Abstract
Background
Cholangiocarcinoma (CC) is the second most common primary malignant liver disease. During the last decades, various novel therapies have been introduced in the field of oncology; nevertheless, the number of treatment options for CC is still limited.
Methods
In this article, current palliative chemotherapy concepts as well as new drug therapies are outlined.
Results
Gemcitabine and cisplatin are the standard treatment of care for patients with inoperable CC. Second-line chemotherapy is not standardized yet and is dependent on the first-line compounds. Antibodies against VEGFR and EGFR showed mixed or negative results. New molecular systemic treatments are not established yet.
Conclusion
Many clinical trials are still ongoing and new therapeutic strategies, including immunotherapies, are under active investigation.
Key Words: Cholangiocarcinoma, Chemotherapy, Survival
Introduction
Cholangiocarcinoma (CC) is a rare but increasing tumor disease, and many patients (80%) present with unresectable intra- and extrahepatic metastases or advanced tumor stage at diagnosis [1,2,3,4,5]. The median survival of untreated patients with unresectable CC is approximately 3-6 months [6]. Therefore, the majority of patients are undergoing palliative systemic chemotherapy. Even though large phase III trials are missing, the role of chemotherapy is evidence-based and has a fixed place in the palliative setting.
Adjuvant Chemotherapy
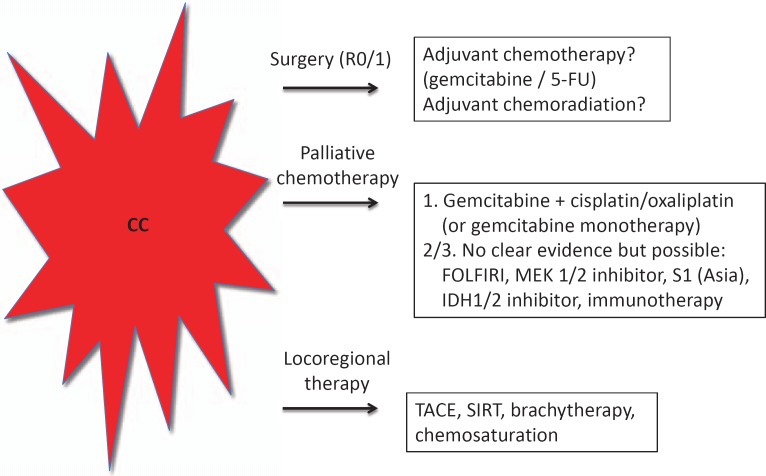
The role of adjuvant chemotherapy or chemoradiation after complete resection is not clear yet (fig. 1). The local recurrence, especially of extrahepatic CC, is high, and the European (European Society for Medical Oncology (ESMO)) as well as the US (National Comprehensive Cancer Network (NCCN)) guidelines mention adjuvant therapy as an option [7,8]. After R0 resection without lymph node involvement, both guidelines recommend chemotherapy with gemcitabine or 5-fluorouracil (5-FU). In contrast, patients with R1 resection or positive lymph nodes could be treated by chemoradiotherapy. The SWOG-S0809 study recently showed that sequential chemotherapy with gemcitabine and capecitabine for 12 weeks followed by radiochemotherapy led to promising results [9]. The further role of adjuvant chemotherapy will be evaluated in three large, currently ongoing phase III studies (BILCAP, PRODIGE-12, ACTICCA-1) in three different European countries (France, Germany, UK).
Fig. 1.
Treatment options for inoperable cholangiocarcinoma.
Palliative Chemotherapy
Retrospectively, systemic chemotherapy with a combination of 5-FU as well as leucovorin with and without etoposide improved survival [10]. Phase II trials with 5-FU demonstrated response rates between 10 and 34% [11,12]. However, the combination of 5-FU/capecitabine and platinum (cisplatin/oxaliplatin) compounds did not improve the tumor response rates significantly [13,14,15,16,17]. So far, different chemotherapeutic drugs have been tested: Gemcitabine as a monotherapy showed response rates between 17.5 and 36% [18,19]. Gemcitabine and capecitabine showed response rates of 25% [20,21,22,23,24], and oxaliplatin and gemcitabine showed response rates of 50% [25,26,27]. Since 2010, a combination therapy with gemcitabine and cisplatin is considered as the standard first-line chemotherapy for non-resectable CC [28]. In this ABC-02 phase III trial, a significantly longer median survival in the combination group compared to gemcitabine monotherapy (11.7 vs. 8.1 months) was achieved (fig. 1). However, patients with impairment of renal function can also be treated with oxaliplatin. Monotherapy with gemcitabine is mainly recommended for elderly patients or patients with impaired ECOG (Eastern Cooperative Oncology Group) performance status or significant additional diseases.
