Abstract
Background
The natural course of traditionally prognostically unfavorable human epidermal growth factor receptor 2 (HER2)-positive breast cancer has been changed by anti-HER2 therapy. It is not clear whether the prognosis for HER2-positive patients treated with adjuvant trastuzumab differs from that of HER2-negative patients.
Methods
We performed a retrospective study including patients with lymph node-negative invasive breast cancer treated at our institution in the period 2000-2009. Disease-free (DFS) and overall survival (OS) were calculated using the Kaplan-Meier method. The Cox proportional hazards model was applied to control for other clinically important variables.
Results
Median follow-up was 90-109 months. The 5-year DFS rates for HER2-negative patients, HER2-positive patients without adjuvant trastuzumab and trastuzumab-treated HER2-positive patients were 88.1% (95% confidence interval (CI) 85.6-90.6%), 73.1% (95% CI 64.3-81.9%) and 90.7% (95% CI 83.1-98.3%), respectively. No significant difference in DFS was observed between trastuzumab-treated HER2-positive patients and HER2-negative patients in multivariate analysis (hazard ratio 1.15; 95% CI 0.53-2.46; p = 0.728). There were no differences in OS among the 3 groups.
Conclusion
Based on our results, the negative prognostic effect of HER2 positivity seen before targeted anti-HER2 treatment has completely disappeared in the era of routine trastuzumab administration in the adjuvant setting.
Keywords: Breast cancer, HER2 status, Adjuvant therapy, Trastuzumab, Prognosis
Introduction
Human epidermal growth factor receptor 2 (HER2) is a transmembrane glycoprotein with intrinsic tyrosine kinase activity that mediates cell growth, differentiation, and survival. The HER2/neu proto-oncogene is amplified in about 20-30% of human breast cancers [1,2]. HER2/neu amplification or HER2 protein overexpression have been consistently found to adversely influence disease-free (DFS) and overall survival (OS) in breast cancer patients [1,2,3,4,5].
Trastuzumab, a humanized monoclonal antibody against the extracellular domain of the HER2 protein, was shown to carry an important survival benefit in combination with chemotherapy versus chemotherapy alone when used as front-line therapy in metastatic, HER2-positive breast cancer patients [6]. Subsequently, several randomized clinical trials indicated improved outcomes in HER2-positive patients treated with the combination of chemotherapy and trastuzumab in the adjuvant setting [7,8,9].
This evidence resulted in the routine use of trastuzumab in metastatic breast cancer and in the adjuvant treatment, which has significantly changed the natural course of HER2-positive disease. In the metastatic setting, HER2-positive patients treated with first-line trastuzumab have even been shown to have better OS rates than HER2-negative patients [10,11]. However, as yet, it has not been clearly established whether the prognosis for HER2-positive patients receiving adjuvant trastuzumab differs to that for HER2-negative patients.
To address this issue, we performed a retrospective study in node-negative breast cancer patients comparing the survival of HER2-negative patients to that of HER2-positive patients who did or did not receive adjuvant trastuzumab treatment.
Patients and Methods
Patients with lymph node-negative invasive breast cancer without distant metastases who had been surgically treated in University Medical Centre Maribor (Slovenia) between 1 January 2000 and 31 December 2009 and for whom HER2 status was known were included in the study. Exclusion criteria were systemic treatment before primary surgery and more than 1 primary cancer. The study was approved by Slovenian National Medical Ethics Committee (Approval No. 55/11/13).
Information on patient diagnosis, treatment and follow-up was obtained from medical records. HER2 status was determined immunohistochemically and additional fluorescent in situ hybridization (FISH) with PathVysion™ HER-2 DNA Probe Kit (Abbott Molecular, Abbott Park, IL, USA) was performed in samples with an immunohistochemical result of 2+. Tumors classified as HER2 positive were those with 3+ staining in immunohistochemistry and those with unequivocal HER2 gene amplification by FISH. Detailed histological information, including HER2 immunohistochemistry, was obtained from original histology reports from the primary surgery. The results of HER2 gene amplification analysis were obtained from our institution's Medical Genetics Laboratory.
The primary surgery was either modified radical mastectomy or breast-conserving surgery, the latter always followed by radiotherapy. Adjuvant systemic treatment was given according to the guidelines followed at our institution at the time. Patients who completed primary treatment were followed-up at our institution at regular intervals.
