula, E. S. Alnemri, R. Fairman, and Y. Shi. 2003. Mechanism of XIAP-mediated inhibition of caspase 9. Mol. Cell 11:519-527. [DOI] [PubMed] [Google Scholar]
- 50.Shuai, K., J. Halpern, J. ten Hoeve, X. Rao, and C. L. Sawyers. 1996. Constitutive activation of STAT5 by the BCR-ABL oncogene in chronic myelogenous leukemia. Oncogene 13:247-254. [PubMed] [Google Scholar]
- 51.Skorski, T. 2002. BCR/ABL regulates response to DNA damage: the role in resistance to genotoxic treatment and in genomic instability. Oncogene 21:8591-8604. [DOI] [PubMed] [Google Scholar]
- 52.Skorski, T., A. Bellacosa, M. Nieborowska-Skorska, M. Majewski, R. Martinez, J. K. Choi, R. Trotta, P. Wlodarski, D. Perrotti, T. O. Chan, M. A. Wasik, P. N. Tsichlis, and B. Calabretta. 1997. Transformation of hematopoietic cells by BCR/ABL requires activation of a PI-3k/Akt-dependent pathway. EMBO J. 16:6151-6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Skorski, T., P. Kanakaraj, M. Nieborowska-Skorska, M. Z. Ratajczak, S. C. Wen, G. Zon, A. M. Gewirtz, B. Perussia, and B. Calabretta. 1995. Phosphatidylinositol-3 kinase activity is regulated by BCR/ABL and is required for the growth of Philadelphia chromosome-positive cells. Blood 86:726-736. [PubMed] [Google Scholar]
- 54.Soengas, M. S., P. Capodieci, D. Polsky, J. Mora, M. Esteller, X. Opitz-Araya, R. McCombie, J. G. Herman, W. L. Gerald, Y. A. Lazebnik, C. Cordon-Cardo, and S. W. Lowe. 2001. Inactivation of the apoptosis effector Apaf-1 in malignant melanoma. Nature 409:207-211. [DOI] [PubMed] [Google Scholar]
- 55.Srinivasula, S. M., M. Ahmad, T. Fernandes-Alnemri, and E. S. Alnemri. 1998. Autoactivation of procaspase 9 by Apaf-1-mediated oligomerization. Mol. Cell 1:949-957. [DOI] [PubMed] [Google Scholar]
- 56.Tashker, J. S., M. Olson, and S. Kornbluth. 2002. Post-cytochrome c protection from apoptosis conferred by a MAPK pathway in Xenopus egg extracts. Mol. Biol. Cell 13:393-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thornberry, N. A., and Y. Lazebnik. 1998. Caspases: enemies within. Science 281:1312-1316. [DOI] [PubMed] [Google Scholar]
- 58.Twiddy, D., D. G. Brown, C. Adrain, R. Jukes, S. J. Martin, G. M. Cohen, M. MacFarlane, and K. Cain. 2004. Pro-apoptotic proteins released from the mitochondria regulate the protein composition and caspase-processing activity of the native Apaf-1/caspase 9 apoptosome complex. J. Biol. Chem. 279:19665-19682. [DOI] [PubMed] [Google Scholar]
- 59.Vyas, S., P. Juin, D. Hancock, Y. Suzuki, R. Takahashi, A. Triller, and G. Evan. 2004. Differentiation dependent sensitivity to apoptogenic factors in PC12 cells. J. Biol. Chem. 279:30983-30993. [DOI] [PubMed] [Google Scholar]
- 60.Wang, X. 2001. The expanding role of mitochondria in apoptosis. Genes Dev. 15:2922-2933. [PubMed] [Google Scholar]
- 61.Zou, H., W. J. Henzel, X. Liu, A. Lutschg, and X. Wang. 1997. Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspase-3. Cell 90:405-413. [DOI] [PubMed] [Google Scholar]