Second-Line Chemotherapy
For the use of second-line chemotherapy, there is currently no clear evidence, while large studies are missing. Thus, the median survival in both a Japanese phase II study as well as in the larger ABC-02 phase III study with gemcitabine and cisplatin was 11.7 and 11.2 months, respectively [29]. Interestingly, in the Japanese study, 75% of the patients received a subsequent therapy, while second-line treatment was only applied in 15% of all patients in the ABC-02 trial. One of the biggest retrospective analyses examined the use of various chemotherapeutic drugs after failure of gemcitabine and showed a better survival compared to best supportive care [30]. A systematic review of second-line chemotherapies in CC analyzed data of 761 patients [31]. The median progression-free survival (PFS) and overall survival (OS) was 3.2 and 7.2 months, respectively. The response rates and the tumor control rates were low with 7.7 and 48%, respectively. However, in daily practice it is not uncommon and probably justified to offer patients with good performance status a second-line chemotherapy.
New Targets and Therapies
New clinical targeted and immunotherapeutic trials with peptide-based vaccines, dendritic cell-based vaccines, and antibodies have been initiated. In more detail, immunotherapeutic approaches are divided into passive and active immunotherapies. For passive immunotherapy, antibodies against epidermal growth factor receptor (EGFR) and vascular endothelial growth factor receptor (VEGFR) were investigated. Currently, the most studied aspect is EGFR inhibition. EGFR expression is frequently observed in intrahepatic CC but only 5-32% of cancers show true overexpression of the EGFR compared to normal tissues. Interestingly, KRAS mutations occur significantly less often in CC compared to other types of cancer [32,33,34,35]. In a single-arm study by Gruenberger et al. [36], the chimeric EGFR antibody cetuximab in combination with GEMOX achieved response rates of 63%. However, the data of the randomized phase II BINGO study were disappointing. This trial did not show an advantage for the combination of cetuximab with GEMOX [37]. It is possible that KRAS mutations are causing resistance to EGFR-targeted therapy. However, a phase II study from Asia found no benefit of EGFR targeting in patients with CC and KRAS wildtype [38]. The German PICCA study also showed no benefit for the treatment with panitumumab [39]. Regarding patients with CC, gallbladder carcinoma, or papillary cancer, another study has tested the combination of GEMOX and the tyrosine kinase inhibitor erlotinib; adding erlotinib showed no benefit in patients with CC, though [40]. In the subgroup analysis, patients treated with erlotinib showed a prolonged PFS; however, it is not yet clear whether overexpression of EGFR is a prerequisite for the efficacy of erlotinib treatment. Erlotinib was also tested in combination with the VEGFR antibody bevacizumab. A tolerable toxicity with response rates of 12% has been demonstrated [41]. The PFS was 4.4 months and the OS 9.9 months. A combination of gemcitabine, oxaliplatin, and bevacizumab (antibody against VEGFR) showed a tumor response measured by fluorodeoxyglucose-positron emission tomography [42].