To analyze the prognostic impact of HER2 status and adjuvant trastuzumab treatment, the patients were divided into 3 groups: HER2 negative, HER2 positive who did not receive adjuvant trastuzumab treatment, and HER2 positive who received adjuvant trastuzumab treatment for 12 months. DFS was calculated from the date of primary surgery until the date of disease recurrence or death from any cause, or the date of the last follow-up visit in case of no recurrence or death. OS was calculated from the date of diagnosis until the date of death from any cause, or the date of the last follow-up visit. The Kaplan-Meier method was used to calculate survival curves and Log-rank test was used to assess the differences between the curves in univariate analysis. Multivariate analyses were performed by applying the multivariate Cox proportional hazards model. All clinically important variables were included in the Cox model. All tests were performed at a significance level of p = 0.05 and a confidence interval (CI) of 95%. All p values were 2-sided. Statistical analysis was performed using the SPSS software package v. 21 (IBM, Armonk, NY, USA).
Results
Patient Characteristics
The final analysis included 761 node-negative breast cancer patients who met the inclusion criteria. 80.2% of the patients had HER2-negative disease, 12.7% were HER2 positive and did not receive adjuvant trastuzumab treatment and 7.1% were HER2 positive and received adjuvant trastuzumab treatment. Patient, tumor, and treatment characteristics for the 3 study groups are presented in table 1. The median age of the patients who received adjuvant trastuzumab (49.5 years; range 34-74 years) was significantly lower than that of the HER2-positive patients who did not receive adjuvant trastuzumab treatment (64 years; range 31-87 years) and HER2-negative patients (62 years; range 24-95 years). The median follow-up time for the 3 groups ranged from 90 to 109 months. 91.9% of all patients received adjuvant systemic therapy. Of these, 126 received adjuvant chemotherapy, 458 adjuvant hormone therapy, and 115 a combination of both. In the trastuzumab-treated group, adjuvant chemotherapy was given in 94.4% of the patients. The reasons for 3 patients not receiving adjuvant chemotherapy were participation in the MINDACT trial with allocation to the ‘no chemotherapy’ arm, very small highly hormone-dependent tumor with no other adverse prognostic features, and refusal of chemotherapy treatment, respectively.
Table 1.
Patient characteristics according to HER2 status and adjuvant trastuzumab treatment (n = 761)a
| HER2− patients | HER2+ patients – trastuzumab | HER2+ patients + trastuzumab | |
|---|---|---|---|
| Total, n | 610 | 97 | 54 |
| Median year of diagnosis, (range) | 2005 (2000–2009) | 2005 (2000–2009) | 2006 (2001–2009) |
| Age at diagnosis, n (%) | |||
| ≥ 40 years | 589 (96.6) | 93 (95.9) | 49 (90.7) |
| < 40 years | 21 (3.4) | 4 (4.1) | 5 (9.3) |
| Pathological tumor size, n (%) | |||
| < 2 cm | 361 (59.9) | 45 (46.4) | 20 (37.0) |
| ≥ 2 cm | 242 (40.1) | 52 (53.6) | 34 (63.0) |
| Pathological tumor type, n (%) | |||
| Ductal invasive | 507 (83.1) | 86 (88.7) | 50 (92.6) |
| Other invasive | 103 (16.9) | 11 (11.3) | 4 (7.4) |
| Histological grade, n (%) | |||
| G1–2 | 419 (73.9) | 47 (50.5) | 21 (40.4) |
| G3 | 148 (26.1) | 46 (49.5) | 31 (59.6) |
| Lymphovascular invasion, n (%) | |||
| No | 517 (92.0) | 82 (89.1) | 43 (79.6) |
| Yes | 45 (8.0) | 10 (10.9) | 11 (20.4) |
| HR status, n (%) | |||
| Positive | 503 (82.5) | 70 (72.2) | 27 (50.0) |
| Negative | 107 (17.5) | 27 (27.8) | 27 (50.0) |
| Surgery type, n (%) | |||
| Breast conserving | 459 (75.2) | 61 (62.9) | 31 (57.4) |
| Mastectomy | 151 (24.8) | 36 (37.1) | 23 (42.6) |
| Adjuvant chemotherapy, n (%) | |||
| No | 456 (74.8) | 61 (62.9) | 3 (5.6) |
| Yes | 154 (25.2) | 36 (37.1) | 51 (94.4) |
−/+ trastuzumab = without/with adjuvant trastuzumab treatment, HR = hormone receptor.