The multikinase inhibitor sorafenib approved for the treatment of hepatocelluar and kidney cancer showed no significant anti-tumor activity [43,44]. Moreover, the combination of sorafenib and gemcitabine showed no improvement of survival in patients with CC [45,46]. The same is true for the combination of sorafenib and erlotinib which did not show a benefit in patients with CC [47]. Another tyrosine kinase inhibitor, cediranib, which was tested in a phase II study in combination with gemcitabine and cisplatin, did not show an advantage [48]. Sunitinib, another multikinase inhibitor tested in the second-line setting, demonstrated a PFS of 1.7 months, while the response rate was 8.9% [49]. Furthermore, the multi-targeted tyrosine kinase inhibitor vandetanib (inhibition of EGFR and VEGFR) in combination with gemcitabine showed no survival benefit [50]. Recently, the MEK 1/2 inhibitor selumetinib was tested in patients with CC and showed response rates of 12%, a PFS of 3.7 months, and an OS of 9.8 months [51]. In addition, the oral prodrug of 5-FU (S1), which is used mainly in Asia, was tested in combination with gemcitabine and cisplatin [52,53]. The combination therapy with gemcitabine resulted in a response rate of 20%, but the combination did not differ from monotherapies. Yet another therapeutic approach, i.e. the combination of a c-MET inhibitor (tivantinib) and gemcitabine, was evaluated and achieved a partial response in 1 patient [54]. In a second study, unselected patients were treated with cabozantinib (inhibitor of c-MET) but did not result in a meaningful response [55]. The incidence of mutations of isocitrate dehydrogenase 1 and 2 (IDH1/2), which is essential for the cellular response to oxidative stress, is approximately 20% for intrahepatic CC [56,57]. IDH inhibitors are also currently being evaluated in clinical trials (fig. 1).
As an active immunotherapeutic approach, Aruga et al. [58] combined different peptides in a palliative setting. Here, the median PFS was 156 days and the OS 380 days. It was also shown that programmed cell death ligand 1 (PD-L1) and human leukocyte antigen (HLA) class I is expressed in human intrahepatic CC [59]. The authors confirmed that positive HLA class I expression in combination with negative/low PD-L1 expression was associated with better clinical disease and that their findings support the usage of immunotherapeutic checkpoint inhibitors. Besides, Gani et al. [60] showed that PD-L1 was expressed in the majority of intrahepatic CC and that PD-L1 expression was associated with decreased survival. However, the role of immune checkpoint inhibitors against CC is not fully investigated yet. Different studies with non-colorectal cancers, including CC, treated with pembrolizumab are not published. Also, the role of defects in mismatch repair genes for CC carcinogenesis is not completely understood. Further immune checkpoint blockade therapies are warranted, and immunotherapeutic approaches for CC are not the standard of care yet.
Disclosure Statement
No conflicts of interest.
References
- 1.Wang Y, Li J, Xia Y, et al. Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol. 2013;31:1188–1195. doi: 10.1200/JCO.2012.41.5984. [DOI] [PubMed] [Google Scholar]
- 2.West J, Wood H, Logan RF, Quinn M, Aithal GP. Trends in the incidence of primary liver and biliary tract cancers in England and Wales 1971-2001. Br J Cancer. 2006;94:1751–1758. doi: 10.1038/sj.bjc.6603127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shaib YH, Davila JA, McGlynn K, El Serag HB. Rising incidence of intrahepatic cholangiocarcinoma in the United States: a true increase? J Hepatol. 2004;40:472–477. doi: 10.1016/j.jhep.2003.11.030. [DOI] [PubMed] [Google Scholar]
- 4.Alvaro D, Crocetti E, Ferretti S, Bragazzi MC, Capocaccia R. Descriptive epidemiology of cholangiocarcinoma in Italy. Dig Liver Dis. 2010;42:490–495. doi: 10.1016/j.dld.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 5.von Hahn T, Ciesek S, Wegener G, Plentz RR, Weismüller TJ, Wedemeyer H, Manns MP, Greten TF, Malek NP. Epidemiological trends in incidence and mortality of hepatobiliary cancers in Germany. Scand J Gastroenterol. 2011;46:1092–1098. doi: 10.3109/00365521.2011.589472. [DOI] [PubMed] [Google Scholar]
- 6.Farley DR, Weaver AL, Nagorney DM. ‘Natural history’ of unresected cholangiocarcinoma: patient outcome after noncurative intervention. Mayo Clin Proc. 1995;70:425–429. doi: 10.4065/70.5.425. [DOI] [PubMed] [Google Scholar]
- 7.Eckel F, Brunner T, Jelic S. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2011;22:vi40–44. doi: 10.1093/annonc/mdr375. [DOI] [PubMed] [Google Scholar]
- 8.Yusuf MA, Kapoor VK, Kamel RR, Kazmi A, Uddin N, Masood N, Al-Abdulkareem A. Modification and implementation of NCCN guidelines on hepatobiliary cancers in the Middle East and North Africa region. J Natl Compr Canc Netw. 2010;8:36–40. doi: 10.6004/jnccn.2010.0123. [DOI] [PubMed] [Google Scholar]
- 9.Ben-Josef E, Guthrie KA, El-Khoueiry AB, Corless CL, Zalupski MM, Lowy AM, Thomas CR, Jr, Alberts SR, Dawson LA, Micetich KC, Thomas MB, Siegel AB, Blanke CD. SWOG S0809: a phase II intergroup trial of adjuvant capecitabine and gemcitabine followed by radiotherapy and concurrent capecitabine in extrahepatic cholangiocarcinoma and gallbladder carcinoma. J Clin Oncol. 2015;33:2617–2622. doi: 10.1200/JCO.2014.60.2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glimelius B, Hoffman K, Sjödén PO, Jacobsson G, Sellström H, Enander LK, Linné T, Svensson C. Chemotherapy improves survival and quality of life in advanced pancreatic and biliary cancer. Ann Oncol. 1996;7:593–600. doi: 10.1093/oxfordjournals.annonc.a010676. [DOI] [PubMed] [Google Scholar]
- 11.Patt YZ, Jones DV, Jr, Hoque A, Lozano R, Markowitz A, Raijman I, Lynch P, Charnsangavej C. Phase II trial of intravenous flourouracil and subcutaneous interferon alfa-2b for biliary tract cancer. J Clin Oncol. 1996;14:2311–2315. doi: 10.1200/JCO.1996.14.8.2311. [DOI] [PubMed] [Google Scholar]
- 12.Harvey JH, Smith FP, Schein PS. 5-Fluorouracil, mitomycin, and doxorubicin (FAM) in carcinoma of the biliary tract. J Clin Oncol. 1984;2:1245–1248. doi: 10.1200/JCO.1984.2.11.1245. [DOI] [PubMed] [Google Scholar]
- 13.Lee GW, Kang JH, Kim HG, Lee JS, Lee JS, Jang JS. Combination chemotherapy with gemcitabine and cisplatin as first-line treatment for immunohistochemically proven cholangiocarcinoma. Am J Clin Oncol. 2006;29:127–131. doi: 10.1097/01.coc.0000203742.22828.bb. [DOI] [PubMed] [Google Scholar]
- 14.Ducreux M, Rougier P, Fandi A, Clavero-Fabri MC, Villing AL, Fassone F, Fandi L, Zarba J, Armand JP. Effective treatment of advanced biliary tract carcinoma using 5-fluorouracil continuous infusion with cisplatin. Ann Oncol. 1998;9:653–656. doi: 10.1023/a:1008241008379. [DOI] [PubMed] [Google Scholar]