Some factors could not be assessed in all tumors.
Disease-Free Survival
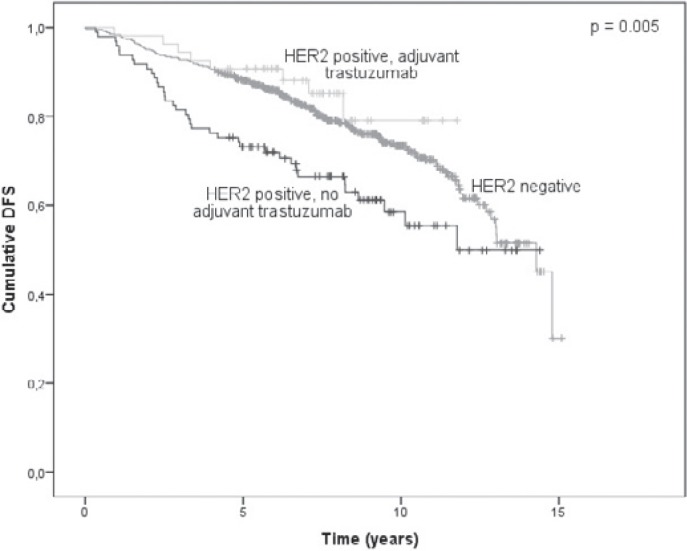
The DFS analysis included 199 events (52 cases of local recurrence, 49 cases of distant organ metastasis, 17 cases of synchronous local and distant recurrence, 3 cases of unspecified recurrence and 78 deaths without evidence of disease recurrence). Of these, 154 occurred in the HER2-negative patients, 37 in the HER2-positive patients who did not receive adjuvant trastuzumab and 8 in the HER2-positive patients who received adjuvant trastuzumab treatment. The 5-year DFS rates in these 3 groups of patients were 88.1%, 73.1%, and 90.7%, respectively. The DFS plots for the 3 groups are shown in figure 1. The difference among all 3 groups was statistically significant (p = 0.005). However, pairwise comparisons revealed statistically significant differences only between HER2-negative and HER2-positive patients without adjuvant trastuzumab treatment (p = 0.003) and between the latter and the patients who received adjuvant trastuzumab (p = 0.010), but not between trastuzumab-treated HER2-positive patients and HER2-negative patients (p = 0.361). The 2- and 5-year DFS rates in 5 subgroups based on HER2 status, anti-HER2 treatment and hormone receptor (HR) status are presented in table 2. After adjusting for year of diagnosis, patient age, tumor size, tumor type, histological grade, lymphovascular invasion, and HR status, HER2-positive patients who did not receive adjuvant trastuzumab treatment had a significantly increased hazard of disease recurrence or death compared with HER2-negative patients (hazard ratio 1.54; 95% CI 1.04-2.28; p = 0.031). No significant difference was observed in trastuzumab-treated HER2-positive patients compared to HER2-negative patients (hazard ratio 1.15; 95% CI 0.53-2.46; p = 0.728; table 3).
Fig. 1.
Kaplan-Meier plot of disease-free survival (DFS) probability according to HER2 status and adjuvant trastuzumab treatment (n = 761).
Table 2.
Disease-free survival (DFS) and overall survival (OS) estimates at 2 and 5 years
| HER2− patients |
HER2+, HR+ patients – trastuzumab |
HER2+, HR− patients trastuzumab |
HER2+, HR+ patients + trastuzumab |
HER2+, HR− patients + trastuzumab |
p* | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | ||
| DFS | 0.002 | ||||||||||
| 2-year | 95.1 | 93.3–96.9 | 92.9 | 86.6–99.0 | 85.2 | 71.9–98.5 | 100 | 92.9–100 | 96.3 | 89.2–100 | |
| 5-year | 88.1 | 85.6–90.6 | 79.9 | 70.5–89.3 | 55.6 | 36.8–74.4 | 88.9 | 77.1–100 | 92.6 | 82.8–100 | |
| OS | 0.069 | ||||||||||
| 2-year | 98.0 | 96.8–99.2 | 95.7 | 91.0–100 | 92.6 | 82.8–100 | 100 | 90.2–100 | 100 | 92.9–100 | |
| 5-year | 92.7 | 90.5–94.9 | 90.0 | 82.9–97.1 | 85.2 | 71.9–98.5 | 92.6 | 82.8–100 | 96.3 | 89.2–100 | |
HR = hormone receptor, −/+ trastuzumab = without/with adjuvant trastuzumab treatment, CI = confidence interval.