- 15.Ducreux M, Van Cutsem E, Van Laethem JL, Gress TM, Jeziorski K, Rougier P, Wagener T, Anak O, Baron B, Nordlinger B, EORTC Gastro Intestinal Tract Cancer Group A randomised phase II trial of weekly high-dose 5-fluorouracil with and without folinic acid and cisplatin in patients with advanced biliary tract carcinoma: results of the 40955 EORTC trial. Eur J Cancer. 2005;41:398–403. doi: 10.1016/j.ejca.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 16.Nehls O, Klump B, Arkenau HT, Hass HG, Greschniok A, Gregor M, Porschen R. Oxaliplatin, fluorouracil and leucovorin for advanced biliary system adenocarcinomas: a prospective phase II trial. Br J Cancer. 2002;87:702–704. doi: 10.1038/sj.bjc.6600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, Koppenhöfer U, Hochhaus A, Stieler J, Trojan J, Gregor M, Klump B. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309–315. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okusaka T, Ishii H, Funakoshi A, Yamao K, Ohkawa S, Saito S, Saito H, Tsuyuguchi T. Phase II study of single-agent gemcitabine in patients with advanced biliary tract cancer. Cancer Chemother Pharmacol. 2006;57:647–653. doi: 10.1007/s00280-005-0095-3. [DOI] [PubMed] [Google Scholar]
- 19.Kubicka S, Rudolph KL, Tietze MK, Lorenz M, Manns M. Phase II study of systemic gemcitabine chemotherapy for advanced unresectable hepatobiliary carcinomas. Hepatogastroenterology. 2001;48:783–789. [PubMed] [Google Scholar]
- 20.Knox JJ, Hedley D, Oza A, Feld R, Siu LL, Chen E, Nematollahi M, Pond GR, Zhang J, Moore MJ. Combining gemcitabine and capecitabine in patients with advanced biliary cancer: a phase II trial. J Clin Oncol. 2005;23:2332–2338. doi: 10.1200/JCO.2005.51.008. [DOI] [PubMed] [Google Scholar]
- 21.Knox JJ, Hedley D, Oza A, Siu LL, Pond GR, Moore MJ. Gemcitabine concurrent with continuous infusional 5-fluorouracil in advanced biliary cancers: a review of the Princess Margaret Hospital experience. Ann Oncol. 2004;15:770–774. doi: 10.1093/annonc/mdh172. [DOI] [PubMed] [Google Scholar]
- 22.Cho JY, Paik YH, Chang YS, Lee SJ, Lee DK, Song SY, Chung JB, Park MS, Yu JS, Yoon DS. Capecitabine combined with gemcitabine (CapGem) as first-line treatment in patients with advanced/metastatic biliary tract carcinoma. Cancer. 2005;104:2753–2758. doi: 10.1002/cncr.21591. [DOI] [PubMed] [Google Scholar]
- 23.Riechelmann RP, Townsley CA, Chin SN, Pond GR, Knox JJ. Expanded phase II trial of gemcitabine and capecitabine for advanced biliary cancer. Cancer. 2007;110:1307–1312. doi: 10.1002/cncr.22902. [DOI] [PubMed] [Google Scholar]
- 24.Koeberle D, Saletti P, Borner M, Gerber D, Dietrich D, Caspar CB, Mingrone W, Beretta K, Strasser F, Ruhstaller T, Mora O, Herrmann R, Swiss Group for Clinical Cancer Research Patient-reported outcomes of patients with advanced biliary tract cancers receiving gemcitabine plus capecitabine: a multicenter, phase II trial of the Swiss Group for Clinical Cancer Research. J Clin Oncol. 2008;26:3702–3708. doi: 10.1200/JCO.2008.16.5704. [DOI] [PubMed] [Google Scholar]
- 25.Verderame F, Russo A, Di Leo R, Badalamenti G, Santangelo D, Cicero G, Valerio MR, Gulotta G, Tomasello G, Gebbia N, Fulfaro F. Gemcitabine and oxaliplatin combination chemotherapy in advanced biliary tract cancers. Ann Oncol. 2006;17(suppl 7):vii68–72. doi: 10.1093/annonc/mdl955. [DOI] [PubMed] [Google Scholar]
- 26.André T, Tournigand C, Rosmorduc O, Provent S, Maindrault-Goebel F, Avenin D, Selle F, Paye F, Hannoun L, Houry S, Gayet B, Lotz JP, de Gramont A, Louvet C, GERCOR Group Gemcitabine combined with oxaliplatin (GEMOX) in advanced biliary tract adenocarcinoma: a GERCOR study. Ann Oncol. 2004;15:1339–1343. doi: 10.1093/annonc/mdh351. [DOI] [PubMed] [Google Scholar]
- 27.André T, Reyes-Vidal JM, Fartoux L, Ross P, Leslie M, Rosmorduc O, Clemens MR, Louvet C, Perez N, Mehmud F, Scheithauer W. Gemcitabine and oxaliplatin in advanced biliary tract carcinoma: a phase II study. Br J Cancer. 2008;99:862–867. doi: 10.1038/sj.bjc.6604628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, Madhusudan S, Iveson T, Hughes S, Pereira SP, Roughton M, Bridgewater J, ABC-02 Trial Investigators Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 29.Valle JW, Furuse J, Jitlal M, Beare S, Mizuno N, Wasan H, Bridgewater J, Okusaka T. Cisplatin and gemcitabine for advanced biliary tract cancer: a meta-analysis of two randomised trials. Ann Oncol. 2014;25:391–398. doi: 10.1093/annonc/mdt540. [DOI] [PubMed] [Google Scholar]
- 30.Walter T, Horgan AM, McNamara M, McKeever L, Min T, Hedley D, Serra S, Krzyzanowska MK, Chen E, Mackay H, Feld R, Moore M, Knox JJ. Feasibility and benefits of second-line chemotherapy in advanced biliary tract cancer: a large retrospective study. Eur J Cancer. 2013;49:329–335. doi: 10.1016/j.ejca.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Lamarca A, Hubner RA, David Ryder W, Valle JW. Second-line chemotherapy in advanced biliary cancer: a systematic review. Ann Oncol. 2014;25:2328–2338. doi: 10.1093/annonc/mdu162. [DOI] [PubMed] [Google Scholar]
- 32.Yoshikawa D, Ojima H, Iwasaki M, Hiraoka N, Kosuge T, Kasai S, Hirohashi S, Shibata T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br J Cancer. 2008;98:418–425. doi: 10.1038/sj.bjc.6604129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harder J, Waiz O, Otto F, Geissler M, Olschewski M, Weinhold B, Blum HE, Schmitt-Graeff A, Opitz OG. EGFR and HER2 expression in advanced biliary tract cancer. World J Gastroenterol. 2009;15:4511–4517. doi: 10.3748/wjg.15.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Andersen JB, Spee B, Blechacz BR, Avital I, Komuta M, Barbour A, Conner EA, Gillen MC, Roskams T, Roberts LR, Factor VM, Thorgeirsson SS. Genomic and genetic characterization of cholangiocarcinoma identifies therapeutic targets for tyrosine kinase inhibitors. Gastroenterology. 2012;142:1021–1031. doi: 10.1053/j.gastro.2011.12.005. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang X, Wang W, Wang C, Wang L, Yang M, Qi M, Su H, Sun X, Liu Z, Zhang J, Qin X, Han B. Characterization of EGFR family gene aberrations in cholangiocarcinoma. Oncol Rep. 2014;32:700–708. doi: 10.3892/or.2014.3261. [DOI] [PubMed] [Google Scholar]
- 36.Gruenberger B, Schueller J, Heubrandtner U, Wrba F, Tamandl D, Kaczirek K, Roka R, Freimann-Pircher S, Gruenberger T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: a phase 2 study. Lancet Oncol. 2010;11:1142–1148. doi: 10.1016/S1470-2045(10)70247-3. [DOI] [PubMed] [Google Scholar]
- 37.Malka D, Cervera P, Foulon S, et al. BINGO investigators Gemcitabine and oxaliplatin with or without cetuximab in advanced biliary-tract cancer (BINGO): a randomised, open-label, non-comparative phase 2 trial. Lancet Oncol. 2014;15:819–828. doi: 10.1016/S1470-2045(14)70212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen JS, Hsu C, Chiang NJ, et al. Taiwan Cooperative Oncology Group A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann Oncol. 2015;26:943–949. doi: 10.1093/annonc/mdv035. [DOI] [PubMed] [Google Scholar]
- 39.Vogel A, Kasper S, Weichert W, Bitzer M, Block A, Riess H, Schulze-Bergkamen H, Moehler MH, Merx KE, Endris V, Schnoy E, Siveke JT, Michl P, Waldschmidt D, Kuhlmann J, Geissler M, Kahl C, Kubicka S. Panitumumab in combination with gemcitabine/cisplatin (GemCis) for patients with advanced KRAS WT biliary tract cancer: a randomized phase II trial of the Arbeitsgemeinschaft Internistische Onkologie (AIO) J Clin Oncol. 2015;33(suppl) abstr 4082. [Google Scholar]