Log-rank test.
Table 3.
Multivariate analysis of disease-free survival (DFS) and overall survival (OS) of node-negative breast cancer patientsa
| DFS |
OS |
|||
|---|---|---|---|---|
| hazard ratio (95% CI) | p | hazard ratio (95% CI) | p | |
| Year of diagnosis | 0.92 | 0.017 | 0.96 | 0.307 |
| (continuous, unit = 1 year) | (0.86–0.99) | (0.88–1.04) | ||
| Age | 1.85 | <0.001 | 2.12 | <0.001 |
| (continuous, unit = 10 years) | (1.60–2.13) | (1.78–2.53) | ||
| Tumor size | 1.08 | 0.618 | 1.14 | 0.477 |
| (≥2 vs. >2 cm) | (0.79–1.48) | (0.80–1.63) | ||
| Tumor type | 1.20 | 0.527 | 0.97 | 0.932 |
| (other invasive vs. ductal invasive) | (0.68–2.12) | (0.49–1.94) | ||
| Grade | 1.47 | 0.037 | 1.54 | 0.043 |
| (G3 vs. G1–2) | (1.02–2.12) | (1.01–2.33) | ||
| Lymphovascular | 1.39 | 0.161 | 1.23 | 0.461 |
| invasion (present vs. absent) | (0.88–2.21) | (0.71–2.16) | ||
| HR | 1.57 | 0.029 | 1.65 | 0.034 |
| status (negative vs. positive) | (1.05–2.35) | (1.04–2.62) | ||
| HER2 status | 0.099 | 0.785 | ||
| Positive – trastuzumab | 1.54 | 0.031 | 1.17 | 0.496 |
| vs. negative | (1.04–2.28) | (0.74–1.86) | ||
| Positive + trastuzumab | 1.15 | 0.728 | 1.12 | 0.811 |
| vs. negative | (0.53–2.46) | (0.43–2.92) | ||
HR = Hormone receptor, −/+ trastuzumab = without/with adjuvant trastuzumab treatment.
The analysis was performed in the 669 patients for whom all data were available.
Overall Survival
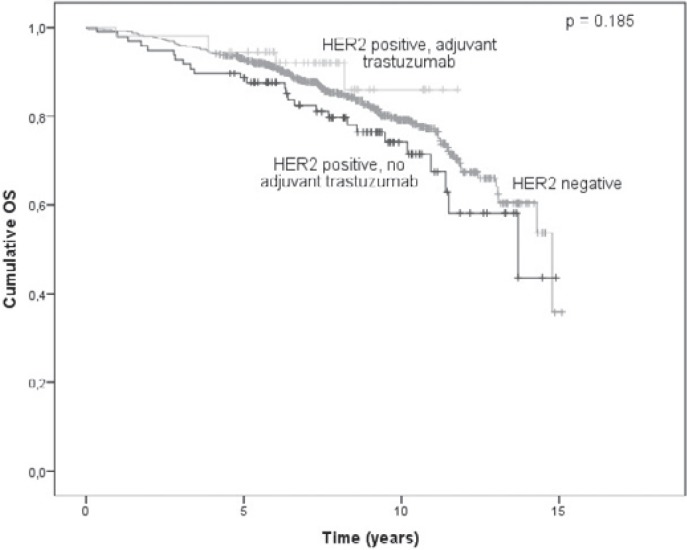
The OS analysis was based on 149 events (61 deaths from breast cancer and 88 deaths from other causes), 118 of which occurred in the HER2-negative group, 26 in the HER2-positive patients who did not receive adjuvant trastuzumab and 5 in the HER2-positive patients who received adjuvant trastuzumab treatment. The 5-year survival rates were 92.7%, 88.6%, and 94.4%, respectively, for the 3 groups. The 2- and 5-year survival rates across subgroups according to HR status are shown in table 2. The OS survival curve is shown in figure 2. The difference in OS among the groups was not statistically significant (p = 0.185), which remained the case after adjusting for other clinically important patient and tumor characteristics (table 3).
Fig. 2.
Kaplan-Meier plot of overall survival (OS) probability according to HER2 status and adjuvant trastuzumab treatment (n = 761).
Discussion
With the introduction of targeted anti-HER2 therapy, HER2 status assumed an important predictive role in addition to its traditional prognostic value in breast cancer patients. Considering the opposing direction of these 2 roles, it has become difficult to interpret the final prognostic significance of HER2 positivity in the era of routine anti-HER2 treatment.
In the metastatic setting, this question was addressed by Dawoood et al. [10] who performed a retrospective study comparing OS of HER2-negative patients with metastatic breast cancer, HER2-positive patients who received first-line trastuzumab treatment, and HER2-positive patients who did not receive first-line trastuzumab treatment. After adjusting for other clinically important variables, they found that trastuzumab-treated HER2-positive patients had a reduced risk of death compared with HER2-negative breast cancer patients (hazard ratio 0.56; 95% CI 0.45-0.69). However, this difference persisted only for the first 2 years after diagnosis of metastatic breast cancer and disappeared afterwards. No patients in this study received trastuzumab in the adjuvant setting.
In a small prospective phase II study, Papaldo et al. [11] compared the overall response rate, time to progression, and OS in metastatic breast cancer patients divided in 2 treatment arms according to HER2 status: HER2-negative patients received weekly vinorelbine, and HER2-positive patients received weekly vinorelbine + trastuzumab. They reported a statistically insignificant trend towards better results in the HER2-positive group for all the observed parameters, with a 5-month difference in median OS (27 months in the vinorelbine + trastuzumab group vs. 22 months in the vinorelbine alone group, p = 0.06).
An open-label phase III trial [12] in HER2-positive patients with newly diagnosed locally advanced or inflammatory breast cancer who were randomized to receive either neoadjuvant trastuzumab plus chemotherapy or neoadjuvant chemotherapy alone, both of which were followed by adjuvant trastuzumab, prospectively included a parallel observational cohort of HER2-negative patients receiving the same neoadjuvant chemotherapy regimen. The survival curves for HER2-negative and combined HER2-positive patients separated after approximately 18 months, HER2-negative patients presenting superior event-free survival. However, hazard ratio and p value could not be calculated because patients were not randomly assigned, and separate survival curves for HER2-negative and HER2-positive patients according to the treatment arm were not reported. A longer follow-up of the same patients [13] revealed 2% higher 5-year event-free, overall, relapse-free, and breast cancer-specific survival rates in HER2-negative patients compared to HER2-positive patients treated with neoadjuvant trastuzumab, but the statistical significance of this difference was not calculated.
In the adjuvant setting, our results show a clear negative prognostic effect of HER2 positivity on DFS, seen in patients who were not treated with adjuvant trastuzumab compared with HER2-negative patients, which persisted even after adjusting for other clinically important cofactors. However, the negative prognostic value of HER2 positivity completely disappeared in patients who received adjuvant trastuzumab treatment whose prognosis was undistinguishable from HER2-negative patients in our series. This is particularly remarkable because patients in the adjuvant trastuzumab group were significantly younger and had more aggressive tumors than HER2-negative patients. On the other hand, no impact of HER2 status or anti-HER2 treatment on OS was seen in our patients. The only determinants of longer OS in multivariate analysis were younger age, positive HR status, and lower tumor grade.
Whereas poor prognosis of untreated HER2-positive patients was expected and in line with numerous other studies [1,2,3,4,5], we have failed to show a prognostic benefit in trastuzumab-treated HER2-positive patients over HER2-negative patients like that reported in the metastatic setting [10,11]. Instead, both groups seemed prognostically equal in our series. A possible explanation for this could be that effective treatment is more important than adverse prognostic features for prolonging survival in metastatic disease where some tumor burden is inevitably present, while this might not be the case in the adjuvant setting. On the other hand, the lack of prognostic superiority of trastuzumab-treated HER2-positive patients in our study might also be associated with the small number of patients and other adverse prognostic features more commonly observed in this group. Larger, prospective studies are needed in order to confirm our findings and further establish the relationship between anti-HER2-treated HER2-positive and HER2-negative breast cancer patients.