- 40.Lee J, Park SH, Chang HM, Kim JS, Choi HJ, Lee MA, Jang JS, Jeung HC, Kang JH, Lee HW, Shin DB, Kang HJ, Sun JM, Park JO, Park YS, Kang WK, Lim HY. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012;13:181–188. doi: 10.1016/S1470-2045(11)70301-1. [DOI] [PubMed] [Google Scholar]
- 41.Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, Philip PA, Picus J, Yong WP, Horvath L, Van Hazel G, Erlichman CE, Holen KD. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol. 2010;28:3491–3497. doi: 10.1200/JCO.2010.28.4075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu AX, Meyerhardt JA, Blaszkowsky LS, Kambadakone AR, Muzikansky A, Zheng H, Clark JW, Abrams TA, Chan JA, Enzinger PC, Bhargava P, Kwak EL, Allen JN, Jain SR, Stuart K, Horgan K, Sheehan S, Fuchs CS, Ryan DP, Sahani DV. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: a phase 2 study. Lancet Oncol. 2010;11:48–54. doi: 10.1016/S1470-2045(09)70333-X. [DOI] [PubMed] [Google Scholar]
- 43.Bengala C, Bertolini F, Malavasi N, Boni C, Aitini E, Dealis C, Zironi S, Depenni R, Fontana A, Del Giovane C, Luppi G, Conte P. Sorafenib in patients with advanced biliary tract carcinoma: a phase II trial. Br J Cancer. 2010;102:68–72. doi: 10.1038/sj.bjc.6605458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.El-Khoueiry AB, Rankin CJ, Ben-Josef E, Lenz HJ, Gold PJ, Hamilton RD, Govindarajan R, Eng C, Blanke CD. SWOG 0514: a phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Invest New Drugs. 2012;30:1646–1651. doi: 10.1007/s10637-011-9719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee JK, Capanu M, O'Reilly EM, Ma J, Chou JF, Shia J, Katz SS, Gansukh B, Reidy-Lagunes D, Segal NH, Yu KH, Chung KY, Saltz LB, Abou-Alfa GK. A phase II study of gemcitabine and cisplatin plus sorafenib in patients with advanced biliary adenocarcinomas. Br J Cancer. 2013;109:915–919. doi: 10.1038/bjc.2013.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Moehler M, Maderer A, Schimanski C, et al. Working Group of Internal Oncology Gemcitabine plus sorafenib versus gemcitabine alone in advanced biliary tract cancer: a double-blind placebo-controlled multicentre phase II AIO study with biomarker and serum programme. Eur J Cancer. 2014;50:3125–3135. doi: 10.1016/j.ejca.2014.09.013. [DOI] [PubMed] [Google Scholar]
- 47.El-Khoueiry AB, Rankin C, Siegel AB, Iqbal S, Gong IY, Micetich KC, Kayaleh OR, Lenz HJ, Blanke CD. S0941: a phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br J Cancer. 2014;110:882–887. doi: 10.1038/bjc.2013.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Valle JW, Wasan H, Jitlal M, Backen AC, Palmer DH, Duggan M, Cunningham D, Anthoney DA, Corrie P, Madhusudan S, Maraveyas A, Ross PJ, Waters JS, Steward WP, Rees C, Beare S, Dive C, Bridgewater JA. ABC-03: a randomized phase II trial of cediranib (AZD2171) or placebo in combination with cisplatin/gemcitabine (CisGem) chemotherapy for patients (pts) with advanced biliary tract cancer (ABC) J Clin Oncol. 2014;32(suppl 5) abstr 4002. [Google Scholar]
- 49.Yi JH, Thongprasert S, Lee J, Doval DC, Park SH, Park JO, Park YS, Kang WK, Lim HY. A phase II study of sunitinib as a second-line treatment in advanced biliary tract carcinoma: a multicentre, multinational study. Eur J Cancer. 2012;48:196–201. doi: 10.1016/j.ejca.2011.11.017. [DOI] [PubMed] [Google Scholar]