Our study has several limitations due to its retrospective nature. First, adjuvant trastuzumab treatment has been implemented in the clinical practice at our institution gradually and for a period of time the choice of patients to whom it was offered was made by a multidisciplinary team. Consequently, the patients in the trastuzumab-treated group were somewhat younger and more often had tumors with other adverse prognostic features. This could imply a bias towards worse prognosis in this study group, but our multivariate model included all the other clinically important prognostic factors and attempted to minimize this risk. Secondly, the number of HER2-positive and especially trastuzumab-treated patients was small compared to the overall study population. This contributed to the low number of events, which was already limited in spite of the long follow-up because of the adjuvant setting and the selection of low-risk node-negative patients. The OS analysis must, therefore, be interpreted with caution. Further obscuring the OS analysis is the fact that many patients received trastuzumab after disease recurrence. Nevertheless, our study is to our knowledge the first to assess the prognostic significance of HER2 status according to anti-HER2 treatment in the adjuvant setting, and compares 3 groups that cannot be compared in a prospective fashion.
In conclusion, our results show that anti-HER2 treatment has indeed changed the natural course of breast cancer, even in the adjuvant setting in node-negative patients. HER2-positive patients who did not receive adjuvant trastuzumab had significantly worse DFS than HER2-negative patients. However, this negative prognostic effect of HER2 positivity was not seen in HER2-positive patients who received adjuvant trastuzumab. In our series, survival of the latter was no different than that of HER2-negative patients.
Disclosure Statement
The authors declare that no conflict of interest exists.
Acknowledgements
The authors would like to thank prof. Janez Stare, PhD, from the Institute for Biostatistics and Medical Informatics, Faculty of Medicine, University of Ljubljana, for advice regarding statistical methods.
References
- 1.Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: Correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 2.Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: Ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist. 2009;14:320–368. doi: 10.1634/theoncologist.2008-0230. [DOI] [PubMed] [Google Scholar]
- 3.Seshadri R, Firgaira FA, Horsfall DJ, et al. Clinical significance of HER-2/neu oncogene amplification in primary breast cancer. The South Australian Breast Cancer Study Group. J Clin Oncol. 1993;11:1936–1942. doi: 10.1200/JCO.1993.11.10.1936. [DOI] [PubMed] [Google Scholar]
- 4.Andrulis IL, Bull SB, Blackstein ME, et al. neu/erbB-2 amplification identifies a poor-prognosis group of women with node-negative breast cancer. Toronto Breast Cancer Study Group. J Clin Oncol. 1998;16:1340–1349. doi: 10.1200/JCO.1998.16.4.1340. [DOI] [PubMed] [Google Scholar]
- 5.Carr JA, Havstad S, Zarbo RJ, et al. The association of HER-2/neu amplification with breast cancer recurrence. Arch Surg. 2000;135:1469–1474. doi: 10.1001/archsurg.135.12.1469. [DOI] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 8.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 9.Joensuu H, Kellokumpu-Lehtinen PL, Bono P, et al. Adjuvant docetaxel or vinorelbine with or without trastuzumab for breast cancer. N Engl J Med. 2006;354:809–820. doi: 10.1056/NEJMoa053028. [DOI] [PubMed] [Google Scholar]
- 10.Dawood S, Broglio K, Buzdar AU, et al. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: An institutional-based review. J Clin Oncol. 2010;28:92–98. doi: 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Papaldo P, Fabi A, Ferretti G, et al. A phase II study on metastatic breast cancer patients treated with weekly vinorelbine with or without trastuzumab according to HER2 expression: Changing the natural history of HER2-positive disease. Ann Oncol. 2006;17:630–636. doi: 10.1093/annonc/mdj110. [DOI] [PubMed] [Google Scholar]
- 12.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): A randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet. 2010;375:377–384. doi: 10.1016/S0140-6736(09)61964-4. [DOI] [PubMed] [Google Scholar]
- 13.Gianni L, Eiermann W, Semiglazov V, et al. Neoadjuvant and adjuvant trastuzumab in patients with HER2-positive locally advanced breast cancer (NOAH): Follow-up of a randomised controlled superiority trial with a parallel HER2-negative cohort. Lancet Oncol. 2014;15:640–647. doi: 10.1016/S1470-2045(14)70080-4. [DOI] [PubMed] [Google Scholar]