- 50.Santoro A, Gebbia V, Pressiani T, Testa A, Personeni N, Arrivas Bajardi E, Foa P, Buonadonna A, Bencardino K, Barone C, Ferrari D, Zaniboni A, Tronconi MC, Cartenì G, Milella M, Comandone A, Ferrari S, Rimassa L. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: the VanGogh study. Ann Oncol. 2015;26:542–547. doi: 10.1093/annonc/mdu576. [DOI] [PubMed] [Google Scholar]
- 51.Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, O'Neil BH, Balsom S, Balint C, Liersemann R, Vasko VV, Bloomston M, Marsh W, Doyle LA, Ellison G, Grever M, Ringel MD, Villalona-Calero MA. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol. 2011;29:2357–2363. doi: 10.1200/JCO.2010.33.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sasaki T, Isayama H, Nakai Y, Ito Y, Yasuda I, Toda N, Kogure H, Hanada K, Maguchi H, Sasahira N, Kamada H, Mukai T, Okabe Y, Hasebe O, Maetani I, Koike K. A randomized phase II study of gemcitabine and S-1 combination therapy versus gemcitabine monotherapy for advanced biliary tract cancer. Cancer Chemother Pharmacol. 2013;71:973–979. doi: 10.1007/s00280-013-2090-4. [DOI] [PubMed] [Google Scholar]
- 53.Li H, Zhang ZY, Zhou ZQ, Guan J, Tong DN, Zhou GW. Combined gemcitabine and S-1 chemotherapy for treating unresectable hilar cholangiocarcinoma: a randomized open-label clinical trial. Oncotarget. 2016;7:26888–26897. doi: 10.18632/oncotarget.8590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pant S, Saleh M, Bendell J, Infante JR, Jones S, Kurkjian CD, Moore KM, Kazakin J, Abbadessa G, Wang Y, Chen Y, Schwartz B, Camacho LH. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann Oncol. 2014;25:1416–1421. doi: 10.1093/annonc/mdu157. [DOI] [PubMed] [Google Scholar]
- 55.Lipka Goyal MBY, Abrams TA, Kwak EL, Cleary JM, Knowles M, Regan E, Gisondi A, Sheehan S, Zheng H, Zhu AX. A phase II trial of cabozantinib (XL-184) in patients with advanced cholangiocarcinoma. J Clin Oncol. 2015;33(suppl 3) abstr 800. [Google Scholar]
- 56.Voss JS, Holtegaard LM, Kerr SE, Fritcher EG, Roberts LR, Gores GJ, Zhang J, Highsmith WE, Halling KC, Kipp BR. Molecular profiling of cholangiocarcinoma shows potential for targeted therapy treatment. Hum Pathol. 2013;44:1216–1222. doi: 10.1016/j.humpath.2012.11.006. [DOI] [PubMed] [Google Scholar]
- 57.Grassian AR, Pagliarini R, Chiang DY. Mutations of isocitrate dehydrogenase 1 and 2 in intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2014;30:295–302. doi: 10.1097/MOG.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 58.Aruga A, Takeshita N, Kotera Y, Okuyama R, Matsushita N, Ohta T, Takeda K, Yamamoto M. Phase I clinical trial of multiple-peptide vaccination for patients with advanced biliary tract cancer. J Transl Med. 2014;12:61. doi: 10.1186/1479-5876-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sabbatino F, Villani V, Yearley JH, Deshpande V, Cai L, Konstantinidis IT, Moon C, Nota S, Wang Y, Al-Sukaini A, Zhu AX, Goyal L, Ting DT, Bardeesy N, Hong TS, Fernandez-del Castillo C, Tanabe KK, Lillemoe KD, Ferrone S, Ferrone CR. PD-L1 and HLA class I antigen expression and clinical course of the disease in intrahepatic cholangiocarcinoma. Clin Cancer Res. 2016;22:470–478. doi: 10.1158/1078-0432.CCR-15-0715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gani F, Nagarajan N, Kim Y, Zhu Q, Luan L, Bhaijjee F, Anders RA, Pawlik TM. Program death 1 immune checkpoint and tumor microenvironment: implications for patients with intrahepatic cholangiocarcinoma. Ann Surg Oncol. 2016;23:2610–2617. doi: 10.1245/s10434-016-5101-y. [DOI] [PubMed] [Google Scholar]